Abstract
The potential of the plasma membrane (Δψ) regulates the electrochemical potential between the outer and inner sides of cell membranes. The opportunistic fungal pathogen, Candida albicans, regulates the membrane potential in response to environmental conditions, as well as the physiological state of the cell. Here we demonstrate a new method for detection of cell membrane depolarization/permeabilization in C. albicans using the potentiometric zwitterionic dye di-4-ANEPPS. Di-4-ANEPPS measures the changes in the cell Δψ depending on the phases of growth and external factors regulating Δψ, such as potassium or calcium chlorides, amiodarone or DM-11 (inhibitor of H+-ATPase). We also demonstrated that di-4-ANEPPS is a good tool for fast measurement of the influence of amphipathic compounds on Δψ.
Keywords: Candida albicans, plasma membrane potential, membrane polarization, di-4-ANEPPS, detergents
1. Introduction
Though the plasma membrane potential (Δψ) is an electrochemical potential difference between extracellular and intracellular compartments in all living cells, the mechanisms maintaining Δψ differ between cell types [1]. Δψ acts as an indicator of the physiological status of the cell; for example, depolarization of the cell membrane in lymphocytes prevents cell proliferation [2]. The influence of the value of Δψ on the lipid lateral localization in the plasma membrane of the yeast Saccharomyces cerevisiae is another example that highlights the importance of the Δψ in cell biology [3].
Candida albicans is a microorganism of human microflora (skin, as well as urinary and gastrointestinal tracts) and the most common cause of opportunistic fungal infections of immunocompromised patients [4]. The value of C. albicans Δψ is ~−120 mV and is comparable to that of pathogenic bacteria, which ranges from ~−130 mV to ~−150 mV [5,6]. Unlike in C. albicans, the value of Δψ of non-pathogenic S. cerevisiae is ~−71 mV and is comparable to the potential of mammalian cells, which is ~−90 mV [5,6].
Highly desirable activities of antifungal compounds include binding to ergosterol and subsequent permeabilization of the cell membrane [7,8]. The loss of cell membrane integrity due to the action of antifungal drugs causes plasma membrane depolarization [9].
Two types of fluorescent probes are commonly used for measurements of C. albicans plasma membrane polarity: slow response potential-sensitive cationic carbocyanines (Dil, DiS and DiO) and anionic bis-barbituric acid oxonols (DiBAC) [10,11,12,13].
Carbocyanines accumulate in hyperpolarized membranes, while bis-oxonol dyes enter depolarized cells [13,14]. Binding to the cell by both groups of dyes results in a red shift of the fluorescence spectrum while a blue shift of fluorescence spectrum is observed when probes are not bound [12,15]. Accumulation of the cationic and anionic dyes in the plasma membrane and changes of Δψ caused by interfering factors require constant monitoring of the time course of the fluorescence spectrum shifts. Additionally, carbocyanines are substrates for C. albicans drug ATP-binding cassette (ABC) transporters (Cdr1 and Cdr2) and are used to measure the activity of these pumps in real time [15]. However, Cadek et al. [16] found that the excretion of carbocyanines by ABC transporters could interfere with the proper measurement of cell membrane potential.
Potentiometric zwitterionic aminonaphthylethenylpyridinium (ANEP) dyes (di-4-ANEPPS and di-8-ANEPPS) were previously used to map the membrane potential along neurons and muscle fibers [16,17,18]. Both probes reduce the excitation fluorescence intensity at ~440 nm and increase it at ~530 nm in response to membrane hyperpolarization [19,20]. In addition, after excitation in the range of ~470 nm to 490 nm, ANEP dyes cause a blue or red fluorescence shift during depolarization or hyperpolarization of membranes, respectively [21,22,23]. Di-4-ANEPPS was also used for measuring membrane potential in S. cerevisiae. The use of this dye in these walled cells showed its lower stability, but faster response, in comparison to previously used cationic and anionic dyes [24].
In this study, we report a new application of monitoring di-4-ANEPPS fluorescence spectral shift in Candida albicans’ Δψ measurement. We developed a straightforward and reliable assay in monitoring de-/hyperpolarization as a result of ion homeostasis disturbance and after treatment with amphipathic compounds, which may provide a better understanding of the physiology of C. albicans.
2. Materials and Methods
2.1. Chemicals, Strains and Growth Conditions
All chemicals and reagents used in this study were purchased from the following sources: 3,3′-Dipropylthiacarbocyanine iodide (diS-C3(3)), sodium dodecyl sulfate (SDS), benzalkonium chloride (BAC), Triton X-100 (Sigma-Aldrich; Poznań, Poland); D-glucose, bacteriological agar, propidium iodide (PI) (manufacturer: Bioshop, distributor: Lab Empire; Rzeszów, Poland); peptone, yeast extract (YE) (manufacturer: BD; distributor: Diag-med; Warszawa, Poland); potassium chloride (KCl) (Chempur; Piekary Śląskie, Poland); calcium chloride (CaCl2) (POCH; Gliwice, Poland); pyridinium, 4-(2-(6-(dibutylamino)-2-naphthalenyl)ethenyl)-1-(3-sulfopropyl), hydroxide inner salt (di-4-ANEPPS) (Thermo Fisher; Warszawa, Poland); 2-dodecanoyloxyethyl-dimethylammonum chloride (DM-11) was a gift from Dr. Łuczyński (Wroclaw, Poland).
C. albicans strain CAF2-1 (genotype: ura3∆::imm434/URA3) was a kind gift from prof. D. Sanglard (Lausanne, Switzerland) [25]. It was routinely grown at 28 °C on YPD medium (2% glucose, 1% peptone, 1% YE) in a shaking incubator (120 rpm). Agar in a final concentration of 2% was used for medium solidification.
To determine growth phases, CAF2-1 was grown in 20 mL of YPD medium for 24 h at 28 °C with shaking (120 rpm). Every two hours, the A600 measurements were performed using a Hach Odyssey DR/2500 spectrophotometer in three independent repetitions.
For specific experiments, CAF2-1 cells were grown until they reached either early (8 h) or late (14 h) logarithmic phase.
