ABSTRACT
Nutrition is a modifiable factor potentially related to aging. Milk and other dairy products may contribute to the prevention of physical and cognitive impairment. We conducted a systematic review to investigate the effectiveness of dairy product intake for preventing cognitive decline, sarcopenia, and frailty in the elderly population. A systematic search for publications in electronic databases [MEDLINE via PubMed, Embase, Scopus, the Cochrane Central Register of Controlled Trials (CENTRAL), and the Cochrane Database of Systematic Reviews] from 2009 to 2018 identified observational and interventional studies in English and Spanish that tested the relation between dairy product consumption and cognitive decline, sarcopenia, and frailty in community-dwelling older people. We assessed the participants, the type of exposure or intervention, the outcomes, and the quality of evidence. We screened a total of 661 records and included 6 studies (5 observational prospective cohort studies and 1 randomized controlled trial). Regarding cognitive impairment, the relation cannot be firmly established. Consumption of milk at midlife may be negatively associated with verbal memory performance. In older women, high intakes of dairy desserts and ice cream were associated with cognitive decline. On the other hand, 1 study demonstrated a significant inverse relation between dairy intake and development of Alzheimer disease among older Japanese subjects. The consumption of dairy products by older people may reduce the risk of frailty, especially with high consumption of low-fat milk and yogurt, and may also reduce the risk of sarcopenia by improving skeletal muscle mass through the addition of nutrient-rich dairy proteins (ricotta cheese) to the habitual diet. Despite the scarcity of evidence on the topic, our systematic review shows that there are some positive effects of dairy products on frailty and sarcopenia, whereas studies concerning cognitive decline have contradictory findings.
Keywords: milk, dairy products, older, cognition, dementia, frailty, sarcopenia
Introduction
A substantial amount of evidence indicates that nutrition is a key factor influencing longevity and age-related diseases. In recent years, frailty has emerged as an interesting consequence of advanced aging that involves multiple physiological systems, both musculoskeletal and cognitive. Frailty is present in ∼10% of people above the age of 65 y (1). Various authors have described frailty as a state characterized by loss of reserve capacity associated with a higher risk of adverse health outcomes, including fractures, hospitalization, disability, and death (2).
Frail older subjects exhibit an increased risk of developing physical and cognitive decline (3). Sarcopenia is the term used to refer to the loss of skeletal muscle mass and function. This concept overlaps with physical frailty. The current idea is that sarcopenia is a key cause of frailty, but not all sarcopenic subjects are necessarily frail. Physical frailty, defined as exhaustion, a low level of physical activity, slowing, weight loss, and impaired grip strength, is related to cognitive decline and incident mild cognitive impairment (3, 4). The general meaning of the term “frailty” is considerably broader and captures other domains, such as cognitive performance. The concept of cognitive decline ranges from the minimal decline associated with normal aging to mild cognitive impairment or severe dementia as the final stage of cognitive impairment.
The etiology of these conditions is not well known, but there are interventions that may slow their onset. A potential therapy of this kind could include nutrition. Nutrition has been associated with frailty syndrome in cross-sectional and prospective studies (5). The associations between dairy product intake and chronic diseases, frailty, and cognitive and physical decline have been under investigation, and the conclusions remain contradictory (6–8). Furthermore, opinions are trending toward reduced dairy product consumption in developed countries. The possibility of reducing the likelihood of frailty and physical and cognitive decline by modifying cardiometabolic health has been described. The composition of dairy products (proteins, minerals, and vitamins) may, in combination or individually, reduce blood pressure (9) and the risk of type 2 diabetes mellitus (10). Thus, there is a need to identify whether dairy intake may be a modifiable risk factor and might even exert a protective effect against frailty and physical and cognitive impairment. The first systematic review that evaluated the association between milk intake and cognitive disorders showed an inverse association between milk consumption and cognitive disorders. It was published by Wu and Sun (11) in 2016, and revealed considerable heterogeneity associated with different categories of milk intake and diverse units of milk consumption. Some of the studies included adjusted only for sociodemographic variables. In 2015, Lana et al. (12) published the first prospective study to examine the association between the consumption of dairy products and the risk of frailty in community-dwelling older adults. Some previous studies found an inverse association with functional disability in older men (13) or better physical performance (14), but the design was cross-sectional and did not assess frailty. The effects of adding protein-rich foods to the habitual diet on lean tissue, muscle mass, and strength had not been studied in nonsarcopenic elderly subjects before the research of Alemán-Mateo et al. (15) reported in 2014. The purpose of our study was to investigate the effectiveness of dairy product intake for preventing frailty, sarcopenia, and cognitive decline in the older population, including a review of the most recent published prospective studies (with better control of residual confounding factors) and interventional studies addressing cognitive disorders, sarcopenia, and frailty.
Methods
Protocol and registration
We performed this systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (16). The systematic review protocol is registered with the International Prospective Register of Systematic Reviews (PROSPERO) as CRD42018099385.
Sources of information and literature search strategy
The literature searches were performed on 1 March, 2018 (first search) and 30 April, 2018 (last search), covering articles published from January, 2009 onward, to assess the most recent evidence. The only systematic review addressing dairy consumption and cognitive performance was published in 2016 and included articles from 2003, some of which included cohorts <60 y of age with incomplete adjustment for possible confounding factors (11). On the other hand, the use of nonspecific global screening tools to analyze cognition domains in early studies limited their interpretation. In our review, we updated the search strategy and included the new related topic of frailty, including its physical correlate of sarcopenia. The selected electronic databases were MEDLINE (via PubMed), Embase, Scopus, the Cochrane Central Register of Controlled Trials (CENTRAL), and the Cochrane Database of Systematic Reviews. A search of the gray literature was also performed using MedNar and worldwidescience.org. In addition, a manual search of the reference sections of the selected articles was completed.
We used Medical Subject Headings (MeSH) terms for PubMed and EMTREE terms for Embase. The following search strategy was applied in Embase as a PICO (Population, Intervention, Comparison, Outcome) search and later adapted to other databases. Population: “aged”/exp OR “aged” OR “aged patient” OR “aged people” OR “aged person” OR “aged subject” OR “elderly” OR “elderly patient” OR “elderly people” OR “elderly person” OR “elderly subject” OR “senior citizen”; Intervention: “dairy product”/exp OR “dairy product” OR “dairy products” OR “fermented milk product”/exp OR “cultured dairy product” OR “cultured milk product” OR “cultured milk products” OR “fermented dairy product” OR “fermented milk product”; Outcome: 1. (“sarcopenia”/exp OR “sarcopenia” OR “muscle weakness”/exp OR “muscle strength loss” OR “muscle weakening” OR “muscle weakness” OR “muscular weakness” OR “weakness, muscle” OR “muscle function”/exp OR “function, muscle” OR “muscle function” OR “muscle performance” OR “muscular function” OR “muscular performance” OR “performance, muscle” OR “muscle mass”/exp OR “muscle mass” OR “muscle volume” OR “muscle weight” OR “weight, muscle”), 2. (“frail elderly”/exp OR “frail elderly” OR “frailty syndrome”/exp OR “frailty phenotype”/exp OR “frailty score”/exp OR “frailty” OR “frail” AND “elderly”), 3. (“memory disorder”/exp OR “memory defect” OR “memory disorder” OR “memory disorders” OR “memory impairment” OR “cognitive decline”/exp OR “mental function”/exp OR “mental function” OR “mental process” OR “mental processes” OR “dementia”/exp OR “dementia” OR “alzheimer disease”/exp OR “alzeimer disease” OR “alzeimers disease” OR “alzheimer dementia” OR “alzheimer disease” OR “alzheimers disease” OR “dementia, alzheimer” OR “late onset alzheimer disease” OR “cognitive defect”/exp OR “cognition disorder” OR “cognition disorders” OR “cognitive defect” OR “cognitive defects” OR “cognitive deficit” OR “cognitive disability” OR “cognitive disorder” OR “cognitive disorders” OR “cognitive dysfunction” OR “cognitive impairment” OR “delirium, dementia, amnestic, cognitive disorders” OR “mental performance”/exp OR “activity, mental” OR “mental activity” OR “mental fitness” OR “mental performance” OR “mental performance assessment” OR “mental performance evaluation” OR “performance, mental” OR “psychologic performance”).
