ABSTRACT
Textbook images of keratin intermediate filament (IF) networks in epithelial cells and the functional compromization of the epidermis by keratin mutations promulgate a mechanical role for this important cytoskeletal component. In stratified epithelia, keratin filaments form prominent radial spokes that are focused onto cell-cell contact sites, i.e. the desmosomes. In this Hypothesis, we draw attention to a subset of keratin filaments that are apposed to the plasma membrane. They form a rim of filaments interconnecting the desmosomes in a circumferential network. We hypothesize that they are part of a rim-and-spoke arrangement of IFs in epithelia. From our review of the literature, we extend this functional role for the subplasmalemmal rim of IFs to any cell, in which plasma membrane support is required, provided these filaments connect directly or indirectly to the plasma membrane. Furthermore, cytoplasmic IF networks physically link the outer nuclear and plasma membranes, but their participation in mechanotransduction processes remain largely unconsidered. Therefore, we also discuss the potential biomechanical and mechanosensory role(s) of the cytoplasmic IF network in terms of such a rim (i.e. subplasmalemmal)-and-spoke arrangement for cytoplasmic IF networks.
KEY WORDS: Desmosomes, Intermediate filaments, Mechanosensory function, Plasma membrane, Nuclear lamins, LINC
Summary: The cytoplasmic intermediate filament comprises a rim and spoke arrangement supporting the plasma membrane and connecting to the nucleus, to deliver their mechanosensory functions.
Identifying the spokes and rim in the intermediate filament wheel
The cytoskeleton delivers a mechanosensory role via its integration with signaling pathways and its direct physical link to and into the nucleus. It facilitates the diversification of cell function and also the integration via its association with cell junctions of individual cells into tissues. Here we focus our attention onto the cytoplasmic cytoskeleton and, in particular, the network of intermediate filaments (IFs). With over 70 functionally distinct IF proteins, they comprise one of the 100 largest gene families in the human genome (Omary, 2009). IFs are distinct from microtubules and actin filaments, the two main filament components of the cytoskeleton. They are structurally diverse and are expressed in tissue- and cell-specific, and subcellular specific patterns, e.g. keratins in epithelia, desmin in muscle, vimentin in mesenchymal cells, lamins in nuclei (Omary, 2009). Mutations in IF genes result in more than 80 human diseases (Human Intermediate Filament Database, www.interfil.org/index.php). Highlighting the diversity of the cytoplasmic network and its subcellular distribution drives mechanistic insight into cell biology as seen for both actin microfilaments (Carlier and Shekhar, 2017) and microtubules (Lancaster and Baum, 2014; van Beuningen and Hoogenraad, 2016) − and the same applies to IFs (Omary, 2009). Here we propose that the cytoplasmic IF network comprises two distinct components, one that forms radial spokes (Baffet et al., 1991; Franke et al., 1987a; Geiger et al., 1983; Hibbs and Clark, 1959; Iwatsuki and Suda, 2007; Katsuma et al., 1988; Koeser et al., 2003; Staehelin, 1974) and another that forms a circumferential rim running parallel to and closely apposed to the plasma membrane (Forbes and Sperelakis, 1975; Franke et al., 1987b; Granger and Lazarides, 1982; Kartenbeck et al., 1983, 1984; McNutt and Fawcett, 1969; Schwarz et al., 2015). The detail and the distribution of these two components are both cell type and cell function specific, and this diversity has in part obscured our appreciation of their individual and collective role(s). We propose that the circumferential rim is contributing to the mechanical stability of the plasma membrane as well as facilitating the mechanosensory function of IFs (Hatzfeld et al., 2017; Jorgens et al., 2017).
In some cell types, the circumferential rim interconnects adherens junctions (Fig. 1). One such example is the IF-desmosome system in epithelial cells, cardiac muscle and arachnoidal tissues (Garrod and Chidgey, 2008; Holthöfer et al., 2007; Jones et al., 2017). IFs are essential to the mechanical, signaling and functional properties of tissues, as revealed by the numerous diseases caused by components of the IF-desmosome complex, for instance skin-blistering diseases (Coulombe et al., 1991; Knöbel et al., 2015; Omary, 2009, 2016; Rugg et al., 1994; Toivola et al., 2015) (www.interfil.org). The textbook view of the desmosome-IF complex in keratinocytes is of IF bundles that interact perpendicular to individual desmosomes, thereby forming a series of radial spokes that physically link the plasma and nuclear compartments in epithelial cells (Farquhar and Palade, 1963; Staehelin, 1974; Troyanovsky et al., 1993) (Fig. 1). As a cell-cell junction, the desmosome provides mechanical strength (Garrod and Chidgey, 2008; Harmon and Green, 2013; Nekrasova and Green, 2013; Vasioukhin et al., 2001). Desmosomes are Ca2+-dependent, cell-adhesion structures (Garrod and Chidgey, 2008) found in tissues and their cells in which significant mechanical forces need to be resisted − such as in skin and the heart − forming what has been termed ‘a mechanical syncytium’ (Harris et al., 2014). The desmosome is a multiprotein complex comprising cadherin-family members, such as the desmogleins and desmocollins, and anchor proteins, including plakoglobin, the plakophilins and desmoplakins (Garrod and Chidgey, 2008). IFs attach specifically to the desmosomal plaques, which provide obvious points of cell-cell contact to focus the cytoplasmic filament networks (Franke et al., 1987a; Geiger et al., 1983; Hibbs and Clark, 1959; Staehelin, 1974). This is achieved through their interaction with a specific IF-binding domain in the C-terminal region of desmoplakins (Kang et al., 2016; Stappenbeck et al., 1993). The conspicuousness of the radial keratin filament component in stratified epithelia has overshadowed the presence of the circumferential rim that interconnects desmosomes and, so, their role has never been addressed in past (Castañón et al., 2013; Schmidt et al., 1994) or recent reviews (Jones et al., 2017; Leube et al., 2015).
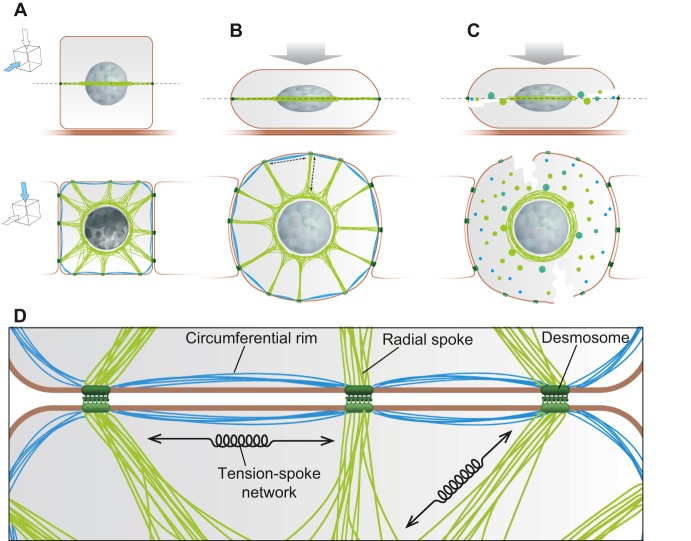
Fig. 1.
Schematic illustration of the spoke-and-rim arrangement of IFs in a basal keratinocyte. The cartoon depicts how radial and cortical keratin filaments are distributed in a single plane of a basal keratinocyte. (A-C) The top panel shows transverse sections and the bottom panel shows views of the horizontal sections indicated in the panel above. (A) In the steady state, the perinuclear keratin network (shown in green) extends out into radial desmosome-anchored filaments (spokes). The subplasmalemmal interdesmosomal filaments are demarcated in blue (rims). (B) Mechanical pressure leads to cell flattening and expansion of the depicted section. This is coupled to tightening and extension of both radial and interdesmosomal filaments. (C) In EBS, the presence of keratin mutations results in keratin aggregate formation, and is accompanied by loss of both the radial and the interdesmosomal, subplasmalemal keratin filaments, while a thickened perinuclear network remains. We suggest that the lack of the subplasmalemmal filaments allows the ‘stretching of the plasma membrane’ and its eventual rupture between desmosomes upon mechanical stress. (D) Arrangement of the two components (circumferential rim and radial spokes) in the keratin IF network; rims connect the desmosomal cell-cell junctions and radial spokes the desmosomes to the nucleus. In the case of those proteins linking IFs to the desmosome − for epithelia and any cell that contains desmosomes and IFs − it is the desmoplakins, plakoglobin and plakophilins that ensure IF attachment. Together they form an adaptable tension-spoke network that is important for maintaining mechanical equilibrium and sensing force changes at the plasma membrane, and transducing these to the nuclear compartment through a direct physical connection mediated by IFs.
