Abstract
Objective
Myocardial infarction remains the number one killer disease worldwide. Cellular therapy using cardiac c-kit cells (CCs) are capable of regenerating injured heart. Previous studies showed mesenchymal stem cell-derived (MSC) extracellular matrices can provide structural support and are capable of regulating stem cell functions and differentiation. This study aimed to evaluate the effects of human MSC-derived matrices for CC growth and differentiation.
Methods
Human Wharton's Jelly-derived MSCs were cultured in ascorbic acid supplemented medium for 14 days prior to decellularisation using two methods. 1% SDS/Triton X-100 (ST) or 20 mM ammonia/Triton X-100 (AT). CCs isolated from 4-week-old C57/BL6N mice were cultured on the decellularised MSC matrices, and induced to differentiate into cardiomyocytes in cardiogenic medium for 21 days. Cardiac differentiation was assessed by immunocytochemistry and qPCR. All data were analysed using ANOVA.
Results
In vitro decellularisation using ST method caused matrix delamination from the wells. In contrast, decellularisation using AT improved the matrix retention up to 30% (p < 0.05). This effect was further enhanced when MSCs were cultured in cardiogenic medium, with a matrix retention rate up to 90%. CCs cultured on cardiogenic MSC matrix (ECMcardio), however, did not significantly improve its proliferation after 3 days (p < 0.05), but the viability of CCs was augmented to 67.2 ± 0.7% after 24-h exposure to H2O2 stress as compared to 42.9 ± 0.5% in control CCs (p < 0.05). Furthermore, CCs cultured on cardiogenic MSC matrices showed 1.7-fold up-regulation in cardiac troponin I (cTnI) gene expression after 21 days (p < 0.05).
Conclusion
Highest matrix retention can be obtained by decellularization using Ammonia/Triton-100 in 2-D culture. ECMcardio could rescue CCs from exogenous hydrogen peroxide and further upregulated the cardiac gene expressions, offering an alternate in vitro priming strategy to precondition CCs which could potentially enhance its survival and function after in vivo transplantation.
Keywords: Cardiac c-kit cells, Mesenchymal stem cells, Extracellular matrices, Cardiomyocyte differentiation
Abbreviations: CC, cardiac c-kit cells; MSC, mesenchymal stem cells; ECM, extracellular matrix; AT, ammonia/triton X-100; ST, SDS/Triton X-100; MI, myocardial infarction; LVEF, left ventricular ejection fraction; αMHC, myosin heavy chain alpha; βMHC, myosin heavy chain beta; cTnI, cardiac troponin I; SMA, smooth muscle actinin; vWF, von Willibrand factor
Highlights
-
•
Ammonia is better than SDS for decellularization of cells in 2D culture.
-
•
ECM confers cardiac c-kit cell resistance towards oxidative stress.
-
•
ECM minimally promotes cardiac c-kit cell cardiomyocyte differentiation.
1. Introduction
The discovery of resident cardiac cells (CCs) has caught the attention of clinicians as these cells could be a promising alternative treatment for treating myocardial infarcted patients. Cardiac c-kit cells (CCs) were first found present in adult rat heart [1], and subsequently being successfully isolated in human [2]. These cells exhibit stem cell characteristics and they are clonogenic, self-renewal and able to differentiate into cardiomyocytes, smooth muscle and endothelial cells [1], [3]. The ability of these CCs in regenerating hearts have been observed in both the rat [1], [4] and mice [5], [6] through the formation of new myocytes and vasculatures. In addition, they protect the pre-existing cardiomyocytes from apoptosis and increased the survival by modulating IGF-1/IGF-1R/Akt pathway in swine model [7], [8]. They also play important roles in cardiomyogenesis in embryonic and neonatal heart development [9], the formation of new cardiomyocytes following myocardial infarction and responsible for cardiac cellular homeostasis in the heart [4], [10].
CCs have been the light of hope for autologous stem cell therapy, which exclude the hurdles in immunogenic rejection following transplantation. Nonetheless, stem cell homing, survival and engraftment are important key factors for successful cell-based therapy. Low oxygen supplied and lack of blood flow in infarcted heart limit the survival of transplanted cells following transplantation [11], [12], which lead to failure in stem cell treatment. Researchers have tried to overcome this problem by preconditioning transplanted cells with growth factors and genetic modifications of stem cell prior to transplantation in order to increase survival of cells. For example, by genetically engineered mesenchymal stem cells (MSCs) overexpressing CXCR4 in combination with SDF-1α, it improved the engraftment of MSCs in rodents’ hearts [13]. However, most of the transplanted cells loss during the first few hours of transplantation and die within a short period [14]. Therefore, higher number of transplanted cells has been suggested to increase the probability of cell engraftment. Intracoronary infusion of 10 million CCs in pigs or equivalent to 40 million CCs in human are proven to be safe [15]. Even though high cell number could be transplanted to increase engraftment efficiency, these cells often underwent another programmed cell death called anoikis, which is induced by weak cell–matrix interaction [16]. Moreover, long term in vitro expansion of CCs in order to obtain sufficient cells for CC transplantation could lead to erosion and replicative senescence. Therefore, a new approach has to be investigated in order to solve these problems.
ECM regulates cell–matrix functions through interaction with ECM proteins and provides mechanical supports to the cells [17], [18]. Natural scaffolds derived from organs is performed by perfusion-decellularisation and then recellularised with suitable stem cell candidate to build a new bioartificial heart [19]. The heart scaffold is decellularised using detergents to omit antigenic properties but preserving the architecture of the heart [19]. However, this technology is not feasible as it is limited by donor availability, scalability and long processing time [20]. Therefore, cell-specific ECM may be useful for cell therapy approaches as a source of biological scaffolds, which offers the advantage in maintaining the structure of their respective tissues and organs.
Cell sheet engineering technology has shown to improve the efficiency and efficacy of cell engraftment through cell–cell interactions [21]. The transplantation of cell sheet composed of rat or human CCs into the infarcted rat hearts were found to improve the CC survival, proliferation, migration and cardiomyocyte differentiation [21]. Lu et al. (2011) demonstrated that cell-derived ECM scaffolds from MSCs, normal human articular chondrocytes and normal human dermal fibroblast-derived ECM support cell adhesion and proliferation differently to their respective cells [22].
