Abstract
Objectives
Nerve growth factor (NGF) has emerged as a key driver of pain in osteoarthritis (OA) and antibodies to NGF are potent analgesics in human disease. Here, we validate a novel vaccine strategy to generate anti-NGF antibodies for reversal of pain behaviour in a surgical model of OA.
Methods
Virus-like particles were derived from the cucumber mosaic virus (CuMV) and coupled to expressed recombinant NGF to create the vaccine. 10-week-old male mice underwent partial meniscectomy to induce OA or sham-surgery. Spontaneous pain behaviour was measured by Linton incapacitance and OA severity was quantified using OARSI histological scoring. Mice (experimental and a sentinel cohort) were inoculated with CuMVttNGF (Vax) or CuMVttctrl (Mock) either before surgery or once pain was established. Efficacy of anti-NGF from the plasma of sentinel vaccinated mice was measured in vitro using a neurite outgrowth assay in PC12 cells.
Results
Anti-NGF titres were readily detectable in the vaccinated but not mock vaccinated mice. Regular boosting with fresh vaccine was required to maintain anti-NGF titres as measured in the sentinel cohort. Both prophylactic and therapeutic vaccination demonstrated a reversal of pain behaviour by incapacitance testing, and a meta-analysis of the two studies showing analgesia at peak anti-NGF titres was highly statistically significant. Serum anti-NGF was able to inhibit neurite outgrowth equivalent to around 150 ug/mL of recombinant monoclonal antibody.
Conclusions
This study demonstrates therapeutic efficacy of a novel NGF vaccine strategy that reversibly alleviates spontaneous pain behaviour in surgically induced murine OA.
Keywords: immunization, vaccine, osteoarthritis, chronic pain, nerve growth factor
Key messages.
What is already known about this subject?
Nerve growth factor (NGF) is a validated target for pain in human and mouse OA.
Neutralising antibodies to NGF show therapeutic efficacy in Phase III clinical studies.
What does this study add?
Here, we demonstrate efficacy of an NGF vaccine that reversibly induces neutralising anti-NGF antibodies and suppresses pain behaviour in murine OA.
How might this impact on clinical practice or future developments?
Vaccination potentially offers a tuneable, cheaper and easier to use alternative to biological therapy in patients.
OA is the most prevalent joint disease costing approximately 1%–2.5% of the gross domestic product of developed countries.1 Greater than 75% of patients experience pain on a daily basis.2 Current standard therapies for pain relief, such as non-steroidal anti-inflammatory drugs (NSAIDs) and opioids are limited by their modest efficacy and long-term safety.3 In the last decade, nerve growth factor (NGF), a key pain sensitiser, has emerged as a promising target for OA pain. In humans, neutralising antibodies to NGF significantly suppress pain associated with late-stage OA.4 However, biological therapy in OA is likely to be limited by cost5 and by treatment failure due to anti-drug antibodies.6 Active immunisation targeting NGF represents an attractive alternative to deliver effective analgesia, while potentially providing a more economically sustainable substitute for patients. The latter is particularly the case as biosimilars replace proprietary products.7
Chronic pain in late OA can be modelled using surgical models of joint destabilisation in mice. Spontaneous pain behaviour is assessed by differential weight distribution of the hind limbs using incapacitance testing. Following joint destabilisation, mice display two phases of pain behaviour: one immediately following surgery (postoperative pain) and a second late phase that starts between weeks 7 and 11 after surgery and which is progressive (online supplementary figure 1a) with worsening joint destruction (online supplementary figure 1b, c).8 9 Both phases of pain behaviour correspond to an increase in NGF expression in the joint (online supplementary figure 1d, e)10 11 and can be neutralised by delivery of NGF’s soluble receptor (TrkAd5).10
annrheumdis-2018-214489supp001.jpeg (257KB, jpeg)
Immunisation against self-proteins can be achieved by displaying the antigen of interest on virus-like particles (VLPs). Due to their optimal size and geometry, VLPs can effectively transit to draining lymph nodes to drive antigen-dependent immunogenicity.11 Antigens are arranged as a repetitive array on the particles’ surfaces via genetic fusion or chemical conjugation to generate good polyclonal antibody responses without breaking T cell tolerance. This means that the antibody response will only occur when the antigen is presented on the VLP.12 13
Here, we describe a novel plant virus derived VLP based on the cucumber mosaic virus,14 that incorporates a tetanus toxoid epitope for T cell help (herein referred to as CuMVtt, figure 1A).15 16 Addition of a non-coding, 3' untranslated region in the VLP expression construct, leads to increased retention of encapsulated RNA suggesting greater particle stability (online supplementary figure 2a). Purified His-tagged NGF was covalently conjugated to CuMVtt (online supplementary figure 2b) as previously described for RNA-phage based VLPs.17 Native conformation of recombinant NGF was tested by its ability to bind a neutralising monoclonal antibody and the interacting domain of the high-affinity receptor (TrkA-d5) (online supplementary figure 2c, d).