The circumferential IF rim is formed from a series of struts that interconnect individual desmosomes. This rim can both position and support desmosomes at the plasma membrane, as suggested by the coordinated desmosomal motility (‘pearls on a string’) observed by time-lapse imaging (Windoffer et al., 2002) and as evidenced in studies on keratin-null embryos, where desmosome positioning was lost in epidermal keratinocytes (Vijayaraj et al., 2009). We suggest that the presence of the circumferential rim lends biomechanical support to the areas of plasma membrane that are located between desmosomes and that are likely to be more sensitive to rupture in disease-based scenarios, such as skin-blistering diseases that involve mutations in keratins 5 and 14 (Coulombe et al., 1991; Thornell et al., 1997). This proposal is consistent with both the ‘fragile network’ (Russell et al., 2004; Werner et al., 2004) and ‘sparse network’ (Beriault et al., 2012) hypotheses that were proposed to help to explain the mechanistic basis to the skin blistering disease epidermolysis bullosa simplex. Both of these hypotheses suggest that the formation of keratin aggregates and, therefore, the loss of the cytoplasmic keratin filament network uncouple the desmosomes and make the epidermis highly vulnerable to mechanical stresses. Here, we draw attention to an important detail, namely that, upon loss of the cytoplasmic keratin network, the desmosomes are not only separated from the nucleus but also from each other, because the circumferential rim of keratin filaments is compromised and no longer there to assist in the positioning and tensioning between desmosomes (Fig. 1).
Although − because of its keratin-desmosome network of filaments and junctions − the epidermal keratinocyte is the most obvious exemplar of our hypothesis, we suggest that the principle of IFs contributing to the mechanical properties of cell membranes is applicable to any situation where cytoplasmic IFs are apposed and linked to membranes in a subplasmalemmal network. IFs also function as signaling platforms (Pallari and Eriksson, 2006) and, therefore, any mechanical function might also be combined with their signaling role. The biological record evidences that, whether it be single cells as, for example, avian erythrocytes (Granger and Lazarides, 1982) or mammalian macrophages (Correia et al., 1999), or cells in tissues (Forbes and Sperelakis, 1975; Franke et al., 1987b; Kartenbeck et al., 1983, 1984; McNutt and Fawcett, 1969) or blastocysts (Schwarz et al., 2015), all possess a circumferential rim of IFs, so any cell missing this subplasmalemmal network will be the exception rather than the rule. The presence of a circumferential rim fits the emerging mechanosensory role(s) for cytoplasmic IFs and their networks. This mechanosensory function is not restricted to the dynamic assembly and disassembly of the filaments. IF subunits freely exchange with the filaments themselves (Herrmann and Aebi, 2016), and both filaments and their subunits associate with signaling molecules, cell cycle factors (Escobar-Hoyos et al., 2015; Pallari and Eriksson, 2006; Pallari et al., 2011; Salas et al., 2016) and transcriptional regulators (Antfolk et al., 2017; Hobbs et al., 2015; Kerns et al., 2016). This function also includes the mechanical properties and connectivity of the filaments themselves (Chung et al., 2013; Hobbs et al., 2016; Jones et al., 2017; Lowery et al., 2015; Nolting et al., 2014; Salas et al., 2016).
Evidence for the subplasmalemmal rim of IFs in epithelia
In epithelia, a radial organization of IFs that emanate from the nucleus to the desmosomes is usually prominent (Franke et al., 1987a; Geiger et al., 1983; Hibbs and Clark, 1959; Koeser et al., 2003; Staehelin, 1974). Desmosomes are also sites at which keratin filament assembly can be initiated (Bologna et al., 1986). In the suprabasal layers of the epidermis, the radial keratin bundles that terminate at desmosomes are the most obvious feature. This is facilitated by desmosomal proteins expressed only in the more-differentiated cells of the epidermis, such as the cytoskeletal protein plakophilin 1 (PKP1) (Moll et al., 1997). In the basal layer of the epidermis, such radial keratin bundles are not as prominent owing to the lower desmosome density (Pieperhoff et al., 2010). There, the circumferential rim of keratin filaments is, therefore, likely to play a more significant biomechanical role (Drochmans et al., 1978; Hibbs and Clark, 1959) and to function together with the radial struts as a single network that has a potential mechanosensory function (Hatzfeld et al., 2017; Jorgens et al., 2017).
Observations made in a study that used blastocysts derived from a knock-in mouse expressing YFP-labeled keratin 8 suggest a crucial role for interdesmosomal keratins in keratin network organization in vivo (Schwarz et al., 2015). The keratin network is the first cytoplasmic IF network to be formed during embryogenesis, and a dotted pattern of keratin fluorescence first appears at the plasma membrane of cell-cell borders (Schwarz et al., 2015). These dotted structures were found to be positive for desmosomal markers and are interconnected by keratin filaments juxtapositioned to the plasma membrane (Fig. 2), thereby comprising the circumferential rim of keratin filaments in these cells (Schwarz et al., 2015).
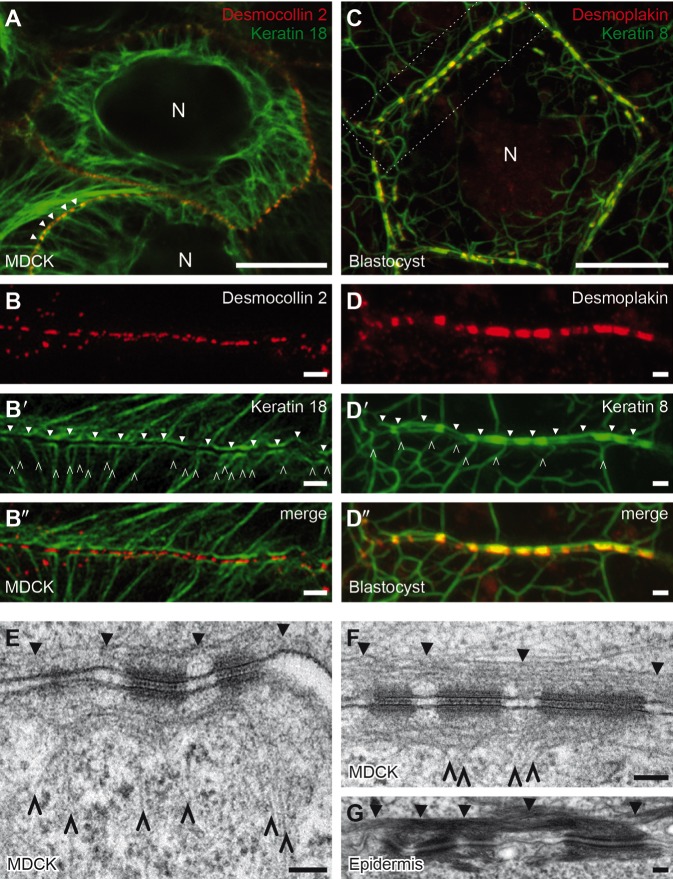
Fig. 2.
A circumferential rim of subplasmalemmal keratins interconnects desmosomes in blastocysts and MDCK cells. (A) Survey fluorescence micrograph of live canine kidney MDCK-derived cells (MDC-2K18r) that expresses YFP-labelled human desmosomal cadherin desmocollin 2 (red channel) and mRFP-labelled human keratin 18 (green channel). The image was recorded with an LSM710 confocal laser scanning microscope equipped with an Airy Scan unit. Note the keratin network surrounding the nucleus (N) that is connected through radial filament bundles (spokes) to desmosomal cell junctions. By using this instrument, keratin filaments that run in parallel to the plasma membrane and connect desmosomal adhesion sites are only just visible (arrowheads), illustrating how easily this particular component of the IF network can be overlooked. (B-B″) In adjacent MDC-2K18r cells, the interdesmosomal subplasmalemmal keratin rim is only clearly identified by using super-resolution structured illumination microscopy (OMX-3D-SIM). Notice that the keratin network includes radial filament spokes (open arrowheads) and the subplasmalemmal keratin network (closed arrowheads). (C,D) Survey projection view (C) and higher magnification fluorescence images of a selected single plane from the boxed area (D-D″) of a fixed murine blastocyst obtained from knock-in mice producing YFP-labeled keratins (green) (Schwarz et al., 2015). The blastocyst was incubated with anti-desmoplakin antibodies (red). Fluorescence images were recorded by using an LSM710 confocal laser scanning microscope equipped with an Airy Scan unit. Note the accumulation of keratin 8 at desmoplakin-positive spots and the interconnecting keratin filaments that run in parallel to the adjacent plasma membrane (closed arrowheads). In addition, thin filaments extend towards the cell interior (open arrowheads) and the nucleus (N). (E,F) Examples of electron microscopy of subplasmalemmal regions of adjacent MDC-2K18r cells derived from MDCK cells. This analysis confirms the rim component of the cytoplasmic IF network. The two distinct desmosome-associated filament systems can be clearly seen: the rims that run in parallel to the plasma membrane (closed arrowheads) and the spokes that loop through the desmosomal plaque from the cell interior (open arrowheads). (G) Electron micrograph of epidermal keratinocytes; also shown here are dense interdesmosomal keratin bundles that run parallel to the plasma membrane (closed arrowheads). Scale bars: 10 µm (A,C), 1 µm (B-B″,D-D″), 100 nm (E-G).
