ABSTRACT
Plant morphogenesis relies on the accurate positioning of the partition (cell plate) between dividing cells during cytokinesis. The cell plate is synthetized by a specialized structure called the phragmoplast, which consists of microtubules, actin filaments, membrane compartments and associated proteins. The phragmoplast forms between daughter nuclei during the transition from anaphase to telophase. As cells are commonly larger than the originally formed phragmoplast, the construction of the cell plate requires phragmoplast expansion. This expansion depends on microtubule polymerization at the phragmoplast forefront (leading zone) and loss at the back (lagging zone). Leading and lagging zones sandwich the ‘transition’ zone. A population of stable microtubules in the transition zone facilitates transport of building materials to the midzone where the cell plate assembly takes place. Whereas microtubules undergo dynamic instability in all zones, the overall balance appears to be shifted towards depolymerization in the lagging zone. Polymerization of microtubules behind the lagging zone has not been reported to date, suggesting that microtubule loss there is irreversible. In this Review, we discuss: (1) the regulation of microtubule dynamics in the phragmoplast zones during expansion; (2) mechanisms of the midzone establishment and initiation of cell plate biogenesis; and (3) signaling in the phragmoplast.
KEY WORDS: Cell plate, Cytokinesis, Microtubule dynamics, Phragmoplast
Summary: Although cytokinesis commonly spans the shortest cell axis, plant development requires divisions across the longest cell axis, which can be up to 1 mm long. This Review addresses the mechanics of this process.
Introduction
In development, animal cells are motile, whereas the rigid oligosaccharide cell walls render plant cells immobile. Therefore, plant development relies on the establishment of correct cell patterns during the early stages of organ formation by precise positioning of partitions (cell plates) between dividing cells. Cell plate construction depends on the pre-prophase band (PPB) and the phragmoplast. Both are plant-specific structures that co-evolved with apical growth and asymmetric cell division (Buschmann and Zachgo, 2016). The PPB forms during late G2 and delineates the position of the division plane by defining the unique molecular composition of the ‘cortical division zone’, the region on the plasma membrane where the cell plate will eventually be attached by the phragmoplast (Rasmussen et al., 2013). The phragmoplast originates during anaphase from the remnants of the central spindle (Segui-Simarro et al., 2004). It consists of microtubules, actin, membrane compartments and proteins that associate with or regulate the above (Boruc and Van Damme, 2015; Lipka et al., 2015). The microtubule component of the phragmoplast consists of two aligned arrays that flank the so-called phragmoplast midzone, where cell plate assembly takes place (Fig. 1). The initial phragmoplast has a disk shape with a diameter that approximately equals that of the daughter nuclei (Fig. 1); however, the parental cell is generally wider. For example, the length of a cambium cell exceeds the diameter of the disk-shaped phragmoplast during late anaphase by up to 100 fold (Larson, 1994). In such cases, the phragmoplast expands through the cytoplasm until the cell plate reaches the mother cell boundary (Fig. 1).
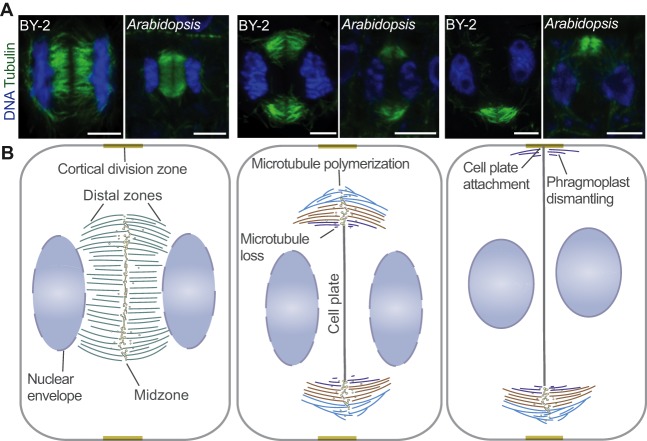
Fig. 1.
Successive stages of phragmoplast expansion. (A) Representative pictures of successive stages of phragmoplast expansion in Tobacco BY-2 and Arabidopsis root meristem cells. In both systems, the phragmoplast expands asymmetrically and first attaches to one side of the mother cell, where microtubules depolymerize. Microtubules were immunostained with anti-tubulin antibody (green) and staining of DNA with DAPI (blue). Scale bars: 5 µm. (B) The phragmoplast is established between daughter nuclei (blue ellipses) during the anaphase-to-telophase transition and at this stage is disk shaped. Cell plate assembly takes place in the midzone. Once the cell plate assembly reaches the tubular network stage, microtubules depolymerize and this results in a ring-shaped phragmoplast. The ring phragmoplast then expands towards the cortical division zone, which was established during prophase by the preprophase band. During the expansion, microtubules depolymerize in the regions where the cell plate reaches a certain degree of maturity (lagging zone, purple) and polymerize at the outermost phragmoplast edge (leading zone, light blue). The leading and lagging zones sandwich transition zone (orange). Phragmoplast expansion is generally asymmetric and the cell plate first fuses with one side of the cortical division zone (Cutler and Ehrhardt, 2002; Lucas and Sack, 2012). The phragmoplast is dismantled once the cell plate attaches to the plasma membrane and the cell wall.
Phragmoplast expansion is driven by the dynamic instability of microtubules. Microtubules depolymerize in the innermost regions – where cell plate synthesis has been accomplished – and re-polymerize at the expanding phragmoplast front – where cell plate synthesis will next take place (Segui-Simarro et al., 2007). Eventually, the cell plate joins the parental cell wall at the site that has been previously determined by the PPB (Lipka et al., 2015) and the phragmoplast disassembles. Animal cells form a cytokinetic structure similar to the phragmoplast, called the midbody (Lloyd and Chan, 2006). However, in contrast to the phragmoplast, the midbody does not expand, remains static during cytokinesis and persists during interphase (Steigemann and Gerlich, 2009). The expansion of the phragmoplast is an important and unique evolutionary advance for plants, because it allows the recycling of tubulin, as well as other phragmoplast proteins, and enables the concomitant correction of any inappropriately positioned division planes.
In this Review, we discuss the regulation of microtubule dynamics in the course of phragmoplast expansion. The first section provides an overview of the relevant phragmoplast components that govern microtubule behavior. The second section focuses on the interplay between phragmoplast components during the expansion. Coordination of the microtubule dynamics with the cell plate assembly results in two asymmetries: (1) the lateral (longitudinal) asymmetry, which is generated by addition of new microtubules on the outer edge and microtubule loss on the inner edge of the expanding phragmoplast; (2) the axial (transverse) asymmetry, which is the consequence of cell plate assembly processes in the midzone. Interaction between these asymmetries generates five functionally distinct zones: distal, leading, transition and lagging zones, and the midzone. We discuss roles of the phragmoplast components in the context of the five zones and provide an overview of the signaling processes that underlie microtubule loss in the lagging zone.
An overview of the main phragmoplast components
A detailed account of phragmoplast proteins has been recently provided in several reviews (Lipka et al., 2015; McMichael and Bednarek, 2013; Müller and Jürgens, 2016). Here, we will focus on highlighting the specific properties of the phragmoplast components that are relevant for the model we discuss.
Microtubule organization and dynamics
Microtubule nucleation
Microtubules in plants are nucleated by the evolutionarily conserved γ-tubulin ring complex (γ-TuRC) comprising six subunits, GCP1–GCP6, where γ-tubulin is GCP1 (Hashimoto, 2013). γ-TuRC can nucleate microtubules in the cytoplasm and on the lattice of extant microtubules. The geometry of microtubule nucleation by γ-TuRC is regulated by the augmin complex, which consists of eight subunits (AUG1–AUG8), as well as by the homologs of mammalian and yeast MOZART1 (MZT1) protein GIP1 and GIP2, which interact with GCP3 (Hashimoto, 2013; Janski et al., 2012). These proteins recruit γ-TuRC to the lattice of extant microtubules and facilitate the nucleation of branched microtubules, whereby a new microtubule forms at an angle relative to the existing one (Hashimoto, 2013; Liu et al., 2014; Walia et al., 2014).
Multiple studies have demonstrated the importance of branched microtubule nucleation by γ-TuRC for plant cytokinesis: γ-tubulin, GCP3, MZT1 (GIP1 and GIP2), and AUG7 colocalize with phragmoplast microtubules (Hotta et al., 2012; Janski et al., 2012; Murata et al., 2013; Nakamura et al., 2012; Nakaoka et al., 2012). Furthermore, the knockout or knockdown of γ-tubulin (Nakaoka et al., 2012; Pastuglia et al., 2006), GCP4 (Kong et al., 2010; Nakaoka et al., 2012), AUG3 (Ho et al., 2011b; Nakaoka et al., 2012), AUG7 (Hotta et al., 2012) or MZT1 (GIP1 and GIP2; Janski et al., 2012) results in disorganized phragmoplasts, abnormal cell divisions and perturbed plant development.
Microtubule polymerization
Several proteins that act on the microtubule plus-ends facilitate microtubule polymerization. The end-binding 1 family of proteins (EB1a, EB1b and EB1c in Arabidopsis) have higher affinity for growing microtubule plus-ends than for the rest of microtubule lattice, where they act as an catastrophe-promoting factor (Akhmanova and Steinmetz, 2008). Although all EB1 proteins colocalize with the phragmoplast, no cytokinetic defects have been observed in eb1a eb1b eb1c triple knockout mutants (Bisgrove et al., 2008; Komaki et al., 2010). CLIP-associating protein (CLASP) binds along the microtubule lattice, but it exhibits its highest affinity for the area behind the tip of the microtubule end that is occupied by EB1 at growing ends (Kirik et al., 2007). However, unlike EB1, CLASP is essential for phragmoplast organization and expansion (Ambrose et al., 2007). The plant-specific protein SPIRAL1 (SPR1) competes with EB1 for the microtubule plus-ends (Galva et al., 2014) and localizes to the phragmoplast (Sedbrook et al., 2004). Therefore, the overlap in the localization of proteins such as SPR1 and CLASP, together with the lack of cytokinetic defects in the eb1a eb1b eb1c, could hint at a compensation by these proteins for the functions of EB1 in the triple knockout plant. The plant homolog of the microtubule-associated protein 215 (XMAP215, also known as CKAP5; in plants known as MOR1 or GEM1), mediates tubulin exchange at the plus end of microtubules and facilitates both polymerization and catastrophe (Twell et al., 2002; Whittington et al., 2001). MOR1 localizes to phragmoplast microtubules and to the midzone (Twell et al., 2002). Mutations in the genes coding for MOR1 perturb phragmoplast structure and cause cytokinetic defects (Kawamura et al., 2006; Twell et al., 2002).
