Abstract
Background/Objectives:
Anthracyclines are used in induction therapy of pediatric acute lymphoblastic leukemia (ALL) and are known to generate oxidative stress; whether this translates into enhanced anti-leukemic activity or hemolytic effects in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency is unknown.
Design/Methods:
Among 726 pediatric newly diagnosed ALL patients treated at St. Jude Children’s Research Hospital, 22 had deficient G6PD activity. We compared the prevalence of positive minimal residual disease (MRD) ≥ 1% at Day 15/Day 19 of induction or ≥ 0.01% at Day 42/ Day 46 (end of induction) and the number of red blood cell (RBC) transfusions after daunorubicin in induction between patients with or without G6PD deficiency, adjusting for ALL risk group, treatment protocol, age, and gender.
Results:
There was no difference in Day 15/19 (p =1) or end of induction MRD (p = 0.76) nor in number of RBC transfusions (p = 0.73); the lack of association with MRD was confirmed in a dataset of 1192 newly diagnosed male patients enrolled in a Children’s Oncology Group trial (p = 0.78).
Conclusion:
We found no evidence that G6PD deficiency affects daunorubicin activity during induction treatment for ALL.
Keywords: leukemia, daunorubicin, doxorubicin, glucose-6-phospate dehydrogenase, pharmacogenetics
Introduction
Anthracyclines are widely used in the treatment of malignancies, including pediatric leukemias. Anthracyclines generate oxidative stress by the production of hydrogen peroxide, which activates the hexose monophosphate shunt, and by the oxidization of glutathione.1 Patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency have diminished activation of the hexose monophosphate shunt and thus have a reduced capacity to regenerate available glutathione for detoxifying reactive oxygen species (ROS) after administration of anthracyclines.2 G6PD deficiency usually manifests itself clinically as hemolytic anemia after exposure to oxidative drugs because the hexose monophosphate shunt is the only means of replenishing glutathione in erythrocytes.3 Although the risk of hemolytic toxicity after exposure to anthracyclines is thought to be low,4,5 there remains some hesitancy to use anthracyclines in patients with G6PD deficiency6 due to the potential magnification of the oxidative stress,7 a concern underscored by a case report in which a G6PD deficient patient developed hemolysis and reticulocytosis after doxorubicin administration. 8
In vitro studies have shown that the carbonyl reductase-mediated metabolism of daunorubicin to its less active metabolite daunorubicinol is decreased in erythrocytes with G6PD deficiency.9 Furthermore, there was less reduced glutathione available and more apoptosis observed in peripheral leukocytes from a single G6PD deficient patient after administration of daunorubicin compared to leukocytes from eight G6PD-normal patients.10 However, there are no large studies evaluating the hemolytic toxicity or the efficacy of anthracyclines in G6PD deficient patients. We investigated the impact of G6PD status on antileukemic efficacy as assessed by minimal residual disease (MRD) and on toxicity (assessed by the number of transfusions received during induction therapy) in pediatric patients with newly diagnosed acute lymphoblastic leukemia (ALL) undergoing remission induction treatment.
Methods
All studies had Institutional Review Board approval. Patients/guardians were assented/consented for therapeutic protocols consistent with the Declaration of Helsinki.
Discovery cohort
Of 1,067 pediatric patients with newly-diagnosed ALL who were enrolled on St. Jude Children’s Research Hospital protocols Total XV (ClinicalTrials.gov #NCT00137111)11 and Total XVI (ClinicalTrials.gov #NCT00549848)12 between August 2000 and September 2016, 734 patients had erythrocyte G6PD activity measured as part of their clinical care. Daunorubicin 25 mg/m2 was administered on days 5 and 12 on Total XV and days 1 and 8 on Total XVI as part of multiagent remission induction therapy including systemic prednisone, vincristine, asparaginase, and (for Total XV only) methotrexate.11,12 Reasons for withholding daunorubicin included mucositis or hyperbilirubinemia. Measurements of MRD were performed by flow cytometry on mononucleated cells from bone marrow aspirates obtained during induction (day 19 of Total XV and day 15 of Total XVI) and at the end of induction (Day 46 on Total XV and Day 42 on Total XVI).11 For purposes of comparing MRD by G6PD status, MRD ≥ 1% at Day 15/19 and ≥ 0.01% at the end of induction were considered positive in this analysis. G6PD activity was measured in blood using a quantitative spectrophotometric assay, and those with 1 or more measures below the lower limit of normal were considered deficient. The lower limit of normal varied over time.13 Red blood cell transfusions administered at St. Jude from the date of the first daunorubicin dose to 14 days after the last dose of daunorubicin were assessed by medical record review. Use of transfusions was not dictated by ALL treatment protocol or by G6PD status and was at physician discretion. We used Fisher’s exact test to compare the frequency of positive MRD during and after induction and the frequency of patients who did vs did not miss doses of daunorubicin in those who were vs were not G6PD deficient. A multivariable Poisson regression analysis, which included risk group assignment at induction, treatment protocol, gender, and age as covariates, was used to determine the effect of G6PD status on the number of transfusions.
