ABSTRACT
Nutrition plays an important role in bone health. The aim of our study was to update the evidence regarding dairy intake, osteoporotic fracture (OF) risk, and prospective bone mass density (BMD) evolution assessed by dual-energy X-ray absorptiometry in Europeans and non-Hispanic whites from North America. A systematic search was conducted in MEDLINE, EMBASE, and Scopus for papers published from 1 January, 2000 to 30 April, 2018. The eligibility criteria were as follows: healthy adults; measurable dairy exposure; hip, vertebral, wrist or OF as outcomes; and cohort or case-control studies. Two independent investigators conducted the search and the data extraction. A pooled analysis was conducted with random-effects models. Publication bias and meta-regression were considered. Ten cohort studies relating to OF risk were selected for meta-analysis. Three papers reporting BMD changes associated with dairy intake could not be aggregated in the meta-analysis. The pooled HRs of the highest compared with the lowest levels of dairy intake were 0.95 (95% CI: 0.87, 1.03; I2 = 82.9%; P-heterogeneity < 0.001) for OF at any site; 0.87 (95% CI: 0.75, 1.01; I2 = 86.7%; P-heterogeneity < 0.001) for hip fractures; and 0.82 (95% CI: 0.68, 0.99; I2 = 0.0%; P-heterogeneity = 0.512) for vertebral fractures. Concerning BMD, the selected studies described a 1.7–3% lower hip BMD in young and postmenopausal women with poor intake of milk in their youth, a positive relationship between baseline milk ingestion and the percentage of trochanter BMD change in elderly people, and a positive correlation between milk consumption and BMD change at the radius in women aged >65 y. In conclusion, in the studied population, the highest consumption of dairy products did not show a clear association with the total OF or hip fracture risks; however, a diminished risk of vertebral fracture could be described. The results regarding BMD change were heterogeneous and did not allow for a definitive conclusion.
Keywords: osteoporotic fractures, hip fracture, vertebral fracture, Colles’ fracture, dairy products, milk, fermented milk, yogurt, cheese, bone mass density
Introduction
Aging is associated with bone loss and frailty and a high risk of osteoporotic fracture (OF). In industrialized countries, the prevalence of osteoporosis has been estimated to be 9–38% for females and 1–8% for males aged >50 y (1). As life expectancy increases, more people will be at risk of suffering hip fracture (HF), vertebral fracture (VF), or wrist fracture (WF), with some described geographic discrepancies in this respect (2). In 2000, the crude estimated prevalence of OF was 9 million worldwide (>1 million for VF, WF, and HF), and the prevalence is expected to reach >2 million for HF in 2020 and >4 million for VF in 2050 (3). The direct impact that OF has on morbidity, mortality, quality of life, and costs (4) has guided the efforts in the search for preventable associated risk factors in this context.
The etiology of OF is complex, involving genetic (5), environmental, hormonal, behavioral, and nutritional factors (6). In addition, comorbid conditions that promote chronic inflammation induce changes in bone health, increasing the susceptibility to low bone mass (7–9). Recently, frailty has also been suggested to be a predictor of OF (10).
Several nutritional factors have been associated with bone mass density (BMD) and OF risk. Nutrients such as calcium, inorganic phosphate, vitamin D, and proteins (11); foods such as milk and dairy products; and, recently, dietary patterns, such as the Mediterranean diet (12), among others (13, 14), have shown some relationship with bone mass and the frequency of OF, but some controversy exists.
In developed countries, there is a trend towards reducing the per-capita consumption of milk in childhood, adolescence (15), and adulthood (16). A wide range of factors underly this tendency: the increased diagnosis of lactose intolerance, the competition from plant-derived drinks, and the substitution of the traditional dietary patterns for other eating behaviors such as veganism are only some of the factors leading to this situation (17). In this scenario, distinguishing between scientific and nonscientific evidence is highly relevant from the consumer perspective, as well as from the perspective of health professionals attending to specific populations.
Studies investigating the association between dairy intake and the risk of low BMD or OF have described discordant results in adults. Regarding OF, 3 meta-analyses of cohort and case-control data have been published, showing no relationship between milk intake and OF (18) or HF risks (18–20), but a lower probability of HF with a higher cheese and yogurt consumption (20). To our knowledge, there have been no randomized controlled trials studying the efficacy of nonfortified or nonmodified dairy in OF prevention. Concerning BMD, cross-sectional studies have described a dose-response association between dairy food intake and higher appendicular bone mass (21, 22), and a meta-analysis of randomized controlled trials demonstrated an increase in BMD associated with the intake of dietary calcium, mostly from fortified dairy sources (23). There is a scientifically proven link between BMD and OF (24), but the normal way in which milk and its derivatives act on bone loss and OF prevention seems to become less clear when searching for strong outcome data.
The objective of this systematic review is to update the latest evidence regarding dairy product consumption in adults and OF risk, including recent epidemiologic data not assessed in previous analyses and covering the most prevalent sites of OF: hip, vertebrae, and wrist. Moreover, we appraise the research relating dairy food intake with BMD progression over time. When possible, a pooled analysis is shown. To avoid the variability imposed by a multiethnic approach, the results are focused on a population from Europe and non-Hispanic whites from North America. The effects of specifically fortified dairy products, out of those consumed by the most individuals in a region, are not included in this research.
Methods
Search strategy
A prospectively developed protocol for this systematic review was registered on PROSPERO, the international prospective register of systematic reviews (CRD42018099433). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (25) was followed in the reporting of this systematic review. The literature search for articles published between 1 January, 2000 and 30 April, 2018 was conducted via MEDLINE (via PubMed), EMBASE, and Scopus. Furthermore, we reviewed the reference lists and abstracts presented at the American Society for Bone and Mineral Research annual meetings from 2012 to 2017, and the gray literature of WorldWideScience (https://worldwidescience.org/). References from the included studies were searched to identify any potential study that had not been captured in our electronic search. All searches were limited to English and Spanish languages and studies conducted with healthy humans ≥18 y of age. The studies had to examine the relationship between dairy product intake and OF (HF, WF, and VF) or BMD change. The following search terms were used: “dairy products,” “milk,” “cheese,” “yogurt,” “butter,” “buttermilk,” “ice cream,” “kefir”; NOT: “food, fortified,” “milk proteins,” “milk, human,” “whey proteins,” “ghee,” “margarine”; AND: “fractures, bone,” “hip fractures,” “Colles' fracture,” “osteoporosis,” “osteoporotic fractures,” “ulna fractures,” “radius fractures.” In addition, the following terms were included: skim milk, whole milk, semiskim milk, whole yogurt, fractures, femur fracture, subtrochanteric fracture, trochanteric fracture, intertrochanteric fracture, femoral neck fracture, vertebral fracture, fragile fracture, fragile fractures, metacarpal fractures, typical radius fracture, wrist fracture, carpal bone fracture, carpal fracture, carpus fractures, trapezoid fracture, and bone mass density.
Study records were organized with the Mendeley reference manager.
Study eligibility criteria and selection
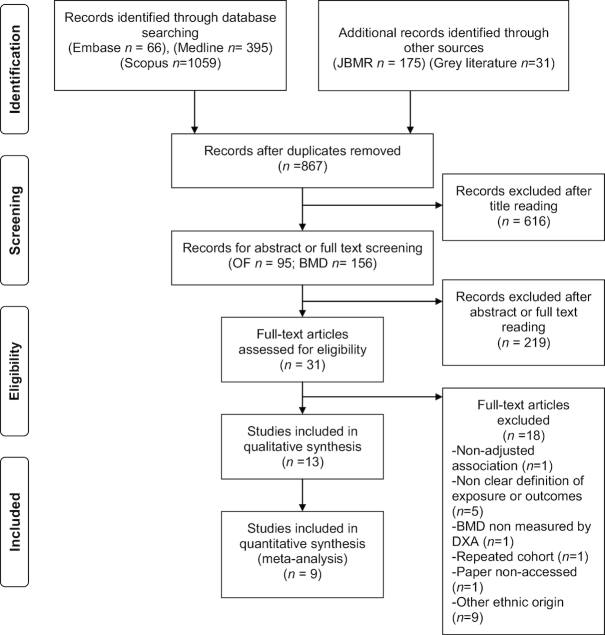
Two researchers oversaw the study selection independently. The eligibility criteria were as follows: prospective cohort and case-control studies relating dairy product intake with OF or BMD, studies conducted in healthy non-Hispanic white populations, studies with a minimum follow-up of 3 y (from the evaluation of the exposure to the outcome assessment), studies in which dairy products were measured quantitatively, studies in which outcomes were reported as incident OF or BMD quantified by dual-energy X-ray absorptiometry (DXA), and studies in which the comparability of groups was assessed by multiple adjustment. We also included some studies with cross-sectional designs that used a retrospective measure for the exposure. Details of the literature search and study selection flow are summarized in Figure 1.
FIGURE 1.
Search strategy and selection of studies. BMD, bone mass density; DXA, dual-energy X-ray absorptiometry; JBMR, Journal of Bone and Mineral Research; OF, osteoporotic fracture.
Data extraction
The following items were evaluated and extracted: author and year of publication; methods (study design, mean follow-up or duration of exposure, geographic area, withdrawals, and date of study); participants (sample size, mean age at exposure, age range, gender, and mean BMI at baseline); exposure assessment [self-reported or interviewed food-frequency questionnaire (FFQ) or other questionnaire]; type of exposure (all dairy products, milk, yogurt, cheese, or cream); dose of exposure (servings/day and servings/week for categoric comparisons of intake; and servings/day, servings/week or servings for each specific increment assessments); portion size of exposure (as specified in each study or, if not, a serving was considered 177 g for total dairy foods, 244 g for milk or yogurt, and 43 g for cheese) (20, 26); outcome assessment (self-report, hospital records, X-ray, or DXA); site of fracture; number of cases; studied site of BMD; measures of exposure effects [multivariate adjusted HR, RR, or OR (95% CI) for fracture outcomes (the highest, or other levels, compared with the lowest consumption, or for each increment in dose of exposure); multivariate-adjusted β coefficient (95% CI) or adjusted mean difference (SD) for BMD outcome]; and adjustments made for comparability of the groups (age, gender, dietary pattern, physical activity, BMI, BMD, alcohol intake, smoking habit, estrogen use, menopausal status, calcium and vitamin D supplementation, daily energy intake, previous fractures, and family history of fractures). We emailed 3 authors in search of missing data, but these data were no longer available (27–29). One author could not be contacted (30). Every study was extracted by 1 investigator and reviewed by a second researcher. Any disagreements were solved via group consensus.
The methodologic quality of the included studies was evaluated using the Newcastle-Ottawa Scale (NOS) (31). Two authors scored each paper independently, and a consensus was reached for inclusion in this report.
Statistical analysis
We estimated the effect size by random-effects meta-analyses, given the foreseeable clinical variability. The HRs were pooled for this estimate. The SEs were calculated after log-transformation of the adjusted HRs and their 95% CIs, approaching a normal distribution. Heterogeneity was assessed with both the Q statistic (significant if the P value <0.10) and the I2 index (low, moderate, and high heterogeneity if this rate approaches 25%, 50%, and 75%, respectively). Subsequently, subgroup analyses were conducted, stratifying for type of dairy and gender.