Studies on the de novo assembly (Troyanovsky et al., 1993) or reassembly of the keratin filament-desmosome networks in epithelial cells take advantage of the Ca2+ dependency of desmosomal junctions (reviewed in Garrod and Chidgey, 2008) to demonstrate the central role played by desmosomes in the organization of the cytoplasmic IF network. By using rat carcinoma cells that were first treated with Ca2+ chelators to break the desmosome and then returned to Ca2+-containing tissue culture medium, keratin network formation can be initiated at the desmosomes (Bologna et al., 1986). This revealed that bundles of keratin filaments emanate from desmosomes, indicative of the spoke-like organization of the cytoplasmic filament network (Koeser et al., 2003). Subplasmalemmal keratin filaments that interconnect desmosomal structures were a prominent feature of epidermoid carcinoma A431 cells transfected with a chimeric desmocollin-connexin construct (Troyanovsky et al., 1993); this demonstrated that the keratin-binding region within the intracellular domain of desmocollin was sufficient to both nucleate desmosome assembly and to facilitate the docking of keratin filaments, whose identity was confirmed by immunoelectron microscopy (Troyanovsky et al., 1993). Taken together, in these examples, the formation of both the spoke and rim components of the cytoplasmic keratin network is a function of desmosomes.
Subplasmalemmal keratin filaments are also present in a variety of other epithelia, as evidenced by electron microscopy (Baffet et al., 1991; Iwatsuki and Suda, 2007; Katsuma et al., 1988). For instance, in hepatocytes, both in situ and in tissue culture, there is a pronounced circumferential rim of keratin filaments (Baffet et al., 1991; Katsuma et al., 1988). Their presence was shown to depend upon the formation of cell-cell contacts in culture systems (Baffet et al., 1991). Removal of the hepatocyte keratins by gene knockout (Baribault et al., 1993) or expression of dominant-negative constructs (Toivola et al., 1998) compromised the mechanical and stress-response of the liver. In bladder umbrella cells (Veranic and Jezernik, 2002) and in the exocrine cells of the duodenum (Iwatsuki and Suda, 2007), there is also a close apposition of keratin filaments with the plasma membrane, although there are only limited details known for this association. It is possible that the keratins themselves interact directly with membranes via amphipathic helices (Ouellet et al., 1988). Alternatively, keratins are present in and bind to lipid droplets within cells of the hepatoma cell line PLC (CRL-8024) and cells of the human epithelial colorectal adenocarcinoma cell line CaCo-2 through perilipin and other lipid-droplet-binding proteins (Heid et al., 2013), indicating the potential diversity in linking keratins and keratin filaments to lipid membranes. All of the above-mentioned cells contain desmosomes − probably the most prominent sites for keratin filament docking at the plasma membrane. Furthermore, in bladder umbrella cells, there is a striking association of keratin filaments with fusiform vesicles (Veranic and Jezernik, 2002) and an intricate network of filaments and membranes that allows the rapid stretching and relaxation of the apical plasma membrane (Lewis, 2000). Whilst umbrella cell stretching is believed to be a trigger for these stretching/relaxation responses (Lewis, 2000; Truschel et al., 2002) and is used to mathematically model the responses (Moulton et al., 2016), any mechanosensory role(s) of the keratin filament network has yet to be addressed in this highly specialized cell (Charrier and Janmey, 2016; Isermann and Lammerding, 2013).
The importance of the subplasmalemmal rim is also evident in the polarized simple epithelium that lines the intestine. Here, the rim is more prominent than the spokes in the cytoplasmic IF network. A dense IF network termed the ‘desmosomal web’ (Brunser and Luft, 1970) is positioned between the desmosomal junctions just underneath the actin-rich, terminal web. Its proximity and links to the actin-based microvillar rootlets (Grimm-Gunter et al., 2009; Hirokawa et al., 1982; Salas et al., 2016; Toivola et al., 2000a,b) suggests a structural function for both keratin and actin in microvilli organization. Indeed, disruption of the keratin-desmosome network by desmoplakin knockout led to a reduction in microvilli length as well as evidencing that desmosomes were not required for keratin organization in the small intestine (Sumigray and Lechler, 2012). This actin-IF organization is also present in the exocrine acinar cells of the pancreas, where a prominent circumferential rim is also present and apposed to an equally prominent actin network beneath the apical plasma membrane (Toivola et al., 2000a). Interestingly, unlike other simple epithelia, disruption of the cytoplasmic keratin networks did not compromise either the organization of the acinar cells or their response to toxins despite being able to selectively remove the radial spokes whilst preserving the circumferential rim (Sumigray and Lechler, 2012; Toivola et al., 2000a). In the epidermis, desmoplakin is required for epidermal integrity, keratin filament organization and membrane-repair-induced actin filament reorganization (Vasioukhin et al., 2001). This diversity in desmoplakin and keratin function with respect to both the radial and circumferential components of the cytoplasmic IF network, as well as the varying compensatory role of the subplasmalemmal actin networks (Toivola et al., 2000a), could explain such differences. The presence of a circumferential rim of IFs is, however, evolutionarily conserved (Coch and Leube, 2016) and, in Caenorhabditis elegans, generates a network below the apical surface of the intestinal cells that form the endotube (Carberry et al., 2012; Geisler et al., 2016). In ciliated epithelia, the subplasmalemmal IF network is also localized just below the actin cortex, where it potentially acts as a counterbalance for cilia movement (Tateishi et al., 2017). This illustrates the cooperativity of the subplasmalemmal IF and actin networks in regulating cell mechanics in a tissue-specific manner (Broussard et al., 2017) and also highlights the importance of the entire cytoskeleton in determining cell shape and function (Pollard and Cooper, 2009).
Compromised subplasmalemmal rim in skin-blistering phenotypes
Keratin mutations account for many of the diseases associated with IFs in humans (Omary, 2009, 2016). In a case of lethal acantholytic epidermolysis bullosa, in which IF connections to desmosomes have been prevented by compound heterozygote mutations in desmoplakin, electron microscopy analyses confirmed that the plasma membrane “was stretched to its limits until intercellular cleavage occurred”, despite the fact that desmosomal connections remained (Jonkman et al., 2005). Here, the biomechanical properties of the epidermis were severely compromised as revealed by its inability to resist the physical stresses associated with giving birth (Jonkman et al., 2005), highlighting the importance of desmosomes as attachment sites for keratin IFs (Vasioukhin et al., 2001). A similar phenotype was reported for a recessive mutation in desmoplakin that lacked the IF-binding domain (Norgett et al., 2000). The observed stretching of the plasma membrane can be explained by the loss of the circumferential subplasmalemmal keratin filament rim that normally interconnects desmosomes in the plane of the plasma membrane. Similar observations were made for mouse models with desmoplakin deficiency (Vasioukhin et al., 2001). Although epidermal desmosome numbers in desmoplakin-knockout mice were similar to those of wild-type littermates, they did not have any keratin filaments attached to them. In addition, keratinocytes cultured from desmoplakin-knockout animals showed a significantly reduced desmosomal content and were compromised in their ability to seal their membranes during epithelial sheet formation (Vasioukhin et al., 2001). In normal, wild-type mice, the basal cells of the epidermis have fewer desmosomes compared to cells in the suprabasal layers (Drochmans et al., 1978; Hibbs and Clark, 1959), with more of the plasma membrane exposed and more prominent adherens junctions. Therefore, we suggest that the biomechanical contribution of the cortical rim of keratin filaments is more important in the basal cells than the suprabasal cells, where the coverage of the plasma membrane with desmosomes is greater. Moreover, the fact that the stretch response of keratin filaments is phosphorylation dependent (Fois et al., 2013) and that IFs are themselves signaling platforms (Pallari and Eriksson, 2006), implicates the phosphorylation of IFs, and the activation of kinase cascades to transduce and amplify the mechanical signals sensed by the IFs. Together with cadherins and plakoglobin, keratins regulate the mechanoresponsive nature of cells (Weber et al., 2012).
Interestingly, desmosomes did not split in half when cells were breaking apart in those rare cases of human lethal acantholytic epidermolysis bullosa that are caused by the loss of the keratin-binding region of desmoplakin (Jonkman et al., 2005), but ended up being partitioned to only one of the two cells. In addition, membrane blebs were observed in the adjacent cell from which the desmosomes had been removed (Jonkman et al., 2005). In another reported case of a naturally occurring keratin 14 knockout in a human patient (Chan et al., 1994; Rugg et al., 1994), cytolysis occurred in the subnuclear region of the basal cells in blister areas, whereas hemidesmosomes that connect the cell to the extracellular matrix (reviewed in Walko et al., 2015) remained intact with whole nuclei floating amongst the debris from the lysed basal cells (Chan et al., 1994; Rugg et al., 1994); this indicates that desmosomes are more resilient to applied forces compared to the intervening and unsupported plasma membrane. Therefore, we interpret these data to suggest that loss of the cytoplasmic keratin network only compromises the cell-cell interface between basal cells of the epidermis, but not their interaction with either the underlying ECM, or the overlying suprabasal cells (Chan et al., 1994; Rugg et al., 1994). In suprabasal layers, such as the spinous layer, desmosomes can comprise 50% or more of the plasma membrane (Pieperhoff et al., 2010) and, therefore, potentially compensate for the absence of a spoke-rim network formed by keratin filaments.