Type of studies, participants, intervention or exposure, and outcomes
We included interventional and observational (cohort, case-control), but not cross-sectional, studies that investigated the relation between dairy products and the development of frailty, sarcopenia, and cognitive decline in community-dwelling individuals aged ≥60 y. The languages included were English and Spanish. The search was limited to the last 9 y to assess the most recent evidence. We excluded studies that considered a combined intervention including diet unless it was possible to analyze the consumption of dairy products specifically (not whey proteins or fortified foods); we also excluded studies with subjective measures of the proposed outcome items, studies that included nonfunctional measures of frailty or sarcopenia in their methodology, and studies that included participants diagnosed with any of the outcomes of interest at the beginning of the follow-up period.
The primary outcomes were cognitive decline, sarcopenia, and frailty. With respect to cognitive impairment, the ordinary validated cognitive scales were accepted for assessing different domains of cognition (episodic memory, semantic memory, working memory, and verbal memory). In addition, the most recent definitions of cognitive impairment or dementia were included. Regarding sarcopenia, this systematic review included studies that used the original definitions (17, 18) or their modified versions. Sarcopenia can be assessed by measuring muscle mass (with different methods) or appendicular lean mass. The classical definition also includes muscle strength (grip strength) and physical performance. This last parameter can be identified using the Short Physical Performance Battery (SPPB) (19) or other validated measures. Clinically, sarcopenia is related to functional disability, which can be studied using the Activities of Daily Living scale, the Instrumental Activities of Daily Living scale, or other validated tests (20). The phenotypic definition of frailty is classically used in a range of studies (21) and includes a combination of the following: fatigue, weakness, weight loss, decreased balance, low levels of physical activity, social withdrawal, slowed motor processing and performance, and increased vulnerability to stressors (22, 23). Some authors have developed modified diagnostic criteria (24).
Selection of studies
Study selection was performed in 2 phases. In phase 1, 2 of the authors (FC-T and CV-B) independently reviewed the titles and abstracts obtained from all the databases. After the removal of duplicate records with a Mendeley database, the titles and abstracts were reviewed. In the case of disagreement about whether a study was eligible, it was necessary to include a third author (FJM-S) to decide which articles would be included. In phase 2, the full texts were read by 2 authors (FC-T and CV-B), and all articles that did not meet the inclusion criteria were excluded.
Data extraction
Data from the selected articles were copied to a Microsoft Excel spreadsheet by 2 authors (FC-T and CV-B) independently. The following information was recorded: author's name, publication year, study country, number of participants, mean age, percentage of male participants, baseline age in years, method of exposure assessment, and outcome definition, describing the selected outcome (cognitive assessment, sarcopenia, or frailty) in this case and the association measure with the corresponding CI.
Methodological quality assessment
The quality of each study was evaluated and scored by 2 authors (FC-T and CV-B) independently, using the Newcastle-Ottawa Scale (NOS) for observational studies (25) and the modified Jadad scale for randomized studies (26). The NOS assigns quality scores ≤9 points. The modified Jadad scale ranged from 0 to 8 points.
Results
Study selection
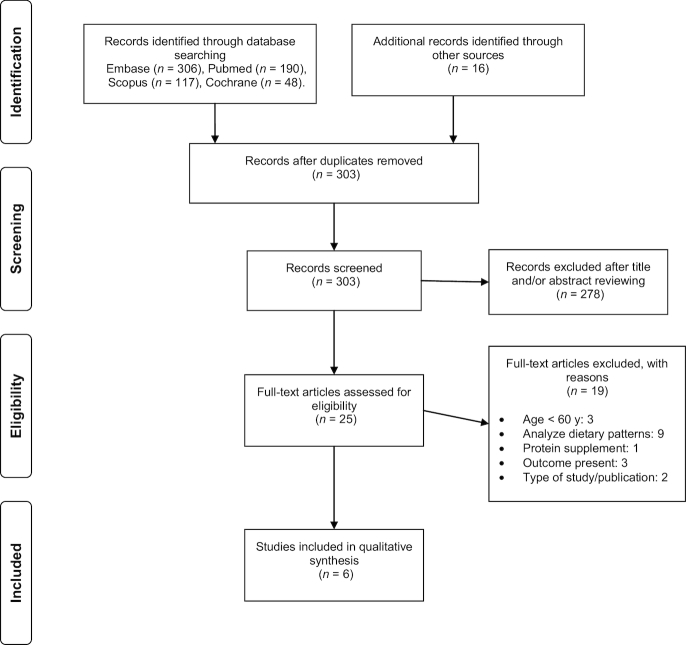
A total of 6 studies (12, 15, 27–30) met the inclusion criteria and were ultimately included in this systematic review (see Figure 1). The database searches provided a total of 661 citations from Scopus (n = 117), MEDLINE/PubMed (n = 190), the Cochrane Library (n = 48), and Embase (n = 306). Sixteen additional studies were identified by checking the references of relevant identified articles and by searching for studies that had cited these articles. Three hundred and three studies remained after duplicates were removed. Of these 303 studies, 278 were discarded because they clearly did not meet the inclusion criteria, as determined from the title and abstract. The full text of the remaining 25 articles was examined, and 19 of those studies did not meet the inclusion criteria (see Supplemental Table 1). All the publications included in this review were of acceptable quality, with a median NOS score of 7.2 out of 9 (range: 6–8) and a modified Jadad scale score of 5.5 out of 8 points. This last scale was employed for the single randomized study (15). The quality assessment of the included studies is shown in Tables 1 and 2.
FIGURE 1.
Flow diagram of studies considered for inclusion in the systematic review.
TABLE 1.
Quality assessment: NOS for cohort studies1
| Selection | Comparability | Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Study, year (ref) | Representativeness of the exposed cohort | Selection of the unexposed cohort | Ascertainment of exposure | Demonstration that the outcome of interest was not present at the start of the study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur? | Adequacy of follow-up of cohorts | Total |
| Vercambre et al., 2009 (27) | * | * | * | ** | * | * | ******* | ||
| Ozawa et al., 2014 (28) | * | * | * | ** | * | * | * | ******** | |
| Lana et al., 2015 (12) | * | * | * | * | * | * | * | * | ******** |
| Kesse-Guyot et al., 2016 (29) | * | * | ** | * | * | ****** | |||
| Petruski-Ivleva et al., 2017 (30) | * | * | * | ** | * | * | ******* | ||
1NOS high-quality choices are identified with a star. A maximum of 1 star is applied for each item within the Selection and Outcome categories, and a maximum of 2 stars for Comparability. NOS, Newcastle-Ottawa Scale; ref, reference.
TABLE 2.
Modified Jadad scale for evaluation of the methodological quality of the randomized clinical trial1
| Study, year (ref) | Was the research described as randomized? | Was the approach to randomization appropriate? | Was the research described as blinded?2 | Was the approach to blinding appropriate? | Was there a presentation of withdrawals and dropouts? | Was there a presentation of the inclusion/exclusion criteria? | Was the approach used to assess adverse effects described? | Was the approach for statistical analysis described? | Total |
|---|---|---|---|---|---|---|---|---|---|
| Alemán-Mateo et al., 2014 (15) | 1 | 1 | 0.5 | 1 | 1 | 0 | 0 | 1 | 5.5 |
1High-quality studies received scores of 4–8; ref, reference.
2Double-blind scores 1; single-blind scores 0.5.
Study characteristics
Five out of the 6 studies (12, 27–30) selected were observational prospective cohort studies, and 1 study (15) was a randomized controlled trial; all 6 were published in English. The main inclusion criteria were community-dwelling adults and the administration of a dietary questionnaire that measured the impact of dairy product intake on cognitive performance, sarcopenia, and frailty. Details of the design, study population, exposure or intervention, outcomes, and follow-up of the individual studies are shown in Tables 3 and 4.
