Abstract
Cyclical formation and regression of the ovarian corpus luteum is required for reproduction. During luteal regression, the microvasculature of the corpus luteum is extensively disrupted. Prostaglandin F2α, a primary signal for luteal regression, induces the expression of transforming growth factor β1 (TGFB1) in the corpus luteum. This study determined the actions of TGFB1 on microvascular endothelial cells isolated from the bovine corpus luteum (CLENDO cells). We hypothesized that TGFB1 participates in the disruption of the microvasculature during luteal regression. TGFB1 activated the canonical SMAD signaling pathway in CLENDO cells. TGFB1 (1 ng/ml) significantly reduced both basal and fetal-calf-serum-stimulated DNA synthesis, without reducing cell viability. TGFB1 also significantly reduced CLENDO cell transwell migration and disrupted the formation of capillary-like structures when CLENDO cells were plated on Matrigel. By contrast, CLENDO cells plated on fibrillar collagen I gels did not form capillary-like structures and TGFB1 induced cell death. Additionally, TGFB1 caused loss of VE-cadherin from cellular junctions and loss of cell–cell contacts, and increased the permeability of confluent CLENDO cell monolayers. These studies demonstrate that TGFB1 acts directly on CLENDO cells to limit endothelial cell function and suggest that TGFB1 might act in the disassembly of capillaries observed during luteal regression.
Key words: TGFB1, Angiogenesis, Luteolysis, Corpus luteum, SMAD, Reproduction, Fertility
Introduction
The corpus luteum is a transient endocrine gland that forms from what remains of the ovulated follicle at the beginning of the luteal phase of the menstrual or estrous cycle (Davis and Rueda, 2002; Niswender et al., 2007; Stocco et al., 2007). The primary function of the corpus luteum is the secretion of progesterone by the steroidogenic luteal cells. Progesterone is required for successful implantation and maintenance of pregnancy. If pregnancy does not occur, the corpus luteum must regress to allow follicular growth, ovulation and a new reproductive cycle. Corpus luteum formation is accompanied by extensive angiogenesis, which is essential to support the steroidogenic capacity of the corpus luteum (Fraser and Duncan, 2005; Plendl, 2000; Robinson et al., 2009). Regression of the corpus lutem (luteolysis) involves an initial loss of the capacity to synthesize and secrete progesterone, followed by loss of cells (Stocco et al., 2007). Luteolysis is characterized by intense angioregression and luteal cell apoptosis (Davis and Rueda, 2002; Davis et al., 2003; Vonnahme et al., 2006), which are accompanied by considerable extracellular matrix (ECM) remodeling characterized by deposition of type I collagen (Duncan, 2000; Irving-Rodgers et al., 2006; Vonnahme et al., 2006). The corpus luteum gradually involutes to form a small scar composed of connective tissue, known as the corpus albicans (Niswender et al., 2000). In most mammalian species, prostaglandin F2α (PGF2α) is accepted as a prominent luteolytic factor. In domestic livestock, the uterus is the main site of PGF2α production, although intraovarian PGF2α production might also contribute to luteal regression in primates and other species (Bogan et al., 2008; Davis and Rueda, 2002).
Transforming growth factor-β1 (TGFB1) is the prototypic member of a large family of evolutionarily conserved secreted cytokines involved in a variety of cellular functions from embryo development to adult tissue homeostasis (Gordon and Blobe, 2008; Knight and Glister, 2006; Wu and Hill, 2009). Most cells, including epithelial, stromal and immune cells such as macrophages, make TGFB1 and have receptors for the ligand. TGFB1 is secreted as an inactive peptide, forming part of a ‘latent complex’ consisting of a mature TGFB1 dimer non-covalently bound to its latency-associated peptide (LAP) and, via LAP, to latent TGFB-binding proteins (LTBPs). Activated TGFB1 binds to ubiquitously expressed cell-surface TGFB1 type I receptors (TGFBRI) and type II receptors (TGFBRII), which are transmembrane serine/threonine kinases. TGFB1 bound to TGFBRII recruits TGFBRI and induces the trans-phosphorylation of TGFBRI. TGFBRI subsequently phosphorylates SMAD proteins 2 and 3. SMAD2 and SMAD3 proteins either interact with SMAD4 and translocate to the nucleus to regulate the expression of target genes, or interact with inhibitory SMAD proteins (i.e. SMAD7) that functionally inhibit the cascade. Once within the nucleus, SMAD2–SMAD3–SMAD4 forms a nuclear complex with transcription factors, co-activators and co-repressors to regulate the transcription of genes (Massagué, 2008).
TGFB1 signaling is vital in blood-vessel morphogenesis and stability (Pardali and ten Dijke, 2009). In humans, various cardiovascular disorders are associated with mutations affecting components of TGFB signaling. Furthermore, studies in mouse models show that the knockout of components of TGFB signaling impairs angiogenesis, resulting in lethal cardiovascular defects (reviewed in ten Dijke and Arthur, 2007). The actions of TGFB1 on the vasculature are highly dependent on the cellular context. For instance, TGFB1 acts as a stimulator or an inhibitor of angiogenesis in vivo and in vitro depending on experimental conditions (Goumans et al., 2009).
Gangrade et al. (Gangrade et al., 1993) reported that TGFB1 is produced by the bovine corpus luteum. Our laboratory (Hou et al., 2008) and others (Stocco et al., 2001; Wang et al., 2003) have shown that a luteolytic dose of PGF2α induces the expression of TGFB1 mRNA in the corpus luteum in vivo. Recent studies by Hou et al. (Hou et al., 2008) indicate that treatment with PGF2α in vitro also induces TGFB1 mRNA expression and protein secretion in primary cultures of bovine luteal cells. In addition, treatment of cultured luteal cells with TGFB1 reduced progesterone secretion, implicating TGFB1 in luteolysis (Hou et al., 2008; Miyamoto et al., 1992).
Endothelial cells comprise 50% of the cells present in the corpus luteum, and are vital to the formation and function of the tissue (Fraser and Duncan, 2005; Robinson et al., 2009; Stouffer et al., 2007). During regression, the microvasculature is extensively disrupted (Henkes et al., 2008). Other than reports on luteal steroidogenic cells, there have been no studies to date that clearly identify the specific cells in the corpus luteum that produce or respond to TGFB1. Additionally, the involvement of TGFB1 in the regulation of tissue remodeling that occurs during luteolysis has not been examined in detail. The aim of our study was to determine the biological functions of TGFB1 in luteal endothelial cells. Our working hypothesis was that TGFB1 participates in the disruption of the microvasculature during luteal regression. We isolated microvascular endothelial cells from the bovine corpus luteum (CLENDO cells) in order to investigate the effects of TGFB1 on the ability of CLENDO cells to proliferate, migrate, form capillary-like structures and maintain the integrity of a monolayer of cells.
Results
Characterization of CLENDO cells
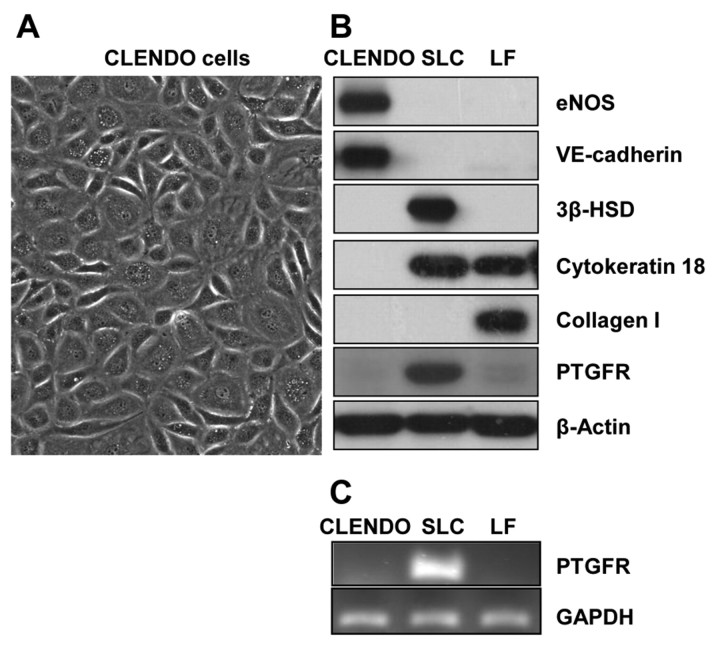
CLENDO cells were isolated using BSL-I lectin-coated magnetic beads. Colonies of endothelial cells were selected and expanded. Although the cells displayed the typical cobblestone morphology characteristic of cultured endothelial cells (Fig. 1A), immunofluorescence revealed that 5–10% of the cells stained positive for α-smooth muscle actin (αSMA), a marker of cells of mesenchymal origin. Therefore, the culture was further purified by fluorescence-activated cell sorting (FACS) with a vascular endothelial (VE)-cadherin (CD144) antibody, a specific endothelial cell marker. Characterization of the purified CLENDO cells by western blot showed that these cells expressed the endothelial cell markers VE-cadherin and endothelial nitric oxide synthase (eNOS), but did not express the receptor for PGF2α (PTGFR), 3-β-hydroxysteroid dehydrogenase (HSD3B; a marker of steroidogenic luteal cells) or collagen type I (a marker of luteal fibroblasts) (Fig. 1B). Purified CLENDO cells did not express cytokeratin 18, consistent with previous reports that demonstrated that the majority of luteal microvascular endothelial cells do not express cytokeratin 18 (Ricken et al., 1995; Tscheudschilsuren et al., 2002) (Fig. 1B). The absence of PTGFR was confirmed by PCR analysis (Fig. 1C). In addition, immunofluorescence staining showed that the amount of αSMA-positive cells in the purified culture was reduced to less than 1% following cell sorting with the CD144 antibody (data not shown).
Fig. 1.
Characterization of CLENDO cells. Microvascular endothelial cells were isolated from bovine corpus luteum and characterized by their morphology and expression of cell markers. (A) CLENDO cells displayed cobblestone morphology. CLENDO cells were characterized by western blot of cell lysates (B) and PCR analysis (C). (B) CLENDO expressed the endothelial cell markers VE-cadherin and eNOS, and did not express cytokeratin 18, PTGFR and the specific markers of steroidogenic luteal cells (SLCs) – HSD3B (3β-HSD) – and of luteal fibroblasts (LFs) – collagen I. (C) The receptor for PGF2α, PTGFR, was detected in SLCs, but was not detectable in CLENDO cells or LFs by PCR analysis.