The cell-deposited ECM from umbilical cord MSCs enhances antioxidant properties and facilitates in vitro cell expansion of the same cell type, which is likely due to in vivo microenvironment mimicking from cell-specific ECM [23]. Implantation of heart tissue-derived decellularised matrix has shown improvement in left ventricular infarcted wall thickening and LVEF after MI in rats [24]. Transplantation of CCs in combination with heart-derived ECM also improved CC survival and engraftment, and preserved heart structure in rats [25]. Human fibroblast-derived ECM enhanced MSC proliferation [26]. Human MSC-derived ECM enhanced Schwann cell proliferation and promoted the secretion of neurotrophic factors for peripheral nerve repair [27].
MSCs were isolated from gelatinous Wharton Jelly from pregnant women [28] which exhibit the characteristics provided by the International Society of Cellular Therapy (ISCT), including the ability for plastic adherence, expressing CD105, CD73 and CD90, and able to differentiate to adipocytes, osteoblasts and chondroblasts [29]. As compared to bone marrow MSCs, WJMSCs have higher proliferation rate [30]. Therefore, in this study, we aim to employ MSCs derived from Wharton Jelly for ECM generation. Wharton Jelly is commonly discarded after birth as biological waste, yet they are readily available and ethically acceptable sources for allogeneic primary MSCs. MSCs obtained from Wharton Jelly possesses high proliferation rates and higher stemness due to the early embryological origin. More importantly, the isolated MSCs are multipotent and exhibit stem cell characteristics. The effect of MSC-ECM on CCs were assessed by proliferation, oxidative stress assay and cardiomyocyte differentiation efficiency.
2. Materials and methods
2.1. Isolation and characterisation of cardiac c-kit cells from mice
The protocol for isolating cardiac c-kit cells (CCs) was adapted from Smits et al. (2009), with slight modification [31]. All procedures were performed according to guidelines approved by the Institutional Animal Care and Use Committee (IACUC) of Universiti Sains Malaysia [USM/Animal Ethics Approval/2014/(91) (547)]. Briefly, whole heart was extracted from 4 to 6 week old C57/BL6N mice immediately following carbon dioxide asphyxiation. Heart tissue was collected in ice-cold Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12), supplemented with 20% foetal bovine serum, and 1× Penicillin and Streptomycin (Gibco®; Thermo Fisher Scientific, Carlsbad, CA, USA). The collected heart was washed in cold-M buffer to remove residual blood by gently pressing with sterile forceps. Upon removal of non-heart tissues, the heart was minced into small pieces of about 1 mm3 and digested in 1 mg/ml Collagenase A (Roche Applied Science, Indianapolis, IN, USA) for 2-hr at 37 °C in water bath. Digested heart tissues were passed through a 40 μm cell strainer, grinded using a syringe plunger and washed in cold M-buffer for five times. Then, the cell filtrate was centrifuged at 300 g for 5 min at room temperature. Cell pellet was re-suspended in 5 mL of incubation medium and counted using a haemocytometer prior to sorting using EasySep® Mouse CD117 (c-kit) selection cocktail (STEMCELL Technologies, Vancouver, Canada) and EasySep® Mouse haematopoietic Progenitor Cell Isolation Cocktail (STEMCELL Technologies, Vancouver, Canada) according to the manufacturer's protocols. Due to CC heterogeneity, positively selected CCs were plated as 0.5 cells/well on 96-well plate to establish clonogenically-expanded CCs. The selected cell colonies with at least 20 cells were cultured on 1.5% (w/v) gelatin-coated (Sigma–Aldrich, St Louis, MO, USA) surface in cardiac cell complete growth medium (CGM) for subsequent clonogenic expansion. CGM was made up of two solutions: Solution 1 and Solution 2. Solution 1 comprised of DMEM/F12 containing 1% (v/v) insulin-transferrin-selenium, 1% (v/v) Penicillin-Streptomycin, 0.1% (v/v) fungizone and 0.1% (v/v) gentamicin. Solution 2 comprised of Neurobasal medium supplemented with 37 mg of l-glutamine, 2% (v/v) B27 supplement and 1% (v/v) N2 supplement. The complete growth medium was prepared by mixing the solutions in the ratio: 45% solution 1, 45% solution 2 and 10% (v/v) embryonic stem cell-qualified FBS (All the above were purchased from Gibco®, Invitrogen Life Technologies Co., CA, USA). Finally, CGM was supplemented with 20 ng/ml epidermal growth factor, 10 ng/ml basal fibroblast growth factor and 10 ng/ml leukemic inhibitory factor (All the growth factors were purchased from Peprotech, Rocky Hill, NG, USA). The media was then sterilised through a 0.22 μm pore filter into a sterile container and store at 4 °C. CCs were then characterised by immunocytochemistry using the antibodies as listed in Table 1.
Table 1.
List of antibodies for CC characterisation and differentiation.
| Antibody | Dilution Factor | Application | Manufacturer |
|---|---|---|---|
| Rabbit Polyclonal Anti-c-kit Antibody (H-300) | 1:50 | ICC/FC | Santa Cruz Biotechnology, Germany (sc-5535) |
| Rabbit Polyclonal Anti-GATA-4 Antibody (H-112) | 1:50 | ICC | Santa Cruz Biotechnology, Germany (sc-9053) |
| Rabbit Polyclonal Anti-NKX2.5 Antibody (H-114) | 1:50 | ICC | Santa Cruz Biotechnology, Germany (sc-14033) |
| Goat Polyclonal Anti-Tryptase Antibody (V-13) | 1:50 | ICC | Santa Cruz Biotechnology, Germany (sc-32473) |
| Goat Polyclonal Anti-Sox2 Antibody (Y-17) |
1:50 | ICC | Santa Cruz Biotechnology, Germany (sc-17320) |
| Mouse Monoclonal Anti-Smooth Mucle Actinin (Clone 5C5) | 1:400 | ICC | Sigma Aldrich, USA (A2172) |
| Rabbit Polyclonal Anti-von Willebrand Factor Antibody | 1:400 | ICC | Dako, USA (A0082) |
| Rabbit Polyclonal anti-Cardiac Troponin I (H-170) | 1:50 | ICC | Santa Cruz Biotechnology, Germany (sc-15368) |
| Alexa Fluor 488 Donkey Anti-rabbit Antibody | 1:500 | ICC | Molecular Probes, CA |
| Alexa Fluor 488 Donkey Anti-goat Antibody | 1:500 | ICC | Molecular Probes, CA |
| Alexa Fluor 568 Donkey Anti-rabbit Antibody | 1:500 | ICC | Molecular Probes, CA |
| Alexa Fluor 568 Donkey Anti-goat Antibody | 1:500 | ICC | Molecular Probes, CA |
Abbreviations; ICC = Immunocytochemistry; FC = Flow cytometry.
