Summary
From the extracellular matrix to the cytoskeleton, a network of molecular links connects cells to their environment. Molecules in this network transmit and detect mechanical forces, which subsequently determine cell behavior and fate. Here, we reconstruct the mechanical pathway followed by these forces. From matrix proteins to actin through integrins and adaptor proteins, we review how forces affect the lifetime of bonds and stretch or alter the conformation of proteins, and how these mechanical changes are converted into biochemical signals in mechanotransduction events. We evaluate which of the proteins in the network can participate in mechanotransduction and which are simply responsible for transmitting forces in a dynamic network. Besides their individual properties, we also analyze how the mechanical responses of a protein are determined by their serial connections from the matrix to actin, their parallel connections in integrin clusters and by the rate at which force is applied to them. All these define mechanical molecular pathways in cells, which are emerging as key regulators of cell function alongside better studied biochemical pathways.
Key words: Cell adhesion, Cytoskeleton, Mechanotransduction
Introduction
Mechanical stimuli that are transmitted between a cell and its environment determine cell functions, such as proliferation (Nelson et al., 2005), differentiation (Engler et al., 2006) and motility (Janmey et al., 2009), as well as key processes in development (Gorfinkiel et al., 2009; Mammoto and Ingber, 2010), tumorigenesis (Kumar and Weaver, 2009; Paszek et al., 2005) and wound healing (Fenteany et al., 2000), among others. Mechanical transmission takes place through different cell structures, but one of the main components, and definitely the best studied one, is that of integrin-based cell adhesions. These adhesions connect extracellular matrix (ECM) proteins to the cell cytoskeleton through the transmembrane integrins, and a molecular complex that can contain over a hundred different types of adaptor molecules (Zaidel-Bar et al., 2007a). Adhesions withstand and exert forces on the ECM even though they and the cytoskeleton are dynamic structures. In addition, adhesions are mechanosensitive elements that convert mechanical stimuli into biochemical responses (Bershadsky et al., 2003; Riveline et al., 2001) by sensing and responding to parameters such as matrix rigidity (Moore et al., 2010). Precisely because of their complexity, however, the mechanisms behind the mechanical function of cell–ECM adhesions have been challenging to unravel. The first step is to dissect where biophysics ends and biochemistry starts; that is, we must identify the molecules that exert, withstand, transmit and respond to forces. For those that respond to force (by changing binding kinetics or by unfolding, for instance) we must then evaluate whether and how this change can lead to true force sensing by triggering biochemical signaling cascades. Only after this is achieved will we be able to distinguish the primary molecular events that detect forces from ensuing biochemical signaling networks.
To address this issue, this Commentary will not focus on signaling networks in adhesions, their assembly and dynamics, or their detailed composition, topics which have recently been reviewed elsewhere (Costa and Parsons, 2010; Dubash et al., 2009; Parsons et al., 2010; Ridley, 2011; Wolfenson et al., 2009). Rather, we will follow the path that force follows as it is transmitted from the ECM to the cytoskeleton, analyzing the molecules likely to be under mechanical stress. We will first briefly review the molecular machinery that is responsible for force generation and the biophysical effects that force has on molecular conformation and binding. We will then analyze the ECM molecules, integrins and adaptor molecules step-by-step. For all the components of this protein network, we will review current knowledge with regard to how they respond to force and the possible links through which they could participate in force transmission throughout the cell. We will then discuss the factors that affect the collective mechanical behavior of all these molecules that are present in adhesions. Finally, we will discuss the signaling events that could be triggered by putative mechanosensors. Owing to space limitations, we will not cover adhesions that are mediated by transmembrane proteins other than integrins (such as syndecans) or cell–cell adhesions.
First step of force transmission – the extracellular matrix
Cellular forces are primarily developed by the acto-myosin cytoskeleton (Box 1) and have a profound effect on molecular conformation and binding (Box 2). Starting at the cell exterior, the first elements that forces encounter in their path are ECM molecules, which form meshworks of polymerized fibers that constitute the major components of the cell microenvironment. Among ECM molecules, fibronectin has been the most studied from a mechanical point of view. Forces of 80–200 pN can stretch all the fibronectin FNIII domains, which contain the integrin-binding sites (Table 1). Upon stretching, fibronectin fibers can extend up to eightfold before breaking (Klotzsch et al., 2009), which leads to unfolding of the FNIII domains and exposure of cryptic sites. This unfolding, however, might account for only a fraction of the fibronectin stretch, which could be mostly determined by a global conformational change (Lemmon et al., 2011). In any case, stretching of FNIII domains is a form of mechanotransduction, because the exposure of the cryptic sites leads to downstream effects, such as the regulation of the fibrillogenesis of fibronectin (Sechler et al., 2001), stimulation of cell growth and contractility (Hocking and Kowalski, 2002), and stimulation of the digestion of gelatin, type IV collagen, α- and β-casein and insulin β-chain (Schnepel and Tschesche, 2000). Force can also change the conformation and distance between the RGD and PHSRN motifs (two synergistic integrin-binding sites within FNIII domains), altering integrin recognition and specificity (Grant et al., 1997; Vogel, 2006). This stretching and unfolding can be achieved in vivo by cell-generated forces (Baneyx et al., 2001; Smith et al., 2007) and is controlled by cell contractility (Baneyx et al., 2002).
Box 1. Force generating machinery.
Polymerization
Upon binding to actin filaments, actin monomers release energy, which the filaments can use to generate force and ‘push’ any structure opposing polymer growth (such as the cell membrane). Indeed, cells migrate and spread through this mechanism. Actin polymerization forces at the cell migrating edge (lamellipodium) are ∼2 nN/µm2 (Heinemann et al., 2011; Prass et al., 2006). Of course, this force generation fluctuates, and the cell also uses a myriad of molecules, including the Arp2/3 complex, Wiskott–Aldrich syndrome protein (WASP), cofilin, formins and many others, to regulate actin dynamics (for a review, see Ridley, 2011). Polymerizing microtubules can also generate forces of up to 4 pN (Dogterom and Yurke, 1997), although their contribution to protrusive forces is much smaller owing to their lower density and their catastrophic disassembly events. Intermediate filaments cannot generate directed forces owing to their lack of polarity.
Molecular motors
Molecular motors bind to cytoskeletal filaments; myosins bind to actin (Sellers, 2000), and kinesins (Hirokawa et al., 2009) and dyneins (Pfister et al., 2006) bind to microtubules. They undergo ATP-fueled conformational changes that generate movement and forces on the filaments. In processive motors such as kinesin 1, the two tubulin-binding heads coordinate to continuously ‘walk’ over the filament (Alonso et al., 2007), generating forces of up to 6 pN (Kuo and Sheetz, 1993; Meyhöfer and Howard, 1995; Svoboda and Block, 1994), transporting organelles and vesicles (Hirokawa et al., 2009). Non-processive motors such as myosin II are not coordinated in the same way and require myosin filaments to generate forces (Harada et al., 1990). Myosin II filaments generate large forces in muscle contraction or fibroblast–ECM adhesion (Cai et al., 2006) of up to 6 pN per myosin head (Finer et al., 1994; Ishijima et al., 1994; Tyska et al., 1999). Their concerted effort can generate stresses of tens of nanonewtons per square meter on the ECM (Balaban et al., 2001; du Roure et al., 2005; Tan et al., 2003).
Box 2. Biophysics of unfolding proteins and breaking bonds.
Forces promote unfolding [a disruption of secondary or tertiary structure that increases the length of the molecule between the pulling ends (Bustamante et al., 2004; Kumar and Suan Li, 2010; Sulkowska and Cieplak, 2008)], bond dissociation (slip bonds), or in some cases even bond strengthening (catch bonds).
Unfolding and slip bonds
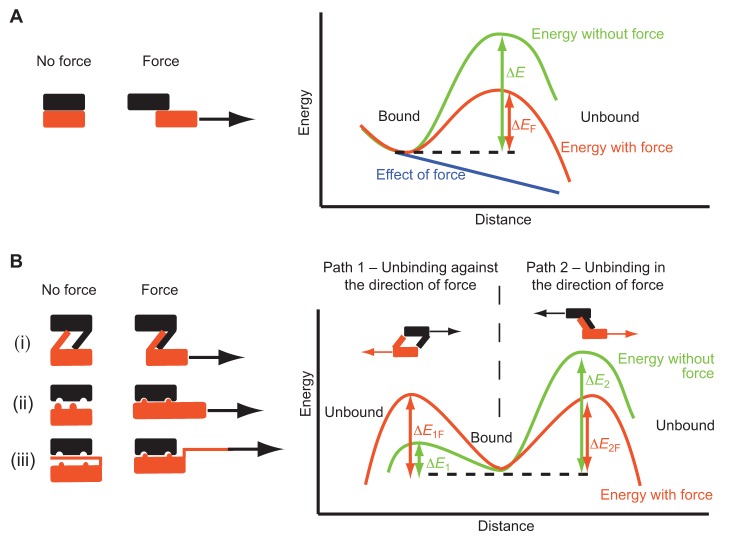
Unfolding and unbinding (Figure panel A) can be modeled by two equilibrium states (folded or bound versus unfolded or unbound) that are separated by a certain distance and an energy barrier (green line, ΔE) (Bustamante et al., 2004). Applied forces ‘tilt’ the energy landscape (blue line), reducing the energy barrier between states (orange line, ΔEF), and thereby decreasing the average time needed for unfolding or unbinding (Bell, 1978). The rate at which force is applied (the loading rate) increases the average force at which unfolding takes place (Evans and Ritchie, 1999; Rico et al., 2007). This is because the time required to reach a given force will be shorter with fast loading, thereby reducing the likelihood of an unfolding event during the process. Thus, loading rates must be considered when comparing measurements.
Catch bonds
Catch bonds (Figure panel B) strengthen, rather than weaken, with force. This could be because force is either opposed by ‘hook-shaped’ molecules (i), the force aligns binding sites by stretching them (ii), or because it displaces an inhibiting region hiding a cryptic binding domain (iii) (Thomas et al., 2008). In the hook example (i), without force application, unbinding is much easier if the hook is separated (path 1, ΔE1) compared with deforming it (path 2, ΔE2). Force applied in the direction of hook deformation, however, impairs unbinding in the opposite direction (ΔE1F>ΔE1), but helps to deform the hook (ΔE2F<ΔE2). As the bond tends to unbind through the path with the lowest barrier, the maximum bond strength is when ΔE1F = ΔE2F, and will decrease for higher forces. Thus, catch bonds have an optimum force range at which they are stable, a very interesting feature, with implications discussed in the main text.
Table 1.
Mechanical parameters of adhesion proteins
| Unfolding | Unbinding | Stiffnessa | |||||||
| Protein | Force (pN) | Speed (µm/s) | Reference | Interaction | Force (pN) | Speed (µm/s) | Reference | pN/nm | Reference |
| ECM | |||||||||
| Fn | 137 | 0.2–0.6 | (Oberhauser et al., 1998) | Fn–α5β1 | 69–93b | 5 | (Li et al., 2003a) | 0.5 | (Oberhauser et al., 1998) |
| 80–200 | 0.6 | (Oberhauser et al., 2002) | Fn–α5β1 | 39 | 0.8 | (Sun et al., 2005) | |||
| Fb or Fib | 150–200 | 1 | (Averett et al., 2009) | Fib–αIIbβ3 | 50–80 | 0.16–1 | (Agnihotri et al., 2009) | 1–3 | (Brown et al., 2007) |
| 100 | (Brown et al., 2007) | Fb–αIIbβ3 | 80–100 | 80 | (Litvinov et al., 2002) | 6 | (Lim et al., 2008a) | ||
| 130 | 0.4–1 | (Lim et al., 2008a) | Fib–αIIbβ3 | 80–90 | 0.8–80 | (Litvinov et al., 2005) | |||
| RGD–αIIbβ3 | 93 | 0.2 | (Lee and Marchant, 2001) | ||||||
| Collagen I | 4–90 | 18000 | (Graham et al., 2004) | Col–α2β1 | 160 | 0.5 | (Niland et al., 2011) | 1.5c | (Thompson et al., 2001) |
| Adaptor proteins | |||||||||
| Talin | 29–51 | 0.4 | (del Rio et al., 2009) | Actin | 2 | 0.06 | (Jiang et al., 2003) | 1.5 | (del Rio et al., 2009) |
| α-Actinin | Actin | 40–78 | 4–50d | (Ferrer et al., 2008) | 1700 | (Paramore et al., 2006) | |||
| Actin | 1.4–44 | ? | (Miyata et al., 1996) | ||||||
| Filamin | 57 | 5 | (Lee et al., 2009) | Actin | 40–65 | 4–50d | (Ferrer et al., 2008) | 200–800 | (Lee et al., 2009) |
| 48 | 6 se | (Chen et al., 2011) | Actin | 70 | 5 | (Lee et al., 2009) | |||
| 50–220 | 0.37 | (Furuike et al., 2001) | |||||||
| Titin kinase | 30 | 1 | (Puchner et al., 2008) | ||||||
Stiffness values were calculated from reported force–extension curves at low extensions, or calculated as first-order approximation from reported worm-like chain parameters at low deformations.
The range from inactive to activated is shown.
Value might correspond to more than one molecule in parallel.
Loading rates in pN/second. To convert retraction speeds into loading rates, the stiffness (spring constant) of the system (studied molecules + measuring probe) must be determined. This value will be dominated by the softest component, and is not always reported.
Time required to unfold protein at constant force.
Fn, fibronectin, Fb, fibrinogen; Fib, fibrin; Col, collagen.
Collagen I fibrils can also be stretched with forces of 1–4 nN, which extrapolates to a force of 4–90 pN per collagen monomer (Graham et al., 2004). The role of force in collagen function is unclear, but many of the key binding sites in collagen, including the GFOGER sequence that can bind to β1 integrin, are cryptic and are only fully exposed upon proteolysis, partial denaturation or force application (Orgel et al., 2011; Perumal et al., 2008). Interestingly, collagens also possess several copies of the RGD sequence (Davis, 1992), which has different integrin specificity and is characteristic of other ECM molecules such as fibronectin (Hynes, 2002). In collagen, the RGD site is only exposed upon denaturation (which can be caused by force), which could change integrin specificity and trigger integrin-mediated wound response (Davis, 1992). The coiled-coil sequences in the fibrinogen α-helices also unfold under forces of ∼100 pN (Table 1), and this molecular unfolding seems to be the main factor determining the elasticity of fibrin fibers in blood clots (Brown et al., 2009). Fibrinogen unfolding can also activate its integrin receptor, macrophage-1 antigen (MAC1 or integrin αMβ2) and promote inflammation, suggesting that there is a possible mechanosensing mechanism (Deng et al., 2011). The relevance of mechanical stimuli for the function of other ECM molecules, including laminins and vitronectin is essentially unexplored. However, the list of cryptic binding sites in these molecules that could be exposed by force is extensive (Davis et al., 2000), opening many intriguing possibilities.