TGFB1 induces phosphorylation of SMAD2 and SMAD3 in CLENDO cells
To gain insight into TGFB1 signaling in CLENDO cells, we performed time-course and concentration-response experiments. Western blot analysis revealed that treatment with TGFB1 produced a concentration-dependent increase in the phosphorylation of SMAD2 and SMAD3 (Fig. 2A). The physiological concentration of 1 ng/ml TGFB1 (Fukuchi et al., 2004; Kiliç et al., 2009; Ouellete et al., 2005; Villar et al., 2009) seemed to be maximally effective for phosphorylation of SMAD2 and SMAD3. Time-course experiments revealed that both SMAD2 and SMAD3 were phosphorylated within 5 minutes following treatment with TGFB1 (1 ng/ml) (Fig. 2B). Phosphorylation of SMAD2 and SMAD3 in response to TGFB1 was transient, with reduced levels of phosphorylation observed 2–4 hours after treatment. The effect of TGFB1 on the phosphorylation of SMAD2 and SMAD3 was prevented by pretreatment of cells with SB-431542, a selective TGFBR1 receptor kinase inhibitor (Inman et al., 2002) (Fig. 2C).
Fig. 2.
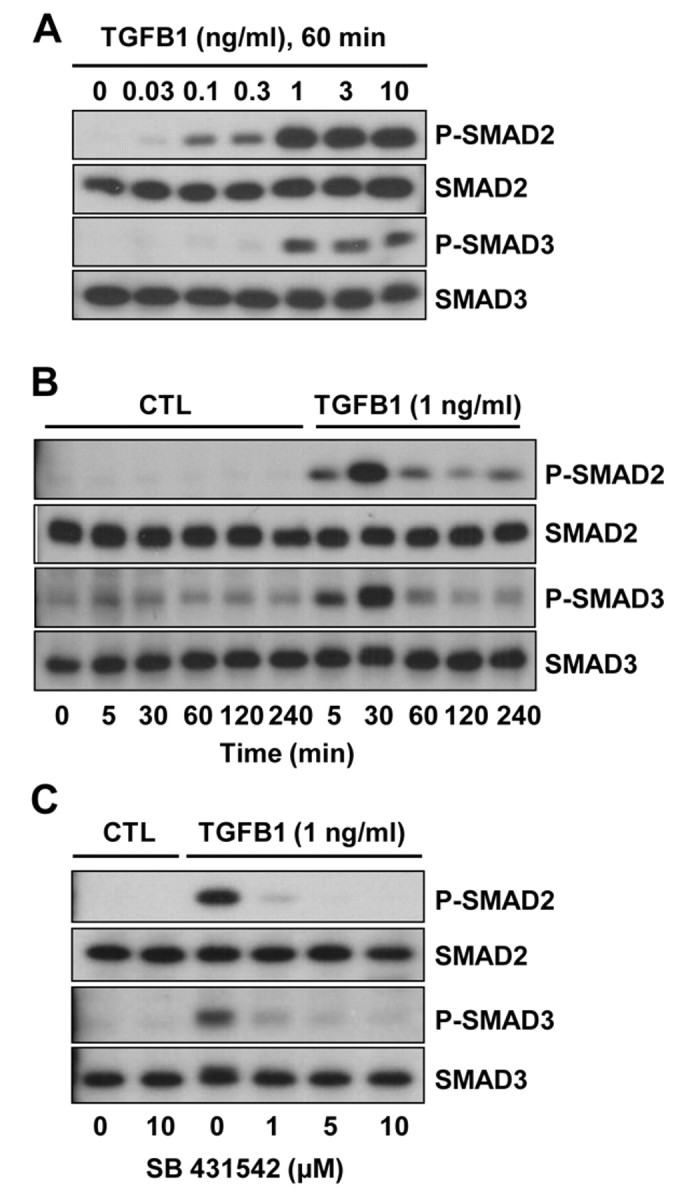
TGFB1 induces phosphorylation of SMAD2 and SMAD3 in CLENDO cells. The time-course response (A) and concentration response (B) to TGFB1 are shown. (A) Cells were serum starved and treated with TGFB1 (0–10 ng/ml) for 60 minutes under serum-free conditions. (B) Cells were serum starved and treated with TGFB1 (1 ng/ml) for up to 4 hours under serum-free conditions. (C) Some cells were pretreated (30 minutes) with the selective TGFBR1 kinase inhibitor SB-431542 (0–10 μM) prior to addition of TGFB1 (1 ng/ml) for 60 minutes. Levels of phosphorylated SMAD2 and SMAD3 (P-SMAD2 and P-SMAD3) and total SMAD2 and SMAD3 were determined by western blot analysis. CTL, control.
TGFB1 reduces DNA synthesis, but does not reduce viability of CLENDO cells plated on plastic
To analyze whether TGFB1 affects CLENDO cell proliferation, we monitored DNA synthesis by measuring [3H]-thymidine incorporation. Treatment of CLENDO cells with 5% fetal calf serum (FCS) routinely caused a fivefold increase in [3H]-thymidine incorporation (Fig. 3A). Treatment with TGFB1 (1 ng/ml) significantly (P<0.05) reduced basal DNA synthesis (54±5% inhibition, mean ± s.e.m., n=6) as well as the stimulatory effect of 5% FCS (Fig. 3A,B). The inhibitory effect of TGFB1 was prevented by pretreatment of cells with the TGFBR1 receptor kinase inhibitor SB-431542 (1 μM) (Fig. 3B). We further examined whether the TGFB1-induced reduction in DNA synthesis was associated with a reduction in cell viability, as measured using the 3,4,5-dimethylthiazol-2-yl-2,5-diphenyl tetrazolium bromide (MTT) assay. Under conditions used for the analysis of [3H]-thymidine incorporation, we found that treatment of CLENDO cells with TGFB1 (1 ng/ml) did not reduce cell viability after 24 or 48 hours when plated on plastic (Fig. 3C). In accordance with previous studies (Friedman et al., 2000; Pru et al., 2003), treatment of CLENDO cells with TNFα for 24 hours resulted in a significant (P<0.05) reduction in cell viability (supplementary material Fig. S1). Under these culture conditions, treatment with both TGFB1 and tumor necrosis factor α (TNFα) did not inhibit or enhance the response to TNFα alone. These results indicate that TGFB1 inhibits CLENDO cell proliferation without reducing cell viability.
Fig. 3.
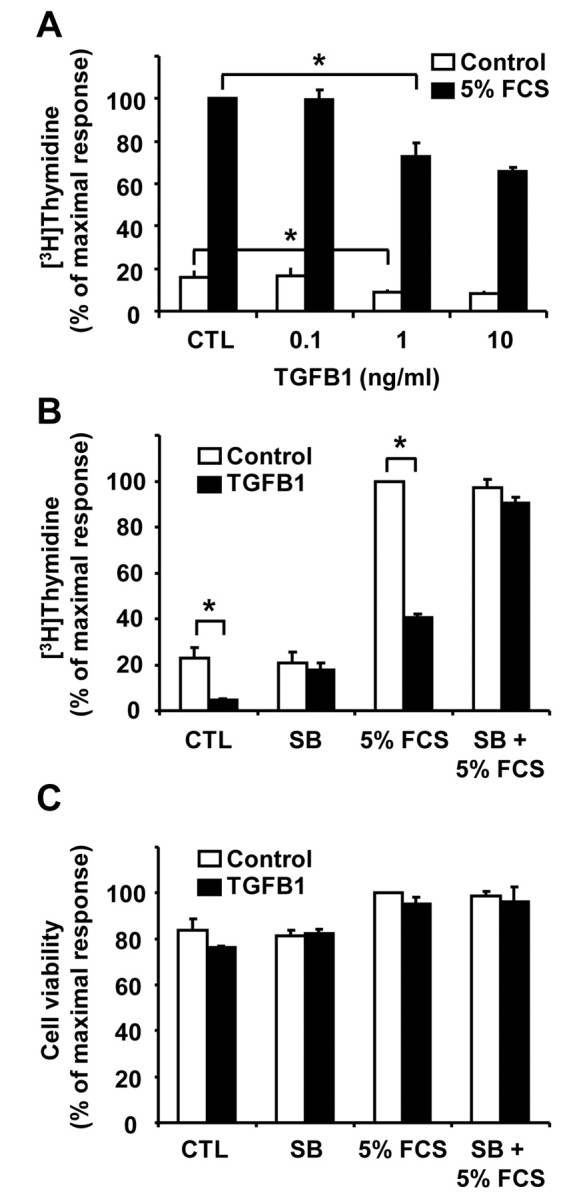
TGFB1 reduces DNA synthesis in CLENDO cells. (A) [3H]-thymidine incorporation assay of cells plated at low density and treated with or without TGFB1 (0–10 ng/ml) in the absence (CTL, control) or presence of 5% FCS for 24 hours. (B) [3H]-thymidine incorporation assay of cells plated at low density and treated for 24 hours with or without TGFB1 (1 ng/ml) in the absence (CTL) or presence of 5% FCS. Some cells were pretreated (30 minutes) with the selective TGFBR1 receptor kinase inhibitor SB-431542 (SB, 1 μM) prior to addition of TGFB1. Data are expressed as the percent incorporation of [3H]-thymidine compared to the maximal response group. Data shown represent three independent experiments each performed in triplicate (mean + s.e.m., n=3, *P<0.05). (C) MTT assay of CLENDO cells plated on plastic at low density and treated for 24 hours with or without TGFB1 (1 ng/ml) in the absence (CTL) or presence of 5% FCS. Some cells were pretreated (30 minutes) with SB-431542 (1 μM) prior to addition of TGFB1. Results are expressed as the percent absorbance observed in the control group. Data shown represent three independent experiments each performed in triplicate (mean ± s.e.m., n=3).
TGFB1 decreases CLENDO cell migration
Endothelial cell migration is another important component in angiogenesis. To determine whether TGFB1 affects CLENDO cell migration, we used a transwell cell culture system with 8 μm pore cell culture inserts. CLENDO cells were highly motile, with nearly 100% of control-treated cells migrating across the membrane within 16 hours. In view of their motility in the present experiments, CLENDO cells were allowed to migrate over a 6 hour time period. We found that treatment of CLENDO cells with TGFB1 (1 ng/ml) significantly reduced the number of cells that migrated over a period of 6 hours (40±3% inhibition, mean ± s.e.m., P<0.05, n=5) (Fig. 4).
