Abstract
Background
Given recent challenges in developing new treatments for Alzheimer dementia (AD), it is vital to explore alternate treatment targets, such as neuromodulation for circuit dysfunction. We previously reported an exploratory Phase IIb double-blind trial of deep brain stimulation targeting the fornix (DBS-f) in mild AD (the ADvance trial). We reported safety but no clinical benefits of DBS-f versus the delayed-on (sham) treatment in 42 participants after one year. However, secondary post hoc analyses of the one-year data suggested a possible DBS-f benefit for participants ≥ 65 years.
Objective
To examine the long-term safety and clinical effects of sustained and delayed-on DBS-f treatment of mild AD after two years.
Methods
42 participants underwent implantation of DBS-f electrodes, with half randomized to active DBS-f stimulation (early on) for two years and half to delayed-on (sham) stimulation after 1 year to provide 1 year of active DBS-f stimulation (delayed on). We evaluated safety and clinical outcomes over the two years of the trial.
Results
DBS-f had a favorable safety profile with similar rates of adverse events across both trial phases (years 1 and 2) and between treatment arms. There were no differences between treatment arms on any primary clinical outcomes. However, post-hoc age group analyses suggested a possible benefit among older (>65) participants.
Conclusion
DBS-f was safe. Additional study of mechanisms of action and methods for titrating stimulation parameters will be needed to determine if DBS has potential as an AD treatment. Future efficacy studies should focus on patients over age 65.
Keywords: Alzheimer’s disease, dementia, treatment, deep brain stimulation, fornix, delayed start
Introduction
The disappointing results of several anti-amyloid trials [1,2] warrant the exploration of alternative Alzheimer disease (AD) treatment strategies. One potential target is the circuit dysfunction known to be part of the AD pathological process [3]. Such dysfunction is thought to lead to disruption in brain networks [4] and may to contribute to cognitive impairment [5]. Deep brain stimulation (DBS) has been shown to modulate the activity of motor circuits in patients with Parkinson’s disease [6], and may have utility for the modulation of dysfunctional neural circuits implicated in AD. Studies in rodents indicate that stimulation of memory circuits increases adult rat hippocampal neurogenesis [7], as well as trophic factors and markers of synaptic plasticity [8]. Further, DBS has been shown to improve memory in a mouse model of Rett Syndrome [9], improve spatial memory in healthy adult mice [10], and reverse the negative memory effects of scopolamine in rats [11].
The phase I open-label trial of DBS targeting the fornix (DBS-f) in 6 adults with mild AD demonstrated a favorable safety profile. In some patients there was increased cerebral glucose metabolism in AD-related brain regions, and possibly attenuated decline in cognitive measures relative to what would have been expected among untreated individuals [12]. The degree of hippocampal atrophy was also lessened compared to a matched AD group [13]. An additional study of one participant reported no significant adverse events, with stabilization of cognitive measures which were previously declining [14]. Following these reports, we undertook the ADvance trial, a phase IIb, randomized, double-blind, sham-controlled, multi-site trial involving 42 participants with mild AD (ClinicalTrials.gov NCT01608061). In order to maintain the blind during the first 12 months of ADvance, all eligible participants underwent electrode implantation surgery after which half were randomly selected to have their electrodes “blindly” activated to DBS-f stimulation (early-on) while the others had a sham treatment (delayed on) for 12 months [15]. All were taking a stable cholinesterase inhibitor medication dose (donepezil, galantamine, or rivastigmine) for at least 2 months prior to study initiation. The rate of acute serious device- or procedure-related adverse events was 7.1%. Of the three long-term study-related serious adverse events which occurred during the first year, none were in the early on (active stimulation) arm [16]. At 6 months, those in the delayed on (control-no stimulation) arm had small (1–5%) decreases in glucose metabolism in all regions. The early on arm demonstrated substantial, statistically significant increases of glucose metabolism in several brain regions in the default mode network at 6 months. These increases continued but were not statistically significant at twelve months [16]. Both arms showed similar declines on the primary cognitive outcomes, but in post-hoc subgroup analyses there was a suggestion of benefit to older (>65) participants [16].
At 12 months, the implanted electrodes were activated in the participants who had been randomized to the delayed on arm, and these participants were followed for an additional “active” stimulation year (12–24 months) along with the early on participants, who continued on active stimulation. In this paper, we compare rates of adverse events and clinical outcome measures in both treatment arms to assess differences between and within arms between the first 12 months (phase 1 with a sham-controlled group) and the second 12 months (phase 2) during which both arms received DBS-f stimulation. Further, we model cognitive outcome trajectories for each treatment arm during both phases and assess whether the trajectories changed after activation in the delayed on arm relative to the early on arm.
Methods and Materials
The methods of the ADvance trial have been described in detail elsewhere [15]. In brief, 42 participants were enrolled at 6 US and 1 Canadian trial sites. Participants were aged 45 to 85, met criteria for mild probable AD (Clinical Dementia Ratings (CDR) of 0.5 or 1 and Alzheimer’s Disease Assessment Scale-11 (ADAS-Cog 11) scores of 12–24 [17], had a caregiver informant, and were on a stable dose of an acetylcholinesterase inhibitor. The study protocol was approved by independent research ethics boards at each site. All participants and their caregivers signed informed consent in person. The trial was overseen by the Food and Drug Administration and Health Canada under Investigational Device Exemption (IDE) G110220, and was registered with clinicaltrials.gov (NCT01608061).
