Abstract
Objective:
High rates of comorbidity and overlapping diagnostic criteria between pediatric Bipolar Disorder (BD) and Attention-Deficit/Hyperactivity Disorder (ADHD) contribute to diagnostic and treatment confusion. To advance what is known about both disorders, we compared affective processing in children with primary BD, primary ADHD and typically developing controls (TDC).
Methods:
Participants included 7–17 year-olds with either “narrow-phenotype” pediatric BD (n=25), ADHD (n=25) or TDC (n=25). Groups were matched on participant age and FSIQ. Affective processing was assessed using the Cambridge Neuropsychological Test Automated Battery Affective Go/NoGo (CANTAB AGN) task.
Results:
We found a group by target valence interaction on commission errors [F(2,71)=5.34, p<0.01, ƞp2=0.13] whereby ADHD, but not TDC participants, made more errors on negative than positive words [t(24)=−2.58, p<0.05, r=0.47]. In contrast, there was a non-significant trend for BD participants to make fewer errors on negative versus positive words compared to ADHD and TDC participants. Between-subjects effects showed that ADHD participants made more errors than TDC, but not BD participants.
Conclusion:
Our main finding advances what is known about the effect of affective processing on response control in in children with ADHD. Our results suggesting a positive affective processing bias in children with ADHD compliment emerging literature showing that difficulties with emotional processing and regulation may be core features of ADHD. Further, given the observed pattern of results in children with ADHD compared to BD children, our behavioral results suggest the importance of examining differences in the brain-behavior mechanisms involved in affective processing in children with ADHD compared to BD children.
Keywords: bipolar disorder, attention-deficit/hyperactivity disorder, affective processing, emotion, child psychiatry
Introduction
During the past two decades, rates of both bipolar disorder (BD) and attention-deficit/hyperactivity disorder (ADHD) in children and adolescents have surged to their highest levels in history. For example, rates of pediatric BD increased 40-fold from 1994–2003, and presently 20% of children and adolescents discharged from psychiatric hospitals are diagnosed with BD.[1,2] Similarly, epidemiologic data indicate that the prevalence of ADHD has risen from 4–6% in 2001 to 8–10% in 2010.[3] Disentangling whether a child has BD, ADHD, or the combination is a common and difficult challenge for clinicians in part due to high rates of comorbidity (e.g., on average 62% of children with BD also meet criteria for ADHD).[4],[5,6] Moreover, criteria for BD and ADHD are often overlapping, and these, overlapping symptoms have been implicated in the rising diagnostic rates of both disorders.[7,8] Specifically, several DSM-V symptoms associated with a manic episode, including distractibility, pressured speech and psychomotor agitation, parallel DSM-V symptoms of ADHD, including being easily distracted, talking excessively, and being often “on the go” or fidgeting.[9] Moreover, while irritability is a diagnostic criteria for mania, irritability is also highly prevent in children with ADHD as 71.6% of children with ADHD (compared to 3.2% of typically developing control [TDC] youth) present with clinically significant irritability. Taken together, these overlapping symptoms suggest that children with BD and children with ADHD may present with similar behavioral phenotypes between the disorders and these overlapping symptoms cross cognitive,motor and affective domains [10,11]. However, there is some evidence for the distinct presentation of BD, namely youth with BD (even those with comorbid ADHD) present with elation, grandiosity, flight of ideas/racing thoughts, decreased need for sleep, and hypersexuality which distinguishes them from children with ADHD.[12] Given this diagnostic and assessment challenge, an alternative approach is needed to address this clinical problem, whereby bio-behavioral markers of BD or ADHD augment clinical history, resulting in better, more specific diagnosis and treatment for children.
Towards this end, studies have begun evaluating the brain-behavior interactions underlying affective processing and regulation in youth with either primary BD or primary ADHD. Affect regulation refers to an “individual’s ability to modify an emotional state so as to promote adaptive, goal-oriented behaviors, and encompasses the processes that allow the individual to select, attend to, and appraise emotionally arousing stimuli”[13]. As such, affective processing and regulation involves a complex set of voluntary and involuntary cognitive processes including both “top down” executive functions, such as attentional and response control (e.g., cognitive control), as well as “bottom up” processes which appraise and identify the emotional salience of a stimulus (e.g., identification of positive vs. negative emotional stimuli, including faces).[14,15]. At a neural level, affective processing and regulation involves the interaction of regions involved in early orienting and perception of emotional stimuli, cognitive control regions involved attentional control, response inhibition and motor planning, and regions involved in the interface between emotional and cognitive control circuitry. [13,16,17] Therefore, affective processing and regulation involves many sub-processing including attentional control, inhibitory control, reward processing and motivation.[18]
Prior work evaluating aspects of affect processing and regulation has shown that both youth with primary BD or with primary ADHD have behavioral deficits on tasks tapping emotional face identification ability vs. typically developing control (TDC) youth.[19–21] When directly compared to either youth with ADHD or TDCs, youth with BD have greater difficulty identifying child happy faces, and are more likely than ADHD youth to make these errors on low-intensity, but not high-intensity happy face expressions.[22] While these results suggest happy face identification deficits as a potentially specific bio-behavioral marker of BD in youth, there is clearly a need for additional studies that evaluate other affect regulation processes in youths with primary BD vs. those with primary ADHD.
Beyond emotional face identification, response control (i.e., selective attention and response inhibition) is another critical aspect of affect regulation because it allows an individual to inhibit irrelevant emotional information while simultaneously attending to goal-oriented information.[15,18,23] Response control helps modulate the effect of an emotional stimuli on an individual’s behavior by helping the individual attend to salient emotional information and regulate their subsequent behavioral responses. While studies have shown that children with either BD or ADHD have deficits on standard measures of response control using basic (e.g., non-emotional) go/no-go paradigms, few studies have examined response control in the context of emotionally salient stimuli.[24–27,10,28,29] This is particularly important as neuroimaging research has shown that children with ADHD have increase neural activation during the processing of emotional stimuli which is distinct from their deficits in cognitive control suggesting that deficits in emotional processing in youth with ADHD go beyond their executive function and cognitive control deficits[30].