Fig. 4.
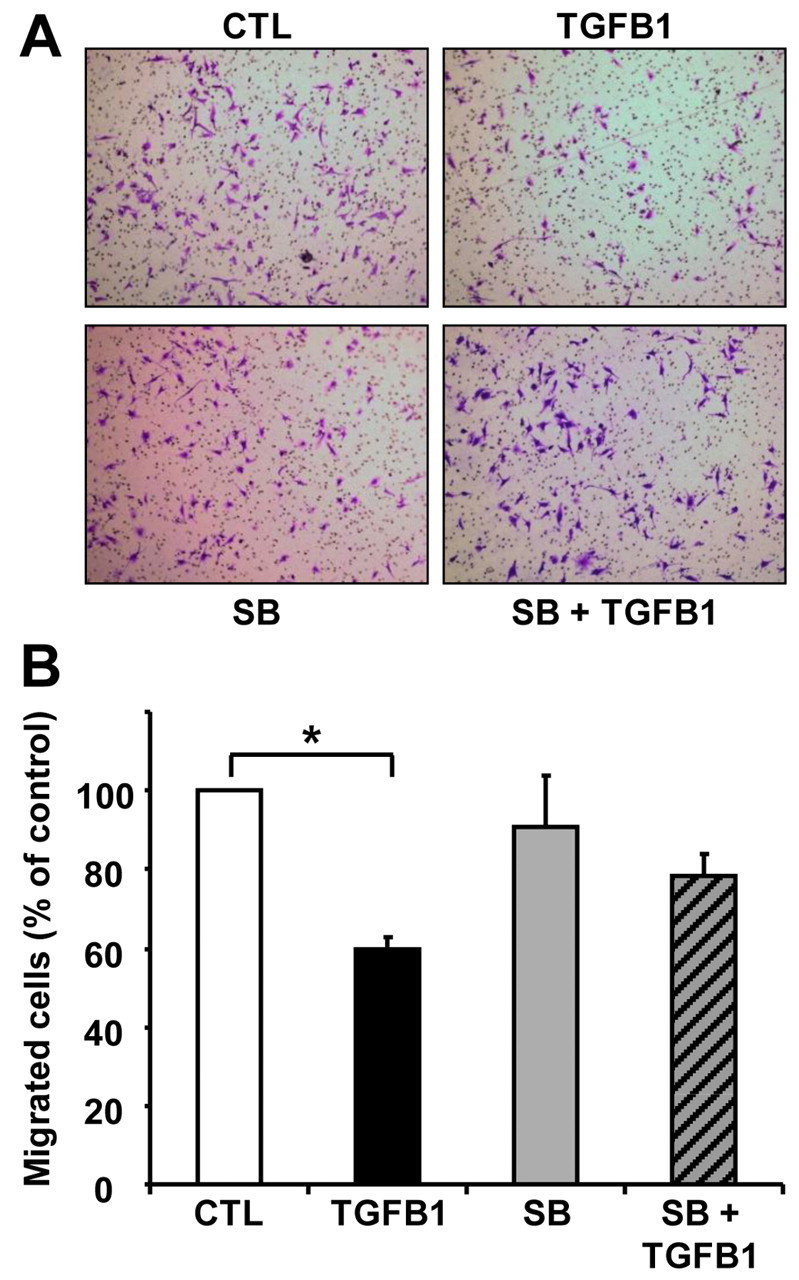
TGFB1 reduces migration of CLENDO cells. CLENDO cells were pretreated with or without SB-431542 (SB, 1 μM) for 30 minutes followed by treatment with TGFB1 (1 ng/ml). Next, cells were plated on transwells and assessed for migration after 6 hours, as described in the Materials and Methods. (A) CLENDO cells found on the bottom of the transwell membrane were fixed, stained with crystal violet and images were obtained for quantification. (B) Quantification of cell migration. Cell migration is represented as a percentage of the number of cells migrated in the control treatment group. Data shown represent five independent experiments each performed in triplicate (mean + s.e.m., n=5, *P<0.05).
TGFB1 disrupts the formation of capillary-like structures
Several studies have shown that TGFB1 supports capillary tube formation by endothelial cells of diverse origin (Bein et al., 2004; Sankar et al., 1996; Serrati et al., 2009). To study the effect of TGFB1 on CLENDO cell capillary morphogenesis, we assayed the in vitro formation of capillary-like structures (CLSs) on Matrigel. We observed that these structures start to form after 2 hours of plating and an interconnected network of CLSs was completely formed after 4 hours on Matrigel-coated wells in growth medium. The network formed by CLENDO cells was stable for 16–24 hours, with spontaneous network involution occurring after longer incubation. In CLENDO cells that were treated with TGFB1 (1 ng/ml) at the time of plating, network formation appeared normal during the first 2 hours, followed by a dramatic collapse of CLENDO capillary-like cords within 8 hours of treatment (Fig. 5A). The TGFB1-induced network regression was characterized by initial rounding of individual CLENDO cells and aggregation that, in time, ended with CLS retraction and dissolution (Fig. 5A). Quantification of the capillary-like network regression induced by TGFB1 is shown as the total CLS length, number of CLSs and number of CLS branch points (Fig. 5B). Treatment with TGFB1 (1 ng/ml) significantly (P<0.001, n=4) reduced the total CLS length (80±5% reduction, mean ± s.e.m.), number of CLSs (82±4% reduction, mean ± s.e.m.) and number of branch points (80±5% reduction, mean ± s.e.m.). The effect of TGFB1 was significantly reduced by pretreatment of cells with SB-431542 (1 μM). Because TGFB1 promotes endothelial cell apoptosis in vitro (Ramsauer and D'Amore, 2007), we determined whether the disruption of capillary morphogenesis was coupled to reduced viability. CLENDO cells plated on thin-layer Matrigel-coated plates were treated with TGFB1 for 24 hours and cell viability was measured by MTT assay. Treatment with TGFB1 alone for 24 hours did not affect cell viability of CLENDO cells plated on Matrigel; however, TGFB1 significantly (P<0.05) enhanced the reduction in viability observed in the presence of TNFα (Fig. 5C). Taken together, these findings suggest that TGFB1 disrupts capillary formation in CLENDO cells, but does not directly reduce cell viability on Matrigel.
Fig. 5.
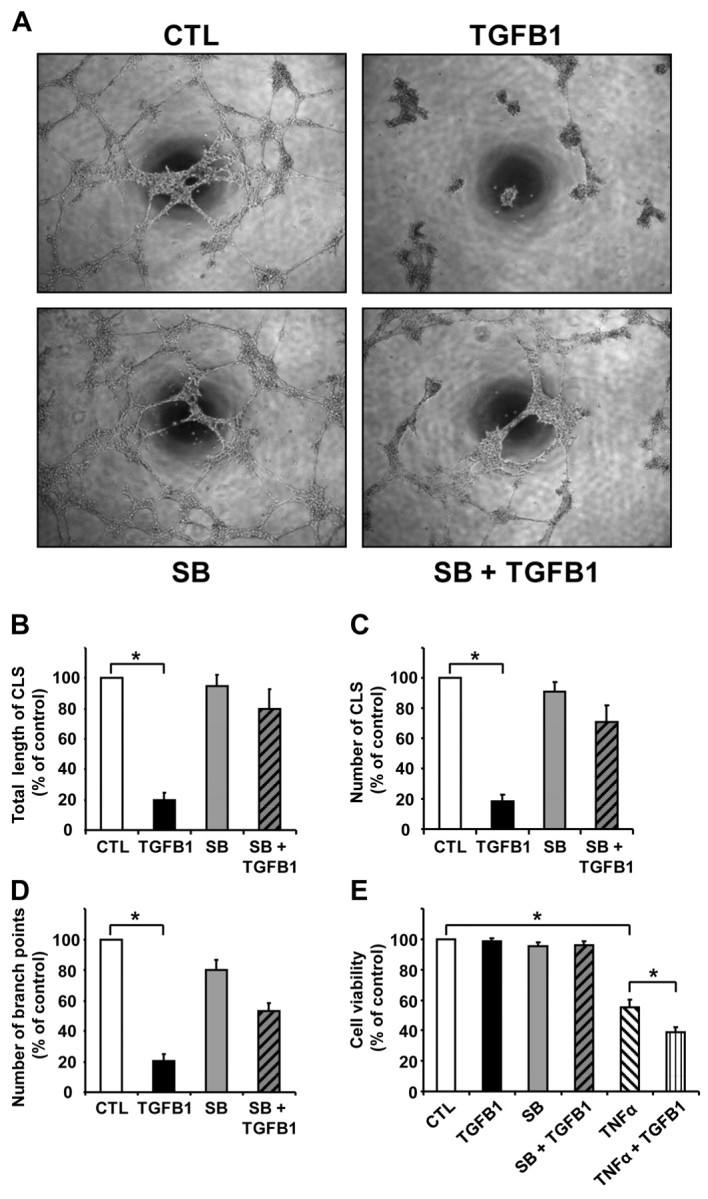
TGFB1 disrupts formation of CLENDO capillary-like structures (CLSs). (A) To perform capillary morphogenesis assays, CLENDO cells were plated (5×104 per well) in growth medium on 48-well plates coated with Matrigel (0.15 ml; 8 mg/ml). Cells were pretreated with or without SB-431542 (SB, 1 μM) for 30 minutes followed by treatment with TGFB1 (1 ng/ml). Pictures were taken under a phase-contrast microscope after 8 hours of incubation at 37°C. CLS formation was quantified as (B) the total length of CLS, (C) the number of CLS and (D) the number of CLS branch points per low-power field. Results are presented as a percentage of measurements obtained in the controls (CTL). Data shown represent four independent experiments each performed in duplicate with similar results (mean ± s.e.m., n=4, *P<0.001). (E) MTT assay of CLENDO cells plated on Matrigel and treated for 24 hours with or without TGFB1 (1 ng/ml) or TNFα (50 ng/ml). Some cells were pretreated (30 minutes) with SB-431542 (1 μM) prior to addition of TGFB1. Data are expressed as the percent cell viability compared to the control group. Data shown represent three independent experiments each performed in triplicate (mean + s.e.m., n=3, *P<0.05).