Spatial organization of microtubules
Microtubule-associated protein 65 (MAP65) belongs to the evolutionary conserved group of microtubule-bundling proteins, which preferentially cross-link anti-parallel microtubules by creating bridges of 25–30 nm width between microtubules (Chan et al., 1999; Gaillard et al., 2008; Smertenko et al., 2004). In addition, MAP65 may also stabilize microtubules (Chan et al., 1999; Mao et al., 2005; Smertenko et al., 2008; Wicker-Planquart et al., 2004). MAP65 is encoded by a family of functionally divergent proteins (Hussey et al., 2002; Kosetsu et al., 2013). In Arabidopsis and Physcomitrella patens, several MAP65 isotypes localize to the midzone where they stabilize the phragmoplast structure by cross-linking anti-parallel microtubules (Kosetsu et al., 2013; Müller et al., 2004; Smertenko et al., 2000; Van Damme et al., 2004b). Consistent with this hypothesis, knockout of the isotype MAP65-3 results in a wider midzone and causes cytokinetic failure, and cells only show cells wall stubs and have multiple nuclei (Caillaud et al., 2008; Ho et al., 2011a; Müller et al., 2004). The mutants also exhibit abnormal post-embryonic development and reduced fertility. In P. patens, failure of cytokinesis was observed upon knockdown of three MAP65 isotypes (Kosetsu et al., 2013). These findings demonstrate the importance of anti-parallel microtubule bundles for cell plate synthesis.
Katanin is the only microtubule-severing protein that so far has been implicated in plant cytokinesis. Live-cell imaging showed that the p60 katanin subunit localized to the phragmoplast (Panteris et al., 2011). Katanin appears to play three functions during cytokinesis: (1) control of the phragmoplast length by preventing microtubule elongation; (2) maintaining orientation of the division plane; and (3) increasing phragmoplast expansion rate (Komis et al., 2017; Panteris et al., 2011). The frequency at which extended phragmoplasts occurred, as reported by Komis et al. (2017), was relatively low, suggesting that this phenotype is conditional (Komis et al., 2017).
Formins
Electron microscopy studies demonstrate an interaction between some phragmoplast microtubules and the cell plate (Austin et al., 2005; Otegui et al., 2001; Samuels et al., 1995; Segui-Simarro et al., 2004). Although the linker proteins remain unknown, this interaction could be facilitated by formins. Formins are capable of connecting cell plate oligosaccharides and the cytoskeleton through their extracellular domain, transmembrane domains or membrane-binding domain, and cytoplasmic microtubule-binding domain (Deeks et al., 2010; Li et al., 2010; Wang et al., 2013). Arabidopsis formins and FH8 and P. patens formin For2A (Ingouff et al., 2005; van Gisbergen et al., 2012) localize to the phragmoplast midzone (Ingouff et al., 2005; Xue et al., 2011; van Gisbergen et al., 2012). Additionally, cytokinetic defects in formin-knockout mutants demonstrate their importance in cell plate assembly (Ingouff et al., 2005).
Kinesin motor proteins
Angiosperm kinesins from groups 5, 7, 12 and 14, as well as the orphan kinesin PAKRP2 localize to the phragmoplast microtubules or the midzone. Furthermore, the transcription of genes encoding kinesins from groups 4 and 13, and several ‘orphan’ kinesins are upregulated during metaphase (Vanstraelen et al., 2006); however, the localization of these kinesins remains unknown. In contrast, the systematic analysis of all kinesins in moss P. patens revealed that 18 kinesins from several groups (4, 7, 8 and 12) and the orphan kinesins KINID1a and KINID1b localized to the phragmoplast midzone, and 14 kinesins of groups 5, 7, 13 and 14 decorate phragmoplast microtubules (Hiwatashi et al., 2008; Miki et al., 2014). The abundance of kinesins in the phragmoplast may hint at their functional significance. Indeed, kinesins could deliver material for cell plate construction, help to maintain phragmoplast structure, regulate microtubule dynamics and direct phragmoplast expansion.
Group 5 kinesins are homotetrameric, plus-end-directed motors with an N-terminal motor domain (Endow et al., 2010). Members of this group localize to microtubules at the phragmoplast midzone of both tobacco and carrot (Asada et al., 1997; Barroso et al., 2000). The Arabidopsis kinesin-5 AtKRP125c interacts with phragmoplast microtubules, and loss of AtKRP125c causes cytokinetic failure (Bannigan et al., 2007). However, the morphology of the phragmoplast is not affected in the absence of AtKRP125c, which suggests that kinesins from group 5 function in phragmoplast expansion rather than maintaining phragmoplast structure (Bannigan et al., 2007).
Group 7 kinesins are plus-end-directed motors capable of transporting signaling molecules to the phragmoplast midzone. Hence, they have been detected both on the phragmoplast microtubules and in the midzone. In tobacco, NACK1 and NACK2 (TETRASPORE and HINKEL, respectively, in Arabidopsis) physically interact with and deliver the mitogen-activated protein kinase (MAPK) kinase kinase (MAPKKK) NPK1 to the phragmoplast midzone (Nishihama et al., 2001, 2002; Soyano et al., 2003). NPK1 mutants exhibit cytokinetic failure, malformed embryos and abnormal microsporogenesis (Nishihama et al., 2002; Söllner et al., 2002; Strompen et al., 2002; Tanaka et al., 2004). In Arabidopsis, TETRASPORE interacts with the protein kinase Two-In-One (TIO), which is essential for cytokinesis (Oh et al., 2014, 2005). Other members of this group in Arabidopsis, including the isoforms Kin7.1, Kin7.3 and Kin7.5, target separase (Elongated Spindle Poles) to the phragmoplast midzone (Moschou et al., 2016a, 2013). Interfering with the interaction between Kin7 and the separase results in cytokinetic failure (Moschou et al., 2013). These data suggest that group-7 kinesins function in controlling the cell plate assembly by facilitating the accumulation of divergent signaling molecules at the phragmoplast midzone.
The group 12 kinesins Phragmoplast-Associated Kinesin-Related Protein 1 (PAKRP1) and PAKRP1L also localize to the phragmoplast midzone (Lee and Liu, 2000; Pan et al., 2004). Although a mutation in PAKRP1L results in an abnormal phragmoplast structure and cytokinetic failure in microspores, the molecular function of these proteins remains poorly understood (Lee et al., 2007). Two members of this group interact with TIO kinase through their tail domain and thus can potentially traffic TIO to the midzone (Oh et al., 2012). Therefore, group 12 kinesins, as those from group 7, play a non-redundant function in the delivery of signaling molecules to the midzone.
The calmodulin-binding kinesin-like protein (KCBP) of the kinesin-14 family decorates microtubules and the midzone in Haemanthus (blood lily) endosperm cells and localizes to the distal zone microtubules in tobacco BY-2 cells (Buschmann et al., 2015). In addition, two other kinesin-14 members, KCA1 and KCA2, localize to the midzone (Vanstraelen et al., 2004), but the functions of these kinesins remain unknown. Finally, the orphan kinesin PAKRP2 localizes to the phragmoplast microtubules and the midzone. Interestingly, pharmacological disruption of the Golgi resulted in a reduction of PAKRP2 localization on phragmoplast microtubules; this kinesin may thus contribute to the delivery of Golgi-derived vesicles to the cell plate (Lee et al., 2001).
A set of diverse microtubule regulators cooperate to control the organization and dynamics of microtubules in the phragmoplast. These microtubules function as a scaffold for the cell plate assembly by providing structural support to the cell plate, as well as serving as transport tracks for the kinesins to deliver vesicles and signaling molecules to the midzone. Such delivery and accumulation of cell plate assembly components in the midzone generates asymmetry between midzone and distal zones.
Signaling components
Cytoskeletal dynamics and cell plate assembly during phragmoplast expansion are coordinated by as yet poorly understood signaling networks. Several classes of serine/threonine protein kinases localize to the phragmoplast microtubules and the midzone, and below we describe the major kinases and their substrates.
The MAPK pathway
The MAPK cascade is the best-understood phragmoplast signaling pathway. In tobacco, this pathways consists of the MAPKKK NPK1, the MAPKK NQK1, and the MAPK NRK1 (Nakashima et al., 1998; Nishihama et al., 2001, 2002; Soyano et al., 2003). NPK1 is delivered to the phragmoplast midzone through interaction with the C-terminus of the group 7 kinesins NACK1 and NACK2. In addition to the delivery, this interaction activates NPK1 (Nishihama et al., 2002). Upon reaching the midzone, NPK1 activates MAPKs (i.e. NRK1 in tobacco; MPK4, MPK6 in Arabidopsis; MKK3 in Alfalfa), which also localize to the midzone and/or phragmoplast microtubules (Beck et al., 2011; Bogre et al., 1999; Kohoutová et al., 2015; Kosetsu et al., 2010; Smekalova et al., 2014; Takahashi et al., 2010). Phosphorylation of MAP65 and EB1 by MAPKs can diminish their affinity to microtubules (Kohoutová et al., 2015; Sasabe et al., 2006; Smertenko et al., 2006). The MAPK pathway appears to be conserved in embryophytes. In P. patens protonemal cells, the NACK orthologs PpNACKa, PpNACKb and PpNACKc also localize to the midzone. Similar to what has been found for NACK mutants in tobacco and Arabidopsis, RNAi-mediated knockdown of the PpNACK orthologs perturbs phragmoplast expansion (Naito and Goshima, 2015).
CDKs, Aurora and TIO
Cyclin-dependent kinases (CDKs) appear to be important for phragmoplast functions because expression of a non-degradable cyclin B mutant, which leads to constitutive activation of Cdc2, results in abnormal cytokinesis (Weingartner et al., 2004). In addition, Cdc2 localizes to both the phragmoplast microtubules and the midzone (Weingartner et al., 2001). CDKs also control microtubule dynamics in the phragmoplast by phosphorylating MAP65 and group 7 kinesins (Sasabe et al., 2011; Weingartner et al., 2004).
Plants have divergent homologs of animal Aurora kinase, which form a separate clade on the Aurora kinase phylogenetic tree (Demidov et al., 2005). The Arabidopsis genome contains three Aurora-encoding genes: Aurora 1, Aurora 2, and Aurora 3. Aurora 1 kinase and, to some extent, Aurora 2 localize to the phragmoplast midzone and the cell plate (Demidov et al., 2005; Van Damme et al., 2004a). Accordingly, downregulation of Aurora kinases results in abnormal cell division (Petrovska et al., 2012; Van Damme et al., 2011). Aurora kinase phosphorylates the C-terminal domain of MAP65 (Boruc et al., 2017; Smertenko et al., 2006), which in cooperation with CDKs and MAPKs, diminishes MAP65 binding to microtubules. Collectively, these pathways increase the cytokinesis rate (Smertenko et al., 2006). Arabidopsis TIO has the same type of kinase domain as does the kinase Fused, a key component of the evolutionary conserved hedgehog signaling pathway of animals, though its substrates remain unknown (Oh et al., 2005). Knockout of TIO or ectopic expression of the wild-type or mutant proteins causes abnormal phragmoplast expansion and positioning, incomplete cytokinesis, and multicellular or polyploid pollen grains (Oh et al., 2012, 2014, 2005). As a consequence, the mutants are sterile. In conclusion, although phragmoplast expansion is guided by multiple signaling pathways, our knowledge about the substrates remains limited. Consequently, the identification of these kinase substrates is essential for our understanding of phragmoplast expansion.
Phragmoplast expansion
Microtubule dynamics in the phragmoplast are coordinated with the cell plate assembly. Consequently, three zones are formed with distinct patterns of microtubule behavior (Figs 1 and 2). The outer leading zone is dominated by the formation of new microtubules. Cell plate assembly initiation occurs in this zone. The transition zone maintains the balance of microtubule polymerization and depolymerization, but also contains some stable microtubules (Murata et al., 2013). Here, the cell plate takes shape through vesicle fusion and tubulation. Third, there is an inner lagging zone, where the cell plate construction achieves a threshold of maturity and microtubules depolymerize. Below, we discuss how microtubule dynamics could be coordinated with the cell plate assembly in each zone.
