ABSTRACT
Proteolytic cleavage of synaptosomal-associated protein 25 by the light chain of botulinum neurotoxin type A (LCA) results in a blockade of neurotransmitter release that persists for several months in motor neurons. The L428A/L429A mutation in LCA is known to significantly shorten both the proteolytic and neuroparalytic effects of the neurotoxin in mice. To elucidate the cellular mechanism for LCA longevity, we studied the effects of L428A/L429A mutation on the interactome, localization and stability of LCA expressed in cultured neuronal cells. Mass spectrometry analysis of the LCA interactome showed that the mutation prevented the interaction of LCA with septins. The wild-type LCA was concentrated in plasma-membrane-associated clusters, colocalizing with septins-2 and septin-7, which accumulated in these clusters only in the presence of LCA. The L428A/L429A mutation decreased co-clustering of LCA and septins and accelerated proteasomal and non-proteasomal degradation of LCA. Similarly, the impairment of septin oligomerization by forchlorfenuron or silencing of septin-2 prevented LCA interaction and clustering with septins and increased LCA degradation. Therefore, the dileucine-mediated LCA–septin co-clustering is crucial for the long-lasting stabilization of LCA-related proteolytic and presumably neuroparalytic activity.
KEY WORDS: Septin, Botulinum toxin A protease, Protein stability, Degradation, Ubiquitylation
INTRODUCTION
Botulinum neurotoxins (BoNTs) inhibit transmitter release from peripheral cholinergic neurons, which underlies both the severe neuroparalytic symptoms of human botulism and the success of using these toxins therapeutically for long-term treatment of a wide variety of neurogenic disorders and cosmetic therapies. BoNTs bind to neurons and deliver their light chains inside the cells, where they specifically cleave one (or, in a single case, two) of the soluble N-ethylmaleimide sensitive factor attachment receptor (SNARE) proteins, resulting in inhibition of neurotransmitter exocytosis (Dolly and Aoki, 2006; Montal, 2010; Popoff and Bouvet, 2009). Seven serotypes of BoNTs, A through G, vary in persistence of their action. The type H BoNT serotype has been recently identified (Dover et al., 2014), but the characteristics of the protein are unknown. The therapeutic effects of BoNT/A last from 3 to 12 months depending on the clinical indication (Dolly and Aoki, 2006), whereas the effects of BoNT/E last less than 4 weeks (Eleopra et al., 1998). The extraordinary longevity of BoNT/A presents a challenge for medical treatment of botulism, but is advantageous for its therapeutic applications. However, the mechanisms that govern the long-lasting action of BoNT/A in neuronal cells are poorly understood.
When expressed in neuroblastoma cells, the light chain of BoNT/A (LCA) is less susceptible to ubiquitylation followed by proteasome degradation than is the light chain of BoNT/E (LCE) (Tsai et al., 2010). Association of LCA with the inner leaflet of the plasma membrane has been suggested to be important for the cellular stability of the LCA. In contrast, the shorter-lived LCE does not associate with the plasma membrane (Fernández-Salas et al., 2004). A double mutation, L428A/L429A, in a dileucine-containing motif (E424FYKLL) in LCA that partially impairs plasma membrane localization of LCA (Fernández-Salas et al., 2004), has been shown to dramatically shorten both the proteolytic and neuroparalytic effects of the neurotoxin in mice (Wang et al., 2011). The results suggest that degradation of LCA is impeded owing to its dileucine-dependent intracellular interactions. Such an interpretation is supported by the finding that fusion of LCE to the dileucine-containing proteolytically inactive LCA, but not to its L428A/L429A mutant, prolongs neuroparalytic effects of BoNT/E in mice (Wang et al., 2011).
Here, by the comparative tandem mass spectrometry (MS/MS) analysis of proteins interacting with the wild-type LCA versus LCE and the L428A/L429A mutant LCA, we have identified membrane cytoskeleton proteins that bind to LCA in a dileucine-dependent manner, but do not interact with LCE. Furthermore, we demonstrate that these interactions are crucial for the remarkable stability of LCA in neuronal cells.
RESULTS
Localization and activity of LCA in cultured neuronal cells
GFP–LCA transiently expressed in non-differentiated SiMa cells was detected by western blot analysis in total cell lysates as a 75 kDa protein (supplementary material Fig. S1A). It is known that LCA catalyzes cleavage of the nine C-terminal amino acid residues in SNAP-25 producing SNAP-25197 (Blasi et al., 1993). Antibodies that specifically interact with the LCA-catalyzed cleavage product (Fernández-Salas et al., 2012) detected a band below 25 kDa in GFP–LCA-expressing SiMa cells (supplementary material Fig. S1A), indicating that GFP–LCA is active in SiMa cells in agreement with the data obtained previously in PC12 cells (Fernández-Salas et al., 2004). Similarly, GFP–LCA expressed in SH-SY5Y cells cleaved endogenous SNAP-25 (data not shown). In addition, recombinant LCA (rLCA) delivered to differentiated PC12 cells using a protein delivery reagent cleaved SNAP-25 in a dose-dependent manner as seen from the gradual increase in the amount of cleaved SNAP-25 by 0.5, 1 and 2 µg of rLCA (supplementary material Fig. S1B). Therefore, LCA introduced in neuronal cells, either by plasmid transfection or by delivering the mature protein, interacts with the endogenous SNAP-25 and cleaves its C-terminal tail.
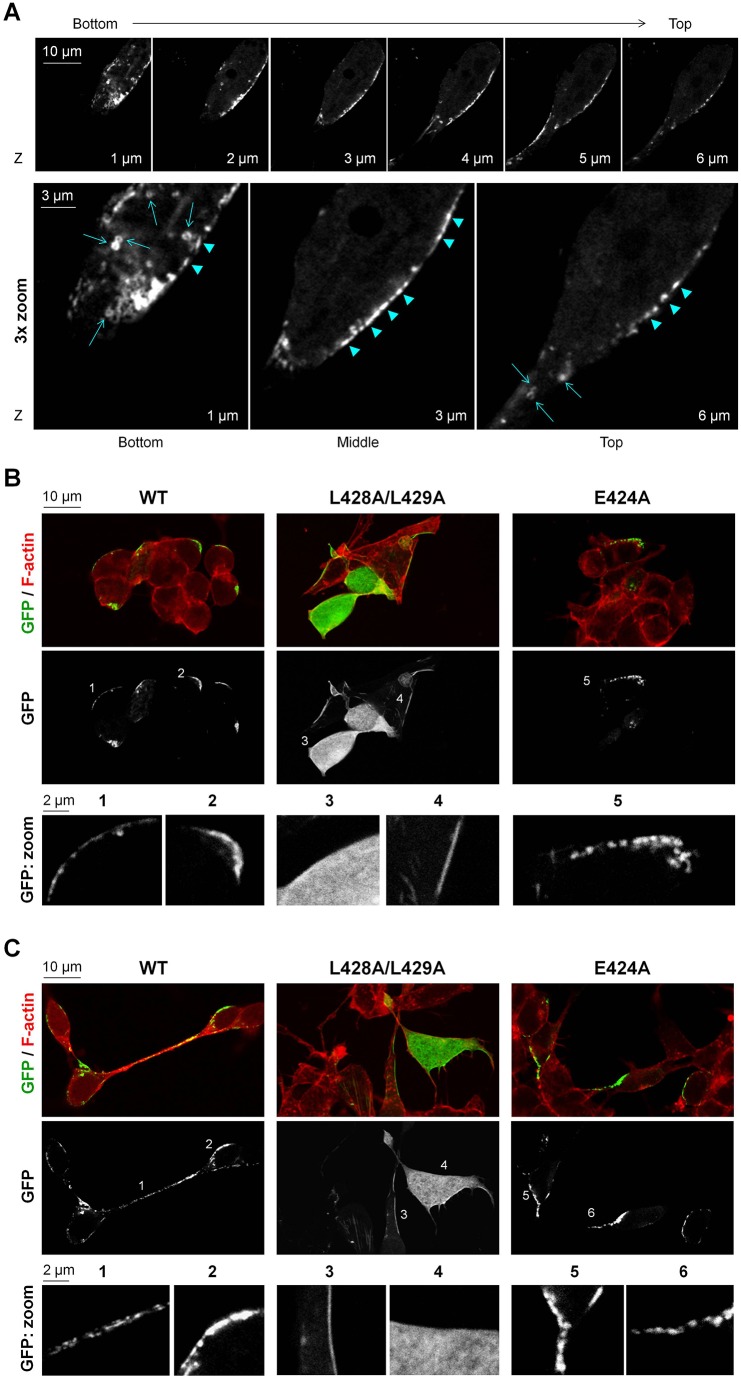
As detected by confocal microscopy, GFP–LCA accumulated in clusters or small aggregates at the plasma membrane in SiMa cells that were both differentiated (Fig. 1A,C) and non-differentiated (Fig. 1B). Similar distribution of GFP–LCA was found in SH-SY5Y cells (supplementary material Fig. S1C). In addition, rLCA introduced into differentiated PC12 cells using the Chariot™ protein delivery reagent was localized to the membrane as detected by immunofluorescence using anti-polyhistidine antibodies (supplementary material Fig. S1D). These results are in agreement with previous data showing punctate plasma membrane localization of GFP–LCA in PC12 cells (Fernández-Salas et al., 2004). Fractionation of differentiated SH-SY5Y cells transfected with rLCA (Chariot™ reagent) by two different methods resulted in detection of rLCA primarily in membrane fractions (supplementary material Fig. S1E). LCA does not possess a transmembrane domain or any other sequence that suggests that it can bind to the plasma membrane. Therefore, the results suggest that LCA might associate with the plasma membrane by protein–protein or by protein–lipid interaction, and that this association is strong enough to remain intact during the fractionation process. By contrast, recombinant LCE (rLCE) was detected mostly in the cytosolic fraction after fractionation of SH-SY5Y cells containing this protein (supplementary material Fig. S1E), in agreement with confocal microscopy detection of GFP–LCE in SiMa cells (supplementary material Fig. S1F) and in PC12 cells (Fernández-Salas et al., 2004). These results contrast with the recent data claiming a similar localization of LCA and LCE in neuronal cells (Tsai et al., 2010).
Fig. 1.
L248A/L249A mutation impairs clustered or small aggregate distribution of LCA at the plasma membrane. (A) Confocal microscopy images showing z-sectioning of a differentiated SiMa cell expressing the wild-type (WT) GFP–LCA. GFP–LCA is seen in ring-like (arrows) and rod-like (arrowheads) clusters or small aggregates at the plasma membrane. The ring-like structures are predominantly seen in the bottom and top sections of the cell, whereas the rod-like structures are mostly seen along the plasma membrane in the middle section of the cell. (B,C) Effect of mutations in the dileucine-based consensus motif on localization of LCA. Confocal microscopy images show loss of clustered or aggregate distribution of LCA owing to the L428A/L429A, but not the E424A, mutation both in non-differentiated (B) and differentiated SiMa cells (C). F-actin (phalloidin) staining was used to show the outlines of cells. The position of the ‘zoom’ images are indicated by numbers.