Effect of TGFB1 and fibrillar collagen type I on CLENDO morphogenesis
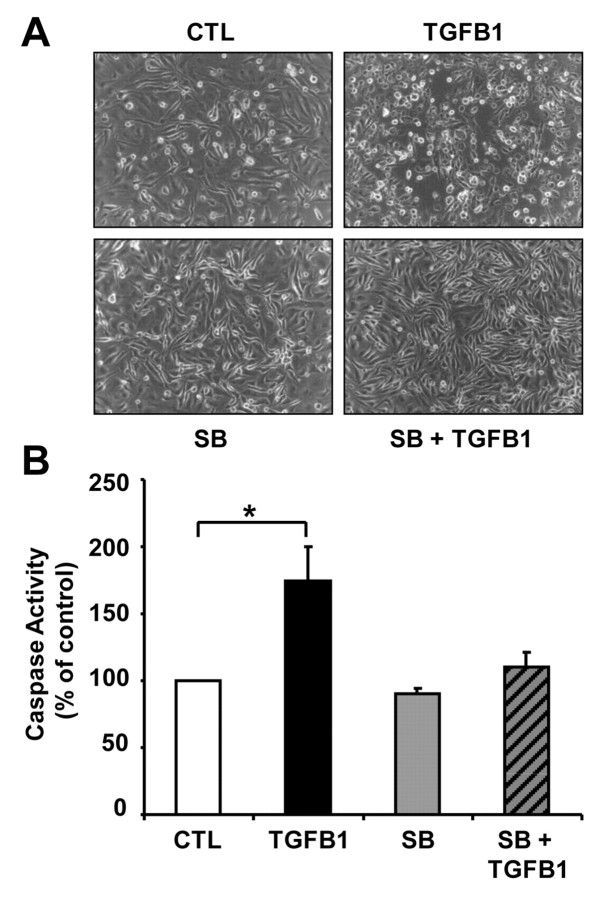
The ECM plays an active role in regulating the behavior and function of cells (Berrier et al., 2007). During luteolysis, the expression of collagen type I increases dramatically (Casey at al., 2005; Vonnahme et al., 2006; Zhao and Luck, 1995). To evaluate the effect of collagen I on CLENDO cell function, we plated CLENDO cells on fibrillar collagen I gels. In contrast to the disruptive effects of TGFB1 on CLENDO morphology after 8 hours when plated on Matrigel (Fig. 5A), TGFB1 had no effect on CLENDO morphology at this time point (supplementary material Fig. S2A). CLENDO cells plated on fibrillar collagen I did not form CLSs and treatment with TGFB1 (1 ng/ml) caused cell rounding and detachment that was evident within 24 hours (Fig. 6A). When the cells were stained with blue fluorescent Hoechst dye at the end of the experiment, we observed a significant increase in the number of condensed nuclei in the TGFB1-treated cells (data not shown). Therefore, to determine whether TGFB1 induced CLENDO cell death when plated on collagen type I gels, we measured caspase-3 activation. CLENDO cells plated on collagen I showed a significant (P<0.05) increase in caspase-3 activity after treatment with TGFB1 for 8 and 24 hours (Fig. 6B; supplementary material Fig. S2B).
Fig. 6.
Effect of TGFB1 on CLENDO viability when plated on fibrillar collagen type I. (A) Morphology of CLENDO cells after plating (5×104 per well) in growth medium on 48-well plates coated with fibrillar collagen type I gels (0.15 ml; ~2.4 mg/ml). Plated cells were pretreated with or without SB-431542 (SB, 1 μM) for 30 minutes followed by control media (CTL) or TGFB1 (1 ng/ml). Pictures were taken under a phase-contrast microscope after 48 hours incubation at 37°C. (B) Caspase-3 and caspase-7 activity was measured after 24 hours of treatment using the Caspase–Glo 3/7 assay kit. Data are expressed as a percentage of the caspase activity observed in controls. Data shown represent three independent experiments each performed in triplicate with similar results (mean + s.e.m., n=3, *P<0.05).
TGFB1 induces VE-cadherin loss from cellular junctions
Cell–cell adhesion mediated by VE-cadherin is crucial for the maintenance of endothelial cell monolayer integrity (Dejana et al., 2008). It has been previously reported that endothelial cell monolayer integrity is altered when VE-cadherin is lost from its localization at cell–cell junctions, resulting in increased monolayer permeability (Alghisi et al., 2009). We tested whether TGFB1 affected VE-cadherin expression and localization. Immunofluorescence analysis showed that, in confluent monolayers of CLENDO cells cultured for 24 hours, VE-cadherin was localized at cell–cell contacts (Fig. 7A, arrows). In confluent monolayers treated with TGFB1 for 24 hours, VE-cadherin localization at cellular junctions was irregular (Fig. 7A, arrowheads). In addition, the loss of VE-cadherin from cellular junctions was associated with the appearance of gaps in the confluent monolayer (Fig. 7A, arrowheads). Western blot analysis showed that the expression of VE-cadherin, as well as other endothelial cell markers such as eNOS and CD31, was not altered by long-term treatment with TGFB1 (Fig. 7B).
Fig. 7.
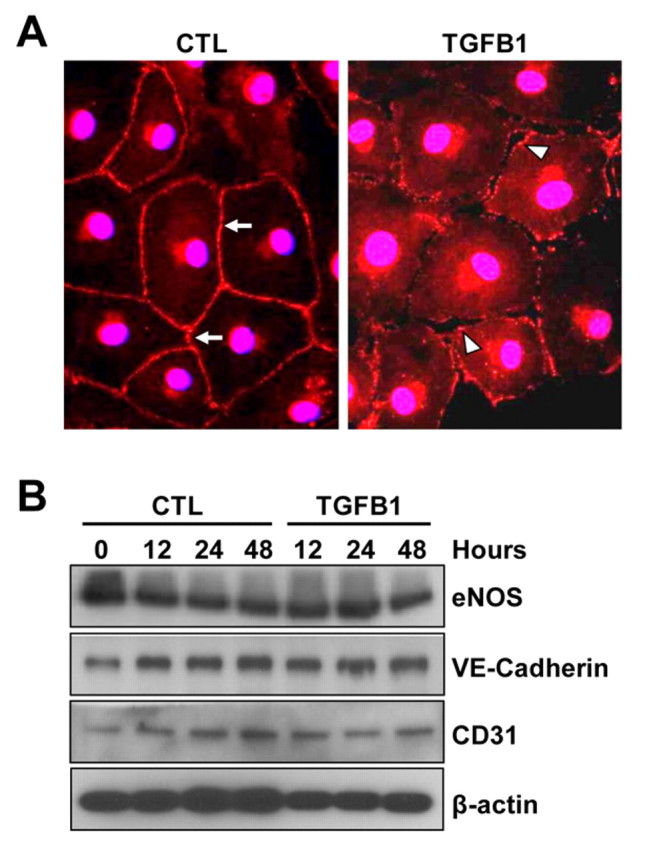
TGFB1 causes loss of VE-cadherin from cellular junctions. (A) Confluent monolayers of CLENDO cells were pretreated with or without SB-431542 (1 μM) for 30 minutes followed by treatment with control medium (CTL) or TGFB1 (1 ng/ml) for 48 hours. VE-cadherin expression (red) was determined by immunofluorescence. In control-treated cells, VE-cadherin was localized at cell–cell contacts (arrows). In TGFB1-treated cells, VE-cadherin localization at cellular junctions was irregular (arrowheads) and gaps between cells appeared in the confluent monolayer (arrowheads). (B) The expression of the endothelial cell markers VE-cadherin, eNOS and CD31 was examined by western blotting. β-actin was used as a loading control.
TGFB1 increases CLENDO monolayer permeability
Based on the above findings that TGFB1 caused loss of cell–cell contacts, we examined whether TGFB1 affects the permeability of CLENDO monolayers. Trans-endothelial permeability was tested by the addition of the fluorescent tracer molecule FITC–dextran to the top chamber and measurement of the fluorescence in the bottom-chamber media over time. CLENDO cells formed a functional barrier when grown to confluence (Fig. 8B) on cell culture membrane inserts (3 μm pore). In controls, the amount of FITC–dextran in the lower chamber increased slowly over a 4 hour period. By contrast, pretreatment with TGFB1 (1 ng/ml) for 24 hours in serum-free medium increased the permeability of CLENDO monolayers by sixfold (Fig. 8A). Pretreatment of cells with SB-431542 abrogated the effect of TGFB1.
Fig. 8.
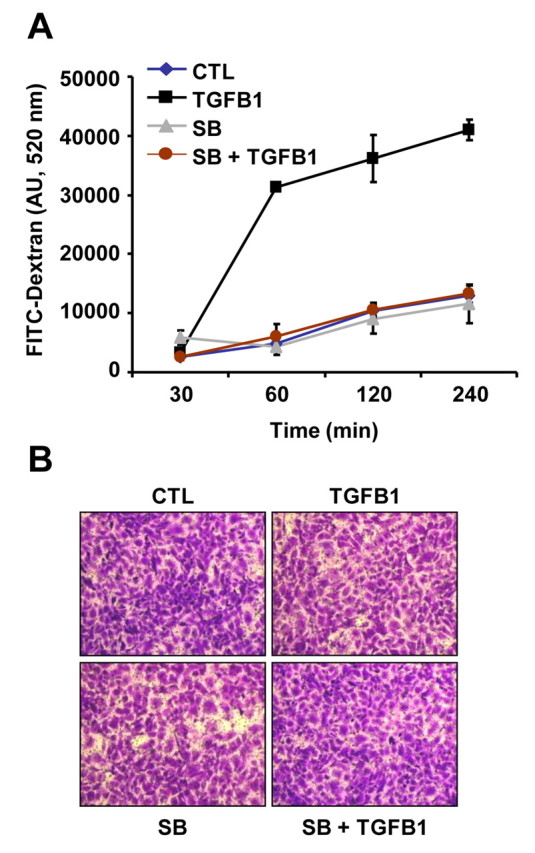
TGFB1 increases the permeability of CLENDO cell monolayers. CLENDO cells were grown to confluence on membrane inserts (3 μm pore). Cells were pretreated with or without SB-431542 (SB, 1 μM) and then treated with control medium (CTL) or TGFB1 (1 ng/ml) for 24 hours. (A) Monolayer permeability was measured by determining the fluorescence of the bottom chamber medium at the indicated times after the addition of the fluorescent tracer molecule FITC–dextran to the top chamber. Data represent mean ± s.e.m. of fluorescence from a representative experiment performed in triplicate. (B) At the end of the assay, monolayers were fixed and stained with crystal violet to show equivalent density of CLENDO monolayers.
