Abstract
Typically, presynaptic terminals form a synapse directly on the surface of postsynaptic processes such as dendrite shafts and spines. However, some presynaptic terminals invaginate—entirely or partially—into postsynaptic processes. We survey these invaginating presynaptic terminals in all animals and describe several examples from the central nervous system, including giant fiber systems in invertebrates, and cup-shaped spines, electroreceptor synapses, and some specialized auditory and vestibular nerve terminals in vertebrates. We then examine mechanoreceptors and photoreceptors, concentrating on the complex of pre- and postsynaptic processes found in basal invaginations of the cell. We discuss in detail the role of vertebrate invaginating horizontal cell processes in both chemical and electrical feedback mechanisms. We also discuss the common presence of indenting or invaginating terminals in neuromuscular junctions on muscles of most kinds of animals, and especially discuss those of Drosophila and vertebrates. Finally, we consider broad questions about the advantages of possessing invaginating presynaptic terminals and describe some effects of aging and disease, especially on neuromuscular junctions. We suggest that the invagination is a mechanism that can enhance both chemical and electrical interactions at the synapse.
Keywords: Drosophila, Horizontal cell, Retina, Motor ending, Electroreceptor, Ephaptic conduction, Lateral inhibition, Subsynaptic reticulum, Subjunctional folds
Introduction
In the nervous system of all animals, neurons transmit impulses most frequently via chemical neurotransmission across synapses (Shepherd 2004). Most of these synapses involve connections between a presynaptic axonal terminal containing synaptic vesicles, some of which concentrate at an active zone where the presynaptic membrane is separated by a synaptic cleft from a specialized patch of postsynaptic membrane. This postsynaptic membrane occurs along the surface of a dendrite (or soma), or the head of a synaptic spine (Shepherd 2004; Petralia et al. 2016). Interestingly, many synapses include invaginating structures that extend into corresponding invaginations in the synaptic partner cell; invaginations are unique synaptic modifications that can isolate or subdivide regions of the synapse to facilitate specialized modulation or signaling functions of the synapse (Petralia et al. 2015, 2016).
Several different invaginating structures are associated with synapses (Fig. 1 and Glossary). One category is protrusions/projections of various sizes that lack active zones, and that extend into an invagination of the opposing cell process, and occur among axons, axon (presynaptic) terminals, dendrite processes including shafts and spines, and associated glial elements (Fig. 1a2; reviewed by Petralia et al. 2015). Another type of invaginating structure is the postsynaptic spine, which invaginates into the presynaptic terminal (Fig. 1a3; reviewed by Petralia et al. 2016). A distinctive feature of these invaginating spines is that they contact one or more synaptic active zones where presynaptic vesicles can release neurotransmitter that binds to receptors on the postsynaptic membrane of the invaginating spine. While most types of invaginating structures associated with synapses fall into these above two categories, a third type of invaginating structure at synapses consists of a presynaptic terminal that entirely or partially invaginates into the postsynaptic process (Fig. 1b1–3). Invaginating presynaptic terminals often are essential to major functions such as mechanoreception, vision, and locomotion. In this review, we describe the varieties of invaginating presynaptic terminals and discuss their functions and significance (Table 1).
Fig. 1.
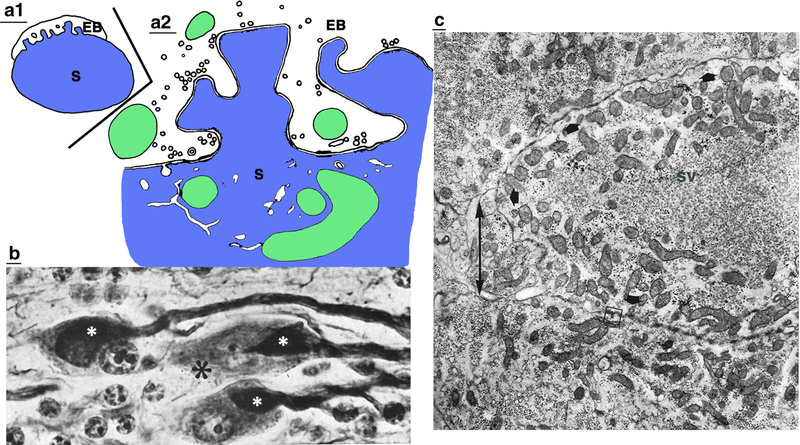
Generalized structures of invaginating synaptic processes, as described in the review trilogy (Petralia et al. 2015, 2016, and this review). a. a1 Standard synapses form directly along the surface of dendrite processes including shafts and spines (spines not shown). a2 Protrusions/projections of various sizes can extend into invaginations, and occur among axons, axon (presynaptic) terminals, dendrite processes including shafts and spines, and associated glial elements. Three examples are shown; reviewed in Petralia et al. (2015). a3 Postsynaptic spines can protrude/project into an invagination of the presynaptic terminal. These are reviewed in Petralia et al. (2016); examples are seen also in figures 6 and 7 of the current review. b1–3 As discussed specifically in the current review, some presynaptic terminals can be invaginated into a postsynaptic structure. The terminal may be fully (b1) or partially (indenting) (b2) invaginating into a postsynaptic invagination, or form an invaginating protrusion (b3). In these structures, the invaginating processes contain presynaptic active zones with concentrations of synaptic vesicles, and associated with postsynaptic specializations (typically a distinctive PSD; red arrow). Also, the axon/terminal orientation can be either mostly perpendicular (as shown) or mostly parallel (not shown; common for neuromuscular junctions) to the postsynaptic process. Often, the terminal has an intermediate orientation, extending in a third dimension at an angle from the plane of the section; for example, in ribbon synapses, the ribbon structure often extends as a ridge in an elongate invagination (see figures 4, 7). The postsynaptic structure can be a dendrite process, or muscle or gland cell (also in the case of the photoreceptor terminal invagination, the terminal membrane may be postsynaptic to horizontal cell processes). Examples illustrated in this review of the structure in figures 1b1, 1b2, and 1b3 are shown in figures 3, 5–9, 11, 12, figures 2, 3, 8–11, and figures 2–6, 11, respectively. See text and other legends for details. Note that all drawings in all figures are original and based on micrographs and drawings in the cited studies. In all drawings, the presynaptic terminals are colorless, postsynaptic processes (usually only the adjacent portion is shown) are blue, mitochondria are green, and Schwann/glial processes are pink (additional structures are yellow) (Color figure online)
Table 1.
Summary of the major groups of animals described in this review, and their indenting/invaginating presynaptic terminals
| Group | Motor terminal synapses | Sensory cell synapses | CNS synapses |
|---|---|---|---|
| Porifera (sponges) | Unknown, but with invaginating processes1 | Unknown, but with invaginating processes | Unknown, but with invaginating processes |
| Ctenophora (comb jellies) | NMJs indented2 | – | Some deeply indented3 |
| Cnidaria (jellyfish, sea anemones, corals, hydroids) | NMJs occasionally indented; also axon wrapped in muscle processes4 | Nematocyte (stinging hair cell) with basal indented efferent5 or basal tunnel with afferents + efferents6 | – |
| Flatworms (Platyhelminthes) | NMJs indented or invaginated; associated with muscle processes7 | – | Some invaginated8 |
| Nematodes (roundworms) and Gastrotricha (hairybacks) | Unknown; synapses at the ends of muscle processes9 | – | – |
| Chaetognatha (arrow worms) and rotifers | Some deeply indented/partly invaginated NMJs10; arrow worms with distinctive subsynaptic apparatus11 | – | – |
| Phoronida (horseshoe worms) | Unknown12 | – | – |
| Entoprocta and Annelida (leeches, earthworms) | Some indented or deeply indented NMJs13 | – | – |
| Mollusca (octopi, squid, snails, mussels, chitons) | NMJs often indented or invaginated14; invaginated terminals in salivary glands (ex) of snails and octopi15, and in dorsal body gland (en) of snails16 | Tunnel fibers and finger twigs invaginate into photoreceptor presynaptic bags/carrots (octopi and squid)17 | Presynaptic processes from afferent giant axons invaginate into efferent giant axons (squid)18 |
| Arthropoda—Chelicerata (spiders, scorpions, mites) | NMJs often indented in spiders and scorpions19; invaginated in a tick20; horseshoe crab with invaginated terminal in muscle evagination21; invaginated terminal in salivary/silk gland (ex) of mite22 | In jumping spiders, retinal terminals invaginated by processes from presynaptic second-order terminals23; also, wolf spiders have invaginating postsynaptic complex in retinal terminals with possible efferent components24 | – |
| Arthropoda—Crustacea (crayfish, lobsters, crabs) | Many deeply invaginated NMJs with elaborate SSR25; double invaginated terminals in labral glands (ex) of water flea26 | Crayfish and lobster have invaginating postsynaptic complex in retinal terminals with possible efferent components27 | Crayfish have indentions/invaginations between giant axons, with combinations of electrical and chemical transmission28 |
| Arthropoda—Insecta (Drosophila, moths, beetles, cicadas, etc.) | Many deeply invaginated NMJs with elaborate SSR29; Indented terminals in prothoracic gland (en) of wax moth30 | – | Invaginating finger projections from giant fibers into interneurons may be presynaptic in Drosophila31 |
| Echinodermata (starfish, sea urchins, sea cucumbers) | Axons may be invaginated in muscle of sea cucumber32; terminals can be invaginated in sea urchin muscle fibers33 | – | – |
| Invertebrate chordates (sea squirts or ascidians, amphioxus or lancelets) | Unknown; in lancelets, synapses at the ends of muscle processes34 | Ascidian coronal organ hair cells with basal groove with afferent + efferent terminals35 | Larval lancelets with juxta-reticular junctions that may invaginate into cell bodies36 |
| Vertebrata—Agnatha (hagfish, lampreys) | Deeply invaginated NMJs with Schwann cell plug in hagfish37; deeply indented in lampreys38 | Invaginating synaptic complex in photoreceptor cells may have some efferents39 | Vestibular nerve spoon endings invaginate into vestibular neuron somas in lamprey larvae40 |
| Vertebrata—sharks and bony fish | Some deeply invaginated NMJs with extensive SJFs in sharks41; indented in bony fish-only a few with prominent SJFs42; some deeply invaginated cardiac NMJs in trout43 | Electroreceptors with presynaptic ribbon/sheet/rod44; in retina, invaginated horizontal cell processes provide negative feedback to photoreceptor cells45 | – |
| Vertebrata—Amphibians (frogs, toads, salamanders, newts) | Some indented NMJs with Schwann fingers and prominent SJFs46; moderately deep indentions of smooth muscle of frog/toad intestines47; indented terminals in pancreatic Islets (en) of toad48, and neuroepithelial body (en) in lung of salamander49 | In salamanders, vesicle-filled invaginating processes in photoreceptor cells may be efferents50; horizontal cell processes provide negative feedback to photoreceptor cells51 | – |
| Vertebrata—reptiles and birds | Indented and occasionally invaginated NMJs in some reptiles52; only shallow indentions in birds53; indented terminals in acinar cells (ex) in pancreas of chicken54 | Indenting efferents in auditory hair cells of pigeon55; in turtles, horizontal cell processes provide negative feedback to photoreceptor cells56 | In birds, reciprocal interdigitations in developing auditory nerve endbulbs on neuron somas57 |
| Vertebrata—mammals | NMJs indented and some with invaginations and/or with extensive SJFs58; also for some NMJs in extraocular59, cardiac60 and smooth muscle61; indented or invaginated terminals in various ex/en glands62 | Invaginating horizontal cell processes provide negative feedback to photoreceptor cells (including structural studies)63 | reciprocal interdigitations in developing auditory nerve endbulbs on neuron somas64; vestibular nerve terminal invaginations into rat lateral vestibular nucleus neuron65; cupshaped spines66; crested dendrites67 |
Indented ≈ presynaptic processes run in a deep pit or groove; invaginated ≈ presynaptic varicosity is embedded within the postsynaptic process. CNS, central nervous system; en, endocrine gland cells; ex, exocrine gland cells; NMJs, neuromuscular junctions; SSR, subsynaptic reticulum; SJFs, subjunctional folds; dash indicates no information found
Graziadei (1966), Barber and Graziadei (1967), Rogers (1969), Økland (1980), Elekes and Ude (1994)
Edwards et al. (1958b), Smith (1960), Rheuben and Reese (1978), Rheuben (1985), Prokop (1999), Wagner et al. (2015)
Flood (1966)
A Variety of Unusual Invaginating Presynaptic Terminals are Found in Animals
The two most notable examples of invaginating presynaptic terminals are those associated with 1) the bases of mechanoreceptor and photoreceptor cells and 2) neuromuscular junctions; these will be described in subsequent sections. In this section, we survey a variety of other examples of invaginating presynaptic terminals; the terminal typically is a varicosity (or bouton; i.e., enlarged structure with presynaptic vesicles). We consider a presynaptic terminal to be invaginating if the varicosity is surrounded entirely by the postsynaptic cell (Fig. 1b1); otherwise, we consider a terminal in a depression to be indenting into the postsynaptic cell (Fig. 1b2); alternatively, the invaginating structure can be a protrusion from the surface of the presynaptic terminal, projecting into the postsynaptic cell (Fig. 1b3). An exact designation is often difficult due to the obliqueness of the entry of the terminal into the postsynaptic cell and the variability in shape of the varicosity, as well as due to the plane of section of the published electron micrographs.
Examples from Invertebrates
The first hint of what could be invaginating synaptic processes in animal evolution is found in a sponge, and we already have discussed this in detail in Petralia et al. (2015, 2016). Briefly sponges (Porifera) do not have definitive neurons or chemical synapses, but the sponge, Tethya lyncurium, has some cells with elongate processes that roughly resemble neurons (Pavans de Ceccatty 1966). These processes can have knob-like structures along their length or at their ends, and these can invaginate into other cells. While the function of these invaginating structures is unknown, it is possible that they represent either presynaptic or postsynaptic portions of the earliest forms of chemical synapses. Alternatively, these structures have only a mechanical function, but this awaits further study.