Integrins
Integrins are heterodimeric transmembrane proteins composed of an α- and β-subunit, and are major molecular links between cells and the ECM. Different combinations of subunits lead to 24 different types of integrins in humans and determine their specificity for ECM molecules (Hynes, 2002). Whereas most of the integrin molecule is located extracellularly, to bind to the ECM, a short cytoplasmic tail binds to adaptor proteins, as discussed below. Although force does not unfold integrins, it can affect them through other mechanisms. First, applied forces can disrupt integrin–ECM bonds. Such rupture forces have been studied extensively for the bonds between fibronectin and α5β1 integrin, and fibrinogen and αIIBβ3 integrin (see Table 1). Given the variety of experimental approaches used, the narrow range of reported unbinding forces (50–100 pN) is remarkable. Integrin–collagen interactions have been far less studied, but the binding strength between the GFOGER binding site in collagen and α2β1 integrin has been reported to be 160 pN (Niland et al., 2011), suggesting that they have a higher resistance to force than integrin–fibronectin adhesions. When rupture forces are measured in live cells containing many integrin–ECM bonds, the calculated force per bond is much lower (below 10 or even 1 pN) than that obtained from single-molecule measurements (Friedland et al., 2009; Roca-Cusachs et al., 2009; Walter et al., 2006). This suggests that in integrin clusters, only a small fraction of molecules withstand forces (Moore et al., 2010).
The bond between fibronectin and α5β1 integrin shows catch bond behavior (i.e. a bond that strengthens when force is applied; see Box 2) in which maximum lifetimes occur at forces of 10–30 pN (Kong et al., 2009). Such catch bond behavior has not been observed for αIIβ3 (Litvinov et al., 2011) or αvβ3 integrin. This could explain the much higher resistance to force of fibronectin–α5β1-integrin bonds compared with those of fibronectin–αvβ3-integrin bonds (Roca-Cusachs et al., 2009) although the latter are required for force sensing. Thus, although stable α5β1 catch bonds can regulate adhesion, more fragile αvβ3 integrin bonds might constitute mechanosensors that undergo frequent unbinding and rebinding events under force, triggering downstream events (Arias-Salgado et al., 2005; von Wichert et al., 2003). In parallel to possible catch bond behaviors, integrins also undergo conformational changes (activation) that prime them for binding to the ECM (Luo et al., 2007; Moser et al., 2009). Activation can also change their affinity for cytoplasmic partners such as α-actinin (Sampath et al., 1998) and catalyze downstream events including tyrosine phosphorylation of different substrates (Legate et al., 2009). Structurally, integrin activation involves separation of all domains except the ECM-binding head, and can be triggered by either ECM binding itself (so-called ‘outside-in activation’) (Kim et al., 2003; Takagi et al., 2002) or binding to its cytoplasmic partner talin (Bouaouina et al., 2008; Calderwood et al., 1999), possibly in combination with kindlin (Moser et al., 2008) (so called ‘inside-out activation’). Integrin activation can also be triggered by force, as shown for α5β1 catch bonds (Friedland et al., 2009) and suggested by results of steered molecular dynamics simulations (Puklin-Faucher et al., 2006). Because integrin-binding partners, such as talin, filamin and α-actinin, bind to the cytoplasmic integrin-β tails (but not α-tails) (Otey et al., 1990; Pfaff et al., 1998), force application from the cytoskeleton through these partner molecules could induce the integrin subunit separation necessary for activation (Puklin-Faucher and Sheetz, 2009). Thus, force-induced integrin activation can also be considered to be a mechanotransduction event.
Adaptor proteins
Integrins, which lack actin-binding domains, are indirectly connected to the actin cytoskeleton through different protein complexes (Fig. 1) that form different types of cell–ECM adhesions, including nascent adhesions, focal complexes, focal adhesions, podosomes and others (Dubash et al., 2009). These complexes can contain over a hundred different types of proteins (Zaidel-Bar et al., 2007a), with many binding in a force-dependent manner (Pasapera et al., 2010; Schiller et al., 2011). Here, we will focus on the path that force must follow from integrins to actin and its effect on the molecules involved.
Fig. 1.
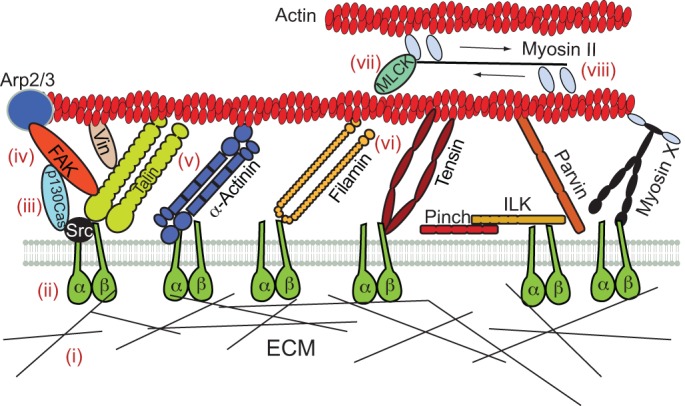
The actin–integrin mechanical connection. Known direct and some indirect connections are shown here. For FAK and p130Cas, possible links connecting them to integrins and actin are shown. Myosin X (MYO10, not discussed in the main text) also binds integrin-β tails. Rather than exerting contractile forces between integrins and actin, however, this myosin binds integrins to transport them to adhesion sites (Zhang et al., 2004). The steps indicated in roman numbers show proposed molecular mechanosensing mechanisms: force could be detected by (i) exposing cryptic domains in ECM molecules, (ii) increasing the lifetime of fibronectin–α5β1-integrin bonds (catch bonds) and activating integrins, (iii) increasing the level of phosphorylation of p130Cas substrate domain, (iv) releasing FAK auto-inhibition and increasing its tyrosine kinase activity, (v) exposing previously buried vinculin-binding sites in talin rod domain, (vi) exposing previously buried binding sites to integrin-β tails or FIP in filamin immunoglobulin-like domains, (vii) releasing MLCK auto-inhibition and activating myosin II, and (viii) increasing the lifetime of myosin-IIA–actin bonds (catch bonds).
The connection between integrin and actin can be mediated by a single molecule, such as talin. Given that the N-terminal head domain of talin binds to and colocalizes with integrin-β tails, and the C-terminal domain binds to and colocalizes with actin (Critchley, 2009; Kanchanawong et al., 2010), talin is ideally positioned in the force path between integrins and actin. Talin-depleted fibroblasts initially spread normally, but then retract and round up instead of developing mature adhesions (Zhang et al., 2008). This behavior is rescued by full-length talin but not by the integrin-binding and -activating talin head alone, suggesting that the force transmitted between integrins and actin by full-length talin has a key role. Indeed, forces of 12 pN can expose cryptic binding sites in talin molecules and cause vinculin binding (del Rio et al., 2009), which might explain why talin is required for adhesion reinforcement and maturation (Jiang et al., 2003; Roca-Cusachs et al., 2009). In cells, talin can stretch cyclically from its folded length of 50 nm to over 400 nm in a vinculin-dependent manner, and the stretching cycles correlate with the lifetime of vinculin in adhesions (Margadant et al., 2011). Thus, vinculin, which has been reported to withstand forces of up to 2.5 pN (Grashoff et al., 2010), might reinforce the mechanical integrin–actin connection by simultaneously binding talin and actin (Burridge and Mangeat, 1984; Menkel et al., 1994). However, the tension level in vinculin molecules has not been directly related to its recruitment to adhesions, and vinculin-null cells have been reported to have both impaired (Diez et al., 2011) and normal (Grashoff et al., 2010) focal adhesions and contractile forces. Therefore, the exact role of vinculin in force transmission and mechanotransduction remains unclear.
Several other molecules can also simultaneously bind to actin and integrins, including α-actinin (Pavalko et al., 1991; Pavalko and LaRoche, 1993), filamin (Hartwig and Stossel, 1975; Loo et al., 1998; Sharma et al., 1995) and tensin (Calderwood et al., 2003; Lo et al., 1994). α-Actinin is an antiparallel dimer and a key actin crosslinker (Sjöblom et al., 2008) that is involved in adhesion maturation (Choi et al., 2008). The crosslinking role of α-actinin is important in adhesions, because normal adhesion elongation is recovered in α-actinin-depleted cells when actin crosslinking is restored through use of a myosin mutant that constitutively binds actin (Choi et al., 2008). However, the transmission of forces between integrins and actin by α-actinin could also have a relevant role. Indeed, inactivating α-actinin in stress fibers leads to their detachment from focal adhesions (Rajfur et al., 2002), and blocking force transmission with exogenous α-actinin fragments that contain only the actin- or the integrin-binding domain, respectively, can lead to the disassembly of stress fibers and focal adhesions (Pavalko and Burridge, 1991; Triplett and Pavalko, 2006). α-Actinin has cryptic sites for binding to the smooth muscle protein smitin within its spectrin-repeat domains (Chi et al., 2005), although these domains might be too stiff to be stretched physiologically (Table 1). Filamin is composed of an actin-binding domain and 24 immunoglobulin-like domains (Popowicz et al., 2006), and also forms dimers that can orthogonally crosslink actin, thus forming three-dimensional meshworks (Flanagan et al., 2001; Hartwig and Shevlin, 1986). This crosslinking activity is crucial for motility, actin architecture, force generation and rigidity sensing (Byfield et al., 2009; Cunningham et al., 1992; Flanagan et al., 2001; Lynch et al., 2011). However, the binding of filamin to integrins is also important, as filamin competes with talin for integrin binding (Kiema et al., 2006), thereby regulating talin-mediated adhesion (Nieves et al., 2010).
Forces of 40–70 pN can both detach filamin from actin and unfold the immunoglobulin-like domains of filamin (Table 1), which contain binding sites to multiple partners. This suggests that different force-loading rates and directions of force application can lead to either unfolding or unbinding, or to unfolding followed by unbinding as proposed for the actin-associated cytoskeletal proteins spectrin and ankyrin in red blood cells (Krieger et al., 2011). Shear strain applied to actin networks crosslinked with filamin increases integrin binding (Ehrlicher et al., 2011), probably by unfolding the 21st immunoglobulin-like domain and exposing a binding site for integrin-β tails (Chen et al., 2009; Pentikäinen and Ylänne, 2009). However, strain also reduces the binding of filamin-A-associated RhoGAP (FILGAP, also known as Rho GTPase-activating protein 24) (Ehrlicher et al., 2011), possibly by separating the two FILGAP-binding domains present in the filamin dimer. This, therefore, is an elegant mechanism by which a single mechanical stimulus could regulate the binding of two proteins in opposite ways. Finally, tensin also forms dimers that have multiple actin-binding sites and a domain that binds to the NPXY motifs on integrin-β tails (Lo, 2004). In fibroblasts, colocalization of tensin with integrins is much more prominent in mature fibrillar adhesions than in early focal contacts (Zamir et al., 2000), pointing towards a role in adhesion maturation. Tensin localization also requires talin and integrin-linked kinase (ILK) in Drosophila (Torgler et al., 2004), suggesting that there are interactions between the three molecules. Expression of a tensin fragment containing the actin homology 2 region can inhibit adhesion formation and the normal rearward translocation of α5β1 integrins (Pankov et al., 2000), indicating that tensin normally transmits force from the cytoskeleton to integrins and is necessary for adhesion maturation. Such a mechanical function is further confirmed by the fact that the defects observed in tensin-null Drosophila (i.e. wing blisters) only appear after mechanical stimulation (Torgler et al., 2004). However, no information is currently available on the mechanical properties of tensin or its molecular interactions.
Links formed by more than one molecule can also connect integrins to actin. For example, ILK binds to integrin-β tails and connects to actin through an interaction with parvin, forming the ILK–pinch–parvin (IPP) complex (Wickström et al., 2010). This complex regulates cell contractility (Wickström et al., 2010) and mechanosensation in touch neurons (Hobert et al., 1999). Kindlin participates in integrin activation (Moser et al., 2008) and could connect to filamin through migfilin (Tu et al., 2003). Given the high degree of complexity in the protein interaction networks in focal adhesions (Zaidel-Bar et al., 2007a), several additional paths connecting actin to integrins are possible. Force transmitted through these paths could be sensed by mechanosensory proteins, because several proteins change enzyme activity or become phosphorylated in response to force (Moore et al., 2010). Several proteins are also recruited by myosin II contractility, including FAK, zyxin, vinculin, α-actinin and LIM proteins such as pinch, zyxin, paxillin and migfilin (Pasapera et al., 2010; Schiller et al., 2011). However, which of the molecules affected by force are true mechanosensors (i.e. they respond directly to forces) and which simply act downstream, remains unclear. For instance, LIM proteins are likely to bind to exposed sites in mechanonsensing proteins but are not mechanosensors themselves (Schiller et al., 2011). Some insight into this question has been obtained by stretching of membrane-stripped cytoskeletons, which should eliminate many downstream biochemical pathways present in live cells. Cytoskeletal stretching leads to increased cytoskeletal binding of paxillin, focal adhesion kinase (FAK), p130Cas (also known as BCAR1), protein kinase B (Akt), Rap guanine nucleotide exchange factor 1 (known as C3G, RAPGEF1 and GRF2) and Crk isoform II (Sawada and Sheetz, 2002; Tamada et al., 2004). Despite these observations, direct evidence for mechanosensing in individual molecules is scarce. Mechanical stretching of the substrate domain of p130Cas increases its phosphorylation by Src family kinases in vitro, enabling the binding of several proteins (Sawada et al., 2006). p130Cas is thus one of the very few demonstrated mechanosensors, although the molecular links through which it would be stretched in cells are unclear. Force application increases the tyrosine kinase activity of FAK (Tomar and Schlaepfer, 2009), possibly by disrupting an inhibitory interaction between its FERM and kinase domains (Cooper et al., 2003). FAK connects indirectly to integrins and actin, through proteins such as talin (Chen et al., 1995), paxillin (Hildebrand et al., 1995), or Arp3 (Serrels et al., 2007). In summary, integrins can be connected to actin through one or more molecules, but, with the exception of talin, it remains unclear which of them experience and respond to forces in vivo.
Myosin
The chain of forces goes from matrix to integrins to adaptor proteins bound to actin, and the actin filament network transmits forces exerted by myosin motors. Myosin I detachment from actin filaments is decreased by up to 75-fold under forces greater than 2 pN, in a mechanism that could contribute to mechanosensing in hair cells (Laakso et al., 2008). Similarly, the myosin-II–actin interaction behaves like a catch bond, with an optimal load of 6 pN (Guo and Guilford, 2006). This is also the maximum force that the motor can exert, indicating that the motor is designed to hold for the longest time at maximal force. External forces might, thus, optimize myosin II binding and promote further force generation by the cell. Additionally, myosin light chain kinase (MLCK) binds both myosin and actin (Hatch et al., 2001; Sellers and Pato, 1984; Yang et al., 2006; Ye et al., 1997) and moves rearward with actin during cell spreading (Giannone et al., 2004). MLCK could therefore be stretched between myosin and rearward flowing actin, resulting in the release of MLCK auto-inhibition and in myosin II activation. Mechanoactivation has indeed already been observed for another member of the MLCK family, the kinase titin, which is unfolded by 10 nm under forces of 30 pN (Gautel, 2011; Puchner et al., 2008).
