Abstract
Autologous biologics, defined as platelet-rich plasma (PRP) and bone marrow aspirate concentrate (BMC), are cell-based therapy treatment options in regenerative medicine practices, and have been increasingly used in orthopedics, sports medicine, and spinal disorders. These biological products are produced at point-of-care; thereby, avoiding expensive and cumbersome culturing and expansion techniques.
Numerous commercial PRP and BMC systems are available but reports and knowledge of bio-cellular formulations produced by these systems are limited. This limited information hinders evaluating clinical and research outcomes and thus making conclusions about their biological effectiveness. Some of their important cellular and protein properties have not been characterized, which is critical for understanding the mechanisms of actions involved in tissue regenerative processes. The presence and role of red blood cells (RBCs) in any biologic has not been addressed extensively. Furthermore, some of the pathophysiological effects and phenomena related to RBCs have not been studied. A lack of a complete understanding of all of the biological components and their functional consequences hampers the development of clinical standards for any biological preparation.
This paper aims to review the clinical implications and pathophysiological effects of RBCs in PRP and BMC; emphasizes hemolysis, eryptosis, and the release of macrophage inhibitory factor; and explains several effects on the microenvironment, such as inflammation, oxidative stress, vasoconstriction, and impaired cell metabolism.
Keywords: Platelet-rich plasma, Bone marrow mesenchymal cells, Plasma free hemoglobin, Eryptosis, Macrophage migration inhibitor factor, Oxidative stress, Inflammation
Abbreviations: BM-MSCs, bone marrow-mesenchymal cells; BMA, bone marrow aspiration; BMC, bone marrow concentrate; Hb, hemoglobin; Hp, haptoglobin; HSCs, hematopoietic stem cells; Hx, hemopexin; MIF, Macrophage migration inhibitory factor; MNCs, mononucleated cells; NO, nitric oxide; OA, osteoarthritis; PAF, platelet activating factor; PFH, plasma free hemoglobin; PRP, platelet-rich plasma; PS, phosphatidylserine; RBC, red blood cell; ROS, reactive oxygen species
Highlights
-
•
Different biological formulations optimize disease specific regenerative treatment protocols.
-
•
Disintegrated RBC's release harmful components to regenerative therapy treatment vials.
-
•
The effectiveness of MSC injection depends on the quality of the bone marrow aspiration procedure.
-
•
PRP and BMC should contain minimal to no erythrocytes.
1. Introduction
Regenerative medicine methods, in particular orthobiologic injections, offer solutions to a number of compelling clinical problems such as tendinopathies and degenerative arthritis which have previously had limited response to medications, rehabilitation, surgery, or joint replacement surgery. Recently, biological therapies have emerged as promising treatment options for many musculoskeletal disorders affecting young adults and the elderly [1]. Autologous biologics prepared at point-of-care, such as platelet-rich plasma (PRP) and bone marrow concentrate (BMC), have become important autologous biological therapeutics in health care strategies for enhanced tissue repair, regenerative processes, and immunomodulation [2], [3].
Within orthobiology, biological therapies utilizing autologous PRP and BMC frequently include the following clinical problem: osteoarthritis (OA), tendon repair, focal chondral lesions, and soft tissue (meniscus, ligaments) repair [4]. In addition, there is early promise in the treatment of nerve conditions and injury [5].
PRP therapies and several related treatment protocols have evolved immensely over the past 20 years. Through laboratory, experimental, and clinical research, followed by meta-analyses, physicians, medical practitioners, and scientists have gained a better understanding of how PRP affects cellular physiology. Notably, they have gained further insight into the functions of some specific biological components in the platelet proteome that affect PRP-treatment outcomes when used to treat various musculoskeletal pathologies [6]. The biological rationale for the clinical use of PRP includes the local delivery of the intra cellular platelet vesicles containing growth factors, cytokines, lysosomes and chemokines [7]. Furthermore, PRP has been recognized to modify inflammatory responses and to stimulate cell proliferation and cell differentiation [8], [9]. The rationale for BMC applications is the abundant and varied bone marrow cell content, such as bone marrow-mesenchymal cells (BM-MSCs), hematopoietic-progenitor cells, platelets, white blood cells, and erythrocytes that are readily accessible and largely dispensable [10]. BM-MSCs are relatively easy to acquire by bone marrow aspiration (BMA) from a variety of anatomic sites with minimal morbidity. An effective BM-MSC injection depends on the quality of the initial BMA procedure, which should minimize trauma to the cellular content of the bone marrow niche while maximizing cellular yields and simultaneously avoiding peripheral blood infiltration [11]. Moreover, the authors believe that a BMA sample should always be followed by a 2-step centrifugation procedure to concentrate the essential cellular content of BMC above the baseline counts, according to the recommendation of Pittenger et al. [12]. BM-MSCs have been found to differentiate into mesodermal lineage cells, such as osteoblasts, endothelial cells, adipose tissue, smooth muscle cells, and multiple musculoskeletal tissue types, including chondrocytes, and tenocytes [13], [14].
These bio-cellular capabilities have led to the use of BM-MSCs as a potential strategy for treating various diseases because they promote biological processes, such as angiogenesis, cell proliferation, and differentiation [15]. Furthermore, the cellular component of BMC can synthesize mediators (cytokines and trophic factors) that participate in tissue repair processes, immune modulation, and the regulation of inflammatory processes [16], [17]. Similar to PRP, viable, autologous prepared BM-MSCs are used to treat a variety of musculoskeletal disorders, such as chondral defects, osteoarthritis, and rotator cuff lesions [18], [19].
However, the discrete characterization of PRP and BMC biological therapies are still in their infancy relative to surgical interventions and pharmaceuticals. One reason for this immaturity may be the lack of standardization of PRP and BMC final product characterization, e.g. the number of the cellular components within the final product delivered to a specific patient [20]. This lack of regulatory standards for clinical practice and the limited consensus on specific formulation characteristics of PRP and BMC products likely contribute to inconsistent patient outcomes, as reported in the literature [21], [22].
RBCs can be damaged as a result of several immune-mediated processes and high shear forces during blood collection for PRP preparation and bone marrow aspiration, or inadequate centrifugation and concentration protocols. As a consequence, the RBC cell membrane will start to disintegrate and hemolysis, with the release of plasma free hemoglobin (PFH) will occur and is characterized by the release of hemoglobin (Hb) and hemin and iron from lysed RBCs [23]. The disintegration of RBC's lead to the development and release of toxic Hb forms, capable of inducing oxidative stress and pro-inflammatory PFH reactions in plasma and tissues. These pathophysiological conditions have the potential of inducing RBC suicidal cell death, eryptosis. During eryptosis, platelet activating factor (PAF) is released from RBCs, exposing phosphatidylserine to the cell surface, affecting in particular RBC-endothelial interaction. This phenomenon is known to contribute to vascular damage or microcirculatory blood flow irregularities [24]. Another significant consequence of PFH is the release of macrophage migration inhibitory factor (MIF), since RBCs contain large concentrations of this enzymatically and chemotactically active cytokine [25]. MIF is identified as a very potent inflammatory cytokine.
