ABSTRACT
The Golgi of mammalian cells is known to be a major microtubule-organizing site that requires microtubules for its organization and protein trafficking. However, the mechanisms underlying the microtubule organization of the Golgi remain obscure. We used immunoprecipitation coupled with mass spectrometry to identify a widely expressed isoform of the poorly characterized muscle protein myomegalin. This newly identified isoform, myomegalin variant 8 (MMG8), localized predominantly to cis-Golgi networks by interacting with AKAP450 (also known as AKAP9), and this interaction with AKAP450 was required for the stability of both proteins. Disrupting MMG8 expression affected endoplasmic reticulum (ER)-to-Golgi trafficking and caused Golgi fragmentation. Furthermore, MMG8 associated with γ-tubulin complexes and with the microtubule plus-end tracking protein EB1 (also known as MAPRE1), and was required for the Golgi localization of these two molecules. On the Golgi, γ-tubulin complexes mediated microtubule nucleation, whereas EB1 functioned in ER-to-Golgi trafficking. These results indicate that MMG8 participates in Golgi microtubule organization and thereby plays a crucial role in the organization and function of the Golgi.
KEY WORDS: Golgi, Microtubule, Myomegalin, Protein trafficking
INTRODUCTION
The Golgi is a membranous organelle that plays a pivotal role in protein post-translational modification, sorting and transport. The assembly, positioning and function of the Golgi require an intact microtubule cytoskeleton (Lippincott-Schwartz, 1998; Rios and Bornens, 2003; Sütterlin and Colanzi, 2010). In interphase animal cells, the Golgi exhibits a crescent-moon-shaped ribbon-like morphology in the perinuclear region that typically surrounds the centrosome. When cells divide, the Golgi undergoes fragmentation and then reassembles during the late stages of mitosis. During reassembly, microtubules derived from the Golgi and centrosomes enable the Golgi to form a continuous ribbon structure featuring Golgi ministacks positioned near the center of cells (Miller et al., 2009). In the secretory pathway used by cells, proteins are transported from the endoplasmic reticulum (ER) to the Golgi, where they are eventually sorted into post-Golgi carriers. The ER-to-Golgi transport of proteins is initiated by the packaging of cargo into COPII-coated vesicles at ER-exit sites, and it is followed by the formation of ER–Golgi cargo carriers that associate with dynein–dynactin to move along microtubules towards the Golgi (Presley et al., 1997; Scales et al., 1997; Watson et al., 2005).
The Golgi serves as a major microtubule-organizing center (Chabin-Brion et al., 2001; Efimov et al., 2007; Miller et al., 2009; Rivero et al., 2009). For example, almost half of all cellular microtubules originate from the Golgi in human retinal pigment epithelial RPE1 cells (Efimov et al., 2007). Moreover, microtubule nucleation at the Golgi does not require centrosomes, and it depends instead on γ-tubulin (Efimov et al., 2007), the principal microtubule nucleator in cells, which exists in the form of γ-tubulin complexes (γTuCs). The cis-Golgi proteins AKAP450 (also known as AKAP9, AKAP350, CG-NAP and hyperion) and GMAP210 (also known as TRIP11) have been proposed to be involved in γ-tubulin recruitment to the Golgi and thus in the Golgi-associated nucleation of microtubules (Ríos et al., 2004; Rivero et al., 2009; Vinogradova et al., 2012). Microtubules originating from the Golgi are required for Golgi ribbon assembly, directional trafficking and cell motility (Miller et al., 2009; Rivero et al., 2009).
The growing tips of microtubules accumulate a diverse group of proteins called plus-end tracking proteins (+TIPs) (Akhmanova and Steinmetz, 2008). To track microtubule plus-ends, almost all +TIPs must interact with the end-binding (EB) proteins, among which EB1 and EB3 (also known as MAPRE1 and MAPRE3, respectively) display similar tip-tracking properties, whereas EB2 (also known as MAPRE2) appears to be distinct from the other two proteins and, relative to them, exhibits considerably weaker tip-tracking activity (Komarova et al., 2009). In one class of +TIPs, an SxIP motif surrounded by basic and serine-rich sequences is present that is required for the interaction of these +TIPs with the end-binding homology (EBH) domain of EB1 (Honnappa et al., 2009). EB1, the prototypic member of the EB family, is detected at all growing microtubule tips, and the +TIPs that are localized in association with EB1 at the microtubule plus-ends perform diverse functions, including regulating microtubule dynamics and microtubule attachment to subcellular targets (Akhmanova and Steinmetz, 2008). Although microtubules associate with the Golgi, whether EB1 and other EB proteins localize and function at the Golgi remains unknown.
In a yeast two-hybrid screen, myomegalin (myomegalin isoform 1, MMG1; GenBank accession NP_055459) was cloned as a protein that interacted with cyclic nucleotide phosphodiesterase 4D and thus was referred to as a phosphodiesterase 4D-interacting protein (Verde et al., 2001). The mmg1 sequence encodes a ∼230-kDa protein that is expressed in heart and skeletal muscles. In GenBank databases, MMG1 is the only protein present that is a homolog of CDK5RAP2, a human microcephaly-related protein that is involved in microtubule organization on centrosomes and in microtubule regulation at growing microtubule tips (Choi et al., 2010; Fong et al., 2008; Fong et al., 2009). Here, we report that a newly identified nonmuscle MMG isoform, MMG8, functions in Golgi microtubule organization and ER-to-Golgi trafficking. Our results reveal that MMG8 is a widely expressed protein that targets to the cis side of the Golgi by interacting with AKAP450. On the Golgi, MMG8 is involved in recruiting γTuCs to promote microtubule nucleation and also in tethering EB1 to enable microtubule-tip capture and efficient ER-to-Golgi trafficking. Therefore, MMG8 plays a key role in Golgi microtubule organization, which is required for efficient ER-to-Golgi trafficking and Golgi organization.
RESULTS
Identification of MMG8
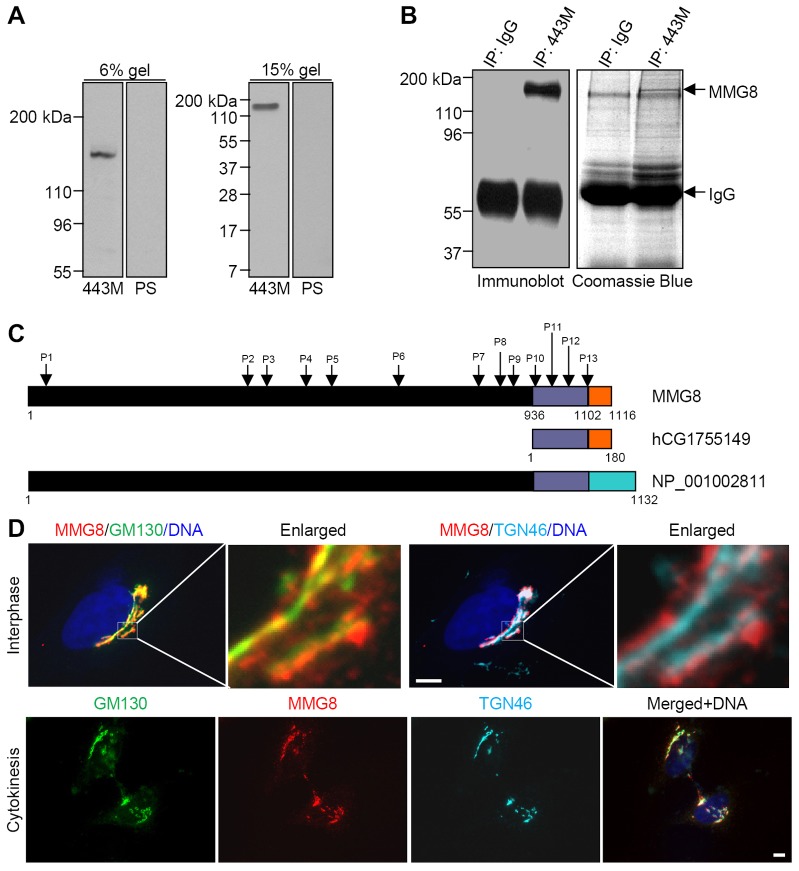
To detect MMG proteins in proliferating cell cultures, RT-PCR was performed using oligonucleotide primers targeting the human mmg1 sequence encoding amino acids 474–762, a region present in other large MMG variants found in gene databases. This MMG sequence was specifically amplified from the total RNA extracted from HeLa cells (supplementary material Fig. S1A), and the amplified product was verified through sequencing. To generate an antibody against this sequence, the RT-PCR product was cloned for expression in bacteria, and the recombinant protein purified from bacteria was used for immunizing rabbits. The resulting antibody, designated as 443M, detected a single band of ∼150 kDa in HeLa extracts. This is substantially smaller than the expected size of MMG1 (Verde et al., 2001) (Fig. 1A). To identify this protein band, we immunoprecipitated the protein from HeLa cells and excised the band from gels to perform mass-spectrometric analysis (Fig. 1B). Tandem mass spectrometry revealed a total of 13 peptide sequences (supplementary material Fig. S1B). Most of the peptide sequences matched the sequences from MMG variant 5 (GenBank accession NP_001002811), and the last four peptides matched sequences encoded by the cDNA sequence hCG1755149 (Fig. 1C). Specifically, peptide 13 extended from the last 9 residues shared by NP_001002811 and hCG1755149 to the sequence unique to hCG1755149.
Fig. 1.