Alternative pathways
Integrins and the cytoskeleton can also transmit forces without involving an integrin–actin link. For instance, increases in membrane tension that result from actin polymerization can activate contractility and exocytosis (Gauthier et al., 2011), or regulate the activity of stretch-sensitive ion channels (Sbrana et al., 2008). Mechanosensitive ion channels also associate with β1 integrins (Chao et al., 2011), and might be activated by forces transmitted through them (Matthews et al., 2010). Force activation of these channels leads to adhesion reinforcement, and affects migration, contractility and recruitment of vinculin (Matthews et al., 2006; Munevar et al., 2004). Besides ion channels, membrane tension (or ECM deformation) might regulate integrin spacing, thereby stretching integrin crosslinking proteins such as receptor-type tyrosine-protein phosphatase alpha (RPTP-α), which can expose a catalytic cleft leading to the activation of Src family kinases (Jiang et al., 2000; von Wichert et al., 2003).
Besides actin, intermediate filaments and microtubules also have a role in force transmission, which is much less explored. The intermediate filament protein vimentin localizes to adhesion sites, and its depletion reduces cell contractility, adhesion size and migration (Eckes et al., 1998; Tsuruta and Jones, 2003). Furthermore, vimentin regulates cell extensions through its interaction with filamin (Kim et al., 2010), depolymerizes upon myosin II inhibition (Johnson et al., 2007), colocalizes and co-precipitates with α2β1 integrins (Kreis et al., 2005), and is required for proper β1 integrin localization at adhesions (Ivaska et al., 2005). Microtubules are also targeted to early adhesion sites (Kaverina et al., 1998), although their depolymerization leads to formation of stress fibers and focal adhesions (Kaverina et al., 1998; Liu et al., 1998), and increased contractility (Stamenović et al., 2002). This result has led to the hypothesis that microtubules normally carry contractile compressive stresses, but it could also be explained by there being increased myosin contractility upon microtubule depolymerization (Kolodney and Elson, 1995). Thus, although cellular forces are mostly generated by actomyosin contractility (Cai et al., 2006), microtubules and intermediate filaments could transmit mechanical forces in special cases.
Putting it all together – integrin clustering
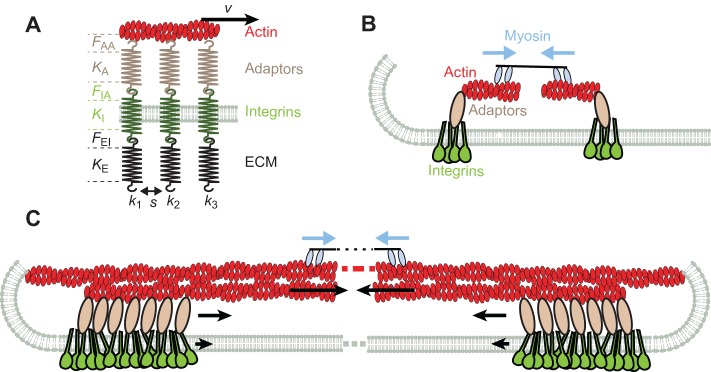
In summary, forces affect a complex series of molecular links that connect the ECM to the actin cytoskeleton. These links can operate in parallel, given that many different proteins can simultaneously support forces (Fig. 2A). The resulting collective behavior naturally depends on the rate of force loading and cannot directly be predicted from individual bond properties. At low loading rates, for instance, when a bond breaks, the force redistributes to the remaining bonds, leading to a faster detachment. The average detachment force per bond can then be smaller in a cluster than in an individual bond (Seifert, 2000). At faster loading rates, rupture is too fast and this effect is not important (Sulchek et al., 2005). However, in live cells, maximum force per bond increases sixfold in pentameric versus monomeric fibronectin–α5β1 bonds (Roca-Cusachs et al., 2009), indicating that, beyond physical factors, protein complexes in integrin clusters stabilize mechanical links. Indeed, integrin cluster formation involves integrin interactions with kindlin, talin and phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P2] (Moser et al., 2009; Saltel et al., 2009), as well as other integrins (Li et al., 2003b). These interactions require a spacing that is smaller than 60 nm because larger distances between integrins in nanopatterned substrates do not sustain cell adhesion (Arnold et al., 2008; Jiang et al., 2003; Schvartzman et al., 2011).
Fig. 2.
Summary of mechanical factors in integrin adhesions. (A) Role of stiffness. Whether and where a single connection between integrins and actin unfolds or breaks under force will depend on the stiffness of the ECM molecule (KE), integrin (KI), and adaptor protein (KA), and the maximum force that can be withstood by the ECM–integrin bond (FEI), the integrin–adaptor bond (FIA) and the adaptor–actin bond (FAA). These stiffness values will generally increase with protein stretching, and change after unfolding or unbinding events. To our knowledge, integrin stiffness (KI) or possible unfolding have not been described. The total stiffness of the chain (k1) will be determined by the softest element in it, and will operate in parallel with other chains in the cluster (k2, k3). The rearward speed v of the actin layer, multiplied by k1, will provide the rate of force loading in the chain, which will affect both individual rupture forces (FEI, FIA, and FAA) and the effectiveness with which the different chains in the cluster cooperate to withstand force. The spacing between integrins (s) will also affect cluster formation. (B) Nascent focal adhesions. In nascent adhesions, adaptor proteins mediate the formation of initial integrin clusters {containing talin, kindlin and PtdIns(4,5)P2}, and might promote actin polymerization from clusters (formins). Myosin II filaments exert local contractile forces between filaments (blue arrows). (C) Focal adhesion maturation. As adhesions mature and grow, they connect to actin fibers moving rearward towards the cell center due to global myosin contractility (blue arrows). Rearward moving actin pulls on bound adaptor proteins, which in turn pull on integrins. Because of transient stick-slip bonds, rearward speeds are reduced with respect to actin to a certain extent in adaptor proteins, and even further in integrins (black arrows).
Once formed, actin filaments grow from clusters, and contractile forces between them bring them together (Rossier et al., 2010; Yu et al., 2011; Ghassemi et al., 2012) (Fig. 2B). This requires a link between adhesion sites and polymerizing actin that could be provided by formins. Formins are found on dorsal stress fibers that emanate from focal adhesions and regulate the distribution of focal adhesions at the lamellum–lamellipodium interface, as well as focal adhesion turnover (Gupton et al., 2007; Hotulainen and Lappalainen, 2006). Formins elongate filament barbed ends at rates of up to 27 nm/second for the formin mDia2 or 120 nm/second for mDia1, as measured in vitro (Chesarone and Goode, 2009; Kovar et al., 2006). These rates are well within the range of rearward flows of actin in vivo (see below). Supporting this hypothesis, formin-mediated polymerization of single actin filaments has been measured to exert forces greater than 1 pN (Kovar and Pollard, 2004). Thus, actin polymerization from clustered integrins could generate forces on adhesions, but details of the interactions need to be worked out. What is clear, however, is that the mechanical behavior of adhesion molecules depends not only on their individual properties but also on their density and spacing in clusters, their interactions and the force loading rate.
Rearward flow and loading rate
Although contractile forces pull initial integrin clusters towards each other, once clusters grow and mature, force is applied mainly by actin flowing rearward from the leading edge to the center of cells (Fig. 2C). External stimuli can also apply forces through the ECM, which can be transmitted to distant points across a cell (Hu et al., 2003), activating factors such as Src (Na et al., 2008) or Rac (Poh et al., 2009). However, endogenous rearward flow appears to regulate adhesion maturation and traction force generation. This flow is mainly driven by myosin II contractility because inhibition of myosin decreases the velocity of actin flow by over two thirds (Cai et al., 2006) and leads to unbalanced actin polymerization forces that dramatically increase membrane tension (Gauthier et al., 2011).
In a variety of cell types and depending on the location within the cell, rearward actin flow is in the range of 2 to 600 nm/second (Fisher et al., 1988; Forscher and Smith, 1988; Gardel et al., 2008; Theriot and Mitchison, 1991). Generally, higher flows are associated with weak or nascent adhesions and low traction forces on the substrate, and vice versa (Chan and Odde, 2008; Kress et al., 2007; Zhang et al., 2008). This suggests that high actin speeds lead to high force loading rates that decrease bond lifetimes (Box 2) and therefore the ability to transmit force from actin to adaptor proteins and integrins (Li et al., 2010). This effect of loading rate would no longer be relevant at speeds below 10 nm/second, at which point the relationship between rearward flow and traction force becomes direct rather than inverse (Gardel et al., 2008). Given the transient nature of the mechanical links, actin flow is coupled best to actin-binding proteins, such as talin and particularly α-actinin, which also move rearward at a speed of ∼5 nm/second, less efficiently to FAK, paxillin or vinculin, and even less efficiently (∼1.3 nm/second) to substrate-bound αv integrins (Hu et al., 2007). The efficiency of this transmission also depends on the ligand density in the ECM (Brown et al., 2006), showing that molecular clustering is a determinant of this process. Thus, in mature adhesions, transient stick–slip bonds seem to couple integrins to adaptor proteins and to the actin cytoskeleton (Margadant et al., 2011).
The rearward speeds of proteins, multiplied by their stiffness (i.e. the ratio between applied force and resulting molecular extension), will determine force-loading rates and thus bond lifetimes (Box 2). Protein stiffness increases as molecules are extended, often following a worm-like chain behavior (Marko and Siggia, 1995), but constant stiffness values can be approximated for small extensions (Table 1). In a serial connection between an ECM molecule, an integrin and an adaptor protein, the total stiffness would be dominated by the softer elements (Fig. 2A). For instance, if fibronectin (0.5 pN/nm) is linked to talin (1.5 pN/nm), both elements are soft and would probably extend and possibly unfold, enabling mechanosensing events. If fibronectin is linked to α-actinin (1700 pN/nm), however, only fibronectin would extend, with α-actinin simply transmitting the force. This difference could explain the reported mechanosensitive unfolding of talin (del Rio et al., 2009) but not of α-actinin. Indeed, talin molecules in adhesions continually extend and relax in concert with the concomitant binding and release of vinculin (Margadant et al., 2011). However, the timescales of talin stretch–relax cycles (6–16 seconds) do not match those of traction forces (30–90 seconds) (Ghassemi et al., 2012). This suggests that actin flow might transmit forces through stiff molecules such as α-actinin, while signaling by repeatedly stretching talin, then unbinding from it, and finally rebinding again. At a loading rate of 2.5 pN/second (0.5 pN/nm and retraction speed of 5 nm/second), for instance, a stretch cycle of 16 seconds would build a force of 40 pN, sufficient to unfold talin and trigger mechanotransduction (Table 1). Given the high retraction speeds used in talin unfolding experiments (400 nm/second, Table 1), in vivo unfolding forces would however be much lower. Thus, it remains unclear whether unfolding or unbinding from actin (measured at 2 pN at 60 nm/second, Table 1) would dominate. The interaction between these two events would define the signaling effectiveness of talin stick–slip cycles. This concept of stick–slip stretching of molecules as a primary force signaling mechanism has yet to be tested in various systems, but it could explain how the cell ‘samples’ adhesions over time and generates integrated signals to the nucleus or other parts of the cell.
Downstream signaling
Downstream signaling from molecular mechanosensing events is mediated by phosphorylation, dephosphorylation, small GTPase signaling and phospholipid signaling, among others (Fig. 3). Whereas the ensuing signaling networks are vast, we will discuss here examples of known signaling cascades that emanate from the specific mechanosensing events discussed above.
Fig. 3.
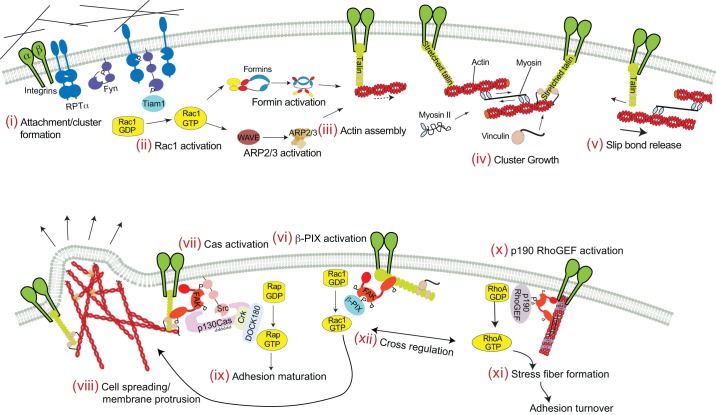
Schematic illustration of the signaling processes downstream of force dectection. The upper panel shows RPTP-α-, Fyn- and talin-dependent signaling events. (i) Cell attachment, integrin clustering and deformation of the extracellular matrix result in RPTP-α activation. This allows the SFK Fyn to access the catalytic site. Fyn is dephosphorylated at tyrosine 531 and switches to the open conformation. (ii) Fyn subsequently phosphorylates Rac1 GEFs, such as Tiam1, that in turn activate Rac1. (iii) Rac1 activity induces actin polymerization from integrin adhesion sites by formin family proteins and/or the ARP2/3 complex. (iv) Rearward actin flow driven by myosin II contractility stretches talin to expose cryptic vinculin-binding sites, which leads to adhesion strengthening and cluster growth. (v) When high forces are generated by actin rearward flow and contractility, the bond between actin and talin slips and talin relaxes. The lower panel depicts FAK- and p130Cas-dependent downstream signaling. (vi) Downstream of integrin clustering, FAK is autophosphorylated at tyrosine 397. Phosphorylated FAK binds to talin and phosphorylates the Rac1 GEF β-PIX (encoded by ARHGEF7) (Karasawa et al., 1982). (vii) FAK also forms a complex with Src to activate p130Cas. p130Cas is hyperphosphorylated and binds to Crk and DOCK180 to activate small Rho GTPases, such as Rap. (viii) Rac1 induces actin polymerization and cell protrusion, whereas Rap enhances adhesion maturation (ix). (x) Additionally, FAK can activate p190GEF (Lim et al., 2008b) to enhance RhoA activity, stress fiber formation (xi) and adhesion turnover. (xii) Cross regulation can alter the region of activity of Rho GTPases.
RPTP-α
The mechanically activated receptor-type tyrosine-protein phosphatase alpha (RPTP-α, encoded by PTPRA) transmits the mechanical signal to Src family kinases (SFKs), which are activated by dephosphorylation (Jiang et al., 2006; Roskoski, 2005; von Wichert et al., 2003; Ylinen, 1996; Zheng et al., 2000). Alternatively, clustering of integrins could also directly activate SFKs (Arias-Salgado et al., 2003; Yu et al., 2011). SFKs then phosphorylate tyrosine residues on Rho GTP exchange factors, such as leukemia-associated Rho GTPase exchange factor (LARG, also known as Rho GEF factor 12) or Tiam1 (Elias et al., 2010; Guilluy et al., 2011), resulting in Rho GTPase activation and cytoskeletal reorganization (Majumdar et al., 1999; Nobes and Hall, 1995; Rottner et al., 1999). During adhesion formation in fibroblasts, RPTP-α also interacts with αvβ3 integrins to activate the SFK Fyn (Kostic and Sheetz, 2006; Su et al., 1999; von Wichert et al., 2003). Although the GTP exchange factors (GEFs) remain unidentified, two Rho GTPases (Rac1 and Cdc42) are activated downstream of RPTP-α, and they are known to regulate actin dynamics and focal adhesion turnover during fibroblast spreading (Arthur and Burridge, 2001; Herrera Abreu et al., 2008; Jones and Katan, 2007; ten Klooster et al., 2006). Rho GTPases can also regulate the region of activity of other Rho GTPases to determine cell morphology (Vega et al., 2011), activate the ARP2/3 complex (Welch, 1999) or directly activate formins. In addition, formins can pass on mechanical signals to the nucleus to induce expression of genes that are under the control of the serum response element (Young and Copeland, 2010). In some, if not most, of these cases, there appears to be crosstalk between different small G proteins (Machacek et al., 2009).