Surgical and Clinical Methods
All participants underwent bilateral implantation of Medtronic 3387 electrodes placed approximately 2mm anterior and parallel to the columns of the fornices. Two weeks after surgery, participants were randomized 1:1 to receive either early stimulation or sham stimulation followed by delayed activation of the implant after 12 months. Participants had clinic visits and safety monitoring one month after implantation and activation (at months 1 and 13), and then every three months. Adverse events were reviewed and adjudicated in real time by a masked internal Clinical Events Committee (CEC), and every 6 months by an unmasked external Data Safety and Monitoring Board.
Neuropsychological Measures
Neuropsychological assessments were obtained at 3-month intervals during the first year and at 6-month intervals during the second year. The primary outcomes were the Alzheimer’s Disease Assessment Scale-cognitive subscale 13 (ADAS-cog 13) [18] and the Clinical Dementia Rating sum of boxes (CDRsb) [19]. Secondary outcomes included the second edition of the California Verbal Learning Test (CVLT-II) [20] sum of first five trials, and the Neuropsychiatric Inventory (NPI) total score [21].
Analytic Plan
For each outcome we fit longitudinal mixed effects models with linear spline terms at 12 months to allow for different slopes following activation in the delayed on arm. These models allowed us to calculate model-based change scores for each study phase and treatment arm. Because post-hoc subgroup analyses of phase 1 data suggested possible age effects, we also fit a model that adjusted for age group at baseline (above or below 65) and that included interactions between age-group-specific phase and treatment arm effects. See the appendix for detailed descriptions of these models.
Analyses were conducted according to intention-to-treat principles, and were adjusted for potential site effects. As this was a phase IIb study, sample size was selected to demonstrate feasibility and safety of DBS-f, rather than efficacy.
Results
At the start of phase 1, 42 participants were randomly assigned to either early on (n=21) or delayed on (n=21) DBS-f treatment arms. All 42 participants were followed to the end of phase 1 (12 months). but 2 participants were not followed after 12 months (both early on), 3 participants were not seen at 18 months, but were seen at 24 (2 early on, 1 delayed on), and 3 subjects were not seen at 24 months (1 early on, 2 delayed on).
Safety Results
Surgical safety [22], and phase 1 safety as of April 30th, 2015 of the trial [16] were reported elsewhere. During phase 2, 15 serious adverse events (SAE) were reported by 8 participants, and 86 non-serious adverse events were reported by 24 participants. Of the 15 SAEs, 7 involved syncope and/or falls (2 early on, 5 delayed on), 2 involved altered mental status (one in each arm), 2 involved seizures or possible seizures (both in the same early on participant pt02002, aged 58), and 1 involved agitation in a delayed on participant. The final 3 occurred in the same early on participant, and included a skin infection, suspected aortic valve endocarditis, and rigidity. None of these phase 2 SAEs were adjudicated by the CEC to be related to study participation. Regarding the non-serious phase 2 adverse events, the most common were neurological (including falls, headache, and muscle spasms), genitourinary (including urinary tract infections, urgency, and incontinence), and pulmonary (including upper respiratory infections and dyspnea). Rates of adverse events were similar in pattern and number comparing phase 1 and phase 2 and were also similar across treatment arms.
Cognitive and Neuropsychiatric Results
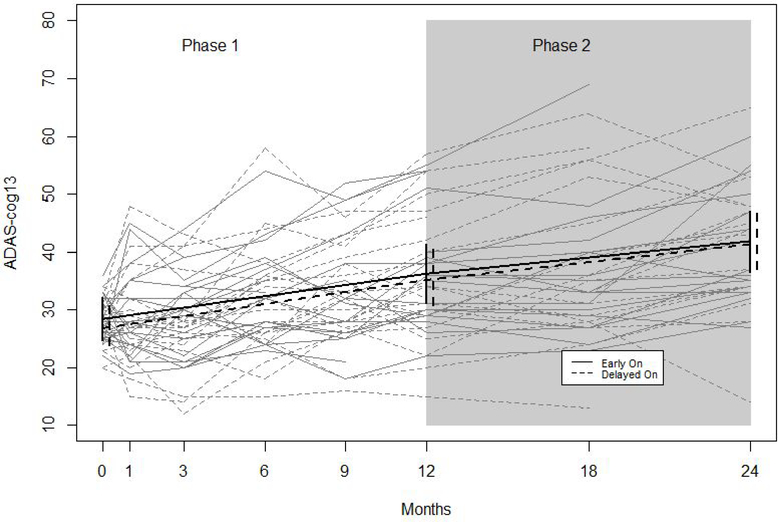
Model-based change scores from the longitudinal mixed effects linear spline models are in table 2. More detailed parameter estimates may be found in supplemental table S1. During phase 1 individuals in both treatment arms worsened over time on all outcomes, as demonstrated by significantly positive model-based change scores for ADAS-cog 13, CDRsb, and NPI and significantly negative change scores for CVLT. Change scores during phase 1 did not vary significantly by treatment arm. In comparing change scores between phase 1 and phase 2, there were no significant differences on any of the outcomes within the delayed on arm, nor within the early on arm. Figure 1 depicts observed and model-based trajectories on the ADAS-cog 13. The trajectories for both treatment arms are parallel during phase 1 and are essentially unchanged during phase 2.
Table 2.
