Abstract
nflammatory and apoptotic caspases mediate two distinct forms of cell death: pyroptosis and apoptosis, respectively. Three independent studies have now demonstrated that the “apoptotic” caspase‐8 can cleave gasdermin D (GSDMD) leading to pyroptosis‐like cell death and IL‐1β release in murine macrophages (Orning et al, 2018; Sarhan et al, 2018; Chen et al, 2019). Orning et al and Chen/Demarco et al also show that the NLRP3 inflammasome is activated downstream of active caspase‐8, but they attribute this inflammasome activation to different pore‐forming proteins, GSDMD and pannexin‐1, respectively (Orning et al, 2018; Chen et al, 2019).
Subject Categories: Autophagy & Cell Death, Immunology
Different forms of programmed cell death are distinguished by distinct morphological changes and the proteins involved (Fig 1A–C; Galluzzi et al, 2018). Apoptosis is well characterised as non‐inflammatory cell death triggered either by extrinsic or intrinsic stimuli, followed by consequential activation of initiator caspases (caspase‐8 or caspase‐9) and executioner caspases (caspase‐3 and caspase‐7). This leads to cell shrinking, membrane blebbing, DNA fragmentation and formation of apoptotic bodies containing cellular contents. Necroptosis, a necrotic form of cell death, can occur upon ligation of death receptors by proapoptotic stimuli, such as TNFα, in the absence of caspase‐8 activation. The pore‐forming protein MLKL mediates plasma membrane disruption, cell swelling and eventual cell lysis. Pyroptosis, another form of necrotic cell death, is typically triggered by activation of pro‐inflammatory caspase‐1/caspase‐4/caspase‐5 in humans (caspase‐1/caspase‐11 in mice), which leads to GSDMD cleavage, pore formation and eventual cell lysis. Caspase‐1 is activated by the formation of the multi‐protein complexes termed inflammasomes consisting of a cytosolic pathogen‐recognition receptor (e.g. NLRP3) and the adaptor protein ASC, which recruits caspase‐1. Pyroptosis promotes inflammation by the release of the cytokines IL‐1β and IL‐18 as well as proteins and nucleic acids acting as danger signals.
Figure 1. Mechanisms of cell death: the connections between caspases and gasdermin D.
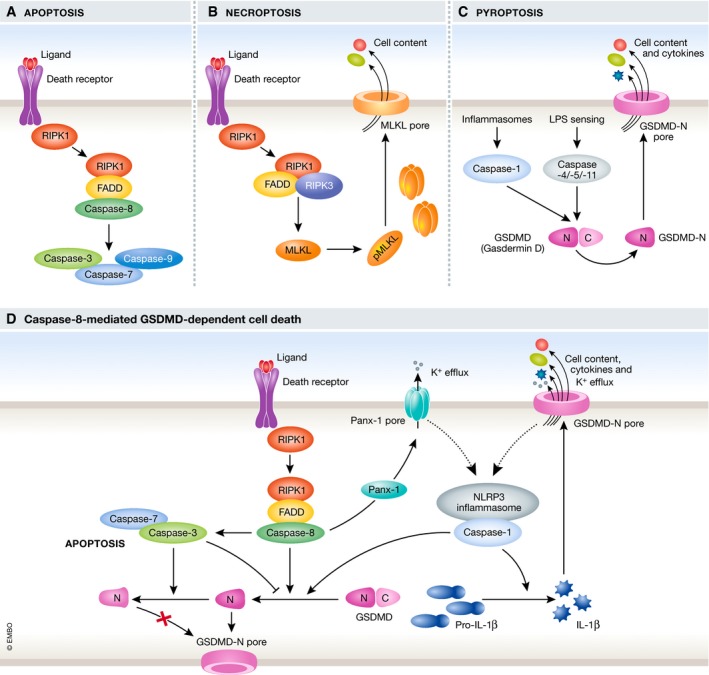
Diverse forms of cell death were traditionally thought to be independent of each other, but emerging evidence is challenging this view. In a variety of scenarios including infection with different Yersinia species such as Y. pestis and Y. pseudotuberculosis, activation of the apoptotic caspase‐8 and inflammatory cell death have been observed to coincide (Philip et al, 2014; Lawlor et al, 2015, 2017). Now, three independent studies have revealed synchronicity between caspase‐8 activity and GSDMD‐mediated cell death in multiple scenarios (Fig 1D). Utilising a combination of the apoptosis trigger TNFα and pharmacological inhibitors blocking pro‐survival signals (e.g. SMAC mimetic or the TAK1 inhibitor 5z‐7‐oxozeaenol), Chen/Demarco et al observe inflammatory caspase‐8‐mediated cell death in murine macrophages (Chen et al, 2019). Sarhan et al and Orning et al also observe caspase‐8‐driven inflammatory cell death studying TNFα/TAK1 inhibitor co‐stimulation or infection with Yersinia, which inhibits TAK1 through the bacterial protein YopJ (Orning et al, 2018; Sarhan et al, 2018). Sarhan et al (2018) established that the necrotic cell death they observed resembled pyroptosis based on the timing of uptake of the nuclear dye PI and Annexin V staining of the plasma membrane. Cells from various genetic knock‐out mice and pharmacological inhibitors were used to dissect this pyroptosis‐like pathway, and Orning et al (2018), Sarhan et al (2018) uniformly concluded that RIPK1‐mediated activation of caspase‐8 drives GSDMD cleavage and consequential pyroptosis, as measured by lactate dehydrogenase (LDH) release. GSDMD−/− cells were not, however, protected from cell death and upon stimulation, but instead underwent apoptosis with release of some LDH which was absent in GSDMD−/−/caspase‐3−/−/‐7−/− cells, suggesting that either GSDMD‐independent apoptosis or GSDMD‐dependent pyroptosis could occur (Orning et al, 2018; Sarhan et al, 2018; Chen et al, 2019).