FAK
Initially identified as a Src kinase substrate (Schaller et al., 1992; Weiner et al., 1993), the tyrosine kinase FAK is a pivotal signaling molecule for focal adhesion dynamics, and acts as both a direct mechanosensor, as discussed above, and downstream of mechanical signaling events (Schober et al., 2007; Wang et al., 2001). FAK activation is initiated by autophosphorylation at tyrosine 397, which can be induced by integrin clustering or binding of tyrosine-phosphorylated peptides (Chen and Chen, 2006; Sieg et al., 1999; Tomar and Schlaepfer, 2009). FAK can also activate integrins, resulting in adhesion strengthening (Michael et al., 2009). The activated FAK alone – or in complex with Src – can then phosphorylate target proteins or act as scaffold to recruit additional SH2-binding proteins to adhesions. Central to its function as regulator of adhesion dynamics, FAK phosphorylates both GEFs and GAPs to modulate the activity of Rho GTPases (Fig. 3) (see Tomar and Schlaepfer, 2009). FAK also phosphorylates paxillin, thereby enhancing focal adhesion turnover (Zaidel-Bar et al., 2007b), and binding of FAK in a complex with Src to p130Cas results in Cas activation (Fonseca et al., 2004).
p130Cas
Cas family proteins are hyperphosphorylated downstream of FAK and SFKs, in a manner that, at least in the case of p130Cas, is enhanced by mechanical stretching (Cary et al., 1998; Sawada et al., 2006; Tamada et al., 2004). During cell migration, phosphorylation of p130Cas at tyrosine 249 acts as a switch for the formation of a complex with Crk and the Rac1 GEF DOCK180 (also known as DOCK1). This complex regulates Rac1 activity and cytoskeletal dynamics (Brugnera et al., 2002; Gustavsson et al., 2004; Webb et al., 2004). Additionally, p130Cas associates with the GEF AND34 (also known as BCAR3) and regulates the activity of the small GTPases Ral, Rap1 and R-Ras in cell motility (Gotoh et al., 2000; Riggins et al., 2003).
Other post-translational modifications
Posttranslational modifications other than phosphorylation are relevant for many cytoskeletal and adhesion proteins, and could be involved in mechanosensing. Acetylation of α-tubulin stabilizes microtubules, which can regulate vesicular trafficking, autophagy or cell motility (Hubbert et al., 2002; Perdiz et al., 2011; Tran et al., 2007). The addition of arginyl from tRNA onto the N-terminus of proteins has been detected in vivo on cytoskeletal proteins including α- and β-actin, myosin and tubulin (Karakozova et al., 2006; Wong et al., 2007). In actin, arginylation changes the interaction dynamics with several binding partners and alters the polymerization properties of the proteins (Karakozova et al., 2006; Saha et al., 2010). Modification of proteins with fatty acid or prenyl residues assists localization to cellular structures and is found on cytoskeletal and adhesion proteins, such as vinculin or several members of the formin family (Han et al., 2009; Harris et al., 2010; Kellie and Wigglesworth, 1987; Resh, 2006).
Nucleocytoplasmic O-GlcNAcylation (i.e. addition of O-linked N-acetylglucosamine) is especially interesting owing to its extensive crosstalk with phosphorylation, as both modifications frequently compete for the same serine or threonine residues (Wang et al., 2007; Wang et al., 2008; Wang et al., 2010). The differences in size and charge between these modifications can alter protein function and stability (Chen et al., 2006; Liang et al., 2006). Several adhesion and actin-binding proteins, such as talin, actin-capping protein, actin-binding LIM proteins (Chalkley et al., 2009; Hagmann et al., 1992; Wang et al., 2010; Zhu et al., 2001) and striated muscle isoforms of actin and myosin (Hédou et al., 2009; Ramirez-Correa et al., 2008) are subjected to O-GlcNAcylation, which might modulate properties of the cytoskeleton, or alter muscle contractility (Cieniewski-Bernard et al., 2009). Increased O-GlcNAcylation is a cellular response to various stress-inducing agents, whereas heat-shock proteins can associate with the glycan to protect the modified proteins (Lefebvre et al., 2001; Zachara et al., 2004). O-GlcNAcylation of adhesion and cytoskeletal proteins could therefore also constitute a protective mechanism against tensile stress. Post-translational modifications such as O-GlcNAcylation can thus be expected to receive further attention, particularly as its deregulation has been correlated with the occurrence of cancer metastasis and invasiveness (Gu et al., 2010; Perdiz et al., 2011).
Conclusions
Despite many recent advances, we still lack a clear picture of the molecular mechanical pathways that transmit and detect forces in adhesions, upstream of biochemical signaling. Simple biophysical considerations regarding molecular stiffness and binding forces (Table 1) can help us to identify probable mechanosensors in soft compared with stiff environments, and separate them from molecules that simply transmit forces. Nevertheless, a systematic analysis of adhesion protein binding and unfolding under physiological pulling rates is required to clarify where and when bonds break and proteins unfold in the network of links that are connected in series from the ECM to actin, and in parallel in integrin clusters. This seems even more pressing after considering that common pulling rates in single-molecule experiments (102–104 nm/second, Table 1) are much higher than physiological rates (1–1×102 nm/second). Such an analysis might unravel simple but elegant biophysical mechanisms; for instance, regulation of the rearward speed of cytoskeletal components and the loading rates might trigger different mechanotransduction cascades by changing the relative resistance to unbinding and unfolding of different proteins. Differences in loading rates and force levels could also help to explain the different behaviors characteristic of fast events, such as initial adhesion formation, as compared to slower tissue or ECM remodeling. Only when this biophysical picture is complete, will we be able to reliably and mechanistically explain how the biophysical inputs (i.e. forces) control the biological outputs of biochemical signaling and effector pathways that comprise cell behaviors.
Acknowledgments
The authors thank Simon Moore for helpful discussions.
Footnotes
Funding
The work of our laboratory was supported in part by a grant by the Spanish Ministry of Economy and Competitiveness [grant number BFU2011-23111].
This article is part of a Minifocus on Mechanotransduction. For further reading, please see related articles: ‘Deconstructing the third dimension – how 3D culture microenvironments alter cellular cues’ by Brendon M. Baker and Christopher S. Chen (J. Cell Sci. 125, 3015-3024). ‘Signalling through mechanical inputs – a coordinated process’ by Huimin Zhang and Michel Labouesse (J. Cell Sci. 125, 3039-3049). ‘United we stand – integrating the actin cytoskeleton and cell–matrix adhesions in cellular mechanotransduction’ by Ulrich S. Schwarz and Margaret L. Gardel (J. Cell Sci. 125, 3051-3060). ‘Mechanosensitive mechanisms in transcriptional regulation’ by Akiko Mammoto et al. (J. Cell Sci. 125, 3061-3073). ‘Molecular force transduction by ion channels – diversity and unifying principles’ by Sergei Sukharev and Frederick Sachs (J. Cell Sci. 125, 3075-3083).
References
- Agnihotri A., Soman P., Siedlecki C. A. (2009). AFM measurements of interactions between the platelet integrin receptor GPIIbIIIa and fibrinogen. Colloids Surf. B Biointerfaces 71, 138–147. 10.1016/j.colsurfb.2009.01.019 [DOI] [PubMed] [Google Scholar]
- Alonso M. C., Drummond D. R., Kain S., Hoeng J., Amos L., Cross R. A. (2007). An ATP gate controls tubulin binding by the tethered head of kinesin-1. Science 316, 120–123. 10.1126/science.1136985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias–Salgado E. G., Lizano S., Sarkar S., Brugge J. S., Ginsberg M. H., Shattil S. J. (2003). Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc. Natl. Acad. Sci. USA 100, 13298–13302. 10.1073/pnas.2336149100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias–Salgado E. G., Lizano S., Shattil S. J., Ginsberg M. H. (2005). Specification of the direction of adhesive signaling by the integrin beta cytoplasmic domain. J. Biol. Chem. 280, 29699–29707. 10.1074/jbc.M503508200 [DOI] [PubMed] [Google Scholar]
- Arnold M., Hirschfeld–Warneken V. C., Lohmüller T., Heil P., Blümmel J., Cavalcanti–Adam E. A., López–García M., Walther P., Kessler H., Geiger B.et al. (2008). Induction of cell polarization and migration by a gradient of nanoscale variations in adhesive ligand spacing. Nano Lett. 8, 2063–2069. 10.1021/nl801483w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur W. T., Burridge K. (2001). RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol. Biol. Cell 12, 2711–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averett L. E., Schoenfisch M. H., Akhremitchev B. B., Gorkun O. V. (2009). Kinetics of the multistep rupture of fibrin ‘A-a’ polymerization interactions measured using atomic force microscopy. Biophys. J. 97, 2820–2828. 10.1016/j.bpj.2009.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban N. Q., Schwarz U. S., Riveline D., Goichberg P., Tzur G., Sabanay I., Mahalu D., Safran S., Bershadsky A., Addadi L.et al. (2001). Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3, 466–472. 10.1038/35074532 [DOI] [PubMed] [Google Scholar]
- Baneyx G., Baugh L., Vogel V. (2001). Coexisting conformations of fibronectin in cell culture imaged using fluorescence resonance energy transfer. Proc. Natl. Acad. Sci. USA 98, 14464–14468. 10.1073/pnas.251422998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baneyx G., Baugh L., Vogel V. (2002). Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc. Natl. Acad. Sci. USA 99, 5139–5143. 10.1073/pnas.072650799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I. (1978). Models for the specific adhesion of cells to cells. Science 200, 618–627. 10.1126/science.347575 [DOI] [PubMed] [Google Scholar]
- Bershadsky A. D., Balaban N. Q., Geiger B. (2003). Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 19, 677–695. 10.1146/annurev.cellbio.19.111301.153011 [DOI] [PubMed] [Google Scholar]
- Bouaouina M., Lad Y., Calderwood D. A. (2008). The N-terminal domains of talin cooperate with the phosphotyrosine binding-like domain to activate beta1 and beta3 integrins. J. Biol. Chem. 283, 6118–6125. 10.1074/jbc.M709527200 [DOI] [PubMed] [Google Scholar]
- Brown A. E., Litvinov R. I., Discher D. E., Weisel J. W. (2007). Forced unfolding of coiled-coils in fibrinogen by single-molecule AFM. Biophys. J. 92, L39–L41. 10.1529/biophysj.106.101261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. E., Litvinov R. I., Discher D. E., Purohit P. K., Weisel J. W. (2009). Multiscale mechanics of fibrin polymer: gel stretching with protein unfolding and loss of water. Science 325, 741–744. 10.1126/science.1172484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. M., Hebert B., Kolin D. L., Zareno J., Whitmore L., Horwitz A. R., Wiseman P. W. (2006). Probing the integrin-actin linkage using high-resolution protein velocity mapping. J. Cell Sci. 119, 5204–5214. 10.1242/jcs.03321 [DOI] [PubMed] [Google Scholar]
- Brugnera E., Haney L., Grimsley C., Lu M., Walk S. F., Tosello–Trampont A. C., Macara I. G., Madhani H., Fink G. R., Ravichandran K. S. (2002). Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat. Cell Biol. 4, 574–582. [DOI] [PubMed] [Google Scholar]
- Burridge K., Mangeat P. (1984). An interaction between vinculin and talin. Nature 308, 744–746. 10.1038/308744a0 [DOI] [PubMed] [Google Scholar]
- Bustamante C., Chemla Y. R., Forde N. R., Izhaky D. (2004). Mechanical processes in biochemistry. Annu. Rev. Biochem. 73, 705–748. 10.1146/annurev.biochem.72.121801.161542 [DOI] [PubMed] [Google Scholar]
- Byfield F. J., Wen Q., Levental I., Nordstrom K., Arratia P. E., Miller R. T., Janmey P. A. (2009). Absence of filamin A prevents cells from responding to stiffness gradients on gels coated with collagen but not fibronectin. Biophys. J. 96, 5095–5102. 10.1016/j.bpj.2009.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y. F., Biais N., Giannone G., Tanase M., Jiang G. Y., Hofman J. M., Wiggins C. H., Silberzan P., Buguin A., Ladoux B.et al. (2006). Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophys. J. 91, 3907–3920. 10.1529/biophysj.106.084806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood D. A., Zent R., Grant R., Rees D. J., Hynes R. O., Ginsberg M. H. (1999). The Talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J. Biol. Chem. 274, 28071–28074. 10.1074/jbc.274.40.28071 [DOI] [PubMed] [Google Scholar]
- Calderwood D. A., Fujioka Y., de Pereda J. M., García–Alvarez B., Nakamoto T., Margolis B., McGlade C. J., Liddington R. C., Ginsberg M. H. (2003). Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc. Natl. Acad. Sci. USA 100, 2272–2277. 10.1073/pnas.262791999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary L. A., Han D. C., Polte T. R., Hanks S. K., Guan J. L. (1998). Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J. Cell Biol. 140, 211–221. 10.1083/jcb.140.1.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkley R. J., Thalhammer A., Schoepfer R., Burlingame A. L. (2009). Identification of protein O-GlcNAcylation sites using electron transfer dissociation mass spectrometry on native peptides. Proc. Natl. Acad. Sci. USA 106, 8894–8899. 10.1073/pnas.0900288106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. E., Odde D. J. (2008). Traction dynamics of filopodia on compliant substrates. Science 322, 1687–1691. 10.1126/science.1163595 [DOI] [PubMed] [Google Scholar]
- Chao J. T., Gui P., Zamponi G. W., Davis G. E., Davis M. J. (2011). Spatial association of the Cav1.2 calcium channel with alpha5beta1-integrin. Am. J. Physiol. Cell Physiol. 300, C477–C489. 10.1152/ajpcell.00171.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Fu H., Zhu X., Cong P., Nakamura F., Yan J. (2011). Improved high-force magnetic tweezers for stretching and refolding of proteins and short DNA. Biophys. J. 100, 517–523. 10.1016/j.bpj.2010.12.3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. C., Appeddu P. A., Parsons J. T., Hildebrand J. D., Schaller M. D., Guan J. L. (1995). Interaction of focal adhesion kinase with cytoskeletal protein talin. J. Biol. Chem. 270, 16995–16999. 10.1074/jbc.270.28.16995 [DOI] [PubMed] [Google Scholar]
