Abstract
Aims/hypothesis
The relationship between BMI and mortality has been extensively investigated in the general population; however, it is less clear in people with type 2 diabetes. We aimed to assess the association of BMI with all-cause and cardiovascular mortality in individuals with type 2 diabetes mellitus.
Methods
We searched electronic databases up to 1 March 2016 for prospective studies reporting associations for three or more BMI groups with all-cause and cardiovascular mortality in individuals with type 2 diabetes mellitus. Study-specific associations between BMI and the most-adjusted RR were estimated using restricted cubic splines and a generalised least squares method before pooling study estimates with a multivariate random-effects meta-analysis.
Results
We included 21 studies including 24 cohorts, 414,587 participants, 61,889 all-cause and 4470 cardiovascular incident deaths; follow-up ranged from 2.7 to 15.9 years. There was a strong nonlinear relationship between BMI and all-cause mortality in both men and women, with the lowest estimated risk from 31–35 kg/m2 and 28–31 kg/m2 (p value for nonlinearity <0.001) respectively. The risk of mortality at higher BMI values increased significantly only in women, whilst lower values were associated with higher mortality in both sexes. Limited data for cardiovascular mortality were available, with a possible inverse linear association with BMI (higher risk for BMI <27 kg/m2).
Conclusions/interpretation
In type 2 diabetes, BMI is nonlinearly associated with all-cause mortality with lowest risk in the overweight group in both men and women. Further research is needed to clarify the relationship with cardiovascular mortality and assess causality and sex differences.
Electronic supplementary material
The online version of this article (doi:10.1007/s00125-016-4162-6) contains peer-reviewed but unedited supplementary material, which is available to authorised users.
Keywords: BMI, Body mass index, Cardiovascular disease, Diabetes, Meta-analysis, Mortality, Nonlinearity, Systematic review
Introduction
Compared with the general population, diabetes mellitus is associated with a higher mortality, mainly attributable to cardiovascular causes [1]. In 2012, an estimated 1.5 million deaths were directly caused by diabetes and by 2030 diabetes is expected to be the seventh leading cause of death worldwide [2]. Being overweight or obese is one of the main modifiable risk factors for type 2 diabetes mellitus, and obesity has been significantly associated with an increased mortality risk in the general population [3]. However, evidence on the association between obesity and mortality in patients with diabetes remains inconclusive with some studies reporting an inverse association between obesity (estimated using the conventional measure of BMI) and mortality [4–6], some reporting U-shaped associations [7, 8], some reporting linear positive associations [9, 10], and some reporting no association [11].
A recent study investigating the relationship between BMI and mortality in 10,568 people with type 2 diabetes found a lower mortality risk in overweight (BMI ≥25 kg/m2), higher mortality risk in underweight (≤18.5 kg/m2), and a similar mortality risk in obese (BMI ≥30 kg/m2) people compared with those with normal weight, indicating a nonlinear association between BMI and all-cause mortality [12]. A recent systematic review including nine studies reported a reduced risk of all-cause mortality in overweight and obese people with type 2 diabetes when compared with normal or non-overweight people and a 5% progressive decrease in mortality for every 5 kg/m2 increase in BMI [13]. This analysis, however, did not explore whether a nonlinear relationship exists between BMI and outcomes. This is particularly relevant, given the possible presence of nonlinearity for BMI values within the category of overweight or obesity. Moreover, a clearer determination of the relationship across BMI values would elucidate the comparative relevance of higher and lower BMI values on mortality risk in people with diabetes.
To help clarify the evidence, we conducted a systematic review and meta-analysis of prospective studies to examine the shape of association of BMI with all-cause and cardiovascular mortality in individuals with type 2 diabetes mellitus.
Methods
This meta-analysis was performed following the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) recommendations [14].
Data sources, searches and study selection
Three independent investigators searched for prospective studies reporting associations between BMI and all-cause and cardiovascular mortality in people with type 2 diabetes mellitus using the databases PubMed, Web of Science, and Scopus. The search strategy combined keywords related to the exposure (i.e. ‘obesity’ OR ‘body mass index’ OR ‘BMI’), population (i.e. ‘diabetes’ OR ‘type 2 diabetes’), outcome (i.e. ‘cardiovascular’ OR ‘vascular’ OR ‘mortality’) and study design (i.e. ‘cohort’ OR ‘longitudinal’) and included articles published in English before 1 March 2016 (details of the search strategy are reported in the electronic supplementary material [ESM]). Reference lists of retrieved articles were also manually scanned for all relevant additional studies and reviews. Prospective studies were included if the RR of cardiovascular or all-cause mortality was reported for at least three BMI categories (one referent and two nonreferent). When multiple publications reported associations from the same cohort, we included the one with the longest follow-up or the largest sample size. We excluded studies including only participants with type 1 diabetes, or only participants with prevalent cardiovascular disease at baseline, or hospitalised patients. If studies reported estimates stratified by prevalent cardiovascular disease at baseline, we used data for participants without baseline cardiovascular disease.
