ABSTRACT
Milk and dairy product consumption has been associated with an increase in prostate cancer risk; however, discrepancies have been observed in the literature. This first overview of systematic reviews and meta-analyses was carried out with the main objective of compiling and discussing the evidence generated to date related to milk and dairy product consumption and prostate cancer risk and mortality. A systematic search in MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, and the Web of Science (from inception to 30 April 2018) was conducted. The inclusion criteria were as follows: adult men, meta-analyses of longitudinal studies, dairy product consumption, and risk of prostate cancer or related outcomes. The AMSTAR2 checklist was used to evaluate methodological quality. The synthesis methods included dairy product exposure (high compared with low consumption or dose-response), dairy product type (total dairy products, milk, cheese, yogurt, and others), and prostate cancer outcomes (total, nonadvanced, and advanced prostate cancer and mortality) displayed in forest plots. Six meta-analyses were identified. These studies reported on the analysis of the 2 to 32 cohorts (up to 848,395 subjects/38,107 cases; 4–28 y of follow-up) and 2 case-control meta-analyses (12,435 subjects). The meta-analysis quality was valued as mostly “good” according to the AMSTAR2 criteria. All RRs of high compared with low consumption (dose-response) for total prostate cancer ranged from 1.68 to 1.09 (1.07 per 400 g/d) for total dairy products, 1.50 to 0.92 (1.06 to 0.98 per 200 g/d) for milk (whole, low-fat, and skim milk considered separately), and 1.18 to 0.74 (1.10 per 50 g/d) for cheese. RRs have decreased since the first meta-analysis. Statistical heterogeneity generates uncertainty in the observed results (up to I2 = 77.1%). In conclusion, although there are some data indicating that higher consumption of dairy products could increase the risk of prostate cancer, the evidence is not consistent. This review was registered with PROSPERO as CRD42018094737.
Keywords: milk, dairy products, prostate cancer, risk, mortality, overview, systematic review, meta-analysis
Introduction
Prostate cancer is a significant public health burden and a major cause of morbidity and mortality among men worldwide (1). According to the latest International Agency for Research on Cancer report, prostate cancer is the second-most common cancer in men worldwide, with more than 1.1 million new cases diagnosed in 2012 and 307,000 deaths recorded (2), accounting for 15% of the cancers diagnosed. However, in more developed regions, prostate cancer is the most common cancer in men (759,000 cases) (3). Prostate cancer is also the most frequently diagnosed cancer, and it is estimated that in just over a decade, prostate cancer will overtake lung cancer as the most common form of cancer in men worldwide (4). Incidence rates of prostate cancer vary more than 25-fold in different parts of the world, with the highest rates consistently observed in North America, Oceania, and Western and Northern Europe, and the lowest rates in Asia (1, 2).
Since the first reviews about prostate cancer epidemiology, diet has been suggested as a possible risk factor in prostate cancer etiology (5). This relation was observed in migration studies in which the incidence and mortality of prostate cancer were increased in immigrants compared to their native homologues (6–8). These observations suggested that changes in lifestyle, including dietary factors, could play an important role in prostate cancer etiology beyond the established risk factors, such as advanced age, black race, family history, and certain genetic polymorphisms.
Ecologic studies were the first studies that reported associations between the intake of milk or dairy products and prostate cancer risk and mortality (9–12). Since then, a large number of epidemiologic studies have been developed worldwide to clarify the influence of dairy products on prostate cancer risk. At the beginning of the century, a compilation of case-control and cohort studies gave rise to the first systematic reviews and meta-analyses (13, 14).
These first studies, in 2004 and 2005, confirmed a positive association of the consumption of milk (13) and dairy products (14) with prostate cancer risk, specifically in men with the highest intakes (14). These findings were later studied (2007) only in Western country cohorts (15), and the results supported the previous conclusions. However, 1 y later, in 2008, a new meta-analysis of cohort studies showed no evidence of an association between dairy or milk consumption and prostate cancer risk (16). An exception existed when homogeneous data were pooled from case-control studies, and a relative risk between dairy consumption and prostate cancer was found (16).
In 2015, a new meta-analysis established that high intakes of dairy products, milk, low-fat milk, and cheese may increase total prostate cancer risk (17). High intakes of whole milk, however, showed a significant inverse association with total prostate cancer risk (17). Although a new meta-analysis in 2016 observed a linear dose-response relation between increased whole milk intake and increased prostate cancer mortality risk (18), in 2018, the World Cancer Research Fund/American Institute for Cancer Research in their Continuous Update Project Expert Report 2018 (Diet, nutrition, physical activity and prostate cancer) concluded that the evidence that a higher consumption of dairy products increases the risk of prostate cancer is limited (19).
Therefore, given the discrepancies observed between the systematic reviews and meta-analysis results, this first overview of systematic reviews and meta-analyses was carried out with the main objective of compiling and discussing the evidence related to milk and dairy product consumption and prostate cancer risk and mortality.
Methods
This review was registered with PROSPERO as CRD42018094737. The Meta-analysis of Observational Studies in Epidemiology (20) and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (21) statements, and the Cochrane Collaboration Handbook (22) recommendations were followed to report this overview of reviews.
Search strategy and study selection
A systematic literature search of MEDLINE, EMBASE, the Cochrane Database of Systematic Reviews, and the Web of Science (from inception to 30 April 2018) was conducted for systematic reviews and meta-analyses addressing the association between dairy product consumption and the risk of prostate cancer. Three search topics were combined using Boolean operators. The first keyword list was related to cancer: cancer OR “prostate cancer.” The second keyword list was related to dairy consumption: dairy OR milk OR yogurt OR cheese OR kefir OR butter OR “dairy products.” The last list of words was related to the type of design: meta OR review. In addition, reference lists of included systematic reviews and meta-analyses were reviewed.
The systematic review was independently performed by 2 reviewers (IC-R and CA-B), and disagreements were resolved by consensus meetings.
Selection criteria
The inclusion criteria were as follows: 1) participants: adult men; 2) study design: meta-analyses including longitudinal studies; 3) exposure: dairy product intake (total dairy, milk, cheese, yogurt, or other dairy products); and 4) outcome: risk of prostate cancer or outcomes related to prostate cancer. The exclusion criteria were as follows: 1) studies written in languages other than English or Spanish; and 2) meta-analyses that did not follow the methodology of a systematic review.
Data extraction
Two researchers (IC-R and CA-B) independently collected the following data from the original studies: 1) author identification and year of publication; 2) number of studies included; 3) length of follow-ups; 4) age of the sample; 5) number of subjects and cases; 6) type of dairy product assessed; 7) risk ratio estimations; 8) heterogeneity reported; and 9) AMSTAR2 risk of bias value (23). In addition, the list of studies included in each meta-analysis and the covariate adjustments used in their analyses were extracted. Disagreements in data collection were resolved by discussion, and a third researcher was involved in case of disagreement (BL-P).