- Chen H. S., Kolahi K. S., Mofrad M. R. (2009). Phosphorylation facilitates the integrin binding of filamin under force. Biophys. J. 97, 3095–3104. 10.1016/j.bpj.2009.08.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. Y., Chen H. C. (2006). Direct interaction of focal adhesion kinase (FAK) with Met is required for FAK to promote hepatocyte growth factor-induced cell invasion. Mol. Cell. Biol. 26, 5155–5167. 10.1128/MCB.02186-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. X., Du J. T., Zhou L. X., Liu X. H., Zhao Y. F., Nakanishi H., Li Y. M. (2006). Alternative O-GlcNAcylation/O-phosphorylation of Ser16 induce different conformational disturbances to the N terminus of murine estrogen receptor beta. Chem. Biol. 13, 937–944. 10.1016/j.chembiol.2006.06.017 [DOI] [PubMed] [Google Scholar]
- Chesarone M. A., Goode B. L. (2009). Actin nucleation and elongation factors: mechanisms and interplay. Curr. Opin. Cell Biol. 21, 28–37. 10.1016/j.ceb.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi R. J., Olenych S. G., Kim K., Keller T. C., 3rd (2005). Smooth muscle alpha-actinin interaction with smitin. Int. J. Biochem. Cell Biol. 37, 1470–1482. 10.1016/j.biocel.2005.02.014 [DOI] [PubMed] [Google Scholar]
- Choi C. K., Vicente–Manzanares M., Zareno J., Whitmore L. A., Mogilner A., Horwitz A. R. (2008). Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat. Cell Biol. 10, 1039–1050. 10.1038/ncb1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieniewski–Bernard C., Montel V., Stevens L., Bastide B. (2009). O-GlcNAcylation, an original modulator of contractile activity in striated muscle. J. Muscle Res. Cell Motil. 30, 281–287. 10.1007/s10974-010-9201-1 [DOI] [PubMed] [Google Scholar]
- Cooper L. A., Shen T. L., Guan J. L. (2003). Regulation of focal adhesion kinase by its amino-terminal domain through an autoinhibitory interaction. Mol. Cell. Biol. 23, 8030–8041. 10.1128/MCB.23.22.8030-8041.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa P., Parsons M. (2010). New insights into the dynamics of cell adhesions. Int Rev Cell Mol Biol 283, 57–91. 10.1016/S1937-6448(10)83002-3 [DOI] [PubMed] [Google Scholar]
- Critchley D. R. (2009). Biochemical and structural properties of the integrin-associated cytoskeletal protein talin. Annu Rev Biophys 38, 235–254. 10.1146/annurev.biophys.050708.133744 [DOI] [PubMed] [Google Scholar]
- Cunningham C. C., Gorlin J. B., Kwiatkowski D. J., Hartwig J. H., Janmey P. A., Byers H. R., Stossel T. P. (1992). Actin-binding protein requirement for cortical stability and efficient locomotion. Science 255, 325–327. 10.1126/science.1549777 [DOI] [PubMed] [Google Scholar]
- Davis G. E. (1992). Affinity of integrins for damaged extracellular matrix: alpha v beta 3 binds to denatured collagen type I through RGD sites. Biochem. Biophys. Res. Commun. 182, 1025–1031. 10.1016/0006-291X(92)91834-D [DOI] [PubMed] [Google Scholar]
- Davis G. E., Bayless K. J., Davis M. J., Meininger G. A. (2000). Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am. J. Pathol. 156, 1489–1498. 10.1016/S0002-9440(10)65020-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio A., Perez–Jimenez R., Liu R. C., Roca–Cusachs P., Fernandez J. M., Sheetz M. P. (2009). Stretching single talin rod molecules activates vinculin binding. Science 323, 638–641. 10.1126/science.1162912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z. J., Liang M., Monteiro M., Toth I., Minchin R. F. (2011). Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat. Nanotechnol. 6, 39–44. 10.1038/nnano.2010.250 [DOI] [PubMed] [Google Scholar]
- Diez G., Auernheimer V., Fabry B., Goldmann W. H. (2011). Head/tail interaction of vinculin influences cell mechanical behavior. Biochem. Biophys. Res. Commun. 406, 85–88. 10.1016/j.bbrc.2011.01.115 [DOI] [PubMed] [Google Scholar]
- Dogterom M., Yurke B. (1997). Measurement of the force-velocity relation for growing microtubules. Science 278, 856–860. 10.1126/science.278.5339.856 [DOI] [PubMed] [Google Scholar]
- du Roure O., Saez A., Buguin A., Austin R. H., Chavrier P., Silberzan P., Ladoux B. (2005). Force mapping in epithelial cell migration. Proc. Natl. Acad. Sci. USA 102, 2390–2395. 10.1073/pnas.0408482102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubash A. D., Menold M. M., Samson T., Boulter E., Garcia–Mata R., Doughman R., Burridge K. (2009). Chapter 1. Focal adhesions: new angles on an old structure. Int. Rev. Cell Mol. Biol. 277, 1–65. 10.1016/S1937-6448(09)77001-7 [DOI] [PubMed] [Google Scholar]
- Eckes B., Dogic D., Colucci–Guyon E., Wang N., Maniotis A., Ingber D., Merckling A., Langa F., Aumailley M., Delouvée A.et al. (1998). Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J. Cell Sci. 111, 1897–1907. [DOI] [PubMed] [Google Scholar]
- Ehrlicher A. J., Nakamura F., Hartwig J. H., Weitz D. A., Stossel T. P. (2011). Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A. Nature 478, 260–263. 10.1038/nature10430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias B. C., Bhattacharya S., Ray R. M., Johnson L. R. (2010). Polyamine-dependent activation of Rac1 is stimulated by focal adhesion-mediated Tiam1 activation. Cell Adhes. Migr. 4, 419–430. 10.4161/cam.4.3.12043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A. J., Sen S., Sweeney H. L., Discher D. E. (2006). Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689. 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- Evans E., Ritchie K. (1999). Strength of a weak bond connecting flexible polymer chains. Biophys. J. 76, 2439–2447. 10.1016/S0006-3495(99)77399-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenteany G., Janmey P. A., Stossel T. P. (2000). Signaling pathways and cell mechanics involved in wound closure by epithelial cell sheets. Curr. Biol. 10, 831–838. 10.1016/S0960-9822(00)00579-0 [DOI] [PubMed] [Google Scholar]
- Ferrer J. M., Lee H., Chen J., Pelz B., Nakamura F., Kamm R. D., Lang M. J. (2008). Measuring molecular rupture forces between single actin filaments and actin-binding proteins. Proc. Natl. Acad. Sci. USA 105, 9221–9226. 10.1073/pnas.0706124105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer J. T., Simmons R. M., Spudich J. A. (1994). Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature 368, 113–119. 10.1038/368113a0 [DOI] [PubMed] [Google Scholar]
- Fisher G. W., Conrad P. A., DeBiasio R. L., Taylor D. L. (1988). Centripetal transport of cytoplasm, actin, and the cell surface in lamellipodia of fibroblasts. Cell Motil. Cytoskeleton 11, 235–247. 10.1002/cm.970110403 [DOI] [PubMed] [Google Scholar]
- Flanagan L. A., Chou J., Falet H., Neujahr R., Hartwig J. H., Stossel T. P. (2001). Filamin A, the Arp2/3 complex, and the morphology and function of cortical actin filaments in human melanoma cells. J. Cell Biol. 155, 511–518. 10.1083/jcb.200105148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca P. M., Shin N. Y., Brábek J., Ryzhova L., Wu J., Hanks S. K. (2004). Regulation and localization of CAS substrate domain tyrosine phosphorylation. Cell. Signal. 16, 621–629. 10.1016/j.cellsig.2003.10.004 [DOI] [PubMed] [Google Scholar]
- Forscher P., Smith S. J. (1988). Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J. Cell Biol. 107, 1505–1516. 10.1083/jcb.107.4.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland J. C., Lee M. H., Boettiger D. (2009). Mechanically activated integrin switch controls alpha5beta1 function. Science 323, 642–644. 10.1126/science.1168441 [DOI] [PubMed] [Google Scholar]
- Furuike S., Ito T., Yamazaki M. (2001). Mechanical unfolding of single filamin A (ABP-280) molecules detected by atomic force microscopy. FEBS Lett. 498, 72–75. 10.1016/S0014-5793(01)02497-8 [DOI] [PubMed] [Google Scholar]
- Gardel M. L., Sabass B., Ji L., Danuser G., Schwarz U. S., Waterman C. M. (2008). Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J. Cell Biol. 183, 999–1005. 10.1083/jcb.200810060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautel M. (2011). Cytoskeletal protein kinases: titin and its relations in mechanosensing. Pflugers Arch. 462, 119–134. 10.1007/s00424-011-0946-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier N. C., Fardin M. A., Roca–Cusachs P., Sheetz M. P. (2011). Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading. Proc. Natl. Acad. Sci. USA 108, 14467–14472. 10.1073/pnas.1105845108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassemi S., Meacci G., Liu S., Gondarenko A. A., Mathur A., Roca–Cusachs P., Sheetz M P., Hone J. (2012). Cells test substrate rigidity by local contractions on sub-micrometer pillars. Proc. Natl. Acad. Sci. USA 109, 5328–5333. 10.1073/pnas.1119886109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannone G., Dubin–Thaler B. J., Döbereiner H. G., Kieffer N., Bresnick A. R., Sheetz M. P. (2004). Periodic lamellipodial contractions correlate with rearward actin waves. Cell 116, 431–443. 10.1016/S0092-8674(04)00058-3 [DOI] [PubMed] [Google Scholar]
- Gorfinkiel N., Blanchard G. B., Adams R. J., Martinez Arias A. (2009). Mechanical control of global cell behaviour during dorsal closure in Drosophila. Development 136, 1889–1898. 10.1242/dev.030866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh T., Cai D., Tian X., Feig L. A., Lerner A. (2000). p130Cas regulates the activity of AND-34, a novel Ral, Rap1, and R-Ras guanine nucleotide exchange factor. J. Biol. Chem. 275, 30118–30123. 10.1074/jbc.M003074200 [DOI] [PubMed] [Google Scholar]
- Graham J. S., Vomund A. N., Phillips C. L., Grandbois M. (2004). Structural changes in human type I collagen fibrils investigated by force spectroscopy. Exp. Cell Res. 299, 335–342. 10.1016/j.yexcr.2004.05.022 [DOI] [PubMed] [Google Scholar]
- Grant R. P., Spitzfaden C., Altroff H., Campbell I. D., Mardon H. J. (1997). Structural requirements for biological activity of the ninth and tenth FIII domains of human fibronectin. J. Biol. Chem. 272, 6159–6166. 10.1074/jbc.272.10.6159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashoff C., Hoffman B. D., Brenner M. D., Zhou R., Parsons M., Yang M. T., McLean M. A., Sligar S. G., Chen C. S., Ha T.et al. (2010). Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266. 10.1038/nature09198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Mi W., Ge Y., Liu H., Fan Q., Han C., Yang J., Han F., Lu X., Yu W. (2010). GlcNAcylation plays an essential role in breast cancer metastasis. Cancer Res. 70, 6344–6351. 10.1158/0008-5472.CAN-09-1887 [DOI] [PubMed] [Google Scholar]
- Guilluy C., Swaminathan V., Garcia–Mata R., O'Brien E. T., Superfine R., Burridge K. (2011). The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat. Cell Biol. 13, 722–727. 10.1038/ncb2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B., Guilford W. H. (2006). Mechanics of actomyosin bonds in different nucleotide states are tuned to muscle contraction. Proc. Natl. Acad. Sci. USA 103, 9844–9849. 10.1073/pnas.0601255103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupton S. L., Eisenmann K., Alberts A. S., Waterman–Storer C. M. (2007). mDia2 regulates actin and focal adhesion dynamics and organization in the lamella for efficient epithelial cell migration. J. Cell Sci. 120, 3475–3487. 10.1242/jcs.006049 [DOI] [PubMed] [Google Scholar]
- Gustavsson A., Yuan M., Fällman M. (2004). Temporal dissection of beta1-integrin signaling indicates a role for p130Cas-Crk in filopodia formation. J. Biol. Chem. 279, 22893–22901. 10.1074/jbc.M309693200 [DOI] [PubMed] [Google Scholar]
- Hagmann J., Grob M., Burger M. M. (1992). The cytoskeletal protein talin is O-glycosylated. J. Biol. Chem. 267, 14424–14428. [PubMed] [Google Scholar]
- Han Y., Eppinger E., Schuster I. G., Weigand L. U., Liang X., Kremmer E., Peschel C., Krackhardt A. M. (2009). Formin-like 1 (FMNL1) is regulated by N-terminal myristoylation and induces polarized membrane blebbing. J. Biol. Chem. 284, 33409–33417. 10.1074/jbc.M109.060699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada Y., Sakurada K., Aoki T., Thomas D. D., Yanagida T. (1990). Mechanochemical coupling in actomyosin energy transduction studied by in vitro movement assay. J. Mol. Biol. 216, 49–68. 10.1016/S0022-2836(05)80060-9 [DOI] [PubMed] [Google Scholar]
- Harris E. S., Gauvin T. J., Heimsath E. G., Higgs H. N. (2010). Assembly of filopodia by the formin FRL2 (FMNL3). Cytoskeleton (Hoboken) 67, 755–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. H., Stossel T. P. (1975). Isolation and properties of actin, myosin, and a new actinbinding protein in rabbit alveolar macrophages. J. Biol. Chem. 250, 5696–5705. [PubMed] [Google Scholar]
- Hartwig J. H., Shevlin P. (1986). The architecture of actin filaments and the ultrastructural location of actin-binding protein in the periphery of lung macrophages. J. Cell Biol. 103, 1007–1020. 10.1083/jcb.103.3.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch V., Zhi G., Smith L., Stull J. T., Craig R., Lehman W. (2001). Myosin light chain kinase binding to a unique site on F-actin revealed by three-dimensional image reconstruction. J. Cell Biol. 154, 611–618. 10.1083/jcb.200105079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hédou J., Bastide B., Page A., Michalski J. C., Morelle W. (2009). Mapping of O-linked beta-N-acetylglucosamine modification sites in key contractile proteins of rat skeletal muscle. Proteomics 9, 2139–2148. 10.1002/pmic.200800617 [DOI] [PubMed] [Google Scholar]
- Heinemann F., Doschke H., Radmacher M. (2011). Keratocyte lamellipodial protrusion is characterized by a concave force-velocity relation. Biophys. J. 100, 1420–1427. 10.1016/j.bpj.2011.01.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera Abreu M. T., Penton P. C., Kwok V., Vachon E., Shalloway D., Vidali L., Lee W., McCulloch C. A., Downey G. P. (2008). Tyrosine phosphatase PTPalpha regulates focal adhesion remodeling through Rac1 activation. Am. J. Physiol. Cell Physiol. 294, C931–C944. 10.1152/ajpcell.00359.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand J. D., Schaller M. D., Parsons J. T. (1995). Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxyl terminal domain of focal adhesion kinase. Mol. Biol. Cell 6, 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Noda Y., Tanaka Y., Niwa S. (2009). Kinesin superfamily motor proteins and intracellular transport. Nat. Rev. Mol. Cell Biol. 10, 682–696. 10.1038/nrm2774 [DOI] [PubMed] [Google Scholar]
