ABSTRACT
Selective autophagy entails cooperation between target recognition and assembly of the autophagic apparatus. Target recognition is conducted by receptors that often recognize tags, such as ubiquitin and galectins, although examples of selective autophagy independent of these tags are emerging. It is less known how receptors cooperate with the upstream autophagic regulators, beyond the well-characterized association of receptors with Atg8 or its homologs, such as LC3B (encoded by MAP1LC3B), on autophagic membranes. The molecular details of the emerging role in autophagy of the family of proteins called TRIMs shed light on the coordination between cargo recognition and the assembly and activation of the principal autophagy regulators. In their autophagy roles, TRIMs act both as receptors and as platforms (‘receptor regulators’) for the assembly of the core autophagy regulators, such as ULK1 and Beclin 1 in their activated state. As autophagic receptors, TRIMs can directly recognize endogenous or exogenous targets, obviating a need for intermediary autophagic tags, such as ubiquitin and galectins. The receptor and regulatory features embodied within the same entity allow TRIMs to govern cargo degradation in a highly exact process termed ‘precision autophagy’.
KEY WORDS: Receptor regulators, TRIMs, Autophagy
Summary: Precision autophagy represents direct recognition of targets by receptors, such as TRIMs, that simultaneously organize core autophagy proteins, coupling recognition to autophagosome formation.
Introduction
Autophagy is a set of diverse processes, often classified as macro-autophagy, micro-autophagy and chaperone-mediated autophagy, which recognize and deliver endogenous cellular constituents or exogenous cargo to lysosomes for degradation (Mizushima et al., 2011). Autophagy can occur in bulk as a response to starvation, whereby portions of the cytoplasm are auto-digested to meet the heightened biogenesis and energy needs of the cell (Rabinowitz and White, 2010; Galluzzi et al., 2014). Autophagy plays equally important roles in homeostasis, acting as a quality and quantity control process by removing protein aggregates (Rubinsztein et al., 2012; Birgisdottir et al., 2013) and defunct or surplus organelles (Maejima et al., 2013; Randow and Youle, 2014), as well as invading microbes and endogenous and exogenous inflammatory agonists (Deretic et al., 2015). The degradation targets for autophagy are not limited to cargo of the cytoplasmic origin, as autophagy can also target nuclear components (Dou et al., 2015). The selectivity of autophagy (Fig. 1) has been a topic of continuing interest; it has been established that a number of receptors can guide autophagic machinery to targets, which are often earmarked for autophagy with ubiquitin or galectins tags (Randow and Youle, 2014; Stolz et al., 2014).
Fig. 1.
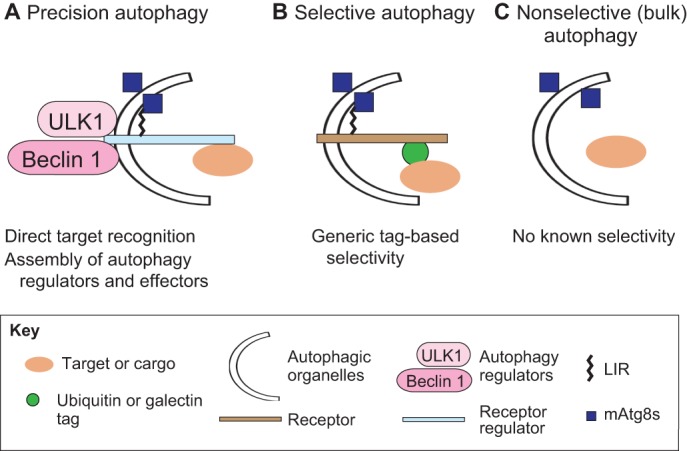
Precision autophagy. (A) Precision autophagy is a receptor-regulator-based form of autophagy. A receptor-regulator protein (such as members of the TRIM family) has the ability to recognize targets directly, often without tags such as ubiquitin or galectins, and assembles regulators and effectors of autophagy to mediate precision (highly selective) autophagy. (B) Conventional selective autophagy is mediated by autophagic receptors that recognize targets through generic tags, such as ubiquitin or galectins, while also binding mammalian (m)Atg8 proteins that connect them to autophagic membranes. (C) Nonselective (bulk) autophagy sequesters mixtures of cytoplasmic components, including cytosol.
A morphologically emblematic event during autophagy is the formation in the cytoplasm of the membranous organelles termed autophagosomes; these are the executors of macro-autophagy (hereafter referred to as autophagy) (Mizushima et al., 2011). Autophagosomes are decorated in yeast with Atg8 and in mammals with LC3B [one of six mammalian (m)Atg8 paralogs – LC3A, LC3B and LC3C (encoded by MAP1LC3A, MAP1LC3B and MAP1LC3C, respectively) and GABARAP, GABARAPL1 and GABARAPL2 (Mizushima et al., 2011; Weidberg et al., 2011)]. mAtg8 proteins are conjugated at their C-terminus to a lipid, phosphatidylethanolamine (PtdEtn), which in turn mediates association with the autophagic membrane (Kabeya et al., 2000). Many other parts of the core autophagy machinery are well-conserved from yeast to humans (Mizushima et al., 2011). In mammals, the key autophagy factors (ATG) include ULK1 (one of four mammalian paralogs of Atg1) (Chan et al., 2007; Kundu and Thompson, 2008) and Beclin 1 (a paralog of yeast Atg6) (Liang et al., 1999). These principal autophagy regulators are controlled by upstream serine and threonine (Ser/Thr) protein kinases 5′-AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) (Egan et al., 2011; Kim et al., 2011, 2013; Russell et al., 2013), as well as E3 ligases and ubiquitylation (Nazio et al., 2013). Lipid kinases, most notably the class III phosphatidylinositol 3-kinase (PI3K) VPS34 (encoded by PIK3C3; which forms a complex with Beclin 1), co-activate autophagy by producing phosphatidylinositol 3-phosphate [PtdIns(3)P], a step that commits them to participating in autophagosome formation (Petiot et al., 2000); however, the roles of other mono-phosphoinositides such as phosphatidylinositol 5-phosphate have recently been suggested (Vicinanza et al., 2015). The protein kinases (ULK1), lipid kinases (Beclin-1–VPS34) and the LC3-conjugation system are all physically connected in their role in controlling autophagy. First, PtdIns(3)P is recognized by WD repeat domain phosphoinositide-interacting protein 2 (WIPI2, the mammalian Atg18), which interacts with ATG16L1 and forms part of the LC3–PtdEtn conjugation system (Dooley et al., 2014). Second, ATG16L1 associates with FIP200 (also known as RB1CC1), a component of the ULK1 complex system (Fujita et al., 2013; Gammoh et al., 2013; Nishimura et al., 2013; Dooley et al., 2014). Autophagy is further coupled to the lysosomal systems. This occurs through the effects on autophagy of members of the key regulator of lysosomal biogenesis ‘microphthalmia/transcription factor E’ (MiT/TFE) family (including transcription factor EB; TFEB) (Settembre et al., 2011; Perera et al., 2015) and through SNAREs (Itakura et al., 2012), which enable the maturation of autophagosomes into the autolysosome. Thus, all parts of the autophagy system are interconnected, thereby enabling spatial and temporal regulation of autophagy.
The homing of the autophagy machinery to intended targets through receptors is an area of intense study (Birgisdottir et al., 2013; Rogov et al., 2014; Khaminets et al., 2015). Frequently, targets exhibit ubiquitin or galectin marks, although this does not appear to be an absolute requirement (Khaminets et al., 2015). Furthermore, the number of proteinaceous, membranous and complex targets for selective autophagy is rapidly increasing. This raises an important question: are the number of receptors and the principles that define them described thus far sufficient to explain all types of selective autophagy? In this Commentary, we will review recent studies on one such family of factors, TRIMs, which appear to be different from conventional receptors because they perform two separate but interlinked duties, by acting both as autophagic receptors and autophagy regulators. Importantly, TRIMs have been shown in several cases to directly recognize their cognate targets that are destined for autophagy without the need for intermediary tags such as ubiquitin and galectins. These functionalities are the basis for a process we have termed ‘precision autophagy’ (Kimura et al., 2015).
Sequestosome-1-like receptors and cargo recognition mediated by tags
As noted above, the cargo entering the autophagosomes is often modified with tags, such as ubiquitin, phosphorylated ubiquitin and galectins (Koyano et al., 2014; Randow and Youle, 2014; Khaminets et al., 2015). These tags are then recognized by sequestosome-1-like receptors (SLRs) named so (Birgisdottir et al., 2013; Deretic et al., 2013) after the founding member of this receptor family, sequestosome 1 (commonly known as p62) (Lamark et al., 2009). SLRs also include other receptors, such as neighbor of BRCA1 gene 1 (NBR1), Ca2+-binding and coiled-coil domain 2 (CALCOCO2, commonly known as NDP52), tax-binding protein 1 (TAXBP1) and optineurin (Bjørkøy et al., 2005; Kirkin et al., 2009; Wild et al., 2011; Newman et al., 2012; Thurston et al., 2012; Deretic et al., 2013; Lazarou et al., 2015). SLRs bind both to mAtg8 proteins through LC3-interacting motifs (LIR) and to ubiquitin through a variety of ubiquitin-binding domains (e.g. UBAN, UBA, UBZ) (Khaminets et al., 2015). Some SLRs bind to galectin tags (Thurston et al., 2012). Besides the above-mentioned group of mammalian proteins, additional receptors fit the SLR criteria. For instance, RPN10, an Arabidopsis receptor that mediates autophagic degradation of proteasome, binds to ubiquitin tags and Atg8s through its ubiquitin-interacting motif (UIM) domains (Marshall et al., 2015). Furthermore, Toll-interacting protein (TOLLIP) in mammals and Cue5 in yeast fit SLR criteria; Cue5 directly binds both to ubiquitin and to Atg8, whereas TOLLIP directly binds to ubiquitin and, in cellular extracts, to LC3 (Lu et al., 2014).
The TRIM protein family
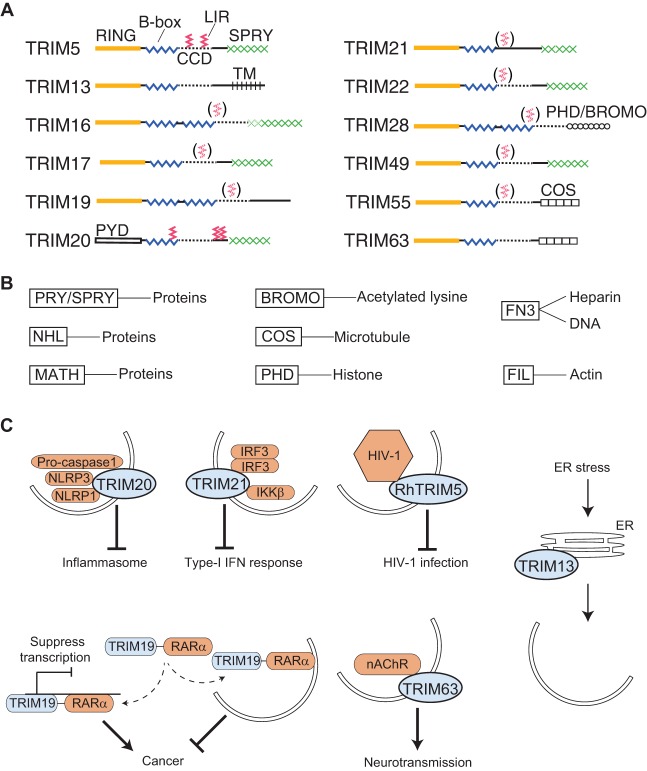
TRIMs were first recognized as a family (Reymond et al., 2001) based on their common tri-partite domain organization, typically comprising an N-terminal RING domain, one or two B-box domains and a coiled-coil domain, although they also have additional features, including a variety of C-terminal domains, inclusive of SPRY domains (Fig. 2A,B; Table S1). TRIMs are conserved in metazoans, and the number of genes encoding them escalates in higher organisms (Sardiello et al., 2008; Boudinot et al., 2011); 82 TRIM genes (excluding the pseudogenes) have been identified in humans (Table S1).
