Abstract
The last 15 years have witnessed the emergence of a new field of research that focuses on the roles played by the intestinal microbiota in health and disease. This research field has produced accumulating evidence indicating that dysregulation of host-microbiota interactions contributes to a range of chronic inflammatory diseases, including inflammatory bowel diseases, colorectal cancer, and metabolic syndrome. Although dysregulation of the microbiota can take complex forms, in some cases, specific bacterial species that can drive specific clinical outcomes have been identified. Among the numerous factors influencing the intestinal microbiota composition, diet is a central actor, wherein numerous dietary factors can beneficially or detrimentally impact the host/microbiota relationship. This review will highlight recent literature that has advanced understanding of microbiota-diet-disease interplay, with a central focus on the following question: Are we ready to use intestinal microbiota composition-based personalized dietary interventions to treat chronic inflammatory diseases?
Keywords: Microbiota, Inflammation, Diet, Personalized Medicine
Abbreviations used in this paper: AIEC, adherent invasive Escherichia coli; CD, Crohn’s disease; CMC, carboxymethylcellulose; FMT, fecal microbiota transplantation; HCC, hepatocellular carcinoma; IBD, inflammatory bowel disease; PD-1, programmed cell death protein 1; P80, polysorbate 80
Summary.
Accumulating evidence supports the orchestrating role of the intestinal microbiota in chronic inflammatory disorders. As more dietary factors are identified as potential mediators of microbiota composition and function, will personal dietary intervention based on microbiota composition soon be part of disease management?
The intestinal microbiota is a vast and complex community of microorganisms that includes 1014 bacteria per intestine and about 100–500 different species per individual.1, 2 Among its numerous functions, the gut microbiota is essential to promote maturation of the intestinal immune system and help digestion by permitting extraction of calories and nutrients that would otherwise be eliminated in feces. Besides these beneficial roles, the gut microbiota can also turn detrimental to its host and, if not well-managed, can lead to the development of inflammatory diseases such as inflammatory bowel disease (IBD) and metabolic syndrome.3, 4, 5 Importantly, the large post–mid-20th century increase in the incidence of IBD and metabolic syndrome highlights the pivotal role of nongenetic factors in determining the extent to which individuals genetically prone to disease actually develop it. Nongenetic factors previously shown to influence the intestinal microbiota and development of colitis are listed in Table 1.
Table 1.
Nongenetic Factors Previously Shown to Influence Intestinal Microbiota in a Way That Triggers Intestinal Inflammation
| Compounds | Model used | Effect on the intestinal microbiota | Effect on the host | Reference |
|---|---|---|---|---|
| Soluble fibers | C57/Bl6 WT treated with DSS C57/Bl6 IL10KO |
Alteration of microbiota composition at the phylum level Impact on bacterial biomass and proinflammatory potential |
Promotion of intestinal inflammation | 55, 56 |
| Aluminum | Colorectal distention in rats | Not studied | Orally administered low-dose aluminum induced visceral hypersensitivity | 79 |
| Maltodextrin | Biofilm formation assay In vitro adhesion to intestinal epithelial cells C57/Bl6 WT treated with DSS |
Increased biofilm formation and adhesion ability of Crohn’s disease–associated adherent and invasive Escherichia coli bacteria No alteration of mucosa-associated microbiota |
Deregulation of intestinal antimicrobial defense Promotion of endoplasmic reticulum stress, mucus depletion |
44, 80, 81 |
| Dietary emulsifier (CMC and P80) | C57/Bl6 WT C57/Bl6 IL10KO C57/Bl6 TLR5KO |
Alteration of microbiota composition Increase of microbiota proinflammatory potential Promotion of microbiota encroachment |
Promotion of low-grade intestinal inflammation and metabolic disorders in WT and TLR5KO mice Promotion of colitis incidence and severity in IL10KO mice |
20, 32, 82, 83 |
| Thickener (carrageenan) | Pig | Microbiota composition alterations at the phylum level Decreased proportion of A muciniphila |
Ulcerations in the large intestine | 84, 85, 86 |
| Artificial sweeteners | SAMP1/YitFc (SAMP) mice | Alteration of microbiota composition, promotion of proteobacteria | Exacerbation of ileal inflammation | 87 |
| Titanium dioxide nanoparticles | Adult zebrafish (Danio rerio) C57/Bl6 WT Wistar rats |
Alteration of microbiota composition | Increase in levels of the inflammatory cytokines Colonic microinflammation |
88, 89, 90 |
DSS, dextran sulfate sodium; WT, wild-type.
During the last 15 years, numerous studies have reported alterations in microbiota composition in both preclinical and clinical models of chronic inflammatory diseases. Although those alterations may, in part, be driven by the disease state, recent work has highlighted that an altered microbiota can also play a role in driving the disease, with the observation that disease can be transferred to germ-free mice by microbiota transplantation. For example, a pioneering study by Turnbaugh et al6 revealed that colonization of germ-free mice with an “obese microbiota” caused the recipient mice to gain more weight than those who received a “lean microbiota.” Mechanistic investigations have highlighted the importance of some specific microbiota members in driving detrimental outcomes, such as intestinal inflammation.7, 8
Altogether, these findings suggest that microbiota could be seen as a next-generation medicine, with the hypothesis that therapeutic/dietary interventions can be based on individual microbiota composition.
