ABSTRACT
Some studies have reported that milk and dairy product consumption reduces bladder cancer incidence, whereas others have reported null or opposite findings. This meta-analysis of 26 cohort and case-control studies has been conducted to pool the risk of the association between milk and dairy products and bladder cancer. A systematic search in MEDLINE, EMBASE, and the Web of Science (from inception to 30 April 2018) was conducted. Random-effects models were used to compute pooled estimates of RR for high or medium compared with low consumption of milk and dairy. Sensitivity analyses were conducted. Subgroup analyses were performed based on type of dairy, gender, geographic location, and type of study design. Random-effects meta-regression was used to evaluate other confounding factors. Overall, medium compared with low consumption was associated with lower pooled risk of bladder cancer for total dairy products (RR = 0.90; 95% CI: 0.81, 0.98), milk (RR = 0.90; 95% CI: 0.82, 0.98), and fermented dairy products (RR = 0.87; 95% CI: 0.79, 0.96). The inverse association for milk consumption was stronger in Asians (RR = 0.79; 95% CI: 0.59, 0.98) and in cohort design studies (RR = 0.85; 95% CI: 0.71, 0.99). Moreover, high compared with low consumption was significantly associated with a lower pooled risk for milk (RR = 0.89; 95% CI: 0.81, 0.98) and fermented dairy products (RR = 0.78; 95% CI: 0.61, 0.94). However, high compared with low consumption of whole milk was significantly associated with a higher risk (RR = 1.21; 95% CI: 1.04, 1.38). The statistical heterogeneity was considerable. In conclusion, the present meta-analysis suggests a decreased risk of bladder cancer associated with medium consumption of total dairy products and with medium and high consumption of milk and fermented dairy products. An increased risk of bladder cancer was observed with high consumption of whole milk. Interpretations of the results should be made with caution. This review was registered at www.crd.york.ac.uk/prospero as CRD42018097020.
Keywords: milk, dairy products, fermented dairy products; whole milk; bladder cancer, meta-analysis; systematic review
Introduction
According to the International Agency for Research on Cancer, bladder cancer is the ninth most common cancer in the world, with 430,000 new cases diagnosed in 2012 (3% of all new cases of cancer) (1).
Bladder cancer becomes more common with increased age and is more common in men than in women. Moreover, the most common risk factor for developing bladder cancer is cigarette smoking. Smokers are 4–7 times more likely to develop bladder cancer than nonsmokers. Other important risk factors are the following: exposure to aromatic amines and 4,4′-methylenebis (2-chloroaniline) used in different types of industries (textile, print, paint, etc.), schistosomiasis parasitic disease, exposure to arsenic in drinking water, certain medications (phenacetin, cyclophosphamide, and chlornaphazine), radiation, and genetic factors (2). However, the occurrence of bladder cancer has not been fully explained by these risk factors. Although the evidence is inconsistent or controversial, dietary habits could also influence the risk of bladder cancer because most metabolites are excreted through the urinary bladder (3).
With regards to dietary factors, different studies have suggested a protective effect of fruit and vegetable intake against bladder cancer and a possible positive association with fat intake (4). The relation between the consumption of milk or dairy products and the risk of bladder cancer has been investigated in several epidemiologic studies since 1980. Some studies have reported that higher intakes of milk or dairy products reduce bladder cancer incidence, whereas other studies observed no significant association. In 2011, the findings of a meta-analysis by Li et al. (5) were not supportive of an independent relation between the intake of milk or dairy products and the risk of bladder cancer, with the exception of inverse associations found in the United States for bladder cancer risk and milk intake and in Japan for bladder cancer risk and dairy product intake. Another meta-analysis conducted in 2011 by Mao et al. (6) suggested a potential protective effect of milk for bladder cancer, but this relation varied widely across geographical regions and specific dairy products. Specifically, they noted a significant association of higher milk consumers with decreased risk of bladder cancer only in Asia and postulated that the different observation may be explained, at least in part, by the variations of milk consumption across the world (6). However, conclusions of these 2 meta-analyses (5, 6) were consistent regarding their findings being based on limited research and future research to confirm these findings is warranted. According to these results, the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) in their Continuous Update Project Expert Report 2018 (Diet, nutrition, physical activity and bladder cancer) (7) concluded that the evidence of a higher consumption of milk and dairy products decreasing the risk of bladder cancer is limited.
Regarding the inconsistent findings on the relation between milk or dairy product intake and bladder cancer risk, this new meta-analysis of prospective cohort and case-control studies was conducted to address this topic and included 3 additional published observational studies. The main goal was to estimate the summary RR of the association between milk and dairy products and bladder cancer and examine potential sources of heterogeneity across studies. Specifically, more detailed analyses were conducted to clarify the relation between milk or dairy product intake and bladder cancer risk, taking into account the type of dairy products, fat content, quantity consumed, geographic location, and the type of study design.
Methods
This study was reported according to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) statements (8) and followed the recommendations of the Cochrane Collaboration Handbook (9). This systematic review and meta-analysis was registered through the international prospective register of systematic reviews (PROSPERO) as CRD42018097020.
Search strategy
We systematically searched the MEDLINE (via PubMed), EMBASE, and Web of Science databases from their inception until April, 2018. Observational studies addressing the association between dairy product consumption and bladder cancer were eligible. The search terms used for the search strategy were: “bladder cancer,” “bladder-cancer,” “bladder,” “urinary tract cancer,” “urinary bladder,” “cancer,” “dairy,” “milk,” “yogurt,” “cheese,” “kefir,” “butter,” “dairy products,” “cohort study,” “population-based,” “case-control study,” “prospective,” and “case control.” The literature search was complemented by screening references included in the articles considered eligible for the systematic review.
Study selection
Inclusion criteria were as follows: 1) participants: adult population; 2) study design: cohort studies or case-control studies with prospective or retrospective data collection; 3) exposure: dairy products [total, milk, (whole milk, low-fat milk, skimmed milk), fermented (i.e., yogurt, fermented milk products, yakult, quark, buttermilk, or sour cream), cheese, or butter] considered as reported by included studies; and 4) outcome: bladder cancer. The criteria for excluding studies were as follows: 1) reports not written in English or Spanish; 2) studies including individuals younger than 18 y old; and 3) ineligible publication types, such as review articles, editorials, comments, guidelines, or case-reports.
When >1 study provided data from the same sample, we only considered the one presenting the most detailed results or providing data for the largest sample size. However, data regarding sample characteristics were extracted from multiple reports to obtain the most complete information.
The literature search was performed independently by 2 reviewers (IC-R and LMB) and disagreements were solved by consensus or involving a third researcher (CS or BL-P).
Data extraction and quality assessment
The following data were extracted from the original reports: 1) year of publication; 2) study characteristics (country, period of data collection, and length of follow-up), 3) sample characteristics (sample size and age distribution), 4) dietary assessment, 5) dairy product assessed, 6) number of bladder cancer events, and 7) methodological quality. Information for case-control and cohort studies was extracted and organized separately into 2 tables.
The Quality in Prognosis Studies tool was used to evaluate the risk of bias in 6 domains: study participation (sampling bias), study attrition (attrition bias), prognostic factor measurement, outcome measurement (ascertainment bias), study confounding, and statistical analysis and reporting (10). Studies were considered to have a low, moderate, or high risk of bias if they satisfied 5–6, 3–4, or 1–2 of the 6 domains, respectively.
Data extraction and quality assessment were independently performed by 2 researchers (IC-R and LMB) and inconsistencies were solved by consensus or involving a third researcher (CS).
Statistical analysis and data synthesis
The lowest (the first quantile reported), medium (quantiles reported between the first and last quantiles), and highest (the last quantile reported) dairy product consumption categories reported from studies were considered as “low,” “medium,” and “high” dairy product consumption, respectively. The DerSimonian and Laird random-effects method was used to compute pooled estimates of RRs and their respective 95% CIs for the risk of bladder cancer associated with dairy product consumption (11). Forest plots were performed separately for high compared with low and medium compared with low dairy product consumption. The heterogeneity of results across studies was evaluated using the I2 statistic (12), and the results were considered as: might not be important (0–40%), may represent moderate heterogeneity (30–60%), may represent substantial heterogeneity (50–90%), and considerable heterogeneity (75–100%) (9). In addition, the corresponding P values were considered.
