ABSTRACT
Pregnancy and lactation are considered critical periods in a female's life. Thus, the maternal diet must provide sufficient energy and nutrients to meet the mother's higher than usual requirements as well as the needs of the growing fetus. The maternal diet must enable the mother to provide stores of nutrients required for adequate fetal development, and good health and quality of life in infancy and later adulthood. Among the food and beverage groups, milk and dairy products can play a very important role in achieving these targets due to their high nutrient density and bioavailability, as well as their availability and widespread consumption.
The objective of this study was to evaluate the influence of maternal milk and dairy consumption on pregnancy and lactation outcomes in healthy women. This report mainly focuses on the effects of the mother's intake of dairy products on infant birth weight and length, fetal femur length, head circumference, gestational weight gain, preterm birth, spontaneous abortion, breast milk consumption, and human milk nutritional value. A systematic review of available studies published up to May 2018 was conducted. A preliminary broad search of the literature yielded 5,695 citations. Four of the investigators independently selected studies for inclusion according to predefined eligibility criteria. Thirty-seven full-text articles were evaluated for potential inclusion, and 17 studies were finally included. Six were prospective cohort studies, 3 were intervention studies, 3 were retrospective cohort studies, 3 were cross-sectional studies, and 2 were case-control studies. Although the number and types of studies prevent definite conclusions, there appears to be a trend that maternal milk intake during pregnancy is positively associated with infant birth weight and length. The lack of studies prevents any conclusions being drawn related to preterm deliveries, spontaneous abortion, and lactation.
Keywords: milk and dairy products, pregnancy, lactation, fetal growth, infant growth, breast milk
Introduction
Pregnancy and lactation are critical periods in a female's life. Due to higher nutritional requirements during pregnancy and lactation, pregnant females are vulnerable (1). The maternal diet during these periods must provide sufficient energy and nutrients to meet the mother's higher than usual requirements and to support adequate fetal development (2). During pregnancy, a woman undergoes rapid and marked physiological changes, including changes to body composition throughout gestation (e.g., higher fat storage during early pregnancy for use in late pregnancy, when demands are highest). Maternal requirements for energy, protein, and most micronutrients are highest during pregnancy and lactation due to fetal and maternal tissue growth, birth, and rapid growth during infancy (3). Furthermore, regarding epigenetics, changes occurring during early gestation are more likely to become ‘fixed’ and have longer-term effects than changes that occur during late gestation (4).
Maternal nutrition is one of the major environmental factors that influences fetal growth, neonatal birth size (5, 6), and the offspring's quality of life (7). It is well established that anthropometric measurements at birth are important predictors of neonatal morbidity and mortality (6). Moreover, highlighting the importance of an adequate quantity and quality of food and beverage groups, studies have shown that fetal thinness and a small head circumference at birth are associated with higher morbidity and mortality from CVD during adult life (8–10) and that a small head circumference at birth predicts lower adulthood quality of life above the age of 50 y (11). Thus, the evaluation and monitoring of dietary intake should be an important part of pregnancy and birth studies, and should also be monitored during lactation.
Among the food and beverage groups, milk and dairy products are most effective for promoting fetal growth and neonatal birth size because they contain various nutrients such as protein, calcium, phosphorus, potassium, iodine, vitamin B12, and riboflavin, among others (12). This food group consistently exhibits a high nutrient density, which is important during physiological states such as pregnancy and lactation. The adequate fulfillment of nutrient requirements is considered important not only during these periods but also in later life. According to the developmental origin of health and disease theory, early nutrition-related factors can be involved in the long-term development of weight changes including obesity, CVDs, diabetes, cancer, and other noncommunicable diseases. Interestingly, maternal weight status during pregnancy has also been linked to adverse birth outcomes, such as low fetal growth, birth defects, and preterm delivery (13, 14). A healthy and varied diet during lactation promotes balanced maternal nutrition and optimal concentrations of some nutrients of human milk (15). The concentrations of many vitamins, iodine, and fatty acids in human milk depend on, or are influenced by, maternal diet (16). In principle, any type of food may be included in the diet of a pregnant woman except in cases of food allergies, gestational mellitus diabetes, preeclampsia, or other conditions/disorders (17). In the Western world, cow milk and associated dairy products are widely consumed by children and adults. Milk is especially recommended for inclusion in the diet of young children because of its nutritive value. Milk and dairy products have high concentrations of nutrients including protein, calcium, phosphorus, potassium, iodine, vitamin B12, and riboflavin (18). In addition, and equally important, milk seems to be an optimal vehicle for enrichment and fortification with some key nutrients for the mother and child, such as calcium, vitamin D, and omega-3 fatty acids, due to its physico-chemical properties, easy accessibility, and widespread consumption.
The aim of this systematic literature review was to evaluate the influence of milk and dairy product consumption on pregnancy and lactation outcomes.
Methods
The research question in this systematic review was ‘Does milk/dairy product consumption impact pregnancy and lactation outcomes?’
Literature search
All available studies up to 15 May 2018 were identified in the PubMed/Medline and Scopus databases. The terms used and the search strategies were: ‘Gestation’ [MeSH (Medical Subject Heading)] OR ‘Pregnancy’ [MeSH] OR ‘Pregnant women’ [MeSH] AND ‘Milk’ [MeSH] OR ‘Dairy products’ [MeSH] OR ‘Cheese’ [MeSH] OR ‘Yoghurt’ [MeSH] OR ‘Kefir’ [MeSH] OR ‘Koumiss’ [MeSH] AND ‘Fetal growth’ [MeSH] OR ‘Fetal development’ [MeSH] OR ‘Body weight’ [MeSH] OR ‘Birth weight’ [MeSH] OR ‘Preterm births’ [MeSH] OR ‘Premature birth’ [MeSH] OR ‘Spontaneous abortion’ [MeSH] OR ‘Miscarriage’ [MeSH] OR ‘Congenital malformations’ [MeSH] OR ‘Congenital abnormalities’ [MeSH] OR ‘Gestational age’ [MeSH] OR ‘Small for gestational age’ [MeSH] OR ‘Extremely low birth weight infant’ [MeSH] OR ‘Very low birth weight infant’ [MeSH] OR ‘Low birth weight infant’ [MeSH] OR ‘Intrauterine growth retardation’ [MeSH] OR ‘Head circumference’ [MeSH]. For lactation, we searched for ‘Lactation’ [MeSH] OR ‘Breastfeeding’ [MeSH] AND ‘Milk’ [MeSH] OR ‘Dairy products’ [MeSH] OR ‘Cheese’ [MeSH] OR ‘Yoghurt’ [MeSH] OR ‘Kefir’ [MeSH] OR ‘Koumiss’ [MeSH] AND ‘Infant growth” [MeSH] OR ‘Infant weight’ [MeSH] OR ‘Infant length’ [MeSH] OR ‘Infant head circumference’ [MeSH] OR ‘Infant body composition’ [MeSH] OR ‘Infant BMI’ [MeSH] OR ‘Milk ejection’ [MeSH] OR ‘Milk, human’ [MeSH] OR ‘Breast milk’ [MeSH] OR ‘Nutritional value’ [MeSH] OR ‘Nutritive value’ [MeSH] AND ‘Breast milk’ [MeSH].
For inclusion, an article had to be published in English or Spanish, but there was no restriction on publication type or sample size.
This review was registered through the International Prospective Register of Systematic Reviews (PROSPERO) (Identification number: CRD42018100907).