Lessons Learned From Cancer Therapy
The concept of microbiota composition-based therapy is most advanced in the research area of cancer therapeutics, particularly in determining drug efficacy. Recent studies have highlighted how intestinal bacteria can impact efficacy of anticancer drugs,9 with the findings that cancer patients can be stratified into responders and non-responders to immunotherapy on the basis of their intestinal microbiota composition. This suggests that microbiota should be considered when assessing therapeutic intervention.10, 11, 12, 13 For example, Gopalakrishnan et al12 identified that fecal microbiota composition differs between patients with metastatic melanoma that responded or not to anti–programmed cell death protein 1 (PD-1) therapy. Patients who responded to this therapy had higher abundance of the Faecalibacterium genus in their fecal microbiota, whereas non-responders harbored a higher abundance of Bacteroidales order.12 This finding importantly highlighted that an initial assessment of microbiota composition could be used as predictor of anti–PD-1 therapy success and could help to determine drug dose and frequency. Similarly, Routy et al11 demonstrated a central role played by the gut microbiota in the therapeutic response induced by immune checkpoint inhibitors targeting the PD-1/PD-L1 axis, with the identification of responder and non-responder patients. Fecal metagenomics revealed that the relative abundance of Akkermansia muciniphila correlated with clinical responses to immune checkpoint inhibitors, and oral supplementation with A muciniphila after fecal microbiota transplantation (FMT) from non-responder feces was sufficient to restore the efficacy of PD-1 blockade in mice model.11 Such studies highlight the future of microbiota-based precision medicine therapies,9, 14 with the concept that patients entering immunotherapy treatment for cancer may first have their microbiota composition analyzed to determine (1) what is the best therapeutic approach and (2) whether some patients could benefit from FMT before therapy to be converted from non-responders to responders. However, although these studies highlight the critical role of microbiota members in defining anti–PD-1 therapy responsiveness, no consensus was reached regarding the specific bacteria associated with therapeutic response, with quite different bacterial species/genus driving the associations reported in each independent study.10, 11, 12 These findings suggest a potential unappreciated heterogeneity and the importance of performing thorough microbiota composition analysis by using uniformed sequencing platform and analysis workflow, as well as precise patient stratification based on cancer type, cancer stage, patient origin, etc.9, 15
Lessons Learned From Research on Dietary Emulsifiers
We and others previously hypothesized that emulsifiers, which are added to most processed foods to aid texture and extend shelf life, might have played a role in the rapid post–mid-20th century increase in the incidence of chronic inflammatory diseases.16, 17, 18, 19 Investigation of this hypothesis led us to observe that emulsifiers induced a chronic intestinal inflammation that promotes development of chronic colitis in susceptible mice and metabolic syndrome in wild-type mice.20 By treating mice with 2 commonly used emulsifiers, namely polysorbate 80 (P80) and carboxymethylcellulose (CMC), at doses seeking to model the broad consumption of the numerous emulsifiers that are incorporated into a large variety of processed foods, we observed changes in species composition of the gut microbiota and increased expression of proinflammatory molecules. Such alterations included increased lipopolysaccharide and flagellin, which can activate host proinflammatory gene expression. Moreover, CMC- and P80-induced alterations in microbiota resulted in enhanced capacity to infiltrate the dense mucus layer that lines the intestine. Mucosa-associated microbiota has been previously studied and is characterized by a distinct composition compared with luminal microbiota,21, 22, 23, 24, 25, 26 with important roles played by this community on maturation of the immune system (for example, the well-documented impact of segmented filamentous bacteria on Th17 cells23, 27). However, the colonic inner mucus layer normally harbors a relatively low bacterial biomass,28, 29 which compositionally differs from luminal and mucosa-associated microbiota.30 CMC and P80 alter this inner mucus environment by promoting microbiota encroachment in a way that triggers chronic colitis in mice genetically prone to this disorder.20 In wild-type mice with normal immune systems, emulsifiers induced low-grade (ie, mild) intestinal inflammation and metabolic syndrome, characterized by increased adiposity and hyperglycemia. As a result of investigation into mechanisms underlying emulsifier-mediated promotion of inflammation, we identified that the effects of their consumption were eliminated in mice lacking a microbiota (germ-free), and that transplantation of microbiota from emulsifier-treated mice to wild-type germ-free recipient mice was sufficient to transfer some parameters of low-grade inflammation and metabolic syndrome, indicating a central role played by the microbiota in mediating the effects.
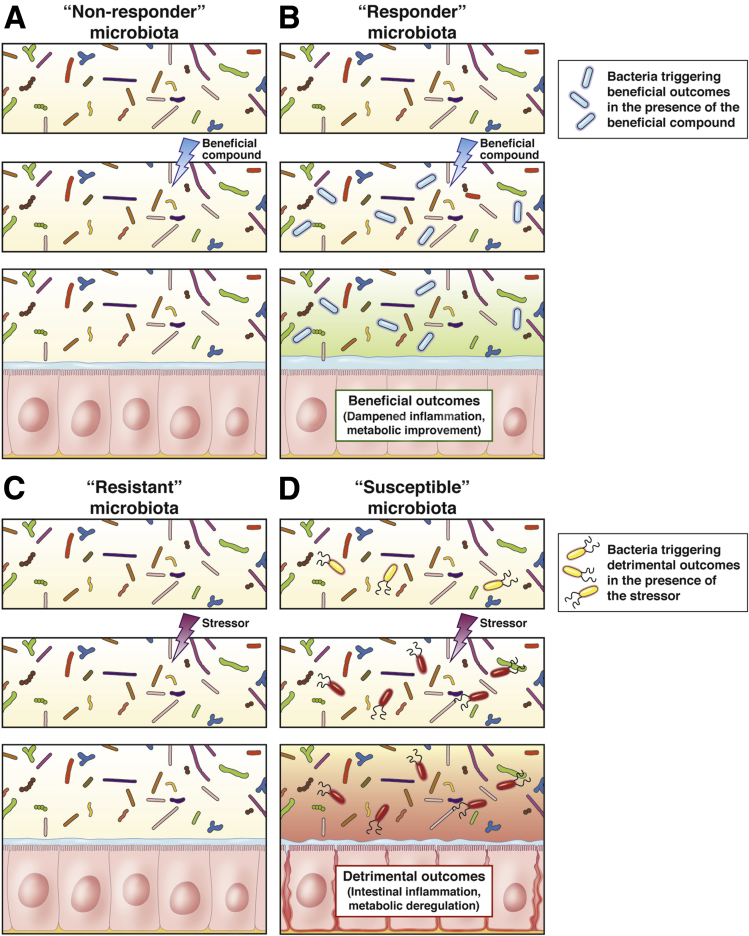
Importantly, we recently demonstrated that a complex microbiota community is required for emulsifier-mediated detrimental effects, with the observation that emulsifier consumption by gnotobiotic mice colonized with a highly restricted microbiota composed of only 8 bacteria, namely altered Schaedler flora (ASF),31 was not sufficient to induce microbiota encroachment, intestinal inflammation, or altered metabolism.32 These findings suggest that a complex microbiota containing some specific species is required to mediate detrimental effects of emulsifier exposure (Figure 1). In the coming years, microbiota composition analysis combined with the evaluation of inter-individual variations in response to emulsifier should identify specific species detrimentally impacted by such compounds. In other words, whereas some individuals may harbor a microbiota that will not be impacted by emulsifier exposure, other individuals may harbor some specific bacteria in their microbiota that make them susceptible to emulsifier-driven detrimental effects such as chronic intestinal inflammation and metabolic deregulations (Figure 1).
Figure 1.
Overview of the resistant and susceptible concept. (A and B) In the presence of a beneficial compound, some microbiota might not respond (A), whereas some others might be responsive (B) by harboring specific bacterial species able to trigger beneficial outcomes. (C and D) In the presence of a detrimental compound, some microbiota might be resistant (C), whereas some others might be susceptible (D) by harboring specific bacterial species able to trigger detrimental outcomes.