To assess whether active caspase‐8 triggered inflammasome formation and subsequent GSDMD cleavage, the three groups assessed different inflammasome‐deficient knock‐out cells. They found that the NLRP3 inflammasome was formed as ASC oligomerised, caspase‐1 was active and cells released IL‐1β. Inflammasome activation, however, unexpectedly appeared to be downstream of GSDMD cleavage (Orning et al, 2018; Sarhan et al, 2018; Chen et al, 2019). Orning et al showed that recombinant mouse GSDMD is cleaved by purified active caspase‐8 (Orning et al, 2018), but although it was less efficient at GSDMD processing in comparison with caspase‐1, its activity was sufficient to trigger pyroptotic cell death in murine macrophages.
Caspase‐1‐mediated GSDMD cleavage generates a pyroptotic p30 fragment, whereas upon Yersinia infection or TNFα/TAK inhibitor treatment, GSDMD was cleaved into p43, p30 and p20 fragments (Orning et al, 2018; Sarhan et al, 2018; Chen et al, 2019). To assess whether caspase‐8 generated the same pyroptosis‐mediating p30 fragment as caspase‐1, Chen/Demarco et al made use of a HEK reconstitution system in which they tested different non‐cleavable GSDMD constructs. Caspase‐8 activity led to cleavage at position D276, the cleavage site used by caspase‐1 generating the p30 fragment (Chen et al, 2019). The p43 and p20 fragment were found to be generated by cleavage of full‐length and the p30 fragment, respectively, at D88 in a caspase‐3‐dependent way (Chen et al, 2019). This suggests inactivation of GSDMD and thereby counteracting pyroptosis during caspase‐3‐mediated apoptosis. In line with this, Chen/Demarco et al reported that cells from a knock‐in mouse bearing a GSDMD D88A mutation showed an increase in pyroptotic cell death (Chen et al, 2019).
Typical pyroptosis is characterised by the release of IL‐1β and IL‐18, and caspase‐8‐induced pyroptosis coincided with NLRP3 activation and IL‐1β secretion (Orning et al, 2018; Chen et al, 2019). Conflicting conclusions emerge from these three studies, however, regarding the pathway leading to NLRP3 inflammasome activation and cytokine secretion. Orning et al suggest that NLRP3 activation is GSDMD‐dependent based on delays in ASC oligomerisation in GSDMD−/− cells. Conversely, Chen/Demarco et al observed normal caspase‐1 processing in GSDMD−/− and/or GSDME−/− cells. Cells from the GSDMD D88A knock‐in mouse also showed no increase of caspase‐1 processing suggesting that GSDMD activation and caspase‐1 processing were not linked (Chen et al, 2019). Chen/Demarco et al suggest that through RIPK3 involvement, the channel‐forming pannexin‐1 was activated and promotes downstream NLRP3 and caspase‐1 activation. IL‐1β release, however, was only partially decreased in pannexin‐1−/− cells (Chen et al, 2019). They speculate that this is due to apoptosis‐driven caspase‐8‐mediated IL‐1β cleavage, which has also been reported by others (Chauhan et al, 2018; Vince et al, 2018). It should be noted, however, that all studies detected rather low levels of released IL‐1β suggesting either inefficient transcription/translation or maturation of pro‐IL‐1β. Sarhan et al (2018) offer an interesting explanation as they report that IL‐1β is not released from TAK1‐inhibited cells, because TAK1 inhibition prevents transcription of pro‐IL‐β. Instead, IL‐1β appeared to be secreted from TAK1‐sufficient cells with the mechanism by which IL‐1β is matured in those cells remaining to be determined. Here, the three studies disagree in how downstream inflammasome activation is mediated post‐caspase‐8 activation with GSDMD and pannexin‐1 both suggested as relevant.