Model-based change scores by treatment arm and phase for primary analysis
| Delayed On | Early On | |||
|---|---|---|---|---|
| Outcome | Phase 1 | Phase 2 | Phase 1 | Phase 2 |
| ADAS-cog 13 | 8.33(1.82) | 6.16(1.97) | 7.83(1.86) | 5.60(1.85) |
| CDRsb | 2.59(.64) | 2.59(.61) | 2.41(.402) | 1.98(.57) |
| CVLT* | −4.37(1.38) | −3.77(1.35) | −4.28(1.02) | −2.61(1.65) |
| NPI** | 6.39(2.22) | 1.40(1.64) | 5.57(1.63) | −.94(1.72) |
Note: values are fitted change per year (SE)
CVLT: California Verbal Learning Test, Trials1 – 5
NPI: Neuropsychiatric Inventory
Figure 1. Observed and Model-based Trajectories of ADAS-cog 13 By Treatment Arm and Study Phase.
Figure 1 shows observed trajectories (grey) and fitted trajectories (black) from a longitudinal mixed effects model with a random intercept and adjustment for site effects. Solid lines denote trajectories of participants randomized to the early on arm, and dashed lines denote trajectories for participants randomized to the delayed on arm. Vertical lines denote 95% confidence intervals.
Subgroup Analyses by Age
We conducted post hoc subgroup analyses to determine if treatment effects varied by age group using > 65 years as the cut-off as reported previously [16]. There were 12 participants under 65 (6 per study arm) and 30 participants over 65 (15 per study arm). No participant was exactly 65 at baseline. Table 3 displays model-based change scores from longitudinal mixed effects linear spline models with additional terms for age group. More detailed parameter estimates are in supplemental table S3.
Table 3.
Model-based change scores by treatment arm and phase, stratified by age
| Under 65 | ||||
|---|---|---|---|---|
| Delayed On | Early On | |||
| Outcome | Phase 1 | Phase 2 | Phase 1 | Phase 2 |
| ADAS-cog 13 | 10.51(3.66) | 7.57(5.51) | 17.13(3.50) | −.26(3.27) |
| CDRsb | .45(.43) | 3.61(1.40) | 3.07(.73) | 4.07(1.40) |
| CVLT | −8.36(3.24) | −3.96(2.34) | −5.28(1.42) | −6.03(2.59) |
| NPI | 7.46(5.52) | 2.59(3.71) | 12.26(3.02) | 2.16(4.06) |
| Over 65 | ||||
| ADAS-cog 13 | 7.41(2.05) | 5.70(1.81) | 3.99(1.14) | 8.14(1.90) |
| CDRsb | 3.45(.78) | 2.20(.62) | 2.13(.45) | 1.38(.46) |
| CVLT | −2.73(1.20) | −3.76(1.66) | −3.83(1.28) | −1.83(1.81) |
| NPI | 5.95(2.17) | .97(1.77) | 2.86(1.37) | −1.32(1.36) |
Note: values are fitted change per year (SE)
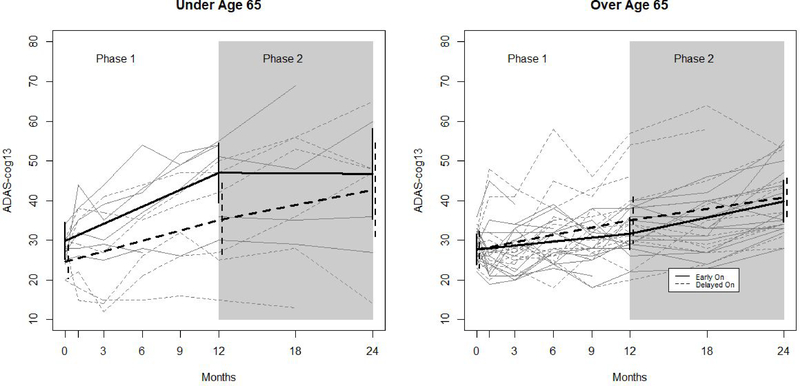
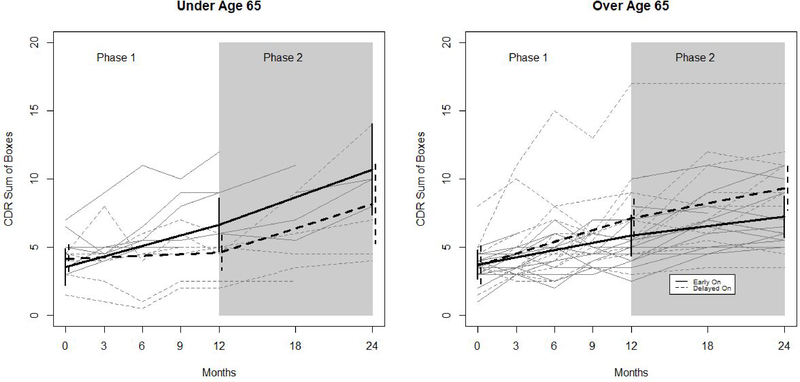
In the younger participants <65 years old, there were no differences in phase 1 model-based change scores between treatment arms for ADAS-cog 13, CVLT, or NPI. In figure 2, this is reflected by the similarly upward slopes of both lines during phase 1 among younger participants. By contrast, younger participants in the early on group worsened more than those in the delayed on group on the CDRsb (change score difference: 2.62 (0.85), p <0.002). In figure 3, this is reflected by the steeper slope among early on participants as compared to delayed on participants during phase 1.
Figure 2. Observed and Model-based Trajectories of ADAS-cog 13 By Age Group, Treatment Arm, and Study Phase.