When a study reported several statistical models, only the one including the largest number of additional covariates was considered. In addition, when studies reported ORs, the RR was calculated using the following equation: RR = OR/(1 − Prevalence) + (Prevalence × OR).
Sensitivity analyses were conducted excluding studies one by one from the pooled effect to assess the robustness of the summary estimates and to detect if any particular study accounted for a large proportion of the heterogeneity.
Subgroup analyses were performed based on the type of dairy products to estimate the risk of bladder cancer associated with dairy product consumption (total dairy products, milk, whole milk, fermented dairy products, cheese, and butter). In addition, subgroup analyses were performed based on gender, geographic location (Americas, Europe, and Asia), and type of study design (case-control and cohort studies) for each dairy product subgroup. Subgroup analyses were performed with ≥3 studies in each subgroup.
In addition, random-effects meta-regression was used to evaluate whether results differed according to the age of participants, percentage of current smokers, or the year when the study started, as these could be considered to be sources of heterogeneity. Random-effects meta-regressions were performed only in dairy product subgroups in which >10 studies were included (13).
Finally, publication bias was evaluated through visual inspection of funnel plots, as well as by using the method proposed by Egger et al. (14). Statistical analyses were performed using StataSE software, version 15 (StataCorp).
Results
Systematic review
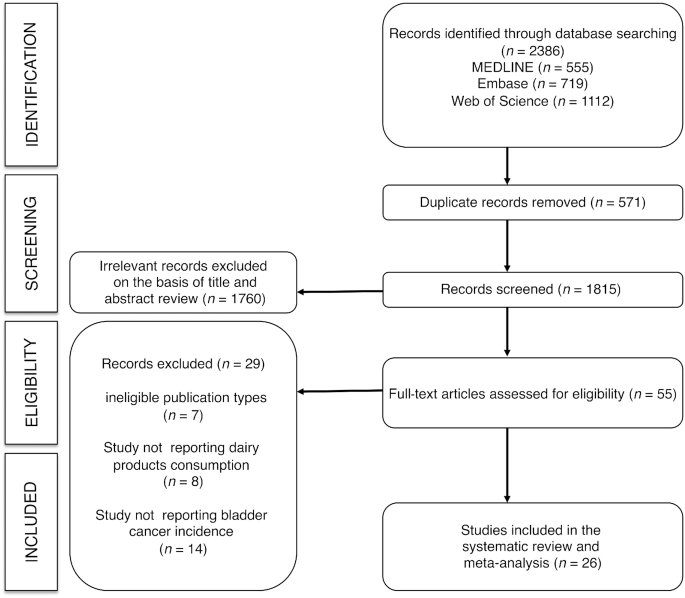
From the 55 full-text articles reviewed, 26 studies [18 case-control (15–32) and 8 cohort studies (33–40)] met the eligibility criteria (Figure 1). The studies were conducted in 8 European countries, 10 countries in the Americas, and 8 Asian countries. The reports were published between 1988 and 2014. Eight studies used a prospective design and 18 utilized a retrospective design. The beginning of data collection in the studies was established between 1942 and 2005 (Tables 1 and 2).
FIGURE 1.
Flow diagram illustrating the identification and selection of studies.
TABLE 1.
Characteristics of the case-control studies included in the systematic review and meta-analysis of the relation between dairy product intake and bladder cancer in the adult population1
| Reference | Country | Study/period of data collection (y) | Age distribution (y) | Sample size | Dietary assessment | Dairy products | Dairy amount comparison | Number of bladder cancer events | Variables of adjustment | Risk of bias2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Mettlin and Graham (15) | United States | 1957–1965 | NA | 1594 | Interview | Milk | Servings per day (5 categories) | 569 | Age | Low |
| Risch et al. (16) | Canada | 1979–1982 | 35–79 | 1618 | Questionnaire | Milk | Servings per day (2 categories) | 826 | Age, sex, area of residence, lifetime smoking consumption, and history of diabetes | High |
| Slattery et al. (17) | United States | 1977–1982 | 21–84 | 1308 | Interview | Milk | Servings per week (4 categories) | 419 | Age, sex, smoking status, diabetes, and bladder infection | High |
| Mettlin et al. (18) | United States | 1982–1990 | 18–97 | 1478 | Questionnaire | High-fat milk 2% fat milk Low-fat milk | Servings per day (3 categories) | 178 | Age, sex, smoking history, education, and county of residence | Low |
| Riboli et al. (19) | Spain | 1985–1986 | <80 | 1224 | Dietary questionnaire | Dairy Butter/cream | Quartiles of intake | 432 | Age, sex, smoking, area of residence, total calories | Moderate |
| Wilkens et al. (20) | United States | 1977–1986 | 30–93 | 783 | FFQ/32 items | Milk | Tertiles of intake | 261 | Age, smoking status, pack-years, employment in a high-risk occupation, consumption of dark green vegetables in men, and total vitamin C consumption in women | High |
| Lu et al. (21) | Taiwan | 1996–1997 | 67.5 | 200 | FFQ | Milk | Nonintake vs. intake | 40 | Age, sex, date of admission, family history, ethnicity, and smoking status | Moderate |
| Wakai et al. (22) | Japan | 1996–1999 | 20–99 | 592 | FFQ/97 items | Dairy | Quartiles of intake | 297 | Age, sex, smoking, and occupational history as a cook | Low |
| Balbi et al. (23) | Uruguay | 1998–1999 | 40–89 | 720 | FFQ/64 items | Dairy Cheese Butter High-fat milk | Tertiles of intake | 144 | Age, BMI, calories | Moderate |
| Ohashi et al. (24) | Japan | 1997–1998 | 49–80 | 625 | Questionnaire | Fermented dairy Yakult | Servings per day (3 categories) | 180 | Age, sex, and smoking | Low |
| Radosavljević et al. (25) | Serbia | 1997–1999 | 26–81 | 260 | FFQ | High-fat milk Low-fat milk Yogurt | Nonintake vs. intake | 130 | Age, sex, smoking, place of residence | Low |
| Wakai et al. (26) | Japan | 1994–2000 | 20–79 | 744 | FFQ | Milk | Quartiles of intake | 124 | Age, sex, cumulative consumption of cigarettes, year of first visit | Low |
| Jiang et al. (27) | United States | 1987–1999 | 25–64 | 3172 | Interview | Milk | Quartiles of intake | 1586 | Age, sex, race, level of education, use of nonsteroidal anti-inflammatory drugs, carotenoid intake, number of years as hairdresser/barber, cigarette smoking status, duration of smoking, and intensity of smoking | Low |
| La Vecchia et al. (28) | Italy | 1985–1987 | 45–74 | 344 | FFQ | Milk Cheese Butter | Tertiles of intake | 163 | Age and sex | Moderate |
| Hemelt et al. (29) | China | 2005–2008 | 65 | 824 | Interview | Milk | Nonintake vs: - Intake - < Daily or daily - 1 cup or >1 cup | 432 | Age, sex, smoking status, smoking frequency, and smoking duration–adjusted ORs | Low |
| Brinkman et al. (30) | Belgium | 1999–2004 | 50–80 | 575 | Validated FFQ/322 items | Milk Cheese | Tertiles of intake | 198 | Sex, age, smoking status, number of cigarettes smoked per day, number of years smoking, occupational exposure to polycyclic aromatic hydrocarbons or aromatic amines, and energy intake | Low |
| Isa et al. (31) | China | 2005–2008 | 40–80 | 956 | FFQ/35 items | Dairy | Servings per week (5 categories) | 487 | Sex, age, smoking status, smoking duration, smoking amount, and other food groups | Low |
| Ronco et al. (32) | Uruguay | 1996–2004 | 30–89 | 1735 | FFQ/64 items | Milk Butter Cheese | Tertiles of intake | 225 | Age, residence, education, BMI, smoking, alcohol drinking, meat consumption, total energy, and total vegetable and fruit intakes | Low |
1NA, not available; PAH, polycyclic aromatic hydrocarbon.
2Risk of bias assessed using the Quality in Prognosis Studies (QUIPS) tool.
TABLE 2.
