ABSTRACT
There is a physiological basis for the roles of selected nutrients, especially proteins, calcium, and vitamin D, in growth and development, which are at a maximum during the pediatric period. Milk and dairy products are particularly rich in this group of nutrients. The present systematic review summarizes the available evidence relating dairy product intake with linear growth and bone mineral content in childhood and adolescence. A search was conducted in the MEDLINE (via PubMed) and SCOPUS databases following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and included intervention-controlled clinical trials with dairy products in children from 1 January, 1926 to 30 June, 2018. The risk of bias for each study was assessed using the Cochrane methodology. The number of study participants, the type of study and doses, the major outcomes, and the key results of the 13 articles included in the review are reported. The present systematic review shows that supplementing the usual diet with dairy products significantly increases bone mineral content during childhood. However, the results regarding a possible relation between dairy product consumption and linear growth are inconclusive.
Keywords: body height, bone density, cheese, children, dairy product, growth and development, milk, yogurt
Introduction
The relation between milk consumption during the pediatric age period and increased linear growth and bone mineralization has been widely hypothesized since the 1920s (1). In addition to fetal development, the pediatric period is when the largest and fastest growth and development occur. This growth is continuous, with rate changes throughout childhood, i.e., accelerated growth during early childhood, stable growth during the preschool and school years, and accelerated growth during puberty (2). Height and bone mineral content (BMC) are known growth markers. Linear growth and bone acquisition are 2 different physiologic processes that do not occur exactly at the same time, although they are related (3). Skeletal mineralization begins during fetal development and continues at different rates until the end of the teenage years. At this point, 90–95% of the total peak bone mass has been reached, of which 40–45% develops during adolescence (4–7). McCormack et al. (3) studied a group of 2014 boys and girls and observed that at 7 y of age they had acquired between 69.5% and 74.5% of their adult height and only between 29.6% and 38.1% of their maximum BMC. At the time of their peak height velocity, these children had acquired almost 90% of their adult height and 57.6–60.2% of their maximum BMC. They also observed that between 6.9% and 10.7% of peak bone mass is gained in late adolescence, after the cessation of linear growth.
Genetic factors, sometimes mediated by hormonal factors, determine ∼70–80% of linear growth and acquisition of BMC (8–10), whereas environmental factors determine ∼20–30%, especially physical activity (11–13), inactivity and sedentarism, and diet (14–17). These exogenous factors are susceptible to change.
Regarding physical activity, it is shown that exercise improves muscle strength, cartilage preservation, and bone remodeling (18, 19). Studies in rat models (20, 21) and clinical trials in osteoporotic patients (18, 19) confirm that aerobic activity plus resistance or strength exercises (like whole body vibration training) are a great tool for improving bone mass.
In relation to the diet, understanding the roles of different food systems and patterns is important for establishing prevention and intervention strategies. Physiology justifies milk and dairy product consumption during the pediatric period, because they are good sources of energy, macronutrients, and micronutrients (proteins, phosphorus, magnesium, vitamin D, and, most importantly, calcium) for growth and development (15, 16). In addition, prospective clinical studies have shown that calcium supplementation can increase the acquisition of bone mass during childhood, adolescence, and early adulthood (17, 22). There is a threshold in the calcium intake, which if exceeded does not affect the bone mass, but if the consumption is below the threshold it results in a negative balance. The level of this threshold depends on the ability to absorb calcium efficiently and decrease urinary losses and varies according to age, ethnic group, and genetic factors (23). Moreover, dairy product consumption increases the secretion of insulin-like growth factor type I, which benefits skeletal development (24). Likewise, dairy products are thought to aid in calcium absorption because of their lactose and casein phosphorylated peptides and because they allow calcium intake to be more homogeneously distributed in relatively small amounts throughout the day (25). Currently, in developed countries, children under the age of 9 y are recommended to use ∼500 mL dairy products and adolescents >600 mL dairy/d (26).
Since the first studies on dietary supplementation with milk (1), the scientific community has continued to study its potential benefit, with the goal of adopting public health policies to optimize pediatric growth and development (27–29). The development of dual-energy X-ray absorptiometry (DXA) equipment since the 1960s has allowed for evaluating BMC as well as density in growth studies (30). This technique showed that children worldwide could improve their bone mass peak via calcium or milk supplementation (14, 27, 31). However, these findings were inconclusive. Two meta-analyses performed by Huncharek et al. (32) in 2008 and Beer (33) in 2012 shed light on this topic. However, current evidence on the association of dairy product consumption with growth and BMC has not been synthesized. Therefore, the present systematic review carried out an updated evaluation of the available evidence from clinical trials that correlate dairy product intake with linear growth and BMC in the pediatric population.
Methods
The present review was prepared following the guidelines for Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (34) and was registered in the International Prospective Register of Systematic Reviews (PROSPERO) as CRD42018100083. The PICOS criteria (Population, Intervention, Comparison, Outcomes, Settings) (35) were used (Table 1) to elaborate the following review question: does the intake of dairy products influence linear growth and BMC in children and adolescents? Controlled intervention studies that evaluated dairy product intake and its relation with BMC and height in children and adolescents were incorporated.
TABLE 1.
PICOS criteria (35) for including studies that evaluate the influence of dairy product intake on linear growth and BMC in children and adolescents
| Parameter | Inclusion criteria |
|---|---|
| Population | <18 y |
| Intervention | Controlled dairy intake |
| Comparison | Control |
| Outcome | Height and BMC |
| Setting | Controlled trials |
1BMC, bone mineral content.