The presence of PFH and RBCs in biological treatment vials is of particular concern when these split products cannot be cleared by natural scavenger proteins and PRP and BMC are applicated in a microenvironment outside of the blood stream.
There is currently almost no research on both the role and consequences of erythrocytes-red blood cells (RBCs) within a final BMC product. Additionally, the concept of bone marrow aspiration (BMA) in autologous prepared biological products has gained minimal to no attention in the clinical and scientific community. This may be related to the fact that the pathophysiological effects of damaged RBCs, PFH and its detrimental mechanisms to tissues have not been widely noted in scientific reports. Therefore, the aim of this article is to elucidate and review the consequences of (damaged) RBCs in biological-based PRP and BMC products when used in musculoskeletal regenerative medicine treatments. This article will focus on RBC disintegration, the release of macrophage inhibitory factor, and PFH influence pro-inflammation, oxidative stress, reactive oxygen species (ROS), cellular dysfunction, eryptosis, and apoptosis.
2. Formulations and bio-cellular components of PRP and BMC
Any PRP or BMC preparation begins by meticulously acquiring a fresh unit of whole blood or bone marrow, respectively, using standard operating procedures according to the specific system used. The next step in the preparation of a biologic is the use of specific, disposable, concentrating device and a dedicated centrifuge system. However, the functional design and methods of PRP and BMC processing systems varies tremendously, and the majority of these systems do not permit full manual control or adjustments to control the production of specific bio-cellular formulations.
2.1. PRP composition
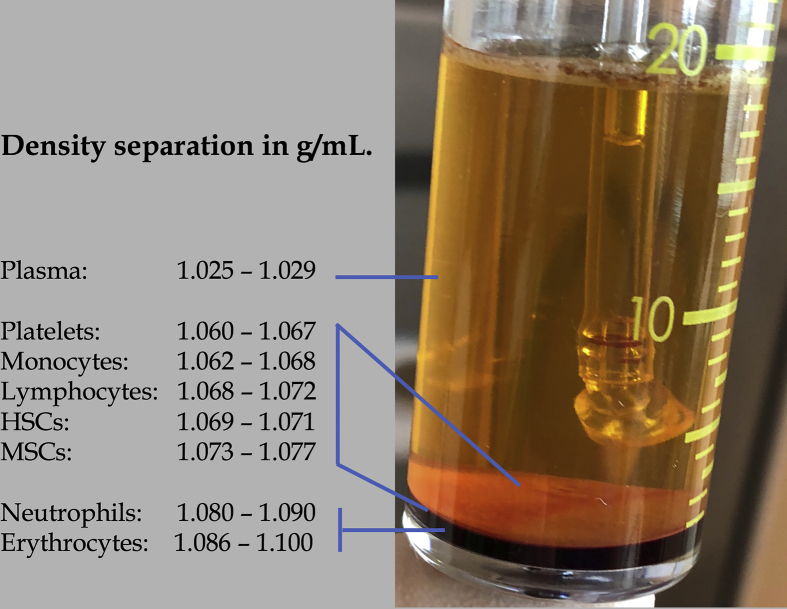
Currently, physicians can choose from more than 30 PRP-processing systems. Therefore, a lack of consensus on standardizing PRP has contributed to the large variation in PRP products. Consequently, different devices produce dissimilar platelet concentrations and cellular compositions [26], [27]. However, optimal whole blood separation is best accomplished by double-spin PRP devices and centrifuges that create a layered buffy coat stratum due to the use of different centrifugal forces and the varying specific gravities and densities of the individual blood components (Fig. 1) [28].
Fig. 1.
Cellular whole blood density separation following the first centrifugation procedure with the EmCyte PurePRP®SP. The whole blood cellular components (indicated by the red lines) are separated in the PurePRP®SP concentrating device as a result of the different cell densities in two basic layers [81]. The top layer is the platelet plasma suspension, consisting of plasma and the multicomponent buffy coat layer, containing platelets, monocytes, lymphocytes, and neutrophils. The second basic layer consists of the erythrocyte cellular pack. The range of the specific cell densities varies between individuals. After a second centrifugation procedure approximately 7 mL of PurePRP is aspirated from the bottom chamber to be used for regenerative therapies. (PurePRP®SP: Pure Platelet-Rich Plasma Supra-Physiologic).
Specific centrifugation design protocols are critical in avoiding agitation, resuspension, and shifting of cellular layers during deceleration. PRP can be characterized as a small volume of plasma with a complex composition of multi-cellular components comprised of platelets and different leukocytes. To induce a functional angiogenic response that stimulates tissue repair mechanisms, via endothelial cell activity. A final PRP product should contain a substantial supraphysiologic concentration of platelets above baseline, greater than 1.5 × 109 platelets/mL [29]. Cavallo et al. reported on the effects of leukocytes. PRP containing fewer leukocytes induced greater cell growth by stimulating chondrocyte anabolism, whereas leukocyte rich PRP stimulated catabolic pathways [30]. In contrast, others demonstrated that leukocyte rich PRP treatment of tendinopathy induced early increased cellularity and new vasculature in an acute inflammatory environment [31]. These studies indicate that different PRP formulations, with varying concentrations of pro-inflammatory leukocytes, are warranted to optimize disease specific treatment protocols. Of note, none of the published PRP classification systems has addressed the presence RBCs in PRP vials [20], [21], [22], [32], [33]. The lack of scientific inquiry into the potential role of RBCs in PRP and its consequences for tissue regeneration or tissue healing is even more concerning. Furthermore, the consequences of damaged RBCs, including the release of RBC content due to inferior whole blood collection or processing, have not yet been reported.
2.2. BMA-BMC composition
The composition and structure of the bone marrow is dynamic, containing a mixture of cellular and non-cellular components (connective tissue), small tissue fragments, and venous blood. Compositional differences in cellularity may occur between individuals [34]. The marrow stroma consists of a heterogeneous population of cells (e.g. fibroblasts, adipocytes, osteoblasts, osteoclasts, macrophages, and endothelial cells) providing a microenvironment for bone marrow stem -and progenitor cells.
According to the authors, similar to blood sampling procedures for PRP preparations, bone marrow harvesting is a very subtle procedure. BMA devices should contain minimally invasive instrumentation to collect a selective bone marrow aspirate containing a high fraction of progenitor stem cells. During device deployment and BMA collection the trabecular bone should be gently penetrated, maintaining a quiescent tissue environment. This technique should lead to reduced tissue activation, incidence of clotting, less peripheral blood contamination, and less RBC destruction, while increasing stem cell yields.
Bone marrow stem cells can be categorized as hematopoietic stem cells (HSCs) and MSCs. HSCs are pluripotent cells that further differentiate into distinctive progenitor cells that mature into blood cells of myeloid (monocyte, granulocyte, megakaryocyte/platelets, and erythrocyte) lineages and lymphoid cells (B, T, and NK cells) through hematopoiesis [35]. MSCs are multipotent adult stem cells that provide a niche for HSCs and, due to their plasticity, have the ability to differentiate into various mesodermal lineages. Such multipotency has the potential to play a valuable therapeutic role in the repair and reconstruction of multiple tissues in musculoskeletal disorders. Furthermore, MSCs have diverse immunomodulatory properties, leading to an expanded interest in their use for various therapeutic applications [36]. Aside from MSCs, human bone marrow contains mononucleated cells (MNCs) with subpopulations of various types of progenitor cells, including endothelial progenitor cells, which are thought to play an important role in angiogenesis. Furthermore, platelets, and erythrocytes are also present in BMA samples [37].
