Abstract
Cancer can be considered the result of a series of genetic variations that lead to a normal cell being transformed into a malignant one while avoiding cell death—atypical characteristics of tumor development. Although a large number of genomics and epigenetic alterations have been identified in cells undergoing apoptotic, autophagic or necrotic cell death, the treatment of cancer remains thought-provoking. Pyroptosis is differentiated from other types of programmed cell death and is mainly activated by Caspase-1. To initiate pyroptosis, cells receive specific “death” messages, produce cytokines, swell, burst, and ultimately die. The deficiency of Caspase-1 expression may lead to inflammation-mediated tumor progression. Hence, the molecular mechanisms for the Caspase-1 activation in tumor tissues are yet to be exploited extensively. This review aims to summarise the latest discoveries about pyroptosis and its new exciting role in inducing cancer cell death.
Introduction
Cancers are diseases caused by aberrant growth of cells that have the metastatic capability hence it can spread to other parts of the body [1]. All tumor cells express the six “hallmarks of cancer”, which are mainly involved in malignant tumor development. These hallmarks are (1) unrestricted cell growth and division in the absence of appropriate signals, (2) constant growth and division even when inhibitory signals are present, (3) evasion of programmed cell death, (4) the ability to undergo an infinite number of cell divisions, (5) induction of angiogenesis and (6) spreading to the encompassing tissue and the formation of metastases [2]. The imbalance of cell proliferation and cell death ratio determines an increase in the number of tumor cells [3]. Apoptosis can be defined as a form of programmed cell death which is actively involving in equalization of the cell balancing process. Damage to and physical disturbance of the cell may elicit accidental cell death, and during the past four decades, it has been found that cells may also actively invoke cell death when needed. These controlled mechanisms of cell death are characteristically linked with human embryonic development, homeostatic maintenance and disease pathology [4].
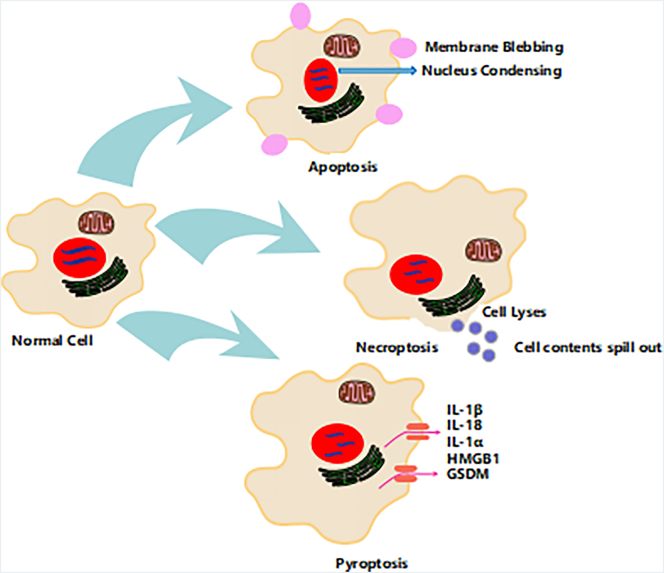
Apoptosis is described as an active (Figure 1), programmed process of self-directed cellular dismantling that does not elicit inflammation [3], [5]. Nevertheless, necrosis has been defined as a passive, accidental form of cell death consequent from the environmental disorder that leads to the uncontrolled release of cellular contents and, subsequently, inflammation (Figure 1). While there are many reports on apoptosis and necrosis as the most investigated cell death processes among cellular biologists, recently, more attention has been paid to study on pyroptosis [6]. Pyroptosis is activated by an early rupture of plasma membrane integrity, which results in the leakage of intracellular components into the extracellular milieu. Pyroptosis has been extensively studied using microbial-pathogenesis mediated immune models; however, there are a growing number of studies looking at its role in inducing cancer cell death. This review presents perspectives on the mechanisms for activating pyroptosis in cancer cells and a discussion on the opportunities it may present for the treatment of cancer.
Figure 1.
Structural characteristics of cell death pathways. Apoptosis, necroptosis, and pyroptosis are programmed forms of cell death. Membrane blebbing followed by the formation of apoptotic bodies are crucial features apoptosis. While necrosis is accompanied by plasma membrane rupture leads to the release of cellular contents. Pyroptosis results from an inflammatory response induced by inflammasome activation, which is tightly associated with IL-1β/IL-18 secretion. Also, GSDM is involved in pyroptosis cell death.