annrheumdis-2018-214489supp002.jpeg (164.4KB, jpeg)
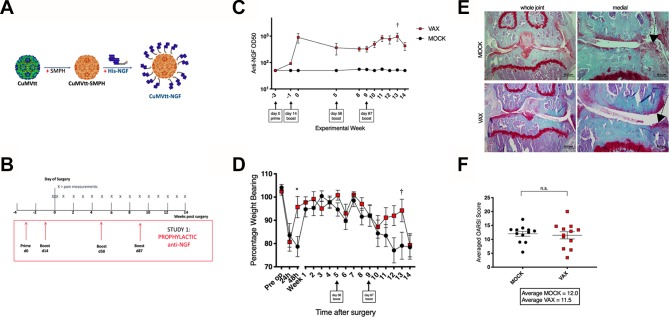
Figure 1.
Prophylactic NGF vaccination blocks murine OA pain. (A) VLP is chemically cross-linked (SMPH) to enable conjugation with His-NGF. (B) Prophylactic vaccination protocol. (C) Anti-NGF titres in sentinel cohort (n=10). (D) Painful behaviour following surgical induction of OA (n=40) measured by Linton incapacitance where 100% represents equal weight distributed across R and L limbs. Repeated-measures two-way ANOVA with Bonferroni multiple comparisons test applied, *adjusted p<0.05. SEM shown. Differences between treatment groups during late OA pain phase were not significant after correcting for multiple testing. † identifies time point of highest anti-NGF titre (see figure 2D). (E) Representative histological sections for (F) cartilage degradation (OARSI) scores 18 weeks after PMX surgery in mice treated with mock or NGF vaccine. Statistical significance is shown by two-tailed t-test. Bars represent mean±SEM, n.s.—non-significant., **p<0.01 by t-test. CuMVtt adapted from EMD: 3855.14 ANOVA, analysis of variance; NGF, nerve growth factor; PMX, partial meniscectomy; VLP, virus-like particle.
To test the therapeutic efficacy of NGF vaccination, mice were immunised with either CuMVttNGF (Vax) or CuMVttctrl (Mock) 2 weeks prior to joint destabilisation (figure 1B). Non-operated sentinel control mice also underwent vaccination to enable regular blood sampling over the experimental course. Immunisation led to seroconversion by week 3, followed by a decline in antibody titres. Additional boosts were necessary to maintain antibody levels (figure 1C). No difference in pain behaviour was detected in NGF immunised animals 24 hours postoperatively (postop), but CuMVttNGF vaccinated animals recovered from pain behaviour faster than mock-vaccinated animals (within 48 hours) (figure 1D). As expected mice were pain free for several weeks, but pain behaviour started to develop from 8 weeks postsurgery. Following a boost at 10 weeks postop, and in keeping with a concomitant rise in the serum levels of anti-NGF antibody, a reversal of pain behaviour was observed. This was maintained for 3 weeks until anti-NGF titres fell and pain behaviour resumed. At termination of the experiment, joints were harvested and scored for OA severity. No difference in disease severity between mock and vaccinated groups was observed (figure 1E,F). Sera were also collected from experimental mice at the end of the study (week 18) to measure general antibody responses. Anti-CuMV IgG levels were elevated in both vaccinated and mock-vaccinated groups compared with non-vaccinated control animals. Total IgG and IgM levels were largely consistent across all groups. There was no evidence of induction of autoantibodies such as rheumatoid factor in any of the groups (online supplementary figure 3).