MMG8 is a novel cis-Golgi protein. (A) HeLa extracts resolved using SDS-PAGE (6% and 15% gels) were probed with an anti-MMG8 antibody (443M) or the preimmune serum (PS). (B) HeLa extracts prepared in RIPA buffer were used for immunoprecipitation (IP). The immunoprecipitates were resolved using SDS-PAGE and immunoblotted with anti-MMG8 or stained with Coomassie Blue. The protein of ∼150 kDa was excised for analysis through mass spectrometry. (C) A schematic representation of the MMG variants. Identical sequence regions are shown in the same color. P1–P13 denote peptides analyzed using mass spectrometry. (D) Representative micrographs (100% of 100 cells) of HeLa cells stained for MMG8 and Golgi proteins. Boxed areas are enlarged on the right. Scale bars: 5 µm.
Based on the protein sequencing data, we cloned a novel MMG isoform, designated as MMG8; we have deposited the sequence of this protein in GenBank under the accession number HQ333476. The coding sequence of MMG8 consists of exons 11–26 and the alternatively spliced exon 27 of the human mmg. Because of the alternative splicing of exon 27, MMG8 possesses a unique carboxy terminus among the MMG isoforms. MMG8 encodes a protein of 1116 amino acids that contains the region 637–925 corresponding to the RT-PCR product, and homologs of MMG8 were detected in Pongo abelii (GenBank accession NP_001126198) and Mus musculus (GenBank accession NP_835181) that exhibited overall sequence similarities of 98% and 92%, respectively. MMG8 homologs were also found in Gallus gallus, Xenopus tropicalis and Danio rerio. The structural features of MMG8 that were predicted based on sequence analysis include coiled-coil domains and an EB1-binding SxIP motif (supplementary material Fig. S1C). A second antibody, 532C, was generated against the carboxy terminus of MMG8. The 443M and 532C antibodies yielded identical results; unless specified otherwise, the results shown below were obtained using 532C.
MMG8 was detected as a single protein band in immunoblots of extracts of proliferating epithelial cells, fibroblasts and neuroblastoma cells (supplementary material Fig. S1D). In C2C12 myotubes, two lower protein bands were detected in addition to the ∼150-kDa species (supplementary material Fig. S1D), suggesting the existence of smaller isoforms or proteolytic products. However, MMG1 was not detected in any of these cell cultures (supplementary material Fig. S1D), although it was recognized by the 443M antibody in rat heart tissue extracts. To determine the subcellular localization of MMG8, we immunostained HeLa cells with the MMG8-specific antibody. MMG8 staining was highly enriched in the Golgi, and a weak general staining was detected in the cytoplasm (Fig. 1D). Moreover, the distribution of the MMG8 signal on the Golgi was similar to that of the cis-Golgi protein GM130 (also known as GOLGA2), but it did not merge with the staining of the trans-Golgi protein TGN46 (also known as TGOLN2) (Fig. 1D), suggesting that MMG8 resides on the cis side of the Golgi. During cytokinesis, MMG8 appeared in both Golgi twins (Fig. 1D).
MMG8 functions in Golgi organization and ER-to-Golgi trafficking
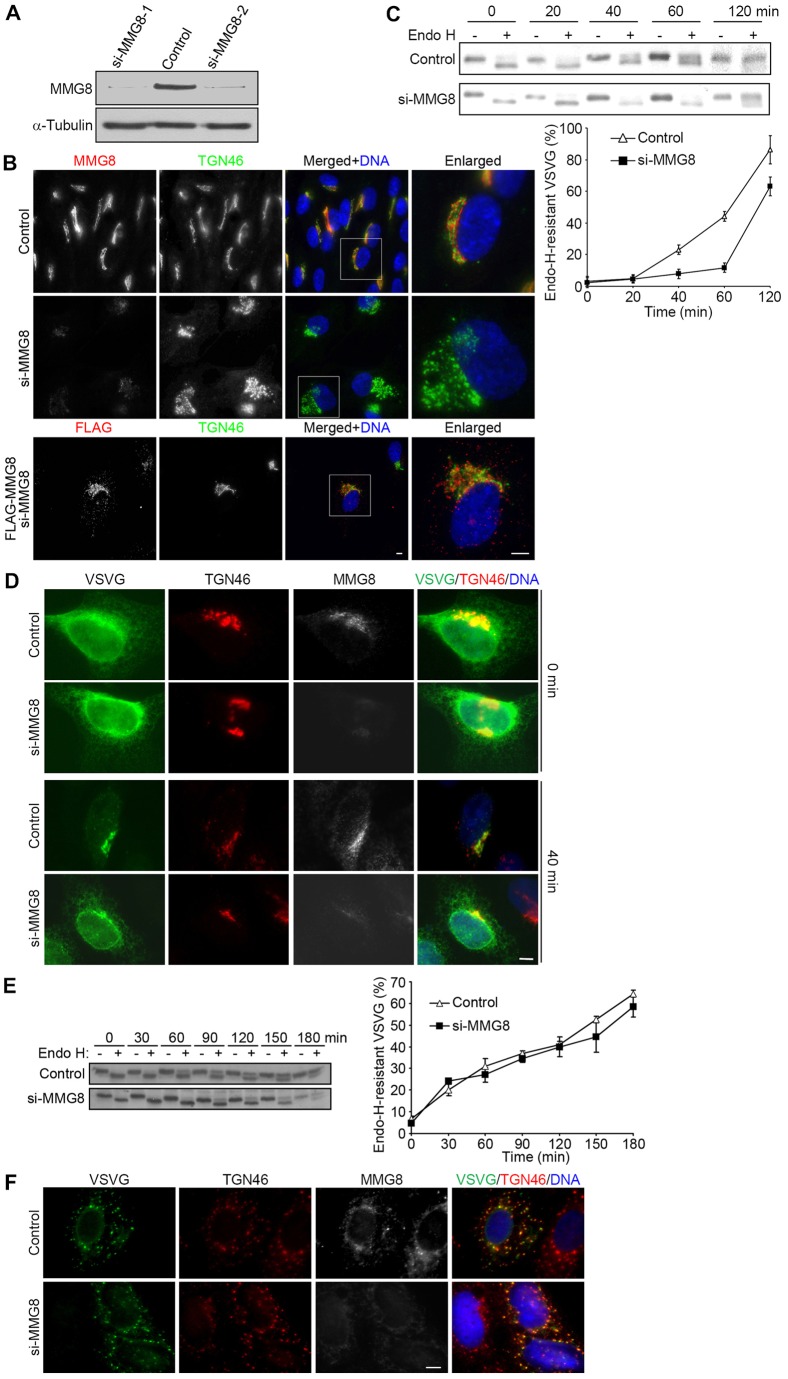
To investigate the function of MMG8, we used RNA interference (RNAi) to suppress MMG8 expression. Two small interfering (si)RNA oligonucleotides were designed to target mmg8, and the transfection of either siRNA into cells effectively depleted the protein (∼85% reduction, Fig. 2A). Cells transfected with mmg8-targeting siRNAs exhibited a weak background in which Golgi patterns were not detected when cells were labeled with the anti-MMG8 antibody (Fig. 2B), which confirmed the specificity of anti-MMG8 staining. In these cells, the Golgi ribbons were broken into patches that overlapped largely with nuclei (Fig. 2B). This Golgi fragmentation could be rescued by the ectopic expression of MMG8 at low levels (Fig. 2B). We also determined that the overexpression of MMG8 affected Golgi structures; when transiently expressed at low levels, MMG8 exhibited a Golgi pattern of distribution, whereas it caused Golgi fragmentation and cytotoxicity even when expressed at moderately high levels (supplementary material Fig. S2). Taken together, these results show that altering MMG8 expression perturbs Golgi organization, and thus they indicate a crucial role for MMG8 in maintaining the structural integrity of the Golgi.
Fig. 2.
MMG8 is required for Golgi structural organization and efficient ER-to-Golgi transport. (A) Immunoblotting of HeLa cells transfected with control or mmg8-targeting siRNAs. The transfection of MMG8 siRNAs (si-MMG8-1 and si-MMG8-2) suppressed the expression of the protein by ∼85%. (B) RPE1 cells transfected with control or mmg8-targeting siRNA were analyzed using immunofluorescence microscopy. The knockdown of MMG8 was rescued by the transfection of an siRNA-resistant construct (FLAG–MMG8). The boxed regions are enlarged on the right. The figure shows micrographs that are representative of ∼1000 cells (n = 3). (C) HeLa cells were transfected with VSVG–YFP. The cells were extracted at various times during incubation at 32°C and were assayed for endo-H resistance. Following the assay, the samples were immunoblotted to check for VSVG. The amounts of the endo-H-resistant form of VSVG relative to the total amounts of VSVG were determined, and quantitative data from three independent experiments are presented here as the mean±s.d. (D) Cells were fixed at 0 or 40 min of 32°C incubation and then immunostained. At least 90% of the 200 cells analyzed for each condition exhibited the phenotypes shown. (E,F) Cells expressing VSVG–YFP were treated with 10 µg/ml nocodazole at 40°C for 6 h and then shifted to 32°C and incubated further. The figure shows the results of the endo-H-resistance assay (E) of cells collected at various times (from three independent experiments; data show the mean±s.d.) and representative images of cells (>90% of 100 cells) that were fixed after incubation for 15 min at 32°C (F). Scale bars: 5 µm.
Next, to test whether MMG8 functions in ER-to-Golgi trafficking, we used the well-characterized cargo VSVG (vesicular stomatitis virus ts045 glycoprotein). At 40°C, VSVG is reversibly misfolded and thus accumulates in the ER; however, shifting cells to 32°C allows the protein to fold correctly, which results in its export from the ER and subsequent transport along microtubules to the Golgi (Presley et al., 1997; Scales et al., 1997). When delivered to the medial Golgi, oligosaccharides linked to VSVG cannot be cleaved by endoglycosidase H (endo-H). In control cells, a substantial amount of VSVG became endo-H resistant after 40 min at 32°C, and approximately half of the total VSVG became resistant after 60 min (Fig. 2C). Disrupting MMG8 expression did not affect the expression and ER accumulation of VSVG at 40°C (Fig. 2C,D). However, MMG8 depletion markedly delayed the acquisition of endo-H resistance (Fig. 2C), which indicates a role of MMG8 in the delivery of VSVG to the Golgi.