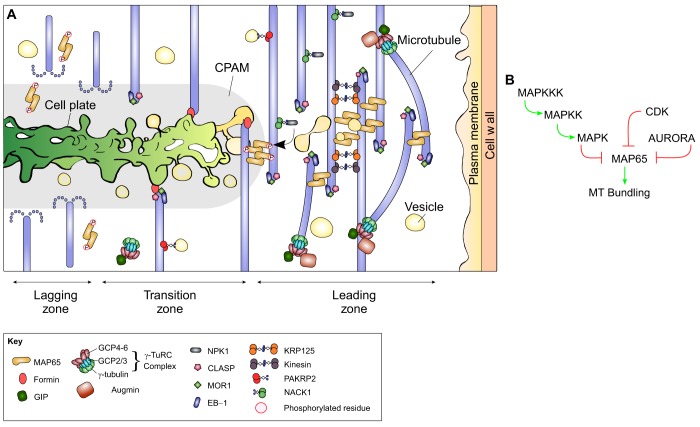
Fig. 2.
Regulation of microtubule processes in the phragmoplast zones. (A) The key events in the phragmoplast zones. In the leading zone, branching and cytoplasmic microtubules are generated by the γ-TuRC-mediated nucleation. Polymerization of microtubules is supported by a family of plus-end-binding proteins. Anti-parallel microtubules are bundled by MAP65 (orange). This overlap of anti-parallel microtubules defines the midzone and recruits nascent cell plate vesicles (pale yellow). In the transition zone, microtubules undergo nucleation, polymerization and depolymerization. Microtubules may be stabilized through formin-mediated contacts with the nascent cell plate membrane or interaction with yet unknown components of the CPAM. Kinesins transport cell plate materials and MAPKKK NPK1 along microtubules. Cell plate vesicles undergo fusion and tubulation, resulting in a tubulo-vesicular network (pale yellow). Callose accumulates in the cell plate (green gradient). In the lagging zone, microtubule-stabilizing proteins become inactivated through phosphorylation and microtubules depolymerize. (B) Multiple kinase pathways regulate the interaction between MAP65 and microtubules. The MAPK cascade, CDKs and Aurora kinases phosphorylate different residues on the C-terminal domain of MAP65. Phosphorylation weakens the affinity of MAP65 for microtubules and inhibits its ability to bundle anti-parallel microtubules. Green lines indicate activation and red lines indicate inhibition.
The leading zone
Addition of new microtubules in the leading zone depends on processes associated with nucleation and elongation. The nucleation events are facilitated by γ-TuRC (Fig. 2). Nucleation on the microtubule-associated γ-TuRCs results in microtubule branching (Murata et al., 2013). Plus-end-binding proteins appear to play a key role in this local microtubule polymerization because mutations in MOR1 or CLASP result in smaller phragmoplasts (Ambrose et al., 2007; Kawamura et al., 2006). Moreover, lack of MOR1 causes fragmented and misshaped phragmoplasts, as well as a premature termination of cell plate synthesis (Twell et al., 2002). The phragmoplast length appears to be controlled by katanin, as the katanin-knockout mutant exhibits extended microtubules (Panteris et al., 2011). Consistent with this activity, katanin localizes to the distal zones during both formation and expansion of the phragmoplast (Panteris et al., 2011).
The majority of the microtubule plus-ends are directed toward the midzone, whereas minus-ends are directed towards the distal edge (Euteneuer et al., 1982; Euteneuer and McIntosh, 1980). Consequently, microtubules that interdigitate in the midzone are mostly of opposite polarities (Euteneuer and McIntosh, 1980; Smertenko et al., 2011). Overlapping anti-parallel microtubules are predicted to be crosslinked by MAP65 (Fig. 3) because MAP65 preferentially binds anti-parallel microtubule bundles (Ho et al., 2011a; Janson et al., 2007). This feature makes MAP65 a marker of the anti-parallel microtubule overlap. The localization of MAP65 suggests that it has a role in the maintenance of the phragmoplast structure by stabilizing microtubule overlaps in the midzone. Such a localization of MAP65 to the phragmoplast midzone in tobacco, Arabidopsis, Norway spruce and Physcomytrella points to evolutionary conservation of the anti-parallel overlap (Kosetsu et al., 2013; Smertenko et al., 2000, 2003, 2004; Van Damme et al., 2004a,b). The physical presence of the midzone overlap has been demonstrated by electron microscopy studies in Arabidopsis (Ho et al., 2011a), Heamanthus (Hepler and Jackson, 1968) and Physcomytrella (Hiwatashi et al., 2008) phragmoplasts. Electron tomography analysis of cryofixed phragmoplasts detected the overlap only during the initial stage of the phragmoplast, but not during the subsequent expansion stages (Austin et al., 2005; Segui-Simarro et al., 2004). The latter findings invoked skepticism about the importance of the anti-parallel microtubule overlap for phragmoplast function, despite apparent cytokinetic defects in MAP65-3 mutants (Caillaud et al., 2008; Ho et al., 2011a; Müller et al., 2004). Austin and colleagues proposed that instead of the overlap, microtubule interactions with the cell plate assembly matrix maintain phragmoplast structure through a stabilization of microtubule plus ends (Austin et al., 2005). However, this model does not explain the commonly observed midzone localization of MAP65, as the electron tomography data suggested that the phragmoplast mostly lacks microtubules in the midzone. Resolving this controversy will require more experimental data.
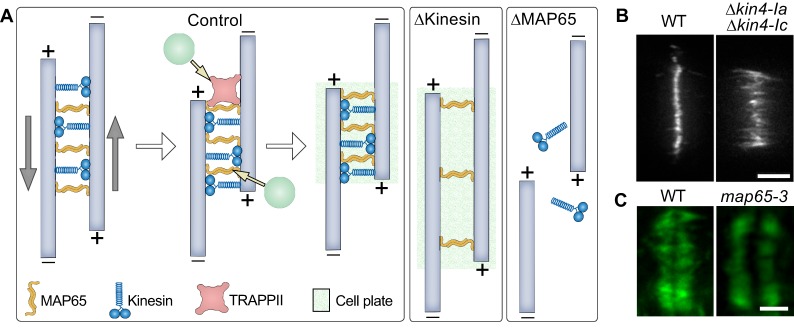
Fig. 3.
Mechanics of the phragmoplast midzone establishment. (A) MAP65 (orange) bundles anti-parallel microtubules (pale blue) in the midzone. Anterograde kinesins (blue) bind to anti-parallel microtubules and slide them apart in opposite directions, as shown by gray arrows. Eventually, the thickness of the midzone is determined by the balance of forces generated by kinesin movements and the affinity of MAP65 for microtubules. MAP65 interacts with the tethering complex TRAPPII. Tethering complexes can initiate cell plate assembly by recruiting vesicles (green circles) to the midzone. In the absence of kinesins (ΔKinesin), both the midzone and cell plate are wider. In the absence of MAP65 (ΔMAP65), kinesins slide microtubules away from each other. Consequently, cell plate formation is abrogated. (B) Example picture of the localization of MAP65a-citrine in Physcomytrella caulonemal tip cell. Reprinted from de Keijzer et al., 2017, with permission from Elsevier. Double knockout of Kin4-Ia and Kin4-Ic results in wider microtubule overlap as evident by MAP65a–citrine localization in living cells. (C) Example picture of how loss of MAP65-3 activity (Arabidopsis ple-6 allele) leads to a wider phragmoplast midzone. Reprinted from Muller et al., 2004, with permission from Elsevier. Microtubules were immunostained with anti-tubulin antibody (green). WT, wild type. Scale bars: 5 μm (B), 2 µm (C).
Importantly, anti-MAP65-3 antibody labels sparse hotspots around the perimeter of the expanding phragmoplast (Ho et al., 2011a, 2012; Müller et al., 2004; Smertenko et al., 2008), and such clusters were also observed in live-cell imaging experiments with MAP65-3–GFP (Smertenko et al., 2008; Van Damme et al., 2004a). The detection of these sparse microtubule overlaps by electron microscopy may be challenging: under the assumption that the phragmoplast structure is stabilized by both anti-parallel microtubule overlaps and by the cell plate assembly machinery, the frequency of the overlaps would depend on the cell plate assembly stage. Furthermore, the mechanical resilience of the overlaps could also play a role and would be proportional to the affinity of MAP65 to microtubules. Thus, the overlap frequency in a given situation could fluctuate as a consequence of: (1) the phragmoplast expansion stage; (2) the phragmoplast size; and (3) the number of available MAP65 molecules per dividing cell and their affinity to microtubules.
The width of the anti-parallel microtubule overlap in vitro is known to be maintained by a module consisting of MAP65 and kinesins (Bieling et al., 2010) (Fig. 3). Consistent with this model, knockout of the kinesins Kin4-Ia and Kin4-Ic results in a wider MAP65-positive domain in the midzone of the moss Physcomitrella patens (de Keijzer et al., 2017). Alteration of normal MAP65 localization in the kin4-Ia kin4-Ib mutant background suggests that the lack of the kinesins results in wider anti-parallel microtubule overlap. Electron microscopy examination of cytokinesis in kin4-Ia kin4-Ib revealed a thicker cell plate (de Keijzer et al., 2017). In contrast to the kin4-Ia kin4-Ib mutant phenotype, the mutation of MAP65 caused disconnection of the phragmoplast halves in Arabidopsis and cell plate assembly failure (Müller et al., 2004; Steiner et al., 2016).
The above findings reveal that there is an intriguing association between the dimensions of the MAP65 domain in the midzone and width of the cell plate. We hypothesize that MAP65 plays a role in midzone establishment by targeting the membrane trafficking machinery. Several recent observations support this view. First, MAP65 can bind vesicles (Zhang et al., 2012). Second, at least two MAP65 isotypes, MAP65-1 and MAP65-3 (PLEIADE), interact with subunits of the TRAPPII vesicle tethering complex subunits TRS130 (CLUB) and TRS120 (Steiner et al., 2016) (Fig. 3). Localization of MAP65-3 is not affected in single trs120 or trs130 mutants, which suggests that targeting of MAP65 to the midzone is independent of the TRAPPII complex. Similarly, the targeting of TRS120 or TRS130 to the midzone was not perturbed in the MAP65-3 mutant ple4 which has a reduced affinity for microtubules (Smertenko et al., 2004; Steiner et al., 2016). It is plausible that TRS120 and TRS130 in a ple4 mutant background are targeted to the midzone through an interaction with MAP65-1 or other midzone-localized MAP65 proteins (e.g. MAP65-2 or MAP65-5; Smertenko et al., 2008). It would be essential to analyze localization of the TRAPPII complex in higher order MAP65-null mutants before the role of MAP65 in TRAPPII targeting to the midzone is conclusively established. However, all available evidence thus far points to an establishment of anti-parallel microtubule overlap by MAP65 and a subsequent accumulation of vesicles in the leading zone, which then results in cell plate initiation.
