Abstract
Objective: This study was conducted to identify the association between rs4804803 polymorphism in DC-SIGN with the susceptibility of severe dengue. Methods: A comprehensive search was conducted to identify all eligible papers in PubMed, Web of Science, China National Knowledge Infrastructure (CNKI), and Google Scholar. Odds ratios (ORs) and corresponding 95% confidence intervals (95% CIs) were used to assess the association. Subgroup analyses were performed by ethnicity. Sensitivity analyses were performed through employing different statistical models (fixed versus random effect model). Results: A total of nine papers and 12 studies, with 1520 severe dengue and 1496 clinical dengue infection were included. The overall meta-analysis revealed significant associations between rs4804803 and severe dengue under the recession (GG versus GA/AA: OR = 0.44, 95%CI, 0.23–0.82) and a codominant model (GG versus AA: OR = 0.43, 95%CI, 0.23–0.81), but sensitivity analysis indicated that the significant pooled ORs were not robust. The subgroup analysis suggested that the carrier of G in rs4804803 was a risk factor for severe dengue under dominant (GG/GA versus AA: OR = 1.86,95%CI, 1.01–3.45), superdominant (GA versus GG/AA: OR = 1.81,95%CI, 1.02–3.21) and a codominant (GA versus AA: OR=1.82,95%CI, 1.02–3.26) models in Asians, while it was a protective factor for severe dengue in South-central Americans under recessive (GG versus GA/AA: OR = 0.27,95%CI, 0.10–0.70) and codominant (GG versus AA: OR=0.24,95%CI, 0.09–0.64) models. The results from subgroup analysis were robust. Conclusions: Dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) promoter-336G/A (rs4804803) polymorphism is association with severe dengue, and it acts in different directions for Asians and South-central Americans.
Keywords: dengue, polymorphism, DC-SIGN, rs4804803, Meta-analysis
1. Introduction
Dengue is the most prevalent mosquito-borne infectious disease, which is endemic more than 125 countries around the world, especially South-East Asia, the Americas, the Western Pacific and Africa. In the past 50 years, the incidence has increased 30 fold and the affected area has extended to new countries and from urban to rural settings [1]. It is estimated that about half of the world’s population is now at risk. The most recently published study about the global burden of dengue indicated that there were 58.4 million (23.6 million–121.9 million) apparent cases with incidence of 810·1 (327.4–1690.8) per 100,000 and 9110 (5630–10,842) deaths with mortality of 1.27 (0.79–1.52) per million in 2013, and dengue was responsible for 1.14 million (0.73–1.98 million) disability-adjusted life-years [2]. The driving forces behind the emergence and reemergence include climate change, poorly planned urbanization, globalization, viral evolution and adaption, and deficiencies in water supply and garbage disposal [3]. Dengue virus (DENV) is a member of the family Flaviviridae under the genus Flavivirus, which also includes Zika virus, Yellow Fever virus and West Nile virus.It is antigenically divided into four serotypes: DENV-1, DENV-2, DENV-3, and DENV-4. The clinical manifestations of DENV infection is protean, from asymptomatic infection, mildly symptomatic disease, to life-threatening severe dengue, including dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS).
According to the World Health Organization (WHO, 2009) dengue case classification, the criteria for severe dengue include severe plasma leakage leading to shock or fluid accumulation with respiratory distress, severe bleeding as evaluated by a clinician, or severe organ impairment involving liver (AST or ALT ≥1000), central nervous system (impaired consciousness), heart and other organs [1,4].The identified risk factors for severe dengue include second infection, age, delay in admission, pregnancy, infection with DENV-2 and certain chronic diseases (such as diabetes, cardiac disorders, asthma) [4,5,6,7,8,9,10,11,12,13,14]. The host-specific genetics is also a contributing factor [15]. Studies indicated that African ancestry was a protective factor for severe dengue [4,16,17]. Patients with AB blood group had higher risk of developing DHF [5]. A large number of association studies between genetic polymorphisms and the severity of dengue have been conducted in the past 20 years. Dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN, also known as CD209) is a type II C-type lectin, which plays an important role in the early interaction of a pathogen with dendritic cell (DC), DC–T cell interaction, DC migration and pathogen uptake [18,19,20]. Other than being involved in viral entry, studies indicated that it also has a role in the progression to severe forms of disease during dengue infection [21,22,23]. rs4804803, a single nucleotide polymorphism (SNP) in the promoter of DC-SIGN, is one of the widely studied loci in the research about the genetic susceptibility of dengue and severe dengue, but the results are quite controversial [19,24,25,26,27,28,29,30,31]. Meta-analysis is the quantitative, scientific synthesis of research results and can establish evidence-based practice and resolve seemingly contradictory research outcomes [32]. So, we performed this meta-analysis to identify the association of rs4804803 with the severity of dengue.
2. Materials and Methods
2.1. Search Strategy and Inclusion Criteria
Electronic searches were conducted in PubMed, Web of Science, China National Knowledge Infrastructure (CNKI) and Google Scholar with the keywords of “dengue” and “CD209 or DC-SIGN” to identify relevant articles published up until 31 December 2018. The references of related articles were also screened to identify papers.
