Abstract
In this study, methodological factors influencing the dissolution of metal(loid)s in simulated lung fluid (SLF) were assessed in order to develop a conservative method for the assessment of inhalation bioaccessibility in PM2.5. To achieve this aim, the effects of solid to liquid (S/L) ratio (1:100 to 1:5000), agitation (no agitation, occasional shaking, orbital and end-over-end rotation), composition of SLF (artificial lysosomal fluid: ALF; phagolysosomal simulant fluid: PSF) and extraction time (1 to 120 h) on metal(loid) bioaccessibility were investigated using PM2.5 from three Australian mining/smelting impacted soils and a certified reference material. The results highlighted that SLF composition significantly (p < 0.001) influenced metal(loid) bioaccessibility and that when a S/L ratio of 1:5000 and end-over-end rotation was used, metal(loid) solubility plateaued after approximately 24 h. Using the methodological parameters that yielded the most conservative estimate of metal(loid) bioaccessibility, PM2.5 was then subjected to simulated gastro-intestinal tract (GIT) solutions to simulate lung clearance and swallowing and the results were compared to extraction using SLF alone. Although metal(loid) bioaccessibility in SLF alone (24 h) varied from simulated GIT solutions alone (p < 0.05), there was no significant difference (p > 0.05) when SLF alone (24 h) was compared to SLF followed by simulated GIT solutions.
Keywords: Bioaccessibility, PM2.5, SLF, ALF, PSF, Inhalation
1. Introduction
Epidemiological studies have consistently linked chronic and short term exposure to fine particulate matter with an aerodynamic diameter of < 2.5 μm (PM2.5) to adverse health outcomes (Fajersztajn et al., 2017; Pinault et al., 2017; Pope and Dockery, 2006; Pun et al., 2017). The main constituents of PM2.5 include sulphate (20%), crustal materials (soil, sand, road and desert dust; 13.4%), equivalent black carbon (11.9%), NH4NO3 (4.7%), sea salt (2.3%), trace element oxides (1%), water (7.2%) and residual matter (40%) (Snider et al., 2016). An increase of 10 μg PM2.5 m3 was linked to increased respiratory illness related hospital admission frequencies and 1.04% in mortality (Atkinson et al., 2014). Recent research highlighted that metals in PM2.5 (e.g. Cu, Fe, Mn and Ni) collected at traffic intersections may have considerable oxidative capabilities (Fujitani et al., 2017). Additionally, metals in aqueous extracts of PM2.5 have been demonstrated to cause lung inflammation and injury, oxidative stress, lipid and protein damage and cardiovascular injury in mouse and rat models (Gavett et al., 2003; Pardo et al., 2016; Shuster-Meiseles et al., 2016). Similarly, metals (Fe, Cu, Ni, Co and Cr) in PM2.5 were also associated with inflammatory responses in mouse type II alveolar cells (He et al., 2017). Therefore, although metal oxides may comprise a small proportion of PM2.5, it may represent substantial potential to cause human health injuries. This is particularly relevant for mining/smelting impacted PM2.5 because fine particulate matter with elevated toxic metal(loid)s from smelter activities may persist in residential areas (e.g. sidewalks) for a long time as reported in northern France by Pelfrêne and Douay (2017). Furthermore, because of its small mass, PM2.5 may stay airborne for extended periods and travel long distances, impacting communities far from point sources.
Instead of using total meta(loid) concentration for human exposure assessment, it is more relevant to use the concentration of metal(loid)s in PM2.5 that may potentially dissolve in the lung fluid and be absorbed into the blood. Although the bioavailable fraction (metal(loid)s absorbed into the systemic circulation) remains the most appropriate, exposure assessment refinement using the bioaccessible fraction (metal(loid) extracted using simulated lung fluid (SLF)) is often more desirable as a rapid and cost effective approach. PM2.5 may stimulate engulfment by lung macrophages in the respiratory system and metal(loid) dissolution may take place within the acidic environment of phagolysosomes. Several SLFs have been developed to simulate phagolysosomal fluid, e.g. simulated intracellular fluid, artificial lysosomal fluid (ALF) and phagolysosomal simulant fluid (PSF), the latter two being more popular in bioaccessibility assays (Kastury et al., 2017). However, the extraction efficiencies of these SLFs have not been compared. Additionally, significant knowledge gaps exist in methods currently used to determine metal(loid) bioaccessibility in PM2.5 (Kastury et al., 2017). For example, the solid to liquid (S/L) ratio used ranges significantly (1:100 –1:1163) (Hamad et al., 2014a; Potgieter-Vermaak et al., 2012; Wiseman and Zereini, 2014) or is not reported because a part of the filter paper with which the particles were collected was used directly in assays (Mukhtar and Limbeck, 2013; Schaider et al., 2007); agitation varies from occasional (Wiseman and Zereini, 2014), continuous (Hamad et al., 2014b; Potgieter-Vermaak et al., 2012) or ultrasonic (Mukhtar and Limbeck, 2013); and extraction time varies from 1 (Mukhtar and Limbeck, 2013) to 120 h (Schaider et al., 2007). Furthermore, approximately 85 to 90% of deposited particles, including those engulfed by macrophages, may be cleared from the lung within 24 h, although clearance from the alveolar region is assumed to be slower (Hofmann, 2011). Because particles cleared from the lungs are transported into the gastrointestinal tract (GIT), several researchers have used dissolution in gastric solution alone to determine metal(loid) bioaccessibility from PM2.5 (Colombo et al., 2008; Okorie et al., 2012; Puls et al., 2012). Evaluation of metal(loid) dissolution in simulated GIT solutions alone or after leaching in SLF has not been investigated.
The overarching aim of this study was to develop a conservative method to assess inhalation bioaccessibility of metal(loid) in PM2.5 from environmental samples. To achieve this aim, the effect of solid to liquid (S/L) ratio, agitation, SLF composition and extraction time on metal(loid) bioaccessibility in PM2.5 was assessed using SLF and compared to the extraction efficiencies using GIT solutions. Seven metal(loid)s (Al, As, Cd, Fe, Mn, Pb, Zn) from three environmental matrices and a certified reference material were used to develop a standardised inhalation bioaccessibility assay for metal(loid)s in PM2.5.
2. Materials and Methods
2.1. Collection of PM2.5
Surface soils (0 to 20 cm) from three Australian mining/smelting impacted sites were collected: historic non-ferrous slag impacted soil from York Peninsula (SH15), smelting impacted soil from Port Pirie (PP) and calcinated mine waste (CMW) from the golden triangle region of Victoria. Soil (< 2 mm) was dried at 40°C and sieved to recover the < 53 μm particle size fraction using an Endecotts Octagon digital shaker. To extract the fine dust fraction (PM2.5), 2 to 5 g of the < 53 μm particle size fraction was applied to a hydrocyclone connected to a three speed vacuum cleaner (Pullman). Upon entering the system, the fine dust fraction travelled towards the top of the cyclone and was collected onto a glass microfiber filter paper (Whatman, grade GF/A, 1.2 μm pore size). The remaining > 2.5 μm particle size fraction was captured in a plastic vessel below the cyclone and discarded. Fine particulate matter (stock PM2.5) depositing on the filter paper was collected, pooled, homogenised (end-over-end rotation for 24 h) and stored at 20°C.