2.2. DiS-C3(3) Assay
The fluorescence assay of Δψ was performed in the early and late logarithmic phase of C. albicans growth as described previously [26]. Δψ measurements using de- and hyperpolarizing compounds (200 mM KCl, 50 μM DM-11; 25 mM CaCl2, 2 μM Amiodarone, respectively) and di-4-ANEPPS (final conc. = 3 x 10−6 M) were performed in the early phase of growth. All reagents were prepared shortly before fluorescence measurements and added at t = 0 min (de- and hyperpolarizing compounds) or at t = 60 min (di-4-ANEPPS).
2.3. Di-4-ANEPPS Assay
Detection of Δψ by di-4-ANEPPS was performed by labelling 3 mL of C. albicans cell suspensions (A600 = 0.1) in citrate phosphate (CP) buffer (pH 6.0). The final concentration of di-4-ANEPPS probe was 3 x 10−6 M. Samples were incubated for 30 min at room temperature (RT). The growth-dependent membrane potential was measured both in the early and late logarithmic phase of C. albicans growth. Membrane potential measurements using de- and hyperpolarizing compounds (200 mM KCl, 50 μM DM-11; 25 mM CaCl2, 2 μM Amiodarone respectively) were performed only in the early phase of growth because of physiological depolarization of plasma membrane in late log phase cells. KCl, DM-11, CaCl2, Amiodarone were added immediately after incubation of cells with di-4-ANEPPS. In all experiments, di-4-ANEPPS was excited at 488 nm (Ex slit = 10 nm) and fluorescence spectra at 520–720 nm (Em slit = 2.5 nm) (PMT voltage = 700 V) were recorded using fluorescence spectrophotometer (HITACHI F-4500) equipped with a xenon lamp. Each experiment was performed in three independent replications and each probe was excited three times. Fluorescence spectra from corresponding experiments were averaged and normalized (value 1 for maximum emission intensity in each case) for comparison of fluorescence maxima shifts.
2.4. Toxicity of di-4-ANEPPS
C. albicans suspensions were treated as described in Section 2.3 (CP buffer; A600 = 0.1; 3 x 10−6 M di-4-ANEPPS; 30 min), washed with CP, and resuspended in CP (100). Suspensions were serially diluted up to 10−3, then 2 µL were spotted onto YPD agar and cultured for 48 h at 28 °C. Afterwards, the plates were photographed using FastGene® B/G GelPic imaging box (Nippon Genetics, Dueren, Germany).
2.5. Influence of Detergents on Δψ
The impact of SDS (0–320 µg/mL), BAC (0–320 µg/mL) and Triton X-100 (0–320 µg/mL) on Δψ was evaluated as described in Section 2.3, with modifications. Fluorescence spectra of di-4-ANEPPS (3 x 10−6 M) solution in CP buffer (pH 6.0) were collected after 30 min incubation in the presence of detergents. Because an interaction between the fluorescent probe and detergents was identified, early log phase C. albicans cells were pretreated with detergents for 30 min at RT, washed three times with CP buffer (pH 6.0), and labelled with di-4-ANEPPS for 30 min. Fluorescence measurements were performed as described above. For data analysis, the red-blue signal ratio (R-B ratio) was calculated by dividing the sum of fluorescence intensity (IF) between 580 and 620 nm by the sum of IF between 540 and 580 nm as shown in the formula below.
| (1) |
Additionally, all results were normalized to the control (value = 1 for the control experiment without the addition of detergents). In this approach, it was assumed that the fluorescence spectra symmetry had a maximum at 580 nm (plasma membrane potential of early log phase cells in control conditions); therefore, blue shift (depolarization) and red shift (hyperpolarization) result in an R-B ratio of <1 and >1, respectively.
2.6. Propidium Iodide (PI) Staining
Assessment of plasma membrane permeability was performed as described before [8], with modifications. Briefly, 3 mL of C. albicans cell suspensions (A600 = 0.1) in CP buffer (pH 6.0) were mixed with SDS (0–320 µg/mL), BAC (0–320 µg/mL) or Triton X-100 (0–320 µg/mL), incubated for 30 min at RT, washed three times with CP buffer, and stained for 5 min with PI to the final dye concentration of 6 x 10−6 M. Next, cell suspensions were washed twice with CP buffer and observed under a Zeiss Axio Imager A2 microscope equipped with a Zeiss Axiocam 503 mono microscope camera and a Zeiss HBO100 mercury lamp. The percentage of permeabilized cells was evaluated by counting PI positive cells out of one hundred cells in three independent repetitions for each experiment. Statistical significance analysis was performed using Student’s t-test (binomial, unpaired).
2.7. Sequences Alignmets
TOK1 gene and Tok1p sequences from S. cerevisiae S288C strain were obtained from Saccharomyces Genome Database (accession ID: SGD:S000003629) [27]. TOK1 gene and Tok1p sequences from C. albicans SC5314 strain were obtained from Candida Genome Database (accession by systematic name: C4_00670W_A) [28]. Sequences alignments were performed by EMBOSS Needle program [29].
3. Results and Discussion
Di-4-ANEPPS dye is widely used in the measurement of the Δψ in tissues [30], but in walled cells it was used only in yeast S. cerevisiae [24]. H+-ATPase forms Δψ in both pathogenic C. albicans and non-pathogenic S. cerevisiae, but its activity in these two species is different. In contrast to S. cerevisiae, C. albicans up-regulates energy reserve metabolism [31] and has a lower acidification activity of H+-ATPase [32]. Our previous investigations indicated that the Δψ of C. albicans measured by diS-C3(3) dye differs from S. cerevisiae [15]. To expand this observation, we used di-4-ANEPPS to measure C. albicans’ Δψ under different conditions and compare this method with the method using diS-C3(3).