Other simple animals only occasionally have structures that might represent invaginating presynaptic terminals. In the ctenophore, Pleurobrachia rhodopis, Hernandez-Nicaise (1973) shows a micrograph (his Fig. 1) of a presynaptic terminal that appears to be at least deeply indenting into a postsynaptic epithelial cell. We also have shown a micrograph of a partly invaginating presynaptic terminal in the brain of a planarian flatworm (figure 2 in Petralia et al. 2015).
Fig. 2.
Drawings of invertebrate giant fiber synapses. a In the squid, presynaptic processes from afferent giant axons (AGA) can extend through layers of Schwann cell (pink) and connective tissue sheaths to invaginate into the efferent giant axons (EGA). A large, dense homogenous mass (dense body; yellow asterisk) may be associated with the presynaptic vesicles (Castejón and Villegas 1964). b In the crayfish, the cytoplasm of one lateral giant fiber (LG1) may bulge into an adjacent one (LG2), forming a presumptive electrical synapse (Heitler et al. 1985); but this synapse can have numerous vesicles on both sides of the synapse, with more on the convex side, suggesting that it can act also as an indenting, presynaptic terminal. c In Drosophila, a giant fiber (GF) can protrude finger-like processes into an adjacent peripherally synapsing interneuron (In), and here it apparently forms an electrical synapse (Blagburn et al. 1999). Yet the inside of the finger is lined with vesicles that can fuse with the presynaptic membrane (where the synaptic cleft widens), suggesting that this also is a chemical synapse. Note that all drawings in all figures are original and based on micrographs and drawings in the cited studies. In all drawings, the presynaptic terminals are colorless, postsynaptic processes (usually only the adjacent portion is shown) are blue, mitochondria are green, and Schwann/glial processes are pink (additional structures are yellow) (Color figure online)
The larval amphioxus, Branchiostoma floridae (a cephalochordate), has some very unusual junctions, called “juxta-reticular” (JR) junctions, that lack synaptic vesicles but have a cisterna of endoplasmic reticulum (ER) on both sides of the junction (Lacalli 2002). These JR junctions may form some crucial links in the circuitry of the larval nervous system, suggesting that they indeed function as synapses, possibly involved with slow locomotion in the larva. Since some of them indent or even invaginate deeply into cell bodies, these may be a special category of invaginating presynaptic terminals.
Invertebrate Giant Fiber Systems
Invaginating presynaptic terminals appear to be associated with the giant axon fiber synapses of squid (mollusk), crayfish (crustacean), and Drosophila (insect); these giant fibers are adapted for rapid responses, most notably the escape response. Castejón and Villegas (1964) describe, in the squid, Sepioteuthis sepioidea, presynaptic processes from afferent giant axons that invaginate into efferent giant axons (Fig. 2a). The terminals contain mitochondria, synaptic vesicles (mostly 50–80 nm plus a few larger dense-cored vesicles) as well as a mass of homogeneous substance (200–600 nm) that the authors suggest may be equivalent to the various presynaptic dense bodies found in specialized synapses, such as the synaptic ribbons in vertebrate retinal photoreceptor synapses and synaptic bodies at the base of many vertebrate hair cells. The pre- and postsynaptic membranes are thickened and there is a 10–13-nm synaptic cleft. The postsynaptic cytoplasm contains some ER and occasional small vesicles.
In crayfish, processes also can protrude or invaginate partially from one giant axon and penetrate an adjacent axon. The patterns are complex and have been studied intensely (Hama 1961; Heitler et al. 1985; Bosch 1990; Leitch 1992). These generally are considered to include various combinations of chemical and electrical (gap junctions) synapses; for the chemical synapses, they are thought to be postsynaptic processes with synaptic vesicles on the presynaptic side. Other regions of contact have narrower clefts and are believed to be gap junctions, even though these can have vesicles lining one or both sides of the cleft. A good example (Leitch et al. 1989; Leitch 1992) is the synapse between medial giant fibers and motor giant fibers in the crayfish, Pacifastacus leniusculus; this synapse appears to be a rectifying (one direction: medial giant to motor giant) electrical type and it mediates an escape reflex that flexes the abdomen to allow the crayfish to escape backward from a frontal attack. The synapse in the newly hatched crayfish appears to be chemical, with thickened densities and a 20–30-nm cleft; presynaptic, 25–40-nm vesicles are pleomorphic and may surround a small presynaptic dense bar. But during subsequent development, these apparent chemical synapses become peripheral to an apparent, central gap junction with large, round, presynaptic, 60–80-nm vesicles and a 4–6-nm cleft. Ultimately, the chemical synapse region forms a thin and poorly developed ring around the large, central gap junction. Another synapse, between the lateral giant fibers and motor giant fibers, shows a roughly similar pattern.
Occasionally, the indenting process in these synapses has a larger accumulation of vesicles within it compared to the other (indented) side of the synapse (Hama 1961; Heitler et al. 1985). Hama (1961) shows examples of these for the giant fiber-motor giant fiber synapses in two species of adult Cambarus. What appear to be definitive synaptic vesicles are fairly rounded and 40–60 nm wide and are mixed with a few smaller vesicular or tubular structures about 20 nm in diameter; similar vesicular components are found on both sides of the synapse, but in some cases, there are distinctly more within the indenting process (supposedly the postsynaptic side). Hama also notes that the cleft of these synapses is ~10 nm. It is tempting to speculate here that if this is a rectifying electrical synapse, then the “postsynaptic” vesicles could represent efferent (retrograde) feedback to modulate the function of the synapse; we will discuss this concept in greater detail in the section on mechanoreceptor/photoreceptor synapses. Heitler et al. (1985) show an example (Fig. 2b; their figure 4) of a deeply indented synapse between two lateral giant synapses in adult P. leniusculus; these synapses have an ~5-nm cleft and 40–90-nm vesicles and are considered to be nonrectifying electrical types, so that impulses can pass in either direction (Leitch et al. 1992). But perhaps the presence of so many vesicles within the indenting process indicates that it has some additional function as a presynaptic terminal of a chemical synapse. The similarities in these two examples, from a rectifying and a non-rectifying synapse, suggest that this is some common functional arrangement in crayfish giant axon synapses.
Fig. 4.
Ampullary electroreceptors of fish. a This example from a sturgeon shows a receptor cell terminal (R) containing synaptic ribbons with their ends (with active zones) invaginating/protruding into the large postsynaptic process (called a nerve terminal by the authors; T; modified from figure 3B in Teeter et al. 1980; reprinted with permission from Springer). Scale bar is 500 nm. b, c Drawings of electroreceptor synapses from a transparent catfish (b; Szamier and Bennett 1973) and a weakly electric gymnotid (c; Szamier and Wachtel 1969). Both have irregular presynaptic rods surrounded by vesicles. In c, the rod branches into the two lobes of the ending of the invaginating terminal protrusion. Note also the postsynaptic cluster of vesicles adjacent to the lobes; most are larger than the presynaptic vesicles. Compare to vesicles of some of the horizontal processes in photoreceptor invaginations in figure 7; are they both examples of presynaptic vesicles in a postsynaptic process?
In Drosophila, mixed synapses between giant fibers and dendrites of tergotrochanteral motor neurons include chemical synapses with a 13–20-nm synaptic cleft and 20–60-nm synaptic vesicles, as well as a presynaptic T-bar structure that is typical of insect chemical synapses (Blagburn et al. 1999); in comparison, the electrical synapses here have a 2–4-nm cleft and 30–55-nm vesicles. These synaptic regions are large and complex and include elongate, invaginating postsynaptic spines as well as some terminal regions that seem to invaginate partially into the postsynaptic dendrite. Perhaps even more intriguing is the synaptic contact between the giant fiber and the peripherally synapsing interneuron. Some of the supposed electrical synapse-gap junctions, with vesicles lining the giant fiber side, invaginate deeply into the interneuron as fingershaped projections or protrusions. In figure 5C from Blagburn et al. (1999), one of the vesicles within the “finger” has fused with the membrane (Fig. 2c). This suggests that this synaptic contact indeed has some function as a chemical synapse; in fact, the synaptic cleft is much wider at the point of fusion of the vesicle. So, these “fingers” probably are another example of an invaginating presynaptic terminal.
Fig. 5.
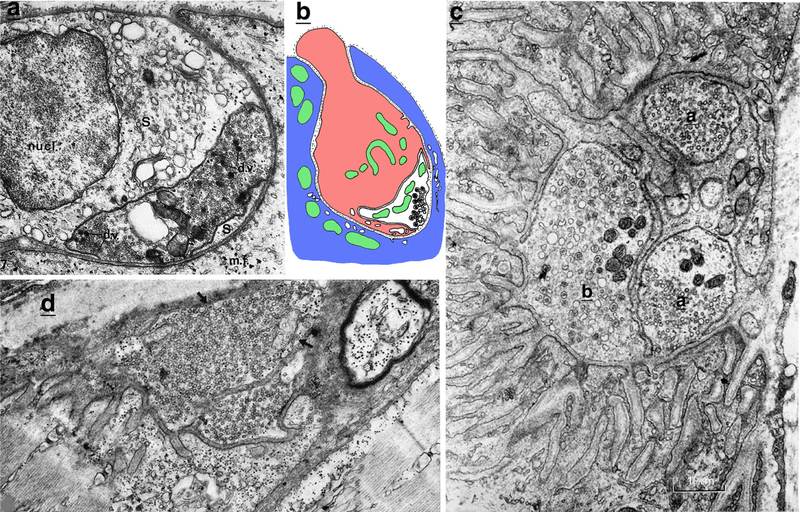
Invaginating terminals of the auditory/vestibular system (8th cranial nerve). a Drawing of endbulb of Held (EB) of newborn kitten; note the reciprocal interdigitations between the endbulb and the soma (S) of a spherical bushy cell neuron (a1 is a low-magnification view of whole cell; a2 is an enlargement of part of endbulb synapse; Ryugo et al. 2006). b, c Micrographs of invaginating spoon synapses between vestibular nerve fibers and vestibular neurons in the ammocoetes larvae of lampreys; note how the large terminals (small, white asterisks in b) invaginate into the neuron somas (modified from figures 2 and 4 in Stefanelli and Caravita 1970; reprinted with permission from Springer). The large asterisk in b marks the emerging end of the vestibular nerve fiber that forms the spoon and passes through the neuron soma. The double-headed arrow in c indicates the gap between the cytoplasmic “lips” of the soma. Short arrows in c indicate distinctive active zones, and sv indicates synaptic vesicles
Examples from Vertebrates
Cup-Shaped Spines
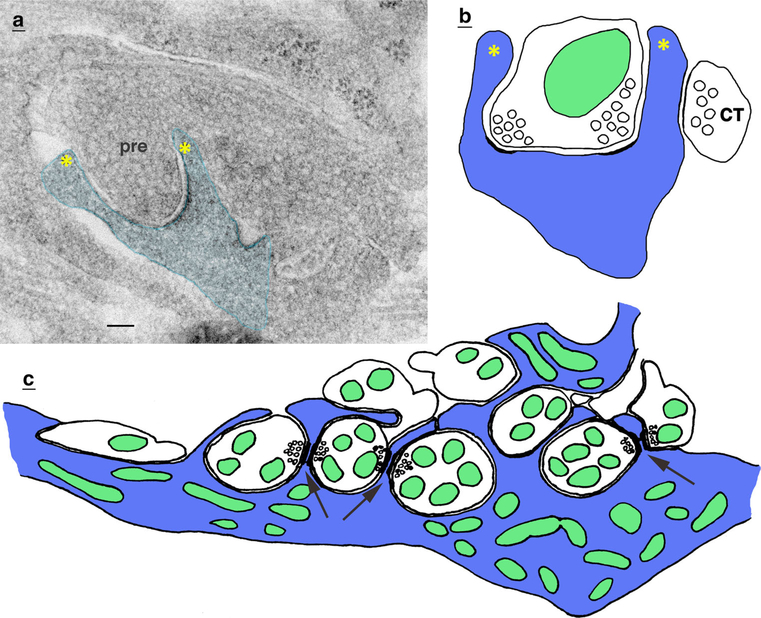
In the cerebral cortex of mammals (rats and cats), it is not uncommon to find cup-shaped or concave synaptic spines with the presynaptic terminal partly indenting into the spine head (cat, Jones and Powell 1969; rat, Peters and Kaiserman-Abramof 1970) (Fig. 3a, b). But the deepest invaginating terminals are found in the dentate gyrus of the hippocampus of the adult rat (Desmond and Levy 1983; Frotscher and Léránth 1986). In some cases, the postsynaptic spine appears to enwrap completely the sides of the terminal (Desmond and Levy 1983). And the latter study found that these concave spines increase in number following high-frequency stimulation of the input from the entorhinal cortex. Several studies have examined cupshaped spines in slice cultures of the CA1 region of the hippocampus of rats (Chang and Greenough 1984) and mice (Roelandse et al. 2003; Nägerl et al. 2008). Live cell imaging using STED (stimulated emission depletion) super-resolution microscopy found that spines often appear more cup-like following chemical induction of long-term potentiation (LTP; slices from P5 to 7 mice and cultured for 2–3 weeks; Nägerl et al. 2008). This seems to be consistent with the findings of Desmond and Levy (1983) for the dentate gyrus. In contrast, Chang and Greenough (1984; slices from adult rats), using high-frequency electrical stimulation to induce LTP, found a decrease in the number of cup-shaped spines (using electron microscopy, as used for all descriptions in this review, unless stated otherwise). Finally, Roelandse et al. (2003) found about half of the spines in the CA1 region of their mouse slice cultures and acute slices (cultured at P8 and incubated for 5 weeks; acute slices from 6-week-old mice) are cup shaped. They describe how in many cases, the spine appears to extend “lamellipodia” around the presynaptic terminals, and sometimes can form “extreme cup-shaped spines,” with the spine appearing to wrap entirely around the sides of the terminal. In comparison with their findings in the slices, they found only about 12% in the CA1 from perfusion-fixed, 6-week-old mice. They argue that the greater number of cup-shaped spines found in slices is more realistic and due to microwave-enhanced fixation of the slices. However, we have noticed commonly that both slices and cultures fixed by immersion also show more cup-shaped spines than are found in perfusion-fixed animals (and often with “lamellipodia”; Mitchell et al. 2012; and unpublished data) (Fig. 3a).