Fig. 2.
Receptor-regulator features of TRIMs and examples of TRIM-mediated precision autophagy. (A) Domain organization of TRIMs and location of LC3-interacting regions (LIRs) of TRIMs. Red solid zig-zag lines represent mapped and mutationally analyzed LIRs. CCD, coiled-coil domain; TM, transmembrane domain. (B) TRIM C-terminal variable domains (see main text) and types of binding partners or cargo recognized by such domains. FN3, fibronectin type-III repeats. (C) Examples of precision autophagy in targeting endogenous (innate immune signal components; mediated by TRIM20 and TRIM21) and exogenous cargo [HIV-1; mediated by Rhesus TRIM5 (RhTRIM5)]. Physiological consequences are indicated. The fusion protein TRIM19–RARα plays a role in cancer. TRIM63 promotes autophagic degradation of nicotinic acetylcholine receptor (nAChR). TRIM13 controls autophagy in response to ER stress (targets presently unknown). TRIM19, also known as PML.
TRIMs are involved in a variety of seemingly disparate cellular processes (Table S1); these include cell survival and death (Grignani et al., 1993), cell cycle and differentiation (Wang et al., 1998), metabolic states (Skurat et al., 2002), cell membrane repair (Cai et al., 2009), synaptic vesicle exocytosis (Li et al., 2001), senescence (Pearson et al., 2000), stem cell pluripotency (Sato et al., 2012) and erythrocyte differentiation (Blaybel et al., 2008; Barde et al., 2013), as well as the control of viral, bacterial and fungal infections (Nisole et al., 2005; Rakebrandt et al., 2014; Cao et al., 2015). These and additional roles are linked to a variety of health conditions. For instance, TRIMs have been implicated in autoimmune and inflammatory disorders (Jefferies et al., 2011; Kawai and Akira, 2011; Kimura et al., 2015) that are associated with genetic polymorphisms (French FMF Consortium, 1997; The International FMF Consortium Guido, 1997), as well as in insulin resistance (Karlberg et al., 2005; Song et al., 2013), urate metabolism (Köttgen et al., 2013), cancer (Hatakeyama, 2011), asthma (Shin et al., 2011; Collison et al., 2013), and neurodegenerative and neuromuscular diseases (Leonhardt et al., 1994; Khan et al., 2014). Among the best-studied roles for TRIMs is their role in cell-autonomous antiviral defense, a trend partially driven by intense research focusing on human immunodeficiency virus (HIV) (Stremlau et al., 2004; Nisole et al., 2005; Pertel et al., 2011; Versteeg et al., 2013).
TRIMs function as autophagic ‘receptor regulators’
A growing number of studies indicate that several TRIMs are linked to autophagy (Fig. 2C; Table S1) (Lipinski et al., 2010; Niida et al., 2010; Perera et al., 2011; McKnight et al., 2012; Tomar et al., 2012; Barde et al., 2013; Pizon et al., 2013; Yang et al., 2013; Khan et al., 2014; Mandell et al., 2014; Choi et al., 2015; Kimura et al., 2015). TRIMs act as autophagy receptor regulators (meaning that they act as both receptors and platforms for assembly of autophagosome machinery) by two means. Firstly, TRIMs recognize their targets (Mandell et al., 2014; Kimura et al., 2015) by finding their cognate cargo (e.g. viral core, inflammasome components) through direct protein–protein binding without a need for ubiquitin or galectin intermediates (Figs 1 and 3A). Secondly, the same TRIMs that function as receptors then also act as platforms for the assembly of the core regulators of autophagy, such as ULK1, Beclin1, ATG16L1 (Mandell et al., 2014; Kimura et al., 2015) (Table S1). These two features distinguish TRIMs from SLRs (Table S2a) and from other unique receptors (Table S2b), and allow the execution of a highly selective form of autophagy, termed precision autophagy (Kimura et al., 2015).
Fig. 3.
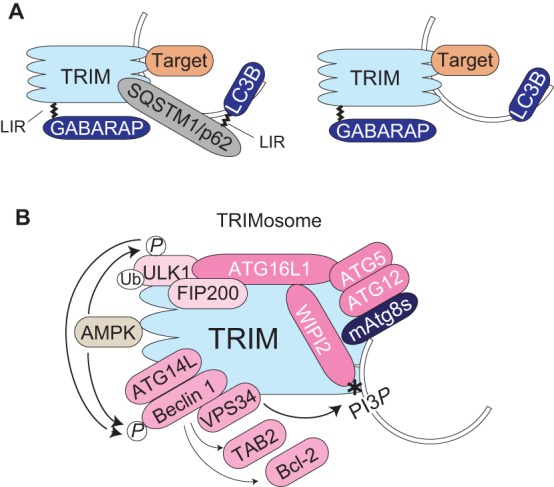
TRIM–SLR interactions and the TRIMosome. (A) TRIMs might link to LC3B directly (right) or indirectly (left) through SLRs, such as sequestosome-1/p62 (SQSTM1/p62). TRIMs interact with mammalian (m)Atg8s through LIRs that show a preference for GABARAPs in vitro. (B) TRIMosome, TRIM protein complex engaged in precision autophagy. Members of the complex and activation events are shown. See text for details. FIP200, also known as RB1CC1; P, phosphorylation; PI3P, phosphatidylinositol 3-phosphate (*); Ub, ubiquitin.
TRIMs as receptors
TRIMs recognize both endogenous and exogenous cargo for autophagy (Fig. 2C). For instance, TRIM20 (also known as MEFV) and TRIM21 have been shown to act as receptors for specific endogenous autophagy targets (Kimura et al., 2015). TRIM20 recognizes the inflammasome components pro-caspase 1, and NLR-family pyrin-domain-containing 1 and 3 proteins (NLRP1 and NLRP3), and presents them for autophagic degradation (Fig. 2C). TRIM21 targets the activated (dimerized) form of interferon regulatory factor 3 (IRF-3), a key transcriptional regulator of type-I interferon (IFN) genes, to mediate autophagy. Both TRIM20 and TRIM21 bind to their targets through their C-terminal SPRY domains. Rhesus TRIM5 recognizes an exogenous target – it binds to the HIV-1 capsid within the incoming viral core directly through its C-terminal SPRY domain (Stremlau et al., 2006; Ganser-Pornillos et al., 2011). This delivers the capsid protein p24 (CA) for TRIM5-directed autophagic degradation (Mandell et al., 2014), contributing to anti-retroviral modalities that are mediated by TRIM5 (Pertel et al., 2011).
The domains present at the C-terminus in the different TRIMs show a considerable variety (Fig. 2A,B; Table S1), suggesting that TRIMs recognize diverse cargo. Domains at the C-termini include fibronectin type-III repeats (FN3) for DNA or heparin binding, PHD fingers for histone binding, BROMO for acetylated lysine residue binding, FIL for actin binding, COS for microtubule binding, and MATH as well as NHL domains, in addition to the abovementioned SPRY (also known as PRY/SPRY) domain used in protein binding (Fig. 2B) (Reymond et al., 2001; Ozato et al., 2008; Sardiello et al., 2008). Moreover, even a single SPRY domain within the same TRIM can interact with many partners and potential substrates (Papin et al., 2007). In addition, the ability of TRIMs to hetero-oligomerize (Cao et al., 1997; Reymond et al., 2001; Bell et al., 2012; Kimura et al., 2015) might allow for a cooperative binding to diverse cargos, targeting them for degradation by autophagy.
TRIM receptors interact with mAtg8s
A common feature of conventional receptors for selective autophagy (Birgisdottir et al., 2013; Randow and Youle, 2014; Rogov et al., 2014; Stolz et al., 2014), such as SLRs, is that they bind to mAtg8s (e.g. LC3B or LC3C), which links them to autophagosomal membranes. TRIM5, TRIM20 and TRIM21 (Mandell et al., 2014; Kimura et al., 2015) bind to mAtg8s (Fig. 2A). Other TRIMs interact with mAtg8s (Table S1) in vitro, as shown for TRIM16, TRIM17, TRIM22, TRIM49 and TRIM55 (Mandell et al., 2014). Colocalization between mAtg8s and the TRIMs listed above in the cytoplasm (Niida et al., 2010; Pizon et al., 2013; Mandell et al., 2014; Kimura et al., 2015), as well as with TRIM63 (Khan et al., 2014), corroborate the biochemical findings. Potential binding to mAtg8 has also been documented for TRIM19 [also known as promyelocytic leukemia (PML) protein] and TRIM28 (Behrends et al., 2010; He et al., 2014). TRIM19 co-immunoprecipitates with LC3B and colocalizes with it in the nucleus (He et al., 2014); this is of interest given that LC3B shuttles between the nucleus and the cytosol upon deacetylation in association with autophagy activation (Huang et al., 2015) and plays a role in autophagic clearance of nuclear components (Dou et al., 2015).
The binding sites for mAtg8 (LIRs; Fig. 2A; Table S1) on several TRIMs have been mapped. TRIM5 has two LIRs, whereas TRIM20 has three LIRs (Mandell et al., 2014; Kimura et al., 2015). Only when all LIRs are mutated or deleted do TRIMs lose their ability to bind to mAtg8s (Mandell et al., 2014; Kimura et al., 2015). The TRIMs show a preference for GABARAPs and LC3A, and do not bind to LC3B when tested in vitro. Nevertheless, LC3B can be found in larger protein complexes with TRIMs when precipitated from cells (Pizon et al., 2013; He et al., 2014; Mandell et al., 2014). Thus, it is likely that LC3B forms larger complexes with TRIMs, possibly indirectly and in a manner that is mediated by an intermediary protein (e.g. p62, discussed below) or following putative post-translational modifications.
Some TRIMs bind to SLRs
Certain TRIMs have the ability to bind to SLRs, such as p62 (Fig. 3A); the known examples include TRIM5 (O'Connor et al., 2010), TRIM50 (Fusco et al., 2012), TRIM55 (Lange et al., 2005; Pizon et al., 2013; Mandell et al., 2014), TRIM13 (Tomar et al., 2012), TRIM17 and TRIM49 (Mandell et al., 2014), TRIM21 (Kimura et al., 2015), as well as TRIM76 (Blandin et al., 2013). Furthermore, p62 can colocalize with TRIM63 in the cytoplasm (Khan et al., 2014) and with TRIM19 in the nucleus within the nuclear PML bodies (Pankiv et al., 2010), although this might not be restricted to the nucleus because TRIM19 functions in the cytoplasm as well (Lin et al., 2004; Dutrieux et al., 2015). The association with p62 might link TRIMs indirectly to LC3B (as discussed above); this is of significance for those TRIMs with no evidence of direct binding to LC3B. In contrast, TRIM16 and TRIM20 do not appear to associate with p62 (Mandell et al., 2014). Whether these TRIMs associate with other SLRs or do not interact with SLRs is unknown.
Similar to p62, some TRIMs can themselves be degraded by autophagy. For instance, TRIM50 (Fusco et al., 2012) and murine TRIM30, a homolog of human TRIM5, are degraded by autophagy (Choi et al., 2015). TRIM20, which mediates autophagic degradation of NLRP3, is degraded in autolysosomes but only in the presence of its target (Kimura et al., 2015).