The objective of concentrating BMA to BMC is to recover the MNCs layer from the bone marrow by density gradient centrifugation (Fig. 2). This is of particular importance because the percentage of MSCs in BMA varies from 0.001 to 0.01% of nucleated cells [38]. Through centrifugation, the bone marrow cell concentrations can be increased 5–8 fold, depending on the quality of the BMA sample and the centrifugation preparation protocol used. The MSCs contained within BMC will potentially provide a direct cellular source for tissue repair and regeneration of the host tissue. In addition, the nucleated cells may have a paracrine effect by delivering various growth factors, cytokines, and chemokines into tissue to orchestrate and direct repair mechanisms [39]. Additionally, concentrated platelets play a key role in BMC, because their paracrine effects are mediated through the secretion of various platelet-derived growth factors and cytokines, such as vascular endothelial growth factor, interleukin-6, platelet-derived growth factor-AB, fibroblast growth factor, stromal cell derived factor-1, and insulin-like growth factor. The combination of MNC and platelets in BMC provide conditions permitting more rapid and effective tissue regenerative potential by MSCs [40]. Critically, when concentrating BMA, the recovery of non-nucleated cells, in particular RBCs, should be significantly reduced.
Fig. 2.
Bone marrow concentrate density separation following EmCyte Aspire ™ BMA harvesting and PureBMC® second spin centrifugation. Anticoagulated aspirated bone marrow was initially injected in the concentration device for the first spin cycle. After the second centrifugation procedure the separation of bone marrow components, according to their different density gradients, follows in the concentrating accessory device. The HSC’s and MSC’s are located on top of the erythrocyte and white blood cell layer [82] and are extracted via the aspirating pipe. (BMA: bone marrow aspirate; PureBMC®: Pure Bone Marrow Concentrate; HSC: hematopoietic stem cell; MSC: mesenchymal stem cell).
Studies have addressed the deleterious effects of RBCs on joints, in particular chondrocyte apoptosis, long term inhibition of proteoglycan synthesis, and damage to cartilage [41], [42]. In addition, it has been shown that hemoglobin in joints stimulated the expression of ADAMTS-5 and -9 by synovial cells, possibly causing cartilage damage [43].
Notably, Dawson and co-workers concluded that RBCs had a negative impact on colony forming unit-fibroblastic growth, indicating that the benefits of using MSCs in regenerative therapies might be limited [44].
3. Hematological composition of prepared biologics
Based on differences in clinical outcomes following biological therapies, we conclude that a full understanding is needed of the true composition of any biologic that is being used in regenerative medicine treatments. With regard to PRP standardization, Chahla et al. reported that preparation protocols were highly inconsistent. In addition, the majority of studies did not describe the complete PRP composition or formulation, thereby hindering the ability to understand the ultimate clinical effects of PRP applications. Sometimes reports about PRP include final platelet and leukocyte concentrations, whereas information about erythrocyte counts and PFH are not provided [45].
Murray and colleagues reported on standards in clinical studies in which BMC was used. In their review, they found that all existing clinical reports that evaluated BMC for orthopedic, musculoskeletal, or sports medicine applications were limited by inadequate reporting of preparation protocols. Remarkably, no studies reported the complete bio-cellular composition of the BMC preparations. Furthermore, they concluded that there are profound deficiencies in reporting the BMC cell characteristics; the total number of cells delivered was reported in only 8% of all reviewed studies [20]. Furthermore, no attention has been given to the consequences of PFH on the microenvironment of treated tissues. We suggest that these deficiencies in reporting hematological variables in autologous biologics may critically influence patient outcomes. Therefore, describing the entire composition and bio-cellular activities of PRP and BMC preparations is of paramount importance because this information might indicate the need to make meaningful protocol adjustments to the preparation of these biologics. To our knowledge, this is the first paper that provides an in-depth analysis of the consequences of PFH and the effect of RBCs on tissues.
3.1. Hematological characteristics of erythrocytes
Erythrocytes develop from HSCs in the red bone marrow in response to erythropoietin, which is produced by the kidneys. A few days after they have entered the circulation, they become mature erythrocytes. During the final maturation process, the nucleus and mitochondria are replaced to maximize the space for hemoglobin [46]. RBCs represent the primary cells in the human circulatory system. Their plasma membrane consists of a specific composition and structure that is highly correlated with their biological functions. It contains a phospholipid bilayer and a membrane skeleton [47]. The membrane components provide RBCs with elasticity, flexibility, and deformability, very important characteristics that maintain RBCs structural integrity and protect them from stress and forces during their passage through microcirculatory capillaries [48]. The cytoskeleton is important because it maintains cellular components, particularly hemoglobin, which is the major protein within RBCs cell membrane. RBCs are responsible for the distribution of oxygen to tissues and for transportation of carbon dioxide to the lungs. Iron and heme moieties inside the RBCs facilitate the binding of oxygen and carbon dioxide and the delivery of oxygen to tissues [49].
RBCs circulate in the body for approximately 120 days before they are removed from the circulatory system by the process of senescence; they are recycled by macrophages. However, under certain conditions, erythrocytes undergo another form of cell death, eryptosis, before reaching their full lifespan. This type of cell death may be caused by an injury to the erythrocyte and may be triggered by a wide variety of factors, such as hemolysis, hyperosmolarity, and oxidative stress. The process of eryptosis is discussed below in more detail [50].
4. Hemolysis and plasma free hemoglobin
Intra-vascular and extra-vascular hemolysis, which releases PFH, is a pathological condition, characterized by the release of Hb and heme from disintegrated RBCs. Several human disease and pathological conditions of with varying etiology are associated with hemolysis, such as paroxysmal nocturnal hemoglobinuria, sickle-cell disease, and thalassemia's [51], [52]. Hemolysis can occur due to a variety of immune-mediated processes. In addition, hemolysis is associated with surgical procedures, hemodialysis, blood transfusion, and other therapies in which mechanical forces can produce red blood cell rupture [53], [54]. In Table 1, a summary of probable causes for hemolysis is presented. In their report, Schaer et al. describe in detail hemoglobin's toxic effects and the body's natural scavenger mechanisms involved in hemolysis [23]. During hemolysis, the RBC cell membranes rupture and free Hb, and its degradation products, heme and iron, are released into the blood stream. The release of Hb from RBCs will lead to specific and significant structural and biochemical changes in the Hb molecule and has adverse clinical effects; Hb toxicity is a fact [55], [56].
Table 1.