Transition zone
Cell plate initiation culminates in the establishment of the cell plate assembly matrix (CPAM) (Segui-Simarro et al., 2004). The exact constituents of the CPAM remain unknown – it appears as a ∼150-nm-wide region around the cell plate that lacks ribosomes (Austin et al., 2005; Segui-Simarro et al., 2004). Some microtubules terminate inside the CPAM and over 50% of these microtubules have blunt ends, which is typical for stable microtubules (Austin et al., 2005). The same work showed a few microtubules terminating at the cell plate (Austin et al., 2005). Stable microtubules are thought provide structural support to the phragmoplast morphology and to the nascent cell plate (Fig. 3A). Live-cell imaging data supports the existence of stable microtubules in the phragmoplast (Murata et al., 2013). In addition, the transition zone contains dynamic microtubules (Murata et al., 2013; Smertenko et al., 2011). The factors that are responsible for microtubule attachment to the CPAM remain unknown. This role could potentially be performed by formins, which are capable of binding both membranes and microtubules.
The population of stable microtubules terminating in the CPAM could be used by kinesins as tracks for the transport of TGN-derived vesicles to the midzone. For example, the Arabidopsis kinesin-12 PAKRP2 binds phragmoplast microtubules and localizes to the midzone (Lee et al., 2001). PAKRP2 also associates with vesicles, as it was found to be enriched in the insoluble fraction of mitotic cell extracts containing endomembrane systems, including vesicles (Lee et al., 2001). Furthermore, disruption of the Golgi and other endomembrane systems with Brefeldin-A causes cytoplasmic localization of AtPAKRP2 (Lee et al., 2001).
The directionality of vesicle trafficking and their subsequent fusion could be driven by Ca2+ signaling. Ca2+ was initially detected in the cell plate following treatment with the Ca2+-precipitating agent antimonate in electron microscopy studies (Wick and Hepler, 1980). These results indicate that the highest Ca2+ concentration is in the midzone and the lowest is in the distal zones. In agreement with this hypothesis, Ca2+ is essential for the catalytic activity of callose synthase, which accumulates in the phragmoplast midzone and produces callose at the cell plate (Aidemark et al., 2009; Him et al., 2001). Furthermore, a Ca2+ gradient appears to control the spatial activity of proteins in the phragmoplast, as evidenced by the localization of the minus-end-directed microtubule motor KCBP. KCBP binding to calmodulin in the presence of Ca2+ inhibits its binding to microtubules (Song et al., 1997). This feature makes KCBP a useful sensor of Ca2+ gradients. Live-cell imaging demonstrates binding of KCBP to microtubules mostly in the distal zone, which suggests that the concentration of Ca2+ is higher in the midzone than in the distal zone (Buschmann et al., 2015).
Fusion of vesicles at the phragmoplast midzone is followed by the formation of tubes (tubulation), the extension of the tubes and their fusion into a network (Samuels et al., 1995; Segui-Simarro et al., 2004). Tubulation is driven by GTPases from the dynamin-related protein 1 and 2 (DRP1/2) groups (Fujimoto et al., 2008; Hong et al., 2003; Konopka et al., 2006; Segui-Simarro et al., 2004).
Vesicle delivery and fusion is accompanied by the synthesis of polysaccharides. Callose is the most abundant polysaccharide that is involved in these early stages of cell plate assembly (Drakakaki, 2015; Thiele et al., 2009). It is synthetized by callose synthase, which might be recruited to the cell plate by DRPs (Hong et al., 2001). Mutant alleles of the callose synthases MASSUE gsl8, gls9 (Chen et al., 2009; Töller et al., 2008), and CHOR (Guseman et al., 2010) exhibit impaired cytokinesis (Chen et al., 2009; Töller et al., 2008; Thiele et al., 2009; Guseman et al., 2010). The deposition of other major cell wall oligosaccharides, such as cellulose, hemicellulose and pectin, occurs simultaneously with callose (Baluška et al., 2005; Dhonukshe et al., 2006; Miart et al., 2014). Several enzymes responsible for the synthesis of cellulose and hemicellulose localize to the midzone (Yokoyama and Nishitani, 2001; Gu et al., 2016; Miart et al., 2014). Pectin synthesis enzymes have not yet been found in the phragmoplast; however, the midzone DRP1 interacts with an enzymatic complex that is responsible for pectin biosynthesis (Atmodjo et al., 2011). Many oligosaccharide-modifying enzymes are also located in the midzone, which indicates that oligosaccharides – like the membrane – undergo remodeling in the course of cell plate shaping. These enzymes include the endo-1,4-β-glucanase KORRIGAN, which digests cellulose fibers (Zuo et al., 2000), and endoxyloglucan transferase that remodels the hemicellulose network by catalyzing splitting and grafting of xyloglucan polymers (Nishitani, 1995), as well as pectin methylesterase, which controls stiffness of the cell wall by modifying pectin (Wang et al., 2016). The main function of the transition zone is therefore to advance cell plate biogenesis from its initiation to the tubular network stage (Segui-Simarro et al., 2004). By this stage, oligosaccharides in the cell plate could provide structural support to the cell plate.
Lagging zone
Microtubule depolymerization at the lagging edge of the phragmoplast appears to be irreversible because de novo formation of microtubules along the cell plate behind the lagging zone has not been reported yet. Therefore, the dismantling of the phragmoplast must be coordinated with the assembly of the cell plate to avoid a premature loss of the microtubule scaffold. Two non-exclusive mechanisms could facilitate the loss of microtubules in the lagging zone: (1) activation of microtubule severing, and (2) inhibition of microtubule nucleation and stabilization. The second mechanism fits better to our current knowledge, because microtubule-destabilizing factors have thus far not been implicated in the processes in the lagging zone. Katanin localizes to the distal zone and appears to control attachment of phragmoplast to the forming nuclear envelope, division plane orientation and the phragmoplast expansion rate (Komis et al., 2017; Panteris et al., 2011), whereas the catastrophe-promoting Arabidopsis kinesin Kin13 associates with the Golgi in root apical meristem cells and plays a role in trichome morphogenesis (Desai et al., 1999; Lu et al., 2005).
Several signaling pathways control microtubule stability in the phragmoplast. As discussed above, the MAPK pathway leads to the phosphorylation of the highly divergent MAP65-1 C-terminal domain (Sasabe et al., 2006; Smertenko et al., 2006). Since the NACK kinesin and NPK1 kinase complex is delivered to the phragmoplast midzone along microtubules, the number of NPK1 molecules delivered to the plus-end of a microtubule would be proportional to the lifetime of a microtubule. Microtubules of a long-life nature would ultimately accumulate more kinase molecules and consequently have higher kinase activity at their plus-ends. This would diminish activity of the plus-end microtubule-stabilizing proteins. Such a ‘clock’ mechanism could define microtubule ‘age’ and stimulate the disassembly of microtubules that have been persisting for a long time.
The MAPK pathway is unlikely to be the sole regulator of MAP65 because mimicking phosphorylation of MAP65 by MAPK results in only a marginal decrease of its affinity to microtubules, indicating the involvement of other signaling pathways (Smertenko et al., 2006). Aurora kinases and Cdc2 also phosphorylate the C-terminus of MAP65 in vitro and in vivo; thus, MAP65 integrates the activity of multiple signaling pathways and has the potential to translate these signals into microtubule stability (Smertenko et al., 2006; Boruc et al., 2017; Fig. 3B). However, the combined activity of all three kinase pathways only accounts for six out of the nine predicted phosphorylation residues of MAP65, and future work thus may reveal additional regulatory mechanisms (Smertenko et al., 2006). For example, TIO kinase interacts with the NACK kinesin homolog TETRASPORE and also with kinesin-12 through two distinct regions located in the non-catalytic C-terminus (Oh et al., 2012, 2014). TIO might therefore phosphorylate MAP65 directly, or by promoting the formation of complexes between the kinesin and MAPKKK that phosphorylate MAP65 or other substrates. Such a combination of regulatory pathways would constitute a truly intricate mechanism to prevent the premature destabilization of phragmoplast microtubules.
In Arabidopsis, the microtubule-associated protein RUNKEL (RUK) contains an apparently inactive serine/threonine kinase domain (Krupnova et al., 2009). Although RUK does not localize to the midzone, a ruk knockout has abnormal phragmoplasts and cytokinesis defects (Krupnova et al., 2009). In addition, expression of a dominant-negative RUK construct caused partial displacement of the kinesin-7 HINKEL (a homolog of TETRASPORE and NACK) from the midzone to the phragmoplast microtubules and resulted in abnormal cytokinesis (Krupnova et al., 2013). These findings raise the intriguing possibility that RUK cooperates with TIO in regulating MAPK activity.
Phosphorylation events also control other microtubule regulators. For example, phosphorylation appears to inactivate the kinesin-7 HINKEL, as the ectopic expression of its phosphomimetic mutant was unable to complement the cytokinetic defects of hinkel (Sasabe et al., 2011). In vitro assays demonstrate that MAPK also may reduce microtubule stability by phosphorylating EB1c (Kohoutová et al., 2015).
Although protein kinases localize to the midzone as early as during initiation of the cell plate assembly, microtubule loss occurs only the in the lagging zone. This suggests the existence of a ‘licensing mechanism’ that prevents microtubule loss before the cell plate assembly reaches a certain threshold. It is conceivable that long-lived microtubules in the lagging zone may be destabilized by the ‘clock’ mechanism mentioned above. However, experimental data shows that a clock mechanism is not sufficient to trigger microtubule depolymerization. Inhibition of cell plate assembly by caffeine or Brefeldin A prevents microtubule depolymerization in the lagging zone (Yasuhara and Shibaoka, 2000; Yasuhara, 2005), giving rise to giant, disk-shaped phragmoplasts, where microtubules persist for hours (Yasuhara and Shibaoka, 2000; Yasuhara, 2005). Therefore, the licensing mechanism, in addition to the microtubule age, would also be expected to rely on the status of cell plate assembly. For example, a certain mechanical resilience threshold must be achieved before microtubule disassembly can proceed. Testing this hypothesis would require the measurement of the impact of oligosaccharide deposition on the mechanical resilience of the cell plate, which is currently not possible with the available tools.
In summary, microtubule loss in the lagging zone appears to be a highly regulated process, which depends on: (1) accumulation of protein kinases from multiple signaling pathways in the midzone; (2) yet unknown chemical or mechanical stimuli related to the deposition of callose or other oligosaccharides in the cell plate; and (3) convergence of multiple signaling pathways on the key microtubule regulators. As such, this zone provides an exciting paradigm to understand the crosstalk between microtubule dynamics and cell wall deposition. Importantly, synchronized microtubule loss in the lagging zone and addition of new microtubules in the leading zone provide the lateral asymmetry that, in combination with the axial asymmetry, drives phragmoplast expansion. Whether these asymmetries act in concert or independently remains to be determined.
Concluding remarks
The complex three-dimensional structure, as well as the cytoplasmic tubulin that surrounds the phragmoplast prevents the tracking of individual microtubules by current microscopy techniques. To date, fluorescence recovery after photobleaching and imaging of drug-treated cells have been used to gain insight into microtubule dynamics in the phragmoplast (Smertenko et al., 2011; Murata et al., 2013). Subsequent validation using models and simulations can increase the value of these indirect experimental approaches (Smertenko et al., 2011). Thus, further progress in unravelling the complex processes governing the functions of the phragmoplast zones will require not only better materials and technologies to measure microtubule dynamics, but also more advanced modeling tools.