To corroborate these findings, we examined the distribution pattern of VSVG. The protein expressed at 40°C was effectively retained in the ER (Fig. 2D). By 40 min after shifting to 32°C, VSVG was predominantly localized at the Golgi in control cells (Fig. 2D). By contrast, VSVG displayed a mixed distribution in MMG8-depleted cells, with a small amount being present at the Golgi and a large amount of the protein being dispersed in the cytoplasm in the form of puncta and ER-like networks (Fig. 2D). Nocodazole-induced microtubule depolymerization fragments the Golgi into functional stacks, which localize at ER-exit sites (Cole et al., 1996). When the trafficking assay was performed using nocodazole-treated cells, the ER-to-Golgi transport of VSVG was unaffected by the silencing of MMG8 expression (Fig. 2E,F). Collectively, these results indicate that MMG8 is involved in the ER-to-Golgi trafficking that occurs in a microtubule-dependent manner.
Association of MMG8 with AKAP450
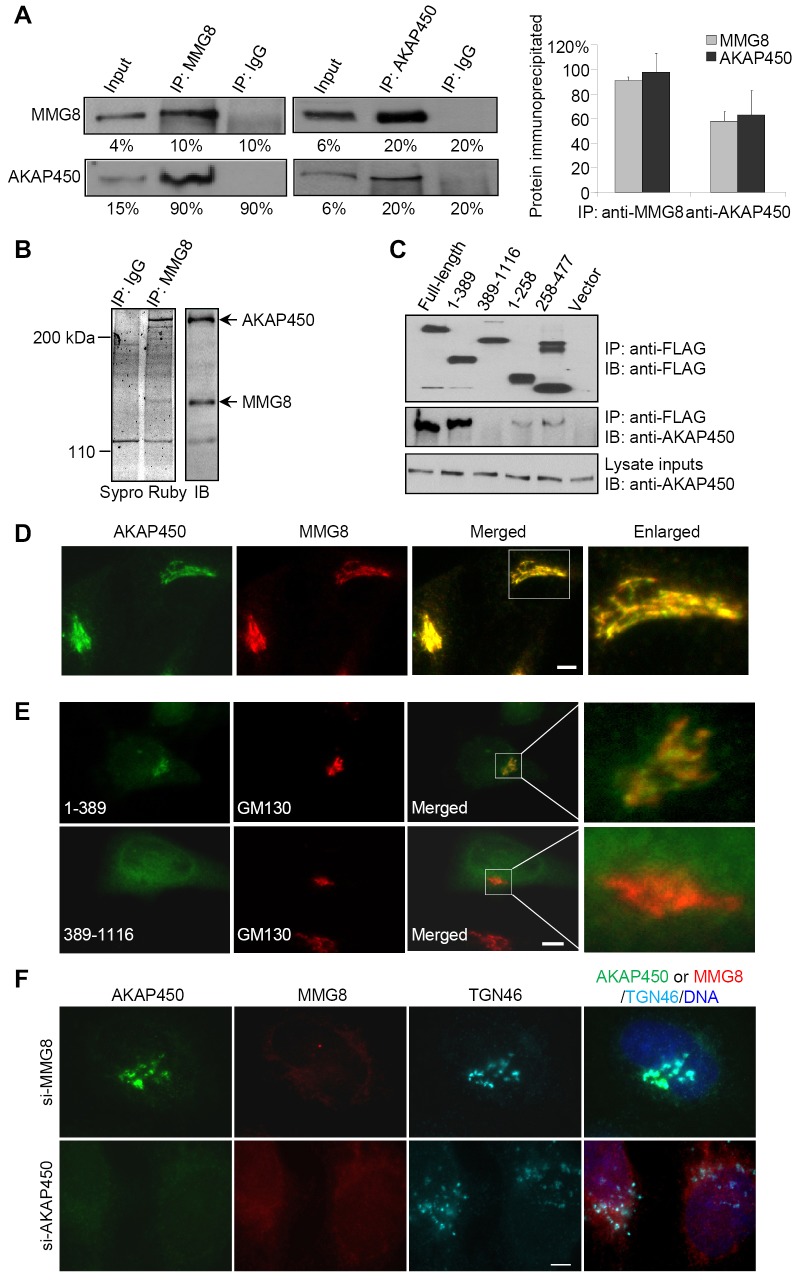
To gain mechanistic insights into MMG8 function, we sought to identify proteins that bind to MMG8. In large-scale immunoprecipitates of MMG8, both the regulatory subunit of protein kinase A (PKA) and EB1 were identified through mass spectrometry. Because AKAP450 is a cis-Golgi protein that anchors PKA (Keryer et al., 1993; Rivero et al., 2009; Schmidt et al., 1999; Takahashi et al., 1999; Witczak et al., 1999), we tested the potential association of MMG8 with AKAP450. Reciprocal immunoprecipitation experiments showed that MMG8 and AKAP450 were coimmunoprecipitated specifically and proportionally (Fig. 3A); quantification of the coimmunoprecipitated AKAP450 and MMG8 in SDS-PAGE gels stained with a fluorescent dye (Fig. 3B) showed that the molar ratio of AKAP450 to MMG8 was 0.98±0.1∶1. Therefore, MMG8 and AKAP450 formed a stoichiometric complex in the cell extracts.
Fig. 3.
MMG8 targets to the Golgi by interacting with AKAP450. (A) Anti-MMG8 and anti-AKAP450 immunoprecipitations (IPs) were performed using HeLa extracts. The immunoprecipitated proteins and inputs were analyzed by immunoblotting and quantifying the samples. The graph shows the amount of the precipitated proteins relative to that of the respective inputs. Data show the mean±s.d. (three independent experiments). (B) Proteins coimmunoprecipitated with MMG8 were stained with Sypro Ruby or detected on immunoblots (IBs). The Sypro Ruby intensities of MMG8 and AKAP450 were quantified. Data from three independent experiments showed that the molar ratio of AKAP450 to MMG8 was 0.98±0.1∶1. (C) HEK293T cells expressing constructs encoding FLAG–MMG8 were subjected to anti-FLAG immunoprecipitation. The immunoprecipitates and inputs were immunoblotted for the MMG8 proteins (anti-FLAG) and AKAP450. (D) RPE1 cells were double-stained for AKAP450 and MMG8. The boxed area is enlarged on the right. (E) MMG8 fragments were ectopically expressed (GFP) in HeLa cells. The cells were examined for the transfected proteins and a Golgi marker. The boxed areas are enlarged on the right. (F) HeLa cells in which MMG8 or AKAP450 was depleted using RNAi were treated with MG132 for 12 h. The cells were then immunostained. The phenotypes shown in E,F were observed in >90% of 100 cells analyzed for each sample. Scale bars: 5 µm.
To map the AKAP450-binding site, various MMG8 fragments were ectopically expressed and their coimmunoprecipitation with AKAP450 was tested. The head region of MMG8 comprising amino acids 1–389 was sufficient for binding to AKAP450, whereas the MMG8 fragment comprising amino acids 389–1116 did not bind to AKAP450 (Fig. 3C); moreover, the deletion of amino acids 1–389 strongly impaired the AKAP450-binding activity of MMG8 (Fig. 3C). Next, we performed double-immunostaining of MMG8 and AKAP450 and verified their prominent colocalization at the Golgi (Fig. 3D). Even after the Golgi was dispersed by treating cells with brefeldin-A or nocodazole, MMG8 colocalized with AKAP450 on Golgi fragments (supplementary material Fig. S3). We also examined the subcellular distribution of the AKAP450-binding and binding-deficient fragments of MMG8. When expressed at low levels, the fragment 1–389 localized specifically at the Golgi (Fig. 3E); by contrast, the expressed 389–1116 fragment did not display any specific distribution pattern (Fig. 3E). These results show that the 1–389 sequence serves as the AKAP450-binding and Golgi-targeting domain of MMG8.
To test whether MMG8 and AKAP450 require each other for Golgi localization, we used RNAi to deplete the proteins one at a time and then checked the Golgi localization of the other protein. Cells depleted of MMG8 or AKAP450 were treated with the proteasome inhibitor MG132 to prevent the degradation of the nontargeted protein (see Fig. 4). MMG8 depletion did not markedly affect the Golgi localization of AKAP450, whereas AKAP450 depletion inhibited MMG8 localization to the Golgi (Fig. 3F). Collectively, these results indicate that MMG8 is targeted to the Golgi through its binding to AKAP450 and that AKAP450 localizes to the Golgi through MMG8-independent mechanisms.
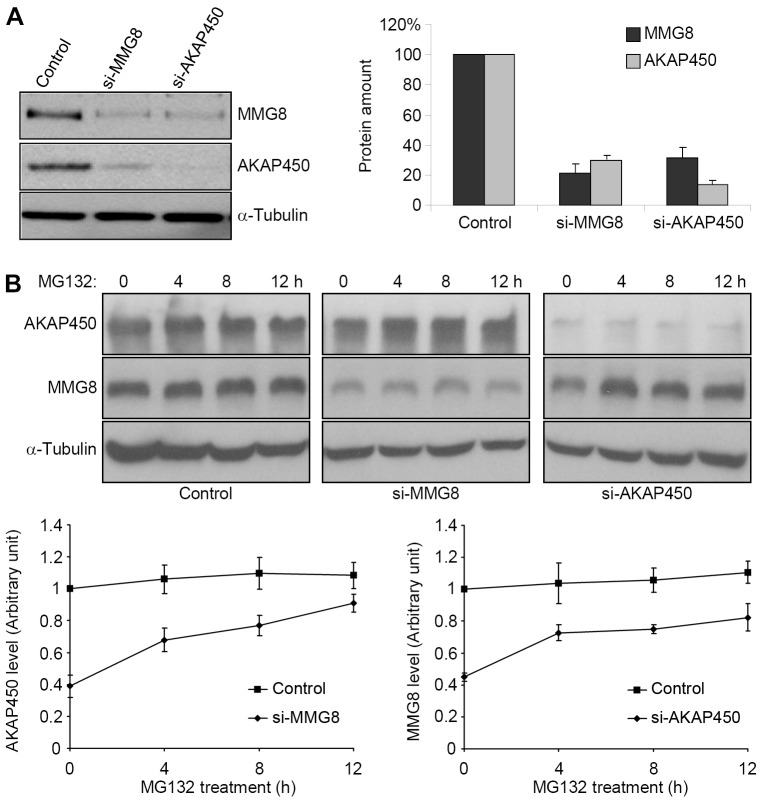
Fig. 4.