Data extraction and quality assessment
We used standardised, pre-defined forms for data extraction and quality assessment. We abstracted data on first author name; year of journal publication; study location and follow-up duration; population age, source, sex distribution; baseline prevalence of cardiovascular disease; exposure definition and assessment; endpoint definition and ascertainment; and adjustment level. For each reported category of BMI, we collected data on: mean or median BMI value; number of participants (or person-years of follow-up); number of cases; and the RR with 95% CI. If category-specific mean or median BMI was not reported, we assumed the BMI value to be the midpoint between lower and upper boundaries; if one boundary was not reported in the first or last category, we assumed the difference between boundaries to be equal to that of the adjacent category. When studies published more than one adjusted RR, we extracted the most-adjusted estimate. When reported, we extracted data separately for men and women. We contacted study authors when it was not possible to extract data from published reports. Study quality was assessed by two authors using the nine-star Newcastle–Ottawa Scale (NOS) [15] and discrepancies were resolved by consensus or independent arbitration.
Data synthesis and analysis
For each outcome, we extracted RRs with 95% CIs for men, women and both. When studies only reported association separately for men and women, a within-study summary estimate was computed by fixed-effect meta-analysis. A similar summary estimate was calculated for one study reporting associations stratified by age. To assess the relationship between BMI and outcomes, we performed a two-stage random-effects dose–response meta-analysis [16–18]. In the first stage, we modelled BMI values using restricted cubic splines with three knots at 10%, 50% and 90% percentile of the BMI distribution. We then estimated study-specific trend between RRs and the two BMI spline transformations with a generalised least squares method, which accounts for the correlation within each set of RRs [16]. In the second stage, the two study-specific regression coefficients were combined in a multivariate random-effects meta-analysis using the restricted maximum likelihood method [19]. We used coefficients obtained from multivariate meta-analysis to perform a Wald-type test for the hypothesis of no exposure–disease association (both regression coefficients equal to zero) and of nonlinearity (second spline coefficient equal to zero) [20]. We assessed heterogeneity between sexes with a multivariate meta-regression. Publication bias was estimated with the Egger’s test [21]. We conducted a sensitivity analysis excluding studies that only report unadjusted effect estimates to assess whether the associations between BMI and mortality change. Furthermore, the association between BMI and several chronic conditions has been reported to vary in different demographic regions; therefore we also assessed whether there was any statistically significant heterogeneity in the association between BMI and mortality by regions (USA, Europe and UK, Asia) using multivariate meta-regression and also stratified the analysis by regions. In addition, use of medications may affect the mortality risk, and considering the changes in the guidelines for the management of diabetes and use of glucose-lowering therapies over the study period we conducted a multivariate meta-regression categorising the study periods as 2003–2010, 2011–2013, 2014–2015) to assess whether there were significant differences in the effect estimates by the time of the study. We also performed additional sensitivity analyses with three knots at different positions (e.g. 25th, 50th and 75th percentile) to assess whether the shape of the association changed.
Analyses were performed using Stata 14.1 [22, 23] and R [24, 25]. Two-sided p value <0.05 was considered statistically significant.
Results
Study characteristics
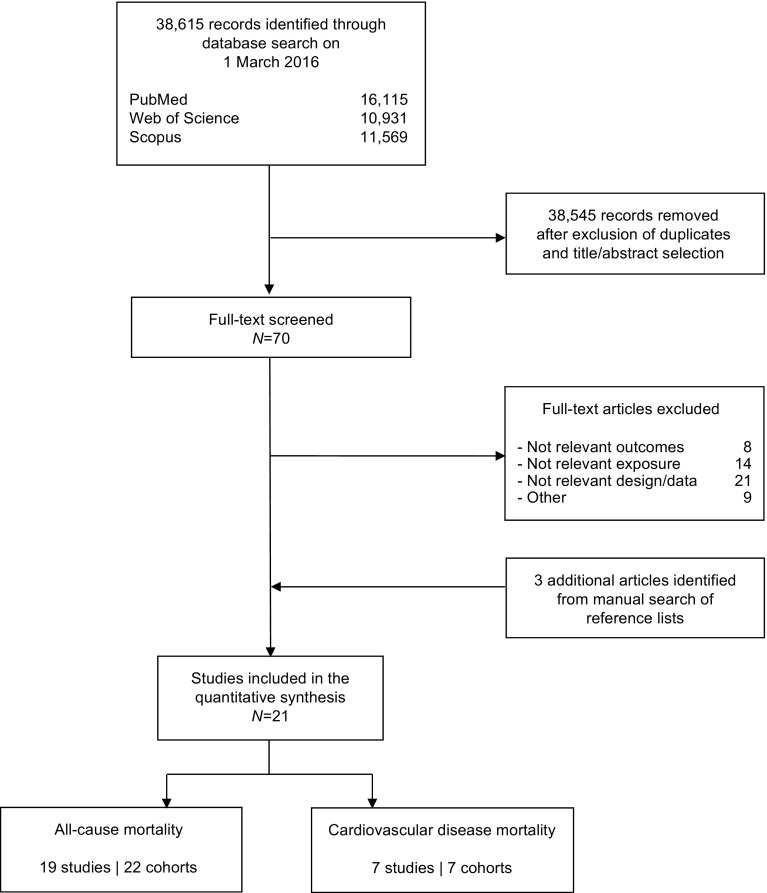
Our initial search yielded 38,615 articles. After screening of title/abstract and exclusion of duplicates, 70 articles remained for further evaluation (Fig. 1). Following detailed assessments, and after the inclusion of three articles identified from manual searches, 21 articles were included in the quantitative analysis (21 studies and 24 unique cohorts; Table 1) [7, 8, 11, 15, 26–42]. Study characteristics and the NOS scores are provided in ESM Tables 1–3. Out of the 21 studies included, ten (48%) had an NOS score of 8, whilst two (10%) studies had NOS scores of 9 or 5, indicating that most of the studies included were of good or high quality. Overall, information was available for 414,587 male and female participants with 61,889 and 4470 incident all-cause and cardiovascular mortality events, respectively. In nine studies associations were reported separately for men and women (Table 1). The mean (or median) duration of follow-up ranged from 2.7 to 15.9 years and the mean baseline age ranged from 40 to 77 years. All but three studies adjusted for age and sex (when appropriate) and diagnosis of diabetes was mainly self-reported or based on biochemical measurements (ESM Tables 1 and 2). Ten studies (47.6%) with 214,694 participants (51.8%) included people from the USA or UK, whilst seven studies were from elsewhere in Europe, one multinational, and one each from Iran, South Korea, and Taiwan.