2.2. Wharton's Jelly-derived mesenchymal stem cells
Umbilical cord samples were collected from mother at full-term pregnancy with informed consent in accordance to ethical committee from Faculty of Medicine and Health Sciences, Universiti Putra Malaysia [32]. The Wharton's Jelly was collected after removal of blood vessels and minced into paste-like tissues. The tissues were incubated with 0.4% collagenase type II and 0.01% DNAse at 37 °C for 30 min with gentle agitation. Equal volume of MSC growth medium, which was composed of DMEM/LG supplemented with 1% (v/v) Penicillin/Streptomycin and 10% (v/v) FBS was added to stop the enzymatic reaction. The digested tissues were then homogenized using handheld cell homogenizer for 5 min. This was followed by filtration through 40 μm cell strainers. The cells were then centrifuged and seeded onto T25 culture flask in MSC growth medium at 1 × 106 cells/cm2.
2.3. Immunofluorescence labelling
Cells were fixed with 4% (w/v) Paraformaldehyde (PFA) (Acros, USA) for 20 min on ice, followed by three times washes with PBS. For nuclear staining, cells were permeabilised with 0.1% (v/v) Triton X-100 (Sigma–Aldrich, St Louis, MO, USA) for 10 min at RT. Upon three washes with 0.1% (v/v) Tween-20 in PBS (PBST), cells were blocked in either 10% (v/v) donkey or goat serum for 30 min at RT. Next, cells were incubated with respective antibodies at 4 °C overnight. Followed by three washes in PBST, cells were stained with secondary antibody conjugated with desired fluorochrome at RT for 1-h. After washing with PBST three times, the nuclei were counterstained with 1 μg/mL DAPI for 14 min at RT. After washed with PBST for three times, the cells were mounted with VECTASHIELD mounting medium. All images were viewed using fluorescence microscope.
2.4. ECM scaffold deposition and decellularisation
The sub-confluent MSC was seeded onto 24-well plate at density 10,000 cells/cm2 in DMEM/LG supplemented with 10% foetal bovine serum (FBS), 1× Penicillin/streptomycin for initial 6 days of culture. On day 6, medium was changed to either 50 μg/mL ascorbic acid-supplemented medium or cardiogenic medium. Medium was changed every 2 days up to day 14. To decellularise the deposited ECM, the cultures were washed twice with PBS and treated with PBS containing 0.5% Triton X-100 and 20 mM NH4OH for 5 min at 37 °C. Lastly, the decellularised MSC ECM was treated with DNAse for 30 min at 37 °C. The ECM was washed with PBS, and stored at 4 °C for up to 1 month.
2.5. Viability assay
Proliferation status of CCs were determined by using Presto Blue®. Briefly, CCs were seeded at 1500 cells/cm2. Different cell numbers (2500, 5000, 10,000, 20,000 cells) were seeded to generate a relative standard curve to quantitate the exact cell number following 3 days of culture. 10% Presto Blue® in serum free medium was added into the culture and incubate for 1-h at 37 °C. The value of fluorescence intensity was measure at 544 nm/590 nm with the use of microplate reader (BMG).
2.6. Oxidative stress assay
The CCs were seeded at 1500 cells/cm2 for 5 days. Cell viability on day 5 was assessed using Presto Blue. The cells were then exposed to 100 μM hydrogen peroxide to induce stress on CCs. After 24-h exposure, CC viability was again assessed by Presto Blue. The value of fluorescence intensity was measure at 544 nm/590 nm with the use of microplate reader (BMG). The cell viability before and after exposure to hydrogen peroxide will be measured.
2.7. Chemical-induced CC cardiac differentiation
Cardiac cells were seeded at 10,000 cells/cm2 in CC complete medium onto surface with or without ECM for 3 days. Thereafter, the medium was changed to cardiogenic medium [8] [Cardiogenic Medium was composed of α-MEM (Sigma–Aldrich, St Louis, MO, USA) supplemented with 10% (v/v) FBS, 1% (v/v) Penicillin-Streptomycin, 0.1% (v/v) gentamicin, 0.1% (v/v) Fungizone, 1 μM dexamethasone (Sigma–Aldrich, St Louis, MO, USA), 50 μg/ml ascorbic acid (Sigma–Aldrich, St Louis, MO, USA) and 1 mM β-glycerophosphate (Sigma–Aldrich, St Louis, MO, USA)] with medium change every 2 days. On day 21 after initiation of differentiation, RNA will be collected and assessed for cardiac markers gene expression (αMHC, βMHC, GATA4, NKX2.5, cTnI) and immunofluorescence staining (cTnI, SMA, vWF).
2.8. RNA extraction and cDNA conversion
Cells were harvested, and RNA was extracted using RNeasy Mini Kit (Qiagen) according to manufacturer's protocol. RNA was stored @−80 °C or use directly for cDNA conversion. To quantitate amount of RNA, 1 μL of sample was loaded onto Nanodrop (Thermo Fisher). 1 μg RNA was then reversed transcribed to cDNA by using QuantiTect Reverse Transcriptase kit (Qiagen) according to manufacturer's protocol. cDNA was either use directly or stored at −80 °C.
2.9. Quantitative real time PCR
Quantitative real time PCR (QPCR) was performed using QuantiNova SYBR Green Kit (Qiagen) and the fluorescence intensity was measured by StepOne Plus (Applied Biosystem) according to manufacturers’ protocols. Briefly, a reaction master mix was prepared by mixing 10 μL 2× QuantiNova SYBR Green PCR Master Mix, 2 μL of QN ROX reference dye, forward and reverse primer at final concentration of 0.7 μM each, and 50 ng of cDNA per one reaction. The cycling condition was i) PCR initial heat activation @95 °C for 2 min, ii) 40 cycles of denaturation @95 °C for 5 s and combined annealing/extension @60 °C for 10 s, iii) Melting curve analysis. The final Ct value was normalised to housekeeping gene and fold changes were analysed using comparative Ct value to a suitable control. Primers were listed in Table 2.
Table 2.