Programmed Cell Death versus Tumor Suppressor Genes
In multicellular organisms, the appropriate regulation of cell death is imperative for processes such as embryonic development and cellular turnover in adult tissues [7]. Therefore, an investigation into the role of pyroptosis in disease conditions is very important, as it not only provides insights into the pathogenesis of a disease but may also provide clues on how the disease can be treated [5]. Cancer results in a loss of equilibrium between cell division and cell death due to alterations in signaling pathways [3]. Mammalian cancers lose the capability to initiate or execute apoptotic cell death programs; hence, they are resistant to several existing chemotherapeutic drugs that target apoptosis [8]. It is, therefore, necessary to discover alternative cell death processes for effective cancer treatments.
Some reports have shown that the inflammasome can trigger both pyroptotic and apoptotic Caspases (Caspases 1 and 11 and Caspases 3, 8, and 9, respectively) under diverse circumstances [5], [9]. A recent study proposed that DNA-dependent inflammasome activation at lower doses of a stimulus lead to the induction of apoptosis over pyroptosis [10]. This evidence clearly demonstrates that apoptotic and pyroptotic processes interact with each other via Caspase activation. The downregulation of tumor suppressor genes, which results in reduced programmed cell death and enhanced tumor growth and inactivation of tumor suppressor genes, leads to many human cancers [11], [12]. However, having dual roles, pyroptosis can be a cause of the problem as well as the solution, as many studies researching new drugs targeting several aspects of pyroptosis have revealed. Nevertheless, the literature examining pyroptosis as cancer treatment is currently limited. This article gives a broad overview of pyroptosis, its mechanisms, and how the pyroptotic pathway can be used as a novel target for the treatment of cancer.
Biochemical and Morphological Features of Pyroptosis
Pyroptosis is induced most commonly by infection with intracellular pathogens and most likely forms part of the antimicrobial response [13]. Here, immune cells distinguish external danger signals within themselves, secrete chemical messengers named pro-inflammatory cytokines that cause cell swelling, bursting and finally cell death [14]. The term “pyroptosis” was originally named in 2001 by Dr. Brad T. Cookson. The Greek word “pyro” means fire, and “ptosis” means falling. The meaning of the combined term “pyroptosis” is, therefore “the falling of fire”. It denotes the pro-inflammatory-mediated bursting of infected cells [15].
The term “pyroptosis” was initially introduced to define a form of cell death that occurs in phagocytes of the myeloid lineage such as macrophages, dendritic cells, and neutrophils, although pyroptosis has also been described in CD4+ T cells, keratinocytes, epithelial cells, endothelial cells and neurons [14], [16]. Pyroptosis is mainly initiated by microbial infection and is followed by Caspase-1 activation and the secretion of pyrogenic interleukins [17], [18]; however, the activation of pyroptosis by nonbacterial or microbial pathological stimuli is still unclear [19]. Additionally, a detailed investigation of this mode of cancer cell death is needed, either alone or alongside other death mechanisms such as apoptosis or necrosis [16], [19]. The following section of this review provides an understanding of how pyroptosis can be induced by other stimuli and detail how its molecular mechanisms differ from other forms of cell death in tumor tissues (Figure 2).
Figure 2.
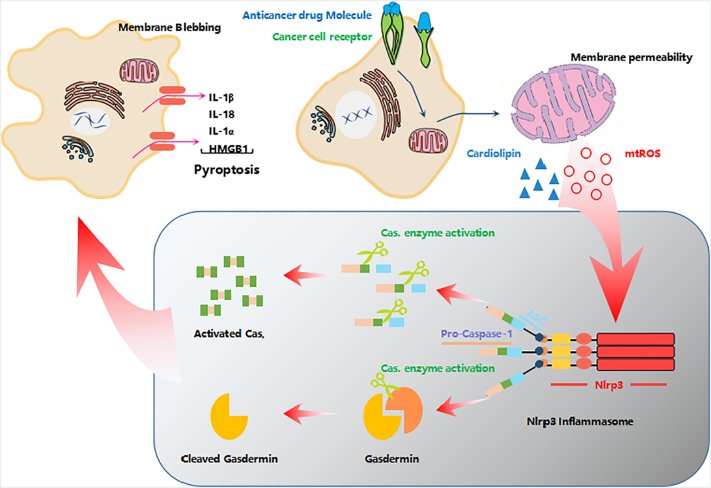
The role of mitochondria for induction of pyroptosis. Drug-treated cells receive signals through cell membrane receptors, and the drug invades the cytoplasm. The drugs altered the significant functions of mitochondria lead to the release of reactive oxygen species (ROS) and cardiolipin, which are actively involved in NLRP inflammasome formation and Caspase-1 activation. Activated Caspase-1 produces cleaved gasdermin to initiate pyroptosis-mediated cell death by cell membrane swelling, pore formation, and eventual lysis.