3.1. Di-4-ANEPPS and DiS-C3(3) Measure Cell Depolarization Depending on the Phases of Growth
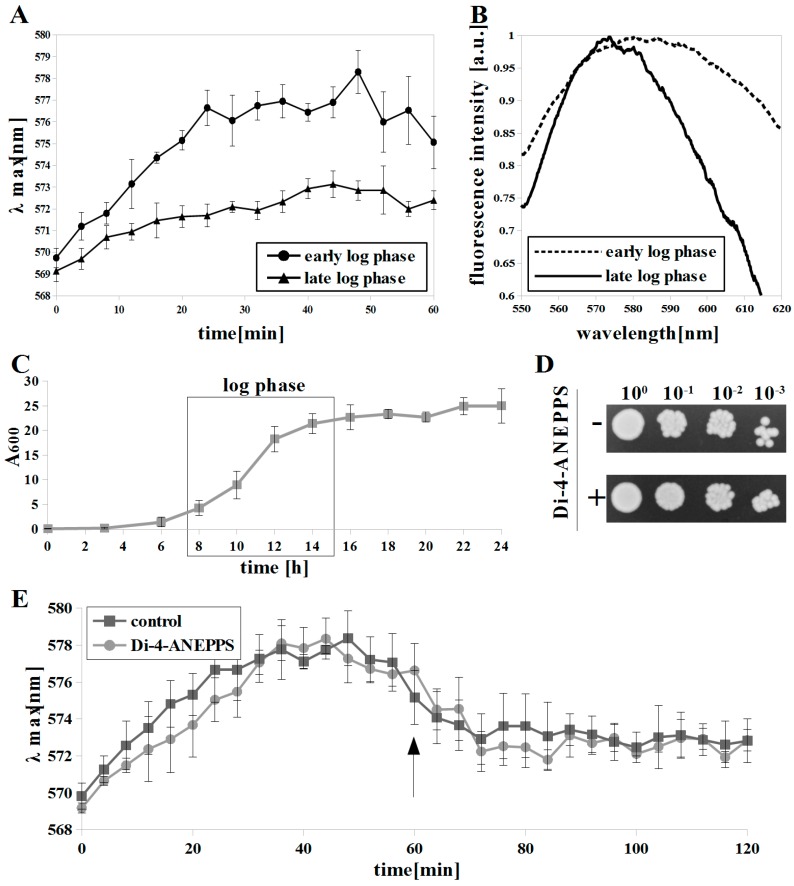
First, we compared diS-C3(3) and di-4-ANEPPS in the detection of growth phase-dependent plasma membrane depolarization for C. albicans (Figure 1A,B). Previously, depolarization of S. cerevisiae plasma membrane resulting from decreased H+-ATPase activity in the late exponential phase was observed using diS-C3(3) [16,33]. In the case of C. albicans, log phase was observed between 8 to 14 h of growth (Figure 1C). Staining with diS-C3(3) in the cells was considerably slower in the late log phase (λmax = ~ 572 nm at 40 min) compared to the early phase (λmax= ~ 577–578 nm at 28 min) of exponential growth (Figure 1A), which indicates membrane depolarization and is in agreement with our previous studies [15].
Figure 1.
Growth phase-dependent depolarization of C. albicans cells measured by: (A) monitoring fluorescence maxima in time by diS-C3(3), means ± SD (n = 4) and (B) fluorescence spectrum shift of di-4-ANEPPS (red-blue signal ratio (R-B) values = 1.043 ± 0.011 and 0.921 ± 0.002 for 8 h and 14 h, respectively), each spectrum is averaged (n = 9); (C) growth curve of C. albicans CAF2-1, strain was grown to 8 h and 14 h (early and late log phases), means ±SD (n = 3); (D) di-4-ANEPPS was not toxic towards C. albicans CAF2-1; (E) ATP-binding cassettes (ABC) mediated diS-C3(3) efflux was not inhibited by addition (arrow) of di-4-ANEPPS, means ±SD (n = 4).
In our study, we used the C. albicans CAF2-1 strain, which has both ABC transporters (Cdr1 and Cdr2). DiS-C3(3) is the substrate for ABC transporters and its efflux is observed at 40–50 min after addition of it to the S. cerevisiae cell suspension [16]. In the case of C. albicans, an efflux of this dye occurs after 60–70 minutes (Figure 1E, control) [15]. For Δψ measurements, we did not monitor diS-C3(3) fluorescence longer than that to avoid ABC transporters interference.
We compared the fluorescence spectrum of di-4-ANEPPS bound to plasma membrane of C. albicans cells in early and late exponential phases of growth (Figure 1B). The di-4-ANEPPS emission maximum (EM) was at ~580 nm for the early exponential phase, whereas in the late exponential cells the EM shifted to ~574 nm, with the spectrum area being noticeably narrowed. Blue-shift of di-4-ANEPPS fluorescence indicates depolarization of the plasma membrane, as previously reported for tissues stained with ANEP dyes [23,34].
In our experiments, di-4-ANEPPS treatment was not toxic in C. albicans (Figure 1D). We conclude that Δψ measurements were not affected by an adverse effect of the dye on C. albicans cells. Additionally, di-4-ANEPPS did not inhibit diS-C3(3) efflux after addition at 60 min. (Figure 1E). The addition of ABC transporter substrate during diS-C3(3) assay results in lower or lack of diS-C3(3) efflux [15], thus di-4-ANEPPS is not an ABC transporter substrate and Δψ measurements were not affected by the ABC transporters efflux activity.
3.2. Di-4-ANEPPS and DiS-C3(3) Measure Cell Depolarization and Hyperpolarization Induced by External Factors
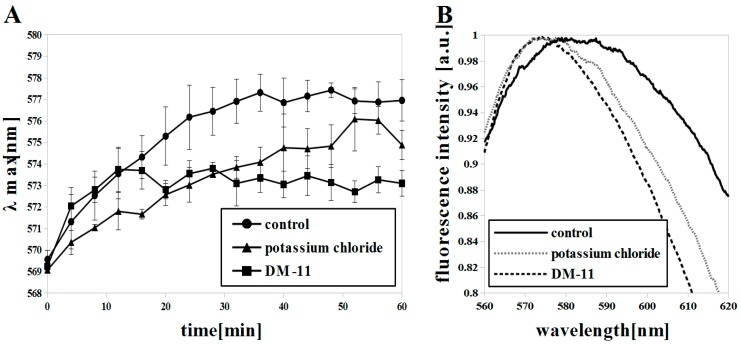
In addition to H+-ATPase, other transmembrane transporters form Δψ in pathogenic and non-pathogenic yeast. K+ ions are transported to the inside of the C. albicans cells by Trk1p uniporter. The single Trk isoform (CaTrk1p) in C. albicans is nearly 60% homologous in four transmembrane motifs to both isoforms of Trkp in S. cerevisiae; this is expected to reflect quantitatively similar functions of these transporters [35]. C. albicans needs a highly efficient K+ uptake system because of low concentration of potassium in the niches of the host organism [36]. The accumulation of potassium ions inside the cell depolarizes the plasma membrane, as demonstrated in S. cerevisiae by Gaskova et al. [37] using diS-C3(3) dye. We used diS-C3(3) and di-4-ANEPPS dyes to measure the C. albicans Δψ after KCl application (Figure 2A,B). A blue shift of λmax (di-4-ANEPPS; EM: 575 nm) and fluorescence intensity kinetics (diS-C3(3); λmax = ~ 575-576 nm at 50 min) were observed (Figure 2A,B), which indicate depolarization of the membrane.