Discussion
The ovarian corpus luteum plays an essential role in reproduction. The developing corpus luteum vascularizes extremely rapidly and, once formed, the maintenance of the vasculature is essential to preserve its functionality (Henkes et al., 2008; Pauli et al., 2005; Plendl, 2000). Therefore, improving our understanding of the factors involved in the development and regression of the luteal microvasculature is necessary for a greater understanding of ovarian cyclicity and fertility. The present study provides the first demonstration that TGFB1, a cytokine produced during luteal regression (Hou et al., 2008; Stocco et al., 2001; Wang et al., 2003), alters the biological functions and fate of luteal endothelial cells. The results suggest that TGFB1 participates in the disassembly of the microvasculature during luteal regression.
TGFB1 plays a central role in the process of angiogenesis (reviewed by Lebrin et al., 2005; ten Dijke and Arthur, 2007). In accordance with its known cell- and context-dependent effects, TGFB1 either inhibits or promotes blood vessel formation in vivo and in vitro (Goumans et al., 2009). We observed that TGFB1 reduced both basal and FCS-stimulated CLENDO cell DNA synthesis. Furthermore, TGFB1 reduced CLENDO cell directional migration. These findings suggest that TGFB1 acts on CLENDO cells to limit angiogenic potential and/or maintain CLENDO cells in a quiescent state.
TGFB1 induces endothelial cell apoptosis in vitro in human umbilical vein endothelial cells (HUVEC) (Ferrari et al., 2009) and in bovine aortic endothelial cells (Pollman et al., 1999). In the present study, TGFB1 did not reduce the viability of CLENDO cells plated on plastic. Previous studies provide evidence that TNFα is capable of inducing apoptosis of bovine (Friedman et al., 2000; Pru et al., 2003) and murine (Henkes et al., 2008) CLENDO cells. Here, we show that TGFB1 did not alter TNFα-induced reductions in CLENDO cell viability when plated on plastic culture plates. Although TGFB1 did not reduce the viability of CLENDO cells when plated on Matrigel-coated culture plates, it enhanced the effect of TNFα in reducing the viability of CLENDO cells plated on Matrigel-coated culture plates. These context-specific actions suggest that TGFB1 might act on the luteal microvasculature to enhance the effect of other cytokines that induce endothelial cell death.
In our studies, bovine CLENDO cells plated on Matrigel in growth medium rapidly formed a network of CLSs. Following TGFB1 treatment, the capillary network became instable, with complete regression at 8 hours after plating, demonstrating that TGFB1 induces the disassembly of luteal capillary-like structures. However, it should be noted that the effect of TGFB1 on endothelial cell capillary morphogenesis is cell-type specific and modified by the context in which the TGFB1 effect is tested. For instance, TGFB1 promoted capillary morphogenesis on Matrigel by microvascular endothelial cells from skin (Serrati et al., 2009) and on collagen type I by rat epididymis fat pad endothelial cells (Sankar et al., 1996). A possible explanation for the cell-type specific effects of TGFB1 is the diversity in endothelial cells derived from different tissues and organs, arteries and veins, large and small vessels and normal and tumor vessels, presumptively as a result of the molecular differences between them and the microenvironment acting in each different tissue (Stevens et al., 2008). A well-studied example of such diversity is illustrated by the endothelial cells of the pulmonary artery and alveolar microvascular endothelium. Pulmonary microvascular endothelial cells have higher proliferative potential and form more blood vessels in both in vitro and in vivo Matrigel angiogenesis assays than do pulmonary artery endothelial cells (Alvarez et al., 2008; King et al., 2004). Pulmonary microvascular cells in vitro also exhibit a more resistant permeability barrier than do pulmonary artery endothelial cells (Kelly et al., 1998; Ofori-Acquah et al., 2008). In addition, they respond distinctively to cytokines. TGFB1 protected against pulmonary artery endothelial cell apoptosis induced by serum deprivation (Lu, 2008), but caused apoptosis of pulmonary microvascular endothelial cells, an effect that was dependent on TGFBRI function (Lu et al., 2009). Likewise, TGFB1 promoted capillary morphogenesis of bovine aortic endothelial cells (Bein et al., 2004), whereas it induced disruption of CLENDO CLSs. In the present studies, the detrimental actions of TGFB1 on CLENDO capillary morphogenesis were inhibited by SB-431542, a selective TGFBR1 receptor kinase inhibitor. The bovine corpus luteum contains multiple endothelial cell populations that differ in their morphology, cytoskeleton proteins and expression of cell adhesion proteins (reviewed in Davis et al., 2003). The CLENDO cells used in the present study, which are cytokeratin negative and VE-cadherin positive, presumptively represent the majority of luteal microvascular endothelial cells (Ricken et al., 1995; Shirasuna et al., 2007; Tscheudschilsuren et al., 2002). It is possible that, as in the examples cited above, subpopulations of luteal endothelial cells respond to TGFB1 in a manner different from that of our CLENDO cells.
Luteolysis is accompanied by considerable ECM remodeling and deposition of collagen type I (Duncan, 2000; Irving-Rodgers et al., 2006; Vonnahme et al., 2006). The predominant collagen is the fibrillar type I, which is found throughout the life of the corpus luteum and is dramatically increased during luteolysis (Zhao and Luck, 1995; Casey et al., 2005). TGFB1 is known to be one of the most potent mediators of wound healing and the fibrotic processes through stimulation of the synthesis of the ECM, including collagen type I, and the inhibition of its breakdown. Of relevance to the role of TGFB1 in CLENDO cell function during regression, we observed that TGFB1 induced apoptosis of CLENDO cells when plated on fibrillar collagen 1 gels, in contrast to CLENDO cells plated on Matrigel-coated plates. This indicates that the actions of TGFB1 on CLENDO cells are context specific. The increased expression of collagen type 1 during luteal regression might create an unfavorable environment for endothelial cells and facilitate the disruption of the microvasculature by TGFB1 and other cytokines. The ECM provides crucial signals to endothelial cells that regulate blood vessel formation and stabilization, and functions as a scaffold for the storage and presentation of many growth factors and cytokines with important roles in vascular remodeling (Davis and Senger, 2008). In addition, the ECM sends integrin-mediated signals through focal adhesions and their associated adaptors and kinases that regulate gene expression and cytoskeleton organization and cell shape (Del Pozo and Schwartz, 2007). Thus, integrins not only provide anchorage for endothelial cells, but also provide information about the microenvironment that affects cell adhesion, proliferation, migration and apoptosis. Endothelial cells express several integrins, which they use to interact with ECM proteins: integrins α1β1, α2β1, α3β1 bind collagen and laminin; integrin α6β1 binds laminin; integrins α4β1 and α5β1 bind fibronectin and fibrin; and integrins αvβ3 and αvβ5 bind vitronectin, fibronectin, fibrin and laminin (Weis, 2007). With relevance to the present studies, TGFB1 regulates the expression of numerous integrins in many cell types, including endothelial cells; the expression of certain integrins influences TGFB1 signaling (reviewed by Margadant and Sonnenberg, 2010). Additional studies are needed to examine the effects of TGFB1, as well as other cytokines that regulate luteolysis, on the expression and functions of integrins in luteal endothelial cells.
One function of endothelial cells is to control the permeation of blood proteins and cells into the vessel wall and the surrounding tissue. Paracellular permeability is controlled by the opening and closing of cell–cell junctions, a function that is crucial to maintain endothelium integrity. In response to vascular damage, endothelial cells retract, increasing the permeability of the vessel, and as a result the vessel is disrupted (Dejana et al., 2008). Bovine endothelial cells express VE-cadherin (Shirasuna et al., 2007), which forms part of adherens junction complexes with proteins p120, β-catenin and plakoglobin. Our results indicate that the exposure of confluent CLENDO cells to TGFB1 resulted in the loss of VE-cadherin from cellular junctions. The loss of VE-cadherin was most probably due to intracellular redistribution, because western blot analysis indicated that total cellular VE-cadherin levels were not changed by TGFB1 treatment. The importance of VE-cadherin to luteal vascularization is underscored by a report by Nakhuda et al. (Nakhuda et al., 2005) showing that administration of the VE-cadherin antibody E4G10 blocked vascularization and function of the mouse corpus luteum. The changes in VE-cadherin in the present study were associated with a significant increase in the permeability of CLENDO cells grown to confluence on porous membranes. Previous reports indicated that TGFB1 causes the rearrangement of adherens junction proteins in pulmonary endothelial cells (Hurst et al., 1999) and increases the permeability of bovine retinal capillary endothelial cells grown to confluent monolayers on porous membranes (Behzadian et al., 2001). Our observations that TGFB1 altered VE-cadherin expression at junctional complexes and increased the permeability of CLENDO cells have important implications for understanding luteal regression. Increased permeability might facilitate the documented influx of circulating immune cells into the regressing corpus luteum (reviewed by Townson and Liptak, 2003).
During luteal regression, alterations in the expression and action of other angiogenic-related factors might work in concert with TGFB1 to effect disruption of the microvasculature and reduce luteal blood flow. The expression of angiogenic factors, such as vascular endothelial growth factor (VEGF) (Berisha et al., 2010; Neuvians et al., 2004; Shirasuna et al., 2010), the angiopoietins (ANPGT1 and ANGPT2) (Berisha et al., 2010; Shirasuna et al., 2010; Tanaka et al., 2004) and cytokines such as TGFB1 (Hou et al., 2008) and TNFα (Henkes et al., 2008), is acutely regulated during regression of the corpus luteum. Angiopoietins play a role in stabilization of capillaries; an elevated ratio of ANGPT2: ANGPT1 decreases capillary stabilization, which, together with a low level of VEGF, results in blood vessel destabilization and regression (Yancopoulos et al., 2000). Following treatment with PGF2α, luteal VEGF protein levels decreased within 2 hours, followed by a reduction in the expression of mRNA encoding ANGPT1 and VEGF (Neuvians et al., 2004; Tanaka et al., 2004). By contrast, ANGPT2 mRNA and protein levels rapidly increased following PGF2α administration (Berisha et al., 2010; Shirasuna et al., 2010; Tanaka et al., 2004). Likewise, PGF2α induced expression of TGFB1 (Hou et al., 2008) and TNFα (Henkes et al., 2008) in luteal tissue. TNFα adversely affects luteal endothelial cells by inducing acid sphingomyelinase and ceramide-induced apoptosis (Pru et al., 2003; Henkes et al., 2008). Collectively, these findings indicate that modulation of vascular stability might be a key component in the cascade of events leading to functional luteolysis. An intriguing observation in the present study was the ability of TGFB1 to augment the cytotoxic effects of TNFα on luteal CLENDO cells on Matrigel. Additional studies are required to determine whether TGFB1 alters the effect of ANGPT2 on CLENDO cell function.