Fig. 1.
Study flow diagram in line with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations
Table 1.
Characteristics of the included studies
| Number of events | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study First author [Ref] |
Country, year | Male (%) | Mean age (years)a | Mean follow-up (years) | Number of participants | All-cause mortality | CVD mortality | Stratified by sex |
| Bozorgmanesh [26] | Iran, 2014 | 44.6 | 53.6 | 9.1b | 1322 | 108 | – | – |
| Chaturvedi [27]c | Multinational, 1995 | 47.3 | 47.2 | 12.0 | 2740 | 544 | – | • |
| Church [28] | US, 2005 | 100 | 50.0 | 15.9 | 2316 | – | 179 | – |
| Costanzo [15] | UK, 2015 | 54.0 | 63.0b | 10.6b | 10,568 | 3744 | – | – |
| Eeg-Olofsson [29] | Sweden, 2009 | 55.7 | 60.3 | 5.6 | 13,087 | 664 | – | – |
| Jackson [30]d | US, 2014 | 40.0 | 57.2 | 9.0e | 2035 | 247 | – | – |
| Khalangot [11] | Ukraine, 2009 | 33.6 | 64.7 | 2.7 | 81,603 | 6570 | 2677 | • |
| Kokkinos [7] | US, 2012 | 100 | 60.0 | 7.5b | 4156 | 1074 | – | – |
| Logue [31]f | Scotland, 2013 | 54.7 | 59.5 | 4.7 | 106,640 | 9631 | – | • |
| Ma [32]f | South Korea, 2012 | 39.2 | 57.1 | 9.2 | 845 | – | 50 | • |
| McEwen [8]f | US, 2007 | 47.0 | 61.0 | 3.7 | 8445 | 758 | 322 | • |
| Menke [33] | US, 2014 | 50.7 | 56.9 | 6.5 | 2543 | 668 | 259 | – |
| Murphy [34] | Iceland, 2014 | 55.3 | 77.4 | 6.7b | 637 | 188 | – | – |
| Perotto [35]f | Italy, 2013 | 43.8 | 68.7 | 10.2b | 1475 | 972 | 498 | – |
| Sluik [36] | Europe, 2011 | 53.8 | 57.3 | 9.3b | 5435 | 641 | – | • |
| Thomas [37] | UK, 2014 | 53.0 | 60.0 | 5.0b | 37,272 | 1762 | – | – |
| Tobias [38] | US, 2014 | 21.5 | 61.4 | 15.8 | 11,427 | 3083 | – | • |
| Tseng [39] | Taiwan, 2012 | 46.0 | 60.7 | 12.0 | 89,056 | 26,951 | – | • |
| Tuomilehto [40] | Malta, 1994 | 40.0 | >40 | 5.0 | 295 | 39 | – | • |
| Zhao [41]f,g | US, 2014 | 37.8 | 52.3 | 8.7 | 29,292 | 3033 | – | – |
| Zoppini [42] | Italy, 2003 | 48.2 | 65.3 | 10.0 | 3398 | 1212 | 485 | – |
aWhen not reported for the overall population, the value has been estimated as weighted mean
bMedian
cThree cohorts
dNon-smokers
eMaximum follow-up
fAdditional data available from correspondence
gTwo cohorts
All-cause mortality
Overall, 18 studies comprising 407,270 participants and 60,815 all-cause death events were included in the analysis; study-specific associations are reported in ESM Figs 1–3. As shown in Fig. 2, there was an overall nonlinear relationship between BMI and all-cause mortality (p value for no exposure–disease association <0.001; p value for nonlinearity <0.001). The lowest risk was at BMI around 33 kg/m2, with an increased mortality risk more evident for lower than higher BMI values. Although no statistically significant heterogeneity was found (p = 0.376), the shape of the relationships differed between men and women. In the analysis restricted to men (nine studies, 141,709 participants/24,230 events), the lowest risk was between 31 and 35 kg/m2, with an increased risk for values lower than 31 kg/m2 and a slightly non-significant increase for values higher than 35 kg/m2 (p value for no exposure–disease association 0.0034; p value for nonlinearity 0.001). Conversely, almost a symmetrical increase was evident for women (eight studies, 168,088 participants/25,061 events) with a BMI nadir around 28–31 kg/m2 (p value for no exposure–disease association <0.001; p value for nonlinearity <0.001).
Fig. 2.