Nonmicrobial Pathological Stimuli for Inducing Pyroptosis
As described earlier, the inflammasome can trigger not only pyroptotic but also apoptotic caspases for cell death. Many chemotherapy drugs and plant metabolic compounds have been investigated and found to destroy cancer cells by activating Caspase-3-mediated apoptosis. Based on this finding, a recent study clearly demonstrated that the cleavage of gasdermin-E by Caspase-3-induced pyroptosis in HeLa, SH-SY5Y and MeWo cells could instead mediate pyroptosis upon treatment with various chemotherapy drugs, such as the DNA-binding/modifying compounds doxorubicin, cisplatin, and actinomycin-D or the topoisomerase inhibitors topotecan, CPT-11, etoposide and mitoxantrone [20]. However, to date, only a few reports have demonstrated the activation of pyroptosis using nonbacterial stimuli. The identification that pyroptosis can be induced through chemotherapy represents a seminal finding and provides a new platform for researchers to focus on pyroptosis-induction in cancer as an alternative therapeutic strategy (Figure 3).
Figure 3.
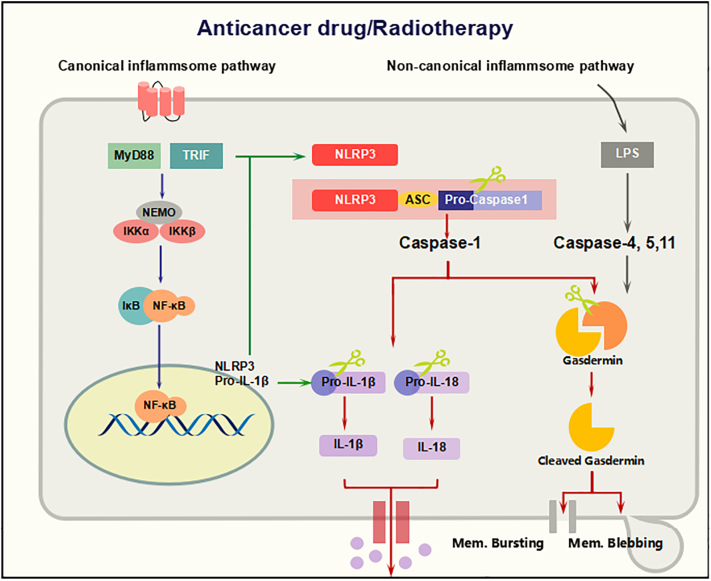
Molecular mechanism of inflammasome activation for pyroptosis. The inflammasome sensors present on the cell membrane detect drug molecules and activate Caspase-1 through the ASC or NLR adaptor. Active Caspase-1 cleaves the gasdermin family and binds to the plasma membrane, generating cell blebbing and membrane rupture and ultimately promoting pyroptosis. These pores can also facilitate the extracellular release of mature IL-1β and IL-18 to induce pyroptosis in cancer cells.
Mitochondria and Pyroptosis
A salient role of mitochondria is the production of ATP energy molecule; hence, it is named the “powerhouse of the cell”. Apart from energy synthesis, mitochondria are involved in other functions, including reactive oxygen species (ROS) generation, redox molecules and metabolites, the regulation of cell signaling and cell death, and biosynthetic metabolism [21]. Mitochondria are crucial regulators of metabolic-redox (meta-redox) modifications in tumor cells by providing a source of DNA-damaging ROS [22]. ROS can also regulate oncogenic signals to inhibit the metastasis of cancer cells [23]. A recent examination concluded that the mode of cancer cell death mediated by radiotherapy using ionizing radiation and existing cancer therapy drug targets was through increased ROS production [24], [25]. Elevated ROS levels were found in the liver carcinoma cells with enhanced autophagy activation [26], [27]. When cancer cells are treated with anticancer drugs, the incomplete reduction of oxygen during mitochondrial oxidative phosphorylation may produce a variety of ROS that may affect both proteins and nucleic acids in cancer cells [28], [29]. mtDNA (mitochondrial DNA) is responsible for targeting cancer cells by causing mutations and is performed for synthesis the variety of proteins to activate key messenger networks for cancer signaling pathways [30], [31]. For example, cardiolipin which is in lipid form and situated inner membrane of mitochondria [32]. Whereas cardiolipin is known for its vital role in mitochondrial oxidative phosphorylation and the initiation of apoptosis, a recent study reported that cardiolipin recruits and activates the NLRP3 inflammasome for pyroptosis-mediated cell death [33]. In addition, damaged mitochondria can facilitate the formation of activating NLRP3 inflammasomes, resulting in Caspase-1-dependent secretion of the interleukin-1b (IL-1b) and IL-18, ultimately directed to the highly inflammatory form of cell death—pyroptosis [18], [33], [34], [35], [36]. Thus, anticancer drug molecules may damage the mitochondria and induce NLRP3 inflammasomes, leading to cancer cell death via pyroptosis (Figure 2).