Probable causes for the development of Red Blood Cell Hemolysis.
| In vivo | Bacteria |
| Parasites | |
| Genetic and autoimmune disorders | |
| Device | Aspiration needle lumen size |
| Needle tip and site hole design and surface area | |
| BMA centrifugation protocols with high g-forces | |
| Surrounding marrow and platelet tissue activation | |
| Device causing turbulence during aspirate collection | |
| High erythrocyte count in aspirate | |
| Physician | Incorrect aspiration technique |
| Excessive syringe suction creating high shear forces | |
| Prolonged storage | |
| Inappropriate, small, needle size | |
| Forceful transfer from syringe into the concentrating tube | |
| High viscosity of injectate |
After RBC hemolysis has occurred, tetrameric and dimeric Hb forms translocate across endothelial barriers to the subendothelial and perivascular space. Secondary mechanisms of Hb toxicity are pro-oxidative and pro-inflammatory PFH reactions in plasma and within tissues. Consequently, these reactions lead to endothelial dysfunction characterized by consumption of endothelial cell nitric oxide (NO). PFH split products reduce and deplete NO, and vasodilatation becomes jeopardized. As a result, heme favors ROS production, leading to the dysregulation of the endothelium vasodilator/vasoconstrictor balance, resulting in severe vasoconstriction and hypertension, with the potential for the development of coagulopathies [57]. Another mechanism of Hb toxicity is PFH forming stable complexes with the acute phase protein haptoglobin (Hp), which is produced and released by the liver. The Hb–Hp complexes are cleared from the circulation. However, when the Hp buffering capacity is overwhelmed, Hb undergoes an instant conversion to metHb (ferric-hemoglobin, Hb-Fe3+). This triggers the release of hemin from Hb-Fe3+, the primary product of the oxidative reactions, transferring reactive porphyrin to cell membranes or soluble plasma proteins, providing free hemin as a ligand for molecular signaling interactions, and finally lead to inflammatory and cytotoxic activities, inducing vascular injury [58], [59]. Another protein, hemopexin (Hx), also produced by the liver and released into circulation, binds to heme to protect against the consequences of the hemolytic damaging effects, especially to prevent from heme entering endothelial cells [60]. Lastly, free hemin can selectively bind to several (cell) receptors, sequence-specific DNA-binding factors, and several enzymes. The significance of this binding potential is an altered cell metabolism and gene transcription. When hemin enters cells, it is neutralized by heme-oxygenase's 1 and 2 that degrade the heme into iron and carbon monoxide, employing anti-inflammatory, antioxidant and anti-apoptotic effects [61].
In summary, when excessive PFH, hemin, and iron are released into the bloodstream and cannot be cleared by natural scavenger proteins, various hemolytic-related sequelae will follow, such as endothelial dysfunction, pro-inflammation, development of reactive oxygen species (ROS), thrombosis, vascular endothelium damage, impaired microcirculation, and organ failure, potentially resulting in morbidity and mortality (Table 2) [62], [63]. The amount of Hb molecules that can be lysed from 1 mL of blood is a 1000-fold higher than the immediate Hp plasma availability, indicating that Hb and heme loads can be massive (60).
Table 2.
Overview of potential physiological consequences following the presence of plasma free hemoglobin in a biological treatment vial.
| Radical oxygen reactions [51] Oxidative stress [51,52] Local vasoconstriction [52,55] Pro-inflammation [51,55,56,68] Impaired cell metabolism [51,55,56] Tissue damage [59] Inflammatory infiltrates [60] Ceramide release [45,63] Endothelial cell dysfunction [54,57,59] Vascular damage [63,64,65] Eryptosis [46,61] Mesenchymal stem cell dysfunction [72] Pain [73] |
Usually, Hb toxicity and PFH's deleterious mechanisms are counteracted by the body's natural occurring homeostatic protein scavengers and clearing mechanisms, provided that they are present and functional. Most importantly, these mechanisms take place in a microenvironment that entails a vascular endothelium milieu, where endothelial cells are layered, with the presence of smooth muscle cells, a functional NO signaling system, and availability of the liver proteins Hp and Hx to interact with the detrimental Hb split products.
5. Eryptosis, erythrocyte suicidal cell death
During their presence in the circulation, RBCs can recuperate from a limited period of applied stress or injury. After they completed their lifecycle, RBCs are removed from the circulation by the process of senescence. Another form of erythrocyte cell death is the phenomenon called eryptosis [50]. This type of RBC cell death may be triggered by a wide variety of factors such as hyperosmolarity, energy depletion, or injury from a prolonged period of cellular stress [49], [64]. High shear forces during specimen collection can liberate harmful hemolytic Hb components from disintegrating RBCs, leading to oxidative stress. This stress is an important accelerant and inducer of eryptosis of vital and intact RBCs that are present in the biologic.
Characteristics of eryptosis are comparable to that of apoptosis, namely, cell shrinkage, membrane blebbing, and exposure of phosphatidylserine on the cell membrane [65]. When cell shrinkage occurs, platelet activating factor (PAF) is liberated from erythrocytes. This is important because PAF plays a role in the control of inflammation and stimulates ceramide release through the disruption of sphingomyelin present in the erythrocyte [49].
Ceramide on the outside of the RBC membrane leads to the exposure of phosphatidylserine (PS), a phospholipid component of the inside of cell membrane where its key role is cell signaling. However, upon RBC damage PS is no longer restricted to the inside of the cell and flips to the outside by enzymatic action. Similarly, PS is a typical feature for apoptotic death of nucleated cells. The importance of PS availability on the cell surface has been characterized as a docking site for factors in the hemostatic system, with significant physiologic consequences [66]. In addition, the presence of PS on the outer cell surface may be a trigger for cell-to-cell interaction, in particular RBC-endothelial interaction. In conditions were PS exposure on RBCs is evident, vascular damage or blood flow complications are observed [67]. Betal and Setty raised concern that PS-RBCs could generate a sub-population of RBCs with enhanced pro-adhesive capabilities via thrombospondin, potentially playing an important role in vascular obstructive complications [68].
6. RBC macrophage migration inhibitory factor
Macrophage migration inhibitory factor (MIF) was first described in 1966 as a soluble, 12.5 kilo-Dalton cytokine released by activated T-lymphocytes [69]. Since its discovery, interest in MIF dynamics has expanded. Both leukocytes and platelets have been identified as sources of MIF [70], [71]. MIF has been reported to inhibit the random migration of monocytes and macrophages. MIF is known to play a central role in promoting inflammation by activating immune and inflammatory cells while promoting the expression of matrix metalloproteinases, nitric oxide, prostaglandin E2 release, and the release of proinflammatory and inflammatory cytokines, such as tumor necrosis factor-⍺, interleukins 1β/2/6/8, and interferon-γ [72].
Reports of a connection of MIF with the most common cell type in blood, the red blood cell, are minimal, in contrast to leukocytes and platelets. Very recently, Karsten et al. extensively studied the presence of MIF in red blood cells and quantified the concentration and activity of this RBC protein. They confirmed that the MIF concentration in whole blood is 1000-fold higher than in plasma and that the relative MIF contribution in platelets and leukocytes is negligible compared to that in RBCs. Furthermore, MIF is functionally active in RBCs [25]. The results of their study confirm that RBCs are a major reservoir of the inflammatory protein MIF, playing a profound role in inflammation. In addition, these findings have significant implications for the ultimate consequences of hemolysis. RBC disintegration will release high concentrations of enzymatically, and thus, chemotactically active MIF.