Figure 2.
Plasma membrane potential (Δψ) reduction and membrane depolarization in C. albicans induced by KCl (200 mM) and DM-11 (50 μM) in early log phase (8 h) shown by: (A) diS-C3(3), means ± SD (n = 4) and (B) di-4-ANEPPS (R-B values = 1.040 ± 0.025, 0.991 ± 0.002 and 0.978 ± 0.010 for control, KCl, DM-11, respectively), each spectrum is averaged (n = 9).
Among transmembrane transporters that contribute to Δψ are regulators of intracellular potassium concentrations, such as the Tok1 channel [38]. Tok1p is a potassium specific channel that releases K+ from the cell and thus regenerates Δψ [39]. Deletion of the TOK1 gene results in the depolarization of plasma membrane, and conversely, its overexpression leads to hyperpolarization of the yeast plasma membrane [40,41]. In our investigations, the C. albicans Δψ was measured with diS-C3(3) dye in real time for 60 min. The Δψ grew more slowly after using KCl (λmax at ~50 min) than in cells not treated with KCl (λmax at ~30 min), but it finally achieved similar λmax values (Figure 2A). By using the di-4-ANEPPS dye and observing the blue shift of fluorescence intensity (control EM: 582 nm; KCl treated EM: 576 nm) we have shown that the plasma membrane of C. albicans’ cells is depolarized (Figure 2B). However, the intensity of this depolarization is lower than after treatment of cells with DM-11 (EM: 574 nm), which is a known H+-ATPase inhibitor in yeast (Figure 2B) [42,43]. We observed reduced staining of cells with diS-C3(3) after 10 min. incubation with DM-11 and no Δψ recovery (Figure 2). DM-11 is a lysosomotropic agent whose deprotonated form penetrates membranes and protonated form accumulates in acidic yeast compartments (e.g., vacuoles). If this compound is used in high concentrations it can cause membrane disruption [44]. Zahumensky et al. [45] have observed the increase of Tok1p activity in S. cerevisiae cells treated with DM-11 and a gradually more extensive Tok1 channel activity with deeper depolarization of the membrane.
Our results indicate a weaker role of Tok1p in Δψ recovery after treatment of cells with DM-11 and deeper membrane depolarization than when using KCl (Figure 2A,B). According to the sequence alignment (Section 2.7), the CaTOK1 gene sequence is identical to the ScTOK1 gene in 48.6% and CaTok1p with ScTok1p in 31.4%, which can indicate partially different functions of these transporters. The staining of C. albicans strains by diS-C3(3) is approximately twice as slow as that of S. cerevisiae [15]. The reason for this difference in the rate of staining could be a lower Δψ in C. albicans cells relative to S. cerevisiae cells. Probably for these reasons, the Δψ reduction and membrane depolarization following the blockage of H+-ATPase by DM-11 in C. albicans are not recovered by Tok1p activity.
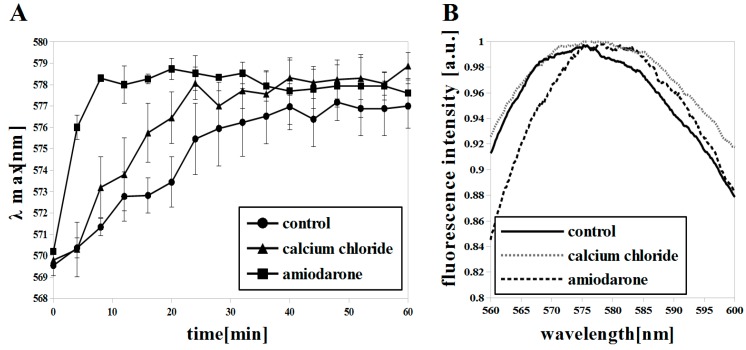
Calcium channels in S. cerevisiae have been identified as high-affinity and low-affinity calcium uptake systems (HACS and LACS). The voltage-gated Cch1p [46] and the stretch-activated Mid1p [47] form a complex that defines the HACS, whereas Fig1p is a component of LACS [48]. The homologs of these genes in C. albicans were found by Brandt et al. [49]. CaCCH1 has a 38.4% identity to its S. cerevisiae homolog while the CaMID1 gene sequence had 36.9% identity to ScMID1 [49]. In S. cerevisiae, HACS is activated by low Ca2+, whereas LACS activity was only revealed under conditions when HACS was inhibited by rich media and its affinity for Ca2+ is 16-fold lower [50]. Brandt et al. [49] observed a similar dependence in C. albicans. The perturbation of calcium homeostasis by the influx of Ca2+ into C. albicans cells leads to their death. This finding has allowed amiodarone (AMD) to be used as an antifungal drug. Maresova et al. [41] and Pena et al. [51] suggested that AMD elicits plasma membrane hyperpolarization by inducing K+ efflux from the cells followed by depolarization resulting in the Ca2+ influx and loss of cell viability.
We used a high concentration of CaCl2 (25 mM) to force the cells to take up Ca2+ through the LACS system and to induce membrane hyperpolarization. The measurements with diS-C3(3) indicated a Δψ increase (λmax = ~578–9 at ~40 min) almost with the same intensity as in the case of cells with a low concentration of CaCl2 (λmax = ~577–8 at ~40 min) (Figure 3A). Di-4-ANEPPS fluorescence showed only a slight red shift in cells with 25 mM CaCl2 (control EM: 575 nm; CaCl2 treated EM: 577 nm) (Figure 3B). Callahora et al. [52] pointed out that agents that did not produce an efflux of K+ also did not produce increased Ca2+ uptake, and those that produced K+ efflux increased Ca2+ uptake. Transport across the plasma membrane in C. albicans cells appears to be reversible. A slight red shift of the di-4-ANEPPS fluorescence indicating low hyperpolarization in our CaCl2 studies may be due to compensation of the negative charge on the outside of membrane by K+ efflux from the cells. On the other hand, when we used AMD we observed a fast Δψ build up (λmax = ~578–9 at 8 min) (Figure 3A) and a red shift of di-4-ANEPPS fluorescence (control EM: 575 nm; AMD treated EM: 580 nm) (Figure 3B) indicating membrane hyperpolarization according to studies on S. cerevisiae by other researchers [41,51].