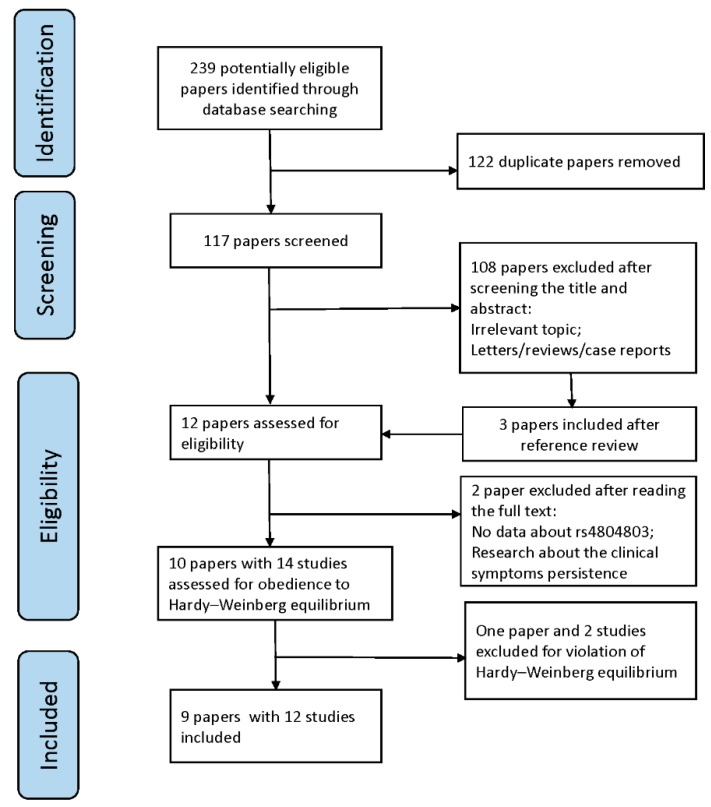
All the studies included in this meta-analysis met the following criteria: (1) a human study with full paper available; (2) a case-control study on the association of rs4804803 polymorphism in DC-SIGN with the development of severe dengue in clinical dengue infection; (3) the original data about the genotype distribution is accessible; (4) published studies;(5) the distributions of genotypes did not differ significantly from the Hardy–Weinberg equilibrium (HWE). Case reports, reviews or articles without full text were excluded. Duplicated studies were carefully screened, then those with bigger sample size, higher quality or published most recently were kept. PRISMA guidelines were followed in the study selection (Figure 1).
Figure 1.
PRISMA flow diagram of systematic search results and study selection.
2.2. Data Extraction
The following information was extracted in each study included: the first author, year of publication, country, WHO criteria for dengue case classification, ethnicity, matching criteria of controls, genotype method, sample size, the numbers of the severe dengue and uncomplicated clinical dengue case (indicated as DF in this paper) with different genotypes.
2.3. Quality Assessment
The quality of each study was assessed independently by two researchers according to the Newcastle–Ottawa Scale (NOS) for case-control study. This criteria include three sectors: selection (a maximum score of four), comparability (a maximum score of two) and exposure (a maximum score of three). The third researcher was asked to conduct the quality assessment if inconsistent existed.
2.4. Statistical Analysis
The deviation from HWE was examined in both severe dengue and DF group with a web tool [33]. Pooled odds ratio (OR) with 95% confidence interval (CI) and z test (p < 0.05 was considered as significant difference) were used to measure the association between rs4804803 polymorphism and the risk of severe dengue under four inheritance models (dominant: GG/GA versus AA; recessive: GG versus GA/AA; codominant: GG versus AA, GA versus AA; superdominant: GA versus GG/AA), respectively. I2 statistic was used to measure the magnitude of heterogeneity and Q test was used to identify whether the heterogeneity was statistical significantly. Fixed effect model was employed in meta-analysis when p value of Q test was more than 0.10 or I2 value was less 50%, otherwise random effect model would be adopted. Pooled OR and its 95%CI were obtained by using the Mantel–Haenszel method in the fixed effect model or by using the Der Simonian and Laird method in the random effect model. When heterogeneity was statistically significantly, meta-regression was conducted to explore the possible source. A funnel plot, Egger’s regression test and Begg’s test were used to identify whether publish bias existed, and p < 0.05 was regarded as statistically significant. Sensitivity analysis was performed through employing different statistical models (fixed effect model versus random effect model). The pooled OR was robust if the result from the fixed and random effect model was consistent. All of the analyses were conducted with Stata 10.0 software (StataCorp, College Station, TS, USA), and two-sided tests with p value less than 0.05 was considered statistically significant unless there was specification.
3. Results
3.1. Study Inclusion and Characteristics
A total of 239 papers were identified through database searching, and 122 of them were excluded for duplication (Figure 1). After reviewing the references and roughly screening the title and abstract, 12 papers were included. The full text of the included papers were accessible. Two papers were removed after reading: one paper had no data about rs4804803 [34]; another paper was a study about the persistence of dengue clinical symptoms [35]. Then, 10 papers with 14 studies were tested for the obedience to HWE, and one paper and two studies were excluded for their violation. Finally, nine papers and 12 studies, with 1520 severe dengue and 1496 DF cases, were included (Table 1). All the papers in this meta-analysis were published during 2005 to 2018, and patients were from Asia or South-central America (SCA).
Table 1.
Characteristics of the individual studies in this meta-analysis.
| First Author | Publish Year | Ethnicity | Country | WHO Criteria | Matching Criteria of Controls | Method | N | Score NOS | |
|---|---|---|---|---|---|---|---|---|---|
| Severe Dengue | DF | ||||||||
| Sakuntabhai [30] | 2005 | Asian | Thailand (RA) | 1997 | Gender | TaqMan | 144 | 50 | 6 |
| Sakuntabhai [30] | 2005 | Asian | Thailand (SI) | 1997 | Gender | TaqMan | 124 | 66 | 6 |
| Sakuntabhai [30] | 2005 | Asian | Thailand (KK) | 1997 | Gender | TaqMan | 87 | 27 | 6 |
| Silva [29] | 2010 | SCA | Brazil | 1997 | Gender and age | BeadArray | 31 | 128 | 9 |
| Wang [19] | 2011 | Asian | China | 1997 | Gender | TaqMan | 106 | 157 | 6 |
| Xavier-Carvalho [28] | 2013 | SCA | Brazil | 2009 | Age | TaqMan. | 52 | 122 | 6 |
| Alagarasu [27] | 2013 | Asian | India | 1999 | Unmentioned | PCR-RFLP | 19 | 64 | 5 |
| Noecker [25] | 2014 | SCA | Mexican | 2009 | Gender | TaqMan | 33 | 51 | 6 |
| Oliveira [26] | 2014 | SCA | Brazil | 2009 | Gender and age | TaqMan | 67 | 74 | 8 |
| Dang [36] | 2016 | Asian | Thailand | 1997 | Gender | TaqMan | 415 | 331 | 6 |
| Oliveira [37] | 2018 | Asian | Vietnam | 1997 | Unmentioned | TaqMan | 20 | 21 | 6 |
| Oliveira [37] | 2018 | Asian | Cambodia | 1997 | Unmentioned | TaqMan | 93 | 57 | 6 |
SCA, South-central American; PCR-RFLP, polymerase chain reaction and restriction fragment length polymorphism.