Quality assessment
After concealment of information about the authors, affiliations, date and source of each manuscript, 2 investigators (IC-R and CA-B) independently assessed the methodological quality of the manuscripts. A standardized checklist, the AMSTAR2 appraisal tool, for reporting systematic reviews and meta-analyses was used (23). This checklist includes 16 criteria, each referring to a relevant methodological aspect of the study. The quality score for each study ranged from 0 to 16 and could be evaluated as excellent, 15–16 items; very good, 12–14 items; good, 9–11 items; acceptable, 6–8 items; and deficient, 3–5 items. Disagreements were resolved by consensus with a third investigator (BL-P).
In addition, an ad hoc table was developed to summarize information regarding the methodology followed in the included systematic reviews and meta-analyses. This table includes information on database, date search, search strategy, inclusion criteria, main outcome, statistical model, sensitivity analyses, subgroup analyses, tool used to assess risk of bias, and quality of included studies.
Data synthesis
To depict the relation between dairy consumption and prostate cancer in each meta-analysis, forest plots were used. They were developed according to dairy product exposure (high compared with low intake or dose-response), and subgroup were based on the type of dairy product (total dairy product, milk, cheese, yogurt, and others). Furthermore, the main prostate cancer outcomes (total, advanced, and nonadvanced prostate cancer, or mortality) were displayed by each type of dairy product. Forest plot graphs were obtained using StataSE software, version 15 (StataCorp) and they displayed data from original meta-analyses without including any additional analyses.
Results
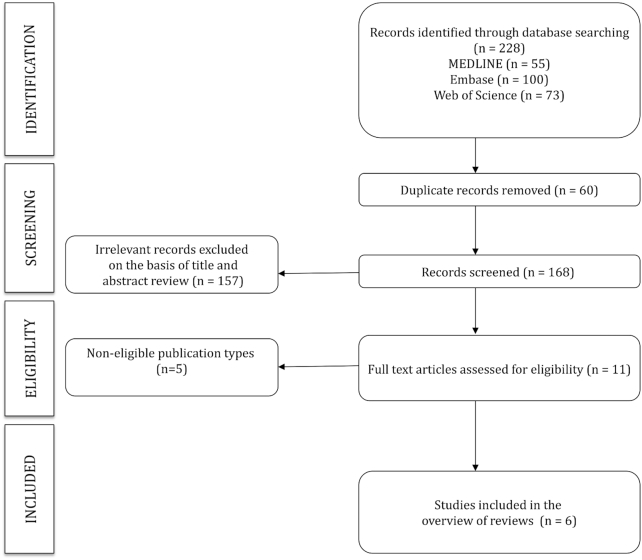
The initial search identified 229 articles, and from those, 6 were included in this overview of reviews (13–18). A flow chart of the study selection process is given in Figure 1. Descriptive information on the included meta-analyses is given in Table 1.
FIGURE 1.
Flow chart.
TABLE 1.
Characteristics of the included meta-analyses1
| Author | n | Age of the population, y | Follow-up, y | N (cases) | Exposure observed | RR (95% CI) | I2 (%) | Risk of bias (AMSTAR) |
|---|---|---|---|---|---|---|---|---|
| Qin et al., 2004 (13) | 11 case controls | 66–72 | — | 4377/5859 | Total dairy products2 | 1.68 (1.34, 2.12)3 | NA | Deficient |
| — | — | — | — | Milk2 | 1.50 (1.25, 1.80)3 | NA | — | |
| — | — | — | — | Milk and dairy products2 | 1.61 (1.22, 2.12)3 | NA | — | |
| Gao et al., 2005 (14) | 10 cohort studies | 25–84 | 5–21 | 282,887 (8383) | Total dairy products2 | 1.11 (1.00, 1.22) | — | Good |
| — | — | — | — | • Total prostate cancer | 1.12 (1.02, 1.24)3 | 37 | — | |
| — | — | — | — | • Advanced prostate cancer | 1.33 (1.00, 1.78) | 0.0 | — | |
| Qin et al., 2007 (15) | 13 cohort studies | NA | NA | 297,119 (7546) | Total dairy products2 | 1.13 (1.02, 1.24)3 | NA | Good |
| — | — | — | — | • Advanced prostate cancer | 1.11 (1.00, 1.24) | NA | — | |
| — | — | — | — | Dairy products2 | 1.18 (1.07, 1.30)3 | NA | — | |
| — | — | — | — | Milk2 | 1.21 (1.00, 1.47) | NA | — | |
| — | — | — | — | Cheese2 | 1.18 (1.03, 1.32)3 | NA | — | |
| Huncharek et al., 2008 (16) | 24 case controls | NA | — | 9293/12,435 | Dairy products2 | 1.14 (1.00, 1.29) | NA | Good |
| — | — | — | — | Milk2 | 1.28 (1.06, 1.55)3 | NA | — | |
| — | — | — | — | Cheese2 | 0.74 (0.62, 0.87)3 | NA | — | |
| 21 cohort studies | 25–99 | 4–21 | NA (18,181) | Dairy products2 | 1.11 (1.03, 1.19) | NA | — | |
| — | — | — | — | Milk2 | 1.06 (0.91, 1.23) | NA | — | |
| — | — | — | — | Cheese2 | 1.11 (0.99, 1.25) | NA | — | |
| Aune et al., 2015 (17) | 32 cohort studies | 25–99 | 6–28 | 848,395 (38,107) | Total dairy products2 | — | — | Good |
| — | — | — | — | • Total prostate cancer | 1.09 (1.02, 1.17)3 | 42.9 | — | |
| — | — | — | — | • Nonadvanced prostate cancer | 1.08 (1.00, 1.18) | 20.5 | — | |
| — | — | — | — | • Advanced prostate cancer | 0.92 (0.79, 1.08) | 34.5 | — | |
| — | — | — | — | • Prostate cancer mortality | 1.11 (0.97, 1.27) | 0.0 | — | |
| — | — | — | — | 400 g/d total dairy products4 | — | — | — | |
| — | — | — | — | • Total prostate cancer | 1.07 (1.02, 1.12)3 | 53 | — | |
| — | — | — | — | • Nonadvanced prostate cancer | 1.09 (1.00, 1.18) | 0.0 | — | |
| — | — | — | — | • Advanced prostate cancer | 0.97 (0.91, 1.05) | 20.1 | — | |
| — | — | — | — | • Prostate cancer mortality | 1.11 (0.92, 1.33) | 21 | — | |
| — | — | — | 566,146 (11,392) | Milk2 | — | — | — | |
| — | — | — | — | • Total prostate cancer | 1.11 (1.03, 1.21)3 | 20.6 | — | |
| — | — | — | — | • Nonadvanced prostate cancer | 1.14 (0.98, 1.32) | 7.8 | — | |
| — | — | — | — | • Advanced prostate cancer | 1.09 (0.86, 1.38) | 0.0 | — | |