This gap in knowledge could be addressed by utilizing affective go/no go (AGN) behavioral tasks that measure how bias in emotional stimuli affect response control; however, few studies have employed the AGN to study individuals with BD vs. those with ADHD. AGN tasks require participants to not only attend and respond to target emotional stimuli (i.e, attentional control), but also inhibit response to distractor information (i.e., response inhibition). The majority of studies using AGN tasks in BD have been conducted in adults. For example, Murphy et al. showed that manic adults with BD had a bias towards positive stimuli (i.e.,faster reaction time to positive vs. negative stimuli), whereas adults with unipolar depression had a bias toward negative stimuli during an AGN task.[31] These results suggest differential attention bias towards emotional stimuli for manic compared to depressed adults.[31] Analogous results have been shown when comparing AGN performance in adults with BD in either depressed or manic mood states, further suggesting a mood-congruent attention bias to emotional stimuli for individuals with BD.[32] Less is known about attentional bias to emotional stimuli in euthymic BD individuals however.
While the CANTAB AGN task has never been used in youth with BD, prior research has assessed affective processing in youth with BD using other tasks, including the self-referent encoding tasks [SRET]). Research examining behavioral performance on a SERT task (i.e., task in which children select yes or no when asked about positive or negative emotional words in relation to themselves) showed that vs. TDCs, youth with BD demonstrated a negative bias (endorsed and recalled more negative than positive words), and this negative bias persisted at 1-year follow-up.[33] Furthermore, at baseline, this negative bias in children with BD was inversely associated with clinician-assessed depressive symptoms again suggesting the possible role of state on affective processing.[33] In another study, children at risk for BD by virtue of having a first-degree relative with BD, recalled more negative than positive words on a SERT vs. TDCs, but also had greater interference from negative words, particularly those with manic-irritable and socially threatening content, on an Emotional Stroop task.[34] Taken together, these studies suggest affective processing deficits, particularly bias for negative stimuli, in children with BD.
In individuals with ADHD, literature utilizing AGN paradigms is also limited. For example, Fried et al. showed that ADHD children made more AGN total commission errors, but not omission errors, than TDC youth. However, this study focused on response control rather than affect components of the task (e.g., interaction between emotional stimuli and response control).[35] Also, a study of adults with ADHD showed that participants treated with memantadine had improved response inhibition (AGN performance) at 6 and 12 week follow-up vs. baseline.[36]. Despite these preliminary studies, there remain considerable gaps in what is known about AGN task performance in children with BD vs. those with ADHD, including the lack of any prior study that directly compared AGN performance in children with either primary BD vs. primary ADHD (whether with or without TDC participants).
To address this gap and advance what is known about response control during affective processing, we directly compared AGN task performance in three mutually-exclusive groups of children ages 7–17 years-old: (1) those with primary BD (n=25), (2) those with primary ADHD (n=25), and (3) TDCs without psychopathology (n=25). While little is known about attentional bias to emotional stimuli in euthymic youth with BD, we hypothesized that BD youth would demonstrate greater bias towards negative emotional stimuli (e.g., faster RT, errors) vs. both ADHD and TDC youth given the prior work in children with BD suggesting attentional bias to negative emotional stimuli [33,34].. However, we hypothesized that overall, youth with ADHD would have greater overall deficits in response control (e.g., more commission errors) irrespective of emotional salience vs. both BD and TDC youth given significant literature citing deficits in response inhibition in ADHD.[28,29]
Methods
Participants
Participants included children and adolescents ages 7–17 years old who were enrolled in an Institutional Review Board approved research study at Bradley Hospital. Written informed parental consent and child assent were obtained prior to participation.
For all participants, psychopathology was assessed using the Child Schedule for Affective Disorders and Schizophrenia, Present and Lifetime Version (K-SADS-PL) administered to parents and children separately by either a board-certified child/adolescent psychiatrist (DPD) or a licensed clinical psychologist (KLK, κ >0.85).[37] Comorbidities were assessed using the KSADS-PL, and within the BD group, comorbid diagnoses were assessed by asking about symptoms and impairments during periods of generally euthymic mood so as to avoid counting manic or depressive symptoms more than once.
Inclusion criteria for all participants was: (1) age between 7–17 years, (2) English fluency, and (3) a consenting parent/guardian. Exclusion criteria were: Wechsler Abbreviated Scales of Intelligence Full Scale IQ (WASI FSIQ)≤70; substance/alcohol abuse or dependence within the last 2 months; primary psychosis or autism spectrum disorders; and medical/neurological conditions that mimic either BD or ADHD.[38]
For the BD group (n=25), inclusion criteria consisted of meeting DSM-IV-TR criteria for BD on the KSADS, including a history of at least one hypomanic (≥ 4 days) or manic (≥ 7 days) episode in which the child exhibited abnormally elevated or expansive mood, and three or more DSM-IV criterion “B” mania symptoms.[39] Children presenting with irritable mood only (i.e., without elated or expansive mood) were not included. Therefore, the BD group meet Leibenluft’s “narrow phenotype” pediatric BD criteria.[40] Youth with BD were not excluded for comorbid behavioral disorders (e.g., ODD, ADHD, etc.) in order to most accurately reflect the pediatric BD population; however, for all children in the BD group, BD was the primary (i.e., most impairing and prevalent symptoms) disorder as assessed via KSADS.
For inclusion in the ADHD group (n=25), participants had to meet DSM-IV-TR criteria for any ADHD subtype. Participants were excluded from the ADHD group for the presence of any current or lifetime mood (i.e., major depression or BD) or anxiety disorders. However, the presence of comorbid behavioral disorders (e.g., ODD, CD) was not exclusionary.
BD and ADHD participants were allowed to remain on their outpatient medication regimens since this was not a treatment study. Participants taking stimulant medications were asked, but not required, to hold these medications for 4 drug half-lives before behavioral testing, which is a common practice in clinical care (e.g., weekends, holidays, etc.).
For inclusion in the TDC group (n=25), participants could not have any history of current or lifetime psychiatric illness or substance abuse/dependence nor could there be a history of psychiatric illness in any first-degree relatives.
To reduce group differences, all groups were matched on participant age and FSIQ (within 1 SD=15 points).