One of the most intriguing questions is what makes phragmoplast a plant-specific microtubule array. The majority of known phragmoplast proteins are highly conserved among eukaryotic lineages. Moreover, these proteins also govern the organization of other plant-specific arrays such as the PPB and cortical microtubules. The identification and functional characterization of plant-specific proteins in the phragmoplast would help to answer this question.
Certainly, the nature of the signaling mechanisms is a significant unknown that remains in phragmoplast biology. Despite our knowledge on the protein kinase pathways in the phragmoplast, the information about the substrates of these kinases remains limited. A novel direction in our understanding of phragmoplast signaling has been highlighted by the discovery of the midzone localization of the caspase-domain protease separase in both angiosperm and gymnosperm species (Moschou et al., 2016b, 2013). Identification of separase substrates could demonstrate how spatially restricted proteolysis contributes to the regulation of phragmoplast expansion. A better understanding of these signaling mechanisms would advance the model of phragmoplast expansion, and, ultimately, facilitate the engineering of phragmoplasts with faster cell plate synthesis.
Acknowledgements
We are grateful to Deirdre Fahy for critical reading of this manuscript and James A. Kilkenny-Roddy for taking part in the discussion on signalling pathways in the phragmoplast.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
Our work in this area is supported by a National Institute of Food and Agriculture hatch grant (WNP00826) and Washington State University startup fund (to A.S.).
References
- Aidemark M., Andersson C.-J., Rasmusson A. G. and Widell S. (2009). Regulation of callose synthase activity in situ in alamethicin-permeabilized Arabidopsis and tobacco suspension cells. BMC Plant Biol. 9, 27 10.1186/1471-2229-9-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmanova A. and Steinmetz M. O. (2008). Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol. 9, 309-322. 10.1038/nrm2369 [DOI] [PubMed] [Google Scholar]
- Ambrose J. C., Shoji T., Kotzer A. M., Pighin J. A. and Wasteneys G. O. (2007). The Arabidopsis CLASP gene encodes a microtubule-associated protein involved in cell expansion and division. Plant Cell 19, 2763-2775. 10.1105/tpc.107.053777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada T., Kuriyama R. and Shibaoka H. (1997). TKRP125, a kinesin-related protein involved in the centrosome-independent organization of the cytokinetic apparatus in tobacco BY-2 cells. J. Cell Sci. 110, 179-189. [DOI] [PubMed] [Google Scholar]
- Atmodjo M. A., Sakuragi Y., Zhu X., Burrell A. J., Mohanty S. S., Atwood J. A., Orlando R. III, Scheller H. V. and Mohnen D. (2011). Galacturonosyltransferase (GAUT)1 and GAUT7 are the core of a plant cell wall pectin biosynthetic homogalacturonan: galacturonosyltransferase complex. Proc. Natl. Acad. Sci. USA 108, 20225-20230. 10.1073/pnas.1112816108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin J. R. 2nd, Segui-Simarro J. M. and Staehelin L. A. (2005). Quantitative analysis of changes in spatial distribution and plus-end geometry of microtubules involved in plant-cell cytokinesis. J. Cell Sci. 118, 3895-3903. 10.1242/jcs.02512 [DOI] [PubMed] [Google Scholar]
- Baluška F., Liners F., Hlavačka A., Schlicht M., Van Cutsem P., McCurdy D. W. and Menzel D. (2005). Cell wall pectins and xyloglucans are internalized into dividing root cells and accumulate within cell plates during cytokinesis. Protoplasma 225, 141-155. 10.1007/s00709-005-0095-5 [DOI] [PubMed] [Google Scholar]
- Bannigan A., Scheible W.-R., Lukowitz W., Fagerstrom C., Wadsworth P., Somerville C. and Baskin T. I. (2007). A conserved role for kinesin-5 in plant mitosis. J. Cell Sci. 120, 2819-2827. 10.1242/jcs.009506 [DOI] [PubMed] [Google Scholar]
- Barroso C., Chan J., Allan V., Doonan J., Hussey P. and Lloyd C. (2000). Two kinesin-related proteins associated with the cold-stable cytoskeleton of carrot cells: characterization of a novel kinesin, DcKRP120-2. Plant J. 24, 859-868. 10.1046/j.1365-313x.2000.00937.x [DOI] [PubMed] [Google Scholar]
- Beck M., Komis G., Ziemann A., Menzel D. and Šamaj J. (2011). Mitogen-activated protein kinase 4 is involved in the regulation of mitotic and cytokinetic microtubule transitions in Arabidopsis thaliana. New Phytol. 189, 1069-1083. 10.1111/j.1469-8137.2010.03565.x [DOI] [PubMed] [Google Scholar]
- Bieling P., Telley I. A. and Surrey T. (2010). A minimal midzone protein module controls formation and length of antiparallel microtubule overlaps. Cell 142, 420-432. 10.1016/j.cell.2010.06.033 [DOI] [PubMed] [Google Scholar]
- Bisgrove S. R., Lee Y.-R. J., Liu B., Peters N. T. and Kropf D. L. (2008). The microtubule plus-end binding protein EB1 functions in root responses to touch and gravity signals in Arabidopsis. Plant Cell 20, 396-410. 10.1105/tpc.107.056846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogre L., Calderini O., Binarova P., Mattauch M., Till S., Kiegerl S., Jonak C., Pollaschek C., Barker P., Huskisson N. S. et al. (1999). A MAP kinase is activated late in plant mitosis and becomes localized to the plane of cell division. Plant Cell 11, 101-113. 10.1105/tpc.11.1.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boruc J. and Van Damme D. (2015). Endomembrane trafficking overarching cell plate formation. Curr. Opin. Plant Biol. 28, 92-98. 10.1016/j.pbi.2015.09.007 [DOI] [PubMed] [Google Scholar]
- Boruc J., Weimer A. K., Stoppin-Mellet V., Mylle E., Kosetsu K., Cedeño C., Jaquinod M., Njo M., De Milde L., Tompa P. et al. (2017). Phosphorylation of MAP65-1 by arabidopsis aurora kinases is required for efficient cell cycle progression. Plant Physiol. 173, 582-599. 10.1104/pp.16.01602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann H. and Zachgo S. (2016). The evolution of cell division: from streptophyte algae to land plants. Trends Plant Sci. 21, 872-883. 10.1016/j.tplants.2016.07.004 [DOI] [PubMed] [Google Scholar]
- Buschmann H., Dols J., Kopischke S., Pena E. J., Andrade-Navarro M. A., Heinlein M., Szymanski D. B., Zachgo S., Doonan J. H. and Lloyd C. W. (2015). Arabidopsis KCBP interacts with AIR9 but stays in the cortical division zone throughout mitosis via its MyTH4-FERM domain. J. Cell Sci. 128, 2033-2046. 10.1242/jcs.156570 [DOI] [PubMed] [Google Scholar]
- Caillaud M.-C., Lecomte P., Jammes F., Quentin M., Pagnotta S., Andrio E., de Almeida Engler J., Marfaing N., Gounon P., Abad P. et al. (2008). MAP65-3 microtubule-associated protein is essential for nematode-induced giant cell ontogenesis in Arabidopsis. Plant Cell 20, 423-437. 10.1105/tpc.107.057422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J., Jensen C. G., Jensen L. C. W., Bush M. and Lloyd C. W. (1999). The 65-kDa carrot microtubule-associated protein forms regularly arranged filamentous cross-bridges between microtubules. Proc. Natl. Acad. Sci. USA 96, 14931-14936. 10.1073/pnas.96.26.14931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.-Y., Liu L., Lee E., Han X., Rim Y., Chu H., Kim S.-W., Sack F. and Kim J.-Y. (2009). The Arabidopsis Callose Synthase Gene GSL8 Is Required for Cytokinesis and Cell Patterning. Plant Physiol. 150, 105-113. 10.1104/pp.108.133918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler S. R. and Ehrhardt D. W. (2002). Polarized cytokinesis in vacuolate cells of Arabidopsis. Proc. Natl. Acad. Sci. USA 99, 2812-2817. 10.1073/pnas.052712299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks M. J., Fendrych M., Smertenko A., Bell K. S., Oparka K., Cvrckova F., Zarsky V. and Hussey P. J. (2010). The plant formin AtFH4 interacts with both actin and microtubules, and contains a newly identified microtubule-binding domain. J. Cell Sci. 123, 1209-1215. 10.1242/jcs.065557 [DOI] [PubMed] [Google Scholar]
- de Keijzer J., Kieft H., Ketelaar T., Goshima G. and Janson M. E. (2017). Shortening of microtubule overlap regions defines membrane delivery sites during plant cytokinesis. Curr. Biol. 27, 514-520. 10.1016/j.cub.2016.12.043 [DOI] [PubMed] [Google Scholar]
- Demidov D., Van Damme D., Geelen D., Blattner F. R. and Houben A. (2005). Identification and dynamics of two classes of aurora-like kinases in Arabidopsis and other plants. Plant Cell 17, 836-848. 10.1105/tpc.104.029710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A., Verma S., Mitchison T. J. and Walczak C. E. (1999). Kin I kinesins are microtubule-destabilizing enzymes. Cell 96, 69-78. 10.1016/S0092-8674(00)80960-5 [DOI] [PubMed] [Google Scholar]
- Dhonukshe P., Baluška F., Schlicht M., Hlavacka A., Šamaj J., Friml J. and Gadella T. W. J. Jr (2006). Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Dev. Cell 10, 137-150. 10.1016/j.devcel.2005.11.015 [DOI] [PubMed] [Google Scholar]
- Drakakaki G. (2015). Polysaccharide deposition during cytokinesis: challenges and future perspectives. Plant Sci. 236, 177-184. 10.1016/j.plantsci.2015.03.018 [DOI] [PubMed] [Google Scholar]
- Endow S. A., Kull F. J. and Liu H. (2010). Kinesins at a glance. J. Cell Sci. 123, 3420-3424. 10.1242/jcs.064113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euteneuer U. and McIntosh J. R. (1980). Polarity of midbody and phragmoplast microtubules. J. Cell Biol. 87, 509-515. 10.1083/jcb.87.2.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euteneuer U., Jackson W. T. and McIntosh J. R. (1982). Polarity of spindle microtubules in Haemanthus endosperm. J. Cell Biol. 94, 644-653. 10.1083/jcb.94.3.644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M., Arimura S.-i., Nakazono M. and Tsutsumi N. (2008). Arabidopsis dynamin-related protein DRP2B is co-localized with DRP1A on the leading edge of the forming cell plate. Plant Cell Rep. 27, 1581-1586. 10.1007/s00299-008-0583-0 [DOI] [PubMed] [Google Scholar]