| — | — | — | — | • Prostate cancer mortality | 1.38 (0.49, 3.86) | 88.1 | — | |
| Aune et al., 2015 (continued) (17) | 32 cohort studies | 25–99 | 6–28 | 566,146 (11,392) | 200 g/d milk4 | — | — | Good |
| — | — | — | — | • Total prostate cancer | 1.03 (1.00, 1.06) | 8.7 | — | |
| — | — | — | — | • Nonadvanced prostate cancer | 1.06 (1.00, 1.13) | 0.0 | — | |
| — | — | — | — | • Advanced prostate cancer | 0.98 (0.89, 1.09) | 0.0 | — | |
| — | — | — | — | • Prostate cancer mortality | 1.04 (0.73, 1.50) | 67.8 | — | |
| — | — | — | 448,719 (19,664) | Whole milk2 | 0.92 (0.85, 0.99)3 | 0.0 | — | |
| — | — | — | — | 200 g/d whole milk4 | — | — | — | |
| — | — | — | — | • Total prostate cancer | 0.98 (0.95, 1.01) | 0.0 | — | |
| — | — | — | — | • Nonadvanced prostate cancer | 0.94 (0.88, 1.00) | 33.7 | — | |
| — | — | — | — | • Advanced prostate cancer | 0.96 (0.87, 1.06) | 0.0 | — | |
| — | — | — | — | • Prostate cancer mortality | 1.29 (0.97, 1.70) | 45.1 | — | |
| — | — | — | 432,943 (19,430) | Low-fat milk2 | 1.14 (1.05, 1.25)3 | 51.0 | — | |
| — | — | — | — | 200 g/d low-fat milk4 | — | — | — | |
| — | — | — | — | • Total prostate cancer | 1.06 (1.01, 1.11)3 | 66.5 | — | |
| — | — | — | — | • Nonadvanced prostate cancer | 1.09 (1.01, 1.17)3 | 77.1 | — | |
| — | — | — | — | • Advanced prostate cancer | 0.99 (0.93, 1.05) | 0.0 | — | |
| — | — | — | — | • Prostate cancer mortality | 1.06 (0.92, 1.22) | 0.0 | — | |
| — | — | — | 887,759 (22,950) | Cheese2 | — | — | — | |
| — | — | — | — | • Total prostate cancer | 1.07 (1.01, 1.13)3 | 0.0 | — | |
| — | — | — | — | • Nonadvanced prostate cancer | 1.03 (0.95, 1.12) | 0.0 | — | |
| — | — | — | — | • Advanced prostate cancer | 1.18 (1.00, 1.41) | 0.0 | — | |
| — | — | — | — | • Prostate cancer mortality | 1.17 (0.75, 1.81) | 20.8 | — | |
| — | — | — | — | 50 g/d cheese4 | — | — | — | |
| — | — | — | — | • Total prostate cancer | 1.10 (1.03, 1.18)3 | 0.0 | — | |
| — | — | — | — | • Nonadvanced prostate cancer | 1.16 (0.96, 1.40) | 39.7 | — | |
| — | — | — | — | • Advanced prostate cancer | 1.06 (0.76, 1.48) | 57.2 | — | |
| — | — | — | — | • Prostate cancer mortality | 1.17 (0.62, 2.23) | 0.0 | — | |
| — | — | — | NA | Yogurt2 | 1.12 (0.97, 1.29) | 67.0 | — | |
| — | — | — | — | 100 g/d yogurt4 | — | — | — | |
| — | — | — | — | • Total prostate cancer | 1.08 (0.93, 1.24) | 81.6 | — | |
| — | — | — | — | • Nonadvanced prostate cancer | 0.97 (0.82, 1.15) | 54.5 | — | |
| — | — | — | — | • Advanced prostate cancer | 0.96 (0.71, 1.30) | 37.8 | — | |
| — | — | — | NA | Skim milk2 | 1.14 (0.88, 1.49) | 88.0 | — | |
| — | — | — | NA | Ice cream2 | 0.95 (0.83, 1.09) | 0.0 | — | |
| — | — | — | NA | Butter2 | 1.03 (0.89, 1.20) | 0.0 | — | |
| Lu et al., 2016 (18) | 2 cohort studies | 18–93 | 06–28 | 315,548 (NA) | Whole milk2 | 1.50 (1.03, 2.17)3 | 0.0 | Very good |
| — | — | — | — | Skim/low-fat milk2 | 1.00 (0.75, 1.33) | 0.0 | — |
1NA, not available.
2High compared with low consumption.
3 P < 0.05.
4Per each increment of cited dairy products.
The meta-analyses were published between 2004 and 2016 and included between 2 and 32 cohort studies of populations ranging from 282,887 to 848,395 subjects and from 7,456 to 38,107 cases of prostate cancer. The cohorts studied were followed for 4–28 y. In addition, 2 meta-analyses considered case-control studies including between 4477 and 12,435 subjects (13, 16).
Most of the studies reported data regarding the relation of the consumption of total dairy products, milk, and cheese with prostate cancer (15–17). Information on the relation between yogurt, ice cream, and butter consumption and prostate cancer was also included in 1 study (17). Separate data for whole, low-fat, and skim milk were reported in 2 studies (17, 18). All studies reported information on the comparison between high and low dairy product consumption (13–18), and 1 reported information on the dose-response relation between dairy products and prostate cancer (17). Total, nonadvanced, advanced, and fatal prostate cancer were observed as outcomes (17).
The heterogeneity of the data in the meta-analyses measured by I2 ranged from 0.0% to 81.6%, but 3 meta-analyses did not provide this information (13, 15, 16). Supplemental Tables 1–3 summarize the case-control studies and the original cohorts included in each meta-analysis and the covariates used for their analysis (Supplemental Tables 1–3).
Risk of bias
As evaluated by the AMSTAR2 tool, only 1 study scored “very good” (18), 4 scored “good” (14–17), and another scored deficient (13). When individual domains of risk of bias were analyzed, all studies had shortcomings in reporting: whether they performed the study selection in duplicate (Q5), the list of excluded studies (Q7), and the funding information of the included studies (Q10) (Table 2).
TABLE 2.