Inclusion and exclusion criteria
Studies were included if they met the following criteria; otherwise they were excluded:
Participants were healthy pregnant or lactating women without diagnosis of a pathological condition.
Participants were aged ≥18 y old.
Participants were classified as normal weight or normal BMI.
The study was focused exclusively on the effects of milk/dairy intake on pregnancy or lactation outcomes and did not address other potentially confounding dietary factors (e.g., intake of legumes, fish, vegetables, or dietary supplements).
All categories of epidemiological studies were included.
Fortified dairy products were not considered in the present review.
The dietary assessment preferably included a validated methodology, regardless of the tool.
The titles and abstracts retrieved (from 5,695 studies in total) were screened for potentially relevant articles. The full texts of potentially relevant articles were reviewed for adherence to the inclusion criteria (n = 37). Our purpose at this phase of screening was to identify articles that described the effects of milk/dairy products on pregnancy and lactation outcomes. Therefore, the full text of any article for which it was not possible to determine relevance from the title and/or abstract was also retrieved for further review. At this point, studies with titles and/or abstracts that clearly did not meet the criteria for inclusion were rejected (n = 20). Four of the investigators (MA, NU, AG-G, and TP) independently decided which studies met the inclusion criteria. Any differences were resolved by consensus or by consulting a fifth reviewer (GV) when consensus could not be reached. A flow diagram of the screening process is depicted in Figure 1. The final number of studies included for the present systematic review was 17.
FIGURE 1.
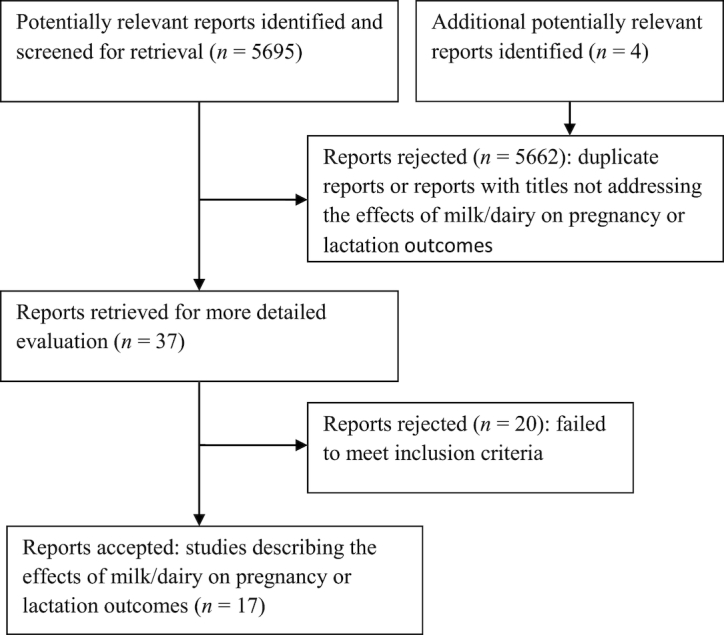
Flow diagram of the screening process.
Outcomes
We focused on the effects of milk/dairy products on gestation and lactation outcomes including fetal growth, birth weight and length, head circumference, body weight gain, premature birth, miscarriage, congenital malformations, gestational age, infant growth, infant weight, infant length, infant head circumference, infant body composition, infant BMI, milk ejection, and human milk nutritional value.
Data extraction and quality assessment (risk of bias)
The data collected from each article included: the authors and year of publication, study design, number of participants, participant age, exposure, method used to record dairy intake, duration of the study, and outcomes of interest (Table 1). Information on the methodological quality of the studies was also abstracted. The risk of bias of all the included studies was assessed by two authors using ‘NHI Study Quality Assessment Tools’ (19), with the appropriate tool selected according to the design of each study. The tools consist of 12 to 14 questions aimed to assess bias based on the research question, study population, recruitment and eligibility criteria, sample size justification, bias in the exposure of interest and outcome assessment, blinding of the process, randomization, participant dropout, and statistical analyses. Studies were rated ‘good’ (A) if the final value was ≥10, ‘fair’ (B) if the final value was between 5 and 9, and ‘poor’ (C) if the value was ≤4. We resolved any differences of opinion by consensus.
TABLE 1.
Characteristics of the included studies on the effects of milk/dairy products on pregnancy and lactation outcomes
| Authors and year | Study design, country | Population, sample size and age (y or range) | Main aim of study | Exposure | Diet method | Time period covered | Outcome measure(s) | Confounder adjustments | Results | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Park et al. (1999) (20) | Crossover dietary intervention trial USA | Lactating women n = 16 32 ± 2 y |
To determine if maternal diet can influence milk rumenic acid concentration | Group A (high-fat dairy period during week 2) Group B (high-fat dairy period during week 3) |
3-d dietary records during the last 3 d of each period and usual intakes of rumenic acid were estimated by using a semi-quantitative FFQ | 3 wk | Nutritive value in breast milk | Consume foods containing minimal amounts of rumenic acid during week 1 (depletion period), supply of food items containing an abundance of rumenic acid (i.e., cheese, yoghurt, whole milk, and ice cream) during the high-fat dairy period | Milk lipid concentration was influenced by diet, such that lipid concentration was greater during the high than the low dairy period. Specifically, milk fat was 18% higher when subjects consumed a high-fat diet | A |
| Di Cintio et al. (2001) (21) | Case/Control study Italy | Pregnant women living in Milan area (Italy) different nationalities n = 2,681 (912 cases and 1,769 controls) 31 y (range 14 y–46 y) |
To explore the association between dietary habits and risk of spontaneous abortion | Milk consumption (per wk) was classified as: ≤3 portions (*) 4–7 portions ≥8 portions Cheese consumption (per wk) was classified as: ≤2 portions (*) 3–4 portions ≥5 portions |
FFQ of 10 selected foods | First trimester of pregnancy | Spontaneous abortion within week 12 of pregnancy | Age, BMI, marital status, education, number of previous miscarriages, and coffee and alcohol intake before pregnancy | Protective association towards risk of abortion was found with high consumption of milk and cheese. Women consuming ≥8 portions per wk of milk had a lower risk of abortion than those consuming ≤3 portions (OR = 0.6, 95% CI: 0.5–0.8) and those consuming ≥5 portions per wk of cheese had a lower risk of abortion than those consuming ≤2 portions (OR = 0.5 95% CI: 0.4–0.6) |
B |
| Chang et al. (2003) (22) | Retrospective cohort, USA | n = 350 African- American pregnant adolescents <17 y | To determine the effect of maternal dairy intake on fetal femur development between 20 and 34 wk of gestation | Dairy intake estimated at first prenatal visit on the basis of number of servings per d and divided in 3 categories: Low <2 servings/d Medium 2–3 servings/d High >3 servings/d |
24-h recall and food frequency methods | First prenatal visit, covering habitual intake | Fetal femur length by ultrasound between 20 and 34 wk of gestation | Gestational age, maternal age, maternal height, prepregnancy BMI, fetal biparietal diameter | Fetal femur length was significantly lower (P < 0.001) in the lowest dairy intake group (<2 servings/d) than in the highest dairy intake group (>3 servings/d), and a dose-response relation was suggested in the intermediate dairy intake group (2–3 servings/d, P = 0.089) | B |
| Ludvigsson and Ludvigsson (2004) (23) | Retrospective cohort, Sweden | Parents of babies born in southeast Sweden, n = 14,000, age not reported | To investigate the relation between milk intake, low birth weight, and intrauterine growth retardation. A second aim was to study the relation between milk intake and preterm birth | Milk consumption in dL/d divided into 4 groups: 0 dL/d ≤2 dL/d 3–10 dL/d >10 dL/d |
FFQ including questions about milk intake | At birth, covering the consumption during pregnancy | Birth weight IUGR risk of preterm birth |
Sex of infant, smoking, alcohol consumption during pregnancy, maternal age, parity, maternal height, maternal weight (prepregnancy), length of education, and whether the mother had a cohabitant | Adjusting for confounders, low milk intake during pregnancy was associated with an increased risk of IUGR (P = 0.019), but not with LBW or preterm birth. The difference in birth weight between women consuming >10 dL/d of milk and women abstaining from milk during pregnancy was 134 g | A |