Inclusion and exclusion criteria
Children and adolescents ≤18 y of age and of any ethnic origin were included. Controlled studies, randomized or not, published from 1 January, 1926 to 30 June, 2018, were incorporated into the review. Studies that used dairy fractions, did not include linear growth or BMC data, or did not compare a control group without dairy products were excluded.
Intervention types
Studies in which the interventions were performed with complete dairy products (not fractions) and were compared with a nonsupplemented group were included. The articles were not restricted by time, type, or amount of dairy in the intervention.
Primary outcome measures
Height in centimeters and changes in height after the intervention in centimeters, centimeters per year, or percentage were included as valid measures for linear growth analysis. To evaluate bone mineralization, the BMC in grams and its modifications in grams per year or percentage were considered.
Literature search
The PUBMED and SCOPUS databases were searched using the Medical Subject Heading (MeSH) terms “dairy products,” “growth,” “development,” “bone density,” and “height.”
In PUBMED the following search strategy was used: “Dairy products” (All Fields) AND [“Growth and development” (All Fields) OR “Growth” (All Fields) OR “development” (All Fields) OR “bone density” (All Fields) OR “body height” (All Fields)] AND {“humans” [MeSH Terms] AND [“infant” (MeSH Terms) OR “child” (MeSH Terms) OR “adolescent” (MeSH Terms)]}. SCOPUS was searched using the following formula, excluding from the results the studies indexed in MEDLINE and those on animals: “Dairy products” AND (“Growth and development” OR “Growth” OR “development” OR “bone density” OR “body height”).
Study selection
Independently, 2 authors (MJdC and CdL) selected the studies from the 56 articles reviewed in full. RL, MLC, AG, and MG-C arbitrated the discrepancies when no consensus on the selection was reached. Finally, 13 articles (36–48) were included in the systematic review.
Data extraction
Two investigators separately extracted the following data from each study: publication year, number of participants by sex, age, study type, intervention characteristics and study duration, and outcomes and conclusions. RL moderated any discrepancies.
Assessment of risk of bias
Two evaluators independently studied the risks of bias following the methodology of the Cochrane Collaboration (49). The articles were analyzed individually, and their risk of bias was classified as high, uncertain, or low depending on random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias). The presence of other additional biases was also analyzed. In cases with a disparity of opinions, a third reviewer arbitrated.
Results
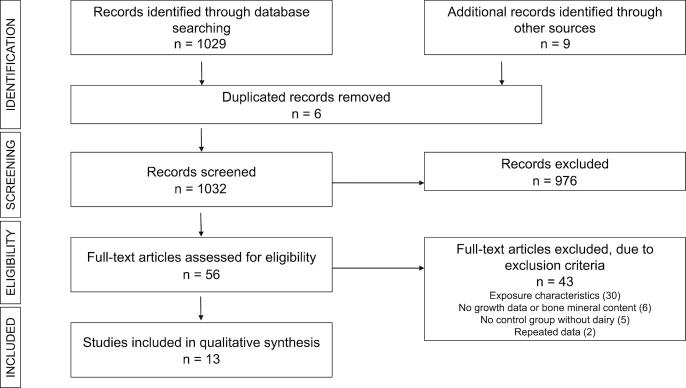
Figure 1 shows the results of each step of the bibliographic search. Of the 1038 results from the first search (PUBMED, 448; SCOPUS, 581; and other sources, 9), 6 duplicate articles and 976 after review of the abstract were eliminated. Fifty-six articles were considered for evaluating the full text. Finally, 13 articles that met the inclusion criteria were included in this systematic review (36–48).
FIGURE 1.
Flow diagram of the literature search process.
Tables 2 and 3 present the main characteristics of the selected clinical trials. The publication dates covered 1926 to 2017, with 7 articles published after 2000. The articles in this review included 3895 children and adolescents (63.67% female). In 5 of these studies (38, 40, 43–45), only girls participated. The sample sizes ranged from 47 to 757 participants, with a mean ± SD of 299 ± 234 participants. The mean age was 9.95 y, ranging from 3 to 18 y. Five articles (36, 38, 39, 43, 46) performed interventions using dairy products (between 0.9 and 1.2 g of calcium-equivalent doses per day) and the other 8 studies exclusively used milk. The intervention period ranged from 14 wk for Morgan et al.’s (48) study in 1926 to 24 mo for those of Merrilees et al. (43), Du et al. (40), and Cheng et al. (38), with a median intervention period of 16 mo.
TABLE 2.