Primer list used in this study.
| Gene/Accession Number | Primer Sequence (5′-3′) |
|---|---|
| GATA4 NM_008092.3 |
Forward: TCTCTGCATGTCCCATACCA Reverse: TGTGTGTGAAGGGGTGAAAA |
| Nkx2.5 NM_008700.2 |
Forward: GCTACAAGTGCAAGCGACAG Reverse: GGGTAGGCGTTGTAGCCATA |
| αMHC NM_010856.4 |
Forward: AAGGTGAAGGCCTACAAGCG Reverse: GGTCTGCTGGAGAGGTTATTCC |
| βMHC NM_080728.2 |
Forward: GCCAACACCAACCTGTCCAAGTTC Reverse: TGCAAAGGCTCCAGGTCTGAGGGC |
| cTnI NM_000353.4 |
Forward: TCTGCCAACTACCGAGCCTAT Reverse: CTCTTCTGCCTCTCGTTCCAT |
| GAPDH NM_008084.2 |
Forward: ACCCAGAAGACTGTGGATGG Reverse: CACATTGGGGGTAGGAACAC |
2.10. Data analysis
All data were expressed as mean ± standard error of mean (SEM). All statistical analyses were performed using SPSS (IBM SPSS Statistics 22). The differences between groups were analysed using independent t-test and One-way ANOVA with Tukey post-hoc test and is considered significant when p < 0.05.
3. Results
3.1. Isolation and characterisation of cardiac c-kit cells
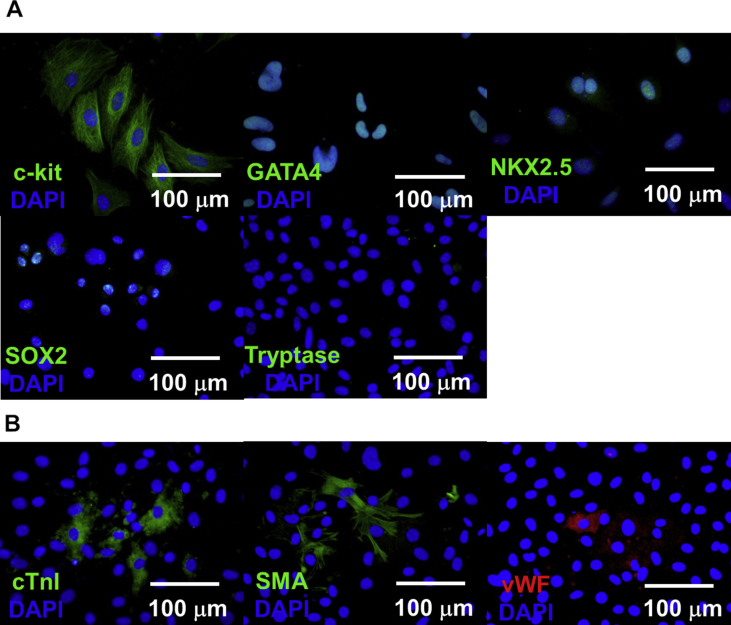
Cardiac c-kit cells isolated from magnetic-associated cell sorting were characterised by immunocytochemistry staining. Cardiac c-kit cells were positive for c-kit, expressed cardiac transcription factors (GATA4, NKX2.5) and SOX2 (Fig. 1a). Tryptase negative suggested no contamination from mast cells, which is also c-kit positive. Dexamethasone-directed cardiac cell differentiation for 21 days showed expression of cardiac troponin I (cTnI), smooth muscle actinin (SMA) and endothelial marker (vWF) (Fig. 1b).
Fig. 1.
Isolation and characterisation of cardiac c-kit cells. (A) Immunocytochemistry staining of cardiac c-kit cells. CCs expressed c-kit, cardiac transcription factors (GATA4, NKX2.5), pluripotent marker (SOX2) but negative for mast cell marker (Tryptase). (B) Dexamethasone-directed cardiac differentiation of CCs for 21 days showed positive expression of cardiac markers (cTnI), smooth muscle actinin (SMA) and endothelial marker (vWF). Nuclei were stained with DAPI (Blue). Scale bar = 100 μm.
3.2. Decellularisation of MSC-derived matrices
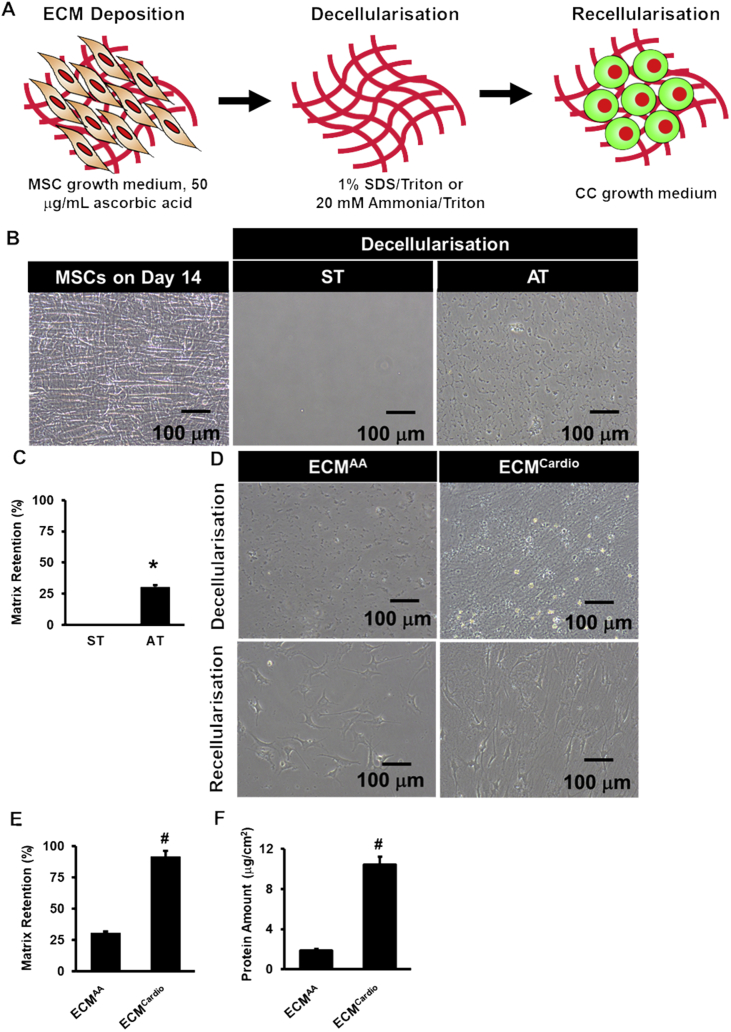
MSCs were cultured in medium containing ascorbic acid on day 6 until day 14 until it reached full cell confluency and morphology on day 14 prior to decellularisation (Fig. 2a). As SDS has been widely used to decellularise organ in tissue engineering study [19], we first decellularised confluent MSCs (Fig. 2b) in 2D culture on day 14 with SDS/Triton X-100 (ST). Complete cell removal was observed, but with no ECM retaining on the surface on the well. To circumvent this, we substituted SDS with ammonia, which is a milder detergent as compared to SDS for decelluarisation. To circumvent this, we substituted SDS with ammonia, which is a milder detergent as compared to SDS for decelluarisation. Although matrix delamination was still observed following AT treatment, 30 ± 1% of the wells with ECM on the surface was observed as compared to ST (p < 0.001) (Fig. 2c). Overall, these results indicated that AT is a better detergent as compared to ST in decellularising cell-derived matrices.