Caspases and Pyroptosis
The group of protease enzymes named Caspases which possess essential roles in the programmed cell death process, such as apoptosis, necroptosis, and pyroptosis [5], [37], [38]. Caspase activation directs to pyroptosis-mediated cell death, which is an emerging function for protecting an organism from aggressive stress signals and pathogenic infection [39], [40]. Caspases have been studied and found to play a variety of roles in processes such as cell proliferation, tumor suppression, cell differentiation, neural development, and axon guidance and aging [40], [41], [42]. The earlier investigations concluded that caspases can be classified into three groups: apoptotic inducer caspases (Caspase-3, 6, and 7), apoptotic originator caspases (Caspase-2, 8, 9, and 10), and inflammatory cytokine activity such as Caspase-1, 4, 5, and 11 [38], [43]. The lack of caspase activity has been recognized as a cause of tumor progression and leads to the deregulation of apoptosis, which is a key feature of most cancers [40], [44], [45], [46]. Hence, the scientific research community has begun to focus more attention on caspases as a key target for cancer therapy to regulate cell death in tumor tissues [37], [43]. Recent research is explicit that cell death caused by pyroptosis is conceded by alterations in osmotic pressure which leads to increased water inflow, cellular swelling and, ultimately, membrane rupture, which has been accredited to Caspase-1 activation [47], [48]. Caspase-1, 4 and 5 expression is directed to form cytokines and cleave gasdermin D by signaling cascades [6], [34], [41], [49], [50], [51]. It is associated with DNA cleavage and nuclear condensation [52], [53], [54]; in pyroptosis cell death, nuclear integrity is uninterrupted which is a distinct characteristic feature from apoptosis. Nevertheless, DNA cleavage and its responsible nuclease during pyroptosis are remained unknown (Figure 3).
Molecular Targets of Pyroptosis
There are five active targets such as cytokines, NLRP3, ASC, Caspases, and Gasdermin family identified for pyroptosis induction. The nucleotide-binding domain, leucine-rich family (NLR), pyrin-containing 3 (NLRP3) inflammasome is currently the most characterized inflammasome, and it consists of a scaffold protein (NLRP3), an apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) adaptor, and caspase 1. Inflammasomes are intracellular multiprotein complexes that act as platforms for the maturation and secretion of the proinflammatory cytokines interleukin (IL)-1b and IL-18. Upon activation of the NLRP3 inflammasome, pro-caspase 1 is recruited to the platform and then auto-catalytically cleaved to its active form, activated caspase-1 processes the cytosolic precursors of the related cytokines IL-1β and IL-18, thus allowing secretion of the biologically active cytokines, finally resulting in pyroptosis [55], [56] On the other hand, caspase-1 activation facilitates the cleavage of gasdermin family to carry out pyroptosis cell death signals [57].
Inflammasomes
Chronic inflammatory responses are not only related to tumor progression but also have important roles in tumor immunity and immunotherapy. The impairment of inflammasome formation may cause many major diseases [58], [59]. The inflammasomes consist of Caspase-1, ASC (apoptosis-associated speck-like protein includes a caspase-recruitment domain (CARD)) and NOD-like receptor (NALP) [35], [60], [61] and they are accumulated in response to invading pathogens and/or threat signals. Inflammasomes are mainly involved in the innate immune pathway for the maturation of active IL-1β and interleukin-18 from pro-IL-1β and IL-18, ultimately inducing pyroptosis [6], [15], [37], [62], [63]. Three NLR family members, Nlrp1, Nlrp3, and Nlrc4, have been targeted to induce the pyroptotic process against pathogenic infections. The NLR family has unique domains with protein–protein interactions [35], [37], [61] due to the presence of an N-terminal PYRIN-PAAD-DAPIN domain (PYD) and a C-terminal CARD. The Nlrp3 inflammasome is predominantly activated against bacterial, viral and fungal agents [64], [65], [66], [67], and it also plays key roles in K+ efflux, lysosomal destabilization, pore formation in the plasma membrane, mitochondrial damage, ROS production, Ca2+ influx, cell swelling and bursting, and pyroptosis-mediated cell death, among others [35], [57], [68], [69]. Unlike Nlrp3, the Nlrp1 and Nlrc4 inflammasomes are activated for a more limited set of bacterial components [47], [64], [65], [70]; hence, Nlrp3 has been extensively studied for therapeutic purposes [45].