MMG8 and AKAP450 are mutually dependent for stability. (A) Extracts of RPE1 cells transfected with the indicated siRNAs were immunoblotted. After quantification, the amounts of MMG8 and AKAP450 in the extracts of si-MMG8- and si-AKAP450-transfected cells were expressed as percentages of the respective amounts in the control extracts. Data from three independent experiments are shown as the mean±s.d. (B) At 72 h after transfection with siRNAs, cells were treated with MG132, after which cell extracts were prepared and immunoblotted. The amounts of MMG8 and AKAP450 are plotted as the mean±s.d. using the data quantified from three independent experiments.
In the RNAi experiments performed on MMG8 and AKAP450, we used immunoblotting to probe the levels of both proteins. Notably, suppressing MMG8 expression led to a proportional reduction in the protein level of AKAP450 and vice versa (Fig. 4A), revealing that the expression of each protein depends on the expression of the other. MG132 was applied next to test whether the proteins were degraded through a proteasome-dependent pathway. In cells depleted of either MMG8 or AKAP450, the level of the other protein increased over the timecourse of MG132 treatment and eventually approached the level in control cells (Fig. 4B). In control-transfected cells, the levels of MMG8 and AKAP450 were not markedly altered following MG132 treatment (Fig. 4B). Thus, in the absence of one of the proteins, the other protein becomes unstable and is degraded by the proteasome.
MMG8 functions in Golgi-based microtubule nucleation
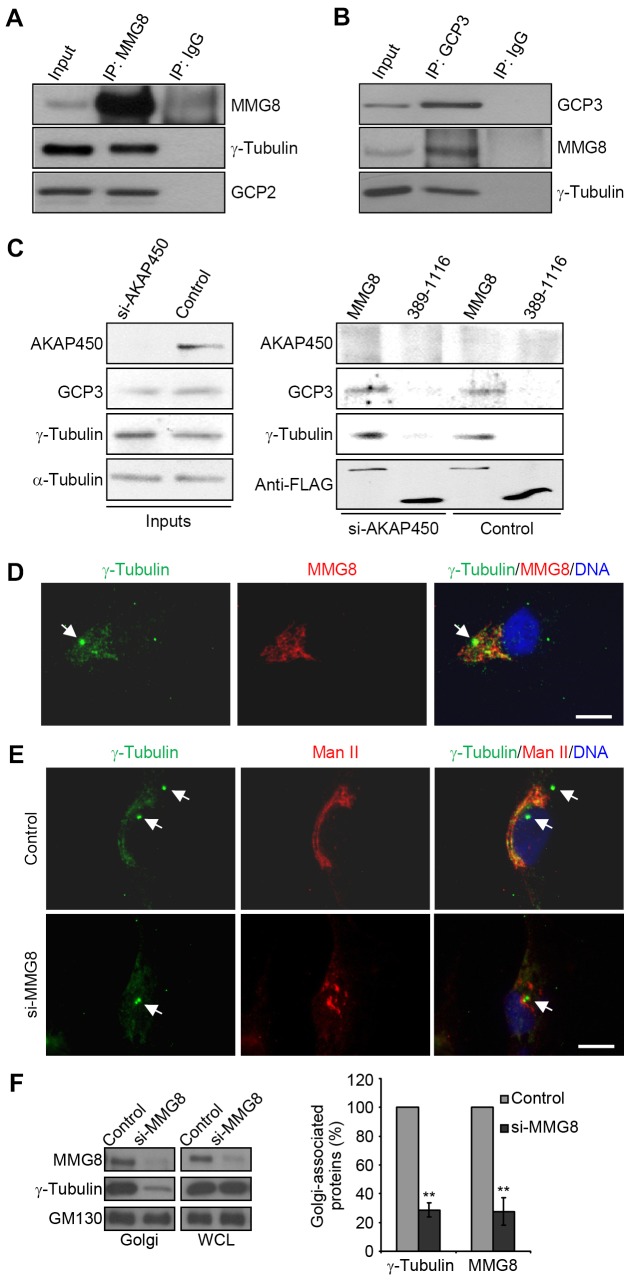
Because AKAP450 is required for microtubule nucleation at the Golgi (Rivero et al., 2009), we explored the potential involvement of MMG8 in microtubule nucleation. Immunoprecipitation of MMG8 coprecipitated γ-tubulin and GCP2, which are γTuC core components that we examined (Fig. 5A). In the reciprocal experiment, MMG8 was specifically coprecipitated with GCP3, another core component of γTuCs (Fig. 5B). To test whether MMG8 can bind to γTuCs in the absence of AKAP450, MMG8 was ectopically expressed in cells and immunoprecipitated in RIPA buffer, in which MMG8 was dissociated from AKAP450 and γTuCs (supplementary material Fig. S4). The immunoprecipitated MMG8 was used in a pull-down assay performed with extracts of control and AKAP450-depleted cells. In both extracts, MMG8 showed similar binding activity towards γTuCs (Fig. 5C), whereas the control MMG8 fragment, 389–1116, did not show any γTuC-binding activity (Fig. 5C). Therefore, we conclude that MMG8 can associate with γTuCs independently of AKAP450 and that the 1–389 sequence of MMG8 is indispensable for its binding to γTuCs.
Fig. 5.
MMG8 associates with γTuCs and is required for γTuC attachment to the Golgi. (A,B) Anti-MMG8 (A) and anti-GCP3 (B) immunoprecipitations (IPs) were performed using HeLa extracts. The immunoprecipitates and inputs were immunoblotted. (C) FLAG–MMG8 and FLAG–MMG8 (389–1116) transiently expressed in HEK293T cells were immunoprecipitated in RIPA buffer. The beads were then used in a pull-down assay together with RPE1 lysates of cells that were transfected with control or AKAP450 siRNA. The pull-downs were examined on immunoblots. (D) RPE1 cells were double-stained for γ-tubulin and MMG8. (E) Cells transfected with siRNAs were immunostained for γ-tubulin and mannosidase II (Man II). Arrows indicate centrosomes. Images shown in D,E are representative (>90%) of 200 cells analyzed for each sample. Scale bars: 5 µm. (F) Golgi membranes were isolated from siRNA-transfected cells. Both the Golgi fractions and the whole cell lysates (WCLs) were analyzed on the immunoblots. The graph shows the protein quantification of the isolated Golgi membranes as the mean±s.d. from three independent experiments; **P <0.01.
To examine whether the Golgi localization of γTuCs requires MMG8, we used RNAi to silence MMG8 expression. To date, the staining of endogenous γ-tubulin at the Golgi has only been observed using an antibody that recognizes multiple epitopes of γ-tubulin (Ríos et al., 2004). We considered the possibility that γ-tubulin is present at the Golgi at such low levels that it cannot be readily detected above background cytosolic staining. Therefore, we tackled this problem by extracting the cytosol before fixation and also by enhancing the staining signal by using tandem secondary antibodies. In cells extracted with a saponin-containing buffer, labeling for MMG8 and GM130 showed that the Golgi was largely intact. γ-tubulin was readily observed at the Golgi in addition to being detected at the centrosomes, and γ-tubulin colocalized with MMG8 at the Golgi (Fig. 5D). MMG8 did not display prominent centrosomal localization in a large population of cells, and RNAi-mediated depletion of MMG8 eliminated the Golgi attachment of γ-tubulin without discernibly affecting the centrosomal staining of γ-tubulin (Fig. 5E). Moreover, the detachment of γ-tubulin from the Golgi membranes was correlated with the removal of Golgi-associated MMG8 (Fig. 5F). These results indicate that MMG8 is required for the Golgi localization of γTuCs but is dispensable for the centrosomal attachment of γTuCs.