Risk of bias assessed with use of the AMSTAR2 tool1
| Author | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Q15 | Q16 | Total |
| Qin et al., 2004 (13) | No | No | Yes | No | No | Yes | No | Yes | No | No | Yes | No | No | Yes | No | Yes | Deficient |
| Gao et al., 2005 (14) | Yes | Yes | Yes | Yes | No | No | No | Yes | No | No | Yes | No | No | Yes | Yes | Yes | Good |
| Qin et al., 2007 (15) | Yes | Yes | Yes | Yes | No | Yes | No | Yes | No | No | Yes | No | No | Yes | Yes | Yes | Good |
| Huncharek et al., 2008 (16) | Yes | Yes | Yes | Yes | No | Yes | No | Yes | No | No | Yes | No | No | Yes | No | Yes | Good |
| Aune et al., 2015 (17) | Yes | Yes | Yes | Yes | No | Yes | No | Yes | No | No | Yes | No | No | Yes | Yes | Yes | Good |
| Lu et al., 2016 (18) | Yes | Yes | Yes | Yes | No | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Very good |
1 Q, Question.
In addition, most of the systematic reviews and meta-analyses conducted the search in 1 database, only 1 included 2 databases (18) and another 1 included gray literature (materials and research produced by organizations outside of the traditional commercial or academic publishing and distribution channels) (16). Four systematic reviews and meta-analyses limited their search strategy to studies published after 1966 (14–16) or 1984 (13). Three of them did not include a population definition among their inclusion criteria (13, 15, 17), and for 1 of them prostate cancer was not the main outcome (18). All the included systematic reviews and meta-analyses, except 2 (16, 17), reported in the methodology the analysis data by comparing the highest category with the lowest. Only 2 studies (13, 17) conducted sensitivity analyses, another (14) conducted metaregressions on variables of interest, and 2 did not perform any subgroup analyses (14, 16). Finally, only 1 assessed the risk of bias of the included original studies (18) (Supplemental Table 4).
Data synthesis
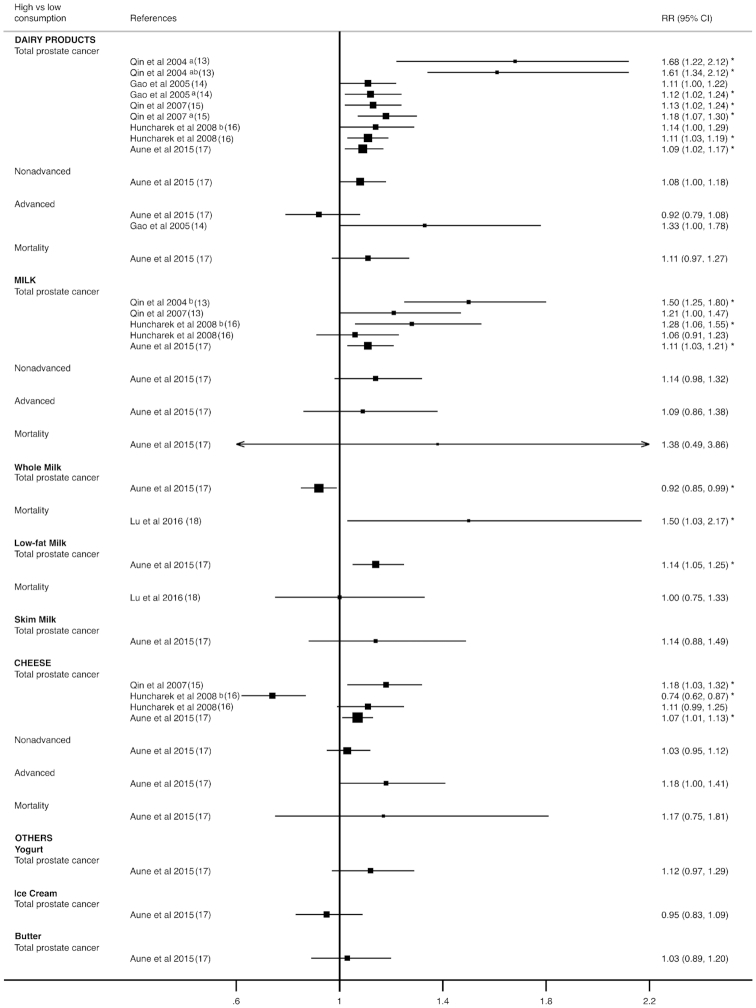
Figure 2 displays a forest plot of high compared with low consumption analyses for each type of dairy product by type of prostate cancer. For total prostate cancer, the RRs ranged from 1.09 to 1.68 for total dairy products, from 1.11 to 1.50 for milk (from 0.92 to 1.50 if whole, low-fat, and skim milk categories were considered separately), from 0.74 to 1.18 for cheese, and from 0.95 to 1.03 for other dairy product consumption. The RRs of advanced prostate cancer were reported as ranging from 0.92 to 1.33 for dairy products, 1.09 (95% CI: 0.86, 1.38) for milk and 1.18 (95% CI: 1.00, 1.41) for cheese consumption. Finally, for prostate cancer mortality, the RRs were reported as 1.11 (95% CI: 0.97, 1.27) for dairy products, 1.38 (95% CI: 0.49–3.86) for milk (from 1 to 1.38 if whole, low-fat, and skim milk categories were considered separately), and 1.17 (95% CI: 0.75, 1.81) for cheese consumption. Only 1 meta-analysis reported a significant decrease in RR for total prostate cancer and cheese consumption (RR: 0.74; 95% CI: 0.62, 0.87; P < 0.05) (16) (see Supplemental Tables 4 and 5).
FIGURE 2.
Forest plot for high compared with low consumption of milk and dairy products. aarticles pooled, bcase-control studies, *P < 0.05.
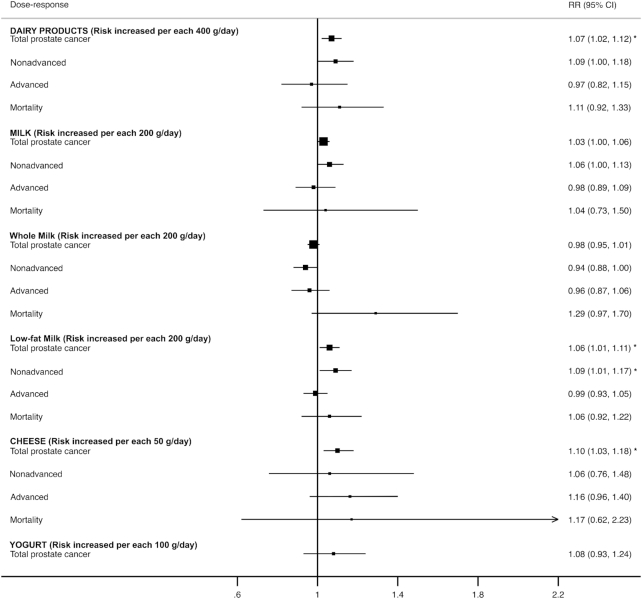
Figure 3 displays a forest plot with dose-response analyses for the consumption of each dairy product type and stage of prostate cancer. For total prostate cancer, the RRs were reported as 1.07 (95% CI: 1.02, 1.12) for each increment of 400 g/d of total dairy products, ranging from 0.98 to 1.06 for each increment of 200 mg/d of milk (whole, low-fat, and skim), 1.10 (95% CI: 1.03, 1.18) for each increment of 50 g/d of cheese, and 1.08 (95% CI: 0.93, 1.24) for each increment of 100 g/d of yogurt. The RRs of nonadvanced prostate cancer were reported as 1.09 (95% CI: 1.00, 1.18) for each increment of 400 g/d of total dairy products, ranging from 0.94 to 1.09 for each increment of 200 g/d of milk (whole, low-fat, and skim), and 1.16 (95% CI: 0.96, 1.40) for each increment of 50 g/d of cheese. The RRs for advanced prostate cancer were reported as 0.97 (95% CI: 0.91, 1.05) for each increment of 400 g/d of total dairy products, ranging from 0.96 to 0.99 for each increment of 200 g/d of milk (whole, low-fat, and skim), and 1.06 (95% CI: 0.76, 1.48) for each increment of 50 g/d of cheese. Finally, the RR for prostate cancer mortality was reported as 1.11 (95% CI: 0.92, 1.33) for each increment of 400 g/d of total dairy products. No statistically significant RR was observed (see Supplemental Tables 4 and 5).