Fig. 3.
Invaginating presynaptic terminals can associate with unusual spine structures. a, b Cup-shaped spines. a This cup-shaped postsynaptic spine from a rat hippocampal cell culture extends projections (yellow asterisks) over the sides of the invaginating protrusion from the terminal (pre), to form a cup-shaped structure. Scale bar is 100 nm (unpublished data; for methods and other examples, see Mitchell et al. 2012). b Drawing of cup-shaped spine around an invaginating presynaptic terminal, from the rat dentate gyrus; this spine also receives a cholinergic terminal (CT; labeled for choline acetyltransferase; for details, see Frotscher and Léránth 1986). c Drawing of crested dendrite from the rat interpeduncular nucleus; unlike simple crest spine synapses, in this case, several crest spines (arrows) along the dendrite join with invaginating presynaptic terminals between each pair of crest spines. For details, see Murray et al. (1979) (Color figure online)
Crested Dendrites
Typically, regular crest synapses do not involve invaginating presynaptic terminals. They have been described already in detail in Petralia et al. (2016). Briefly, they are an unusual type of flattened spine-like synapse with either two terminals on the two flattened sides, or alternatively, the crest invaginates into one terminal that covers both sides; the two thickened postsynaptic densities (PSDs) of the two sides are joined by a central, flattened array of “subjunctional bodies.” However, Murray et al. (1979) describe a more complex structure called a “crested dendrite” in the rat interpeduncular nucleus. Electron micrographs show up to seven presynaptic terminals that appear to be invaginating along one side of the dendrite, and crests are evident in the dendritic processes between several of the invaginating terminals (Fig. 3c).
Invaginations into Developing Spinal Motoneurons
Invaginating processes surrounded by a postsynaptic subsurface cistern, found on somas of motoneurons in the spinal cords of developing cats and rats (also the bases of dendrites, at least in cats), appear to be derived from synaptic terminals with postsynaptic subsurface cisterns (cat—Conradi and Skoglund 1969; Ronnevi 1977, 1979; rat—Li et al. 1995). The invagination typically is filled with vesicles and appears to involve the entire portion of the terminal that bears the postsynaptic subsurface cistern; these invaginations are most common in the first postnatal week. Studies in rats suggest that the synaptic terminals with subsurface cisterns (called “C-terminals”) are cholinergic (Li et al. 1995). In the cat, the bottom membrane of the invaginated terminal cistern often is lined on the outside (i.e., facing the somal cytoplasm) with “small electron-dense and vesicular bodies...,” and the cistern can be continuous with the rough endoplasmic reticulum (RER; Ronnevi 1979). In the rat (Li et al. 1995), the subsurface cistern of the invaginated terminal has “electron-dense fibrous elements linking it to the underlying endoplasmic reticulum.” Ronnevi (1979) provides some evidence that these invaginating terminals are being phagocytosed by the neuron; a somewhat similar phagocytosis by glial cells appears to occur for some other kinds of terminals on the postnatal motoneuron (Ronnevi 1977). Phagocytosis seems to be a likely explanation for these structures, as the invagination with its surrounding cistern generally resembles phagophore formation in early autophagy (Ylä-Anttila et al. 2009; Biazik et al. 2015). Also, some similar examples are seen of glial processes invaginating into neurons (Li et al. 2005; Fedorenko and Uzdensky 2009). Phagocytosis of terminals by neurons and glia is probably part of the normal pruning and refinement of synaptic connections of the motoneuron during development (Ronnevi 1977, 1979). It is not known whether these invaginating terminals also function in neurotransmission. A probably similar phenomenon of invagination/phagocytosis is found for motoneurons in adult cats after intracellular recording and filling of the neuron with the dye Procion Yellow (Berthold et al. 1979).
CCK-Interneuron Invaginated Synapse
In Petralia et al. (2015), we discuss non-synaptic projections/protrusions from CCK-positive, GABAergic interneuron synapses in the amygdala (Yoshida et al. 2011; Omiya et al. 2015) and hippocampus (Léránth and Frotscher 1986; Acsády et al. 2000). Technically, these are not considered invaginating presynaptic terminals because the invaginating axonal protrusion lacks active zones. But it deserves a brief mention here, because of its precise and complex organization for retrograde suppression of neurotransmitter release via endocannabinoids (eCBs; at least in the amygdala system): a type of a reverse active zone arrangement. The GABA receptors are on the flat synaptic surface ringing around the central invagination, but the synthetic enzyme to make the eCBs is on the postsynaptic side of the invagination. The presynaptic surface of both the terminal and the invaginating processes bears the eCB receptors.
Electroreceptor Synapses
Electroreceptive sensory organs are used to sense electric fields and function for prey detection, identification of objects in the environment and social communication; they are found in many kinds of fish and a few amphibians, as well as in monotreme mammals (Jørgensen 2005). In the ampullary electroreceptor cells of many fish, the presynaptic terminal bears a synaptic ribbon or rod or bar that often looks very much like the one in vertebrate photoreceptors; however, unlike in photoreceptor synapses, a protrusion of the electroreceptor presynaptic terminal along with part of the synaptic ribbon invaginates into the postsynaptic process (Fig. 4). The invaginating presynaptic terminal protrusion in the electroreceptor cells of the electric fish, Gymnarchus niloticus, has an elongate presynaptic ribbon or “sheet” that is about 1 micrometer tall and about 2.5 micrometers long—parallel to the plane of the synapse (Mullinger 1969); up to two-thirds of the sheet is within the invagination. In other words, the presynaptic invagination is ridge shaped, extending between two “longitudinal ridges” of the postsynaptic process (afferent nerve terminal). It usually is covered in a single layer of 30–50-nm synaptic vesicles, while other similar vesicles are nearby in the cell base. The sheet actually is composed of a stack of thin lamellae; and the U-shaped end of the presynaptic membrane invagination is highly organized, supported by a series of curved synaptic “rodlets.” A similar invaginating presynaptic terminal protrusion (but usually not as deep), with definitive synaptic ribbon covered by a single layer of synaptic vesicles, is found in the electroreceptor cells in several other fish, such as the lungfish, Protopterus dolloi (Roth and Tscharntke 1976), the fresh water ray, Potamotrygon sp. (Szamier and Bennett 1980), and the sturgeon, Scaphirhyncus platorynchus (Teeter et al. 1980) (Fig. 4a).
In the transparent catfish, Kryptopterus bicirrhus, the presynaptic invagination has an enlarged, bulbous end, and the presynaptic “rod” appears “dumbbell”-shaped in section (1 × 0.3 micrometers), with enlargements at the end of the invagination and at its base (Szamier and Bennett 1973) (Fig. 4b). As for the ribbon/sheet in the previous species, a single layer of synaptic vesicles covers the rod, while additional clusters of vesicles are found nearby in the cell base. In the weakly electric, South American gymnotid fish, Eigenmannia virescens, the presynaptic rod is up to 3 micrometers long, and about the last third of it extends into the presynaptic invagination. This last part of the rod has two bulbous expansions, one at the base and one in the enlarged ending of the invaginating presynaptic terminal protrusion (Szamier and Wachtel 1969). However, the presynaptic terminal ending has two lobes and the bulbous expansion of the rod extends a branch into each one (Fig. 4c). The small vesicles lining the rod are 20–30 nm; interestingly, there also is a cluster of larger postsynaptic vesicles, 40–60 nm, in this species (Szamier and Wachtel 1969; see also Lissmann and Mullinger 1968), suggesting a possible efferent synaptic function. The end of the presynaptic invagination also has two lobes in the South American blind catfish, Pseudocetopsis sp. (Andres et al. 1988).
Overall, the structure of the invaginating presynaptic terminal protrusion of the electroreceptor cell, especially in those where the presynaptic dense structure is ribbon/ sheet shaped, is remarkably like the ribbon synapses of vertebrate rod and cone photoreceptor cell synapses, except that the terminals in the latter do not invaginate into the postsynaptic processes. Instead, there are 3–4 small postsynaptic processes in the latter, surrounded by the end of the presynaptic terminal. Perhaps even more interesting, in some cases, the postsynaptic process can appear to extend as two ridges that define the sides of the membrane contact with the invaginating presynaptic terminal of the electroreceptor cell. This arrangement bears a superficial resemblance to that of the photoreceptor cells; thus, the presynaptic ribbon of the photoreceptor synapse often extends into a cell protrusion within a larger invagination that houses several postsynaptic processes; in particular, the horizontal cell processes typically extend up and surround the photoreceptor cell presynaptic protrusion. We will return to the possible functions of these structures in a later section.
Specialized Auditory/Vestibular Nerve Terminals in the Vertebrate Brain
It is common for fibers of the eighth cranial nerve, originating from the peripheral ganglion neurons of the auditory and vestibular end organs, to form very large presynaptic terminal structures called endbulbs or spoon endings, on neurons in the brain near the point where these fibers enter the brain, and in a few cases, can become indenting or invaginating into the postsynaptic neuron.
Auditory fibers form such endings, called endbulbs of Held, in the anteroventral cochlear nucleus of mammals (Neises et al. 1982; Wang et al. 1998; Ryugo et al. 2006) and in the nucleus magnocellularis of reptiles (alligator lizard, Gerrhonotus multicarinatus; Szpir et al. 1990) and birds (Parks 1981; Jhaveri and Morest 1982; Carr and Boudreau 1996). Typically, these endbulbs can enwrap a large part of the neuron soma and contain multiple active zones with presynaptic vesicles, as well as attachment plaques (puncta adherentia; these lack synaptic vesicles and have symmetrical membrane densities). During the early development of these endbulbs, their contacts with the soma are highly convoluted, often forming reciprocal interdigitations between the endbulb and neuron soma, i.e., with processes appearing to invaginate in both directions (Jhaveri and Morest 1982; Neises et al. 1982; Carr and Boudreau 1996; Ryugo et al. 2006). Neises et al. (1982) call those in the rat, endbulb “processes” and somatic “appendages,” and explain how these grow in opposite directions to form the interdigitations. Ryugo et al. (2006) describe them in the cat as alternating endbulb “protrusions” or “feet” and somatic “appendages” (Fig. 5a). Many of the active zones in rats and cats at these early stages are found on the regions of the somatic membrane that are not invaginated into the endbulb, or described in another way, on the ends of protrusions from the endbulb into the soma (Neises et al. 1982; Ryugo et al. 2006). Thus, viewed in this way, these protrusions can be considered invaginating presynaptic terminal processes. In contrast, in endbulbs of adult rats and cats, invaginating pre- or post-synaptic processes are not common, although there may be an occasional stubby, invaginating postsynaptic spine (Wang et al. 1998; Ryugo et al. 2006). Development of endbulb synapses is roughly similar in birds, although the endbulb at early stages also can invaginate some processes more deeply into the neuron soma (Jhaveri and Morest 1982; Carr and Boudreau 1996).
Endbulbs in mature birds and mammals cover much of the relatively flat surface of the neuron soma. These endbulbs are designed largely as relay synapses, “...built for high-fidelity and high-frequency synaptic transmission…,” although they also are capable of synaptic plasticity (Oleskevich et al. 2004). Endbulb synapses go through a series of modifications during development, improving their accuracy and reliability in neurotransmission (Brenowitz and Trussell 2001). It is likely that the interdigitations and invaginations in the early endbulb synapse reflect a stage of greater developmental plasticity; an increase in invaginating processes appears to be a common occurrence in synaptic plasticity, as reviewed in Petralia et al. (2015).
Vestibular fibers form large spoon-shaped (“spoon”) endings on neurons of lampreys (Johnston 1902; Stefanelli 1937; Stefanelli and Caravita 1970), reptiles (the common wall lizard, Lacerta muralis [now Podarcis muralis]; Beccari 1911), and birds (Hinojosa and Robertson 1967; Peusner 1984; Petralia and Peusner 1990). In reptiles and birds, spoon endings form in the tangential nucleus, close to the entry of the vestibular nerve into the brain. Goldfish (Carassius auratus) have a similar tangential nucleus, but the large terminal endings are not spoon shaped (Hinojosa 1973). These various animals, known for their highly developed locomotion, i.e., swimming fish and flying birds (and running birds and reptiles), have well-developed, large terminals covering a large part of a relatively flat, somal surface. The specialized synapses are probably optimized for rapid vestibular-ocular and postural reflexes (Wilson and Wylie 1970; Suwa et al. 1999; Shao et al. 2004). All these large vestibular fiber endings on the somas of tangential nucleus neurons have a combination of vesicular active zones, attachment plaques, and gap junctions; the latter are indicative of electrical synapses. Similar synapses are found in the lamprey, in which spoon endings form on vestibular neurons in three groups near the entry of the vestibular nerve. Illustrations in light microscope studies in the adult lamprey seem to show typical spoon-shaped structures (Petromyzon fluviatilis and P. marinus; Stefanelli 1937), although illustrations in Johnston (Lampetra wilderi; 1902) seem to show spoon endings that are partly indenting in the neuron somas. In comparison, an ultrastructural study of the larval stage of the lamprey (ammocoetes stage of Lampetra planeri) reveals that the vestibular presynaptic “spoon” actually invaginates into the neuron soma: “In other words, it is the cell and not the fiber that forms the spoon.” (Stefanelli and Caravita 1970) (Fig. 5b, c). As for the large vestibular fiber synapses on tangential nucleus neurons of the fish and bird tangential nucleus, those in larval lampreys have vesicular active zones, attachment plaques, and gap junctions. But unlike in the larval lamprey, the spoon ending in the chicken at least does not appear to invaginate into the soma in its early development, although the spoon ending does go through some substantial morphological changes in development (Peusner 1984; Petralia and Peusner 1990). The difference may be related to the developmental situation; the early changes in spoon ending development of the chicken occur in the embryo, while the larval lamprey is a free-living active stage. Formation of the distinctive invaginating “spoon” ending on the vestibular neurons of the larval lamprey, and its apparent changes occurring in maturation of the lamprey, likely reflects the synaptic plasticity correlated with the profound change in lamprey form and behavior as it matures (Stefanelli and Caravita 1970).