Characteristics of cohort studies included in the systematic review and meta-analysis of the relation between dairy product intake and bladder cancer in the adult population1
| Reference | Country | Study/period of data collection (y) | Age distribution (y) | Sample size | Dietary assessment | Dairy products | Dairy amount comparison | Number of bladder cancer events | Variables of adjustment | Risk of bias2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Ursin et al. (33) | Norway | 1967–1978 | 35–74 | 15,914 | Dietary questionnaire | Milk | Servings per day (3 categories) | 91 | Age, smoking, and residence | High |
| Chyou et al. (34) | United States | 1942–1968 | 49–68 | 7995 | FFQ/17 items | Milk Ice cream | Servings per week (3 categories) | 96 | Age and smoking | High |
| Michaud et al. (35) | United States | 1986–1996 | 40–75 | 47,909 | FFQ/131 items | Milk | Increase of 240 mL | 252 | Age, vegetables, smoking, energy, geographic region, fruits, and other beverages | Low |
| Nagano et al. (36) | Japan | Life-Span study/ 1979–1993 | 50–80 | 38,540 | FFQ/22 items | Milk Butter/cheese | Servings per day (3 categories) | 114 | Age, BMI, gender, radiation, smoking, calendar time, and education | Moderate |
| Sakauchi et al. (37) | Japan | JACC study/1988–1997 | 40–79 | 65,184 | FFQ/32 items | Milk Yogurt Cheese Butter | Servings per week (3 categories) | 115 | Sex, age, and smoking index | Moderate |
| Larsson et al. (38) | Sweden | Swedish mammography cohort/1987–1997 | 45–83 | 82,002 | FFQ/96 items | Dairy Cheese Milk Fermented dairy | Quartiles of intake | 485 | Age, smoking, sex, total energy intake, and education | Low |
| Keszei et al. (39) | Netherlands | Netherlands cohort study/1986–2002 | 55–69 | 120,852 | Validated FFQ | Dairy Nonfermented dairy Fermented dairy Cheese Butter | Quintiles of intake | 1549 | Age, sex, smoking, fat, energy, meat, fruits, vegetables, and beverages | Low |
| Ros et al. (40) | Europe | EPIC/1992–2000 | 53.3 | 233,236 | FFQ | Milk | Tertiles of intake | 513 | Age, sex, smoking status, duration of smoking, lifetime intensity of smoking, energy intake from fat and nonfat sources | Low |
1JACC, Japan Collaborative Cohort; EPIC, European Prospective Investigation into Cancer and Nutrition.
2Risk of bias assessed using the Quality in Prognosis Studies (QUIPS) tool.
The age of included participants ranged between 18.0 and 99.0 y old, with sample sizes ranging from 200 to 233,236 participants; 595,698 participants were included in the meta-analysis (18,752 from case-control studies and 576,946 from cohort studies). The number of bladder cancer events observed were between 40 and 1586 across the studies. Most of the studies measured dairy product consumption with an FFQ, 4 studies used an interview, and 4 studies used a nonspecified dietary questionnaire (dairy product items considered in the dietary assessments in the studies are reported in Supplemental Table 1). The dairy products reported were: total dairy products, milk, cheese, butter, yogurt, cream, and 1 fermented milk (yakult). One study considered cocoa milk and pudding as dairy products in dietary assessments (39). Most of the studies reported data by categories of dairy product consumption, and only 1 study reported a dose–response analysis of dairy product consumption.
The studies included in the systematic review and meta-analysis presented approximate mean values of milk consumption as follows: low, ∼50 mL/d (∼4 mL/d in Asia, ∼57 mL/d in the United States, and ∼83 mL/d in Europe); medium, ∼227 mL/d (∼107 mL/d in Asia, ∼229 mL/d in the United States, and ∼306 mL/d in Europe); and high, ∼336 mL/d (∼200 mL/d in Asia, ∼348 mL/d in the United States, and ∼452 mL/d in Europe). The approximate mean values of whole milk consumption were low, ∼0 mL/d; medium, ∼110 mL/d; and high, ∼220 mL/d. Moreover, for fermented dairy products, the approximate mean values were low, ∼4 g/d (∼8.3 g/d in Asia and ∼0.0 g/d in Europe); medium, ∼67 g/d (∼36 g/d in Asia and ∼94 g/d in Europe); and high, ∼160 g/d (∼71 g/d in Asia and ∼249 g/d in Europe). Finally, for total dairy products, the approximate mean values were low, ∼201 g/d (∼16 g/d in Asia and ∼304 g/d in Europe); medium, ∼345 g/d (∼92 g/d in Asia and ∼515 g/d in Europe); and high, ∼545 g/d (∼195 g/d in Asia and ∼779 g/d in Europe).
All studies reported models adjusted for several covariates. All studies reported models adjusted by age and most studies included sex and smoking status. Other common adjustments were residence and other food groups. No study included calcium supplement consumption in the adjustments.
Study quality
As assessed by the Quality in Prognosis Studies tool (Supplemental Table 2), 58% of the studies obtained a total score corresponding to a low risk of bias, 23% had a moderate risk of bias, and only 19% had a high risk of bias. The study attrition domain showed a moderate or high risk of bias in most studies (65%). Conversely, 85% of the studies showed a low risk of bias in the statistical analysis and reporting domain. No study scored a high risk of bias in the outcome measurement or statistical analysis and reporting domains.
Meta-analyses
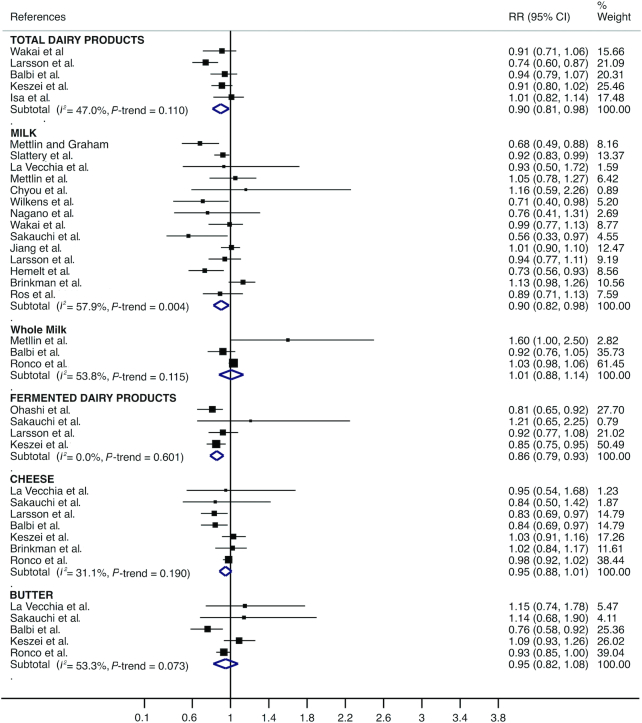
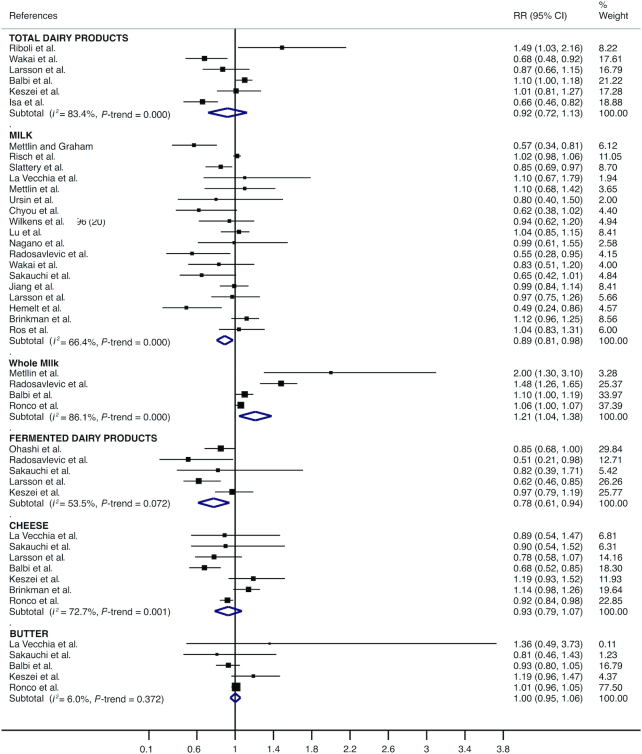
For total dairy products, medium compared with low consumption was significantly associated with a lower pooled risk estimate for bladder cancer (RR = 0.90; 95% CI: 0.81, 0.98); the same was true for milk (RR = 0.90; 95% CI: 0.82, 0.98) and fermented dairy products (RR = 0.87; 95% CI: 0.79, 0.96). Heterogeneity in the RR estimates was not significant for dairy products (I2 = 47.0%; P = 0.110) or for fermented dairy products (I2 = 0.0%; P = 0.539) and was moderate for milk (I2 = 57.9%; P = 0.004) (Figure 2). Moreover, high compared with low consumption was significantly associated with a lower pooled risk estimate for bladder cancer for milk (RR = 0.89; 95% CI: 0.81, 0.98) and for fermented dairy products (RR = 0.78; 95% CI: 0.61, 0.94). However, high compared with low consumption was significantly associated with a higher pooled risk estimate for bladder cancer for whole milk (RR = 1.21; 95% CI: 1.04, 1.38). Heterogeneity in the RR estimates was moderate for fermented dairy products (I2 = 53.5%; P = 0.072) and substantial for milk (I2 = 66.4%; P < 0.001) and whole milk (I2 = 86.1%; P < 0.001) (Figure 3).