Possible mechanosensory function of the spoke-and-rim arrangement for cytoplasmic IF networks
So far, it has been difficult to determine the relative contribution of the radial spokes compared to that of the circumferential rim of keratin filaments in regard to mechanosensory properties of individual cells. The spokes traverse the cytoplasmic space and, as shown recently in 3D culture of mammary epithelial cells, these keratin IFs cause deep nuclear invaginations and tunnels that pass through the nucleus. The connection of the keratin IFs to the outer nuclear envelope illustrate how cytoplasmic IFs correlate with localized nuclear deformations (Jorgens et al., 2017). The fact that mechanical strain at the cell periphery alters the nuclear morphology (Isermann and Lammerding, 2013; Maniotis et al., 1997; Osorio and Gomes, 2014) demonstrates that the nucleus is mechanically linked via the cytoplasmic cytoskeleton to the plasma membrane. The nesprins, plectin and LINC complex connect the radial IF component to the nuclear membrane (Ketema and Sonnenberg, 2011; Meinke and Schirmer, 2015), linking physically the cytoplasmic and nuclear IF networks (Cho et al., 2017). Specifically, nesprin 1 is required for nuclear anchorage of desmin (Chapman et al., 2014), whereas nesprin 3 anchors both keratins (Wilhelmsen et al., 2005) and vimentin (Palmisano et al., 2015) via plectin to the nucleus. This supports a role for cytoplasmic IFs in mechanotransduction via the nuclear IF cytoskeleton (Almeida et al., 2015; Aureille et al., 2016). When we are able to manipulate independently the spoke and the rim components of the keratin network, their relative contribution to any proposed functions in mechanotransduction and/or mechanosensing will become apparent (Hatzfeld et al., 2017; Jorgens et al., 2017). This will require the careful characterization of the subcellular distribution for individual IF proteins, and their partitioning between the spoke and rim components of the cytoplasmic IF network as an experimental approach to manipulate each component independently (Lovering et al., 2011; Toivola et al., 2000a). Nevertheless, the first step has been taken in this article by identifying the rim and spoke IFs as distinct − albeit linked − components in the cytoplasmic IF network.
Keratin IFs are a main cytoplasmic, mechanical component in epithelial cells (Lulevich et al., 2010; Ramms et al., 2013), and the subplasmalemmal keratin network forms as soon as desmosomes are present during development and before any radial IF spokes are apparent (Schwarz et al., 2015). The subplasmalemmal rim of filaments is, therefore, independent of the formation of the filament spokes. From in vitro systems, it is quite clear that the partnership between membranes and the cytoskeleton is essential (Ko and McCulloch, 2000; Loiseau et al., 2016; Vogel and Schwille, 2012) and, whilst these synthetic biology approaches have largely focused on the actomyosin systems (Vogel and Schwille, 2012), consideration of IF-membrane networks is essential to complete our understanding given the evidence from cellular systems (Broussard et al., 2017). However, only once we are able to selectively remove or modify the circumferential rim of keratin filaments whilst maintaining the radial spokes, will we be able to fully explain basal cell cytolysis in the keratin 14 knockout phenotype (Chan et al., 1994; Rugg et al., 1994) and fully describe the mechanotransduction properties of the cytoplasmic IF network. By analogy, compromising the nuclear lamina renders the nuclear envelope susceptible to mechanical stress, specifically at the points on the membrane where lamin support is absent (Denais et al., 2016; Funkhouser et al., 2013). We predict that compromising the subplasmalemmal rim of IFs will have analogous consequences at the plasma membrane, thereby threatening its mechanical properties and altering the mechanosensory response of the cell.
Evidence from non-epithelial cells and tissues for a subplasmalemmal IF belt
Desmosomes are not restricted to epithelia (Garrod and Chidgey, 2008); they are also prominent features of the myocardium and Purkinje fibers (Kartenbeck et al., 1983), as well as meningeal cells (Kartenbeck et al., 1984). In these cell types, filaments of desmin and vimentin, rather than of keratins, attach to the desmosomes (Kartenbeck et al., 1983, 1984). Electron microscopy data from meningeal cells (Kartenbeck et al., 1984), the myocardium and Purkinje fibers (Kartenbeck et al., 1983) demonstrated that IFs are closely apposed and often parallel to the plasma membranes between the desmosomes in these various cells and tissues. For cardiomyocytes (Forbes and Sperelakis, 1975; McNutt and Fawcett, 1969), the confirmation that these filaments were, indeed, IFs was delivered by immunoelectron microscopy (Kartenbeck et al., 1983, 1984). These examples suggest that IF composition per se is not important for desmosomal attachment, rather a more important question is whether the desmosome is an absolute requirement for cytoplasmic IFs to be organized in a spoke and rim arrangement.
The term ‘subplasmalemmal’ was initially used to describe filaments apposed to the plasma membrane in lens fiber cells (Franke et al., 1987b). The eye lens has IFs comprising vimentin and the beaded filament proteins (Sandilands et al., 1995a,b) and its plasma membranes are enriched in plakoglobin, a key desmosomal component (Cowin et al., 1986; Straub et al., 2003). Lens fiber cells are, however, special in that they have no cell-cell junctions with the morphological features of desmosomes. They also do not retain their nuclei and, thus, only have an IF network at the plasma membrane (reviewed in Song et al., 2009). Accordingly, compromising this IF network affects the biomechanical properties of the lens itself, which became softer and more resilient to deformation by increased elasticity (Fudge et al., 2011). Taken together, it is clear that there is considerable diversity in potential plasma membrane attachment sites for IFs. Note, too, that there is also a blurring of the conventional categorization of cell-cell junctions on the basis of morphology, subcellular location and the tissues/cells in which they are expressed. In the context of the eye lens, it is the subplasmalemmal apposition of IFs in the lens fiber cells that is important (Franke et al., 2013, 2015; Straub et al., 2011).
Therefore, although the plasma membrane attachment sites for cytoplasmic IFs depend on the cellular context, the overall concept is generic and desmosomes are not an absolute requirement for organizing the cytoplasmic IF network. The avian erythrocyte is just such an example and illustrates that even in a cell that is not incorporated into a tissue, there is a prominent association of IFs with the plasma membrane (Granger and Lazarides, 1982). Unlike their mammalian counterpart, avian erythrocytes remain nucleated and have an IF network comprising vimentin and synemin (Granger et al., 1982; Woodcock, 1980). Ankyrin has been implicated as a possible plasma membrane docking site for these IFs (Georgatos and Blobel, 1987b; Georgatos and Marchesi, 1985). Although the LINC complex was uncharacterized at the time of these studies (Isermann and Lammerding, 2013), lamin B was already then identified as a potential nuclear envelope docking site for vimentin (Georgatos and Blobel, 1987a). Therefore, these are some of the first lines of evidence that IFs are physically connected to the plasma and nuclear membranes. Although the attachment sites at the nuclear and plasma membranes are different, this nevertheless points to a common biological principle – lipid bilayers have to be supported, for example, by vimentin, keratins (Ramms et al., 2013) or lamins (Denais et al., 2016).
Conclusions and perspectives
Here we have extended the concept of desmosomes as focus points for IFs by highlighting the, thus far unappreciated, element of the IF ‘wheel’– namely the circumferential rim of IFs. Although this subplasmalemmal rim has been described in the literature for some time, its significance has not been appreciated, neither in terms of its mechanobiological potential, nor its presence in diverse cell types, such as cardiomyocytes and their derivatives (Purkinje fibers), epithelia and meninges. Here, we have also provided new, complementary electron and high resolution, fluorescence microscopy data (Fig. 2) to help draw attention to the subplasmalemmal cytoplasmic IF network and the experimental approaches needed to study this system. Therefore, our hypothesis is that there are two distinct components to the cytoplasmic IF network found in both epithelial and non-epithelial cells – one that is parallel to the plane of the plasma membrane (the ‘rim’) and the other (the ‘spokes’) that emanates from the nucleus and connects to the plasma membrane at discrete sites such as, but not limited to, desmosomes. This is what we term the rim-and-spoke hypothesis to describe the mechanosensory network of cytoplasmic IFs.
As the IF spokes are typically prominent in most cells, the comparably thinner circumferential rim is, therefore, often overlooked. IFs by virtue of their structure, however, are resilient cytoskeletal filaments (Charrier and Janmey, 2016; Fudge et al., 2008), and the networks they form bend and buckle in response to forces (Nolting et al., 2014) in a phosphorylation-dependent manner (Fois et al., 2013) as key aspects of their connectivity and, therefore, their potential mechanosensory role. A number of questions follow. For instance, do these inter-desmosomal, subplasmalemmal filaments interact directly or indirectly with the plasma membrane? Are any such interactions mediated through linkers, such as plectin (Geerts et al., 1999; Osmanagic-Myers et al., 2015b), or other cytoskeletal elements, such as actin (Green et al., 1987; Werner et al., 2004)? Furthermore, how do the IF and actin networks complement each other at the plasma membrane? The rim-and-spoke hypothesis provides a framework in which to begin to address these questions and, importantly, identify subplasmalemmal IFs and the circumferential rim as a distinct element in the mechanosensory IF network (Isermann and Lammerding, 2013).