TABLE 3.
Characteristics of the studies evaluating the association between dairy intake and cognitive performance1
| Exposure | Outcome definition: cognitive performance | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors (ref), country | Study design | Participants (n) | Age (y) | Follow-up (y) | Male (%) | Baseline age (y) | Method of assessment | Category | Type | Method of assessment | Association measure | Controlled variables |
| Vercambre et al. (27), France | Cohort | 4809 women from the Aging subcohort of the Etude Epidémiologique de Femmes de la Mutuelle Générale de l'Education Nationale | 65.5 ± 1.8 | 13 | 0 | 45–64, 65–79, >80 | Self-administered questionnaires at baseline only. Use of photographs to facilitate estimation of portion size. | Ts of food intake | Cognitive decline | DECO scale,2 range: 0–38 (cutoff 33, sensitivity 89%, specificity 67%). | Milk and yogurt: 234.8 ± 191.9, P for trend 0.182. T2 vs. 1: OR: 1.21* (0.97, 1.50); T3 vs. 1 OR: 1.17* (0.93, 1.46). Dairy desserts:3 22.89 ± 35.78, P- trend 0.010.* Group 2 vs. 1: OR: 1.02* (0.82, 1.28); Group 3 vs. 1: OR: 1.33* (1.07, 1.65). | Age, education, physical activity, hypertension, diabetes mellitus, BMI; smoking habits, energy intake, supplement consumption, postmenopausal hormones use, hypercholesterolemia, coronary heart disease, stroke, cancer, depression. |
| Ozawa et al. (28), Japan | Prospective cohort, community-based | 1081 subjects from the Hisayama study | Q1: 68.6 ± 6.4, Q2: 69.8 ± 6.4, Q3: 68.9 ± 6.1, Q4: 70.4 ± 6.8 | 17 | 42.2 | 60 | 70 semiquantitative FFQ items only at baseline. 1-d average intake of dairy products: 84.6 g. | Qs (g/d): women: <45, 45–96, 97–197, ≥198; men: <20, 20–75, 76–173, ≥174 | Dementia (Alzheimer or vascular dementia) | DSM-III-R to define the diagnosis of dementia. Criteria NINCDS-ADRDA to define subjects with Alzheimer disease. NINDS to determine vascular dementia. Autopsy (74.7%). | All-cause dementia: HR (95% CI), (P-trend 0.09). Q2: 0.85 (0.62, 1.18); Q3: 0.69 (0.50, 0.96); Q4: 0.80 (0.57, 1.11). Alzheimer disease: HR(95% CI), (P-trend 0.03). *Q2: 0.64 (0.41, 0.99); Q3: 0.57 (0.37, 0.87); Q4: 0.63 (0.41, 0.98). Vascular dementia: HR (95% CI), (P-trend 0.14). Q2: 1.02 (0.59, 1.77); Q3: 0.74 (0.42, 1.33); Q4: 0.69 (0.37, 1.29). | Age; sex; education; physical activity; stroke; hypertension; diabetes mellitus; total cholesterol; BMI; smoking habits; energy intake; vegetable, fruit, fish, and meat intake; alcohol consumption. |
| Kesse-Guyot et al. (29), France | Cohort | 3076 subjects from the SU.VI.MAX 2 study after excluding participants aged <45 y at baseline and those with missing data | Age of participants at cognitive evaluation: 65.5 ± 4.6 | 13 | T1: 59.9, T2: 52.1, T3: 55.6 | 52 (range: 45–65) | Computerized 24-h dietary records bimonthly. Self-reporting with validated photographs to assess portion size. Food composition table. | Ts: (g/d): T1: <191.6; T2: 191.6–327.2; T3 >327.2. Mean ± SD consumption (g/d): men: 280.7 ± 165.4; women: 277.2 ± 160.6 | Cognitive functioning. Clinical examination, neuropsychological evaluation by trained neuropsychologists | Episodic memory.4 Lexical-semantic memory.5 Working memory.6 Mental flexibility.7 Verbal memory. | Total dairy product (adjusted mean differences): Medium-T2: 0.05 (−0.78, 0.89); High-T3: −0.29 (−1.15, 0.57). Milk: Medium-T2: −0.09 (−0.94, 0.76)*; High-T3: −0.99 (−1.83, −0.15).* Cream: Medium-T2: 0.85 (−0.01, 1.70); High-T3: 0.47 (−0.37, 1.31). Yogurt: Medium-T2: 0.46 (−0.38, 1.31)*; High-T3: 0.64 (−0.30, 1.57).* Cheese: Medium-T2: 0.65 (−0.19, 1.49); High-T3: 0.63 (−0.21, 1.47). | Age, gender, education, physical activity, stroke, hypertension, diabetes mellitus, BMI, energy intake, occupation, alcohol intake, depression, self-rated health, adherence to the Mediterranean diet, Western and healthy pattern score. |
| Petruski-Ivleva et al. (30), United States | Cohort | 13,752 subjects from theARIC cohort | Not included in the article | 20 | 44 | 45–64 | FFQ on 2 occasions during the follow-up period | Milk intake: almost never (11%); <1 glass8/d (50%); 1 glass/d (15%); >1 glass/d (24%). Serving of dairy.9 | Cognitive functioning | Verbal learning10 (DWRT). Executive function11 (DSST). Expressive language12 (WFT). All test scores were converted to z scores. | Global results (20-y decline)-adjusted mean differences-: Almost never: −0.94 (−1.00, −0.88); <1 glass/d (5%): −0.99 (−1.01, −0.96); 1 glass/d (6%): −1.00 (−1.05, −0.95); >1 glass/d (11%): −1.04 (−1.08, −1.01). | Age; gender; education; physical activity; hypertension; diabetes mellitus; BMI; smoking habits; energy intake; vegetable, fruit, and alcohol intake; CHD; cancer; adherence to the Mediterranean diet. |
1Values are means ± SDs or measures (95% CIs) unless otherwise indicated. *Significant association. ARIC, Atherosclerosis Risk in Communities; CHD, coronary heart disease; DECO, Detérioration Cognitive Observée (observed cognitive deterioration); DSM-III-R, Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition; DSST, Digit Symbol Substitution Test; DWRT, Delayed Word Recall Test; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association; NINDS, National Institute of Neurological Disorders and Stroke; Q, quartile; ref, reference; SU.VI.MAX, SUpplémentation en Vitamines et Minéraux AntioXydants; T, tertile; WFT, Word Fluency Test.
2The DECO scale is a 19-item Likert scale that allows the evaluation of recent cognitive decline based on alterations of the capacity to perform specific tasks related to memory, attention, visuospatial, and language skills. A DECO score <33 constituted a group of recent cognitive decliners.
3Group 1, group 2, and group 3 were defined as no consumption, consumption less than or equal to the median, and consumption above the median for dairy desserts and ice cream, respectively.
4Episodic memory was evaluated with the RI-48 test (delayed cued recall test comprising a list of 48 words).
5Lexical-semantic memory was assessed by 2 verbal fluency tasks (semantic fluency and verbal fluency).
6Working memory was assessed with the forward and backward digit span tests.
7The Delis-Kaplan trail-making test was used to assess mental flexibility.
8236.5-mL glass.
9Intake of all dairy (including skim or low-fat and whole milk, yogurt, ice cream, cottage cheese, other cheese, and butter) in servings per day. One serving of dairy was equal to an 8-ounce (236.5 mL) cup of milk, 1 cup of yogurt, half a cup of ice cream, half a cup of cottage cheese, 1 slice of hard cheese, or 1 pat of butter.
10Verbal learning and short-term memory were assessed via the DWRT.
11Executive function was assessed via the DSST.
12Expressive language was assessed via the WFT.
TABLE 4.