The NLRP3 inflammasome has a protective role against the development of cancer [56], [71], [72]. For instance, the NLRP3 inflammasome is significantly down-regulated in liver cancer tissues when compared with non-cancerous liver tissues and that patients with more progressive hepatocellular carcinoma have lower expression levels of the NLRP3 inflammasome components, indicating that the down-regulation of the multiprotein NLRP3 inflammasome platform is likely involved in HCC progression. Furthermore IL-18 was previously assigned an antitumor function in a variety of experimental tumor models [73], [74] and notably mice lacking caspase-1 are defective in the maturation and secretion of IL-1β and IL-18 [75], [76]. Moreover, IL-18 was reported to inhibit tumor growth and angiogenesis [77], [78]. In summary, inflammasome components and pathways suppress tumor growth by activating tumor-associated inflammatory responses and may provide clues for cancer therapy (Figure 3).
Gasdermin Family
The six members of gasdermin family have found in most of all vertebrates they are GSDMA, GSDMB, GSDMC, GSDMD, DFNA5, and DFNB59 [79]. The gasdermin group mostly activated in epithelial tissues and appears to show a fundamental function in the regulation of epithelial proliferation and differentiation. Moreover, GSDMA, GSDMC, GSDMD, and DFNA5 also have been studied to act as tumor suppressors in cancer tissues [80]. Among them, GSDMD has been identified as a substrate of caspase activation and performs pyroptosis in infected cells [36], [81]. Activated Caspase-1 and Caspase-11/4/5 produce cleaved gasdermin D, releasing its N domain, which induces cell swelling and bursting of the plasma membrane. Finally, cell death occurs via pyroptosis [20], [82], [83], [84], [85], [86]. Further studies must analyze the formation of GSDMD pores in cancer cell membranes both in vitro and in vivo and how these mechanisms contribute to pyroptosis-mediated programmed cell death of cancer cells (Figure 3). Similarly, more investigations are required to determine the biochemical basis of pyroptosis cell death in cancer therapy.
Conclusion
Pyroptosis is a lytic type of programmed cell death used to destroy pathogens that occurs by activating signaling pathways to induce cell swelling and plasma membrane rupture. Recent studies have mainly focused on the pyroptosis pathway as an alternative mode of cell death in the treatment of many diseases. However, the mode of action for the induction of pyroptosis-mediated cancer cell death for cancer therapy remains unclear. Further research is still required in order to uncover the mechanisms of pyroptosis and its precise functions in vitro and in vivo. Moreover, this review suggests that the activation of various signaling pathways in combination with existing chemotherapeutic agents in order to boost the anticancer immune response would be a novel therapeutic target for cancer pathogenesis.
Acknowledgments
Acknowledgment
The authors are very grateful to Henan Provincial People's Hospital, Zhengzhou, Henan Province, China for the financial support provided.
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Dongxiao Li, Email: 651215540@qq.com.
Deyu Li, Email: lidy0408@sohu.com.