Measures
Overall functional impairment in the BD and ADHD participants was assessed using clinician ratings on the Children’s Global Assessment Scale (CGAS).[41] Additionally, ADHD symptoms were assessed using the Conner’s ADHD Rating Scale Parent report (T-scores from the Inattentive subscale, Hyperactivity/Impulsivity subscale, and ADHD index).[42] For the BD group, current symptoms (e.g., week of testing) of mania and depression were assessed using the Young Mania Rating Scale (YMRS) and Children’s Depression Rating Scale (CDRS) respectively.[43,44] The Children’s Behavior Checklist (CBCL) was also completed by parents to assess various internalizing and externalizing difficulties.[45]
CANTAB Affective Go/No Go Task
The Affective Go/No Go task (AGN; Cambridge Neuropsychological Test Automated Battery [CANTAB])[46] was used to assess information processing bias and response control in the context of positive and negative emotional stimuli. None of the participants in this study had been engaged in prior research using CANTAB. During the task, words (positive valence, negative valence, or neutral) are rapidly presented in the center of the screen. Examples of positively-valenced words include “warmth” or “joyful” while negatively-valenced words include “mistake” or “burden”. Each word is displayed for 300 ms, and there is a 900ms interval between words. Participants are given a target valence (e.g., positive or negative) and instructed to response to target words of the specified valence by hitting the response button as quickly as possible. In addition to target words, participants are also instructed to withhold response to distractor stimuli (words of neutral or opposite valence than that assigned).
The task consists of 20 blocks total including 2 practice blocks. Each block contains 18 trials, 9 of which include the target valance and 9 of which include the distractor. The 18 non-practice blocks are equally split between having the target valence be positive, negative or neutral. Therefore, there are 6 block (54 trials of target valence; 54 trials of distractor) in which the target valence is positive; 6 blocks (54 trials of target valence; 54 trials of distractor) in which the target valence is negative, and 6 blocks (54 trials of target valence; 54 trials of distractor) in which the target valence is neutral. Further, the 18 non-practice blocks can be divided evenly into “shift” blocks (n=9) in which the participant is required to inhibit responding to previous targets, and instead respond to previous distractors (e.g., Negative – Positive), and “non-shift blocks” (n=9) which require the participant to continue responding to the same target as in the previous trial (e.g., Positive – Positive).
Outcome measures include: (1) correct response latency or Response Time (RT); (2) commission errors (responses to distractor valence) and (3) omission errors (non-response to target valences).
Analytic Strategy:
Statistical Package for Social Sciences (version 21.0, SPSS Inc., Chicago, IL) was used for all analyses. Examination of dependent variables (e.g., RT, commission errors, omission errors) showed a normal distribution (Shaprio-Wilks p>0.05); therefore, parametric tests were used.[47] Specifically, each dependent variable (RT, commissions, omissions) was analyzed in a 2×2×3 general linear model repeated-measures analysis of variance (GLM ANOVA) with “shift condition” (shift, non-shift) and target valence (positive, negative) as within subjects variables and group (Bipolar, ADHD, TDC) as a between subjects factor. Main effects, interaction effects and subsequent pairwise comparisons were examined. Significance was set at p<0.05 with Bonferonni correction for multiple comparisons. Effect sizes for GLM ANOVAs were calculated using partial eta squared (ƞp2), and for paired t-tests using the equation r =√ (t²/t² + df) in which 0.10 = small effect, 0.30 = medium effect and 0.50 = large effect. [47,48] Post-hoc analyses were conducted to explore the effects of comorbid ADHD on results (see Supplement).
Results
Participants
During the week of AGN testing, the BD group was overall euthymic by clinician-administered mood ratings (YMRS: 9.20±7.78, CDRS: 29.28±8.90). Specifically, within the BD group, 68% (17/25) were euthymic (YMRS<12, CDRS<40), 4% (1/25) were depressed (YMRS<12, CDRS≥40), 16% (4/25) were hypomanic (YMRS=13–24, CDRS<40) and 12% (3/25) were mixed (YMRS>12, CDRS≥40). No participants were actively manic at the time of assessment (YMRS>25, CDRS<40).
Regarding ADHD symptoms, BD youth had greater levels of parent-reported ADHD symptoms than youth with ADHD [F(1,47)=4.82, p<0.05] (ADHD Index t-scores: BD: 73.60±11.60; ADHD: 66.83±9.87). Of the 25 BD participants, 18 (72%) also met diagnostic criteria for ADHD.
Youth in the BD group demonstrated higher levels of overall impairment on the clinician-administered CGAS than youth in the ADHD group [F(1,48)=13.61, p≤0.001] (BD: 61.52±13.70; ADHD: 75.80±13.67). At the time of testing, 1 (4%) participant in the BD group and 3 (12%) participants in the ADHD group were taking stimulant medication.
Given that participants were matched on age and Full Scale IQ, there were no between group differences on these variables (Table 1). Groups did not significantly differ either child gender [χ2 (2, N=75)=2.24, p=0.33]n or ethnicity [χ2 (2, N=75)=1.32, p=0.52].
Table 1.
Participant Characteristics
| Variable | Pediatric BD (n=25) | ADHD (n=25) | Control (n=25) | Significance |
|---|---|---|---|---|
| Age (years) | 12.92 (3.00) | 12.92 (2.57) | 12.64 (3.35) | F(2,72)=0.07 |
| Sex: male | 14 (56%) | 15 (60%) | 10 (40%) | χ2(2,N=75)=2.24 |
| FSIQ | 108.36 (9.51) | 110.04(10.25) | 110.72 (9.54) | |
| Ethnicity: Caucasian | 20 (80%) | 17 (68%) | 20 (80%) | χ2(2,N=75)=1.32 |
| KSADS Diagnosis (present in past 6 months) | ||||
| Manic episode | 23 (92%) | 0 (0%) | 0 (0%) | |
| Depressive episode | 13 (52%) | 0 (0%) | 0 (0%) | |
| ADHD | 18 (72%) | 25 (100%) | 0 (0%) | |
| Oppositional Defiant Disorder | 20 (80%) | 4 (16%) | 0 (0%) | |
| Generalized Anxiety Disorder | 3 (12%) | 0 (0%) | 0 (0%) | |
| YMRS score | 9.20 (7.78) | -- | -- | |
| CDRS score | 29.28 (8.90) | -- | -- | |
| CGAS score | 61.52 (13.70) | 75.80 (13.67) | -- | F(1,48) = 13.61** |
| Conners Rating Scale, ADHD Index t-scoreq | 73.60 (11.60) | 66.83 (9.87) | -- | F(1,47)= 4.82* |
| Medication | ||||
| Lithium | 8 (32%) | 0 (0%) | -- | |
| Atypical neuroleptic | 16 (64%) | 0 (0%) | -- | |
| Anti-epileptic | 5 (20%) | 0 (0%) | -- | |
| Anti-depressant | 4 (16%) | 0 (0%) | -- | |
| Stimulantb | 7 (28%) | 22 (88%) | -- | |
| Alpha agonist ADHD medication | 3 (12%) | 4 (16%) | -- |
Note. Results presented as M(SD) or as n(%). ADHD=Attention Deficit/Hyperactivity Disorder; BD=Bipolar Disorder; CDRS= Children’s Depression Rating Scale; CGAS=Children’s Global Assessment Scale; FSIQ=Full-Scale IQ; KSADS=Schedule for Affective Disorders interview; SES=Socio-economic status; YMRS=Young Mania Rating Scale.