A fascinating consequence of the hypothesis proposed here is that the cortical interjunctional, subplasmalemmal IFs are part of a tension-spoke network that physically links the plasma membrane compartment to the nucleus (Aureille et al., 2016; Jorgens et al., 2017). With such a rim of interjunctional filaments in place, the desmosome-filament network can not only help mitigate any compressive and tensile forces experienced by the cell, but could also act as a mechanosensor (Hatzfeld et al., 2017; Jorgens et al., 2017) to amplify and transduce these mechanical inputs from localized plasma membrane regions directly to the LINC complex and nucleus (Meinke and Schirmer, 2015; Osmanagic-Myers et al., 2015a) through the keratin spokes. Thus, just as the nuclear membrane and LINC complex provide the interface for cellular mechanosensory functions (Cho et al., 2017), the plasma membrane and cortical cytoskeleton could constitute the cell-cell and/or cell-ECM interface. The identification of the cortical subplasmalemmal rim of IFs, therefore, calls for a re-evaluation of IF function in normal and diseased tissues, as well as for updating their potential mechanosensory role(s).
Acknowledgements
We thank Sabine Eisner for excellent technical support and Adam Breitscheidel for preparing the artwork. We also thank the referees for their valuable input and constructive suggestions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
The work was supported by a COFUND Senior Fellowship at Durham University (REL), the Deutsche Forschungsgemeinschaft (LE 566/18-2, LE 566/20-1 and LE 566/22-1;REL), the Interdisciplinary Center for Clinical Research (IZKF) within the Faculty of Medicine at RWTH Aachen University and a Boost Fund by RWTH Aachen University. The financial support of the Fight for Sight UK (grant number 1585), the Leverhulme Trust (RPG-2012-554) and the Royal Society (IE140736) are gratefully acknowledged. K.J.G is supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R37 AR043380 and RO1 AR041836), the National Cancer Institute (R01 CA122151) and the Joseph L. Mayberry Endowment. J.A.B. was supported by a National Cancer Institute training grant (Post Graduate Program in Cutaneous Biology; T32 AR060710).
References
- Almeida F. V., Walko G., McMillan J. R., McGrath J. A., Wiche G., Barber A. H. and Connelly J. T. (2015). The cytolinker plectin regulates nuclear mechanotransduction in keratinocytes. J. Cell Sci. 128, 4475-4486. 10.1242/jcs.173435 [DOI] [PubMed] [Google Scholar]
- Antfolk D., Sjöqvist M., Cheng F., Isoniemi K., Duran C. L., Rivero-Muller A., Antila C., Niemi R., Landor S., Bouten C. V. C. et al. (2017). Selective regulation of Notch ligands during angiogenesis is mediated by vimentin. Proc. Natl. Acad. Sci. USA 114, E4574-E4581. 10.1073/pnas.1703057114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aureille J., Belaadi N. and Guilluy C. (2016). Mechanotransduction via the nuclear envelope: a distant reflection of the cell surface. Curr. Opin. Cell Biol. 44, 59-67. 10.1016/j.ceb.2016.10.003 [DOI] [PubMed] [Google Scholar]
- Baffet G., Loyer P., Glaise D., Corlu A., Etienne P. L. and Guguen-Guillouzo C. (1991). Distinct effects of cell-cell communication and corticosteroids on the synthesis and distribution of cytokeratins in cultured rat hepatocytes. J. Cell Sci. 99, 609-615. [DOI] [PubMed] [Google Scholar]
- Baribault H., Price J., Miyai K. and Oshima R. G. (1993). Mid-gestational lethality in mice lacking keratin 8. Genes Dev. 7, 1191-1202. 10.1101/gad.7.7a.1191 [DOI] [PubMed] [Google Scholar]
- Beriault D. R., Haddad O., McCuaig J. V., Robinson Z. J., Russell D., Lane E. B. and Fudge D. S. (2012). The mechanical behavior of mutant K14-R125P keratin bundles and networks in NEB-1 keratinocytes. PLoS ONE 7, e31320 10.1371/journal.pone.0031320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologna M., Allen R. and Dulbecco R. (1986). Organization of cytokeratin bundles by desmosomes in rat mammary cells. J. Cell Biol. 102, 560-567. 10.1083/jcb.102.2.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard J. A., Yang R., Huang C., Nathamgari S. S. P., Beese A. M., Godsel L. M., Hegazy M. H., Lee S., Zhou F., Sniadecki N. J. et al. (2017). The desmoplakin/intermediate filament linkage regulates cell mechanics. Mol. Biol. Cell. 10.1091/mbc.E16-07-0520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunser O. and Luft J. H. (1970). Fine structure of the apex of absorptive cells from rat small intestine. J. Ultrastruct. Res. 31, 291-311. 10.1016/S0022-5320(70)90133-4 [DOI] [PubMed] [Google Scholar]
- Carberry K., Wiesenfahrt T., Geisler F., Stocker S., Gerhardus H., Uberbach D., Davis W., Jorgensen E., Leube R. E. and Bossinger O. (2012). The novel intestinal filament organizer IFO-1 contributes to epithelial integrity in concert with ERM-1 and DLG-1. Development 139, 1851-1862. 10.1242/dev.075788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M.-F. and Shekhar S. (2017). Global treadmilling coordinates actin turnover and controls the size of actin networks. Nat. Rev. Mol. Cell Biol. 18, 389-401. 10.1038/nrm.2016.172 [DOI] [PubMed] [Google Scholar]
- Castañón M. J., Walko G., Winter L. and Wiche G. (2013). Plectin-intermediate filament partnership in skin, skeletal muscle, and peripheral nerve. Histochem. Cell Biol. 140, 33-53. 10.1007/s00418-013-1102-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y., Anton-Lamprecht I., Yu Q. C., Jackel A., Zabel B., Ernst J. P. and Fuchs E. (1994). A human keratin 14 “knockout”: the absence of K14 leads to severe epidermolysis bullosa simplex and a function for an intermediate filament protein. Genes Dev. 8, 2574-2587. 10.1101/gad.8.21.2574 [DOI] [PubMed] [Google Scholar]
- Chapman M. A., Zhang J., Banerjee I., Guo L. T., Zhang Z., Shelton G. D., Ouyang K., Lieber R. L. and Chen J. (2014). Disruption of both nesprin 1 and desmin results in nuclear anchorage defects and fibrosis in skeletal muscle. Hum. Mol. Genet. 23, 5879-5892. 10.1093/hmg/ddu310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier E. E. and Janmey P. A. (2016). Mechanical properties of intermediate filament proteins. Methods Enzymol. 568, 35-57. 10.1016/bs.mie.2015.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S., Irianto J. and Discher D. E. (2017). Mechanosensing by the nucleus: from pathways to scaling relationships. J. Cell Biol. 216, 305-315. 10.1083/jcb.201610042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung B.-M., Rotty J. D. and Coulombe P. A. (2013). Networking galore: intermediate filaments and cell migration. Curr. Opin. Cell Biol. 25, 600-612. 10.1016/j.ceb.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coch R. A. and Leube R. E. (2016). Intermediate filaments and polarization in the intestinal epithelium. Cells 5, 32 10.3390/cells5030032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia I., Chu D., Chou Y. H., Goldman R. D. and Matsudaira P. (1999). Integrating the actin and vimentin cytoskeletons. adhesion-dependent formation of fimbrin-vimentin complexes in macrophages. J. Cell Biol. 146, 831-842. 10.1083/jcb.146.4.831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe P. A., Hutton M. E., Letal A., Hebert A., Paller A. S. and Fuchs E. (1991). Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: genetic and functional analyses. Cell 66, 1301-1311. 10.1016/0092-8674(91)90051-Y [DOI] [PubMed] [Google Scholar]
- Cowin P., Kapprell H.-P., Franke W. W., Tamkun J. and Hynes R. O. (1986). Plakoglobin: a protein common to different kinds of intercellular adhering junctions. Cell 46, 1063-1073. 10.1016/0092-8674(86)90706-3 [DOI] [PubMed] [Google Scholar]
- Denais C. M., Gilbert R. M., Isermann P., McGregor A. L., te Lindert M., Weigelin B., Davidson P. M., Friedl P., Wolf K. and Lammerding J. (2016). Nuclear envelope rupture and repair during cancer cell migration. Science 352, 353-358. 10.1126/science.aad7297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drochmans P., Freudenstein C., Wanson J. C., Laurent L., Keenan T. W., Stadler J., Leloup R. and Franke W. W. (1978). Structure and biochemical composition of desmosomes and tonofilaments isolated from calf muzzle epidermis. J. Cell Biol. 79, 427-443. 10.1083/jcb.79.2.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Hoyos L. F., Shah R., Roa-Pena L., Vanner E. A., Najafian N., Banach A., Nielsen E., Al-Khalil R., Akalin A., Talmage D. et al. (2015). Keratin-17 promotes p27KIP1 nuclear export and degradation and offers potential prognostic utility. Cancer Res. 75, 3650-3662. 10.1158/0008-5472.CAN-15-0293 [DOI] [PubMed] [Google Scholar]