Fig. 2.
Extracellular matrix generation. (A) Schematic diagram illustrated the process of ECM generation. (B) MSCs cultured on day 14 were decellularised using SDS/Triton X-100 (ST) or ammonia/Triton X-100 (AT). (C) The number of well-retaining ECM following treatments with ST or AT. (D) Representative images observed under phase contrast microscopy following decellularisation and recellularization of ascorbic acid- and cardiogenic-treated MSCs. (E) The number of well-retaining ECM following treatment with AT. (F) Protein quantification of ECMAA and ECMCardio. *p < 0.05 vs. ST, #p < 0.05 vs. ECMAA.
3.3. MSC-matrix retention after cell decellularisation in 2-dimensional culture
Dexamethasone-based medium have been proposed to be able to direct cardiac cell differentiation towards cardiac lineages [3]. Therefore, as culture medium may affect ECM secretion we cultured MSCs in dexamethasone-based cardiogenic medium instead of ascorbic-acid supplemented medium to induce deposition of matrices that mimicking cardiomyocyte microenvironment. Interestingly, we observed 92 ± 5% of wells retaining matrices on the surface following decellularisation using AT, as shown in Fig. 2e(p < 0.001 vs. ascorbic acid). Protein quantification confirmed the presence of higher protein level in cardiogenic- ECM (ECMCardio) than ascorbic acid-treated ECM (ECMAA) (10.4 ± 0.8 μg/ml vs. 1.9 ± 0.2 μg/ml, p < 0.01) (Fig. 2f). The difference between ECMAA and ECMCardio was that ECMCardio preserved the ultrastructure of MSCs and retained higher ECM protein on the surface as demonstrated in Fig. 2d. Taken together, these results suggest that ECM retention was dependant on decellularisation technique and induction medium for ECM deposition.
3.4. The effects of MSC-derived ECM on CC proliferation and resistance to oxidative stress
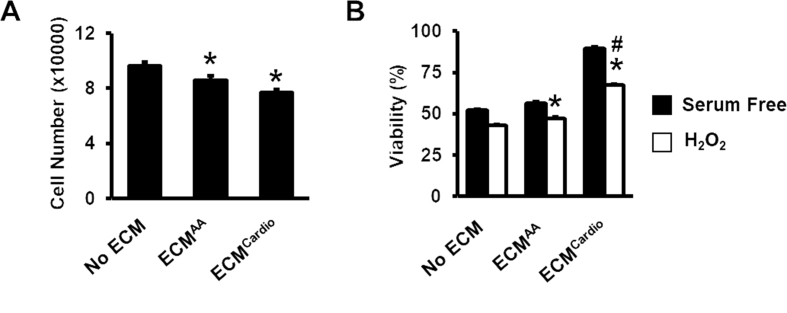
To elucidate if ECMAA or ECMCardio improved CC proliferation, we seeded the cells on the ECM and cultured for 3 days. We found that the cells culturing on ECM did not showed any improvement in CC proliferation as compared to CCs cultured without ECM (Fig. 3a) (77,552 ± 802 on ECMCardio vs. 86,312 ± 3201 cells on ECMAA, p > 0.05). Highest cell viability was found on CC cultured on ECMCardio following exposure to 100 μM hydrogen peroxide (H2O2) for 24 h in serum free condition (67.2 ± 0.7%), p < 0.05 vs. control), followed by ECMAA (Fig. 3b) (47.1 ± 0.9%, p < 0.05 vs. control) and control (42.9 ± 0.5%). These results suggest that ECM confers CC resistance towards exogenous oxidative stress.
Fig. 3.
The effect of decellularised MSC-ECM on CC proliferation and resistance to oxidative stress. (A) The cell number of CC cultured on surface with or without ECM for 3 days were assessed using Presto Blue ®. (B) Viability of CCs post-H2O2 treatment. *p < 0.05 vs. No ECM; #p < 0.05 vs. ECMAA
3.5. The effects of MSC-derived ECMs on CC cardiomyocyte differentiation
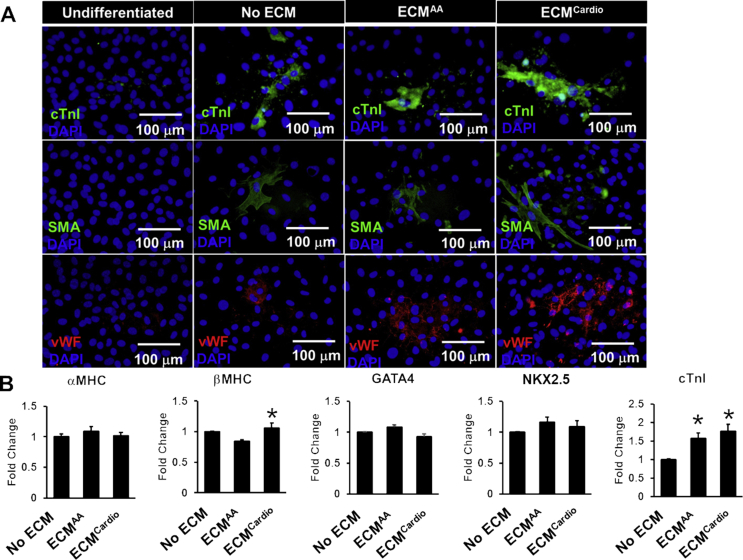
Next, cardiac differentiation of CCs was tested after cultured on both ECMs after differentiated using dexamethasone-based medium for 21 days. Immunocytochemistry showed that the differentiated cells were positive for cardiac troponin I, smooth muscle and endothelial cells for all the groups with or without ECM (Fig. 4a). Gene expression analysis showed up-regulation in αMHC (1.2-fold, p < 0.05 vs. control) and cTnI (1.8-fold, p < 0.05 vs. control) genes in CCs after cultured on ECMcardio (Fig. 4b). CCs cultured on ECMAA also exhibited up-regulation of cTnI (1.6-fold, p < 0.05 vs. control) gene expression. No significant differences were observed for αMHC, GATA4 and NKX2.5 gene expressions among groups.