Replication cohort
Day 29 MRD data were retrieved on December 2, 2013 for 2,598 newly-diagnosed pediatric ALL patients who were enrolled on Children’s Oncology Group (COG) protocol AALL0232 between March 1, 2004 and January 18, 2011. Next generation sequencing of the G6PD gene as previously described14 was done for 2,139 of these patients, using DNA from blood obtained at remission. Daunorubicin 25 mg/m2 was administered on Days 1, 8, 15, and 22 as part of a multi-agent induction regimen including systemic vincristine, dexamethasone or prednisone, and peg-asparaginase.14 Bone marrow aspirates were done on Day 29 to assess MRD by flow cytometry.15 For purposes of comparing MRD between G6PD deficient and non-deficient patients, MRD ≥ 0.1% was considered positive and MRD < 0.1% was considered negative. G6PD activity level and red blood cell transfusion data were not available; thus, G6PD phenotype was assigned based on the patient’s genotype according to the WHO classification system and the CPIC guidelines.16 We used the Fisher’s exact test to compare the frequencies of Day 29 MRD < vs ≥ 0.1% between male patients with or without G6PD deficiency (based on genotype).
For all patients included in both St Jude and COG cohorts, genetic ancestry was inferred using Affymetrix 500K or Mapping SNP6.0 data as previously described.17
Results and Discussion
St. Jude cohort
Excluded from the analysis were one patient who died during induction, one without risk group assignment at the start of induction, and six with conflicting G6PD activity measurements.13 Of the 726 evaluable patients, 22 had deficient G6PD activity and 704 had normal G6PD activity (Supporting Information Figure S1). MRD results at Day 15/19 and end of induction were missing in six and eight patients, respectively. There was no difference in age, presenting white blood cell count, DNA index, or immunophenotype between the two G6PD groups (Supplemental Information Table S1); race was the only feature that differed by G6PD status (p = 2.6 × 10−6), with most deficient patients being black.
There was no difference in the frequency of MRD ≥ 1% at Day 15/19 between G6PD deficient patients and G6PD normal patients (30% vs. 28.7%, p = 1) (Fig. 1). This held true when the analysis was restricted to each protocol (Total XV: 16.7% vs. 22.6%, p = 1; Total XVI: 35.7% vs. 30.6%, p = 0.77), to risk groups (Standard/High risk: 50% vs. 42.8%, p = 0.73; Low risk:16.7% vs. 16.8%, p = 1) and to black patients only (33.3% vs. 32.2%, p = 1), when the analysis was separated by gender (Males: 33.3% vs. 30.2%, p = 0.76; Females: 25.0% vs. 26.7%, p = 1), and when the MRD threshold was set to 0.1% (Supporting Information Table S2). There was also no difference in the frequency of MRD ≥ 0.01% at the end of induction between G6PD deficient patients and G6PD normal patients (9.1% vs. 14.9%, p = 0.76) (Supporting Information Table S3).
Figure 1. G6PD status did not impact Day 15/19 MRD in daunorubicin-containing ALL induction therapy.