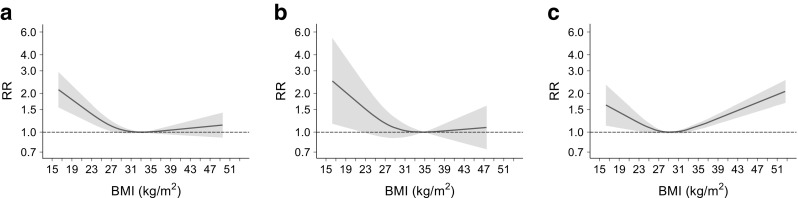
Relationship between BMI and all-cause mortality for (a) both sexes, (b) men and (c) women. Grey shading indicates 95% CI
For the three analyses, there was evidence of heterogeneity across studies (ESM Table 4) but not of publication bias (p values 0.403; 0.521; and 0.550, respectively).
Cardiovascular mortality
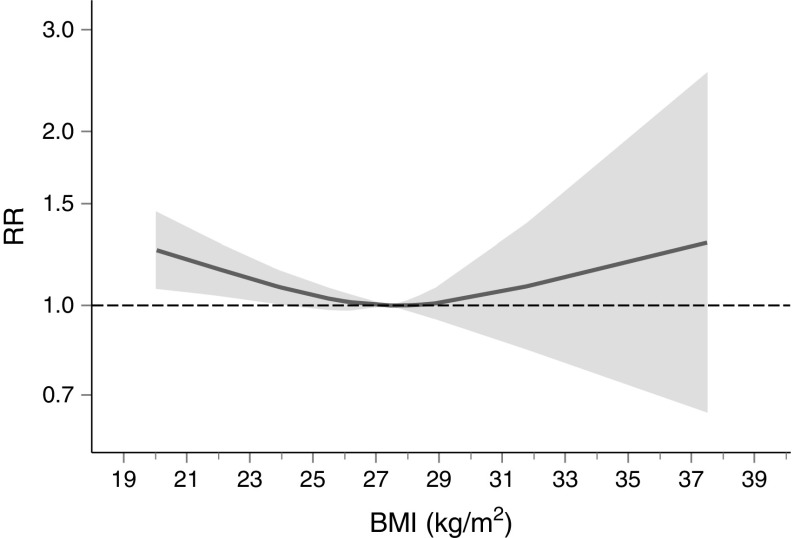
Six studies comprising 98,309 participants reported data on BMI and 4291 cardiovascular incident deaths for both men and women (Table 1 and ESM Fig. 4). The overall shape of the association suggested an increased risk of cardiovascular mortality for values lower than 27 kg/m2 and a less clear association for higher values (p value for no exposure–disease association 0.0176; p value for nonlinearity 0.140) (Fig. 3). However, a reduced risk for values greater than 27 kg/m2 and a possible linear negative trend cannot be excluded (RR per unit increase of BMI: 0.98; 95%CI: 0.96, 0.99; p = 0.013). No publication bias was present (p = 0.258). Owing to the limited number of studies, it was not possible to stratify the analysis by sex.
Fig. 3.
Relationship between BMI and cardiovascular mortality (both sexes). Grey shading indicates 95% CI
Sensitivity analyses
The results were consistent with those of the main analysis after the exclusion of two studies reporting unadjusted estimates, yet the risk of all-cause death for high values of BMI in women was less pronounced (ESM Table 5 and ESM Fig. 5). No heterogeneity was found in stratified analyses by region (USA, Europe and UK, Asia; p = 0.069) or period (2003–2009, 2011–2013, 2014–2015; p = 0.117) (ESM Table 6 and ESM Fig. 6). Results were substantially similar in sensitivity analyses considering alternative knot locations (ESM Fig. 7 and ESM Fig. 8).
Discussion
Results of this meta-analysis indicate a clear nonlinear relationship between BMI and all-cause mortality, with the lowest risk found around 33 kg/m2 and an increased risk more evident for lower compared with higher values. The shape of the association between BMI and all-cause mortality, however, was found to be different between male and female participants such that the risk did not increase for values higher than 35 kg/m2 in men whilst a symmetrical rise was found in women above and below a nadir around 28–31 kg/m2. Conversely, the limited data availability for cardiovascular mortality precluded a clear interpretation of the relationship, particularly for high values of BMI.
Our meta-analysis is the largest to-date to assess the association between BMI and mortality among people with diabetes. However, there are certain limitations of this study. First, most of the included studies adjusted risk estimates for a number of potential confounders; only five, however, adjusted for alcohol consumption, which is closely associated with mortality and BMI [43, 44]. Cardiorespiratory fitness has been reported as a potential effect modifier in the association of BMI and mortality, suggesting a ‘fit and fat’ phenomenon whereby higher cardiorespiratory fitness attenuates the increased mortality risk associated with higher BMI [45]. Given the available data, we could not explore this hypothesis in our meta-analysis. Similarly, we could not assess the impact of fat distribution, another possible effect modifier in the relationship between BMI and outcomes. Indeed, recent research has suggested that fat distribution is more strongly associated than BMI to future cardiovascular events risk [46]. Possible confounders of the association are drug treatment (as some glucose-lowering drugs are associated with both body weight and mortality [47]) and smoking, which is negatively correlated with BMI and positively with mortality [48, 49]. This association is conditioned on the effect of having diabetes such that the inverse association between smoking and BMI is exaggerated, which may potentially lead to an underestimation of the mortality risk associated with obesity. This may result in a collider bias, requiring detailed adjustment for smoking [50]. Almost all studies included in the meta-analysis, however, adjusted for smoking status. We were unable to conduct a stratified analysis by smoking status as only two studies reported the risk of mortality by BMI separately for each smoking category. Zhao and colleagues found that the risk estimates for different BMI groups compared with a reference group (30–34.9 kg/m2) were slightly higher in never smokers in comparison with current smokers in both Black and White participants [41]. In comparison Jackson and colleagues did not find a variation in the association between BMI and mortality by smoking status [30].