| Olsen et al. (2007) (24) | Prospective cohort study, Denmark | Pregnant women, n = 50,177, mean age ranged between 28.2 ± 4.3 y and 30.4 ± 4.7 y in different exposure groups | To examine whether milk consumption during pregnancy is associated with greater infant size at birth | Milk consumption in glasses (200 mL)/d divided into 8 categories: 0 glasses/d 0–1 glasses/d 1–2 glasses/d 2–3 glasses/d 3–4 glasses/d 4–5 glasses/d 5–6 glasses/d >6 glasses/d |
A 360-item FFQ | 25 wk of gestation referred to the previous 4 wk | Birth weight, birth length, head circumference, abdominal circumference, placental weight |
Infant gestational age, infant sex, mother´s parity (nulliparous versus multiparous), age, height, prepregnant BMI, gestational weight gain (in quintiles), smoking status, and total energy intake; father´s height and family´s socio-economic status | Mean birth weight was ∼100 g higher among the group that consumed 4–5 glasses of milk/d compared with those who consumed no milk. The odds of being small for gestational age (SGA) declined with increasing consumption of milk and the large for gestational age (LGA) odds increased with exposure | A |
| Xue et al. (2008) (25) | Retrospective cohort, USA | Nurses’ mother's cohort n = 34,063 26.3 y for mothers of babies with birth weight <2,500 g and 27.2 y for mothers of babies with birthweight ≥4,000 g |
To investigate parental predictors of birth weight | Milk consumption in glasses/d (*) divided into 4 categories: ≤4 glasses/wk 5 glasses/wk – 1 glass/d 2–3 glasses/d ≥4 glasses/d |
A questionnaire was mailed to the mothers asking about consumption of common energy- and nutrient- dense foods during their pregnancy decades earlier | Recall of diet during pregnancy decades earlier | Birth weight IUGR |
Parental anthropometric characteristics, pregnancy conditions, parental behavior, maternal diet during index pregnancy, parental demographic and socio-economic characteristics, maternal reproductive factors | Daily consumption of each additional glass of milk was associated with an increase of ≈6 g in birth weight (P for trend = 0.01) | A |
| Heppe et al. 2011 (26) | Prospective cohort, The Netherlands | Pregnant women n = 3,405 31.4 ± 4.4 y |
Assess the association of first trimester maternal milk consumption and its constituents with fetal growth and the risks of neonatal complications | Glasses of milk (150 mL)/d; 4 categories: 0–1 glass/d 1–2 glass/d 2–3 glass/d >3 glass/d |
Validated semi-quantitative FFQ administered at study incorporation to follow-up, which ranged from early pregnancy (<18 wk) to birth | Previous 3 mo, covering intake within the first trimester | Fetal growth: head circumference (mm), femur length (mm), estimated fetal weight (g) Birth outcomes: gestational age (wk), weight (g) and length (mm), head circumference (mm) Neonatal complications: preterm (%); small for gestational age (%); large for gestational age (%) |
Maternal age, height, weight, and weight before gestation; parity; socio-economic characteristics and education level; smoking and alcohol use, vomiting and nausea during pregnancy; folic acid supplementation and several characteristics of the diet such as energy intake and consumption of fruit, vegetables, fish, meat, and coffee | Maternal milk consumption was positively associated with birth weight (P for trend < 0.01). The birth weight difference between the highest and the lowest categories of milk consumption was 88 g (95% CI: 39–135) | A |
| Borazjani et al. (2013) (27) | Cross-sectional study, India | Pregnant women n = 156 28 y |
Assess the effects of daily intake of milk and protein by pregnant women on fetal growth and determine the growth pattern and velocity of growth | Group 1: ≤155.64 mL milk/d Group 2: 155.65–465.17 mL/d Group 3: ≥465.18 mL/d |
24-h recall and FFQ | Weeks 16 to 38 | Head circumference Abdomen circumference Biparietal diameter Femur length Birth weight |
Maternal age, weight, height, and BMI; parity; socio-economic characteristics and education level; other components of the diet and total caloric intake and total protein intake | Better fetal growth was observed in Group 2 (in head circumference, biparietal diameter, and femur length) | B |
| Hrolfsdottir et al. (2013) (28) | Prospective cohort (The Aarhus Birth Cohort), Denmark | Pregnant women n = 809 29.1 ± 4.2 y |
Examine whether milk consumption during pregnancy is associated with infant size at birth and offspring height- and growth-related biomarkers at ∼20 y of age | Maternal milk consumption ≥150 mL/d versus <150 mL/d Predominantly low-fat milk |
FFQ | Gestational week 30 | Birth weight Birth length |
Maternal age, height, prepregnancy BMI, and weight gain until week 30 of gestation; parity; socio-economic characteristics and education level; smoking status; total energy intake | Maternal milk consumption of ≥150 mL/d was associated with higher birth weight and birth length | A |
| Malhotra et al. (2014) (29) | Cross-sectional, India | Pregnant women n = 124,385 15–49 y |
Examine the role of maternal diet in determining low birth weight in infants | Food frequency consumption stratified by never/occasionally, daily, and weekly | Survey of diet and food consumption frequency (7 groups) | Not clearly specified | Birth weight | Maternal age at the time of birth; twin births; gender of the infant and mother's height; socio-economic data (caste) | Infants whose mothers consumed milk and curd daily (OR = 1.17; 95% CI: 1.06–1.29) had higher odds of not having a low birth weight infant | B |
| Li et al. (2014) (30) | Intervention parallel group, ‘Project of a glass of milk’, China | Pregnant women n = 3,526 19–43 y |
Determine whether daily consumption of liquid milk alone can increase the blood folate concentration in pregnant women and whether there are differences in blood folate concentrations between Han and Mongolian women after cessation of folic acid supplementation | Maternal groups: folic acid supplement + milk (243 mL); folic acid supplement; milk (243 mL); control |
Periodical records by organizers | From confirmation of pregnancy (5–7 wk) to parturition | Birth weight Birth length Serum folate |
Folic acid supplementation; ethnicity | Maternal supplementation with milk resulted in increased average birth weight and height of newborns. The percentage of infants of low birth weight (<2,500 g) without maternal milk supplementation was higher than that of the infants born to mothers with milk supplementation |
B |
| Yahvah et al. (2015) (31) | Randomized, crossover, dietary intervention trial, USA |
Lactating women n = 15 27 ± 1 y |
Investigate the effects of increased maternal dairy fat intake on lipid-related gene expression in the lactating human mammary gland | Group 1: 4 servings of full-fat dairy products Group 2: 4 servings of nonfat dairy products |
Diet records on 2 weekdays and 1 weekend day prior to the first dietary intervention and again during each of the intervention periods | 2 treatments each lasting 14 d and a 2-wk washout period | Nutritive value in breast milk | To help control for confounding effects, subjects were asked to refrain for consuming all other dairy products, including butter | Variation in maternal lipid intake quickly alters milk lipid content and the fatty acid profile, and these changes are not associated with major shifts in gene expression in the lactating human mammary epithelial cell | C |
| Olmedo-Requena et al. (2016) (32) | Prospective cohort, Spain | Pregnant women n = 973 18 y and older |