2.2. Physicochemical characterisation of PM2.5
Particle size distribution was analysed by dispersing 5 mg of PM2.5 in 0.1 M NaCl overnight (end-over-end rotation), followed by analysis using a Particle Sizer 380 ZLS (NICOPM). The BET surface area in samples was determined using an ASAP 2420 (Micrometritics) after thermal degassing at 200°C overnight. Total metal(loid) concentration was determined using ICP-MS (ASX-500 series), following aqua-regia digestion (1:3 – 70% HNO3: 36.5% HCl) (MARS-6 microwave (CEM) and USEPA method 3051 (USEPA, 1998)). X-ray absorption spectroscopy (XAS) was utilised to determine As, Fe and Pb speciation (MRCAT beamlines 10-BM (As and Fe) and 10-ID (Pb)), Sector 10, at the Advanced Photon Source of the Argonne National Laboratory, U.S following methods described in (Kropf et al., 2010; Segre et al., 2000).
2.3. Formulation of simulated lung fluids
Artificial lysosomal fluid (ALF) and phagolysosomal simulant fluid (PSF) were freshly made by dissolving the following in 1 L of MilliQ water and adjusting the pH to 4.5:
ALF:
3.21 g Sodium chloride, 6.0 g Sodium hydroxide, 20.8 g Citric acid, 0.128 g Calcium chloride (dihydrate), 0.071 g Sodium hydrogen phosphate dibasic (anhydrous), 0.039 g Sodium sulphate, 0.05 g Magnesium chloride (anhydrous), 0.059 g Glycine, 0.077 g Sodium citrate (dihydrate), 0.09 g Sodium tartrate (dihydrate), 0.085 g Sodium lactate, 0.086 g Sodium pyruvate, 0.05 g Benzalkonium chloride.
PSF:
6.65 g Sodium chloride, 0.029 g Calcium chloride (dihydrate), 0.142 g Sodium hydrogen phosphate dibasic (anhydrous), 0.071 g Sodium sulphate, 0.45 g Glycine, 4.085 g Potassium hydrogen phthalate, 0.05 g Benzalkonium chloride.
2.4. Assessment of metal(loid) bioaccessibility using SLF
2.4.1. The effect of solid to liquid (S/L) ratio on metal(loid) bioaccessibility
Artificial lysosomal fluid (ALF) was utilised in this assay as it is the most commonly used SLF to assess the bioaccessibility of metal(loid)s in PM2.5. The solid (g) to liquid (mL) ratios (S/L) tested in this assay were 1:100, 1:500, 1:1000 and 1:5000. When necessary, the pH was re-adjusted to 4.5 ± 0.5 at the start of the assay using 1 M NaOH, which was performed at 37°C for up to 120 h using end-over-end rotation at 45 rpm. Samples were collected at 1, 8, 24, 48, 72, 96 and 120 h and centrifuged at 13,000 rpm (18 g) for 3 minutes to separate the solid from liquid. The supernatant was diluted with 0.1 M HNO3 and stored at 4°C until analysis using ICP-MS (ASX-500 series).
2.4.2. The effect of agitation on metal(loid) bioaccessibility
Using an S/L ratio of 1:5000, metal(loid) bioaccessibility in PM2.5 was assessed using ALF with no agitation, occasional agitation (15 minutes of orbital rotation at 150 rpm, once a day), orbital rotation (150 rpm) and end-over-end rotation (45 rpm). Assays were conducted for up to 120 h at 37°C, with sample collection, separation and metal(loid) analysis performed according to section 2.3.1.
2.4.3. The effect of SLF composition on metal(loid) bioaccessibility
The extraction efficiencies of ALF and PSF were investigated in this assay as they are the two most widely used SLFs. Using an S/L ratio of 1:5000 and end-over-end rotation (45 rpm), PM2.5 was assessed for up to 120 h at 37°C. The starting pH was readjusted to 4.5 ± 0.5 in ALF and PSF using 1 M NaOH and KOH respectively. Sample collection and separation was performed according to section 2.3.1. Metal(loid)s were analysed by ICP-MS using matrix matched calibration to avoid matrix bias.
2.5. The effect of simulated GIT solutions on metal(loid) bioaccessibility
This assay was conducted in two stages at 37°C to investigate the combined effect of particle deposition in the lung, followed by simulation of lung clearance and passage through the GIT. In stage 1, PM2.5 was added to ALF (S/L ratio 1:5000), pH adjusted to 4.5 ± 0.1 and agitated for 24 h using end-over-end rotation (45 rpm) in a 50 mL Falcon tube. At the end of the assay, samples were centrifuged at 4000 rpm for 10 minutes to separate the solid from liquid. The supernatant was decanted, reserving 10 mL for metal(loid) analysis (lung phase sample), which was acidified using 0.1 M HNO3 and stored at 4°C until analysis. Stage 2 was conducted using the SBRC method (Kelley et al., 2002) with modification described by? Juhasz et al, (2009a). Simulated gastric solution (40 ml, 0.4 M glycine, pH adjusted to 1.5 ± 0.05 using concentrated HCl) was added to the residual PM2.5 from step 1 and rotated end-over-end (45 rpm). After 1 hour, gastric phase samples (4 mL) were collected, syringe filtered (0.45 μm), acidified with 0.1 M HNO3 and stored at 4°C until analysis. Bovine bile (70 mg) and porcine pancreatin (20 mg), dissolved in 3 mL MilliQ water, was then added to each tube to simulate the conditions in the small intestines, while the pH was adjusted to 7.0 ± 0.1 (using 50% and 5% NaOH; ~ 1 ml). Samples were rotated end-over-end for 4 h, after which, intestinal phase samples (10 mL) were collected by syringe filtration (0.45 μm), acidified with 0.1 M HNO3 and stored at 4°C until analysis. To avoid matrix bias, matrix matched calibration was used during the analysis of metal(loid)s by ICP-MS.
2.6. Quality assuarance, quality control and statistical analysis
The accuracy of the aqua-regia digestion method was confirmed by including duplicate analysis, check values and a quantitative average As and Pb recovery from NIST 2710a (1540 ± 100 mg As kg−1 and 5522 ± 30 mg Pb kg−1). The average deviation between duplicate samples (n = 3) was 4.8% for As and 4.7% for Pb. The average As and Pb recovery from spiked samples (n = 3) was 93.8% and 107.8% respectively, whereas average check value recoveries (n = 3) for As was 99.8% and Pb was 104.3%.
Statistical significance of differences among metal(loid) bioaccessibility determined in sections 2.4.1, 2.4.2 and 2.4.3 were conducted using Two-way ANOVA (α = 0.05), treating each time point independently. Statistical significance in section 2.5 for metal(loid) bioaccessibility was conducted using One-way ANOVA (α = 0.05).