FIGURE 3.
Forest plot for dose-response analyses of milk and dairy product consumption. *P < 0.05.
Discussion
Taking into account the systematic reviews and meta-analyses of case-control and cohort studies included in this first overview of reviews, it is possible to infer that there is some evidence pointing to a higher consumption of dairy products increasing the risk of prostate cancer. However, the evidence is still not conclusive, mainly because of statistical heterogeneity, the reduced number of studies included in each analysis, and weak control of confounding factors in primary studies, which generate uncertainty in the results observed.
In 2004, Qin et al. published the first systematic review and meta-analysis of case-control studies and found a positive and significant association between dairy product consumption and prostate cancer risk, when all studies were combined and when milk or milk and dairy products were pooled (13). However, this association was not supported by a later systematic review and meta-analysis of case-control studies, which included 13 additional case-control studies (16). Although Qin et al. found an increase in prostate cancer risk of 68% in men with higher dairy product consumption, Huncharek et al. demonstrated no association between dairy exposure and prostate cancer risk when homogeneous data were pooled (RR: 1.14; 95% CI: 0.76, 1.48). No association was determined even when data from population-based studies were analyzed (RR: 1.08; 95% CI: 0.90, 1.30), which have a great advantage over hospital-based control studies.
In both meta-analyses, higher milk consumption was significantly associated with increased prostate cancer risk. However, the relative risk was reduced from 50% in the first meta-analysis to 28% in the second meta-analysis, which included 4 of the case-control studies used by its predecessor. Ten case-control studies were pooled to determine an RR of 1.28 (95% CI: 1.06, 1.55) by Huncharek et al., which indicated a significant heterogeneity between studies (Qtest, P = 0.04).
Different from the other dairy products, a higher cheese consumption was associated with a significant reduction of prostate cancer risk, with an RR of 0.74 (95% CI: 0.62, 0.87) using heterogeneous data (Qtest, P = 0.02). After dropping the heterogeneous studies, this relation disappeared (RR: 0.73; 95% CI: 0.51, 1.04).
Thus, neither total dairy products nor cheese have a relation with higher prostate cancer risk. Milk consumption also shows no relation with prostate cancer risk; the RR for milk consumption was reduced from the first meta-analysis to the second, but the conclusions came from heterogeneous data. In this regard, case-control studies determine whether the exposure is associated with the outcome; they may prove an association but do not demonstrate causation (24). They are also vulnerable to recall and selection bias derived from the design, which may have resulted in an overestimation of the association. As a statistical method, a meta-analysis cannot solve these inconveniences. On the other hand, cohort studies are more effective than case-control studies (25).
Because of the limitations of case-control studies, systematic reviews and meta-analyses of cohort studies were conducted (14–17). The first systematic review and meta-analysis of cohort studies found an association between dairy product consumption and prostate cancer risk (RR: 1.11; 95% CI: 1.00, 1.22; P = 0.047; I2 = 28%) (14). However, when the analysis was limited to studies using a validated FFQ (RR: 1.08; 95% CI: 0.92, 1.28) or adjusted for energy intake (RR: 1.09; 95% CI: 0.89, 1.44), this association disappeared. When the RRs of the total prostate cancer studies were pooled (ns = 8), an increased risk associated with higher dairy product consumption was observed (RR: 1.12; 95% CI: 1.02, 1.24; P < 0.05). Nonetheless, a moderate heterogeneity between studies was also detected (I2 = 37%). An I2 value of <25% signifies low heterogeneity (26).
Three more systematic reviews and meta-analyses from cohort studies have been published since the previous study, and all found that higher dairy product consumption increased prostate cancer risk (15–17). However, the RR was reduced from 18% in the first meta-analysis to 9% in the latest meta-analysis published in 2015, which is closer to the actual effect size (15, 17). This reduction indicates that with more evidence available, the association between dairy product consumption and prostate cancer risk is smaller and better defined. In this regard, even though the direction of the effect seems to be clear, moderate heterogeneity is still present between the primary studies selected (I2 = 43%; Qtest, P = 0.04) (17). In addition, the RRs of both greater-weight meta-analyses lost their significance when cohort studies adjusted for dietary calcium intake were pooled ([RR: 1.06; 95% CI: 0.92–1.22 (16)]; [RR: 0.99; 95% CI: 0.92–1.07; I2 = 0% (17)]). Other drawbacks identified by the authors were variations in the definition of dairy products across the studies (16), publication bias, measurement errors in the dietary assessment (17), or differences among prostate cancer stages (15). Taking into account this last drawback, some systematic reviews and meta-analyses from cohort studies conducted stratified analyses for advanced and nonadvanced prostate cancers and prostate cancer mortality (14, 15, 17). However, even though they all found a significant rise in the RRs for total prostate cancer (from 1.18 to 1.09), none showed a significant association with dairy product consumption when analyses stratified by prostate cancer stage were carried out (from 0.92 to 1.33). However, the number of studies was lower when the analyses were stratified by stage so the lack of association could be a result of low statistical power. The associations in the meta-analysis by Aune et al. (17) were slightly stronger for prostate cancer mortality than for total prostate cancer; however, again most likely because of the limited number of studies, the results were not significant.
There was 1 meta-analysis of cohort studies carried out by Aune et al. (17) that reported a dose-response analysis (ns = 15). These authors observed a significant 7% increase in prostate cancer risk for every 400 g of total dairy products consumed per day. However, a moderate heterogeneity was found (I2 = 44%; Qtest, P = 0.04), and a study effect using Egger's test (P = 0.08) and Begg's test (P = 0.02) was reported. When the trim-and-fill method was used to assess the publication bias, the increase in prostate cancer risk from dairy product consumption disappeared (RR: 1.04; 95% CI: 0.99, 1.09; 5 studies added). Unexpectedly, when the dose-response meta-analysis was stratified by prostate cancer stage, no significant associations were observed.