Lessons Learned From Patient Cohort Studies
FMT has recently gained interest as a possible novel treatment option for chronic inflammatory diseases including IBD. Indeed, even though treatments of such disorders by FMT are quite exploratory at present, the interest in its potential is attested to by the increasing number of publications describing FMT as therapy for IBD.33 However, the clinical outcomes in the various studies are excessively heterogenous, with studies reporting no improvement, deterioration, improvement, improvement but no remission, and/or clinical remission.34, 35, 36, 37, 38 These studies once again demonstrated heterogeneity in therapeutic outcomes and suggest that personalized intervention based on microbiota composition of the donor and/or the recipient microbiota may be needed, even if coming with quite some challenges. Currently, the notion that FMT can successfully treat diseases characterized by gut inflammation is best supported by the successful use of FMT in treatment of recurrent Clostridium difficile infection, which results in severe colitis. This approach allows a donor microbiota to recolonize the gastrointestinal tract of a patient with a complex microbial community, thus conferring “colonization resistance” to C difficile infection and preventing pathogenesis.39 Although specific approaches to FMT treatment of recurrent C difficile vary across institutions, it generally uses clinical stratification of both donors and recipients to transplant microbiota that will reach high success rates of engraftment.40 Analogously, a proper diagnosis strategy and clinical classification of IBD patients, and perhaps fecal donors, would seem warranted for successful FMT-based IBD therapy.
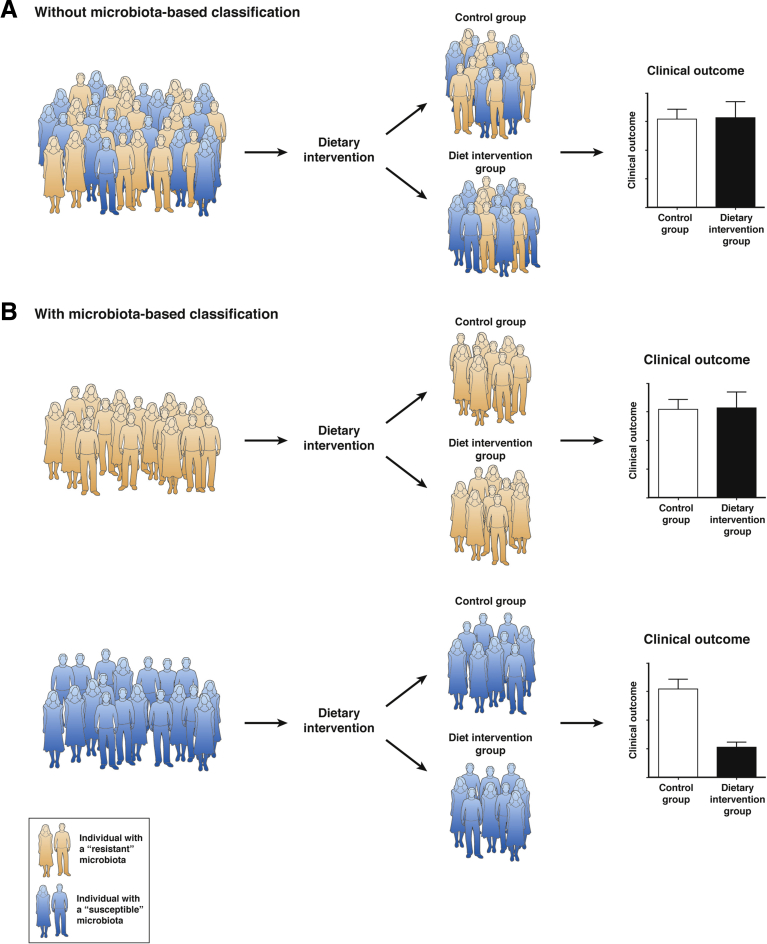
Similarly, another approach to beneficially manipulate the intestinal microbiota in the IBD population is through dietary modulation, which may also highly benefit from considering inter-individual variations in microbiota composition. Association study between dietary intake and incidence of IBD has pointed to food components as impacting IBD development. Indeed, certain food components such as polyunsaturated fatty acids, omega-6 fatty acids, and meat seem to predispose for IBD, whereas fibers and fruits or vegetables were associated with a decreased risk of Crohn’s disease (CD) and ulcerative colitis, respectively.41 Results from dietary therapy, such as exclusive enteral nutrition (which is using exclusive liquid feeding with either elemental or polymeric formulas) for IBD, remain inconclusive.42, 43 Because IBDs are multifactorial diseases and IBD population is highly heterogeneous, performing dietary intervention in the IBD population to investigate the role of a food component or a food additive without considering this inter-individual variation might not provide conclusive results. An example highlighting this concept is a study demonstrating that the ubiquitous dietary component maltodextrin enhanced CD-associated adherent invasive Escherichia coli (AIEC) biofilm formation.44 Hence, on the basis of the observation that only 30%–40% of the CD population harbor AIEC bacteria,4, 45, 46 we can hypothesize that if a research clinical trial using maltodextrin-free diet were to be performed at the IBD population level, results will show different results for AIEC+ and AIEC– subjects (Figure 2A). On the other hand, if such maltodextrin-free diet clinical trial were to focus on the CD population carrying AIEC bacteria, we can expect much clearer beneficial clinical outcomes (Figure 2B).
Figure 2.
Importance of microbiota-based classification in research clinical trial. (A) Without a classification based on the resistant and susceptible status of the individuals, a dietary intervention aiming to supplement a beneficial compound or withdraw a detrimental one will lead to the absence of significant clinical outcome between the treated and the control groups. (B) By classifying the participants on the basis of their microbiota status (resistant or susceptible), significant clinical outcomes can be observed in the susceptible population, whereas no effect is observed in the population harboring a resistant microbiota.
Other important investigations focusing on the obese population have highlighted the importance of the intestinal microbiota in clinical trials, particularly in regard to dietary intervention. In an elegant study by Clément and Cani groups, the authors found that baseline of A muciniphila abundance within the intestinal microbiota associated with better clinical outcomes after a 6-week dietary intervention in the obese population.47 By stratifying the population on the basis of A muciniphila level, they indeed found that patients harboring a higher level of this specific bacteria had greatest benefits from the dietary intervention.47 Hence, one can speculate that such observation may apply to other bacteria, and that the success of dietary intervention aiming to improve metabolic health may highly benefit from individualization based on microbiota composition.47, 48
In a similar vein of research, a recent study demonstrated that colonization of the gastrointestinal tract by probiotic is dependent on and can be predicted by microbiota composition,49 suggesting that some microbiota might be more predisposed to benefit from probiotic treatment. Importantly, although patients can be stratified by their ability to be colonized, functional outcomes were not addressed in this study, and numerous reports actually suggest that a daily probiotic does not necessarily need to colonize to exert its beneficial effect.50, 51 Interestingly, probiotic treatment was also recently found to have a detrimental impact on microbiota recolonization after antibiotic use, highlighting that stable colonization of the gastrointestinal tract by such species may also be detrimental.52
Lessons Learned From Preclinical Research on Purified Soluble Fiber
In a recent study, we unexpectedly observed that in mice, enriching purified diets with soluble fiber inulin led to icteric hepatocellular carcinoma (HCC), with up to 40% penetrance rate in male mice and 20% in female mice.53 In this model, we identified a central role played by the intestinal microbiota, with the observation that HCC susceptibility was transferred to normally unaffected mice by co-housing. Mechanistically, soluble fiber–induced HCC was prevented by inhibition of microbial fermentation. This study also revealed that an analysis of the microbiota composition could predict the likelihood of an animal to develop soluble fiber–induced HCC.53 Moreover, HCC penetrance was impacted by the animal provider, highlighting further that a specific microbiota is required for the development of soluble fiber–driven HCC and suggesting that in this case, the intestinal microbiota could be more determinant than host genetics for precision medicine.54
Similarly, we identified that although soluble fiber supplementation was beneficial in a model of diet-induced obesity in wild-type mice,55 it was associated with exacerbation of colitis in genetically susceptible mice (IL10KO).56 Altogether, these findings suggest that precision dietary supplementation based on personal gut microbiota might be a promising future direction to manage intestinal and extraintestinal disorders. For example, Prevotella genus is known to be an effective producer of short-chain fatty acid through dietary fiber degradation,57, 58 and one can imagine dietary recommendation for daily fiber intake based on Prevotella relative abundance in the gastrointestinal tract to avoid detrimental impacts on the liver.