Figure 2. shows observed trajectories (grey) and fitted trajectories (black) based on a longitudinal mixed effects model with a random intercept and adjustment for site effects. Trajectories are shown separately for younger (N=12; 6 per arm) and older participants (N=30; 15 per group). Solid lines denote trajectories of participants randomized to the early on arm, and dashed lines denote trajectories for participants randomized to the delayed on arm. Vertical lines denote 95% confidence intervals
Figure 3. Observed and Model-based Trajectories of CDR Sum of Boxes By Age Group, Treatment Arm, and Study Phase.
Figure 3 shows observed trajectories (grey) and fitted trajectories (black) based on a longitudinal mixed effects model with a random intercept and adjustment for site effects. Trajectories are shown separately for younger (N=12; 6 per arm) and older participants (N=30; 15 per group). Solid lines denote trajectories of participants randomized to the early on arm, and dashed lines denote trajectories for participants randomized to the delayed on arm. Vertical lines denote 95% confidence intervals.
There were no statistically significant differences in change scores between phase 1 and phase 2 among younger delayed on participants, suggesting no effect of DBS-f activation on cognitive trajectory. However, these participants did worsen more on CDRsb during phase 2 (stimulation on) than during phase 1 (3.60 (1.40) vs. .45 (.43); p=0.054), though this difference was not statistically significant. This is reflected in figure 3 by the upward turn in trajectory of CDRsb for the delayed on arm. Younger early on participants worsened less during phase 2 than during phase 1 on ADAS-cog13 (−.26 (3.27) vs 17.13 (3.50); p<0.001). This is reflected in figure 2 by the downward turn in trajectory of ADAS-cog13 for the early on arm.
Among older (>65) participants, those in the delayed on group worsened significantly on all outcomes during phase 1. Older participants in the early on group also worsened in phase 1, but less so. This is reflected in figures 2 and 3 by the steeper initial trajectories of the delayed on group, relative to the early on group, for both ADAS-cog13 and CDRsb. These phase 1 treatment arm effects were not statistically significantly different from 0 among older participants on any outcome. There were no statistically significant differences in change scores between phase 1 and phase 2 among older participants in either treatment arm. This is reflected in figures 2 and 3 by the essentially unchanged trajectories in both treatment arms.
There were some differences in change scores as a function of age group. Among individuals in the early on arm during phase 1, younger participants worsened on the ADAS-cog13 significantly more than older participants (3.99 (1.14) vs. 17.13 (3.50); p<0.001), but the between age group difference in phase 1 treatment arm differences was not statistically significant (−3.42 (2.35) vs. 6.61 (5.05), p=0.072). The pattern was also present on the CDRsb, and the between age group difference in phase 1 treatment arm differences was statistically significant (−1.32 (.90) vs. 2.62 (.85); p=0.001). This is reflected in figures 2 and 3 where we see that among younger subjects, the early on arm fares worse than the delayed on arm, but among older subjects, the early on arm fares better.
Among younger early on participants, ADAS-cog 13 change scores decreased less between phase 1 and phase 2 (possibly indicating slowing of progression), but among older early on participants, ADAS-cog 13 changes scores increased (indicating cognitive worsening; 4.15 (2.42) vs. −17.40 (3.83); p<0.001). Among younger delayed on participants, CDRsb change scores increased between phase 1 and phase 2, but among older delayed on participants, CDRsb change scores decreased (−1.26 (.99) vs. 3.15 (1.64); p=0.021).
Discussion
We present the safety and clinical outcome data from the second experimental phase (12–24 months) of a clinical trial of DBS-f for treatment of mild AD dementia. All participating subjects received active DBS-f stimulation from months 12–24. During phase 2, severe adverse events were rare (0.33/person), did not differ as a function of treatment arm assignment (early-on versus delayed-on), and none were adjudicated to be related to study participation. Other adverse events did not differ by treatment arm assignment, and followed an expected pattern given the age and condition of the participants. The observed safety profile in this study is consistent with long-term follow-up of individuals receiving DBS of other brain regions for the treatment of movement disorders [23,24].
The primary efficacy analyses showed no differences between treatment arms with regard to change on clinical outcomes in either phase of the study. This finding was consistent with the previous report of the phase 1 (first 12 months) results [16], and may have reflected placebo effects of the sham surgery. As noted, post hoc subgroup analyses by age group in phase 1 suggested a possible DBS-f treatment benefit in participants over age 65. Consequently, we undertook additional secondary analyses of the phase 2 data (12–24 months) to examine age group effects. During phase 1, younger participants in the early on arm may have fared worse than those in the delayed on arm, though this pattern was not statistically significant and was not seen consistently across individual outcomes. By contrast, the early on arm showed less worsening compared to the delayed on arm in the older participants (>65 years old at the time of study entry) suggesting a possible sustained benefit of DBS-f. Again, these post-hoc results were neither statistically significant nor consistent across all four outcomes.
In comparing phase 1 and phase 2 among younger participants, the delayed on arm fared worse on the CDRsb in phase 2, suggesting a deleterious effect of DBS-f activation, though this between phase difference was not statistically significant. In looking at the CDRsb change scores across treatment arms and phases and age groups (table 3), we see that this between-phase difference in the younger delayed on participants may have resulted from a flat trajectory during phase 1, rather than a steep trajectory during phase 2. By contrast, younger early on participants fared better on the ADAS-cog13 during phase 2 as compared to phase 1, suggesting a delayed beneficial effect of DBS-f activation. Neither finding was consistent across outcomes.