Remarkably, LCA was accumulated in patches or clusters along the plasma membrane rather than being evenly distributed at the membrane (Fig. 1A). These clusters had ring-like shapes at the bottom and top planes of the cells and rod-like shapes in the middle plane of the cell (Fig. 1A). Some of the rod-like structures seen in horizontal sections along the plasma membranes might be projections of the ring-like structures located in the plane of the lateral membrane.
The L428A/L429A mutation prevents clustered distribution of LCA at the membrane
Previously, the L428A/L429A mutant had been found to be loosely associated with the membrane and partially distributed throughout the cytoplasm in PC-12 cells (Fernández-Salas et al., 2004). Along with these data, in fractionated SH-SY5Y cells transfected with the recombinant (r)L428A/L429A mutant, the protein was detected in both membrane and cytosolic fractions, in contrast to almost exclusive detection of the wild-type LCA in membrane fractions (supplementary material Fig. S1E). When expressed in SiMa cells, the L428A/L429A mutant was either predominantly found in the cytoplasm (Fig. 1B, zoom field 3; Fig. 1C, zoom field 4) or distributed between the cytoplasm and the plasma membrane (Fig. 1B, zoom field 4; Fig. 1C, zoom field 3). In a minority of cells, the L428A/L429A mutant was found predominantly at the plasma membrane. Importantly, the distribution of the L428A/L429A mutant in the membrane was not clustered, but rather even and homogeneous, in contrast to that of the wild type. The differences in distribution of the wild-type LCA and its L428A/L429A mutant were observed in both non-differentiated (Fig. 1B) and differentiated (Fig. 1C) SiMa cells. L428 and L429 reside in a putative acidic dileucine motif, (D/E)xxxL(L/I), known to be important for protein trafficking. To determine whether the acidic residue in the E424FYKLL motif is involved in trafficking and localization of LCA, we mutated the E424 to alanine. Similar to wild-type LCA, the E424A mutant had clustered distribution along the plasma membrane (Fig. 1B, zoom field 5; Fig. 1C, zoom fields 5 and 6), suggesting that L428 and L429 are the amino acids that are crucial for the clustered or small aggregate localization of LCA at the plasma membrane.
The L428A/L429A mutation decreases stability of LCA in neuronal cells
Recent studies have shown that the double mutation L428A/L429A in LCA dramatically shortens both proteolytic and neuroparalytic effects of the neurotoxin in vivo (Wang et al., 2011). To test whether this mutation affects the stability of LCA expressed in SiMa cells, we compared the stability of the wild-type and mutated proteins during prolonged cell growth. To decrease the rate of cell proliferation, normal medium was replaced by low-serum (0.5% FBS) medium 24 h after transfection of non-differentiated SiMa cells. This allowed cell survival without overgrowth for up to 20 days after transfection. Transfected cells grown in separate wells were lysed after the indicated time periods following transfection, and the amount of GFP–LCA and β-actin in cell lysates were analyzed by western blotting. The initial levels of expression of the wild-type LCA and the L428A/L429A mutant were similar. The decrease in the amount of the wild-type GFP–LCA was slower than that of the L428A/L429A mutant (Fig. 2A), suggesting that the mutation decreases stability of LCA.
Fig. 2.
The L428A/L429A mutation decreases the life time of LCA. (A,B) Non-differentiated cells expressing the wild-type (WT) LCA or the L428A/L428A mutant (A) and the H227Y or H227Y/L428A/L428A mutants (B) were lysed after the indicated time periods of cell incubation in low-serum medium following transfection, and the amount of GFP–LCA and β-actin was determined by immunoblotting. Densitometry quantification of the GFP–LCA signal normalized by β-actin signal show that the L428A/L429A mutation shortens the life span of LCA. (C) Confocal microscopy images showing that the H227Y mutation did not change the clustered or aggregate distribution of LCA, whereas the additional mutation of L428 and L429 to alanine residues in the inactive mutant of LCA resulted in a loss of clustered distribution. Non-differentiated SiMa cells incubated for 3 days in a low-serum medium are shown. F-actin (phalloidin) staining was used to show the outlines of cells. Results are mean±s.d. (n = 3). *P<0.01 compared with the wild-type LCA (A) or H227Y (B), Student's t-test.
To exclude the possibility that LCA-catalyzed cleavage of the endogenous SNAP-25 interfered with this cell-based assay of LCA persistence, we performed the same assay in cells expressing proteolytically inactive LCA mutants, H227Y and H227Y/L428A/L429A (Fig. 2B). The H227Y mutant, similar to the wild-type LCA, predominantly accumulated in clusters or small aggregates along the plasma membrane (Fig. 2C, zoom field 1). The H227Y/L428A/L429A mutant was either evenly distributed in the membrane (Fig. 2C, zoom field 2) or predominantly found in the cytoplasm (Fig. 2C, zoom field 3), similar to that of the L428A/L429A mutant (Fig. 1B,C). Even though cells were not differentiated prior to transfection, they look similar to differentiated cells (compare Fig. 2C and Fig. 1C), presumably owing to their incubation in low-serum medium. The initial levels of expression of H227Y and H227Y/L428A/L429A mutants were similar to those of the wild-type LCA and its L428A/L429A mutant. Interestingly, the inactive mutants of both LCA and L428A/L429A persisted for a longer time than their respective catalytically active counterparts (Fig. 2A,B), suggesting that cleavage of SNAP-25 by LCA might inhibit cell growth resulting in preferential growth and enrichment of cells that express lower levels of or no LCA. Importantly, LCA H227Y was detected in cells longer than LCA H227Y/L428A/L429A (Fig. 2B), confirming that the L428A/L429A mutation increases the rate of LCA degradation in SiMa cells.
The increase in degradation rate of LCA by the L428A/L429A mutation was also confirmed in differentiated PC12 containing the recombinant light chains delivered to the cells by the protein transfection reagent Chariot™ (supplementary material Fig. S2). The persistence of light chains in differentiated PC12 cells was determined by detecting SNAP-25197 (LCA-cleaved SNAP-25) or SNAP-25180 (LCE-cleaved SNAP-25) for 14 days. The proteolytic activity of wild-type rLCA persisted almost unchanged on average for 14 days, while the activity of rLCE declined by 80% after 3 days and disappeared after 14 days of cell incubation (supplementary material Fig. S2). The activity of the L428A/L429A mutant was decreased by 40% after 14 days. Removal of 22 C-terminal residues that include L428 and L429 resulted in a more dramatic decrease in proteolytic activity of LCA over time (supplementary material Fig. S2), confirming that the L428/L429 dileucine is important for LCA stability.
The L428A/L429A mutation accelerates LCA proteolysis by both proteasomal and non-proteasomal proteases in neuronal cells
To determine whether the L428A/L429A mutation increases the susceptibility of LCA to intracellular ubiquitylation and/or proteasomal degradation, we used the irreversible proteasome inhibitor lactacystin (Fenteany et al., 1995). Non-transfected SiMa cells were used as a negative control, and LCE, which has been reported to be ubiquitylated and degraded by the proteasomal pathway in neuronal cells (Tsai et al., 2010), was used as a positive control. Transfected SiMa cells were pre-incubated with or without lactacystin. The expressed proteins were immunoprecipitated using an anti-GFP antibody and analyzed by immunoblotting using an ubiquitin antibody (detection of polyubiquitylated forms) or a GFP antibody (detection of total amount). In the absence of the inhibitor, the ubiquitylated forms were detected in both the wild-type LCA and wild-type LCE at similar levels, but were absent in non-transfected cells (Fig. 3A). The results indicate that not only LCE but also LCA is subject to polyubiquitylation. Cell exposure to lactacystin did not change the amount of polyubiquitylated forms of the wild-type LCA (Fig. 3A). These results suggest that the polyubiquitylated forms of LCA are only slightly degraded by the proteasome, explaining why a proteasome inhibitor has no effect. In contrast, the amount of the polyubiquitylated forms of the L428A/L429A mutant were increased by 50% by adding lactacystin, indicating that the mutation increases the rate of proteasomal degradation of polyubiquitylated forms of LCA, which was inhibited by lactacystin. Similarly, the amount of polyubiquitylated LCE was increased by adding lactacystin, but to a greater degree (by 150%). Thus, these results are consistent with the longer life of the L428A/L429A mutant as compared to that of LCE (supplementary material Fig. S2). If the L428A/L429A mutation also increased the rate of formation of ubiquitylated forms, one would expect a mutation-induced increase in the amount of polyubiquitylated forms of LCA in the absence of the proteasomal inhibitor. However, in the absence of lactacystin, the amount of polyubiquitylated forms of LCA is decreased by the L428A/L429A mutation (Fig. 3A). Remarkably, even in the presence of lactacystin, the level of polyubiquitylation was lower for the mutant than for the wild-type LCA (Fig. 3A). The results suggest that the L428A/L429A mutation increases the rate of degradation of polyubiquitylated forms, but not the rate of their formation.
Fig. 3.
The L428A/L429A mutation increases the sensitivity of LCA to intracellular degradation. Non-differentiated SiMa cells expressing the wild-type (WT) LCA, or L428A/L429A mutant, or wild-type LCE were exposed to lactacystin (proteasome inhibitor), bafilomycin (inhibitor of lysosomal V-type ATPase) or MG-132 (inhibitor of proteasomal, lysosomal, and calpain proteases), and the effects of inhibitors on the light chains (LCs) were measured. (A) Cells were incubated with or without lactacystin for 16 h and lysed. GFP-tagged LCs were immunoprecipitated with GFP antibody. Polyubiquitylated LCs were detected with an ubiquitin-specific antibody. The membrane was stripped and re-probed with GFP monoclonal antibody to detect total amounts of the various LCs. Densitometric quantification of the results shows lactacystin-induced accumulation of the polyubiquitylated forms of LCE and the L428A/L429A mutant, but no effect on the wild-type LCA polyubiquitylated forms. (B) Effect of the inhibitors of degradation on the total amount of LCs. Cells expressing the respective GFP-tagged LCs were incubated with cycloheximide in the absence or in the presence of degradation inhibitors for 8 h. The amount of LCs and β-actin in total cell lysates was determined by immunoblotting. Quantification of the results indicates that both MG-132 and bafilomycin protect the L428A/L429A mutant and LCE, but not the wild-type LCA. (C) Downregulation of septin-2 by siRNA increases the rate of LCA degradation. Non-differentiated cells expressing GFP–LCA were treated with septin-2 or control siRNA as described in the Materials and Methods and were incubated with cycloheximide in the absence or presence of MG-132. The amount of LCA, septin-2 and β-actin in total cell lysates was determined by immunoblotting. Quantification of the results indicates that downregulation of septin-2 decreases the amount of mature GFP–LCA in the absence, but not in the presence, of the degradation inhibitor MG-132. Results are mean±s.d. (n = 3). *P<0.01 compared with control (no inhibitors), Student's t-test. WB, western blot analysis; IP, immunoprecipitation; NT, non-transfected cells; ?, unidentified bands; IgG HCh, the heavy chain of the antibody used for immunoprecipitation.