annrheumdis-2018-214489supp003.jpeg (124.4KB, jpeg)
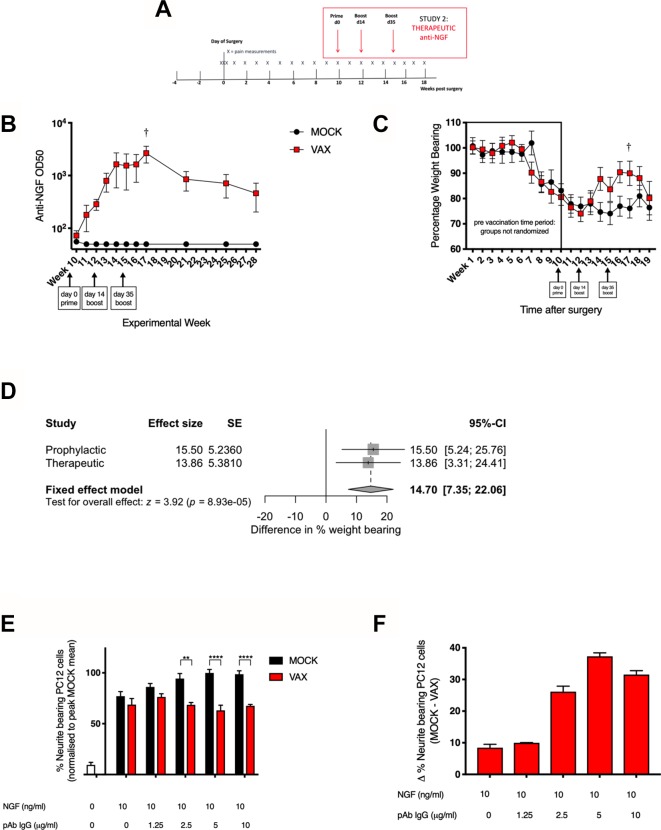
A second experiment was carried out to establish whether analgesia could be induced by immunisation after induction of pain behaviour i.e. therapeutic vaccination (figure 2A). When pain behaviour was established (10 weeks postop) mice were randomised into two groups: vaccinated and mock-vaccinated. Vaccine boosts were delivered at weeks 12 and 15 postop to maintain titres. Higher titre anti-NGF levels at the end of the experiment (around OD50 103) appeared to be associated with an analgesic response between weeks 14 and 18 postop (figure 2B,C). A subsequent spontaneous reduction in titres was associated with resumption of pain behaviour. Direct correlation between antibody levels and pain behaviour during the experiment was not possible as titres were only measured in the sentinel and not the experimental group. A meta-analysis comparing the analgesic effects across both studies at the point of highest titre in the sentinel group (week 13 for the prophylactic study and week 17 for the therapeutic study, marked by †) yielded a significant difference (p=8.93e-05) between mock and vaccinated cohorts (figure 2D). No heterogeneity of effect was detected between the two studies (I2=0, p=0.827). The sentinel cohort was maintained to follow the fall in antibody titres over the following 10 weeks, which was similar to that observed in previous studies.17 IgG purified from the serum of CuMVttNGF vaccinated, but not control mice was able to dose-dependently inhibit NGF induced neurite outgrowth in PC-12 cells (figure 2E,F), to a level similar to that seen with 150 ug/mL monoclonal anti-NGF antibody (online supplementary figure 4). The effect appeared to plateau after 5 ug/mL.
annrheumdis-2018-214489supp004.jpeg (212.3KB, jpeg)
Figure 2.
Therapeutic NGF vaccination reduces murine OA pain. (A) Therapeutic vaccination protocol. (B) Anti-NGF titres in sentinel cohort (n=10). (C) Painful behaviour measured by incapacitance testing where 100% represents equal weight distributed across R and L Limbs (n=40). Mice were randomised to receive mock or NGF vaccine at 10 weeks postsurgery. Repeated-measures two-way ANOVA with Bonferroni multiple comparisons test applied. SEM shown. Differences between treatment groups during late OA pain phase were not significant after correcting for multiple testing (D) Forest plot of meta-analysis comparing the effect size of analgesic response between mock and vaccinated cohorts at points of highest titre in the sentinel groups (week 13 for the prophylactic study, week 17 for the therapeutic study, marked by †). (E) Neurite outgrowth inhibition with increasing concentrations of IgG isolated from serum of vaccinated animals and (F) their normalised difference compared with mock-vaccinated animals. Bars represent mean±SEM, *p<0.05, ***p<0.001, ****p<0.0001 by t-test. ANOVA, analysis of variance; NGF, nerve growth factor.