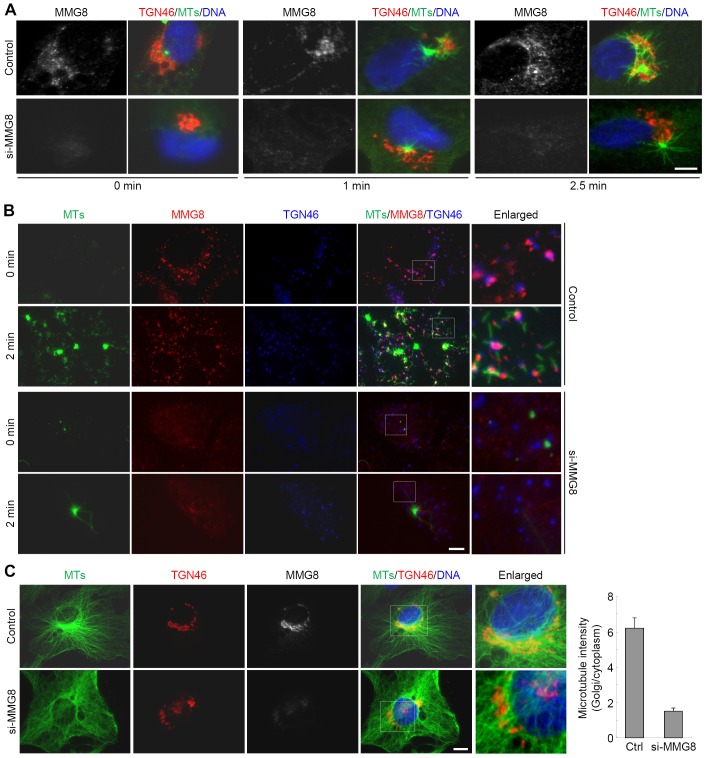
To investigate whether MMG8 functions in microtubule nucleation, microtubule regrowth assays were performed in RPE1 cells after cold-induced microtubule depolymerization. Before the assays, the cells were transfected with either a control or an mmg8-targeting siRNA. In cold-treated control cells, short microtubules appeared at the Golgi and as a centrosomal aster after the cells were rewarmed for 1 min (Fig. 6A). At 2.5 min of rewarming, prominent microtubule filaments were observed to emanate from the Golgi and also from the centrosomes (Fig. 6A). By contrast, the MMG8-depleted cells did not exhibit microtubule growth from the Golgi even after 2.5 min of rewarming; the centrosomal regrowth was not affected by MMG8 depletion (Fig. 6A). Thus, MMG8 depletion inhibits microtubule nucleation at the Golgi but does not affect centrosomal nucleation. Similar results were obtained using nocodazole-treated RPE1 cells, which showed that Golgi ministacks were dispersed in the cytoplasm. Following nocodazole washout, microtubules grew from the ministacks that were co-stained for MMG8 in the control cells; however, the microtubules failed to grow from the ministacks in the MMG8-depleted cells (Fig. 6B).
Fig. 6.
MMG8 is required for microtubule nucleation at the Golgi. (A) RPE1 cells were transfected with the indicated siRNAs. After cold-induced depolymerization, microtubule regrowth was initiated by warming the cells to 37°C. The cells were then stained for MMG8, TGN46 and microtubules (anti-α-tubulin). MTs, microtubules. (B) Microtubule regrowth was performed after nocodazole-induced depolymerization. In A,B, the images shown represent >90% of 100–200 cells analyzed for each condition. (C) Immunofluorescence micrographs of cells transfected with siRNAs. The boxed areas are enlarged on the right. The fluorescence intensities of microtubules were measured at the Golgi area and in the cytoplasm. After subtracting the background, the ratios of Golgi∶cytoplasm were derived. Data show the mean±s.d. (n = 30 cells for each quantification). Ctrl, control. Scale bars: 5 µm.
In RPE1 cells, microtubules emanated from the Golgi and centrosomes and were distributed in a radial pattern, and the Golgi region harbored a high density of microtubules (Fig. 6C). Suppressing MMG8 expression substantially reduced the microtubule density at the Golgi (Fig. 6C) and the microtubules did not radiate from the Golgi region (Fig. 6C). These data further support the conclusion that MMG8 is required for microtubule growth and organization at the Golgi.
Association of MMG8 with EB1 and EB3
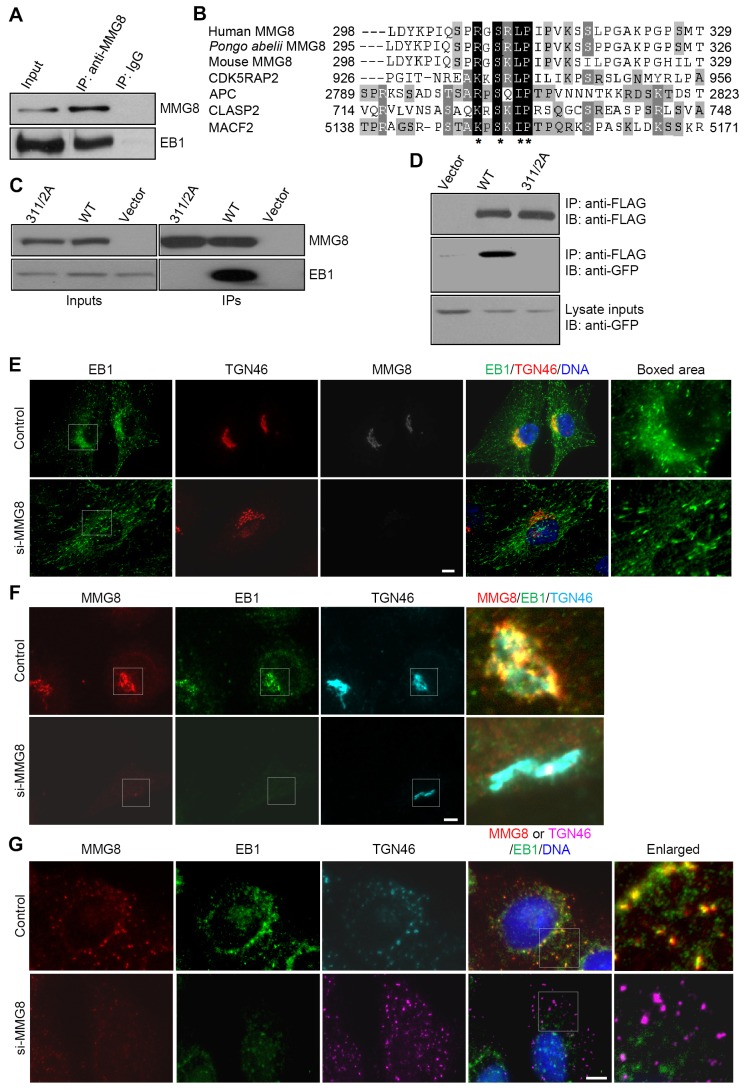
Mass spectrometry revealed that the immunoprecipitates of MMG8 contained EB1 in addition to PKA. The coimmunoprecipitation of EB1 with MMG8 was verified by means of anti-EB1 immunoblotting (Fig. 7A). MMG8 contains a putative SxIP motif within its 298–329 sequence region, and this motif is conserved in the Pongo abelii and Mus musculus counterparts of MMG8 (Fig. 7B). We constructed an MMG8 mutant in which two crucial residues of the SxIP motif, Leu311 and Pro312, were changed to alanines. In striking contrast to wild-type MMG8, the L311A/P312A mutant exhibited no binding activity towards EB1 and EB3 (Fig. 7C,D). Notably, these substitutions did not affect the binding of MMG8 to AKAP450.
Fig. 7.
MMG8 binds to EB1 and mediates its recruitment to the Golgi. (A) Immunoprecipitation of MMG8 was performed and the immunoprecipitates (IPs) and inputs were immunoblotted with the indicated antibodies. (B) MMG8 contains the SxIP (x denotes any amino acid residue) motif that is required for interaction with EB1; residues highlighted in black are crucial for EB1 binding (asterisks). Pongo abelii MMG8, NP_001126198; mouse MMG8, NP_835181. (C) MMG8 and its L311A/P312A mutant (311/2A) were ectopically expressed. After immunoprecipitating the proteins by targeting the tag (anti-FLAG IP), the immunoprecipitates and inputs were immunoblotted. Vector, FLAG vector; WT, wild-type MMG8. (D) HEK293T cells were doubly transfected with GFP–EB3 and the MMG8 constructs. The immunoprecipitates and inputs were immunoblotted (IB) for EB3 (anti-GFP) and MMG8 (anti-FLAG). (E,F) HeLa cells transfected with the indicated siRNAs were triple-stained for MMG8, EB1 and TGN46. The cells were either left untreated (E) or were extracted with a saponin-containing buffer (F) before fixation. The boxed areas are enlarged on the right. (G) Cells transfected with the indicated siRNAs were treated with nocodazole to depolymerize microtubules, after which the cells were immunostained. The boxed areas are enlarged on the right. The images shown for all immunofluorescence experiments represent >90% of ∼500 cells analyzed for each condition. Scale bars: 5 µm.
In RPE1 cells, EB1 was immunostained throughout the Golgi, in addition to being detected as microtubule tip-tracking speckles (Fig. 7E). Inhibiting MMG8 expression eliminated the Golgi-associated pattern of EB1, but it did not markedly affect the tip-tracking behavior of EB1 (Fig. 7E). To visualize the Golgi localization of EB1 clearly, cells were extracted with a buffer containing saponin to remove cytosolic proteins but preserve Golgi networks. In the extracted cells, EB1 clearly localized at the Golgi in an MMG8-dependent manner (Fig. 7F). Similar results were obtained using cells treated with nocodazole, which induced Golgi fragmentation (Fig. 7G).