- Hobert O., Moerman D. G., Clark K. A., Beckerle M. C., Ruvkun G. (1999). A conserved LIM protein that affects muscular adherens junction integrity and mechanosensory function in Caenorhabditis elegans. J. Cell Biol. 144, 45–57. 10.1083/jcb.144.1.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking D. C., Kowalski K. (2002). A cryptic fragment from fibronectin's III1 module localizes to lipid rafts and stimulates cell growth and contractility. J. Cell Biol. 158, 175–184. 10.1083/jcb.200112031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotulainen P., Lappalainen P. (2006). Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol. 173, 383–394. 10.1083/jcb.200511093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K., Ji L., Applegate K. T., Danuser G., Waterman–Storer C. M. (2007). Differential transmission of actin motion within focal adhesions. Science 315, 111–115. 10.1126/science.1135085 [DOI] [PubMed] [Google Scholar]
- Hu S. H., Chen J. X., Fabry B., Numaguchi Y., Gouldstone A., Ingber D. E., Fredberg J. J., Butler J. P., Wang N. (2003). Intracellular stress tomography reveals stress focusing and structural anisotropy in cytoskeleton of living cells. Am. J. Physiol. Cell Physiol. 285, C1082–C1090. [DOI] [PubMed] [Google Scholar]
- Hubbert C., Guardiola A., Shao R., Kawaguchi Y., Ito A., Nixon A., Yoshida M., Wang X. F., Yao T. P. (2002). HDAC6 is a microtubule-associated deacetylase. Nature 417, 455–458. 10.1038/417455a [DOI] [PubMed] [Google Scholar]
- Hynes R. O. (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687. 10.1016/S0092-8674(02)00971-6 [DOI] [PubMed] [Google Scholar]
- Ishijima A., Harada Y., Kojima H., Funatsu T., Higuchi H., Yanagida T. (1994). Single-molecule analysis of the actomyosin motor using nano-manipulation. Biochem. Biophys. Res. Commun. 199, 1057–1063. 10.1006/bbrc.1994.1336 [DOI] [PubMed] [Google Scholar]
- Ivaska J., Vuoriluoto K., Huovinen T., Izawa I., Inagaki M., Parker P. J. (2005). PKCepsilon-mediated phosphorylation of vimentin controls integrin recycling and motility. EMBO J. 24, 3834–3845. 10.1038/sj.emboj.7600847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey P. A., Winer J. P., Murray M. E., Wen Q. (2009). The hard life of soft cells. Cell Motil. Cytoskeleton 66, 597–605. 10.1002/cm.20382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G., den Hertog J., Hunter T. (2000). Receptor-like protein tyrosine phosphatase alpha homodimerizes on the cell surface. Mol. Cell. Biol. 20, 5917–5929. 10.1128/MCB.20.16.5917-5929.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G. Y., Giannone G., Critchley D. R., Fukumoto E., Sheetz M. P. (2003). Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature 424, 334–337. 10.1038/nature01805 [DOI] [PubMed] [Google Scholar]
- Jiang G. Y., Huang A. H., Cai Y. F., Tanase M., Sheetz M. P. (2006). Rigidity sensing at the leading edge through alphavbeta3 integrins and RPTPalpha. Biophys. J. 90, 1804–1809. 10.1529/biophysj.105.072462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. P., Tang H. Y., Carag C., Speicher D. W., Discher D. E. (2007). Forced unfolding of proteins within cells. Science 317, 663–666. 10.1126/science.1139857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. P., Katan M. (2007). Role of phospholipase Cgamma1 in cell spreading requires association with a beta-Pix/GIT1-containing complex, leading to activation of Cdc42 and Rac1. Mol. Cell. Biol. 27, 5790–5805. 10.1128/MCB.00778-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanchanawong P., Shtengel G., Pasapera A. M., Ramko E. B., Davidson M. W., Hess H. F., Waterman C. M. (2010). Nanoscale architecture of integrin-based cell adhesions. Nature 468, 580–584. 10.1038/nature09621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakozova M., Kozak M., Wong C. C., Bailey A. O., Yates J. R., 3rd, Mogilner A., Zebroski H., Kashina A. (2006). Arginylation of beta-actin regulates actin cytoskeleton and cell motility. Science 313, 192–196. 10.1126/science.1129344 [DOI] [PubMed] [Google Scholar]
- Karasawa J., Kuriyama Y., Kuro M., Kikuchi H., Sawada T., Mitsugi T. (1982). [Monitoring system of cerebral blood flow and cerebral metabolism. Part II. Relationship between internal jugular O2 tention and cerebral blood flow (author's transl)]. No To Shinkei 34, 239–245. [PubMed] [Google Scholar]
- Kaverina I., Rottner K., Small J. V. (1998). Targeting, capture, and stabilization of microtubules at early focal adhesions. J. Cell Biol. 142, 181–190. 10.1083/jcb.142.1.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellie S., Wigglesworth N. M. (1987). The cytoskeletal protein vinculin is acylated by myristic acid. FEBS Lett. 213, 428–432. 10.1016/0014-5793(87)81536-3 [DOI] [PubMed] [Google Scholar]
- Kiema T., Lad Y., Jiang P. J., Oxley C. L., Baldassarre M., Wegener K. L., Campbell I. D., Ylänne J., Calderwood D. A. (2006). The molecular basis of filamin binding to integrins and competition with talin. Mol. Cell 21, 337–347. 10.1016/j.molcel.2006.01.011 [DOI] [PubMed] [Google Scholar]
- Kim H., Nakamura F., Lee W., Hong C., Pérez–Sala D., McCulloch C. A. (2010). Regulation of cell adhesion to collagen via beta1 integrins is dependent on interactions of filamin A with vimentin and protein kinase C epsilon. Exp. Cell Res. 316, 1829–1844. 10.1016/j.yexcr.2010.02.007 [DOI] [PubMed] [Google Scholar]
- Kim M., Carman C. V., Springer T. A. (2003). Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science 301, 1720–1725. 10.1126/science.1084174 [DOI] [PubMed] [Google Scholar]
- Klotzsch E., Smith M. L., Kubow K. E., Muntwyler S., Little W. C., Beyeler F., Gourdon D., Nelson B. J., Vogel V. (2009). Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. Proc. Natl. Acad. Sci. USA 106, 18267–18272. 10.1073/pnas.0907518106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodney M. S., Elson E. L. (1995). Contraction due to microtubule disruption is associated with increased phosphorylation of myosin regulatory light chain. Proc. Natl. Acad. Sci. USA 92, 10252–10256. 10.1073/pnas.92.22.10252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F., García A. J., Mould A. P., Humphries M. J., Zhu C. (2009). Demonstration of catch bonds between an integrin and its ligand. J. Cell Biol. 185, 1275–1284. 10.1083/jcb.200810002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic A., Sheetz M. P. (2006). Fibronectin rigidity response through Fyn and p130Cas recruitment to the leading edge. Mol. Biol. Cell 17, 2684–2695. 10.1091/mbc.E05-12-1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar D. R., Pollard T. D. (2004). Insertional assembly of actin filament barbed ends in association with formins produces piconewton forces. Proc. Natl. Acad. Sci. USA 101, 14725–14730. 10.1073/pnas.0405902101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar D. R., Harris E. S., Mahaffy R., Higgs H. N., Pollard T. D. (2006). Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell 124, 423–435. 10.1016/j.cell.2005.11.038 [DOI] [PubMed] [Google Scholar]
- Kreis S., Schönfeld H. J., Melchior C., Steiner B., Kieffer N. (2005). The intermediate filament protein vimentin binds specifically to a recombinant integrin alpha2/beta1 cytoplasmic tail complex and co-localizes with native alpha2/beta1 in endothelial cell focal adhesions. Exp. Cell Res. 305, 110–121. 10.1016/j.yexcr.2004.12.023 [DOI] [PubMed] [Google Scholar]
- Kress H., Stelzer E. H., Holzer D., Buss F., Griffiths G., Rohrbach A. (2007). Filopodia act as phagocytic tentacles and pull with discrete steps and a load-dependent velocity. Proc. Natl. Acad. Sci. USA 104, 11633–11638. 10.1073/pnas.0702449104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger C. C., An X., Tang H. Y., Mohandas N., Speicher D. W., Discher D. E. (2011). Cysteine shotgun-mass spectrometry (CS-MS) reveals dynamic sequence of protein structure changes within mutant and stressed cells. Proc. Natl. Acad. Sci. USA 108, 8269–8274. 10.1073/pnas.1018887108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Weaver V. M. (2009). Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 28, 113–127. 10.1007/s10555-008-9173-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Suan Li M. (2010). Biomolecules under mechanical force. Phys. Rep. 486, 1–74. 10.1016/j.physrep.2009.11.001 [DOI] [Google Scholar]
- Kuo S. C., Sheetz M. P. (1993). Force of single kinesin molecules measured with optical tweezers. Science 260, 232–234. 10.1126/science.8469975 [DOI] [PubMed] [Google Scholar]
- Laakso J. M., Lewis J. H., Shuman H., Ostap E. M. (2008). Myosin I can act as a molecular force sensor. Science 321, 133–136. 10.1126/science.1159419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I., Marchant R. E. (2001). Force measurements on the molecular interactions between ligand (RGD) and human platelet αIIbβ3 receptor system. Surf. Sci. 491, 433–443. 10.1016/S0039-6028(01)01309-7 [DOI] [Google Scholar]
- Lee H., Pelz B., Ferrer J. M., Kim T., Lang M. J., Kamm R. D. (2009). Cytoskeletal deformation at high strains and the role of cross-link unfolding or unbinding. Cell. Mol. Bioeng. 2, 28–38. 10.1007/s12195-009-0048-8 [DOI] [Google Scholar]
- Lefebvre T., Cieniewski C., Lemoine J., Guerardel Y., Leroy Y., Zanetta J. P., Michalski J. C. (2001). Identification of N-acetyl-d-glucosamine-specific lectins from rat liver cytosolic and nuclear compartments as heat-shock proteins. Biochem. J. 360, 179–188. 10.1042/0264-6021:3600179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legate K. R., Wickström S. A., Fässler R. (2009). Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 23, 397–418. 10.1101/gad.1758709 [DOI] [PubMed] [Google Scholar]
- Lemmon C. A., Ohashi T., Erickson H. P. (2011). Probing the folded state of fibronectin type III domains in stretched fibrils by measuring buried cysteine accessibility. J. Biol. Chem. 286, 26375–26382. 10.1074/jbc.M111.240028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Y., Redick S. D., Erickson H. P., Moy V. T. (2003a). Force measurements of the alpha5beta1 integrin-fibronectin interaction. Biophys. J. 84, 1252–1262. 10.1016/S0006-3495(03)74940-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R. H., Mitra N., Gratkowski H., Vilaire G., Litvinov R., Nagasami C., Weisel J. W., Lear J. D., DeGrado W. F., Bennett J. S. (2003b). Activation of integrin alphaIIbbeta3 by modulation of transmembrane helix associations. Science 300, 795–798. 10.1126/science.1079441 [DOI] [PubMed] [Google Scholar]
- Li Y., Bhimalapuram P., Dinner A. R. (2010). Model for how retrograde actin flow regulates adhesion traction stresses. J. Phys. Condens. Matter 22, 194113 10.1088/0953-8984/22/19/194113 [DOI] [PubMed] [Google Scholar]
- Liang F. C., Chen R. P., Lin C. C., Huang K. T., Chan S. I. (2006). Tuning the conformation properties of a peptide by glycosylation and phosphorylation. Biochem. Biophys. Res. Commun. 342, 482–488. 10.1016/j.bbrc.2006.01.168 [DOI] [PubMed] [Google Scholar]
- Lim B. B., Lee E. H., Sotomayor M., Schulten K. (2008a). Molecular basis of fibrin clot elasticity. Structure 16, 449–459. 10.1016/j.str.2007.12.019 [DOI] [PubMed] [Google Scholar]
- Lim Y., Lim S. T., Tomar A., Gardel M., Bernard–Trifilo J. A., Chen X. L., Uryu S. A., Canete–Soler R., Zhai J., Lin H.et al. (2008b). PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J. Cell Biol. 180, 187–203. 10.1083/jcb.200708194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinov R. I., Shuman H., Bennett J. S., Weisel J. W. (2002). Binding strength and activation state of single fibrinogen-integrin pairs on living cells. Proc. Natl. Acad. Sci. USA 99, 7426–7431. 10.1073/pnas.112194999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinov R. I., Bennett J. S., Weisel J. W., Shuman H. (2005). Multi-step fibrinogen binding to the integrin (alpha)IIb(beta)3 detected using force spectroscopy. Biophys. J. 89, 2824–2834. 10.1529/biophysj.105.061887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinov R. I., Barsegov V., Schissler A. J., Fisher A. R., Bennett J. S., Weisel J. W., Shuman H. (2011). Dissociation of bimolecular αIIbβ3-fibrinogen complex under a constant tensile force. Biophys. J. 100, 165–173. 10.1016/j.bpj.2010.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B. P., Chrzanowska–Wodnicka M., Burridge K. (1998). Microtubule depolymerization induces stress fibers, focal adhesions, and DNA synthesis via the GTP-binding protein Rho. Cell Adhes. Commun. 5, 249–255. 10.3109/15419069809040295 [DOI] [PubMed] [Google Scholar]
- Lo S. H. (2004). Tensin. Int. J. Biochem. Cell Biol. 36, 31–34. 10.1016/S1357-2725(03)00171-7 [DOI] [PubMed] [Google Scholar]
- Lo S. H., Janmey P. A., Hartwig J. H., Chen L. B. (1994). Interactions of tensin with actin and identification of its three distinct actin-binding domains. J. Cell Biol. 125, 1067–1075. 10.1083/jcb.125.5.1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo D. T., Kanner S. B., Aruffo A. (1998). Filamin binds to the cytoplasmic domain of the beta1-integrin. Identification of amino acids responsible for this interaction. J. Biol. Chem. 273, 23304–23312. 10.1074/jbc.273.36.23304 [DOI] [PubMed] [Google Scholar]
- Luo B. H., Carman C. V., Springer T. A. (2007). Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 25, 619–647. 10.1146/annurev.immunol.25.022106.141618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch C. D., Gauthier N. C., Biais N., Lazar A. M., Roca–Cusachs P., Yu C. H., Sheetz M. P. (2011). Filamin depletion blocks endoplasmic spreading and destabilizes force-bearing adhesions. Mol. Biol. Cell 22, 1263–1273. 10.1091/mbc.E10-08-0661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machacek M., Hodgson L., Welch C., Elliott H., Pertz O., Nalbant P., Abell A., Johnson G. L., Hahn K. M., Danuser G. (2009). Coordination of Rho GTPase activities during cell protrusion. Nature 461, 99–103. 10.1038/nature08242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar M., Seasholtz T. M., Buckmaster C., Toksoz D., Brown J. H. (1999). A rho exchange factor mediates thrombin and Galpha(12)-induced cytoskeletal responses. J. Biol. Chem. 274, 26815–26821. 10.1074/jbc.274.38.26815 [DOI] [PubMed] [Google Scholar]