3.2. Quantitative Data Synthesis
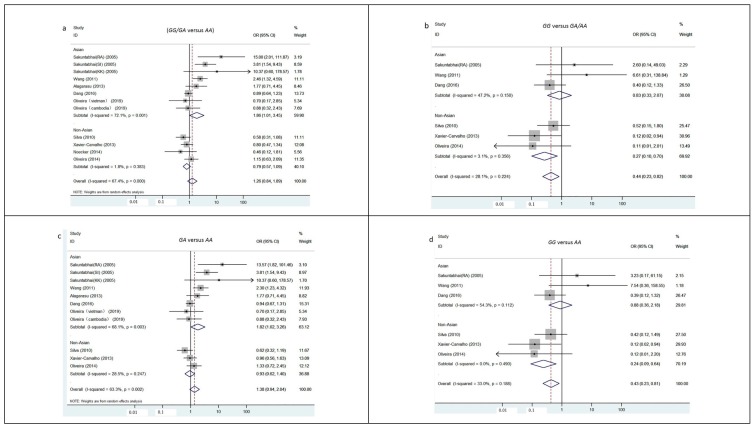
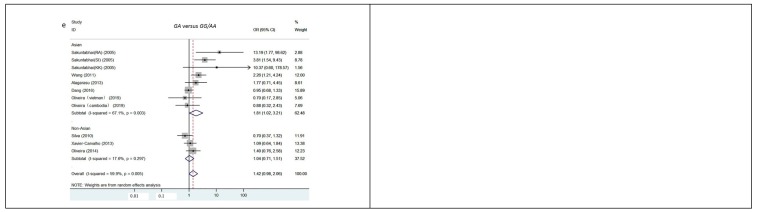
The results from the pooled meta-analyses showed that, except under the recession and a codominant model, no significant associations were found under any other inheritance models (GG/GA versus AA: OR = 1.26, 95%CI, 0.84–1.19; GA versus AA: OR = 1.38, 95%CI, 0.94–2.04; GA versus GG/AA: OR = 1.42, 95%CI, 0.98–2.06, Figure 2). The G allele of rs4804803 in DC-SIGN reduced the severity of clinical dengue infections under the recession (GG versus GA/AA: OR = 0.44, 95%CI, 0.23–0.82) and a codominant model (GG versus AA: OR = 0.43, 95%CI, 0.23–0.81). The results from subgroup meta-analysis uncovered the different role of rs4804803 in the development of severe dengue for different ethnicity. The carrying of G in rs4804803 acted as a risk factor when progression to severe dengue under dominant (GG/GA versus AA: OR = 1.86, 95%CI, 1.01–3.45), superdominant (GA versus GG/AA: OR = 1.81, 95%CI, 1.02–3.21) and a codominant (GA versus AA: OR = 1.82, 95%CI, 1.02–3.26) model in Asians, while it reduced the risk of severe dengue in SCA under recessive (GG versus GA/AA: OR = 0.27, 95%CI, 0.10–0.70) and a codominant (GG versus AA: OR = 0.24, 95%CI, 0.09–0.64) models (Figure 2).
Figure 2.
Subgroup meta-analysis by ethnicity on rs4804803 polymorphism and severity of dengue. Five studies, which were Sakuntabhai(SI) and Sakuntabhai(KK) published in 2005, Alagarasu published in 2013, Oliveira(vietman) and Oliveira(Cambodia) published in 2018, were automatically excluded in genotype analysis of GG versus GA/AA and GG versus AA due to absence of GG individuals in both groups; Other than the analysis under dominant inheritance model, the study conducted by Noecker et al. was automatically excluded in all the genotype analysis due to no information about the distribution of GG and GA genotype obtained. (a) Subgroup meta-analysis under dominant inheritance model (GG/GA versus AA). (b) Subgroup meta-analysis under recessive inheritance model (GG versus GA/AA). (c) Subgroup meta-analysis under codominant inheritance model (GA versus AA). (d) Subgroup meta-analysis under codominant inheritance model (GG versus AA). (e) Subgroup meta-analysis under superdominant inheritance model(GA versus GG/AA).
3.3. Heterogeneity and Publication Bias
The result from the Q test and I2 test implied that there were significant heterogeneities among studies under dominant (GG/GA versus AA: I2 = 67.4%, p < 0.001), superdominant (GA versus GG/AA: I2 = 59.9%, p = 0.005) and codominant (GA versus AA: I2 = 63.3%, p = 0.002) models. To explore the source of heterogeneities, meta-regression analyses by ethnicity and matching criteria of controls were conducted. The result suggested that ethnicity might be one of the reasons for heterogeneity under the dominant model (p = 0.044, Table 2).
Table 2.