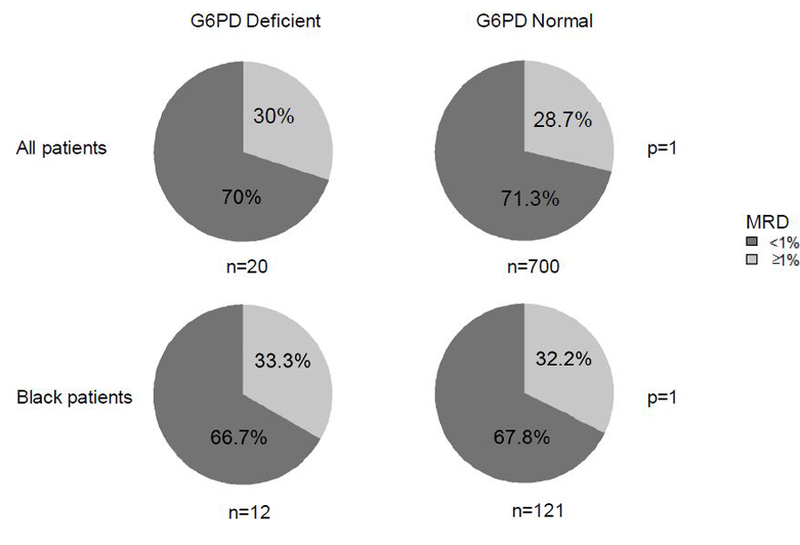
Minimal residual disease (MRD) was assessed from bone marrow aspirates at Day 19 (TOTAL XV) and Day 15 (TOTAL XVI). There was no difference in frequency of MRD≥1% at Day 15/19 between G6PD deficient and G6PD normal patients (30 vs. 28.7%, p =1). This held true when the analysis was restricted to only black patients (33.3 vs 32.2%, p =1).
G6PD deficient patients did not receive more red blood cell transfusions after daunorubicin administration than those with normal G6PD activity (mean, 4.2 [SD 2.7] vs 3.9 [SD 2.8], p = 0.73 by multivariable Poisson regression; Fig. 2). We did not compare G6PD groups for other non-hemolytic toxicities, such as infection, myelosuppression, or mucositis. However, there was no difference in the frequency of receiving only 1 dose of daunorubicin (rather than the scheduled 2 doses) between patients with deficient G6PD activity (9.1%; 2 out of 22) versus those with normal G6PD activity (11.4%; 80 out of 704, p = 1).
Figure 2. The number of red blood cell transfusions received from the date of the first dose of daunorubicin up to 14 days after the second dose was not affected by G6PD status.
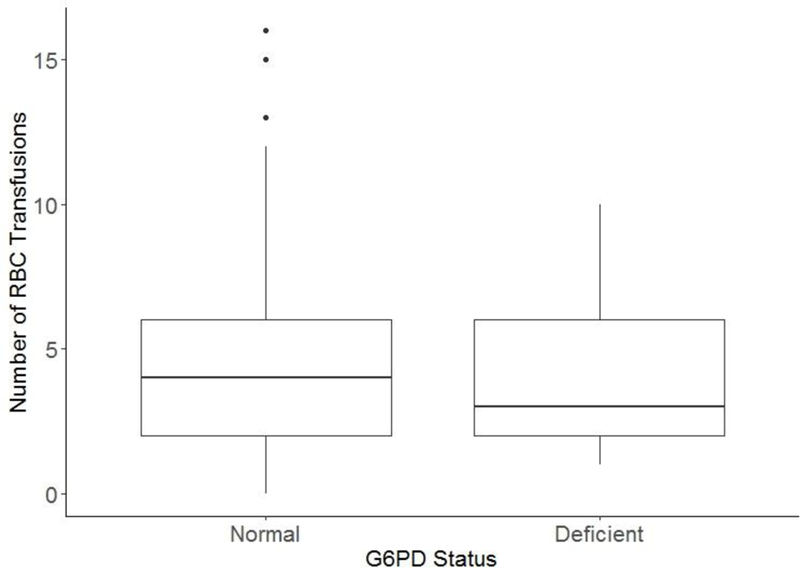
The mean (SD) number of red blood cell transfusions were 4.2 (2.7) in patients with deficient G6PD activity and was 3.9 (2.8) in patients with normal G6PD activity (p = 0.73, by multivariable Poisson regression analysis).
COG AALL0232 cohort
The cohort of 2,139 patients consisted 44% female, and 5% black, 26% Hispanic, 55% white and 14% of other admixed races. There was no difference in age, presenting white blood count, or DNA index between the two G6PD groups (Supplemental Information Table S4); the only feature that differed by G6PD status was race (p = 4.3×10−15), with most deficient patients being black. We observed 16 polymorphic single nucleotide polymorphisms (SNPs) in G6PD, including 7 nonsynonymous exonic SNPs with an assigned WHO classification and 9 nonsynonymous exonic SNPs with unknown clinical function (Supporting Information Table S5 and S6). 21 patients were considered deficient (20 males, 1 female). Twenty-five female patients were heterozygous for a Class II-III allele, and thus G6PD status could not be assigned based on genotype. Patients with a nonsynonymous SNP with unknown clinical significance were removed from further analysis.