The publication bias was studied by visual inspection of the funnel plot symmetry as well as by Egger's test (asymmetry considered if P < 0.05) (32).
A metaregression analysis was conducted, when possible, to evaluate the impact of other variables on the estimated effect size (age at exposure; geographic area; sample size; outcome assessment; and adjustment by dietary pattern, physical activity, BMD, smoking, calcium and vitamin D supplementation, and daily energy intake).
All the tests were conducted with Stata version 12 (StataCorp LP), and used the following commands: metan, metafunnel, and metabias.
Results
Overview of the included studies
After the systematic search, we found 10 cohort studies related to risk of OF [summarized in Table 1 (n = 374,476); age at baseline 53–77 y; BMI 24.8–27.6 kg/m2; 4 from Europe (29, 33–35) and 6 from North America (27, 28, 30, 36–38)]. In 2 of the studies, the exposure was measured during childhood (30) and adolescence (30, 36). Regarding exposure, 3 studies investigated any type of dairy product (29, 34, 37), 9 studies investigated milk (27, 28, 30, 33–38), 5 studies investigated yogurt (27, 28, 33, 34, 37), 6 studies investigated cheese (27, 28, 33, 34, 36, 37), and 2 studies investigated cream (27, 28). The reported outcomes were fractures at any site (30, 33, 34), HF (27–29, 33, 34, 36, 37), VF (34, 35, 38), and WF (34).
TABLE 1.
Characteristics of the studies evaluating the association between dairy intake and osteoporotic fractures1
| Reference | Population | n | Baseline age, y | BMI, kg/m2 | Dairy product | Dairy dose2 | Follow-up (years) | Outcome/outcome assessment | Adjustment main effect | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Feskanich, 2018 (37) | Health Professionals Follow-up Study: menThe Nurses’ Health Study: women USA | 123,906; HPFS: 43,306 NHS: 80,600 | Men: 50–75/57.7 Women: 34–60/53.6 | Men: 25.5–25.9 Women: 25.3–25.9 | — | FFQ (self-reported) | Men: 17.5 Women: 20.8 | Hip fracture, n = 2832 (men = 694; women = 2138) Self-reported; demonstrated in a small validation study by confirmation with medical records | Age, follow-up cycle, total energy intake, calcium and vitamin D intake, protein, retinol, vitamin K, caffeine, alcohol, milk during teenage years, BMI, height, physical activity, smoking, use of postmenopausal hormones, diuretics and oral steroids; diagnosis of cancer, diabetes and cardiovascular disease. Milk, cheese and yogurt: adjusted for one another | Multivariable-adjusted RR3 (95% CI). Reference: the lowest intake Results for categories of dairy consumption (lowest-highest) |
| Milk | <1 serving/wk (ref), 1 serving/wk, 2–4 servings/wk, 5–6 servings/wk, 1 serving/d, ≥2 servings/d, per 1 serving/d | — | — | — | Men: 1.00, 1.00 (0.72–1.37), 0.81 (0.63–1.04), 0.79 (0.59–1.05), 0.73 (0.58–0.96), 0.77 (0.58–1.05), 0.91 (0.82–1.02) Women: 1.00, 0.89 (0.73–1.08), 0.94 (0.81–1.09), 0.90 (0.76–1.07), 0.88 (0.75–1.03), 0.77 (0.64–0.94), 0.92 (0.86–0.98) | |||||
| Cheese | <1 serving/wk (ref), 1 serving/wk, 2–4 servings/wk, 5–6 servings/wk, ≥1 serving/d, per 1 serving/d | — | — | — | Men: 1.00, 0.89 (0.67–1.19), 0.96 (0.75–1.24), 0.72 (0.51–1.01), 1.08 (0.77–1.51), 1.04 (0.85–1.28) Women: 1.00, 1.02 (0.83–1.26), 0.93 (0.77–1.12), 0.90 (0.74–1.11), 0.85 (0.68–1.06), 0.88 (0.79–0.99) | |||||
| Yogurt | Never (ref), <1 serving/wk, 2–4 servings/wk, ≥5 servings/wk, per 1 serving/wk | — | — | — | Men: 1.00, 1.12 (0.93–1.34), 1.29 (1.00–1.66), 0.92 (0.67–1.25), 1.04 (0.97–1.11) Women: 1.00, 0.97 (0.87–1.08), 0.97 (0.84–1.12), 1.08 (0.93–1.26), 0.77 (0.53–1.12), 1.00 (0.97–1.04) | |||||
| Feskanich, 2014 (36) | Health Professionals Follow-up Study: men The Nurses’ Health Study: women USA | 96,927; HPFS: 35,349 NHS: 61,578 | Men: 42–77 Women: 40–65 Exposure intake: teenage (13–18 y) | Men: 25.6–25.9 Women: 25.9–26.1 | — | FFQ (self-reported) teenage consumption | 22 | Hip fracture, n = 1226 (men = 490; women = 1226) Outcome median age: 78 y (men) and 73 y (women) Self-reported | Age, adult milk consumption in each period, intakes of calcium, retinol, and vitamin D from supplements, protein, alcohol and caffeine intakes, total energy intake, physical activity, BMI, smoking, use of diuretics and oral steroids (men); hormone replacement (women); incident diagnoses of osteoporosis and cancer | Multivariable-adjusted RR3 (95% CI) Reference: 1 serving/d Results for categories of dairy consumption (lowest-highest) |
| Milk | <2 servings/wk, 2–6 servings/wk, 1 serving/d (ref), 2–3 servings/d, ≥4 servings/d | — | — | — | Men: 0.75 (0.50–1.12), 0.97 (0.69–1.35), 1, 1.07 (0.84–1.39), 1.21 (0.86–1.69) Women: 1.03 (0.85–1.24), 0.89 (0.73–1.07, 1, 0.95 (0.81–1.12), 1.01 (0.78–1.31) | |||||
| Cheese | Per increase in 2 servings/wk | — | — | — | Men: 0.96 (0.84–1.03) Women: 1.01 (0.96–1.05) | |||||
| Michaëlsson, 2014 (33) | Swedish Mammography Cohort: women Cohort of Swedish Men: men Sweden | 106,772; Men: 45,339 Women: 61,433 | Men: 45–79 Women: 39–74 y | Men: 25.0–26.4 Women: 24.5–25.0 | — | FFQ (self-reported) | 20.1 Men: 13 Women: 22 | Any fracture Men = 5379 Women = 17252 Hip fracture Men = 1166 Women = 4259 Swedish national patient registry (ICD-10 codes) | Age, BMI, height, total energy and alcohol intake, healthy dietary pattern, calcium, and vitamin D supplements, ever use of cortisone, educational level, living alone, physical activity level, smoking status, and Charlson's comorbidity index estrogen replacement therapy and nulliparity (women) | Multivariable-adjusted HR (95% CI). Reference: the lowest intake Results for categories of dairy consumption (lowest-highest/per 1-serving increment) |
| Milk | g/d: <200 (ref), 200–399, 400–599, ≥600, continuous (per 200) | — | — | — | Swedish Mammography Cohort Any fractures:1.00,1.07 (1.04–1.11), 1.16 (1.11–1.21), 1.16 (1.08–1.25), 1.02 (1.00–1.04) Hip fractures: 1.00, 1.19 (1.11–1.28), 1.55 (1.41–1.69), 1.60 (1.39–1.84), 1.09 (1.05–1.13) Cohort of Swedish Men Any fractures: 1.00, 1.02 (0.96–1.10), 1.01 (0.93–1.08), 1.03 (0.94–1.11), 1.01 (0.99–1.03) Hip fractures: 1.00, 0.95 (0.82–1.11), 1.13 (0.97–1.31), 1.01 (0.85–1.20), 1.03 (0.99–1.07) | |||||
| Cheese | g/d: <20 (ref), 20–39, 40–59, ≥60, continuous (per 20) | — | — | — | Swedish Mammography Cohort Any fractures: 1.00, 0.86 (0.64–0.96), 0.92 (0.88–0.96), 0.85 (0.81–0.90), 0.96 (0.94–0.97) Hip fracture: 1.00, 0.69 (0.64–0.74), 0.81 (0.75–0.88), 0.57 (0.50–0.64), 0.86 (0.83–0.89) Cohort of Swedish Men Any fractures: 1.00, 0.98 (0.89–1.08), 1.00 (0.92–.09), 0.95 (0.88–1.04), 1.00 (0.98–1.01) Hip fracture: 1.00, 0.77 (0.63–0.94), 0.91 (0.77–1.08), 0.80 (0.68–0.95), 1.00 (0.97–1.03). | |||||
| Fermented milk/yogurt | g/d: <1 (ref), 1–199, 200–399, ≥400, continuous (per 200) | — | — | — | Swedish Mammography Cohort Any fractures: 1.00, 0.88 (0.85–0.92), 0.92 (0.88–0.96), 0.90 (0.82–0.99), 0.97 (0.95–1.00) Hip fracture: 1.00, 0.72 (0.66–0.77), 0.81 (0.74–0.88), 0.64 (0.53–0.78), 0.89 (0.84–0.94) Cohort of Swedish Men Any fractures: 1.00, 0.93 (0.87–1.00), 0.92 (0.86–0.99), 0.95 (0.87–1.03), 1.00 (0.97–1.03) Hip fracture: 1.00, 0.77 (0.66–0.90), 0.76 (0.65–0.88), 0.77 (0.65–0.92), 0.97 (0.92–1.03) | |||||
| Sahni, 2014 (28) | Framingham Original Cohort (men and women) USA | 764 (men and women) | 68–96/77 ± 4.9 | 25.6 ± 4.5 | — | FFQ (self-reported and reviewed with clinic staff) | 11.6 | Hip fracture, n = 97 Self-reported hip fractures were confirmed by review of medical records, radiographic and operative reports | Age, gender, weight, height, and total energy intake; subsequent models were adjusted for calcium and vitamin D supplement use and current smoking Final models were adjusted for baseline BMD at the femoral neck | — |
| Milk | Servings/wk: low ≤1, medium >1 and <7, high ≥7 | — | — | — | HR (95% CI); P (per servings/wk): 0.995 (0.962–1.029); 0.779 HR (95% CI); P Reference: the lowest intake: Highest intake: 0.58 (0.31–1.06); 0.078 Medium intake: 0.61 (0.36–1.08); 0.071 | |||||