Meta-regression by ethnicity and matching criteria of controls under different inheritance models.
| Model of inheritance | Factor | Β (95%CI) | t | p | |
|---|---|---|---|---|---|
| Dominant | GG/GA versus AA | Matching criteria of controls | 0.53 (−0.44, 1.49) | 1.23 | 0.249 |
| Ethnicity | 1.35 (0.04, 2.66) | 2.33 | 0.044 | ||
| Recessive | GG versus GA/AA | Matching criteria of controls | 0.98 (−4.41, 6.37) | 0.58 | 0.603 |
| Ethnicity | 2.20 (−3.00, 7.39) | 1.35 | 0.271 | ||
| Codominant | GG versus AA | Matching criteria of controls | 0.86 (−4.69, 6.40) | 0.49 | 0.657 |
| Ethnicity | 2.31 (−3.02, 7.65) | 1.38 | 0.262 | ||
| GA versus AA | Matching criteria of controls | 0.47 (−0.60, 1.55) | 1.02 | 0.338 | |
| Ethnicity | 1.13 (−0.39, 2.66) | 1.71 | 0.125 | ||
| Superdominant | GA versus GG/AA | Matching criteria of controls | 0.44 (−0.61, 1.50) | 0.97 | 0.359 |
| Ethnicity | 0.99 (−0.50, 2.49) | 1.53 | 0.165 | ||
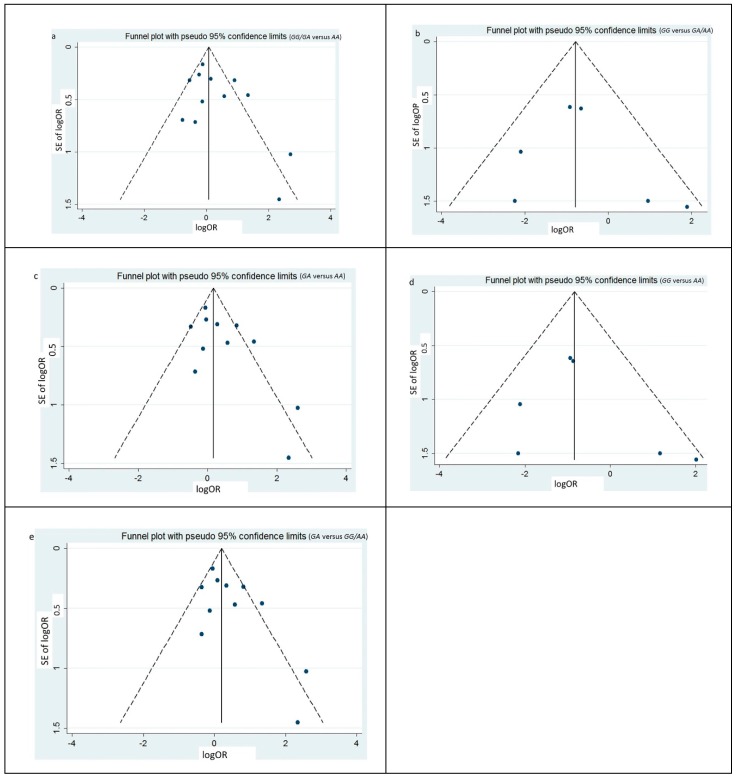
The shape of the funnel plots under four models were symmetrical, which suggested no publication bias existing (Figure 3). The result from Egger’s regression test and Begg’s test confirmed that there was no publication bias (p < 0.05, Table 3).
Figure 3.
Funnel plots for publication bias of the meta-analysis on rs4804803 polymorphism and severity of dengue. Data from all the eligible studies, both from Asians and SCA, were included. (a) Funnel plots for publication bias under dominant inheritance model (GG/GA versus AA). (b) Funnel plots for publication bias under recessive inheritance model (GG versus GA/AA). (c) Funnel plots for publication bias under codominant inheritance model (GA versus AA). (d) Funnel plots for publication bias under codominant inheritance model (GG versus AA). (e) Funnel plots for publication bias under superdominant inheritance model (GA versus GG/AA).
Table 3.
Test of publication bias under different inheritance models.
| Model of inheritance | Egger’s | Begg’s | |||||
|---|---|---|---|---|---|---|---|
| t | p | z | p | ||||
| Dominant | GG/GA versus AA | 1.56 | 0.150 | 1.17 | 0.244 | ||
| Recessive | GG versus GA/AA | 0.54 | 0.616 | 1.13 | 0.260 | ||
| Codominant | GG versus AA | 0.74 | 0.499 | 0.75 | 0.452 | ||
| GA versus AA | 1.96 | 0.081 | 1.25 | 0.213 | |||
| Superdominant | GA versus GG/AA | 2.05 | 0.071 | 1.09 | 0.276 | ||
3.4. Sensitivity Analysis
The sensitivity analysis showed that, except under dominant model, the pooled ORs and 95% CIs were inconsistent between the fixed effect model and random effect model under any other inheritance models (Table 4). It indicated that the significant pooled ORs were not robust under those three models. In consideration of the possible different role of rs4804803 during the development of severe dengue in Asian and SCA, and ethnicity recognized as a possible source of heterogeneity under the dominant model, subgroup sensitivity analyses by ethnicity were conducted. The ORs and 95% CIs were consistent between the fixed effect model and random effect model under the four inheritance models in Asians and SCA, respectively, which indicated that the results from the subgroup meta-analysis were robust (Table 4).
Table 4.
Sensitivity analysis under different inheritance models through adopting different effect models.