3. Results and Discussion
The complex nature of PM2.5 composition, deposition and clearance has contributed to the variability in the in-vitro methods used for bioaccessibility assessment. This study focused on investigating the effects of the methodological factors that influence metal(loid) bioaccessibility using SLF (e.g. sample matrix, methodological parameters, effect of ingestion following inhalation), in order to standardise a conservative method that is biologically relevant to a human inhalation scenario. Upon inhalation, PM2.5 deposition in the lung stimulates engulfment by epithelial and alveolar macrophages, creating a phagosome. The fusion of a lysosome to the phagosome creates a phagolysosome with an internal pH of 4.5, where metal(loid) dissolution may occur depending on its mineralogy and speciation. For the purposes of optimising a protocol for bioaccessibility testing, it was assumed that once inhaled, 100% of the PM2.5 was deposited in the lung and was engulfed by macrophages. Furthermore, it was also assumed that upon clearance from the lung by the mucociliary action, PM2.5 passed through the GIT. Although PM2.5 may contain fine (0.1–2.5 μm) and ultrafine (≤ 0.1 μm) particles, those with aerodynamic sizes between 0.1– 2 μm stimulate macrophage engulfment most efficiently (Black, 1999), while ultrafine particles may be absorbed into the pulmonary interstitium and exert additional toxicity, such as the generation of reactive oxygen species (Oberdörster, 2000). Because metal(loid) dissolution alone is assessed in a bioaccessibility assay, additional toxicity caused by fine and ultrafine particles was considered to be out of scope for this study. In discussing the results, particular focus was given to As and Pb as these two metal(loid)s have been identified by the Agency for Toxic Substances and Disease Registry as the number 1 and 2 contaminants in the Priority List of Hazardous Substances (2017).
3.1. Physico-chemical characteristics of mining/smelting impacted PM2.5
The total concentration of nine trace elements (As, Cd, Cr, Co, Cu, Mn, Ni, Pb and Zn) and six major elements (Al, Ca, Fe, K, Mg and P) in PM2.5 is given in Table 1. The concentration of As was the highest in CMW (15240±190 mg/kg), followed by SH15 (2010±17.2 mg/kg) and PP (160±0.8 mg/kg). Similar to CMW, elevated As concentrations in soil samples from gold mining regions have been reported previously (Meunier et al., 2010) and may be associated with the sulphidic phases in gold ores (Ollson et al., 2016). The results of speciation analysis demonstrated that the majority of the As present in all three samples was sorbed species (70–90%) with the remainder present as scorodite and beudantite (Table 2). The concentration of Pb was highest in PP (7450±490 mg/kg) as a result of historic Pb smelting activity with Pb present predominantly as sorbed / bound phases (93%; Table 2). Similar to PP, Pb in CMW (1800±10 mg/kg) was also present as sorbed / bound phases, however, tertiary Pb phosphate, hydroxypyromorphite and litharge were observed in SH15 (1330±16 mg/kg).
Table 1.
Total metal(loid) concentration (mean ± SEM), particle size distribution (mean ± SEM) and BET surface area in PM2.5 from mining and smelting impacted materials.
| Minor element concentration (mg/kg) | Major element concentration (mg/kg) | Mean particle diameter (μm) | BET surface area (m2/g) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Co | Cr | Cu | Mn | Ni | Pb | Zn | Al | Ca | Fe | K | Mg | P | |||
| SH15 | 2011 ± 17.2 | 45.5 ± 0.4 | 16.9 ± 0.15 | 46.9 ± 0.19 | 175 ± 0.66 | 1319 ± 14.7 | 26.3 ± 0.34 | 1330 ± 16.2 | 17578 ± 245 | 26747 ± 187 | 113848 ± 743 | 40486 ± 415 | 6832 ± 18.6 | 23330 ± 209 | 3447 ± 17 | 1.2 ± 0.05 | 4.4 |
| PP | 156 ± 0.8 | 37.2 ± 0.2 | 17.6 ± 0.7 | 46.1 ± 1.5 | 350 ± 21.5 | 1180 ± 85.7 | 27.1 ± 0.99 | 7454 ± 485 | 9873 ± 377 | 36726 ± 2353 | 57612 ± 2532 | 35044 ± 913 | 10616 ± 556 | 15777 ± 567 | 1286 ± 19.5 | 2.17 ± 0.65 | 32.1 |
| CMW | 15242 ± 192 | 5.02 ± 0.07 | 225 ± 2.66 | 70 ± 0.59 | 551 ± 5.89 | 763 ± 9.01 | 533 ± 4.59 | 1803 ± 10.0 | 1592 ± 17.4 | 14178 ± 573 | 9913 ± 188 | 384934 ± 5659 | 4136 ± 226 | 8307 ± 59 | 508 ± 3.24 | 1.6 ± 0.05 | 18.7 |
Table 2.
Arsenic and lead speciation in SH15, PP and CMW PM2.5.
| As Speciation Weighted Percentage | Pb Speciation Weighted Percentage | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Mineral Sorbed As(V) | Mineral Sorbed As(III) | Scorodyte | Jarosite – As (V) | Beudantite | Mineral Sorbed Pb | Organic Bound Pb | Ter. Pb Phosphate | Hydroxypyromorphite | Litherage | Plumbojarosite |
| SH15 | 73 | 17 | 9 | 66 | 17 | 11 | 6 | ||||
| PP | 70 | 5 | 17 | 7 | 65 | 28 | 7 | ||||
| CMW | 80 | 5 | 15 | 42 | 58 | ||||||
The concentration of As and Pb in particles with less than 10 μm in aerodynamic diameter (PM10) in SH15, PP and CMW has been reported elsewhere (Kastury et al.) (Submitted manuscript in 2018). There was no significant difference (p > 0.05) in As concentration between PM10 and PM2.5, indicating that As was not enriched in the smaller particle size fraction. However, compared to PM10, Pb in PM2.5 was enriched by up to 1.7 fold. A similar enrichment in the concentrations of other trace elements (e.g. Co, Cr, Cu, Mn, Ni and Zn) in PM2.5 suggests that this fraction may act as a sink for toxic metals. Although the mean particle diameter was similar in PM2.5 across the three samples (1.2±0.05 μm in SH15, 2.17±0.65 μm in PP and 1.6±0.05 μm in CMW), noteworthy differences in BET surface area were observed between PP (32.1 m2/g), CMW (18.7 m2/g) and SH15 (4.4 m2/g). The high BET surface area observed in PP PM2.5 may be attributed to the emission of fine particles during the smelting which may contribute to the enrichment of Pb in the small particle size fraction.