Similarly, the amount of the non-ubiquitylated wild-type LCA in total cell lysates was not affected by the reversible proteasome inhibitor MG-132 (Fig. 3B). In contrast, the amount of the L428A/L429A mutant was increased by MG-132 (Fig. 3B). A minor but statistically significant increase in the amount of the L428A/L429A mutant was also observed in the presence of bafilomycin, which inhibits the lysosomal V-type-ATPase (Yoshimori et al., 1991), thus preventing lysosomal acidification and hence the activity of lysosomal proteases (Fig. 3B). Given that MG-132, unlike the more specific lactacystin, inhibits not only proteasomal, but also some lysosomal and non-lysosomal cysteine proteases (Lee and Goldberg, 1998; Tawa et al., 1997), the results suggest that, in the absence of inhibitors, the non-ubiquitylated forms of the mutant are degraded by non-proteasomal proteases. The greater protection of the mutant by MG-132 than by bafilomycin, which specifically inhibits lysosomal degradation, suggests the involvement of both lysosomal and cytosolic proteases. Similarly, LCE was protected by both MG-132 and bafilomycin, but not by lactacystin (Fig. 3B), suggesting that non-ubiquitylated LCE is also degraded by lysosomal and cytosolic proteases.
In summary, the results show that both LCA and LCE undergo intracellular polyubiquitylation. However, the polyubiquitylated forms of LCE rapidly undergo proteasomal degradation, consistent with previously published data (Tsai et al., 2010), whereas the polyubiquitylated forms of LCA are highly resistant to proteasomal proteolysis. In addition, the non-ubiquitylated LCE is more susceptible than LCA to proteolysis by non-proteasomal proteases. The L428A/L429A mutation induces rapid proteasomal degradation of the ubiquitylated LCA and accelerates degradation of the non-modified LCA by both lysosomal and cytosolic proteases.
Identification of proteins that interact with LCA through the longevity-essential dileucine motif
Given that the L428A/L429A mutation increases the susceptibility of LCA to degradation, these two leucine residues might be important for the interaction of this protease with particular proteins that protect it from intracellular degradation. Therefore, the proteins that bind to the wild type, but not to the L428A/L429A mutant, can be considered as the LCA-interacting proteins that might determine the stability of LCA. To identify these binding partners, we performed a comparative nano-liquid chromatography (nLC)-MS/MS analysis of the proteins co-immunoprecipitated with GFP-fused constructs of either the wild-type LCA or its L428A/L429A mutant from 1% NP-40 and 0.5% sodium deoxycholate (DOC) lysates of SiMa cells transiently transfected with a respective construct. As a negative control, a parallel nLC-MS/MS analysis was performed on the proteins co-immunoprecipitated with GFP alone, so only the proteins that were not detected in GFP immunoprecipitates were considered as specific LCA interactors. The analysis of the data by Mascot software showed that the L428A/L429A mutation impairs the interaction of LCA with several septin proteins (Table 1). As a rough approximation, protein score and sequence coverage calculated by Mascot software can be considered as proportional to the amount of the protein present in the immunoprecipitate. The highest values of the scores and percentage of sequence coverage were found for septin-2, septin-7 and septin-9, implicating them as major interacting partners of LCA (Table 1). Several other septins, namely septin-6, septin-11, septin-3 and septin-5, which were detected with lower score and coverage, appear to be minor interacting partners. Septin-2, septin-6, septin-11, septin-3 and septin-5 were detected exclusively in the wild-type LCA immunoprecipitates, suggesting that the interaction of LCA with septins is mediated by L428 and L429. Septin-7 and septin-9 were detected with both the wild-type LCA and its mutant. However, both the protein score and peptide coverage were significantly lower in the mutant immunoprecipitates as compared to those in the wild-type LCA immunoprecipitates (Table 1), suggesting that the mutation severely impaired the interaction of LCA with these proteins. The nLC-MS/MS analysis of the proteins immunoprecipitated by the GFP antibody from the cell lysates of SiMa cells expressing GFP–LCE using the same protocol shows that GFP–LCE was immunoprecipitated with a high efficiency as can be inferred for the high Mascot scores for both BoNT/E and GFP (Table 1). However, none of the septins were identified as interacting partners for GFP–LCE by nLC-MS/MS, confirming the specificity of the LCA–septin interaction. Septins belong to a protein family that includes at least 14 highly related GTP-binding cytoskeletal proteins that play important roles in various cellular processes (Hall and Russell, 2012; Mostowy and Cossart, 2012; Nakahira et al., 2010; Saarikangas and Barral, 2011).
Table 1. Proteins immunoprecipitated by anti-GFP antibody from 1% NP40 and 0.5% DOC total lysates of cells expressing the wild-type or L428A/L429A GFP-LCA or wild-type GFP-LCE.
Only the proteins detected in three different experiments and not detected in GFP immunoprecipitates are shown, unless specified otherwise. The values of the protein score and the percentage of sequence coverage from one of the three experiments are shown. ND, not determined.
Detected in one of the three experiments.
Surprisingly, SNAP-25 or other SNARE proteins were not detected in GFP–LCA immunoprecipitates by nLC-MS/MS (Table 1) in spite of the fact that LCA cleaves SNAP-25 in SiMa cells (supplementary material Fig. S1A,B). To determine whether SNARE proteins co-immunoprecipitate with GFP–LCA under milder extracting conditions, we performed an nLC-MS/MS analysis of GFP–LCA immunoprecipitates isolated from 0.2% DOC membrane extracts. Similar to that found in total cell lysates (Table 1), septin-2, septin-7, septin-9, septin-11 and septin-5 were detected in GFP–LCA immunoprecipitates, but not in GFP immunoprecipitates, in 0.2% DOC membrane extracts (supplementary material Table S1), confirming them as specific LCA-interacting proteins. Several SNARE proteins and SNARE-interacting proteins, but not SNAP-25, were detected (supplementary material Table S1). However, only two of them, syntaxin-1a and syntaxin 16, were found exclusively in GFP–LCA immunoprecipitates. The values of the protein score and the percentage of sequence coverage indicate that the abundance of these proteins in GFP–LCA immunoprecipitates is very low when compared to the GFP–LCA protein amount. Consistent with the nLC-MS/MS results, co-immunoprecipitation of syntaxin-1, but not of SNAP-25, with GFP–LCA was detected in 0.2% DOC extracts by western blot analysis (supplementary material Fig. S3A). The location of L428 and L429 in a putative acidic dileucine motif, E424FYKLL, suggests the involvement of this motif in LCA interaction with adaptor protein complexes (Fernández-Salas et al., 2004). However, nLC-MS/MS analysis of GFP–LCA immunoprecipitates isolated from total cell lysates of GFP–LCA-expressing SiMa cells also did not detect any subunits from adaptor protein complexes (AP-1, AP-2, AP-3 or AP-4) (Table 1). One of the subunits was detected in GFP–LCA immunoprecipitates isolated from 0.2% DOC membrane extracts (supplementary material Table S1), and one subunit was found in a single experiment in L428A/L429A immunoprecipitate from total cell lysates (Table 1), but both subunits were also detected in GFP immunoprecipitates (supplementary material Table S1), indicating the lack of a specific interaction of these subunits with LCA.
The results indicate that association of GFP–LCA with SNARE proteins is weak and/or transient and, hence, is unlikely to be responsible for membrane tethering of LCA as was previously suggested (Chen and Barbieri, 2011). The data provide no evidence for a specific interaction between LCA and adaptor proteins complexes. By contrast, the stable interaction of LCA with septins suggests a role for these membrane cytoskeleton proteins in stable attachment of LCA to the specific plasma membrane regions. Given that the L248A/L429A mutation, which decreased LCA stability (Figs 2 and 3), prevented the interaction of LCA with septins (Table 1), the results also suggest that septins might protect LCA from intracellular degradation.
Partial colocalization of LCA with SNARE proteins in SiMa cells
Immunofluorescence of total endogenous SNAP-25 showed its partial colocalization with LCA in SiMa cells, both non-differentiated and differentiated (supplementary material Fig. S3B). Earlier studies have demonstrated colocalization of LCA with SNAP-25197 in neuronal PC12 cells (Fernández-Salas et al., 2004). The differences are probably due to the fact that the antibody used for immunostaining of SiMa cells recognizes both intact SNAP-25 and SNAP-25197. Similarly, only partial colocalization with syntaxin-1 was found in both non-differentiated and differentiated SiMa cells (supplementary material Fig. S3C). Both SNAP-25 and syntaxin-1 are evenly distributed along the plasma membrane as opposed to clustered distribution of GFP–LCA in restricted regions at the plasma membrane (supplementary material Fig. S3B,C).
Downregulation of septin-2 increases LCA degradation
Two consecutive transfections of SiMa cells with the mixture of two small interfering RNAs (siRNAs) targeting septin-2, performed 48 and 24 h prior to GFP–LCA transfection, resulted in a decrease in septin-2 expression by 50%, as detected by western blot analysis (Fig. 3C). At 24 h after GFP–LCA transfection, cells were incubated in the presence or in the absence of MG-132 for 8 h. To look specifically at the mature GFP–LCA, all samples were incubated with cycloheximide for 2 h before and also during this 8-h incubation. The amount of GFP–LCA was decreased by septin-2 siRNA by 53% (Fig. 3C). This effect was partially prevented by MG-132, indicating that septin-2 protects LCA from degradation.