Controlled trials with dairy product interventions to evaluate the effects on body height in 3895 children and adolescents1
| Reference | n | Age,2 y | Intervention | Type and time of intervention | Outcome | Results3 | Conclusion |
|---|---|---|---|---|---|---|---|
| Vogel et al. (36) | 240 (154F) | 11.8 ± 1.5 | Dairy (3 servings/d—0.9 g Ca/d) | RCT, 18 mo | Linear growth (cm)/year | Linear growth (cm)/y: IG: 4.43 ± 0.23; CG: 4.95 ± 0.25 | Significantly less increase in annual growth |
| Lien et al. (37) | 454 (237F) | 7–8 | Cow milk (500 mL/d; 6 d/wk) | RCT, 6 mo | Linear growth (cm) | Linear growth (cm): IG: 3.6 ± 4.8; CG: 3.2 ± 5.6 | Significant increase in age-adjusted height |
| Height-for-age change | HAZ: IG: 0.18 ± 0.92; CG: −0.25 ± 1.67 | ||||||
| Cheng et al. (38) | 195 (195F) | 10–12 | Low fat cheese (1 g Ca/d) | RCT, 24 mo | Linear growth (cm) | Linear growth (cm): IG: 9.1 ± 0.3; CG: 9.1 ± 0.3 | No significant differences |
| He et al. (39) | 402 (217F) | 3–5 | Yogurt (125 mL/d; 5 d/wk) | RCT, 9 mo | Linear growth (cm) | Linear growth (cm): IG: 5.43 ± 0.69; CG: 5.24 ± 0.76 | Significant increase |
| Du et al. (40) | 757 (757F) | 10 | Cow milk (300 mL/d) | CT, 24 mo | Linear growth (cm) | Linear growth (cm): IG: 13.4; CG: 12.2 | Significant increase in percentage of change |
| Lau et al. (41) | 344 (143F) | 9–10 | Skimmed milk powder (40 g/d) | RCT, 18 mo | Linear growth (cm) | Linear growth (cm): IG: 7.11 ± 0.19; CG: 7.06 ± 0.17 | No significant differences |
| Grillenberger (42) | 554 (241F) | 7.1 | Cow milk (200 mL/d) | CT, 23 mo | Linear growth (cm) | Linear growth (cm): IG: 10.31 ± 0.33; CG: 10.04 ± 0.34 | Significant increase in children with a baseline HAZ below the median |
| Height-for-age change | HAZ: IG: 0.55 ± 0.05; CG: 0.4 ± 0.06 | ||||||
| Merrilees et al. (43) | 105 (105F) | 15–18 | Dairy (1 g Ca/d) | RCT, 24 mo | Linear growth (cm) | Linear growth (cm): IG: 1.3; CG: 1.6 | No significant differences |
| Cadogan et al. (44) | 82 (82F) | 12.2 ± 0.3 | Cow milk (568 mL/d) | RCT, 18 mo | Linear growth (cm) | Linear growth (cm): IG: 8.8 ± 3.78; CG: 8.2 ± 2.95 | No significant differences |
| Chan et al. (45) | 48 (48F) | 9–13 | Dairy (1.2 g Ca/d) | RCT, 12 mo | Linear growth (cm) | Linear growth (cm): IG: 5.2 ± 1.6; CG: 5.3 ± 1.2 | No significant differences |
| Baker et al. (46) | 581 (253F) | 7–8 | Cow milk (190 mL/d) | RCT, 21.5 mo | Linear growth (cm) | Linear growth (cm): IG: 9.46 ± 1.68; CG: 9.18 ± 1.67 | Significant increase in percentage of change |
| Lampl and Johnston (47) | 86 (24F) | 7.7–13 | Cow milk (250 mL/d) | RCT, 8 mo | Linear growth (cm) | Linear growth (cm): IG: 3.45 ± 0.14; CG: 1.75 ± 0.17 | Significant increase in age-adjusted height |
| Height-for-age change | HAZ: IG: 0.87 ± 0.13; CG: −1.37 ± 0.1 | ||||||
| Morgan et al. (48) | 47 (24F) | 7–15 | Cow milk (284 mL/d) | CT, 14 wk | Linear growth (cm) | No data | No significant differences |
1CG, control group; CT, controlled trial; F, female; HAZ, height-for-age z score; IG, intervention group; RCT, randomized controlled trial.
2Values are ranges, means, or means ± SDs, as reported in the studies.
3Values are means, or means ± SDs, as reported in the studies.
TABLE 3.
Controlled trials of dairy product interventions to evaluate effects on BMC in 1771 children and adolescents1
| Reference | n | Age,2 y | Intervention | Type and time of intervention | Outcome | Results3 | Conclusion |
|---|---|---|---|---|---|---|---|
| Vogel et al. (36) | 240 (154F) | 11.8 ± 1.5 | Dairy (3 servings/d—0.9 g Ca/d) | RCT, 18 mo | Change (g/y) | Total BMC: no data; spine: no data; femur: no data; radius: no data; tibia: no data | Significant increase in BMC at the tibia |
| Cheng et al. (38) | 195 (195F) | 10–12 | Cheese (1 g Ca/d) | RCT, 24 mo | Change (%) | Total BMC: IG: 38.1; CG: 35; total femur: IG: 25.9; CG: 26.2; Lumbar spine: IG: 34.2; CG: 34 | No significant differences |
| Du et al. (40) | 757 (757F) | 10 | Milk (300 mL/d) | CT, 24 mo | Grams | Total BMC: IG: 1875.6 ± 281.3; CG: 1854.4 ± 268.4 | Significant increase in percentage of change in total body BMC |
| Lau et al. (41) | 344 (143F) | 9–10 | Milk (250 mL/d) | RCT, 18 mo | Change (%) | Total BMC: IG: 18.46 ± 0.67; CG: 16.88 ± 0.6; total hip: IG: 25.89; CG: 22.77; femoral neck: IG: 13.16; CG: 10.64; spine: IG: 21.51; CG: 19.23 | Significant increase in BMC at the hip |
| Merrilees et al. (43) | 105 (105F) | 15–18 | Dairy (1 g Ca/d) | RCT, 24 mo | Change (grams) | Total BMC: IG: 168.9 ± 24.7; CG: 167.4 ± 16.2; lumbar spine: IG: 3.83; CG: 2.58; femoral neck: IG: 0.12; CG: 0.06; trochanter: IG: 0.75; CG: 0.24 | Significant increase in BMC at the trochanter |
| Cadogan et al. (44) | 82 (82F) | 12.2 ± 0.3 | Cow milk (568 mL/d) | RCT, 18 mo | Change (%) | Total BMC (%): IG: 27; CG: 24.1; total BMC (g): IG: 428; CG: 391 | Significant increase in percentage of change in total body BMC |
| Chan et al. (45) | 48 (48F) | 9–13 | Dairy (1.2 g Ca/d) | RCT, 12 mo | Change (%) | Total BMC (g): IG 1695 ± 317; CG: 1617 ± 152; total BMC (%): IG: 14.2 ± 7.0; CG: 7.6 ± 6.0 | Significant increase in percentage of change in total body BMC |
1BMC, bone mineral content; CG, control group; CT, controlled trial; F, female; IG, intervention group; RCT, randomized controlled trial.