Our findings showed a nonlinear relationship between BMI and all-cause mortality in people with diabetes, with the lowest risk around 33 kg/m2. This is in line with a large meta-analysis of 141 studies assessing the association between BMI and mortality in the general population which found a 6% statistically significant reduction in mortality risk associated with being overweight (BMI 25–30 kg/m2), a 5% non-significant reduction associated with being modestly obese (BMI 30–35 kg/m2), and a 29% increase in the risk associated with a BMI of over 35 kg/m2 when compared with normal weight (BMI 18–25 kg/m2) [3]. In contrast, a recent dose–response meta-analysis assessing the relationship in type 2 diabetes reported a progressive linear 5% reduction of all-cause mortality for every 5 kg/m2 increase in the BMI (six studies and eight cohorts) [13]. However, this meta-analysis also included studies reporting on populations with pre-existing cardiovascular morbidities, such as heart failure [51, 52], whilst some large population-based studies were omitted. Moreover, in view of previous knowledge about the shape of the association between BMI and mortality in the general population, we did not assume the dose–response relationship to be linear and did not use conventional BMI categories but rather investigated possible nonlinearity in the BMI–mortality association. This enabled us to clarify the shape of the association across a wide range of BMI and assess whether there was a significant departure from linearity and a difference between male and female participants. Our results, indeed, clearly showed a nonlinear relationship for all-cause mortality, whilst the association with cardiovascular mortality is less clear (and potentially linear). We found, in particular, the mortality curve to be steeper in women compared with men, with a considerably higher risk of mortality at higher BMIs in women than men, and the nadir to be lower in women than men. Of note, in the sensitivity analysis excluding studies reporting unadjusted estimates, the risk for higher BMI was less pronounced: whether this is relates to a reduced statistical power or a true effect could not be ascertained. However, our findings are line with a general population study including over 12 million adults from South Korea, which found the optimal BMI for women to be lower than men, especially at younger ages [53]. This may be attributed to a positive and strong association between obesity and sex-specific cancer incidence and mortality in women [54–57].
The progressively increasing risk of all-cause mortality for values of BMI ≤25 kg/m2 in both men and women may have a number of explanations. Sarcopenic obesity is a condition characterised by relative increase in fat mass and reduction in muscle mass, thus resulting in a different total body fat composition for the same weight (and therefore BMI). Typically, people with sarcopenic obesity have visceral fat accumulation [58], a well-known risk factor for cardiovascular mortality [46]. The prevalence of sarcopenic obesity has been reported to be higher in type 2 diabetes compared with non-diabetes (15.7% vs 6.9% respectively in the Korean Sarcopenic Obesity Study) [59] and it has also been linked to an increased risk of falls [59], physical disability [60] and cardiovascular and all-cause mortality [61–64]. Another possible explanation could be reverse causation. Underlying conditions may result in loss of appetite or increased metabolic demands with subsequent unintentional weight loss. This phenomenon has been reported to be stronger in people with diabetes as they have higher rates of underlying illness compared with the general population [50]. This may partly explain the shape of association in our meta-analysis, with an increased risk for low values of BMI. In this analysis we were able to include data from eight studies that excluded the initial 2 years of follow-up to reduce the risk of reverse causation. A third possible reason is genetics. Some single nucleotide polymorphism (SNP) variants are more strongly associated with type 2 diabetes in lean compared with obese subsets [65]. Among such SNPs, variants at TCF7L2 and CDKAL1 have also been associated with increased risk of cancer in people with diabetes [66], potentially explaining the higher mortality risk for low BMI values. However, stronger evidence (ideally with Mendelian randomisation studies) is needed to support a genetic explanation in people with diabetes. Finally, potentially improved diagnosis and care of obese people with type 2 diabetes (i.e. screen-detected diabetes, more intensive control of risk factors) is another plausible explanation [67].
Conclusion
Findings from this meta-analysis of prospective cohort studies demonstrated a nonlinear relationship between BMI and all-cause mortality, with lower risks between 31 and 35 kg/m2 for men and 28 and 31 kg/m2 for women. Further research is needed to decipher whether this association is truly causal and different from that between BMI and cardiovascular mortality. Furthermore, to better clarify the link between excess fat and outcomes in people with diabetes, multiple assessments (including overall and region-specific body fat accumulation) over time are warranted. The results of our study, along with available previous knowledge in this field, do not downgrade the importance of weight control and appropriate lifestyle as cornerstones for the prevention and management of cardiometabolic diseases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 1220 kb)
Acknowledgements
We acknowledge the support from the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care – East Midlands (NIHR CLAHRC – EM), the Leicester Clinical Trials Unit and the NIHR Leicester–Loughborough Diet, Lifestyle and Physical Activity Biomedical Research Unit, which is a partnership between University Hospitals of Leicester NHS Trust, Loughborough University and the University of Leicester. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
The authors would like to express their gratitude to the following authors for providing additional data from their published manuscripts for this meta-analysis: G. Bruno (Universita’ di Torino, Turin, Italy); G. Hu (Pennington Biomedical Research Center, Baton Rouge, LA, USA); J. Logue (University of Glasgow, Glasgow, UK); L. McEwen (University of Michigan, Ann Arbor, MI, USA); S. Park (Seoul National University College of Medicine, Seoul, Korea); D. K. Tobias (Harvard Medical School and Brigham and Women’s Hospital, Boston, MA, USA). The authors also thank A. Crippa (Karolinska Institutet, Stockholm, Sweden) for helpful suggestions.