| Yogurt | None, any intake >0 servings/wk | — | — | — | HR (95% CI); P (per servings/wk): 0.948 (0.796, 1.113); 0.554HR (95% CI); P Reference: the lowest intake: Any intake: 1.09 (0.65, 1.81); 0.746 | |||||
| Cheese | servings/wk: minimal ≤1, some >1 | — | — | — | HR (95% CI); P (per servings/wk): 0.975 (0.901, 1.055); 0.526 HR (95% CI); P Reference: the lowest intake: Some intake: 0.72 (0.48, 1.08); 0.117 | |||||
| Cream | servings/wk: low <0.5, medium ≥0.5 and <3, high ≥3 | — | — | — | HR (95% CI); P (per servings/wk): 1.024 (0.989, 1.060); 0.182 HR (95% CI); P Reference: the lowest intake: Medium intake: 0.86 (0.47, 1.58); 0.626 High intake: 1.04 (0.59, 1.86); 0.881 | |||||
| Milk + yogurt | servings/wk: low ≤ 1, medium >1 and <10, high ≥10 | — | — | — | HR (95% CI); P (per servings/wk): 0.993 (0.961, 1.026); 0.689 HR (95% CI); P Reference: the lowest intake: Highest intake: 0.63 (0.34, 1.15); 0.133 Medium intake: 0.65 (0.37, 1.14); 0.136 | |||||
| Sahni, 2013 (27) | Framingham Offspring Study (men and women) USA | 3212 (men and women) | 26–85/55 ± 9.6 | 27.3 | — | FFQ (self-reported and reviewed with clinic staff) | 12 | Hip fracture, n = 43 Self-reported (confirmed by review of medical records and radiographic and operative reports) | Age, gender, total energy intake, height and weight. Subsequent models were further adjusted for current cigarette smoking, calcium and vitamin D supplements; in women menopause and estrogen use | Multivariable-adjusted HR (95% CI); P Highest intakes (2–3) vs lowest intakes (1) None of the other dairy groups showed a significant association (P-trend = 0.25–0.61, data not shown) |
| Milk/fluid dairy | Values in tertiles without specifying quantity | — | — | — | Milk (tertiles) T2: 0.78 (0.37, 1.63); 0.09 T3: 0.50 (0.22, 1.13); 0.09 Fluid dairy (tertiles) T2: 0.92 (0.46, 1.87); 0.06 T3: 0.40 (0.17, 0.99); 0.06 | |||||
| Yogurt | servings/wk: no intake, ≤4, >4 | — | — | — | Yogurt (categories) C2: 0.39 (0.15, 1.02); 0.10 C3: 0.57 (0.19, 1.68); 0.10 | |||||
| Feart, 2013 (34) | Three City Prospective Cohort Study France | 1482 (men: 550; women: 932) | 67.7–94.9/75.9 | Reported as categories according to type of fracture | — | Interviewed FFQ + 24-h recall | 8 | Hip fracture,n = 57Vertebral fracture,n = 43 Wrist fracture, n = 73 Self-reported | Age, gender, physical activity, total energy intake, educational level, marital status, BMI, osteoporosis self-reported diagnosis and treatment, calcium and vitamin D; each individual food group item of Medi Score | HR (95% CI); P Reference: the highest intake |
| — | — | — | — | — | Dairy products | servings/wk: men </≥1; women </≥17.9 | — | — | — | HF: 0.95 (0.54, 1.68); 0.86 VF: 1.53 (0.79, 2.95); 0.21 WF: 2.03 (1.22, 2.39); 0.007 FAS: 1.51 (1.07, 2.11); 0.02 |
| — | — | — | — | — | Yogurt | servings/wk: women </≥7; men </≥6 | — | — | — | HF: 1.11 (0.62, 1.99); 0.72 VF: 0.85 (0.42, 1.70); 0.64 WF: 1.98 (1.22, 3.21); 0.75 FAS: 1.29 (0.92, 1.81); 0.15 |
| — | — | — | — | — | Milk | </≥0.25 servings/wk | — | — | — | HF: 1.16 (0.67, 2.02); 0.60 VF: 1.15 (0.60, 2.20); 0.68 WF: 0.96 (0.59, 1.56); 0.88 FAS: 1.10 (0.97, 1.53); 0.57 |
| — | — | — | — | — | Cheese | </≥7 servings/wk | — | — | — | HF: 1.28 (0.72, 2.28); 0.4 VF: 1.55 (0.80, 2.99); 0.68 WF: 0.96 (0.59, 1.56); 0.88 FAS: 1.14 (0.81, 1.61); 0.46 |
| Benetou, 2011 (29) | European Prospective Investigation into Cancer and Nutrition Study (elderly European volunteers ≥60 y old) Europe | 29,122 (men 10,538; women: 18,584) | 60–86 Men: 64.0 ± 4.1 Women: 64.5 ± 3.7 | Men: 27.5 ± 3.8 Women: 27.7 ± 7.7 | Dairy products | Self-administered or interviewer-administered FFQ Country-specific quintile | 8 | Hip fractures Women = 222 Men = 53 Telephone interviews/mailed questionnaires eliciting self-reported information Record linkage (with hospital discharge records using ICD-10 codes S72.0–S72.2) | Stratified by center and adjusted for gender, age at recruitment, educational level, smoking status, BMI, height, physical activity in leisure time, dietary supplement use, diabetes mellitus, and total energy intake | HR (95% CI); P, per quintile of dairy intake: 1.02 (0.93, 1.12); 0.62 |
| Nevitt, 2005 (35) | Study of Osteoporotic Fractures (metropolitan areas women) USA | 7238 women | 65–99 | 25.2 | Milk | Interviewed FFQ Milk consumption when pregnant or teen </≥1 glass/d | 3.7 | Vertebral fracture, n = 181 Women aged 65–69 y: 118 Women aged ≥50 y: 60 Quantitative vertebral morphometry | Adjusted for age, clinical center, and BMD | OR (95% CI) Reference: the highest intake1.49 (1.09, 2.04) |
| Roy, 2003 (38) | European Vertebral Osteoporosis Study: patients recruited from 36 European centers from the European Prospective Osteoporosis Study Europe | 6575 (men: 3173; women: 3402) | Men: 63.1 ± 7.8 Women: 62.2 ± 7.6 | Men: 27.1 ± 3.4 Women: 27 ± 4.4 | Milk | Interviewer administered questionnaire Milk consumption in 3 age periods (<25 y; 26–49 y; >50 y) </≥1 glass/d | 3.8 | Vertebral fracture, n = 224 Men = 80 Women = 144 Morphometric and qualitative (radiologist-assessed) | Adjusted for age and center | RR3 (95% CI) Reference: the lowest intake ≤25 y Men: 1.02 (0.63, 1.64) Women: 1.02 (0.71, 1.46) 26–49 y Men: 1.01 (0.62, 1.64) Women: 0.89 (0.61, 1.30) ≥50 y Men: 0.75 (0.44, 1.26) Women: 1.04 (0.71, 1.50) |
| Kalkwarf, 2003 (30) | NHANES III (non-Hispanic, white women) USA | 1880 women | 1371 (42.2%) aged 20–49 y1880 (57.8%) aged ≥50 y Exposure: milk intake during childhood (5–12 y) and adolescence (13–17 y) | 25.1 ± 0.6, aged 20–49 y 27.3 ± 0.5, aged ≥50 y | Milk | Interviewer administered questionnaire | From exposure measurement to outcome assessment, at least 33 y | Osteoporotic fracture (≥50 y)n = 158 Self-reported | Age, weight, and estrogenic deficiency | OR (95% CI) (>50 y) Reference: the highest intake Results for categories of milk consumption (lowest-highest) |
| <1 serving/wk, 1–6 servings/wk, 1 serving/d, > 1 serving/d (ref) | — | — | — | Child intake 2.25 (1.26, 4) 1.00 (0.67, 1.49) 1.39 (0.67, 1.49) 1 Adolescent intake 1.29 (0.75, 2.19) 0.87 (0.57, 1.29) 1.59 (0.84, 3.04) 1 | ||||||
| Categorization: low intake: <1 serving/wk, high intake: >1 serving/d (ref) | — | — | — | Childhood & adolescence (≤/>1 serving/wk) 1.19 (0.83, 1.70) |
1BMD, bone mass density; FAS, fractures at any site; FFQ, food-frequency questionnaire; HF, hip fracture; Medi, Mediterranean diet; ref, reference; T, tertile; VF, vertebral fracture; WF, wrist fracture.
2Serving size: Feskanich, 2014, 2018 (36, 37), milk 240 mL; cheese 1 oz (28 g) for hard cheeses and cream cheese and 0.5 cup (120 mL) for cottage or ricotta cheese; yogurt 1 cup (240 mL). Michaëlsson, 2014 (33), as shown in the table. Sahni, 2013, 2014 (27, 28), milk: 237 mL, ice milk: ½ cup = 120 mL, cottage or ricotta cheese = 120 mL, other cheese = 28 g, cream = 1 Tbs, sour cream = 1 Tbs, ice cream = 120 mL, cream cheese = 28 g, yogurt = 240 mL. Feart, 2013 (34), not described by the authors. Benetou, 2011 (29), not described by the authors. Nevitt, 2005 (35), milk 1 glass. Roy, 2003 (38), milk 1 glass. Kalkwarf, 2003 (30), milk 1 glass.
3As stated by the authors, Cox proportional hazards models were adjusted in the study.
4Regression model.
The measured exposure levels for comparisons were different among the included papers (Table 1). The outcome assessment was self-reported in 5 studies (29, 30, 34, 36, 37), and well-validated methods were used in the remaining studies (27, 28, 33, 35, 38).
Concerning the longitudinal data of BMD measured with DXA, 3 papers matched our search strategy (Table 2). Two studies assessed the exposition in adulthood (milk, fermented dairy, cheese, and cream) (39, 40), and 1 during childhood and adolescence (milk) (30). The researchers used interviewed FFQs in all of the studies.
TABLE 2.