In contrast to the vestibular system in these other vertebrates, the mammalian vestibular system has neither spoon endings nor a definitive tangential nucleus (mammals do have a small interstitial nucleus of the vestibular nerve). However, large terminals on neuron somas in the rat lateral vestibular nucleus bear a similar combination of vesicular active zones, attachment plaques, and gap junctions (Sotelo and Palay 1968, 1970); these almost certainly originate from the vestibular nerve (Nagy et al. 2013). Interestingly, these large terminals typically are indenting into the somal surface, forming a “perikaryal cup,” with somal spines forming synapses on the upper sides; also, some of these terminals appear to invaginate more deeply into the soma. In contrast, neither invaginating terminals nor gap junctions have been described in the lateral vestibular nucleus of the cat (although interestingly, various terminals receive invaginating postsynaptic spines from somas or dendrites; Mugnaini et al. 1967). Thus, curiously, invaginating presynaptic terminals occur in two different branches of evolution of vertebrate vestibular circuitry, i.e., in jawless fish and rats. How might this have occurred? Of all the examples of animals with these specialized eighth nerve synapses discussed here, the larval lamprey and rat probably are the least dependent on rapid and accurate orientation responses. The case of the larval lamprey is special, since as discussed above, the invaginating terminal may represent development plasticity occurring in a free-living animal (e.g., as opposed to a chick embryo); perhaps its complex invaginating spoon ending reflects the combination of developmental plasticity and active function as a relay synapse. On the other hand, it is less apparent why the vestibular nerve terminals in the rat lateral vestibular nucleus are indenting/invaginate into the neuron soma. There are vestibular nerve terminals with a similar combination of vesicular active zones, attachment plaques, and gap junctions (but no invaginations into the postsynaptic cell) on lateral vestibular neurons of the toadfish (Opsanus tau; Korn et al. 1977), and both the toadfish and rat show electrotonic coupling between neurons, presumably via the gap junctions in these terminals (Korn et al. 1973, 1977). And as noted, spoon endings of lampreys, goldfish, and chickens also have the same combination of three types of junctions, although it is not clear whether gap junctions in chicken spoon endings are functional (Peusner and Giaume 1994; Shao et al. 2004). We only can speculate that larval lampreys and rats may require less coordination and postural control than adult fish and birds, and so the absence of terminal invaginations into the postsynaptic cell of the latter animals may, if anything, help ensure efficiency of signal transmission.
The Base of Some Mechanoreceptor, and Many Kinds of Photoreceptor Cells Can Have a Complex of Invaginating Pre- and Postsynaptic Processes
The afferent synaptic complexes at the bases of mechanoreceptor and photoreceptor cells can be highly specialized and show many commonalities. We previously discussed these already in detail in terms of non-synaptic projections (Petralia et al. 2015) and invaginating postsynaptic spines (Petralia et al. 2016). Here we discuss these in terms of the efferent functions that serve generally to modulate the main excitatory synaptic output of the receptor cell synapses, and we show how these often are a presynaptic component of the invaginating afferent synaptic complexes.
Hair Cells
In the Cnidaria, the nematocyte is the well-known stinging cell used for prey capture and defense, and it is a modified hair cell. In the nematocyte of Hydra littoralis, Westfall et al. (1971) show a micrograph of a presynaptic neurite forming an efferent synapse at the base of the nematocyte. The terminal is indenting into the base and has 2–3 large, clear synaptic vesicles (100 + nm). In contrast to this simple efferent basal synapse, the base of the nematocyte in Coryne tubulosa contains a distinctive invagination called a “basal tunnel” containing a complex of several neurites (Holtmann and Thurm 2001a, b). Most of these appear to be afferents, and there are one or two of them that form a synaptic cleft with a presynaptic “magno-vesicle” (rarely there are two of them), 250–600 nm in diameter. But in one micrograph, the neurite complex in the basal tunnel also has a neurite-filled with dense-cored vesicles about 100–140 nm in diameter and presumably is a presynaptic efferent terminal (assumed to be neurosecretory, as typified by the dense-cored vesicles); it sits more than 500 nm from the presynaptic active zone (Fig. 6a). In addition, although not described by the authors, another neurite makes an apparent efferent synaptic contact, with thickening of the pre- and postsynaptic membranes, directly to the right of one of the postsynaptic afferents that form a direct synapse with the magno-vesicle. This neurite contains three small dense-cored vesicles, about 80 nm in diameter, at the contact, and thus, this is likely another efferent terminal in the basal tunnel. Ventrally, the neurite bundle in the basal tunnel is bordered by a pair of supporting cells, and these are bound to the nematocyte base on each side by desmosomes; and the desmosome region extends through the supporting cells to continue as hemidesmosomes that bind to the underlying mesoglea.
Fig. 6.
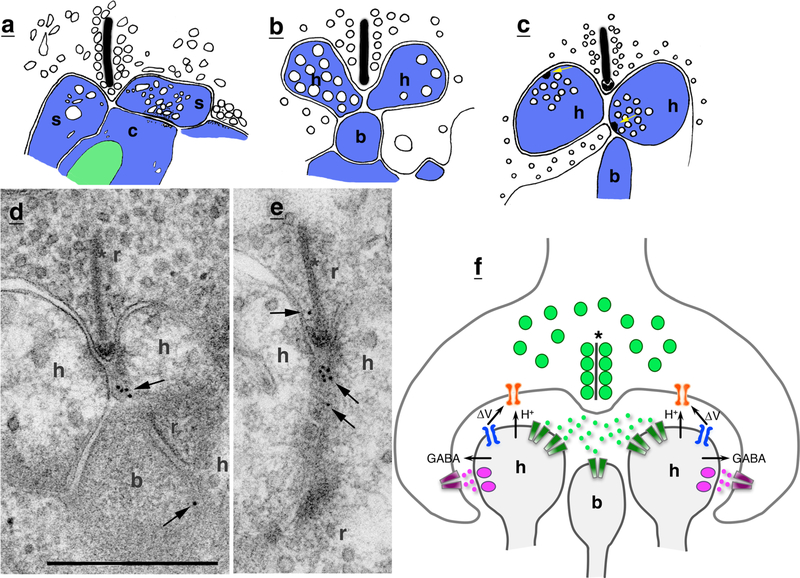
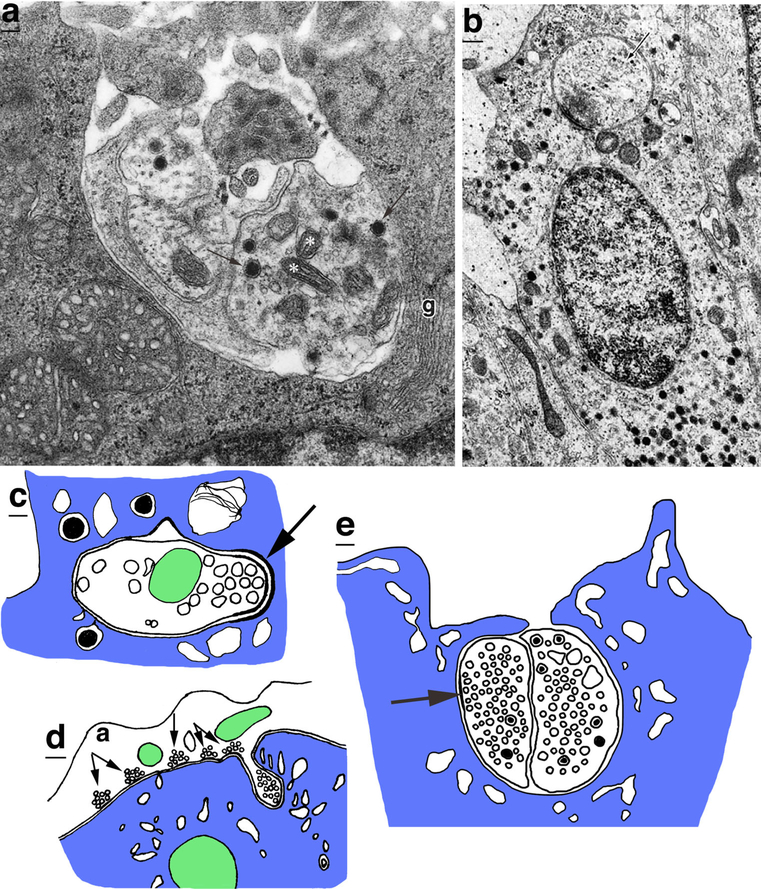
Drawings of invertebrate receptor terminal complexes. a The basal tunnel of nematocytes from the cnidarian, Coryne tubulosa (Holtmann and Thurm 2001a, b), contains pre- and postsynaptic processes, like the invaginations of vertebrate photoreceptor rods and cones. Apparent presynaptic terminals (pre) in the tunnel have densecored vesicles. The nematocyte terminal area has a single magnovesicle (v). b The retinula (photoreceptor) axon terminals of crayfish can have invaginating complexes (Hafner 1974) that look very like those of vertebrate photoreceptor rods and cone synapses. c The photoreceptor terminals in the octopus form large bags (b) or carrots that contain three kinds of invaginating processes, including postsynaptic spines, tunnel fibers (t), which are nerve trunks of small neurons, and vesicle-filled finger twigs (asterisks) that extend from adjacent bags; the spines are blue and have a presynaptic cluster of vesicles (Dilly et al. 1963; Case et al. 1972) (c1 is a low magnification of a whole bag and parts of 2 adjacent bags; c2 is an enlargement of upper left part of central bag) (Color figure online)
Hair cells of the coronal organ, a mechanoreceptor structure found in the oral siphon of ascidians or sea squirts (urochordates) have one or two deep grooves on their bases, in which run a group of neurites. In sections, the groove appears to be a deep invagination with its outside boundary covered by the basal lamina of the hair cell (Burighel et al. 2003; Manni et al. 2006). Many of these neurites are definitive postsynaptic afferents, with presynaptic vesicles on the hair cell side of the cleft; we already have illustrated this in Petralia et al. 2016, as an example of multiple, postsynaptic processes in an invagination. But the former authors also describe presynaptic efferent terminals within these invaginations, forming synapses with the base of the hair cell, as well as with the neurites that form the afferent synapses. While they describe these efferents in several kinds of ascidians, the micrographs that they included in the papers do not show distinctive vesicles in these efferents that synapse on the hair cell base. Overall, this hair cell groove with invaginating neurites resembles the nematocyte (hair cell) basal tunnel with invaginating neurites, found in Cnidaria; in both cases, the neurite bundle in the invagination includes both postsynaptic afferents and presynaptic efferents.
Presynaptic efferent terminals at the base of vertebrate hair cells do not typically invaginate into the hair cell. However, these terminals do form in indentions in the base of tall hair cells in the basilar papilla (hearing) of the pigeon (Takasaka and Smith 1971). Interestingly, these efferent terminals are relatively small, while those on the short hair cell bases are large and do not indent into the base; both types have a subsynaptic cistern in the hair cell base. These tall and short hair cells are believed to be analogous to the inner and outer hair cells, respectively, of mammals; as in these mammalian equivalents, afferent innervation predominates in the tall hair cells and efferent innervation predominates in the short hair cells (Fischer 1992; Tan et al. 2013).
Invertebrate Photoreceptors
There is relatively little evidence for invaginating presynaptic terminals associated with the synapses of invertebrate photoreceptors. A distinctive example is found in the retinal axon terminals of two kinds of jumping spiders; Oberdorfer (1977) describes how “The surface of the retinal terminals is deeply invaginated by processes of second-order terminals.” The illustration of this (his figure 16) shows one of these invaginated presynaptic second-order terminals making a synapse (with presynaptic vesicles) deep within the invagination into the retinal axon terminal. However, the electron micrographs in the paper do not show clear examples of these synapses. In the illustration, other second-order terminals receive synapses from the retinal axon terminal and also invaginate thin, non-synaptic processes into the retinal axon terminal (see also Eakin and Brandenburger 1971). The entire glomerular structure, with central retinal axon terminal and surrounding second-order terminals, is encapsulated in glial processes. The wolf spider has a partially invaginating postsynaptic complex of processes, and the presynaptic process contains an elongate dense bar that resembles the ribbon of vertebrate rod and cone synapses (Trujillo-Cenóz and Melamed 1967). In the crayfish (Procambarus sp.), sections through the large retinula (retinal axon) terminals show deeply invaginating postsynaptic complexes that also closely resemble those of the rods and cones of vertebrate retinas (Hafner 1974) (Fig. 6b). The presynaptic process projects into the middle of a triad of postsynaptic processes, and this short, presynaptic protrusion contains a short, presynaptic bar density and a cluster of synaptic vesicles. Sometimes the postsynaptic processes can contain numerous vesicles that resemble the presynaptic vesicles. A somewhat similar synaptic arrangement appears to be present in the lobster (Hámori and Horridge 1966). It is not known whether the wolf spider and crustacean invaginating synapse complexes include an invaginating presynaptic component. However, the remarkable similarity of these invaginating retinal synaptic complexes to those of vertebrates suggests that the complex in these arthropods functions like those in the vertebrate retina, including perhaps some efferent functions, as we will discuss more specifically in later sections.