References
- 1.Cancer Fact sheet N°297. World Health Organization; 2018. [Retrieved 21 March 2018] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Bohm I, Schild H. Apoptosis: the complex scenario for a silent cell death. Mol Imaging Biol. 2003;5:2–14. doi: 10.1016/s1536-1632(03)00024-6. [DOI] [PubMed] [Google Scholar]
- 4.Hao Z. Specific ablation of the apoptotic functions of cytochrome c reveals a differential requirement for cytochrome c and Apaf-1 in apoptosis. Cell. 2005;121:579–591. doi: 10.1016/j.cell.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Taabazuing CY, Okondo MC, Bachovchin DA. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem Biol. 2017;24:507–514. doi: 10.1016/j.chembiol.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He WT, Wan H, Lu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jorgensen I, Rayamajhi M, Miao EA. Programmed cell death as a defence against infection. Nat Rev Immunol. 2017;17:151–164. doi: 10.1038/nri.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitt CA, Fridman JS, Yang M, Baranov E, Hoffman RM, Lowe SW. Dissecting p53 tumor suppressor functions in vivo. Cancer Cell. 2002;1:289–298. doi: 10.1016/s1535-6108(02)00047-8. [DOI] [PubMed] [Google Scholar]
- 9.Pierini R, Juruj C, Perret M, Jones CL, Mangeot P, Weiss DS, Henry T. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ. 2012;19:1709–1721. doi: 10.1038/cdd.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sagulenko V, Thygesen SJ, Sester DP, Idris A, Cridland JA, Vajjhala PR, Roberts TL, Schroder K, Vince JE, Hill JM, Silke J, Stacey KJ. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cel Death Differ. 2013;20:1149–1160. doi: 10.1038/cdd.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauer JH, Hefand SL. New tricks of an old molecule: lifespan regulation by p53. Aging Cell. 2006;5:437–440. doi: 10.1111/j.1474-9726.2006.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong RSY. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauernfeind F, Hornung V. Of inflammasomes and pathogens —sensing of microbes by the inflammasome. EMBO Mol Med. 2013;5:814–826. doi: 10.1002/emmm.201201771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 16.Walle LV, Lamkanfi M. Pyroptosis. Curr Biol. 2016;26:543–576. doi: 10.1016/j.cub.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 17.Guey B, Bodnar M, Manie SN, Tardivel A, Petrilli V. Caspase-1 autoproteolysis is differentially required for NLRP1b and NLRP3 inflammasome function. Proc Natl Acad Sci U S A. 2014;111:17254–17259. doi: 10.1073/pnas.1415756111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aglietti RA, Dueber EC. Recent insights into the molecular mechanisms underlying pyroptosis and gasdermin family functions. Trends Immunol. 2017;38:261–271. doi: 10.1016/j.it.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Oliver K, Lorenzo G, Laurence Z, Guido K. Pyroptosis – a cell death modality of its kind? Eur J Immunol. 2010;40:595–653. doi: 10.1002/eji.200940160. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 21.Sejal V, Elma Z, Marcia CH. Mitochondria and Cancer. Cell. 2016;166:555–566. doi: 10.1016/j.cell.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bause AS, Haigis MC. SIRT3 regulation of mitochondrial oxidative stress. Exp Gerontol. 2013;48:634–639. doi: 10.1016/j.exger.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Porporato PE, Payen VL, Pérez-Escuredo J, De Saedeleer CJ, Danhier P, Copetti T, Dhup S, Tardy M, Vazeille T, Bouzin C. A mitochondrial switch promotes tumor metastasis. Cell Rep. 2014;8:754–766. doi: 10.1016/j.celrep.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 24.Tong L, Chuang CC, Wu S, Zuo L. Reactive oxygen species in redox cancer therapy. Cancer Lett. 2015;367:18–25. doi: 10.1016/j.canlet.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Zhengzhi Z, Haocai C, Haolong L, Songmao W. Induction of reactive oxygen species: an emerging approach for cancer therapy. Apoptosis. 2017;22:1321–1335. doi: 10.1007/s10495-017-1424-9. [DOI] [PubMed] [Google Scholar]
- 26.Yu Y, Duan J, Yu Y, Li Y, Liu X, Zhou X, Ho KF, Tian L, Sun Z. Silica nanoparticles induce autophagy and autophagic cell death in HepG2 cells triggered by reactive oxygen species. J Hazard Mater. 2014;270:176–186. doi: 10.1016/j.jhazmat.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 27.Khan M, Mohammad A, Patil G, Naqvi SA, Chauhan LK, Ahmad I. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials. 2012;33:1477–1488. doi: 10.1016/j.biomaterials.2011.10.080. [DOI] [PubMed] [Google Scholar]
- 28.Szewczyk A, Wojtczak L. Mitochondria as a pharmacological target. Pharmacol Rev. 2002;54:101–127. doi: 10.1124/pr.54.1.101. [DOI] [PubMed] [Google Scholar]
- 29.Kanvah S. Oxidation of DNA: damage to nucleobases. Acc Chem Res. 2010;43:280–287. doi: 10.1021/ar900175a. [DOI] [PubMed] [Google Scholar]