| Model of inheritance | Group | I2 (%) | Fixed effect model | Random effect model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| z | p | OR(95%CI) | z | p | OR(95%CI) | |||||
| Dominant | GG /GA versus AA | Overall | 67.4 | 1.67 | 0.096 | 1.17 (0.97, 1.42) | 1.12 | 0.263 | 1.26 (0.84, 1.89) | |
| Asian | 72.1 | 3.12 | 0.002 | 1.46 (1.15, 1.85) | 1.98 | 0.048 | 1.86 (1.01, 3.45) | |||
| SCA | 1.8 | 1.48 | 0.140 | 0.79 (0.57, 1.08) | 1.43 | 0.153 | 0.79 (0.57, 1.09) | |||
| Recessive | GG versus GA/AA | Overall | 28.1 | 2.57 | 0.010 | 0.44 (0.23, 0.82) | 1.59 | 0.112 | 0.47 (0.19, 1.19) | |
| Asian | 47.2 | 0.40 | 0.691 | 0.83 (0.33, 2.07) | 0.21 | 0.834 | 1.22 (0.20, 7.51) | |||
| SCA | 3.1 | 2.67 | 0.008 | 0.27 (0.10, 0.70) | 2.29 | 0.022 | 0.30 (0.11, 0.84) | |||
| Codominant | GG versus AA | Overall | 33.0 | 2.63 | 0.009 | 0.43 (0.23, 0.81) | 1.54 | 0.122 | 0.46 (0.18, 1.23) | |
| Asian | 54.3 | 0.27 | 0.786 | 0.88 (0.36, 2.18) | 0.34 | 0.732 | 1.41 (0.20, 10.26) | |||
| SCA | 0.0 | 2.84 | 0.005 | 0.24 (0.09, 0.64) | 2.57 | 0.010 | 0.27 (0.10, 0.73) | |||
| GA versus AA | Overall | 63.3 | 2.46 | 0.014 | 1.28 (1.05, 1.56) | 1.62 | 0.104 | 1.38 (0.94, 2.04) | ||
| Asian | 68.1 | 3.24 | 0.001 | 1.49 (1.17, 1.90) | 2.02 | 0.043 | 1.82 (1.02, 3.26) | |||
| SCA | 28.5 | 0.37 | 0.709 | 0.94 (0.67, 1.32) | 0.32 | 0.746 | 0.94 (0.62, 1.40) | |||
| Superdominant | GA versus GG/AA | Overall | 59.9 | 2.80 | 0.005 | 1.32 (1.09, 1.61) | 1.84 | 0.065 | 1.42 (0.98, 2.06) | |
| Asian | 67.1 | 3.26 | 0.001 | 1.50 (1.17, 1.91) | 2.04 | 0.042 | 1.81 (1.02, 3.21) | |||
| SCA | 17.6 | 0.21 | 0.833 | 1.04 (0.74, 1.45) | 0.19 | 0.850 | 1.04 (0.71, 1.51) | |||
4. Discussion
DC-SIGN is preferentially expressed on myeloid DCs [18]. It is composed of 404 amino acids and four domains: a cytoplasmic domain, a transmembrane domain, an extracellular neck domain, and a carbohydrate recognition domain (CRD) [18,20]. The cytoplasmic domain is responsible for receptor signaling for the binding, phagocytosis and intra-cellular trafficking of ligand molecules. The transmembrane domain, consisting of 15 amino acids, anchors the proteins to the cytoplasmic membrane. The neck domains, completed of seven complete repeats and one incomplete repeat, stabilizes the tetramer of the extra-cellular portion, enhances the affinity between DC-SIGN and its ligand and amplifies the signal. The CRD in DC-SIGN is Ca2+ dependent and can selectively recognize and bind to high-mannose oligosaccharides. Generally, DC-SIGN performs cell-adhesion and pathogen recognition functions [18]. The DC-SIGN gene is located on chromosome 19p13.2-3. rs4804803, a SNP in the promoter of DC-SIGN, is one of the most frequently studied genetic locus. In vitro, rs4804803 affected a Sp1 binding site and the level of transcription of DC-SIGN, with lower DC-SIGN expression for G allele in rs4804803 [30]. The population-based genetic association studies indicated that the polymorphism of rs4804803 was related to the susceptibility of HIV-1, tuberculosis, Chikungunya [38,39,40,41].
Naturally, DENV is introduced into human skin by an infected mosquito vector. Its primary target cells in the skin are Langerhans cells or immature DCs. Studies indicated that DC-SIGN not only acts as an attachment factor during DENV infection but also plays a role in virus entry [42]. Immature DENV, non-infectious without antibodies, could infect immature DCs through interaction with DC-SIGN. It suggested that immature DENV particles could contribute to dengue pathogenesis through DC-SIGN during primary infection [43]. Although a study indicated that a carrier of the G allele in rs4804803 expressed lower level of DC-SIGN in vitro, monocyte-derived DCs from individuals with AG genotype in rs4804803 had a higher DC-SIGN expression than those with the AA genotype in response to dengue infection, and higher TNF-a, IL-12p40, and IP-10 production, lower viral replication were also noted [19]. The reason for this contradiction should be explored further. The genetic association studies showed that the polymorphism of rs4804803 might be related to the susceptibility of dengue, but it remains controversial. The studies conducted in the Taiwan and Mexican populations indicated that the G allele in rs4804803 increased the risk of clinical dengue infections, but a studies from Thailand population implied it as a protective factor [19,25,30]. No association was found in studies from Brazil and Western India [26,27].
Recently, studies suggested that the DC-SIGN might also have a role in the progression to severe dengue. Antibody-dependent enhancement (ADE) is the phenomenon that heterotypic antibodies do not neutralize virions of the subsequent infecting DENV type, but facilitate virus entry, replication in target immune cells, and consequently lead to higher viremia [44]. It is widely regarded as one of the reason for the development of severe dengue [45]. A study indicated that ADE was inversely correlated with surface expression of DC-SIGN in vitro: Mature DC, which expresses lower level of DC-SIGN, exhibited ADE, while immature DC, expressing higher levels of DC-SIGN, did not undergo ADE [21]. Platelets from DENV-infected patients present signs of activation, mitochondrial dysfunction, and activation of apoptosis caspase cascade, which may contribute to the genesis of thrombocytopenia. DC-SIGN, as a critical receptor, was involved in this progress of DENV-dependent platelet activation [22,23]. Expression of DC-SIGN was downregulated on platelets in patients with dengue infection, and the decreased receptor expression diminishes platelet activation [46]. Inconsistency was also noted when researchers tried to explore the role of rs4804803 in the development of severe dengue. GG genotype of rs4804803 was recognized protectively to severe dengue in a studies from Brazil [28], but higher risk was found from a study in Thailand and in Taiwan, China, respectively [19,30]. No associations were recognized in studies from Mexican, Western India, and another two studies from Brazil and Thailand [25,26,27,29,36].