Characteristics of studies evaluating the association between dairy intake, sarcopenia, and frailty1
| Exposure | Outcome definition | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors (ref), country | Study design | Participants (n) | Age (y) | Follow-up (y) | Male (%) | Baseline age (y) | Method of assessment | Category | Type | Method of assessment | Association measure | Controlled variables |
| Alemán-Mateo et al. (15), Mexico | Single-blind randomized clinical trial | 100 healthy volunteers recruited through home visits and telephone calls | 70.2 ± 7 | 3 mo | 50 | 60 | IG: ricotta cheese 210 g/d. CG: habitual diet | — | Sarcopenia | Body composition (ASMM): DXA. Hand grip strength (handgrip dynamometer). Physical performance: SPPB, SCPT. | Mean relative change (%)±SD:ASMM (kg/m2): IG: +0.7 ± 3.43; CG: −1.1 ± 2.6 (P = 0.004).* Grip strength (kg): IG: −0.6 ± 10.8; CG: −4.5 ± 10.8 (P = 0.07). SPPB (global score): IG: +2.4 ± 9.9; CG: +1.2 ± 9.3 (P = 0.55). SPPB (balance score): IG: +3.7 ± 17.1; CG: −2.4 ± 12.7 (P ≤ 0.05).* | — |
| Lana et al. (12), Spain | Prospective cohort, community-based | 1871 community-dwelling adults (free of frailty) from Seniors-ENRICA | 68.8 ± 6.7 | 3.5 | 48.4 | 60 | Computerized diet history.2 Includes sets of photographs. Consumption of dairy products: mean daily intake (±SD) 306.3 ± 177.5 g | Servings/wk. Whole milk or yogurt: <1: 50%; 1–6: 25.8%; ≥7: 20.4%. Low-fat milk or yogurt: <1: 22.3%; 1–6: 34.4%; ≥7: 4.3%. Cheese: <1: 29.2%; 1–6: 47.8%; ≥7: 23%. | Frailty, cumulative incidence: 7.2% | Modified frailty definition for CHS (3 of 5 Fried criteria): exhaustion, weakness, low physical activity, slow walking speed, and weight loss. Cohort-specific quintiles. | 1–6 serving/wk; ≥7 serving/wk.3Whole milk or yogurt (P-trend = 0.10): OR: 1.26 (0.75, 2.13); OR: 1.53 (0.9, 2.6).Whole milk (P- trend = 0.12): OR: 1.49 (0.89, 2.49); OR: 1.50 (0.65, 3.44). Whole yogurt (P- trend = 0.12): OR: 0.87 (0.47, 1.61); OR: 1.76 (1.01, 3.14). Low-fat milk or yogurt (P- trend = 0.03): OR: 0.55 (0.32, 0.97); OR: 0.52 (0.29, 0.90).* Low-fat milk (P- trend = 0.02): OR: 0.39 (0.24, 0.68); OR: 0.57 (0.32, 0.99).* Low-fat yogurt (P- trend = 0.53): OR: 0.80 (0.45, 1.40); OR: 0.87 (0.47, 1.60). Cheese (P- trend = 0.61): OR: 0.66 (0.41, 1.07); OR: 0.91 (0.52, 1.61). | Age, education, physical activity, diabetes mellitus, BMI, smoking habits, energy intake, alcohol intake, sleep time, CHD, depression, protein, calcium, saturated fat intake, chronic obstructive lung disease, musculoskeletal disorder, diet prescribed by a physician, independency in IADL, self-rated health, living alone, adherence to the Mediterranean diet. |
1Values are means ± SDs or measures (95% CIs) unless otherwise indicated. *Significant association. ASMM, appendicular skeletal muscle mass; CG, control group; CHD, coronary heart disease; CHS, Cardiovascular Health Study; IADL, Instrumental Activities of Daily Living scale; IG, intervention group; ref, reference; SCPT, stair-climb power test; Seniors-ENRICA, older cohort of the Study on Nutrition and Cardiovascular Risk in Spain; SPPB, Short Physical Performance Battery (0–12).
2One standard serving of milk = 250 mL, 1 standard serving of yogurt = 125 mL, 1 standard serving of cheese = 40 g.
3Reference = 1 serving/wk.
Impact on cognitive function
In 4 large long-term observational prospective cohort studies (27–30), the aim was to study the association between the intake of dairy products and cognitive impairment or dementia. These studies involved 22,718 older adults, although evaluation at baseline was performed in middle age in the studies by Kesse-Guyot et al. (29) and Petruski-Ivleva et al. (30). The study by Vercambre et al. (27) included only women. One study was conducted in the United States (30), 1 in Japan (28), and 2 in France (27, 29). The dietary assessment was self-reported (27, 29) or administered by an interviewer (28, 30). It was performed once at baseline (27, 28), twice (30), or bimonthly (29). The studies assessed the intake of milk and other dairy products. Some of them included ice cream as a dairy dessert (27), others assessed both the total and specific (milk, cream, yogurt, milk-based desserts, and cheese) intake of dairy products (29), whereas 1 quantified total milk and included a dairy product food group to establish a daily diet quality score (skim or low-fat and whole milk, yogurt, ice cream, cottage cheese, other cheese, and butter) (30). The timing of outcome measures was variable and included 3 investigations (30), evaluations every 1–2 y (28), or a single final evaluation at the end of the follow-up (27, 29). The follow-up period varied between 13 and 20 y. Petruski-Ivleva et al. (30) and Kesse-Guyot et al. (29) evaluated cognitive performance through neuropsychological assessment, whereas Vercambre et al. (27) assessed cognitive performance based on informant self-response to a validated questionnaire (Detérioration Cognitive Observée scale). Ozawa et al. (28) determined dementia and subtype diagnoses based on established clinical and neuropathological criteria; these assessments were performed in subjects who underwent autopsy (74.7% of patients who died).
One study (28) demonstrated a significant inverse relation between dairy intake and the development of Alzheimer disease (AD) among older Japanese subjects after adjusting for potential confounders. The development of AD was significantly reduced among subjects in the second, third, and fourth quartiles of dairy intake compared with those in the first quartile (adjusted HR: 0.64; 95% CI: 0.41, 0.99 for the second quartile; adjusted HR: 0.57; 95% CI: 0.37, 0.87 for the third quartile; adjusted HR: 0.63; 95% CI: 0.41, 0.98 for the fourth quartile). In Vercambre et al.’s study (27), higher consumption of dairy desserts and ice cream was associated with cognitive decline in older women (consumption less than or equal to the median compared with no consumption OR: 1.02; 95% CI: 0.82, 1.28; and consumption above the median compared with no consumption OR: 1.33, 95% CI: 1.07, 1.65; P-trend = 0.010). High consumption of milk at midlife was negatively associated with verbal memory (mean difference tertile 3 compared with tertile 1: −0.99; 95% CI: −1.83, −0.15), and the association was not changed after adjustment for saturated fat intake; skimmed milk was rarely consumed in this sample (29). Along the same lines, another investigation (30) suggested that greater milk intake at midlife may be associated with a higher rate of cognitive decline over a 20-y period. This response was graded across milk intake categories (the difference in global z scores between those who reported almost never drinking milk and those who reported drinking >1 glass/d was −0.10; 95% CI: −0.16, −0.03) and was shown to be equivalent to a 10% additional decline. Most participants reported drinking skim or low-fat milk, which accounted for 75% of total milk intake. Those who reported drinking more total milk also reported consuming more of other dairy products and thus exhibited greater overall dairy consumption. The association of skim or low-fat milk intake and all dairy products with the change in cognitive function was similar to the association observed with total milk intake alone. Other prospective studies (27, 31), conducted in Western countries, have reported a conflicting association increasing the risk of cognitive decline in those subjects with higher consumption of full-cream milk, milk, dairy desserts, and ice cream.