The results presented here on the activities of TGFB1 in CLENDO cells constitute an important contribution to understanding the mechanisms involved in luteolysis. These studies provide the first information on the actions of TGFB1 on microvascular endothelial cells of the corpus luteum. The results demonstrate that TGFB1 acts directly on CLENDO cells to limit endothelial cell growth, and disrupt capillary morphogenesis and endothelial barrier function. These studies suggest that TGFB1 might participate in the disassembly of luteal capillaries in vivo, thereby contributing to the regression of the corpus luteum. Understanding the mechanisms of normal luteal function could lead to possible therapeutic targets for treating infertility or for contraception.
Materials and Methods
Materials
DMEM was obtained from Mediatech (Manassas, VA) and FCS was from Cambrex (Walkersville, MD). Type II collagenase was obtained from Atlantic Biologicals (Lawrenceville, GA). Dynabeads M-450 Epoxy was purchased from Invitrogen (Camarillo, CA). BSL-I lectin and mounting medium VECTASHIELD were from Vector Laboratories (Burlingame, CA). Endothelial cell growth supplement (ECGS) was purchased from Millipore (Bedford, MA). Human TGFB1 and recombinant human TNFα were from R&D Systems (Minneapolis, MN). SB-431542, MTT, FITC–dextran (Mr 40,000) and monoclonal anti-β-actin antibody were from Sigma (St Louis, MO). P-SMAD2, P-SMAD3, SMAD2 and SMAD3 antibodies were purchased from Cell Signaling Technology (Beverly, MA), Collagen I antibody was from Rockland (Gilbertsville, PA). Cytokeratin 18 antibody was from Millipore (Billerica, MA). eNOS antibody was from BD Transduction Laboratories (San Jose, CA) and VE-cadherin antibody was obtained from Pierce (Rockford, IL). PTGFR antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-rabbit Alexa-Fluor-488-conjugated IgG was from Molecular Probes (Eugene, OR). Thymidine [methyl-3H] was from MP Biomedicals (Santa Ana, CA). Bovine collagen type I, Matrigel basement membrane growth factor reduced, Matrigel matrix thin layer multiwell plates and cell culture inserts (transparent PET membrane, 3 and 8 μm pore) were purchased from BD Biosciences (Bedford, MA). The HSD3B antibody was a gift from Ian Mason, University of Texas Southwestern Medical Center, Dallax, TX (Hou et al., 2010). Western Lightning ECL was from PerkinElmer Life Sciences (Waltham, MA). Kodak X-ray film was purchased from Fisher Scientific (Hampton, NH).
Cell isolation and culture
Bovine ovaries were collected at a local slaughterhouse from first trimester pregnant cows (fetal crown-rump length <15 cm). CLENDO cells were isolated in our laboratory from the corpus luteum by enzymatic digestion with type II collagenase, followed by affinity purification with magnetic beads. The magnetic beads (Dynabeads M-450 Epoxy) were coated with BSL-I lectin following the manufacturer's instructions. The bead-adherent cells were washed and cultured in growth medium [DMEM supplemented with 10% FCS, 20 μg/ml ECGS and antibiotics (100 UI/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml amphotericin)]. Colonies of endothelial cells identified by their morphology were subcultured and expanded for further purification by FACS using an endothelial-cell-specific antibody against VE-cadherin. FACS-purified CLENDO cells were maintained in growth medium and used for all experiments between passages 6 and 12.
FACS
Single-cell suspensions of CLENDO cells were washed once with staining buffer (PBS-2% BSA), centrifuged and resuspended in staining buffer. For surface staining, cells (5×105 in 100 μl staining buffer) were incubated with VE-cadherin antibody (0.3 μg in 100 μl) for 30 minutes at 4°C, in the dark, washed with staining buffer and centrifuged. Next, cells were incubated with anti-rabbit Alexa-Fluor-488-conjugated IgG (1:100 dilution in staining buffer) for 30 minutes at 4°C, in the dark, washed with staining buffer and centrifuged. Cells were resuspended in DMEM-1% FCS and sorted in a FACScalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
Western blot analysis
Cell monolayers were harvested from well plates with ice-cold cell lysis buffer [20 mM Tris-HCl (pH 7), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton X-100, and protease and phosphatase inhibitor cocktails]. Equal amounts of protein from cell lysates were subjected to electrophoresis under reducing conditions on 10% SDS-PAGE and electrotransferred to PVDF membranes. The membranes were blocked with TBS-T (Tris-buffered saline, 0.1% Tween 20) containing 5% nonfat dried milk at room temperature for 1 hour and incubated overnight at 4°C with a 1:1000 dilution of primary antibodies in TBS-T with 5% nonfat dried milk. After extensive washing in TBS-T, membranes were incubated for 1 hour with horseradish peroxidase (HRP)-coupled secondary antibody diluted 1:2500 in TBS-T with 2% dried milk at room temperature. After washing in TBS-T, the membranes were developed with Western Lightning ECL detection system. Detection was performed by exposure to blue-light-sensitive autoradiography film.
RNA isolation and PCR analysis
RNA from CLENDO cells, bovine steroidogenic luteal cells and bovine luteal fibroblasts was extracted with an AbsolutelyRNA kit (Agilent, Santa Clara, CA). One microgram of RNA was reverse transcribed in a total volume of 20 μl with SuperScript II reverse transcriptase (Invitrogen) and oligo(dT)15 primers. PCR primers were designed based on bovine PTGFR and GAPDH cDNA sequences using the Primer3 online primer tool (http://frodo.wi.mit.edu/primer3/input.htm). PCR was performed on 1 μl of the generated cDNA under the following conditions: 5 minutes at 95°C; (30 seconds at 95°C, 30 seconds at 55°C and 30 seconds at 72°C) for 35 cycles, and 10 minutes at 72°C. The sequences for forward and reverse primers are as follows. PTGFR: forward, 5′-GTTGAGTGGGGTGTGCTTTT-3′; reverse, 5′-ATGGCATTGCAAACAAATGA-3′. GAPDH: forward, 5′-TCTGCACCTTCTGCCGATG-3′; reverse, 5′-AGCAGTTGGTGGTGCAGGA-3′.
[3H]-thymidine incorporation
CLENDO cells were seeded in 24-well plates at a density of 2×104/well in growth medium (DMEM supplemented with 5% FCS and 20 μg/ml ECGS). After 24 hours, cells were rinsed and serum starved in DMEM for 2 hours. Cells were treated in serum-free DMEM with control or TGFB1 (0–10 ng/ml) for 24 hours and [3H]-thymidine (4 μCi/ml) was added 6 hours before the end of the incubation period. To terminate the incubations, unincorporated radioactivity was removed by washing cells with ice-cold PBS followed by the addition of 10% trichloroacetic acid for 30 minutes at 4°C. Next, wells were washed with ice-cold PBS and solubilized with 0.2 M NaOH at room temperature. The radioactivity was determined by liquid scintillation counting.
MTT assay for cell viability
CLENDO cells were seeded in 48-well plates at low (2×104/well) or high (4×104/well) density in growth medium (DMEM supplemented with 5% FCS and 20 μg/ml ECGS). For some experiments, cells were plated on Matrigel matrix thin layer multiwell plates. After 24 hours, cells were rinsed and serum starved in DMEM for 2 hours. Cells were treated in serum-free DMEM for 24 hours, as indicated in the figure legends. MTT (0.5 mg/ml) was added to each well 4 hours before the end of the incubation period. Next, the medium was removed and DMSO was added to each well. Optical density was read in a spectrophotometer (SPECTRAmax PLUS; Molecular devices, Union City, CA) using a wavelength of 570 nm.
Cell migration
Cell migration was assayed using the Boyden chamber method. CLENDO cells (5×104 in 250 μl serum-free DMEM) were seeded in cell culture inserts (transparent PET membrane, 8 μm pores) placed in 24-well plates. The lower chamber was filled with 800 μl DMEM containing 5% FCS. Cells were pretreated with SB-431542 (1 μM, 30 minutes) followed by TGFB1 (1 ng/ml) and allowed to migrate for 6 hours at 37°C. Migrated cells were fixed, stained in methanol-0.04% crystal violet and photographed. Cells migrating in the central area of the insert were photographed (100× magnification). Quantification of cell numbers was done using MicroSuite FIVE software for imaging applications from Soft Imaging System Corporation (Lakewood, CO). The experiment was repeated on five separate occasions and three inserts were used for each treatment.
In vitro capillary morphogenesis assay
Capillary morphogenesis experiments were performed using Matrigel and bovine collagen type I gels. Matrigel (0.15 ml/well; ~8 mg/ml) was pipetted into 48-well plates and polymerized for 1 hour at 37°C. Bovine collagen I was diluted in DMEM and pipetted into 48-well plates (0.15 ml/well; ~2.4 mg/ml), polymerized for 1 hour at 37°C and dried overnight at room temperature. CLENDO cells were plated (5×104/well) in growth medium (DMEM supplemented with 5% FCS and 20 μg/ml ECGS). Cells were pretreated at the time of plating in their growth medium with SB-431542 (1 μM) for 30 minutes, followed by TGFB1 (1 ng/ml). The effects on morphogenesis of endothelial cells were photographed after 8 hours for Matrigel gels, and 8 and 24 hours for collagen I gels with an inverted microscope. One picture (40× magnification) was taken in the central area of each well and three wells were used for each condition tested. Quantification of the tubule formation activity was done using MicroSuite FIVE software for imaging applications from Soft Imaging System Corporation (Lakewood, CO). In the collagen I gel experiments, viability was examined by staining cells with blue fluorescent Hoechst 33258 at 1 μg/ml for 30 minutes.