- 30.Carew JS, Huang P. Mitochondrial defects in cancer. Mol Cancer. 2002;1:9. doi: 10.1186/1476-4598-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerard GM, Souza D, Mayura AW, Vaibhav S, Shah A. Approaches for targeting mitochondria in cancer therapy. Biochim Biophys Acta. 2011;1807:689–696. doi: 10.1016/j.bbabio.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Nowicki M, Frentzen M. Cardiolipin synthase of Arabidopsis thaliana. FEBS Lett. 2005;579:2161–2165. doi: 10.1016/j.febslet.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Prajwal G, Lukens JR, Thirumala-Devi K. Mitochondria: diversity in the regulation of the NLRP3 inflammasome. Trends Mol Med. 2015;21:193–201. doi: 10.1016/j.molmed.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev. 2015;265:6–21. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Man SM, Kanneganti TD. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol. 2016;16:7–21. doi: 10.1038/nri.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins JG. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 39.Kang SJ, Wang S, Hara H, Peterson EP, Namura S, Hanjani SA, Huang Z, Srinivasan A, Tomaselli KJ, Thornberry NA. Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J Cell Biol. 2000;149:613–622. doi: 10.1083/jcb.149.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KS, McIntire CR, LeBlanc PM, Meunier C, Turbide C, Gros P, Beauchemin N. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Viganò E, Diamond CE, Spreafico R, Balachander A, Sobota RM, Mortellaro A. Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nat Commun. 2015;6:8761. doi: 10.1038/ncomms9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shalini S, Dorstyn L, Dawar S, Kumar S. Old, new and emerging functions of caspases. Cell Death Differ. 2015;22:526–539. doi: 10.1038/cdd.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mcllwain DR, Berger T, Mak TW. Caspase Functions in Cell Death and Disease. Cold Spring Harb Perspect Biol. 2013;5:1–28. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McIntire CR, Yeretssian G, Saleh M. Inflammasomes in infection and inflammation. Apoptosis. 2009;14:522–535. doi: 10.1007/s10495-009-0312-3. [DOI] [PubMed] [Google Scholar]
- 45.Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, Herfarth HH, Jobin C, Ting JP. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brough D, Rothwell NJ. Caspase-1-dependent processing of pro-interleukin-1β is cytosolic and precedes cell death. J Cell Sci. 2007;120:772–781. doi: 10.1242/jcs.03377. [DOI] [PubMed] [Google Scholar]
- 48.Helen MB, Douglas RG. Vol. 10. 2017. Immunologic Repercussions of Cell Death; pp. 418–448. (Kell. & Firestein's Textbook of Rheum). [Google Scholar]
- 49.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russo HM. Active caspase-1 induces plasma membrane pores that precede pyroptotic lysis and are blocked by lanthanides. J Immunol. 2016;197:1353–1367. doi: 10.4049/jimmunol.1600699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruhl S, Broz P. The gasdermin-D pore: executor of pyroptotic cell death. Oncotarget. 2016;7:57481–57482. doi: 10.18632/oncotarget.11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 53.Elkon KB. 2013. Apoptosis, Necrosis, and Autophagy (Eighth Edition), Dubois' Lupus Erythematosus and Related Syndromes; pp. 115–126. [Google Scholar]
- 54.Vince JE, Silke J. The intersection of cell death and inflammasome activation. Cell Mol Life Sci. 2016;73:2349–2367. doi: 10.1007/s00018-016-2205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vincent C, Sánchez FM, Mazo AB, Castejón GL, Gomez AI, Verkhratsky A, Brough D, Pelegrín P. Apoptosis-associated speck-like protein containing CARD forms specks but does not activate caspase-1 in the absence of NLRP3 during macrophage swelling. J Immunol. 2015;194(3):1261–1273. doi: 10.4049/jimmunol.1301676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei Q, Mu K, Li T, Zhang Y, Yang Z, Jia X, Zhao W, Huai W, Guo P, Han L. Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression. Lab Invest. 2014;94(1):52. doi: 10.1038/labinvest.2013.126. [DOI] [PubMed] [Google Scholar]