Colocalization of LCA with septin-2 and septin-7 in SiMa cells
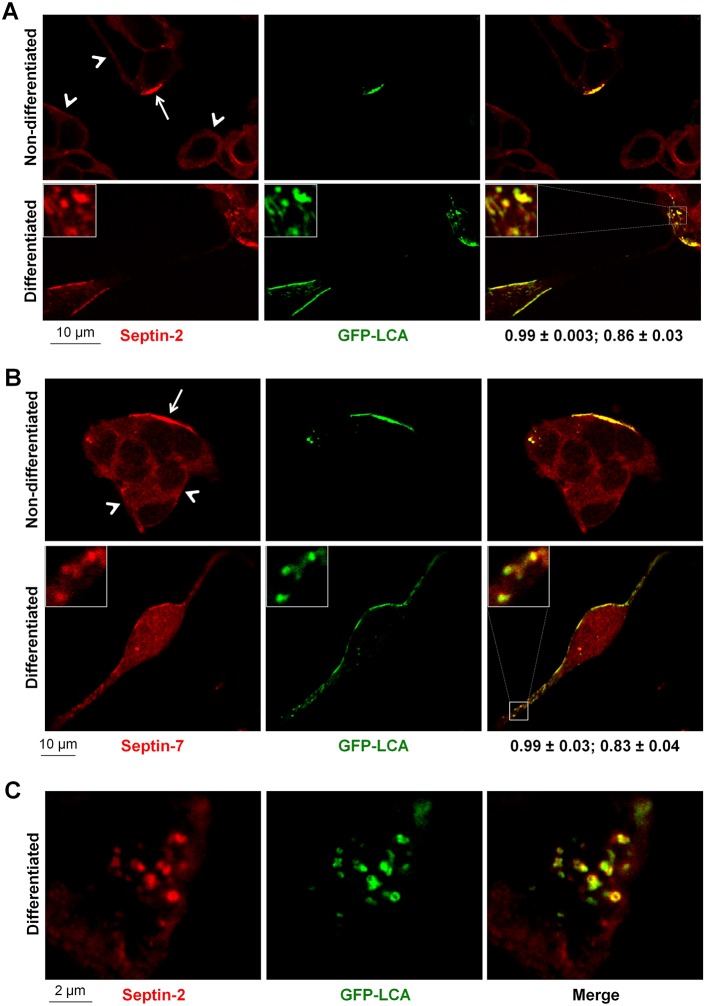
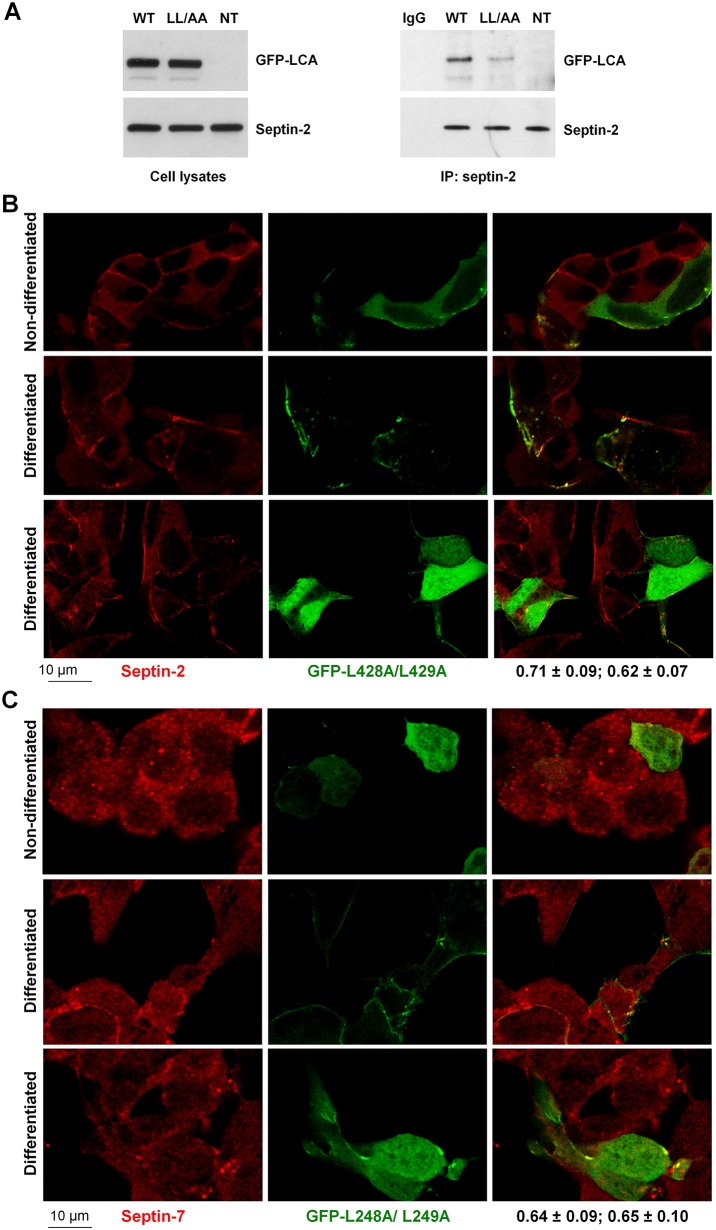
Both septin-2 and septin-7 were precisely colocalized with LCA in clusters distributed along the plasma membrane (Fig. 4A,B). These clusters have ring-like shapes as seen from high-resolution images of the basal sections of LCA-expressing cells (Fig. 4C). Remarkably, both septin-2 and septin-7 displayed much more intense accumulation in LCA-positive regions at the plasma membrane as compared to other membrane regions in LCA-expressing cells or to the plasma membrane of non-transfected cells (Fig. 4A,B), suggesting that LCA presence results in a re-organization of the septin cytoskeleton. The L428A/L429A mutation dramatically decreased co-immunoprecipitation of LCA with septin-2 as detected by immunoblotting (Fig. 5A) and decreased colocalization of LCA with both septin-2 and septin-7 (Fig. 5B,C), consistent with the impairment of LCA interaction with septins by this mutation (Table 1). Importantly, the mutation decreased the accumulation of both septin-2 and septin-7 in LCA-positive regions at the membrane, so that distribution of these cytoskeletal proteins in the mutant-expressing cells was similar to that in non-transfected cells (Fig. 5B,C). The results are consistent with the involvement of septins in clustering of LCA at the plasma membrane. Furthermore, the results suggest that the presence of LCA induces the assembly of septin oligomeric structures.
Fig. 4.
LCA recruits septin-2 and septin-7 to membrane-associated clusters or small aggregates. (A,B) LCA is colocalized with septin-2 (A) and septin-7 (B) in clusters at the plasma membrane both in non-differentiated and differentiated SiMa cells. Immunofluorescence of both septin-2 and septin-7 in LCA-positive regions at the plasma membrane (arrows) is much more intense compared to that at the plasma membrane in non-transfected cells (arrowheads). Insets show magnified images of the boxed regions. The coefficient of colocalization with septins (mean±s.d., n = 12) followed by the overlap coefficient (mean±s.d., n = 12) is indicated below the merged images. (C) High-magnification images of the basal sections of non-differentiated LCA-transfected cells showing colocalization of LCA and septin-2 in the ring-like structures.
Fig. 5.
Both the interaction and co-clustering of LCA with septins is impaired by the L428A/L429A mutation. (A) The L428A/L429A mutation decreases the amount of LCA that co-immunoprecipitates with septin-2. (B,C) The L428A/L429A mutation decreases colocalization of LCA with septin-2 (B) and septin-7 (C) both in non-differentiated and differentiated cells. The mutation also decreased accumulation of both septin-2 and septin-7 in LCA-positive regions. The coefficient of colocalization with septins (mean±s.d., n = 12) followed by the overlap coefficient (mean±s.d., n = 12) is indicated below the merged images. WT, wild-type GFP–LCA; NT, non-transfected cells; IP, immunoprecipitation; LL/AA, GFP–LCA-L428A/L429A mutation.
To determine whether LCA-induced septin clustering occurs after neuronal cell exposure to BoNT/A, we used differentiated SiMa cells that we previously demonstrated to be as sensitive to BoNT/A as primary motor neurons (Fernández-Salas et al., 2012). In these settings, the LCA will be delivered to the plasma membrane of the neuronal cells following receptor binding, internalization, and translocation as it occurs in vivo. We exposed SiMa cells to 1 or 10 nM BoNT/A overnight and performed septin-2 immunofluorescence. The representative high- and low-magnification images show clustering of septin-2 in BoNT/A-exposed cells (supplementary material Fig. S4). Statistical analysis of the fluorescence intensity profiles demonstrated that BoNT/A exposure increased the mean fluorescence intensity of septin-2 at the plasma membrane of SiMa cells, consistent with the LCA-induced recruitment of septin-2 to clusters in the membrane. BoNT/A exposure also increased the frequency of the sites with high fluorescence intensity in the membrane in a dose-dependent manner, consistent with clustering of septin-2 at the plasma membrane upon treatment with BoNT/A.
Effect of an inhibitor of septin assembly on localization and stability of LCA
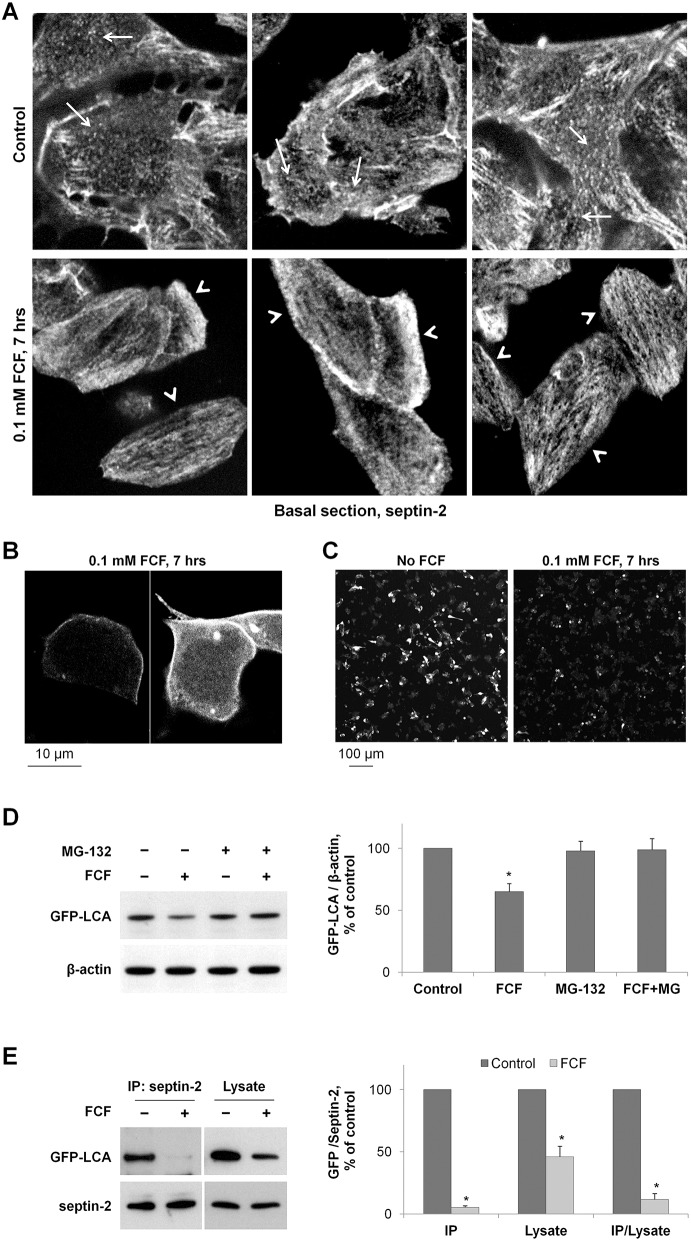
To assess whether septins are important for stability of LCA, we used an inhibitor of septin oligomerization, forchlorfenuron (FCF). This compound has been recently found to affect septin assembly, organization and dynamics, both in yeast and mammalian cells (Hu et al., 2008; Iwase et al., 2004). FCF impaired normal septin assembly and induced the formation of abnormally large septin structures (DeMay et al., 2010; Hu et al., 2008). The effects of FCF on mitosis, cell migration and cell adhesion were similar to those induced by depletion of septin-2 by siRNA (Hu et al., 2008; Sidhaye et al., 2011).