Figure 3.
Δψ increase and membrane hyperpolarization in C. albicans induced with calcium chloride (CaCl2) (25 mM) and amiodarone (AMD) (2 μM) in early log phase (8 h) shown by: (A) diS-C3(3), means ±SD (n = 4) and (B) di-4-ANEPPS (R-B values = 0.978 ± 0.011, 0.996 ± 0.006 and 1.033 ± 0.023 for control, CaCl2, AMD, respectively), each spectrum is averaged (n = 9).
3.3. Di-4-ANEPPS Is a Suitable Tool for Fast Measuring of the Influence of Detergents on Δψ
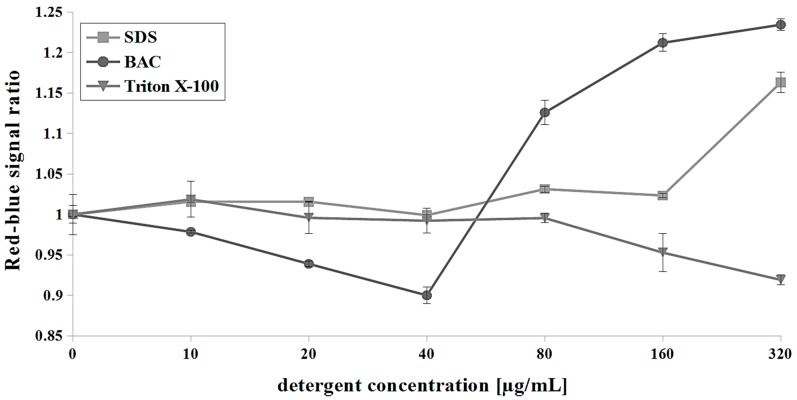
In Figure 1, Figure 2 and Figure 3 we show the validation of the usage of di-4-ANEPPS in Δψ measurements in comparison to already known diS-C3(3) dye. The di-4-ANEPPS assay is more rapid and reliable due to lack of toxicity towards C. albicans cells (Figure 1D) and unlike diS-C3(3), the di-4-ANEPPS Δψ measurements are not interfered with by ABC transporters activity (Figure 1E). Here, we wanted to show the vast potential of the di-4-ANEPPS dye for rapid screening of Δψ in C. albicans as a result of cell physiology changes. We selected the influence of amphipathic compounds on C. albicans’ membranes using three detergents: cationic benzalkonium chloride (BAC), anionic sodium dodecyl sulfate (SDS) and non-ionic Triton X-100. Additionally, for more clear presentation of di-4-ANEPPS fluorescence shifts we used an R-B ratio formula, described in Section 2.5.
The mechanism of antifungal action of commonly used detergents is often not well understood. Kodedova et al. [53] showed that detergents at high concentrations cause membrane permeabilization in S. cerevisiae and outflow of cations from the inside of the cell. Permeabilized cells cannot maintain Δψ and there is a massive outflow of cations from the inside of the cell. Gaskova et al. [37] noted that this outflow of cations enhances diS-C3(3) binding capacity of the cytosolic components and this leads to a fast increase of λmax.
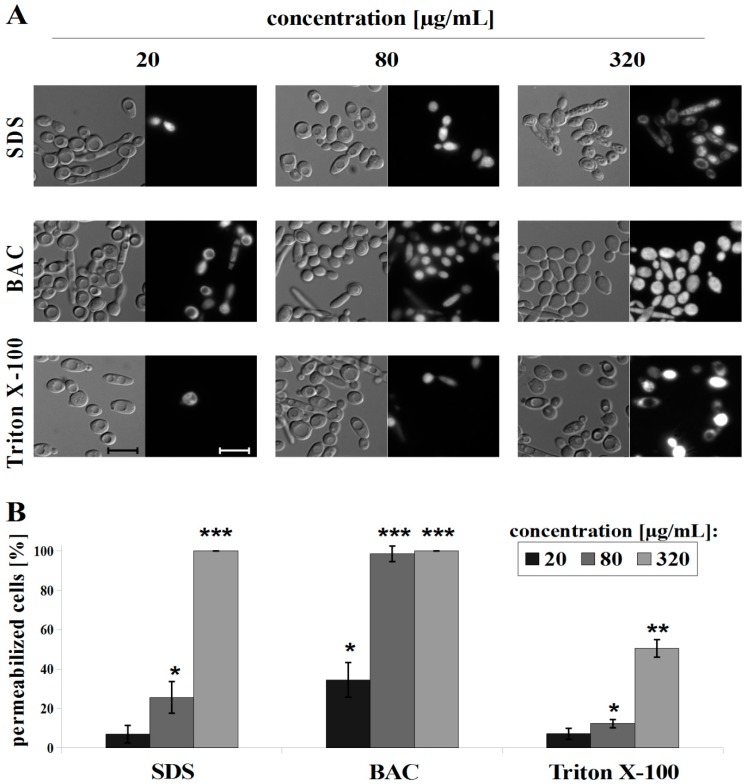
SDS is an efficient solubilizer of integral membrane proteins [54]. At low concentrations, SDS increased the permeability of the S. cerevisiae plasma membrane, as demonstrated by Kodedova et al. [53], using diS-C3(3), whereas at a higher concentration (1.44 mg/mL), SDS caused a very rapid red shift of diS-C3(3) indicating fully permeabilized membranes. The intensity of antifungal activity of SDS depends on the time of incubation with cells and the concentration used. After a 30 min treatment with SDS, we observed hyperpolarization of the plasma membrane in a range of 80–320 μg/mL SDS (R-B ratio increase of up to ~1.15 at 320 μg/mL) (Figure 4). PI measurements indicate a 25% permeabilization at a concentration of 80 μg/mL SDS and fully permeabilized cells in higher concentrations (Figure 5).
Figure 4.
The influence of detergents sodium dodecyl sulfate (SDS) (0–320 µg/mL), benzalkonium chloride (BAC) (0–320 µg/mL) and Triton X-100 (0–320 µg/mL) on Δψ. For data analysis, red-blue signal ratio (R-B ratio) was calculated as described in Section 2.5, means ± SD (n = 9).
Figure 5.