TRIMs as autophagy regulators
TRIMs affect autophagy as a whole process (Lipinski et al., 2010; Perera et al., 2011; McKnight et al., 2012; Tomar et al., 2012; Barde et al., 2013; Pizon et al., 2013; Khan et al., 2014; Pineda et al., 2015) and interact with ATG factors (Behrends et al., 2010; Yang et al., 2013; Mandell et al., 2014; Kimura et al., 2015). Of the 82 human TRIMs, 49 TRIMs have an effect on autophagy (Table S1). The first screens of TRIMs (Mandell et al., 2014) assessed the role of the entire TRIM family on autophagy that had been induced by inhibition of mTOR, a classic way of activating autophagosome formation. Using this approach and pp242 as an mTOR inhibitor, 32 TRIMs were identified as regulating autophagy and TRIM5 was among those hits (Mandell et al., 2014). Another screen (Kimura et al., 2015) that aimed to identify TRIMs affecting immunologically induced autophagy utilized IFN-γ, which is known from earlier studies to induce autophagy but with incompletely understood molecular mechanisms. These studies have revealed that 24 TRIMs (including TRIM8, TRIM20 and TRIM21) are required for optimal induction of autophagy through IFN-γ (Kimura et al., 2015). Several genome-wide screens, albeit not aimed at studying TRIMs, indicate that a number of additional TRIMs (TRIM29, TRIM51 and TRIM69) modulate autophagy (Lipinski et al., 2010; McKnight et al., 2012). Furthermore, TRIM13, TRIM28, TRIM55, TRIM56 and TRIM63 have been reported in separate studies to affect autophagy (Tomar et al., 2012; Barde et al., 2013; Pizon et al., 2013; Yang et al., 2013; Khan et al., 2014; Kimura et al., 2015; Pineda et al., 2015).
Two recent studies show that TRIMs directly assemble the cardinal autophagy regulators ULK1 and Beclin 1 into a protein complex that we have called the TRIMosome (Fig. 3B). Within a TRIMosome, individual TRIMs (e.g. TRIM5, TRIM6, TRIM17, TRIM20, TRIM21, TRIM22 and TRIM49) form protein complexes with both ULK1 and Beclin 1 (Mandell et al., 2014; Kimura et al., 2015). Induction of autophagy depends on a phosphorylation cascade that includes activation of ULK1 by AMPK-mediated phosphorylation at several sites, including Ser317 and Ser555 (Egan et al., 2011; Kim et al., 2011). TRIMosomes contain detectable amounts of AMPK (Kimura et al., 2015) and are enriched for the activated form of ULK1 – i.e. phosphorylated by AMPK at Ser317 (Mandell et al., 2014; Kimura et al., 2015) and Ser555 (Kimura et al., 2015). Beclin 1 within the TRIMosome complexes is phosphorylated at Ser15 (Mandell et al., 2014), an indication of its activation by ULK1 (Russell et al., 2013), and at Ser91 and Ser94, indicative of activation by AMPK (Kim et al., 2013). These molecular events are reminiscent of the assembly of activated ULK1 and Beclin 1, which are anchored by immunity-related GTPase M (IRGM) (Chauhan et al., 2015), another type of platform for the assembly of autophagic machinery.
TRIM8, identified in a screen for IFN-γ-inducible autophagy regulators (Kimura et al., 2015), ubiquitylates and activates TGFβ-activated kinase 1 (TAK1; also known as NR2C2) (Li et al., 2011), which in turn activates AMPK (Xie et al., 2006), and so links to the key roles of AMPK described above. Several TRIMs (e.g. TRIM13 and TRIM5) show spatial juxtaposition to double FYVE-containing protein 1 (DFCP1) (Tomar et al., 2012; Mandell et al., 2014), a marker for the omegasome, which is believed to be the cradle for autophagosome formation from the endoplasmic reticulum (ER) (Axe et al., 2008). Other autophagy factors, FIP200 (an ULK1 interactor), VPS34, ATG14L and UVRAG (Beclin-1-interacting proteins), WIPI2 [a PtdIns(3)P-binding protein interacting with ATG16L1], ATG16L1, ATG5 and ATG12 (an ‘E3 ligase for mAtg8 lipidation), and activating molecule in BECN1-regulated autophagy protein 1 (AMBRA1) have been detected in complexes with TRIMs (Behrends et al., 2010; Yang et al., 2013; Mandell et al., 2014; Kimura et al., 2015). TRIM5 activates this complex by promoting dissociation of the negative regulators Bcl-2 and TAB2 (Wei et al., 2008; Criollo et al., 2011; Takaesu et al., 2012) from Beclin 1 (Mandell et al., 2014); this is likely to occur in conjunction with the Beclin 1 phosphorylation events described above.
TRIM-dependent regulation can both accelerate and decelerate autophagy. TRIM17, a negative regulator of pp242-induced autophagy, sequesters the autophagy machinery away from bulk autophagy (Mandell et al., 2014). Furthermore, even a specific TRIM protein might have positive and negative effects on autophagy, as in the case of TRIM28 (Barde et al., 2013; Yang et al., 2013; Mandell et al., 2014; Pineda et al., 2015). For its positive effect, TRIM28 activates VPS34 by modulating binding to Beclin 1 (Yang et al., 2013). For its negative effect, TRIM28, as an E3 ligase, has been reported to mediate proteasomal degradation of AMPK, potentially downregulating autophagy (Pineda et al., 2015). Splice variants of TRIM55 have different activities in the proteasome and autophagy systems during muscle cell differentiation (Pizon et al., 2013), increasing one or the other.
Precision autophagy
The definition of precision autophagy is based on the twofold principle that the same entity (e.g. a TRIM) recognizes directly its cognate autophagic cargo without a need for tags such as ubiquitin or galectins, and at the same time mediating the assembly of core ATG factors into autophagy-activating complexes (Kimura et al., 2015).
An important question arises as to whether the previously described conventional receptors could eventually fit into the broader definition of precision autophagy (Birgisdottir et al., 2013; Randow and Youle, 2014; Rogov et al., 2014; Stolz et al., 2014). These receptors, such as p62, associate with other factors, including autophagy-linked FYVE protein (ALFY; also known as WDFY3) (Clausen et al., 2010), which in turn binds to ATG5, ATG12 and ATG16L1 (Filimonenko et al., 2010). ULK1 and p62 colocalize on the ER in the early stages of autophagy initiation (Itakura and Mizushima, 2010), and p62 binds to ULK1 and ULK2 (Pridgeon et al., 2003; Ro et al., 2014). NDP52 interacts with FIP200 (Wang et al., 2011), and recent studies with optineurin and NDP52 hint that these proteins affect the distribution of autophagy regulators such as ULK1, WIPI1 and DFCP1 (Lazarou et al., 2015). These observations suggest that SLRs, either alone or in combination with other platforms, could act in precision autophagy akin to TRIMs, but more work is needed to establish such relationships. There are also other platform-forming complexes that, similar to TRIMs, assemble ATG factors, such as the exocyst complex (Bodemann et al., 2011) and IRGM (Chauhan et al., 2015); however, cargo receptors within such complexes have not been defined. Thus, a broader definition of precision autophagy might emerge that encompasses additional new or previously studied receptors and molecular assemblies.
Other autophagy receptors
Beyond the receptor classes of TRIMs (Table S1) and SLRs (Table S2a), unique autophagy receptors have also been described (Table S2b) that participate in specific forms of autophagy. Atg32 is a receptor for mitophagy in yeast (Kanki et al., 2009; Okamoto et al., 2009) and its equivalent, Bcl2-L-13, has been identified in mammals (Murakawa et al., 2015). During reticulocyte development, Nix (also known as BNIP3L) appears to be the receptor for mitophagy (Sandoval et al., 2008; Schwarten et al., 2009; Novak et al., 2010). FUNDC1 (Liu et al., 2012) and BNIP3 (Zhang et al., 2008; Zhu et al., 2013) contribute to mitophagy during hypoxia in mammals. Nevertheless, there is evidence that subsets of SLRs (Lazarou et al., 2015; Matsumoto et al., 2015) play a role in mammalian mitophagy in conjunction with the PINK1–Parkin–phosphorylated-ubiquitin system (Koyano et al., 2014; Lazarou et al., 2015), which is activated in response to mitochondrial damage. Of note, the mitochondria-specific phospholipid cardiolipin is directly recognized by LC3B (Chu et al., 2013), potentially short-circuiting or aiding receptor recognition. A further implication of lipid recognition in mitophagy comes from the contribution to this process of SMURF1 (Orvedahl et al., 2011) – a C2-domain-containing protein (indicative of a lipid recognition) – and IRGM, which binds to cardiolipin (Singh et al., 2010). How all of these receptors and factors connect with the upstream regulators of autophagy remains to be determined.
Other organelles are recognized by unique receptors in order to be targeted for autophagy. In yeast and Pichia, pexophagy occurs through the peroxisome-anchored receptors Atg30 and Atg36 (Farré et al., 2008; Motley et al., 2012); albeit pexophagy that is mediated by the SLR NBR1 has been described in mammalian cells (Deosaran et al., 2013). ER-phagy is facilitated by membrane-associated receptors, such as FAM134B in mammals (Khaminets et al., 2015) and Atg40 (the functional counterpart of FAM134B in yeast); these function as receptors for autophagy of peripheral ER, whereas an additional receptor, Atg39 acts on peri-nuclear ER (Mochida et al., 2015).
Various proteinaceous targets and complexes are recognized not through ubiquitin but through unique receptors. NCOA4 has a receptor role in autophagy of ferritin (Dowdle et al., 2014; Mancias et al., 2014); NCOA4 binds to mAtg8 proteins in a manner similar to that of TRIMs with the exception of LC3B and recruits the large 450-kDa ferritin complex to the autolysosome (Dowdle et al., 2014; Mancias et al., 2014), thereby contributing to iron homeostasis. STBD1, which binds to GABARAP and GABARAPL1, and glycogen synthase, which binds to Atg8 in Drosophila, could be receptors that recognise glycogen for autophagic degradation (Jiang et al., 2011; Zirin et al., 2013).
Role of TRIM-directed precision autophagy in disease
TRIMs have a role in infectious, autoimmune and neoplastic diseases (Ozato et al., 2008; Hatakeyama, 2011; Jefferies et al., 2011; Kawai and Akira, 2011). In the context of inflammatory etiology of disease, autophagy can suppress inflammasome output either indirectly (Saitoh et al., 2008; Schroder and Tschopp, 2010; Nakahira et al., 2011; Zhou et al., 2011), or directly through selective autophagy of assembled inflammasomes (Shi et al., 2012) or inflammasome components, such as that mediated by TRIM20 (Kimura et al., 2015). TRIM20, also known as PYRIN, is encoded by the MEFV gene, which is the risk locus for familial Mediterranean fever (FMF). TRIM20 has 308 FMF-associated variants (http://fmf.igh.cnrs.fr/ISSAID/infevers/), and these polymorphisms commonly occur in its PRY/SPRY domain (Masters et al., 2009). TRIM20 variants with mutations in the PRY/SPRY domain show deficiency in the autophagic degradation of NLRP3 (Kimura et al., 2015). Additional FMF-associated polymorphisms fall within the TRIM20 LIR motifs (E403K in the first LIR motif, Y471X and E474K in the second LIR motif). Of note, TRIM20 expression is strongly induced by IFN-γ (Carthagena et al., 2009; Chae et al., 2011). Thus, TRIM expression is regulated, which in turn determines the outcome – i.e. the IFN-γ–TRIM20–autophagy axis might suppress excessive inflammasome activation, in keeping with the notion that inflammasome activation can be inhibited by IFN-γ (Chae et al., 2011). This capacity could be compromised in FMF owing to TRIM20 polymorphisms, which reduces the autophagic degradation of inflammasome components.
One of the hallmarks of autoimmune diseases, such as systemic lupus erythematosus (SLE) and Sjögren syndrome, is the activation of type-I IFN (Banchereau and Pascual, 2006). The autophagic receptor regulator TRIM21 directs precision autophagy of activated (dimerized) IRF-3, thereby reducing activation of type-I IFN (Kimura et al., 2015). This is also reflected in the related ability of TRIM21 to degrade inhibitor of NF-κB kinase (IKKβ, encoded by IKBKB) through autophagy (Niida et al., 2010). TRIM21 also targets other factors that are responsible for type-I IFN activation, including IRF-5, IRF-7 and IRF-8, for degradation through proteasomal or, thus far, unknown mechanisms (Higgs et al., 2008, 2010; Espinosa et al., 2009; Yoshimi et al., 2009; Young et al., 2011; Lazzari et al., 2014). TRIMs are often described as autoantigens in autoimmune diseases and cancers, including TRIM21 and TRIM68 in SLE and Sjögren syndrome (Der et al., 1998; Billaut-Mulot et al., 2001), TRIM33 in dermatomyositis and the associated paraneoplastic phenomena (Targoff et al., 2006; Fujimoto et al., 2012), TRIM19 in primary biliary cirrhosis (Stinton et al., 2011), and TRIM28 in colon cancer (Kijanka et al., 2010; Hector et al., 2012) and dermatomyositis (Satoh et al., 2012). Furthermore, TRIMs as a protein family have been associated with the regulation of type-I IFN responses (Versteeg et al., 2013), in keeping with relationships described for TRIM21 above (Kimura et al., 2015).