2Values are ranges, means, or means ± SDs, as reported in the studies.
3Values are means, or means ± SDs, as reported in the studies.
Dairy intake and height
All studies included in the review provided linear growth data (Table 2). Six studies showed significant changes in height adjusted for age (which relates the height with that corresponding to the age of the child in a reference population) or percentage change in height favoring the intervention group (37, 39, 40, 42, 46, 47). Among these, Grillenberger's study (42) found significant differences in z-score changes for height adjusted for age, but only in patients who were short in stature for their age at the baseline. The remaining articles showed no statistically significant differences in height after the intervention (36, 38, 41, 43–45, 48). Four (38, 43–45) of the 5 articles that included only girls (38, 40, 43–45) found no statistically significant differences in height; however, in the studies that included both boys and girls, 5 (37, 39, 42, 46, 47) of 8 (36, 37, 39, 41, 42, 46–48) found significant differences in height. Nevertheless, the studies that analyzed the subgroups by sex demonstrated no significant differences in height change between the sexes (36, 37, 39, 41, 42, 46–48). None of the articles that used dairy products rather than milk (36, 38, 39, 43, 48) showed significant differences favoring the intervention in height. In addition, the study with dairy products published by Vogel et al. (36) concluded that individuals in the intervention group grew significantly less than those in the control group.
Dairy intake and BMC
Changes in BMC were evaluated in 7 articles (36, 38, 40, 41, 43–45) (Table 3). BMC was assessed by DXA in all of them. All articles included data on total body BMC (36, 38, 40, 41, 43–45); 4 of the 7 studies (36, 38, 41, 43) included femur determinations, 2 total femur (36, 46), 1 femoral neck (41), and 1 femoral neck and trochanter (43); and 3 (38, 41, 43) included column measurements, 1 total (41), and 2 (38, 43) of the lumbar spine. One study included measurements in total hip (41) and another in radius and tibia (36). Six articles (36, 40, 41, 43–45) found significant differences in BMC levels: total BMC (40, 44, 45), tibia (36), total hip (41), or trochanter (43). One of these studies, which recruited 10- to 12-y-old girls, found no significant differences in total body, total femur, or lumbar spine BMC after the intervention. However, statistically significant differences in bone mineral density were found at the femur level (38).
Assessment of risk of bias
Fifty-four percent of the articles presented low risks of selection bias (random sequence generation) and attrition bias (incomplete outcome data); 25% had low risks of performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessment). In 77% of the articles, the risk of information bias (reporting bias) was uncertain, because they published nonsignificant data. The risk of selection bias (allocation concealment) was high in all participating articles. A high risk of attrition bias was considered when there were no references in the study to the data lost during the intervention and an evaluation of whether they were relevant, when the proportion of missing data was sufficient to have a clinically significant effect, or the methods of imputation for the treatment of missing data were used inappropriately. Other risks of bias included a short intervention time (48) of 14 wk and stratification through self-reported physical activity level questionnaires (38).
The article published by Lien et al. (37) presented the lowest risk of bias, although its mechanism of random assignment by collectivities and not individuals implies a significant risk of bias. The studies with the highest risks of presenting biased results were those of Morgan et al. (48) and Grillenberger (42). Additional information on the risk of bias analysis (a risk of bias graph and summary) of the analyzed articles is included in Supplemental Figures 1 and 2.
Discussion
The present systematic review of controlled trials on the effects of dairy product consumption on linear growth and BMC during the pediatric period shows that supplementing the usual diet with these foods significantly increases BMC. However, the results regarding a possible relation between dairy product intake and height are inconclusive.