C. albicans CAF2-1 strain staining with propidium iodide (PI) after treatment with detergents: SDS, BAC and Triton X-100, presented as: (A) microscopic observations, scale bar = 10 µm and (B) histograms of the counted % of permeabilized cells, means ± SD (n = 3), statistical analysis at each concentration was performed relative to control experiment without detergent (* p < 0.05; ** p < 0.01; *** p < 0.001).
BAC is a quaternary ammonium compound which has been used in clinical applications since 1935 [55]. Kodedova et al. [53] found that 18 μg/mL BAC caused a red shift in S. cerevisiae cells stained with diS-C3(3), which indicated partial permeabilization of cells while others have been depolarized. At 0.36 μg/mL BAC the cells were depolarized [53]. Our results with di-4-ANEPPS show a similar trend in BAC interaction with C. albicans cells. We observed depolarization of cells in the range of 10–40 μg/mL BAC (R-B ratio drop of up to 0.9 at 40 μg/mL) and hyperpolarization when the concentrations of 80–320 μg/mL BAC were used (R-B ratio increase of up to ~1.25 at 320 μg/mL) (Figure 4). As we show in Figure 5, BAC induced the highest permeability of membranes among the used detergents in the concentration of 20 μg/mL (>90% permeabilized cells) and from a concentration of 40 μg/mL we observed a full permeabilization of cells.
The nonionic detergent Triton X-100 was previously used for permeabilization as a tool for the assay of yeast intracellular enzymes in whole cells [56], but the information on the Triton X-100 effect of yeast plasma membrane is scarce. Using di-4-ANEPPS and PI, we observed the weakest effect of Triton X-100 among the detergents used. Triton X-100 induced a blue shift of di-4-ANEPPS (R-B = 0.95 and 0.9 at 160 and 320 μg/mL, respectively), which means depolarization of the C. albicans plasma membrane only at the highest concentrations used (Figure 4). We also observed approximately 50% permeabilization of C. albicans cells at 320 μg/mL Triton X-100 (Figure 5).
4. Conclusions
In this study, we reported the use of di-4-ANEPPS dye on Δψ measurements of C. albicans. For the development of the method, we tested different conditions disturbing ion homeostasis, such as cell aging or de- and hyperpolarising agents (KCl and DM-11; CaCl2 and AMD) and compared results with the known diS-C3(3) assay. We provided new information on the response of C. albicans under those conditions and discussed our data based on the findings reported by other research groups using non-pathogenic S. cerevisiae. Due to the advantages of di-4-ANEPPS over diS-C3(3), we developed an R-B ratio formula for rapid Δψ calculations and proposed it as a method for detection of C. albicans physiology disturbances on the example of the influence of commonly used detergents (SDS, BAC and Triton X-100).
Author Contributions
J.S.; conception, design, analysis, and interpretation of data for the work, preparation of the figures. A.K.; conception, interpretation of data for the work, writing and editing the manuscript.
Funding
This work was supported by the National Science Centre, Poland, NCN Grants: 2016/23/B/NZ1/01928, 2017/25/N/NZ1/00050 and by Wrocław Centre of Biotechnology, Program: the Leading National Research Centre (KNOW) for 2014–2018.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gásková D., Brodská B., Herman P., Vecer J., Malínský J., Sigler K., Benada O., Plásek J. Fluorescent probing of membrane potential in walled cells: diS-C3(3) assay in Saccharomyces cerevisiae. Yeast. 2007;14:1189–1197. doi: 10.1002/(SICI)1097-0061(19980930)14:13<1189::AID-YEA320>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 2.Leonard R.J., Garcia M.L., Slaughter R.S., Reuben J.P. Selective blockers of voltage-gated K+ channels depolarize human T lymphocytes: mechanism of the antiproliferative effect of charybdotoxin. Proc. Nat. Acad. Sci. USA. 1992;89:10094–10098. doi: 10.1073/pnas.89.21.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grossmann G., Opekarová M., Malinsky J., Weig-Meckl I., Tanner W. Membrane potential governs lateral segregation of plasma membrane proteins and lipids in yeast. EMBO J. 2007;26:1–8. doi: 10.1038/sj.emboj.7601466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kett D.H., Azoulay E., Echeverria P.M., Vincent J.L. Candida bloodstream infections in intensive care units: Analysis of the extended prevalence of infection in intensive care unit study. Crit. Care Med. 2011;39:665–670. doi: 10.1097/CCM.0b013e318206c1ca. [DOI] [PubMed] [Google Scholar]
- 5.Vacata V., Kotyk A., Sigler K. Membrane potentials in yeast cells measured by direct and indirect methods. Biochim. Biophys. Acta. 1981;643:265–268. doi: 10.1016/0005-2736(81)90241-8. [DOI] [PubMed] [Google Scholar]
- 6.Prasad R., Hofer M. Tetraphenylphosphonium is an indicator of negative membrane potential in Candida albicans. Biochim. Biophys. Acta. 1986;861:377–380. doi: 10.1016/0005-2736(86)90442-6. [DOI] [PubMed] [Google Scholar]