Vaccines to self-antigens have been developed for other non-communicable diseases over the years. Early studies showed preclinical success but with limited clinical efficacy, which may have been due to poor immunogenicity of the vaccine platform, requiring the use of codelivery of adjuvant in preclinical models. Recent studies using refined vaccine platforms have demonstrated translatable efficacy from mouse to large animals including humans.18–20 Our results show that CuMVttNGF vaccination produces analgesia in mice when delivered both before and after pain behaviour has become established. A unique aspect of this study is to combine a novel VLP-based therapeutic vaccine with measures of spontaneous pain behaviour in murine OA; its success confirming NGF as a valid target for OA related pain.21
Implementation of this type of strategy to treat OA pain has additional benefits. It induces a polyclonal response that might be more effective than a recombinant monoclonal antibody as it will stimulate antigen removal mediated by Fc-dependent clearance mechanisms.7 It should also prevent a reduction in efficacy over time by anti-idiotypic antibodies. However, safety is also a concern. Accelerated arthropathy (rapidly progressive OA, RPOA) has been described in a small proportion of patients receiving high dose anti-NGF therapy, especially in combination with NSAIDs.3 The mechanism for this is unclear and may be related to loss of joint protection when pain is abrogated or due to, as yet, undefined disease modifying actions of NGF.3 It is therefore reassuring that this vaccination strategy does not induce long-lived antibody responses and requires regular boosting to maintain titres. While we did not observe accelerated disease in our NGF-vaccinated cohort, we recognise that safety remains a significant issue, and this would need to be monitored carefully in any future clinical development. This proof of concept study has significant translational potential; in the first instance within veterinary practice where activity measures are validated pain outcomes.22 Ultimately, this has the potential to reduce the burden of disease in humans (online supplementary files 5–7).
annrheumdis-2018-214489supp005.docx (12.2KB, docx)
annrheumdis-2018-214489supp006.docx (22.4KB, docx)
annrheumdis-2018-214489supp007.docx (14.3KB, docx)
Acknowledgments
We would like to thank Til Röhn for technical advice on NGF production, Victoria Batchelor for help during tissue harvesting, Marcia Curtinha for histological support and Florian Olomski for gifting the PC-12 cells.
Footnotes
ISvL and AE-T contributed equally.
Handling editor: Josef S Smolen
Contributors: AE-T, ISvL and TLV designed the studies. MFB originated the concept of the vaccine. JM-Z and ISvL conducted the mouse surgery and ISvL conducted the behavioural studies. AZ provided VLP constructs and developed purification strategies. AE-T produced and characterised the CuMVttNGF vaccine. AET performed the vaccinations and immunological assays. IP performed the histological preparation and in conjunction with ISvL conducted the histological analysis. LJ conducted and approved of the statistical analysis. JM-A conducted the ELISA experiment.
Funding: This work was supported by the Arthritis Research UK Centre for OA Pathogenesis (Grant 20205) and Arthritis Research UK project grant to AET and MFB (Grant 21185). ISvL is supported by a Prize studentship awarded by the Kennedy Trust for Rheumatological Research (KTRR).
Competing interests: MFB is founder of SAIBA GmbH and Hypopet AG, that own the VLP-platform IP and is involved in the development of therapeutic VLP-based vaccines for commercial purposes.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data supporting this study are available on reasonable request from the corresponding authors.