- Gaillard J., Neumann E., Van Damme D., Stoppin-Mellet V., Ebel C., Barbier E., Geelen D. and Vantard M. (2008). Two microtubule-associated proteins of arabidopsis MAP65s promote antiparallel microtubule bundling. Mol. Biol. Cell 19, 4534-4544. 10.1091/mbc.E08-04-0341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galva C., Kirik V., Lindeboom J. J., Kaloriti D., Rancour D. M., Hussey P. J., Bednarek S. Y., Ehrhardt D. W. and Sedbrook J. C. (2014). The microtubule plus-end tracking proteins SPR1 and EB1b interact to maintain polar cell elongation and directional organ growth in Arabidopsis. Plant Cell 26, 4409-4425. 10.1105/tpc.114.131482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F., Bringmann M., Combs J. R., Yang J., Bergmann D. C. and Nielsen E. (2016). Arabidopsis CSLD5 Functions in Cell Plate Formation in a Cell Cycle-Dependent Manner. Plant Cell. 28, 1722-1737. 10.1105/tpc.16.00203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guseman J. M., Lee J. S., Bogenschutz N. L., Peterson K. M., Virata R. E., Xie B., Kanaoka M. M., Hong Z. L. and Torii K. U. (2010). Dysregulation of cell-to-cell connectivity and stomatal patterning by loss-of-function mutation in Arabidopsis CHORUS (GLUCAN SYNTHASE-LIKE 8). Development 137, 1731-1741. 10.1242/dev.049197 [DOI] [PubMed] [Google Scholar]
- Hashimoto T. (2013). A ring for all: gamma-tubulin-containing nucleation complexes in acentrosomal plant microtubule arrays. Curr. Opin. Plant Biol. 16, 698-703. 10.1016/j.pbi.2013.09.002 [DOI] [PubMed] [Google Scholar]
- Hepler P. K. and Jackson W. T. (1968). Microtubules and early stages of cell-plate formation in the endosperm of Haemanthus katherinae Baker. J. Cell Biol. 38, 437-446. 10.1083/jcb.38.2.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Him J. L. K., Pelosi L., Chanzy H., Putaux J.-L. and Bulone V. (2001). Biosynthesis of (1 -> 3)-beta-D-glucan (callose) by detergent extracts of a microsomal fraction from Arabidopsis thaliana. Eur. J. Biochem. 268, 4628-4638. 10.1046/j.1432-1327.2001.02382.x [DOI] [PubMed] [Google Scholar]
- Hiwatashi Y., Obara M., Sato Y., Fujita T., Murata T. and Hasebe M. (2008). Kinesins are indispensable for interdigitation of phragmoplast microtubules in the moss Physcomitrella patens. Plant Cell 20, 3094-3106. 10.1105/tpc.108.061705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C.-M. K., Hotta T., Guo F., Roberson R. W., Lee Y.-R. J. and Liu B. (2011a). Interaction of antiparallel microtubules in the phragmoplast is mediated by the microtubule-associated protein MAP65-3 in Arabidopsis. Plant Cell 23, 2909-2923. 10.1105/tpc.110.078204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C. M., Hotta T., Kong Z., Zeng C. J., Sun J., Lee Y. R. and Liu B. (2011b). Augmin plays a critical role in organizing the spindle and phragmoplast microtubule arrays in Arabidopsis. Plant Cell 23, 2606-2618. 10.1105/tpc.111.086892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C.-M. K., Lee Y.-R. J., Kiyama L. D., Dinesh-Kumar S. P. and Liu B. (2012). Arabidopsis microtubule-associated protein MAP65-3 cross-links antiparallel microtubules toward their plus ends in the phragmoplast via its distinct C-terminal microtubule binding domain. Plant Cell 24, 2071-2085. 10.1105/tpc.111.092569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z. L., Delauney A. J. and Verma D. P. S. (2001). A cell plate-specific callose synthase and its interaction with phragmoplastin. Plant Cell 13, 755-768. 10.1105/tpc.13.4.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z., Geisler-Lee C. J., Zhang Z. and Verma D. P. S. (2003). Phragmoplastin dynamics: multiple forms, microtubule association and their roles in cell plate formation in plants. Plant Mol. Biol. 53, 297-312. 10.1023/B:PLAN.0000006936.50532.3a [DOI] [PubMed] [Google Scholar]
- Hotta T., Kong Z., Ho C.-M. K., Zeng C. J. T., Horio T., Fong S., Vuong T., Lee Y.-R. J. and Liu B. (2012). Characterization of the Arabidopsis augmin complex uncovers its critical function in the assembly of the acentrosomal spindle and phragmoplast microtubule arrays. Plant Cell 24, 1494-1509. 10.1105/tpc.112.096610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey P. J., Hawkins T. J., Igarashi H., Kaloriti D. and Smertenko A. (2002). The plant cytoskeleton: recent advances in the study of the plant microtubule-associated proteins MAP-65, MAP-190 and the Xenopus MAP215-like protein, MOR1. Plant Mol. Biol. 50, 915-924. 10.1023/A:1021236307508 [DOI] [PubMed] [Google Scholar]
- Ingouff M., Fitz Gerald J. N., Guerin C., Robert H., Sorensen M. B., Van Damme D., Geelen D., Blanchoin L. and Berger F. (2005). Plant formin AtFH5 is an evolutionarily conserved actin nucleator involved in cytokinesis. Nat. Cell Biol. 7, 374-380. 10.1038/ncb1238 [DOI] [PubMed] [Google Scholar]
- Janski N., Masoud K., Batzenschlager M., Herzog E., Evrard J.-L., Houlne G., Bourge M., Chaboute M.-E. and Schmit A.-C. (2012). The GCP3-interacting proteins GIP1 and GIP2 are required for gamma-tubulin complex protein localization, spindle integrity, and chromosomal stability. Plant Cell 24, 1171-1187. 10.1105/tpc.111.094904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson M. E., Loughlin R., Loïodice I., Fu C., Brunner D., Nédélec F. J. and Tran P. T. (2007). Crosslinkers and motors organize dynamic microtubules to form stable bipolar arrays in fission yeast. Cell 128, 357-368. 10.1016/j.cell.2006.12.030 [DOI] [PubMed] [Google Scholar]
- Kawamura E., Himmelspach R., Rashbrooke M. C., Whittington A. T., Gale K. R., Collings D. A. and Wasteneys G. O. (2006). MICROTUBULE ORGANIZATION 1 regulates structure and function of microtubule arrays during mitosis and cytokinesis in the Arabidopsis root. Plant Physiol. 140, 102-114. 10.1104/pp.105.069989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik V., Herrmann U., Parupalli C., Sedbrook J. C., Ehrhardt D. W. and Hulskamp M. (2007). CLASP localizes in two discrete patterns on cortical microtubules and is required for cell morphogenesis and cell division in Arabidopsis. J. Cell Sci. 120, 4416-4425. 10.1242/jcs.024950 [DOI] [PubMed] [Google Scholar]
- Kohoutová L., Kourová H., Nagy S. K., Volc J., Halada P., Mészáros T., Meskiene I., Bögre L. and Binarová P. (2015). The Arabidopsis mitogen-activated protein kinase 6 is associated with gamma-tubulin on microtubules, phosphorylates EB1c and maintains spindle orientation under nitrosative stress. New Phytol. 207, 1061-1074. 10.1111/nph.13501 [DOI] [PubMed] [Google Scholar]
- Komaki S., Abe T., Coutuer S., Inze D., Russinova E. and Hashimoto T. (2010). Nuclear-localized subtype of end-binding 1 protein regulates spindle organization in Arabidopsis. J. Cell Sci. 123, 451-459. 10.1242/jcs.062703 [DOI] [PubMed] [Google Scholar]
- Komis G., Luptovčiak I., Ovečka M., Samakovli D., Šamajová O. and Šamaj J. (2017). Katanin effects on dynamics of cortical microtubules and mitotic arrays in arabidopsis thaliana revealed by advanced live-cell imaging. Frontiers in plant science 8, 866 10.3389/fpls.2017.00866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Z., Hotta T., Lee Y.-R. J., Horio T. and Liu B. (2010). The {gamma}-tubulin complex protein GCP4 is required for organizing functional microtubule arrays in Arabidopsis thaliana. Plant Cell 22, 191-204. 10.1105/tpc.109.071191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka C. A., Schleede J. B., Skop A. R. and Bednarek S. Y. (2006). Dynamin and cytokinesis. Traffic 7, 239-247. 10.1111/j.1600-0854.2006.00385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosetsu K., Matsunaga S., Nakagami H., Colcombet J., Sasabe M., Soyano T., Takahashi Y., Hirt H. and Machida Y. (2010). The MAP kinase MPK4 is required for cytokinesis in Arabidopsis thaliana. Plant Cell 22, 3778-3790. 10.1105/tpc.110.077164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosetsu K., de Keijzer J., Janson M. E. and Goshima G. (2013). MICROTUBULE-ASSOCIATED PROTEIN65 is essential for maintenance of phragmoplast bipolarity and formation of the cell plate in physcomitrella patens. Plant Cell. 25, 4479-4492. 10.1105/tpc.113.117432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnova T., Sasabe M., Ghebreghiorghis L., Gruber C. W., Hamada T., Dehmel V., Strompen G., Stierhof Y.-D., Lukowitz W., Kemmerling B. et al. (2009). Microtubule-associated kinase-like protein RUNKEL needed [corrected] for cell plate expansion in Arabidopsis cytokinesis. Curr. Biol. 19, 518-523. 10.1016/j.cub.2009.02.021 [DOI] [PubMed] [Google Scholar]
- Krupnova T., Stierhof Y.-D., Hiller U., Strompen G. and Müller S. (2013). The microtubule-associated kinase-like protein RUNKEL functions in somatic and syncytial cytokinesis. Plant J. 74, 781-791. 10.1111/tpj.12160 [DOI] [PubMed] [Google Scholar]
- Larson P. (1994). The Vascular Cambium: Development and Structure. Springer-Verlag. [Google Scholar]
- Lee Y. R., and Liu B. (2000). Identification of a phragmoplast-associated kinesin-related protein in higher plants. Curr. Biol. 10, 797-800. 10.1016/S0960-9822(00)00564-9 [DOI] [PubMed] [Google Scholar]
- Lee Y.-R. J., Giang H. M. and Liu B. (2001). A novel plant kinesin-related protein specifically associates with the phragmoplast organelles. Plant Cell 13, 2427-2439. 10.1105/tpc.13.11.2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. R., Li Y. and Liu B. (2007). Two Arabidopsis phragmoplast-associated kinesins play a critical role in cytokinesis during male gametogenesis. Plant Cell. 19, 2595-2605. 10.1105/tpc.107.050716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Shen Y., Cai C., Zhong C., Zhu L., Yuan M. and Ren H. (2010). The type II Arabidopsis formin14 interacts with microtubules and microfilaments to regulate cell division. Plant Cell 22, 2710-2726. 10.1105/tpc.110.075507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka E., Herrmann A. and Mueller S. (2015). Mechanisms of plant cell division. Wiley Interdiscip Rev. Dev. Biol. 4, 391-405. 10.1002/wdev.186 [DOI] [PubMed] [Google Scholar]
- Liu T., Tian J., Wang G., Yu Y., Wang C., Ma Y., Zhang X., Xia G., Liu B. and Kong Z. (2014). Augmin triggers microtubule-dependent microtubule nucleation in interphase plant cells. Curr. Biol. 24, 2708-2713. 10.1016/j.cub.2014.09.053 [DOI] [PubMed] [Google Scholar]