TRIMs play a role in defense against viral pathogens (Nisole et al., 2005), which at least in the case of TRIM5 involves precision autophagy (Mandell et al., 2014). The Rhesus TRIM5 can execute precision autophagy of the HIV-1 capsid. In contrast, the weak affinity of human TRIM5 for the HIV-1 capsid precludes effective precision autophagy. As a consequence, Rhesus TRIM5, but not human TRIM5, contributes to defense against HIV-1 through precision autophagy. Antimicrobial roles of TRIMs that are mediated through autophagy might extend to a defense against other intracellular pathogens; this includes bacteria (Rakebrandt et al., 2014) and possibly fungi. For instance, the antifungal activity of TRIM62 that is conveyed by CARD9, a mediator of innate immunity signals, which although not yet formally linked to autophagy (Cao et al., 2015), is known to play a role in autophagy through the interactions it has with RUBICON (encoded by KIAA0226) (Yang et al., 2012).
A number of mutations and polymorphisms in TRIMs are known to be associated with cancer, neurodegeneration, neuro-muscular diseases, immune disease (e.g. FMF as described above), infections (as in the case of HIV-1 susceptibility), metabolic traits, neurodegeneration, psychiatric disorders, heart disease and aging processes (Table S1). Several TRIMs are also involved in oncogenesis (Table S1). A classic example of this is TRIM19, which is fused with retinoic acid receptor α (RARα) in acute PML disease (de Thé et al., 1991; Kakizuka et al., 1991). Furthermore, fusions between TRIM24 and B-Raf are found in liver cancer (Le Douarin et al., 1995), between TRIM27 and RET in papillary thyroid cancer (Hasegawa et al., 1996), and of TRIM4 with B-Raf in lung cancer (Zheng et al., 2014) or with MET in melanoma (Yeh et al., 2015). Among these, the TRIM19–RARα fusion protein is already known to be degraded by autophagy (Isakson et al., 2010; Wang et al., 2014), whereas clearance of TRIM19–RARα is a therapeutic target in acute PML disease (Nasr et al., 2008). Thus, precision autophagy of TRIM19–RARα and other oncogenic fusions could be beneficial in cancer treatment (Fig. 2C).
A recent sequencing of tumor tissues has revealed that TRIM23 is a previously unappreciated gene involved in cancer progression (Lawrence et al., 2014). TRIMs are also known to regulate tumor suppressor proteins such as p53 (encoded by TP53) (Hatakeyama, 2011). Indeed, TRIM24 (Allton et al., 2009), TRIM39 (Zhang et al., 2012a,b) and TRIM59 (Zhou et al., 2014) help to degrade p53. In addition, TRIM8, TRIM13, TRIM19, TRIM21 and TRIM25 also affect p53 stability (Bernardi et al., 2004; Joo et al., 2011; Caratozzolo et al., 2012; Reddy et al., 2014; Zhang et al., 2015). TRIM28, TRIM29 and TRIM32 antagonize p53 function (Wang et al., 2005; Yuan et al., 2010; Liu et al., 2014). In turn, TRIMs and p53 affect each other transcriptionally. TRIM3 increases the level of p53 (Cheung et al., 2010), and the expression of TRIM22 is increased by p53 (Obad et al., 2004), whereas that of TRIM9 is increased by mutated p53 (Okaichi et al., 2013). The relationship between TRIMs and p53 fit with the dual role of p53 in autophagy (Tasdemir et al., 2008; Tavernarakis et al., 2008).
A recent study has linked TRIM-directed autophagy with certain types of neurodegeneration (Khan et al., 2014). TRIM63 mediates autophagy to promote the turnover of nicotinic acetylcholine receptor in muscles to remodel neuromuscular junctions (Fig. 2C) (Kim et al., 2014). Thus, TRIMs and precision autophagy could play a wider role in neurodegenerative diseases.
Conclusion and future perspectives
The role of the TRIM proteins as autophagic receptors widens our understanding of the repertoire, versatility and capacity for selective autophagy in mammalian cells. In their role as assembly platforms, TRIMs provide a new paradigm for the organization, localization and homing of the autophagic apparatus in mammalian cells. The TRIM family has numerous members (82 members in humans), suggesting that they account for the regulation of a significant portion of selective autophagy. Moreover, the TRIM family has expanded over the course of evolution, suggesting that this family co-evolves with the expanding need for clearance of more complex or emerging targets. Given the breadth of the role of TRIMs in various diseases (Table S1), it will be important to explore precision autophagy – in addition to bulk autophagy – as a therapeutic target.
The integration of TRIMs with autophagy is multi-tiered. When TRIMs act as selective autophagy receptors, they also assemble the principal core autophagy regulators. This represents a key feature of precision autophagy. The physical ‘home’ or an embodiment of this dual role as both receptors and regulators is the TRIMosome (Fig. 3B), which furthermore might act as a mammalian equivalent of the phagophore-assembly site (PAS; also known as pre-autophagosomal structure) for precision autophagy. The TRIMosome proteinaceous organelle, or apparatus, initiates and executes autophagy in coordination with the recognition of its intended substrate.
The above concepts and relationships raise questions, such as how many TRIMs act as autophagic receptors and what are their specific targets? Do different TRIMs cooperate through their known heterotypic interactions in clearance of more complex (multivalent) targets? How is the autophagic role of TRIMs integrated with the other functions of TRIMs, including regulation of gene expression and pro-inflammatory signaling? What is the interplay between the E3 ligase activity of TRIMs and precision (or other forms of) autophagy? Do TRIMs regulate autophagic maturation in addition to the initiation of the process? Can TRIMs also negatively regulate autophagy through competition or by direct inhibitory activities, and does this reflect the need for a homeostatic ‘off switch’ in order to limit autophagy after it has been initiated? Finally, are there receptors other than TRIMs that fit the concept of receptor regulators and precision autophagy? Answering these questions and gaining a further understanding of the TRIMs promises to enlighten us about the specifics of how autophagy is deployed in mammalian cells.
Acknowledgements
We thank Drs Terje Johansen and Ashish Jain for important collaborative contributions to TRIM studies. We thank Drs Beth Levine and Ivan Dikic for input regarding the definition of precision autophagy.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by the National Institutes of Health [grant numbers AI042999 and AI111935]. T.K. was supported by Manpei Suzuki Diabetes Foundation and Uehara Memorial Foundation. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.163758/-/DC1
References
- Allton K., Jain A. K., Herz H.-M., Tsai W.-W., Jung S. Y., Qin J., Bergmann A., Johnson R. L. and Barton M. C. (2009). Trim24 targets endogenous p53 for degradation. Proc. Natl. Acad. Sci. USA 106, 11612-11616. 10.1073/pnas.0813177106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axe E. L., Walker S. A., Manifava M., Chandra P., Roderick H. L., Habermann A., Griffiths G. and Ktistakis N. T. (2008). Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 182, 685-701. 10.1083/jcb.200803137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J. and Pascual V. (2006). Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 25, 383-392. 10.1016/j.immuni.2006.08.010 [DOI] [PubMed] [Google Scholar]
- Barde I., Rauwel B., Marin-Florez R. M., Corsinotti A., Laurenti E., Verp S., Offner S., Marquis J., Kapopoulou A., Vanicek J. et al. (2013). A KRAB/KAP1-miRNA cascade regulates erythropoiesis through stage-specific control of mitophagy. Science 340, 350-353. 10.1126/science.1232398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrends C., Sowa M. E., Gygi S. P. and Harper J. W. (2010). Network organization of the human autophagy system. Nature 466, 68-76. 10.1038/nature09204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. L., Malyukova A., Holien J. K., Koach J., Parker M. W., Kavallaris M., Marshall G. M. and Cheung B. B. (2012). TRIM16 acts as an E3 ubiquitin ligase and can heterodimerize with other TRIM family members. PLoS ONE 7, e37470 10.1371/journal.pone.0037470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi R., Scaglioni P. P., Bergmann S., Horn H. F., Vousden K. H. and Pandolfi P. P. (2004). PML regulates p53 stability by sequestering Mdm2 to the nucleolus. Nat. Cell Biol. 6, 665-672. 10.1038/ncb1147 [DOI] [PubMed] [Google Scholar]
- Billaut-Mulot O., Cocude C., Kolesnitchenko V., Truong M.-J., Chan E. K. L., Hachula E., de la Tribonnière X., Capron A. and Bahr G. M. (2001). SS-56, a novel cellular target of autoantibody responses in Sjögren syndrome and systemic lupus erythematosus. J. Clin. Invest. 108, 861-869. 10.1172/JCI200113469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgisdottir A. B., Lamark T. and Johansen T. (2013). The LIR motif - crucial for selective autophagy. J. Cell Sci. 126, 3237-3247. [DOI] [PubMed] [Google Scholar]
- Bjørkøy G., Lamark T., Brech A., Outzen H., Perander M., Øvervatn A., Stenmark H. and Johansen T. (2005). p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 171, 603-614. 10.1083/jcb.200507002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandin G., Marchand S., Charton K., Danièle N., Gicquel E., Boucheteil J.-B., Bentaib A., Barrault L., Stockholm D., Bartoli M. et al. (2013). A human skeletal muscle interactome centered on proteins involved in muscular dystrophies: LGMD interactome. Skelet. Muscle 3, 3 10.1186/2044-5040-3-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaybel R., Théoleyre O., Douablin A. and Baklouti F. (2008). Downregulation of the Spi-1/PU.1 oncogene induces the expression of TRIM10/HERF1, a key factor required for terminal erythroid cell differentiation and survival. Cell Res. 18, 834-845. 10.1038/cr.2008.68 [DOI] [PubMed] [Google Scholar]
- Bodemann B. O., Orvedahl A., Cheng T., Ram R. R., Ou Y.-H., Formstecher E., Maiti M., Hazelett C. C., Wauson E. M., Balakireva M. et al. (2011). RalB and the exocyst mediate the cellular starvation response by direct activation of autophagosome assembly. Cell 144, 253-267. 10.1016/j.cell.2010.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudinot P., van der Aa L. M., Jouneau L., Du Pasquier L., Pontarotti P., Briolat V., Benmansour A. and Levraud J.-P. (2011). Origin and evolution of TRIM proteins: new insights from the complete TRIM repertoire of zebrafish and pufferfish. PLoS ONE 6, e22022 10.1371/journal.pone.0022022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C., Masumiya H., Weisleder N., Matsuda N., Nishi M., Hwang M., Ko J.-K., Lin P., Thornton A., Zhao X. et al. (2009). MG53 nucleates assembly of cell membrane repair machinery. Nat. Cell Biol. 11, 56-64. 10.1038/ncb1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao T., Borden K. L., Freemont P. S. and Etkin L. D. (1997). Involvement of the rfp tripartite motif in protein-protein interactions and subcellular distribution. J. Cell Sci. 110, 1563-1571. [DOI] [PubMed] [Google Scholar]