In comparing phase 1 and phase 2 among older participants, there were no statistically significant differences in change scores on any of the outcomes in either the delayed on or early on treatment arms. We note nevertheless that the CDRsb scores in this older age group deteriorated less in patients receiving DBS for both Phase 1 and Phase 2 (Table 3). In addition the between-phase differences in change scores within the delayed on arm were in the same direction and of similar magnitude as between-treatment arm differences in changes scores during phase 1. Though this phase IIb trial was not powered for efficacy, it is possible that a future larger trial in AD patients over age 65 might detect statistically significant beneficial effects of DBS-f.
Given the favorable safety profile and possible treatment benefits among older participants, there is an argument to be made for continuing to explore DBS-f as a treatment for late onset AD. In view of the projected increases worldwide in AD incidence and prevalence, the costs (both monetary and human) that such increases will entail [25], and the current lack of any disease-modifying treatments, it is vital that potentially efficacious treatments be fully explored. One explanation for the possible age-related treatment effect differences noted in this study may be that the younger individuals had comparatively more severe pretreatment brain pathology, reflected by greater structural and functional neuroimaging deficits than the older individuals [26,27]. Some or all of the younger participants in our trial may have already progressed, in a neuropathological sense, past the mild stage of AD that was the designated target population for this treatment modality. We noted that variability in illness trajectory was greatest among the younger study participants throughout the 2 year follow up period, and there were only 12 participants under the age of 65. Future DBS-f trials may elect to limit enrollment to a more homogeneous mild AD population, perhaps enrolling only older individuals where both the diagnosis and treatment progression are better documented prior to randomization.
There are a number of characteristics of DBS-f itself that might enhance its efficacy. Potentially modifiable parameters include frequency, pulse width, voltage, and pattern of stimulation. When DBS is used for the treatment of Parkinson’s disease, stimulation parameters are titrated based on the immediately observable effect on motor symptoms [28]. There is currently no analogous short-term signal of benefit in the context of AD. Identification of such a signal, for example, through EEG, could guide the choice of stimulation parameters to be tested [16]. Exploration of other sites of stimulation may also be fruitful. In a recent open-label trial of DBS of the nucleus basalis of Meynert, four of six participants were considered treatment responders [29], and DBS of the frontal lobes has also been explored [30].
Further investigation into the underlying mechanism of action, as well as identification of a metric to guide, in real time, the choice of stimulation parameters is needed to determine if DBS-f has potential as a treatment for AD. Additionally, DBS-f may be a model for understanding mechanisms by which brain stimulation can improve outcomes in AD, and we may be able to build on these results to use less invasive brain stimulation methods such as repetitive transcranial magnetic stimulation, transcranial direct current stimulation, or other noninvasive methods yet to be developed that can effectively target deep brain regions such as the fornix. Also, identification of a reliable imaging marker to track progression would be useful for future studies.
In conclusion, DBS-f appeared to be safe when given to patients with mild AD over a 2-year period. It must be noted that this study was not powered to assess efficacy, and therefore any interpretations of efficacy analyses must be made with caution. However, based on the post hoc subgroup analyses, if there is benefit, it is most likely to be found among individuals over 65.
Supplementary Material
Table 1.
Summary of Adverse Events by Category and Treatment Group in Phase 2
| All Adverse Events | Serious Adverse Events | |||
|---|---|---|---|---|
| Delayed On | Early On | Delayed On | Early On | |
| Programming | 6 (11%) | 0 | 0 | 0 |
| Psychiatric | 9 (17%) | 14 (27%) | 1 (14%) | 0 |
| General Medical | 38 (72%) | 38 (73%) | 6 (86%) | 8 (100%) |
| Event Subcategory | ||||
| Auditory/Ocular/Oral (HEENT) | 0 | 1 | 0 | 0 |
| Cardiovascular | 6 | 5 | 4 | 2 |
| Constitutional | 1 | 1 | 0 | 0 |
| Dermatological | 5 | 4 | 0 | 1 |
| Endocrine/Metabolic (Lab Abnormalties) | 1 | 0 | 0 | 0 |
| Gastrointestinal | 2 | 1 | 0 | 0 |
| Genitourinary | 4 | 1 | 0 | 0 |
| Hematology/Oncology | 0 | 2 | 0 | 0 |
| Infectious Disease | 1 | 0 | 0 | 0 |
| Neurological | 13 | 15 | 2 | 5 |
| Ortho/Musculoskeletal | 2 | 4 | 0 | 0 |
| Pulmonary/Upper Respiratory | 3 | 5 | 0 | 0 |
| Total | 53 | 52 | 7 | 8 |
Acknowledgements
This research is supported by the National Institute on Aging (R01AG042165), Federal Economic Development Agency for Southern Ontario, and Functional Neuromodulation Ltd., the sponsor of the ADvance study. The ADvance Study team includes:
Functional Neuromodulation: Todd Langevin, Lisa Fosdick, Kristen Drake, Donald E. Reymers, Robyn Moxon, Dan O’Connell, Vince Owens, Cara Pendergrass, Susan Klees, Steven D. Targum, and the seven participating clinical trial sites:
Chair’s Office at Johns Hopkins University and University of Toronto: Constantine G. Lyketsos, MD, MHS, Co-PI, Elizabeth Plank Althouse Professor and Chair of Psychiatry and Behavioral Sciences at Johns Hopkins Bayview; Andres M. Lozano, MD, PhD, FRCSC, FACS, Co-PI, Professor, and Chair of Neurosurgery, Tasker Chair of Functional Neurosurgery; Gwenn Smith, PhD, Imaging Core Director, Richman Family Professor of Psychiatry and Behavioral Sciences, Johns Hopkins University; Cynthia A. Munro, PhD, Neuropsychologist, Associate Professor of Psychiatry and Behavioral Sciences, Johns Hopkins University; Esther S. Oh, MD, PhD, Medical Monitor and CEC Chair, Associate Professor of Geriatric Medicine, Johns Hopkins University; Jeannie-Marie Leoutsakos, PhD, Biostatistics and Methodology Core Leader, Associate Professor of Psychiatry Behavioral Sciences, Johns Hopkins University.