- Farquhar M. G. and Palade G. E. (1963). Junctional complexes in various epithelia. J. Cell Biol. 17, 375-412. 10.1083/jcb.17.2.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fois G., Weimer M., Busch T., Felder E. T., Oswald F., von Wichert G., Seufferlein T., Dietl P. and Felder E. (2013). Effects of keratin phosphorylation on the mechanical properties of keratin filaments in living cells. FASEB J. 27, 1322-1329. 10.1096/fj.12-215632 [DOI] [PubMed] [Google Scholar]
- Forbes M. S. and Sperelakis N. (1975). The “imaged-desmosome”: a component of intercalated discs in embryonic guinea pig myocardium. Anat. Rec. 183, 243-257. 10.1002/ar.1091830203 [DOI] [PubMed] [Google Scholar]
- Franke W. W., Cowin P., Schmelz M. and Kapprell H. P. (1987a). The desmosomal plaque and the cytoskeleton. Ciba Found Symp. 125, 26-48. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Kapprell H. P. and Cowin P. (1987b). Plakoglobin is a component of the filamentous subplasmalemmal coat of lens cells. Eur. J. Cell Biol. 43, 301-315. [PubMed] [Google Scholar]
- Franke W. W., Heid H., Zimbelmann R., Kuhn C., Winter-Simanowski S., Dörflinger Y., Grund C. and Rickelt S. (2013). Transmembrane protein PERP is a component of tessellate junctions and of other junctional and non-junctional plasma membrane regions in diverse epithelial and epithelium-derived cells. Cell Tissue Res. 353, 99-115. 10.1007/s00441-013-1645-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Rickelt S., Zimbelmann R., Dörflinger Y., Kuhn C., Frey N., Heid H. and Rosin-Arbesfeld R. (2015). Striatins as plaque molecules of zonulae adhaerentes in simple epithelia, of tessellate junctions in stratified epithelia, of cardiac composite junctions and of various size classes of lateral adherens junctions in cultures of epithelia- and carcinoma-derived cells. Cell Tissue Res. 359, 779-797. 10.1007/s00441-014-2053-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge D., Russell D., Beriault D., Moore W., Lane E. B. and Vogl A. W. (2008). The intermediate filament network in cultured human keratinocytes is remarkably extensible and resilient. PLoS ONE 3, e2327 10.1371/journal.pone.0002327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge D. S., McCuaig J. V., Van Stralen S., Hess J. F., Wang H., Mathias R. T. and FitzGerald P. G. (2011). Intermediate filaments regulate tissue size and stiffness in the murine lens. Invest. Ophthalmol. Vis. Sci. 52, 3860-3867. 10.1167/iovs.10-6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funkhouser C. M., Sknepnek R., Shimi T., Goldman A. E., Goldman R. D. and Olvera de la Cruz M. (2013). Mechanical model of blebbing in nuclear lamin meshworks. Proc. Natl. Acad. Sci. USA 110, 3248-3253. 10.1073/pnas.1300215110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod D. and Chidgey M. (2008). Desmosome structure, composition and function. Biochim. Biophys. Acta 1778, 572-587. 10.1016/j.bbamem.2007.07.014 [DOI] [PubMed] [Google Scholar]
- Geerts D., Fontao L., Nievers M. G., Schaapveld R. Q. J., Purkis P. E., Wheeler G. N., Lane E. B., Leigh I. M. and Sonnenberg A. (1999). Binding of integrin alpha6beta4 to plectin prevents plectin association with F-actin but does not interfere with intermediate filament binding. J. Cell Biol. 147, 417-434. 10.1083/jcb.147.2.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Schmid E. and Franke W. W. (1983). Spatial distribution of proteins specific for desmosomes and adhaerens junctions in epithelial cells demonstrated by double immunofluorescence microscopy. Differentiation 23, 189-205. 10.1111/j.1432-0436.1982.tb01283.x [DOI] [PubMed] [Google Scholar]
- Geisler F., Gerhardus H., Carberry K., Davis W., Jorgensen E., Richardson C., Bossinger O. and Leube R. E. (2016). A novel function for the MAP kinase SMA-5 in intestinal tube stability. Mol. Biol. Cell 27, 3855-3868. 10.1091/mbc.E16-02-0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos S. D. and Blobel G. (1987a). Lamin B constitutes an intermediate filament attachment site at the nuclear envelope. J. Cell Biol. 105, 117-125. 10.1083/jcb.105.1.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos S. D. and Blobel G. (1987b). Two distinct attachment sites for vimentin along the plasma membrane and the nuclear envelope in avian erythrocytes: a basis for a vectorial assembly of intermediate filaments. J. Cell Biol. 105, 105-115. 10.1083/jcb.105.1.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos S. D. and Marchesi V. T. (1985). The binding of vimentin to human erythrocyte membranes: a model system for the study of intermediate filament-membrane interactions. J. Cell Biol. 100, 1955-1961. 10.1083/jcb.100.6.1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger B. L. and Lazarides E. (1982). Structural associations of synemin and vimentin filaments in avian erythrocytes revealed by immunoelectron microscopy. Cell 30, 263-275. 10.1016/0092-8674(82)90032-0 [DOI] [PubMed] [Google Scholar]
- Granger B. L., Lazarides E. and Repasky E. A. (1982). Synemin and vimentin are components of intermediate filaments in avian erythrocytes. J. Cell Biol. 92, 299-312. 10.1083/jcb.92.2.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. J., Geiger B., Jones J. C., Talin J. C. and Goldman R. D. (1987). The relationship between intermediate filaments and during the formation of desmosomes and adherens-type junctions in mouse epidermal keratinocytes. J. Cell Biol. 104, 1389-1402. 10.1083/jcb.104.5.1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm-Gunter E.-M. S., Revenu C., Ramos S., Hurbain I., Smyth N., Ferrary E., Louvard D., Robine S. and Rivero F. (2009). Plastin 1 binds to keratin and is required for terminal web assembly in the intestinal epithelium. Mol. Biol. Cell 20, 2549-2562. 10.1091/mbc.E08-10-1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon R. M. and Green K. J. (2013). Structural and functional diversity of desmosomes. Cell Commun. Adhes. 20, 171-187. 10.3109/15419061.2013.855204 [DOI] [PubMed] [Google Scholar]
- Harris A. R., Daeden A. and Charras G. T. (2014). Formation of adherens junctions leads to the emergence of a tissue-level tension in epithelial monolayers. J. Cell Sci. 127, 2507-2517. 10.1242/jcs.142349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld M., Keil R. and Magin T. M. (2017). Desmosomes and Intermediate Filaments: Their Consequences for Tissue Mechanics. Cold Spring Harb. Perspect. Biol. 9, a029157 10.1101/cshperspect.a029157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heid H., Rickelt S., Zimbelmann R., Winter S., Schumacher H. and Dörflinger Y. (2013). Lipid droplets, perilipins and cytokeratins--unravelled liaisons in epithelium-derived cells. PLoS ONE 8, e63061 10.1371/journal.pone.0063061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann H. and Aebi U. (2016). Intermediate filaments: structure and assembly. Cold Spring Harb. Perspect. Biol. 8 10.1101/cshperspect.a018242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs R. G. and Clark W. H. Jr (1959). Electron microscope studies of the human epidermis: the cell boundaries and topography of the stratum malpighii. J. Biophys. Biochem. Cytol. 6, 71-76. 10.1083/jcb.6.1.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Tilney L. G., Fujiwara K. and Heuser J. E. (1982). Organization of actin, myosin, and intermediate filaments in the brush border of intestinal epithelial cells. J. Cell Biol. 94, 425-443. 10.1083/jcb.94.2.425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs R. P., DePianto D. J., Jacob J. T., Han M. C., Chung B.-M., Batazzi A. S., Poll B. G., Guo Y., Han J., Ong S. F. et al. (2015). Keratin-dependent regulation of Aire and gene expression in skin tumor keratinocytes. Nat. Genet. 47, 933-938. 10.1038/ng.3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs R. P., Jacob J. T. and Coulombe P. A. (2016). Keratins are going nuclear. Dev. Cell 38, 227-233. 10.1016/j.devcel.2016.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthöfer B., Windoffer R., Troyanovsky S. and Leube R. E. (2007). Structure and function of desmosomes. Int. Rev. Cytol. 264, 65-163. 10.1016/S0074-7696(07)64003-0 [DOI] [PubMed] [Google Scholar]