- 7.Sanglard D., Ischer F., Parkinson T., Falconer D., Bille J. Candida albicans mutations in the ergosterol biosynthetic pathway and resistance to several antifungal agents. Antimicrob. Agents Chemother. 2003;47:2404–2412. doi: 10.1128/AAC.47.8.2404-2412.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suchodolski J., Feder-Kubis J., Krasowska A. Antifungal activity of ionic liquids based on (−)-menthol: A mechanism study. Microbiol. Res. 2017;197:56–64. doi: 10.1016/j.micres.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Thevelein J.M., Beullens M., Honshoven F., Hoebeeck G., Detremerie K., Den Hollander J.A., Jans A.W.H. Regulation of the cAMP level in the yeast Saccharomyces cerevisiae: Intracellular pH and the effect of membrane depolarizing compounds. J. Gen. Microbiol. 1987;133:2191–2196. doi: 10.1099/00221287-133-8-2191. [DOI] [PubMed] [Google Scholar]
- 10.Liao R.S., Rennie R.P., Talbot J.A. Assessment of the effect of amphotericin B on the vitality of Candida albicans. Antimicrob. Agents Chemother. 1999;43:1034–1041. doi: 10.1128/AAC.43.5.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang B., Hwang J.S., Lee J., Lee D.G. The antimicrobial peptide, psacotheasin induces reactive oxygen species and triggers apoptosis in Candida albicans. Biochem. Biophys. Res. Commun. 2011;405:267–271. doi: 10.1016/j.bbrc.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Epps D.E., Wolfe M.L., Groppi V. Characterization of the steady-state and dynamic fluorescence properties of the potential-sensitive dye bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC4(3)) in model systems and cells. Chem. Phys. Lipids. 1994;69:137–150. doi: 10.1016/0009-3084(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 13.Cabrini G., Verkman A.S. Potential-sensitive response mechanism of diS-C3-(5) in biological membranes. J. Membr. Biol. 1986;92:171–182. doi: 10.1007/BF01870706. [DOI] [PubMed] [Google Scholar]
- 14.Novo D., Perlmutter N.G., Hunt R.H., Shapiro H.M. Accurate flow cytometric membrane potential measurement in bacteria using diethyloxacarbocyanine and a ratiometric technique. Cytometry. 1999;35:55–63. doi: 10.1002/(SICI)1097-0320(19990101)35:1<55::AID-CYTO8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Szczepaniak J., Łukaszewicz M., Krasowska A. Detection of inhibitors of Candida albicans Cdr transporters using a diS-C3(3) fluorescence. Front. Microbiol. 2015;6:176. doi: 10.3389/fmicb.2015.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Čadek R., Chládková K., Sigler K., Gášková D. Impact of the growth phase on the activity of multidrug resistance pumps and membrane potential of S. cerevisiae: Effect of pump overproduction and carbon source. BBA Biomembr. 2004;1665:111–117. doi: 10.1016/j.bbamem.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Schaffer P., Ahammer H., Müller W., Koidl B., Windisch H. Di-4-ANEPPS causes photodynamic damage to isolated cardiomyocytes. Pflug. Arch. Eur. J. Phys. 1994;426:548–551. doi: 10.1007/BF00378533. [DOI] [PubMed] [Google Scholar]
- 18.Fromherz P., Müller C.O. Voltage-sensitive fluorescence of amphiphilic hemicyanine dyes in neuron membrane. Biochim. Biophys. Acta. 1993;1150:111–122. doi: 10.1016/0005-2736(93)90079-F. [DOI] [PubMed] [Google Scholar]
- 19.Gross E., Bedlack R.S., Loew L.M. Dual-wavelength ratiometric fluorescence measurement of the membrane dipole potential. Biophys. J. 1994;67:208–216. doi: 10.1016/S0006-3495(94)80471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montana V., Farkas D.L., Loew L.M. Dual-wavelength ratiometric fluorescence measurements of membrane potential. Biochemistry. 1989;28:4536–4539. doi: 10.1021/bi00437a003. [DOI] [PubMed] [Google Scholar]
- 21.McGahren E.D., Beach J.M., Duling B.R. Capillaries demonstrate changes in membrane potential in response to pharmacological stimuli. Am. J. Physiol. 1998;274:60–65. doi: 10.1152/ajpheart.1998.274.1.H60. [DOI] [PubMed] [Google Scholar]
- 22.Beach J.M., McGahren E.D., Xia J., Duling B.R. Ratiometric measurement of endothelial depolarization in arterioles with a potential-sensitive dye. Am. J. Physiol. 1996;270:2216–2227. doi: 10.1152/ajpheart.1996.270.6.H2216. [DOI] [PubMed] [Google Scholar]
- 23.Kao W.Y., Davis C.E., Kim Y.I., Beach J.M. Fluorescence emission spectral shift measurements of membrane potential in single cells. Biophys. J. 2001;81:1163–1170. doi: 10.1016/S0006-3495(01)75773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaloupka R., Plášek J., Slavík J., Siglerová V., Sigler K. Measurement of membrane potential in Saccharomyces cerevisiœ by the electrochromic probe di-4-ANEPPS: Effect of intracellular probe distribution. Folia Microbiol. 1997;42:451–456. doi: 10.1007/BF02826552. [DOI] [PubMed] [Google Scholar]
- 25.Fonzi W.A., Irwin M.Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szczepaniak J., Łukaszewicz M., Krasowska A. Estimation of Candida albicans ABC transporter behavior in real-time via fluorescence. Front. Microbiol. 2015;6:1382. doi: 10.3389/fmicb.2015.01382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saccharomyces Genome Database. [(accessed on 2 March 2019)]; Available online: https://www.yeastgenome.org/locus/S000003629.
- 28.Candida Genome Database. [(accessed on 2 March 2019)]; Available online: http://www.candidagenome.org/cgi-bin/locus.pl?locus=C4_00670W_A&organism=C_albicans_SC5314.
- 29.EMBOSS Needle. [(accessed on 2 March 2019)]; Available online: https://www.ebi.ac.uk/Tools/psa/emboss_needle/nucleotide.html.