Because not all dairy products are similar, systematic reviews and meta-analyses of cohort studies have analyzed dairy products by category. Until 2008, all meta-analyses of cohort studies had not found any relation between higher milk consumption and an increase in prostate cancer risk (15, 16). However, the most recent, carried out in 2015, observed an increased risk of 11% (17). This analysis included 15 cohort studies (n = 11,392) with low heterogeneity (I2 = 21%; Qtest, P = 0.22). Nevertheless, it is important to note that most primary studies included in this analysis were not adjusted for potential confounding factors, such as BMI, weight, or waist-to-hip ratio (ns = 10/15), physical activity (ns = 12/15), diabetes (ns = 14/15), or PSA test results (ns = 15/15), and showed no association when they were taken into account separately (17); however, P-heterogeneity within each subgroup or between subgroups was not significant. Analysis from studies carried out in Europe (ns = 6/15), where heterogeneity between studies was 0, did not find an association between milk consumption and prostate cancer risk (RR: 1.04; 95% CI: 0.94–1.14; I2 = 0%) (17). In this regard, when the dose-response analysis was carried out, the RR for a 200 g/d increase in milk intake showed no significant relation with higher prostate cancer risk, with no evidence of heterogeneity (I2 = 9%).
The relation between different kinds of milk and prostate cancer risk or mortality was analyzed by 2 systematic reviews and meta-analyses of cohort studies (17, 18). In the first, Aune et al. observed that greater whole milk consumption reduced prostate cancer risk by 8% (RR: 0.92; 95% CI: 0.85, 0.99; P < 0.05); ns = 6; with no heterogeneity between studies (I2 = 0%) (17). However, the second study suggested that consumption of whole milk increases mortality risk by up to 50% if the disease is already present (RR: 1.50; 95% CI: 1.03, 2.17; P < 0.05; I2 = 0%) (18). The evidence in the second study was taken from only 2 cohort studies, and when the dose-response analysis was carried out by Aune et al., no significant association was observed between consumption of 200 g/day of whole milk and total prostate cancer risk or mortality (17).
Unlike whole milk intake, higher low-fat milk consumption showed a significant 14% increase in prostate cancer risk (RR: 1.14; 95% CI: 1.05, 1.25; P < 0.05; I2 = 51%) and a 6% increase when a dose-response meta-analysis was carried out [(RR: 1.06; 95% CI: 1.01, 1.11; P < 0.05) per 200 g/d; I2 = 67%]. However, both findings had substantial heterogeneity between studies (17).
It has been reported that higher cheese consumption, another important dairy product, increases prostate cancer risk in men (15, 17). However, not all systematic reviews and meta-analyses have reported the same results, with Huncharek et al. reporting no relation between higher cheese consumption and prostate cancer risk (16). In addition, when a meta-analysis stratified by prostate cancer stage was carried out, the association was not clear. In any case, it is relevant that the RR was reduced from 18% in the first meta-analysis cohorts to 7% in the latest meta-analysis (15, 17). When a dose-response analysis was carried out, a 10% increase in prostate cancer risk for every 50 g/d of cheese consumption was found [(RR: 1.10; 95% CI: 1.03, 1.18; P < 0.05) ns = 11], with no heterogeneity between studies (I2 = 0%). However, as in high compared with low consumption, when a dose-response meta-analysis by prostate cancer stage was conducted, no association was observed. Consumption of other dairy products, such as yogurt, ice cream, and butter, did not show any association with prostate cancer risk.
Several mechanisms have been proposed to explain the relation between milk and dairy product consumption and prostate cancer risk. It had been speculated that high calcium intake, largely from dairy products, could increase prostate cancer risk by reducing bioactive vitamin D (27), which impedes proliferation and induces apoptosis of cancer cells (28). However, this mechanism has lost support as changes in vitamin D concentration in response to calcium intake are not substantial enough to influence the proliferation and differentiation of prostate cancer cells (29). One of the most studied mechanisms is related to concentrations of insulin like growth factor I (IGF-I). This hormone is produced mainly by the liver and is regulated by IGF binding protein-3 (IGFBP-3) (30). IGF-I modulates growth and cellular development and promotes proliferation, differentiation, and survival. In addition, it inhibits cellular apoptosis, facilitating cell growth (31). IGF-I has also been recently related to a 9% increase in prostate cancer risk [(RR: 1.09; 95% CI: 1.03, 1.16; P < 0.05) ns = 51] (32). In this regard, there is some evidence suggesting that the consumption of milk, but not dairy products (33), may increase IGF-I blood concentrations (32). However, Harrison et al. also found that IGFBP-3 increases with milk consumption (32). IGFBP-3 is a strong antiproliferative protein that provokes apoptosis and inhibits cell proliferation in prostate cancer (34). Indeed, this same systematic review and meta-analysis observed that prostate cancer risk decreased with IGFBP-3 [(RR: 0.90; 95% CI: 0.83, 0.98; P < 0.05) ns = 39] (32). In addition, IGF-I is an established positive risk factor for breast cancer (35). However, in premenopausal women, milk and dairy consumption has been identified as having a possible protective effect (36). Therefore, the mechanism is not completely understood.
One important limitation of the systematic reviews and meta-analyses included in this overview is the presence of statistical heterogeneity in the results. Statistical heterogeneity comes from clinical heterogeneity (population included, interventions compared, outcomes collected, etc.) and methodological heterogeneity (study designs, degrees of control over bias, etc.) (37), and as meta-analyses bring together diverse studies, heterogeneity is expected. However, heterogeneity measures the degree of inconsistency in the results of the studies (38), therefore describing the total variation across studies that results from heterogeneity rather than chance (39). These particularities make interpretation difficult, reduce the confidence, and limit the application and validity of the results obtained. The heterogeneity reflects the wide variability of the observed results (from 0 to 77.1% in all studies where it was calculated and the RR was significant) and shows that the evidence for milk and dairy product consumption being related to an increase in prostate cancer risk is still not consistent.
The total quality of the systematic reviews and meta-analyses published to date and evaluated here were valued mostly as “good.” Therefore, the risk of bias could be considered relatively low, although the AMSTAR2 tool was not designed to generate an overall score (23). In this regard, although some results indicated that dairy product consumption increases prostate cancer risk, many epidemiologists consider that an increased risk of <20% (RR = 1.0–1.2) or a decreased risk of <10% (RR = 0.9–1.0) has a strength of association classified as weak or no association (40, 41). They argue that confounding factors can lead to a weak association between exposure and a result, and it is usually not possible to identify an adequate measure or control for weak confounding characteristics, which adds uncertainty to the results obtained. Regarding potential confounding or moderator variables, only 3 systematic reviews and meta-analysis performed subgroup analysis and only 1 performed metaregressions. Aune et al. (17) performed both analyses, including in these analyses the adjustments for potential confounding factors included in original articles. These analyses showed that adjustments mitigating the association between dairy products and prostate cancer were alcohol consumption for total dairy products, and adiposity parameters, physical activity, and diabetes mellitus for milk. These analyses should be considered with caution as they were not separately measured, and other confusing factors could moderate these associations.