Apoptosis assay
Capillary morphogenesis was tested for CLENDO cells plated on collagen I gels and treated with TGFB1 as described above. Caspase-3 and caspase-7 activity was determined after 8 and 24 hours incubation using the Caspase–Glo 3/7 assay kit (catalog number G8091; Promega, Madison, WI) following the manufacturer's instructions. Briefly, Caspase–Glo 3/7 reagent was added to each well in a 1:1 ratio and incubated with gentle shaking for 30 minutes at room temperature before measuring luminescence using a FLUOstar OPTIMA microplate reader (BMG LABTECH, Cary, NC).
Immunofluorescence
CLENDO cells were cultured on glass cover slips and treated in serum-free DMEM with control or TGFB1 (1 ng/ml) for 24 hours. After treatment, cells were washed twice with cold PBS and fixed with ice-cold 4% paraformaldehyde for 30 minutes. Cells were permeabilized in PBS and 0.4% Triton X-100 for 10 minutes at room temperature and then blocked for 1 hour in blocking buffer (PBS, 0.2% Triton X-100, 10% FBS). Primary antibody was diluted 1:200 in blocking buffer and incubated overnight at 4°C. Cells were washed with PBS and incubated for 1 hour at room temperature with appropriate secondary fluorophore-conjugated antibodies diluted 1:400 in blocking buffer. Cells were next washed three times with PBS and mounted with VECTASHIELD.
In vitro permeability assay
CLENDO cells were seeded on cell culture inserts (transparent PET membrane, 3 μm pores, 6×104 cells/insert), placed in 24-well plates and grown to confluency in a total volume of 250 μl and 800 μl of complete growth medium (DMEM supplemented with 5% FBS and 20 μg/ml ECGS) for the upper and lower chambers, respectively. Cells were washed and pretreated with SB-431542 (1 μM, 30 minutes) followed by TGFB1 (1 ng/ml) for 24 hours in serum-free DMEM. After 24 hours, FITC–dextran (0.5 mg/ml final concentration) was added to the upper chamber. At given time points, 50 μl aliquots from the lower chamber were removed for measurement and replaced with 50 μl of fresh medium in order to maintain the hydrostatic equilibrium. The fluorescence of each sample diluted 1:20 in PBS was measured at 485/520 nm excitation/emission wavelengths using a FLUOstar OPTIMA microplate reader (BMG LABTECH Inc, Cary, NC). After the last time point, cells were fixed and stained in methanol-0.04% crystal violet, and photographed.
Statistical analyses
All data are represented as mean ± s.e.m. Unless specified in the figure legends, at least three experiments were conducted on separate occasions (n=3) and each experiment was performed in triplicate for each condition tested. Comparisons for differences between groups were made by ANOVA with Tukey's post test. In all analyses, a value of P<0.05 was considered significant.
Acknowledgments
This work was supported by grants from the Department of Veterans Affairs Biomedical Laboratory Research and Development Merit Review Research Program (J.S.D.), the Olson Center for Women's Health at the University of Nebraska Medical Center (J.S.D.) and a University of Nebraska Medical Center Graduate Studies Fellowship (D.M.).
Footnotes
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.084558/-/DC1
References
- Alghisi G. C., Ponsonnet L., Rüegg C. (2009). The integrin antagonist cilengitide activates alphaVbeta3, disrupts VE-cadherin localization at cell junctions and enhances permeability in endothelial cells. PLoS ONE 4, e4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez D. F., Huang L., King J. A., ElZarrad M. K., Yoder M. C., Stevens T. (2008). Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J. Physiol. Lung Cell. Mol. Physiol. 294, L419-L430. [DOI] [PubMed] [Google Scholar]
- Behzadian M. A., Wang X. L., Windsor L. J., Ghaly N., Caldwell R. B. (2001). TGF-β increases retinal endothelial cell permeability by increasing MMP-9: possible role of glial cells in endothelial barrier function. Invest. Ophthalmol. Vis. Sci. 42, 85385-85389. [PubMed] [Google Scholar]
- Bein K., Odell-Fiddler E. T., Drinane M. (2004). Role of TGF-beta1 and JNK signaling in capillary tube patterning. Am. J. Physiol. Cell Physiol. 287, C1012-C1022. [DOI] [PubMed] [Google Scholar]
- Berisha B., Meyer H. H. D., Schams D. (2010). Effect of prostaglandin F2 Alpha on local luteotropic and angiogenic factors during induced functional luteolysis in the bovine corpus luteum. Biol. Reprod. 82, 940-947. [DOI] [PubMed] [Google Scholar]
- Berrier A. L., Yamada K. M. (2007). Cell-matrix adhesion. J. Cell. Physiol. 213, 565-573. [DOI] [PubMed] [Google Scholar]
- Bogan R. L., Murphy M. J., Stouffer R. L., Hennebold J. D. (2008). Prostaglandin synthesis, metabolism, and signaling potential in the rhesus macaque corpus luteum throughout the luteal phase of the menstrual cycle. Endocrinology 149, 5861-5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey O. M., Morris D. G., Powell R., Sreenan J. M., Fitzpatrick R. (2005). Analysis of gene expression in non-regressed and regressed bovine corpus luteum tissue using a customized ovarian cDNA array. Theriogenology 64, 1963-1976. [DOI] [PubMed] [Google Scholar]
- Davis G. E., Senger D. R. (2008). Extracellular matrix mediates a molecular balance between vascular morphogenesis and regression. Curr. Opin. Hematol. 15, 197-203. [DOI] [PubMed] [Google Scholar]
- Davis J. S., Rueda B. R. (2002). The corpus luteum: an ovarian structure with maternal instincts and suicidal tendencies. Front. Biosci. 7, 1949-1978. [DOI] [PubMed] [Google Scholar]
- Davis J. S., Rueda B. R., Spanel-Borowski K. (2003). Microvascular endothelial cells of the corpus luteum. Reprod. Biol. Endocrinol. 10, 89-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Orsenigo F., Lampugnani M. G. (2008). The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 121, 2115-2122. [DOI] [PubMed] [Google Scholar]
- Del Pozo M. A., Schwartz M. A. (2007). Rac, membrane heterogeneity, caveolin and regulation of growth by integrins. Trends Cell Biol. 17, 246-250. [DOI] [PubMed] [Google Scholar]
- Duncan W. C. (2000). The human corpus luteum: remodeling during luteolysis and maternal recognition of pregnancy. Rev. Reprod. 5, 12-17. [DOI] [PubMed] [Google Scholar]
- Ferrari G., Cook B. D., Terushkin V., Pintucci G., Mignatti P. (2009). Transforming growth factor-beta 1 (TGF-beta1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosis. J. Cell. Physiol. 219, 449-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser H. M., Duncan W. C. (2005). Vascular morphogenesis in the primate ovary. Angiogenesis 8, 101-116. [DOI] [PubMed] [Google Scholar]
- Friedman A., Weiss S., Levy N., Meidan R. (2000). Role of tumor necrosis factor alpha and its type I receptor in luteal regression: induction of programmed cell death in bovine corpus luteum-derived endothelial cells. Biol. Reprod. 63, 1905-1912. [DOI] [PubMed] [Google Scholar]
- Fukuchi M., Miyazaki T., Fukai Y., Nakajima M., Sohda M., Masuda N., Manda R., Tsukada K., Kato H., Kuwano H. (2004). Plasma level of transforming growth factor beta1 measured from the azygos vein predicts prognosis in patients with esophageal cancer. Clin. Cancer Res. 10, 2738-2741. [DOI] [PubMed] [Google Scholar]
- Gangrade B. K., Gotcher E. D., Davis J. S., May J. V. (1993). The secretion of transforming growth factor-beta by bovine luteal cells in vitro. Mol. Cell. Endocrinol. 93, 117-123. [DOI] [PubMed] [Google Scholar]
- Gordon K. J., Blobe G. C. (2008). Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim. Biophys. Acta 1782, 197-228. [DOI] [PubMed] [Google Scholar]
- Goumans M. J., Liu Z., ten Dijke P. (2009). TGF-β signaling in vascular biology and dysfunction. Cell Res. 19, 116-127. [DOI] [PubMed] [Google Scholar]
- Henkes L. E., Sullivan B. T., Lynch M. P., Kolesnick R., Arsenault D., Puder M., Davis J. S., Rueda B. R. (2008). Acid sphingomyelinase involvement in tumor necrosis factor alpha-regulated vascular and steroid disruption during luteolysis in vivo. Proc. Natl. Acad. Sci. USA 105, 7670-7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X., Arvisais E. W., Jiang C., Chen D. B., Roy S. K., Pate J. L., Hansen T. R., Rueda B. R., Davis J. S. (2008). Prostaglandin F2alpha stimulates the expression and secretion of transforming growth factor B1 via induction of the early growth response 1 gene (EGR1) in the bovine corpus luteum. Mol. Endocrinol. 22, 403-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X., Arvisais E. W., Davis J. S. (2010). Luteinizing hormone stimulates mammalian target of rapamycin signaling in bovine luteal cells via pathways independent of AKT and mitogen-activated protein kinase: modulation of glycogen synthase kinase 3 and AMP-activated protein kinase. Endocrinology 151, 2846-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst V., 5th, Goldberg P. L., Minnear F. L., Heimark R. L., Vincent P. A. (1999). Rearrangement of adherens junctions by transforming growth factor-beta1: role of contraction. Am. J. Physiol. 276, L582-L595. [DOI] [PubMed] [Google Scholar]
- Inman G. J., Nicolás F. J., Callahan J. F., Harling J. D., Gaster L. M., Reith A. D., Laping N. J., Hill C. S. (2002). SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 62, 65-74. [DOI] [PubMed] [Google Scholar]
- Irving-Rodgers H. F., Roger J., Luck M. R., Rodgers R. J. (2006). Extracellular matrix of the corpus luteum. Semin. Reprod. Med. 24, 242-250. [DOI] [PubMed] [Google Scholar]
- Kelly J. J., Moore T. M., Babal P., Diwan A. H., Stevens T., Thompson W. J. (1998). Pulmonary microvascular and macrovascular endothelial cells: differential regulation of Ca2+ and permeability. Am. J. Physiol. 274, L810-L819. [DOI] [PubMed] [Google Scholar]