The overall meta-analyses showed that the carrier of G in rs4814803 was a significant protector for severe dengue under the recession (GG versus GA/AA, OR < 1) and a codominant model (GG versus AA, OR < 1). However the significant pooled ORs were not robust and the heterogeneities between different studies were noted under four inheritance models. Ethnicity was identified as a possible source of heterogeneity. The further subgroup meta-analysis indicated that rs4804803 might play a different role in progression to severe dengue in different ethnicities. The carrier of G in rs4804803 acted as a risk factor for the development of severe dengue under dominant (GA/GG versus AA: OR > 1), superdominant (GA versus AA/GG: OR > 1) and a codominant (GA versus AA: OR > 1) models in Asians, while it reduced the risk in SCA under recessive (GG versus GA/AA: OR < 1) and a codominant (GG versus AA: OR < 1) model. The results from subgroup analysis were robust according the sensitivity analysis. The meta-analysis conducted by Xavier-Carvalho et al. [28] also indicated the different association of rs4804803 with severe dengue: the G allele of rs4804803 was associated with higher risk of severe dengue in Asians, but was a protective factor in Brazilians. Another study from Pabalan et al. [24] also suggested it is a protective factor for DHF in the SCA population when compared with healthy and asymptomatic control, but no association was conferred in Asians. Studies conducted in Brazil indicated that the G carrier was a protective factor for the symptoms of headache and arthralgia in dengue fever [26]. Besides dengue, the polymorphism of rs4804803 was also associated with the severity of tuberculosis, severe acute respiratory syndromes and tick-borne encephalitis [37,38,47]. The opposite effect of rs4804803 on the development of severe dengue is not inexplicable in consideration the role of DC- SIGN in ADE and DENV-dependent platelet activation: the expression of DC-SIGN was inversely correlated with ADE, but positively correlated with DENV-dependent platelet activation [21,22,46]. The other possible reasons for the different role of rs4804803 might be related to: the different frequency of G allele in rs4804803 between ethnicities; the different distribution of the serotype and the ratio of secondary globally; other factors, including genetic factors, that might determine the different role of rs4804803 in DC-SIGN during dengue infection.
5. Conclusions
In summary, the G carrier of rs4804803 in DC-SIGN has a higher risk for progression to severe dengue in Asians and a lower risk in SCA. Regarding the multiple factor for the development to severe dengue, there are limitations in this meta-analysis. Globally, the epidemic status of dengue across the whole world and in different years are different, so the ratio of secondary infection, one of the important reasons for severe dengue, might be inconsistent between studies. However, this was not included in the meta-analysis. Information on serotypes was not available from all of the included papers, so this was also not included in the analysis.
Author Contributions
Conceptualization, J.R. and E.C.; methodology, J.R., Z.W. and E.C.; formal analysis and writing-original draft preparation, J.R.
Funding
This study was supported by the medical research program of Zhejiang Province (2019ZD003) and the state project for scientific & technological development of the 13th five-year plan (2017ZX10303404003006, 2017ZX10303404005004, 2017ZX10303404008002).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization Dengue Guidelines for Diagnosis, Treatment, Prevention and Control [EB] [(accessed on 1 December 2018)]; Available online: https://www.who.int/neglected_diseases/resources/9789241547871/en/
- 2.Stanaway J.D., Shepard D.S., Undurraga E.A., Halasa Y.A., Coffeng L.E., Brady O.J., Hay S.I., Bedi N., Bensenor I.M., Castañeda-Orjuela C.A., et al. The global burden of dengue: An analysis from the global burden of disease study 2013. Lancet Infect. Dis. 2016;6:712–723. doi: 10.1016/S1473-3099(16)00026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., Drake J.M., Brownstein J.S., Hoen A.G., Sankoh O., et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xavier-Carvalho C., Cardoso C.C., de Souza Kehdy F., Pacheco A.G., Moraes M.O. Host genetics and dengue fever. Infect. Genet. Evol. 2017;56:99–110. doi: 10.1016/j.meegid.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Murugananthan K., Subramaniyam S., Kumanan T., Owens L., Ketheesan N., Noordeen F. Blood group ab is associated with severe forms of dengue virus infection. VirusDisease. 2018;29:103–105. doi: 10.1007/s13337-018-0426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nunes P.C.G., de Filippis A.M.B., Lima M.Q.D.R., Faria N.R.D.C., de Bruycker-Nogueira F., Santos J.B., Heringer M., Chouin-Carneiro T., Couto-Lima D., de Santis Gonçalves B., et al. 30 years of dengue fatal cases in Brazil: A laboratorial-based investigation of 1047 cases. BMC Infect. Dis. 2018;18:346. doi: 10.1186/s12879-018-3255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suppiah J., Ching S., Amin-Nordin S., Mat-Nor L., Ahmad-Najimudin N., Low G.K., Abdul-Wahid M., Thayan R., Chee H. Clinical manifestations of dengue in relation to dengue serotype and genotype in Malaysia: A retrospective observational study. PLoS Negl. Trop. Dis. 2018;12:e6817. doi: 10.1371/journal.pntd.0006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huy N.T., Van Giang T., Thuy D.H., Kikuchi M., Hien T.T., Zamora J., Hirayama K. Factors associated with dengue shock syndrome: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2013;7:e2412. doi: 10.1371/journal.pntd.0002412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moraes G.H., de Fatima Duarte E., Duarte E.C. Determinants of mortality from severe dengue in Brazil: A population-based case-control study. Am. J. Trop. Med. Hyg. 2013;88:670–676. doi: 10.4269/ajtmh.11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agrawal V.K., Prusty B., Reddy C.S., Mohan R.G., Agrawal R.K., Sekher S.B.V. Clinical profile and predictors of severe dengue disease: A study from South India. Casp. J. Intern. Med. 2018;9:334–340. doi: 10.22088/cjim.9.