- Isermann P. and Lammerding J. (2013). Nuclear mechanics and mechanotransduction in health and disease. Curr. Biol. 23, R1113-R1121. 10.1016/j.cub.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki H. and Suda M. (2007). Keratin 20 expressed in the endocrine and exocrine cells of the rabbit duodenum. Acta Histochem. Cytochem. 40, 123-130. 10.1267/ahc.07007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. C. R., Kam C. Y., Harmon R. M., Woychek A. V., Hopkinson S. B. and Green K. J. (2017). Intermediate filaments and the plasma membrane. Cold Spring Harb. Perspect. Biol. 9, a025866 10.1101/cshperspect.a025866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman M. F., Pasmooij A. M. G., Pasmans S. G. M. A., van den Berg M. P., ter Horst H. J., Timmer A. and Pas H. H. (2005). Loss of desmoplakin tail causes lethal acantholytic epidermolysis bullosa. Am. J. Hum. Genet. 77, 653-660. 10.1086/496901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgens D. M., Inman J. L., Wojcik M., Robertson C., Palsdottir H., Tsai W.-T., Huang H., Bruni-Cardoso A., López C. S., Bissell M. J. et al. (2017). Deep nuclear invaginations are linked to cytoskeletal filaments - integrated bioimaging of epithelial cells in 3D culture. J. Cell Sci. 130, 177-189. 10.1242/jcs.190967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H., Weiss T. M., Bang I., Weis W. I. and Choi H.-J. (2016). Structure of the intermediate filament-binding region of desmoplakin. PLoS ONE 11, e0147641 10.1371/journal.pone.0147641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartenbeck J., Franke W. W., Moser J. G. and Stoffels U. (1983). Specific attachment of desmin filaments to desmosomal plaques in cardiac myocytes. EMBO J. 2, 735-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartenbeck J., Schwechheimer K., Moll R. and Franke W. W. (1984). Attachment of vimentin filaments to desmosomal plaques in human meningiomal cells and arachnoidal tissue. J. Cell Biol. 98, 1072-1081. 10.1083/jcb.98.3.1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuma Y., Marceau N., Ohta M. and French S. W. (1988). Cytokeratin intermediate filaments of rat hepatocytes: different cytoskeletal domains and their three-dimensional structure. Hepatology 8, 559-568. 10.1002/hep.1840080321 [DOI] [PubMed] [Google Scholar]
- Kerns M. L., Hakim J. M. C., Lu R. G., Guo Y., Berroth A., Kaspar R. L. and Coulombe P. A. (2016). Oxidative stress and dysfunctional NRF2 underlie pachyonychia congenita phenotypes. J. Clin. Invest. 126, 2356-2366. 10.1172/JCI84870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketema M. and Sonnenberg A. (2011). Nesprin-3: a versatile connector between the nucleus and the cytoskeleton. Biochem. Soc. Trans. 39, 1719-1724. 10.1042/BST20110669 [DOI] [PubMed] [Google Scholar]
- Knöbel M., O'Toole E. A. and Smith F. J. D. (2015). Keratins and skin disease. Cell Tissue Res. 360, 583-589. 10.1007/s00441-014-2105-4 [DOI] [PubMed] [Google Scholar]
- Ko K. S. and McCulloch C. A. G. (2000). Partners in protection: interdependence of cytoskeleton and plasma membrane in adaptations to applied forces. J. Membr. Biol. 174, 85-95. 10.1007/s002320001034 [DOI] [PubMed] [Google Scholar]
- Koeser J., Troyanovsky S. M., Grund C. and Franke W. W. (2003). De novo formation of desmosomes in cultured cells upon transfection of genes encoding specific desmosomal components. Exp. Cell Res. 285, 114-130. 10.1016/S0014-4827(03)00016-8 [DOI] [PubMed] [Google Scholar]
- Lancaster O. M. and Baum B. (2014). Shaping up to divide: coordinating actin and microtubule cytoskeletal remodelling during mitosis. Semin. Cell Dev. Biol. 34, 109-115. 10.1016/j.semcdb.2014.02.015 [DOI] [PubMed] [Google Scholar]
- Leube R. E., Moch M. and Windoffer R. (2015). Intermediate filaments and the regulation of focal adhesion. Curr. Opin. Cell Biol. 32, 13-20. 10.1016/j.ceb.2014.09.011 [DOI] [PubMed] [Google Scholar]
- Lewis S. A. (2000). Everything you wanted to know about the bladder epithelium but were afraid to ask. Am. J. Physiol. Renal. Physiol. 278, F867-F874. [DOI] [PubMed] [Google Scholar]
- Loiseau E., Schneider J. A. M., Keber F. C., Pelzl C., Massiera G., Salbreux G. and Bausch A. R. (2016). Shape remodeling and blebbing of active cytoskeletal vesicles. Sci. Adv. 2, e1500465 10.1126/sciadv.1500465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering R. M., O'Neill A., Muriel J. M., Prosser B. L., Strong J. and Bloch R. J. (2011). Physiology, structure, and susceptibility to injury of skeletal muscle in mice lacking keratin 19-based and desmin-based intermediate filaments. Am. J. Physiol. Cell Physiol. 300, C803-C813. 10.1152/ajpcell.00394.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery J., Kuczmarski E. R., Herrmann H. and Goldman R. D. (2015). Intermediate filaments play a pivotal role in regulating cell architecture and function. J. Biol. Chem. 290, 17145-17153. 10.1074/jbc.R115.640359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lulevich V., Yang H.-Y., Isseroff R. R. and Liu G.-Y. (2010). Single cell mechanics of keratinocyte cells. Ultramicroscopy 110, 1435-1442. 10.1016/j.ultramic.2010.07.009 [DOI] [PubMed] [Google Scholar]
- Maniotis A. J., Chen C. S. and Ingber D. E. (1997). Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl. Acad. Sci. USA 94, 849-854. 10.1073/pnas.94.3.849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt N. S. and Fawcett D. W. (1969). The ultrastructure of the cat myocardium. II. Atrial muscle. J. Cell Biol. 42, 46-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinke P. and Schirmer E. C. (2015). LINC'ing form and function at the nuclear envelope. FEBS Lett. 589, 2514-2521. 10.1016/j.febslet.2015.06.011 [DOI] [PubMed] [Google Scholar]
- Moll I., Kurzen H., Langbein L. and Franke W. W. (1997). The distribution of the desmosomal protein, plakophilin 1, in human skin and skin tumors. J. Invest. Dermatol. 108, 139-146. 10.1111/1523-1747.ep12332388 [DOI] [PubMed] [Google Scholar]
- Moulton D. E., Sulzer V., Apodaca G., Byrne H. M. and Waters S. L. (2016). Mathematical modelling of stretch-induced membrane traffic in bladder umbrella cells. J. Theor. Biol. 409, 115-132. 10.1016/j.jtbi.2016.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekrasova O. and Green K. J. (2013). Desmosome assembly and dynamics. Trends Cell Biol. 23, 537-546. 10.1016/j.tcb.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolting J.-F., Möbius W. and Köster S. (2014). Mechanics of individual keratin bundles in living cells. Biophys. J. 107, 2693-2699. 10.1016/j.bpj.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgett E. E., Hatsell S. J., Carvajal-Huerta L., Cabezas J. C., Common J., Purkis P. E., Whittock N., Leigh I. M., Stevens H. P. and Kelsell D. P. (2000). Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum. Mol. Genet. 9, 2761-2766. 10.1093/hmg/9.18.2761 [DOI] [PubMed] [Google Scholar]
- Omary M. B. (2009). “IF-pathies”: a broad spectrum of intermediate filament-associated diseases. J. Clin. Invest. 119, 1756-1762. 10.1172/JCI39894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary M. B. (2016). Cell biology to disease and back. Nat. Rev. Mol. Cell Biol. 17, 4 10.1038/nrm.2015.9 [DOI] [PubMed] [Google Scholar]
- Osmanagic-Myers S., Dechat T. and Foisner R. (2015a). Lamins at the crossroads of mechanosignaling. Genes Dev. 29, 225-237. 10.1101/gad.255968.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmanagic-Myers S., Rus S., Wolfram M., Brunner D., Goldmann W. H., Bonakdar N., Fischer I., Reipert S., Zuzuarregui A., Walko G. et al. (2015b). Plectin reinforces vascular integrity by mediating crosstalk between the vimentin and the actin networks. J. Cell Sci. 128, 4138-4150. 10.1242/jcs.172056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio D. S. and Gomes E. R. (2014). Connecting the nucleus to the cytoskeleton for nuclear positioning and cell migration. Adv. Exp. Med. Biol. 773, 505-520. 10.1007/978-1-4899-8032-8_23 [DOI] [PubMed] [Google Scholar]
- Ouellet T., Levac P. and Royal A. (1988). Complete sequence of the mouse type-II keratin EndoA: its amino-terminal region resembles mitochondrial signal peptides. Gene 70, 75-84. 10.1016/0378-1119(88)90106-0 [DOI] [PubMed] [Google Scholar]
- Pallari H.-M. and Eriksson J. E. (2006). Intermediate filaments as signaling platforms. Sci. STKE 2006, pe53 10.1126/stke.3662006pe53 [DOI] [PubMed] [Google Scholar]
- Pallari H.-M., Lindqvist J., Torvaldson E., Ferraris S. E., He T., Sahlgren C. and Eriksson J. E. (2011). Nestin as a regulator of Cdk5 in differentiating myoblasts. Mol. Biol. Cell 22, 1539-1549. 10.1091/mbc.E10-07-0568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmisano M. G., Bremner S. N., Hornberger T. A., Meyer G. A., Domenighetti A. A., Shah S. B., Kiss B., Kellermayer M., Ryan A. F. and Lieber R. L. (2015). Skeletal muscle intermediate filaments form a stress-transmitting and stress-signaling network. J. Cell Sci. 128, 219-224. 10.1242/jcs.142463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieperhoff S., Borrmann C., Grund C., Barth M., Rizzo S. and Franke W. W. (2010). The area composita of adhering junctions connecting heart muscle cells of vertebrates. VII. The different types of lateral junctions between the special cardiomyocytes of the conduction system of ovine and bovine hearts. Eur. J. Cell Biol. 89, 365-378. 10.1083/jcb.42.1.46 [DOI] [PubMed] [Google Scholar]