- Mammoto T., Ingber D. E. (2010). Mechanical control of tissue and organ development. Development 137, 1407–1420. 10.1242/dev.024166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margadant F., Chew L. L., Hu X., Yu H., Bate N., Zhang X., Sheetz M. P. (2011). Mechanotransduction in vivo by repeated talin stretch-relaxation events depends upon vinculin. PLoS Biol. 9, e1001223 10.1371/journal.pbio.1001223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marko J. F., Siggia E. D. (1995). Stretching DNA. Macromolecules 28, 8759–8770. 10.1021/ma00130a008 [DOI] [Google Scholar]
- Matthews B. D., Overby D. R., Mannix R., Ingber D. E. (2006). Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J. Cell Sci. 119, 508–518. 10.1242/jcs.02760 [DOI] [PubMed] [Google Scholar]
- Matthews B. D., Thodeti C. K., Tytell J. D., Mammoto A., Overby D. R., Ingber D. E. (2010). Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integr Biol (Camb) 2, 435–442. 10.1039/c0ib00034e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkel A. R., Kroemker M., Bubeck P., Ronsiek M., Nikolai G., Jockusch B. M. (1994). Characterization of an F-actin-binding domain in the cytoskeletal protein vinculin. J. Cell Biol. 126, 1231–1240. 10.1083/jcb.126.5.1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyhöfer E., Howard J. (1995). The force generated by a single kinesin molecule against an elastic load. Proc. Natl. Acad. Sci. USA 92, 574–578. 10.1073/pnas.92.2.574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael K. E., Dumbauld D. W., Burns K. L., Hanks S. K., García A. J. (2009). Focal adhesion kinase modulates cell adhesion strengthening via integrin activation. Mol. Biol. Cell 20, 2508–2519. 10.1091/mbc.E08-01-0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata H., Yasuda R., Kinosita K., Jr (1996). Strength and lifetime of the bond between actin and skeletal muscle alpha-actinin studied with an optical trapping technique. Biochim. Biophys. Acta 1290, 83–88. 10.1016/0304-4165(96)00003-7 [DOI] [PubMed] [Google Scholar]
- Moore S. W., Roca–Cusachs P., Sheetz M. P. (2010). Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Dev. Cell 19, 194–206. 10.1016/j.devcel.2010.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M., Nieswandt B., Ussar S., Pozgajova M., Fässler R. (2008). Kindlin-3 is essential for integrin activation and platelet aggregation. Nat. Med. 14, 325–330. 10.1038/nm1722 [DOI] [PubMed] [Google Scholar]
- Moser M., Legate K. R., Zent R., Fässler R. (2009). The tail of integrins, talin, and kindlins. Science 324, 895–899. 10.1126/science.1163865 [DOI] [PubMed] [Google Scholar]
- Munevar S., Wang Y. L., Dembo M. (2004). Regulation of mechanical interactions between fibroblasts and the substratum by stretch-activated Ca2+ entry. J. Cell Sci. 117, 85–92. 10.1242/jcs.00795 [DOI] [PubMed] [Google Scholar]
- Na S., Collin O., Chowdhury F., Tay B., Ouyang M., Wang Y., Wang N. (2008). Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc. Natl. Acad. Sci. USA 105, 6626–6631. 10.1073/pnas.0711704105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C. M., Jean R. P., Tan J. L., Liu W. F., Sniadecki N. J., Spector A. A., Chen C. S. (2005). Emergent patterns of growth controlled by multicellular form and mechanics. Proc. Natl. Acad. Sci. USA 102, 11594–11599. 10.1073/pnas.0502575102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieves B., Jones C. W., Ward R., Ohta Y., Reverte C. G., LaFlamme S. E. (2010). The NPIY motif in the integrin beta1 tail dictates the requirement for talin-1 in outside-in signaling. J. Cell Sci. 123, 1216–1226. 10.1242/jcs.056549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niland S., Westerhausen C., Schneider S. W., Eckes B., Schneider M. F., Eble J. A. (2011). Biofunctionalization of a generic collagenous triple helix with the α2β1 integrin binding site allows molecular force measurements. Int. J. Biochem. Cell Biol. 43, 721–731. 10.1016/j.biocel.2011.01.013 [DOI] [PubMed] [Google Scholar]
- Nobes C. D., Hall A. (1995). Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62. 10.1016/0092-8674(95)90370-4 [DOI] [PubMed] [Google Scholar]
- Oberhauser A. F., Marszalek P. E., Erickson H. P., Fernandez J. M. (1998). The molecular elasticity of the extracellular matrix protein tenascin. Nature 393, 181–185. 10.1038/30270 [DOI] [PubMed] [Google Scholar]
- Oberhauser A. F., Badilla–Fernandez C., Carrion–Vazquez M., Fernandez J. M. (2002). The mechanical hierarchies of fibronectin observed with single-molecule AFM. J. Mol. Biol. 319, 433–447. 10.1016/S0022-2836(02)00306-6 [DOI] [PubMed] [Google Scholar]
- Orgel J. P., Antipova O., Sagi I., Bitler A., Qiu D., Wang R., Xu Y., San Antonio J. D. (2011). Collagen fibril surface displays a constellation of sites capable of promoting fibril assembly, stability, and hemostasis. Connect. Tissue Res. 52, 18–24. 10.3109/03008207.2010.511354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey C. A., Pavalko F. M., Burridge K. (1990). An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J. Cell Biol. 111, 721–729. 10.1083/jcb.111.2.721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankov R., Cukierman E., Katz B. Z., Matsumoto K., Lin D. C., Lin S., Hahn C., Yamada K. M. (2000). Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J. Cell Biol. 148, 1075–1090. 10.1083/jcb.148.5.1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramore S., Ayton G. S., Mirijanian D. T., Voth G. A. (2006). Extending a spectrin repeat unit. I: linear force-extension response. Biophys. J. 90, 92–100. 10.1529/biophysj.105.066969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons J. T., Horwitz A. R., Schwartz M. A. (2010). Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 11, 633–643. 10.1038/nrm2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasapera A. M., Schneider I. C., Rericha E., Schlaepfer D. D., Waterman C. M. (2010). Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J. Cell Biol. 188, 877–890. 10.1083/jcb.200906012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek M. J., Zahir N., Johnson K. R., Lakins J. N., Rozenberg G. I., Gefen A., Reinhart–King C. A., Margulies S. S., Dembo M., Boettiger D.et al. (2005). Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254. 10.1016/j.ccr.2005.08.010 [DOI] [PubMed] [Google Scholar]
- Pavalko F. M., Burridge K. (1991). Disruption of the actin cytoskeleton after microinjection of proteolytic fragments of alpha-actinin. J. Cell Biol. 114, 481–491. 10.1083/jcb.114.3.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko F. M., LaRoche S. M. (1993). Activation of human neutrophils induces an interaction between the integrin beta 2-subunit (CD18) and the actin binding protein alpha-actinin. J. Immunol. 151, 3795–3807. [PubMed] [Google Scholar]
- Pavalko F. M., Otey C. A., Simon K. O., Burridge K. (1991). Alpha-actinin: a direct link between actin and integrins. Biochem. Soc. Trans. 19, 1065–1069. [DOI] [PubMed] [Google Scholar]
- Pentikäinen U., Ylänne J. (2009). The regulation mechanism for the auto-inhibition of binding of human filamin A to integrin. J. Mol. Biol. 393, 644–657. 10.1016/j.jmb.2009.08.035 [DOI] [PubMed] [Google Scholar]
- Perdiz D., Mackeh R., Poüs C., Baillet A. (2011). The ins and outs of tubulin acetylation: more than just a post-translational modification? Cell. Signal. 23, 763–771. 10.1016/j.cellsig.2010.10.014 [DOI] [PubMed] [Google Scholar]
- Perumal S., Antipova O., Orgel J. P. (2008). Collagen fibril architecture, domain organization, and triple-helical conformation govern its proteolysis. Proc. Natl. Acad. Sci. USA 105, 2824–2829. 10.1073/pnas.0710588105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff M., Liu S., Erle D. J., Ginsberg M. H. (1998). Integrin beta cytoplasmic domains differentially bind to cytoskeletal proteins. J. Biol. Chem. 273, 6104–6109. 10.1074/jbc.273.11.6104 [DOI] [PubMed] [Google Scholar]
- Pfister K. K., Shah P. R., Hummerich H., Russ A., Cotton J., Annuar A. A., King S. M., Fisher E. M. (2006). Genetic analysis of the cytoplasmic dynein subunit families. PLoS Genet. 2, e1 10.1371/journal.pgen.0020001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh Y. C., Na S., Chowdhury F., Ouyang M., Wang Y., Wang N. (2009). Rapid activation of Rac GTPase in living cells by force is independent of Src. PLoS ONE 4, e7886 10.1371/journal.pone.0007886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popowicz G. M., Schleicher M., Noegel A. A., Holak T. A. (2006). Filamins: promiscuous organizers of the cytoskeleton. Trends Biochem. Sci. 31, 411–419. 10.1016/j.tibs.2006.05.006 [DOI] [PubMed] [Google Scholar]
- Prass M., Jacobson K., Mogilner A., Radmacher M. (2006). Direct measurement of the lamellipodial protrusive force in a migrating cell. J. Cell Biol. 174, 767–772. 10.1083/jcb.200601159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchner E. M., Alexandrovich A., Kho A. L., Hensen U., Schäfer L. V., Brandmeier B., Gräter F., Grubmüller H., Gaub H. E., Gautel M. (2008). Mechanoenzymatics of titin kinase. Proc. Natl. Acad. Sci. USA 105, 13385–13390. 10.1073/pnas.0805034105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puklin–Faucher E., Sheetz M. P. (2009). The mechanical integrin cycle. J. Cell Sci. 122, 179–186. 10.1242/jcs.042127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puklin–Faucher E., Gao M., Schulten K., Vogel V. (2006). How the headpiece hinge angle is opened: New insights into the dynamics of integrin activation. J. Cell Biol. 175, 349–360. 10.1083/jcb.200602071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajfur Z., Roy P., Otey C., Romer L., Jacobson K. (2002). Dissecting the link between stress fibres and focal adhesions by CALI with EGFP fusion proteins. Nat. Cell Biol. 4, 286–293. 10.1038/ncb772 [DOI] [PubMed] [Google Scholar]
- Ramirez–Correa G. A., Jin W., Wang Z., Zhong X., Gao W. D., Dias W. B., Vecoli C., Hart G. W., Murphy A. M. (2008). O-linked GlcNAc modification of cardiac myofilament proteins: a novel regulator of myocardial contractile function. Circ. Res. 103, 1354–1358. 10.1161/CIRCRESAHA.108.184978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M. D. (2006). Trafficking and signaling by fatty-acylated and prenylated proteins. Nat. Chem. Biol. 2, 584–590. 10.1038/nchembio834 [DOI] [PubMed] [Google Scholar]
- Rico F., Roca–Cusachs P., Sunyer R., Farré R., Navajas D. (2007). Cell dynamic adhesion and elastic properties probed with cylindrical atomic force microscopy cantilever tips. J. Mol. Recognit. 20, 459–466. 10.1002/jmr.829 [DOI] [PubMed] [Google Scholar]
- Ridley A. J. (2011). Life at the leading edge. Cell 145, 1012–1022. 10.1016/j.cell.2011.06.010 [DOI] [PubMed] [Google Scholar]
- Riggins R. B., Quilliam L. A., Bouton A. H. (2003). Synergistic promotion of c-Src activation and cell migration by Cas and AND-34/BCAR3. J. Biol. Chem. 278, 28264–28273. 10.1074/jbc.M303535200 [DOI] [PubMed] [Google Scholar]
- Riveline D., Zamir E., Balaban N. Q., Schwarz U. S., Ishizaki T., Narumiya S., Kam Z., Geiger B., Bershadsky A. D. (2001). Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 153, 1175–1186. 10.1083/jcb.153.6.1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca–Cusachs P., Gauthier N. C., Del Rio A., Sheetz M. P. (2009). Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proc. Natl. Acad. Sci. USA 106, 16245–16250. 10.1073/pnas.0902818106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R., Jr (2005). Src kinase regulation by phosphorylation and dephosphorylation. Biochem. Biophys. Res. Commun. 331, 1–14. 10.1016/j.bbrc.2005.03.012 [DOI] [PubMed] [Google Scholar]
- Rossier O. M., Gauthier N., Biais N., Vonnegut W., Fardin M. A., Avigan P., Heller E. R., Mathur A., Ghassemi S., Koeckert M. S.et al. (2010). Force generated by actomyosin contraction builds bridges between adhesive contacts. EMBO J. 29, 1055–1068. 10.1038/emboj.2010.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottner K., Hall A., Small J. V. (1999). Interplay between Rac and Rho in the control of substrate contact dynamics. Curr. Biol. 9, 640 10.1016/S0960-9822(99)80286-3 [DOI] [PubMed] [Google Scholar]
- Saha S., Mundia M. M., Zhang F., Demers R. W., Korobova F., Svitkina T., Perieteanu A. A., Dawson J. F., Kashina A. (2010). Arginylation regulates intracellular actin polymer level by modulating actin properties and binding of capping and severing proteins. Mol. Biol. Cell 21, 1350–1361. 10.1091/mbc.E09-09-0829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltel F., Mortier E., Hytönen V. P., Jacquier M. C., Zimmermann P., Vogel V., Liu W., Wehrle–Haller B. (2009). New PI(4,5)P2- and membrane proximal integrin-binding motifs in the talin head control beta3-integrin clustering. J. Cell Biol. 187, 715–731. 10.1083/jcb.200908134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath R., Gallagher P. J., Pavalko F. M. (1998). Cytoskeletal interactions with the leukocyte integrin beta2 cytoplasmic tail. Activation-dependent regulation of associations with talin and alpha-actinin. J. Biol. Chem. 273, 33588–33594. 10.1074/jbc.273.50.33588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y., Sheetz M. P. (2002). Force transduction by Triton cytoskeletons. J. Cell Biol. 156, 609–615. 10.1083/jcb.200110068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y., Tamada M., Dubin–Thaler B. J., Cherniavskaya O., Sakai R., Tanaka S., Sheetz M. P. (2006). Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127, 1015–1026. 10.1016/j.cell.2006.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbrana F., Sassoli C., Meacci E., Nosi D., Squecco R., Paternostro F., Tiribilli B., Zecchi–Orlandini S., Francini F., Formigli L. (2008). Role for stress fiber contraction in surface tension development and stretch-activated channel regulation in C2C12 myoblasts. Am. J. Physiol. Cell Physiol. 295, C160–C172. 10.1152/ajpcell.00014.2008 [DOI] [PubMed] [Google Scholar]
- Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. (1992). pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc. Natl. Acad. Sci. USA 89, 5192–5196. 10.1073/pnas.89.11.5192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller H. B., Friedel C. C., Boulegue C., Fässler R. (2011). Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 12, 259–266. 10.1038/embor.2011.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnepel J., Tschesche H. (2000). The proteolytic activity of the recombinant cryptic human fibronectin type IV collagenase from E. coli expression. J. Protein Chem. 19, 685–692. 10.1023/A:1007104420017 [DOI] [PubMed] [Google Scholar]
- Schober M., Raghavan S., Nikolova M., Polak L., Pasolli H. A., Beggs H. E., Reichardt L. F., Fuchs E. (2007). Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J. Cell Biol. 176, 667–680. 10.1083/jcb.200608010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schvartzman M., Palma M., Sable J., Abramson J., Hu X. A., Sheetz M. P., Wind S. J. (2011). Nanolithographic control of the spatial organization of cellular adhesion receptors at the single-molecule level. Nano Lett. 11, 1306–1312. 10.1021/nl104378f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechler J. L., Rao H., Cumiskey A. M., Vega–Colón I., Smith M. S., Murata T., Schwarzbauer J. E. (2001). A novel fibronectin binding site required for fibronectin fibril growth during matrix assembly. J. Cell Biol. 154, 1081–1088. 10.1083/jcb.200102034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert U. (2000). Rupture of multiple parallel molecular bonds under dynamic loading. Phys. Rev. Lett. 84, 2750–2753. 10.1103/PhysRevLett.84.2750 [DOI] [PubMed] [Google Scholar]
- Sellers J. R. (2000). Myosins: a diverse superfamily. Biochim. Biophys. Acta 1496, 3–22. 10.1016/S0167-4889(00)00005-7 [DOI] [PubMed] [Google Scholar]
- Sellers J. R., Pato M. D. (1984). The binding of smooth muscle myosin light chain kinase and phosphatases to actin and myosin. J. Biol. Chem. 259, 7740–7746. [PubMed] [Google Scholar]
- Serrels B., Serrels A., Brunton V. G., Holt M., McLean G. W., Gray C. H., Jones G. E., Frame M. C. (2007). Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat. Cell Biol. 9, 1046–1056. 10.1038/ncb1626 [DOI] [PubMed] [Google Scholar]
- Sharma C. P., Ezzell R. M., Arnaout M. A. (1995). Direct interaction of filamin (ABP-280) with the beta 2-integrin subunit CD18. J. Immunol. 154, 3461–3470. [PubMed] [Google Scholar]
- Sieg D. J., Hauck C. R., Schlaepfer D. D. (1999). Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J. Cell Sci. 112, 2677–2691. [DOI] [PubMed] [Google Scholar]
- Sjöblom B., Salmazo A., Djinović–Carugo K. (2008). Alpha-actinin structure and regulation. Cell. Mol. Life Sci. 65, 2688–2701. 10.1007/s00018-008-8080-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. L., Gourdon D., Little W. C., Kubow K. E., Eguiluz R. A., Luna–Morris S., Vogel V. (2007). Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol. 5, e268 10.1371/journal.pbio.0050268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenović D., Mijailovich S. M., Tolić–Nørrelykke I. M., Chen J. X., Wang N. (2002). Cell prestress. II. Contribution of microtubules. Am. J. Physiol. Cell Physiol. 282, C617–C624. [DOI] [PubMed] [Google Scholar]
- Su J., Muranjan M., Sap J. (1999). Receptor protein tyrosine phosphatase alpha activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr. Biol. 9, 505–511. 10.1016/S0960-9822(99)80234-6 [DOI] [PubMed] [Google Scholar]
- Sulchek T. A., Friddle R. W., Langry K., Lau E. Y., Albrecht H., Ratto T. V., DeNardo S. J., Colvin M. E., Noy A. (2005). Dynamic force spectroscopy of parallel individual Mucin1-antibody bonds. Proc. Natl. Acad. Sci. USA 102, 16638–16643. 10.1073/pnas.0505208102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sułkowska J. I., Cieplak M. (2008). Stretching to understand proteins - a survey of the protein data bank. Biophys. J. 94, 6–13. 10.1529/biophysj.107.105973 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sun Z., Martinez–Lemus L. A., Trache A., Trzeciakowski J. P., Davis G. E., Pohl U., Meininger G. A. (2005). Mechanical properties of the interaction between fibronectin and alpha5beta1-integrin on vascular smooth muscle cells studied using atomic force microscopy. Am. J. Physiol. Heart Circ. Physiol. 289, H2526–H2535. 10.1152/ajpheart.00658.2004 [DOI] [PubMed] [Google Scholar]
- Svoboda K., Block S. M. (1994). Force and velocity measured for single kinesin molecules. Cell 77, 773–784. 10.1016/0092-8674(94)90060-4 [DOI] [PubMed] [Google Scholar]
- Takagi J., Petre B. M., Walz T., Springer T. A. (2002). Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110, 599–11. 10.1016/S0092-8674(02)00935-2 [DOI] [PubMed] [Google Scholar]
- Tamada M., Sheetz M. P., Sawada Y. (2004). Activation of a signaling cascade by cytoskeleton stretch. Dev. Cell 7, 709–718. 10.1016/j.devcel.2004.08.021 [DOI] [PubMed] [Google Scholar]
- Tan J. L., Tien J., Pirone D. M., Gray D. S., Bhadriraju K., Chen C. S. (2003). Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc. Natl. Acad. Sci. USA 100, 1484–1489. 10.1073/pnas.0235407100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Klooster J. P., Evers E. E., Janssen L., Machesky L. M., Michiels F., Hordijk P., Collard J. G. (2006). Interaction between Tiam1 and the Arp2/3 complex links activation of Rac to actin polymerization. Biochem. J. 397, 39–45. 10.1042/BJ20051957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriot J. A., Mitchison T. J. (1991). Actin microfilament dynamics in locomoting cells. Nature 352, 126–131. 10.1038/352126a0 [DOI] [PubMed] [Google Scholar]
- Thomas W. E., Vogel V., Sokurenko E. (2008). Biophysics of catch bonds. Annu. Rev. Biophys. 37, 399–416. 10.1146/annurev.biophys.37.032807.125804 [DOI] [PubMed] [Google Scholar]
- Thompson J. B., Kindt J. H., Drake B., Hansma H. G., Morse D. E., Hansma P. K. (2001). Bone indentation recovery time correlates with bond reforming time. Nature 414, 773–776. 10.1038/414773a [DOI] [PubMed] [Google Scholar]
- Tomar A., Schlaepfer D. D. (2009). Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Curr. Opin. Cell Biol. 21, 676–683. 10.1016/j.ceb.2009.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgler C. N., Narasimha M., Knox A. L., Zervas C. G., Vernon M. C., Brown N. H. (2004). Tensin stabilizes integrin adhesive contacts in Drosophila. Dev. Cell 6, 357–369. 10.1016/S1534-5807(04)00055-3 [DOI] [PubMed] [Google Scholar]
- Tran A. D., Marmo T. P., Salam A. A., Che S., Finkelstein E., Kabarriti R., Xenias H. S., Mazitschek R., Hubbert C., Kawaguchi Y.et al. (2007). HDAC6 deacetylation of tubulin modulates dynamics of cellular adhesions. J. Cell Sci. 120, 1469–1479. 10.1242/jcs.03431 [DOI] [PubMed] [Google Scholar]
- Triplett J. W., Pavalko F. M. (2006). Disruption of alpha-actinin-integrin interactions at focal adhesions renders osteoblasts susceptible to apoptosis. Am. J. Physiol. Cell Physiol. 291, C909–C921. 10.1152/ajpcell.00113.2006 [DOI] [PubMed] [Google Scholar]
- Tsuruta D., Jones J. C. (2003). The vimentin cytoskeleton regulates focal contact size and adhesion of endothelial cells subjected to shear stress. J. Cell Sci. 116, 4977–4984. 10.1242/jcs.00823 [DOI] [PubMed] [Google Scholar]
- Tu Y., Wu S., Shi X., Chen K., Wu C. (2003). Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell 113, 37–47. 10.1016/S0092-8674(03)00163-6 [DOI] [PubMed] [Google Scholar]
- Tyska M. J., Dupuis D. E., Guilford W. H., Patlak J. B., Waller G. S., Trybus K. M., Warshaw D. M., Lowey S. (1999). Two heads of myosin are better than one for generating force and motion. Proc. Natl. Acad. Sci. USA 96, 4402–4407. 10.1073/pnas.96.8.4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega F. M., Fruhwirth G., Ng T., Ridley A. J. (2011). RhoA and RhoC have distinct roles in migration and invasion by acting through different targets. J. Cell Biol. 193, 655–665. 10.1083/jcb.201011038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V. (2006). Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu. Rev. Biophys. Biomol. Struct. 35, 459–488. 10.1146/annurev.biophys.35.040405.102013 [DOI] [PubMed] [Google Scholar]
- von Wichert G., Jiang G., Kostic A., De Vos K., Sap J., Sheetz M. P. (2003). RPTP-alpha acts as a transducer of mechanical force on alphav/beta3-integrin-cytoskeleton linkages. J. Cell Biol. 161, 143–153. 10.1083/jcb.200211061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter N., Selhuber C., Kessler H., Spatz J. P. (2006). Cellular unbinding forces of initial adhesion processes on nanopatterned surfaces probed with magnetic tweezers. Nano Lett. 6, 398–402. 10.1021/nl052168u [DOI] [PubMed] [Google Scholar]
- Wang H. B., Dembo M., Hanks S. K., Wang Y. L. (2001). Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc. Natl. Acad. Sci. USA 98, 11295–11300. 10.1073/pnas.201201198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Pandey A., Hart G. W. (2007). Dynamic interplay between O-linked N-acetylglucosaminylation and glycogen synthase kinase-3-dependent phosphorylation. Mol. Cell. Proteomics 6, 1365–1379. 10.1074/mcp.M600453-MCP200 [DOI] [PubMed] [Google Scholar]
- Wang Z., Gucek M., Hart G. W. (2008). Cross-talk between GlcNAcylation and phosphorylation: site-specific phosphorylation dynamics in response to globally elevated O-GlcNAc. Proc. Natl. Acad. Sci. USA 105, 13793–13798. 10.1073/pnas.0806216105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Udeshi N. D., Slawson C., Compton P. D., Sakabe K., Cheung W. D., Shabanowitz J., Hunt D. F., Hart G. W. (2010). Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates cytokinesis. Sci. Signal. 3, ra2 10.1126/scisignal.2000526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb D. J., Donais K., Whitmore L. A., Thomas S. M., Turner C. E., Parsons J. T., Horwitz A. F. (2004). FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 6, 154–161. 10.1038/ncb1094 [DOI] [PubMed] [Google Scholar]
- Weiner T. M., Liu E. T., Craven R. J., Cance W. G. (1993). Expression of focal adhesion kinase gene and invasive cancer. Lancet 342, 1024–1025. 10.1016/0140-6736(93)92881-S [DOI] [PubMed] [Google Scholar]
- Welch M. D. (1999). The world according to Arp: regulation of actin nucleation by the Arp2/3 complex. Trends Cell Biol. 9, 423–427. 10.1016/S0962-8924(99)01651-7 [DOI] [PubMed] [Google Scholar]
- Wickström S. A., Lange A., Montanez E., Fässler R. (2010). The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J. 29, 281–291. 10.1038/emboj.2009.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfenson H., Henis Y. I., Geiger B., Bershadsky A. D. (2009). The heel and toe of the cell's foot: a multifaceted approach for understanding the structure and dynamics of focal adhesions. Cell Motil. Cytoskeleton 66, 1017–1029. 10.1002/cm.20410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C. C., Xu T., Rai R., Bailey A. O., Yates J. R., 3rd, Wolf Y. I., Zebroski H., Kashina A. (2007). Global analysis of posttranslational protein arginylation. PLoS Biol. 5, e258 10.1371/journal.pbio.0050258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. X., Chen H. Q., Chen C., Yu W. P., Zhang W. C., Peng Y. J., He W. Q., Wei D. M., Gao X., Zhu M. S. (2006). Microfilament-binding properties of N-terminal extension of the isoform of smooth muscle long myosin light chain kinase. Cell Res. 16, 367–376. 10.1038/sj.cr.7310047 [DOI] [PubMed] [Google Scholar]
- Ye L. H., Hayakawa K., Kishi H., Imamura M., Nakamura A., Okagaki T., Takagi T., Iwata A., Tanaka T., Kohama K. (1997). The structure and function of the actin-binding domain of myosin light chain kinase of smooth muscle. J. Biol. Chem. 272, 32182–32189. 10.1074/jbc.272.51.32182 [DOI] [PubMed] [Google Scholar]
- Ylinen P. (1996). Acetabular cup loosening–peeling off of the plasma-sprayed porous coating in 2 cases. Acta Orthop. Scand. 67, 508–510. 10.3109/17453679608996678 [DOI] [PubMed] [Google Scholar]
- Young K. G., Copeland J. W. (2010). Formins in cell signaling. Biochim. Biophys. Acta 1803, 183–190. 10.1016/j.bbamcr.2008.09.017 [DOI] [PubMed] [Google Scholar]
- Yu C. H., Law J. B., Suryana M., Low H. Y., Sheetz M. P. (2011). Early integrin binding to Arg-Gly-Asp peptide activates actin polymerization and contractile movement that stimulates outward translocation. Proc. Natl. Acad. Sci. USA 108, 20585–20590. 10.1073/pnas.1109485108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachara N. E., O'Donnell N., Cheung W. D., Mercer J. J., Marth J. D., Hart G. W. (2004). Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J. Biol. Chem. 279, 30133–30142. 10.1074/jbc.M403773200 [DOI] [PubMed] [Google Scholar]
- Zaidel–Bar R., Itzkovitz S., Ma'ayan A., Iyengar R., Geiger B. (2007a). Functional atlas of the integrin adhesome. Nat. Cell Biol. 9, 858–867. 10.1038/ncb0807-858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel–Bar R., Milo R., Kam Z., Geiger B. (2007b). A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J. Cell Sci. 120, 137–148. 10.1242/jcs.03314 [DOI] [PubMed] [Google Scholar]
- Zamir E., Katz M., Posen Y., Erez N., Yamada K. M., Katz B. Z., Lin S., Lin D. C., Bershadsky A., Kam Z.et al. (2000). Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat. Cell Biol. 2, 191–196. 10.1038/35008607 [DOI] [PubMed] [Google Scholar]
- Zhang H., Berg J. S., Li Z., Wang Y., Lång P., Sousa A. D., Bhaskar A., Cheney R. E., Strömblad S. (2004). Myosin-X provides a motor-based link between integrins and the cytoskeleton. Nat. Cell Biol. 6, 523–531. 10.1038/ncb1136 [DOI] [PubMed] [Google Scholar]
- Zhang X., Jiang G., Cai Y., Monkley S. J., Critchley D. R., Sheetz M. P. (2008). Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat. Cell Biol. 10, 1062–1068. 10.1038/ncb1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X. M., Resnick R. J., Shalloway D. (2000). A phosphotyrosine displacement mechanism for activation of Src by PTPalpha. EMBO J. 19, 964–978. 10.1093/emboj/19.5.964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Leber B., Andrews D. W. (2001). Cytoplasmic O-glycosylation prevents cell surface transport of E-cadherin during apoptosis. EMBO J. 20, 5999–6007. 10.1093/emboj/20.21.5999 [DOI] [PMC free article] [PubMed] [Google Scholar]