In recent years, milk and dairy products have been constantly under scrutiny, and some important institutions have recommended limiting their consumption, arguing that research has shown little benefit and considerable potential for harm (42, 43). However, milk and dairy product consumption has demonstrated neutral associations with cardiovascular diseases and all-cause mortality (44) and there is evidence suggesting that dairy consumption may protect against metabolic diseases such as type 2 diabetes (45, 46). Indeed, milk and dairy product consumption has also been associated with a reduction in colorectal cancer risk (47, 48), and organizations such as the World Cancer Research Fund/American Institute for Cancer Research have reported that there is not sufficient evidence to recommend reducing milk and dairy consumption to reduce the risk of cancer (19).
Accepting the results of a single systematic review involves risks; therefore, the strength of this overview of reviews is that it has compiled, discussed, and summarized the evidence generated on milk and dairy products and prostate cancer risk from all systematic reviews and meta-analyses of observational studies published to date into 1 comprehensive, accessible, and usable document. Observations obtained in this first overview of systematic reviews and meta-analyses will contribute to decisions related to milk and dairy product consumption and prostate cancer risk and mortality. An overview of reviews does not include new evidence from primary studies, which could be considered a limitation. Since the last meta-analysis of case-control studies, 3 additional primary studies have been carried out. In general terms, because they all used a different methodology, they all observed that milk or dairy product consumption increases prostate cancer risk (49–51). However, primary studies of cohorts observed that milk consumption did not show an association with total prostate cancer risk in adult men (52, 53). Therefore, new lines of investigation should include the analysis of the newest evidence generated related to prostate cancer risk mainly in some subtypes of dairy products and subgroup analyses where there is not yet an adequate statistical power to detect significant associations, of the association of IGF-I and IGFBP-3 with types of milk and dairy products, as well as with prostate cancer stages, and of the relation between milk and dairy product consumption and prostate cancer risk related to polymorphisms.
In conclusion, although there are some data indicating that higher dairy product consumption could increase prostate cancer risk, the total evidence generated to date is still not conclusive, mainly because of statistical heterogeneity, the number of studies included in each analysis, and weak control of confounding factors in primary studies, all of which generate uncertainty in the observed results. Therefore, there is currently not sufficient evidence to justify a reduction in daily milk and dairy product consumption. Daily intake of milk and dairy products should follow the dietary recommendations put forth by the competent authorities of each country.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—BL-P: wrote the original manuscript; IC-R and CA-B: performed the search strategy, study selection, data extraction, and quality assessment; BL-P: was involved in case of disagreement in data extraction and quality assessment; IC-R: conducted statistical analyses; LMB and CS: participated in the interpretation and analysis of the data obtained; CG-C: had primary responsibility for the final content of the manuscript; and all authors: read and approved the final paper.
Notes
Sponsored by the Interprofessional Dairy Organization, (INLAC), Spain. The sponsor had no role in the design of the studies included in the supplement, in the collection, analyses, or interpretation of the data; in the writing of the manuscripts, and in the decision to publish the results. Publication costs for this supplement were defrayed in part by the payment of page charges. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
Author disclosures: BL-P, LMB, CS, IC-R, CA-B, and CG-C, no conflicts of interest.
Supplementary Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
References
- 1. Zhou CK, Check DP, Lortet-Tieulent J, Laversanne M, Jemal A, Ferlay J, Bray F, Cook MB, Devesa SS. Prostate cancer incidence in 43 populations worldwide: an analysis of time trends overall and by age group. Int J Cancer. 2016;138:1388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 3. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 4. American Cancer Society. Cancer Facts & Figures 2014. Cancer Facts Fig. [Internet]. 2014;1–72.. Available from: http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. [Google Scholar]
- 5. Flanders WD. Review: prostate cancer epidemiology. Prostate. 1984;5:621–9. [DOI] [PubMed] [Google Scholar]
- 6. Maskarinec G, Noh JJ. The effect of migration on cancer incidence among Japanese in Hawaii. Ethn Dis. 2004;14:431–9. [PubMed] [Google Scholar]
- 7. Lee J, Demissie K, Lu S-E, Rhoads GG. Cancer incidence among Korean-American immigrants in the United States and native Koreans in South Korea. Cancer Control. 2007;14:78–85. [DOI] [PubMed] [Google Scholar]
- 8. Kumar NB, Yu D, Akinremi TO, Odedina FT. Comparing dietary and other lifestyle factors among immigrant Nigerian men living in the US and indigenous men from Nigeria: potential implications for prostate cancer risk reduction. J Immigr Minor Heal. 2009;11:391–9. [DOI] [PubMed] [Google Scholar]
- 9. Tominaga S, Kuroishi T. An ecological study on diet/nutrition and cancer in Japan. Int J Cancer. 1997;(Suppl 10):2–6. [DOI] [PubMed] [Google Scholar]
- 10. Grant WB. An ecologic study of dietary links to prostate cancer. Altern Med Rev. 1999;4:162–9. [PubMed] [Google Scholar]
- 11. Ganmaa D, Li X-M, Wang J, Qin L-Q, Wang P-Y, Sato A. Incidence and mortality of testicular and prostatic cancers in relation to world dietary practices. Int J Cancer. 2002;98:262–7. [DOI] [PubMed] [Google Scholar]
- 12. Grant WB. A multicountry ecologic study of risk and risk reduction factors for prostate cancer mortality. Eur Urol. 2004;45:271–9. [DOI] [PubMed] [Google Scholar]
- 13. Qin L-Q, Xu J-Y, Wang P-Y, Kaneko T, Hoshi K, Sato A. Milk consumption is a risk factor for prostate cancer: meta-analysis of case-control studies. Nutr Cancer. 2004;48:22–7. [DOI] [PubMed] [Google Scholar]
- 14. Gao X, LaValley MP, Tucker KL. Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. J Natl Cancer Inst. 2005;97:1768–77. [DOI] [PubMed] [Google Scholar]
- 15. Qin L-Q, Xu J-Y, Wang P-Y, Tong J, Hoshi K. Milk consumption is a risk factor for prostate cancer in Western countries: evidence from cohort studies. Asia Pac J Clin Nutr. 2007;16:467–76. [PubMed] [Google Scholar]
- 16. Huncharek M, Muscat J, Kupelnick B. Dairy products, dietary calcium and vitamin D intake as risk factors for prostate cancer: a meta-analysis of 26,769 cases from 45 observational studies. Nutr Cancer. 2008;60:421–41. [DOI] [PubMed] [Google Scholar]