- Cao Z., Conway K. L., Heath R. J., Rush J. S., Leshchiner E. S., Ramirez-Ortiz Z. G., Nedelsky N. B., Huang H., Ng A., Gardet A. et al. (2015). Ubiquitin ligase TRIM62 regulates CARD9-mediated anti-fungal immunity and intestinal inflammation. Immunity 43, 715-726. 10.1016/j.immuni.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caratozzolo M. F., Micale L., Turturo M. G., Cornacchia S., Fusco C., Marzano F., Augello B., D'Erchia A. M., Guerrini L., Pesole G. et al. (2012). TRIM8 modulates p53 activity to dictate cell cycle arrest. Cell Cycle 11, 511-523. 10.4161/cc.11.3.19008 [DOI] [PubMed] [Google Scholar]
- Carthagena L., Bergamaschi A., Luna J. M., David A., Uchil P. D., Margottin-Goguet F., Mothes W., Hazan U., Transy C., Pancino G. et al. (2009). Human TRIM gene expression in response to interferons. PLoS ONE 4, e4894 10.1371/journal.pone.0004894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae J. J., Cho Y.-H., Lee G.-S., Cheng J., Liu P. P., Feigenbaum L., Katz S. I. and Kastner D. L. (2011). Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1beta activation and severe autoinflammation in mice. Immunity 34, 755-768. 10.1016/j.immuni.2011.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E. Y. W., Kir S. and Tooze S. A. (2007). siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J. Biol. Chem. 282, 25464-25474. 10.1074/jbc.M703663200 [DOI] [PubMed] [Google Scholar]
- Chauhan S., Mandell M. A. and Deretic V. (2015). IRGM governs the core autophagy machinery to conduct antimicrobial defense. Mol. Cell 58, 507-521. 10.1016/j.molcel.2015.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C. C., Yang C., Berger T., Zaugg K., Reilly P., Elia A. J., Wakeham A., You-Ten A., Chang N., Li L. et al. (2010). Identification of BERP (brain-expressed RING finger protein) as a p53 target gene that modulates seizure susceptibility through interacting with GABA(A) receptors. Proc. Natl. Acad. Sci. USA 107, 11883-11888. 10.1073/pnas.1006529107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi U. Y., Choi W. Y., Hur J. Y. and Kim Y.-J. (2015). Polyubiquitin chain-dependent protein degradation in TRIM30 cytoplasmic bodies. Exp. Mol. Med. 47, e159 10.1038/emm.2015.12 [DOI] [PubMed] [Google Scholar]
- Chu C. T., Ji J., Dagda R. K., Jiang J. F., Tyurina Y. Y., Kapralov A. A., Tyurin V. A., Yanamala N., Shrivastava I. H., Mohammadyani D. et al. (2013). Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat. Cell Biol. 15, 1197-1205. 10.1038/ncb2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T. H., Lamark T., Isakson P., Finley K., Larsen K. B., Brech A., Øvervatn A., Stenmark H., Bjørkøy G., Simonsen A. et al. (2010). p62/SQSTM1 and ALFY interact to facilitate the formation of p62 bodies/ALIS and their degradation by autophagy. Autophagy 6, 330-344. 10.4161/auto.6.3.11226 [DOI] [PubMed] [Google Scholar]
- Collison A., Hatchwell L., Verrills N., Wark P. A., de Siqueira A. P., Tooze M., Carpenter H., Don A. S., Morris J. C., Zimmermann N. et al. (2013). The E3 ubiquitin ligase midline 1 promotes allergen and rhinovirus-induced asthma by inhibiting protein phosphatase 2A activity. Nat. Med. 19, 232-237. 10.1038/nm.3049 [DOI] [PubMed] [Google Scholar]
- Criollo A., Niso-Santano M., Malik S. A., Michaud M., Morselli E., Mariño G., Lachkar S., Arkhipenko A. V., Harper F., Pierron G. et al. (2011). Inhibition of autophagy by TAB2 and TAB3. EMBO J. 30, 4908-4920. 10.1038/emboj.2011.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Thé H., Lavau C., Marchio A., Chomienne C., Degos L. and Dejean A. (1991). The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell 66, 675-684. 10.1016/0092-8674(91)90113-D [DOI] [PubMed] [Google Scholar]
- Deosaran E., Larsen K. B., Hua R., Sargent G., Wang Y., Kim S., Lamark T., Jauregui M., Law K., Lippincott-Schwartz J. et al. (2013). NBR1 acts as an autophagy receptor for peroxisomes. J. Cell Sci. 126, 939-952. 10.1242/jcs.114819 [DOI] [PubMed] [Google Scholar]
- Der S. D., Zhou A., Williams B. R. G. and Silverman R. H. (1998). Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95, 15623-15628. 10.1073/pnas.95.26.15623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V., Saitoh T. and Akira S. (2013). Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 13, 722-737. 10.1038/nri3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V., Kimura T., Timmins G., Moseley P., Chauhan S. and Mandell M. (2015). Immunologic manifestations of autophagy. J. Clin. Invest. 125, 75-84. 10.1172/JCI73945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley H. C., Razi M., Polson H. E. J., Girardin S. E., Wilson M. I. and Tooze S. A. (2014). WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12–5-16L1. Mol. Cell 55, 238-252. 10.1016/j.molcel.2014.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Z., Xu C., Donahue G., Shimi T., Pan J.-A., Zhu J., Ivanov A., Capell B. C., Drake A. M., Shah P. P. et al. (2015). Autophagy mediates degradation of nuclear lamina. Nature 527, 105-109. 10.1038/nature15548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle W. E., Nyfeler B., Nagel J., Elling R. A., Liu S., Triantafellow E., Menon S., Wang Z., Honda A., Pardee G. et al. (2014). Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat. Cell Biol. 16, 1069-1079. 10.1038/ncb3053 [DOI] [PubMed] [Google Scholar]
- Dutrieux J., Maarifi G., Portilho D. M., Arhel N. J., Chelbi-Alix M. K. and Nisole S. (2015). PML/TRIM19-dependent inhibition of retroviral reverse-transcription by Daxx. PLoS Pathog. 11, e1005280 10.1371/journal.ppat.1005280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan D. F., Shackelford D. B., Mihaylova M. M., Gelino S., Kohnz R. A., Mair W., Vasquez D. S., Joshi A., Gwinn D. M., Taylor R. et al. (2011). Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331, 456-461. 10.1126/science.1196371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa A., Dardalhon V., Brauner S., Ambrosi A., Higgs R., Quintana F. J., Sjostrand M., Eloranta M.-L., Ni Gabhann J., Winqvist O. et al. (2009). Loss of the lupus autoantigen Ro52/Trim21 induces tissue inflammation and systemic autoimmunity by disregulating the IL-23-Th17 pathway. J. Exp. Med. 206, 1661-1671. 10.1084/jem.20090585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré J. C., Manjithaya R., Mathewson R. D. and Subramani S. (2008). PpAtg30 tags peroxisomes for turnover by selective autophagy. Dev. Cell 14, 365-376. 10.1016/j.devcel.2007.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filimonenko M., Isakson P., Finley K. D., Anderson M., Jeong H., Melia T. J., Bartlett B. J., Myers K. M., Birkeland H. C. G., Lamark T. et al. (2010). The selective macroautophagic degradation of aggregated proteins requires the PI3P-binding protein Alfy. Mol. Cell 38, 265-279. 10.1016/j.molcel.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French FMF Consortium (1997). A candidate gene for familial Mediterranean fever. Nat. Genet. 17, 25-31. 10.1038/ng0997-25 [DOI] [PubMed] [Google Scholar]
- Fujimoto M., Hamaguchi Y., Kaji K., Matsushita T., Ichimura Y., Kodera M., Ishiguro N., Ueda-Hayakawa I., Asano Y., Ogawa F. et al. (2012). Myositis-specific anti-155/140 autoantibodies target transcription intermediary factor 1 family proteins. Arthritis Rheum. 64, 513-522. 10.1002/art.33403 [DOI] [PubMed] [Google Scholar]
- Fujita N., Morita E., Itoh T., Tanaka A., Nakaoka M., Osada Y., Umemoto T., Saitoh T., Nakatogawa H., Kobayashi S. et al. (2013). Recruitment of the autophagic machinery to endosomes during infection is mediated by ubiquitin. J. Cell Biol. 203, 115-128. 10.1083/jcb.201304188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco C., Micale L., Egorov M., Monti M., D'Addetta E. V., Augello B., Cozzolino F., Calcagni A., Fontana A., Polishchuk R. S. et al. (2012). The E3-ubiquitin ligase TRIM50 interacts with HDAC6 and p62, and promotes the sequestration and clearance of ubiquitinated proteins into the aggresome. PLoS ONE 7, e40440 10.1371/journal.pone.0040440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L., Pietrocola F., Levine B. and Kroemer G. (2014). Metabolic control of autophagy. Cell 159, 1263-1276. 10.1016/j.cell.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammoh N., Florey O., Overholtzer M. and Jiang X. (2013). Interaction between FIP200 and ATG16L1 distinguishes ULK1 complex–dependent and –independent autophagy. Nat. Struct. Mol. Biol. 20, 144-149. 10.1038/nsmb.2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganser-Pornillos B. K., Chandrasekaran V., Pornillos O., Sodroski J. G., Sundquist W. I. and Yeager M. (2011). Hexagonal assembly of a restricting TRIM5alpha protein. Proc. Natl. Acad. Sci. USA 108, 534-539. 10.1073/pnas.1013426108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignani F., Ferrucci P. F., Testa U., Talamo G., Fagioli M., Alcalay M., Mencarelli A., Grignani F., Peschle C., Nicoletti I. et al. (1993). The acute promyelocytic leukemia-specific PML-RARalpha fusion protein inhibits differentiation and promotes survival of myeloid precursor cells. Cell 74, 423-431. 10.1016/0092-8674(93)80044-F [DOI] [PubMed] [Google Scholar]
- Hasegawa N., Iwashita T., Asai N., Murakami H., Iwata Y., Isomura T., Goto H., Hayakawa T. and Takahashi M. (1996). A RING finger motif regulates transforming activity of the rfp/ret fusion gene. Biochem. Biophys. Res. Commun. 225, 627-631. 10.1006/bbrc.1996.1221 [DOI] [PubMed] [Google Scholar]
- Hatakeyama S. (2011). TRIM proteins and cancer. Nat. Rev. Cancer 11, 792-804. 10.1038/nrc3139 [DOI] [PubMed] [Google Scholar]
- He W., Hu C.-X., Hou J.-K., Fan L., Xu Y.-W., Liu M.-H., Yan S.-Y., Chen G.-Q. and Huang Y. (2014). Microtubule-associated protein 1 light chain 3 interacts with and contributes to growth inhibiting effect of PML. PLoS ONE 9, e113089 10.1371/journal.pone.0113089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector S., Chen H., Kijanka G., Murray F. and Prehn J. H. H. (2012). A reverse-ELISA for the detection of TRIM28/KAP1 serum autoantibodies in colorectal cancer patients. Acta Oncol. 51, 394-396. 10.3109/0284186X.2011.652742 [DOI] [PubMed] [Google Scholar]
- Higgs R., Ni Gabhann J., Ben Larbi N., Breen E. P., Fitzgerald K. A. and Jefferies C. A. (2008). The E3 ubiquitin ligase Ro52 negatively regulates IFN-beta production post-pathogen recognition by polyubiquitin-mediated degradation of IRF3. J. Immunol. 181, 1780-1786. 10.4049/jimmunol.181.3.1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs R., Lazzari E., Wynne C., Ní Gabhann J., Espinosa A., Wahren-Herlenius M. and Jefferies C. A. (2010). Self protection from anti-viral responses--Ro52 promotes degradation of the transcription factor IRF7 downstream of the viral Toll-Like receptors. PLoS ONE 5, e11776 10.1371/journal.pone.0011776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R., Xu Y., Wan W., Shou X., Qian J., You Z., Liu B., Chang C., Zhou T., Lippincott-Schwartz J. et al. (2015). Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol. Cell 57, 456-466. 10.1016/j.molcel.2014.12.013 [DOI] [PubMed] [Google Scholar]
- Isakson P., Bjoras M., Boe S. O. and Simonsen A. (2010). Autophagy contributes to therapy-induced degradation of the PML/RARA oncoprotein. Blood 116, 2324-2331. 10.1182/blood-2010-01-261040 [DOI] [PubMed] [Google Scholar]