Data were not available for all participants for these variables: for BD participants (n=25); for ADHD participants (n=24).
One participants in the BD group and 3 in the ADHD group were taking stimulant medication the day of the assessment.
p≤ 0.05
p ≤ 0.001
CANTAB AGN
All behavioral outcome measures are presented in Table 2.
Table 2.
AGN outcome measures by Group
| Groups | |||
|---|---|---|---|
| BD (n=25) | ADHD (n= 25) | TDC (n=25) | |
| Commission Errors | |||
| Shift-positive | 14.88±6.37 | 14.84±6.63 | 10.20±6.10 |
| Non-shift positive | 11.92±7.20 | 13.20±6.51 | 8.96±6.16 |
| Shift negative | 11.58±6.39 | 14.56±6.74 | 10.04±7.00 |
| Non-shift negative | 12.08±7.14 | 14.96±7.46 | 10.36±6.84 |
| Omission Errors | |||
| Shift-positive | 5.83±5.04 | 5.00±3.63 | 6.56±4.43 |
| Non-shift positive | 5.42±5.41 | 5.76±3.46 | 7.08±5.24 |
| Shift negative | 4.29±4.40 | 4.64±3.68 | 5.76±5.25 |
| Non-shift negative | 5.21±4.90 | 4.52±3.77 | 6.12±4.74 |
| Response Time (ms) | |||
| Shift-positive | 441.26±105.10 | 396.85±116.56 | 464.82±133.73 |
| Non-shift positive | 435.05±97.86 | 392.68±122.32 | 487.71±114.44 |
| Shift negative | 466.31±96.33 | 418.62±120.84 | 480.04±126.66 |
| Non-shift negative | 453.30±119.57 | 415.73±117.68 | 477.96±137.21 |
Note. Results presented as M±SD. ADHD= attention deficit/hyperactivity disorder, BD= bipolar disorder, TDC= typically developing control
Commission Errors (Responses to distractor valence)
While the three-way interaction between shift condition (e.g., same vs. different emotional valence of target between blocks), target valence (i.e., positive or negative emotional stimuli) and group was not significant, the two-way interaction between target valence and group was significant [F(2,71)=5.34, p<0.01, ƞp2=0.13] (Figure 1). Follow-up paired t-tests showed that participants in the ADHD made significantly more commissions on negative vs. positive words [t(24)=−2.58, p<0.05, r=0.47]. Further, there was a trend for BD participants to make fewer errors on negative compared to positive valence words [t(24)=1.91, p=0.07,r=0.36 ]. There were no differences in errors between positive and negative words for the TDC group.
Figure 1.
Group by Target Valence Interaction for Commission Errors.

Note. ADHD=attention-deficit/hyperactivity disorder; BD bipolar disorder; TDC=typically-developing Control.
We also found a significant two-way interaction between shift condition and target valence [F(1,72)=14.97, p<0.001,ƞp2=0.17]. Follow-up paired t-tests showed that in the non-shift condition all participants made more commission errors for negative valence vs. positive valence targets [t(74)=−3.014, p<0.01 r=0.88]). However, in the shift condition, all participants made more errors for positive valence targets vs. negative valence targets [t(74)=2.21, p<0.05, r=0.25)]).
There was a significant main effect of shift condition on commission errors [F(1,72)=9.36,p≤0.01,ƞp2=0.12] such that participants made more errors in the shift (26.31±12.83) vs. the non-shift (24.87±13.39) condition. There was no main effect of target valence on commission errors.
Tests of between subjects effects for the model showed a significant effect of group [F(2,72)=4.60, p<0.05,ƞp2=0.11). Pair-wise comparisons showed that ADHD made significantly more overall commission errors vs. TDC youth (p=0.02), but not more than BD youth. BD participants and TDC participants were not significantly different.
Omission Errors (Non-response to target valences)
The three-way interaction between shift condition, group and target valence was not significant. Additionally, there were no significant interactions between shift condition and group, group by target valence or shift condition by target valence.
There was a significant main effect of target valence on omission errors [F(1,72)=4.96,p<0.05,,ƞp2=0.06] whereby all participants made more omission errors for positive (12.13± 8.44) vs. negative valence targets (10.72±8.54). There was no main effect of shift condition. The between subjects effect was not significant.
Response Time
The three-way and two-way interactions for response time were not significant. Results showed a significant main effect for response time as a function of target valence [F(1,72)=4.53, p<0.05,ƞp2=0.06] such that for all participants response times were faster for positive (435.55±111.50) vs. negative emotional stimuli (448.28±116.21). The between subjects effect was not significant.
Post-Hoc Analyses Examining the Effect of Comorbid ADHD within the BD group (see Supplement for full details)
To explore potential effects of comorbid ADHD on BD group on results, we compared BD participants with comorbid ADHD (BD+ADHD, n=18), BD participants without comorbid ADHD (BD-ADHD; n=7), youth with ADHD only (n=25), and TDC youth (n=25).
Briefly, for commission errors, results continued to show a significant two-way interaction between target valence and group [F(3,71)=3.55, p<.05]. Follow-up paired t-tests for the BD groups did not reveal a statistically significant group differences for the BD-ADHD group [t(6)=0.77, p=0.47, r=0.30] regarding errors on positive versus negative valence targets. However, for the BD+ADHD group [t(17)=1.74, p=0.10,r=0.39] a non-significant trend emerged in which greater errors were observed for positive versus negative valence words (Supplement Figure 1).
Post-Hoc Examination of Relationship Between AGN Variables and Dimensional Measures of Psychopathology
To better understand our findings related to AGN commission errors, we examined the relationship between AGN positive and negative valence commission errors and dimensional measures of psychopathology including: Conners Total ADHD, Inattentive and Hyperactive/Impulsive Indices (t-scores), CGAS, YMRS, CDRS, and CBCL Depressed-Withdrawn subscale.
Commissions
Commission errors on positively-valenced words were significantly positively related to Conners ADHD index (r=0.35, p<0.01), Conners Inattention (r=0.43, p<0.001), Conners Hyperactivity/Impulsivity (r=0.28, p<0.05), and CBCL Depressed/Withdrawn (r=0.31, p<0.01), scores.