Regarding the impact of dairy consumption on linear growth, 6 studies (37, 39, 40, 42, 46, 47) revealed statistically significant increases in the sizes of children whose ages ranged from 3 to 10 y. Five of these studies had the largest sample sizes, between 454 and 757 participants (37, 39, 40, 42, 46). However, 7 studies observed no significant differences (36, 38, 41, 43–45, 48), although the sample size was very low in 5 of them (43–45, 47, 48), 105, 82, 48, 86, and 47 participants, respectively, which could decrease the statistical power of the data analysis, and these included older children (9–18 y of age). Therefore, the linear growth rates, amount of milk supplemented (specifically calcium), and duration of the intervention were too heterogeneous to draw firm conclusions. These inconclusive results are consistent with the scientific literature that assesses the relation between calcium supplementation (the main micronutrient in milk involved in bone metabolism) and bone length, which has not evidenced a clear benefit of calcium intake on size during growth periods (15, 16, 24). In 2011, a meta-analysis was published addressing this question, which suggested an additional 0.4 cm/y increase in height for every 245 mL of milk consumed per day, although the degree of evidence was considered of moderate quality because most of the included studies presented serious limitations in their design and execution (33).
In selected populations with high malnutrition indexes (40, 42) and mainly vegetarian diets, an increased final size was observed after dairy product supplementation, which could be explained by the contributions of additional energy and high-biological-value proteins. One group (42) evidenced this effect specifically in the subset of children who started with a lower height-for-age z score at the beginning of the intervention, which could support this hypothesis, although this may not have been adequately explored in the articles cited. Nevertheless, 1 cohort study by Marshall et al. (31), not included in the review, also suggested a relation between milk consumption and height increase, although in this case the children who participated belonged to families of medium socioeconomic status, with low risks of malnutrition. However, this relation was nonlinear; therefore, the mechanism that could explain the beneficial effect could not be attributed exclusively to this dietary measure but may link to a more favorable environment involving other unevaluated factors. In this sense, recent studies (50, 51) relate dairy product consumption to a healthier diet. Thus, a study conducted in Australia in 2012 on 222 children between 8 and 10 y old and involving 3 food recalls concluded that adequate dairy product consumption was associated with highly nutritious diets (50). Moreover, 1 study followed 1991 children from 8 European countries for 4 y and concluded consuming dairy products (milk, yogurt, and cheese) as snacks is associated with higher diet quality. Consuming dairy products outside of regular meals may be a good strategy for improving energy balance throughout childhood (51).
Regarding bone mineralization and dairy product consumption, 7 controlled trials were evaluated (36, 38, 40, 41, 43–45), of which 6 (36, 38, 41, 43–45) were randomized. Six of the 7 mentioned studies, 1 nonrandomized, showed positive relations with bone mineralization (36, 40, 41, 43–45), although this was measured in different locations: 3 at the total body level (40, 44, 45), 1 in the pelvis (41), 1 in the trochanter (43), and 1 in the tibia (36). These results were consistent with other investigations, which, conversely, studied the effect of avoiding milk consumption for prolonged periods. Black et al. (52) recruited 50 children between 3 and 10 y of age, who, for different reasons, ingested no milk (lactose intolerance or lifestyle) and observed that these children had significantly lower bone mineral densities and more fractures than did controls that consumed 200 mL of milk daily. However, the blood calcium concentrations in this group were lower, as an association between these concentrations and the z scores, as shown by DXA, was found only at some skeletal sites, suggesting that intakes of both calcium and other nutrients in milk are essential to properly mineralize bones. Studies supporting the benefits of milk or dairy products on bone show a significant inverse association between dairy food intake and bone turnover markers as well as a positive association with BMC (25, 53).
One study in the present systematic review further explored this hypothesis by comparing the effect of calcium supplementation on girls between 10 and 12 y old, either by administering pills or by increasing cheese intake (38). Both measures increased bone mass compared with the placebo, and this effect was greater in the group that received the food, although it was only statistically significant at the level of the tibial cortical bone. In addition, studies conducted on adults have also supported the hypothesis that dairy products ingested during childhood improve bone mineralization, and adults who consumed more milk in childhood had better bone density (17, 22).
However, demonstrating the true effects of improved BMC on individual health has not been possible. A 22-y prospective observational study concluded that high dairy intake during adolescence did not appear to prevent bone fractures in women during adulthood. In men, it appeared to be more of a risk factor, although the association was attenuated when weight was added to the model (54). Although observational studies only suggest hypotheses, and multiple factors influence the risk of fractures, knowing the long-term results of dairy supplementation interventions would be interesting. Likewise, the studies included in this review seemed to have short intervention times (between 14 wk and 2 y) compared with a study that evaluated dairy intake over 5 y (54).
Regarding sex, the present review found that studies on girls obtained less significant results for height than did those on both boys and girls, but studies using both sexes showed no differences between the sexes. Some cohort studies that included both boys and girls found statistically significant differences in height relative to dairy intake but also found no differences between subgroups by sex (31, 55). However, a study on height in adult males from 48 European countries found that the ratio of high-quality protein intake, especially from dairy products, to low-quality proteins from wheat was the most important factor in the secular improvement of height (56). Therefore, more studies including men or larger studies including both sexes should be designed to better evaluate the possible differences.
In the present review, we analyzed evidence from the last 92 y of intervention studies on the effect of milk and dairy products on linear growth and BMC in children. When evaluating the risk of bias in each of the studies and assessing their results, not all biases should be considered equally relevant. For example, for the intervention characteristics and results evaluated, we believe that performance bias is less important because height and BMC are objective data (objective measures). Conversely, the most relevant bias risks in this review appeared to be random sequence generation or selection bias, detection bias, and attrition bias.
Further studies should include male populations, unify anatomical sites for determining bone mineralization, and improve the blinding methods, although it is difficult to blind dairy food supplementation, but the risk of bias would be lower. Research comparing the effects of calcium from milk with those of isolated calcium supplementation should be continued.