To analyze the risk of having an SGA infant according to the mother´s dairy intake during the first half of pregnancy | Total intake of dairy products (g/d) | FFQ | Weeks 20 to 22 | SGA (neonates weighing < the 10th percentile adjusted for gestational age) | Maternal age; smoking status and alcohol consumption; physical activity; pregnancy-induced hypertension; pregnancy weight gain; prepregnancy BMI; educational level; social class; energy intake and consumption of fruits, vegetables, and fish | An increase intake of dairy products by 100 g/d during the first half of pregnancy decreased the risk of having an SGA infant by 11%, with an OR = 0.89; 95% CI: 0.83– 0.96. An inadequate intake of dairy products was associated with a higher risk of SGA |
B |
| Abreu et al. (2017) (33) | Prospective, Portugal | Pregnant women n = 98 18–40 y |
Determine the association between dairy product consumption during pregnancy and neonatal and maternal outcomes | Total intake of dairy products (g/d): First trimester: 350.1 ± 149.5 Second trimester: 340.6 ± 228.9 |
A 3-d food diary for each trimester | First and second trimesters of pregnancy | Birth weight Birth length Head circumference Placental weight Weight gain during pregnancy |
Mother's weight, height, and prepregnancy BMI and weight gain during gestation; smoking status; neonatal sex; gestation age; socio-economic characteristics; educational level; energy intake and compliance to Mediterranean diet (score) | Total dairy and yoghurt intake in the first trimester were positively associated with head circumference and placental weight, respectively. Change in total dairy intake between the second and first trimester was negatively associated with maternal weight gain during pregnancy |
A |
| Ahmadi et al.(2017) (34) | Case-control study, Iran | Pregnant women n = 662 (331 cases and 331 controls) Cases: 27.8 ± 5.3 y Controls: 27.3 ± 4.4 y |
Study the associations between nutrient deficiencies and the risk of spontaneous abortion | Primary: diet composition Secondary: food subgroup intake Number of dairy portions/d: <2 portions 2–3 portions >3 portions |
Validated semi-quantitative FFQ | Previous 3 mo, covering intake within the first trimester | Spontaneous abortion | Cases and controls were matched on maternal age; duration from last delivery; BMI; occupation and educational status | Consumption of fewer than 2 dairy portions was statistically associated with cases (52.3% versus 36.3%), whereas the consumption of >3 portions of dairy products was more frequent in the control group (6.3% versus 2.1%; P < 0.001). | A |
| Mukhopadhyay et al. (2018) (35) | Prospective, observational cohort study, India | Pregnant women n = 2,036 24.4 ± 3.8 y |
To examine the relations between birth weight and maternal intake of milk, protein from milk and vitamin B12 from milk | Median consumption of 310 g/d milk in the first trimester | Validated FFQ | Three trimesters of pregnancy | Birth weight Birth length Gestational weight gain |
Maternal age, weight and height; gestational age at delivery; educational level; parity and energy intake | Birth weight was positively associated with the intake of milk products in the first trimester. Intake of milk products in the third trimester was positively associated with gestational weight gain between the second and third trimester |
A |
| Hjertholm et al. (2018) (36) | Cross-sectional, Malawi | Pregnant women n = 203 |
Examine the association between maternal dietary intake during pregnancy and infant birth size | Food frequency consumption in 7 d. For each food group, the number of days on which at least one food from it was consumed was counted | Quantified recall using a 3-d repeated interactive multi-pass 24-h recall and semi-quantified recall using a 4-d repeated single-pass 24-h recall in which details about the foods and time of consumption were recorded | Weeks 28 to 35 | Birth weight Birth length Head circumference Abdominal circumference |
Maternal age, weight, height; marital status; socio-economic characteristics; educational level; parity; gestational age and energy intake | Each additional day of milk consumption within the 7 measurement days was associated with a 75.3 g increase in birth weight (P = 0.02). | B |
(*) Precise volume not reported.
Data analysis/statistics
Due to the heterogeneity among studies, few studies met the inclusion criteria; therefore, it was not possible to perform a meta-analysis. The overall effects of milk and dairy products on pregnancy and lactation within each study are described below. The point estimates and statistical tests described in the original studies are also summarized.
Results
The effects and associations of maternal milk and dairy product consumption on normal pregnancy and lactation are summarized for the 17 studies that were included in the review (20–36). Six of these studies were prospective cohort studies, 3 were intervention studies, 3 were retrospective cohort studies, 3 were cross-sectional studies, and 2 were case-control studies. Most studies reported variables considered to be potential confounders, such as socio-demographic factors and lifestyle characteristics of the subjects. The 17 studies involved >237,555 women, of which 53.3% were from India, 21.5% were from Denmark, 14.5% were from the USA, 5.9% from Sweden, 1.5% from China, 1.4% from The Netherlands, 1.1% from Italy, 0.4% from Spain, and the remainder were from Iran, Malawi, Portugal, and Russia. The intake of milk and dairy products was evaluated through dietary information with appropriate and validated questionnaires in most cases.
According to the quality scale used, 9 trials were classified as good (A), 7 trials were classified as fair (B), and 1 trial was classified as poor (C). The main bias identified pertained to missing information regarding the participation rate of eligible persons and the blinding of the assessors to the exposure status of the participants. The characteristics of the studies that were analyzed are described in Table 1.
Effects of maternal milk and dairy product consumption on pregnancy outcomes
We retrieved 15 articles that evaluated the effects and associations of maternal milk and dairy product consumption during normal pregnancy. Twelve studies used birth weight to evaluate normal fetal growth; 7 used birth length, 5 used head circumference, and 3 used femur length as additional indicators of normal fetal growth.
Infant birth weight, small for gestational age (SGA), and intrauterine growth retardation (IUGR)
Six prospective cohort studies showed positive correlations of maternal milk consumption during pregnancy on adequate infant birth weight.
A prospective cohort study in Spain (32) was carried out to analyze the associated risk of having an SGA infant according to the mother's dairy intake. The results showed that an increased intake of dairy products by 100 g/d during the first half of pregnancy was associated with a decreased risk of having an SGA infant by 11% (OR = 0.89; 95% CI: 0.83–0.96). The authors also found that an inadequate intake of dairy products was associated with a higher risk of SGA.
In a cohort of Danish pregnant women (28), maternal milk consumption of ≥150 mL/d versus <150 mL/d was associated with a 0.32 increase in z-score for birth weight (95% CI: 0.06–0.58). Based on their follow-up results, the authors suggested that a maternal milk consumption of ≥150 mL/d may have a growth-promoting effect that may persist into early adulthood. Also in Denmark, a prospective study in The Danish National Birth Cohort (24) showed that milk intake in pregnancy was associated with higher birth weight for gestational age and lower risk of SGA (49%, 95% CI: 35%, 61%). Mean birth weight was ∼100 g higher among the group that consumed 4–5 glasses of milk/d (1 glass = 200 mL) compared with those who consumed no milk.