Lessons Learned From General Population-based Studies
Because of recent advances in nucleic acid sequencing methods that have dramatically increased the cost-effectiveness of microbiota composition analyses techniques, important progress has recently been made by investigating microbiota composition at the population level together with detailed characterization of clinical outcomes. Among these recent studies, one assessed factor can contribute to postprandial blood glucose level elevation after a meal, a defining parameter of prediabetes and a major risk factor for type 2 diabetes. Although inter-individual variations in postprandial blood glucose level had been previously described, they were yet not well-understood. In this research, Zeevi et al59 monitored glucose levels of 800 participants in response to almost 50,000 meals, and they importantly found high inter-individual variability of blood glucose levels after an identical meal, suggesting that dietary recommendations at the population level may have limited utility. In addition, the authors used machine-learning algorithm that integrated multiple parameters, including gut microbiota composition, and they demonstrated that such algorithm can accurately predict postprandial glycemic response to a specific meal. Such findings elegantly demonstrate the microbiota power in determining clinical outcomes and further support the concept of integrating microbiota inter-individual variations for clinical research.60 A tool based on multiple factors, including microbiota composition, that is able to precisely predict glycemic response could be highly beneficial for the type 1 diabetic population by providing personalized dietary recommendations as well as optimized dosing of insulin to precisely control blood glucose levels.60
Discussion and Perspective
The recent and rapid evolution of the microbiome field of research has demonstrated the importance of the microbiome in determining health and disease outcomes. Furthermore, it suggests that microbiome analysis should be added to clinical trials, especially as relates to dietary research, because this information may be essential for correctly appreciating clinical outcomes (Figure 2). Indeed, although a dietary intervention aiming to withdraw and/or supplement specific macro/micronutrients to treat/prevent a chronic inflammatory disease may fail at the population level, the identification of subpopulation harboring specific bacteria that can mediate the beneficial effect may reveal positive outcome of such dietary interventions (Figure 2). Hence, the importance that the intestinal microbiota may have on the outcome of clinical intervention in chronic inflammatory diseases, either drug-based or dietary-based, should now be appreciated and routine feces collection considered. Furthermore, it is estimated that one-third of clinical trials are terminated as a result of hepatotoxicity in subset of patients. Microbiota composition should be investigated in such scenarios because it may allow for those drugs to be used by the population carrying resistant microbiota, whereas they should be avoided by the population harboring a susceptible microbiota that will lead to hepatotoxicity.48, 61 Other factors known to have an impact on microbiota composition, such as gender, genetic factors, and circadian rhythm, will also need to be considered in future studies investigating microbiota-based therapeutic interventions.62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73 Regarding the latter, for example, microbiota shows host-synchronized circadian variations in its composition,74 and recent studies highlighted deregulation and disconnection of such microbiota rhythm in chronic inflammatory disease.75 Hence, microbiota-based therapy will also need to consider multiple important variables including circadian rhythm, for example by targeting a deleterious bacterium at the time of the day when it is the most abundant or by aiming to reconnect microbiota and host rhythms.
Importantly, microbiota composition–based individual classification can be investigated before dietary intervention as well as retrospectively. Indeed, although the ultimate goal of such approach is to classify patients on the basis of their microbiota composition before dietary intervention, further research is needed to better define what are a “resistant” and a “susceptible” microbiota. As an example highlighting this concept, although we have started to identify some specific bacteria triggering emulsifier detrimental effects, fully defining resistant and susceptible microbiota will likely need to be done retrospectively after dietary intervention, with individuals being classified on the basis of their therapeutic response. We envision that such data can be used to identify, within the general population, individuals harboring a microbiota more likely to be beneficially impacted by emulsifier withdraw. Moreover, with the impact of the intestinal microbiota on drug metabolism,76, 77 microbiota composition analysis should now be considered in patient pharmacogenomics analysis.
Conclusion
We submit that deeper understanding of the human microbiota will lead to the identification and comprehension of resistant and susceptible microbiota, allowing health care providers to provide precise, tailored dietary and therapeutic recommendations for their patients. Clearly, additional research is warranted to uncover the determinants and mechanisms by which gut microbiome composition would render a human subject as resistant or susceptible to a particular intervention. To date, most studies have relied on analyzing microbiota composition via 16S rRNA gene sequencing, but accurately predicting responses may require analysis of metagenomes (all the genomic content of a microbiota) and/or metatranscriptomes (all the genes actively expressed by a microbiota) to successfully identify responders and non-responders. Moreover, in addition to the species level, identification of microbiota members at the strain level may need to be investigated.78 In addition, it appears important to determine which microbiota subpopulation is to be used to properly stratify patient populations: fecal microbiota, luminal microbiota, mucosa-associated microbiota, or inner mucus–associated microbiota, especially because these microbiota subtypes are not communicating with the host or responding to dietary intervention in the same way. Our recently developed technique of laser capture microdissection to specifically collect and identify inner mucus–associated bacteria could help in this endeavor.30 Moreover, even such extensive analysis may not yield optimal predictive power, and hence we envision that developing functional assays to test how individual microbiotas respond to a particular treatment will be needed. Such need for further studies notwithstanding, routine sampling of stool in dietary-based or drug-based clinical intervention should already be considered to broadly assess microbiota composition in clinical trials, including retrospective analysis. Such approaches may soon begin to unlock the potential of the microbiota in serving as a predictor of clinical outcome in dietary intervention.
Footnotes
Author contributions E.V., A.T.G., and B.C. wrote the manuscript.
Conflicts of interest The authors disclose no conflicts.
Funding E.V. is a recipient of a Career Development Award from the Crohn’s and Colitis Foundation. B.C. is a recipient of a Career Development Award from the Crohn’s and Colitis Foundation, an Innovator Award from the Kenneth Rainin Foundation and a Seed Grant from the Brain and Behavior program at Georgia State University. A.T.G. is supported by NIH grants DK099071 and DK083890.