Impact on sarcopenia
One trial (15) investigated the effect of adding milk protein to the habitual diet on skeletal muscle mass, strength, and physical performance in Mexican elders without sarcopenia. This study was a single-blind randomized clinical trial that included 100 nonsarcopenic adults aged ≥60 y (50 men and 50 women) who were assigned in a 1:1 ratio to the intervention (adding 210 g of ricotta cheese daily to their habitual diet) or control group (habitual diet) for a period of 12 wk. The primary outcomes were relative changes in appendicular skeletal muscle mass (ASMM) as measured via DXA, handgrip strength as measured by a handheld dynamometer, and physical performance as measured using the SPPB and the stair-climb power test. The addition of 210 g of ricotta cheese (18 g of protein) improved ASMM. The relative change in ASMM was positive in the intervention group (0.7 ± 3.43 kg/m2) and negative in the control group (−1.1 ± 2.6 kg/m2) (P = 0.004). The improvement in the balance-test score was significant in the intervention group (3.7 ± 17) and negative in the control group (−2.4 ± 12.7) (P ≤ 0.05).
Impact on frailty
In 1 study (12), the objective was to determine the effect of dairy products on frailty. This was an observational prospective cohort study conducted in Spain that included 1871 community-dwelling subjects aged ≥60 y. For the diet history, information on the consumption of dairy products, including milk (whole and low-fat in different proportions, 0–2%), yogurt (whole and low-fat), and cheese, during the previous year was collected. The mean follow-up time was 3.5 y. Frailty was assessed based on a modified version of the Fried criteria (22). Consuming ≥7 servings of low-fat milk or yogurt per week was associated with a lower risk of frailty (OR: 0.52; 95% CI: 0.29, 0.90; P-trend = 0.03) than was found in individuals consuming <1 serving/wk. The results were similar for low-fat milk considered separately (OR: 0.57; 95% CI: 0.32, 0.99; P-trend = 0.02). Consumption of whole-milk dairy or cheese did not seem to affect frailty status after adjustment for several confounding factors.
Discussion
This systematic review identified 6 studies that included 24,689 community-dwelling older people >60 y of age who were followed for a minimum of 3.5 y, including some cohorts that were studied for 20 y, allowing midlife exposure to dairy products to be isolated from the different outcomes. The identified studies considered dairy intake as a component of dietary intake and were longitudinal. This systematic review showed that consumption of milk at midlife may be negatively associated with verbal memory performance. A greater rate of cognitive decline was probable after greater milk intake at midlife over a 20-y period. In the subgroup of older women, higher intake of dairy desserts and ice cream was associated with cognitive decline. In older Japanese subjects, a significant inverse relation between dairy intake and the development of AD was found. The addition of nutrient-rich dairy proteins (210 g of ricotta cheese) may improve physical performance while attenuating the loss of muscle strength. Finally, high consumption of low-fat milk and yogurt is related to some positive effects on frailty. Lifestyle factors such as physical activity or adherence to dietary patterns could induce residual confounding in the relation between dairy product intake and cognitive decline. All the selected studies have carefully controlled for multiple variables, including educational level, cardiovascular disease risk factors, energy intake, ischemic heart disease, alcohol consumption, or even adherence to a Mediterranean diet (12, 29, 30); all the controlled variables are described in Tables 3 and 4.
Cognitive decline outcome
In the systematic review, a longitudinal study (27) suggested no significant association between consumption of dairy products (including milk, yogurt, and cheese) and cognitive decline in French women. The women who suffered cognitive decline reported previous higher intake of dairy desserts and ice cream. The time interval between the dietary and cognitive assessments was >10 y, which was sufficient to explore the long-term effect of dietary habits. Several studies (27, 28) used only a baseline FFQ, where the assumption that dairy intake is stable throughout the follow-up period is a limitation of this design. Ozawa et al.’s long-term prospective study (28) demonstrated a significant inverse association between dairy intake and the risk of developing Alzheimer dementia in a Japanese population, after adjusting for potential confounders. In Western countries, some prospective studies (27, 31) revealed an increased risk of cognitive decline with higher consumption of whole milk, dairy desserts, and ice cream. The Kesse-Guyot et al. study (29) indicates that dairy product consumption is associated with better performance in specific cognitive domains after adjustment for lifestyle factors, health status markers, and dietary patterns. An inverse U-shaped relation between dairy product intake and working memory was observed in women. In this study, high consumption of milk was negatively associated with verbal memory, and this association was not altered after adjustment for saturated fat intake. Better verbal memory performance was suggested in those participants with high yogurt consumption, but this finding may depend on the underlying healthy dietary patterns. One strength of this study was the analysis of compliance with dietary recommendations regarding dairy and cognitive performance. Another longitudinal study (27) investigating the specific role of yogurt did not identify any association. Petruski-Ivleva et al. (30) analyzed multiple measures of cognitive function over time, considering milk intake categories, suggesting that a milk intake >1 glass/d at midlife may be associated with a greater rate of cognitive decline over a 20-y period. This study was the only one to determine lactase persistence in the sample, but owing to the small number of subjects included, the analysis lacked power to detect differences. Lactose intolerance is due to incomplete digestion of lactose, with consequent gastrointestinal discomfort that may lead to avoidance of dairy foods. This limited intake may be related to adverse health effects. Dairy products such as yogurt or cheese may be better tolerated by these subjects. In some cases, it is necessary to use exogenous lactase supplements or isolated milk proteins. Further longitudinal studies are needed to elucidate the possible link between milk intake and changes in cognitive performance in adults.
We have considered diverse explanations for the relation between dairy product intake and cognitive decline. Investigations linking cognitive performance decline and midlife dietary exposure have illustrated that vascular risk factors can increase the risk of dementia in aged people (32–34). Longitudinal studies describe a lower risk of these risk factors in subjects with higher intake of dairy products (9, 35). Another explanation is based on the nutritional components of dairy products (vitamin B-12, calcium, and vitamin D). Full-fat dairy products contain vitamin D, which has been associated with neuroprotective, antioxidant, and anti-inflammatory effects (36). The mechanisms implicated in the different outcomes are described in Table 5.
TABLE 5.
Suggested mechanisms underlying the relation between dairy product intake and different outcomes
| Cognitive performance | Frailty | Sarcopenia | |
|---|---|---|---|
| High-biologic-value proteins (essential amino acids). | Whey protein–derived bioactive compounds contribute to weight loss. | Protein intake and calcium may delay sarcopenia and bone loss (both related to frailty) (39). Bioactive peptides have shown a beneficial effect on blood pressure. | Gains in total lean tissue are related to the amount of added protein. Dietary protein supplementation boosts muscle protein synthesis and improves nitrogen balance (40). The muscle response to protein can be influenced by antioxidant intake (41). Several studies have investigated the combined effect of dairy protein with exercise. |
| Minerals (calcium, phosphorus, magnesium). | Modulation of vascular function. Antioxidant and anti-inflammatory properties (42). | Milk minerals (magnesium, phosphorus, potassium, calcium) may be responsible for the positive effect on hypertension (9). | Lactose enhances calcium absorption. Magnesium is involved in protein synthesis; its intake is positively associated with appendicular lean mass (43). The role of calcium in sarcopenia seems to be related to calcium intake in older adults. |
| Vitamins (B group, D) | Antioxidant and anti-inflammatory effects (36). | — | Variable evidence of increased muscle strength after correction of vitamin D deficiency (44). |
| Fat content | — | The saturated fat content could influence the risk of frailty. This fact should be confirmed in future research. | — |
| Lower risk of cardiovascular disease risk factors (9, 35):• Diabetes mellitus • Hypertension • Dyslipidemia • Obesity. | Vascular hypothesis of dementia (32). Weight loss (increased satiety) (45).↓ Metabolic syndrome (46, 47).↑ Fat excretion (48).↓ Insulin resistance (49). Effect of lactose on oxidative stress. | Dairy product consumption lowers the likelihood of cardiometabolic conditions (50). Positive effects on blood pressure, with reduction in the incidence of hypertension (especially low-fat dairy products) (51). Reduction of inflammatory markers.↑ insulin sensitivity and lower plasma insulin (52). | — |
Sarcopenia outcome
The second outcome analyzed was the relation between dairy product consumption and the incidence of sarcopenia, which involved the only selected interventional study. The addition of 210 g of ricotta cheese improved ASMM and balance-test scores, with attenuation of the loss of muscle strength. It is important to emphasize that there was no increase in total or truncal fat during the study, nor was there renal function impairment. The main effect on lean tissue could be explained by the amount of daily protein added to the diet, which was equivalent to 18.12 g of protein. Increasing total protein intake improves nitrogen balance. The classic RDA was 0.8 g · kg–1 · d –1 of protein, but this recommendation did not include the amount of daily protein necessary to prevent functional decline, especially with aging. New evidence suggests that dietary protein supplementation above the RDA (1–1.5 g · kg–1 · d –1) may be an important intervention to prevent sarcopenia (37); protein ingestion has been demonstrated to attenuate age-dependent muscle loss and is related to quality of life (38). Thus, the authors estimated that adding ricotta cheese increased protein intake from 0.9 g to 1.2 g of protein · kg–1 · d–1, assuming regular protein intake with meals. Perhaps the nonsarcopenic subjects included in this study were more sensitive to the anabolic stimulus of a high protein intake and they were probably more active. Sarcopenia syndrome is a multifactorial disorder in which nondietary factors such as physical activity are involved. These results reinforce the preventive role of the intervention in earlier phases, before the development of sarcopenia. Clinically, this better muscular condition implies an improvement in balance, which is a measure included in the SPPB evaluation. Although this evaluation is not part of the Fried criteria, it may provide additional information about the risk of falls and lower-extremity strength.