The effect of the inhibitor on septin organization in SiMa cells is evident from the septin-2 immunofluorescence performed in the cells incubated with 100 µM FCF for 7 h (Fig. 6A). In the bottom section of control cells, the ring-like and punctuated structures are evident (Fig. 6A, arrows). The inhibitor disrupted these structures and induced the formation of elongated linear filaments (Fig. 6A, arrowheads). In addition, in the presence of the inhibitor, cells became more elongated (Fig. 6A). Incubation of non-differentiated SiMa cells with 100 µM FCF, which was added to the cells 14 h after LCA transfection, increased cytosolic localization of LCA and decreased clustered distribution of LCA at the plasma membrane (Fig. 6B), resulting in a more-even and homogeneous distribution of LCA, which resembled the pattern seen for the L428A/L429A mutant (Fig. 5). Exposure to FCF also caused a substantial decrease in LCA cellular content (Fig. 6C), suggesting that FCF either inhibits biosynthesis or increases degradation of LCA. To determine the effect of FCF specifically on degradation, cells were incubated with cycloheximide for 2 h before and for 8 h during cell incubation with or without FCF. Western blot analysis of cell lysates showed a significant decrease in LCA amounts, indicating that FCF increases the degradation rate of the mature LCA. Addition of the degradation inhibitor, MG-132, prevented the FCF-induced degradation of LCA (Fig. 6D). To evaluate whether FCF decreases LCA degradation by preventing the interaction between LCA and septin, we performed immunoprecipitation of septin-2 from GFP–LCA-expressing cells incubated with or without 100 µM FCF for 24 h. The exposure to FCF did not change the amount of septin-2 in total cell lysates or immunoprecipitated fractions, but significantly decreased the amount of GFP–LCA in both total cell lysates and the septin-2 immunoprecipitates (Fig. 6E). Importantly, the decrease in the immunoprecipitated fraction was greater than in cell lysates, as seen from a dramatic decrease in the amount of the co-immunoprecipitated GFP–LCA that was normalized to its amount in the total cell lysates (Fig. 6E, ‘IP/Lysate’ on the graph). The results indicate that disruption of septin oligomerization by FCF impairs the interaction between LCA and septins, which, in turn, accelerates LCA degradation. Therefore, normal septin dynamics are required for the interaction of LCA with septins, which protects LCA from degradation.
Fig. 6.
Both downregulation of septin-2 and disruption of septin organization by FCF increase LCA degradation. (A) FCF disrupts the ring-like and punctuated structures of septin 2 (arrows) and induces the formation of elongated filaments (arrowheads) in SiMa cells. (B) High-resolution/high-magnification confocal microscopy images of non-differentiated GFP-LCA-expressing SiMa cells exposed to 100 µM FCF showing increased cytoplasmic localization of LCA and relatively even/homogeneous distribution of LCA along the plasma membrane. (C) Non-differentiated SiMa cells plated with similar density were transfected with GFP–LCA and 14 h later were incubated with or without FCF for 7 h. Low magnification confocal microscopy images of these cells show a substantial decrease in GFP fluorescence in FCF-exposed cells. (D) Disruption of septin organization by FCF induces degradation of GFP–LCA. Non-differentiated cells expressing GFP–LCA were incubated with cycloheximide in the absence or in the presence of 100 µM FCF or 20 µM MG-132. The amount of LCA and β-actin in total cell lysates was determined by immunoblotting. Quantification of the results indicates that exposure of cells to FCF decreases the amount of mature GFP–LCA in the absence, but not in the presence, of the degradation inhibitor MG-132. (E) FCF impairs the interaction between septin-2 and LCA. Septin-2 immunoprecipitation was performed after incubation of GFP–LCA-expressing cells with or without FCF for 24 h, and the amount of both septin-2 and GFP–LCA was determined by immunoblotting in immunoprecipitated fraction and total cell lysates. Densitometry quantification of the results shows that FCF decreases the amount of GFP–LCA in the septin-2 immunoprecipitated fractions more dramatically than in cell lysates, indicating its disruptive effect on septin-2-LCA interaction. Results are mean±s.d. (n = 3); *P<0.01 compared with control (no inhibitors), Student's t-test.
In conclusion, the dileucine-mediated interaction of LCA with septins is required for both the clustered or aggregate distribution at the plasma membrane and the remarkable stability of LCA in neuronal cells.
DISCUSSION
New properties of LCA dileucine motif
Previous studies have shown that the L428A/L429A mutation decreases association of LCA with the plasma membrane in PC12 cells (Fernández-Salas et al., 2004) and shortens the effects of BoNT/A in vivo (Wang et al., 2011). Here we demonstrate that the L428A/L429A mutation abolishes the interaction between LCA and septins, prevents the clustered or aggregate distribution of LCA at the plasma membrane, and decreases the stability of LCA in SiMa cells (Figs 2, 3, 5; Table 1). These results demonstrate that the LCA–septin interaction through the dileucine motif in LCA is essential for subcellular localization and persistence of LCA in neuronal cells.
L428 and L429 are located in a dileucine-containing (D/E)xxxL(L/I) consensus motif near the C-terminus of LCA. Such motifs, present in the cytoplasmic domains of many transmembrane proteins, are known to mediate the interaction of these proteins with the adaptor protein complexes AP-1, AP-2 or AP-3 (Bonifacino and Traub, 2003; Janvier et al., 2003; Mattera et al., 2011; Sitaram et al., 2012; Traub, 2009), which is important for intracellular trafficking. Our nLC-MS/MS analysis did not find dileucine-specific interaction of LCA with any of the subunits of adaptor protein complexes (Table 1). The E424A mutation did not change the localization (Fig. 1B,C) or stability (data not shown) of LCA. By contrast, our results clearly demonstrate that the L428A/L429A mutation impairs the interaction of LCA with septins (Table 1; Fig. 5), suggesting that the L428/L429 dileucine maybe part of a new septin-binding motif. Further studies are required to elucidate whether these two leucine residues are involved in direct interaction with septins and to identify other amino acid residues that are crucial for this interaction. Interestingly, the mutation of LI/AA in a dileucine-containing motif of lysosome membrane protein 2 (LIMP-II, also known as SCARB2), ERAPLI, abolished the interaction between LIMP-II and septin-7 (Baust et al., 2008). No other residues, particularly the glutamine residue, in this motif had been mutated in LIMP-II. The authors suggested that the interaction between LIMP-II and septin-7 was mediated by AP-3 adaptor protein complex that binds to LIMP-II in a LI-motif-dependent manner (Baust et al., 2008). However, no experimental evidence for septin–AP-3 binding was provided, so it is possible that septin-7 directly interacted with the dileucine motif of LIMP-II.
Membrane cytoskeleton proteins rather than SNARE proteins are preferred LCA-interacting partners
Recent studies have suggested that the interaction of LCA and SNAP-25 is responsible for stable association of LCA with the plasma membrane in neurons (Chen and Barbieri, 2011). However, our results from a global analysis of the LCA interactome in SiMa cells (Table 1; supplementary material Table S1) indicate that association of GFP–LCA with SNAP-25 is weak and transient and hence is unlikely to be responsible for the stabilization of LCA. This conclusion is consistent with the enzyme–substrate nature of the LCA–SNAP-25 interaction, implying that SNAP-25 constantly associates with and dissociates from LCA. In addition, the results suggest that the interaction with other SNARE proteins, particularly with syntaxin, is weak and transient. These data and the highly dynamic nature of SNARE complexes (Yu et al., 2011) mean that a stabilizing effect of SNARE proteins on LCA is doubtful.
By contrast, the results presented here indicate that a stable interaction of LCA with membrane cytoskeletal proteins of the septin protein family is important for both plasma membrane tethering and stabilization of LCA. Of 14 known septins, which form a family of highly related GTP-binding proteins, seven were detected as interacting partners of the wild-type LCA, but not of the L428A/L429A mutant (Table 1). Septins are membrane cytoskeletal proteins that associate with the plasma membrane through their interaction with phosphatidylinositol 4,5-bisphosphate (Bretscher et al., 2002; Mostowy and Cossart, 2012; Stamenkovic and Yu, 2010). By also interacting with actins and tubulins, septins serve as linkers between the plasma membrane and the intracellular cytoskeleton (Gilden and Krummel, 2010). In addition, by binding to other proteins, they act as diffusion barriers important for defining specific membrane compartments and for localizing interacting proteins at specific intracellular sites (Gilden and Krummel, 2010; Hall and Russell, 2012; Mostowy and Cossart, 2012; Nakahira et al., 2010; Saarikangas and Barral, 2011; Spiliotis et al., 2008). Septins are known to form membrane-associated hetero-oligomeric structures, such as rings and filaments (Gilden and Krummel, 2010; Hall and Russell, 2012; Kinoshita, 2003; Mostowy and Cossart, 2012). Accordingly, LCA, septin-2 and septin-7 are found colocalized in ring-like structures associated with the plasma membrane (Fig. 4). Moreover, LCA recruits septins to the membrane, both when overexpressed in cells by transfection (Fig. 4) and when introduced into cells by exposure to BoNT/A (supplementary material Fig. S4).
Dileucine-mediated formation of LCA/septins clusters at the membrane protects LCA from degradation
The formation of LCA-containing clusters at the plasma membrane requires the interaction of LCA with septins. When this interaction is prevented by the L428A/L429A mutation, the formation of LCA–septin hetero-oligomeric structures at the plasma membrane is abolished (Fig. 5), and LCA becomes more susceptible to degradation (Fig. 2A,B; Fig. 3A,B). Similarly, co-clustering and interaction of LCA with septins, as well as LCA stability are impaired by inhibiting normal septin oligomerization (Fig. 6). Importantly, LCA appears to actively recruit septins and to induce LCA–septin ring-like structures in restricted zones of the plasma membrane.
The lack of interaction of LCE with septins (Table 1) is probably, at least in part, responsible for the lower stability of this protein as compared to LCA. The results presented in our study suggest that lack of sensitivity of polyubiquitylated forms of LCA to lactacystin as compared to that of LCE (Fig. 3A) is due to a direct or indirect effect of the septins that interact with LCA, but not with LCE, and protect the polyubiquitylated forms of LCA from proteasomal degradation. Similarly, non-ubiquitylated LCA, but not LCE, interacts with septins that protect LCA from lysosomal and cytosolic degradation, explaining why LCA, unlike LCE, is insensitive to bafilomycin and MG-132 (Fig. 3B).
In motor neurons, LCA probably accumulates at the presynaptic membrane where it interacts with septins and cleaves SNAP-25, thus inhibiting neurotransmission. The presence of septins in presynaptic membranes has been demonstrated in several studies (Ihara et al., 2007; Kinoshita et al., 2000; Tsang et al., 2011; Xue et al., 2004; Yang et al., 2010). The conformation of SNAP-25 within a SNARE complex (Chen et al., 2002) differs drastically from the conformation of the LCA-bound SNAP-25 (Breidenbach and Brunger, 2004) (Fig. 7A), so SNAP-25 cannot bind LCA and SNARE proteins simultaneously. This is consistent with the fact that SNARE proteins in a SNARE complex cannot be cleaved by any of the botulinum neurotoxins (Pantano and Montecucco, 2014). Therefore, LCA likely binds to and cleaves free SNAP-25 prior to the formation of SNARE complexes in the vicinity of an active zone. Importantly, superposition of known crystal structures of a truncated LCA bound to SNAP-25 (PDB ID 1XTG) with a full-length LCA (PDB ID 2W2D) shows that the LCA-bound SNAP-25 does not conceal the L428/L429 dileucine motif (Fig. 7B), providing likely LCA binding to septins through this motif (Fig. 7C). LCA could possibly recruit septins outside the active zone of the presynaptic membrane. The free SNAP-25 is delivered to this portion of the membrane either by a biosynthetic route or by lateral diffusion after release from SNARE complexes formed during a vesicle–membrane fusion event in the active zone (Fig. 7C). LCA-catalyzed cleavage of the nine C-terminal amino acid residues generates SNAP-25197. The cleaved SNAP-25197 is able to form SNARE complexes, but these complexes are not competent for exocytosis (Pantano and Montecucco, 2014). As full-length SNARE-complex-bound SNAP-25 molecules dissociate, they will be cleaved by LCA, as will be newly synthesized SNAP-25 molecules as long as LCA persists in the presynaptic membrane. The association of LCA with septins that protects LCA from intracellular degradation (Figs 2, 3 and 6) ensures the remarkable longevity of both proteolytic and, probably, the neuroparalytic effects of LCA.