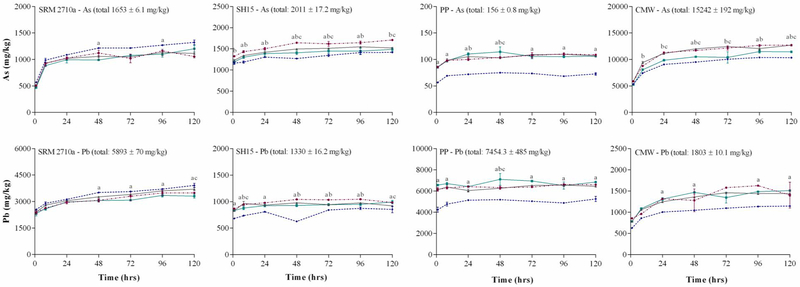
3.2. The effect of S/L ratio on PM2.5 metal(loid) bioaccessibility
Figure 1 shows the influence of S/L ratio on As and Pb bioaccessibility in PM2.5. For both As and Pb, dissolution plateaued within 24 h. Although As concentration varied significantly between samples, percentage As bioaccessibility after 24 h was similar across the four matrices (PP: 70.5%; SH15: 69.1%: CMW: 64.7%; NIST2710a: 60.1%). The similarity in As bioaccessibility between the environmental samples stems from the similarity in As speciation (e.g. 70–90% of the As was present as sorbed species). In contrast, Pb bioaccessibility varied among the matrices, with values ranging from 50.6% (NIST2710a) to 81.2% (PP). The high As and Pb bioaccessibility in PP can be attributed to the predominance of sorbed species and the high BET surface area (32.1 m2/g) which will both influence metal(loid) dissolution.
Figure 1: Effect of solid to liquid ratio ( 1:100,
1:100,  1:500,
1:500,  1:1000 &
1:1000 &  1:5000) on As and Pb bioaccessibility (mg/kg) (37°C, ALF, end-over-end rotation) (mean ± SEM, n = 3).
1:5000) on As and Pb bioaccessibility (mg/kg) (37°C, ALF, end-over-end rotation) (mean ± SEM, n = 3).
SRM2710a = Standard reference material from the National Institute of Standards and Technology, SH15 = non-ferrous slag impacted PM2.5, PP = smelter impacted PM2.5 and CMW = calcinated mine waste impacted PM2.5. Significant difference (ANOVA, α = 0.05) between 1:5000 & 1:100 = a, 1:5000 & 1: 500 = b, 1:5000 & 1:1000 = c.
S/L ratio did not significantly affect As and Pb bioaccessibility in SRM 2710a after 24 h (p > 0.05). This result is similar to a recent study conducted by Pelfrêne et al. (2017) who reported no significant difference in Cd, Pb and Zn bioaccessibility in three certified reference materials (BCR-723, SRM NIST 2710a and SRM NIST 1648a) using ALF, a S/L ratio of 1:1000 – 1:10,000 and a 24 h assessment period. In the present study, As and Pb bioaccessibility at 24 h was significantly higher (p < 0.05) in PP, SH15 and CMW when a S/L ratio of 1:5000 was used compared to 1:100. However, As bioaccessibility decreased by 8% in SH15 and between 12–14% in CMW when S/L ratio of 1:5000 was used compared to 1:500 and 1:1000 (p < 0.05). A similar decrease in the bioaccessibility of other elements (Al, Fe, Mn and Zn) was also observed in these samples (Figure S1). Schaider et al. (2007) suggested that phosphate may form insoluble Zn3(PO4)2 during SLF extractions at pH 4.5, which may account for decreased Zn bioaccessibility with increasing S/L ratio. Arsenic and Pb have also been shown to form complexes with inorganic and organic constituents in SLF (Marschner et al., 2006), decreasing the fraction that remains in solution over time.
Although the concentration of PM2.5 in air may vary significantly depending on location (1–217 μg/m3) (WHO, 2016), the acceptable concentration of PM2.5 is 10 μg/m3 (Apte et al., 2015) (with a 2015 world average PM2.5 of 44 μg/m3 (Bank, 2017)). Using an air intake value of 20 m3/day (Julien et al., 2011), the mass of PM2.5 that may be inhaled using PM2.5 concentrations of 10 to 217 μg/m3 falls between 0.2 to 4.34 mg. With a total lung fluid volume of 20 mL (Julien et al., 2011), the biologically relevant S/L ratio may be estimated to be 1:4650 – 1:100,000, although, it may be assumed that the total volume of phagolysosomal liquid within the macrophages would be lower than lung lining fluid. The lowest biologically relevant particle loading used in this study was 1:5000, because concentrations of several metals were below the level of quantification when a lower S/L ratio was used. Also, at lower particle loading, scaling up becomes impracticable as it would require large volumes of SLF. As a consequence, a S/L ratio of 1:5000 was used as a biologically relevant particle loading for further studies assessing the influence of other operational parameters on inhalation bioaccessibility.
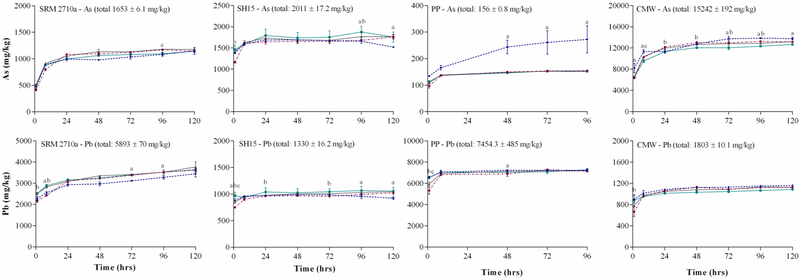
3.3. The effect of agitation on PM2.5 metal(loid) bioaccessibility
It has been suggested that certain agitation methods may result in particle agglomeration and reduce the available surface area for metal-chelator interaction in-vitro (Julien et al., 2011). Therefore, the effect of four different agitation methods was assessed for their influence on metal(loid) bioaccessibility: no agitation, occasional orbital, end-over-end rotation and magnetic stirring. Figure 2 demonstrates that with the exception of magnetic stirring, As and Pb bioaccessibility appears to be unaffected by the choice of agitation, plateauing after 24 h. This result agrees with findings of (Kastury et al.) (Submitted manuscript in 2018), where using a S/L ratio of 1:5000 and Gamble’s solution (pH 7.4), there was no significant difference in the bioaccessibility of metal(loid)s as a result of agitation types other than magnetic stirring. As strong mechanical mixing is not congruent to the mixing processes in the lung, magnetic stirring was concluded as not being biologically relevant. Particles were visually the most dispersed using end-over-end shaking and for this reason, end-over-end shaking was selected as the mixing approach for sample agitation.
Figure 2:
Effect of agitation (magnetic stirring , orbital rotation
, orbital rotation , occasional stirring
, occasional stirring , end-over-end rotation
, end-over-end rotation  ) on As and Pb bioaccessibility (mg/kg) (37°C, ALF, S/L ratio of 1:5000) (mean ± SEM, n = 3). SRM2710a = Standard reference material from the National Institute of Standards and Technology, SH15 = non-ferrous slag impacted PM2.5, PP = smelter impacted PM2.5 and CMW = calcinated mine waste impacted PM2.5. Significant difference between end over end vs magnetic stirring = a, end over end vs occasional stirring = b and end over end vs orbital rotation = c).