Golgi-associated EB1 functions in ER-to-Golgi trafficking
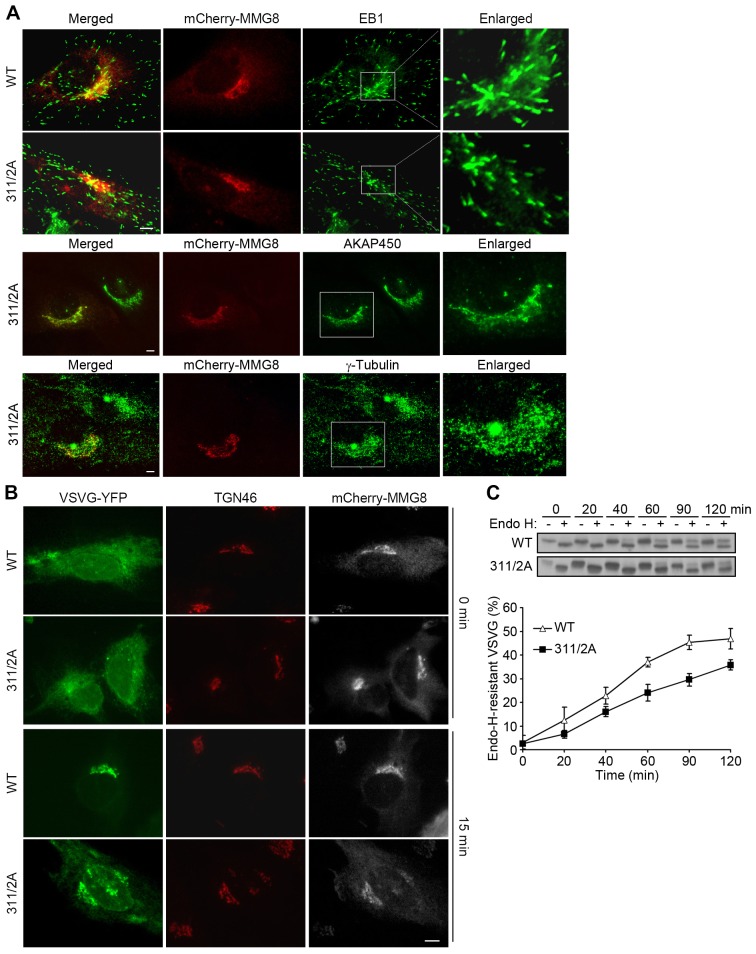
EB1 might depend on its interaction with MMG8 for attachment to the Golgi. To test this possibility, we expressed the EB1-binding-deficient mutant version of MMG8 (L311A/P312A) and the wild-type protein in cells. The cells that expressed the proteins at moderate levels and did not exhibit obvious changes in Golgi morphology were selected for analysis. Both the wild-type and the mutant protein showed prominent localization at the Golgi, and the transfections did not markedly affect the microtubule tip-tracking of EB1 (Fig. 8A). The Golgi localization of EB1 was substantially diminished or even eliminated in cells transfected with the mutant protein, as compared with the EB1 localization in the wild-type MMG8-transfected cells (Fig. 8A). By contrast, the Golgi localization of AKAP450 and γ-tubulin was not markedly affected by the expression of the mutant (Fig. 8A). Therefore, the expression of the MMG8 mutant detached EB1 specifically from the Golgi. To test the function of Golgi-associated EB1, we performed the VSVG trafficking assays using cells that were transfected with the MMG8 constructs. At 15 min after shifting to 32°C, the majority of VSVG was localized to the Golgi in cells expressing wild-type MMG8, but VSVG was still dispersed in the cytoplasm of cells expressing the EB1-binding-deficient mutant (Fig. 8B). Consequently, VSVG acquired endo-H resistance more slowly in cells transfected with the EB1-binding-deficient mutant than in cells transfected with wild-type MMG8 (Fig. 8C). These results indicate that the removal of EB1 from the Golgi impairs the ER-to-Golgi transport of VSVG.
Fig. 8.
Golgi-associated EB1 is required for efficient ER-to-Golgi trafficking. (A) RPE1 cells transfected with MMG8 and its mutant were immunostained. WT, wild-type MMG8; 311/2A, MMG8 (L311A/P312A). The boxed regions are enlarged on the right. Every analyzed cell (n = 100) showed the presented phenotypes. (B,C) VSVG–YFP was coexpressed with the MMG8 constructs to evaluate protein trafficking. The representative images shown in B are of cells (>80% of 100 cells) at 0 or 15 min after transfer to 32°C, as indicated. Scale bars: 5 µm. (C) Endo-H resistance of VSVG was quantified using data from three separate experiments and is shown as the mean±s.d.
DISCUSSION
In mammalian cells, the assembly of cisternal stacks into an integrated Golgi and the subcellular positioning of the Golgi depend on Golgi-associated microtubules (Ríos et al., 2004; Rivero et al., 2009; Vinogradova et al., 2012). In this study, we identified and characterized a novel protein, MMG8, and demonstrated its crucial role in microtubule organization on the Golgi. MMG8 homologs were identified in chicken, frog and zebrafish in addition to those in mammals, suggesting that this protein is conserved in vertebrates. In the proliferating epithelial and fibroblast cultures examined, MMG8 was the only detected isoform encoded by the MMG gene, which is multiply spliced. MMG1, a protein that is homologous to CDK5RAP2 and is expressed in muscles (Verde et al., 2001), was not detected in epithelial cells and fibroblasts (supplementary material Fig. S1D). Like CDK5RAP2, MMG8 contains the SxIP motif that interacts with EB1 and EB3. However, MMG8 does not contain the conserved γTuC-binding domain and the centrosome-targeting domain that are present at the amino and carboxy terminus, respectively, of CDK5RAP2 (Fong et al., 2008; Wang et al., 2010); instead, MMG8 interacts with γTuCs and localizes to the Golgi through its amino-terminal region, which is not homologous to CDK5RAP2. Whereas CDK5RAP2 is involved in centrosomal organization and functions, MMG8 functions at the Golgi in microtubule organization and efficient ER-to-Golgi trafficking.
MMG8 is a cis-Golgi protein that localizes at the Golgi through its interaction with AKAP450. Supporting this view, MMG8 colocalized with AKAP450 at ER-exit sites after brefeldin-A treatment and at Golgi ministacks after nocodazole-induced microtubule depolymerization (supplementary material Fig. S3). Remarkably, MMG8 and AKAP450 were mostly present in the same subcellular complexes and their stability was mutually dependent. Therefore, these proteins likely form a functional complex when performing various functions. One such function involves mediating microtubule nucleation at cis-Golgi networks. AKAP450 is known to associate with γTuCs (Hurtado et al., 2011; Takahashi et al., 2002), whereas MMG8 can interact with γTuCs independently of AKAP450 (Fig. 5C). Taken together, this study and those of others (Hurtado et al., 2011; Rivero et al., 2009) indicate that the MMG8–AKAP450 complex anchors γTuCs to cis-Golgi networks, where they nucleate microtubules. One of the effects of eliminating AKAP450 and, thus, Golgi-derived microtubules is impaired directional cell migration (Rivero et al., 2009); in agreement with this finding, we observed defective migration of MMG8-depleted cells in a scratch-wounding assay. However, whether AKAP450 binds to γTuCs through MMG8 remains unclear. One potential scenario is that by functioning as a scaffold protein on the Golgi, AKAP450 recruits MMG8 and CDK5RAP2, together with their bound γTuCs. Another scenario is that both AKAP450 and MMG8 bind directly to γTuCs; such interactions of γTuCs within the complex could promote the Golgi attachment of the γTuCs. In contrast to its essential role in the Golgi attachment of γTuCs, MMG8 was dispensable for the centrosomal localization of γTuCs (Fig. 5E). Therefore, γTuCs are tethered to the Golgi through a mechanism that is distinct from that underlying their centrosomal localization.
We determined that EB1 localized to the Golgi in an MMG8-dependent manner. This is supported by the observation that the expression of the EB1-binding-deficient mutant of MMG8 delocalized EB1 from the Golgi (Fig. 8A). These observations suggest that MMG8 tethers EB1 and EB3 together with microtubule tips to Golgi membranes. Such a function is reminiscent of the binding of EB1 to STIM1, an SxIP-motif-containing protein that associates with ER membranes (Grigoriev et al., 2008). Therefore, our results agree with the notion that EB1 complexes formed with other +TIPs mediate the attachment of microtubule tips to subcellular targets.
Efficient ER-to-Golgi trafficking requires microtubules on which secretory cargos move towards the Golgi in association with the minus-end-directed motor dynein–dynactin (Presley et al., 1997; Scales et al., 1997; Watson et al., 2005). Our results showed that MMG8 is required for microtubule-dependent ER-to-Golgi trafficking (Fig. 2C,E). Interestingly, this function of MMG8 required its binding to EB1 or EB3 and thus the association of EB1 or EB3 with the Golgi (Fig. 8). We envision that the EB1/EB3-mediated attachment of microtubules to the Golgi plays a crucial role in ER-to-Golgi trafficking. The transport efficiency was decreased in response to MMG8 silencing because of the disruption of Golgi-nucleated microtubules, although a direct role of MMG8 in trafficking cannot be excluded. Collectively, these observations suggest that MMG8 participates in various Golgi-associated activities by regulating Golgi-associated microtubules.
MMG8 is involved in several activities that contribute to ensuring the structural integrity of the Golgi. First, MMG8 is required for recruiting γTuCs to, and thus enabling microtubule nucleation on, the Golgi. Second, MMG8 interacts with EB1 and EB3 and thereby attaches microtubule tips to the Golgi. During Golgi biogenesis, microtubules originating at the Golgi and centrosome are required for facilitating the assembly of Golgi ministacks into larger clusters and for gathering Golgi fragments for central positioning (Miller et al., 2009; Vinogradova et al., 2012). Third, the knockdown of MMG8 affects ER-to-Golgi transport and thus perturbs the delivery of proteins and membranes that are required for the assembly and maintenance of the Golgi structure (Diao et al., 2008; Marra et al., 2007). Fourth, MMG8 is required for stabilizing AKAP450, which recruits several regulatory molecules such as PKA (Schmidt et al., 1999; Takahashi et al., 1999; Witczak et al., 1999). Notably, PKA is required for Golgi biogenesis and structural reorganization (Bejarano et al., 2006; Mavillard et al., 2010). We observed that the overexpression of MMG8 caused a disorganization of the Golgi (supplementary material Fig. S2). Therefore, ensuring proper structural organization of the Golgi appears to require maintaining the homeostasis of Golgi-localized MMG8.
Collectively, the data presented herein support a model in which MMG8 regulates microtubule organization on the Golgi by forming functional complexes with AKAP450, γTuCs and EB1 or EB3. These MMG8 functions are required for efficient ER-to-Golgi trafficking and structural organization of the Golgi.