In the octopus (Octopus and Eledone spp.), the optic nerve axons from the photoreceptors enter the optic lobe and pass through a layer of outer granule cells and then expand into large, en passant presynaptic structures described as “elongated varicose presynaptic bags” (Dilly et al. 1963) (Fig. 6c). Case et al. (1972) describe these structures as “carrots” due to their unusual shape. The bags/carrots are full of synaptic vesicles and form synaptic active zones with numerous invaginating postsynaptic spines. In addition to these spines, the “carrots” are invaginated by nerve fibers, called “tunnel fibers” that pass through the bags/carrots singly or in small bundles and are in direct contact with the membrane of the carrot surrounding them (Dilly et al. 1963; Case et al. 1972). Tunnel fibers represent the nerve trunks of small neurons of the outer granule cell layer called “microneurons,” and it is not clear whether these trunks are axons or dendrites (Case et al. 1972). Tunnel fibers often run approximately at right angles to the invaginating postsynaptic spines; they contain mitochondria but no distinctive synaptic vesicles. In addition, the bags/carrots invaginate processes called “finger twigs” into adjacent bags/carrots; these contain synaptic vesicles (Dilly et al. 1963). Similar invaginating contacts, without specialized junctions, occur between adjacent axons or axon terminals in the nervous systems of a variety of animals and may suggest ephaptic conduction (discussed in a later section); we have reviewed these in Petralia et al. (2015) and will not discuss them in this review. However, vesicle-filled “finger-like invaginations” are found between adjacent presynaptic bags/carrots in the squid, Loligo pealei, which is a cephalopod like the octopi, and invaginations can have very close junctions (possibly gap junctions), with submembrane cisternae on each side (Cohen 1973; Haghighat et al. 1984). Both papers discuss how this could indicate electrical communication between the bags—either via gap junctions or ephaptic conduction. Overall, neither the tunnel fibers of octopi nor the finger twigs of octopi and squid appear to form definitive chemical synapses in the bags/carrots. We suggest that invaginating tunnel fibers and finger twigs form efferent communication with the bags/carrots, either chemical or electrical or both, like the arrangement for vertebrate photoreceptors, as we will discuss next.
Vertebrate Photoreceptors
Most of the examples of mechanoreceptors, electroreceptors, and invertebrate photoreceptors that we have described so far show a common pattern of complex synaptic interactions that involves invaginating processes and synaptic communication that appears to go both ways. But this complex has been studied most thoroughly in vertebrate photoreceptors. Thus, we describe here how a complex of postsynaptic processes invaginates into the axonal end (base) of a vertebrate photoreceptor cell; but also, that some processes in this complex send feedback communication to the photoreceptor and thus can act as invaginating presynaptic terminal processes (Fig. 7).
Fig. 7.
Horizontal cell process synapses in photoreceptor invaginations. a-c Drawings of photoreceptor terminals showing evidence that horizontal cell processes are presynaptic. a In the mudpuppy (amphibian), photoreceptor terminal complexes are partly invaginated. The side processes (s), probably horizontal cell processes, form chemical synapses on the central process (c) and these side processes have pleomorphic vesicles (compared to the larger, round vesicles at the ribbon synapse; Dowling and Werblin 1969). b In the macaque monkey ribbon synapses of the cone pedicles (Raviola and Gilula 1975), the horizontal cell processes (h) can have numerous large, round vesicles. b, bipolar cell process. c In the human, the horizontal processes of rod spherules can have large vesicles and make distinctive synapses (including a small, presynaptic dense body; yellow arrows) with the rod spherule cell membrane or with the bipolar cell processes (Linberg and Fisher 1988). Synapses with the rod membrane may be either on the side of the ribbon or on small projections of rod cytoplasm within the invagination, including just below the ribbon. d, e In rats, we show immunogold localization (10 nm) of GABA-A receptor within the invaginations of rod spherules (sections examined in two 150-g rats; Chemicon mouse antibody MAB341 made against the receptor beta chain#). Some labeling is seen in various processes (including horizontal and bipolar cell processes) in the invaginations of rod spherules and cone pedicles. For example, prevalent labeling is seen between the ribbon (asterisk) synapse active zone and the horizontal cell processes; the latter often have some large, round vesicles, like those of the monkey shown in b. Labeling (arrows) shown here is associated with a central process between the horizontal cell processes. These processes between the horizontal cell processes appear to be projections from the rod (r) cytoplasm (based on examination of the structure of these processes in many rod spherules). Somewhat similar projections have been shown in rod spherules of eel (Klooster and Kamermans 2016), rabbit (Hirano et al. 2005), monkey (Harvey and Calkins 2002), and human (Linberg and Fisher 1988); in the latter study, these projections are shown to be postsynaptic to definitive synapses with horizontal cell processes (as illustrated in c). In our examples, it is difficult to identify which side of the contact has the gold-labeled antigenic sites. Scale bar is 500 nm (unpublished data). #Antibody fully characterized and widely used; see Häring et al. 1985; Hering et al. 2003; Ohkawa et al. 2014; Arama et al. 2015; controls lacking the primary antibody showed only rare gold in both animals. f Diagram illustrating three theories for the feedback mechanism of horizontal cell processes on the retinal terminal synapse: GABA, protons (H+ is pH) and ephaptic transmission (ΔV; an electrical field effect at close range; Gardner et al. 2015; Kramer and Davenport 2015). The involvement of GABA is supported by recent studies (Hirano et al. 2016; also Liu et al. 2013), but they suggest that GABA from horizontal cell processes activates autoreceptors directly on the horizontal cell processes, probably indirectly mediating the pH-based feedback mechanism (Color figure online)
While we have described previously the invaginating postsynaptic processes at vertebrate photoreceptor synapses (Petralia et al. 2016), here we will examine these same synapses, but now from the point of view of invaginating presynaptic or efferent processes, i.e., processes within the same invagination, but that mediate neurotransmission retrograde, to the photoreceptor cell terminal. The presynaptic active zone in the base of photoreceptor cells of jawless fish varies in structure, from clusters of presynaptic vesicles lacking any presynaptic dense body, seen in the Atlantic hagfish, Myxine glutinosa, to various round to irregular dense bodies surrounded by clusters of vesicles, seen in the Pacific hagfish, Polistotrema stouti and Eptatretus burgeri, finally to distinctive presynaptic ribbons lined with vesicles in the lamprey, Lampetra fluviatilis; the latter structure remains the standard for most other vertebrates (Holmberg 1970, 1971; Holmberg and Öhman 1976). Postsynaptic processes in hagfish include two general kinds: Type 1 processes have many agranular vesicles and type 2 processes only have at most a few vesicles. The vesicles in type 1 processes range from 30 to 50 nm in the Atlantic hagfish and from 40 to 50 nm in the Pacific hag-fish; in both cases, the size range is the same as that in the presynaptic active zone of the receptor cell base. In contrast, the postsynaptic processes in the lamprey synapse lack any large numbers of vesicles, although they often have a few vesicles of various sizes; these postsynaptic processes of the lamprey are considered to be true horizontal and bipolar cell processes, as found in other vertebrates. No presynaptic active zones are described in the postsynaptic processes of any of these jawless fish, but it seems likely that some function as presynaptic terminals, especially the type 1 processes of hagfish, with such a high density of vesicles; note also that Holmberg (1971) assumes the these must represent efferent terminals.
In other vertebrates (fish, amphibians, reptiles, birds, mammals; i.e., those with jaws), the postsynaptic processes (i.e., postsynaptic to the ribbon synapse) in the photoreceptor invagination generally lack any appreciable accumulation of vesicles or evidence of presynaptic active zones (Attwell et al. 1993; Sterling and Demb 2004; Kramer and Davenport 2015); but there are exceptions. In the retina of the mudpuppy, Necturus maculosus, postsynaptic processes commonly form chemical synapses with adjacent postsynaptic processes (Dowling and Werblin 1969); these synapses form in the partially invaginated complexes of processes (i.e., they “...usually just dent the surface of the terminal...” of the photoreceptor cell), while more deeply invaginated complexes seem to lack these synapses. In the images (their figure 8), the “presynaptic” vesicles in the “postsynaptic” process appear to be a little smaller and less rounded than those of the photoreceptor ribbon (Fig. 7a). These processes often have microtubules and they form the sides of the postsynaptic triad of the ribbon, and the authors note that they are likely horizontal cell processes. In the retina of the larval tiger salamander, Ambystoma tigrinum, some vesicle-filled processes, possibly from horizontal cells, can invaginate into the base of the photoreceptor cell (Lasansky 1973). Dowling and Boycott (1966) illustrate an unidentified small process with vesicles that contacts the cone base (pedicle) of a young rhesus macaque monkey on the terminal membrane but not in the deep invaginating complex. Some published micrographs of the invaginating complex of processes at the ribbon synapses of the cone pedicle of macaque monkeys show horizontal processes with large numbers of vesicles (Raviola and Gilula 1975; Sterling and Matthews 2005) (Fig. 7b). In the complex of processes in the deep invagination at the ribbon synapses of the base (rod spherule) of rod photoreceptor cells in humans, horizontal cell presynaptic terminal active zones form synapses with the invaginated membrane of the rod cell (Linberg and Fisher 1988) (Fig. 7c). This is probably the most definitive case for ultrastructural evidence for a presynaptic chemical synapse function for horizontal cell processes within the invagination, but it should be noted that the latter study examined normal areas of retina from two adult human males with retinoblastomas (methods described in Linberg and Fisher 1986).
Fig. 8.
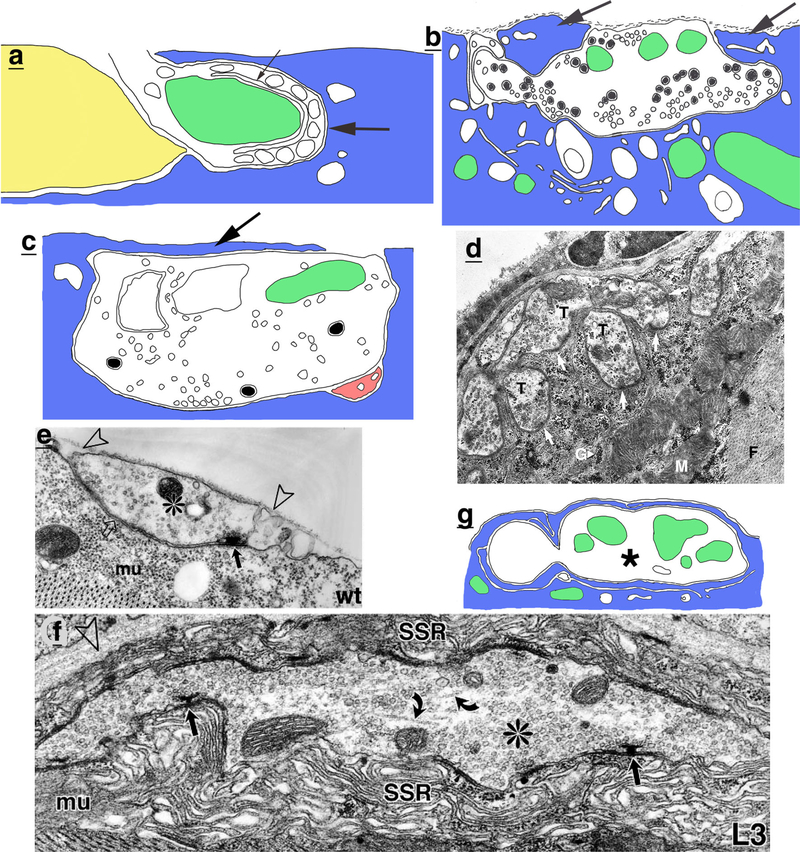
NMJs of invertebrates. a Drawing of invaginated NMJ of a ctenophore. Note the unusual terminal structure that is characteristic of the ctenophore synapse (large arrow), including a single row of vesicles, flat reticular cisternum (small arrow), and adjacent mitochondrion (Horridge 1965). b Drawing of an NMJ from the snail, Helix aspersa, showing how muscle processes (arrows) enclose the terminal in a partial tunnel-like invagination in the muscle fiber; other examples noted in the study (Rogers 1969) may be complete tunnels. The terminal contains small, clear and larger granular vesicles (including dense-cored ones). c Drawing of an NMJ of the octopus, showing how the deeply indenting terminal is covered by a narrow prolongation of the muscle (arrow); the synaptic active zone with clustered vesicles is evident on bottom, center (Ducros 1972). The small process in pink is probably glial and has cytoplasm like that of a presumptive glial cell that covers the NMJ (not shown). d Micrograph of a tick NMJ showing deeply invaginated terminals (T) with synapses (arrows) in a bulge of muscle sarcoplasm, containing myofibrils (F), glycogen granules (G), and mitochondria (M) (modified from figure 1a in Hart et al. 1980; reprinted with permission from Springer). e-g Development of the NMJ of Drosophila, from late embryonic (e) to late larval (f; third instar larva (L3); Prokop 1999), and then to adult (g; Wagner et al. 2015). e In the late embryo (stage 17), the terminal (*) is in a shallow indention in the muscle fiber (mu) and is covered over by a basement membrane (arrowheads). Note the non-junctional areas of contact (open arrow) and the T-bar synapse (arrow). f In contrast, in the late larval stage (third instar), the terminal is invaginating into the muscle fiber and surrounded by a thick subsynaptic reticulum (SSR). The terminal is filled with synaptic vesicles, but also has regions with microtubules (bent arrows) and a few mitochondria. g Drawing of the NMJ in the one-day-old adult; note the great reduction in SSR (the abundant presynaptic vesicles are excluded for clarity). Figures e and f modified from figures 2F and 2G in Prokop 1999; reprinted with permission from Springer
Invaginating Processes from Vertebrate Horizontal Cells Have Both Pre- and Postsynaptic Functions
Horizontal cells are unique neurons with several unusual features. In many mammals, such as the cat and rabbit, there are two kinds of horizontal cells, type A and type B (Kolb 1977; Peichl and González-Soriano 1994; Sterling and Demb 2004); both kinds send processes (“dendrites”) to invaginate into cone photoreceptor cell pedicles (terminals; Kolb 1977; Sterling and Demb 2004). Type B also has a fine axon that ends in an elaborate arborization that invaginates processes into thousands of rod terminals; yet this axon arborization appears to be electrically isolated from the soma and “dendrites,” so it presumably does not function as a conventional neuron axon. In comparison, there are four kinds of horizontal cells in turtles; the large type 1 is like the mammalian type B, with a large axon arborization that connects to rods as well as to some cones, while the type 1 “dendrites” and the processes of the other three types of horizontal cells (like the A cells of mammals) connect to different subsets of cones (Ammermüller and Kolb 1996). On the other hand, nocturnal rodents such as rats and mice appear to have only one kind of horizontal cell, with large dendritic and axonal arborizations like the type B (Peichl and González-Soriano 1994; He et al. 2000). In addition to invaginating processes into the terminal bases of rods and cones, horizontal cells also make chemical synapses (not involving invaginations) within the neuropil of the outer plexiform layer just below the rod and cone terminal bases; the presynaptic structures originate from both the “dendritic” and axonal arborizations, and the postsynaptic processes of these synapses vary in different vertebrates but include bipolar neurons and other horizontal neurons, as well as processes extending from the bases of the photoreceptor cells in some cases (Dowling and Werblin 1969; Lasansky 1973; Fisher and Boycott 1974; Sakai and Naka 1983, 1986; Kolb and Jones 1984; Linberg and Fisher 1988). In addition to chemical synapses, horizontal cells are coupled via gap junctions (He et al. 2000; Hombach et al. 2004; Klooster and Kamermans 2016; Greb et al. 2017) and desmosomes (Haverkamp et al. 2000). The desmosomes, which contain glutamate receptors, are found below the base of the cone pedicle and probably are formed between horizontal cell processes. Another unusual structure is associated with the invaginated processes of horizontal cells in cone pedicles of fish. These form thin extensions deep into the cone terminal, called fish retinal spinules; typically, they have dense material at their tip resembling a modified PSD. These spinules usually are common in the day but mostly disappear at night, and are affected by several factors such as dopamine. While it is possible that these spinules have a presynaptic function (Weiler and Wagner 1984; Popova 2014), there seems to be more evidence that they are primarily postsynaptic structures (reviewed in Petralia et al. 2015) and they will not be discussed further in this review.