Commission errors on negatively-valenced words were significantly positively related to Conners ADHD index (r=0.29, p≤0.01) and Conners Inattention (r=0.37, p≤0.001) scores.
Discussion
The main finding of our study, the first to compare performance on an AGN task in youth with BD, youth with ADHD and TDCs, revealed a significant diagnosis by target valence interaction in which children with ADHD made significantly more commission errors on negative vs. positively valances words compared to both BD and TDC participants. In contrast, there was a trend for BD participants to make fewer errors on negative vs. positive words compared to ADHD and TDC participants. Furthermore, when the BD group was divided into those with (BD+ADHD) and without ADHD (BD-ADHD), results showed that BD+ADHD participants, but not BD-ADHD participants were likely driving this trend for fewer commission errors on negative vs. positive emotional stimuli (Supplement materials). In addition to the group by target valence interaction, tests of between subjects effects revealed a significant main effect of group such that individuals in the ADHD made significantly more overall commission errors (regardless of target valence) than TDC, but not BD, participants. No group main or interaction effects were found for omission errors or response time. Taken together, our results suggest different behavioral patterns of affective processing bias in children with ADHD versus those with BD.
Our primary findings is that children with ADHD made significantly more commission errors on negative versus positively valances words compared to both BD and TDC participants. While deficits in response control in children with ADHD are well-documented within the literature, few studies have examined response control in the context of emotionally salient stimuli.[49,10,50] To our knowledge, only Fried and colleagues have compared ADHD and TDC children using an affective go/no-go paradigm (CANTAB AGN task).[35] Similar to our results, this study found that children with ADHD made significantly more overall commission errors than TDC participants; however, the role of target valence was not reported. Therefore, our work adds to the literature on response control in children with ADHD by suggesting the importance of emotional processing under conditions in which response control is required. Specifically, children with ADHD, but not TDC or BD participants, made more errors when target stimuli were negatively-valence with positive words as distractors than when target stimuli were positive with negative words as distractors. Therefore, in our study children with ADHD showed a bias towards positively-valenced emotional stimuli.
Our results suggesting affective processing bias in children with ADHD are interesting in light of recent work arguing that difficulties with emotion regulation and processing may be a core feature of ADHD.[13] [15,51] From an affective neuroscience viewpoint, emotional processing and regulation involve both top-down attentional and inhibitory control which involve prefrontal regions as well as bottom-up processes such as the identification and appraisal of an emotional stimuli involving subcortical regions (e.g., amygdala, insula, striatum). For example, a prior study examining behavioral inhibition in boys with and without ADHD using a stop signal task showed that response inhibition accounted for 11% of the variance in “dysregulated” behavior during an observed frustration task.[52] At a neural level, research by Posner et al. showed that disturbances in emotional processing in ADHD stem from underlying neural alterations that are independent of those associated with impairments in cognitive control[30]. These results suggest that youth with ADHD have emotional processing deficits that are independent of their other deficits in response control. In this study, while behavioral task data on the Emotional Stroop did not elicit group differences between ADHD and TDC participants, increased neural reactivity in the medial prefrontal cortex, a neural area associated with regulation of affect, in adolescents with ADHD was related to emotional processing deficits even after controlling for differences in cognitive control. In the context of prior work, our results may suggest emotional processing deficits in children with ADHD involve alterations in prefrontal-striatal regions. Future research is needed to extend our behavioral findings and examine differences in neural processing of affective stimuli in children with ADHD compared to TDC children and those with other forms of psychopathology.
While not wanting to over-interpret a non-significant trend, our results suggest a trend of small effect for BD participants to demonstrate a greater bias towards negative emotional stimuli than positive emotional stimuli. This pattern is consistent with prior behavioral studies of AGN tasks in BD adults. Specifically, using the CANTAB AGN, Murphy and colleagues showed that manic patients demonstrated a bias to positive affective stimuli (e.g., faster response time) whereas depressed patients showed a bias towards negative affective stimuli suggesting possible differences in response control and affective processing between mania and depression.[31] As a follow-up study, Garcia-Blanco et al. compared AGN performance in BD adults who were either depressed (n=22), euthymic (n=28) or manic (n=30) and healthy controls (HC).[32] Results showed a target valence by group interaction in which depressed participants showed faster RT for negative vs. positive words while manic patients demonstrated faster RTs for positive rather than negative words.[32] The authors suggested that attentional biases to emotional stimuli may be related to state rather than trait functioning. Furthermore, in a subsequent study, Roiser et al. tested this possibility by examining the effect of mood state on AGN performance in adults with BD vs. HCs by using positive mood induction prior to AGN performance.[53] Results showed that after positive mood induction, adults with BD made significantly more commission errors to negative emotional stimuli than positive emotional stimuli than HCs, indicating a positive emotional bias.[53] Interestingly, even when adults with BD are considered to have stable mood (i.e., no increase in depressive or manic symptoms), AGN performance compared to healthy controls suggests a tendency for adults with BD to attend more readily to negatively valenced information compared to material with less emotional content (e.g., neutral stimuli), and that positively valenced stimuli may be process less efficiently (i.e., worse ability to discriminate and response accurately to positive stimuli and increased reaction time).[54] Furthermore, it should be noted that when we compared those with BD and ADHD to those with BD alone (Supplement), it was the comorbid BD and ADHD group, rather than the BD only group, that appeared to drive the results suggesting that greater positive compared to negative valence errors. As youth in the BD+ADHD group were more likely to be symptomatic rather than euthymic, mood state may have influences these results. Thus, further work is needed to examine the role of mood state in AGN performance in youth with BD, although this is beyond the scope of our current study since the majority of our BD participants were euthymic.
Regarding clinical applications, our results suggest the importance of emotional processing deficits in children with ADHD. Over the past-decade, there has been a growing recognition that children with ADHD demonstrate increased emotional lability and decreased emotion regulation ability compared to TDC children.[13] Further, emotion regulation deficits mediate the relationship between ADHD and depressive symptoms in children suggesting the importance of comprehensive assessment and treatment for difficulties with emotional processing and regulation in children with ADHD.[55,56] As children with ADHD made significantly more overall commission errors than TDC children, it may be that in the wake of emotionally salient information, children with ADHD have increased difficulty attending to task relevant information. In the context of their daily lives, such difficulties with attending and response control in the presence of emotional stimuli could result in missed information and poor decision-making by children with ADHD. In contrast, in children with BD, a negative processing bias even when euthymic may suggest the need for intensive cognitive behavioral treatment (CBT) such as cognitive restructuring of automatic negative thoughts regardless of current mood state.