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang J., Hsu J.P., Yeo T.W., Leo Y.S., Lye D.C. Diabetes, cardiac disorders and asthma as risk factors for severe organ involvement among adult dengue patients: A matched case-control study. Sci. Rep. 2017;7:39872. doi: 10.1038/srep39872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thanachartwet V., Oer-areemitr N., Chamnanchanunt S., Sahassananda D., Jittmittraphap A., Suwannakudt P., Desakorn V., Wattanathum A. Identification of clinical factors associated with severe dengue among Thai adults: A prospective study. BMC Infect. Dis. 2015;15:420. doi: 10.1186/s12879-015-1150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machado C.R., Machado E.S., Rohloff R.D., Azevedo M., Campos D.P., de Oliveira R.B., Brasil P. Is pregnancy associated with severe dengue? A review of data from the Rio De Janeiro surveillance information system. PLoS Neglect. Trop. Dis. 2013;7:e2217. doi: 10.1371/journal.pntd.0002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinto R.C., Castro D.B., Albuquerque B.C., Sampaio V.D.S., Passos R.A., Costa C.F., Sadahiro M., Braga J.U. Mortality predictors in patients with severe dengue in the state of Amazonas, Brazil. PLoS ONE. 2016;11:e161884. doi: 10.1371/journal.pone.0161884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaturvedi U., Nagar R., Shrivastava R. Dengue and dengue haemorrhagic fever: Implications of host genetics. FEMS Immunol. Med. Microbiol. 2006;47:155–166. doi: 10.1111/j.1574-695X.2006.00058.x. [DOI] [PubMed] [Google Scholar]
- 16.Carabali M., Hernandez L.M., Arauz M.J., Villar L.A., Ridde V. Why are people with dengue dying? A scoping review of determinants for dengue mortality. BMC Infect. Dis. 2015;15:301. doi: 10.1186/s12879-015-1058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanton R.E., Silva L.K., Morato V.G., Parrado A.R., Dias J.P., Melo P.R., Reis E.A., Goddard K.A., Nunes M.R., Rodrigues S.G., et al. Genetic ancestry and income are associated with dengue hemorrhagic fever in a highly admixed population. Eur. J. Hum. Genet. 2008;16:762–765. doi: 10.1038/ejhg.2008.4. [DOI] [PubMed] [Google Scholar]
- 18.Zhang F., Ren S., Zuo Y. DC-SIGN, DC-SIGNR and LSECtin: C-type lectins for infection. Int. Rev. Immunol. 2014;33:54–66. doi: 10.3109/08830185.2013.834897. [DOI] [PubMed] [Google Scholar]
- 19.Wang L., Chen R.F., Liu J.W., Lee I.K., Lee C.P., Kuo H.C., Huang S.K., Yang K.D. DC-SIGN (CD209) promoter −336 A/G polymorphism is associated with dengue hemorrhagic fever and correlated to DC-SIGN expression and immune augmentation. PLoS Negl. Trop. Dis. 2011;5:e934. doi: 10.1371/journal.pntd.0000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell D.A., Fadden A.J., Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. J. Biol. Chem. 2001;276:28939–28945. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- 21.Boonnak K., Slike B.M., Burgess T.H., Mason R.M., Wu S., Sun P., Porter K., Rudiman I.F., Yuwono D., Puthavathana P., et al. Role of dendritic cells in antibody-dependent enhancement of dengue virus infection. J. Virol. 2008;82:3939–3951. doi: 10.1128/JVI.02484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hottz E.D., Oliveira M.F., Nunes P.C.G., Nogueira R.M.R., Valls-de-Souza R., Da Poian A.T., Weyrich A.S., Zimmerman G.A., Bozza P.T., Bozza F.A. Dengue induces platelet activation, mitochondrial dysfunction and cell death through mechanisms that involve DC-SIGN and caspases. J. Thromb. Haemost. 2013;11:951–962. doi: 10.1111/jth.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon A.Y., Sutherland M.R., Pryzdial E.L.G. Dengue virus binding and replication by platelets. Blood. 2015;126:378–385. doi: 10.1182/blood-2014-09-598029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pabalan N., Chaisri S., Tabunhan S., Phumyen A., Jarjanazi H., Steiner T.S. Associations of DC-SIGN (CD209) promoter-336G/A polymorphism (rs4804803) with dengue infection: A systematic review and meta-analysis. Acta Trop. 2018;177:186–193. doi: 10.1016/j.actatropica.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Noecker C.A., Amaya-Larios I.Y., Galeana-Hernandez M., Ramos-Castaneda J., Martinez-Vega R.A. Contrasting associations of polymorphisms in FcγRIIa and DC-SIGN with the clinical presentation of dengue infection in a mexican population. Acta Trop. 2014;138:15–22. doi: 10.1016/j.actatropica.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira L.F., Lima C.P., Azevedo R.S., Mendonça D.S., Rodrigues S.G., Carvalho V.L., Pinto E.V., Maia A.L., Maia M.H., Vasconcelos J.M., et al. Polymorphism of DC-SIGN (CD209) promoter in association with clinical symptoms of dengue fever. Viral Immunol. 2014;27:245–249. doi: 10.1089/vim.2013.0119. [DOI] [PubMed] [Google Scholar]