Fig. 7.
A postulated model showing that stabilization of LCA by septins at the presynaptic membrane results in persistent cleavage of the free pool of SNAP-25 and prolonged inhibition of neurotransmission. (A) Superposition of high resolution structures of SNAP-25 within a SNARE complex (PDB ID 1KIL) and SNAP-25 bound to LCA (PDB ID 1XTG) showing significant differences in the conformation of the C-terminal domain of SNAP-25 in these two protein complexes. The tight interaction of the C-terminal domain of SNAP-25 with its N-terminal domain and with other SNAREs, syntaxin (yellow) and synaptobrevin (orange), within the SNARE complex is not compatible with LCA-catalyzed cleavage of SNARE-complex-bound SNAP-25. (B) Superposition of high-resolution structures of a truncated LCA (gray surface) bound to the C-terminal core domain of SNAP-25 (PDB ID 1XTG) with a full-length LCA (PDB ID 2W2D, thin purple lines) shows no hindrance of L428 and L429 (purple spheres) by bound SNAP-25, indicating that the dileucine-mediated interaction of LCA with septins is permissive for the LCA proteolytic activity. (C) A putative model showing that the dileucine-mediated interaction with septin filaments tethers LCA to the presynaptic membrane in the vicinity of an active zone, where it cleaves SNAP-25 monomers that are either newly synthesized or released from SNARE complexes after synaptic vesicle fusion. After the cleavage of the nine C-terminal amino acid residues by LCA, SNAP-25197 forms SNARE complexes that are not competent for exocytosis, resulting in a blockage of synaptic vesicle fusion and neurotransmitter release. Association of LCA with septins protects LCA from intracellular degradation, providing an explanation for the remarkable longevity of proteolytic and, probably, paralytic effects of LCA in motor neurons.
In neurons, septin-2, septin-5 and septin-8 have been found to interact with syntaxin-1 and to inhibit membrane fusion (Amin et al., 2008; Beites et al., 2005; Beites et al., 1999; Ito et al., 2009). These results suggest that LCA might interact with SNAP-25–syntaxin-1 heterodimers, in which the N-terminal of SNAP-25 is bound to a single SNARE domain of syntaxin-1, leaving the C-terminal domain available for binding to LCA. Stabilization of LCA might result from its physical binding to the relatively stable cytoskeletal proteins. Interestingly, GFP-tagged septins exhibit slower turnover than some other cortical proteins that were analyzed (e.g. actin, syntaxin-1A and a glutamate-aspartate transporter; Hagiwara et al., 2011). It is also possible that septins attenuate degradation of LCA owing to their interaction with other proteins. Septins are known to form scaffolds for protein recruitment and regulate multiple signaling events (Amin et al., 2008; Baust et al., 2008; Brand et al., 2012; Sharma et al., 2013; Xue et al., 2004). The specific signaling pathways involved in stabilization of LCA by septins remain to be identified.
In conclusion, an integrative microscopic, biochemical and proteomic analysis of LCA-interacting proteins indicates that the dileucine-mediated interaction of LCA with septins is required for engagement of LCA into septin-containing membrane-associated cytoskeleton complexes, which protects LCA from intracellular degradation.
MATERIALS AND METHODS
Expression of LCA and LCE in neuronal cells
Human neuroblastoma SiMa cells (DSMZ, Braunschweig, Germany; Marini et al., 1999) were grown in RPMI 1640 with 10% fetal bovine serum and 0.1 mM non-essential amino acids. PC12 rat pheochromocytoma cells (ATCC, Manassas, VA) were grown in RPMI medium with 2 mM GlutaMAX™, 5% fetal bovine serum (FBS, heat-inactivated) and 10% equine serum. SH-SY5Y human neuroblastoma cells (ECACC, HPA, Porton Down, UK) were grown in minimum essential medium/F12 with 2 mM GlutaMAX™ I and Earle's salts, 10% fetal bovine serum and 0.1 mM non-essential amino acids. The growth medium for all three cell lines were supplemented by 10 mM HEPES, 1 mM sodium pyruvate, 100 U/ml penicillin and 100 µg/ml streptomycin (Sigma-Aldrich, St. Louis, MO). SiMa and SH-SY5Y cells were maintained at 37°C under 5.0% CO2, and PC12 cells were maintained at 37°C under 7.5% CO2. SiMa and SH-SY5Y cells were differentiated in RPMI medium with 2 mM GlutaMAX™, 1× B27 supplement and 1× N2 supplement. PC12 cells were differentiated in RPMI media with 2 mM GlutaMAX™, 1× B27 supplement, 1× N2 supplement and 50 ng/ml NGF (Promega, Madison, WI). All tissue culture reagents were from Life Technologies (Carlsbad, CA) unless specified otherwise.
The wild-type and L428A/L429A mutated LCA with the GFP at the N-terminus (GFP–LCA) and the wild-type LCE with the GFP at the N-terminus (GFP–LCE) were constructed as described previously (Fernández-Salas et al., 2004). The H227Y, E424A and H227Y/L428A/L429A mutants of GFP–LCA were constructed by site-directed mutagenesis using the QuikChange mutagenesis kit (Agilent Technologies, Cedar Creek, TX, USA). For the E424A mutant, the following primers were used with the wild-type plasmid as a template: 5′-CTGGATTGTTTGCATTTTATAAGTTGCTATGTGTAAGAGGG-3′ (forward) and 5′-CCCTCTTACACATAGCAACTTATAAAATGCAAACAATCCAG-3′ (reverse). For the H227Y and H227Y/L428A/L429A mutants, the following primers were used with the wild-type plasmid and the L428A/L429A mutant, respectively, as a template: 5′-GCACATGAACTTATATATGCTGGACATAGATTATATGGAATAGCAATT-3′ (forward) and 5′-AATTGCTATTCCATATAATCTATGTCCAGCATATATAAGTTCATGTGC-3′ (reverse).
The wild-type and mutated proteins were expressed in neuronal cells by transient transfection using Lipofectamine 2000 transfection reagent (Life Technologies, Carlsbad, CA). Where indicated, cells were differentiated for at least 3 days prior to transfection (Fernández-Salas et al., 2012). The recombinant His-tagged LCA (rLCA), its L428A/L429A mutant, its truncated variant without the 22 C-terminal residues (LCA ΔC), and the recombinant His-tagged LCE (rLCE) were synthesized and purified as described previously (Fernández-Salas et al., 2004). The proteins were delivered to 7-day differentiated PC12 (ATCC) cells using the Chariot™ protein delivery reagent (Active Motif, Carlsbad, CA, USA) according to manufacturer's protocol. Briefly, the light chains (6 µg/well) were mixed with Chariot™ reagent (6 µl) in 0.5× Dulbecco's PBS (D-PBS, 200 µl/well) and incubated at room temperature for 30 min. The cells were washed once with 1× D-PBS and the light chain/Chariot mixture was added with 0.5 ml serum-free medium. After 2 h incubation, the medium was replaced with differentiation medium. The cells were harvested at various times after transfection and presence of cleaved SNAP-25 was analyzed by western blotting.
Fractionation of SH-SY5Y cells transfected with rLCA and rLCE
SH-SY5Y cells were plated overnight, differentiated for 3 days, and transfected with rLCA or rLCE (20 µg protein per 10 cm dish) using the Chariot™ reagent. The cells were fractionated 24 h later with the ProteoExtract Subcellular Proteome Extraction Kit, S-PEK, or with the ProteoExtract Native Membrane Protein Extraction Kit, M-PEK (Calbiochem, EMD Biosciences, San Diego, CA) following the manufacturer's protocols. Correct fractionation of the cells was confirmed by western blot analysis of specific subcellular markers: cytochrome P450 reductase for membranes, tubulin for the cytoplasm, and PARP for the nuclear fraction, using polyclonal antibodies against cytochrome P450 reductase (StressGen, Victoria, BC, Canada) and monoclonal anti-tubulin and anti-PARP antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA) (data not shown).
Primary antibodies
A polyclonal antibody against GFP (Clontech, Mountain View, CA, USA), and polyclonal antibody against septin-2 (Sigma-Aldrich, St. Louis, MO, USA) were used for immunoprecipitation. The following monoclonal antibodies were used for western blot analysis or for immunofluorescence staining: against GFP, clones 7.1 and 13.1 (Roche Diagnostics, Indianapolis, IN), ubiquitin (P4D1; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), NSF (EMD Millipore, Billerica, MA), and syntaxin-1 (Sigma-Aldrich, St. Louis, MO). The following polyclonal antibodies were used: against septin-2 and against SNAP-25 (Sigma-Aldrich, St. Louis, MO), septin-7 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), His-tag (ab1187, Abcam Biotechnology, Cambridge, UK), and β-actin (Cell Signaling, Danvers, MA). Monoclonal antibodies that specifically recognize the LCA cleavage product of SNAP-25, SNAP-25197, were used for immunoblotting in SiMa cells (Fernández-Salas et al., 2012). Rabbit polyclonal antibodies that specifically recognize SNAP-25197, and LCE cleavage product SNAP-25180 (Fernández-Salas et al., 2004) were used in PC12 cells. These antibodies do not crossreact with the full-length SNAP-25, SNAP-25206.
Immunofluorescence staining
Fixation and immunostaining of GFP–LCA-transfected SiMa cells was performed as described previously (Tokhtaeva et al., 2012a) using appropriate primary antibodies and Alexa-Fluor-633-conjugated anti-mouse or anti-rabbit IgG secondary antibodies (Life Technologies, Carlsbad, CA, USA). F-actin was visualized by using Alexa-Fluor-633–phalloidin (Life Technologies, Carlsbad, CA, USA). PC12 cells containing rLCA were fixed and immunostained as described previously (Fernández-Salas et al., 2004) using the polyclonal anti-His-tag primary antibody and the Alexa-Fluor-488-conjugated secondary antibody (Life Technologies, Carlsbad, CA, USA).