In post-cardiac arrest patients, acute hemolysis could cause a spike in MIF levels and may explains why cellular damage has been reported before signs of an inflammatory syndrome [73]. However, if MIF is not deactivated or modulated, it exerts its pro-inflammatory signaling activity to surrounding tissues. In addition, Zhang et al. reported that MIF levels in synovial fluids, but not in plasma, correlated with self-reported pain scores in patients with knee OA [74]. They concluded that the MIF signaled inflammatory cytokines in inflamed osteoarthritic joints have an important pathophysiological role in the generation and maintenance of OA-induced pain by acting on nociceptive nerve cells. Additionally, MIF synovial fluid and plasma levels were reported to be directly related to disease severity in knee OA, comparing by Kellgren and Lawrence grading system [75].
7. Potential consequences of RBCs within PRP or BMC preparations
The natural occurring biological homeostatic regulations of blood vessels should not be compared with cellular behavior in treatment syringes containing BMA, BMC, or PRP preparations. The endothelial single cell lining in all blood vessels and its constant regulation of exchange between the blood stream and the surrounding tissues provides these interior blood vessels walls with a unique blood-tissue barrier with a unique signaling mechanism [76]. Together with the smooth muscle cell layer below the endothelial cell layer, both cell layers regulate a variety of homeostatic mechanisms that control vascular relaxation, enzyme substance release that controls blood coagulation, immune function, platelet adhesion, and contribute to regulation of the consequences of RBC hemolysis via nitric oxide.
Polymer-based disposable sterile treatment syringes are routinely used to collect autologous prepared biologics for use in regenerative medicine. The polymers that are used in the health care industry and medical device industry have demanding requirements, such as profound biocompatible and low cytotoxic characteristics [77].
Due to the different cellular -and polymer characteristics, the presence of natural occurring scavengers and compensatory mechanisms are neither present nor feasible within syringes.
8. Discussion
RBCs can be damaged during blood/bone marrow collection or during centrifugation due to high shear forces, used collection and concentrating materials, and/or inadequate centrifugation protocols. If this occurs, the RBC components cannot be cleared because the native in-vivo environment has changed to an ex-vivo polymer-based milieu. In a treatment syringe NO reactions and hemolysis can take place with no counteracting haptoglobin Hb sequestration, or hemin blocking by hemopexin. Therefore, in these circumstances, PFH is an active and harmful component of any biological treatment vial. Furthermore, non-scavenged oxidative stress reactions, resulting from the consequences of hemolysis, may cause non-damaged RBCs present in the treatment vial to ultimately undergo the process of eryptosis, leading to PAF release, contributing to pro-inflammation and the stimulation of ceramide release. In addition, eryptotic RBCs will bind with PS, potentially enhancing pro-adhesion and clot formation through thrombospondin, which is a major protein and present in platelet α-granules.
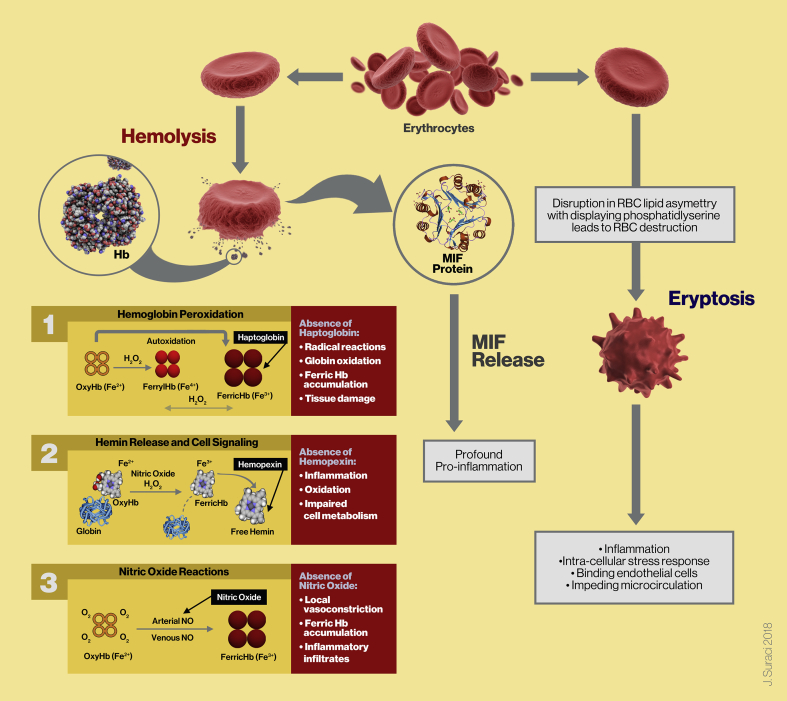
Following centrifugation procedures to produce PRP and BMC, concentrated cells are suspended in a small volume of plasma. Ideally, the formulation of these biologics should contain minimal to no RBCs. Hypothetically, when intact RBCs are part of a biological cell composition, and the biologics are delivered outside the blood stream to a local tissue microenvironment, the RBCs will undergo eryptosis because they are not able to leave the body in a natural way through the process of senescence. This might ultimately lead to secondary inflammatory conditions. In addition, the enormous MIF cytokine reservoir within RBCs are accountable for further increased levels of inflammation (Fig. 3).
Fig. 3.
Schematic summary illustration showing the pathophysiological effects and reactions of RBC hemolysis and eryptosis. The pathophysiological consequences of RBC hemolysis and PFH development in a biological treatment vial. Under normal circumstances PFH and its split products oxyHb (Fe2+), ferric Hb (Fe3+), and free hemin are released into plasma where they are cleared by natural occurring scavengers and compensatory mechanisms like Hp, Hx, and NO vascular reactions. However, in their absence and due to excessive PFH, a build-up of ferric and heme products continues, potentially leading to toxic consequences like direct pro-inflammation and pro-oxidant effects, endothelial cell dysfunction, and vasoconstriction. A biologic formulation which contains a high concentration of RBCs, combined with oxidative and hemolytic components, applied to tissue microenvironments, will lead to RBC cell membrane asymmetry and membrane disruption. This will lead to eryptosis, while displaying PS, leading to inflammation, and endothelial cell reactions with decreased microcirculatory activity. Another consequence of RBC disintegration and PFH is an abundant release of MIF cytokines, playing a profound role in pro-inflammatory processes (Adapted in part and modified from Schaer et al. [23]).