- Pollard T. D. and Cooper J. A. (2009). Actin, a central player in cell shape and movement. Science 326, 1208-1212. 10.1126/science.1175862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramms L., Fabris G., Windoffer R., Schwarz N., Springer R., Zhou C., Lazar J., Stiefel S., Hersch N., Schnakenberg U. et al. (2013). Keratins as the main component for the mechanical integrity of keratinocytes. Proc. Natl. Acad. Sci. USA 110, 18513-18518. 10.1073/pnas.1313491110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg E. L., McLean W. H., Lane E. B., Pitera R., McMillan J. R., Dopping-Hepenstal P. J., Navsaria H. A., Leigh I. M. and Eady R. A. (1994). A functional “knockout” of human keratin 14. Genes Dev. 8, 2563-2573. 10.1101/gad.8.21.2563 [DOI] [PubMed] [Google Scholar]
- Russell D., Andrews P. D., James J. and Lane E. B. (2004). Mechanical stress induces profound remodelling of keratin filaments and cell junctions in epidermolysis bullosa simplex keratinocytes. J. Cell Sci. 117, 5233-5243. 10.1242/jcs.01407 [DOI] [PubMed] [Google Scholar]
- Salas P. J., Forteza R. and Mashukova A. (2016). Multiple roles for keratin intermediate filaments in the regulation of epithelial barrier function and apico-basal polarity. Tissue Barriers 4, e1178368 10.1080/21688370.2016.1178368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandilands A., Prescott A. R., Carter J. M., Hutcheson A. M., Quinlan R. A., Richards J. and FitzGerald P. G. (1995a). Vimentin and CP49/Filensin form distinct networks in the lens which are independantly modulated during lens fibre cell differentiation. J. Cell Sci. 108, 1397-1406. [DOI] [PubMed] [Google Scholar]
- Sandilands A., Prescott A. R., Hutcheson A. M., Quinlan R. A., Casselman J. T. and FitzGerald P. G. (1995b). Filensin is proteolytically processed during lens fiber cell differentiation by multiple independant pathways. Eur. J. Cell Biol. 67, 238-253. [PubMed] [Google Scholar]
- Schmidt A., Heid H. W., Schafer S., Nuber U. A., Zimbelmann R. and Franke W. W. (1994). Desmosomes and cytoskeletal architecture in epithelial differentiation: cell type-specific plaque components and intermediate filament anchorage. Eur. J. Cell Biol. 65, 229-245. [PubMed] [Google Scholar]
- Schwarz N., Windoffer R., Magin T. M. and Leube R. E. (2015). Dissection of keratin network formation, turnover and reorganization in living murine embryos. Sci. Rep. 5, 9007 10.1038/srep09007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Landsbury A., Dahm R., Liu Y., Zhang Q. and Quinlan R. A. (2009). Functions of the intermediate filament cytoskeleton in the eye lens. J. Clin. Invest. 119, 1837-1848. 10.1172/JCI38277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L. A. (1974). Structure and function of intercellular junctions. Int. Rev. Cytol. 39, 191-283. 10.1016/S0074-7696(08)60940-7 [DOI] [PubMed] [Google Scholar]
- Stappenbeck T. S., Bornslaeger E. A., Corcoran C. M., Luu H. H., Virata M. L. and Green K. J. (1993). Functional analysis of desmoplakin domains: specification of the interaction with keratin versus vimentin intermediate filament networks. J. Cell Biol. 123, 691-705. 10.1083/jcb.123.3.691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub B. K., Boda J., Kuhn C., Schnoelzer M., Korf U., Kempf T., Spring H., Hatzfeld M. and Franke W. W. (2003). A novel cell-cell junction system: the cortex adhaerens mosaic of lens fiber cells. J. Cell Sci. 116, 4985-4995. 10.1242/jcs.00815 [DOI] [PubMed] [Google Scholar]
- Straub B. K., Rickelt S., Zimbelmann R., Grund C., Kuhn C., Iken M., Ott M., Schirmacher P. and Franke W. W. (2011). E-N-cadherin heterodimers define novel adherens junctions connecting endoderm-derived cells. J. Cell Biol. 195, 873-887. 10.1083/jcb.201106023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumigray K. D. and Lechler T. (2012). Desmoplakin controls microvilli length but not cell adhesion or keratin organization in the intestinal epithelium. Mol. Biol. Cell 23, 792-799. 10.1091/mbc.E11-11-0923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi K., Nishida T., Inoue K. and Tsukita S. (2017). Three-dimensional organization of layered apical cytoskeletal networks associated with mouse airway tissue development. Sci. Rep. 7, 43783 10.1038/srep43783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornell L.-E., Carlsson L., Li Z., Mericskay M. and Paulin D. (1997). Null mutation in the desmin gene gives rise to a cardiomyopathy. J. Mol. Cell. Cardiol. 29, 2107-2124. 10.1006/jmcc.1997.0446 [DOI] [PubMed] [Google Scholar]
- Toivola D. M., Omary M. B., Ku N.-O., Peltola O., Baribault H. and Eriksson J. E. (1998). Protein phosphatase inhibition in normal and keratin 8/18 assembly- incompetent mouse strains supports a functional role of keratin intermediate filaments in preserving hepatocyte integrity. Hepatology 28, 116-128. 10.1002/hep.510280117 [DOI] [PubMed] [Google Scholar]
- Toivola D. M., Baribault H., Magin T., Michie S. A. and Omary M. B. (2000a). Simple epithelial keratins are dispensable for cytoprotection in two pancreatitis models. Am. J. Physiol. Gastrointest. Liver Physiol. 279, G1343-G1354. [DOI] [PubMed] [Google Scholar]
- Toivola D. M., Ku N.-O., Ghori N., Lowe A. W., Michie S. A. and Omary M. B. (2000b). Effects of keratin filament disruption on exocrine pancreas-stimulated secretion and susceptibility to injury. Exp. Cell Res. 255, 156-170. 10.1006/excr.1999.4787 [DOI] [PubMed] [Google Scholar]
- Toivola D. M., Boor P., Alam C. and Strnad P. (2015). Keratins in health and disease. Curr. Opin. Cell Biol. 32, 73-81. 10.1016/j.ceb.2014.12.008 [DOI] [PubMed] [Google Scholar]
- Troyanovsky S. M., Eshkind L. G., Troyanovsky R. B., Leube R. E. and Franke W. W. (1993). Contributions of cytoplasmic domains of desmosomal cadherins to desmosome assembly and intermediate filament anchorage. Cell 72, 561-574. 10.1016/0092-8674(93)90075-2 [DOI] [PubMed] [Google Scholar]
- Truschel S. T., Wang E., Ruiz W. G., Leung S. M., Rojas R., Lavelle J., Zeidel M., Stoffer D. and Apodaca G. (2002). Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol. Biol. Cell 13, 830-846. 10.1091/mbc.01-09-0435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beuningen S. F. B. and Hoogenraad C. C. (2016). Neuronal polarity: remodeling microtubule organization. Curr. Opin. Neurobiol. 39, 1-7. 10.1016/j.conb.2016.02.003 [DOI] [PubMed] [Google Scholar]
- Vasioukhin V., Bowers E., Bauer C., Degenstein L. and Fuchs E. (2001). Desmoplakin is essential in epidermal sheet formation. Nat. Cell Biol. 3, 1076-1085. 10.1038/ncb1201-1076 [DOI] [PubMed] [Google Scholar]
- Veranic P. and Jezernik K. (2002). Trajectorial organisation of cytokeratins within the subapical region of umbrella cells. Cell Motil. Cytoskeleton 53, 317-325. 10.1002/cm.10077 [DOI] [PubMed] [Google Scholar]
- Vijayaraj P., Kröger C., Reuter U., Windoffer R., Leube R. E. and Magin T. M. (2009). Keratins regulate protein biosynthesis through localization of GLUT1 and -3 upstream of AMP kinase and Raptor. J. Cell Biol. 187, 175-184. 10.1083/jcb.200906094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. K. and Schwille P. (2012). Minimal systems to study membrane-cytoskeleton interactions. Curr. Opin. Biotechnol. 23, 758-765. 10.1016/j.copbio.2012.03.012 [DOI] [PubMed] [Google Scholar]
- Walko G., Castañón M. J. and Wiche G. (2015). Molecular architecture and function of the hemidesmosome. Cell Tissue Res. 360, 529-544. 10.1007/s00441-015-2216-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G. F., Bjerke M. A. and DeSimone D. W. (2012). A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev. Cell 22, 104-115. 10.1016/j.devcel.2011.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner N. S., Windoffer R., Strnad P., Grund C., Leube R. E. and Magin T. M. (2004). Epidermolysis bullosa simplex-type mutations alter the dynamics of the keratin cytoskeleton and reveal a contribution of actin to the transport of keratin subunits. Mol. Biol. Cell 15, 990-1002. 10.1091/mbc.E03-09-0687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen K., Litjens S. H. M., Kuikman I., Tshimbalanga N., Janssen H., van den Bout I., Raymond K. and Sonnenberg A. (2005). Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J. Cell Biol. 171, 799-810. 10.1083/jcb.200506083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windoffer R., Borchert-Stuhltrager M. and Leube R. E. (2002). Desmosomes: interconnected calcium-dependent structures of remarkable stability with significant integral membrane protein turnover. J. Cell Sci. 115, 1717-1732. [DOI] [PubMed] [Google Scholar]
- Woodcock C. L. F. (1980). Nucleus-associated intermediate filaments from chicken erytrocytes. J. Cell Biol. 85, 881-889. 10.1083/jcb.85.3.881 [DOI] [PMC free article] [PubMed] [Google Scholar]