FIGURE 2.
Forest plot including risk ratio of medium compared with low dairy product intake for bladder cancer in the adult population.
FIGURE 3.
Forest plot including risk ratio of high compared with low dairy product intake for bladder cancer in the adult population.
Sensitivity analysis
When the impact of individual studies was examined by removing studies from the analysis 1 at a time, a significantly higher risk of bladder cancer associated with medium compared with low whole milk consumption was found after removing the Ronco et al. study (32). Conversely, the significantly lower risk of bladder cancer associated with medium compared with low milk consumption disappeared after removing the Hemelt et al. (29), Mettlin et al. (18), and Wilkens et al. (20) studies. Finally, for medium compared with low and for high compared with low cheese consumption, a significantly lower risk of bladder cancer was found after removing the Brinkman et al. (30) and Keszei et al. (39) studies.
Subgroup analyses and meta-regression
When analyses were performed based on gender, geographic location, and type of study design, there were enough studies to perform an analysis of milk consumption only. The risk of bladder cancer associated with medium compared with low consumption was significantly protective in the Asian region (RR = 0.79; 95% CI: 0.59, 0.98, I2 = 56.8%) and for the cohort design studies (RR = 0.85; 95% CI: 0.71, 0.99, I2 = 19.7%) (Table 3).
TABLE 3.
Subgroup analyses for the risk of bladder cancer for high compared with low and medium compared with low milk intake in the adult population, based on gender, region, and type of study design1
| Subgroup | n | High vs. low consumption RR (95% CI) | I 2 | P | Medium vs. low consumption RR (95% CI) | I 2 | P |
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Male | 4 | 0.86 (0.65, 1.07) | 78.7 | 0.003 | 0.93 (0.77, 1.08) | 34.6 | 0.217 |
| Female | 3 | 0.97 (0.85, 1.08) | 68.2 | 0.043 | — | — | |
| Geographical location | |||||||
| Americas | 7 | 0.88 (0.76, 1.01) | 74.9 | 0.001 | 0.90 (0.79, 1.02) | 59.2 | 0.031 |
| Europe | 6 | 0.96 (0.79, 1.13) | 51.2 | 0.068 | 1.00 (0.87, 1.13) | 37.2 | 0.189 |
| Asia | 5 | 0.80 (0.57, 1.04) | 69.4 | 0.011 | 0.79 (0.59, 0.98) | 56.8 | 0.074 |
| Type of design | |||||||
| Case-control | 12 | 0.90 (0.80, 1.01) | 72.4 | <0.001 | 0.92 (0.82, 1.02) | 67.3 | 0.002 |
| Cohort | 6 | 0.86 (0.70, 1.02) | 31.6 | 0.199 | 0.85 (0.71, 0.99) | 19.7 | 0.289 |
1 P < 0.05 indicates the presence of heterogeneity between studies.
The random-effects meta-regression model could be performed only for milk consumption, showing that the age of participants (P = 0.744 for high compared with low and P = 0.442 for medium compared with low), percentage of current smokers (P = 0.841 for high compared with low and P = 0.078 for medium compared with low), and the year when the study started (P = 0.218 for high compared with low and P = 0.353 for medium compared with low) were not related to the pooled RR estimates (Figures 4 and 5).
FIGURE 4.
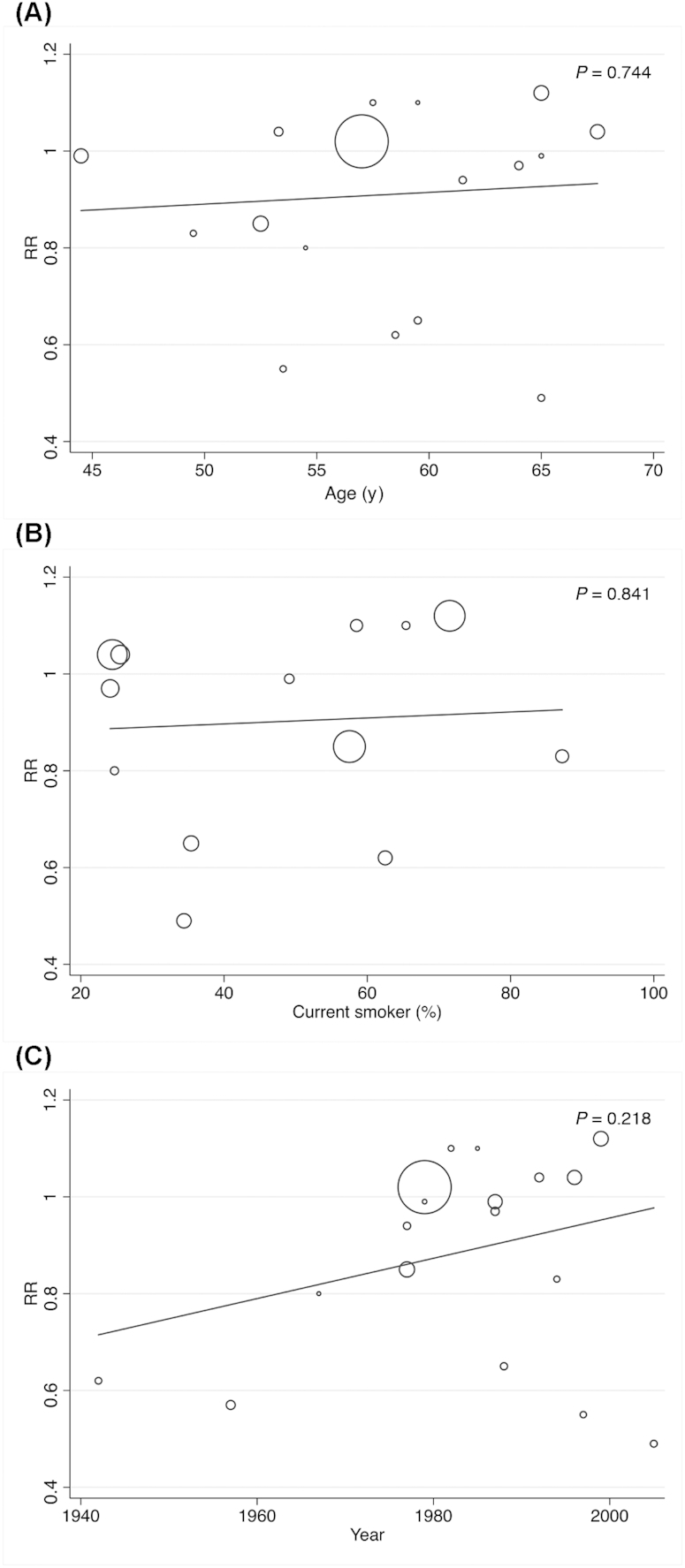
Random-effects meta-regression analyses for the moderator effect of age, current smoker, and the year of beginning of the studies in the relation between high compared with low milk intake and bladder cancer in the adult population.
FIGURE 5.