- Kiliç Z. M., Ayaz S., Ozin Y., Nadir I., Cakal B., Ulker A. (2009). Plasma transforming growth factor-beta1 level in inflammatory bowel disease. Turk. J. Gastroenterol. 20, 165-170. [DOI] [PubMed] [Google Scholar]
- King J., Hamil T., Creighton J., Wu S., Bhat P., McDonald F., Stevens T. (2004). Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc. Res. 67, 139-151. [DOI] [PubMed] [Google Scholar]
- Knight P. G., Glister C. (2006). TGF-beta superfamily members and ovarian follicle development. Reproduction 132, 191-206. [DOI] [PubMed] [Google Scholar]
- Lebrin F., Deckers M., Bertolino P., ten Dijke P. (2005). TGF-beta receptor function in the endothelium. Cardiovasc. Res. 65, 599-608. [DOI] [PubMed] [Google Scholar]
- Lu Q. (2008). Transforming growth factor-beta1 protects against pulmonary artery endothelial cell apoptosis via ALK5. Am. J. Physiol. Lung Cell. Mol. Physiol. 295, L123-L133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q., Patel B., Harrington E. O., Rounds S. (2009). Transforming growth factor-beta1 causes pulmonary microvascular endothelial cell apoptosis via ALK5. Am. J. Physiol. Lung Cell. Mol. Physiol. 296, L825-L838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margadant C., Sonnenberg A. (2010). Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 11, 97-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. (2008). TGFβ in cancer. Cell 134, 215-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto A., Okuda K., Schweigert F. J., Schams D. (1992). Effects of basic fibroblast growth factor, transforming growth factor-beta and nerve growth factor on the secretory function of the bovine corpus luteum in vitro. J. Endocrinol. 135, 103-114. [DOI] [PubMed] [Google Scholar]
- Nakhuda G. S., Zimmermann R. C., Bohlen P., Liao F., Sauer M. V., Kitajewski J. (2005). Inhibition of the vascular endothelial cell (VE)-specific adhesion molecule VE-cadherin blocks gonadotropin-dependent folliculogenesis and corpus luteum formation and angiogenesis. Endocrinology 146, 1053-1059. [DOI] [PubMed] [Google Scholar]
- Neuvians T. P., Berisha B., Schams D. (2004). Vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) expression during induced luteolysis in the bovine corpus luteum. Mol. Reprod. Dev. 67, 389-395. [DOI] [PubMed] [Google Scholar]
- Niswender G. D., Juengel J. L., Silva P. J., Rollyson M. K., McIntush E. W. (2000). Mechanisms controlling the function and life span of the corpus luteum. Physiol. Rev. 80, 1-29. [DOI] [PubMed] [Google Scholar]
- Niswender G. D., Davis T. L., Griffith R. J., Bogan R. L., Monser K., Bott R. C., Bruemmer J. E., Nett T. M. (2007). Judge, jury and executioner: the auto-regulation of luteal function. Soc. Reprod. Fertil. 64 Suppl, 191-206. [DOI] [PubMed] [Google Scholar]
- Ofori-Acquah S. F., King J., Voelkel N., Schaphorst K. L., Stevens T. (2008). Heterogeneity of barrier function in the lung reflects diversity in endothelial cell junctions. Microvasc. Res. 75, 391-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette Y., Price C. A., Carrière P. D. (2005). Follicular fluid concentration of transforming growth factor-beta1 is negatively correlated with estradiol and follicle size at the early stage of development of the first-wave cohort of bovine ovarian follicles. Domest. Anim. Endocrinol. 29, 623-633. [DOI] [PubMed] [Google Scholar]
- Pardali E., ten Dijke P. (2009). Transforming growth factor-beta signaling and tumor angiogenesis. Front. Biosci. 14, 4848-4861. [DOI] [PubMed] [Google Scholar]
- Pauli S. A., Tang H., Wang J., Bohlen P., Posser R., Hartman T., Sauer M. V., Kitajewski J., Zimmermann R. C. (2005). The vascular endothelial growth factor (VEGF)/VEGF receptor 2 pathway is critical for blood vessel survival in corpora lutea of pregnancy in the rodent. Endocrinology 146, 1301-1311. [DOI] [PubMed] [Google Scholar]
- Plendl J. (2000). Angiogenesis and vascular regression in the ovary. Anat. Histol. Embryol. 29, 257-266. [DOI] [PubMed] [Google Scholar]
- Pollman M. J., Naumovski L., Gibbons G. H. (1999). Vascular cell apoptosis: cell type-specific modulation by transforming growth factor-beta1 in endothelial cells versus smooth muscle cells. Circulation 99, 2019-2026. [DOI] [PubMed] [Google Scholar]
- Pru J. K., Lynch M. P., Davis J. S., Rueda B. R. (2003). Signaling mechanisms in tumor necrosis factor alpha-induced death of microvascular endothelial cells of the corpus luteum. Reprod. Biol. Endocrinol. 1, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsauer M., D'Amore P. A. (2007). Contextual role for angiopoietins and TGFbeta1 in blood vessel stabilization. J. Cell Sci. 120, 1810-1817. [DOI] [PubMed] [Google Scholar]
- Ricken A. M., Spanel-Borowski K., Saxer M., Huber P. R. (1995). Cytokeratin expression in bovine corpora lutea. Histochem. Cell Biol. 103, 345-354. [DOI] [PubMed] [Google Scholar]
- Robinson R. S., Woad K. J., Hammond A. J., Laird M., Hunter M. G., Mann G. E. (2009). Angiogenesis and vascular function in the ovary. Reproduction 138, 869-881. [DOI] [PubMed] [Google Scholar]
- Sankar S., Mahooti-Brooks N., Bensen L., McCarthy T. L., Centrella M., Madri J. A. (1996). Modulation of transforming growth factor beta receptor levels on microvascular endothelial cells during in vitro angiogenesis. J. Clin. Invest. 97, 1436-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serratì S., Margheri F., Pucci M., Cantelmo A. R., Cammarota R., Dotor J., Borràs-Cuesta F., Fibbi G., Albini A., Del Rosso M. (2009). TGFβ1 antagonistic peptides inhibit TGFβ1-dependent angiogenesis. Biochem. Pharmacol. 77, 813-825. [DOI] [PubMed] [Google Scholar]
- Shirasuna K., Watanabe S., Nagai K., Sasahara K., Shimizu T., Ricken A. M., Spanel-Borowski K., Miyamoto A. (2007). Expression of mRNA for cell adhesion molecules in the bovine corpus luteum during the estrous cycle and PGF2alpha-induced luteolysis. J. Reprod. Dev. 53, 1319-1328. [DOI] [PubMed] [Google Scholar]
- Shirasuna K., Sasahara K., Matsui M., Shimizu T., Miyamoto A. (2010). Prostaglandin F2alpha differentially affects mRNA expression relating to angiogenesis, vasoactivation and prostaglandins in the early and mid corpus luteum in the cow. J. Reprod. Dev. 56, 428-436. [DOI] [PubMed] [Google Scholar]
- Stevens T., Phan S., Frid M. G., Alvarez D., Herzog E., Stenmark K. R. (2008). Lung vascular cell heterogeneity: endothelium, smooth muscle, and fibroblasts. Proc. Am. Thorac. Soc. 5, 783-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco C., Callegari E., Gibori G. (2001). Opposite effect of prolactin and prostaglandin F(2α) on the expression of luteal genes as revealed by rat cDNA expression array. Endocrinology 142, 4158-4161. [DOI] [PubMed] [Google Scholar]
- Stocco C., Telleria C., Gibori G. (2007). The molecular control of corpus luteum formation, function, and regression. Endocr. Rev. 28, 117-149. [DOI] [PubMed] [Google Scholar]
- Stouffer R. L., Xu F., Duffy D. M. (2007). Molecular control of ovulation and luteinization in the primate follicle. Front. Biosci. 12, 297-307. [DOI] [PubMed] [Google Scholar]
- Tanaka J., Acosta T. J., Berisha B., Tetsuka M., Matsui M., Kobayashi S., Schams D., Miyamoto A. (2004). Relative changes in mRNA expression of angiopoietins and receptors tie in bovine corpus luteum during estrous cycle and prostaglandin F2alpha-induced luteolysis: a possible mechanism for the initiation of luteal regression. Reprod. Dev. 50, 619-626. [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Arthur H. M. (2007). Extracellular control of TGFbeta signalling in vascular development and disease. Nat. Rev. Mol. Cell Biol. 8, 857-869. [DOI] [PubMed] [Google Scholar]
- Townson D. H., Liptak A. R. (2003). Chemokines in the corpus luteum: implications of leukocyte chemotaxis. Reprod. Biol. Endocrinol. 1, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscheudschilsuren G., Aust G., Nieber K., Schilling N., Spanel-Borowski K. (2002). Microvascular endothelial cells differ in basal and hypoxia-regulated expression of angiogenic factors and their receptors. Microvasc. Res. 63, 243-251. [DOI] [PubMed] [Google Scholar]
- Villar A. V., Cobo M., Llano M., Montalvo C., González-Vílchez F., Martín-Durán R., Hurlé M. A., Nistal J. F. (2009). Plasma levels of transforming growth factor-b1 reflect left ventricular remodeling in aortic stenosis. PLoS ONE 4, e8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonnahme K. A., Redmer D. A., Borowczyk E., Bilski J. J., Luther J. S., Johnson M. L., Reynolds L. P., Grazul-Bilska A. T. (2006). Vascular composition, apoptosis, and expression of angiogenic factors in the corpus luteum during prostaglandin F2alpha-induced regression in sheep. Reproduction 131, 1115-1126. [DOI] [PubMed] [Google Scholar]
- Wang Z., Tamura K., Yoshie M., Tamura H., Imakawa K., Kogo H. (2003). Prostaglandin F2alpha-induced functional regression of the corpus luteum and apoptosis in rodents. J. Pharmacol. Sci. 92, 19-27. [DOI] [PubMed] [Google Scholar]
- Weis S. M. (2007). Evaluating integrin function in models of angiogenesis and vascular permeability. Methods Enzymol. 426, 505-528. [DOI] [PubMed] [Google Scholar]
- Wu M. Y., Hill C. S. (2009). TGF-beta superfamily signaling in embryonic development and homeostasis. Dev. Cell. 16, 329-343. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Davis S., Gale N. W., Rudge J. S., Wiegand S. J., Holash J. (2000). Vascular-specific growth factors and blood vessel formation. Nature 407, 242-248. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Luck M. R. (1995). Gene expression and protein distribution of collagen, fibronectin and laminin in bovine follicles and corpora lutea. J. Reprod. Fertil. 104, 115-123. [DOI] [PubMed] [Google Scholar]