References
- 1.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., Mende D.R., Li J., Xu J., Li S., Li D., Cao J., Wang B., Liang H., Zheng H., Xie Y., Tap J., Lepage P., Bertalan M., Batto J.M., Hansen T., Le Paslier D., Linneberg A., Nielsen H.B., Pelletier E., Renault P., Sicheritz-Ponten T., Turner K., Zhu H., Yu C., Li S., Jian M., Zhou Y., Li Y., Zhang X., Li S., Qin N., Yang H., Wang J., Brunak S., Dore J., Guarner F., Kristiansen K., Pedersen O., Parkhill J., Weissenbach J., Meta H.I.T.C., Bork P., Ehrlich S.D., Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Price J., Abu-Ali G., Huttenhower C. The healthy human microbiome. Genome Med. 2016;8:51. doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belkaid Y., Hand T.W. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chassaing B., Darfeuille-Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1720–1728. doi: 10.1053/j.gastro.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 5.Chassaing B., Aitken J.D., Gewirtz A.T., Vijay-Kumar M. Gut microbiota drives metabolic disease in immunologically altered mice. Adv Immunol. 2012;116:93–112. doi: 10.1016/B978-0-12-394300-2.00003-X. [DOI] [PubMed] [Google Scholar]
- 6.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 7.Devkota S., Wang Y., Musch M.W., Leone V., Fehlner-Peach H., Nadimpalli A., Antonopoulos D.A., Jabri B., Chang E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagao-Kitamoto H., Shreiner A.B., Gillilland M.G., 3rd, Kitamoto S., Ishii C., Hirayama A., Kuffa P., El-Zaatari M., Grasberger H., Seekatz A.M., Higgins P.D., Young V.B., Fukuda S., Kao J.Y., Kamada N. Functional characterization of inflammatory bowel disease-associated gut dysbiosis in gnotobiotic mice. Cell Mol Gastroenterol Hepatol. 2016;2:468–481. doi: 10.1016/j.jcmgh.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jobin C. Precision medicine using microbiota. Science. 2018;359:32–34. doi: 10.1126/science.aar2946. [DOI] [PubMed] [Google Scholar]
- 10.Matson V., Fessler J., Bao R., Chongsuwat T., Zha Y., Alegre M.L., Luke J.J., Gajewski T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Routy B., Le Chatelier E., Derosa L., Duong C.P.M., Alou M.T., Daillere R., Fluckiger A., Messaoudene M., Rauber C., Roberti M.P., Fidelle M., Flament C., Poirier-Colame V., Opolon P., Klein C., Iribarren K., Mondragon L., Jacquelot N., Qu B., Ferrere G., Clemenson C., Mezquita L., Masip J.R., Naltet C., Brosseau S., Kaderbhai C., Richard C., Rizvi H., Levenez F., Galleron N., Quinquis B., Pons N., Ryffel B., Minard-Colin V., Gonin P., Soria J.C., Deutsch E., Loriot Y., Ghiringhelli F., Zalcman G., Goldwasser F., Escudier B., Hellmann M.D., Eggermont A., Raoult D., Albiges L., Kroemer G., Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 12.Gopalakrishnan V., Spencer C.N., Nezi L., Reuben A., Andrews M.C., Karpinets T.V., Prieto P.A., Vicente D., Hoffman K., Wei S.C., Cogdill A.P., Zhao L., Hudgens C.W., Hutchinson D.S., Manzo T., Petaccia de Macedo M., Cotechini T., Kumar T., Chen W.S., Reddy S.M., Szczepaniak Sloane R., Galloway-Pena J., Jiang H., Chen P.L., Shpall E.J., Rezvani K., Alousi A.M., Chemaly R.F., Shelburne S., Vence L.M., Okhuysen P.C., Jensen V.B., Swennes A.G., McAllister F., Marcelo Riquelme Sanchez E., Zhang Y., Le Chatelier E., Zitvogel L., Pons N., Austin-Breneman J.L., Haydu L.E., Burton E.M., Gardner J.M., Sirmans E., Hu J., Lazar A.J., Tsujikawa T., Diab A., Tawbi H., Glitza I.C., Hwu W.J., Patel S.P., Woodman S.E., Amaria R.N., Davies M.A., Gershenwald J.E., Hwu P., Lee J.E., Zhang J., Coussens L.M., Cooper Z.A., Futreal P.A., Daniel C.R., Ajami N.J., Petrosino J.F., Tetzlaff M.T., Sharma P., Allison J.P., Jenq R.R., Wargo J.A. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vetizou M., Pitt J.M., Daillere R., Lepage P., Waldschmitt N., Flament C., Rusakiewicz S., Routy B., Roberti M.P., Duong C.P., Poirier-Colame V., Roux A., Becharef S., Formenti S., Golden E., Cording S., Eberl G., Schlitzer A., Ginhoux F., Mani S., Yamazaki T., Jacquelot N., Enot D.P., Berard M., Nigou J., Opolon P., Eggermont A., Woerther P.L., Chachaty E., Chaput N., Robert C., Mateus C., Kroemer G., Raoult D., Boneca I.G., Carbonnel F., Chamaillard M., Zitvogel L. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrosino J.F. The microbiome in precision medicine: the way forward. Genome Med. 2018;10:12. doi: 10.1186/s13073-018-0525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodrich J.K., Di Rienzi S.C., Poole A.C., Koren O., Walters W.A., Caporaso J.G., Knight R., Ley R.E. Conducting a microbiome study. Cell. 2014;158:250–262. doi: 10.1016/j.cell.2014.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I., Panaccione R., Ghosh S., Wu J.C.Y., Chan F.K.L., Sung J.J.Y., Kaplan G.G. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 17.Roberts C.L., Rushworth S.L., Richman E., Rhodes J.M. Hypothesis: increased consumption of emulsifiers as an explanation for the rising incidence of Crohn's disease. J Crohns Colitis. 2013;7:338–341. doi: 10.1016/j.crohns.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Roberts C.L., Keita A.V., Duncan S.H., O'Kennedy N., Soderholm J.D., Rhodes J.M., Campbell B.J. Translocation of Crohn's disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers. Gut. 2010;59:1331–1339. doi: 10.1136/gut.2009.195370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swidsinski A., Ung V., Sydora B.C., Loening-Baucke V., Doerffel Y., Verstraelen H., Fedorak R.N. Bacterial overgrowth and inflammation of small intestine after carboxymethylcellulose ingestion in genetically susceptible mice. Inflamm Bowel Dis. 2009;15:359–364. doi: 10.1002/ibd.20763. [DOI] [PubMed] [Google Scholar]