In summary, three studies show that Yersinia infection or extrinsic apoptosis triggers can lead to GSDMD cleavage and subsequent pyroptosis‐like cell death in murine macrophages, which is mediated by the apoptotic caspase‐8 (Orning et al, 2018; Sarhan et al, 2018; Chen et al, 2019; Fig 1D). Downstream of GSDMD processing, the NLRP3 inflammasome becomes activated leading to cytokine maturation and release, thereby promoting inflammation. Another apoptotic caspase, caspase‐3, appears to counteract the cell death observed by processing the pyroptotic‐p30 fragment and full‐length GSDMD further into the inactive p20 and p43 fragments, respectively. This caspase‐3 activity together with the observation that, in the absence of GSDMD, cells underwent apoptosis demonstrates that several cell death pathways are triggered upon stimulation and that they do not operate exclusively. This raises the question of what shifts the balance between caspase‐8‐mediated GSDMD cleavage and caspase‐3 activation and the subsequent GSDMD inactivation. Intriguingly, caspase‐3 inhibition of pyroptosis has also been shown to cleave another Gasdermin family member, Gasdermin E (GSDME), leading to secondary necrosis (Rogers et al, 2017; Wang et al, 2017). Sarhan et al and Chen/Demarco et al also detected GSDME cleavage (Sarhan et al, 2018; Chen et al, 2019), which was mediated by caspase‐3 (Sarhan et al, 2018). GSDME−/− cells, however, did not show a significant decrease in cell death. Understanding the role of caspase‐3 in the crosstalk between cell death pathways as well as the consequential downstream effects of separate pathways should be a focus of future studies. Determining the physiological relevance of the described pathway is important, especially with the suggestion that this pathway does not occur in human macrophages under the same conditions (Sarhan et al, 2018). Further work should explore the balance of caspase‐3 in inhibition and activation of pyroptosis and apoptosis, respectively, and how the crosstalk between the pathways mediates the response to infection or inflammation in vivo.
Acknowledgements
CEB is supported by a Wellcome Trust Investigator Award 108045/Z/15/Z.
The EMBO Journal (2019) 38: e102065
See also: KW Chen et al (May 2019)
[The copyright line of this article was changed on 23 April 2019 after original online publication.]
References
- Chauhan D, Bartok E, Gaidt MM, Bock FJ, Herrmann J, Seeger JM, Broz P, Beckmann R, Kashkar H, Tait SWG et al (2018) BAX/BAK‐induced apoptosis results in Caspase‐8‐dependent IL‐1beta maturation in macrophages. Cell Rep 25: 2354–2368. e2355 [DOI] [PubMed] [Google Scholar]
- Chen KW, Demarco B, Heilig R, Shkarina K, Boettcher A, Farady CJ, Pelczar P, Broz P (2019) Extrinsic and intrinsic apoptosis activate pannexin‐1 to drive NLRP3 inflammasome assembly. EMBO J 38: e101638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW et al (2018) Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25: 486–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D'Cruz AA, Hall C, Kaur Spall S, Anderton H, Masters SL et al (2015) RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun 6: 6282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor KE, Feltham R, Yabal M, Conos SA, Chen KW, Ziehe S, Grass C, Zhan Y, Nguyen TA, Hall C et al (2017) XIAP loss triggers RIPK3‐ and Caspase‐8‐driven IL‐1beta activation and cell death as a consequence of TLR‐MyD88‐induced cIAP1‐TRAF2 degradation. Cell Rep 20: 668–682 [DOI] [PubMed] [Google Scholar]
- Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, Brooks A, Xia S, Wu H, Kelliher MA et al (2018) Pathogen blockade of TAK1 triggers caspase‐8‐dependent cleavage of gasdermin D and cell death. Science 362: 1064–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NH, Dillon CP, Snyder AG, Fitzgerald P, Wynosky‐Dolfi MA, Zwack EE, Hu B, Fitzgerald L, Mauldin EA, Copenhaver AM et al (2014) Caspase‐8 mediates caspase‐1 processing and innate immune defense in response to bacterial blockade of NF‐kappaB and MAPK signaling. Proc Natl Acad Sci USA 111: 7385–7390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C, Fernandes‐Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES (2017) Cleavage of DFNA5 by caspase‐3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun 8: 14128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, Rongvaux A, Bunnell SC, Shao F, Green DR et al (2018) Caspase‐8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci USA 115: E10888–E10897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince JE, De Nardo D, Gao W, Vince AJ, Hall C, McArthur K, Simpson D, Vijayaraj S, Lindqvist LM, Bouillet P et al (2018) The mitochondrial apoptotic effectors BAX/BAK activate Caspase‐3 and ‐7 to trigger NLRP3 inflammasome and Caspase‐8 driven IL‐1beta activation. Cell Rep 25: 2339–2353. e2334 [DOI] [PubMed] [Google Scholar]
- Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F (2017) Chemotherapy drugs induce pyroptosis through caspase‐3 cleavage of a gasdermin. Nature 547: 99–103 [DOI] [PubMed] [Google Scholar]