A large body of evidence suggests that the invaginating processes of horizontal cells act as both pre- and postsynaptic structures (Fig. 7). Most of this work involves cones, but similar processes probably occur in rods (Thoreson et al. 2008). The cone pedicle (terminal) is very complex and has postsynaptic processes at two or three levels (Vardi et al. 1998; Haverkamp et al. 2000; DeVries et al. 2006; Sterling and Demb 2004). Glutamate released from the ribbon synapse first contacts ionotropic glutamate receptors on the adjacent invaginated horizontal processes and then, a little lower, reaches glutamate receptors on the invaginated bipolar processes (note that bipolar cells relay the signal to ganglion cells whose axons form the optic nerve to the brain). Glutamate may continue to diffuse via spillover out of the invagination, to affect glutamate receptors on bipolar cells just below the pedicle; finally, within about 1 ms the diffusing glutamate may reach as far as the glutamate-receptor-bearing desmosomes noted above. Thus, the positioning of the postsynaptic processes at different levels within and outside the invagination allows for complex control of responses. There also are various kinds of ionotropic and metabotropic glutamate receptors at the different levels, allowing for more precise control and variation of the responses (Vardi et al. 1998; Harvey and Calkins 2002; Puthussery et al. 2014). The invagination is a crucial part of this mechanism, resulting in “...a longer path for diffusion of transmitter and electrical current out of the cleft” (Kramer and Davenport 2015). It should be noted that the vision mechanism is a little counterintuitive (and actually too complicated to describe here in detail): Roughly speaking, glutamate is released continuously in the dark, and light hyperpolarizes the cones and reduces their release of glutamate, resulting in hyperpolarization of the horizontal cells, which send a feedback to the cone terminals; ultimately this leads to modulation of the response (Kamermans and Fahrenfort 2004; Sterling and Demb 2004). Horizontal cell processes send a feed-forward signal to the bipolar cell processes and a feedback signal to the cone cell terminal membrane. The feedback mechanism makes the invaginating horizontal processes a kind of “reciprocal synapse” (Kramer and Davenport 2015). This mechanism allows for lateral inhibition of diffuse light stimulation from surrounding cones, whereby the horizontal cell feedback modulates the voltage-gated calcium channels of the cone; this then regulates glutamate release, ultimately leading to contrast enhancement and high acuity vision. The negative feedback has been shown to occur between horizontal cells and cones in a wide variety of vertebrates, including fish, salamanders, turtles, and mammals (e.g., see Tatsukawa et al. 2005 (turtle), and various references listed in Babai and Thoreson 2009), as well with rods in salamanders and mice (Babai and Thoreson 2009). A large amount of evidence indicates that horizontal cells signal through the inhibitory neurotransmitter, GABA. We already have noted several examples of vesicular active zones in horizontal cell processes, and these likely contain GABA. Physiology studies on the carp, Cyprinus carpio, show that GABA seems to mediate the negative feedback from horizontal cells to cones (Murakami et al. 1982). Several studies show evidence of GABA receptors in the cones of various vertebrates (Yazulla et al. 1989; Liu et al. 2005). In mammals, GABA receptors within the photoreceptor terminal invagination are found in bipolar cell processes, especially opposite contacts with horizontal cell processes (Greferath et al. 1994; Vardi and Sterling 1994; Vardi et al. 1998), and in cone terminals (although the evidence is more limited; Vardi et al. 1998; Pattnaik et al. 2000). Thus, horizontal cell processes can be presynaptic to two different structures in the invagination: bipolar cell processes and the photoreceptor terminal membrane of the invagination (Fig. 7). Hirano et al. (2016) show that vesicular release of GABA from mouse horizontal cells appears to be necessary for the modulation of calcium channels of the photoreceptor terminals (see also Hirano et al. 2005). But we also have noted that most of the invaginating horizontal processes in vertebrates do not seem to contain many vesicles, and several studies suggest that GABA is released from these invaginated horizontal cell processes via non-vesicular GABA transport (Haverkamp et al. 2000; Gardner et al. 2015; Kramer and Davenport 2015). In fact, currently there are three competing theories for the feedback mechanism: GABA, protons (pH), and ephaptic transmission (an electrical field effect at close range; Thoreson and Mangel 2012; Gardner et al. 2015; Kramer and Davenport 2015) (Fig. 7f). In the ephaptic model, ion exchange in the narrow cleft between the horizontal process and the cone terminal membrane alters the gating of calcium channels on the cone cell membrane, mediating a negative feedback effect. This possible ephaptic transmission may be enhanced by a zone of connexin (and possibly pannexin) hemichannels at the point of close contact between the invaginating horizontal cell process and the cone terminal membrane (Klaassen et al. 2011; Gardner et al. 2015; Kramer and Davenport 2015; Klooster and Kamermans 2016; Greb et al. 2017). Most likely, two or more of these mechanisms work together for horizontal cell feedback (Klaassen et al. 2011; Gardner et al. 2015; Kramer and Davenport 2015). For example, Kramer and Davenport (2015) favor the proton/pH mechanism, at least for mammals, perhaps in combination with the ephaptic mechanism; in contrast, Gardner et al. (2015) favor the ephaptic mechanism, perhaps in combination with the GABA mechanism. And as noted, Hirano et al. (2016) show evidence supporting a GABA mechanism. However, this latter mechanism seems to be indirect, with GABA released from horizontal cells and activating autoreceptors on the horizontal cells, possibly driving a pH-based feedback mechanism (Liu et al. 2013; Hirano et al. 2016); a GABA autoreceptor-based mechanism also might be present in lower vertebrates (Klooster et al. 2004; Endeman et al. 2012).
We suggest that the relative importance of the different mechanisms varies among different vertebrates, based on the wide variations in retinal structure that we have described here. For example, we have described examples where there are definitive chemical synapses within the invagination and other cases where there are invaginating processes that are filled with vesicles that resemble synaptic vesicles; perhaps these are the best cases of feedback via chemical neurotransmission. On the other hand, in cases where there are no accumulations of vesicles in invaginating processes, feedback may rely mainly on ephaptic and/or proton/pH mechanisms within the invagination.
Do Similar Photoreceptor Inhibitory Mechanisms Occur in Invertebrates?
Similar structures also may indicate some kind of feedback mechanism in invertebrate photoreceptor terminals, i.e., we have described invaginating vesicle-filled “postsynaptic” processes as well as enigmatic, invaginating processes such as the tunnel fibers in photoreceptor terminals of the octopus. Lateral and efferent inhibition has been described in various invertebrates (Gur et al. 1972; Suzuki and Tasaki 1983; Barlow et al. 2001). In flies and other insects, as in vertebrates, lateral inhibition may involve GABA neurotransmission, ephaptic effects, and/or local electrochemical gradients (Shaw 1975; Weckström and Laughlin 2010; Freifeld et al. 2013). For example, GABAergic input is a component of the first part of visual circuitry of Drosophila, involving the photoreceptor cell terminals and processes from several kinds of neurons, including at least one kind that probably is equivalent to vertebrate bipolar cells, and others that are GABAergic (Freifeld et al. 2013). Alternatively, this complex is described by Weckström and Laughlin (blowfly; 2010) as a lamina cartridge surrounded by basement membrane and epithelial glial cells to optimize local extracellular field potentials. Thus, these insect visual neuronal complexes are compacted and closely organized systems that appear to mediate feedback readily by one or more mechanisms. While, in this case, they probably do not involve invaginating presynaptic terminals, we suggest that invaginating processes may be one optimal way to achieve this kind of integration for some neuronal arrangements in various invertebrates, as in vertebrates. In fact, as we described above, crayfish (also lobsters and wolf spiders) photoreceptor terminals can have invaginating complexes that look very similar to vertebrate ones (Hámori and Horridge 1966; Trujillo-Cenóz and Melamed 1967; Hafner 1974), and functional studies suggest that there is lateral inhibition mediated by GABA at the photoreceptor terminal complexes of crayfish (Glantz and Bartels 1994; Glantz et al. 2000).
A different mechanism of inhibition at photoreceptor terminals occurs in the octopus. The large photoreceptor terminals, called bags or carrots, appear to send inhibitory signals to adjacent bags via acetylcholine neurotransmission (Piscopo et al. 2007). This seems to be consistent with the occurrence of vesicle-filled, finger-like (or called finger twigs) invaginations found between adjacent bags in octopi and squid (Dilly et al. 1963; Cohen 1973; Haghighat et al. 1984), as we described above. This inhibitory cholinergic signal modulates the activity at retinal terminals, which may utilize glutamate as an excitatory neurotransmitter, at least in the retinal terminal processes that extend past the bags (Piscopo et al. 2007).
Most Kinds of Neuromuscular Terminals Indent and Sometimes Fully Invaginate into the Muscle Cell
Muscle structure varies among animals and will not be described in detail in this review (see reviews of Hanson and Lowy 1957; Smith 1966; Page 1968). Broadly speaking, some animals have “striated” muscles in which the contractile elements are organized into myofibrils that have repeating subunits called sarcomeres, usually associated with a complex arrangement of tubular elements that will be described later; other animals have less well-organized muscle fibers called “smooth.” Striated muscle fibers have been described best in arthropods and vertebrates. They also occur in some invertebrate chordates and some of the muscle fibers in simple animals. In fact, striated muscle may have evolved independently two to four times, in Ctenophora, in Cnidaria (once or twice), and in higher animals (Burton 2008; Steinmetz et al. 2012; Achim and Arendt 2014). For example, while most anthozoans (sea anemones/Cnidaria) and ctenophores have mainly smooth muscle fibers, striated muscle fibers seem to be employed for relatively rapid motions, including in a group of swimming anthozoans (in this case, they are epitheliomuscular cells), and in special tentacles used for prey capture, found in a group of ctenophores (Burton 2008; Achim and Arendt 2014). Other animals may have forms of striated muscle fibers for special uses, such as the adductor muscle of scallops (Mollusca; Nunzi and Franzini Armstrong 1981), in the pedicellariae (small, claw-like appendages) of some echinoderms (Pentreath and Cobb 1972), the body wall muscles of the earthworm (Mill and Knapp 1970b), and the trunk musculature of the arrow worm (Chaetognatha; Duvert and Savineau 1986). Otherwise, many invertebrates have various kinds of unstriated = smooth muscle fibers (Hanson and Lowy 1957; Smith 1966) or are believed to have intermediate fibers (leech—Pucci and Afzelius 1962; nematode—Rosenbluth 1965; flatworm—Sulbarán et al. 2015).
It is common for neuromuscular terminals to indent into the surface of muscle cells of animals; many kinds of animals show this to some degree. However, deep invaginations are less common; there are many good examples in arthropods, and this is best known and studied in Drosophila, as well as a few distinctive examples in vertebrates. These invaginating neuromuscular junctions (NMJs) also typically have complex structural arrangements on the postsynaptic side.
Invertebrate NMJs
In the ctenophores (comb jellies), NMJs typically show at least a small indention into the muscle cell (Horridge 1965; Hernandez-Nicaise et al. 1980), and in one figure (Fig. 8a; his figure 11A; Horridge 1965), the terminal is almost fully invaginated: At least the entire active zone is within the invagination. On the postsynaptic side, the muscle cytoplasm has some irregular reticula and vesicles, but nothing definitive. At this point, it is interesting to note that the typical ctenophore presynaptic terminal is different from that of most other animals; it features a complex called a presynaptic triad, consisting of 1) a single row of vesicles along the presynaptic membrane, 2) a flat reticular cisternum against the other side of the row of vesicles, and 3) one or more mitochondria against the cisternum (Hernandez-Nicaise 1973). So, the NMJ shows an indent into the muscle like what we will describe for many other animals, despite the unusual presynaptic structure.
Fig. 11.