Abbreviations
- NOS
Newcastle–Ottawa Scale
- SNP
Single nucleotide polymorphism
Funding
FZ is a Clinical Research Fellow funded with an unrestricted Educational Grant from Sanofi-Aventis to the University of Leicester. The funding source had no involvement in this study.
Data availability
Statistical codes and datasets are available from the corresponding author.
Duality of interest
DRW has received grant in support of investigator initiated studies and honoraria from Sanofi-Aventis and Novo Nordisk. MJD has acted as consultant, advisory board member and speaker for Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, AstraZeneca and Janssen and as a speaker for Mitsubishi Tanabe Pharma Corporation. She has received grants in support of investigator and investigator initiated trials from Novo Nordisk, Sanofi-Aventis and Lilly. KK has acted as a consultant and speaker for Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Servier and Merck Sharp & Dohme. He has received grants in support of investigator and investigator initiated trials from Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Pfizer, Boehringer Ingelheim and Merck Sharp & Dohme. KK has received funds for research, honoraria for speaking at meetings and has served on advisory boards for Lilly, Sanofi-Aventis, Merck Sharp & Dohme and Novo Nordisk. All other authors declare there is no duality of interest in connection with their involvement in this study.
Contribution statement
NND and FZ conceived the study; FZ, NND and DP conducted the literature search and extracted the data; ND and FZ wrote the first draft; FZ designed the study, statistical plan and data analysis; DW, GM, MJD and KK supervised the study and critically reviewed the manuscript for important intellectual content. All authors contributed to the interpretation of data, revision of the manuscript and provided final approval of the version to be published. NND and FZ had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Global health estimates: deaths by cause, age, sex and country, 2000–2012. Geneva: World Health Organization; 2014. [Google Scholar]
- 3.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories. A systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doehner W, Erdmann E, Cairns R, et al. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: an analysis of the PROactive study population. Int J Cardiol. 2012;162:20–26. doi: 10.1016/j.ijcard.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 5.Kokkinos P, Myers J, Faselis C, Doumas M, Kheirbek R, Nylen E. BMI-mortality paradox and fitness in African American and Caucasian men with type 2 diabetes. Diabetes Care. 2012;35:1021–1027. doi: 10.2337/dc11-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEwen LN, Kim C, Karter AJ, et al. Risk factors for mortality among patients with diabetes – The Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes Care. 2007;30:1736–1741. doi: 10.2337/dc07-0305. [DOI] [PubMed] [Google Scholar]
- 7.So WY, Yang X, Ma RC, et al. Risk factors in V-shaped risk associations with all-cause mortality in type 2 diabetes: the Hong Kong Diabetes Registry. Diabetes Metab Res Rev. 2008;24:238–246. doi: 10.1002/dmrr.792. [DOI] [PubMed] [Google Scholar]
- 8.Khalangot M, Tronko M, Kravchenko V, Kulchinska J, Hu G. Body mass index and the risk of total and cardiovascular mortality among patients with type 2 diabetes: a large prospective study in Ukraine. Heart. 2009;95:454–460. doi: 10.1136/hrt.2008.150524. [DOI] [PubMed] [Google Scholar]
- 9.Hu G, Jousilahti P, Barengo NC, Qiao Q, Lakka TA, Tuomilehto J. Physical activity, cardiovascular risk factors, and mortality among Finnish adults with diabetes. Diabetes Care. 2005;28:799–805. doi: 10.2337/diacare.28.4.799. [DOI] [PubMed] [Google Scholar]
- 10.Katzmarzyk PT, Hu G, Cefalu WT, Mire E, Bouchard C. The importance of waist circumference and BMI for mortality risk in diabetic adults. Diabetes Care. 2013;36:3128–3130. doi: 10.2337/dc13-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEwen LN, Karter AJ, Waitzfelder BE, et al. Predictors of mortality over 8 years in type 2 diabetic patients: Translating Research Into Action for Diabetes (TRIAD) Diabetes Care. 2012;35:1301–1309. doi: 10.2337/dc11-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costanzo P, Cleland JGF, Pellicori P, et al. The obesity paradox in type 2 diabetes mellitus: relationship of body mass index to prognosis: a cohort study. Ann Intern Med. 2015;162:610–618. doi: 10.7326/M14-1551. [DOI] [PubMed] [Google Scholar]
- 13.Liu XM, Liu YJ, Zhan J, He QQ. Overweight, obesity and risk of all-cause and cardiovascular mortality in patients with type 2 diabetes mellitus: a dose–response meta-analysis of prospective cohort studies. Eur J Epidemiol. 2015;30:35–45. doi: 10.1007/s10654-014-9973-5. [DOI] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology – a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.Wells G, Shea B, O'Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Available from: www.ohri.ca/programs/clinical_epidemiology/oxford.asp, accessed 31 Oct 2016