Fig. 4.
The effect of decellularised MSC-ECM on CC cardiac differentiation. (A) Immunofluorescence staining of differentiated CCs on ECM (cTnI: cardiac troponin I); SMA: smooth muscle actinin; vWF: von Willibrand factor. (B) Cardiac gene expressions were assessed by qPCR. *p < 0.05 vs. No ECM.
4. Discussions
Cardiac cell therapy has been proven to be a promising strategy for treating patients with myocardial infarction [4], [33]. However, cellular therapy is often limited by low cell number, poor cell engraftment and cell differentiation post transplantation [6], [14]. Following myocardial infarction, infarcted region was replaced by scar tissue, which also involves changes in extracellular matrices [34]. These deleterious events could limit cell engraftment and viability post-transplantation. Decellularised extracellular matrix (ECM) obtained by removal of cellular components, has recently emerged as a cell culture technology for maintaining primary cell phenotype during expansion. Decellularised ECM can be obtained from in vivo tissue ECM or ECM fabricated by cells cultured in vitro. The ECM is composed of many types of collagens, proteoglycans, glycoproteins, and glycosaminoglycans that form a complex structure [35]. The protein components of the ECM vary for different tissues and organs [36] and the composition of the ECM is determined by developmental and pathological conditions [34], [37].
In this study, MSCs were cultured on plastic surface in normal growth medium for 6 days, and changed the medium to normal growth medium supplemented with ascorbic acid to increase the ECM deposition from cells [38], [39]. Sodium dodecyl sulphate (SDS) is an ionic detergent which has been widely used in decellularising organs ECM to solubilise both nuclear and cytoplasmic cellular membranes [19]. In this study SDS was combined with Triton X-100 (ST), a non-ionic surfactant which offers only mild decellularization effects on tissue structure [40]. However with ST, matrices were delaminated from the well surface, attributed to SDS which potentially compromised the ECM content and mechanical strength [41], and thus not suitable for decellularization on 2D cell monolayer. Ammonium hydroxide is another mild detergent which have been reported to efficiently decellularising cell-derived ECM [22], [42], [43], [44]. Decellularization by substituting ST with AT yielded 30% of wells with matrix retention on plastic surface. However, the matrix ultrastructure following AT treatment was distorted despite having intact protein matrices on the surface, of which also prone to delamination after washing. Strategy to minimize delamination following 2-D culture decellularisation includes coating with ethanolamine- [23], [45], or fibronectin [42], [46] to chemically rigidify the prepared matrices. However, coating with another ECM may alter the composition of secreted ECM, MSC and CC functions, and thus affects the interpretation of the results in this study. Nonetheless, we resolved it by culturing the MSCs in the cardiogenic medium containing dexamethasone, ascorbic acid, and β-glyceralphosphate for 14 days, with high rates of matrix retention on the wells was observed. This result suggests that choice of culture medium and decellularisation technique affect the quality of produced ECM.
Previous research determined that the ECM influences stem cell differentiation and the maintenance of stemness [47], [48]. Decellularized matrices from MSCs cultured in osteogenic- or adipogenic-stimulating medium have shown to improves its osteogenesis and adipogenesis [49], suggesting that the stimulation also induces secretion of specific ECM that facilitates specific differentiation process. Although MSC-ECM has been shown to improve proliferation and differentiation of cultured adult MSCs [44], proliferation was not seen in CCs cultured on cardiogenic ECM in this study. This propose that the secreted matrices were more likely to be pro-differentiation than pro-proliferation.
Oxidative stress has been observed in the heart following myocardial infarction (10–12). Although cells exhibit antioxidant system that could minimise the effect of ROS, the deregulation of the antioxidant-oxidant systems can result in decompensated cardioprotective effect (13, 14). H2O2 has been demonstrated to increase reactive oxygen species (ROS) in cells [15], [16]. The increased ROS level can damage cell membranes and cellular constituents, which then lead to cell death [17], [18]. Here, we observed MSC-ECM confers CC resistance to exogenous H2O2, showing higher cell viability compared to control, a similar observation with a previous study [23]. Future study will examine if the observed effect is due to the capability of MSC-ECM in modulating H2O2 degradation in and redox signalling in CCs, as seen in endothelial cells (15), the role of PI3K/Akt signalling (16) or the influence in caspase 8 and 9 activities that dictate the apoptosis and necrosis of CCs (17).
Recently, the identity and cardiac commitment of endogenous cardiac c-kit cells has been questioned and hugely debated, which begins with a study by van Berlo et al.(2014) who challenged the cardiac commitment of c-kit cells through tracked the development of c-kit cells in the growing heart using Kit-Cre lineage tracing in mice [50]. They found that only 0.03% of the endogenous c-kit cells become cardiomyocytes, and number may reduce to 0.008% if cell fusion event is considered [50]. Majority of the c-kit was found to be CD31 + endothelial cells. The same conclusion was also drawn from two independent studies [51], [52]. However, a study argued that myogenic cardiac c-kit cells express low yet detectable c-kit mRNA and protein, the low level of Cre expression in the c-kit cells from KitCre knock-in mice could affect the efficiency of loxP recombination, and thus question the capability of the system in identifying the myogenic c-kit population [53]. These myogenic c-kit cells are single-cells derived clones from the cohort of lineage negative CD45 negative population, which constitute only 1–2% of the total c-kit myocardial cells, with only 10% of them can robustly form contracting cardiomyocytes after stimulated with TGF-β/Wnt molecules [54]. Nonetheless, autologous human cardiac c-kit cell therapy showed improvement in heart performance in patients with ischemic cardiomyopathy [55], suggesting the capability of cardiac c-kit cells in regenerating diseased heart, albeit via a mechanism that remains to be unravelled.
In conclusions, the retention of MSC-secreted ECM in 2D culture may be affected by culture medium and decellularisation solution, and AT is a better decellularising agent than ST in this instance. ECMCardio protects CCs from exogenous oxidative stress and further upregulated the cardiac gene expressions, offering an alternate in vitro priming strategy to precondition CCs which could potentially enhance its survival and function after in vivo transplantation.
Conflicts of interest
JJT received a research grant from CryoCord Sdn Bhd. All funders do not involve in conceiving the experimental design and data analysis of this project. Others declare no conflict of interest.