Although no conclusive data exist relating the influence of milk and dairy products to linear growth, data related to improving bone mineralization after milk and milk product supplementation indicate that dairy products are important for proper bone health beginning in childhood.
Conclusion
The data obtained in the present review support the dietary guidelines (26) on the importance of children regularly consuming dairy products to ensure or improve their bone health. An increase of BMC is observed when the usual diet is supplemented with dairy products. This is especially important during this period of life at a time when consumption of this traditionally basic food in children's diets, particularly in Western countries, is decreasing due to lifestyle changes that favor intake of fast food, soft drinks, or plant seed–based beverages. Future research should be oriented towards realizing randomized controlled trials of appropriate sample size and adequate power, long-term interventions, and deep analyses of cohort studies in children beginning in early life.
Supplementary Material
Acknowledgments
The authors’ contributions were as follows—all authors: contributed to the design, analysis, and presentation of the results; drafted the manuscript; discussed and revised the manuscript; and read and approved the final manuscript.
Notes
This supplement was sponsored by the Interprofessional Dairy Organization (INLAC), Spain. The sponsor had no role in the design of the studies included in the supplement; in the collection, analyses, or interpretation of the data; in the writing of the manuscripts; or in the decision to publish the results. This study was partially funded by the University of Granada Plan Propio de Investigación 2016, Excellence actions: Unit of Excellence on Exercise and Health (UCEES), Plan Propio de Investigación 2018, Programa Contratos-Puente, the Junta de Andalucía, Consejería de Conocimiento, Investigación y Universidades, and European Regional Development Funds (ref. SOMM17/6107/UGR). The opinions expressed in this publication are those of the author(s) and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
The Centro de Investigación Biomédica en Red de la Fisiopatología de la Obesidad y Nutrición (CIBERobn) is an initiative of the Instituto de Salud Carlos III (ISCIII) of Spain which is supported by funding from the Fondo Europeo de Desarrollo Regional (FEDER).
Author disclosures: CdL, MJdC, MG-C, AG, MLC, and RL, no conflicts of interest.
Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: BMC, bone mineral content; DXA, dual-energy X-ray absorptiometry; MeSH, Medical Subject Headings.
References
- 1. Corry-Mann HC. Diets for boys during the school age. Medical Research Council Special Report Series No. 105. London: HMSO; 1926. [Google Scholar]
- 2. Carrascosa A, Yeste D, Gussinyer M. Masa ósea en la infancia y adolescencia. In: Pombo M, editor. Tratado De Endocrinología Pediátrica. 4th ed Madrid, Spain: McGraw-Hill-Interamericana; 2009. pp. 457–72. [Google Scholar]
- 3. McCormack SE, Cousminer DL, Chesi A, Mitchell JA, Roy SM, Kalkwarf HJ, Lappe JM, Gilsanz V, Oberfield SE, Shepherd JA et al.. Association between linear growth and bone accrual in a diverse cohort of children and adolescents. JAMA Pediatr. 2017;171(9):e171769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marcus R. Endogenous and nutritional factors affecting bone. Bone. 1996;18(1 Suppl):11S–13S. [DOI] [PubMed] [Google Scholar]
- 5. Heaney RP, Abrams S, Dawson-Hughes B, Looker A, Marcus R, Matkovic V, Weaver C. Peak bone mass. Osteoporos Int. 2000;11:985–1009. [DOI] [PubMed] [Google Scholar]
- 6. Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the University of Saskatchewan Bone Mineral Accrual Study. J Bone Miner Res. 1999;14:1672–9. [DOI] [PubMed] [Google Scholar]
- 7. Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res. 2011;26:1729–39. [DOI] [PubMed] [Google Scholar]
- 8. Seeman E, Hopper JL, Bach LA, Cooper ME, Parkinson E, McKay J, Jerums J. Reduced bone mass in daughters of women with osteoporosis. N Engl J Med. 1989;320:554–8. [DOI] [PubMed] [Google Scholar]
- 9. Ferrari S, Rizzoli R, Slosman D, Bonjour JP. Familial resemblance for bone mineral mass is expressed before puberty. J Clin Endocrinol Metab. 1998;83:358–61. [DOI] [PubMed] [Google Scholar]
- 10. Duren DL, Sherwood RJ, Choh AC, Czerwinski SA, Chumlea WC, Lee M, Sun SS, Demerath EW, Siervogel RM, Towne B. Quantitative genetics of cortical bone mass in healthy 10-year-old children from the Fels Longitudinal Study. Bone. 2007;40:464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nikander R, Sievanen H, Heinonen A, Daly RM, Uusi-Rasi K, Kannus P. Targeted exercise against osteoporosis: a systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2010;8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tan VP, Macdonald HM, Kim S, Nettlefold L, Gabel L, Ashe MC, McKay HA. Influence of physical activity on bone strength in children and adolescents: a systematic review and narrative synthesis. J Bone Miner Res. 2014;29:2161–81. [DOI] [PubMed] [Google Scholar]