- 16.Orsini N, Li RF, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose–response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose–response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 18.Orsini N, Bellocco R. Generalized least squares for trend estimation of summarized dose–response data. Stata J. 2006;6:40–57. [Google Scholar]
- 19.Jackson D, Riley R, White IR. Multivariate meta-analysis: potential and promise. Stat Med. 2011;30:2481–2498. doi: 10.1002/sim.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desquilbet L, Mariotti F. Dose–response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–1057. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.StataCorp . Stata Statistical Software: Release 14. College Station: StataCorp LP; 2015. [Google Scholar]
- 23.White IR. Multivariate random-effects meta-regression: updates to mvmeta. Stata J. 2011;11:255–270. [Google Scholar]
- 24.Dosresmeta CA. Performing multivariate dose–response meta-analysis. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 25.Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag; 2009. [Google Scholar]
- 26.Bozorgmanesh M, Arshi B, Sheikholeslami F, Azizi F, Hadaegh F. No obesity paradox-BMI incapable of adequately capturing the relation of obesity with all-cause mortality: an inception diabetes cohort study. Int J Endocrinol. 2014;2014:282089. doi: 10.1155/2014/282089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaturvedi N, Stevens LK, Fuller JH, et al. Mortality and morbidity associated with body-weight in people with IDDM – the WHO Multinational Study of Vascular-Disease in Diabetes. Diabetes Care. 1995;18:761–765. doi: 10.2337/diacare.18.6.761. [DOI] [PubMed] [Google Scholar]
- 28.Church TS, LaMonte MJ, Barlow CE, Blair SN. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med. 2005;165:2114–2120. doi: 10.1001/archinte.165.18.2114. [DOI] [PubMed] [Google Scholar]
- 29.Eeg-Olofsson K, Cederholm J, Nilsson P, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia. 2009;52:65–73. doi: 10.1007/s00125-008-1190-x. [DOI] [PubMed] [Google Scholar]
- 30.Jackson CL, Yeh HC, Szklo M, et al. Body-mass index and all-cause mortality in US adults with and without diabetes. J Gen Intern Med. 2014;29:25–33. doi: 10.1007/s11606-013-2553-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logue J, Walker JJ, Leese G, et al. Association between BMI measured within a year after diagnosis of type 2 diabetes and mortality. Diabetes Care. 2013;36:887–893. doi: 10.2337/dc12-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma SH, Park B-Y, Yang JJ, et al. Interaction of body mass index and diabetes as modifiers of cardiovascular mortality in a cohort study. J Prev Med Public Health. 2012;45:394–401. doi: 10.3961/jpmph.2012.45.6.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menke A, Casagrande SS, Cowie CC. The relationship of adiposity and mortality among people with diabetes in the US general population: a prospective cohort study. BMJ Open. 2014;4:e005671. doi: 10.1136/bmjopen-2014-005671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy RA, Reinders I, Garcia ME, et al. Adipose tissue, muscle, and function: potential mediators of associations between body weight and mortality in older adults with type 2 diabetes. Diabetes Care. 2014;37:3213–3219. doi: 10.2337/dc14-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perotto M, Panero F, Gruden G, et al. Obesity is associated with lower mortality risk in elderly diabetic subjects: the Casale Monferrato study. Acta Diabetol. 2013;50:563–568. doi: 10.1007/s00592-011-0338-1. [DOI] [PubMed] [Google Scholar]
- 36.Sluik D, Boeing H, Montonen J, et al. Associations between general and abdominal adiposity and mortality in individuals with diabetes mellitus. Am J Epidemiol. 2011;174:22–34. doi: 10.1093/aje/kwr048. [DOI] [PubMed] [Google Scholar]
- 37.Thomas G, Khunti K, Curcin V, et al. Obesity paradox in people newly diagnosed with type 2 diabetes with and without prior cardiovascular disease. Diabetes Obes Metab. 2014;16:317–325. doi: 10.1111/dom.12217. [DOI] [PubMed] [Google Scholar]
- 38.Tobias DK, Pan A, Jackson CL, et al. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. 2014;370:233–244. doi: 10.1056/NEJMoa1304501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tseng CH. Obesity paradox: differential effects on cancer and noncancer mortality in patients with type 2 diabetes mellitus. Atherosclerosis. 2013;226:186–192. doi: 10.1016/j.atherosclerosis.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Tuomilehto J, Schranz A, Aldana D, Pitkaniemi J. The effect of diabetes and impaired glucose-tolerance on mortality in Malta. Diabet Med. 1994;11:170–176. doi: 10.1111/j.1464-5491.1994.tb02015.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhao WH, Katzmarzyk PT, Horswell R, et al. Body mass index and the risk of all-cause mortality among patients with type 2 diabetes mellitus. Circulation. 2014;130:2143–2151. doi: 10.1161/CIRCULATIONAHA.114.009098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zoppini G, Verlato G, Leuzinger C, et al. Body mass index and the risk of mortality in type II diabetic patients from Verona. Int J Obes. 2003;27:281–285. doi: 10.1038/sj.ijo.802199. [DOI] [PubMed] [Google Scholar]