Frailty outcome
The third outcome, the risk of frailty, is close to the definition of sarcopenia and was analyzed in 1 study (12). The criteria for defining frailty are well defined: a modified definition of frailty used in the Cardiovascular Health Study, considering frailty with 3 of 5 criteria, was employed. The authors included different dairy products with sufficient detail (yogurt, milk, and cheese), with several fat proportions and a follow-up period long enough for the outcome to be accurately assessed. The authors adjusted the results for different important confounders. Higher consumption of low-fat milk or yogurt (≥7 servings/wk) was associated with a lower risk of frailty (specifically, slow walking speed and weight loss items). Gait speed is an objective component of frailty syndrome, also associated with cardiovascular mortality (53). This important fact provides additional valuable prognostic information for different pathologies beyond traditional scoring methods. Although a recent systematic review (54) concluded that greater adherence to a Mediterranean diet is associated with a significantly lower risk of frailty incidence in community-dwelling older people, there is scant evidence regarding the effect of particular foods on the risk of frailty (8, 55). Another study (27) included in our review did not identify a relation between dairy consumption (milk, yogurt, and dairy desserts) and functional impairment, but in this case, a less sensitive, simplified Instrumental Activities of Daily Living scale was used.
Several mechanisms may indirectly explain the effect of frailty risk acting over related disorders, such as sarcopenia and cardiometabolic conditions (see Table 5). The protein and calcium included in dairy products are an essential component of sarcopenia and osteopenia intervention (39). Preclinical atherosclerosis and inflammation states are potentially modifiable risk factors for frailty (56). The inflammation theory explains the existence of a systemic proinflammatory state that has been termed “inflammaging” in the elderly (57, 58). On the other hand, yogurt naturally contains minerals and vitamins that have been associated with improved measures of frailty (59, 60).
Methodological considerations and limitations
The cognitive decline outcome was analyzed by different methods. The classic Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition criteria were used to define the possibility of a dementia diagnosis; for the Alzheimer or vascular dementia subtype, the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria or National Institute of Neurological Disorders and Stroke criteria were used. This approach was a clinical means of assessing the incidence of cognitive impairment in Ozawa et al.’s study (28). On the other hand, scales such as Detérioration Cognitive Observée were used in 1 study (27) to identify the subject's capacity to perform specific tasks related to memory, attention, visuospatial, and language skills based on the subject's self-response to the questionnaire. This type of analysis could be imprecise because it is complicated to identify the effect of dairy products on different cognitive domains. Kesse-Guyot et al.’s study (29) was based on clinical examination, with an additional neuropsychological evaluation by a trained neuropsychologist with a special interest in the different memory domains. This approach allowed the authors to perform a more detailed evaluation that could help to detect early signs of cognitive impairment. However, baseline comprehensive cognitive performance data were not available, so causal inference was limited, and the participant volunteers could have been particularly compliant in this case. Some studies only included women, which limited the generalization of the results to men (27). In general, these details explain the difficulty in assessing global cognitive function.
Second, most of the studies used FFQs (quantitative or semiquantitative) to assess dietary patterns, with estimation of portion sizes. The time interval between the dietary and cognitive assessments was >10 y, which is sufficient to explore the long-term effect of dietary habits. An important limitation of the studies using a baseline FFQ to predict a change in health outcome (cognitive performance in this case) over a prolonged period is the possibility that dietary habits change over time in some populations and the assumption that the assessment of average dairy product intake at different visits reflects long-term habitual intake throughout adulthood. In 2 studies (27, 28) the FFQ was administered only at baseline. In Petruski-Ivleva et al.’s study (30), an FFQ was administered over 2 visits during the follow-up period. In Kesse-Guyot et al.’s study (29), the participants completed a 24-h dietary record bimonthly. Some authors have investigated the accuracy of nutrient intake estimation by FFQ and have concluded that memory could influence the validity of these questionnaires (61), although they are a reliable method of assessing long-term intake. One study included 2 groups, consisting of dairy products (milk and yogurt, dairy desserts and ice cream) and cheese (27), whereas another study assessed total dairy products and 4 individual dairy foods (milk, cream, yogurt, and cheese) (29). One study only studied milk in general (30), combining skim or low-fat and whole milk. One study included dairy products without specifying the particular included foods (28). This point is of interest because different dairy products may exhibit different associations with cognitive disorders. There were different categories of milk intake, most of which were described in tertiles or quartiles (27–29); the number of glasses (236.5 mL) was employed in 1 case (30). A standardized measurement should be used in future prospective studies. In addition, the type of design of most of the included studies is particularly prone to reverse causality because demented patients may have modified their diet as a consequence of their cognitive impairment.
Third, Ozawa et al.’s study (28) was based on a Japanese population, and Japanese individuals have historically consumed approximately half the amount of dairy products compared with Western populations. This fact could explain the discrepancy in the influence of dairy foods on the risk of dementia. In such populations with low dairy intake, high consumption is considered to reduce the risk of dementia. Fourth, the data on fat contents are not clear. One study provided results after adjustment for saturated fat intake, and there was no change in the association (29). Another study (30) provided percentages of skim or low-fat milk intake, and the observed association with the change in cognitive function was similar to that observed for total milk. Finally, 1 study (27) measured dietary intake over an extensive period of time, such that the consumption of full-fat dairy products was likely, because this kind of dairy product was consumed frequently at that time.
In the single-blind randomized clinical trial (15), the methodology for analyzing the sarcopenia outcome was based on the classical European consensus, using DXA to study fat mass, lean tissue, and bone mineral contents, a dynamometer for assessing hand grip strength, and the SPPB for evaluating physical performance. It is important to also assess muscle strength using a leg press, to detect possible changes in leg muscle strength. The duration of the study could have been too short and the number of participants too small to demonstrate a clear effect on muscle mass. The study did not include biopsies of skeletal muscle or assessment of biomarkers to improve the physiological explanation of the results. The amount of protein given to participants could also have been insufficient; other studies offer supplementation with 30–40 g protein/d, with special interest in considering the protein distribution during the day (62). In clinical practice, a combined intervention including not only protein but also resistance exercise would be recommended (63).
The longitudinal study of frailty risk concluded that the association between dairy products and frailty differs depending on the fat content. A higher risk of frailty was not found in consumers of higher amounts of saturated fat, so a possible confounding factor could be present in individuals consuming low-fat dairy products. On the other hand, most robust individuals survived until the end of the follow-up period, which may have led to underestimation of the frailty incidence, although study mortality was low.