A prospective cohort study of pregnant women in The Netherlands (26) found that a high maternal milk consumption during pregnancy (>3 glasses/d compared with <3 glasses/d, 1 glass = 150 mL) was associated with greater fetal weight gain, particularly in the third trimester of pregnancy, resulting in a higher birth weight. The birth weight difference between the highest and lowest categories of milk consumption was 88 g (95% CI: 39–135). According to the authors, the reported effect was associated with a higher intake of protein, but not fats or carbohydrates, from milk.
In Portugal (33), it was reported that yoghurt intake (128.6 ± 99.2 g/d) in the first trimester was positively associated with placental weight (P = 0.012). In the second trimester, there was a trend of a positive association between yoghurt intake and higher birth weight (P = 0.06).
Birth weight was also positively associated with the intake of milk products in the first trimester (median consumption 310 g/d) (β = 86.8, 95% CI: 29.1–144.6; P < 0.001) in another prospective observational cohort study of pregnant women in India (35).
Two observational cross-sectional studies obtained similar results, linking maternal dairy intake during pregnancy to birth weight. Malhotra et al. (29) and Hjertholm et al. (36) studied Indian and Malawian pregnant women, respectively, and reported that mothers that consumed milk and curd daily had higher odds (OR = 1.17; 95% CI: 1.06–1.29) of not having a low birth-weight infant (29) and that each additional day of milk consumption was associated with a 75.3 g increase in birth weight (P = 0.02) (36).
A large retrospective cohort study (25) reported a significant positive association between maternal milk intake and infant birth weight. It was the Nurses’ Mother's Cohort study, involving 34,063 nurses and their mothers. Maternal consumption of 2–3 and 4+ glasses of milk (volume not reported) per d by the mother during pregnancy was associated with a 16 g (P = 0.007) and a 19 g (P = 0.13) increase, respectively, in birth weight, when compared with consumption of ≤4 glasses per wk (P for trend = 0.01). In this study, IUGR was not significantly related to maternal consumption of milk.
Another retrospective cohort study (23) aimed to investigate the relation between milk intake, low birth weight, and IUGR. Adjusting for confounders, low milk intake during pregnancy was associated with an increased risk of IUGR (P = 0.019). Furthermore, they found that the difference in birth weight between women consuming >10 dL/d of milk and women abstaining from milk during pregnancy was 134 g.
The only intervention study evaluating the possible effects of milk and dairy product consumption on pregnancy (30) was carried out in China. Pregnant mothers received a 243 mL supplement of milk, and this intervention resulted in an increase in the birth weight of newborns by 1.9% (P < 0.05). In addition, the percentage of low birth-weight infants (<2,500 g) without maternal milk supplementation was 1.8%, which was significantly higher (P < 0.05) than the percentage of low birth-weight infants born to mothers with milk supplementation (0.8%). The authors stated that the frequency of low birth weight was significantly decreased by maternal supplementation with milk.
Infant birth length, fetal femur length, and head circumference
Nine studies reported results regarding maternal dairy or milk consumption in relation to birth length and/or fetal femur length and head circumference. Six studies reported superior fetal growth in terms of fetal length or femur length and head circumference associated with maternal dairy intake during pregnancy. One cross-sectional study (27) reported that pregnant women with an intake of 155.7–465.2 mL milk per d showed increased fetal growth, specifically with regards to femur length, head circumference, and biparietal diameter. Additionally, in the above-mentioned prospective cohort of Danish pregnant women (28), maternal milk consumption of ≥150 mL/d compared with <150 mL/d was associated with a 0.34 increase in z-score for birth length (95% CI: 0.04–0.64). The other study carried out in Denmark (24) showed the odds of having a large for gestational age (LGA) infant increased with exposure, and reported that women who consumed >6 glasses of milk/d had a 59% (95% CI: 16%, 116%) higher odds of having an LGA infant. Head circumference also showed increases across the whole range of milk intake (P < 0.001). In the intervention study with pregnant women carried out in China, maternal supplementation with 243 mL milk resulted in an increased average height of newborns (P < 0.05) (30). In a retrospective cohort study in African-American pregnant adolescents (22), fetal femur length was significantly lower (P < 0.001) in the lowest dairy intake group (<2 servings/d) compared with the highest dairy intake group (>3 servings/d), and a dose-response relation was suggested in the intermediate dairy intake group (2–3 servings/d, P = 0.089).
A prospective cohort in Portugal (33) showed that total dairy intake (350.1 ± 149.5 g/d) in the first trimester was positively associated with head circumference (P = 0.014). The remaining 3 studies did not find a clear association between maternal milk or dairy consumption and birth length, femur length, or head circumference (26, 35, 36).
Gestational weight gain
Two prospective cohort studies evaluated gestational weight gain in response to maternal milk and dairy product intake. For pregnant women in India, milk product intake in the third trimester was positively associated (P < 0.001) with gestational weight gain between the second and third trimester (35). In a study of pregnant Portuguese women (33), a reduction in total dairy intake between the first (350.1 ± 149.5 g/d) and second trimester (340.6 ± 228.9 g/d) was negatively associated with maternal weight gain during pregnancy (β = −0.007, P = 0.020).
Preterm birth and spontaneous abortion
Two studies examined the relation between milk consumption and the risk of spontaneous abortion, both being case-control studies and both reporting potential positive preventive effects of the consumption of milk to avoid spontaneous abortion (21, 34).
Di Cintio et al. (21) explored the association between dietary habits and the risk of miscarriage, at 12 wk of pregnancy, using data from a case-control study conducted in Milan (northern Italy). The results showed a preventive association between milk and cheese intake and risk of abortion with ORs of 0.5 for cheese and 0.8 for milk, comparing the highest with the lowest volume of intake. Similar results were found in Iran where, as a part of a broader case-control study aimed at exploring the impact of diet on the risk of spontaneous abortion, the authors reported significantly lower consumption of dairy products (P < 0.001) in women who experienced a spontaneous abortion before 14 wk of pregnancy than in matched controls (34).
Preterm birth was considered as an outcome in 2 of the prospective cohorts analyzed. In the Swedish (23) and Dutch cohort (26), no impact of maternal dairy intake on the risk of neonatal complications, such as preterm birth, was observed. Finally, no study was found that evaluated the effect of maternal dairy product intake during pregnancy on the risk of congenital malformations.
Effect of milk and dairy product consumption on lactation outcomes
Considering that adequate infant growth is one of the primary outcome indicators of lactation, we specifically addressed the question of maternal consumption of dairy products during breastfeeding and its possible relation to differential infant growth. However, even though we identified an important number of potentially relevant articles to address the question (n = 2,687), only 2 were finally included in this review, since most of them did not adhere to the inclusion criteria. All studies evaluating infant growth were focused on human milk or formula milk intake by the infant, but maternal dairy intake impact was not evaluated.
Park et al. (20), in a crossover dietary intervention study with 16 breastfeeding women, reported that women who consumed a low-fat dairy diet produced lower fat human milk, compared with the milk they produced when they consumed more fat from dairy products. Specifically, milk lipid concentration was greater during the high-fat versus low-fat dairy period (46.6 ± 5.0 compared with 38.3 ± 1.6 mg/g milk, respectively; P < 0.05). In this sense, 1 study had a randomized, crossover design involving a dietary intervention trial with 15 lactating women with diets enriched in full-fat or nonfat dairy products for 14 d. It was observed that an elevated maternal lipid intake from full-fat dairy products was associated with increased lipids and an altered fatty acid profile in the milk produced by the mother, but these changes were not associated with significant changes in gene expression in the human mammary epithelial cells of the mother (31).