) on As and Pb bioaccessibility (mg/kg) (37°C, ALF, S/L ratio of 1:5000) (mean ± SEM, n = 3). SRM2710a = Standard reference material from the National Institute of Standards and Technology, SH15 = non-ferrous slag impacted PM2.5, PP = smelter impacted PM2.5 and CMW = calcinated mine waste impacted PM2.5. Significant difference between end over end vs magnetic stirring = a, end over end vs occasional stirring = b and end over end vs orbital rotation = c).
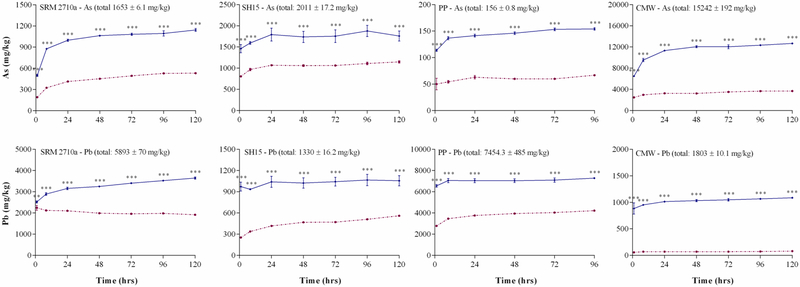
3.4. The effect of SLF composition on PM2.5 metal(loid) bioaccessibility
A recent review by Kastury et al. (2017) identified four fluid compositions simulating the environment inside a phagolysosome: Simulated intracellular fluid (Thelohan and De Meringo, 1994), two versions of artificial lysosomal fluid (ALF) (Midander et al., 2007; Stopford et al., 2003) and phagolysosomal simulant fluid (PSF) (Stefaniak et al., 2005). Simulated intracellular fluid has not been used in recent metal(loid) bioaccessibility studies (Kastury et al., 2017), while preliminary experiments demonstrated that there was no significant difference in metal(loid) extraction efficiencies between the two versions of ALFs (data not shown). Due to their extensive use, metal(loid) bioaccessibility using ALF and PSF was assessed in order to compare extraction efficacies (Figure 3 and S3). As with previous assessments, As and Pb solubility plateaued at 24 h, indicating that this timeframe may be adequate for the assessment of metal(loid) bioaccessibility in PM2.5.
Figure 3: Effect of SLF composition (ALF , PSF
, PSF ) on As and Pb bioaccessibility (mg/kg) (37°C, S/L ratio of 1:5000 and end-over-end rotation: 45 rpm) (mean ± SEM, n = 3).
) on As and Pb bioaccessibility (mg/kg) (37°C, S/L ratio of 1:5000 and end-over-end rotation: 45 rpm) (mean ± SEM, n = 3).
SRM2710a = Standard reference material from the National Institute of Standards and Technology, SH15 = non-ferrous slag impacted PM2.5, PP = smelter impacted PM2.5 and CMW = calcinated mine waste impacted PM2.5. Significant differences (two-way ANOVA, α = 0.05) in bioaccessibility between the two simulated lung fluids are indicated as * = P < 0.05, ** = p < 0.01 and *** = p < 0.001.
When the 24 h time point was assessed, both As and Pb bioaccessibility was significantly higher (p < 0.001) when assessed using ALF compared to PSF. Arsenic bioaccessibility was 1.6 (SH15) to 3.4 fold higher (CMW) while Pb bioaccessibility was 1.5 (SRM 2710a) to 3.9 fold higher (CMW) using ALF compared to PSF. The bioaccessibility of other elements (Al, Cd, Fe, Mn and Zn; Figure S4) was also higher in ALF, with the values plateauing within 24 h. Although both ALF and PSF methodologies are conducted under the same pH conditions, there are important differences in fluid composition with respect to metal chelation capacity, e.g., amino acids and organic molecules. Glycine was the only metal chelator present in PSF while ALF contains a mixture of glycine, citrate, tartrate, lactate and pyruvate, which may contribute to the higher metal(loid) extraction capacity. Consequently, for a conservative estimate of metal(loid) inhalation bioaccessibility, ALF should be utilised as the in-vitro fluid.
3.5. The effect of simulated GIT solutions on PM2.5 metal(loid) bioaccessibility
Macrophage engulfment (including alveolar and epithelial) is most effectively stimulated by particle sizes of 0.1–2 μm (Black, 2005), which predominantly deposit in the head airways and only 10% in both the alveolar region and tracheobronchial region (Smith, 1994). Approximately 90% of the particles depositing in the respiratory tract are thought to be transported to the pharynx by mucociliary action, although clearance is assumed to be slower from terminal alveoli (Kastury et al., 2017). It is therefore likely that particles engulfed by epithelial lung macrophages in the tracheobronchial region are transported to the GIT, where metal(loid) dissolution and absorption may occur. Several researchers have assessed PM2.5 metal(loid) bioaccessibility using simulated gastric solutions on the presumption that this would yield the most conservative bioaccessibility estimates. In this study, metal(loid) bioaccessibility in SLF alone (24 h) was compared to bioaccessibility outcomes in simulated GIT solutions alone, as well as following a 24 h SLF phase in order to glean differences between assessment approaches.
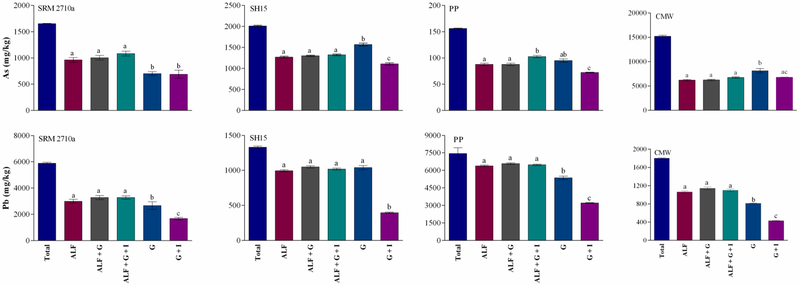
3.5.1. Effect of transitioning PM2.5 to simulated GIT solution following extraction in ALF
Figure 4 shows a comparison of As and Pb bioaccessibility when assessed using ALF alone for 24 h (ALF) or when PM2.5 was transitioned into gastric or intestinal phases of the SBRC assay following 24 h extraction using ALF when transitioned to gastrointestinal extraction (either ALF+G or ALF+G+I). With the exception of PP, As bioaccessibility in ALF alone did not significantly differ from values obtained using ALF+G or ALF+G+I (p>0.05). For example, in SH15, As bioaccessibility was 63.3 ± 0.9%, 64.7 ± 0.8% and 65.9 ± 0.9% when assessed using ALF, ALF+G and ALF+G+I respectively. Although a significant increase in As bioaccessibility was observed in PP using ALF+G+I compared to ALF alone or ALF+G (p < 0.05), the increase was small (1.2 fold). Similarly, there was no significant difference (p > 0.05) in Pb bioaccessibility when assessed using ALF, ALF+G and ALF+G+I for all matrices. Lead bioaccessibility in these assays ranged from 50.8 to 55.6% (SRM 2710a), 74.8 to 78.9% (SH15), 85.6 to 88.3% (PP) and 58.8 to 63.3% (CMW). Likewise, no significant increase in PM2.5 metal(loid) bioaccessibility was also observed for Cd, Fe and Mn when ALF was utilised alone or in combination with GIT extraction (Figure S4). This result is in contrast to findings from PM10 studies (i.e. PM10 samples sourced from SH15, PP and CMW) (Kastury et al. submitted manuscript), where As and Pb bioaccessibility increased up to 3 fold, when bioaccessibility assays were transitioned from SLF (Hatch’s solution, pH 7.4) into simulated GIT solutions. The reason for this difference was attributed to the neutral pH (7.4) of Hatch’s solution used to assess PM10 inhalation bioaccessibility where metal(loid) bioaccessibility was low compared to the acidic pH of ALF (4.5) where maximum dissolution occurred.