Conclusions
The relation between dairy product intake and cognitive decline is complex and probably depends on the type of dairy product and the quantity ingested. Studies concerning cognitive decline have produced contradictory findings. Increased consumption of dairy desserts and ice cream was observed to be associated with cognitive decline in women, and high consumption of milk at midlife was negatively associated with cognitive domains such as verbal memory after adjustment for saturated fat intake. A greater rate of cognitive decline over a 20-y period was probable after greater milk intake at midlife, although the response was graded across milk intake categories. The association of skim or low-fat milk and all dairy with change in cognitive function was similar to that of total milk consumption. A significant inverse relation between dairy intake and the development of AD was detected but seemed to be limited to the Asian population. Methodological variability and the difficulty of assessing global cognitive function prevent conclusions from being drawn about the optimal dairy intake for the older population. Future long-term intervention trials are needed, with detailed assessment of dairy intake, fat content, and cognition domains several times during the follow-up period. Attention to the overall dietary composition seems to be a more useful approach for the prevention and management of AD risk. The addition of nutrient-rich dairy proteins may improve physical performance and attenuate loss of muscle strength, thereby helping to prevent sarcopenia syndrome in the elderly population. Our systematic review showed that the available evidence is limited, but there are some positive effects of dairy product intake on frailty, especially with high consumption of low-fat milk and yogurt.
Supplementary Material
Acknowledgments
We thank Vincenzo Malafarina and Letizia Suescun (Complejo Hospitalario, Navarra) for technical consultation during the publication process. All authors have read and approved the final manuscript.
Notes
This supplement was sponsored by the Interprofessional Dairy Organization (INLAC) of Spain. The sponsor had no role in the design of the studies included in the supplement; in the collection, analyses, or interpretation of the data; in the writing of the manuscripts; or in the decision to publish the results. Instituto de Salud Carlos III is supported by funds from the Spanish Ministry of Health and FEDER (Fondo Europeo de Desarrollo Regional) (PI15/00773). Publication costs for this supplement were defrayed in part by the payment of page charges. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
Author disclosures: FC-T, CV-B, CF-P, and FJM-S, no conflicts of interest.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: AD, Alzheimer disease; ASMM, appendicular skeletal muscle mass; NOS, Newcastle-Ottawa Scale; SPPB, Short Physical Performance Battery.
References
- 1. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(08):1487–92. [DOI] [PubMed] [Google Scholar]
- 2. McGuigan FE, Bartosch P, Åkesson KE. Musculoskeletal health and frailty. Best Pract Res Clin Rheumatol. 2017;31(2):145–59. [DOI] [PubMed] [Google Scholar]
- 3. Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA. Frailty is associated with incident Alzheimer's disease and cognitive decline in the elderly. Psychosom Med. 2007;69(5):483–9. [DOI] [PubMed] [Google Scholar]
- 4. Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. 2010;58(2):248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yannakoulia M, Ntanasi E, Anastasiou CA, Scarmeas N. Frailty and nutrition: from epidemiological and clinical evidence to potential mechanisms. Metabolism. 2017;68:64–76. [DOI] [PubMed] [Google Scholar]
- 6. Bauer JM, Morley JE. The relevance of healthy diets for the prevention of frailty and cognitive impairment. Curr Opin Clin Nutr Metab Care. 2018;21(1):1–3. [DOI] [PubMed] [Google Scholar]
- 7. Dominguez LJ, Barbagallo M. The relevance of nutrition for the concept of cognitive frailty. Curr Opin Clin Nutr Metab Care. 2017;20(1):61–8. [DOI] [PubMed] [Google Scholar]
- 8. Lo Y-L, Hsieh Y-T, Hsu L-L, Chuang S-Y, Chang H-Y, Hsu C-C, Chen C-Y, Pan W-H. Dietary pattern associated with frailty: results from Nutrition and Health Survey in Taiwan. J Am Geriatr Soc. 2017;65(9):2009–15. [DOI] [PubMed] [Google Scholar]
- 9. Soedamah-Muthu SS, Verberne LDM, Ding EL, Engberink MF, Geleijnse JM. Dairy consumption and incidence of hypertension: a dose-response meta-analysis of prospective cohort studies. Hypertension. 2012;60(5):1131–7. [DOI] [PubMed] [Google Scholar]
- 10. Tong X, Dong J-Y, Wu Z-W, Li W, Qin L-Q. Dairy consumption and risk of type 2 diabetes mellitus: a meta-analysis of cohort studies. Eur J Clin Nutr. 2011;65(9):1027–31. [DOI] [PubMed] [Google Scholar]
- 11. Wu L, Sun D. Meta-analysis of milk consumption and the risk of cognitive disorders. Nutrients. 2016;8(12):824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lana A, Rodriguez-Artalejo F, Lopez-Garcia E. Dairy consumption and risk of frailty in older adults: a prospective cohort study. J Am Geriatr Soc. 2015;63(9):1852–60. [DOI] [PubMed] [Google Scholar]
- 13. Kim J, Lee Y. Frequency of dairy consumption and functional disability in older persons. J Nutr Health Aging. 2011;15(9):795–800. [DOI] [PubMed] [Google Scholar]
- 14. Radavelli-Bagatini S, Zhu K, Lewis JR, Dhaliwal SS, Prince RL. Association of dairy intake with body composition and physical function in older community-dwelling women. J Acad Nutr Diet. 2013;113(12):1669–74. [DOI] [PubMed] [Google Scholar]
- 15. Alemán-Mateo H, Carreón VR, Macías L, Astiazaran-García H, Gallegos-Aguilar AC, Enríquez JR. Nutrient-rich dairy proteins improve appendicular skeletal muscle mass and physical performance, and attenuate the loss of muscle strength in older men and women subjects: a single-blind randomized clinical trial. Clin Interv Aging. 2014;9:1517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel J-P, Rolland Y, Schneider SM et al.. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D et al.. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roedl KJ, Wilson LS, Fine J. A systematic review and comparison of functional assessments of community-dwelling elderly patients. J Am Assoc Nurse Pract. 2016;28(3):160–9. [DOI] [PubMed] [Google Scholar]
- 21. Chin A Paw MJM, Dekker JM, Feskens EJ, Schouten EG, Kromhout D. How to select a frail elderly population? A comparison of three working definitions. J Clin Epidemiol. 1999;52(11):1015–21. [DOI] [PubMed] [Google Scholar]
- 22. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G et al.. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–57. [DOI] [PubMed] [Google Scholar]
- 23. Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Walston JD; Interventions on Frailty Working Group. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52(4):625–34. [DOI] [PubMed] [Google Scholar]
- 24. Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16(7):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Internet]. Ottawa: Ottawa Hospital Research Institute; 2013; [cited 30 Jun, 2018]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 26. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Control Clin Trials. 1996;17(1):1–12. [DOI] [PubMed] [Google Scholar]
- 27. Vercambre M-N, Boutron-Ruault M-C, Ritchie K, Clavel-Chapelon F, Berr C. Long-term association of food and nutrient intakes with cognitive and functional decline: a 13-year follow-up study of elderly French women. Br J Nutr. 2009;102(3):419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ozawa M, Ohara T, Ninomiya T, Hata J, Yoshida D, Mukai N, Nagata M, Uchida K, Shirota T, Kitazono T et al.. Milk and dairy consumption and risk of dementia in an elderly Japanese population: the Hisayama study. J Am Geriatr Soc. 2014;62(7):1224–30. [DOI] [PubMed] [Google Scholar]