Banner Alzheimer’s Institute, Phoenix: Anna Burke, MD, Geriatric Psychiatrist, Dementia Specialist; Francisco A. Ponce, MD, Associate Professor of Neurosurgery, Director Barrow Center for Neuromodulation. Banner Sun Health Research Institute, Sun City: Marwan Sabbagh, MD, Director, Banner Sun Health Research Institute; Francisco A. Ponce, MD, Associate Professor of Neurosurgery, Director Barrow Center for Neuromodulation.
Brown University, Rhode Island Hospital, Butler Hospital: Stephen Salloway, MD/MS Professor of Neurology, Director of Neurology and Memory and Aging Program; Rees Cosgrove, MD/PhD (Currently at Brigham and Women’s Hospital, Department of Neurosurgery); Wael Asaad, MD/PhD, Assistant Professor of Neurosurgery, Director of Functional Neurosurgery and Neuromodulation.
Johns Hopkins University School of Medicine, Baltimore MD: Paul Rosenberg, MD, Associate Professor, Associate Director, Memory and Alzheimer’s Treatment Center; William S. Anderson, MD, PhD, Associate Professor of Neurosurgery, Zoltan Mari, MD, Associate Professor of Neurology, Ned Sacktor, MD, Professor of Neurology.
University of Florida – Gainesville: Michael S. Okun, MD, Professor of Neurology, Co-Director of the Center for Movement Disorders and Neurorestoration; Kelly D. Foote, MD, Professor of Neurosurgery, Co-Director for Center for Movement Disorders and Neurorestoration. University of Pennsylvania: David A. Wolk, MD, Associate Professor of Neurology, Assistant Director Penn Memory Center; Gordon Baltuch, MD/PhD, Professor of Neurosurgery, Director Center for Functional and Neurorestorative Neurosurgery.
University of Toronto/Toronto Western Hospital: Andres M. Lozano, MD, PhD, FRCSC, FACS, Professor of Neurosurgery, Tasker Chair of Functional Neurosurgery; David F. Tang-Wai, MDCM FRCPC, Associate Professor of Neurology & Geriatric Medicine; Mary Pat McAndrews, PhD, Professor of Psychology; Peter Giacobbe, MD, Assistant Professor of Psychiatry. The study was conceived, designed and conducted by the investigators. The funding sources (National Institutes of Health, Federal Economic Development Agency for Southern Ontario, Functional Neuromodulation) helped in the collection, verification and storage of the data but not in data interpretation or writing the manuscript. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
William S. Anderson
Consulting Fees:
I serve as a compensated consultant for Longeviti, LLC. This is a company that makes custom fit cranioplasty implants.
Wael F. Asaad
Patents/Royalties
Patent Pending: “Improved Intracranial Fixation Device” for DBS electrodes and other intracranial probes.
Anna Burke
Consulting Fees:
Consulting-Eli Lilly
Grants
Agency: Banner Alzheimer’s Institute has received research support from FNM for the ADVance Study.
Dates: 2013–2016
Kristen E. Drake
Equity:
Stock options held in Functional Neuromodulation.
Sponsors:
Employed by Functional Neuromodulation (as Senior Clinical Manager) for duration of the study.
Kelly D. Foote
Grants
Agency: Medtronic
Dates: 2007-present
Agency: ANS/St. Jude
Dates: 2008–2014
Agency: Functional Neuromodulation
Dates: 2012–2015
Agency: Neuropace
Dates: 2010–2013
Agency: Boston Scientific
Dates: 2013-present
Lisa Fosdick
Equity:
I have stock options as part of my employment.
Sponsors:
Senior Operations Manager and also provided data management and analytic help
Peter Giacobbe
Lecture Fees:
Speakers Bureau/Honoraria: BMS, Lundbeck
Andres M. Lozano
Consulting Fees:
Medtronic St Jude Boston Scientific Aleva Functional Neuromodulation Equity:
Functional Neuromodulation
Lecture Fees:
Occasional Lecture fees for Medtronic, St Jude. Boston Scientific, Aleva
Patents/Royalties
Patents in the field of deep brain stimulation
Constantine G. Lyketsos
Consulting Fees:
Limited consulting or advising for the following over almost two decades: Astra-Zeneca, Glaxo-Smith Kline, Eisai, Novartis, Forest, Supernus, Adlyfe, Takeda, Wyeth, Lundbeck, Merz, Lilly, Pfizer, Genentech, Elan, NFL Players Association, NFL Benefits Office, Avanir, Zinfandel, BMS, Abvie, Janssen, Orion, Otsuka, Astellas
Grants
Agency: fNMI
Dates: 2010–2016
Zoltan Mari
Consulting fees: AbbVie, Revance, Ipsen, Lundbeck, GLG
Grants: NIH, MJFF, NPF, AVID, AbbVie, Sunovion
Occasional expert witness testimony
Michael S. Okun
Consulting Fees:
Dr. Okun serves as a consultant for the National Parkinson Foundation, and has received research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. Dr. Okun’s DBS research is supported by: R01 NR014852 and R01NS096008. Dr. Okun has previously received honoraria, but in the past >60 months has received no support from industry. Dr. Okun has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, and Cambridge (movement disorders books). Dr. Okun is an associate editor for New England Journal of Medicine Journal Watch Neurology. Dr. Okun has participated in CME and educational activities on movement disorders (in the last 36) months sponsored by PeerView, Prime, QuantiaMD, WebMD, Medicus, MedNet, Henry Stewart, and by Vanderbilt University. The institution and not Dr. Okun receives grants from Medtronic, Abbvie, Allergan, and ANS/St. Jude, and the PI has no financial interest in these grants. Dr. Okun has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria.