In most countries, PRP and BMC biologics for regenerative medicine treatment procedures are approved and safe procedures, based on indication-specific pathologies. Nevertheless, there are currently no viable therapies designed to attenuate the adverse effects of PFH and hemin within such biologics. Replacement, or supraphysiologic dosing of Hp and/or Hx is not a feasible option to counteract ongoing hemolysis in a treatment vial. Increasing IL-6 and TNF-alpha concentrations in vivo, to trigger liver Hp and/or Hx synthesis, is likely counter-productive to inflammatory environments [78]. However, minimizing or eliminating RBCs in a biologic is a more feasible and realistic goal that depends on the choice of a device system and concomitant preparation protocols. Ultimately, physicians should choose the preparation method that safeguards the biologic by targeting the required significant cell numbers. We encourage further in-depth clinical trials that evaluate clinical outcomes and emphasize the pathophysiological effects of RBCs in autologous biological preparations, as demonstrated in various in-vitro studies. In an in-vitro study by Harrison et al. a significant negative effect of RBCs was demonstrated leading to suppressed fibroblasts proliferation and fibroblast cell growth [79].
In another study, cartilage end-plate derived stem cells (CESCs) were cultured and the proinflammatory cytokine MIF interfered with the homing of MSCs and they suggested that intervertebral disk repair and regeneration might be impaired [80]. Therefore, well designed clinical studies should confirm the finding from in-vitro studies. Laboratory and clinical studies should be directed towards a complete understanding of the ultimate bio-cellular formulation to potentially reduce patient morbidity and improve measurable outcomes. The authors are aware that this recommendation poses exciting basic scientific, clinical, and regulatory challenges. Simultaneously, during this development process, outcomes of these studies can contribute to accepted standards for musculoskeletal regenerative medicine treatment protocols.
9. Conclusions
A lack of a complete understanding of all of the biological components and their consequences hampers the development of clinical standards for any biological preparation. In this review, the clinical implications and pathophysiological effects of RBCs in PRP and BMC, with emphasis on hemolysis, eryptosis, and the release of macrophage inhibitory factor have been addressed. Furthermore, the effects of RBCs to the joint microenvironment have been examined, with suggested improvements provided to address these issues during the harvesting and processing of PRP and BMC.
Acknowledgement
The authors thank Mr. Joel Suraci for the preparation of figure 3.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2019.03.009.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Yelin E., Weinstein S., King T. The burden of musculoskeletal diseases in the United States. Semin Arthritis Rheum. 2016 doi: 10.1016/j.semarthrit.2016.07.013. https://doi:10.1016/jsemarthrit201607013 [DOI] [PubMed] [Google Scholar]
- 2.Andia I., Rubio-Azpeitia E., Maffulli N. Platelet-rich Plasma modulates the secretion of inflammatory/angiogenic proteins by inflamed tenocytes. Clin Orthop Relat Res. 2015;473:1624–1634. doi: 10.1007/s11999-015-4179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugaya K. Potential use of stem cells in neuroreplacement therapies for neurodegenerative diseases. Int Rev Cytol. 2003;228:1–30. doi: 10.1016/s0074-7696(03)28001-3. [DOI] [PubMed] [Google Scholar]
- 4.Malanga G.A. Regenerative treatment for orthopedic conditions. PM R. 2015 doi: 10.1016/j.pmrj.2015.01.016. https://doi: 10.1016/j.pmrj.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 5.Sanchez M., Garate A., Delgado D., Padilla S. Platelet-rich plasma, an adjuvant biological therapy to assist peripheral nerve repair. Neural Regen Res. 2017;12:47–52. doi: 10.4103/1673-5374.198973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Senzel L., Gnatenko D., Bahou W. The platelet proteome. Curr Opin Hematol. 2009;16:329–333. doi: 10.1097/MOH.0b013e32832e9dc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everts P.A., Knape J.T., Weibrich G., Schönberger J.P., Hoffmann J.J., Overdevest E.P. Platelet rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38:174–187. [PMC free article] [PubMed] [Google Scholar]
- 8.Kovtun A., Bergdolt S., Wiegner R., Radermacher P., Huber-Lang M., Ignatius A. The crucial role of neutrophil granulocytes in bone fracture healing. Eur Cell Mater. 2016;32:152–162. doi: 10.22203/ecm.v032a10. [DOI] [PubMed] [Google Scholar]
- 9.Fitzpatrick J., Bulsara M., Zheng M. The effectiveness of platelet-rich plasma in the treatment of tendinopathy. a meta-analysis of randomized controlled clinical trials. Am J Sports Med. 2016 doi: 10.1177/0363546516643716. https://doi:10.1177/0363546516643716 [DOI] [PubMed] [Google Scholar]
- 10.Jorgensen C., Noel D. Mesenchymal stem cells in osteoarticular diseases. Regen Med. 2011;6:44–51. doi: 10.2217/rme.11.80. [DOI] [PubMed] [Google Scholar]
- 11.Oh M., Nor J. The perivascular niche and self-renewal of stem cells. Front Physiol. 2015 doi: 10.3389/fphys.2015.00367. https://doi: 10.3389/fphys.2015.00367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 13.Otero-Vinas M., Falanga V. Mesenchymal stem cells in chronic wounds: the spectrum from basic to advanced therapy. Adv Wound Care (New Rochelle) 2016;5:149–163. doi: 10.1089/wound.2015.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mithoefer K., McAdams T., Williams R.J., Kreuz P.C., Mandelbaum B.R. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37:2053–2063. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]
- 15.da Silva Meirelles L., Fontes A., Covas D., Caplan A. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Bashir J., Sherman A., Lee H., Kaplan L., Hare J.M. Mesenchymal stem cell therapies in the treatment of musculoskeletal diseases. PMR. 2014;6:61–69. doi: 10.1016/j.pmrj.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Das A., Sinha M., Datta S., Abas M., Chaffee S., Sen C.K. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015;185:2596–2606. doi: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orozco L., Munar A., Soler R., Alberca M., Soler F., Huguet M. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: Two-year follow-up results. Transplantation. 2014;97:e66–e68. doi: 10.1097/TP.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 19.Hernigou P., Flouzat Lachaniette C., Delambre J., Duffiet P., Chevallier N. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38:1811–1818. doi: 10.1007/s00264-014-2391-1. [DOI] [PubMed] [Google Scholar]