Figure 4: Comparison of As and Pb bioaccessibility when assessed using ALF alone or in combination with GIT extraction (either gastric [G] or gastric and intestinal [G+I] extraction) (mean ± SEM, n = 3).
Total metal(loid) concentration ( ), ALF (
), ALF ( ), ALF + gastric solution (
), ALF + gastric solution ( ), ALF + gastric + intestinal solution (
), ALF + gastric + intestinal solution ( ), gastric solution (
), gastric solution ( ), gastric + intestinal solution (
), gastric + intestinal solution ( ). SRM2710a = Standard reference material from the National Institute of Standards and Technology, SH15 = non-ferrous slag impacted PM2.5, PP = smelter impacted PM2.5 and CMW = calcinated mine waste impacted PM2.5. PM2.5 was assessed in ALF for 24 hours, followed by gastric (G) and intestinal (I) solutions (SBRC method). Additionally, PM2.5 was assessed in gastric (G) and intestinal (I) solutions only to determine the difference between inhalation + ingestion and ingestion only pathways. Statistically significant differences (ANOVA, α = 0.05) between metal(loid) bioaccessibility is indicated by dissimilar letters.
). SRM2710a = Standard reference material from the National Institute of Standards and Technology, SH15 = non-ferrous slag impacted PM2.5, PP = smelter impacted PM2.5 and CMW = calcinated mine waste impacted PM2.5. PM2.5 was assessed in ALF for 24 hours, followed by gastric (G) and intestinal (I) solutions (SBRC method). Additionally, PM2.5 was assessed in gastric (G) and intestinal (I) solutions only to determine the difference between inhalation + ingestion and ingestion only pathways. Statistically significant differences (ANOVA, α = 0.05) between metal(loid) bioaccessibility is indicated by dissimilar letters.
3.5.2. Comparative extraction efficiencies of ALF and simulated GIT solutions
PM2.5 metal(loid) bioaccessibility in ALF alone was also compared to bioaccessibility outcomes obtained in simulated gastric [G] and small intestinal solutions [G+I] alone (Figure 4). When As was assessed, bioaccessibility was 16.6 – 29.4% lower following G+I assessment compared to G. Similar findings have been reported for other contaminated materials (Juhasz et al., 2009b; Li et al., 2015) using the SBRC assay (used in this study), physiologically based extraction test (PBET), in vitro gastro-intestinal method (IVG) and Deutches Institut fur Normung (DIN). Similarly, compared to gastric phase alone, Pb bioaccessibility following G+I extraction decreased significantly (18.8 to 61.9%), which occurs as a result of the increase in pH from 1.5 to 7 (Smith et al., 2011). The mechanisms responsible for the decrease in As and Pb bioaccessibility under G+I conditions include co-precipitation with amorphous Fe or re-adsorption into the sample matrix as a result of the increase in pH (Martínez and McBride, 2001; O’Reilly and Hochella, 2003; Ruby et al., 1996).
Metal(loid) bioaccessibility using ALF alone was significantly different from that using simulated GIT solutions alone (P < 0.05). Arsenic bioaccessibility was higher when assessed using gastric phase conditions (G) compared to ALF for PP (1.08 fold; p > 0.05), SH15 (1.2 fold; p < 0.05) and CMW (1.3 fold; p < 0.05). Higher As bioaccessibility when assessed using simulated gastric solution was expected as the extent of the solubility of As (V), which is the principle form that As was present in PM2.5 samples, is pH dependent (Gersztyn et al., 2013). In contrast, Pb bioaccessibility in ALF was 1.12, 1.20 and 1.30 fold higher (P < 0.05) in SRM 2710a, PP and CMW respectively compared to gastric phase alone; with the exception of SH15 where no significant difference (p > 0.05) was observed between methodologies. This result differs to results obtained by Mukhtar and Limbeck (2013), who reported higher Pb bioaccessibility using simulated gastric solutions compared to ALF. However, Mukhtar and Limbeck (2013) conducted extraction using both gastric solutions and ALF over a 1 h period, whereas in this study, a more biologically relevant extraction time was used (e.g. 24 h extraction using ALF and 1 h extraction using gastric solution). It is likely that the higher Pb bioaccessibility in ALF was a combination of increased contact time and the presence of metal chelators in ALF (e.g. citrate, tartrate, lactate, pyruvate). Similar to Pb, higher Fe and Mn bioaccessibility (Figure S4) was also observed in ALF alone, compared to simulated GIT solutions.
4. Conclusion
The results of this study demonstrate that S/L ratio, fluid composition, as well as extraction time significantly influences metal(loid) bioaccessibility in PM2.5. Furthermore, when a S/L ratio of 1:5000, end-over-end rotation (45 rpm) and 24-hour extraction time was used, metal(loid) bioaccessibility in ALF was higher than in simulated GIT solutions. Because the average thickness of air-blood barrier is estimated to be 1.3 μm in diameter, soluble ions and small molecules are thought to be rapidly absorbed into blood (Kanapilly, 1977). As the majority of metal(loid) solubilisation was observed to take place in the SLF within 24 h, it may be presumed that dissolved metal(loid)s would potentially be absorbed into the systemic circulation via the pulmonary interstitium. This suggests that it may not be necessary to undertake further investigations using extractions that simulate gastric solution alone or in conjunction with ALF extraction to estimate PM2.5 inhalation bioaccessibility.
Supplementary Material
5. Acknowledgements
Farzana Kastury acknowledges the Commonwealth Government of Australia, Research Training program scholarship (RTPd), University of South Australia for the VC and President’s Scholarship and the MF & MH Joyner Scholarship in Science. Ranju Karna was supported by Internship/Research Participation Program at the National Risk Management Research Laboratory, U.S. Environmental Protection Agency, administered by the Oak Ridge Institute for Science and Education via an interagency agreement between the U.S. Department of Energy and EPA. Although EPA contributed to this article, the research presented was not performed by or funded by EPA and was not subject to EPA’s quality system requirements. Consequently, the views, interpretations, and conclusions expressed in this article are solely those of the authors and do not necessarily reflect or represent EPA’s views or policies. MRCAT operations are supported by the Department of Energy and the MRCAT member institutions. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02–06CH11357.