Discussion
In this systematic review of studies published up to May 2018, we first focused on infant birth weight, SGA, and IUGR. Low birth weight not only affects infant mortality and morbidity but also increases risk factors for several chronic diseases (37). Eleven studies showed positive associations of milk or dairy product consumption on birth weight or a reduced risk of having an SGA infant. Only one of these studies reported no significant association between dairy product intake during the first and second trimesters of pregnancy and birth weight, although a trend of a positive association between yoghurt intake and birth weight was observed. Of the 11 studies, 5 were prospective cohort studies, 3 were retrospective cohort studies, 2 were cross-sectional studies, and 1 was an intervention study. A higher milk or dairy product intake was associated with an increase in birth weight of ∼100 g (26, 35, 36) or 1.9% (30) or with a 0.32 increase in z-score (28). An increased intake of dairy products by 100 g/d during the first half of pregnancy decreased the risk of having an SGA infant by 11% (32), and women consuming >6 glasses/d (>1,200 mL) had a 49% (95% CI: 35%, 61%) lower adjusted odds of having an SGA infant (24). Furthermore, mothers that consumed milk and curd daily had higher odds (OR = 1.17; 95% CI: 1.06–1.29) of not having a low birth-weight infant (29). Ludvigsson et al. (23) reported that low milk intake during pregnancy was associated with an increased risk of IUGR. The large cohort study of the Nurses’ Mother's Cohort reported that higher maternal milk consumption was associated with increased birth weight, but it is interesting to note that due to the retrospective design and long recall period of exposures, the participants had to answer questions on their own dietary habits from decades earlier, related to early life exposures of their daughters, with the memory limitations that this involves. The authors themselves state that the magnitude of the birth weight increase was modest (≈6 g per each daily additional glass of milk). These results are consistent with those previously reported by Brantsaeter et al. (38) in a systematic review of studies published between 2000 and 2011 that included only Western populations. The present review included studies reporting positive associations in African and Asian populations; thus, there is evidence of a positive association between birth weight and milk and dairy product intake in both developed and developing countries.
There was large heterogeneity among the included studies, e.g., with regard to the assessment of maternal milk and dairy intake. Several studies (n = 9) used a validated FFQ, whereas the remaining administered surveys on diet and food consumption frequency (29) or used periodical records (30). According to Clark et al. (39), the healthcare infrastructure and therefore the nutritional assessment resources can be limited in developing countries. Furthermore, all of the studies included potential confounding factors in their analyses. Characteristics of the mother were included in a comprehensive manner in 8 studies, and BMI and gestational weight gain, which are both related to maternal dietary behavior and infant growth measures, were considered in 6 articles. Birth weight is highly correlated with gestational weight gain [revised by Brantsaeter et al. (38)], thus, we recommend that this parameter is measured in future research, to allow the associations between milk consumption, fetal growth, and infant birth weight to be properly assessed.
Nine studies reported the results of maternal dairy or milk consumption in relation to birth length, fetal femur length, or head circumference, of which 6 reported a significant association. The intervention study in the ‘Project of a glass of milk’ in China, suggests that drinking milk during pregnancy may enhance birth outcomes in terms of birth height (30). The prospective cohort in Denmark (28) also showed an increased birth length of infants whose mothers consumed at least 150 mL milk/d. The other study carried out in Denmark (24) reported women who consumed >6 glasses of milk/d (>1,200 mL) had a 59% (95% CI: 16%, 116%) higher odds of having an LGA infant. A positive association between maternal milk consumption and head circumference was suggested in 1 prospective cohort study and 1 cross-sectional study (24, 27, 33). Femur length was positively related to maternal milk consumption in the prospective cohort study in India and retrospective cohort study in the USA (27).
It is important to note that these studies involved the maternal intake of at least 150 mL milk/d and that the increases in infant length with milk intake involved milk consumption at different stages during pregnancy, i.e., at its confirmation (30), throughout pregnancy, and up to the last weeks (27, 28, 30, 33), regardless of the time covered by the method used to record dietary intake. For the dietary assessment, 5 of the studies used FFQ (27, 28); the intervention study used periodical records (30), and the Portuguese study included 3-d food diaries for each trimester of pregnancy (33). Although they contribute to study heterogeneity, these types of surveys are widely used in nutritional epidemiology to determine food, energy, and nutrient intake in cross-sectional and cohort studies, as well as individual assessments and evaluations of total diet (40). No randomized controlled trials involving healthy pregnant women were identified among the studies considered in this systematic review. Regarding pregnancy, only 1 intervention trial was retrieved (30). A parallel group design was planned for that study, and pregnant mothers were assigned to 4 different groups according firstly to their ethnic groups and secondly to their assigned supplement regimen, but the authors did not report whether random classification was used. Moreover, the authors did not specify in this study if the mothers included milk or dairy products, or not, in their habitual diets, and if they did, what was the amount. This study evaluates the effect of including a glass of milk a day, together with a folic acid supplement, or not, depending on the experimental group. To the best of our knowledge, no randomized controlled studies have evaluated the relation between maternal intake of milk or dairy products and infant birth length, at least in the last 40 years.
Two prospective studies (26, 35) and 1 cross-sectional study (36) reported no association of milk intake with infant birth length, femur length, or head circumference. In a recent narrative review on maternal dietary patterns and the risk of low birth-weight offspring, the data were similarly inconclusive regarding maternal consumption of milk and yoghurt and fetal length (39). In the above-mentioned systematic review (38), the authors found 3 studies reported no association between dairy product intake and fetal length or infant birth length, and 2 studies reported positive associations in healthy subjects of Western populations. In that review, the authors reported large heterogeneity among the studies with regard to the dietary method, time covered by the dietary method, and the range of exposure. However, they reported that a beneficial increase in fetal growth was most pronounced for those women at the lower end of the consumption rate spectrum that increased milk intake. Similarly, in the present review, we can conclude that although the evidence is limited, it suggests that moderate maternal milk consumption during pregnancy, compared with no or very low intake, is positively associated with birth length.
Regarding other gestational outcomes, very few studies evaluated the possible impact of milk and dairy product consumption on gestation or neonatal complications, such as spontaneous abortion, preterm birth, or congenital malformations.
We found no study addressing the relation between milk consumption itself and the risk of spontaneous abortion. However, we included the results from 2 studies (21, 34), exploring the relation between the nutritional content of the whole diet and the risk of spontaneous abortion, that assessed the independent impacts of different food groups, including milk and cheese. Both studies found a positive impact of milk consumption and dairy products to prevent abortion. Nevertheless, the observed association should be carefully interpreted as the retrospective nature of case-control studies make them particularly susceptible to bias; disease and exposure have already occurred at the outset of a case-control study, and there may be differential reporting of exposure information between cases and controls based on their disease status. Furthermore, there is no way to discriminate if final conclusions refer to the additive effects of the whole diet rather than the individual effects of the different food groups, as Ahmadi and colleagues point out in their article. As an additional limitation, studies included only women with spontaneous abortion requiring hospital admission, with the consequent exclusion of women with subclinical abortions or very early pregnancy losses (34).