Francisco A. Ponce
Consulting Fees:
Consultant for Medtronic
Paul B. Rosenberg
Consulting Fees:
< $5000 annually from Abbvie, Merck, INSYS, GLG
Marwan N. Sabbagh
Consulting Fees:
consulting-Axovant Biogen FORUM Pharmaceuticals Fujirebio Diagnostics Humana Lilly Pharmaceuticals Sanofi vTv Therapeutics
Equity:
Stock ownership-Brain Health, Inc, Muses Labs, Inc, Versanum Inc.
Steven D. Targum
Consulting fees or vender grants: Acadia Pharmaceuticals, Alkermes Inc., Alkermes Inc., Axovant, BrainCells Inc., Forum Pharmaceuticals, Functional Neuromodulation Inc., Intracellular Therapies, Inc., Johnson and Johnson PRD, Methylation Sciences Inc., Navitor, Neurim Pharmaceuticals, Prana Biotechnology Ltd., Pfizer Inc., Resilience Therapeutics, and Sunovion Pharmaceuticals. Equity: Bracket Global LLC, Prana, Methylation sciences, functional Neuromodulation Inc.
Patents/Royalties
Royalties-Tenspeed/Random House
Grants
Agency: AstraZeneca
Agency: Avid Pharmaceuticals
Agency: Axovant
Agency: Genetech
Agency: Lilly Pharmaceuticals
Agency: Merck & Co
Agency: Pfizer
Agency: Piramal Imaging
Agency: Roche Diagnostics Corporation
Agency: vTv Therapeutics
Stephen Salloway
Consulting Fees:
Biogen, Merck, Roche, Genentech, Lilly
Grants
Agency: Functional Neuromodulation
Dates: 2013–2016
Agency: Novartis
Dates: 2016
Agency: Biogen
Dates: 2013–2016
Agency: Merck
Dates: 2013–2016
Agency: Roche
Dates: 2012–2016
Agency: Avid
Dates: 2013–2016
Agency: Lilly
Dates: 2014–2016
Agency: Genentech
Dates: 2013–2016
Gwenn S. Smith
Grants
Agency: Functional Neuromodulation, Incorporated
Dates: 2012–2015
Steven D. Targum
Consulting Fees:
Forum Pharmaceuticals (advisory) Equity: stock in Methylation Sciences Inc., Prana Biotechnology Ltd., Functional Neuromodulation, Bracket Global
David Wolk
Grants
Agency: Merck
Dates: 6/1/14 to present
Agency: Avid Radiopharmaceuticals
Dates: 6/1/14 to present
Agency: Biogen
Dates: 11/17/15 to present
References
- [1].Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, Grundman M, Yuen E, Black R, Brashear HR, Bapineuzumab 301 and 302 Clinical Trial Investigators (2014) Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N. Engl. J. Med 370, 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, Raman R, Sun X, Aisen PS, Siemers E, Liu-Seifert H, Mohs R, Alzheimer’s Disease Cooperative Study Steering Committee, Solanezumab Study Group (2014) Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N. Engl. J. Med 370, 311–321. [DOI] [PubMed] [Google Scholar]
- [3].Vickers JC, Mitew S, Woodhouse A, Fernandez-Martos CM, Kirkcaldie MT, Canty AJ, McCormack GH, King AE (2016) Defining the earliest pathological changes of Alzheimer’s disease. Curr. Alzheimer Res 13, 281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Palop JJ, Mucke L (2010) Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat. Neurosci 13, 812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dennis EL, Thompson PM (2014) Functional brain connectivity using fMRI in aging and Alzheimer’s disease. Neuropsychol. Rev 2 4, 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Perestelo-Pérez L, Rivero-Santana A, Pérez-Ramos J, Serrano-Pérez P, Panetta J, Hilarion P (2014) Deep brain stimulation in Parkinson’s disease: meta-analysis of randomized controlled trials. J. Neurol 261, 2051–2060. [DOI] [PubMed] [Google Scholar]
- [7].Toda H, Hamani C, Fawcett AP, Hutchison WD, Lozano AM (2008) The regulation of adult rodent hippocampal neurogenesis by deep brain stimulation. J. Neurosurg. 108, 132–138. [DOI] [PubMed] [Google Scholar]
- [8].Gondard E, Chau HN, Mann A, Tierney TS, Hamani C, Kalia SK, Lozano AM (2015) Rapid Modulation of Protein Expression in the Rat Hippocampus Following Deep Brain Stimulation of the Fornix. Brain Stimul. 8, 1058–1064. [DOI] [PubMed] [Google Scholar]
- [9].Hao S, Tang B, Wu Z, Ure K, Sun Y, Tao H, Gao Y, Patel AJ, Curry DJ, Samaco RC, Zoghbi HY, Tang J (2015) Forniceal deep brain stimulation rescues hippocampal memory in Rett syndrome mice. Nature 526, 430–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stone SSD, Teixeira CM, Devito LM, Zaslavsky K, Josselyn SA, Lozano AM, Frankland PW (2011) Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J. Neurosci 31, 13469–13484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hescham S, Lim LW, Jahanshahi A, Steinbusch HWM, Prickaerts J, Blokland A, Temel Y (2013) Deep brain stimulation of the forniceal area enhances memory functions in experimental dementia: the role of stimulation parameters. Brain Stimul. 6, 72–77. [DOI] [PubMed] [Google Scholar]