- Itakura E. and Mizushima N. (2010). Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins. Autophagy 6, 764-776. 10.4161/auto.6.6.12709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura E., Kishi-Itakura C. and Mizushima N. (2012). The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 151, 1256-1269. 10.1016/j.cell.2012.11.001 [DOI] [PubMed] [Google Scholar]
- Jefferies C., Wynne C. and Higgs R. (2011). Antiviral TRIMs: friend or foe in autoimmune and autoinflammatory disease? Nat. Rev. Immunol. 11, 617-625. 10.1038/nri3043 [DOI] [PubMed] [Google Scholar]
- Jiang S., Wells C. D. and Roach P. J. (2011). Starch-binding domain-containing protein 1 (Stbd1) and glycogen metabolism: identification of the Atg8 family interacting motif (AIM) in Stbd1 required for interaction with GABARAPL1. Biochem. Biophys. Res. Commun. 413, 420-425. 10.1016/j.bbrc.2011.08.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo H. M., Kim J. Y., Jeong J. B., Seong K. M., Nam S. Y., Yang K. H., Kim C. S., Kim H. S., Jeong M., An S. et al. (2011). Ret finger protein 2 enhances ionizing radiation-induced apoptosis via degradation of AKT and MDM2. Eur. J. Cell Biol. 90, 420-431. 10.1016/j.ejcb.2010.12.001 [DOI] [PubMed] [Google Scholar]
- Kabeya Y., Mizushima N., Ueno T., Yamamoto A., Kirisako T., Noda T., Kominami E., Ohsumi Y. and Yoshimori T. (2000). LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720-5728. 10.1093/emboj/19.21.5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizuka A., Miller W. H. Jr, Umesono K., Warrell R. P. Jr, Frankel S. R., Murty V. V. V. S., Dmitrovsky E. and Evans R. M. (1991). Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RARalpha with a novel putative transcription factor, PML. Cell 66, 663-674. 10.1016/0092-8674(91)90112-C [DOI] [PubMed] [Google Scholar]
- Kanki T., Wang K., Cao Y., Baba M. and Klionsky D. J. (2009). Atg32 is a mitochondrial protein that confers selectivity during mitophagy. Dev. Cell 17, 98-109. 10.1016/j.devcel.2009.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlberg N., Jalanko H., Kallijarvi J., Lehesjoki A.-E. and Lipsanen-Nyman M. (2005). Insulin resistance syndrome in subjects with mutated RING finger protein TRIM37. Diabetes 54, 3577-3581. 10.2337/diabetes.54.12.3577 [DOI] [PubMed] [Google Scholar]
- Kawai T. and Akira S. (2011). Regulation of innate immune signalling pathways by the tripartite motif (TRIM) family proteins. EMBO Mol. Med. 3, 513-527. 10.1002/emmm.201100160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaminets A., Heinrich T., Mari M., Grumati P., Huebner A. K., Akutsu M., Liebmann L., Stolz A., Nietzsche S., Koch N. et al. (2015). Regulation of endoplasmic reticulum turnover by selective autophagy. Nature 522, 354-358. 10.1038/nature14498 [DOI] [PubMed] [Google Scholar]
- Khan M. M., Strack S., Wild F., Hanashima A., Gasch A., Brohm K., Reischl M., Carnio S., Labeit D., Sandri M. et al. (2014). Role of autophagy, SQSTM1, SH3GLB1, and TRIM63 in the turnover of nicotinic acetylcholine receptors. Autophagy 10, 123-136. 10.4161/auto.26841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijanka G., Hector S., Kay E. W., Murray F., Cummins R., Murphy D., MacCraith B. D., Prehn J. H. M. and Kenny D. (2010). Human IgG antibody profiles differentiate between symptomatic patients with and without colorectal cancer. Gut 59, 69-78. 10.1136/gut.2009.178574 [DOI] [PubMed] [Google Scholar]
- Kim J., Kundu M., Viollet B. and Guan K.-L. (2011). AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132-141. 10.1038/ncb2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Kim Y. C., Fang C., Russell R. C., Kim J. H., Fan W., Liu R., Zhong Q. and Guan K.-L. (2013). Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell 152, 290-303. 10.1016/j.cell.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-J., Syed G. H., Khan M., Chiu W.-W., Sohail M. A., Gish R. G. and Siddiqui A. (2014). Hepatitis C virus triggers mitochondrial fission and attenuates apoptosis to promote viral persistence. Proc. Natl. Acad. Sci. USA 111, 6413-6418. 10.1073/pnas.1321114111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Jain A., Choi S. W., Mandell M. A., Schroder K., Johansen T. and Deretic V. (2015). TRIM-mediated precision autophagy targets cytoplasmic regulators of innate immunity. J. Cell Biol. 210, 973-989. 10.1083/jcb.201503023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkin V., Lamark T., Sou Y.-S., Bjørkøy G., Nunn J. L., Bruun J.-A., Shvets E., McEwan D. G., Clausen T. H., Wild P. et al. (2009). A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol. Cell 33, 505-516. 10.1016/j.molcel.2009.01.020 [DOI] [PubMed] [Google Scholar]
- Köttgen A., Albrecht E., Teumer A., Vitart V., Krumsiek J., Hundertmark C., Pistis G., Ruggiero D., O'Seaghdha C. M., Haller T. et al. (2013). Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat. Genet. 45, 145-154. 10.1038/ng.2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyano F., Okatsu K., Kosako H., Tamura Y., Go E., Kimura M., Kimura Y., Tsuchiya H., Yoshihara H., Hirokawa T. et al. (2014). Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature 510, 162-166. 10.1038/nature13392 [DOI] [PubMed] [Google Scholar]
- Kundu M. and Thompson C. B. (2008). Autophagy: basic principles and relevance to disease. Annu. Rev. Pathol. 3, 427-455. 10.1146/annurev.pathmechdis.2.010506.091842 [DOI] [PubMed] [Google Scholar]
- Lamark T., Kirkin V., Dikic I. and Johansen T. (2009). NBR1 and p62 as cargo receptors for selective autophagy of ubiquitinated targets. Cell Cycle 8, 1986-1990. 10.4161/cc.8.13.8892 [DOI] [PubMed] [Google Scholar]
- Lange S., Xiang F., Yakovenko A., Vihola A., Hackman P., Rostkova E., Kristensen J., Brandmeier B., Franzen G., Hedberg B. et al. (2005). The kinase domain of titin controls muscle gene expression and protein turnover. Science 308, 1599-1603. 10.1126/science.1110463 [DOI] [PubMed] [Google Scholar]
- Lawrence M. S., Stojanov P., Mermel C. H., Robinson J. T., Garraway L. A., Golub T. R., Meyerson M., Gabriel S. B., Lander E. S. and Getz G. (2014). Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505, 495-501. 10.1038/nature12912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarou M., Sliter D. A., Kane L. A., Sarraf S. A., Wang C., Burman J. L., Sideris D. P., Fogel A. I. and Youle R. J. (2015). The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524, 309-314. 10.1038/nature14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzari E., Korczeniewska J., Ní Gabhann J., Smith S., Barnes B. J. and Jefferies C. A. (2014). TRIpartite Motif 21 (TRIM21) differentially regulates the stability of Interferon Regulatory Factor 5 (IRF5) isoforms. PLoS ONE 9, e103609 10.1371/journal.pone.0103609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin B., Zechel C., Garnier J. M., Lutz Y., Tora L., Pierrat P., Heery D., Gronemeyer H., Chambon P. and Losson R. (1995). The N-terminal part of TIF1, a putative mediator of the ligand-dependent activation function (AF-2) of nuclear receptors, is fused to B-raf in the oncogenic protein T18. EMBO J. 14, 2020-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt E. A., Kapp L. N., Young B. R. and Murnane J. P. (1994). Nucleotide sequence analysis of a candidate gene for ataxia-telangiectasia group D (ATDC). Genomics 19, 130-136. 10.1006/geno.1994.1022 [DOI] [PubMed] [Google Scholar]
- Li Y., Chin L.-S., Weigel C. and Li L. (2001). Spring, a novel RING finger protein that regulates synaptic vesicle exocytosis. J. Biol. Chem. 276, 40824-40833. 10.1074/jbc.M106141200 [DOI] [PubMed] [Google Scholar]
- Li Q., Yan J., Mao A.-P., Li C., Ran Y., Shu H.-B. and Wang Y.-Y. (2011). Tripartite motif 8 (TRIM8) modulates TNFalpha- and IL-1beta-triggered NF-kappaB activation by targeting TAK1 for K63-linked polyubiquitination. Proc. Natl. Acad. Sci. USA 108, 19341-19346. 10.1073/pnas.1110946108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X. H., Jackson S., Seaman M., Brown K., Kempkes B., Hibshoosh H. and Levine B. (1999). Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402, 672-676. 10.1038/45257 [DOI] [PubMed] [Google Scholar]
- Lin H.-K., Bergmann S. and Pandolfi P. P. (2004). Cytoplasmic PML function in TGF-beta signalling. Nature 431, 205-211. 10.1038/nature02783 [DOI] [PubMed] [Google Scholar]
- Lipinski M. M., Zheng B., Lu T., Yan Z., Py B. F., Ng A., Xavier R. J., Li C., Yankner B. A., Scherzer C. R. et al. (2010). Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer's disease. Proc. Natl. Acad. Sci. USA 107, 14164-14169. 10.1073/pnas.1009485107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Feng D., Chen G., Chen M., Zheng Q., Song P., Ma Q., Zhu C., Wang R., Qi W. et al. (2012). Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 14, 177-185. 10.1038/ncb2422 [DOI] [PubMed] [Google Scholar]
- Liu J., Zhang C., Wang X. L., Ly P., Belyi V., Xu-Monette Z. Y., Young K. H., Hu W. and Feng Z. (2014). E3 ubiquitin ligase TRIM32 negatively regulates tumor suppressor p53 to promote tumorigenesis. Cell Death Differ. 21, 1792-1804. 10.1038/cdd.2014.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K., Psakhye I. and Jentsch S. (2014). Autophagic clearance of polyQ proteins mediated by ubiquitin-Atg8 adaptors of the conserved CUET protein family. Cell 158, 549-563. 10.1016/j.cell.2014.05.048 [DOI] [PubMed] [Google Scholar]
- Maejima I., Takahashi A., Omori H., Kimura T., Takabatake Y., Saitoh T., Yamamoto A., Hamasaki M., Noda T., Isaka Y. et al. (2013). Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J. 32, 2336-2347. 10.1038/emboj.2013.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancias J. D., Wang X., Gygi S. P., Harper J. W. and Kimmelman A. C. (2014). Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 509, 105-109. 10.1038/nature13148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell M. A., Jain A., Arko-Mensah J., Chauhan S., Kimura T., Dinkins C., Silvestri G., Münch J., Kirchhoff F., Simonsen A. et al. (2014). TRIM proteins regulate autophagy and can target autophagic substrates by direct recognition. Dev. Cell 30, 394-409. 10.1016/j.devcel.2014.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R. S., Li F., Gemperline D. C., Book A. J. and Vierstra R. D. (2015). Autophagic degradation of the 26S proteasome is mediated by the dual ATG8/ubiquitin receptor RPN10 in arabidopsis. Mol. Cell 58, 1053-1066. 10.1016/j.molcel.2015.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters S. L., Simon A., Aksentijevich I. and Kastner D. L. (2009). Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease. Annu. Rev. Immunol. 27, 621-668. 10.1146/annurev.immunol.25.022106.141627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto G., Shimogori T., Hattori N. and Nukina N. (2015). TBK1 controls autophagosomal engulfment of polyubiquitinated mitochondria through p62/SQSTM1 phosphorylation. Hum. Mol. Genet. 24, 4429-4442. 10.1093/hmg/ddv179 [DOI] [PubMed] [Google Scholar]
- McKnight N. C., Jefferies H. B. J., Alemu E. A., Saunders R. E., Howell M., Johansen T. and Tooze S. A. (2012). Genome-wide siRNA screen reveals amino acid starvation-induced autophagy requires SCOC and WAC. EMBO J. 31, 1931-1946. 10.1038/emboj.2012.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N., Yoshimori T. and Ohsumi Y. (2011). The role of atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 27, 107-132. 10.1146/annurev-cellbio-092910-154005 [DOI] [PubMed] [Google Scholar]