- 57.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dunn JH, Ellis LZ, Fujita M. Inflammasomes as molecular mediators of inflammation and cancer: potential role in melanoma. Cancer Lett. 2012;314:24–33. doi: 10.1016/j.canlet.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Kolb R, Liu GH, Janowski AM, Sutterwala FS, Zhang W. Inflammasomes in cancer: a double-edged sword. Protein Cell. 2014;5:12–20. doi: 10.1007/s13238-013-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ippagunta SK, Malireddi RK, Shaw PJ, Neale GA, Vande Walle L, Green DR, Fukui Y, Lamkanfi M, Kanneganti TD. The inflammasome adaptor ASC regulates the function of adaptive immune cells by controlling Dock2-mediated Rac activation and actin polymerization. Nat Immunol. 2011;12:1010–1016. doi: 10.1038/ni.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 62.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Melvin K, Beichu G. Inflammasomes and Cancer: The Dynamic Role of the inflammasome in Tumor Development. Front Immunol. 2017;8:1–9. doi: 10.3389/fimmu.2017.01132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 65.Chen KW. The neutrophil NLRC4 inflammasome selectively promotes IL-1 maturation without pyroptosis during acute Salmonella challenge. Cell Rep. 2014;8:570–582. doi: 10.1016/j.celrep.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 66.Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265:130–142. doi: 10.1111/imr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu S, Ding S, Wang P, Wei Z, Pan W, Palm NW, Yang Y, Yu H, Li HB, Wang G. Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature. 2017;546:667–670. doi: 10.1038/nature22967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martel J, Lai HC, Ko YF, Young JD, Ojcius DM. Alternative functions for the multifarious inflammasome. Biom J. 2016;39:183–187. doi: 10.1016/j.bj.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmid-Burgk JL, Gaidt MM, Schmidt T, Ebert TS, Bartok E, Hornung V. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur J Immunol. 2015;45:2911–2917. doi: 10.1002/eji.201545523. [DOI] [PubMed] [Google Scholar]
- 71.Anthony K. Immunology: the inflammasome protects? Nat Rev Cancer. 2010;10:383. doi: 10.1038/nrc2862. [DOI] [PubMed] [Google Scholar]
- 72.Zaki MH, Lamkanfi M, Kanneganti TD. The Nlrp3 inflammasome: contributions to intestinal homeostasis. Trends Immunol. 2011;32:171–179. doi: 10.1016/j.it.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Micallef MJ, Tanimoto T, Kohno K, Ikeda M, Kurimoto M. Interleukin 18 induces the sequential activation of natural killer cells and cytotoxic T lymphocytes to protect syngeneic mice from transplantation with Meth A sarcoma. Cancer Res. 1997;57:4557–4563. [PubMed] [Google Scholar]
- 74.Osaki T, Hashimoto W, Gambotto A, Okamura H, Robbins PD, Kurimoto M, Lotze MT, Tahara H. Potent antitumor effects mediated by local expression of the mature form of the interferon-g inducing factor, interleukin-18 (IL-18) Gene Ther. 1999;6:808–815. doi: 10.1038/sj.gt.3300908. [DOI] [PubMed] [Google Scholar]
- 75.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-g production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 76.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1β converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 77.Cao E, Zang X, Ramagopal UA, Mukhopadhaya A, Fedorov A, Fedorov E, Zencheck WD, Lary JW, Cole JL, Deng H. T cell immunoglobulin mucin-3 crystal structure reveals a galectin-9-independent ligand binding surface. Immunity. 2007;26:311–321. doi: 10.1016/j.immuni.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 78.Hegardt P, Widegren B, Li L, Sjo¨gren B, Kjellman C, Sur I, Sjo¨gren HO. Nitric oxide synthase inhibitor and IL-18 enhance the antitumor immune response of rats carrying an intrahepatic colon carcinoma. Cancer Immunol Immunother. 2001;50:491–501. doi: 10.1007/s002620100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Erivan S, Ramos J, Ana Carolina M. Gasdermin: A new player to the inflammasome game. Biom J. 2017;40:313–316. doi: 10.1016/j.bj.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.GSDMD gasdermin D [Homo sapiens (human)] - Gene - NCBI. www.ncbi.nlm.nih.gov
- 81.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Vol. 526. 2015. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death; pp. 660–665. (Nature). [DOI] [PubMed] [Google Scholar]
- 82.Meunier E, Dick MS, Dreier RF, Schürmann N, Kenzelmann Broz D, Warming S, Roose-Girma M, Bumann D, Kayagaki N, Takeda K. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature. 2014;509:366–370. doi: 10.1038/nature13157. [DOI] [PubMed] [Google Scholar]
- 83.Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT. Caspase-11 cleaves gasdermin D for noncanonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 84.Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Müller DJ, Broz P, Hiller S. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35:1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jianjin WG, Feng S. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci. 2017;42:245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 86.Rathkey JK, Benson BL, Chirieleison SM, Yang J, Xiao TS, Dubyak GR, Huang AY, Abbott DW. Live-cell visualization of gasdermin D-driven pyroptotic cell death. J Biol Chem. 2017;292:14649–14685. doi: 10.1074/jbc.M117.797217. [DOI] [PMC free article] [PubMed] [Google Scholar]