- 27.Alagarasu K., Damle I.M., Bachal R.V., Mulay A.P., Shah P.S., Dayaraj C. Association of promoter region polymorphisms of CD209 gene with clinical outcomes of dengue virus infection in Western India. Infect. Genet. Evol. 2013;17:239–242. doi: 10.1016/j.meegid.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 28.Xavier-Carvalho C., Gibson G., Brasil P., Ferreira R.X., de Souza Santos R., Gonçalves Cruz O., de Oliveira S.A., de Sá Carvalho M., Pacheco A.G., Kubelka C.F., et al. Single nucleotide polymorphisms in candidate genes and dengue severity in children: A case–control, functional and meta-analysis study. Infect. Genet. Evol. 2013;20:197–205. doi: 10.1016/j.meegid.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Silva L.K., Blanton R.E., Parrado A.R., Melo P.S., Morato V.G., Reis E.A., Dias J.P., Castro J.M., Vasconcelos P.F., Goddard K.A., et al. Dengue hemorrhagic fever is associated with polymorphisms in JAK1. Eur. J. Hum. Genet. 2010;18:1221–1227. doi: 10.1038/ejhg.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakuntabhai A., Turbpaiboon C., Casadémont I., Chuansumrit A., Lowhnoo T., Kajaste-Rudnitski A., Kalayanarooj S.M., Tangnararatchakit K., Tangthawornchaikul N., Vasanawathana S., et al. A variant in the CD209 promoter is associated with severity of dengue disease. Nat. Genet. 2005;37:507–513. doi: 10.1038/ng1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lozach P., Burleigh L., Staropoli I., Navarro-Sanchez E., Harriague J., Virelizier J., Rey F.A., Desprès P., Arenzana-Seisdedos F., Amara A. Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN)-mediated enhancement of dengue virus infection is independent of DC-SIGN internalization signals. J. Biol. Chem. 2005;280:23698–23708. doi: 10.1074/jbc.M504337200. [DOI] [PubMed] [Google Scholar]
- 32.Gurevitch J., Koricheva J., Nakagawa S., Stewart G. Meta-analysis and the science of research synthesis. Nature. 2018;555:175–182. doi: 10.1038/nature25753. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez S., Gaunt T.R., Day I.N. Hardy-weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am. J. Epidemiol. 2009;169:505–514. doi: 10.1093/aje/kwn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wibawa T., Wijayanti N., Arguni E., Laksono I.S. DC-SIGN (CD209) carbohydrate recognition domain is not polymorphic in dengue virus-infected Indonesian patients. Trop. Med. Health. 2015;43:101–105. doi: 10.2149/tmh.2015-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dettogni R.S., Tristao-Sa R., Dos S.M., Da S.F., Louro I.D. Single nucleotide polymorphisms in immune system genes and their association with clinical symptoms persistence in dengue-infected persons. Hum. Immunol. 2015;76:717–723. doi: 10.1016/j.humimm.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 36.Dang T.N., Naka I., Sa-Ngasang A., Anantapreecha S., Wichukchinda N., Sawanpanyalert P., Patarapotikul J., Tsuchiya N., Ohashi J. Association of BAK1 single nucleotide polymorphism with a risk for dengue hemorrhagic fever. BMC Med. Genet. 2016;17:43. doi: 10.1186/s12881-016-0305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliveira M., Saraiva D.P., Cavadas B., Fernandes V., Pedro N., Casademont I., Koeth F., Alshamali F., Harich N., Cherni L., et al. Population genetics-informed meta-analysis in seven genes associated with risk to dengue fever disease. Infect. Genet. Evol. 2018;62:60–72. doi: 10.1016/j.meegid.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 38.Vannberg F.O., Chapman S.J., Khor C.C., Tosh K., Floyd S., Jackson-Sillah D., Crampin A., Sichali L., Bah B., Gustafson P., et al. CD209 genetic polymorphism and tuberculosis disease. PLoS ONE. 2008;3:e1388. doi: 10.1371/journal.pone.0001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan K., Xu M., Ching J., So T., Lai S., Chu C., Yam L., Wong A., Chung P., Chan V., et al. CD209 (DC-SIGN) −336A>G promoter polymorphism and severe acute respiratory syndrome in Hong Kong Chinese. Hum. Immunol. 2010;71:702–707. doi: 10.1016/j.humimm.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selvaraj P., Alagarasu K., Swaminathan S., Harishankar M., Narendran G. CD209 gene polymorphisms in south Indian HIV and HIV-TB patients. Infect. Genet. Evol. 2009;9:256–262. doi: 10.1016/j.meegid.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Chaaithanya I.K., Muruganandam N., Surya P., Anwesh M., Alagarasu K., Vijayachari P. Association of oligoadenylate synthetase gene cluster and DC-SIGN (CD209) gene polymorphisms with clinical symptoms in chikungunya virus infection. DNA Cell Biol. 2016;35:44–50. doi: 10.1089/dna.2015.2819. [DOI] [PubMed] [Google Scholar]
- 42.Liu P., Ridilla M., Patel P., Betts L., Gallichotte E., Shahidi L., Thompson N.L., Jacobson K. Beyond attachment: Roles of DC-SIGN in dengue virus infection. Traffic. 2017;18:218–231. doi: 10.1111/tra.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richter M.K., Da S.V.J., Torres P.S., Hoornweg T.E., van de Pol D.P., Rodenhuis-Zybert I.A., Wilschut J., Smit J.M. Immature dengue virus is infectious in human immature dendritic cells via interaction with the receptor molecule DC-SIGN. PLoS ONE. 2014;9:e98785. doi: 10.1371/journal.pone.0098785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katzelnick L.C., Gresh L., Halloran M.E., Mercado J.C., Kuan G., Gordon A., Balmaseda A., Harris E. Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017;358:929–932. doi: 10.1126/science.aan6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuczera D., Assolini J.P., Tomiotto-Pellissier F., Pavanelli W.R., Silveira G.F. Highlights for dengue immunopathogenesis: Antibody-dependent enhancement, cytokine storm, and beyond. J. Interferon Cytokine Res. 2018;38:69–80. doi: 10.1089/jir.2017.0037. [DOI] [PubMed] [Google Scholar]
- 46.Tomo S., Mohan S., Ramachandrappa V.S., Samadanam D.M., Suresh S., Pillai A.B., Tamilarasu K., Ramachandran R., Rajendiran S. Dynamic modulation of DC-SIGN and FcYR2A receptors expression on platelets in dengue. PLoS ONE. 2018;13:e206346. doi: 10.1371/journal.pone.0206346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barkhash A.V., Perelygin A.A., Babenko V.N., Brinton M.A., Voevoda M.I. Single nucleotide polymorphism in the promoter region of the CD209 gene is associated with human predisposition to severe forms of tick-borne encephalitis. Antivir. Res. 2012;93:64–68. doi: 10.1016/j.antiviral.2011.10.017. [DOI] [PubMed] [Google Scholar]