- 20.Mautner K., Malanga G.A., Smith J., Shiple B., Ibrahim V., Sampson S. A call for a standard classification system for future biologic research: the rationale for new PRP nomenclature. PM R. 2015 doi: 10.1016/j.pmrj.2015.02.005. https://doi.org: 10.1016/j.pmrj.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 21.Murray I., Robinson P., West Ch, Goudie E.B., Yong L.Y., White T.O. Reporting standards in clinical studies evaluating bone marrow aspirate concentrate: a systematic review. Arthroscopy. 2018;34:1366–1375. doi: 10.1016/j.arthro.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 22.Magalon J., Chateau A., Bertrand B., Louis M.L., Silvestre A., Giraudo L. DEPA classification: a proposal for standardizing PRP use and a retrospective application of available devices. BMJ Open Sport Exerc Med. 2016;2:1–6. doi: 10.1136/bmjsem-2015-000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaer D., Buehler P., Alayash A., Belcher J.D., Vercellotti G.M. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121:1276–1284. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klatt Ch, Krüger I., Zey S., Krott K.J., Spelleken M., Gowert N.S. Platelet-RBC interaction mediated by FasL/FasR induces procoagulant activity important for thrombosis. J Clin Invest. 2018;128:3906–3925. doi: 10.1172/JCI92077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karsten E., Hill C., Herbert B. Red blood cells: The primary reservoir of macrophage migration inhibitory factor in whole blood. Cytokine. 2018;102:34–40. doi: 10.1016/j.cyto.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Mazzucco L., Balbo V., Cattana E., Borzini P. Not every PRP-gel is born equal. Evaluation of growth factor availability for tissues through four PRP-gel preparations: Fibrinet, RegenPRP-Kit, Plateltex and one manual procedure. Vox Sang. 2009;97:110–118. doi: 10.1111/j.1423-0410.2009.01188.x. [DOI] [PubMed] [Google Scholar]
- 27.Everts P., Hoffmann J., Weibrich G., Mahoney C.B., Schönberger J.P., van Zundert A. Differences in platelet growth factor release and leucocyte kinetics during autologous platelet gel formation. Transfus Med. 2006;16:363–368. doi: 10.1111/j.1365-3148.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- 28.Degen R., Bernard J., Oliver K., Dines J. Commercial separation systems designed for preparation of platelet-rich plasma yield differences in cellular composition. HSS J. 2017;13:75–80. doi: 10.1007/s11420-016-9519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giusti I., Rughetti A., D’Ascenzo S. Identification of an optimal concentration of platelet gel for promoting angiogenesis in human endothelial cells. Transfusion. 2009;49:771–778. doi: 10.1111/j.1537-2995.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 30.Cavallo C., Filardo G., Mariani E., Kon E., Marcacci M., Pereira Ruiz M.T. Comparison of platelet rich plasma formulations for cartilage healing: an in vitro study. J Bone Joint Surg Am. 2014;96:423–429. doi: 10.2106/JBJS.M.00726. [DOI] [PubMed] [Google Scholar]
- 31.Dragoo J.L., Braun H.J., Durham J.L., Ridley B.A., Odegaard J.I., Luong R. Comparison of the acute inflammatory response of two commercial platelet-rich plasma systems in healthy rabbit tendons. Am J Sports Med. 2012;40:1274–1281. doi: 10.1177/0363546512442334. [DOI] [PubMed] [Google Scholar]
- 32.DeLong J., Russell R., Mazzocca A. Platelet-rich plasma: The PAW classification system. Arthroscopy. 2012;28:998–1009. doi: 10.1016/j.arthro.2012.04.148. [DOI] [PubMed] [Google Scholar]
- 33.Dohan Ehrenfest D., Andia I., Zumstein M., Zhang C.Q., Pinto N.R., Bielecki T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014;4:3–9. [PMC free article] [PubMed] [Google Scholar]
- 34.Muschler G., Nitto H., Boehm C., Easley K. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001;19:117–125. doi: 10.1016/S0736-0266(00)00010-3. [DOI] [PubMed] [Google Scholar]
- 35.Pontikoglou C., Deschaseaux F., Sensebé L., Papadaki H.A. Bone marrow mesenchymal stem cells: biological properties and their role in hematopoiesis and hematopoietic stem cell transplantation. Stem Cell Rev. 2011;7:569–589. doi: 10.1007/s12015-011-9228-8. [DOI] [PubMed] [Google Scholar]
- 36.Chamberlain G., Fox J., Ashton B., Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. J. Stem Cell. 2007;2739:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 37.Moatsche G., Morris E., Cinque M., Pascual-Garrido C., Chahla J., Engebretsen L. Biological treatment of the knee with platelet-rich plasma or bone marrow aspirate concentrates A review of the current status. Acta Orthop. 2017;88:670–674. doi: 10.1080/17453674.2017.1368899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 39.Fong L., Chan C., Goodman S. Stem cell homing in musculoskeletal injury. Biomaterials. 2011;32:395–409. doi: 10.1016/j.biomaterials.2010.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imam M., Holton J., Ernstbrunner E., Pepke W., Grubhofer F., Narvani A. A systematic review of the clinical applications and complications of bone marrow aspirate concentrate in management of bone defects and nonunions. SICOT J. 2017 doi: 10.1007/s00264-017-3597-9. https://doi.org:10.1051/sicotj/2017007 [DOI] [PubMed] [Google Scholar]
- 41.Braun H.J., Kim H.J., Chu C.R., Dragoo J.L. The effect of platelet rich plasma formulations and blood products on human synoviocytes: Implications for intra-articular injury and therapy. Am J Sports Med. 2014;42:1204–1210. doi: 10.1177/0363546514525593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hooiveld M., Roosendal G., Wenting M., van den Berg M., Bijlsma J., Lafeber F. Short term exposure of cartilage to blood results in apoptosis. Am J Pathol. 2003;162:943–951. doi: 10.1016/S0002-9440(10)63889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tajima T., Sekimoto T., Yamaguchi N., Taniguchi N., Kurogi S., Maruyama M. Hemoglobin stimulates the expression of ADAMTS-5 and ADAMTS-9 by synovial cells: a possible cause of articular cartilage damage after intra-articular hemorrhage. BMC Muskuloskelet Disord. 2017;18:449–455. doi: 10.1186/s12891-017-1815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dawson J., Smith J., Aarvold A., Ridgway J.N., Curran S.J., Dunlop D.G. Enhancing the osteogenic efficacy of human bone marrow aspirate: concentrating osteoprogenitors using wave-assisted filtration. Cytotherapy. 2013;15:242–252. doi: 10.1016/j.jcyt.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Chahla J., Cinque M., Piuzzi N., Mannava S., Geeslin A.G., Murray I.R. A call for standardization in platelet-rich plasma preparation protocols and composition reporting. A systematic review of the clinical orthopaedic literature. J Bone Joint Surg Am. 2017;99:1769–1779. doi: 10.2106/JBJS.16.01374. [DOI] [PubMed] [Google Scholar]
- 46.Finch C. Erythropoiesis, erythropoietin, and iron. Blood. 1982;60:1241–1246. [PubMed] [Google Scholar]
- 47.Mohandas N., Gallagher P.G. Red cell membrane: past, present, and future. Blood. 2008;112:3939–3948. doi: 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park Y., Best C., Badizadegan K. Measurement of red blood cell mechanics during morphological changes. Proc Natl Acad Sci. 2010;107:6731–6736. doi: 10.1073/pnas.0909533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lang K., Lang P., Bauer C., Duranton C., Wieder T., Huber S.M. Mechanisms of suicidal erythrocyte death. Cell Physiol Biochem. 2005;15:195–202. doi: 10.1159/000086406. [DOI] [PubMed] [Google Scholar]
- 50.Ghashghaeinia M., Cluitmans J., Akel A., Dreischer P., Toulany M., Köberle M. The impact of erythrocyte age on eryptosis. British J Haemat. 2012;157:606–614. doi: 10.1111/j.1365-2141.2012.09100.x. [DOI] [PubMed] [Google Scholar]