Immunoprecipitation of GFP–LCA or GFP–LCE
At 48 h after transfection, SiMa cells were rinsed twice with ice-cold PBS and lysed by incubation with 50 mM Tris-HCl pH 7.5 containing 150 mM NaCl, 1% Nonidet P40, 0.5% sodium deoxycholate (DOC) and Complete Protease Inhibitor Cocktail, 1 tablet/50 ml (Roche Diagnostics, Indianapolis, IN) at 4°C for 30 min followed by scraping cells. Cell extracts were clarified by centrifugation (15,000 g, 10 min) at 4°C. Alternatively, microsomal membranes were isolated by differential centrifugation as described previously (Vagin et al., 2008), and membrane proteins were extracted by incubation of 2 mg microsome membranes with 1 ml of 50 mM Tris-HCl pH 7.5 containing 0.2% DOC and Complete Protease Inhibitor Cocktail, 1 tablet/50 ml at 4°C for 30 min. Membrane extracts were clarified by centrifugation (100,000 g, 1 h) at 4°C. GFP-linked LCA or LCE were immunoprecipitated from total cell lysates (1–2 mg protein) or membrane extracts (0.5–1 mg protein) by using GFP antibody as described previously (Tokhtaeva et al., 2012b). The adherent proteins were eluted from the beads by incubation in 35 µl of SDS-PAGE sample buffer (4% SDS, 0.05% Bromophenol Blue, 20% glycerol, 1% β-mercaptoethanol in 0.1 M Tris pH 6.8) for 5 min at 80°C.
Confocal microscopy
Confocal microscopy images were acquired using the Zeiss LSM 510 laser scanning confocal microscope (Carl Zeiss MicroImaging GmbH, Germany) or a Leica confocal (Leica Microsystems Inc., Buffalo Grove, IL) microscope using appropriate laser settings. Coefficients of colocalization with septins (means±s.d.) and overlap coefficients (mean±s.d.) were calculated by using ZEN 2009 software (Carl Zeiss MicroImaging GmbH, Germany). Statistical analysis of the images showing immunofluorescence of septin-2 (supplementary material Fig. S4) was performed by collecting profiles of the fluorescence intensity along the plasma membrane of randomly selected cells using Zen2009 software. The mean fluorescence intensity and the percentage of the sites with the fluorescence intensity greater than a certain threshold in the membrane were calculated by using MS Excel. The statistical difference in the calculated parameters for different experimental conditions was determined by a Student's t-test.
Determination of ubiquitylation levels of GFP–LCA or GFP–LCE
At 48 h after transfection, cells were incubated with or without 2.5 µM lactacystin (Sigma-Aldrich, St. Louis, MO) for 16 h to enrich polyubiquitylated forms of LCA and LCE by inhibiting their proteasomal degradation. Cells were lysed in the lysis buffer described above, which was supplemented by 10 mM iodoacetamide and 50 µM MG-132 (UBPBio, Aurora, CO, USA) to preserve ubiquitylated forms of LCA and LCE by inhibiting deubiquitylating enzymes, and both proteasomal and non-proteasomal proteases. Insoluble materials were removed by centrifugation (15,000 g, 10 min) at 4°C, and the clarified lysate was used for immunoprecipitation of GFP-tagged proteins performed as described above, with the exception that 10 mM iodoacetamide and 50 µM MG-132 were present in all washing buffers.
Exposure of SiMa cells to inhibitors of protein degradation and the inhibitor of septin organization
SiMa cells were transiently transfected with GFP–LCA, the L428A/L429A mutant or GFP–LCE in suspension and split in two wells of a six-well plate. Inhibitors of protein degradation, 50 nM bafilomycin (Sigma-Aldrich, St. Louis, MO), 20 µM MG-132 or 2.5 µM lactacystin, were added in one of these two wells 48 h after transfection. To study the degradation of the mature light chains, but not of the newly synthesized light chains, protein synthesis was inhibited by adding 20 µg/ml cycloheximide (Sigma-Aldrich, St. Louis, MO, USA) for 2 h before and for 8 h during cell incubation with the inhibitors of degradation. Control cells were incubated for 10 h with cycloheximide alone. To avoid any possible effects of LCA-induced cleavage of SNAP-25 on cell growth during the prolonged cell incubation with the inhibitors, the inactive variants of both LCA and the L428A/L429A mutant, H227Y and H227Y/L428A/L429A, were used in all the experiments with degradation inhibitors. However, for the sake of simplicity, the mutation H227Y is omitted in labeling in Fig. 3 and in the corresponding figure legend.
To study the role of septins on stability of LCA in SiMa cells, the inhibitor of septin oligomerization, forchlorfenuron (FCF, Sigma-Aldrich, St. Louis, MO) was added to the cells as indicated.
Silencing of septin-2
Septin-2 was downregulated by using a mixture of two Ambion pre-designed siRNA duplexes, sense, 5′-GAAAAUCGACUCUCAUAAATT-3′ and antisense, 5′-UUUAUGAGAGUCGAUUUUCCT-3′ (duplex 1); and sense, 5′-CAAUCAAGUUCACCGAAAATT-3′ and antisense, 5′-UUUUCGGUGAACUUGAUUGGG-3′ (duplex 2). Ambion Silencer® Negative Control No. 1 siRNA (Life Technologies, Carlsbad, CA, USA) was used as a negative control. Cells were transfected twice with the same mixture of duplexes, at 48 and 24 h prior to the transfection with GFP–LCA according to the manufacturer's protocol.
Western blot analysis
Immunoprecipitated proteins eluted from the beads, and proteins of cell lysates or membrane extracts were separated by SDS-PAGE, transferred onto a nitrocellulose membrane (BioRad, Hercules, CA, USA) and detected by western blot analysis as described previously (Tokhtaeva et al., 2012b). Immunoblots were quantified by densitometry using Zeiss LSM 510 software, version 3.2.
nLC-MS/MS of immunoprecipitated proteins
To analyze proteins co-immunoprecipitated with LCA by nLC-MS/MS, immunoprecipitation was performed as described above with the exception that, prior to adding to cell lysates, the rabbit polyclonal antibody against GFP (Clontech, Mountain View, CA, USA) was cross-linked to the protein-A–agarose beads (Tokhtaeva et al., 2011). Immunoprecipitated proteins were separated by 4–12% reducing SDS-PAGE. Then each lane was excised, and sliced into 12 pieces. Proteins contained in each gel slice were trypsinized (Promega, Madison, WI, USA) and analyzed by nLC-MS/MS as described previously (Doolittle et al., 2009; Tokhtaeva et al., 2011) and in supplementary material Table S1.
Statistical analysis
Statistical analysis was performed using Student's t-test (GraphPad Prism 4 software and Microsoft Excel). Statistical significance and number of experiments are specified in the figure legends.
Footnotes
Competing interests
P.E.G, R.L, L.W., R.A and E.F.-S. are employees of Allergan Inc. and hold Allergan stock. G.S. is a consultant for Allergan Inc.
Author contributions
O.V. and E.F.-S. led the project, designed experiments, analyzed and interpreted results and wrote the manuscript; O.V., E.T., P.E.G., P.S., S.B., J.P.W., and R.L executed experiments and analyzed data; J.P.W., G.S., L.W. and R.A. contributed to conception of the study, discussion of the results and writing of the manuscript.
Funding
This work was supported by a grant from Allergan, Inc. to O.V.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.146324/-/DC1
References
- Amin N. D., Zheng Y. L., Kesavapany S., Kanungo J., Guszczynski T., Sihag R. K., Rudrabhatla P., Albers W., Grant P., Pant H. C. (2008). Cyclin-dependent kinase 5 phosphorylation of human septin SEPT5 (hCDCrel-1) modulates exocytosis. J. Neurosci. 28, 3631–3643. 10.1523/JNEUROSCI.0453-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baust T., Anitei M., Czupalla C., Parshyna I., Bourel L., Thiele C., Krause E., Hoflack B. (2008). Protein networks supporting AP-3 function in targeting lysosomal membrane proteins. Mol. Biol. Cell 19, 1942–1951. 10.1091/mbc.E08-02-0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beites C. L., Campbell K. A., Trimble W. S. (2005). The septin Sept5/CDCrel-1 competes with alpha-SNAP for binding to the SNARE complex. Biochem. J. 385, 347–353. 10.1042/BJ20041090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beites C. L., Xie H., Bowser R., Trimble W. S. (1999). The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat. Neurosci. 2, 434–439. 10.1038/8100 [DOI] [PubMed] [Google Scholar]
- Blasi J., Chapman E. R., Link E., Binz T., Yamasaki S., De Camilli P., Südhof T. C., Niemann H., Jahn R. (1993). Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature 365, 160–163. 10.1038/365160a0 [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Traub L. M. (2003). Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72, 395–447. 10.1146/annurev.biochem.72.121801.161800 [DOI] [PubMed] [Google Scholar]
- Brand F., Schumacher S., Kant S., Menon M. B., Simon R., Turgeon B., Britsch S., Meloche S., Gaestel M., Kotlyarov A. (2012). The extracellular signal-regulated kinase 3 (mitogen-activated protein kinase 6 [MAPK6])-MAPK-activated protein kinase 5 signaling complex regulates septin function and dendrite morphology. Mol. Cell. Biol. 32, 2467–2478. 10.1128/MCB.06633-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidenbach M. A., Brunger A. T. (2004). Substrate recognition strategy for botulinum neurotoxin serotype A. Nature 432, 925–929. 10.1038/nature03123 [DOI] [PubMed] [Google Scholar]
- Bretscher A., Edwards K., Fehon R. G. (2002). ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 3, 586–599. 10.1038/nrm882 [DOI] [PubMed] [Google Scholar]
- Chen S., Barbieri J. T. (2011). Association of botulinum neurotoxin serotype A light chain with plasma membrane-bound SNAP-25. J. Biol. Chem. 286, 15067–15072. 10.1074/jbc.M111.224493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Tomchick D. R., Kovrigin E., Araç D., Machius M., Südhof T. C., Rizo J. (2002). Three-dimensional structure of the complexin/SNARE complex. Neuron 33, 397–409. 10.1016/S0896-6273(02)00583-4 [DOI] [PubMed] [Google Scholar]
- DeMay B. S., Meseroll R. A., Occhipinti P., Gladfelter A. S. (2010). Cellular requirements for the small molecule forchlorfenuron to stabilize the septin cytoskeleton. Cytoskeleton (Hoboken) 67, 383–399. [DOI] [PubMed] [Google Scholar]
- Dolly J. O., Aoki K. R. (2006). The structure and mode of action of different botulinum toxins. Eur. J. Neurol. 13 Suppl. 4, 1–9. 10.1111/j.1468-1331.2006.01648.x [DOI] [PubMed] [Google Scholar]
- Doolittle M. H., Ben-Zeev O., Bassilian S., Whitelegge J. P., Péterfy M., Wong H. (2009). Hepatic lipase maturation: a partial proteome of interacting factors. J. Lipid Res. 50, 1173–1184. 10.1194/jlr.M800603-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover N., Barash J. R., Hill K. K., Xie G., Arnon S. S. (2014). Molecular characterization of a novel botulinum neurotoxin type H gene. J. Infect. Dis. 209, 192–202. 10.1093/infdis/jit450 [DOI] [PubMed] [Google Scholar]