Characteristics of the studies evaluating the association between dairy intake and bone mass density change1
| Reference | Population | n | Baseline age, y | BMI, kg/m2 | Dairy product | Dairy dose2 | Duration of the follow-up, y | Outcome/outcome assessment | Adjustment main effect | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Biver, 2018 (39) | Geneva Retirees Cohort (healthy postmenopausal women) Switzerland | 482 | ≥65 | By levels of fermented dairy products intake: 26.3 ± 5.7, 25.1 ± 4.2, 25.1 ± 4.7 | Fermented dairy products (yogurts, fresh cheese, “petit-suisse” cheese, quark, kefir) Milk Ripened cheese | Interviewed FFQ <1 serving/wk 1–6 servings/wk ≥1 servings/day Continuous: servings/wk | 3.0 ± 0.5 | Longitudinal change (annual percentage of BMD change): aBMD, DXA Volumetric BMD and microstructure variables, for total bone, cortical, and trabecular compartments: HR-pQCT | Energy, calcium, and protein intakes (only fermented dairy) | There was no significant correlation between annual change (%) in aBMD (total hip and spine) and dairy intake (servings/week) Significant correlation between annual change (%) in aBMD (radius) and milk intake (servings/week)—nonadjustedr = 0.15; P < 0.001 Cortical bone (HR-pQCT) loss was attenuated at nonbearing bone sites in fermented dairy product consumers, not in milk or ripened cheese consumers, independently of total energy, calcium, and protein intakes |
| Sahni, 2017 (40) | Framingham Original Cohort Framingham Osteoporosis Study USA | 628 men and women (27% used vitamin D supplements) | 75 (69–96) | Nonvitamin D users: 26.9 ± 4.5 Vitamin D users: 25.9 ± 4.6 | Milk Yogurt Cheese Cream Milk + yogurt Milk + yogurt + cheese | FFQ (self-reported and reviewed with clinic staff) Continuous: servings/wk | 3.9 (range: 2.1–5.1) | Femur, spine, and radius BMD (DXA) Percentage BMD change | Baseline BMD, age, weight, height, sex (men, women who never used estrogen or used it formerly, and women currently using estrogen), total energy intake, calcium supplement use, and smoking | Vitamin D supplement nonusers: no association between dairy intake and percentage BMD change Vitamin D supplement users (per servings/wk β ± SE;P) Trochanter BMD: Milk: 0.2084 ± 0.101; 0.040 Milk + yogurt: 0.2127 ± 0.097; 0.030 Milk + yogurt + cheese: 0.2352 ± 0.089; 0.009 No associations with BMD at other sites or with different dairy exposure. |
| Kalkwarf, 2003 (30) | NHANES III (non-Hispanic, white women) USA | 3251 | 1371 (42.2%) aged 20–49 1880 (57.8%) aged ≥50 Exposure: milk intake during: childhood (5–12) adolescence (13–17) | 25.16 ± 0.6 aged 20–49 y 27.30 ± 0.5 aged ≥50 y | Milk | Interviewer-administered questionnaire <1 serving/wk 1–6 servings/wk 1 serving/d >1 serving/d Categorization Low intake: <1 serving/wk High intake: >1 serving/d | From exposure measurement to outcome assessment, at least 33 y | Mean BMD Total hip DXA | BMD 20–49 y: current calcium intake, age, weight, estrogen deficiency, and physical activity BMD ≥50 y: current calcium intake, age, weight, estrogen deficiency, physical activity, ever a smoker, and alcohol intake | 20–49 y: childhood milk intake and hip BMD: No association (overall P = 0.31) 20–49 y: Adolescence milk intake and hip BMD: Positive association (P ≤ 0.02) Mean BMD of consumers <1 serving/wk was ∼3% lower than those who consumed >1 serving/d 20–49 y: combined childhood and adolescence milk intake and hip BMD: 1.7% lower in women with low intake in childhood and adolescence 2.1% lower in women with high intake in childhood and low intake in adolescence Compared with women with high intake in childhood and adolescence (P ≤ 0.05) BMD ≥50 y: childhood milk intake and hip BMD: Mean BMD of consumers <1 serving/wk was 2.1% lower than those who consumed >1 serving/d (P = 0.10) BMD ≥50 y: adolescence milk intake and hip BMD: Mean BMD of consumers <1 serving/wk was 2.2% lower than those who consumed >1 serving/d. (P < 0.10) BMD ≥50 y: combined childhood and adolescence milk intake and hip BMD: No association in (i) women with low intake in childhood and adolescence; or (ii) women with high intake in childhood and low intake in adolescence, compared with women with high intake in childhood and adolescence (P = 0.98) |
1aBMD, areal bone mass density; BMD, bone mass density; DXA, dual-energy X-ray absorptiometry; FFQ, food-frequency questionnaire; HR-pQCT, high-resolution peripheral quantitative computerized tomography.
The quality scores of the selected studies are presented in Tables 3 and 4. An interclass coefficient of 0.81 (P = 0.008) for the NOS score was obtained when the global interobserver reliability was evaluated.
TABLE 3.
Quality score: Newcastle-Ottawa quality assessment scale for cohort studies—outcome: osteoporotic fractures1
| Reference | Year | Selection: Representativeness of the exposed cohort | Selection: Selection of the non-exposed cohort | Selection: Ascertainment of exposure | Selection: Demonstration that outcome of interest was not present at start of study | Comparability: Comparability of cohorts on the basis of the design or analysis | Outcome: Assessment of outcome | Outcome: Was follow-up long enough for outcomes to occur | Outcome: Adequacy of follow-up of cohorts | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Kalkwarf (30) | 2003 | ★ | ★ | ★ | — | — | — | ★ | — | ★★★★ (4) |
| Roy (38) | 2003 | ★ | ★ | ★ | — | — | ★ | ★ | ★ | ★★★★★★ (6) |
| Nevitt (35) | 2005 | ★ | ★ | — | ★ | ★ | ★ | ★ | ★ | ★★★★★★★ (7) |
| Benetou (29) | 2011 | — | ★ | — | — | ★★ | — | ★ | — | ★★★★ (4) |
| Sahni (27) | 2013 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | — | ★★★★★★★ (7) |
| Feart (34) | 2013 | ★ | ★ | ★ | — | ★★ | — | ★ | ★ | ★★★★★★★ (7) |
| Feskanich (36) | 2014 | — | ★ | — | — | ★★ | — | ★ | ★ | ★★★★★ (5) |
| Michaëlsson (33) | 2014 | ★ | ★ | — | ★ | ★★ | ★ | ★ | — | ★★★★★★★ (7) |
| Sahni (28) | 2014 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | — | ★★★★★★★ (7) |
| Feskanich (37) | 2018 | — | ★ | — | — | ★★ | — | ★ | ★ | ★★★★★ (5) |
1The Newcastle-Ottawa Scale (NOS) identifies high-quality choices with a star. A maximum of 1 star is applied for each item within the Selection and Outcome categories, and a maximum of 2 stars for Comparability. The NOS assigns quality scores up to 9 points. All the publications included in the osteoporotic fractures review were of acceptable quality, with a median NOS score of 5.9 out of 9 (range: 4–7).
TABLE 4.
Quality score: Newcastle-Ottawa quality assessment scale for cohort studies—outcome: bone mass density1
| Reference | Year | Selection: Representativeness of the exposed cohort | Selection: Selection of the non-exposed cohort | Selection: Ascertainment of exposure | Selection: Demonstration that outcome of interest was not present at start of study | Comparability: Comparability of cohorts on the basis of the design or analysis | Outcome: Assessment of outcome | Outcome: Was follow-up long enough for outcomes to occur | Outcome: Adequacy of follow-up of cohorts | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Kalkwarf (30) | 2003 | ★ | ★ | ★ | — | ★ | ★ | ★ | — | ★★★★★★ (6) |
| Sahni (40) | 2017 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | — | ★★★★★★★ (7) |
| Biver (39) | 2018 | ★ | ★ | ★ | ★ | — | ★ | ★ | — | ★★★★★★ (6) |
1The Newcastle-Ottawa Scale (NOS) identifies high-quality choices with a star. A maximum of 1 star is applied for each item within the Selection and Outcome categories, and a maximum of 2 stars for Comparability. The NOS assigns quality scores up to 9 points. All the publications included in the bone mass density review were of acceptable quality, with a median NOS score of 6.3 out of 9 (range: 6–7).
Dairy intake and risk of osteoporotic fracture at any site
Three studies assessed the risk of OF at any site (22,944 cases) in terms of baseline dairy product intake. Michaëlsson et al. (33) stratified the results by gender and by dairy type (milk, cheese, and yogurt). In men, no association was observed between dairy consumption and OF risk when the highest level of intake was compared with the lowest level of intake. In women, the corresponding HRs were 1.16 (95% CI: 1.08, 1.25), 0.85 (95% CI: 0.81, 0.9), and 0.9 (95% CI: 0.82, 0.99), with a higher risk of fracture associated with greater milk exposure, and a lower risk associated with higher cheese and yogurt intake. Kalkwarf et al. (30) studied the odds of OF in women >50 y of age based on milk consumption during childhood and adolescence. The OR of OF when comparing the highest with the lowest intakes of milk was 0.84 (95% CI: 0.59, 1.2). In the Feart et al. (34) cohort with men and women, the HRs for OF at the highest level of consumption compared with the lowest level of consumption were 0.66 (95% CI: 0.47, 0.93), 0.78 (95% CI: 0.55, 1.09), 0.91 (95% CI: 0.65–1.27), and 0.88 (95% CI: 0.62, 1.23) for any dairy type, cheese, milk, and yogurt, respectively.
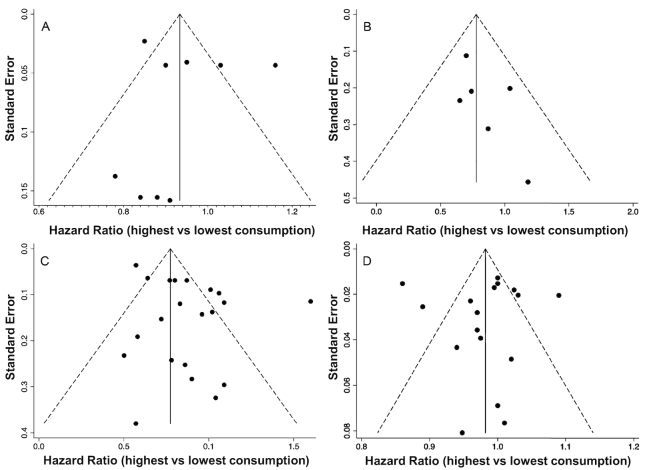
The random-effects meta-analysis (n = 109,134) did not show any association between total dairy intake and OF at any site, with high heterogeneity (pooled mean risk: 0.95; 95% CI: 0.87, 1.03; I2 = 82.9%; P-heterogeneity < 0.001). For cheese and yogurt, a risk reduction of OF at any site was observed in the pooled analysis, with moderate heterogeneity for cheese (pooled mean risk: 0.89; 95% CI: 0.81, 0.98; I2 = 59%; P-heterogeneity = 0.087) and no heterogeneity for yogurt (pooled mean risk: 0.92; 95% CI: 0.87, 0.9); I2 = 0.0%; P-heterogeneity = 0.437). For milk, the relationship with OF risk was not significant (pooled mean risk: 1.05; 95% CI: 0.94, 1.18; I2 = 61.3%; P-heterogeneity = 0.051) (Figure 2). The funnel plot showed asymmetry (Egger's test P = 0.099), indicating publication bias (Figure 3A).
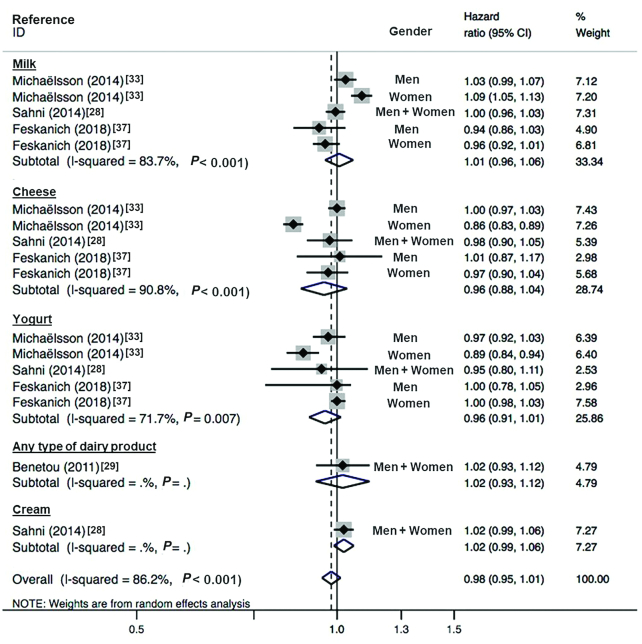
FIGURE 2.
Forest plot showing pooled HR (95% CI) of incident fractures at any site related to highest compared with lowest dairy intake (n = 109,134; 3 cohort studies) and stratified by the type of dairy. With the use of a random-effects model, the overall HR was estimated, with an I2 statistic of 82.9%. The HRs after stratification for dairy type are shown.
FIGURE 3.