- 20.Chassaing B., Koren O., Goodrich J.K., Poole A.C., Srinivasan S., Ley R.E., Gewirtz A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92–96. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H., Limenitakis J.P., Fuhrer T., Geuking M.B., Lawson M.A., Wyss M., Brugiroux S., Keller I., Macpherson J.A., Rupp S., Stolp B., Stein J.V., Stecher B., Sauer U., McCoy K.D., Macpherson A.J. The outer mucus layer hosts a distinct intestinal microbial niche. Nature Communications. 2015;6:8292. doi: 10.1038/ncomms9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duerkop B.A., Vaishnava S., Hooper L.V. Immune responses to the microbiota at the intestinal mucosal surface. Immunity. 2009;31:368–376. doi: 10.1016/j.immuni.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Ivanov, Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U., Wei D.G., Goldfarb K.C., Santee C.A., Lynch S.V., Tanoue T., Imaoka A., Itoh K., Takeda K., Umesaki Y., Honda K., Littman D.R. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carroll I.M., Ringel-Kulka T., Keku T.O., Chang Y.H., Packey C.D., Sartor R.B., Ringel Y. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2011;301:G799–G807. doi: 10.1152/ajpgi.00154.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galley J.D., Yu Z., Kumar P., Dowd S.E., Lyte M., Bailey M.T. The structures of the colonic mucosa-associated and luminal microbial communities are distinct and differentially affected by a prolonged murine stressor. Gut Microbes. 2014;5:748–760. doi: 10.4161/19490976.2014.972241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinsen F.A., Knecht H., Neulinger S.C., Schmitz R.A., Knecht C., Kuhbacher T., Rosenstiel P.C., Schreiber S., Friedrichs A.K., Ott S.J. Dynamic changes of the luminal and mucosa-associated gut microbiota during and after antibiotic therapy with paromomycin. Gut Microbes. 2015;6:243–254. doi: 10.1080/19490976.2015.1062959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaboriau-Routhiau V., Rakotobe S., Lecuyer E., Mulder I., Lan A., Bridonneau C., Rochet V., Pisi A., De Paepe M., Brandi G., Eberl G., Snel J., Kelly D., Cerf-Bensussan N. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 28.Johansson M.E., Phillipson M., Petersson J., Velcich A., Holm L., Hansson G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansson G.C., Johansson M.E. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Gut Microbes. 2010;1:51–54. doi: 10.4161/gmic.1.1.10470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chassaing B., Gewirtz A.T. Identification of inner mucus-associated bacteria by laser capture microdissection. Cell Mol Gastroenterol Hepatol. 2019;7:157–160. doi: 10.1016/j.jcmgh.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dewhirst F.E., Chien C.C., Paster B.J., Ericson R.L., Orcutt R.P., Schauer D.B., Fox J.G. Phylogeny of the defined murine microbiota: altered Schaedler flora. Appl Environ Microbiol. 1999;65:3287–3292. doi: 10.1128/aem.65.8.3287-3292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chassaing B., Van de Wiele T., De Bodt J., Marzorati M., Gewirtz A.T. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017;66:1414–1427. doi: 10.1136/gutjnl-2016-313099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Syal G., Kashani A., Shih D.Q. Fecal microbiota transplantation in inflammatory bowel disease: a primer for internists. Am J Med. 2018;131:1017–1024. doi: 10.1016/j.amjmed.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Vermeire S., Joossens M., Verbeke K., Hildebrand F., Machiels K., Van den Broeck K., Van Assche G., Rutgeerts P.J., Raes J. Pilot study on the safety and efficacy of faecal microbiota transplantation in refractory Crohn's disease. Gastroenterology. 2012;142:S-360. [Google Scholar]
- 35.Kump P.K., Grochenig H.P., Lackner S., Trajanoski S., Reicht G., Hoffmann K.M., Deutschmann A., Wenzl H.H., Petritsch W., Krejs G.J., Gorkiewicz G., Hogenauer C. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013;19:2155–2165. doi: 10.1097/MIB.0b013e31829ea325. [DOI] [PubMed] [Google Scholar]
- 36.Vaughn B.P., Gevers D., Ting A., Korzenik J.R., Robson S.C., Moss A.C. Fecal microbiota transplantation induces early improvement in symptoms in patients with active Crohn's disease. Gastroenterology. 2014;146 S-591–S-592. [Google Scholar]
- 37.Landy J., Al-Hassi H.O., Mann E.R., Peake S.T., McLaughlin S.D., Ciclitira P.J., Clark S.K., Knight S.C., Hart A.L. A prospective controlled pilot study of fecal microbiota transplantation for chronic refractory pouchitis. Gastroenterology. 2013;144:S-897. [Google Scholar]
- 38.Colman R.J., Rubin D.T. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2014;8:1569–1581. doi: 10.1016/j.crohns.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao K., Young V.B. Fecal microbiota transplantation for the management of Clostridium difficile infection. Infect Dis Clin North Am. 2015;29:109–122. doi: 10.1016/j.idc.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hvas C.L., Jorgensen S.M.D., Jorgensen S.P., Storgaard M., Lemming L., Hansen M.M., Erikstrup C., Dahlerup J.F. Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent Clostridium difficile infection. Gastroenterology. 2019;156:1324–1332. doi: 10.1053/j.gastro.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 41.Hou J.K., Abraham B., El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563–573. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 42.Verma S., Brown S., Kirkwood B., Giaffer M.H. Polymeric versus elemental diet as primary treatment in active Crohn's disease: a randomized, double-blind trial. Am J Gastroenterol. 2000;95:735–739. doi: 10.1111/j.1572-0241.2000.01527.x. [DOI] [PubMed] [Google Scholar]
- 43.Gorard D.A., Hunt J.B., Payne-James J.J., Palmer K.R., Rees R.G., Clark M.L., Farthing M.J., Misiewicz J.J., Silk D.B. Initial response and subsequent course of Crohn's disease treated with elemental diet or prednisolone. Gut. 1993;34:1198–1202. doi: 10.1136/gut.34.9.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nickerson K.P., McDonald C. Crohn's disease-associated adherent-invasive Escherichia coli adhesion is enhanced by exposure to the ubiquitous dietary polysaccharide maltodextrin. PLoS One. 2012;7:e52132. doi: 10.1371/journal.pone.0052132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darfeuille-Michaud A., Boudeau J., Bulois P., Neut C., Glasser A.L., Barnich N., Bringer M.A., Swidsinski A., Beaugerie L., Colombel J.F. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004;127:412–421. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 46.Darfeuille-Michaud A., Neut C., Barnich N., Lederman E., Di Martino P., Desreumaux P., Gambiez L., Joly B., Cortot A., Colombel J.F. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology. 1998;115:1405–1413. doi: 10.1016/s0016-5085(98)70019-8. [DOI] [PubMed] [Google Scholar]