MATERIALS AND METHODS
Plasmids and oligonucleotides
The human MMG clone NP_001002811 (MMG variant 5) was obtained from the Kazusa DNA Research Institute (Kisarazu, Chiba, Japan). The coding sequence of MMG8 was constructed by replacing a carboxy-terminal sequence of NP_001002811 with the corresponding MMG8 sequence amplified using RT-PCR. The protein and nucleotide sequences of MMG8 have been deposited in GenBank under accession number HQ333476. Mutations were introduced into MMG8 by means of PCR-based site-directed mutagenesis. Two siRNA duplexes were synthesized to target MMG8 (si-MMG8-1, AACCUCCAGUGGCUGAAAGAA; si-MMG8-2, AAGCAGAGAGACAGCUCUAUA); an siRNA duplex was also synthesized against human AKAP450 (si-AKAP450, AACUUUGAAGUUAACUAUCAA) (Rivero et al., 2009).
Antibodies
Two MMG8 fragments, containing amino acids 637–925 and 926–1116, were cloned for bacterial expression in fusion with a hexahistidine (His6) tag. The recombinant proteins were purified using Ni2+–nitrilotriacetic-acid resins (Qiagen, Valencia, CA) in the presence of 6 M urea and were then dialyzed against phosphate-buffered saline (PBS). After dialysis, the proteins were used for immunizing rabbits. Antisera generated against amino acids 637–925 and 926–1116 were designated as 443M and 532C, respectively. Antibodies were purified from the sera by using the respective antigens immobilized on nitrocellulose membranes. Using similar methods, an antibody was generated against the AKAP450 fragment containing amino acids 1924–2170 and was purified. The generation of anti-GCP2 and anti-GCP3 antibodies has been described previously (Choi et al., 2010; Fong et al., 2008). The following monoclonal antibodies were from commercial sources: anti-GM130, anti-AKAP450 and anti-EB1 from BD Biosciences (Franklin Lakes, NJ); and anti-α-tubulin, anti-γ-tubulin and anti-FLAG (M2) from Sigma Aldrich (St Louis, MO). The polyclonal antibodies purchased were anti-FLAG (Sigma Aldrich), anti-GFP (FL, Santa Cruz Biotechnology, Dallas, TX), anti-mannosidase II (Millipore, Billerica, MA) and anti-TGN46 (sheep polyclonal, Serotec, Kidlington, Oxford, UK).
Cell culture
Mammalian cells were cultured in medium containing 10% fetal bovine serum and were maintained at 37°C under 5% CO2. HeLa, HEK293T, MCF-7, IMR-5 (a human neuroblastoma cell line) and C2C12 (a mouse myoblast line) cells were grown in Dulbecco's modified Eagle's medium (DMEM); human retinal pigment epithelial cells hTERT-RPE1 (RPE1) were grown in DMEM∶Ham's F12 (1∶1) (Life Technologies, Carlsbad, CA); and MRC-5 human fetal-lung fibroblasts were grown in MEM (Life Technologies). C2C12 myoblasts were differentiated into myotubes by using DMEM supplemented with 2% horse serum. Brefeldin-A, nocodazole, cycloheximide and MG132 were purchased from Sigma Aldrich.
Immunoprecipitation and pull-down assays
Transfected proteins were immunoprecipitated (using antibodies against their peptide tags) from cell extracts prepared in lysis buffer [20 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1 mM dithiothreitol and a protease-inhibitor cocktail (Roche, Basel, Switzerland)]. To immunoprecipitate endogenous MMG8, cell extracts were prepared in RIPA buffer (25 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate and 0.1% SDS) supplemented with the protease-inhibitor cocktail and then clarified. After preclearing with Protein-A–agarose (Life Technologies), the extracts were incubated with agitation at 4°C for 4 h with an MMG8-specific antibody bound to Protein-A-conjugated beads. After extensive washing, the immunoprecipitates were eluted by boiling the beads in SDS-PAGE sample buffer. To prepare samples for mass-spectrometric analysis, protein bands visualized by means of Coomassie Blue staining were excised and subjected to in-gel tryptic digestion. The peptides recovered in this manner were introduced through a nanoelectrospray ion source into a quadrupole/time-of-flight mass spectrometer (QSTAR-Pulsar, Applied Biosystems/Sciex, Foster City, CA). Protein identity was revealed by searching a nonredundant sequence database with tandem mass spectra.
In the pull-down assay of γTuCs, FLAG–MMG8 transiently expressed in HEK293T cells was immunoprecipitated using anti-FLAG-coupled beads (M2, Sigma Aldrich) in RIPA buffer. After immunoprecipitation, the beads were washed with RIPA buffer and with lysis buffer supplemented with 0.1% Tween-20. The beads were then tested for binding to γTuCs in extracts of RPE1 cells prepared using the lysis buffer containing 0.1% Tween-20. After sedimenting the beads, bound proteins were examined by means of immunoblotting.
Immunofluorescence microscopy
To perform immunostaining, cells were fixed either with cold methanol for 5 min at −20°C or with 4% paraformaldehyde in PBS for 15 min at room temperature. After staining, cell images were acquired using an epifluorescence microscope (Eclipse TE2000, Nikon, Tokyo, Japan) or a confocal microscope (LSM510 META, Carl Zeiss, Jena, Germany). To visualize γ-tubulin at the Golgi, cells were extracted for 30 min in a saponin-containing buffer (0.1 M 1,4-piperazinediethanesulfonic acid-KOH pH 6.9, 2 M glycerol, 5 mM MgCl2, 2 mM EGTA and 0.1% saponin) before methanol fixation. To enhance staining signals, cells were stained sequentially with two secondary antibodies – Alexa-Fluor-labeled goat secondary antibodies and then donkey anti-goat-IgG secondary antibodies labeled with the same dye (Life Technologies).
VSVG trafficking assay
At 72 h after transfection with siRNAs, cells were transfected with a plasmid encoding VSVG–YFP and then cultured at 40°C overnight. To initiate the trafficking assays, the cells were changed to a medium that contained 100 µg/ml cycloheximide and was prewarmed to 32°C; the cells were incubated at 32°C for various times and then either analyzed for endo-H resistance or immunostained. Endo-H resistance was assayed according the manufacturer's protocol (New England Biolabs, Ipswich, MA). Briefly, cells were collected in SDS-PAGE sample buffer and boiled for 10 min. After boiling, the samples were mixed with 1 U/µl endo-H and then incubated at 37°C for 4 h before immunoblotting for VSVG (by using anti-YFP). To analyze the subcellular distribution of VSVG, the cells were fixed with methanol at −20°C for 5 min and then immunostained.
Isolation of Golgi membranes
The protocol used for isolating Golgi membranes was adapted from methods described elsewhere (Malhotra et al., 1989). Cells were homogenized in ice-cold homogenization buffer (10 mM Tris-HCl pH 7.4, 0.5 M sucrose, 5 mM EDTA and the protease-inhibitor cocktail). The homogenates were centrifuged at 900 g for 10 min at 4°C to remove nuclei and intact cells. The postnuclear supernatant (1 ml) was layered on top of 1.25 M sucrose (1 ml; dissolved in 10 mM Tris-HCl pH 7.4) and centrifuged at 90,000 g for 90 min at 4°C in an ultracentrifuge by using a TLS-55 swinging-bucket rotor (Beckman Coulter, Brea, CA). The crude smooth-membrane fraction, which appeared as a white band immediately above the interface with the 1.25-M-sucrose layer, was collected through aspiration and adjusted to 1.2 M sucrose. This membrane fraction (0.6 ml) was sequentially overlaid with 1.1, 1.0 and 0.5 M sucrose (0.5 ml of each sucrose solution, prepared in 10 mM Tris-HCl pH 7.4) and then centrifuged at 90,000 g for 2.5 h at 4°C in the TLS-55 rotor. The Golgi membranes, which were enriched at the interface of 0.5 M–1.0 M sucrose, were collected, diluted 1∶3 with 10 mM Tris-HCl (pH 7.4) and pelleted by centrifugation (180,000 g for 60 min at 4°C) and then used in immunoblotting analysis.
Microtubule regrowth
Cellular microtubules were completely depolymerized by placing cells on ice for 1 h or by treating them with 10 µg/ml nocodazole for 2 h. To initiate microtubule regrowth, cold-treated cells were transferred to a 37°C water bath. Nocodazole-treated cells were washed several times with ice-cold PBS and then incubated in medium that was prewarmed to 37°C. To reduce background cytoplasmic staining, cells were extracted briefly before fixation by using a cytoskeleton-stabilizing buffer (50 mM imidazole pH 6.8, 50 mM KCl, 0.5 mM MgCl2, 1 mM EGTA, 0.1 mM EDTA, 4% PEG4000 and 0.1% saponin) (Svitkina et al., 1996). Microtubules were analyzed using immunofluorescence microscopy.
Acknowledgments
We thank Wanjin Hong (Institute of Molecular and Cell Biology, Singapore), Adam D. Linstedt (Carnegie Mellon University, Pittsburgh, PA) and Sigurd Ørstavik (Oslo University Hospital, Norway) for reagents. We also thank Yanzhuang Wang (University of Michigan, Ann Arbor, MI) for valuable discussions and Jason Tam and Xulun Sun (The Hong Kong University of Science and Technology, Hong Kong, China) for technical assistance.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
Z.W., C.Z. and R.Z.Q. designed the experiments. Z.W. and C.Z. conducted the experiments. Z.W., C.Z. and R.Z.Q. analyzed the data. Z.W. and R.Z.Q. wrote the paper.
Funding
This work was supported by grants from the Research Grants Council [General Research Fund 662511 and 662612 and Theme-based Research Scheme T13-607/12R] of Hong Kong; the National Key Basic Research Program of China [grant number 2013CB530900]; the University Grants Committee [Area of Excellence Scheme AoE/M-06/08 and Special Equipment Grant SEG_HKUST05] of Hong Kong; the Innovation and Technology Commission [grant number ITCPD/17-9] of Hong Kong; and the TUYF Charitable Trust [grant number TUYF12SC05].