- Lloyd C. and Chan J. (2006). Not so divided: the common basis of plant and animal cell division. Nat. Rev. Mol. Cell Biol. 7, 147-152. 10.1038/nrm1831 [DOI] [PubMed] [Google Scholar]
- Lu L., Lee Y. R. J., Pan R. Q., Maloof J. N. and Liu B. (2005). An internal motor kinesin is associated with the golgi apparatus and plays a role in trichome morphogenesis in Arabidopsis. Mol. Biol. Cell 16, 811-823. 10.1091/mbc.E04-05-0400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. R. and Sack F. D. (2012). Polar development of preprophase bands and cell plates in the Arabidopsis leaf epidermis. Plant J. 69, 501-509. 10.1111/j.1365-313X.2011.04809.x [DOI] [PubMed] [Google Scholar]
- Mao T., Jin L., Li H., Liu B. and Yuan M. (2005). Two microtubule-associated proteins of the Arabidopsis MAP65 family function differently on microtubules. Plant Physiol. 138, 654-662. 10.1104/pp.104.052456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael C. M. and Bednarek S. Y. (2013). Cytoskeletal and membrane dynamics during higher plant cytokinesis. New Phytol. 197, 1039-1057. 10.1111/nph.12122 [DOI] [PubMed] [Google Scholar]
- Miart F., Desprez T., Biot E., Morin H., Belcram K., Höfte H., Gonneau M. and Vernhettes S. (2014). Spatio-temporal analysis of cellulose synthesis during cell plate formation in Arabidopsis. Plant J. 77, 71-84. 10.1111/tpj.12362 [DOI] [PubMed] [Google Scholar]
- Miki T., Naito H., Nishina M. and Goshima G. (2014). Endogenous localizome identifies 43 mitotic kinesins in a plant cell. Proc. Natl. Acad. Sci. USA 111, E1053-E1061. 10.1073/pnas.1311243111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschou P. N., Smertenko A. P., Minina E. A., Fukada K., Savenkov E. I., Robert S., Hussey P. J. and Bozhkov P. V. (2013). The caspase-related protease separase (extra spindle poles) regulates cell polarity and cytokinesis in Arabidopsis. Plant Cell 25, 2171-2186. 10.1105/tpc.113.113043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschou P. N., Gutierrez-Beltran E., Bozhkov P. V. and Smertenko A. (2016a). Separase promotes microtubule polymerization by activating CENP-E-related kinesin Kin7. Dev. Cell 37, 350-361. 10.1016/j.devcel.2016.04.015 [DOI] [PubMed] [Google Scholar]
- Moschou P. N., Savenkov E. I., Minina E. A., Fukada K., Reza S. H., Gutierrez-Beltran E., Sanchez-Vera V., Suarez M. F., Hussey P. J., Smertenko A. P. et al. (2016b). EXTRA SPINDLE POLES (Separase) controls anisotropic cell expansion in Norway spruce (Picea abies) embryos independently of its role in anaphase progression. New Phytol. 212, 232-243. 10.1111/nph.14012 [DOI] [PubMed] [Google Scholar]
- Müller S. and Jürgens G. (2016). Plant cytokinesis-No ring, no constriction but centrifugal construction of the partitioning membrane. Semin. Cell Dev. Biol. 53, 10-18. 10.1016/j.semcdb.2015.10.037 [DOI] [PubMed] [Google Scholar]
- Müller S., Smertenko A., Wagner V., Heinrich M., Hussey P. J. and Hauser M.-T. (2004). The plant microtubule-associated protein AtMAP65-3/PLE is essential for cytokinetic phragmoplast function. Curr. Biol. 14, 412-417. 10.1016/j.cub.2004.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T., Sano T., Sasabe M., Nonaka S., Higashiyama T., Hasezawa S., Machida Y. and Hasebe M. (2013). Mechanism of microtubule array expansion in the cytokinetic phragmoplast. Nat. Commun. 4, 1967 10.1038/ncomms2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito H. and Goshima G. (2015). NACK kinesin is required for metaphase chromosome alignment and cytokinesis in the moss physcomitrella patens. Cell Struct. Funct. 40, 31-41. 10.1247/csf.14016 [DOI] [PubMed] [Google Scholar]
- Nakamura M., Yagi N., Kato T., Fujita S., Kawashima N., Ehrhardt D. W. and Hashimoto T. (2012). Arabidopsis GCP3-interacting protein 1/MOZART 1 is an integral component of the gamma-tubulin-containing microtubule nucleating complex. Plant J. 71, 216-225. 10.1111/j.1365-313X.2012.04988.x [DOI] [PubMed] [Google Scholar]
- Nakaoka Y., Miki T., Fujioka R., Uehara R., Tomioka A., Obuse C., Kubo M., Hiwatashi Y. and Goshima G. (2012). An inducible RNA interference system in Physcomitrella patens reveals a dominant role of augmin in phragmoplast microtubule generation. Plant Cell 24, 1478-1493. 10.1105/tpc.112.098509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M., Hirano K., Nakashima S., Banno H., Nishihama R. and Machida Y. (1998). The expression pattern of the gene for NPK1 protein kinase related to mitogen-activated protein kinase kinase kinase (MAPKKK) in a tobacco plant: correlation with cell proliferation. Plant Cell Physiol. 39, 690-700. 10.1093/oxfordjournals.pcp.a029423 [DOI] [PubMed] [Google Scholar]
- Nishihama R., Ishikawa M., Araki S., Soyano T., Asada T. and Machida Y. (2001). The NPK1 mitogen-activated protein kinase kinase kinase is a regulator of cell-plate formation in plant cytokinesis. Genes Dev. 15, 352-363. 10.1101/gad.863701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihama R., Soyano T., Ishikawa M., Araki S., Tanaka H., Asada T., Irie K., Ito M., Terada M., Banno H. et al. (2002). Expansion of the cell plate in plant cytokinesis requires a kinesin-like protein/MAPKKK complex. Cell 109, 87-99. 10.1016/S0092-8674(02)00691-8 [DOI] [PubMed] [Google Scholar]
- Nishitani K. (1995). Endo-xyloglucan transferase, a new class of transferase involved in cell-wall construction. J. Plant Res. 108, 137-148. 10.1007/BF02344317 [DOI] [Google Scholar]
- Oh S. A., Johnson A., Smertenko A., Rahman D., Park S. K., Hussey P. J. and Twell D. (2005). A divergent cellular role for the FUSED kinase family in the plant-specific cytokinetic phragmoplast. Curr. Biol. 15, 2107-2111. 10.1016/j.cub.2005.10.044 [DOI] [PubMed] [Google Scholar]
- Oh S. A., Allen T., Kim G. J., Sidorova A., Borg M., Park S. K. and Twell D. (2012). Arabidopsis Fused kinase and the Kinesin-12 subfamily constitute a signalling module required for phragmoplast expansion. Plant J. 72, 308-319. 10.1111/j.1365-313X.2012.05077.x [DOI] [PubMed] [Google Scholar]
- Oh S. A., Bourdon V., Dickinson H. G., Twell D. and Park S. K. (2014). Arabidopsis Fused kinase TWO-IN-ONE dominantly inhibits male meiotic cytokinesis. Plant Reprod 27, 7-17. 10.1007/s00497-013-0235-6 [DOI] [PubMed] [Google Scholar]
- Otegui M. S., Mastronarde D. N., Kang B. H., Bednarek S. Y. and Staehelin L. A. (2001). Three-dimensional analysis of syncytial-type cell plates during endosperm cellularization visualized by high resolution electron tomography. Plant Cell 13, 2033-2051. 10.1105/tpc.13.9.2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan R., Lee Y. R., and Liu B. (2004). Localization of two homologous Arabidopsis kinesin-related proteins in the phragmoplast. Planta 220, 156-164. 10.1007/s00425-004-1324-4 [DOI] [PubMed] [Google Scholar]
- Panteris E., Adamakis I.-D. S., Voulgari G. and Papadopoulou G. (2011). A role for katanin in plant cell division: microtubule organization in dividing root cells of fra2 and lue1 arabidopsis thaliana mutants. Cytoskeleton 68, 401-413. 10.1002/cm.20522 [DOI] [PubMed] [Google Scholar]
- Pastuglia M., Azimzadeh J., Goussot M., Camilleri C., Belcram K., Evrard J.-L., Schmit A. C., Guerche P. and Bouchez D. (2006). Gamma-tubulin is essential for microtubule organization and development in Arabidopsis. Plant Cell 18, 1412-1425. 10.1105/tpc.105.039644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovská B., Cenklová V., Pochylová Z., Kourová H., Doskočilová A., Plíhal O., Binarová L. and Binarová P. (2012). Plant Aurora kinases play a role in maintenance of primary meristems and control of endoreduplication. New Phytol. 193, 590-604. 10.1111/j.1469-8137.2011.03989.x [DOI] [PubMed] [Google Scholar]
- Rasmussen C. G., Wright A. J. and Müller S. (2013). The role of the cytoskeleton and associated proteins in determination of the plant cell division plane. Plant J. 75, 258-269. 10.1111/tpj.12177 [DOI] [PubMed] [Google Scholar]
- Samuels A. L., Giddings T. H. and Staehelin L. A. (1995). Cytokinesis in tobacco BY-2 and root-tip cells - a new model of cell plate formation in higher-plants. J. Cell Biol. 130, 1345-1357. 10.1083/jcb.130.6.1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabe M., Soyano T., Takahashi Y., Sonobe S., Igarashi H., Itoh T. J., Hidaka M. and Machida Y. (2006). Phosphorylation of NtMAP65-1 by a MAP kinase down-regulates its activity of microtubule bundling and stimulates progression of cytokinesis of tobacco cells. Genes Dev. 20, 1004-1014. 10.1101/gad.1408106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabe M., Boudolf V., De Veylder L., Inze D., Genschik P. and Machida Y. (2011). Phosphorylation of a mitotic kinesin-like protein and a MAPKKK by cyclin-dependent kinases (CDKs) is involved in the transition to cytokinesis in plants. Proc. Natl. Acad. Sci. USA 108, 17844-17849. 10.1073/pnas.1110174108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedbrook J. C., Ehrhardt D. W., Fisher S. E., Scheible W. R. and Somerville C. R. (2004). The Arabidopsis sku6/spiral1 gene encodes a plus end-localized microtubule-interacting protein involved in directional cell expansion. Plant Cell 16, 1506-1520. 10.1105/tpc.020644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segui-Simarro J. M., Austin J. R. 2nd, White E. A. and Staehelin L. A. (2004). Electron tomographic analysis of somatic cell plate formation in meristematic cells of Arabidopsis preserved by high-pressure freezing. Plant Cell. 16, 836-856. 10.1105/tpc.017749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segui-Simarro J. M., Otegui M. S., Austin J. R. 2nd and Staehelin L. A. (2007). Plant cytokinesis - insights gained from electron tomography studies. In Cell Division Control in Plants, Vol. 9 pp. 251-287. (ed. Verma D. P. S. and Hong Z.). Berlin Heidelberg: Springer-Verlag. [Google Scholar]
- Smekalova V., Luptovčiak I., Komis G., Šamajová O., Ovečka M., Doskočilová A., Takáč T., Vadovič P., Novák O., Pechan T. et al. (2014). Involvement of YODA and mitogen activated protein kinase 6 in Arabidopsis post-embryogenic root development through auxin up-regulation and cell division plane orientation. New Phytol. 203, 1175-1193. 10.1111/nph.12880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smertenko A., Saleh N., Igarashi H., Mori H., Hauser-Hahn I., Jiang C.-J., Sonobe S., Lloyd C. W. and Hussey P. J. (2000). A new class of microtubule-associated proteins in plants. Nat. Cell Biol. 2, 750-753. 10.1038/35036390 [DOI] [PubMed] [Google Scholar]