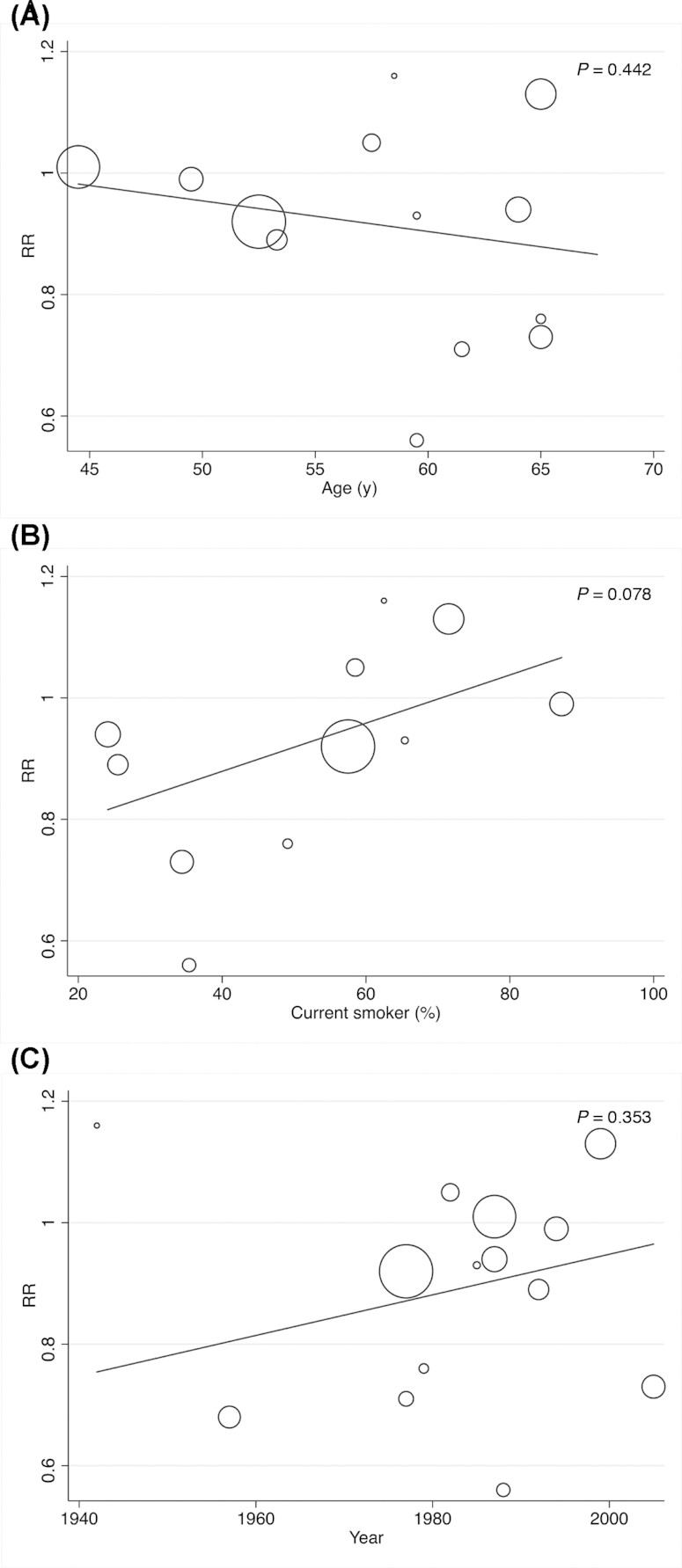
Random-effects meta-regression analyses for the moderator effect of age, current smoker, and the year of beginning of the studies in the relation between medium compared with low milk intake and bladder cancer in the adult population.
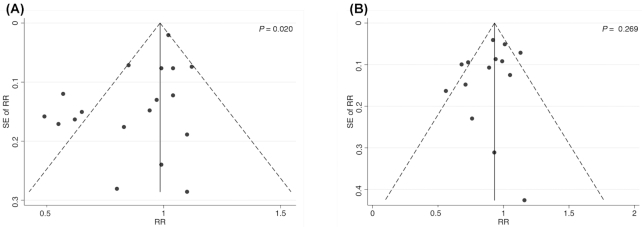
Publication bias
Publication bias was analyzed for only milk consumption because ≥10 articles addressing milk were included in this systematic review and meta-analysis. Evidence of publication bias was found by funnel plot asymmetry and Egger's test for only high compared with low milk consumption (P = 0.020) (Figure 6).
FIGURE 6.
Assessment of potential publication bias by Egger test for studies addressing the relation between high compared with low and medium compared with low milk intake and bladder cancer in the adult population.
Discussion
The present meta-analysis summarized the evidence to date regarding the association between milk and dairy productconsumption and bladder cancer risk, representing a pooled total of 26 epidemiologic studies (8 cohort and 18 case-control) with a total sample size of ≤595,698 to obtain more stable results. The study results suggested that medium compared with low consumption of total dairy products, milk, and fermented milk decreased the risk of bladder cancer. Moreover, high compared with low consumption of milk and fermented milk products also decreased the risk. In contrast, high compared with low consumption of whole milk was significantly associated with a higher risk of bladder cancer. No association was observed with butter and cheese consumption or with high compared with low consumption of any other type of dairy products.
The results of medium fermented milk consumption demonstrated no heterogeneity. However, as in both previous meta-analyses (5, 6), considerable heterogeneity was detected in the other significant results, especially in whole milk, for which heterogeneity was substantial. Different factors could influence the heterogeneity, including age, gender, geographic location, type of design, smoking, and year when the study started. Most studies included in the present meta-analysis adjusted the results by ≥3 of the most important factors. Moreover, based on the subgroup analyses by gender, geographic location, and type of study design, the present meta-analysis indicated that a significant proportion of the observed heterogeneity in the milk results may be explained by differences in study location and study design. In addition, the random-effects meta-regression model showed that the age of the participants, the percentage of current smokers, and the year when the study started were not related to the pooled RR estimates. Furthermore, the sensitivity analysis showed that the significantly lower risk of bladder cancer associated with medium compared with low milk consumption disappeared with the removal of 3 studies. Finally, publication bias was found for milk consumption. Therefore, the results should be interpreted with caution.
In spite of the heterogeneity and the risk of bias detected in the present meta-analysis, 3 important aspects warrant further discussion.
First, the results showed that the relation between consumption of milk and dairy products and bladder cancer risk varied significantly by the type of dairy product. The present meta-analysis observed a decrease in the risk of bladder cancer associated with medium consumption of total dairy products and with medium and high consumption of milk and fermented dairy products but no associations with cheese or butter consumption. Of the previous meta-analyses, Mao et al. (6) suggested a potential protective effect of high milk consumption for bladder cancer and Li et al. (5) only observed an inverse association in the United States for bladder cancer risk and high milk consumption, and in Japan for bladder cancer risk and high total dairy product consumption with a limited study population. Several biological mechanisms have been proposed in order to explain the relation between milk and dairy product consumption and bladder cancer risk. Milk and dairy products contain several bioactive constituents that are potentially protective against cancer, one of which is vitamin D. Although they do not contain vitamin D naturally, enriched/fortified milk and dairy products can constitute a significant source of vitamin D (41). Vitamin D not only impedes proliferation and induces apoptosis in tumor cells but also regulates metabolism-related tumor suppressors and oncogenes (42). In this regard, an association between vitamin D deficiency status and increased risk of bladder cancer has been observed (43). Indeed, the latest related meta-analyses have demonstrated that maintaining sufficient serum 25-hydroxyvitamin D concentrations is associated with decreased bladder cancer risk (44, 45). However, the observational studies included in the present meta-analysis do not specify whether the dairy products they registered were fortified with vitamin D. Other bioactive compounds present in milk and dairy products, such as calcium (46, 47), casein, and lactose as promoters of calcium bioavailability (48) and vitamin A (49), have also been related with a decreased risk of some kinds of cancers.