- [12].Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, Wherrett J, Naglie G, Hamani C, Smith GS, Lozano AM (2010) A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann. Neurol. 68, 521–534. [DOI] [PubMed] [Google Scholar]
- [13].Sankar T, Chakravarty MM, Bescos A, Lara M, Obuchi T, Laxton AW, McAndrews MP, Tang-Wai DF, Workman CI, Smith GS, Lozano AM (2015) Deep Brain Stimulation Influences Brain Structure in Alzheimer’s Disease. Brain Stimul. 8, 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fontaine D, Deudon A, Lemaire JJ, Razzouk M, Viau P, Darcourt J, Robert P (2013) Symptomatic treatment of memory decline in Alzheimer’s disease by deep brain stimulation: a feasibility study. J. Alzheimers. Dis 34, 315–323. [DOI] [PubMed] [Google Scholar]
- [15].Holroyd K, Fosdick L, Smith G, Leoutsakos J (2015) Deep brain stimulation targeting the fornix for mild Alzheimer dementia. Access Journal of …. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lozano A, Fosdick L, Chakravarty MM, Leoutsakos JM (2016) A Phase II Study of Fornix Deep Brain Stimulation in Mild Alzheimer’s Disease. Journal of Alzheimer’s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jack CR Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, Thies B, Phelps CH (2011/5) Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers. Dement. 7, 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rosen WG, Mohs RC, Davis KL (1984) A new rating scale for Alzheimer’s disease. Am. J. Psychiatry 141, 1356–1364. [DOI] [PubMed] [Google Scholar]
- [19].Morris JC (1997) Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int. Psychogeriatr 9 Suppl 1, 173–6; discussion 177–8. [DOI] [PubMed] [Google Scholar]
- [20].Woods SP, Delis DC, Scott JC, Kramer JH, Holdnack JA (2006) The California Verbal Learning Test--second edition: test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Arch. Clin. Neuropsychol 21, 413–420. [DOI] [PubMed] [Google Scholar]
- [21].Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J (1994) The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 44, 2308–2314. [DOI] [PubMed] [Google Scholar]
- [22].Ponce FA, Asaad W, Foote KD, Anderson WS Cosgrove GR Baltuch GH, Beasley K, Fosdick L, Oh ES, Targum SD, Smith GS, Lyketsos CG, Lozano AM Bilateral deep brain stimulation of the fornix for Alzheimers disease: surgical safety in the ADvance Trial. J Neurosurgery 125 (1): 75–84, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kenney C, Simpson R, Hunter C, Ondo W, Almaguer M, Davidson A, Jankovic J (2007) Short-term and long-term safety of deep brain stimulation in the treatment of movement disorders. J. Neurosurg 106, 621–625. [DOI] [PubMed] [Google Scholar]
- [24].Lyons KE, Koller WC, Wilkinson SB, Pahwa R (2001) Long term safety and efficacy of unilateral deep brain stimulation of the thalamus for parkinsonian tremor. J. Neurol. Neurosurg. Psychiatry 71, 682–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Prince MJ (2015) World Alzheimer Report 2015: the global impact of dementia: an analysis of prevalence, incidence, cost and trends.
- [26].Migliaccio R, Agosta F, Possin KL, Canu E, Filippi M, Rabinovici GD, Rosen HJ, Miller BL, Gorno-Tempini ML (2015) Mapping the Progression of Atrophy in Early- and Late-Onset Alzheimer’s Disease. J. Alzheimers. Dis 46, 351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kim EJ, Cho SS, Jeong Y, Park KC, Kang SJ, Kang E, Kim SE, Lee KH, Na DL (2005) Glucose metabolism in early onset versus late onset Alzheimer’s disease: an SPM analysis of 120 patients. Brain 128, 1790–1801. [DOI] [PubMed] [Google Scholar]
- [28].Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ Jr, Rothlind J, Sagher O, Reda D, Moy CS, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein J, Stoner G, Heemskerk J, Huang GD, CSP 468 Study Group (2009) Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 301, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kuhn J, Hardenacke K, Lenartz D, Gruendler T, Ullsperger M, Bartsch C, Mai JK, Zilles K, Bauer A, Matusch A, Schulz R-J, Noreik M, Bührle CP, Maintz D, Woopen C, Häussermann P, Hellmich M, Klosterkötter J, Wiltfang J, Maarouf M, Freund H-J, Sturm V (2015) Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer’s dementia. Mol. Psychiatry 20, 353–360. [DOI] [PubMed] [Google Scholar]
- [30].Scharre D, Weichart E, Nielson D, Zhang J, Agrawal P, Sederberg P, Knopp M, Rezai A (2016) Deep Brain Stimulation of Frontal Lobe Networks to Treat Alzheimer’s Disease (P2. 222). Neurology 86, P2–222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.