NMJs in rats. A, B. Most skeletal muscles in mammals consist of variations of slow (a) and fast (b) twitch muscles. For example, the rat extensor digitorum longus (b1, b2; low and high magnifications) is made up of mostly fast twitch fibers innervated by a broad nerve terminal with multiple bulblike expansions (protrusions) that indent deeply into the muscle fiber and have deep subjunctional folds (f); part of one expansion is drawn in b2 (Ellisman et al. 1976). In comparison, in the broad terminals of NMJs of the rat soleus muscle, made of mostly slow twitch fibers, the expansions and their subjunctional folds are less regular and deep (a1, a2; low and high magnifications). c, d In rat extraocular muscles, there are some slow, tonic muscle fibers. NMJs of these fibers tend to have simple terminals, mostly with little or no indention or subjunctional folds (Teräväinen 1968a). Examples in c and d include one terminal with a few subjunctional folds at the postsynaptic membrane (PSM, c), and another (d) with a terminal (TA) almost completely covered by projections (MP) from the muscle, so that it appears to be completely invaginated. ASM indicates the amorphous basement membrane in the synaptic cleft and PSM indicates the postsynaptic density; some vesiculate sarcoplasmic reticulum is evident below the density. Arrows in c indicate dense-cored vesicles. TE indicates a thin glial (teloglial or Schwann) sheath between the synaptic membranes (and between the fold regions of the PSM). c and d are modified from figures 15 and 19, respectively, of Teräväinen (1968a; reprinted with permission from Springer). e Drawing of deeply indented NMJ of a smooth muscle fiber from the rat vas deferens (Taxi 1964)
We have not found good examples in the literature of indented/invaginated NMJs in Cnidaria, despite their closer homology to higher animals compared to ctenophores. NMJs in hydras (Westfall et al. 1980) and an anemone, Aiptasia pallida (Westfall et al. 2002) show little if any indention. On the other hand, Spencer (1979) shows an indented NMJ in the anthomedusan, Polyorchis penicillatus (a kind of jellyfish form); the presynaptic side appears to have a couple of small vesicles but nothing definitive. The author calls this a neuromuscular synapse, but it is a synapse between a radial nerve axon and an epithelial cell that is connected via gap junctions to the subumbrella swimming muscle (i.e., functionally neuromuscular). But even more interesting is a contact between the sphincter muscle, which reduces the jellyfish bell opening, and axons of the outer nerve ring; this muscle extends processes that form a complex sheath around the axon. Perhaps this is only for insulation like myelin, but the author notes that this could be a means of electrical conduction to activate the muscle; this would be a similar mechanism to the ephaptic transmission mechanisms that we have discussed for photoreceptors. In other words, and using a broad definition, these ensheathed axons could represent a kind of presynaptic invaginating process.
Flatworm NMJs commonly are formed on small processes that project a short distance from the surface of the muscle cell and are called by various name combinations: “sarcoplasmic/myoplasmic processes/extensions/projections/outpocketings” (MacRae 1963; Chien and Koopowitz 1972; Pan 1980; Cousin and Dorsey 1991); for example, in the polyclad flatworm, Notoplana articola, these sarcoplasmic extensions can be as long as 10 micrometers, although the ones cut longitudinally in the figures seem to be about 1–1.5 micrometers long (Chien and Koopowitz 1972). In the miracidium (free-living swimming stage) of the trematode flatworm, Schistosoma mansoni (human parasitic fluke), the sarcoplasmic “outpocketings” can be more elaborate and the nerve terminal appears to form in a “pocket” or “depression” or even a deeper invagination that can surround >¾ of the presynaptic terminal surface (Pan 1980); the terminal contains small, clear vesicles and sometimes also larger, dense-cored ones, the cleft is ~15 nm, and there is an ~30-nm PSD.
Like flatworms, roundworms (nematodes; Reger 1965) and gastrotrichs (Teuchert 1977) typically form NMJs at the ends of processes that extend from the muscle cells; but they are particularly well developed in roundworms. We have not found published examples of indenting terminals at these NMJs. Among various small groups of invertebrates, we did not find examples of indented NMJs in larval phoronid worms (Lacalli 1990), but images of deeply indenting/partly invaginating NMJ terminals have been published for an entoproct, Barentsia gracilis (Reger 1969), a rotifer, Trichocerca rattus (Clément 1977), and an arrow worm or chaetognath, Sagitta setosa (Duvert and Barets 1983). The chaetognath NMJs are particularly interesting because some NMJs have a distinctive “subsynaptic apparatus” below the postsynaptic membrane, containing deep plasma membrane infoldings (most are probably part of a subsynaptic reticulum rather than true folds), sometimes interspersed with mitochondria; this is perhaps like what we will describe in many arthropod NMJs.
Among the segmented worms (Annelida), NMJs often are indented to various extents. In leeches, the terminals sometimes are deeply indented into the muscle cell (Rosenbluth 1973; Yaksta-Sauerland and Coggeshall 1973). Terminal indentions in the muscle fibers of oligochaete annelids, including the earthworm (Lumbricus terrestris; Benedeczky et al. 1990) and Branchiobdella pentodonta (Farnesi and Vagnetti 1975), typically are not as deep. In other studies of earthworms (Mill and Knapp 1970a; Rosenbluth 1972) and archiannelids (Rieger and Rieger 1975), the terminals do not seem to show a distinct indention. Interestingly, earthworm NMJs can have postsynaptic “muscle tails” that extend from the muscle fiber (Mill and Knapp 1970a; Rosenbluth 1972), and may be like sarcoplasmic extensions that we have described in some simpler kinds of worms. Also, in some NMJ synapses in leeches (Rosenbluth 1973; Yaksta-Sauerland and Coggeshall 1973) and oligochaetes (Rosenbluth 1972; Farnesi and Vagnetti 1975), there are unusual postsynaptic membrane structures, typically concave, with a regular array of fine projections that may be hexagonal. These NMJs also usually have a thickened laminar material or basement membrane in the cleft, and in the region of the concavity, the cleft can be up to 100 nm wide.
In the NMJs of mollusks, typically the presynaptic terminals are indenting or invaginating into the muscle; examples are described from all the major groups, including chitons (Polyplacophora; Økland 1980), snails (Gastropoda; Rogers 1969), mussels (Bivalvia; McKenna and Rosenbluth 1973), and squid (Cephalopoda; Florey 1969). In most cases, there does not appear to be any particular specialization on the postsynaptic side, except that mitochondria often come close to the postsynaptic membrane. But the NMJs are more elaborate in the foot of the snail, Helix aspersa; sometimes the presynaptic terminal can be almost completely invaginated into the muscle, “...enclosed in a tunnel produced by the overlapping of the muscle cell flanges.” (Rogers 1969). In the author’s figure 20, the large terminal contains numerous small, clear and larger, densecored (granular) vesicles, and the muscle cytoplasm (sarcoplasm) just under the postsynaptic membrane has some very large vesiculate structures as well as some smaller vesicles and mitochondria (Fig. 8b). In comparison, some terminals in muscles of the intestine and hepatopancreas of the snail, H. pomatia, also may be largely covered by muscle processes and show a similar range of vesicles (small, clear and larger granular [dense-cored to fully dense]), but show little postsynaptic specializations (Elekes and Ude 1994). In the channeled whelk, Busycon canaliculatum or B. canaliculatus, a large marine snail, some images in Hill and Sanger (1974) of NMJs of muscles associated with feeding structures appear to be indented to invaginated by processes of the muscle fiber and show only a few postsynaptic vesiculate structures and portions of sarcoplasmic reticulum (SR); one example has mostly small, clear vesicles, while another has mostly large granular vesicles. In cephalopods (Octopus, Sepia), terminals can be deeply indenting to completely invaginating in the muscle fiber; and the synaptic active zones can have a small presynaptic dense body and dense postsynaptic membrane (Graziadei 1966). In the cephalic aorta of the octopus, Octopus vulgaris, some presynaptic terminals can invaginate completely in the longitudinal muscle fibers; they are filled with 30–40-nm synaptic vesicles as well as a few larger vesiculate structures, but there are neither distinctive subsynaptic structures nor thickenings of the pre-/postsynaptic membranes (Barber and Graziadei 1967). Some terminals on muscle fibers of the salivary glands of O. vulgaris can be deeply indenting and then covered over most of their top by “prolongements étroits” (narrow prolongations) of the muscle fiber (Ducros 1972) (Fig. 8c). In all of the above examples of snail and cephalopod NMJs, the terminals seem to become invaginated by the extension of a thin process/flange of the muscle fiber that can cover over most of the top of the terminal.
There is relatively little evidence of NMJ invagination among the deuterostomes (animals in which the blastopore becomes the anus in embryonic development). Several studies of the NMJs of tunicates (a group of invertebrate chordates) do not show any images of indenting or invaginating terminals (Bone and Ryan 1974; Dolder 1975; Cavey and Cloney 1976). Generally, the NMJs in echinoderms (starfish, sea urchins, sea cucumbers, etc.) are not well defined (Dolder 1975; Cobb 1978). In the sea cucumber, Cucumaria frondosa, Doyle (1967) shows an image of a fine process, which the author identifies as an axon that invaginates into a muscle fiber; but this process does not appear to have any vesicles. The author also shows images of tubular invaginations within muscle fibers, and each invagination has a small axon profile containing various vesicles and vesiculate structures. An even more distinctive tubular structure is found in the sea urchin, Echinus esculentus (Cobb and Laverack 1966). In the NMJ, the presynaptic terminal forms a deep indention in the muscle fiber; the walls of this indention contain mitochondria; in addition, “wing-like processes” extend from the muscle cell membrane and can envelope the terminal completely in a tubular extension.
Arthropod NMJs
Chelicerates
Chelicerates include the Arachnida (spiders, scorpions, mites and ticks, etc.), Merostomata (horseshoe crabs), and Pycnogonida (sea spiders). As is typical of skeletal muscles in arthropods (as well as in vertebrates), chelicerates have striated skeletal muscles consisting of contractile units called sarcomeres that are deeply invaginated by a transverse tubular system extending from the surface membrane, with the tubules interspersed with elongate elements of the SR, together forming a “dyad.” Chelicerate NMJs show some interesting variations in presynaptic invaginating terminals. The NMJs of two arachnid groups, spiders (Aranae) and scorpions (Scorpiones), typically consist of several axon terminals enwrapped by glial processes, and the whole complex fits into a groove or indention on the muscle surface (Zebe and Rathmayer 1968; Melamed and Trujillo-Cenóz 1971; Sherman and Luff 1971; Smith 1971; Fourtner and Sherman 1973). Images in publications of NMJs of the retinal muscles of wolf spiders (Melamed and Trujillo-Cenóz 1971) and of leg muscles of the scorpion, Hadrurus hirsutus (Smith 1971), show a small, presynaptic dense body in the center of the active zone; it is roughly triangular and about the size of a synaptic vesicle. The small, clear synaptic vesicles range from 35 to 60 nm, with occasional dense-cored (granular) vesicles about 100 nm (Fourtner and Sherman 1973). The postsynaptic region does not usually have many specialized structures. The tubule/SR dyads often are shown to form on the sides of the synaptic terminals, but in addition, examples are shown for both the wolf spider and scorpion, of a vertical line of postsynaptic SR arising near the center of the terminal contact (Melamed and Trujillo-Cenóz 1971; Smith 1971). In contrast to the NMJs of spiders and scorpions, the NMJ of a tick, Amblyomma variegatum (Acari; mites and ticks), can include several deeply invaginating presynaptic terminals in a bulge of sarcoplasm on the side of the muscle (Hart et al. 1980) (Fig. 8d).
The NMJs of leg muscles of the horseshoe crab, Limulus polyphemus, are formed on large evaginations of the muscle sarcoplasm, up to 20 micrometers in diameter and extending up to 40 micrometers from the muscle fiber surface (Sherman and Fourtner 1972; Fourtner and Sherman 1973). Several presynaptic terminals can invaginate, some deeply, into a sarcoplasmic evagination. In addition, the evagination has slender invaginations of its plasma membrane as well as a postsynaptic “large complex of membranous tubules and vesicles.” The small, clear synaptic vesicles range from 35 to 45 nm, with occasional dense-cored (granular) vesicles from 70 to 100 nm.
Thus, the range in structure of presynaptic invaginating terminals in chelicerate NMJs of skeletal muscles varies from a relatively shallow groove or indention for spiders and scorpions, to more deeply invaginating ones in ticks and horseshoe crabs (and the latter in a sarcoplasmic evagination). How can one explain these differences? Fourtner and Sherman (1973) compare structure and function of NMJs in spiders/scorpions versus horseshoe crabs. They point out several differences: Muscles of spiders and scorpions are innervated by fewer neurons than those of horseshoe crabs, but the former may show more specializations in motor axon types. They also note that spiders and scorpions do not seem to have peripheral inhibition of muscles, unlike horseshoe crabs. However, more recent studies indeed show peripheral inhibition in the former (e.g., Wolf and Harzsch 2002). Overall, they suggest that the unusual arrangements of the NMJs in horseshoe crabs reflect their earlier appearance in chelicerate evolution as well as their lack of further evolution since early prehistoric times. So perhaps the interesting arrangement of multiple invaginating terminals in a sarcoplasmic evagination is a primitive feature of chelicerate NMJ evolution.
Cardiac muscle NMJs also have been studied and they show a similar range of structure in spiders (Sherman 1973) and horseshoe crabs (Lang 1972); these include some NMJs in which the presynaptic terminals are invaginated into a sarcoplasmic evagination, and other simpler ones in which the NMJs just contact the muscle surface. There also is another kind of NMJ in horseshoe crabs, in which the presynaptic terminal invaginates directly into an enlargement (i.e., not an evagination) of the sarcoplasm on the muscle surface.
Finally, little is known about the NMJs of sea spiders (Pycnogonida), but Fahrenbach (1994) states that the presynaptic terminals indent into the surface of the muscle.