- 17. Aune D, Navarro Rosenblatt DA, Chan DS, Vieira AR, Vieira R, Greenwood DC, Vatten LJ, Norat T. Dairy products, calcium, and prostate cancer risk: a systematic review and meta-analysis of cohort studies. Am J Clin Nutr. 2015;101:87–117. [DOI] [PubMed] [Google Scholar]
- 18. Lu W, Chen H, Niu Y, Wu H, Xia D, Wu Y. Dairy products intake and cancer mortality risk: a meta-analysis of 11 population-based cohort studies. Nutr J. 2016;15:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, physical activity and prostate cancer. [Internet]. 2018;1–16.. Available from: www.wcrf.org/dietandcancer/about. [Google Scholar]
- 20. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group, JAMA. 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 21. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Altman DG, Booth A et al.. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JPT, Green S (editors). Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. [Internet]. Available from: www.cochrane-handbook.org. [Google Scholar]
- 23. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E et al.. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lewallen S, Courtright P. Epidemiology in practice: case-control studies. Community Eye Health. 1998;11:57–8. [PMC free article] [PubMed] [Google Scholar]
- 25. Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg. 2010;126:2234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan JM, Giovannucci EL. Dairy products, calcium, and vitamin D and risk of prostate cancer. Epidemiol Rev. 2001;23:87–92. [DOI] [PubMed] [Google Scholar]
- 28. Abu El Maaty MA, Wölfl S. Vitamin D as a novel regulator of tumor metabolism: insights on potential mechanisms and implications for anti-cancer therapy. Int J Mol Sci. 2017;18(10):2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bonjour J-P, Chevalley T, Fardellone P. Calcium intake and vitamin D metabolism and action, in healthy conditions and in prostate cancer. Br J Nutr. 2007;97:611. [DOI] [PubMed] [Google Scholar]
- 30. Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev. 1997;18:801–31. [DOI] [PubMed] [Google Scholar]
- 31. Jones JI, Clemmons DR.. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. [DOI] [PubMed] [Google Scholar]
- 32. Harrison S, Lennon R, Holly J, Higgins JPT, Gardner M, Perks C, Gaunt T, Tan V, Borwick C, Emmet P et al.. Does milk intake promote prostate cancer initiation or progression via effects on insulin-like growth factors (IGFs)? A systematic review and meta-analysis. Cancer Causes Control. 2017;28:497–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qin L-Q, He K, Xu J-Y. Milk consumption and circulating insulin-like growth factor-I level: a systematic literature review. Int J Food Sci Nutr. 2009;60:(Suppl 7):330–40. [DOI] [PubMed] [Google Scholar]
- 34. Shahjee H, Bhattacharyya N, Zappala G, Wiench M, Prakash S, Rechler MM. An N-terminal fragment of insulin-like growth factor binding protein-3 (IGFBP-3) induces apoptosis in human prostate cancer cells in an IGF-independent manner. Growth Horm IGF Res. 2008;18:188–97. [DOI] [PubMed] [Google Scholar]
- 35. Key TJ, Appleby PN, Reeves GK, Roddam AW; Endogenous Hormones and Breast Cancer Collaborative Group. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11:530–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dong J-Y, Zhang L, He K, Qin L-Q. Dairy consumption and risk of breast cancer: a meta-analysis of prospective cohort studies. Breast Cancer Res Treat. 2011;127:23–31. [DOI] [PubMed] [Google Scholar]
- 37. Higgins J, Thompson S, Deeks J, Altman D. Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy. 2002;7:51–61. [DOI] [PubMed] [Google Scholar]
- 38. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 39. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Craun GF, Calderon RL. How to interpret epidemiological associations. Environ Prot. 1979;108–15. [Google Scholar]
- 41. Moson R. Occupational epidemiology. 2nd edition Boca Raton (FL): CRC Press Inc; 1990. [Google Scholar]
- 42. Skerrett PJ. Harvard to USDA: check out the healthy eating plate—Harvard health blog—Harvard health publishing. [Internet]. 2011. Available from: https://www.health.harvard.edu/blog/harvard-to-usda-check-out-the-healthy-eating-plate-201109143344. [Google Scholar]
- 43. Harvard Health Publishing. Harvard researchers continue to support their healthy eating plate—Harvard Health. [Internet]. 2017. Available from: https://www.health.harvard.edu/plate/harvard-researchers-launch-healthy-eating-plate. [Google Scholar]
- 44. Guo J, Astrup A, Lovegrove JA, Gijsbers L, Givens DI, Soedamah-Muthu SS. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose–response meta-analysis of prospective cohort studies. Eur J Epidemiol. 2017;32:269–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aune D, Norat T, Romundstad P, Vatten LJ. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr. 2013;98:1066–83. [DOI] [PubMed] [Google Scholar]
- 46. Gao D, Ning N, Wang C, Wang Y, Li Q, Meng Z, Liu Y, Li Q. Dairy products consumption and risk of type 2 diabetes: systematic review and dose-response meta-analysis. PLoS One. 2013;8:e73965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Aune D, Lau R, Chan DSM, Vieira R, Greenwood DC, Kampman E, Norat T. Dairy products and colorectal cancer risk: a systematic review and meta-analysis of cohort studies. Ann Oncol. 2012;23:37–45. [DOI] [PubMed] [Google Scholar]
- 48. Huncharek M, Muscat J, Kupelnick B. Colorectal cancer risk and dietary intake of calcium, vitamin D, and dairy products: a meta-analysis of 26,335 cases from 60 observational studies. Nutr Cancer. 2009;61:47–69. [DOI] [PubMed] [Google Scholar]
- 49. Raimondi S, Mabrouk JB, Shatenstein B, Maisonneuve P, Ghadirian P. Diet and prostate cancer risk with specific focus on dairy products and dietary calcium: a case-control study. Prostate. 2010;70:1054–65. [DOI] [PubMed] [Google Scholar]
- 50. Deneo-Pellegrini H, Ronco AL, De Stefani E, Boffetta P, Correa P, Mendilaharsu M, Acosta G. Food groups and risk of prostate cancer: a case-control study in Uruguay. Cancer Causes Control. 2012;23:1031–8. [DOI] [PubMed] [Google Scholar]
- 51. Lassed S, Deus CM, Lourenço N, Dahdouh A, Rizvanov AA, Oliveira PJ, Zama D. Diet, lifestyles, family history, and prostate cancer incidence in an East Algerian patient group. Biomed Res Int. 2016;2016:5730569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Torfadottir JE, Steingrimsdottir L, Mucci L, Aspelund T, Kasperzyk JL, Olafsson O, Fall K, Tryggvadottir L, Harris TB, Launer L et al.. Milk intake in early life and risk of advanced prostate cancer. Am J Epidemiol. 2012;175:144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lane JA, Oliver SE, Appleby PN, Lentjes MAH, Emmett P, Kuh D, Stephen A, Brunner EJ, Shipley MJ, Hamdy FC et al.. Prostate cancer risk related to foods, food groups, macronutrients and micronutrients derived from the UK Dietary Cohort Consortium food diaries. Eur J Clin Nutr. 2017;71:274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.