Nevertheless, the prognostic factor measurement of the primary studies included in the present meta-analysis did not allow us to obtain data about nutrient intakes, so it was not possible to establish an association to support the contribution of dairy products to the previous biological mechanism described. In addition to the bioactive compounds, fermented dairy products contain probiotics, live microorganisms whose beneficial effects for human health have been widely described (50). The mechanism of action of probiotic microorganisms can be explained by the enhancement of the nonspecific and specific immune responses of the host, production of antimicrobial substances, and competition with pathogens for binding sites (51). To date, no evidence has been generated about the possible relation of fermented dairy product consumption with the risk of bladder cancer. However, probiotics have shown a suppressing effect on superficial bladder cancer (52). Moreover, in a prospective study of a Swedish adult population, it was observed that a high intake of fermented milk may lower the risk of developing bladder cancer (38). In this regard, it has been observed that probiotics are capable of altering host immune function, conferring protection from localized and excessive inflammatory responses (53), and improving rates of bladder cancer recurrence (54). In addition, some studies have supported the hypothesis that habitual consumption of lactic acid bacteria can promote anticancer immunity (24).
The second important result relates to the fat content in milk. According with the results observed by Mao et al. (6), in the current study high whole milk consumption seemed to increase the risk of developing bladder cancer. Nevertheless, the heterogeneity in these data was substantial, and this result should be considered with caution. Moreover, other fatty dairy products, such as cheese and butter, did not present this adverse association. Total fat intake has been related to bladder cancer risk (55). The main hypothesis supporting a possible effect of fat on cancer risk is based on the intraluminal effect of products of fat digestion, such as secondary bile acids; however, human data supporting this hypothesis are weak (56). In this regard, milk and dairy products are an important dietary source of total and saturated fats (57). Indeed, the main portion of milk lipids (97–98%) is TGs or esters of fatty acids (58). Nevertheless, milk fat also contains conjugated linoleic acid (CLA), a geometrical and positional stereoisomer of linoleic acid, which is present in milk and dairy products and derived from ruminants (59). It has been observed that CLA exerts antineoplastic activity and may have antiproliferative or proapoptotic properties. Indeed, a strong inhibition by CLA of malignant bladder cancer cell lines has been observed (60). This is possible mainly because CLA, with its t10c12 isomer, inhibits insulin-like growth factor receptor (IGF-IR) signaling, which contributes to decreased cell proliferation and increased apoptosis of cancerous bladder cells (61). Indeed, whole milk consumption has been associated with a decreased risk of some cancers, such as prostate cancer. In contrast, total milk and low-fat milk have been related to higher risk of prostate cancer (62). The diverging results for fat in dairy products suggest that further studies are needed to clarify the influence of fat content on bladder cancer risk. Currently, the recommendation provided by some of the most important institutions in the field of nutrition is that low-fat dairy varieties should be encouraged (63, 64).
The last highlighted issue indicated that the quantity of dairy products consumed might play an important role in their relation with bladder cancer risk. To date, the meta-analyses published to evaluate the relation between milk and dairy products and bladder cancer have measured the highest compared with the lowest quantiles of consumption (5, 6). To our knowledge, this is the first meta-analysis to evaluate not only high compared with low consumption but also medium compared with low consumption. The present study observed a reduced risk of bladder cancer with medium consumption of total dairy products, milk, and fermented products and with high consumption of milk and fermented products. In contrast, Mao et al. (6) observed only high compared with low milk consumption was significantly associated with a reduced risk of bladder cancer and Li et al. (5) only observed an inverse association in the United States for bladder cancer risk and high milk consumption, and in Japan for bladder cancer risk and high total dairy product consumption with a limited study population. In the current meta-analysis, it was not possible to carry out a dose–response analysis. To elucidate the amounts of milk and total dairy product consumption that were included in the low, medium, or high categories, approximate mean values were calculated. These data indicated that the medium consumption of milk (∼227 mL/d) and total dairy products (∼345 g/d) was similar to the minimum servings recommended in the most important food guides (64, 65). For example, The “Dietary Guidelines for Americans” issued by the USDA and US Department of Health and Human Services recommends 3 servings from the “milk, yogurt and cheese” food group each day as part of a healthy, balanced diet. Examples of 1 serving include 200 mL of milk, 125 g of yogurt, or 25 g of hard cheese (64), so the minimum serving recommended for total dairy products, including “milk, yogurt and cheese,” is 350 g/d. Therefore, in regards to the results of the present meta-analysis, the combination of a serving of milk and fermented dairy products might be a suitable option to obtain benefits related to decreased bladder cancer risk. However, in the subgroup analysis of milk consumption by geographical location, a reduction in bladder cancer risk was observed for medium consumption only in the Asia region. The approximate mean calculated in the Asia population was lower than in America or Europe, so the results reported in the present study may require us to reflect on the adequate daily recommendation for milk. Nevertheless, additional factors related to the Asian population also need to be taken into account, such as the possible role of their healthy dietary and lifestyle habits and other genetic differences (66). For example, lactose intolerance occurs in ∼25% of people in Europe; 50–80% of people of Hispanic origin, people from south India, and black people; and almost 100% of people in Asia (67).
Finally, in relation to dairy products, it is important to highlight that milk consumption in developed countries has been declining slightly in the last few decades. A recent update from the USDA (68) confirmed that dairy milk consumption declined 25% from 1996 to 2016 (68). This observed reduction in consumption of milk may be a result of campaigns promoting the idea of cow milk consumption being unsuitable for humans (69). In recent years, influential groups have criticized milk and dairy products and recommend limiting consumption of dairy-based foods due to little benefit and potential harm to humans (70, 71). However, the available scientific evidence supports the intake of milk and dairy products contributing to meeting nutrient recommendations and that it may protect against the most prevalent chronic diseases and the risk of all-cause mortality, whereas very few adverse effects have been reported (72–74). The results of the present meta-analysis contribute to broadening this evidence.
As a systematic review and meta-analysis of previously published studies, our study has several limitations that need to be taken into account when considering its contributions. First, because both case-control and cohort studies were included, a wide variation exists across studies and the methodological differences in the study designs could bias the results because of the great variety included in the analysis. In addition, the results of total milk and dairy product intake were based on responses to a single questionnaire that was administered only once. A second limitation is that heterogeneity may be due to milk and dairy product intakes including a collection of several products and reported dairy items which may have varied across studies and some items were combined. Lastly, other important dietary and lifestyle factors may have influenced the results because they were not considered in most of the primary studies included. Therefore, these results should be interpreted with caution.
In conclusion, the results of the present meta-analysis suggest a decreased risk of bladder cancer associated with medium consumption of total dairy products (∼345 g/d) and with medium and high consumption of milk (∼227 mL/d, ∼336 mL/d) and fermented dairy products (∼67 g/d, ∼160 g/d). Moreover, an increased risk of bladder cancer was observed with high whole milk consumption (∼220 mL/d), although the results should be interpreted with caution. Currently, the intake of milk and dairy products should follow the dietary recommendations put forth by the competent authorities of each country. The daily combination of milk and fermented dairy products might be a healthy option to reduce bladder cancer risk. Future research is warranted to clarify the adequate serving size and the role of fat content and the different geographical locations to establish conclusive recommendations for reducing the risk of bladder cancer.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—LMB: wrote the original manuscript; IC-R and LMB: performed the search strategy, study selection, data extraction, and quality assessment; CS and BL-P: were involved in case of disagreement during data extraction and quality assessment; IC-R: conducted the statistical analyses; BL-P: participated in the interpretation and analysis of the data obtained; CG-C: had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript.
Notes
This supplement was sponsored by the Interprofessional Dairy Organization (INLAC), Spain.