- Mochida K., Oikawa Y., Kimura Y., Kirisako H., Hirano H., Ohsumi Y. and Nakatogawa H. (2015). Receptor-mediated selective autophagy degrades the endoplasmic reticulum and the nucleus. Nature 522, 359-362. 10.1038/nature14506 [DOI] [PubMed] [Google Scholar]
- Motley A. M., Nuttall J. M. and Hettema E. H. (2012). Pex3-anchored Atg36 tags peroxisomes for degradation in Saccharomyces cerevisiae. EMBO J. 31, 2852-2868. 10.1038/emboj.2012.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakawa T., Yamaguchi O., Hashimoto A., Hikoso S., Takeda T., Oka T., Yasui H., Ueda H., Akazawa Y., Nakayama H. et al. (2015). Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat. Commun. 6, 7527 10.1038/ncomms8527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K., Haspel J. A., Rathinam V. A. K., Lee S.-J., Dolinay T., Lam H. C., Englert J. A., Rabinovitch M., Cernadas M., Kim H. P. et al. (2011). Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 12, 222-230. 10.1038/ni.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr R., Guillemin M.-C., Ferhi O., Soilihi H., Peres L., Berthier C., Rousselot P., Robledo-Sarmiento M., Lallemand-Breitenbach V., Gourmel B. et al. (2008). Eradication of acute promyelocytic leukemia-initiating cells through PML-RARA degradation. Nat. Med. 14, 1333-1342. 10.1038/nm.1891 [DOI] [PubMed] [Google Scholar]
- Nazio F., Strappazzon F., Antonioli M., Bielli P., Cianfanelli V., Bordi M., Gretzmeier C., Dengjel J., Piacentini M., Fimia G. M. et al. (2013). mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat. Cell Biol. 15, 406-416. 10.1038/ncb2708 [DOI] [PubMed] [Google Scholar]
- Newman A. C., Scholefield C. L., Kemp A. J., Newman M., McIver E. G., Kamal A. and Wilkinson S. (2012). TBK1 kinase addiction in lung cancer cells is mediated via autophagy of Tax1bp1/Ndp52 and non-canonical NF-kappaB signalling. PLoS ONE 7, e50672 10.1371/journal.pone.0050672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niida M., Tanaka M. and Kamitani T. (2010). Downregulation of active IKKbeta by Ro52-mediated autophagy. Mol. Immunol. 47, 2378-2387. 10.1016/j.molimm.2010.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T., Kaizuka T., Cadwell K., Sahani M. H., Saitoh T., Akira S., Virgin H. W. and Mizushima N. (2013). FIP200 regulates targeting of Atg16L1 to the isolation membrane. EMBO Rep. 14, 284-291. 10.1038/embor.2013.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisole S., Stoye J. P. and Saïb A. (2005). TRIM family proteins: retroviral restriction and antiviral defence. Nat. Rev. Microbiol. 3, 799-808. 10.1038/nrmicro1248 [DOI] [PubMed] [Google Scholar]
- Novak I., Kirkin V., McEwan D. G., Zhang J., Wild P., Rozenknop A., Rogov V., Löhr F., Popovic D., Occhipinti A. et al. (2010). Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 11, 45-51. 10.1038/embor.2009.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obad S., Brunnström H., Vallon-Christersson J., Borg A., Drott K. and Gullberg U. (2004). Staf50 is a novel p53 target gene conferring reduced clonogenic growth of leukemic U-937 cells. Oncogene 23, 4050-4059. 10.1038/sj.onc.1207524 [DOI] [PubMed] [Google Scholar]
- O'Connor C., Pertel T., Gray S., Robia S. L., Bakowska J. C., Luban J. and Campbell E. M. (2010). p62/sequestosome-1 associates with and sustains the expression of retroviral restriction factor TRIM5alpha. J. Virol. 84, 5997-6006. 10.1128/JVI.02412-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okaichi K., Izumi N., Takamura Y., Fukui S. and Kudo T. (2013). Transcriptional specificity in various p53-mutant cells. Anticancer Res. 33, 923-928. [PubMed] [Google Scholar]
- Okamoto K., Kondo-Okamoto N. and Ohsumi Y. (2009). Mitochondria-anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Dev. Cell 17, 87-97. 10.1016/j.devcel.2009.06.013 [DOI] [PubMed] [Google Scholar]
- Orvedahl A., Sumpter R. Jr, Xiao G., Ng A., Zou Z., Tang Y., Narimatsu M., Gilpin C., Sun Q., Roth M. et al. (2011). Image-based genome-wide siRNA screen identifies selective autophagy factors. Nature 480, 113-117. 10.1038/nature10546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K., Shin D.-M., Chang T.-H. and Morse H. C. III (2008). TRIM family proteins and their emerging roles in innate immunity. Nat. Rev. Immunol. 8, 849-860. 10.1038/nri2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiv S., Lamark T., Bruun J.-A., Overvatn A., Bjorkoy G. and Johansen T. (2010). Nucleocytoplasmic shuttling of p62/SQSTM1 and its role in recruitment of nuclear polyubiquitinated proteins to promyelocytic leukemia bodies. J. Biol. Chem. 285, 5941-5953. 10.1074/jbc.M109.039925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papin S., Cuenin S., Agostini L., Martinon F., Werner S., Beer H.-D., Grütter C., Grütter M. and Tschopp J. (2007). The SPRY domain of Pyrin, mutated in familial Mediterranean fever patients, interacts with inflammasome components and inhibits proIL-1beta processing. Cell Death Differ. 14, 1457-1466. 10.1038/sj.cdd.4402142 [DOI] [PubMed] [Google Scholar]
- Pearson M., Carbone R., Sebastiani C., Cioce M., Fagioli M., Saito S., Higashimoto Y., Appella E., Minucci S., Pandolfi P. P. et al. (2000). PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature 406, 207-210. 10.1038/35021000 [DOI] [PubMed] [Google Scholar]
- Perera S., Holt M. R., Mankoo B. S. and Gautel M. (2011). Developmental regulation of MURF ubiquitin ligases and autophagy proteins nbr1, p62/SQSTM1 and LC3 during cardiac myofibril assembly and turnover. Dev. Biol. 351, 46-61. 10.1016/j.ydbio.2010.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera R. M., Stoykova S., Nicolay B. N., Ross K. N., Fitamant J., Boukhali M., Lengrand J., Deshpande V., Selig M. K., Ferrone C. R. et al. (2015). Transcriptional control of autophagy–lysosome function drives pancreatic cancer metabolism. Nature 524, 361-365. 10.1038/nature14587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertel T., Hausmann S., Morger D., Züger S., Guerra J., Lascano J., Reinhard C., Santoni F. A., Uchil P. D., Chatel L. et al. (2011). TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature 472, 361-365. 10.1038/nature09976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petiot A., Ogier-Denis E., Blommaart E. F. C., Meijer A. J. and Codogno P. (2000). Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J. Biol. Chem. 275, 992-998. 10.1074/jbc.275.2.992 [DOI] [PubMed] [Google Scholar]
- Pineda C. T., Ramanathan S., Fon Tacer K., Weon J. L., Potts M. B., Ou Y.-H., White M. A. and Potts P. R. (2015). Degradation of AMPK by a cancer-specific ubiquitin ligase. Cell 160, 715-728. 10.1016/j.cell.2015.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizon V., Rybina S., Gerbal F., Delort F., Vicart P., Baldacci G. and Karsenti E. (2013). MURF2B, a novel LC3-binding protein, participates with MURF2A in the switch between autophagy and ubiquitin proteasome system during differentiation of C2C12 muscle cells. PLoS ONE 8, e76140 10.1371/journal.pone.0076140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridgeon J. W., Geetha T. and Wooten M. W. (2003). A method to identify p62's UBA domain interacting proteins. Biol. Proced. Online 5, 228-237. 10.1251/bpo66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz J. D. and White E. (2010). Autophagy and metabolism. Science 330, 1344-1348. 10.1126/science.1193497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakebrandt N., Lentes S., Neumann H., James L. C. and Neumann-Staubitz P. (2014). Antibody- and TRIM21-dependent intracellular restriction of Salmonella enterica. Pathog. Dis. 72, 131-137. 10.1111/2049-632X.12192 [DOI] [PubMed] [Google Scholar]
- Randow F. and Youle R. J. (2014). Self and nonself: how autophagy targets mitochondria and bacteria. Cell Host Microbe 15, 403-411. 10.1016/j.chom.2014.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy B. A., van der Knaap J. A., Bot A. G. M., Mohd-Sarip A., Dekkers D. H. W., Timmermans M. A., Martens J. W. M., Demmers J. A. A. and Verrijzer C. P. (2014). Nucleotide biosynthetic enzyme GMP synthase is a TRIM21-controlled relay of p53 stabilization. Mol. Cell 53, 458-470. 10.1016/j.molcel.2013.12.017 [DOI] [PubMed] [Google Scholar]
- Reymond A., Meroni G., Fantozzi A., Merla G., Cairo S., Luzi L., Riganelli D., Zanaria E., Messali S., Cainarca S. et al. (2001). The tripartite motif family identifies cell compartments. EMBO J. 20, 2140-2151. 10.1093/emboj/20.9.2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro S.-H., Semple I. A., Park H., Park H., Park H.-W., Kim M., Kim J. S. and Lee J. H. (2014). Sestrin2 promotes Unc-51-like kinase 1 mediated phosphorylation of p62/sequestosome-1. FEBS J. 281, 3816-3827. 10.1111/febs.12905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogov V., Dötsch V., Johansen T. and Kirkin V. (2014). Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol. Cell 53, 167-178. 10.1016/j.molcel.2013.12.014 [DOI] [PubMed] [Google Scholar]
- Rubinsztein D. C., Codogno P. and Levine B. (2012). Autophagy modulation as a potential therapeutic target for diverse diseases. Nat. Rev. Drug Discov. 11, 709-730. 10.1038/nrd3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. C., Tian Y., Yuan H., Park H. W., Chang Y.-Y., Kim J., Kim H., Neufeld T. P., Dillin A. and Guan K.-L. (2013). ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 15, 741-750. 10.1038/ncb2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T., Fujita N., Jang M. H., Uematsu S., Yang B.-G., Satoh T., Omori H., Noda T., Yamamoto N., Komatsu M. et al. (2008). Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 456, 264-268. 10.1038/nature07383 [DOI] [PubMed] [Google Scholar]
- Sandoval H., Thiagarajan P., Dasgupta S. K., Schumacher A., Prchal J. T., Chen M. and Wang J. (2008). Essential role for Nix in autophagic maturation of erythroid cells. Nature 454, 232-235. 10.1038/nature07006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardiello M., Cairo S., Fontanella B., Ballabio A. and Meroni G. (2008). Genomic analysis of the TRIM family reveals two groups of genes with distinct evolutionary properties. BMC Evol. Biol. 8, 225 10.1186/1471-2148-8-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Okumura F., Ariga T. and Hatakeyama S. (2012). TRIM6 interacts with Myc and maintains the pluripotency of mouse embryonic stem cells. J. Cell Sci. 125, 1544-1555. 10.1242/jcs.095273 [DOI] [PubMed] [Google Scholar]
- Satoh M., Chan J. Y. F., Ross S. J., Li Y., Yamasaki Y., Yamada H., Vazquez-del Mercado M., Petri M. H., Jara L. J., Saavedra M. A. et al. (2012). Autoantibodies to transcription intermediary factor TIF1beta associated with dermatomyositis. Arthritis Res. Ther. 14, R79 10.1186/ar3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K. and Tschopp J. (2010). The inflammasomes. Cell 140, 821-832. 10.1016/j.cell.2010.01.040 [DOI] [PubMed] [Google Scholar]
- Schwarten M., Mohrlüder J., Ma P., Stoldt M., Thielmann Y., Stangler T., Hersch N., Hoffmann B., Merkel R. and Willbold D. (2009). Nix directly binds to GABARAP: a possible crosstalk between apoptosis and autophagy. Autophagy 5, 690-698. 10.4161/auto.5.5.8494 [DOI] [PubMed] [Google Scholar]