- 29. Kesse-Guyot E, Assmann KE, Andreeva VA, Ferry M, Hercberg S, Galan P. Consumption of dairy products and cognitive functioning: findings from the SU.VI.MAX 2 study. J Nutr Heal Aging. 2016;20(2):128–37. [DOI] [PubMed] [Google Scholar]
- 30. Petruski-Ivleva N, Kucharska-Newton A, Palta P, Couper D, Meyer K, Graff M, Haring B, Sharrett R, Heiss G. Milk intake at midlife and cognitive decline over 20 years. The Atherosclerosis Risk In Communities (ARIC) study. Nutrients. 2017;9(10):1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Almeida OP, Norman P, Hankey G, Jamrozik K, Flicker L. Successful mental health aging: results from a longitudinal study of older Australian men. Am J Geriatr Psychiatry. 2006;14(1):27–35. [DOI] [PubMed] [Google Scholar]
- 32. Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Monette MCE, Baird A, Jackson DL. A meta-analysis of cognitive functioning in nondemented adults with type 2 diabetes mellitus. Can J Diabetes. 2014;38(6):401–8. [DOI] [PubMed] [Google Scholar]
- 34. Gifford KA, Badaracco M, Liu D, Tripodis Y, Gentile A, Lu Z, Palmisano J, Jefferson AL. Blood pressure and cognition among older adults: a meta-analysis. Arch Clin Neuropsychol. 2013;28(7):649–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aune D, Norat T, Romundstad P, Vatten LJ. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr. 2013;98(4):1066–83. [DOI] [PubMed] [Google Scholar]
- 36. Annweiler C, Schott AM, Rolland Y, Blain H, Herrmann FR, Beauchet O. Dietary intake of vitamin D and cognition in older women: a large population-based study. Neurology. 2010;75(20):1810–6. [DOI] [PubMed] [Google Scholar]
- 37. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, Phillips S, Sieber C, Stehle P, Teta D et al.. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14(08):542–59. [DOI] [PubMed] [Google Scholar]
- 38. Wolfe RR. The role of dietary protein in optimizing muscle mass, function and health outcomes in older individuals. Br J Nutr. 2012;108(S2):S88–93. [DOI] [PubMed] [Google Scholar]
- 39. Bonjour JP, Kraenzlin M, Levasseur R, Warren M, Whiting S. Dairy in adulthood: from foods to nutrient interactions on bone and skeletal muscle health. J Am Coll Nutr. 2013;32(4):251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Robinson SM, Reginster JY, Rizzoli R, Shaw SC, Kanis JA, Bautmans I, Bischoff-Ferrari H, Bruyère O, Cesari M, Dawson-Hughes B et al.. Does nutrition play a role in the prevention and management of sarcopenia?. Clin Nutr. 2018;37(4):1121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chaput JP, Lord C, Cloutier M, Aubertin Leheudre M, Goulet EDB, Rousseau S, Khalil A, Dionne IJ. Relationship between antioxidant intakes and class I sarcopenia in elderly men and women. J Nutr Health Aging. 2007;11(4):363–9. [PubMed] [Google Scholar]
- 42. Berridge MJ. Calcium hypothesis of Alzheimer's disease. Pflügers Arch. 2010;459(3):441–9. [DOI] [PubMed] [Google Scholar]
- 43. van Dronkelaar C, van Velzen A, Abdelrazek M, van der Steen A, Weijs PJM, Tieland M. Minerals and sarcopenia; the role of calcium, iron, magnesium, phosphorus, potassium, selenium, sodium, and zinc on muscle mass, muscle strength, and physical performance in older adults: a systematic review. J Am Med Dir Assoc. 2018;19(1):6–11. [DOI] [PubMed] [Google Scholar]
- 44. Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, Petermans J, Reginster J-Y, Bruyère O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014;99(11):4336–45. [DOI] [PubMed] [Google Scholar]
- 45. Major GC, Chaput J-P, Ledoux M, St-Pierre S, Anderson GH, Zemel MB, Tremblay A. Recent developments in calcium-related obesity research. Obes Rev. 2008;9(5):428–45. [DOI] [PubMed] [Google Scholar]
- 46. Pereira MA, Jacobs DR, Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: the CARDIA Study. JAMA. 2002;287(16):2081–9. [DOI] [PubMed] [Google Scholar]
- 47. Pfeuffer M, Schrezenmeir J. Milk and the metabolic syndrome. Obes Rev. 2007;8(2):109–18. [DOI] [PubMed] [Google Scholar]
- 48. Astrup A, Chaput J-P, Gilbert J-A, Lorenzen JK. Dairy beverages and energy balance. Physiol Behav. 2010;100(1):67–75. [DOI] [PubMed] [Google Scholar]
- 49. Turner KM, Keogh JB, Clifton PM. Dairy consumption and insulin sensitivity: a systematic review of short- and long-term intervention studies. Nutr Metab Cardiovasc Dis. 2015;25(1):3–8. [DOI] [PubMed] [Google Scholar]
- 50. Bouillon K, Batty GD, Hamer M, Sabia S, Shipley MJ, Britton A, Singh-Manoux A, Kivimäki M. Cardiovascular disease risk scores in identifying future frailty: the Whitehall II prospective cohort study. Heart. 2013;99(10):737–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Toledo E, Delgado-Rodríguez M, Estruch R, Salas-Salvadó J, Corella D, Gomez-Gracia E, Fiol M, Lamuela-Raventós RM, Schröder H, Arós F et al.. Low-fat dairy products and blood pressure: follow-up of 2290 older persons at high cardiovascular risk participating in the PREDIMED study. Br J Nutr. 2009;101(1):59–67. [DOI] [PubMed] [Google Scholar]
- 52. Rice BH, Quann EE, Miller GD. Meeting and exceeding dairy recommendations: effects of dairy consumption on nutrient intakes and risk of chronic disease. Nutr Rev. 2013;71(4):209–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chainani V, Shaharyar S, Dave K, Choksi V, Ravindranathan S, Hanno R, Jamal O, Abdo A, Abi Rafeh N. Objective measures of the frailty syndrome (hand grip strength and gait speed) and cardiovascular mortality: a systematic review. Int J Cardiol. 2016;215:487–93. [DOI] [PubMed] [Google Scholar]
- 54. Kojima G, Avgerinou C, Iliffe S, Walters K. Adherence to Mediterranean diet reduces incident frailty risk: systematic review and meta-analysis. J Am Geriatr Soc. 2018;66(4):783–8. [DOI] [PubMed] [Google Scholar]
- 55. Chan R, Leung J, Woo J. Dietary patterns and risk of frailty in Chinese community-dwelling older people in Hong Kong: a prospective cohort study. Nutrients. 2015;7(08):7070–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chang C-C, Hsu C-Y, Huang P-H, Liu L-K, Chen L-K, Chen J-W, Lin S-J. Association between frailty and carotid intima media thickness and inflammatory marker in an elderly population. Geriatr Gerontol Int. 2017;17(12):2449–54. [DOI] [PubMed] [Google Scholar]
- 57. Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–54. [DOI] [PubMed] [Google Scholar]
- 58. Draganidis D, Karagounis LG, Athanailidis I, Chatzinikolaou A, Jamurtas AZ, Fatouros IG. Inflammaging and skeletal muscle: can protein intake make a difference?. J Nutr. 2016;146(10):1940–52. [DOI] [PubMed] [Google Scholar]
- 59. Matteini AM, Walston JD, Fallin MD, Bandeen-Roche K, Kao WHL, Semba RD, Allen RH, Guralnik J, Fried LP, Stabler SP. Markers of B-vitamin deficiency and frailty in older women. J Nutr Health Aging. 2008;12(5):303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shardell M, Hicks GE, Miller RR, Kritchevsky S, Andersen D, Bandinelli S, Cherubini A, Ferrucci L. Association of low vitamin D levels with the frailty syndrome in men and women. J Gerontol A Biol Sci Med Sci. 2009;64A(1):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bowman GL, Shannon J, Ho E, Traber MG, Frei B, Oken BS, Kaye JA, Quinn JF. Reliability and validity of food frequency questionnaire and nutrient biomarkers in elders with and without mild cognitive impairment. Alzheimer Dis Assoc Disord. 2011;25(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tomé D. Muscle protein synthesis and muscle mass in healthy older men. J Nutr. 2017;147(12):2209–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lee S-Y, Tung H-H, Liu C-Y, Chen L-K. Physical activity and sarcopenia in the geriatric population: a systematic review. J Am Med Dir Assoc. 2018;19(5):378–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.