The sponsor had no role in the design of the studies included in the supplement; in the collection, analyses, or interpretation of the data; in the writing of the manuscripts; or in the decision to publish the results. Publication costs for this supplement were defrayed in part by the payment of page charges. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
Author disclosures: LMB, BL-P, CS, IC-R, and CG-C, no conflicts of interest.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 2. Letašiová S, Medveďová A, Šovčíková A, Dušinská M, Volkovová K, Mosoiu C, Bartonová A. Bladder cancer, a review of the environmental risk factors. Environ Heal. 2012;11:S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Research. WCRFII for C. Diet, Nutrition, Physical Activity and Bladder Cancer. 2015. [Google Scholar]
- 4. Silberstein JL, Parsons JK. Evidence-based principles of bladder cancer and diet. Urology. 2010;75:340–6. [DOI] [PubMed] [Google Scholar]
- 5. Li F, An S, Zhou Y, Liang Z, Jiao Z, Jing Y, Wan P, Shi X, Tan W. Milk and dairy consumption and risk of bladder cancer: a meta-analysis. Urology. 2011;78:1298–305. [DOI] [PubMed] [Google Scholar]
- 6. Mao Q-Q, Dai Y, Lin Y-W, Qin J, Xie L-P, Zheng X-Y. Milk consumption and bladder cancer risk: a meta-analysis of published epidemiological studies. Nutr Cancer. 2011;63:1263–71. [DOI] [PubMed] [Google Scholar]
- 7. World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and bladder cancer. In: Continuous Update Project Expert Report 2018: Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. London: WCRF; 2018. p. 1–38.. Available from: https://www.wcrf.org/sites/default/files/Bladder-cancer-report.pdf. [Google Scholar]
- 8. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 9. Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. [Internet] The Cochrane Collaboration; 2011. Available from: http://handbook.cochrane.org. [Google Scholar]
- 10. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–6. [DOI] [PubMed] [Google Scholar]
- 11. DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–14. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 13. Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18:2693–708. [DOI] [PubMed] [Google Scholar]
- 14. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mettlin C, Graham S. Dietary risk factors in human bladder cancer. Am J Epidemiol. 1979;110:255–63. [DOI] [PubMed] [Google Scholar]
- 16. Risch HA, Burch JD, Miller AB, Hill GB, Steele R, Howe GR. Dietary factors and the incidence of cancer of the urinary bladder. Am J Epidemiol. 1988;127:1179–91. [DOI] [PubMed] [Google Scholar]
- 17. Slattery ML, West DW, Robison LM. Fluid intake and bladder cancer in Utah. Int J Cancer. 1988;42:17–22. [DOI] [PubMed] [Google Scholar]
- 18. Mettlin CJ, Schoenfeld ER, Natarajan N. Patterns of milk consumption and risk of cancer. Nutr Cancer. 1990;13:89–99. [DOI] [PubMed] [Google Scholar]
- 19. Riboli E, González CA, López-Abente G, Errezola M, Izarzugaza I, Escolar A, Nebot M, Hémon B, Agudo A. Diet and bladder cancer in Spain: a multi-centre case-control study. Int J Cancer. 1991;49:214–19. [DOI] [PubMed] [Google Scholar]
- 20. Wilkens LR, Kadir MM, Kolonel LN, Nomura AM, Hankin JH. Risk factors for lower urinary tract cancer: the role of total fluid consumption, nitrites and nitrosamines, and selected foods. Cancer Epidemiol Biomarkers Prev. 1996;5:161–6. [PubMed] [Google Scholar]
- 21. Lu CM, Lan SJ, Lee YH, Huang JK, Huang CH, Hsieh CC. Tea consumption: fluid intake and bladder cancer risk in southern Taiwan. Urology. 1999;54:823–8. [DOI] [PubMed] [Google Scholar]
- 22. Wakai K, Takashi M, Okamura K, Yuba H, Suzuki K, Murase T, Obata K, Itoh H, Kato T, Kobayashi M et al.. Foods and nutrients in relation to bladder cancer risk: a case-control study in Aichi Prefecture, central Japan. Nutr Cancer. 2000;38:13–22. [DOI] [PubMed] [Google Scholar]
- 23. Balbi JC, Larrinaga MT, De Stefani E, Mendilaharsu M, Ronco AL, Boffetta P, Brennan P. Foods and risk of bladder cancer: a case-control study in Uruguay. Eur J Cancer Prev. 2001;10:453–8. [DOI] [PubMed] [Google Scholar]
- 24. Ohashi Y, Nakai S, Tsukamoto T, Masumori N, Akaza H, Miyanaga N, Kitamura T, Kawabe K, Kotake T, Kuroda M et al.. Habitual intake of lactic acid bacteria and risk reduction of bladder cancer. Urol Int. 2002;68:273–80. [DOI] [PubMed] [Google Scholar]
- 25. Radosavljević V, Janković S, Marinković J, Djokić M. Fluid intake and bladder cancer. A case control study. Neoplasma. 2003;50:234–8. [PubMed] [Google Scholar]
- 26. Wakai K, Hirose K, Takezaki T, Hamajima N, Ogura Y, Nakamura S, Hayashi N, Tajima K. Foods and beverages in relation to urothelial cancer: case-control study in Japan. Int J Urol. 2004;11:11–19. [DOI] [PubMed] [Google Scholar]
- 27. Jiang X, Castelao JE, Groshen S, Cortessis VK, Shibata DK, Conti DV, Gago-Dominguez M. Water intake and bladder cancer risk in Los Angeles County. Int J Cancer. 2008;123:1649–56. [DOI] [PubMed] [Google Scholar]
- 28. La Vecchia C, Negri E, Decarli A, D'Avanzo B, Liberati C, Franceschi S. Dietary factors in the risk of bladder cancer. Nutr Cancer. 1989;12:93–101. [DOI] [PubMed] [Google Scholar]
- 29. Hemelt M, Hu Z, Zhong Z, Xie L-P, Wong YC, Tam P-C, Cheng KK, Ye Z, Bi X, Lu Q et al.. Fluid intake and the risk of bladder cancer: results from the South and East China case-control study on bladder cancer. Int J Cancer. 2010;127:638–45. [DOI] [PubMed] [Google Scholar]
- 30. Brinkman MT, Buntinx F, Kellen E, Dagnelie PC, Van Dongen MCJM, Muls E, Zeegers MP. Dietary intake of micronutrients and the risk of developing bladder cancer: results from the Belgian case-control study on bladder cancer risk. Cancer Causes Control. 2011;22:469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Isa F, Xie L-P, Hu Z, Zhong Z, Hemelt M, Reulen RC, Wong YC, Tam P-C, Yang K, Chai C et al.. Dietary consumption and diet diversity and risk of developing bladder cancer: results from the South and East China case-control study. Cancer Causes Control. 2013;24:885–95. [DOI] [PubMed] [Google Scholar]
- 32. Ronco AL, Mendilaharsu M, Boffetta P, Deneo-Pellegrini H, De Stefani E. Meat consumption, animal products, and the risk of bladder cancer: a case-control study in Uruguayan men. Asian Pac J Cancer Prev. 2014;15:5805–9. [DOI] [PubMed] [Google Scholar]
- 33. Ursin G, Bjelke E, Heuch I, Vollset SE. Milk consumption and cancer incidence: a Norwegian prospective study. Br J Cancer. 1990;61:454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chyou PH, Nomura AM, Stemmermann GN. A prospective study of diet, smoking, and lower urinary tract cancer. Ann Epidemiol. 1993;3:211–16. [DOI] [PubMed] [Google Scholar]
- 35. Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Curhan GC, Willett WC, Giovannucci EL. Fluid intake and the risk of bladder cancer in men. N Engl J Med. 1999;340:1390–7. [DOI] [PubMed] [Google Scholar]
- 36. Nagano J, Kono S, Preston DL, Moriwaki H, Sharp GB, Koyama K, Mabuchi K. Bladder-cancer incidence in relation to vegetable and fruit consumption: a prospective study of atomic-bomb survivors. Int J Cancer. 2000;86:132–8. [DOI] [PubMed] [Google Scholar]
- 37. Sakauchi F, Mori M, Washio M, Watanabe Y, Ozasa K, Hayashi K, Miki T, Nakao M, Mikami K, Ito Y et al.. Dietary habits and risk of urothelial cancer incidence in the JACC Study. J Epidemiol. 2005;15(Suppl 2):S190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Larsson SC, Andersson S-O, Johansson J-E, Wolk A. Cultured milk, yogurt, and dairy intake in relation to bladder cancer risk in a prospective study of Swedish women and men. Am J Clin Nutr. 2008;88:1083–7. [DOI] [PubMed] [Google Scholar]