Regarding the possible impact of milk consumption on preterm birth, most of the evaluated studies did not distinguish between the intake of milk and dairy products from other components of the diet and were therefore excluded from the present review (41). Only 2 studies clearly reported the exposure of interest; 1 was the Generation R Study, a population-based prospective cohort study following subjects from fetal life until young adulthood in the city of Rotterdam, The Netherlands (26), which did not find any statistical relation between milk intake and percent of preterm births. The other was a retrospective cohort in Sweden (23) that also showed no association between milk intake and preterm birth. Furthermore, no studies were found that specifically evaluated the effect of milk and dairy product intake during pregnancy on the risk of congenital malformations.
Only 2 crossover dietary intervention trials assessed the effects of the consumption of dairy products on the nutritional value of human milk. Specifically, one study revealed that a change in maternal lipid consumption rapidly alters the milk lipid content and fatty acid profile (31). In this regard, two articles not included in this review for not adhering to the inclusion criteria, reported that women who consumed low-fat dairy diets had a lower fat content in their human milk than women who consumed more fat from dairy products (20, 42). It is well known (43) that human milk must provide most of its total energy content to the breastfeeding child as fatty acids. Long chain polyunsaturated fatty acids are essential and play vital roles in the health of infants by performing structural and physiological functions (44). To our knowledge, no other studies have described the relation between the maternal intake of dairy products and human milk nutritional value or milk production (volume).
Considering all the above-mentioned issues, it could be suggested that the most obvious effects found from milk and dairy product intake, mainly during pregnancy, seem to be in accordance with the common recommendations of 2–3 servings/d (45, 46), especially compared with no or very low milk intake.
Conclusions
To summarize, although the number and types of studies provide insufficient evidence to offer definite conclusions, there appears to be an important trend of positive associations between moderate maternal milk intake during pregnancy and both infant birth weight and length. Randomized controlled trials are needed that examine the relations between maternal dairy product intake and the main pregnancy and lactation outcomes to provide women with specific dietary advice during these critical physiological periods regarding both themselves and their offspring.
Acknowledgments
The authors’ contributions were as follows—GV-M: conceived the review; MA, NU, AG-G, and TP: developed and conducted the search strategy, the data extraction, and the qualitative synthesis of the findings; MA: led the drafting of the manuscript with contributions from NU, AG-G, TP, and GV-M. All authors provided critical input, read and reviewed the manuscript for important content, and approved its submitted version.
Notes
This supplement was sponsored by the Interprofessional Dairy Organization, (INLAC), Spain. The sponsor had no role in the design of the studies included in the supplement; in the collection, analyses, or interpretation of the data; in the writing of the manuscripts; or in the decision to publish the results. Publication costs for this supplement were defrayed in part by the payment of page charges. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
Author disclosures: MA, NU, AG-G, TP, and GV-M, no conflicts of interest.
Abbreviations used: IUGR, intrauterine growth retardation; PROSPERO, International Prospective Register of Systematic Reviews; SGA, small for gestational age.
References
- 1. Harnisch JM, Harnisch PH, Harnisch DR Sr. Family medicine obstetrics: pregnancy and nutrition. Prim Care. 2012;39(1):39–54. [DOI] [PubMed] [Google Scholar]
- 2. Kaiser L, Allen LH; American Dietetic Association. Position of the American Dietetic Association: nutrition and lifestyle for a healthy pregnancy outcome. J Am Diet Assoc. 2008;108(3):553–61. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. WHO recommendations on antenatal care for a positive pregnancy experience. Geneva: WHO Guidelines Approved by the Guidelines Review Committee; 2016. [PubMed] [Google Scholar]
- 4. Mathers JC. Diet and epigenetics. In: Geissler CPH, editor Human nutrition. 2017. [Google Scholar]
- 5. Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, Li N, Hu G, Corrado F, Rode L et al.. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. 2017;317(21):2207–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li N, Liu E, Guo J, Pan L, Li B, Wang P, Liu J, Wang Y, Liu G, Baccarelli AA et al.. Maternal prepregnancy body mass index and gestational weight gain on pregnancy outcomes. PLoS One. 2013;8(12):e82310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koletzko B, Brands B, Chourdakis M, Cramer S, Grote V, Hellmuth C, Kirchberg F, Prell C, Rzehak P, Uhl O et al.. The power of programming and the EarlyNutrition project: opportunities for health promotion by nutrition during the first thousand days of life and beyond. Ann Nutr Metab. 2014;64(3–4):187–96. [DOI] [PubMed] [Google Scholar]
- 8. Barker DJ, Osmond C, Simmonds SJ, Wield GA. The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. BMJ. 1993;306(6875):422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martyn CN, Barker DJ, Osmond C. Mothers' pelvic size, fetal growth, and death from stroke and coronary heart disease in men in the UK. Lancet. 1996;348(9037):1264–8. [DOI] [PubMed] [Google Scholar]
- 10. Risnes KR, Nilsen TI, Romundstad PR, Vatten LJ. Head size at birth and long-term mortality from coronary heart disease. Int J Epidemiol. 2009;38(4):955–62. [DOI] [PubMed] [Google Scholar]
- 11. Xu T, Zhang ZX, Han SM, Hu HT, Xiao XH, Gong XM, Chen X, Wang ZS, Liu AM. Relationship between birth head circumference and adulthood quality of life in Chinese people. J Paediatr Child Health. 2010;46(11):642–6. [DOI] [PubMed] [Google Scholar]
- 12. Melnik BC, John SM, Schmitz G. Milk consumption during pregnancy increases birth weight, a risk factor for the development of diseases of civilization. J Transl Med. 2015;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27(5):358–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanson M, Gluckman P. Developmental origins of noncommunicable disease: population and public health implications. Am J Clin Nutr. 2011;94(6 Suppl):1754S–8S. [DOI] [PubMed] [Google Scholar]
- 15. James DC, Lessen R; American Dietetic Association. Position of the American Dietetic Association: promoting and supporting breastfeeding. J Am Diet Assoc. 2009;109(11):1926–42. [DOI] [PubMed] [Google Scholar]
- 16. Haug A, Høstmark AT, Harstad OM. Bovine milk in human nutrition–a review. Lipids Health Dis. 2007;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Plećas D, Plesinac S, Kontić Vucinić O. Nutrition in pregnancy: basic principles and recommendations. Srp Arh Celok Lek. 2014;142(1–2):125–30. [DOI] [PubMed] [Google Scholar]