- 13. Bounds W, Skinner J, Carruth BR, Ziegler P. The relationship of dietary and lifestyle factors to bone mineral indexes in children. J Am Diet Assoc. 2005;105:735–41. [DOI] [PubMed] [Google Scholar]
- 14. Iuliano-Burns S, Stone J, Hopper JL, Seeman E. Diet and exercise during growth have site-specific skeletal effects: a co-twin control study. Osteoporos Int. 2005;16:1225–32. [DOI] [PubMed] [Google Scholar]
- 15. Alexy U, Remer T, Manz F, Neu CM, Schoenau E. Long-term protein intake and dietary potential renal acid load are associated with bone modeling and remodeling at the proximal radius in healthy children. Am J Clin Nutr. 2005;82:1107–14. [DOI] [PubMed] [Google Scholar]
- 16. Moyer-Mileur LJ, Xie B, Ball SD, Pratt T. Bone mass and density response to a 12-month trial of calcium and vitamin D supplement in preadolescent girls. J Musculoskelet Neuronal Interact. 2003;3:63–70. [PubMed] [Google Scholar]
- 17. Vatanparast H, Bailey DA, Baxter-Jones AD, Whiting SJ. The effects of dietary protein on bone mineral mass in young adults may be modulated by adolescent calcium intake. J Nutr. 2007;137:2674–9. [DOI] [PubMed] [Google Scholar]
- 18. Musumeci G. The use of vibration as physical exercise and therapy. J Funct Morphol Kinesiol. 2017;2:17. [Google Scholar]
- 19. Castrogiovanni P, Trovato FM, Szychlinska MA, Nsir H, Imbesi R, Musumeci G. The importance of physical activity in osteoporosis. From the molecular pathways to the clinical evidence. Histol Histopathol. 2016;31(11):1183–94. [DOI] [PubMed] [Google Scholar]
- 20. Pichler K, Loreto C, Leonardi R, Reuber T, Weinberg AM, Musumeci G. In rat with glucocorticoid-induced osteoporosis, RANKL is downregulated in bone cells by physical activity (treadmill and vibration stimulation training). Histol Histopathol. 2013;28:1185–96. [DOI] [PubMed] [Google Scholar]
- 21. Musumeci G, Loreto C, Leonardi R, Castorina S, Giunta S, Carnazza ML, Trovato FM, Pichler K, Weinberg AM. The effects of physical activity on apoptosis and lubricin expression in articular cartilage in rats with glucocorticoid-induced osteoporosis. J Bone Miner Metab. 2013;31(3):274–84. [DOI] [PubMed] [Google Scholar]
- 22. Matkovic V, Goel PK, Badenhop-Stevens NE, Landoll JD, Li B, Ilich JZ, Skugor M, Nagode LA, Mobley SL, Ha EJ et al.. Calcium supplementation and bone mineral density in females from childhood to young adulthood: a randomized controlled trial. Am J Clin Nutr. 2005;81:175–88. [DOI] [PubMed] [Google Scholar]
- 23. Marx SJ, Bourdeau JE. Calcium metabolism. In: Narins RG, editor. Maxwell and Kleeman's Clinical Disorders of Fluid and Electrolyte Metabolism. International ed McGraw-Hill; 1994. pp. 269–74. [Google Scholar]
- 24. Caroli A, Poli A, Ricotta D, Banfi G, Cocchi D. Invited review: dairy intake and bone health: a viewpoint from the state of the art. J Dairy Sci. 2011;94:5249–62. [DOI] [PubMed] [Google Scholar]
- 25. Heaney RP. Calcium, dairy products and osteoporosis. J Am Coll Nutr. 2000;19(Suppl):83S–99S. [DOI] [PubMed] [Google Scholar]
- 26. Dror DK, Allen LH. Dairy product intake in children and adolescents in developed countries: trends, nutritional contribution, and a review of association with health outcomes. Nutr Rev. 2014;72(2):68–81. [DOI] [PubMed] [Google Scholar]
- 27. Cook J, Irwig LM, Chinn S, Altman DG, Florey CD. The influence of availability of free school milk on the height of children in England and Scotland. J Epidemiol Community Health. 1979;33(3):171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baten J. Protein supply and nutritional status in nineteenth century Bavaria, Prussia and France. Econ Hum Biol. 2009;7(2):165–80. [DOI] [PubMed] [Google Scholar]
- 29. Elwood PC, Haley TJ, Hughes SJ, Sweetnam PM, Gray OP, Davies DP. Child growth (0–5 years), and the effect of entitlement to a milk supplement. Arch Dis Child. 1981;56(11):831–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cameron JR, Sorenson J. Measurement of bone mineral in vivo: an improved method. Science. 1963;142:230–2. [DOI] [PubMed] [Google Scholar]
- 31. Marshall TA, Curtis AM, Cavanaugh JE, Warren JJ, Levy SM. Higher longitudinal milk intakes are associated with increased height in a birth cohort followed for 17 years. J Nutr. 2018;148(7):1144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huncharek M, Muscat J, Kupelnick B. Impact of dairy products and dietary calcium on bone-mineral content in children: results of a meta-analysis. Bone. 2008;43:312–21. [DOI] [PubMed] [Google Scholar]
- 33. Beer H. Dairy products and physical stature: a systematic review and meta-analysis of controlled trials. Econ Hum Biol. 2012;10(3):299–309. [DOI] [PubMed] [Google Scholar]