- 39. Keszei AP, Schouten LJ, Goldbohm RA, van den Brandt PA. Dairy intake and the risk of bladder cancer in the Netherlands Cohort Study on Diet and Cancer. Am J Epidemiol. 2010;171:436–46. [DOI] [PubMed] [Google Scholar]
- 40. Ros MM, Bas Bueno-de-Mesquita HB, Büchner FL, Aben KKH, Kampman E, Egevad L, Overvad K, Tjønneland A, Roswall N, Clavel-Chapelon F et al.. Fluid intake and the risk of urothelial cell carcinomas in the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer. 2011;128:2695–708. [DOI] [PubMed] [Google Scholar]
- 41. Spiro A, Buttriss JL. Vitamin D: an overview of vitamin D status and intake in Europe. Nutr Bull. 2014;39:322–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abu el Maaty M, Wölfl S. Vitamin D as a novel regulator of tumor metabolism: insights on potential mechanisms and implications for anti-cancer therapy. Int J Mol Sci. 2017;18:2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang H, Zhang H, Wen X, Zhang Y, Wei X, Liu T. Vitamin D deficiency and increased risk of bladder carcinoma: a meta-analysis. Cell Physiol Biochem. 2015;37:1686–92. [DOI] [PubMed] [Google Scholar]
- 44. Zhao Y, Chen C, Pan W, Gao M, He W, Mao R, Lin T, Huang J. Comparative efficacy of vitamin D status in reducing the risk of bladder cancer: a systematic review and network meta-analysis. Nutrition. 2016;32:515–23. [DOI] [PubMed] [Google Scholar]
- 45. Liao Y, Huang J-L, Qiu M-X, Ma Z-W. Impact of serum vitamin D level on risk of bladder cancer: a systemic review and meta-analysis. Tumor Biol. 2015;36:1567–72. [DOI] [PubMed] [Google Scholar]
- 46. Cho E, Smith-Warner SA, Spiegelman D, Beeson WL, van den Brandt PA, Colditz GA, Folsom AR, Fraser GE, Freudenheim JL, Giovannucci E et al.. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst. 2004;96:1015–22. [DOI] [PubMed] [Google Scholar]
- 47. Chen P, Hu P, Xie D, Qin Y, Wang F, Wang H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010;121:469–77. [DOI] [PubMed] [Google Scholar]
- 48. Guéguen L, Pointillart A. The bioavailability of dietary calcium. J Am Coll Nutr. 2000;19:119S–36S. [DOI] [PubMed] [Google Scholar]
- 49. Park Y, Spiegelman D, Hunter DJ, Albanes D, Bergkvist L, Buring JE, Freudenheim JL, Giovannucci E, Goldbohm RA, Harnack L et al.. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control. 2010;21:1745–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. George Kerry R, Patra JK, Gouda S, Park Y, Shin H-S, Das G. Benefaction of probiotics for human health: a review. J Food Drug Anal. 2018;26:927–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Singh VP, Sharma J, Babu S, Rizwanulla, Singla A. Role of probiotics in health and disease: a review. J Pak Med Assoc. 2013;63:253–7. [PubMed] [Google Scholar]
- 52. Aso Y, Akazan H. Prophylactic effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer. Urol Int. 1992;49:125–9. [DOI] [PubMed] [Google Scholar]
- 53. Mombelli B, Gismondo MR. The use of probiotics in medical practice. Int J Antimicrob Agents. 2000;16:531–6. [DOI] [PubMed] [Google Scholar]
- 54. Feyisetan O, Tracey C, Hellawell GO. Probiotics, dendritic cells and bladder cancer. BJU Int. 2012;109:1594–7. [DOI] [PubMed] [Google Scholar]
- 55. La Vecchia C, Negri E. Nutrition and bladder cancer. Cancer Causes Control. 1996;7:95–100. [DOI] [PubMed] [Google Scholar]
- 56. Newmark HL, Wargovich MJ, Bruce WR. Colon cancer and dietary fat, phosphate, and calcium: a hypothesis. J Natl Cancer Inst. 1984;72:1323–5. [PubMed] [Google Scholar]
- 57. Nettleton JA, Brouwer IA, Geleijnse JM, Hornstra G. Saturated fat consumption and risk of coronary heart disease and ischemic stroke: a science update. Ann Nutr Metab. 2017;70:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Norat T, Riboli E. Dairy products and colorectal cancer. A review of possible mechanisms and epidemiological evidence. Eur J Clin Nutr. 2003;57:1–17. [DOI] [PubMed] [Google Scholar]
- 59. Belury MA. Inhibition of carcinogenesis by conjugated linoleic acid: potential mechanisms of action. J Nutr. 2002;132:2995–8. [DOI] [PubMed] [Google Scholar]
- 60. Maggiora M, Bologna M, Cerù MP, Possati L, Angelucci A, Cimini A, Miglietta A, Bozzo F, Margiotta C, Muzio G et al.. An overview of the effect of linoleic and conjugated-linoleic acids on the growth of several human tumor cell lines. Int J Cancer. 2004;112:909–19. [DOI] [PubMed] [Google Scholar]
- 61. Jung JI, Cho HJ, Kim J, Kwon DY, Park JHY. trans-10,cis-12 conjugated linoleic acid inhibits insulin-like growth factor-I receptor signaling in TSU-Pr1 human bladder cancer cells. J Med Food. 2010;13:13–19. [DOI] [PubMed] [Google Scholar]
- 62. Aune D, Navarro Rosenblatt DA, Chan DS, Vieira AR, Vieira R, Greenwood DC, Vatten LJ, Norat T. Dairy products, calcium, and prostate cancer risk: a systematic review and meta-analysis of cohort studies. Am J Clin Nutr. 2015;101:87–117. [DOI] [PubMed] [Google Scholar]
- 63. Roth N, Knai C. Food based dietary guidelines in the WHO European Region. [Internet]. Copenhagen: World Health Organization; 2003. p. 1–38.. Available from: http://www.euro.who.int/__data/assets/pdf_file/0017/150083/E79832.pdf. [Google Scholar]
- 64. US Department of Health and Human Services and USDA. 2015–2020 dietary guidelines for Americans. 8th ed December2015. Available from: http://health.gov/dietaryguidelines/2015/guidelines/. [Google Scholar]
- 65. FAO/WHO. Food-based dietary guidelines. [Internet]. [cited 20 Sep, 2018]. Rome: FAO; 2018. Available from: http://www.fao.org/nutrition/education/food-based-dietary-guidelines/en/. [Google Scholar]
- 66. Kelly M. The nutrition transition in developing Asia: dietary change, drivers and health impacts. [Internet]. In: Jackson P, Spiess WEL, Sultana F, editors. Eating, drinking: surviving: The International year of global understanding – IYGU. Cham: Springer; 2016; [cited 12 Jul, 2018]. p. 83–90.. Available from: http://link.springer.com/10.1007/978-3-319-42468-2_9. [Google Scholar]
- 67. Bhatnagar S, Aggarwal R. Lactose intolerance. BMJ. 2007;334:1331–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. USDA, Economic Research Service. Dairy Data. [Internet]. 2018; [cited 29 Jun, 2018]. Available from: https://www.ers.usda.gov/data-products/dairy-data/. [Google Scholar]
- 69. Fischer WJ, Schilter B, Tritscher AM, Stadler RH. Contaminants of milk and dairy products: contamination resulting from farm and dairy practices. In: Fuquay JW, editor Encyclopedia of dairy sciences. 2nd ed San Diego, CA: Elsevier; 2011. p. 887–897. [Google Scholar]
- 70. Skerrett PJ. Harvard to USDA: check out the healthy eating plate. [Internet]. Harvard Health Blog. Cambridge, MA: Harvard Health Publishing; 2016; [cited 29 Jun, 2018]. Available from: https://www.health.harvard.edu/blog/harvard-to-usda-check-out-the-healthy-eating-plate-201109143344. [Google Scholar]
- 71. Harvard researchers continue to support their healthy eating plate. [Internet]. Cambridge, MA: Harvard Health Publishing; 2017; [cited 29 Jun, 2018]. Available from: https://www.health.harvard.edu/plate/harvard-researchers-launch-healthy-eating-plate. [Google Scholar]
- 72. Thorning TK, Raben A, Tholstrup T, Soedamah-Muthu SS, Givens I, Astrup A. Milk and dairy products: good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr Res. 2016;60:32527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Guo J, Astrup A, Lovegrove JA, Gijsbers L, Givens DI, Soedamah-Muthu SS. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose–response meta-analysis of prospective cohort studies. Eur J Epidemiol. 2017;32:269–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mullie P, Pizot C, Autier P. Daily milk consumption and all-cause mortality, coronary heart disease and stroke: a systematic review and meta-analysis of observational cohort studies. BMC Public Health. 2016;16:1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.