- 47.Dao M.C., Everard A., Aron-Wisnewsky J., Sokolovska N., Prifti E., Verger E.O., Kayser B.D., Levenez F., Chilloux J., Hoyles L., Consortium M.I.-O., Dumas M.E., Rizkalla S.W., Dore J., Cani P.D., Clement K. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 48.Nayak R.R., Turnbaugh P.J. Mirror, mirror on the wall: which microbiomes will help heal them all? BMC Med. 2016;14:72. doi: 10.1186/s12916-016-0622-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zmora N., Zilberman-Schapira G., Suez J., Mor U., Dori-Bachash M., Bashiardes S., Kotler E., Zur M., Regev-Lehavi D., Brik R.B., Federici S., Cohen Y., Linevsky R., Rothschild D., Moor A.E., Ben-Moshe S., Harmelin A., Itzkovitz S., Maharshak N., Shibolet O., Shapiro H., Pevsner-Fischer M., Sharon I., Halpern Z., Segal E., Elinav E. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018;174:1388–1405 e21. doi: 10.1016/j.cell.2018.08.041. [DOI] [PubMed] [Google Scholar]
- 50.Kristensen N.B., Bryrup T., Allin K.H., Nielsen T., Hansen T.H., Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. 2016;8:52. doi: 10.1186/s13073-016-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farnworth E.R. The evidence to support health claims for probiotics. J Nutr. 2008;138:1250S–1254S. doi: 10.1093/jn/138.6.1250S. [DOI] [PubMed] [Google Scholar]
- 52.Suez J., Zmora N., Zilberman-Schapira G., Mor U., Dori-Bachash M., Bashiardes S., Zur M., Regev-Lehavi D., Ben-Zeev Brik R., Federici S., Horn M., Cohen Y., Moor A.E., Zeevi D., Korem T., Kotler E., Harmelin A., Itzkovitz S., Maharshak N., Shibolet O., Pevsner-Fischer M., Shapiro H., Sharon I., Halpern Z., Segal E., Elinav E. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174:1406–1423 e16. doi: 10.1016/j.cell.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 53.Singh V., Yeoh B.S., Chassaing B., Xiao X., Saha P., Aguilera Olvera R., Lapek J.D., Jr., Zhang L., Wang W.B., Hao S., Flythe M.D., Gonzalez D.J., Cani P.D., Conejo-Garcia J.R., Xiong N., Kennett M.J., Joe B., Patterson A.D., Gewirtz A.T., Vijay-Kumar M. Dysregulated microbial fermentation of soluble fiber induces cholestatic liver cancer. Cell. 2018;175:679–694 e22. doi: 10.1016/j.cell.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wan Y.Y., Jena P.K. Precision dietary supplementation based on personal gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16:204–206. doi: 10.1038/s41575-019-0108-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chassaing B., Miles-Brown J., Pellizzon M., Ulman E., Ricci M., Zhang L., Patterson A.D., Vijay-Kumar M., Gewirtz A.T. Lack of soluble fiber drives diet-induced adiposity in mice. Am J Physiol Gastrointest Liver Physiol. 2015;309:G528–G541. doi: 10.1152/ajpgi.00172.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miles J.P., Zou J., Kumar M.V., Pellizzon M., Ulman E., Ricci M., Gewirtz A.T., Chassaing B. Supplementation of low- and high-fat diets with fermentable fiber exacerbates severity of DSS-induced acute colitis. Inflamm Bowel Dis. 2017;23:1133–1143. doi: 10.1097/MIB.0000000000001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schroeder B.O., Backhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22:1079–1089. doi: 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
- 58.Chen T., Long W., Zhang C., Liu S., Zhao L., Hamaker B.R. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci Rep. 2017;7:2594. doi: 10.1038/s41598-017-02995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeevi D., Korem T., Zmora N., Israeli D., Rothschild D., Weinberger A., Ben-Yacov O., Lador D., Avnit-Sagi T., Lotan-Pompan M., Suez J., Mahdi J.A., Matot E., Malka G., Kosower N., Rein M., Zilberman-Schapira G., Dohnalova L., Pevsner-Fischer M., Bikovsky R., Halpern Z., Elinav E., Segal E. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163:1079–1094. doi: 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 60.Jumpertz von Schwartzenberg R., Turnbaugh P.J. Siri, what should I eat? Cell. 2015;163:1051–1052. doi: 10.1016/j.cell.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 61.Wang L., McLeod H.L., Weinshilboum R.M. Genomics and drug response. N Engl J Med. 2011;364:1144–1153. doi: 10.1056/NEJMra1010600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taneja V. Microbiome: impact of gender on function & characteristics of gut microbiome. In: Legato M.J., editor. Principles of gender-specific medicine: gender in the genomic era. 3rd ed. Elsevier; New York: 2017. pp. 569–583. [Google Scholar]
- 63.Goodrich J.K., Davenport E.R., Clark A.G., Ley R.E. The relationship between the human genome and microbiome comes into view. Annu Rev Genet. 2017;51:413–433. doi: 10.1146/annurev-genet-110711-155532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blekhman R., Goodrich J.K., Huang K., Sun Q., Bukowski R., Bell J.T., Spector T.D., Keinan A., Ley R.E., Gevers D., Clark A.G. Host genetic variation impacts microbiome composition across human body sites. Genome Biol. 2015;16:191. doi: 10.1186/s13059-015-0759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davenport E.R. Elucidating the role of the host genome in shaping microbiome composition. Gut Microbes. 2016;7:178–184. doi: 10.1080/19490976.2016.1155022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goodrich J.K., Waters J.L., Poole A.C., Sutter J.L., Koren O., Blekhman R., Beaumont M., Van Treuren W., Knight R., Bell J.T., Spector T.D., Clark A.G., Ley R.E. Human genetics shape the gut microbiome. Cell. 2014;159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goodrich J.K., Davenport E.R., Beaumont M., Jackson M.A., Knight R., Ober C., Spector T.D., Bell J.T., Clark A.G., Ley R.E. Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe. 2016;19:731–743. doi: 10.1016/j.chom.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goodrich J.K., Davenport E.R., Waters J.L., Clark A.G., Ley R.E. Cross-species comparisons of host genetic associations with the microbiome. Science. 2016;352:532–535. doi: 10.1126/science.aad9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonder M.J., Kurilshikov A., Tigchelaar E.F., Mujagic Z., Imhann F., Vila A.V., Deelen P., Vatanen T., Schirmer M., Smeekens S.P., Zhernakova D.V., Jankipersadsing S.A., Jaeger M., Oosting M., Cenit M.C., Masclee A.A., Swertz M.A., Li Y., Kumar V., Joosten L., Harmsen H., Weersma R.K., Franke L., Hofker M.H., Xavier R.J., Jonkers D., Netea M.G., Wijmenga C., Fu J., Zhernakova A. The effect of host genetics on the gut microbiome. Nat Genet. 2016;48:1407–1412. doi: 10.1038/ng.3663. [DOI] [PubMed] [Google Scholar]
- 70.Turpin W., Espin-Garcia O., Xu W., Silverberg M.S., Kevans D., Smith M.I., Guttman D.S., Griffiths A., Panaccione R., Otley A., Xu L., Shestopaloff K., Moreno-Hagelsieb G., Consortium G.E.M.P.R., Paterson A.D., Croitoru K. Association of host genome with intestinal microbial composition in a large healthy cohort. Nat Genet. 2016;48:1413–1417. doi: 10.1038/ng.3693. [DOI] [PubMed] [Google Scholar]