- 34. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, PRISMA-P Group . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sackett DL, Strauss SE, Richardson WS. Evidence-based Medicine: How to Practice and Teach EBM. London: Churchill-Livingstone; 2000. [Google Scholar]
- 36. Vogel KA, Martin BR, McCabe LD, Peacock M, Warden SJ, McCabe GP, Weaver CM. The effect of dairy intake on bone mass and body composition in early pubertal girls and boys: a randomized controlled trial. Am J Clin Nutr. 2017;105(5):1214–29. [DOI] [PubMed] [Google Scholar]
- 37. Lien DTK, Nhung BT, Khan NC, Hop LT, Nga NTQ, Hung NT, Kiers J, Shigeru Y, te Biesebeke R. Impact of milk consumption on performance and health of primary school children in rural Vietnam. Asia Pac J Clin Nutr. 2009;18(3):326–44. [PubMed] [Google Scholar]
- 38. Cheng S, Lyytikäinen A, Kröger H, Lamberg-Allardt C, Alén M, Koistinen A, Wang QJ, Suuriniemi M, Suominen H, Mahonen A et al.. Effects of calcium, dairy product, and vitamin D supplementation on bone mass accrual and body composition in 10–12-y-old girls: a 2-y randomized trial. Am J Clin Nutr. 2005;82(5):1115–26. [DOI] [PubMed] [Google Scholar]
- 39. He M, Yang YX, Han H, Men JH, Bian LH, Wang GD. Effects of yogurt supplementation on the growth of preschool children in Beijing suburbs. Biomed Environ Sci. 2005;18(3):192–7. [PubMed] [Google Scholar]
- 40. Du X, Zhu K, Trube A, Zhang Q, Ma G, Hu X, Fraser DR, Freenfield H. School-milk intervention trial enhances growth and bone mineral accretion in Chinese girls aged 10–12 years in Beijing. Br J Nutr. 2004;92:159–68. [DOI] [PubMed] [Google Scholar]
- 41. Lau EM, Lynn H, Chan YH, Lau W, Woo J. Benefits of milk powder supplementation on bone accretion in Chinese children. Osteoporos Int. 2004;15(08):654–8. [DOI] [PubMed] [Google Scholar]
- 42. Grillenberger M. Food supplements have a positive impact on weight gain and addition of animal source foods increases lean body mass of Kenyan schoolchildren. J Nutr. 2003;133(11):3957S–64S. [DOI] [PubMed] [Google Scholar]
- 43. Merrilees MJ, Smart EJ, Gilchrist NL, Frampton C, Turner JG, Hooke E, March RL, Maguire P. Effects of dairy food supplements on bone mineral density in teenage girls. Eur J Nutr. 2000;39(6):256–62. [DOI] [PubMed] [Google Scholar]
- 44. Cadogan J, Eastell R, Jones N, Barker M. Milk intake and bone mineral acquisition in adolescent girls: randomized, controlled intervention trial. BMJ. 1997;315:1255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chan GM, Hoffman K, McMurry M. Effects of dairy products on bone and body composition in pubertal girls. J Pediatr. 1995;126(4):551–5. [DOI] [PubMed] [Google Scholar]
- 46. Baker IA, Elwood PC, Hughes J, Jones M, Moore F, Sweetnam PM. A randomized controlled trial of the effect of the provision of free school milk on the growth of children. J Epidemiol Community Health. 1980;34:31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lampl M, Johnston F. The effects of protein supplementation on the growth and skeletal maturation of New Guinean school children. Ann Hum Biol. 1978;5(3):219–27. [DOI] [PubMed] [Google Scholar]
- 48. Morgan AF, Hatfield GD, Tanner MA. A comparison of the effects of supplementary feeding of fruits and milk on the growth of children. Am J Dis Child. 1926;32:839–49. [Google Scholar]
- 49. Higgins JPT, Altman DG, Sterne JAC. Chapter 8. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 London, UK: The Cochrane Collaboration; 2011. Available from: https://handbook-5-1.cochrane.org/. [Google Scholar]
- 50. Rangan AM, Flood VM, Denyer G, Webb K, Marks GB, Gill TP. Dairy consumption and diet quality in a sample of Australian children. J Am Coll Nutr. 2012;31(3):185–93. [DOI] [PubMed] [Google Scholar]
- 51. Iglesia I, Intemann T, De Miguel-Etayo P, Pala V, Hebestreit A, Wolters M, Eiben G, Moreno LA. Dairy consumption at snack meal occasions and overall diet quality during childhood. IDEFICS / I. Family cohort. Ann Nutr Metab. 2018;73(Suppl. 2):1–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Black RE, Williams SM, Jones IE, Goulding A. Children who avoid drinking cow milk have low dietary calcium intakes and poor bone health. Am J Clin Nutr. 2002;76(3):675–80. [DOI] [PubMed] [Google Scholar]
- 53. Rizzoli R. Dairy products, yogurts, and bone health. Am J Clin Nutr. 2014;99(5):1256S–62S. [DOI] [PubMed] [Google Scholar]
- 54. Feskanich D, Bischoff-Ferrari HA, Frazier L, Willett W. Milk consumption during teenage years and risk of hip fractures in older adults. JAMA Pediatr. 2014;168(1):54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nezami M, Segovia-Sipaco G, Beeson WL, Sabaté J. Associations between consumption of dairy foods and anthropometric indicators of health in adolescents. Nutrients. 2016;8(7):427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Grasgruber P, Cacek J, Kalina T, Sebera M. The role of nutrition and genetics as key determinants of the positive height trend. Econ Hum Biol. 2014;15:81–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.