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.155408/-/DC1
References
- Akhmanova A., Steinmetz M. O. (2008). Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol. 9, 309–322. 10.1038/nrm2369 [DOI] [PubMed] [Google Scholar]
- Bejarano E., Cabrera M., Vega L., Hidalgo J., Velasco A. (2006). Golgi structural stability and biogenesis depend on associated PKA activity. J. Cell Sci. 119, 3764–3775. 10.1242/jcs.03146 [DOI] [PubMed] [Google Scholar]
- Chabin-Brion K., Marceiller J., Perez F., Settegrana C., Drechou A., Durand G., Poüs C. (2001). The Golgi complex is a microtubule-organizing organelle. Mol. Biol. Cell 12, 2047–2060. 10.1091/mbc.12.7.2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. K., Liu P., Sze S. K., Dai C., Qi R. Z. (2010). CDK5RAP2 stimulates microtubule nucleation by the gamma-tubulin ring complex. J. Cell Biol. 191, 1089–1095. 10.1083/jcb.201007030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole N. B., Sciaky N., Marotta A., Song J., Lippincott-Schwartz J. (1996). Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. Mol. Biol. Cell 7, 631–650. 10.1091/mbc.7.4.631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao A., Frost L., Morohashi Y., Lowe M. (2008). Coordination of golgin tethering and SNARE assembly: GM130 binds syntaxin 5 in a p115-regulated manner. J. Biol. Chem. 283, 6957–6967. 10.1074/jbc.M708401200 [DOI] [PubMed] [Google Scholar]
- Efimov A., Kharitonov A., Efimova N., Loncarek J., Miller P. M., Andreyeva N., Gleeson P., Galjart N., Maia A. R., McLeod I. X. et al. (2007). Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev. Cell 12, 917–930. 10.1016/j.devcel.2007.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong K. W., Choi Y. K., Rattner J. B., Qi R. Z. (2008). CDK5RAP2 is a pericentriolar protein that functions in centrosomal attachment of the gamma-tubulin ring complex. Mol. Biol. Cell 19, 115–125. 10.1091/mbc.E07-04-0371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong K. W., Hau S. Y., Kho Y. S., Jia Y., He L., Qi R. Z. (2009). Interaction of CDK5RAP2 with EB1 to track growing microtubule tips and to regulate microtubule dynamics. Mol. Biol. Cell 20, 3660–3670. 10.1091/mbc.E09-01-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriev I., Gouveia S. M., van der Vaart B., Demmers J., Smyth J. T., Honnappa S., Splinter D., Steinmetz M. O., Putney J. W., Jr, Hoogenraad C. C. et al. (2008). STIM1 is a MT-plus-end-tracking protein involved in remodeling of the ER. Curr. Biol. 18, 177–182. 10.1016/j.cub.2007.12.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honnappa S., Gouveia S. M., Weisbrich A., Damberger F. F., Bhavesh N. S., Jawhari H., Grigoriev I., van Rijssel F. J., Buey R. M., Lawera A. et al. (2009). An EB1-binding motif acts as a microtubule tip localization signal. Cell 138, 366–376. 10.1016/j.cell.2009.04.065 [DOI] [PubMed] [Google Scholar]
- Hurtado L., Caballero C., Gavilan M. P., Cardenas J., Bornens M., Rios R. M. (2011). Disconnecting the Golgi ribbon from the centrosome prevents directional cell migration and ciliogenesis. J. Cell Biol. 193, 917–933. 10.1083/jcb.201011014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keryer G., Rios R. M., Landmark B. F., Skalhegg B., Lohmann S. M., Bornens M. (1993). A high-affinity binding protein for the regulatory subunit of cAMP-dependent protein kinase II in the centrosome of human cells. Exp. Cell Res. 204, 230–240. 10.1006/excr.1993.1029 [DOI] [PubMed] [Google Scholar]
- Komarova Y., De Groot C. O., Grigoriev I., Gouveia S. M., Munteanu E. L., Schober J. M., Honnappa S., Buey R. M., Hoogenraad C. C., Dogterom M. et al. (2009). Mammalian end binding proteins control persistent microtubule growth. J. Cell Biol. 184, 691–706. 10.1083/jcb.200807179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J. (1998). Cytoskeletal proteins and Golgi dynamics. Curr. Opin. Cell Biol. 10, 52–59. 10.1016/S0955-0674(98)80086-0 [DOI] [PubMed] [Google Scholar]
- Malhotra V., Serafini T., Orci L., Shepherd J. C., Rothman J. E. (1989). Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell 58, 329–336. 10.1016/0092-8674(89)90847-7 [DOI] [PubMed] [Google Scholar]
- Marra P., Salvatore L., Mironov A., Jr, Di Campli A., Di Tullio G., Trucco A., Beznoussenko G., Mironov A., De Matteis M. A. (2007). The biogenesis of the Golgi ribbon: the roles of membrane input from the ER and of GM130. Mol. Biol. Cell 18, 1595–1608. 10.1091/mbc.E06-10-0886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavillard F., Hidalgo J., Megias D., Levitsky K. L., Velasco A. (2010). PKA-mediated Golgi remodeling during cAMP signal transmission. Traffic 11, 90–109. 10.1111/j.1600-0854.2009.01007.x [DOI] [PubMed] [Google Scholar]
- Miller P. M., Folkmann A. W., Maia A. R., Efimova N., Efimov A., Kaverina I. (2009). Golgi-derived CLASP-dependent microtubules control Golgi organization and polarized trafficking in motile cells. Nat. Cell Biol. 11, 1069–1080. 10.1038/ncb1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley J. F., Cole N. B., Schroer T. A., Hirschberg K., Zaal K. J., Lippincott-Schwartz J. (1997). ER-to-Golgi transport visualized in living cells. Nature 389, 81–85. 10.1038/38891 [DOI] [PubMed] [Google Scholar]
- Rios R. M., Bornens M. (2003). The Golgi apparatus at the cell centre. Curr. Opin. Cell Biol. 15, 60–66. 10.1016/S0955-0674(02)00013-3 [DOI] [PubMed] [Google Scholar]
- Ríos R. M., Sanchís A., Tassin A. M., Fedriani C., Bornens M. (2004). GMAP-210 recruits gamma-tubulin complexes to cis-Golgi membranes and is required for Golgi ribbon formation. Cell 118, 323–335. 10.1016/j.cell.2004.07.012 [DOI] [PubMed] [Google Scholar]
- Rivero S., Cardenas J., Bornens M., Rios R. M. (2009). Microtubule nucleation at the cis-side of the Golgi apparatus requires AKAP450 and GM130. EMBO J. 28, 1016–1028. 10.1038/emboj.2009.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales S. J., Pepperkok R., Kreis T. E. (1997). Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell 90, 1137–1148. 10.1016/S0092-8674(00)80379-7 [DOI] [PubMed] [Google Scholar]
- Schmidt P. H., Dransfield D. T., Claudio J. O., Hawley R. G., Trotter K. W., Milgram S. L., Goldenring J. R. (1999). AKAP350, a multiply spliced protein kinase A-anchoring protein associated with centrosomes. J. Biol. Chem. 274, 3055–3066. 10.1074/jbc.274.5.3055 [DOI] [PubMed] [Google Scholar]
- Sütterlin C., Colanzi A. (2010). The Golgi and the centrosome: building a functional partnership. J. Cell Biol. 188, 621–628. 10.1083/jcb.200910001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina T. M., Verkhovsky A. B., Borisy G. G. (1996). Plectin sidearms mediate interaction of intermediate filaments with microtubules and other components of the cytoskeleton. J. Cell Biol. 135, 991–1007. 10.1083/jcb.135.4.991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Shibata H., Shimakawa M., Miyamoto M., Mukai H., Ono Y. (1999). Characterization of a novel giant scaffolding protein, CG-NAP, that anchors multiple signaling enzymes to centrosome and the golgi apparatus. J. Biol. Chem. 274, 17267–17274. 10.1074/jbc.274.24.17267 [DOI] [PubMed] [Google Scholar]
- Takahashi M., Yamagiwa A., Nishimura T., Mukai H., Ono Y. (2002). Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring gamma-tubulin ring complex. Mol. Biol. Cell 13, 3235–3245. 10.1091/mbc.E02-02-0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde I., Pahlke G., Salanova M., Zhang G., Wang S., Coletti D., Onuffer J., Jin S. L., Conti M. (2001). Myomegalin is a novel protein of the golgi/centrosome that interacts with a cyclic nucleotide phosphodiesterase. J. Biol. Chem. 276, 11189–11198. 10.1074/jbc.M006546200 [DOI] [PubMed] [Google Scholar]
- Vinogradova T., Paul R., Grimaldi A. D., Loncarek J., Miller P. M., Yampolsky D., Magidson V., Khodjakov A., Mogilner A., Kaverina I. (2012). Concerted effort of centrosomal and Golgi-derived microtubules is required for proper Golgi complex assembly but not for maintenance. Mol. Biol. Cell 23, 820–833. 10.1091/mbc.E11-06-0550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wu T., Shi L., Zhang L., Zheng W., Qu J. Y., Niu R., Qi R. Z. (2010). Conserved motif of CDK5RAP2 mediates its localization to centrosomes and the Golgi complex. J. Biol. Chem. 285, 22658–22665. 10.1074/jbc.M110.105965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P., Forster R., Palmer K. J., Pepperkok R., Stephens D. J. (2005). Coupling of ER exit to microtubules through direct interaction of COPII with dynactin. Nat. Cell Biol. 7, 48–55. 10.1038/ncb1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witczak O., Skålhegg B. S., Keryer G., Bornens M., Taskén K., Jahnsen T., Orstavik S. (1999). Cloning and characterization of a cDNA encoding an A-kinase anchoring protein located in the centrosome, AKAP450. EMBO J. 18, 1858–1868. 10.1093/emboj/18.7.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]