Funnel plots of dairy consumption and osteoporotic fracture risk. (A) Highest compared with lowest dairy intake and osteoporotic fracture risk at any site. (B) Highest compared with lowest dairy intake and vertebral fracture risk. (C) Highest compared with lowest dairy intake and hip fracture risk. (D) Risk of hip fracture by each increment in dairy intake.
Michaëlsson et al. (33) described a dose-response relationship for women when the exposure was reported for each increment in cheese and yogurt intake (HR: 0.85; 95% CI: 0.81, 0.90 and HR: 0.90; 95% CI: 0.82, 0.99, respectively) and for each increment in milk consumption (HR: 1.16; 95% CI: 1.08, 1.25). No meta-analysis could be conducted to assess a dose increment association between dairy consumption and risk of OF at any site.
Dairy intake and risk of hip fracture
Seven of the selected studies explored the association between the intake of dairy products and the risk of HF (10,445 cases); 3 of the studies were from Europe (29, 33, 34) and 4 were from North America (27, 28, 36, 37). Michaëlsson et al. (33) described a diminished risk associated with cheese and yogurt consumption for men and women (HR: 0.80; 95% CI: 0.68, 0.95; HR: 0.77; 95% CI: 0.65, 0.92; HR: 0.57; 95% CI: 0.50, 0.64; and HR: 0.64; 95% CI: 0.53, 0.78). Although no association was found between milk and HF in men, a higher risk of HF was shown in women (HR: 1.60; 95% CI: 1.39, 1.84). In the Framingham Original Cohort (28), no relationship was described between milk, cheese, yogurt, and cream consumption and the risk of HF, with a tendency towards a lower risk for the highest level of milk intake (HR: 0.58; 95% CI: 0.31, 1.06). The data from the Framingham Offspring Cohort (27) showed a potential statistical association between HF and milk and yogurt ingestion with a tendency towards a diminished risk for both milk and yogurt (HR: 0.50; 95% CI: 0.22, 1.13 and HR: 0.57; 95% CI: 0.19, 1.68, respectively). The study by Feskanich et al. (37) did not show a relationship with the risk for HF when the exposure (total dairy, milk, cheese, and yogurt) was assessed at baseline. When dairy exposure was analyzed as cumulative intake over time, a risk reduction associated with only the highest milk ingestion was observed in women (HR: 0.77; 95% CI: 0.64, 0.94) (data not shown in the pooled analysis). In 2014, Feskanich et al. (36) researched the association between teenage intake of milk and cheese and HF outcome. A link between dairy exposure in youth and adulthood HF could not be established (HR: 1.02; 95% CI: 0.93, 1.12). As this study could contain repeated samples from the cohorts included in the paper published by the same author in 2018, the data were not included in the pooled analysis. In the cohort of Feart et al. (34), a relationship between total dairy, yogurt, milk, and cheese was not found.
In the pooled analysis (5 studies; n = 236,136) comparing the highest with the lowest levels of dairy intake, the random-effects meta-analysis showed a tendency towards a diminished risk but without significance and with high heterogeneity (0.87; 95% CI: 0.75, 1.01; I2 = 86.7%, P-heterogeneity < 0.001) (Figure 4). In the stratified analysis by type of dairy (cheese, milk, and yogurt), an association with HF risk could not be defined, and the interstudy heterogeneity was moderate or high. The meta-regression analysis did not detect any influence of the studied variables in the estimation of the effect size. The funnel plot also revealed asymmetry, although Egger's test showed a P value of 0.463 (Figure 3C).
FIGURE 4.
Forest plot showing pooled HR (95% CI) of incident hip fractures related to highest compared with lowest dairy intake (n = 236,136; 5 cohort studies) and stratified by the type of dairy. With the use of a random-effects model, the overall HR was estimated, with an I2 statistic of 86.7%. The HRs after stratification for dairy type are shown.
Regarding the association of the increments of dairy intake with HF risk, Benetou et al. (29) reported no association between HF and each quintile increment in total dairy intake (HR: 1.02; 95% CI: 0.93, 1.12). In the Michaëlsson et al. (33) cohorts, no relation with HF risk was observed for each increment in dairy exposure in men. However, in women, each increment in milk intake was related to a higher risk of HF (HR: 1.09; 95% CI 1.05, 1.13), and each increment in cheese and yogurt was related to a lower risk of HF (HR: 0.86; 95% CI: 0.83, 0.89 and HR: 0.89; 95% CI: 0.84, 0.94). Sahni et al. (28) did not observe an association between HF risk and each increment in servings/week of milk, yogurt, cheese, cream, and milk + yogurt. In the study by Feskanich et al. (37), when total dairy was combined in men and women, a lower risk of HF was observed for each increment of 1 serving/d (HR: 0.97: 95% CI: 0.95, 0.99 concerning intake at baseline, and HR: 0.94; 95% CI: 0.90, 0.98 regarding cumulative consumption). For each increment in cumulative milk intake, a decreased HF risk was described in women (HR: 0.92; 95% CI: 0.86, 0.98) and in both genders (HR: 0.92; 95% CI: 0.87, 0.97). When these increments were analyzed in teenagers, no association with HF risk was observed (36).
For each increment in dairy consumption, the pooled analysis (4 studies; n = 231,442) showed a tendency towards a lower risk of HF, which was not significant and had high interstudy heterogeneity (0.98; 95% CI: 0.95, 1.01; I2 = 86.2%; P-heterogeneity < 0.001) (Figure 5). The associations were not significant when yogurt and cheese were evaluated. In the funnel plot, some asymmetry could be observed; however, the P value of the Egger's test was 0.982 (Figure 3D).
FIGURE 5.
Forest plot showing pooled HR (95% CI) of incident hip fractures related to an increment in dairy intake (n = 231,442; 4 cohort studies) and stratified by the type of dairy. With the use of a random-effects model, the overall HR was estimated, with an I2 statistic of 86.2%. The HRs after stratification for dairy type are shown.
Dairy intake and risk of vertebral fracture
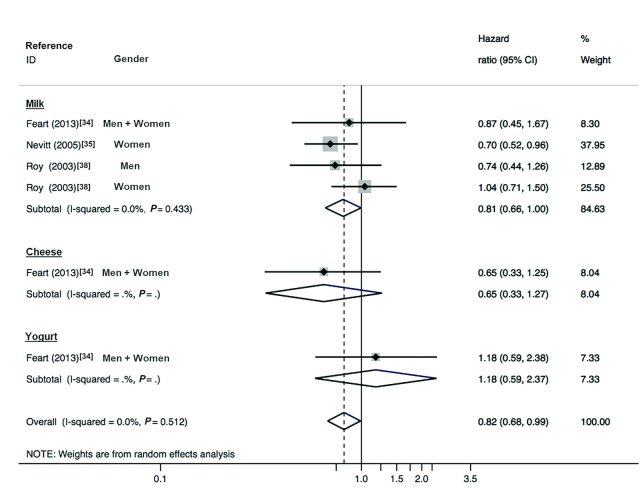
Three studies (267 cases) reported data relating dairy consumption with VF risk. The study by Feart et. al (34) did not show a significant association between total dairy, milk, cheese, and yogurt exposure, and the incidence of VF in men and women, but the results showed a tendency towards a lower risk for all dairy types except for yogurt (HRs: 0.65, 0.87, 0.65, and 1.18 for total dairy, milk, cheese, and yogurt, respectively). Nevitt et al. (35) described a risk reduction associated with the highest level of milk intake (HR: 0.70; 95% CI: 0.52, 0.96) in women; however, Roy et al. (38) did not find a relationship between exposure (measured in childhood, adolescence, and early and late adulthood) and VF risk in men and women. In this study, and for homogeneity purposes, only the data relative to the last exposure were introduced into the pooled analysis.
The random-effects meta-analysis (n = 11,893) showed, with no heterogeneity, a risk reduction in the incidence of VF linked to a higher intake of dairy products (pooled risk: 0.82; 95% CI: 0.68, 0.99; I2 = 0.0%; P-heterogeneity = 0.512) (Figure 6). The funnel plot did not show asymmetry (Egger's test P = 0.068) (Figure 3B).
FIGURE 6.
Forest plot showing pooled HR (95% CI) of incident vertebral fractures related to highest compared with lowest dairy intake (n = 11,893; 3 cohort studies) and stratified by the type of dairy. With the use of a random-effects model, the overall HR was estimated, with an I2 statistic of 0.0%. The HRs after stratification for dairy type are shown.
Dairy intake and risk of wrist fracture
Only 1 study (34) assessed the relationship between dairy consumption and WF (73 cases) in men and women. The highest level of total dairy and yogurt intake was associated with a lower risk of WF (HR: 0.49; 95% CI: 0.29, 0.82 and HR: 0.51: 95% CI: 0.31, 0.82, respectively). An association with milk and cheese ingestion could not be demonstrated.
Dairy intake and BMD changes
The data reported were heterogeneous [annual percentage of change in BMD (39), percentage of BMD change over time (40), and mean percentage difference among groups of consumers (26)], and thus a meta-analysis was not possible.
Kalkwarf et al. (30) described the differences in total hip BMD at 20–40 and ≥50 y of age in non-Hispanic white women included in the Third National Health and Nutrition Examination Survey (n = 3251). Milk intake was retrospectively measured based on childhood (5–12 y) and adolescence (13–17 y) milk consumption. For the comparison of milk consumption and BMD, 2 levels of exposure were considered: low intake (<1 serving/wk) and high intake (>1 serving/d). In the youngest women, there was no difference in BMD related to childhood milk consumption, but BMD was ∼3% lower in those whose intakes were <1 serving/d during adolescence (P < 0.02). Combining the reported intakes from childhood and adolescence, the women with a low consumption during the 2 periods combined and those with a low intake during adolescence and a high intake during childhood had a BMD 1.7% and 2.1% lower, respectively, when compared with women with a high intake of milk during both childhood and adolescence (P ≤ 0.05). In women >50 y of age, BMD was 2.1% (P = 0.10) and 2.2% lower in those with a low intake of milk during childhood and adolescence, respectively, but there was no association among groups when the milk consumptions during childhood and adolescence were combined.
In the Framingham Osteoporosis Study cohort (40), a positive relationship was described between the baseline consumption of milk and the combined consumption of milk + yogurt and milk + yogurt + cheese and the percentage of change in trochanter BMD after 4 y of follow-up in patients who supplemented with vitamin D [β coefficients (per 1 serving/wk) ± SE: 0.2084 ± 0.101, P = 0.040; 0.2127 ± 0.097, P = 0.030; 0.2352 ± 0.089, P = 0.009), but no relationship was observed in patients who did not supplement. The models were adjusted for baseline BMD, age, weight, height, sex (men, women who never used estrogen or used it previously, and women currently using estrogen), total energy intake, calcium supplement use, and smoking. It was not possible to find any other association at different sites of BMD (femoral neck and lumbar spine), even after stratification for vitamin D supplementation.
The most recent study on this topic (39) explored the correlation between dairy consumption (milk, ripened cheese, and fermented dairy assessed by the mean of 2 FFQ assessments during 3 y of follow-up) and the annual percentage of change in BMD in European women >65 y old. There was no association between the intakes of fermented dairy and cheese and the change in BMD, but a positive correlation was described between milk consumption and change in BMD at the radius site (r = 0.15; P < 0.001; not adjusted).