- Settembre C., Di Malta C., Polito V. A., Arencibia M. G., Vetrini F., Erdin S., Erdin S. U., Huynh T., Medina D., Colella P. et al. (2011). TFEB links autophagy to lysosomal biogenesis. Science 332, 1429-1433. 10.1126/science.1204592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C.-S., Shenderov K., Huang N.-N., Kabat J., Abu-Asab M., Fitzgerald K. A., Sher A. and Kehrl J. H. (2012). Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 13, 255-263. 10.1038/ni.2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S. W., Oh T. J., Park S.-M., Park J. S., Jang A. S., Park S. W., Uh S. T., An S. and Park C.-S. (2011). Asthma-predictive genetic markers in gene expression profiling of peripheral blood mononuclear cells. Allergy Asthma Immunol. Res. 3, 265-272. 10.4168/aair.2011.3.4.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. B., Ornatowski W., Vergne I., Naylor J., Delgado M., Roberts E., Ponpuak M., Master S., Pilli M., White E. et al. (2010). Human IRGM regulates autophagy and cell-autonomous immunity functions through mitochondria. Nat. Cell Biol. 12, 1154-1165. 10.1038/ncb2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurat A. V., Dietrich A. D., Zhai L. and Roach P. J. (2002). GNIP, a novel protein that binds and activates glycogenin, the self-glucosylating initiator of glycogen biosynthesis. J. Biol. Chem. 277, 19331-19338. 10.1074/jbc.M201190200 [DOI] [PubMed] [Google Scholar]
- Song R., Peng W., Zhang Y., Lv F., Wu H.-K., Guo J., Cao Y., Pi Y., Zhang X., Jin L. et al. (2013). Central role of E3 ubiquitin ligase MG53 in insulin resistance and metabolic disorders. Nature 494, 375-379. 10.1038/nature11834 [DOI] [PubMed] [Google Scholar]
- Stinton L. M., Swain M., Myers R. P., Shaheen A. A. and Fritzler M. J. (2011). Autoantibodies to GW bodies and other autoantigens in primary biliary cirrhosis. Clin. Exp. Immunol. 163, 147-156. 10.1111/j.1365-2249.2010.04288.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz A., Ernst A. and Dikic I. (2014). Cargo recognition and trafficking in selective autophagy. Nat. Cell Biol. 16, 495-501. 10.1038/ncb2979 [DOI] [PubMed] [Google Scholar]
- Stremlau M., Owens C. M., Perron M. J., Kiessling M., Autissier P. and Sodroski J. (2004). The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427, 848-853. 10.1038/nature02343 [DOI] [PubMed] [Google Scholar]
- Stremlau M., Perron M., Lee M., Li Y., Song B., Javanbakht H., Diaz-Griffero F., Anderson D. J., Sundquist W. I. and Sodroski J. (2006). Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc. Natl. Acad. Sci. USA 103, 5514-5519. 10.1073/pnas.0509996103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaesu G., Kobayashi T. and Yoshimura A. (2012). TGFbeta-activated kinase 1 (TAK1)-binding proteins (TAB) 2 and 3 negatively regulate autophagy. J. Biochem. 151, 157-166. 10.1093/jb/mvr123 [DOI] [PubMed] [Google Scholar]
- Targoff I. N., Mamyrova G., Trieu E. P., Perurena O., Koneru B., O'Hanlon T. P., Miller F. W. and Rider L. G. (2006). A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 54, 3682-3689. 10.1002/art.22164 [DOI] [PubMed] [Google Scholar]
- Tasdemir E., Maiuri M. C., Galluzzi L., Vitale I., Djavaheri-Mergny M., D'Amelio M., Criollo A., Morselli E., Zhu C., Harper F. et al. (2008). Regulation of autophagy by cytoplasmic p53. Nat. Cell Biol. 10, 676-687. 10.1038/ncb1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernarakis N., Pasparaki A., Tasdemir E., Maiuri M. C. and Kroemer G. (2008). The effects of p53 on whole organism longevity are mediated by autophagy. Autophagy 4, 870-873. 10.4161/auto.6730 [DOI] [PubMed] [Google Scholar]
- The International FMF Consortium Guido (1997). Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell 90, 797-807. 10.1016/S0092-8674(00)80539-5 [DOI] [PubMed] [Google Scholar]
- Thurston T. L. M., Wandel M. P., von Muhlinen N., Foeglein A. and Randow F. (2012). Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 482, 414-418. 10.1038/nature10744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar D., Singh R., Singh A. K. and Pandya C. D. (2012). TRIM13 regulates ER stress induced autophagy and clonogenic ability of the cells. Biochim. Biophys. Acta 1823, 316-326. 10.1016/j.bbamcr.2011.11.015 [DOI] [PubMed] [Google Scholar]
- Versteeg G. A., Rajsbaum R., Sánchez-Aparicio M. T., Maestre A. M., Valdiviezo J., Shi M., Inn K.-S., Fernandez-Sesma A., Jung J. and García-Sastre A. (2013). The E3-ligase TRIM family of proteins regulates signaling pathways triggered by innate immune pattern-recognition receptors. Immunity 38, 384-398. 10.1016/j.immuni.2012.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicinanza M., Korolchuk V. I., Ashkenazi A., Puri C., Menzies F. M., Clarke J. H. and Rubinsztein D. C. (2015). PI(5)P regulates autophagosome biogenesis. Mol. Cell 57, 219-234. 10.1016/j.molcel.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. G., Delva L., Gaboli M., Rivi R., Giorgio M., Cordon-Cardo C., Grosveld F. and Pandolfi P. P. (1998). Role of PML in cell growth and the retinoic acid pathway. Science 279, 1547-1551. 10.1126/science.279.5356.1547 [DOI] [PubMed] [Google Scholar]
- Wang C., Ivanov A., Chen L., Fredericks W. J., Seto E., Rauscher F. J. III and Chen J. (2005). MDM2 interaction with nuclear corepressor KAP1 contributes to p53 inactivation. EMBO J. 24, 3279-3290. 10.1038/sj.emboj.7600791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Huo K., Ma L., Tang L., Li D., Huang X., Yuan Y., Li C., Wang W., Guan W. et al. (2011). Toward an understanding of the protein interaction network of the human liver. Mol. Syst. Biol. 7, 536 10.1038/msb.2011.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Cao L., Kang R., Yang M., Liu L., Zhao Y., Yu Y., Xie M., Yin X., Livesey K. M. et al. (2014). Autophagy regulates myeloid cell differentiation by p62/SQSTM1-mediated degradation of PML-RARα oncoprotein. Autophagy 7, 401-411. 10.4161/auto.7.4.14397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Pattingre S., Sinha S., Bassik M. and Levine B. (2008). JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell 30, 678-688. 10.1016/j.molcel.2008.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidberg H., Shpilka T., Shvets E., Abada A., Shimron F. and Elazar Z. (2011). LC3 and GATE-16 N termini mediate membrane fusion processes required for autophagosome biogenesis. Dev. Cell 20, 444-454. 10.1016/j.devcel.2011.02.006 [DOI] [PubMed] [Google Scholar]
- Wild P., Farhan H., McEwan D. G., Wagner S., Rogov V. V., Brady N. R., Richter B., Korac J., Waidmann O., Choudhary C. et al. (2011). Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 333, 228-233. 10.1126/science.1205405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M., Zhang D., Dyck J. R. B., Li Y., Zhang H., Morishima M., Mann D. L., Taffet G. E., Baldini A., Khoury D. S. et al. (2006). A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc. Natl. Acad. Sci. USA 103, 17378-17383. 10.1073/pnas.0604708103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.-S., Rodgers M., Min C.-K., Lee J.-S., Kingeter L., Lee J.-Y., Jong A., Kramnik I., Lin X. and Jung J. U. (2012). The autophagy regulator Rubicon is a feedback inhibitor of CARD9-mediated host innate immunity. Cell Host Microbe 11, 277-289. 10.1016/j.chom.2012.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Fiskus W., Yong B., Atadja P., Takahashi Y., Pandita T. K., Wang H.-G. and Bhalla K. N. (2013). Acetylated hsp70 and KAP1-mediated Vps34 SUMOylation is required for autophagosome creation in autophagy. Proc. Natl. Acad. Sci. USA 110, 6841-6846. 10.1073/pnas.1217692110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh I., Botton T., Talevich E., Shain A. H., Sparatta A. J., de la Fouchardiere A., Mully T. W., North J. P., Garrido M. C., Gagnon A. et al. (2015). Activating MET kinase rearrangements in melanoma and Spitz tumours. Nat. Commun. 6, 7174 10.1038/ncomms8174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimi R., Chang T.-H., Wang H., Atsumi T., Morse H. C. III and Ozato K. (2009). Gene disruption study reveals a nonredundant role for TRIM21/Ro52 in NF-kappaB-dependent cytokine expression in fibroblasts. J. Immunol. 182, 7527-7538. 10.4049/jimmunol.0804121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. A., Sermwittayawong D., Kim H.-J., Nandu S., An N., Erdjument-Bromage H., Tempst P., Coscoy L. and Winoto A. (2011). Fas-associated death domain (FADD) and the E3 ubiquitin-protein ligase TRIM21 interact to negatively regulate virus-induced interferon production. J. Biol. Chem. 286, 6521-6531. 10.1074/jbc.M110.172288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z., Villagra A., Peng L., Coppola D., Glozak M., Sotomayor E. M., Chen J., Lane W. S. and Seto E. (2010). The ATDC (TRIM29) protein binds p53 and antagonizes p53-mediated functions. Mol. Cell. Biol. 30, 3004-3015. 10.1128/MCB.01023-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Bosch-Marce M., Shimoda L. A., Tan Y. S., Baek J. H., Wesley J. B., Gonzalez F. J. and Semenza G. L. (2008). Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 283, 10892-10903. 10.1074/jbc.M800102200 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang L., Huang N.-J., Chen C., Tang W. and Kornbluth S. (2012a). Ubiquitylation of p53 by the APC/C inhibitor Trim39. Proc. Natl. Acad. Sci. USA 109, 20931-20936. 10.1073/pnas.1212047110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Mei Y., Fu N.-y., Guan L., Xie W., Liu H.-h., Yu C.-d., Yin Z., Yu V. C. and You H. (2012b). TRIM39 regulates cell cycle progression and DNA damage responses via stabilizing p21. Proc. Natl. Acad. Sci. USA 109, 20937-20942. 10.1073/pnas.1214156110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Elabd S., Hammer S., Solozobova V., Yan H., Bartel F., Inoue S., Henrich T., Wittbrodt J., Loosli F. et al. (2015). TRIM25 has a dual function in the p53/Mdm2 circuit. Oncogene 34, 5729-5738. 10.1038/onc.2015.21 [DOI] [PubMed] [Google Scholar]
- Zheng Z., Liebers M., Zhelyazkova B., Cao Y., Panditi D., Lynch K. D., Chen J., Robinson H. E., Shim H. S., Chmielecki J. et al. (2014). Anchored multiplex PCR for targeted next-generation sequencing. Nat. Med. 20, 1479-1484. 10.1038/nm.3729 [DOI] [PubMed] [Google Scholar]
- Zhou R., Yazdi A. S., Menu P. and Tschopp J. (2011). A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221-225. 10.1038/nature09663 [DOI] [PubMed] [Google Scholar]
- Zhou Z., Ji Z., Wang Y., Li J., Cao H., Zhu H. H. and Gao W.-Q. (2014). TRIM59 is up-regulated in gastric tumors, promoting ubiquitination and degradation of p53. Gastroenterology 147, 1043-1054. 10.1053/j.gastro.2014.07.021 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Massen S., Terenzio M., Lang V., Chen-Lindner S., Eils R., Novak I., Dikic I., Hamacher-Brady A. and Brady N. R. (2013). Modulation of serines 17 and 24 in the LC3-interacting region of Bnip3 determines pro-survival mitophagy versus apoptosis. J. Biol. Chem. 288, 1099-1113. 10.1074/jbc.M112.399345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirin J., Nieuwenhuis J. and Perrimon N. (2013). Role of autophagy in glycogen breakdown and its relevance to chloroquine myopathy. PLoS Biol. 11, e1001708 10.1371/journal.pbio.1001708 [DOI] [PMC free article] [PubMed] [Google Scholar]