- Smertenko A. P., Bozhkov P. V., Filonova L. H., von Arnold S. and Hussey P. J. (2003). Re-organisation of the cytoskeleton during developmental programmed cell death in Picea abies embryos. Plant J. 33, 813-824. 10.1046/j.1365-313X.2003.01670.x [DOI] [PubMed] [Google Scholar]
- Smertenko A. P., Chang H.-Y., Wagner V., Kaloriti D., Fenyk S., Sonobe S., Lloyd C., Hauser M. T. and Hussey P. J. (2004). The Arabidopsis microtubule-associated protein AtMAP65-1: molecular analysis of its microtubule bundling activity. Plant Cell 16, 2035-2047. 10.1105/tpc.104.023937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smertenko A. P., Chang H.-Y., Sonobe S., Fenyk S. I., Weingartner M., Bögre L. and Hussey P. J. (2006). Control of the AtMAP65-1 interaction with microtubules through the cell cycle. J. Cell Sci. 119, 3227-3237. 10.1242/jcs.03051 [DOI] [PubMed] [Google Scholar]
- Smertenko A. P., Kaloriti D., Chang H.-Y., Fiserova J., Opatrny Z. and Hussey P. J. (2008). The C-terminal variable region specifies the dynamic properties of Arabidopsis microtubule-associated protein MAP65 isotypes. Plant Cell 20, 3346-3358. 10.1105/tpc.108.063362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smertenko A. P., Piette B. and Hussey P. J. (2011). The origin of phragmoplast asymmetry. Curr. Biol. 21, 1924-1930. 10.1016/j.cub.2011.10.012 [DOI] [PubMed] [Google Scholar]
- Söllner R., Glässer G., Wanner G., Somerville C. R., Jürgens G. and Assaad F. F. (2002). Cytokinesis-defective mutants of Arabidopsis. Plant Physiol. 129, 678-690. 10.1104/pp.004184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H., Golovkin M., Reddy A. S. N. and Endow S. A. (1997). In vitro motility of AtKCBP, a calmodulin-binding kinesin protein of Arabidopsis. Proc. Natl. Acad. Sci. USA 94, 322-327. 10.1073/pnas.94.1.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyano T., Nishihama R., Morikiyo K., Ishikawa M. and Machida Y. (2003). NQK1/NtMEK1 is a MAPKK that acts in the NPK1 MAPKKK-mediated MAPK cascade and is required for plant cytokinesis. Genes Dev. 17, 1055-1067. 10.1101/gad.1071103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigemann P. and Gerlich D. W. (2009). Cytokinetic abscission: cellular dynamics at the midbody. Trends Cell Biol. 19, 606-616. 10.1016/j.tcb.2009.07.008 [DOI] [PubMed] [Google Scholar]
- Steiner A., Rybak K., Altmann M., McFarlane H. E., Klaeger S., Nguyen N., Facher E., Ivakov A., Wanner G., Kuster B. et al. (2016). Cell cycle-regulated PLEIADE/AtMAP65-3 links membrane and microtubule dynamics during plant cytokinesis. Plant J. 88, 531-541. 10.1111/tpj.13275 [DOI] [PubMed] [Google Scholar]
- Strompen G., El Kasmi F., Richter S., Lukowitz W., Assaad F. F., Jurgens G. and Mayer U. (2002). The Arabidopsis HINKEL gene encodes a kinesin-related protein involved in cytokinesis and is expressed in a cell cycle-dependent manner. Curr. Biol. 12, 153-158. 10.1016/S0960-9822(01)00655-8 [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Soyano T., Kosetsu K., Sasabe M. and Machida Y. (2010). HINKEL kinesin, ANP MAPKKKs and MKK6/ANQ MAPKK, which phosphorylates and activates MPK4 MAPK, constitute a pathway that is required for cytokinesis in Arabidopsis thaliana. Plant Cell Physiol. 51, 1766-1776. 10.1093/pcp/pcq135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Ishikawa M., Kitamura S., Takahashi Y., Soyano T., Machida C. and Machida Y. (2004). The AtNACK1/HINKEL and STUD/TETRASPORE/AtNACK2 genes, which encode functionally redundant kinesins, are essential for cytokinesis in Arabidopsis. Genes Cells 9, 1199-1211. 10.1111/j.1365-2443.2004.00798.x [DOI] [PubMed] [Google Scholar]
- Thiele K., Wanner G., Kindzierski V., Jüergens G., Mayer U., Pachl F. and Assaad F. F. (2009). The timely deposition of callose is essential for cytokinesis in Arabidopsis. Plant J. 58, 13-26. 10.1111/j.1365-313X.2008.03760.x [DOI] [PubMed] [Google Scholar]
- Töller A., Brownfield L., Neu C., Twell D. and Schulze-Lefert P. (2008). Dual function of Arabidopsis glucan synthase-like genes GSL8 and GSL10 in male gametophyte development and plant growth. Plant J. 54, 911-923. 10.1111/j.1365-313X.2008.03462.x [DOI] [PubMed] [Google Scholar]
- Twell D., Park S. K., Hawkins T. J., Schubert D., Schmidt R., Smertenko A. and Hussey P. J. (2002). MOR1/GEM1 has an essential role in the plant-specific cytokinetic phragmoplast. Nat. Cell Biol. 4, 711-714. 10.1038/ncb844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme D., Bouget F.-Y., Van Poucke K., Inzé D. and Geelen D. (2004a). Molecular dissection of plant cytokinesis and phragmoplast structure: a survey of GFP-tagged proteins. Plant J. 40, 386-398. 10.1111/j.1365-313X.2004.02222.x [DOI] [PubMed] [Google Scholar]
- Van Damme D., Van Poucke K., Boutant E., Ritzenthaler C., Inze D. and Geelen D. (2004b). In vivo dynamics and differential microtubule-binding activities of MAP65 proteins. Plant Physiol. 136, 3956-3967. 10.1104/pp.104.051623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme D., De Rybel B., Gudesblat G., Demidov D., Grunewald W., De Smet I., Houben A., Beeckman T. and Russinova E. (2011). Arabidopsis alpha Aurora kinases function in formative cell division plane orientation. Plant Cell 23, 4013-4024. 10.1105/tpc.111.089565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gisbergen P. A., Li M., Wu S. Z. and Bezanilla M. (2012). Class II formin targeting to the cell cortex by binding PI(3,5)P(2) is essential for polarized growth. J. Cell Biol. 198, 235-250. 10.1083/jcb.201112085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanstraelen M., Torres Acosta J. A., De Veylder L., Inze D. and Geelen D. (2004). A plant-specific subclass of C-terminal kinesins contains a conserved a-type cyclin-dependent kinase site implicated in folding and dimerization. Plant Physiol. 135, 1417-1429. 10.1104/pp.104.044818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanstraelen M., Inzé D. and Geelen D. (2006). Mitosis-specific kinesins in Arabidopsis. Trends Plant Sci. 11, 167-175. 10.1016/j.tplants.2006.02.004 [DOI] [PubMed] [Google Scholar]
- Walia A., Nakamura M., Moss D., Kirik V., Hashimoto T. and Ehrhardt D. W. (2014). GCP-WD mediates gamma-TuRC recruitment and the geometry of microtubule nucleation in interphase arrays of Arabidopsis. Curr. Biol. 24, 2548-2555. 10.1016/j.cub.2014.09.013 [DOI] [PubMed] [Google Scholar]
- Wang J., Zhang Y., Wu J., Meng L. and Ren H. (2013). AtFH16, [corrected] an Arabidopsis type II formin, binds and bundles both microfilaments and microtubules, and preferentially binds to microtubules. J Integr. Plant Biol. 55, 1002-1015. 10.1111/jipb.12089 [DOI] [PubMed] [Google Scholar]
- Wang H., Zhuang X., Wang X., Law A. H., Zhao T., Du S., Loy M. M. and Jiang L. (2016). A distinct pathway for polar exocytosis in plant cell wall formation. Plant Physiol. 172, 1003-1018. 10.1104/pp.16.00754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartner M., Binarova P., Drykova D., Schweighofer A., David J. P., Heberle-Bors E., Doonan J. and Bogre L. (2001). Dynamic recruitment of Cdc2 to specific microtubule structures during mitosis. Plant Cell 13, 1929-1943. 10.1105/tpc.13.8.1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartner M., Criqui M.-C., Mészáros T., Binarova P., Schmit A.-C., Helfer A., Derevier A., Erhardt M., Bögre L. and Genschik P. (2004). Expression of a nondegradable cyclin B1 affects plant development and leads to endomitosis by inhibiting the formation of a phragmoplast. Plant Cell 16, 643-657. 10.1105/tpc.020057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington A. T., Vugrek O., Wei K. J., Hasenbein N. G., Sugimoto K., Rashbrooke M. C. and Wasteneys G. O. (2001). MOR1 is essential for organizing cortical microtubules in plants. Nature 411, 610-613. 10.1038/35079128 [DOI] [PubMed] [Google Scholar]
- Wick S. M. and Hepler P. K. (1980). Localization of Ca++-containing antimonate precipitates during mitosis. J. Cell Biol. 86, 500-513. 10.1083/jcb.86.2.500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker-Planquart C., Stoppin-Mellet V., Blanchoin L. and Vantard M. (2004). Interactions of tobacco microtubule-associated protein MAP65-1b with microtubules. Plant J. 39, 126-134. 10.1111/j.1365-313X.2004.02115.x [DOI] [PubMed] [Google Scholar]
- Xue X. H., Guo C. Q., Du F., Lu Q. L., Zhang C. M. and Ren H. Y. (2011). AtFH8 is involved in root development under effect of low-dose latrunculin B in dividing cells. Mol. Plant 4, 264-278. 10.1093/mp/ssq085 [DOI] [PubMed] [Google Scholar]
- Yasuhara H., and Shibaoka H. (2000). Inhibition of cell-plate formation by brefeldin A inhibited the depolymerization of microtubules in the central region of the phragmoplast. Plant Cell Physiol. 41, 300-310. 10.1093/pcp/pci121 [DOI] [PubMed] [Google Scholar]
- Yasuhara H. (2005). Caffeine inhibits callose deposition in the cell plate and the depolymerization of microtubules in the central region of the phragmoplast. Plant Cell Physiol. 46, 1083-1092. 10.1093/pcp/pci121 [DOI] [PubMed] [Google Scholar]
- Yokoyama R., and Nishitani K. (2001). Endoxyloglucan transferase is localized both in the cell plate and in the secretary pathway destined for the apoplast in tobacco cells. Plant Cell Physiol. 42, 292-300. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Lin F., Mao T., Nie J., Yan M., Yuan M. and Zhang W. (2012). Phosphatidic acid regulates microtubule organization by interacting with MAP65-1 in response to salt stress in Arabidopsis. Plant Cell 24, 4555-4576. 10.1105/tpc.112.104182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J. R., Niu Q.-W., Nishizawa N., Wu Y., Kost B. and Chua N.-H. (2000). KORRIGAN, an arabidopsis endo-1,4-beta-glucanase, localizes to the cell plate by polarized targeting and is essential for cytokinesis. Plant Cell 12, 1137-1152. 10.1105/tpc.12.7.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]