Discussion
This systematic review and meta-analysis showed a tendency towards a 5% and 13% risk reduction in incident total OF and HF, respectively, and a significant 18% lower risk of VF associated with the highest level of dairy intake. The dose increment analysis did not show a clear relationship between HF and each unit increase in dairy intake. Concerning BMD change as measured with DXA, the results were scarce and heterogeneous, and a meta-analysis could not be conducted. One study observed a 1.7–3% lower hip BMD in young and postmenopausal women with lower milk intake during childhood and adolescence. A second paper described a positive relationship between baseline milk consumption and milk combined with yogurt or cheese and the percentage of trochanter BMD change, but only in vitamin D–supplemented men and women. The last study showed a positive correlation when the baseline milk intake and BMD at the radius were studied in women aged >65 y, but no relationship could be described when fermented dairy or cheese were analyzed.
Dairy and fracture risk
When considering the OF at any site and milk consumption, the results of our study are in accordance with those of another meta-analysis published in 2005 based on 3 cohorts in Europe, Australia, and Canada (18). The authors did not find a clear relationship between milk intake and the studied event in women or men (RR for low milk intake: 1.09 and 1.11, respectively, without significance), and although a higher risk of OF was observed in people >80 y of age when milk consumption was low, the significant difference was lost when adjusting for BMD. In our meta-analysis, no included study adjusted for BMD [except Nevitt et al. (35)], and an effect attenuation could have been observed if that variable had been involved in the estimated HR. In the meta-analysis by Kanis et al. (18), the fact that other calcium sources (yogurt and cheese, among others) were not accounted for in the dietary questionnaire was briefly discussed. Indeed, we did observe a differential effect from cheese and yogurt, in which the highest levels of intake were significantly associated with an 11% and 8% lower risk of OF, respectively. Given the different effect size estimates of milk, cheese, and yogurt, the pooled analysis for total dairy did not reach significance. The total heterogeneity of the meta-analysis was high, but when the subgroups of dairy products were analyzed, some differences between studies disappeared (yogurt) or were reduced (cheese). The visual inspection of the funnel plot permitted us to ascertain that the women's cohort data from Michaëlsson et al. (33) were the cause of potential bias. This did not seem to be related to publication, citation, or poor methodologic analysis (which obtained good-quality scores on the NOS). A possible explanation was the existence of differences in underlying risk or exposure in this specific population from Northern Europe. As the number of studies in the meta-analysis was low, a meta-regression analysis could not be conducted.
When we studied the relationship between dairy intake and HF risk, a firm conclusion could not be drawn, but a tendency towards a lower risk when comparing the highest with the lowest intake levels was described, without differences after stratifying by type of dairy. Two previous meta-analyses addressed the same question and obtained comparable results. Kanis et al. (18) observed a nonsignificant higher risk of HF associated with a low consumption of milk in all age ranges, as did Bian et al. (20) in their pooled analysis of cohort studies. In fact, their global HR for levels of milk intake was identical to ours. However, when the data were obtained from case-control designs, a significant 29% risk reduction could be reported. This is the only previous meta-analysis to have considered the effect of different dairy products on HF risk. Considering cohort studies, the authors found a diminished risk of HF when comparing high and low cheese (32% lower) and yogurt (25% lower) consumption. Although we described a risk reduction of 20% and 13%, respectively, for cheese and yogurt, our results did not reach significance. The heterogeneity was high when all studies were combined and milk intake was evaluated as the exposure (20), but in our work, the heterogeneity was moderate or high in all the strata. When we compare the cohort data from Bian et al. (20) with our data, it is prudent to discuss some issues. We did not include papers published before 2000 in order to explore the most recent evidence about the topic, assuming an improvement in methodologic quality in research over time. Second, we excluded cohort studies not ethnically related to Caucasians, with the purpose of making the assumptions linked to genetic, cultural, social, and behavioral factors in assessing fracture risk uniform (2, 41). Lastly, we updated the information with a recent cohort from Europe (37) with a weight similar to that of the study by Michaëlsson et al. (33), although the recent study used a more conservative approach when reporting the highest levels of exposure [e.g., ≥2 servings/d (37) versus ≥3 servings/d (33) when milk ingestion was evaluated] and, to some degree, demonstrated diverging estimated HRs for each dairy exposure.
Another important point is that, in the subgroup analysis, Bian et al. (20) did not describe any differences in the pooled estimate of RRs of HF from milk exposure when they stratified by several adjusted variables. Nevertheless, when the total energy intake was considered, a significant 31% risk reduction was observed in nonadjusted studies, whereas no association could be described in those that were adjusted. All the studies included in our meta-analysis used total dietary kilocalories as a covariate in the multivariate Cox models, so we could not evaluate this term. Our meta-regression analysis did not show any difference when the other prespecified variables were studied.
Our search strategy did not find case-control studies, as some of them were published before 2000 or, if not, the studied population was not of European or North American origin (non-Hispanic whites) (42–46).
After exploring the funnel plot, a clear asymmetry was verified, although Egger's test was not significant. The cause of this estimated bias was, again, the study of Michaëlsson et al. (33), particularly from the data of women and milk exposure, whose HRs were >1.5. In addition, the HRs related to cheese and yogurt consumption, from the women in the same study, deviated to the left. As the same author reported, this bias could be linked to the different exposure ranges used in this article in comparison with the rest of the studies (47).
The global estimated HR for HF associated with each increment in dairy intake (milk, cheese, or yogurt) was not significant in our study. Neither of the 2 previously published meta-analyses could demonstrate a dose-response relationship between milk intake and HF risk (19, 20). In the analysis of bias, the asymmetry worked in the same way that it did in the pooled HF HR estimation when exposure was measured as the highest compared with the lowest levels of intake.
The pooled risk of a VF was reduced by 18% in our meta-analysis, and this estimate was significant. Despite the small number of papers and the found precision, the funnel plot did not show asymmetry, and a publication bias could be rejected. To our knowledge, this is the first article describing such a pooled relationship between dairy and VF risk.
Dairy and BMD
Through our search strategy (prospective studies published after the year 2000), we did not find many studies exploring whether a higher dairy intake is related to time-dependent changes in BMD measured by DXA. We chose this outcome assessment because it is the most used in the clinical setting and is a validated tool for osteoporosis diagnoses (24). In this way, DXA changes can be interpreted from a physician's perspective. Although the information shown in the 3 papers is not comparable, given the different ages at exposure, outcome assessments, and ways of estimating the main effect [correlations (39), adjusted β coefficients (40) and mean percentage of differences between groups (30)], they describe, in women, a positive association between the consumption of milk during infancy and adolescence and hip BMD in adulthood (30) and between the intake of milk after 65 y of age and BMD change at the radius (39). In men and women, the same positive association was found between dairy intake and trochanter BMD in vitamin D–supplemented participants but not in the nonsupplemented participants (40). Although recent research has questioned the efficacy of calcium and vitamin D supplementation in the prevention of fractures in the elderly (48), these interactions with diet have to be taken into account.
Other studies have contributed to the research on BMD progression related to dairy consumption. In the prospective cohort of the Canadian Multicentre Osteoporosis Study (n = 6510) (49), a higher percentage of total energy intake in the form of dairy protein attenuated bone loss at the hip in postmenopausal women. In addition, in women aged >50 y, a negative relationship was described between percentages of calories ingested from plant-derived proteins and changes in spine BMD. Nevertheless, there was no description of dairy servings, so we could not include this study in our systematic review.
Most of our previous knowledge about dairy products and BMD comes from cross-sectional studies (21, 27), and based on these studies, a positive association has been established. However, different relationships have been described relating BMD sites and types of dairy. Currently, the study of dietary patterns has reached the field of bone health. From a prospective perspective, some healthy and nutrient-dense dietary patterns containing low-fat dairy have been associated with improved evolution of BMD at different sites (50–52).
A recent randomized controlled trial meta-analysis (23) described an improvement in BMD associated with higher intakes of calcium from the diet 1 and 2 y after the intervention. The authors conclude that the effect of dietary calcium or calcium supplements on BMD is modest (1–2% increase after 1–2 y) and not cumulative over time. They speculate that this percentage of change is unlikely to prevent an OF. However, if the yearly aging-associated decline in BMD has been estimated at 1–2% (53), this effect could be relevant. In fact, a 1% improvement in spine bone mineral density was associated with a 0.03 decrease in the RR of vertebral fracture in patients taking antiresorptive drugs (54), but the mean increase in BMD after treatment with the modern drugs to prevent fractures is higher than that of dairy (55).
Recently, novel techniques have begun to be used in research to ascertain other properties linked to bone health. Quantitative computed tomography (QCT) permits the assessment of trabecular bone separately from the cortical portion of bone, and allows researchers to study the microarchitecture of cancellous bone in vivo (56). A study by Biver et al. (39) describes, in detail, other relevant correlations between dairy and bone microstructure, as assessed by high-resolution peripheral QCT (HR-pQCT). Another recent Canadian paper (57) focused on the role of the intake of milk and milk alternatives during childhood and adolescence in bone structure in early adulthood in men and women (n = 116). In women, after adjustment, a higher intake of milk and milk alternatives (3.8 compared with 1.3 servings/d) was positively associated with total bone area, cortical area, and cortical content in radius shaft, but no relationship was found with trabecular bone, bone strength in torsion, or the structure of the tibia. Nevertheless, the sample size was probably too small to obtain enough statistical power. Cross-sectional studies have also described such a positive association between dairy and the microarchitecture of bone (58).
Proposed links between dairy, bone health, and fractures
Some nutrients contained in dairy foods play a leading role in bone health. Studies that have assessed the relationship between protein in the diet and the BMD (49) or the risk of fracture (59) have described a differential effect of plant, animal, and dairy proteins. Secondary effects of milk on the endogenous insulin-like growth factor system have been reported (60). Classic arguments regarding the calcium, phosphorous, and vitamin D contents of dairy products are subject to debate (61). However, more recent knowledge has enhanced the role of not only nutrient content but also dairy type in bone health. In this way, vitamin K from cheese and fermented dairy acts as a cofactor for posttranslational conversion of glutamyl to γ-carboxyglutamyl residues in osteocalcin, leading to the binding of calcium to bone proteins and enabling the mineralization process (62). The effects of fermented milk are of relevance as a source of prebiotics and probiotics that modify the gut microbiota composition. An increase in calcium gut absorption and an attenuation of sex hormone deficiency–induced bone loss have been linked to yogurt and fermented milk (39), explaining some differences in the OF risk estimation that we found in our meta-analysis when stratification by dairy type was performed. Furthermore, some foods have been related to a healthier dietary pattern. At this point, yogurt and fermented milk consumption has been associated not only with nutrient-dense diets but with healthy behaviors (63).
Nonetheless, it is necessary to point out that the variability in BMD and OF risk is only partially explained by dietary factors. With aging, sex steroid deficiency, fattening of the bone marrow, frailty, and risk of falls may play a greater role in OF risk than diet (64). Moreover, genetic factors seem to explain ∼60% of the variance in peak bone mass. One of the studied genes is the vitamin D receptor gene promoter (VDRp), which presents with diverse genotypes across populations. The polymorphism at the –1012 loci has been implicated in different responses of bone mass accrual to the dairy ingested during adolescence, with genotypes associated with better bone health in postmenarcheal girls in relation to milk and nonmilk dairy and genotypes showing no change in bone health or bone markers associated with dairy intake (30% of the European population) (41). In the papers included in this review, there are no data related to specific genotyping, preventing the use of a stratified analysis in this regard.