Our study has a number of limitations, including some mood state heterogeneity and concomitant medication use within the BD group, and absence of comorbid mood/anxiety disorders in ADHD participants. First, as noted previously, there was heterogeneity in the mood state of our BD participants. While the BD sample was euthymic overall, some participants were in mixed, depressed or hypomanic states by rating scales, with none manic; therefore, complicating the effect of mood state on our results. A second limitation is that the majority of our BD participants (84%; 21/25) were taking their usual psychotropic medications at the time of assessment, and potential post-hoc analyses of unmedicated BD youths would be underpowered. Prior studies have not examined the effect of medication on AGN performance. However, further work is needed to determine if affective processing on the AGN is improved when mood symptoms treated. Finally, as noted previously, our ADHD sample did not have comorbid mood and anxiety disorders because it was meant as a psychiatric control group to balance the high rates of comorbid ADHD in the BD sample. As such, our ADHD findings may be specific to ADHD, though they may not generalize to ADHD participants more broadly given high rates of psychiatric comorbidity associated with ADHD. Future studies should examine the role of comorbid mood and anxiety problems in children with ADHD in relation to performance on behavioral affective processing tasks in order to clarify this question.
In sum, our results comparing AGN performance in youth with BD, youth with ADHD and TDCs suggest a positive processing bias in ADHD children compared to TDCs, and a non-significant trend for a negative processing bias in children with BD. Interestingly, our results showed this negative processing bias in BD youth who were mostly euthymic suggesting the presence of these deficits regardless of mood state in contrast to prior literature. Taken together, our behavioral results coupled with prior neuroimaging studies of affective processing in youth with ADHD vs. BD, may suggest that different neural mechanisms are implicated in the pathophysiology of these disorders.
Supplementary Material
Figure 2.
Total Commision Errors by Group
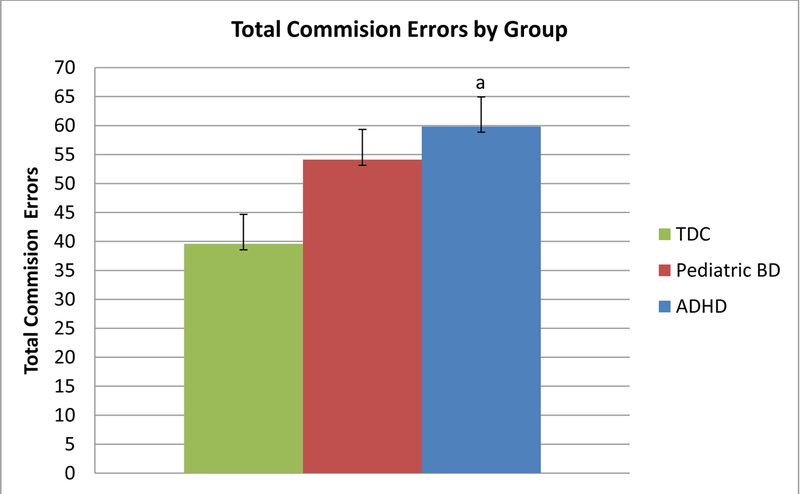
Note. ADHD=Attention-Deficit/Hyperactivity Disorder; BD Bipolar Disorder; TDC=Typically Developing Control. a= ADHD > TDC, p≤0.05
Acknowledgement:
This work was supported by NIMH (5K22MH074945 and 3K22MH074945–02S1 PI=DP Dickstein) and Bradley Hospital.
Footnotes
Conflict of Interest Statement:
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Contributor Information
Karen E. Seymour, Johns Hopkins University School of Medicine Division of Child and Adolescent Psychiatry..
Kerri L. Kim, Bradley Hospital’s Pediatric, Mood, Imaging and NeuroDevelopmental (PediMIND) Program and the Alpert Medical School of Brown University when this work was prepared..
Grace K. Cushman, Bradley Hospital’s Pediatric, Mood, Imaging and NeuroDevelopmental (PediMIND) Program and the Alpert Medical School of Brown University when this work was prepared.
Megan E. Puzia, Bradley Hospital’s Pediatric, Mood, Imaging and NeuroDevelopmental (PediMIND) Program and the Alpert Medical School of Brown University when this work was prepared..
Alexandra B. Weissman, Bradley Hospital’s Pediatric, Mood, Imaging and NeuroDevelopmental (PediMIND) Program and the Alpert Medical School of Brown University when this work was prepared..
Daniel P. Dickstein, Bradley Hospital’s Pediatric, Mood, Imaging and NeuroDevelopmental (PediMIND) Program and the Alpert Medical School of Brown University when this work was prepared..