Author contribution
JJT conceived research design. WHN performed the experiments, analysed data and drafted the manuscript. JJT, BS, SHN, YKY and RR interpreted and analysed data. JJT, BS revised, proofread and approved the final draft manuscript.
Funding
This study was funded by Sciencefund from Malaysian Ministry of Science Technology and Innovation (02-01-05-SF0684). JJT is also a recipient of Universiti Sains Malaysia Research University Grant (Individual: 1001/CIPPT/811226) and CryoCord Sdn. Bhd. Research Fund.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Beltrami A.P., Barlucchi L., Torella D., Baker M., Limana F., Chimenti S. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 2.Bearzi C., Rota M., Hosoda T., Tillmanns J., Nascimbene A., De Angelis A. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104(35):14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith A.J., Lewis F.C., Aquila I., Waring C.D., Nocera A., Agosti V. Isolation and characterization of resident endogenous c-Kit+ cardiac stem cells from the adult mouse and rat heart. Nat Protoc. 2014;9(7):1662–1681. doi: 10.1038/nprot.2014.113. [DOI] [PubMed] [Google Scholar]
- 4.Ellison G.M., Vicinanza C., Smith A.J., Aquila I., Leone A., Waring C.D. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154(4):827–842. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 5.Messina E., De Angelis L., Frati G., Morrone S., Chimenti S., Fiordaliso F. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95(9):911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 6.Hong K.U., Guo Y., Li Q.H., Cao P., Al-Maqtari T., Vajravelu B.N. c-kit+ Cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0096725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellison G.M., Torella D., Dellegrottaglie S., Perez-Martinez C., Perez de Prado A., Vicinanza C. Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J Am Coll Cardiol. 2011;58(9):977–986. doi: 10.1016/j.jacc.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Kawaguchi N., Smith A.J., Waring C.D., Hasan M.K., Miyamoto S., Matsuoka R. c-kitpos GATA-4 high rat cardiac stem cells foster adult cardiomyocyte survival through IGF-1 paracrine signalling. PLoS One. 2010;5(12) doi: 10.1371/journal.pone.0014297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira-Martins J., Ogórek B., Cappetta D., Matsuda A., Signore S., D'Amario D. Cardiomyogenesis in the developing heart is regulated by C-Kit–Positive cardiac stem cells novelty and significance. Circ Res. 2012;110(5):701–715. doi: 10.1161/CIRCRESAHA.111.259507. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Nadal-Ginard B., Ellison G.M., Torella D. The cardiac stem cell compartment is indispensable for myocardial cell homeostasis, repair and regeneration in the adult. Stem Cell Res. 2014;13(3 Pt B):615–630. doi: 10.1016/j.scr.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Muller-Ehmsen J., Whittaker P., Kloner R.A., Dow J.S., Sakoda T., Long T.I. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34(2):107–116. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M., Methot D., Poppa V., Fujio Y., Walsh K., Murry C.E. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33(5):907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 13.Zhang D., Fan G.-C., Zhou X., Zhao T., Pasha Z., Xu M. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2008;44(2):281–292. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dow J., Simkhovich B.Z., Kedes L., Kloner R.A. Washout of transplanted cells from the heart: a potential new hurdle for cell transplantation therapy. Cardiovasc Res. 2005;67(2):301–307. doi: 10.1016/j.cardiores.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Keith M.C.L., Tang X.-L., Tokita Y., Li Q-h, Ghafghazi S., Moore Iv J. Safety of intracoronary infusion of 20 million C-kit positive human cardiac stem cells in pigs. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0124227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frisch S.M., Screaton R.A. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13(5):555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 17.Wilgus T.A. Growth factor–extracellular matrix interactions regulate wound repair. Adv Wound Care. 2012;1(6):249–254. doi: 10.1089/wound.2011.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S.-H., Turnbull J., Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209(2):139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- 19.Ott H.C., Matthiesen T.S., Goh S.K., Black L.D., Kren S.M., Netoff T.I. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14(2):213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 20.Guyette J.P., Charest J.M., Mills R.W., Jank B.J., Moser P.T., Gilpin S.E. Bioengineering human myocardium on native extracellular matrix. Circ Res. 2016;118(1):56–72. doi: 10.1161/CIRCRESAHA.115.306874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zakharova L., Mastroeni D., Mutlu N., Molina M., Goldman S., Diethrich E. Transplantation of cardiac progenitor cell sheet onto infarcted heart promotes cardiogenesis and improves function. Cardiovasc Res. 2010;87(1):40–49. doi: 10.1093/cvr/cvq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu H., Hoshiba T., Kawazoe N., Koda I., Song M., Chen G. Cultured cell-derived extracellular matrix scaffolds for tissue engineering. Biomaterials. 2011;32(36):9658–9666. doi: 10.1016/j.biomaterials.2011.08.091. [DOI] [PubMed] [Google Scholar]
- 23.Liu X., Zhou L., Chen X., Liu T., Pan G., Cui W. Culturing on decellularized extracellular matrix enhances antioxidant properties of human umbilical cord-derived mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2016;61:437–448. doi: 10.1016/j.msec.2015.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai W., Gerczuk P., Zhang Y., Smith L., Kopyov O., Kay G.L. Intramyocardial injection of heart tissue-derived extracellular matrix improves postinfarction cardiac function in rats. J Cardiovasc Pharmacol Ther. 2013;18(3):270–279. doi: 10.1177/1074248412472257. [DOI] [PubMed] [Google Scholar]
- 25.Jiang D.Q., Gu T.X., Xu Z.F., Liu S., Li X.Y. Effect of implantation of cardiosphere-derived cells combined with rat heart tissue-derived extracellular matrix on acute myocardial infarction in rats. South Med J. 2016;36(10):1316–1321. [PubMed] [Google Scholar]
- 26.Zhou Y., Zimber M., Yuan H., Naughton G.K., Fernan R., Li W.J. Effects of human fibroblast-derived extracellular matrix on mesenchymal stem cells. Stem Cell Rev. 2016;12(5):560–572. doi: 10.1007/s12015-016-9671-7. [DOI] [PubMed] [Google Scholar]