To test for an association of MRD and G6PD status, we focused first on the 1192 males, given the difficulty in assigning G6PD phenotype to females. There was no difference in the frequency of MRD ≥ 0.1% at Day 29 between the 20 G6PD deficient males based on genotype and the 1172 G6PD normal males (15% vs. 20.7%, p = 0.78) (Supporting Information Figure S3, Supporting Information Table S7). This held true when the analysis was restricted to non-white males (15% vs. 20.6%, p = 0.78) (Supporting Information Fig. 3, Supporting Information Table S7). For females, there was only one G6PD deficient patient who had homozygous Asahi genotype, and she was negative for D29 MRD. We further compared female patients who were heterozygous for a Class II-III allele and those who were homozygous for Class IV alleles. There was no difference in the frequency of MRD ≥ 0.1% at Day 29 between the 25 heterozygous females and the 911 G6PD normal females (20% vs. 16.2%, p = 0.58) (Fig.3, Supporting Information Table S7). This held true when the analysis was restricted to non-white females (20.8% vs. 19.1%, p = 0.79) (Supporting Information Table S7).
Conclusions
In an ALL remission induction regimen that included two doses of daunorubicin, G6PD activity phenotype did not affect efficacy or toxicity. There was no difference in MRD, missing daunorubicin doses, or number of red blood cell transfusions between patients with normal or deficient G6PD activity. Likewise, in a separate protocol which included four doses of daunorubicin, G6PD genotype was not associated with MRD. Although other agents (glucocorticoids, vincristine, asparaginase, with or without methotrexate) were given during ALL remission induction that could have affected these phenotypes (MRD, missing doses, or number of transfusions), these other agents are not likely to be affected by G6PD status.4,5,18–20 In the clinic, anthracyclines are almost always used as part of multi-agent regimens.
One limitation of our study is that the majority of our patients with G6PD deficiency were black; thus, we did not have the full range of international diversity of G6PD variants in our cohort. It is possible that some Class II-III variants that are more common in other ancestral groups may be associated with more severe defects than those we observed in this cohort. Therefore, the results of this study may not be applicable to patients with more severe G6PD deficiency. Another related limitation of this study was the small number of patients with G6PD deficiency, which limited our power. For example, assuming a baseline level of 30% patients with positive MRD (e.g. mid-induction at Day 15/19), we had 80% power to detect a difference in the frequency of positive MRD if there was at least a 64% difference in MRD positivity between G6PD deficient vs normal patients. Regardless, the prevalence of MRD positivity in G6PD deficient and normal patients was nearly identical (30% vs 28.7%) (Table 1), and the findings were replicated in a much larger independent cohort. Therefore, it is unlikely that anthracycline-containing remission induction therapy exerted increased leukemia cytoreduction in G6PD-deficient patients. Although it is possible that patients received transfusions prior to their admission to St. Jude, the influence of this confounder was minimized by focusing on transfusions given after the daunorubicin treatment, when transfusions were adequately documented.
In conclusion, we found no evidence that G6PD status affects treatment efficacy or risk of red blood cell toxicity in daunorubicin-containing ALL induction therapy regimens. Our findings should allow clinicians to discount drug/gene interaction warnings against the use of anthracyclines in patients with G6PD deficiency.
Supplementary Material
Acknowledgements
This study was supported by NIH grants CA 21765, CA 142665, CA 36401, P50 GM 115279, U10 CA98543 and U10 CA180886 (COG Chair’s grants), U10 CA98413 and U10 CA180899 (COG Statistics and Data Center grants), U24 CA114766 (COG Specimen Banking), St Baldrick’s Foundation funding, and by ALSAC. MLL is the Benioff Chair of Children’s Health and the Deborah and Arthur Ablin Endowed Chair for Pediatric Molecular Oncology at Benioff Children’s Hospital. EAR is a KiDS of NYU Foundation Professor at NYU Langone Health. SPH is the Jeffrey E. Perelman Distinguished Chair in Pediatrics at The Children’s Hospital of Philadelphia.