- Eleopra R., Tugnoli V., Rossetto O., De Grandis D., Montecucco C. (1998). Different time courses of recovery after poisoning with botulinum neurotoxin serotypes A and E in humans. Neurosci. Lett. 256, 135–138. 10.1016/S0304-3940(98)00775-7 [DOI] [PubMed] [Google Scholar]
- Fenteany G., Standaert R. F., Lane W. S., Choi S., Corey E. J., Schreiber S. L. (1995). Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science 268, 726–731. 10.1126/science.7732382 [DOI] [PubMed] [Google Scholar]
- Fernández-Salas E., Steward L. E., Ho H., Garay P. E., Sun S. W., Gilmore M. A., Ordas J. V., Wang J., Francis J., Aoki K. R. (2004). Plasma membrane localization signals in the light chain of botulinum neurotoxin. Proc. Natl. Acad. Sci. USA 101, 3208–3213. 10.1073/pnas.0400229101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Salas E., Wang J., Molina Y., Nelson J. B., Jacky B. P., Aoki K. R. (2012). Botulinum neurotoxin serotype A specific cell-based potency assay to replace the mouse bioassay. PLoS ONE 7, e49516 10.1371/journal.pone.0049516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden J., Krummel M. F. (2010). Control of cortical rigidity by the cytoskeleton: emerging roles for septins. Cytoskeleton (Hoboken) 67, 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara A., Tanaka Y., Hikawa R., Morone N., Kusumi A., Kimura H., Kinoshita M. (2011). Submembranous septins as relatively stable components of actin-based membrane skeleton. Cytoskeleton (Hoboken) 68, 512–525. 10.1002/cm.20528 [DOI] [PubMed] [Google Scholar]
- Hall P. A., Russell S. E. (2012). Mammalian septins: dynamic heteromers with roles in cellular morphogenesis and compartmentalization. J. Pathol. 226, 287–299. 10.1002/path.3024 [DOI] [PubMed] [Google Scholar]
- Hu Q., Nelson W. J., Spiliotis E. T. (2008). Forchlorfenuron alters mammalian septin assembly, organization, and dynamics. J. Biol. Chem. 283, 29563–29571. 10.1074/jbc.M804962200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara M., Yamasaki N., Hagiwara A., Tanigaki A., Kitano A., Hikawa R., Tomimoto H., Noda M., Takanashi M., Mori H. et al. (2007). Sept4, a component of presynaptic scaffold and Lewy bodies, is required for the suppression of alpha-synuclein neurotoxicity. Neuron 53, 519–533. 10.1016/j.neuron.2007.01.019 [DOI] [PubMed] [Google Scholar]
- Ito H., Atsuzawa K., Morishita R., Usuda N., Sudo K., Iwamoto I., Mizutani K., Katoh-Semba R., Nozawa Y., Asano T. et al. (2009). Sept8 controls the binding of vesicle-associated membrane protein 2 to synaptophysin. J. Neurochem. 108, 867–880. 10.1111/j.1471-4159.2008.05849.x [DOI] [PubMed] [Google Scholar]
- Iwase M., Okada S., Oguchi T., Toh-e A. (2004). Forchlorfenuron, a phenylurea cytokinin, disturbs septin organization in Saccharomyces cerevisiae. Genes Genet. Syst. 79, 199–206. 10.1266/ggs.79.199 [DOI] [PubMed] [Google Scholar]
- Janvier K., Kato Y., Boehm M., Rose J. R., Martina J. A., Kim B. Y., Venkatesan S., Bonifacino J. S. (2003). Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. J. Cell Biol. 163, 1281–1290. 10.1083/jcb.200307157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A., Noda M., Kinoshita M. (2000). Differential localization of septins in the mouse brain. J. Comp. Neurol. 428, 223–239. [DOI] [PubMed] [Google Scholar]
- Kinoshita M. (2003). Assembly of mammalian septins. J. Biochem. 134, 491–496. 10.1093/jb/mvg182 [DOI] [PubMed] [Google Scholar]
- Lee D. H., Goldberg A. L. (1998). Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 8, 397–403. 10.1016/S0962-8924(98)01346-4 [DOI] [PubMed] [Google Scholar]
- Marini P., MacLeod R. A., Treuner C., Bruchelt G., Böhm W., Wolburg H., Schweizer P., Girgert R. (1999). SiMa, a new neuroblastoma cell line combining poor prognostic cytogenetic markers with high adrenergic differentiation. Cancer Genet. Cytogenet. 112, 161–164. 10.1016/S0165-4608(98)00269-6 [DOI] [PubMed] [Google Scholar]
- Mattera R., Boehm M., Chaudhuri R., Prabhu Y., Bonifacino J. S. (2011). Conservation and diversification of dileucine signal recognition by adaptor protein (AP) complex variants. J. Biol. Chem. 286, 2022–2030. 10.1074/jbc.M110.197178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montal M. (2010). Botulinum neurotoxin: a marvel of protein design. Annu. Rev. Biochem. 79, 591–617. 10.1146/annurev.biochem.051908.125345 [DOI] [PubMed] [Google Scholar]
- Mostowy S., Cossart P. (2012). Septins: the fourth component of the cytoskeleton. Nat. Rev. Mol. Cell Biol. 13, 183–194. [DOI] [PubMed] [Google Scholar]
- Nakahira M., Macedo J. N., Seraphim T. V., Cavalcante N., Souza T. A., Damalio J. C., Reyes L. F., Assmann E. M., Alborghetti M. R., Garratt R. C. et al. (2010). A draft of the human septin interactome. PLoS ONE 5, e13799 10.1371/journal.pone.0013799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantano S., Montecucco C. (2014). The blockade of the neurotransmitter release apparatus by botulinum neurotoxins. Cell. Mol. Life Sci. 71, 793–811. 10.1007/s00018-013-1380-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoff M. R., Bouvet P. (2009). Clostridial toxins. Future Microbiol. 4, 1021–1064. 10.2217/fmb.09.72 [DOI] [PubMed] [Google Scholar]
- Saarikangas J., Barral Y. (2011). The emerging functions of septins in metazoans. EMBO Rep. 12, 1118–1126. 10.1038/embor.2011.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Quintana A., Findlay G. M., Mettlen M., Baust B., Jain M., Nilsson R., Rao A., Hogan P. G. (2013). An siRNA screen for NFAT activation identifies septins as coordinators of store-operated Ca2+ entry. Nature 499, 238–242. 10.1038/nature12229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhaye V. K., Chau E., Breysse P. N., King L. S. (2011). Septin-2 mediates airway epithelial barrier function in physiologic and pathologic conditions. Am. J. Respir. Cell Mol. Biol. 45, 120–126. 10.1165/rcmb.2010-0235OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaram A., Dennis M. K., Chaudhuri R., De Jesus-Rojas W., Tenza D., Setty S. R., Wood C. S., Sviderskaya E. V., Bennett D. C., Raposo G. et al. (2012). Differential recognition of a dileucine-based sorting signal by AP-1 and AP-3 reveals a requirement for both BLOC-1 and AP-3 in delivery of OCA2 to melanosomes. Mol. Biol. Cell 23, 3178–3192. 10.1091/mbc.E11-06-0509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliotis E. T., Hunt S. J., Hu Q., Kinoshita M., Nelson W. J. (2008). Epithelial polarity requires septin coupling of vesicle transport to polyglutamylated microtubules. J. Cell Biol. 180, 295–303. 10.1083/jcb.200710039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenkovic I., Yu Q. (2010). Merlin, a “magic” linker between extracellular cues and intracellular signaling pathways that regulate cell motility, proliferation, and survival. Curr. Protein Pept. Sci. 11, 471–484. 10.2174/138920310791824011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawa N. E., Jr, Odessey R., Goldberg A. L. (1997). Inhibitors of the proteasome reduce the accelerated proteolysis in atrophying rat skeletal muscles. J. Clin. Invest. 100, 197–203. 10.1172/JCI119513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokhtaeva E., Clifford R. J., Kaplan J. H., Sachs G., Vagin O. (2012a). Subunit isoform selectivity in assembly of Na,K-ATPase α-β heterodimers. J. Biol. Chem. 287, 26115–26125. 10.1074/jbc.M112.370734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokhtaeva E., Sachs G., Souda P., Bassilian S., Whitelegge J. P., Shoshani L., Vagin O. (2011). Epithelial junctions depend on intercellular trans-interactions between the Na,K-ATPase β1 subunits. J. Biol. Chem. 286, 25801–25812. 10.1074/jbc.M111.252247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokhtaeva E., Sachs G., Sun H., Dada L. A., Sznajder J. I., Vagin O. (2012b). Identification of the amino acid region involved in the intercellular interaction between the β1 subunits of Na+/K+ -ATPase. J. Cell Sci. 125, 1605–1616. 10.1242/jcs.100149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub L. M. (2009). Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat. Rev. Mol. Cell Biol. 10, 583–596. 10.1038/nrm2751 [DOI] [PubMed] [Google Scholar]
- Tsai Y. C., Maditz R., Kuo C. L., Fishman P. S., Shoemaker C. B., Oyler G. A., Weissman A. M. (2010). Targeting botulinum neurotoxin persistence by the ubiquitin-proteasome system. Proc. Natl. Acad. Sci. USA 107, 16554–16559. 10.1073/pnas.1008302107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang C. W., Estey M. P., DiCiccio J. E., Xie H., Patterson D., Trimble W. S. (2011). Characterization of presynaptic septin complexes in mammalian hippocampal neurons. Biol. Chem. 392, 739–749. 10.1515/BC.2011.077 [DOI] [PubMed] [Google Scholar]
- Vagin O., Tokhtaeva E., Yakubov I., Shevchenko E., Sachs G. (2008). Inverse correlation between the extent of N-glycan branching and intercellular adhesion in epithelia. Contribution of the Na,K-ATPase beta1 subunit. J. Biol. Chem. 283, 2192–2202. 10.1074/jbc.M704713200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zurawski T. H., Meng J., Lawrence G., Olango W. M., Finn D. P., Wheeler L., Dolly J. O. (2011). A dileucine in the protease of botulinum toxin A underlies its long-lived neuroparalysis: transfer of longevity to a novel potential therapeutic. J. Biol. Chem. 286, 6375–6385. 10.1074/jbc.M110.181784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J., Tsang C. W., Gai W. P., Malladi C. S., Trimble W. S., Rostas J. A., Robinson P. J. (2004). Septin 3 (G-septin) is a developmentally regulated phosphoprotein enriched in presynaptic nerve terminals. J. Neurochem. 91, 579–590. 10.1111/j.1471-4159.2004.02755.x [DOI] [PubMed] [Google Scholar]
- Yang Y. M., Fedchyshyn M. J., Grande G., Aitoubah J., Tsang C. W., Xie H., Ackerley C. A., Trimble W. S., Wang L. Y. (2010). Septins regulate developmental switching from microdomain to nanodomain coupling of Ca(2+) influx to neurotransmitter release at a central synapse. Neuron 67, 100–115. 10.1016/j.neuron.2010.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimori T., Yamamoto A., Moriyama Y., Futai M., Tashiro Y. (1991). Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J. Biol. Chem. 266, 17707–17712. [PubMed] [Google Scholar]
- Yu W., Kawasaki F., Ordway R. W. (2011). Activity-dependent interactions of NSF and SNAP at living synapses. Mol. Cell. Neurosci. 47, 19–27. 10.1016/j.mcn.2011.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]