- 27.Xiao B., Rao F., Guo Z.Y., Sun X., Wang Y.G., Liu S.Y. Extracellular matrix from human umbilical cord-derived mesenchymal stem cells as a scaffold for peripheral nerve regeneration. Neural Regen Res. 2016;11(7):1172–1179. doi: 10.4103/1673-5374.187061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subramanian A., Fong C.Y., Biswas A., Bongso A. Comparative characterization of cells from the various compartments of the human umbilical cord shows that the Wharton's Jelly compartment provides the best source of clinically utilizable mesenchymal stem cells. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0127992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 30.Batsali A., Kouvidi E., Damianaki A., Stratigi A., Kastrinaki M.-C., Papadaki H.A. Comparative analysis of bone marrow and Wharton's Jelly mesenchymal stem/stromal cells. Blood. 2013;122(21):1212. [Google Scholar]
- 31.Smits A.M., van Vliet P., Metz C.H., Korfage T., Sluijter J.P., Doevendans P.A. Human cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: an in vitro model for studying human cardiac physiology and pathophysiology. Nat Protoc. 2009;4(2):232–243. doi: 10.1038/nprot.2008.229. [DOI] [PubMed] [Google Scholar]
- 32.Tong C.K., Vellasamy S., Tan B.C., Abdullah M., Vidyadaran S., Seow H.F. Generation of mesenchymal stem cell from human umbilical cord tissue using a combination enzymatic and mechanical disassociation method. Cell Biol Int. 2011;35(3):221–226. doi: 10.1042/CBI20100326. [DOI] [PubMed] [Google Scholar]
- 33.Bolli R., Chugh A.R., D'Amario D., Loughran J.H., Stoddard M.F., Ikram S. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378(9806):1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Sullivan K.E., Quinn K.P., Tang K.M., Georgakoudi I., Black L.D. Extracellular matrix remodeling following myocardial infarction influences the therapeutic potential of mesenchymal stem cells. Stem Cell Res Ther. 2014;5(1):14. doi: 10.1186/scrt403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hynes R.O. Extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manabe R., Tsutsui K., Yamada T., Kimura M., Nakano I., Shimono C. Transcriptome-based systematic identification of extracellular matrix proteins. Proc Natl Acad Sci U S A. 2008;105(35):12849–12854. doi: 10.1073/pnas.0803640105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Y., Puperi D.S. Ascorbic acid promotes extracellular matrix deposition while preserving valve interstitial cell quiescence within 3D hydrogel scaffolds. J Tissue Eng Regenerat Med. 2017;11(7):1963–1973. doi: 10.1002/term.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei F.L., Qu C.Y., Song T.L., Ding G., Fan Z.P., Liu D.Y. Vitamin C treatment promotes mesenchymal stem cell sheet formation and tissue regeneration by elevating telomerase activity. J Cell Physiol. 2012;227(9):3216–3224. doi: 10.1002/jcp.24012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilbert T.W. Strategies for tissue and organ decellularization. J Cell Biochem. 2012;113(7):2217–2222. doi: 10.1002/jcb.24130. [DOI] [PubMed] [Google Scholar]
- 41.Xing Q., Yates K., Tahtinen M., Shearier E., Qian Z., Zhao F. Decellularization of fibroblast cell sheets for natural extracellular matrix scaffold preparation. Tissue Eng C Methods. 2015;21(1):77–87. doi: 10.1089/ten.tec.2013.0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prewitz M.C., Seib F.P., von Bonin M., Friedrichs J., Stiszel A., Niehage C. Tightly anchored tissue-mimetic matrices as instructive stem cell microenvironments. Nat Methods. 2013;10(8):788–794. doi: 10.1038/nmeth.2523. [DOI] [PubMed] [Google Scholar]
- 43.Lu H., Hoshiba T., Kawazoe N., Chen G. Comparison of decellularization techniques for preparation of extracellular matrix scaffolds derived from three-dimensional cell culture. J Biomed Mater Res A. 2012;100(9):2507–2516. doi: 10.1002/jbm.a.34150. [DOI] [PubMed] [Google Scholar]
- 44.Ng C.P., Mohamed Sharif A.R., Heath D.E., Chow J.W., Zhang C.B.Y., Chan-Park M.B. Enhanced ex vivo expansion of adult mesenchymal stem cells by fetal mesenchymal stem cell ECM. Biomaterials. 2014;35(13):4046–4057. doi: 10.1016/j.biomaterials.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 45.Franco-Barraza J., Beacham D.A., Amatangelo M.D., Cukierman E. Curr protoc cell biol. John Wiley & Sons, Inc.; 2001. Preparation of extracellular matrices produced by cultured and primary fibroblasts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kusuma G.D., Brennecke S.P., O'Connor A.J., Kalionis B., Heath D.E. Decellularized extracellular matrices produced from immortal cell lines derived from different parts of the placenta support primary mesenchymal stem cell expansion. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bi Y., Ehirchiou D., Kilts T.M., Inkson C.A., Embree M.C., Sonoyama W. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13(10):1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 48.Bi Y., Stuelten C.H., Kilts T., Wadhwa S., Iozzo R.V., Robey P.G. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J Biol Chem. 2005;280(34):30481–30489. doi: 10.1074/jbc.M500573200. [DOI] [PubMed] [Google Scholar]
- 49.Hoshiba T., Kawazoe N., Tateishi T., Chen G. Development of stepwise osteogenesis-mimicking matrices for the regulation of mesenchymal stem cell functions. J Biol Chem. 2009;284(45):31164–31173. doi: 10.1074/jbc.M109.054676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Berlo J.H., Kanisicak O., Maillet M., Vagnozzi R.J., Karch J., Lin S.C. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509(7500):337–341. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sultana N., Zhang L., Yan J., Chen J., Cai W., Razzaque S. Resident c-kit(+) cells in the heart are not cardiac stem cells. Nat Commun. 2015;6:8701. doi: 10.1038/ncomms9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Q., Yang R., Huang X., Zhang H., He L., Zhang L. Genetic lineage tracing identifies in situ Kit-expressing cardiomyocytes. Cell Res. 2016;26(1):119–130. doi: 10.1038/cr.2015.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vicinanza C., Aquila I., Cianflone E., Scalise M., Marino F., Mancuso T. Kit(cre) knock-in mice fail to fate-map cardiac stem cells. Nature. 2018;555(7697):e1–e5. doi: 10.1038/nature25771. [DOI] [PubMed] [Google Scholar]
- 54.Vicinanza C., Aquila I., Scalise M., Cristiano F., Marino F., Cianflone E. Adult cardiac stem cells are multipotent and robustly myogenic: c-kit expression is necessary but not sufficient for their identification. Cell Death Differ. 2017 doi: 10.1038/cdd.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chugh A.R., Beache G.M., Loughran J.H., Mewton N., Elmore J.B., Kajstura J. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126(11 Suppl 1):S54–S64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]