Strengths and limitations
Our study addressed the association between OF and dairy intake in a population of Caucasian origin, stratifying by outcome site (any OF, HF, VF, and WF) and dairy type. Moreover, to our knowledge, this is the first report estimating the VF pooled risk related to dairy consumption. We updated the evidence, adding new information from large cohorts not previously included in a meta-analysis and accounted for the best-quality research on this topic. Longitudinal data of BMD were systematically searched and summarized.
Notwithstanding, several limitations must be mentioned. We limited our study to the non-Hispanic white population living in Europe and North America. In these geographic areas, the ingested dietary calcium is higher than in other countries from the Asia-Pacific region, South America, the Far East, and North Africa (65), and it is possible to hypothesize that the effect of dairy on bone health could be greater in these regions. In a cohort from China (65), a positive association was shown between high milk consumption and hip BMD progression over time. A case-control study from Taiwan (42) reported a higher risk of HF in patients with low milk intake, and case-control data from the Fourth Korea National Health and Nutrition Examination Survey (43) revealed increased odds of osteoporosis in participants consuming no milk or milk products. In Iranian and Indian women, a regular intake of milk was protective against osteoporosis (44), and other case-control studies have reported a similar positive relation in those geographic areas (45, 46). Similarly, consumption in a state of deficiency appears to be more effective than augmentation above a normal intake. Therefore, examining the relation between dairy and bone in other ethnic populations will be important in future studies. Another consequence of this restricted search was the low number of studies analyzed, which precluded forming more subgroups and conducting sensitivity and meta-regression analyses. Some original data could not be received from the contacted authors, and difficulties were found in the standardization of the exposure measurements. Overall, the conclusions are limited to the investigators’ purposes in the studied population, with most of the participants not meeting the public recommendations for dairy intake, making it difficult to observe different levels of intake among groups. In this regard, the exposition assessment could be a source of bias. Most included studies used FFQs. This method of assessment is cost effective in epidemiologic cohort studies, but the estimate of the portions ingested can be limited by the type of form used (semiquantitative or quantitative), and this term is not explained in all the papers. Also, the way the FFQ is administered changes the precision of the measurement: interviewed subjects (27–30, 34, 35, 38–40) yield higher-quality data than from those who self-report (29, 33, 36, 37). A combination of methods, such as the FFQ with 24-h recall, can produce more accurate data about diet (34). On the other hand, those studies with retrospective assessment of the exposure (during teenage, pregnancy, or childhood) are exposed to recall errors (30, 35, 36, 38). Other potential confounders have probably not been considered in the meta-analysis; for example, an adjustment for comorbidity was made only in the Michaëlsson et al. (33) cohort. Recently, a nested substudy of the PREDIMED trial (66) associated diets with a high glycemic index and high glycemic load with a greater risk of OF. As dairy products are a versatile component of the human diet, the way in which these foods are consumed could condition the effects observed. In this way, the interaction exerted by added sugars has not been discussed in the papers included in this study.
In conclusion, this systematic review and meta-analysis could not confirm an association between the risk for OF at any site or HF and the intake of dairy products in non-Hispanic whites from Europe and North America, showing a moderate to high heterogeneity in the pooled analysis. However, a clear relation was shown when the evaluated outcome was VF, as a significant 18% risk reduction was reported. Although this meta-analysis did not show an association between risk of OF and intake of dairy products as a whole, it did show that yogurt and cheese may affect OF risk. The systematic review of BMD change over time pointed to a lower value linked with a lower dairy consumption, but the results were confined to some bone sites, and more studies are needed.
Further research in bone health must consider the fat content of dairy, the added sugar, and the ways in which these foods are integrated into dietary habits, as well as the genetic factors acting as effect modifiers in the relation between dairy and bone response.
Acknowledgments
We thank Ángel Gil Hernández, Natalia Pérez Ferre, Clara Marcuello Foncillas, María Gemma Hernández Núñez, and Ane Azcutia for their support during the manuscript preparation.
All authors have read and approved the final manuscript.
Notes
This supplement was sponsored by the Interprofessional Dairy Organization (INLAC), Spain. The sponsor had no role in the design of the studies included in the supplement; in the collection, analyses, or interpretation of the data; in the writing of the manuscripts; and in the decision to publish the results. Publication costs for this supplement were defrayed in part by the payment of page charges. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
This study was partially supported by grants from GenObIA-CM with reference (S2017/BMD-3773), financed by the Comunidad de Madrid and cofinanced with Structural Funds of the European Union; from Instituto de Salud Carlos III supported with funds from the Spanish Ministry of Health and FEDER (PI17/1732); and from Fundación de Investigación en Nutrición y Metabolismo (FINUMET).
Author disclosures: PM-M, MT-E, AL-S, CF-P, FC-T, and MÁR-H, no conflicts of interest.
Abbreviations used: aBMD, areal bone mass density; BMD, bone mass density; DXA, dual-energy X-ray absorptiometry; FFQ, food-frequency questionnaire; HF, hip fracture; HR-pQCT, high-resolution peripheral quantitative computerized tomography; NOS, Newcastle-Ottawa Scale; OF, osteoporotic fracture; QCT, quantitative computerized tomography; VF, vertebral fracture; WF, wrist fracture.
References
- 1. Wade SW, Strader C, Fitzpatrick LA, Anthony MS, O'Malley CD. Estimating prevalence of osteoporosis: examples from industrialized countries. Arch Osteoporos. 2014;9(1):182. [DOI] [PubMed] [Google Scholar]
- 2. Cauley JA, Chalhoub D, Kassem AM, Fuleihan GE-H. Geographic and ethnic disparities in osteoporotic fractures. Nat Rev Endocrinol. 2014;10(6):338–51. [DOI] [PubMed] [Google Scholar]
- 3. El-Hajj Fuleihan G, Chakhtoura M, Cauley JA, Chamoun N. Worldwide fracture prediction. J Clin Densitom. 2017;20(3):397–424. [DOI] [PubMed] [Google Scholar]
- 4. Si L, Winzenberg TM, Palmer AJ. A systematic review of models used in cost-effectiveness analyses of preventing osteoporotic fractures. Osteoporos Int. 2014;25(1):51–60. [DOI] [PubMed] [Google Scholar]
- 5. Rizzoli R, Bianchi ML, Garabédian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46(2):294–305. [DOI] [PubMed] [Google Scholar]
- 6. Brennan-Olsen SL, Vogrin S, Leslie WD, Kinsella R, Toombs M, Duque G, Hosking SM, Holloway KL, Doolan BJ, Williams LJ et al.. Fractures in indigenous compared to non-indigenous populations: a systematic review of rates and aetiology. Bone Reports. 2017;6:145–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghodsi M, Larijani B, Keshtkar AA, Nasli-Esfahani E, Alatab S, Mohajeri-Tehrani MR. Mechanisms involved in altered bone metabolism in diabetes: a narrative review. J Diabetes Metab Disord. 2016;15(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Okazaki R, Watanabe R, Inoue D. Osteoporosis associated with chronic obstructive pulmonary disease. J Bone Metab. 2016;23(3):111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldring SR. Inflammatory signaling induced bone loss. Bone. 2015;80:143–9. [DOI] [PubMed] [Google Scholar]
- 10. Li G, Thabane L, Papaioannou A, Ioannidis G, Levine MAH, Adachi JD. An overview of osteoporosis and frailty in the elderly. BMC Musculoskelet Disord. 2017;18(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bonjour J-P, Kraenzlin M, Levasseur R, Warren M, Whiting S. Dairy in adulthood: from foods to nutrient interactions on bone and skeletal muscle health. J Am Coll Nutr. 2013;32(4):251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Byberg L, Bellavia A, Larsson SC, Orsini N, Wolk A, Michaëlsson K. Mediterranean diet and hip fracture in Swedish men and women. J Bone Miner Res. 2016;31(12):2098–105. [DOI] [PubMed] [Google Scholar]
- 13. de Jonge E Al, Kiefte-de Jong JC, Hofman A, Uitterlinden AG, Kieboom BC, Voortman T, Franco OH, Rivadeneira F. Dietary patterns explaining differences in bone mineral density and hip structure in the elderly: the Rotterdam Study. Am J Clin Nutr. 2017;105(1):203–11. [DOI] [PubMed] [Google Scholar]
- 14. Langsetmo L, Hanley DA, Prior JC, Barr SI, Anastassiades T, Towheed T, Goltzman D, Morin S, Poliquin S, Kreiger N et al.. Dietary patterns and incident low-trauma fractures in postmenopausal women and men aged ≥50 y: a population-based cohort study. Am J Clin Nutr. 2011;93(1):192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dror DK, Allen LH. Dairy product intake in children and adolescents in developed countries: trends, nutritional contribution, and a review of association with health outcomes. Nutr Rev. 2014;72(2):68–81. [DOI] [PubMed] [Google Scholar]
- 16. Canadian Dairy Information Centre. Government of Canada. [Internet]. Available from: http://www.dairyinfo.gc.ca (accessed 7 July, 2018). [Google Scholar]
- 17. McCarthy KS, Parker M, Ameerally A, Drake SL, Drake MA. Drivers of choice for fluid milk versus plant-based alternatives: what are consumer perceptions of fluid milk?, J Dairy Sci. 2017;100(08):6125–38. [DOI] [PubMed] [Google Scholar]
- 18. Kanis JA, Johansson H, Oden A, De Laet C, Johnell O, Eisman JA, McCloskey E, Mellstrom D, Pols H, Reeve J et al.. A meta-analysis of milk intake and fracture risk: low utility for case finding. Osteoporos Int. 2005;16(7):799–804. [DOI] [PubMed] [Google Scholar]
- 19. Bischoff-Ferrari HA, Dawson-Hughes B, Baron JA, Kanis JA, Orav EJ, Staehelin HB, Kiel DP, Burckhardt P, Henschkowski J, Spiegelman D et al.. Milk intake and risk of hip fracture in men and women: a meta-analysis of prospective cohort studies. J Bone Miner Res. 2011;26(4):833–9. [DOI] [PubMed] [Google Scholar]
- 20. Bian S, Hu J, Zhang K, Wang Y, Yu M, Ma J. Dairy product consumption and risk of hip fracture: a systematic review and meta-analysis. BMC Public Health. 2018;18(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Radavelli-Bagatini S, Zhu K, Lewis JR, Prince RL. Dairy food intake, peripheral bone structure, and muscle mass in elderly ambulatory women. J Bone Miner Res. 2014;29(7):1691–700. [DOI] [PubMed] [Google Scholar]