References
- 1.Moreno C, Laje G, Blanco C, Jiang H, Schmidt AB, Olfson M (2007) National Trends in outpatient diagnosis and treatment of bipolar disorder in youth. Archives of general psychiatry 64 (9):1032–1039 [DOI] [PubMed] [Google Scholar]
- 2.Blader JC, Carlson GA (2007) Increased rates of bipolar disorder diagnoses among U.S. child, adolescent, and adult inpatients, 1996–2004. Biological psychiatry 62 (2):107–114. doi: 10.1016/j.biopsych.2006.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Getahun D, Jacobsen SJ, Fassett MJ, Chen W, Demissie K, Rhoads GG (2013) Recent trends in childhood attention-deficit/hyperactivity disorder. JAMA pediatrics 167 (3):282–288. doi: 10.1001/2013.jamapediatrics.401 [DOI] [PubMed] [Google Scholar]
- 4.Hurtig T, Ebeling H, Taanila A, Miettunen J, Smalley S, McGough J, Loo S, Jarvelin MR, Moilanen I (2007) ADHD and comorbid disorders in relation to family environment and symptom severity. European child & adolescent psychiatry 16 (6):362–369. doi: 10.1007/s00787-007-0607-2 [DOI] [PubMed] [Google Scholar]
- 5.Wozniak J, Biederman J, Kiely K, Ablon JS, Faraone SV, Mundy E, Mennin D (1995) Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. Journal of the American Academy of Child and Adolescent Psychiatry 34 (7):867–876. doi: 10.1097/00004583-199507000-00010 [DOI] [PubMed] [Google Scholar]
- 6.Kowatch RA, Youngstrom EA, Danielyan A, Findling RL (2005) Review and meta-analysis of the phenomenology and clinical characteristics of mania in children and adolescents. Bipolar disorders 7 (6):483–496. doi: 10.1111/j.1399-5618.2005.00261.x [DOI] [PubMed] [Google Scholar]
- 7.Youngstrom EA, Arnold LE, Frazier TW (2010) Bipolar and ADHD Comorbidity: Both Artifact and Outgrowth of Shared Mechanisms. Clinical psychology : a publication of the Division of Clinical Psychology of the American Psychological Association 17 (4):350–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galanter CA, Leibenluft E (2008) Frontiers between attention deficit hyperactivity disorder and bipolar disorder. Child and adolescent psychiatric clinics of North America 17 (2):325–346, 10.1016/j.chc.2007.11.001 [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric A (2013) Diagnostic and statistical manual of mental disorders 5th edn. American Psychiatric Publishing, Arlington, VA [Google Scholar]
- 10.Walshaw PD, Alloy LB, Sabb FW (2010) Executive function in pediatric bipolar disorder and attention-deficit hyperactivity disorder: in search of distinct phenotypic profiles. Neuropsychology review 20 (1):103–120. doi: 10.1007/s11065-009-9126-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rucklidge JJ (2006) Impact of ADHD on the neurocognitive functioning of adolescents with bipolar disorder. Biological psychiatry 60 (9):921–928. doi: 10.1016/j.biopsych.2006.03.067 [DOI] [PubMed] [Google Scholar]
- 12.Geller B, Zimerman B, Williams M, Delbello MP, Bolhofner K, Craney JL, Frazier J, Beringer L, Nickelsburg MJ (2002) DSM-IV mania symptoms in a prepubertal and early adolescent bipolar disorder phenotype compared to attention-deficit hyperactive and normal controls. Journal of child and adolescent psychopharmacology 12 (1):11–25. doi: 10.1089/10445460252943533 [DOI] [PubMed] [Google Scholar]
- 13.Shaw P, Stringaris A, Nigg J, Leibenluft E (2014) Emotion Dysregulation in Attention Deficit Hyperactivity Disorder. The American journal of psychiatry doi: 10.1176/appi.ajp.2013.13070966 [DOI] [PMC free article] [PubMed]
- 14.LeDoux J (2012) Rethinking the emotional brain. Neuron 73 (4):653–676. doi: 10.1016/j.neuron.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips ML, Drevets WC, Rauch SL, Lane R (2003) Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological psychiatry 54 (5):504–514. doi: 10.1016/s0006-3223(03)00168-9 [DOI] [PubMed] [Google Scholar]
- 16.Phillips ML, Ladouceur CD, Drevets WC (2008) A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular psychiatry 13 (9):829, 833–857. doi: 10.1038/mp.2008.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochsner KN, Gross JJ (2005) The cognitive control of emotion. Trends in cognitive sciences 9 (5):242–249. doi: 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 18.Rothbart M, Posner M (2006) Temperament, attention, and developmental psychopathology. In: Cicchetti D, Cohen D (eds) Developmental Psychopathology: Vol 2. Developmental Neuroscience, vol 2 John Wiley & Sons, Hoboken, NJ, pp 465–501 [Google Scholar]
- 19.Rosen HR, Rich BA (2010) Neurocognitive correlates of emotional stimulus processing in pediatric bipolar disorder: a review. Postgraduate medicine 122 (4):94–104. doi: 10.3810/pgm.2010.07.2177 [DOI] [PubMed] [Google Scholar]
- 20.Brotman MA, Guyer AE, Lawson ES, Horsey SE, Rich BA, Dickstein DP, Pine DS, Leibenluft E (2008) Facial emotion labeling deficits in children and adolescents at risk for bipolar disorder. The American journal of psychiatry 165 (3):385–389. doi: 10.1176/appi.ajp.2007.06122050 [DOI] [PubMed] [Google Scholar]
- 21.Dickstein DP, Castellanos FX (2012) Face processing in attention deficit/hyperactivity disorder. Current topics in behavioral neurosciences 9:219–237. doi: 10.1007/7854_2011_157 [DOI] [PubMed] [Google Scholar]
- 22.Seymour KE, Pescosolido MF, Reidy BL, Galvan T, Kim KL, Young M, Dickstein DP (2013) Emotional face identification in youths with primary bipolar disorder or primary attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 52 (5):537–546 10.1016/j.jaac.2013.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnsten AF, Rubia K (2012) Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. Journal of the American Academy of Child and Adolescent Psychiatry 51 (4):356–367. doi: 10.1016/j.jaac.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 24.Alderson RM, Rapport MD, Kofler MJ (2007) Attention-deficit/hyperactivity disorder and behavioral inhibition: a meta-analytic review of the stop-signal paradigm. Journal of abnormal child psychology 35 (5):745–758. doi: 10.1007/s10802-007-9131-6 [DOI] [PubMed] [Google Scholar]
- 25.Passarotti AM, Sweeney JA, Pavuluri MN (2010) Differential engagement of cognitive and affective neural systems in pediatric bipolar disorder and attention deficit hyperactivity disorder. Journal of the International Neuropsychological Society : JINS 16 (1):106–117. doi: 10.1017/S1355617709991019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leibenluft E, Rich BA, Vinton DT, Nelson EE, Fromm SJ, Berghorst LH, Joshi P, Robb A, Schachar RJ, Dickstein DP, McClure EB, Pine DS (2007) Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. The American journal of psychiatry 164 (1):52–60. doi: 10.1176/appi.ajp.164.1.52 [DOI] [PubMed] [Google Scholar]
- 27.Rubia K, Smith A, Taylor E (2007) Performance of children with attention deficit hyperactivity disorder (ADHD) on a test battery of impulsiveness. Child neuropsychology : a journal on normal and abnormal development in childhood and adolescence 13 (3):276–304. doi: 10.1080/09297040600770761 [DOI] [PubMed] [Google Scholar]