References
- 1. Reginster JY. The prevalence and burden of arthritis. Rheumatology 2002;41(Suppl 1):3–6. 10.1093/rheumatology/41.S1.3 [DOI] [PubMed] [Google Scholar]
- 2. Care A. Oa arthritis nation 2014 report. Arthritis Care 2014;2014:1–56. [Google Scholar]
- 3. Lane NE, Corr M. Osteoarthritis in 2016: Anti-NGF treatments for pain - two steps forward, one step back? Nat Rev Rheumatol 2017;13:76–8. 10.1038/nrrheum.2016.224 [DOI] [PubMed] [Google Scholar]
- 4. Lane NE, Schnitzer TJ, Birbara CA, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med 2010;363:1521–31. 10.1056/NEJMoa0901510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Losina E, Michl G, Collins JE, et al. Model-based evaluation of cost-effectiveness of nerve growth factor inhibitors in knee osteoarthritis: impact of drug cost, toxicity, and means of administration. Osteoarthritis Cartilage 2016;24:776–85. 10.1016/j.joca.2015.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aubin F, Carbonnel F, Wendling D. The complexity of adverse side-effects to biological agents. J Crohns Colitis 2013;7:257–62. 10.1016/j.crohns.2012.06.024 [DOI] [PubMed] [Google Scholar]
- 7. Bachmann MF, Whitehead P. Active immunotherapy for chronic diseases. Vaccine 2013;31:1777–84. 10.1016/j.vaccine.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 8. Inglis JJ, McNamee KE, Chia S-L, et al. Regulation of pain sensitivity in experimental osteoarthritis by the endogenous peripheral opioid system. Arthritis Rheum 2008;58:3110–9. 10.1002/art.23870 [DOI] [PubMed] [Google Scholar]
- 9. Driscoll C, Chanalaris A, Knights C, et al. Nociceptive sensitizers are regulated in damaged joint tissues, including articular cartilage, when osteoarthritic mice display pain behavior. Arthritis Rheumatol 2016;68:857–67. 10.1002/art.39523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McNamee KE, Burleigh A, Gompels LL, et al. Treatment of murine osteoarthritis with TrkAd5 reveals a pivotal role for nerve growth factor in non-inflammatory joint pain. Pain 2010;149:386–92. 10.1016/j.pain.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 11. Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol 2010;10:787–96. 10.1038/nri2868 [DOI] [PubMed] [Google Scholar]
- 12. Chackerian B, Frietze KM. Moving towards a new class of vaccines for non-infectious chronic diseases. Expert Rev Vaccines 2016;15:561–3. 10.1586/14760584.2016.1159136 [DOI] [PubMed] [Google Scholar]
- 13. Jennings GT, Bachmann MF. Immunodrugs: therapeutic VLP-based vaccines for chronic diseases. Annu Rev Pharmacol Toxicol 2009;49:303–26. 10.1146/annurev-pharmtox-061008-103129 [DOI] [PubMed] [Google Scholar]
- 14. Zeltins A, West J, Zabel F, et al. Incorporation of tetanus-epitope into virus-like particles achieves vaccine responses even in older recipients in models of psoriasis, Alzheimer's and cat allergy. NPJ Vaccines 2017;2 10.1038/s41541-017-0030-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol 2013;14:428–36. 10.1038/ni.2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goncalves L, Albarran B, Salmen S, et al. The nonresponse to hepatitis B vaccination is associated with impaired lymphocyte activation. Virology 2004;326:20–8. 10.1016/j.virol.2004.04.042 [DOI] [PubMed] [Google Scholar]
- 17. Röhn TA, Ralvenius WT, Paul J, et al. A virus-like particle-based anti-nerve growth factor vaccine reduces inflammatory hyperalgesia: potential long-term therapy for chronic pain. J Immunol 2011;186:1769–80. 10.4049/jimmunol.1000030 [DOI] [PubMed] [Google Scholar]
- 18. Tissot AC, Maurer P, Nussberger J, et al. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: a double-blind, randomised, placebo-controlled phase IIA study. Lancet 2008;371:821–7. 10.1016/S0140-6736(08)60381-5 [DOI] [PubMed] [Google Scholar]
- 19. Fettelschoss-Gabriel A, Fettelschoss V, Thoms F, et al. Treating insect-bite hypersensitivity in horses with active vaccination against IL-5. J Allergy Clin Immunol 2018;142:1194–205. 10.1016/j.jaci.2018.01.041 [DOI] [PubMed] [Google Scholar]
- 20. Cavelti-Weder C, Timper K, Seelig E, et al. Development of an interleukin-1β vaccine in patients with type 2 diabetes. Mol Ther 2016;24:1003–12. 10.1038/mt.2015.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mantyh PW, Koltzenburg M, Mendell LM, et al. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology 2011;115:189–204. 10.1097/ALN.0b013e31821b1ac5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lascelles BDX, Brown DC, Maixner W, et al. Spontaneous painful disease in companion animals can facilitate the development of chronic pain therapies for humans. Osteoarthritis Cartilage 2018;26:175–83. 10.1016/j.joca.2017.11.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2018-214489supp001.jpeg (257KB, jpeg)
annrheumdis-2018-214489supp002.jpeg (164.4KB, jpeg)
annrheumdis-2018-214489supp003.jpeg (124.4KB, jpeg)
annrheumdis-2018-214489supp004.jpeg (212.3KB, jpeg)
annrheumdis-2018-214489supp005.docx (12.2KB, docx)
annrheumdis-2018-214489supp006.docx (22.4KB, docx)
annrheumdis-2018-214489supp007.docx (14.3KB, docx)