- 18. Valentine CJ, Wagner CL. Nutritional management of the breastfeeding dyad. Pediatr Clin North Am. 2013;60(1):261–74. [DOI] [PubMed] [Google Scholar]
- 19. National Heart Lung and Blood Institute. Study Quality Assessment Tools U.S. Department of Health & Human Services; 2018. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. [Google Scholar]
- 20. Park Y, McGuire MK, Behr R, McGuire MA, Evans MA, Shultz TD. High-fat dairy product consumption increases δ9c,11t-18:2 (rumenic acid) and total lipid concentrations of human milk. Lipids. 1999;34(6):543–9. [DOI] [PubMed] [Google Scholar]
- 21. Di Cintio E, Parazzini F, Chatenoud L, Surace M, Benzi G, Zanconato G, La Vecchia C. Dietary factors and risk of spontaneous abortion. Eur J Obstet Gynecol Reprod Biol. 2001; 95(1):132–6. [DOI] [PubMed] [Google Scholar]
- 22. Chang SC, O'Brien KO, Nathanson MS, Caulfield LE, Mancini J, Witter FR. Fetal femur length is influenced by maternal dairy intake in pregnant African American adolescents. Am J Clin Nutr. 2003;77(5):1248–54. [DOI] [PubMed] [Google Scholar]
- 23. Ludvigsson JF, Ludvigsson J. Milk consumption during pregnancy and infant birthweight. Acta Paediatr. 2004;93(11):1474–8. [DOI] [PubMed] [Google Scholar]
- 24. Olsen SF, Halldorsson TI, Willett WC, Knudsen VK, Gillman MW, Mikkelsen TB, Olsen J; NUTRIX Consortium. Milk consumption during pregnancy is associated with increased infant size at birth: prospective cohort study. Am J Clin Nutr. 2007;86(4):1104–10. [DOI] [PubMed] [Google Scholar]
- 25. Xue F, Willett WC, Rosner BA, Forman MR, Michels KB. Parental characteristics as predictors of birthweight. Hum Reprod. 2008;23(1):168–77. [DOI] [PubMed] [Google Scholar]
- 26. Heppe DH, van Dam RM, Willemsen SP, den Breeijen H, Raat H, Hofman A, Steegers EA, Jaddoe VW. Maternal milk consumption, fetal growth, and the risks of neonatal complications: the Generation R Study. Am J Clin Nutr. 2011;94(2):501–9. [DOI] [PubMed] [Google Scholar]
- 27. Borazjani F, Angali KA, Kulkarni SS. Milk and protein intake by pregnant women affects growth of foetus. J Health Popul Nutr. 2013;31(4):435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hrolfsdottir L, Rytter D, Hammer Bech B, Brink Henriksen T, Danielsen I, Steingrimsdottir L, Olsen SF, Halldorsson TI. Maternal milk consumption, birth size and adult height of offspring: a prospective cohort study with 20 years of follow-up. Eur J Clin Nutr. 2013;67(10):1036–41. [DOI] [PubMed] [Google Scholar]
- 29. Malhotra N, Upadhyay RP, Bhilwar M, Choy N, Green T. The role of maternal diet and iron-folic acid supplements in influencing birth weight: evidence from India's National Family Health Survey. J Trop Pediatr. 2014;60(6):454–60. [DOI] [PubMed] [Google Scholar]
- 30. Li YF, Hu NS, Tian XB, Li L, Wang SM, Xu XB, Wang N, Shi CG, Zhu JC, Sun JS et al.. Effect of daily milk supplementation on serum and umbilical cord blood folic acid concentrations in pregnant Han and Mongolian women and birth characteristics in China. Asia Pac J Clin Nutr. 2014;23(4):567–74. [DOI] [PubMed] [Google Scholar]
- 31. Yahvah KM, Brooker SL, Williams JE, Settles M, McGuire MA, McGuire MK. Elevated dairy fat intake in lactating women alters milk lipid and fatty acids without detectible changes in expression of genes related to lipid uptake or synthesis. Nutr Res. 2015;35(3):221–8. [DOI] [PubMed] [Google Scholar]
- 32. Olmedo-Requena R, Amezcua-Prieto C, Luna-Del-Castillo Jde D, Lewis-Mikhael AM, Mozas-Moreno J, Bueno-Cavanillas A, Jiménez-Moleón JJ. Association between low dairy intake during pregnancy and risk of small-for-gestational-age infants. Matern Child Health J. 2016;20(6):1296–304. [DOI] [PubMed] [Google Scholar]
- 33. Abreu S, Santos PC, Montenegro N, Mota J. Relationship between dairy product intake during pregnancy and neonatal and maternal outcomes among Portuguese women. Obes Res Clin Pract. 2017;11(3):276–86. [DOI] [PubMed] [Google Scholar]
- 34. Ahmadi R, Ziaei S, Parsay S. Association between nutritional status with spontaneous abortion. Int J Fertil Steril. 2017;10(4):337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mukhopadhyay A, Dwarkanath P, Bhanji S, Devi S, Thomas A, Kurpad AV, Thomas T. Maternal intake of milk and milk proteins is positively associated with birth weight: a prospective observational cohort study. Clin Nutr ESPEN. 2018;25:103–9. [DOI] [PubMed] [Google Scholar]
- 36. Hjertholm KG, Iversen PO, Holmboe-Ottesen G, Mdala I, Munthali A, Maleta K, Ferguson E, Kamudoni P. Maternal dietary intake during pregnancy and its association to birth size in rural Malawi: a cross-sectional study. Matern Child Nutr. 2018;14(1–9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fall CHD. Fetal malnutrition and long-term outcomes. Nestlé Nutr Inst Workshop Ser. 2013;74:11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brantsaeter AL, Olafsdottir AS, Forsum E, Olsen SF, Thorsdottir I. Does milk and dairy consumption during pregnancy influence fetal growth and infant birthweight? A systematic literature review. Food Nutr Res. 2012;56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Clark DC. Dairy and growth, latest findings, and lessons learned. Food Nutr Bull. 2016;37:(Suppl 1):S22–8. [DOI] [PubMed] [Google Scholar]
- 40. Salvador Castell G, Serra-Majem L, Ribas-Barba L. What and how much do we eat? 24-hour dietary recall method. Nutr Hosp. 2015;31:(Suppl 3):46–8. [DOI] [PubMed] [Google Scholar]
- 41. Chia AR, de Seymour JV, Colega M, Chen LW, Chan YH, Aris IM, Tint MT, Quah PL, Godfrey KM, Yap F et al.. A vegetable, fruit, and white rice dietary pattern during pregnancy is associated with a lower risk of preterm birth and larger birth size in a multiethnic Asian cohort: the Growing Up in Singapore Towards healthy Outcomes (GUSTO) cohort study. Am J Clin Nutr. 2016;104(5):1416–23. [DOI] [PubMed] [Google Scholar]
- 42. Anderson NK, Beerman KA, McGuire MA, Dasgupta N, Griinari JM, Williams J, McGuire MK. Dietary fat type influences total milk fat content in lean women. J Nutr. 2005; 135(3):416–21. [DOI] [PubMed] [Google Scholar]
- 43. Koletzko B, Lien E, Agostoni C, Böhles H, Campoy C, Cetin I, Decsi T, Dudenhausen JW, Dupont C, Forsyth S et al.. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med. 2008;36(1):5–14. [DOI] [PubMed] [Google Scholar]
- 44. Delgado-Noguera MF, Calvache JA, Bonfill Cosp X, Kotanidou EP, Galli-Tsinopoulou A. Supplementation with long chain polyunsaturated fatty acids (LCPUFA) to breastfeeding mothers for improving child growth and development. Cochrane Database Syst Rev. 2015;14(7):CD007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th edition December2015. Available from: http://health.gov/dietaryguidelines/2015/guidelines/. [Google Scholar]
- 46. Aranceta J, Arija V, Maíz E, Martínez de Victoria E, Ortega RM, Pérez-Rodrigo C, Quiles J, Rodríguez A, Román B, Salvador GGrupo Colaborativo de la Sociedad Española de Nutrición Comunitaria (SENC); et al.; Grupo Colaborativo de la Sociedad Española de Nutrición Comunitaria (SENC) Guías alimentarias para la población española (SENC, diciembre 2016); la nueva pirámide de la alimentación saludable. Nutr Hosp. 2016;33(Suppl 8):1–48. [Google Scholar]