- 51.Frei A., Guo Y., Jones D., Pritchard K.A., Jr., Fagan K.A., Hogg N. Vascular dysfunction in a murine model of severe hemolysis. Blood. 2008;112:398–405. doi: 10.1182/blood-2007-12-126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gozzelino R., Jeney V., Soares M. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 53.Jones S. A relationship between Reynolds stresses and viscous dissipation: implications to red cell damage. Ann Biomed Eng. 1995;23:21–38. doi: 10.1007/BF02368297. [DOI] [PubMed] [Google Scholar]
- 54.Meyer C., Heiss C., Drexhage C., Kehmeier E.S., Balzer J., Mühlfeld A. Hemodialysis-induced release of hemoglobin limits nitric oxide bioavailability and impairs vascular function. J Am Coll Cardiol. 2010;55:454–459. doi: 10.1016/j.jacc.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 55.Figueiredo R., Fernandez P., Mourao-Sa D., Porto B.N., Dutra F.F., Alves L.S. Characterization of heme as activator of toll-like receptor 4. J Biol Chem. 2007;282:20221–20229. doi: 10.1074/jbc.M610737200. [DOI] [PubMed] [Google Scholar]
- 56.Kumar S., Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett. 2005;157:175–188. doi: 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 57.Vinchi F., Tolosano E. Therapeutic approaches to limit hemolysis-driven endothelial dysfunction: scavenging free heme to preserve vasculature homeostasis. Oxid Med Cell Long. 2013 doi: 10.1155/2013/396527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jeney V., Balla J., Yachie A., Varga Z., Vercellotti G.M., Eaton J.W. Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002;100:879–887. doi: 10.1182/blood.v100.3.879. [DOI] [PubMed] [Google Scholar]
- 59.Nagy E., Eaton J.W., Jeney V., Soares M.P., Varga Z., Galajda Z. Red cells, hemoglobin, heme, iron, and atherogenesis. Arterioscler Thromb Vasc Biol. 2010;30:1347–1353. doi: 10.1161/ATVBAHA.110.206433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith A., McCulloh R. Hemopexin and haptoglobin: allies against heme toxicity from hemoglobin not contenders. Front Physiol. 2015 doi: 10.3389/fphys.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seixas E., Gozzelino R., Chora A., Ferreira A., Silva G., Larsen R. Heme oxygenase-1 affords protection against noncerebral forms of severemalaria. Proc Natl Acad Sci USA. 2009;106:15837–15842. doi: 10.1073/pnas.0903419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ogawa K., Sun J., Taketani S., Nakajima O., Nishitani C., Sassa S. Heme mediates derepression of Maf recognition element through direct binding to transcription repressor Bach1. EMBO J. 2001;20:283543. doi: 10.1093/emboj/20.11.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alayash A. Oxygen therapeutics: can we tame haemoglobin? Nat Rev Drug Discov. 2004;3:152–159. doi: 10.1038/nrd1307. [DOI] [PubMed] [Google Scholar]
- 64.Rother R., Bell L., Hillmen P., Gladwin M. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 65.Repsold L., Joubert A. Eryptosis: An erythrocyte’s suicidal type of cell death. BioMed Res Int. 2018 doi: 10.1155/2018/9405617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berg C., Engels I., Rothbart A., Lauber K., Renz A., Schlosser S.F. Human mature red blood cells express caspase-3 and caspase-8, but are devoid of mitochondrial regulators of apoptosis. Cell Death Differ. 2015;8:1197–1206. doi: 10.1038/sj.cdd.4400905. [DOI] [PubMed] [Google Scholar]
- 67.Esmon Ch. The interactions between inflammation and coagulation. Br. J Haematol. 2005;131:417–430. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 68.Betal S., Setty B. Phosphatidylserine-positive erythrocytes bind to immobilized and soluble thrombospondin-1 via its heparin binding domain. Trans Res. 2008;152:165–177. doi: 10.1016/j.trsl.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baugh J., Bucala R. Macrophage migration inhibitory factor. Critical Care Med. 2002;30:S27–S35. [PubMed] [Google Scholar]
- 70.Lehmann L., Weber S., Fuchs D., Klaschik S., Schewe J.C., Book M. Intracellular detection of macrophage migration inhibitory factor in peripheral blood leukocytes. Free Radic Biol Med. 2005;38:1170–1179. doi: 10.1016/j.freeradbiomed.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 71.Jensen M., de Nully Brown D., Lund B., Nielsen O., Hasselbalch H. Increased circulating platelet-leukocyte aggregates in myeloproliferative disorders is correlated to previous thrombosis, platelet activation and platelet count. Eur J Haematol. 2001;66:143–151. doi: 10.1034/j.1600-0609.2001.00359.x. [DOI] [PubMed] [Google Scholar]
- 72.Calandra T., Bernhagen J., Metz C.N., Spiegel L.A., Bacher M., Donelly T. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 73.Pohl J., Rammos C., Totzeck M., Stock P., Kelm M., Rassaf T. MIF reflects tissue damage rather than inflammation in post-cardiac arrest syndrome in a real-life cohort. Resuscitation. 2016;100:32–37. doi: 10.1016/j.resuscitation.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 74.Zhang P., Liu J., Xu L., Sun Y., Sun X. Synovial fluid macrophage migration inhibitory factor levels correlate with the severity of self-reported pain in knee osteoarthritis patients. Med Sci Monit. 2016;22:2182–2186. doi: 10.12659/MSM.895704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu M., Hu C. Association of MIF serum and synovial fluid with severity of knee osteoarthritis. Clin Biochem. 2012;45:737–739. doi: 10.1016/j.clinbiochem.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 76.Michiels C. Endothelial cell functions. J Cell Physiol. 2003;196:430–443. doi: 10.1002/jcp.10333. [DOI] [PubMed] [Google Scholar]
- 77.Maitz M. Applications of synthetic polymers in clinical medicine. Biosurf Biotribology. 2015;1:161–176. [Google Scholar]
- 78.Nakagawa-Tosa N., Morimatsu M., Kawasaki M., Nakatsuji H., Syuto B., Saito M. Stimulation of haptoglobin synthesis by interleukin-6 and tumor necrosis factor, but not by interleukin-1, in bovine primary cultured hepatocytes. J Vet Med Sci. 1995;57:219–223. doi: 10.1292/jvms.57.219. [DOI] [PubMed] [Google Scholar]
- 79.Harrison S., Vavken P., Murray M. Erythrocytes inhibit ligament fibroblast proliferation in a collagen scaffold. J Orthop Res. 2011;29:1361–1366. doi: 10.1002/jor.21321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiong C., Huang B., Zhou Y., Cun Y.P., Liu L.T., Wang J. Macrophage migration inhibitory factor inhibits the migration of cartilage end plate-derived stem cells by reacting with CD74. PLoS One. 2012 doi: 10.1371/journal.pone.0043984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alves R., Grimalt R. A review of platelet-rich plasma: history, biology, mechanisms of action, and classification. Skin Appendage Disord. 2018;4:18–24. doi: 10.1159/000477353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grisendi G., Anneren C., Cafarelli L., Sternieri R., Veronesi E., Cervo G.L. GMP-manufactured density gradient media for optimized mesenchymal stromal/stem cell isolation and expansion. Cytotherapy. 2010;12:466–477. doi: 10.3109/14653241003649510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.