- 22. Laird E, Molloy AM, McNulty H, Ward M, McCarroll K, Hoey L, Hughes CF, Cunningham C, Strain JJ, Casey MC. Greater yogurt consumption is associated with increased bone mineral density and physical function in older adults. Osteoporos Int. 2017;28(08):2409–19. [DOI] [PubMed] [Google Scholar]
- 23. Tai V, Leung W, Grey A, Reid IR, Bolland MJ. Calcium intake and bone mineral density: systematic review and meta-analysis. BMJ. 2015;351:h4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW, Kemper AR, Kubik M et al.. Screening for osteoporosis to prevent fractures. JAMA. 2018;319(24):2521–31. [DOI] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. [DOI] [PubMed] [Google Scholar]
- 26. United States Department of Agriculture. USDA National Nutrient Database for Standard Reference: USDA ARS. [Internet]. Available from: https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/nutrient-data-laboratory/docs/usda-national-nutrient-database-for-standard-reference/ (accessed 7 July, 2018). [Google Scholar]
- 27. Sahni S, Tucker KL, Kiel DP, Quach L, Casey VA, Hannan MT. Milk and yogurt consumption are linked with higher bone mineral density but not with hip fracture: the Framingham Offspring Study. Arch Osteoporos. 2013;8(1–2):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sahni S, Mangano KM, Tucker KL, Kiel DP, Casey VA, Hannan MT. Protective association of milk intake on the risk of hip fracture: results from the Framingham Original Cohort. J Bone Miner Res. 2014;29(08):1756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Benetou V, Orfanos P, Zylis D, Sieri S, Contiero P, Tumino R, Giurdanella MCC, Peeters PHMHM, Linseisen J, Nieters A et al.. Diet and hip fractures among elderly Europeans in the EPIC cohort. Eur J Clin Nutr. 2011;65(1):132–9. [DOI] [PubMed] [Google Scholar]
- 30. Kalkwarf HJ, Khoury JC, Lanphear BP. Milk intake during childhood and adolescence, adult bone density, and osteoporotic fractures in US women. Am J Clin Nutr. 2003;77(1):257–65. [DOI] [PubMed] [Google Scholar]
- 31. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. Ottawa Hospital Research Institute [Internet]. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2013. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 30 June, 2018). [Google Scholar]
- 32. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Michaëlsson K, Wolk A, Langenskiöld S, Basu S, Lemming EW, Melhus H, Byberg L. Milk intake and risk of mortality and fractures in women and men: cohort studies. BMJ. 2014;349(1):g6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feart C, Lorrain S, Ginder Coupez V, Samieri C, Letenneur L, Paineau D, Barberger-Gateau P. Adherence to a Mediterranean diet and risk of fractures in French older persons. Osteoporos Int. 2013;24(12):3031–41. [DOI] [PubMed] [Google Scholar]
- 35. Nevitt MC, Cummings SR, Stone KL, Palermo L, Black DM, Bauer DC, Genant HK, Hochberg MC, Ensrud KE, Hillier TA et al.. Risk factors for a first-incident radiographic vertebral fracture in women > or = 65 years of age: the study of osteoporotic fractures. J Bone Miner Res. 2005;20(1):131–40. [DOI] [PubMed] [Google Scholar]
- 36. Feskanich D, Bischoff-Ferrari HA, Frazier AL, Willett WC. Milk consumption during teenage years and risk of hip fractures in older adults. JAMA Pediatr. 2014;168(1):54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Feskanich D, Meyer HE, Fung TT, Bischoff-Ferrari HA, Willett WC. Milk and other dairy foods and risk of hip fracture in men and women. Osteoporos Int. 2018;29(2):385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roy DK, O'Neill TW, Finn JD, Lunt M, Silman AJ, Felsenberg D, Armbrecht G, Banzer D, Benevolenskaya LI, Bhalla A et al.. Determinants of incident vertebral fracture in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int. 2003;14(1):19–26. [DOI] [PubMed] [Google Scholar]
- 39. Biver E, Durosier-Izart C, Merminod F, Chevalley T, van Rietbergen B, Ferrari SL, Rizzoli R. Fermented dairy products consumption is associated with attenuated cortical bone loss independently of total calcium, protein, and energy intakes in healthy postmenopausal women. Osteoporos Int. 2018;29(08):1771–82. [DOI] [PubMed] [Google Scholar]
- 40. Sahni S, Mangano KM, Kiel DP, Tucker KL, Hannan MT. Dairy intake is protective against bone loss in older vitamin D supplement users: the Framingham Study. J Nutr. 2017;147(4):645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Esterle L, Jehan F, Sabatier J-P, Garabedian M. Higher milk requirements for bone mineral accrual in adolescent girls bearing specific Caucasian genotypes in the VDR promoter. J Bone Miner Res. 2009;24(08):1389–97. [DOI] [PubMed] [Google Scholar]
- 42. Lan T-Y, Hou S-M, Chen C-Y, Chang W-C, Lin J, Lin C-C, Liu W-J, Shih T-F, Tai T-Y. Risk factors for hip fracture in older adults: a case-control study in Taiwan. Osteoporos Int. 2010;21(5):773–84. [DOI] [PubMed] [Google Scholar]
- 43. Kim M-H, Lee JS, Johnson MA. Poor socioeconomic and nutritional status are associated with osteoporosis in Korean postmenopausal women: data from the Fourth Korea National Health and Nutrition Examination Survey (KNHANES) 2009. J Am Coll Nutr. 2015;34(5):400–7. [DOI] [PubMed] [Google Scholar]
- 44. Keramat A, Patwardhan B, Larijani B, Chopra A, Mithal A, Chakravarty D, Adibi H, Khosravi A. The assessment of osteoporosis risk factors in Iranian women compared with Indian women. BMC Musculoskelet Disord. 2008;9(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jitapunkul S, Yuktananandana P, Parkpian V. Risk factors of hip fracture among Thai female patients. J Med Assoc Thai. 2001;84(11):1576–81. [PubMed] [Google Scholar]
- 46. Jha RM, Mithal A, Malhotra N, Brown EM. Pilot case-control investigation of risk factors for hip fractures in the urban Indian population. BMC Musculoskelet Disord. 2010;11(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Michaëlsson K, Byberg L. Interpretation of milk research results, J Dairy Sci. 2018;29:773–5. [DOI] [PubMed] [Google Scholar]
- 48. Zhao J-G, Zeng X-T, Wang J, Liu L. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults. JAMA. 2017;318(24):2466–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Langsetmo L, Barr SI, Berger C, Kreiger N, Rahme E, Adachi JD, Papaioannou A, Kaiser SM, Prior JC, Hanley DA et al.. Associations of protein intake and protein source with bone mineral density and fracture risk: a population-based cohort study. J Nutr Health Aging. 2015;19(08):861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rogers TS, Harrison S, Judd S, Orwoll ES, Marshall LM, Shannon J, Langsetmo L, Lane NE, Shikany JM, Osteoporotic Fractures in Men (MrOS) Study Research Group . Dietary patterns and longitudinal change in hip bone mineral density among older men. Osteoporos Int. 2018;29(5):1135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Langsetmo L, Poliquin S, Hanley DA, Prior JC, Barr S, Anastassiades T, Towheed T, Goltzman D, Kreiger N, CaMos Research Group . Dietary patterns in Canadian men and women ages 25 and older: relationship to demographics, body mass index, and bone mineral density. BMC Musculoskelet Disord. 2010;11(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van den Hooven EH, Ambrosini GL, Huang R-C, Mountain J, Straker L, Walsh JP, Zhu K, Oddy WH. Identification of a dietary pattern prospectively associated with bone mass in Australian young adults. Am J Clin Nutr. 2015;102(5):1035–43. [DOI] [PubMed] [Google Scholar]
- 53. Berger C, Langsetmo L, Joseph L, Hanley DA, Davison KS, Josse R, Kreiger N, Tenenhouse A, Goltzman D, Canadian Multicentre Osteoporosis Study Research Group . Change in bone mineral density as a function of age in women and men and association with the use of antiresorptive agents. Can Med Assoc J. 2008;178(13):1660–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cummings SR, Karpf DB, Harris F, Genant HK, Ensrud K, LaCroix AZ, Black DM. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002;112(4):281–9. [DOI] [PubMed] [Google Scholar]
- 55. Anastasilakis AD, Polyzos SA, Makras P. Therapy of endocrine disease: denosumab versus bisphosphonates for the treatment of postmenopausal osteoporosis. Eur J Endocrinol. 2018;179(1):R31–45. [DOI] [PubMed] [Google Scholar]
- 56. Wong AKO, Manske SL. A comparison of peripheral imaging technologies for bone and muscle quantification: a review of segmentation techniques. J Clin Densitom. 2018, 10.1016/j.jocd.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 57. Z Movassagh E, Kontulainen S, Baxter-Jones ADG, Whiting S, Szafron M, Papadimitropoulos M, Vatanparast H. Are milk and alternatives and fruit and vegetable intakes during adolescence associated with cortical and trabecular bone structure, density, and strength in adulthood?, Osteoporos Int. 2017;28(2):609–19. [DOI] [PubMed] [Google Scholar]
- 58. van Dongen LH, Kiel DP, Soedamah-Muthu SS, Bouxsein ML, Hannan MT, Sahni S. Higher dairy food intake is associated with higher spine quantitative computed tomography (QCT) bone measures in the Framingham Study for men but not women. J Bone Miner Res. 2018;33(7):1283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fung TT, Meyer HE, Willett WC, Feskanich D. Protein intake and risk of hip fractures in postmenopausal women and men age 50 and older. Osteoporos Int. 2017;28(4):1401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hoeflich A, Meyer Z. Functional analysis of the IGF-system in milk. Best Pract Res Clin Endocrinol Metab. 2017;31(4):409–18. [DOI] [PubMed] [Google Scholar]
- 61. Bolland MJ, Leung W, Tai V, Bastin S, Gamble GD, Grey A, Reid IR. Calcium intake and risk of fracture: systematic review. BMJ. 2015;351:h4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van Ballegooijen AJ, Pilz S, Tomaschitz A, Grübler MR, Verheyen N. The synergistic interplay between vitamins D and K for bone and cardiovascular health: a narrative review. Int J Endocrinol. 2017;2017:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mena-Sánchez G, Babio N, Martínez-González M, Corella D, Schröder H, Vioque J, Romaguera D, Martínez JA, Lopez-Miranda J, Estruch R et al.. Fermented dairy products, diet quality, and cardio-metabolic profile of a Mediterranean cohort at high cardiovascular risk. [Internet].Nutr Metab Cardiovasc Dis. 2018. Available from: 10.1016/j.numecd.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 64. Demontiero O, Vidal C, Duque G. Aging and bone loss: new insights for the clinician. Ther Adv Musculoskelet Dis. 2012;4(2):61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen Y, Xiang J, Wang Z, Xiao Y, Zhang D, Chen X, Li H, Liu M, Zhang Q. Associations of bone mineral density with lean mass, fat mass, and dietary patterns in postmenopausal Chinese women: a 2-year prospective study. PLoS One. 2015;10(9):e0137097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. García-Gavilán JF, Bulló M, Camacho-Barcia L, Rosique-Esteban N, Hernández-Alonso P, Basora J, Martínez-González MA, Estruch R, Fitó M, Salas-Salvadó J. Higher dietary glycemic index and glycemic load values increase the risk of osteoporotic fracture in the PREvención con DIeta MEDiterránea (PREDIMED)-Reus trial. Am J Clin Nutr. 2018;107(6):1035–42. [DOI] [PubMed] [Google Scholar]