- 71.Wang J., Thingholm L.B., Skieceviciene J., Rausch P., Kummen M., Hov J.R., Degenhardt F., Heinsen F.A., Ruhlemann M.C., Szymczak S., Holm K., Esko T., Sun J., Pricop-Jeckstadt M., Al-Dury S., Bohov P., Bethune J., Sommer F., Ellinghaus D., Berge R.K., Hubenthal M., Koch M., Schwarz K., Rimbach G., Hubbe P., Pan W.H., Sheibani-Tezerji R., Hasler R., Rosenstiel P., D'Amato M., Cloppenborg-Schmidt K., Kunzel S., Laudes M., Marschall H.U., Lieb W., Nothlings U., Karlsen T.H., Baines J.F., Franke A. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet. 2016;48:1396–1406. doi: 10.1038/ng.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Igartua C., Davenport E.R., Gilad Y., Nicolae D.L., Pinto J., Ober C. Host genetic variation in mucosal immunity pathways influences the upper airway microbiome. Microbiome. 2017;5:16. doi: 10.1186/s40168-016-0227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thaiss C.A., Levy M., Korem T., Dohnalova L., Shapiro H., Jaitin D.A., David E., Winter D.R., Gury-BenAri M., Tatirovsky E., Tuganbaev T., Federici S., Zmora N., Zeevi D., Dori-Bachash M., Pevsner-Fischer M., Kartvelishvily E., Brandis A., Harmelin A., Shibolet O., Halpern Z., Honda K., Amit I., Segal E., Elinav E. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell. 2016;167:1495–1510 e12. doi: 10.1016/j.cell.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 74.Voigt R.M., Forsyth C.B., Green S.J., Engen P.A., Keshavarzian A. Circadian rhythm and the gut microbiome. Int Rev Neurobiol. 2016;131:193–205. doi: 10.1016/bs.irn.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 75.Thaiss C.A., Zeevi D., Levy M., Zilberman-Schapira G., Suez J., Tengeler A.C., Abramson L., Katz M.N., Korem T., Zmora N., Kuperman Y., Biton I., Gilad S., Harmelin A., Shapiro H., Halpern Z., Segal E., Elinav E. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159:514–529. doi: 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 76.Spanogiannopoulos P., Bess E.N., Carmody R.N., Turnbaugh P.J. The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Nat Rev Microbiol. 2016;14:273–287. doi: 10.1038/nrmicro.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saad R., Rizkallah M.R., Aziz R.K. Gut pharmacomicrobiomics: the tip of an iceberg of complex interactions between drugs and gut-associated microbes. Gut Pathog. 2012;4:16. doi: 10.1186/1757-4749-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Greenblum S., Carr R., Borenstein E. Extensive strain-level copy-number variation across human gut microbiome species. Cell. 2015;160:583–594. doi: 10.1016/j.cell.2014.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Esquerre N., Basso L., Dubuquoy C., Djouina M., Chappard D., Blanpied C., Desreumaux P., Vergnolle N., Vignal C., Body-Malapel M. Aluminum ingestion promotes colorectal hypersensitivity in rodents. Cell Mol Gastroenterol Hepatol. 2019;7:185–196. doi: 10.1016/j.jcmgh.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nickerson K.P., Chanin R., McDonald C. Deregulation of intestinal anti-microbial defense by the dietary additive, maltodextrin. Gut Microbes. 2015;6:78–83. doi: 10.1080/19490976.2015.1005477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Laudisi F., Di Fusco D., Dinallo V., Stolfi C., Di Grazia A., Marafini I., Colantoni A., Ortenzi A., Alteri C., Guerrieri F., Mavilio M., Ceccherini-Silberstein F., Federici M., MacDonald T.T., Monteleone I., Monteleone G. The food additive maltodextrin promotes endoplasmic reticulum stress-driven mucus depletion and exacerbates intestinal inflammation. Cell Mol Gastroenterol Hepatol. 2019;7:457–473. doi: 10.1016/j.jcmgh.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Holder M.K., Peters N.V., Whylings J., Fields C.T., Gewirtz A.T., de Vries G.J., Chassaing B. Dietary emulsifiers affect the intestinal microbiota and alter anxiety and social behaviors in a sex-dependent manner. Sci Rep. 2019;9:172. doi: 10.1038/s41598-018-36890-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Viennois E., Merlin D., Gewirtz A.T., Chassaing B. Dietary emulsifier-induced low-grade inflammation promotes colon carcinogenesis. Cancer Res. 2017;77:27–40. doi: 10.1158/0008-5472.CAN-16-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watt J., Marcus R. Carrageenan-induced ulceration of the large intestine in the guinea pig. Gut. 1971;12:164–171. doi: 10.1136/gut.12.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martino J.V., Van Limbergen J., Cahill L.E. The role of carrageenan and carboxymethylcellulose in the development of intestinal inflammation. Front Pediatr. 2017;5:96. doi: 10.3389/fped.2017.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shang Q., Sun W., Shan X., Jiang H., Cai C., Hao J., Li G., Yu G. Carrageenan-induced colitis is associated with decreased population of anti-inflammatory bacterium, Akkermansia muciniphila, in the gut microbiota of C57BL/6J mice. Toxicol Lett. 2017;279:87–95. doi: 10.1016/j.toxlet.2017.07.904. [DOI] [PubMed] [Google Scholar]
- 87.Rodriguez-Palacios A., Harding A., Menghini P., Himmelman C., Retuerto M., Nickerson K.P., Lam M., Croniger C.M., McLean M.H., Durum S.K., Pizarro T.T., Ghannoum M.A., Ilic S., McDonald C., Cominelli F. The artificial sweetener Splenda promotes gut Proteobacteria, dysbiosis, and myeloperoxidase reactivity in Crohn's disease-like ileitis. Inflamm Bowel Dis. 2018;24:1005–1020. doi: 10.1093/ibd/izy060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen L., Guo Y., Hu C., Lam P.K.S., Lam J.C.W., Zhou B. Dysbiosis of gut microbiota by chronic coexposure to titanium dioxide nanoparticles and bisphenol A: implications for host health in zebrafish. Environ Pollut. 2018;234:307–317. doi: 10.1016/j.envpol.2017.11.074. [DOI] [PubMed] [Google Scholar]
- 89.Bettini S., Boutet-Robinet E., Cartier C., Comera C., Gaultier E., Dupuy J., Naud N., Tache S., Grysan P., Reguer S., Thieriet N., Refregiers M., Thiaudiere D., Cravedi J.P., Carriere M., Audinot J.N., Pierre F.H., Guzylack-Piriou L., Houdeau E. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci Rep. 2017;7:40373. doi: 10.1038/srep40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nogueira C.M., de Azevedo W.M., Dagli M.L., Toma S.H., Leite A.Z., Lordello M.L., Nishitokukado I., Ortiz-Agostinho C.L., Duarte M.I., Ferreira M.A., Sipahi A.M. Titanium dioxide induced inflammation in the small intestine. World J Gastroenterol. 2012;18:4729–4735. doi: 10.3748/wjg.v18.i34.4729. [DOI] [PMC free article] [PubMed] [Google Scholar]