- 30.Bachtel A.D., Gray R.A., Stohlman J.M., Bourgeois E.B., Pollard A.E., Rogers J.M. A novel approach to dual excitation ratiometric optical mapping of cardiac action potentials with di-4-ANEPPS using pulsed LED excitation. IEEE Trans. BioMed. Eng. 2011;58:2120–2126. doi: 10.1109/TBME.2011.2148719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodaki A., Bohovych I.M., Enjalbert B., Young T., Odds F.O., Gow N.A.R., Brown A.J.P. Glucose promotes stress resistance in the fungal pathogen Candida albicans. Mol. Biol. Cell. 2009;20:4845–4855. doi: 10.1091/mbc.e09-01-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calahorra M., Sanchez N.S., Pena A. Characterization of glycolytic metabolism and ion transport of Candida albicans. Yeast. 2012;29:357–370. doi: 10.1002/yea.2915. [DOI] [PubMed] [Google Scholar]
- 33.Nso E., Goffeau A., Dufour J.P. Fluctuations during growth of the plasma membrane H(+)-ATPase activity of Saccharomyces cerevisiae and Schizosaccharomyces pombe. Folia Microbiol. 2002;47:401–406. doi: 10.1007/BF02818697. [DOI] [PubMed] [Google Scholar]
- 34.Tsutsui H., Jinno Y., Tomita A., Okamura Y. Rapid evaluation of a protein-based voltage probe using a field-induced membrane potential change. BBA Biomembr. 2014;1838:1730–1737. doi: 10.1016/j.bbamem.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Miranda M., Bashi E., Vylkova S., Edgerton M., Slayman C., Rivetta A. Conservation and dispersion of sequence and function in fungal TRK potassium transporters: Focus on Candida albicans. FEMS Yeast Res. 2009;9:278–292. doi: 10.1111/j.1567-1364.2008.00471.x. [DOI] [PubMed] [Google Scholar]
- 36.Hušeková B., Elicharová H., Sychrová H. Pathogenic Candida species differ in the ability to grow at limiting potassium concentrations. Can. J. Microbiol. 2016;62:394–401. doi: 10.1139/cjm-2015-0766. [DOI] [PubMed] [Google Scholar]
- 37.Gaskova D., Cadek R., Chaloupka R., Plasek J., Sigler K. Factors underlying membrane potential-dependent and –independent fluorescence responses of potentiometric dyes in stressed cells: diS-C3(3) in yeast. Biochim. Biophys. Acta. 2001;1511:74–79. doi: 10.1016/S0005-2736(00)00383-7. [DOI] [PubMed] [Google Scholar]
- 38.Ariño J., Ramos J., Sychrová H. Alkali metal cation transport and homeostasis in yeasts. Microbiol. Mol. Biol. Rev. 2010;74:95–120. doi: 10.1128/MMBR.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertl A., Bilher H., Reid J.D., Kettner C., Slayman C.I. Physiological characterization of the yeast plasma membrane outward rectifying K+ channel, DIK1 (TOK1) in situ. J. Membr. Biol. 1998;162:67–80. doi: 10.1007/s002329900343. [DOI] [PubMed] [Google Scholar]
- 40.Maresova J., Urbankova E., Gaskova D., Sychrova H. Measurements of plasma membrane potential changes in Saccharomyces cerevisiae cells reveal the importance of the Tok1 channel in membrane potential maintenance. FEMS Yeast Res. 2006;6:1039–1046. doi: 10.1111/j.1567-1364.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- 41.Maresova L., Muend S., Zhang Y.Q., Sychrova H., Rao R. Membrane hyperpolarization drives cation influx and fungicidal activity of amiodarone. J. Biol. Chem. 2009;284:2795–2802. doi: 10.1074/jbc.M806693200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hendrych T., Kodedova M., Sigler K., Gaskova D. Characterization of the kinetics and mechanisms of inhibition of drugs interacting with the S. cerevisiae multidrug resistance pumps Pdr5p and Snq2p. Biochim. Biophys. Acta. 2009;1788:717–723. doi: 10.1016/j.bbamem.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Lachowicz T.M., Krasowska A., Łuczyński J., Witek S. Plasma membrane H+ATPase activity in wild type and mutants of yeast Saccharomyces cerevisiae treated by some lysosomotropic drugs. Folia Microbiol. 1998;43:203–205. doi: 10.1007/BF02816514. [DOI] [PubMed] [Google Scholar]
- 44.Krasowska A., Chmielewska L., Łuczyński J., Witek S., Sigler K. Dual mechanism of the antifungal effect of new lysosomotropic agents on Saccharomyces cerevisiae RXII strain. Cell. Mol. Biol. Lett. 2003;8:111–120. [PubMed] [Google Scholar]
- 45.Zahumenský J., Jančíková I., Drietomská A., Švenkrtová A., Hlaváček O., Hendrych T., Plášek J., Sigler K., Gášková D. Yeast Tok1p channel is a major contributor to membrane potential maintenance under chemical stress. BBA Biomembr. 2017;1859:1974–1985. doi: 10.1016/j.bbamem.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 46.Paidhungat M., Garrett S. A homolog of mammalian, voltage-gated calcium channels mediates yeast pheromone-stimulated Ca2+ uptake and exacerbates the cdc1(Ts) growth defect. Mol. Cell. Biol. 1997;17:6339–6347. doi: 10.1128/MCB.17.11.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iida H., Nakamura H., Ono T., Okumura M., Anraku Y. MID1, a novel Saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating. Mol. Cell. Biol. 1994;14:8259–8271. doi: 10.1128/MCB.14.12.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muller E.M., Mackin N.A., Erdman S.E., Cunningham K.W. Fig1p facilitates Ca2+ influx and cell fusion during mating of Saccharomyces cerevisiae. J. Biol. Chem. 2003;278:38461–38469. doi: 10.1074/jbc.M304089200. [DOI] [PubMed] [Google Scholar]
- 49.Brand A., Shanks S., Duncan V.M.S., Yang M., Mackenzie K., Gow N.A.R. Hyphal orientation of Candida albicans is regulated by a calcium-dependent mechanism. Curr. Biol. 2007;17:347–352. doi: 10.1016/j.cub.2006.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muller E.M., Locke E.G., Cunningham K.W. Differential regulation of two Ca2+ influx systems by pheromone signaling in Saccharomyces cerevisiae. Genetics. 2001;159:1527–1538. doi: 10.1093/genetics/159.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pena A., Calahorra M., Michel B., Ramirez J., Sanchez N.S. Effects of amiodarone on K+, internal pH and Ca2+ homeostasis in Saccharomyces cerevisiae. FEMS Yeast Res. 2009;9:832–848. doi: 10.1111/j.1567-1364.2009.00538.x. [DOI] [PubMed] [Google Scholar]
- 52.Calahorra M., Sanchez N.S., Peña A. Effects of acridine derivatives on Ca2+ uptake by Candida albicans. Bioenergetics. 2017;6:152. doi: 10.4172/2167-7662.1000152. [DOI] [Google Scholar]
- 53.Kodedova M., Sigler K., Lemire B.D., Gaskova D. Fluorescence method for determining the mechanism and speed of action of surface-active drugs on yeast cells. Biotechniques. 2011;50:58–63. doi: 10.2144/000113568. [DOI] [PubMed] [Google Scholar]
- 54.Maite M., Champeil P., Moller J.V. Interaction of membrane proteins and lipids with solubilizing detergents. Biochim. Biophys. Acta. 2000;1508:86–111. doi: 10.1016/s0304-4157(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 55.Block S.S. Disinfection, Sterilization and Preservation. 4th ed. Lea & Febiger; Philadelphia, PA, USA: 1991. [Google Scholar]
- 56.Laouar L., Muligan B.J., Lowe K.C. Yeast permeabilization with surfactants. Biotechnol. Lett. 1992;14:719–720. doi: 10.1007/BF01021649. [DOI] [Google Scholar]