- 28.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF (2005) Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological psychiatry 57 (11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006 [DOI] [PubMed] [Google Scholar]
- 29.Nigg JT (2005) Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient challenges for the coming decade. Biological psychiatry 57 (11):1424–1435. doi: 10.1016/j.biopsych.2004.11.011 [DOI] [PubMed] [Google Scholar]
- 30.Posner J, Maia TV, Fair D, Peterson BS, Sonuga-Barke EJ, Nagel BJ (2011) The attenuation of dysfunctional emotional processing with stimulant medication: an fMRI study of adolescents with ADHD. Psychiatry research 193 (3):151–160. doi: 10.1016/j.pscychresns.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES (1999) Emotional bias and inhibitory control processes in mania and depression. Psychological medicine 29 (6):1307–1321 [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Blanco AC, Perea M, Livianos L (2013) Mood-congruent bias and attention shifts in the different episodes of bipolar disorder. Cognition & emotion 27 (6):1114–1121. doi: 10.1080/02699931.2013.764281 [DOI] [PubMed] [Google Scholar]
- 33.Whitney J, Joormann J, Gotlib IH, Kelley RG, Acquaye T, Howe M, Chang KD, Singh MK (2012) Information processing in adolescents with bipolar I disorder. Journal of child psychology and psychiatry, and allied disciplines 53 (9):937–945. doi: 10.1111/j.1469-7610.2012.02543.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gotlib IH, Traill SK, Montoya RL, Joormann J, Chang K (2005) Attention and memory biases in the offspring of parents with bipolar disorder: indications from a pilot study. Journal of child psychology and psychiatry, and allied disciplines 46 (1):84–93. doi: 10.1111/j.1469-7610.2004.00333.x [DOI] [PubMed] [Google Scholar]
- 35.Fried R, Hirshfeld-Becker D, Petty C, Batchelder H, Biederman J (2012) How Informative Is the CANTAB to Assess Executive Functioning in Children With ADHD? A Controlled Study. Journal of attention disorders doi: 10.1177/1087054712457038 [DOI] [PubMed]
- 36.Surman CB, Hammerness PG, Petty C, Spencer T, Doyle R, Napolean S, Chu N, Yorks D, Biederman J (2013) A pilot open label prospective study of memantine monotherapy in adults with ADHD. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry 14 (4):291–298. doi: 10.3109/15622975.2011.623716 [DOI] [PubMed] [Google Scholar]
- 37.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N (1997) Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry 36 (7):980–988. doi: 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- 38.Wechsler D (2005) Wechsler abbreviated scale of intelligence The Psychological Corporation, San Antonio, TX [Google Scholar]
- 39.American Psychiatric A (2000) Diagnostics and statistics manual of mental disorders 4th edition text-revision (DSM-IV-TR) 4th edn. American Psychiatric Association, Washington, DC [Google Scholar]
- 40.Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS (2003) Defining clinical phenotypes of juvenile mania. The American journal of psychiatry 160 (3):430–437 [DOI] [PubMed] [Google Scholar]
- 41.Shaffer D, Gould M, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia S (1983) A Children’s Global Assessment Scale (CGAS). Archives of general psychiatry 40 (11):1228–1231 [DOI] [PubMed] [Google Scholar]
- 42.Conners CK, Wells KC, Parker JD, Sitarenios G, Diamond JM, Powell JW (1997) A new self-report scale for assessment of adolescent psychopathology: factor structure, reliability, validity, and diagnostic sensitivity. Journal of abnormal child psychology 25 (6):487–497 [DOI] [PubMed] [Google Scholar]
- 43.Young RC, Biggs JT, Ziegler VE, Meyer DA (1978) A rating scale for mania: reliability, validity, and sensitivity. The British journal of psychiatry : the journal of mental science 133:429–435 [DOI] [PubMed] [Google Scholar]
- 44.Poznanski EO, Cook SC, Carroll BJ (1979) A depression rating scale for children. Pediatrics 64 (4):442–450 [PubMed] [Google Scholar]
- 45.Achenbach TM, Rescorla LA (2001) Manual for the ASEBA school age forms & profiles University of Vermont: Research Center for Children, Youth & Families, Burlington, VT [Google Scholar]
- 46.Cambridge Neuropsychological Test Automated Battery (CANTAB) (2013). Cambridge Cognition, Cambridge, UK [Google Scholar]
- 47.Field A (2013) Discovering Statistics Using IBM SPSS Statistics 4th edn. SAGE Publications Ltd, Los Angeles, CA [Google Scholar]
- 48.Rosnow RL (2003) Effect sizes for experimenting psychologists. Canadian journal of experimental psychology = Revue canadienne de psychologie experimentale 57 (3):221–237 [DOI] [PubMed] [Google Scholar]
- 49.Rubia K, Smith A, Taylor E (2007) Performance of children with attention deficit hyperactivity disorder (ADHD) on a test battery of impulsiveness. Child neuropsychology : a journal on normal and abnormal development in childhood and adolescence 13 (3):276–304. doi: 10.1080/09297040600770761 [DOI] [PubMed] [Google Scholar]
- 50.Willcutt E, Doyle AE, Nigg JT, Faraone SV, Pennington BF (2005) Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological psychiatry 57 (11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006 [DOI] [PubMed] [Google Scholar]
- 51.Phillips ML, Drevets WC, Rauch SL, Lane R (2003) Neurobiology of emotion perception II: implications for major psychiatric disorders. Biological psychiatry 54 (5):515–528. doi: 10.1016/s0006-3223(03)00171-9 [DOI] [PubMed] [Google Scholar]
- 52.Walcott CM, Landau S (2004) The relation between disinhibition and emotion regulation in boys with attention deficit hyperactivity disorder. Journal of clinical child and adolescent psychology : the official journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53 33 (4):772–782. doi: 10.1207/s15374424jccp3304_12 [DOI] [PubMed] [Google Scholar]
- 53.Roiser J, Farmer A, Lam D, Burke A, O’Neill N, Keating S, Smith GP, Sahakian B, McGuffin P (2009) The effect of positive mood induction on emotional processing in euthymic individuals with bipolar disorder and controls. Psychological medicine 39 (5):785–791. doi: 10.1017/S0033291708004200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gopin CB, Burdick KE, Derosse P, Goldberg TE, Malhotra AK (2011) Emotional modulation of response inhibition in stable patients with bipolar I disorder: a comparison with healthy and schizophrenia subjects. Bipolar disorders 13 (2):164–172. doi: 10.1111/j.1399-5618.2011.00906.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seymour KE, Chronis-Tuscano A, Halldorsdottir T, Stupica B, Owens K, Sacks T (2012) Emotion regulation mediates the relationship between ADHD and depressive symptoms in youth. Journal of abnormal child psychology 40 (4):595–606. doi: 10.1007/s10802-011-9593-4 [DOI] [PubMed] [Google Scholar]
- 56.Seymour KE, Chronis-Tuscano A, Iwamoto DK, Kurdziel G, Macpherson L (2013) Emotion Regulation Mediates the Association Between ADHD and Depressive Symptoms in a Community Sample of Youth. Journal of abnormal child psychology doi: 10.1007/s10802-013-9799-8 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