- 43.Howie EK, Sui X, Lee DC, Hooker SP, Hebert JR, Blair SN. Alcohol consumption and risk of all-cause and cardiovascular disease mortality in men. J Aging Res. 2011;2011:805062. doi: 10.4061/2011/805062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gatineau M, Mathrani S. Obesity and alcohol: an overview. Oxford: National Obesity Observatory; 2012. [Google Scholar]
- 45.McAuley PA, Beavers KM. Contribution of cardiorespiratory fitness to the obesity paradox. Prog Cardiovasc Dis. 2014;56:434–440. doi: 10.1016/j.pcad.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Neeland IJ, Turer AT, Ayers CR, et al. Body fat distribution and incident cardiovascular disease in obese adults. J Am Coll Cardiol. 2015;65:2150–2151. doi: 10.1016/j.jacc.2015.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holman RR, Sourij H, Califf RM. Cardiovascular outcome trials of glucose-lowering drugs or strategies in type 2 diabetes. Lancet. 2014;383:2008–2017. doi: 10.1016/S0140-6736(14)60794-7. [DOI] [PubMed] [Google Scholar]
- 48.Rasky E, Stronegger WJ, Freidl W. The relationship between body weight and patterns of smoking in women and men. Int J Epidemiol. 1996;25:1208–1212. doi: 10.1093/ije/25.6.1208. [DOI] [PubMed] [Google Scholar]
- 49.Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87:801–809. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 50.Preston SH, Stokes A. Obesity paradox: conditioning on disease enhances biases in estimating the mortality risks of obesity. Epidemiology. 2014;25:454–461. doi: 10.1097/EDE.0000000000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waring ME, Saczynski JS, McManus D, et al. Weight and mortality following heart failure hospitalization among diabetic patients. Am J Med. 2011;124:834–840. doi: 10.1016/j.amjmed.2011.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiss A, Boaz M, Beloosesky Y, Kornowski R, Grossman E. Body mass index and risk of all-cause and cardiovascular mortality in hospitalized elderly patients with diabetes mellitus. Diabet Med. 2009;26:253–259. doi: 10.1111/j.1464-5491.2009.02672.x. [DOI] [PubMed] [Google Scholar]
- 53.Yi S-W, Ohrr H, Shin S-A, Yi J-J. Sex-age-specific association of body mass index with all-cause mortality among 12.8 million Korean adults: a prospective cohort study. Int J Epidemiol. 2015;44:1696–1705. doi: 10.1093/ije/dyv138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taghizadeh N, Boezen HM, Schouten JP, Schroder CP, de Vries EGE, Vonk JM. BMI and lifetime changes in BMI and cancer mortality risk. PLoS One. 2015;10:e0125261. doi: 10.1371/journal.pone.0125261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arnold M, Pandeya N, Byrnes G, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16:36–46. doi: 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134–1139. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384:755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kohara K. Sarcopenic obesity in aging population: current status and future directions for research. Endocrine. 2014;45:15–25. doi: 10.1007/s12020-013-9992-0. [DOI] [PubMed] [Google Scholar]
- 59.Kim TN, Park MS, Yang SJ, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS) Diabetes Care. 2010;33:1497–1499. doi: 10.2337/dc09-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 61.Miller MD, Crotty M, Giles LC, et al. Corrected arm muscle area: an independent predictor of long-term mortality in community-dwelling older adults? J Am Geriatr Soc. 2002;50:1272–1277. doi: 10.1046/j.1532-5415.2002.50316.x. [DOI] [PubMed] [Google Scholar]
- 62.Heitmann BL, Erikson H, Ellsinger BM, Mikkelsen KL, Larsson B. Mortality associated with body fat, fat-free mass and body mass index among 60-year-old Swedish men – a 22-year follow-up. The study of men born in 1913. Int J Obes. 2000;24:33–37. doi: 10.1038/sj.ijo.0801082. [DOI] [PubMed] [Google Scholar]
- 63.Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68:1001–1007. doi: 10.1038/ejcn.2014.117. [DOI] [PubMed] [Google Scholar]
- 64.Atkins JL, Whincup PH, Morris RW, Lennon LT, Papacosta O, Wannamethee SG. Sarcopenic obesity and risk of cardiovascular disease and mortality: a population-based cohort study of older men. J Am Geriatr Soc. 2014;62:253–260. doi: 10.1111/jgs.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perry JRB, Voight BF, Yengo L, et al. Stratifying type 2 diabetes cases by BMI identifies genetic risk variants in LAMA1 and enrichment for risk variants in lean compared to obese cases. Plos Genet. 2012;8:e1002741. doi: 10.1371/journal.pgen.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma RCW, So WY, Tam CHT, et al. Genetic variants for type 2 diabetes and new-onset cancer in Chinese with type 2 diabetes. Diabetes Res Clin Pract. 2014;103:328–337. doi: 10.1016/j.diabres.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 67.Schenkeveld L, Magro M, Oemrawsingh RM, et al. The influence of optimal medical treatment on the 'obesity paradox', body mass index and long-term mortality in patients treated with percutaneous coronary intervention: a prospective cohort study. BMJ Open. 2012;2:e000535. doi: 10.1136/bmjopen-2011-000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1220 kb)
Data Availability Statement
Statistical codes and datasets are available from the corresponding author.