6. References
- Apte JS, Marshall JD, Cohen AJ, Brauer M, 2015. Addressing global mortality from ambient PM2. 5. Environmental science & technology 49, 8057–8066. [DOI] [PubMed] [Google Scholar]
- Atkinson R, Kang S, Anderson H, Mills I, Walton H, 2014. Epidemiological time series studies of PM2. 5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax, thoraxjnl-2013–204492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank, T.W., 2017. PM2.5 air pollution, mean annual exposure (micrograms per cubic meter), PM2.5 air pollution.
- Black J, 1999. Biological Performance of Materials: Fundamentals of Biocompatibility, Third ed. CRC Press. [Google Scholar]
- Black J, 2005. Biological performance of materials: fundamentals of biocompatibility. CRC Press. [Google Scholar]
- Colombo C, Monhemius AJ, Plant JA, 2008. The estimation of the bioavailabilities of platinum, palladium and rhodium in vehicle exhaust catalysts and road dusts using a physiologically based extraction test. Science of The Total Environment 389, 46–51. [DOI] [PubMed] [Google Scholar]
- Fajersztajn L, Saldiva P, Pereira LAA, Leite VF, Buehler AM, 2017. Short-term effects of fine particulate matter pollution on daily health events in Latin America: a systematic review and meta-analysis. International Journal of Public Health 62, 729–738. [DOI] [PubMed] [Google Scholar]
- Fujitani Y, Furuyama A, Tanabe K, Hirano S, 2017. Comparison of oxidative abilities of PM2. 5 collected at traffic and residential sites in Japan. Contribution of transition metals and primary and secondary aerosols. Aerosol and Air Quality Research 17, 574–587. [Google Scholar]
- Gavett SH, Haykal-Coates N, Copeland LB, Heinrich J, Gilmour MI, 2003. Metal composition of ambient PM2. 5 influences severity of allergic airways disease in mice. Environmental health perspectives 111, 1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersztyn L, Karczewska A, Gałka B, 2013. Influence of pH on the solubility of arsenic in heavily contaminated soils/Wpływ pH na rozpuszczalność arsenu w glebach silnie zanieczyszczonych. Ochrona Srodowiska i Zasobów Naturalnych 24, 7–11. [Google Scholar]
- Hamad SH, Schauer JJ, Shafer MM, Al-Rheem EA, Skaar PS, Heo J, Tejedor-Tejedor I, 2014a. Risk assessment of total and bioavailable potentially toxic elements (PTEs) in urban soils of Baghdad–Iraq. Science of The Total Environment 494, 39–48. [DOI] [PubMed] [Google Scholar]
- Hamad SH, Schauer JJ, Shafer MM, Al-Rheem EA, Skaar PS, Heo J, Tejedor-Tejedor I, 2014b. Risk assessment of total and bioavailable potentially toxic elements (PTEs) in urban soils of Baghdad–Iraq. Science of The Total Environment 494–495, 39–48. [DOI] [PubMed] [Google Scholar]
- He M, Ichinose T, Yoshida S, Ito T, He C, Yoshida Y, Arashidani K, Takano H, Sun G, Shibamoto T, 2017. PM2. 5-induced lung inflammation in mice: Differences of inflammatory response in macrophages and type II alveolar cells. Journal of Applied Toxicology. [DOI] [PubMed] [Google Scholar]
- Hofmann W, 2011. Modelling inhaled particle deposition in the human lung—A review. Journal of Aerosol Science 42, 693–724. [Google Scholar]
- Juhasz AL, Weber J, Smith E, Naidu R, Marschner B, Rees M, Rofe A, Kuchel T, Sansom L, 2009a. Evaluation of SBRC-gastric and SBRC-intestinal methods for the prediction of in vivo relative lead bioavailability in contaminated soils. Environmental science & technology 43, 4503–4509. [DOI] [PubMed] [Google Scholar]
- Juhasz AL, Weber J, Smith E, Naidu R, Rees M, Rofe A, Kuchel T, Sansom L, 2009b. Assessment of four commonly employed in vitro arsenic bioaccessibility assays for predicting in vivo relative arsenic bioavailability in contaminated soils. Environmental science & technology 43, 9487–9494. [DOI] [PubMed] [Google Scholar]
- Julien C, Esperanza P, Bruno M, Alleman LY, 2011. Development of an in vitro method to estimate lung bioaccessibility of metals from atmospheric particles. Journal of Environmental Monitoring 13, 621–630. [DOI] [PubMed] [Google Scholar]
- Kanapilly G, 1977. Alveolar microenvironment and its relationship to the retention and transport into blood of aerosols deposited in the alveoli. Health Physics 32, 89–100. [DOI] [PubMed] [Google Scholar]
- Kastury F, Smith E, Juhasz AL, 2017. A critical review of approaches and limitations of inhalation bioavailability and bioaccessibility of metal (loid) s from ambient particulate matter or dust. Science of The Total Environment 574, 1054–1074. [DOI] [PubMed] [Google Scholar]
- Kastury F, Smith E, Karna RR, Scheckel KG, Juhasz AL, 2018. An inhalation-ingestion bioaccessibility assay (IIBA) for the assessment of exposure to metal(loid)s in PM10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley ME, Brauning S, Schoof R, Ruby M, 2002. Assessing oral bioavailability of metals in soil. Battelle Press. [Google Scholar]
- Kropf A, Katsoudas J, Chattopadhyay S, Shibata T, Lang E, Zyryanov V, Ravel B, McIvor K, Kemner K, Scheckel K, 2010. The new MRCAT (Sector 10) bending magnet beamline at the advanced photon source, AIP Conference Proceedings. AIP, pp. 299–302. [Google Scholar]
- Li H-B, Li J, Zhu Y-G, Juhasz AL, Ma LQ, 2015. Comparison of arsenic bioaccessibility in housedust and contaminated soils based on four in vitro assays. Science of The Total Environment 532, 803–811. [DOI] [PubMed] [Google Scholar]
- Marschner B, Welge P, Hack A, Wittsiepe J, Wilhelm M, 2006. Comparison of soil Pb in vitro bioaccessibility and in vivo bioavailability with Pb pools from a sequential soil extraction. Environmental science & technology 40, 2812–2818. [DOI] [PubMed] [Google Scholar]
- Martínez CE, McBride MB, 2001. Cd, Cu, Pb, and Zn coprecipitates in Fe oxide formed at different pH: Aging effects on metal solubility and extractability by citrate. Environmental Toxicology and Chemistry 20, 122–126. [PubMed] [Google Scholar]
- Meunier L, Walker SR, Wragg J, Parsons MB, Koch I, Jamieson HE, Reimer KJ, 2010. Effects of soil composition and mineralogy on the bioaccessibility of arsenic from tailings and soil in gold mine districts of Nova Scotia. Environmental science & technology 44, 2667–2674. [DOI] [PubMed] [Google Scholar]