Footnotes
Conflicts of Interest
The authors have no competing interests.
References
- 1.Henderson CA, Metz EN, Balcerzak SP & Sagone AL Jr. Adriamycin and daunomycin generate reactive oxygen compounds in erythrocytes. Blood 52, 878–885 (1978). [PubMed] [Google Scholar]
- 2.Sagone AL Jr. & Burton GM The effect of BCNU and adriamycin on normal and G6PD deficient erythrocytes. Am J Hematol 7, 97–106 (1979). [DOI] [PubMed] [Google Scholar]
- 3.Sivilotti ML Oxidant stress and haemolysis of the human erythrocyte. Toxicol Rev 23, 169–188 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Youngster I et al. Medications and glucose-6-phosphate dehydrogenase deficiency: an evidence-based review. Drug Saf 33, 713–726, 10.2165/11536520-000000000-00000 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Luzzatto L,MA, Vulliamy T in The Metabolic and Molecular Bases of Inherited Disease (ed Beaudet AL Scriver CR, Sly WS, Valle D) Ch. 179, 4517–4553 (McGraw-Hill, 2001). [Google Scholar]
- 6.La Verde N et al. Safe chemotherapy and hormone therapy for treating early breast cancer in a glucose 6-phosphate dehydrogenase-deficient patient: case report. Anticancer Drugs 23, 758–760, 10.1097/CAD.0b013e3283556bbe (2012). [DOI] [PubMed] [Google Scholar]
- 7.McDonagh EM et al. PharmGKB summary: very important pharmacogene information for G6PD. Pharmacogenet Genomics 22, 219–228, 10.1097/FPC.0b013e32834eb313 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doll DC Oxidative haemolysis after administration of doxorubicin. Br Med J (Clin Res Ed) 287, 180–181 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amitai Y, Bhooma T & Frischer H Glucose-6-phosphate dehydrogenase deficiency severely restricts the biotransformation of daunorubicin in human erythrocytes. J Lab Clin Med 127, 588–598 (1996). [DOI] [PubMed] [Google Scholar]
- 10.Efferth T, Fabry U, Glatte P & Osieka R Increased induction of apoptosis in mononuclear cells of a glucose-6-phosphate dehydrogenase deficient patient. J Mol Med (Berl) 73, 47–49 (1995). [DOI] [PubMed] [Google Scholar]
- 11.Pui CH et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 360, 2730–2741, 10.1056/NEJMoa0900386 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y et al. Genome-Wide Study Links PNPLA3 Variant With Elevated Hepatic Transaminase After Acute Lymphoblastic Leukemia Therapy. Clin Pharmacol Ther, 10.1002/cpt.629 (2017). [DOI] [PMC free article] [PubMed]
- 13.Robinson KM et al. Concordance between glucose-6-phosphate dehydrogenase (G6PD) genotype and phenotype and rasburicase use in patients with hematologic malignancies. Pharmacogenomics J, 10.1038/s41397-018-0043-3 (2018). [DOI] [PMC free article] [PubMed]
- 14.Liu C et al. Clinical and Genetic Risk Factors for Acute Pancreatitis in Patients With Acute Lymphoblastic Leukemia. J Clin Oncol 34, 2133–2140, 10.1200/JCO.2015.64.5812 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borowitz MJ et al. Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children’s Oncology Group study AALL0232. Blood 126, 964–971, 10.1182/blood-2015-03-633685 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Relling MV et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for rasburicase therapy in the context of G6PD deficiency genotype. Clin Pharmacol Ther 96, 169–174, 10.1038/clpt.2014.97 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez CA et al. Genome-wide analysis links NFATC2 with asparaginase hypersensitivity. Blood 126, 69–75, 10.1182/blood-2015-02-628800 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lichtman MA,BE, Kipps TJ, et al. Williams hematology (McGraw-Hill, 2006). [Google Scholar]
- 19.Kliegman RM,BR, Jenson HB, et al. Nelson textbook of pediatrics . 18 edn, (WB Saunders Co, 2007). [Google Scholar]
- 20.Fauci AS,BE, Kasper DL, et al. . Harrison’s principles of internal medicine . 17 edn, (McGraw-Hill, 2008). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