- Midander K, Pan J, Wallinder IO, Leygraf C, 2007. Metal release from stainless steel particles in vitro—influence of particle size. Journal of Environmental Monitoring 9, 74–81. [DOI] [PubMed] [Google Scholar]
- Mukhtar A, Limbeck A, 2013. Comparison of the extraction efficiencies of different leaching agents for reliable assessment of bio-accessible trace metal fractions in airborne particulate matter, E3S Web of Conferences. EDP Sciences. [Google Scholar]
- O’Reilly SE, Hochella MF, 2003. Lead sorption efficiencies of natural and synthetic Mn and Fe-oxides. Geochimica et Cosmochimica Acta 67, 4471–4487. [Google Scholar]
- Oberdörster G, 2000. Pulmonary effects of inhaled ultrafine particles. International archives of occupational and environmental health 74, 1–8. [DOI] [PubMed] [Google Scholar]
- Okorie A, Entwistle J, Dean JR, 2012. Estimation of daily intake of potentially toxic elements from urban street dust and the role of oral bioaccessibility testing. Chemosphere 86, 460–467. [DOI] [PubMed] [Google Scholar]
- Ollson CJ, Smith E, Scheckel KG, Betts AR, Juhasz AL, 2016. Assessment of arsenic speciation and bioaccessibility in mine-impacted materials. Journal of hazardous materials 313, 130–137. [DOI] [PubMed] [Google Scholar]
- Pardo M, Porat Z, Rudich A, Schauer JJ, Rudich Y, 2016. Repeated exposures to roadside particulate matter extracts suppresses pulmonary defense mechanisms, resulting in lipid and protein oxidative damage. Environmental Pollution 210, 227–237. [DOI] [PubMed] [Google Scholar]
- Pelfrêne A, Cave M, Wragg J, Douay F, 2017. In Vitro Investigations of Human Bioaccessibility from Reference Materials Using Simulated Lung Fluids. International Journal of Environmental Research and Public Health 14, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelfrêne A, Douay F, 2017. Assessment of oral and lung bioaccessibility of Cd and Pb from smelter-impacted dust. Environmental Science and Pollution Research. [DOI] [PubMed] [Google Scholar]
- Pinault LL, Weichenthal S, Crouse DL, Brauer M, Erickson A, Donkelaar A.v., Martin RV, Hystad P, Chen H, Finès P, Brook JR, Tjepkema M, Burnett RT, 2017. Associations between fine particulate matter and mortality in the 2001 Canadian Census Health and Environment Cohort. Environmental Research 159, 406–415. [DOI] [PubMed] [Google Scholar]
- Pope CA, Dockery DW, 2006. Health Effects of Fine Particulate Air Pollution: Lines that Connect. Journal of the Air & Waste Management Association 56, 709–742. [DOI] [PubMed] [Google Scholar]
- Potgieter-Vermaak S, Rotondo G, Novakovic V, Rollins S, Van Grieken R, 2012. Component-specific toxic concerns of the inhalable fraction of urban road dust. Environmental Geochemistry and Health 34, 689–696. [DOI] [PubMed] [Google Scholar]
- Puls C, Limbeck A, Hann S, 2012. Bioaccessibility of palladium and platinum in urban aerosol particulates. Atmospheric Environment 55, 213–219. [Google Scholar]
- Pun VC, Kazemiparkouhi F, Manjourides J, Suh HH, 2017. Long-Term PM2. 5 Exposures and Respiratory, Cancer and Cardiovascular Mortality in American Older Adults. American journal of epidemiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Registry, A.f.T.S.a.D., 2017. Priority List of Hazardous Substances. [Google Scholar]
- Ruby MV, Davis A, Schoof R, Eberle S, Sellstone CM, 1996. Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environmental science & technology 30, 422–430. [Google Scholar]
- Schaider LA, Senn DB, Brabander DJ, McCarthy KD, Shine JP, 2007. Characterization of zinc, lead, and cadmium in mine waste: implications for transport, exposure, and bioavailability. Environmental science & technology 41, 4164–4171. [DOI] [PubMed] [Google Scholar]
- Segre C, Leyarovska N, Chapman L, Lavender W, Plag P, King A, Kropf A, Bunker B, Kemner K, Dutta P, 2000. The MRCAT insertion device beamline at the Advanced Photon Source, AIP Conference Proceedings. AIP, pp. 419–422. [Google Scholar]
- Shuster-Meiseles T, Shafer MM, Heo J, Pardo M, Antkiewicz DS, Schauer JJ, Rudich A, Rudich Y, 2016. ROS-generating/ARE-activating capacity of metals in roadway particulate matter deposited in urban environment. Environmental Research 146, 252–262. [DOI] [PubMed] [Google Scholar]
- Smith E, Weber J, Naidu R, McLaren RG, Juhasz AL, 2011. Assessment of lead bioaccessibility in peri-urban contaminated soils. Journal of hazardous materials 186, 300–305. [DOI] [PubMed] [Google Scholar]
- Smith H, 1994. Human respiratory tract model for radiological protection. ICRP Publication 66. [Google Scholar]
- Snider G, Weagle CL, Murdymootoo KK, Ring A, Ritchie Y, Stone E, Walsh A, Akoshile C, Anh NX, Balasubramanian R, Brook J, Qonitan FD, Dong J, Griffith D, He K, Holben BN, Kahn R, Lagrosas N, Lestari P, Ma Z, Misra A, Norford LK, Quel EJ, Salam A, Schichtel B, Segev L, Tripathi S, Wang C, Yu C, Zhang Q, Zhang Y, Brauer M, Cohen A, Gibson MD, Liu Y, Martins JV, Rudich Y, Martin RV, 2016. Variation in global chemical composition of PM2.5: emerging results from SPARTAN. Atmospheric Chemistry and Physics 16, 9629–9653. [Google Scholar]
- Stefaniak A, Guilmette R, Day G, Hoover M, Breysse P, Scripsick R, 2005. Characterization of phagolysosomal simulant fluid for study of beryllium aerosol particle dissolution. Toxicology in vitro 19, 123–134. [DOI] [PubMed] [Google Scholar]
- Stopford W, Turner J, Cappellini D, Brock T, 2003. Bioaccessibility testing of cobalt compounds. Journal of Environmental Monitoring 5, 675–680. [DOI] [PubMed] [Google Scholar]
- Thelohan S, De Meringo A, 1994. In vitro dynamic solubility test: influence of various parameters. Environmental health perspectives 102, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA, 1998. Microwave assissted acid digestion of sediments, slidges, soils, and oils.
- WHO, 2016. WHO Global Urban Ambient Air Pollution Database, Public health, environmental and social determinants of health (PHE).
- Wiseman CL, Zereini F, 2014. Characterizing metal (loid) solubility in airborne PM 10, PM 2.5 and PM 1 in Frankfurt, Germany using simulated lung fluids. Atmospheric Environment 89, 282–289. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.