Crustacea
Crustacean NMJ structure and function have been studied widely, especially those of the limb muscles of crayfish, crabs, and lobsters. There are many variations, and for example, different muscles show different compositions of excitatory phasic (fast) and tonic (slow) and inhibitory innervation (Atwood 1976; Msghina et al. 1998). In general, excitatory terminals have round synaptic vesicles and inhibitory ones have slightly irregular synaptic vesicles; both have only occasional dense-cored vesicles, and both sometimes have a presynaptic “dense body” or “dense bar” about the size of a vesicle (as we described above for some chelicerates); inhibitory terminals are GABAergic, and excitatory terminals at least for limb muscles are glutamatergic. Some synapses associated with the NMJ complex are axoaxonic, with an inhibitory presynaptic terminal and excitatory postsynaptic terminal; these often form close to where the terminals are forming synapses on the muscle (crayfish—Atwood and Morin 1970; Jahromi and Atwood 1974; Govind et al. 1995; crab—Sherman and Atwood 1972). Phasic neurons fire in short bursts for rapid movements, while tonic neurons fire continuously during locomotion, and they show some characteristic differences. In crayfish leg muscles, phasic axons have few mitochondria and their terminals are thin, while tonic axons have many mitochondria and thicker terminals that look like a chain of varicosities running along the muscle surface (King et al. 1996; Bradacs et al. 1997; Msghina et al. 1998). Various measurements of synapse active zones and dense bars revealed only relatively minor differences between phasic and tonic terminals (Msghina et al. 1998).
Deeply invaginating presynaptic terminals are found in several studies of NMJs of various limb muscles (several species of crayfish—Atwood and Morin 1970; Jahromi and Atwood 1974; Govind et al. 1995; the striped or lined shore crab, Pachygrapsus crassipes—Atwood and Johnston 1968; the great spider crab, Hyas araneus—Sherman and Atwood 1972). In some cases, the invagination also contains prominent glial processes (Sherman and Atwood 1972; Jahromi and Atwood 1974). Jahromi and Atwood (1974) illustrate examples of NMJ invaginating terminal complexes containing both excitatory and inhibitory terminals, and other examples in which the invaginating terminals form synapses in a “semi-isolated outgrowth of the muscle fiber.” Atwood and Morin (1970) show an example in which the postsynaptic muscle fiber forms “...a series of extensions from the main body of the fiber...” and these extensions form “…a complex series of folds about the nerve terminals.” In other cases, the postsynaptic area can have an elaborate subsynaptic reticulum (SSR) with many large folds and distinct openings to the surface facing the terminal (Jahromi and Atwood 1974; Govind et al. 1995). In studies comparing phasic and tonic excitatory terminals in NMJs of a limb extensor muscle in the freshwater crayfish, Procambarus clarkii, both the large tonic and the small phasic terminals are indenting into the series of wide folds of the muscle SSR; in some images, the small phasic terminal is sitting below the tonic terminal and so is surrounded by the SSR and the tonic terminal (King et al. 1996; Bradacs et al. 1997; Msghina et al. 1998).
Examples of NMJ structure of other muscles include the eyestalk levator muscle of the long-eyed swimming or sentinel crab, Podophthalmus vigil (Hoyle and McNeill 1968), and abdominal extensor muscles of the lobster, Homarus americanus (Govind et al. 1985). Hoyle and McNeill (1968) found that the “fast” terminals tend to be in an indention (“groove”) on the muscle surface, while the “slow” terminals (and/or possibly inhibitory terminals) can be either near the surface or in deep invaginations; the deeply invaginating terminals are accompanied by “Schwann cell” processes. Note that “fast” and “slow” are used for both axons and muscle fibers and are relative terms, both for describing the eyestalk muscle function and comparing to the swifter reactions of limb muscles of this and other crustaceans. The postsynaptic structure in these synapses of the eyestalk muscle is termed by the authors a “subsynaptic pad” containing “…twisted tubules representing invaginations from the surface membrane, and part of the transverse tubular system…” Finally, Govind et al. (1985) show an image of an NMJ in an abdominal extensor muscle of an adult lobster that contains two excitatory and one inhibitory terminals formed together in a deep indention on the muscle surface; as we have noted for the limb NMJs of crustaceans, both the excitatory and inhibitory terminal active zones can have a presynaptic dense bar, and the difference in vesicle shape is readily distinguishable (round versus irregular, respectively).
Insects
NMJs have been described in several different kinds of insects; they are similar in many ways to those that we have described in Crustacea. Deeply invaginating presynaptic terminals are found in some NMJs in the mealworm beetle, Tenebrio molitor (Smith 1960). In other insects, the terminal can sit in a fairly deep indention or groove, as in the cicada (Tibicen linnei; Edwards et al. 1958b), cockroaches (Edwards 1959; O’Connor et al. 1965; Miller and Adams 1974), larval stage of the blowfly (Phormia terraenovae; Osborne 1967), and Carolina sphinx moth, Manduca sexta (Rheuben and Reese 1978; Rheuben 1985). NMJs in the femoral muscle of the leg of the yellow jacket wasp (Vespula carolina) sit in a deep indention or groove, but the upper two-thirds of the terminal often is wrapped closely by a glial cell (nerve sheath cell or “lemnoblast”) so that it forms a “lid”; thus, the terminal effectively is sealed between the muscle fiber and lemnoblast cell membranes (Edwards et al. 1958a). Something similar appears to be found in NMJs of the sphinx moth, in which a glial cell can cover the terminal and extend into the sides of the groove (Rheuben and Reese 1978). The SSR is well developed in the NMJs of some insects (Osborne 1967; Rheuben 1985). In the cicada flight muscle NMJs, the postsynaptic structure seems to be unusually complex and is called a “rete synapticum” (Edwards et al. 1958b); it probably is a variation of SSR. As they describe it: “The plasma membrane in the postsynaptic area may show considerable vesiculation and occasional deep infolding. The deep folds may form spirals of several turns, or break up into numerous vesicles, or be continuous with the layered membranes.” In contrast to a definitive SSR, Smith (1960) describes, in the flight muscle NMJs of the mealworm beetle, a postsynaptic “…series of membrane folds enclosing cytoplasm containing many vesicles…” that represent “…complex convolutions of the plasma membrane of the muscle fiber…”
Titmus (1981) compared phasic (fast) and tonic (slow) excitatory, and inhibitory NMJs in muscles of the jumping leg of the locust Schistocerca (gregaria or americana); as we described above for the crayfish, phasic terminals tend to be smaller and thinner than tonic terminals. Excitatory terminals often form the NMJ in a muscle groove, while inhibitory terminals often seem to form on the flat muscle surface without a groove and with little or no sarcoplasm between the synaptic cleft and the myofilaments. Also, as in Crustacea, excitatory and inhibitory terminals have round and irregular synaptic vesicles, respectively. And like Crustacea, insect NMJs can have presynaptic dense bodies or bars (Osborne 1967; Rheuben and Reese 1978; Titmus 1981); in blowfly larvae (Osborne 1967), some of these show the classic structure of insect T-bars, seen in many other insect synapses (Budnik et al. 1990; Blagburn et al. 1999; Rivlin et al. 2004; Prokop and Meinertzhagen 2006).
Drosophila
NMJs of the common fruit fly, Drosophila (wild D. melanogaster and various strains), have been studied intensely, especially those of the larval stage, and are used as a major model of synapse growth and plasticity, membrane trafficking, and human neurological diseases (Deshpande and Rodal 2016) (Fig. 8e–g); we only can highlight here a few of the many studies. In the late embryo, the NMJ sits in a shallow groove on the muscle fiber, without any apparent SSR (Fig. 8e), but by the late larval stage (postembryonic; posthatch), the large presynaptic terminals usually are invaginated completely in the muscle fiber and are surrounded by a thick, highly developed SSR (Fig. 8f) (Prokop 1999; Prokop and Meinertzhagen 2006). In adults, the SSR becomes reduced, and the invagination is incomplete; images of the invaginating presynaptic terminals show about one-half to two-thirds of the plasma membrane covered by the muscle sarcoplasm (Budnik et al. 1990; Rivlin et al. 2004; Beramendi et al. 2007; Wagner et al. 2015) (Fig. 8g). In both late larva and adults, the terminals are large and filled with glutamatergic synaptic vesicles and mitochondria; typically, the active zones contain clusters of vesicles surrounding a T-bar density, and the SSR contains disk large, dorsal and cactus, proteins involved in synaptic function/plasticity (Budnik et al. 1990; Prokop and Meinertzhagen 2006; Beramendi et al. 2007; Wagner et al. 2015).
The larval NMJ is one of the best studied and understood models of the specialized function of invaginating presynaptic terminals. The larvae of Drosophila grow rapidly and so must regulate closely the co-development of muscles and their innervation. Muscle area increases 100x during larval development and this is accompanied by increased arborization of the innervation, with an increase in numbers of the presynaptic varicosities (terminals or boutons; reviewed in Deshpande and Rodal 2016). Basically, this regulation involves the exchange of various factors back and forth in a specialized enclosed area formed by the invaginating presynaptic terminal; it consists of a synaptic cleft plus adjacent perisynaptic areas connected to channels formed into the postsynaptic sarcoplasm by the SSR. First of course, there is the release of the neurotransmitter, glutamate, from the presynaptic vesicles that then activates the postsynaptic glutamate receptors. The presynaptic terminal also secretes the developmental regulating factor, Wg (Wingless; a Wnt; Korkut et al. 2009, 2013). Wg is secreted, along with its binding protein, a transmembrane protein called Evi (Everness interrupted/Wntless/Sprinter), in small vesicles called exosomes. Exosomes probably are released via fusion of multivesicular bodies with the presynaptic terminal membrane. Wg binds to its postsynaptic receptor, DFz2 (Drosophila Frizzled-2). Binding of Wg here may be aided by the heparin sulfate proteoglycan, perlecan/trol. All three proteins may associate in the SSR (Kamimura et al. 2013). Wg binding to the postsynaptic DFz2 induces “Frizzled nuclear import” in which the cleaved C-terminal of DFz2 is transported to the muscle nucleus, inducing postsynaptic differentiation of the NMJ. Evi also is produced by the muscle fiber and it targets the Wg-associated protein, dGRIP, to the SSR where the latter protein assists in the Frizzled nuclear import process (Korkut et al. 2009). Wg secreted in exosomes also binds to DFz2 on the presynaptic, perisynaptic membrane, where it may help regulate the proliferation of the presynaptic structures (Kamimura et al. 2013).
In addition, Wg can be secreted by glial processes to affect postsynaptic differentiation and to modulate spontaneous postsynaptic potentials (mEJPs; Kerr et al. 2014). Note that glial processes typically are not evident in electron micrographs of the deeply invaginating presynaptic terminals. However, light microscope studies show that glial processes can extend deeply into the invagination intermittently (Fuentes-Medel et al. 2009). This is not surprising, as we have noted above how electron microscopy reveals that glial processes can extend into invaginations in the NMJs of some other insects. Some factors such as Gbb and Syt4 are involved in retrograde signaling from the postsynaptic region to the presynaptic terminals. Syt4 (synaptotagmin 4) originates in the presynaptic terminal and is transported to the postsynaptic muscle fiber in exosomes like Wg and Evi. It then regulates a retrograde signal that mediates “...activity-dependent presynaptic growth and potentiation of quantal release” (Korkut et al. 2013). Finally, another retrograde signal, the TGF-β/BMP (bone morphogenetic protein) homolog called Gbb (glass bottom boat), is secreted by the muscle (after induction by a glia-derived factor) into the synaptic cleft and binds to its receptor on the presynaptic terminal where it may control synaptic growth (McCabe et al. 2003; Fuentes-Medel et al. 2012); Gbb also is secreted by the presynaptic terminals in dense-cored vesicles and helps to control synaptic transmission (James et al. 2014; Deshpande and Rodal 2016). One BMP presynaptic receptor, Wit (Wishful Thinking) appears to require a trans-synaptic complex of neurexin and neuroligin for its proper localization and stability, and mutants of the latter two proteins have distinctive ultrastructural defects in the synapse (Banerjee et al. 2017). There are numerous other components to NMJ regulation in Drosophila (reviewed in Deshpande and Rodal 2016; Van Vactor and Sigrist 2017); for example, APPL, the Drosophila homolog of p-amyloid precursor protein (APP) helps regulate synapse formation at NMJs (Torroja et al. 1999). In summary, the invaginating presynaptic terminal of the larval Drosophila NMJ may be designed specifically to facilitate the precise exchange of several factors that coordinate the rapid growth and function of these NMJs, and this is an essential component of rapid larval growth.
Vertebrate NMJs
Like arthropod muscles, vertebrate skeletal muscle is striated, with contractile units called sarcomeres (reviewed in Page 1968; Morgan and Proske 1984; Mescher 2016). In twitch (fast) fibers, which can propagate an action potential, the sarcomeres are invested with two systems of tubules that regularly contact each other in complexes of two sacs of SR with a transverse (T) tubule in between, forming a “triad.” The position of the triads in relation to the sarcomeres differs in different vertebrates (Smith 1966; Page 1968). The T-tubules conduct current from the surface membrane to the SR, which regulates the calcium concentration responsible for muscle contraction. In contrast to twitch muscle fibers, tonic (slow) fibers have few contacts between the SR and T-tubules and thus form few triads (Page 1968; Hess 1970; Morgan and Proske 1984). The sarcomeres also tend to be less well-organized in tonic fibers.
Typical NMJs of vertebrate skeletal muscles consist of a large presynaptic terminal that sits in a “gutter” or “primary fold,” basically an indention as we have described for invertebrates, on the muscle fiber (reviewed in Sanes and Lichtman 1999; Shi et al. 2012; Mescher 2016). The terminal is capped with a Schwann cell and this is covered with an extrasynaptic basal lamina that is continuous with that of the muscle fiber. Acetylcholine is released from the terminal synaptic vesicles at multiple active zones. Directly opposite these active zones, the postsynaptic membrane is extended into deep junctional (“secondary” or subjunctional) folds, about one micrometer long. Acetylcholine receptors are found on the unfolded part of the postsynaptic membrane and along the top sections (“crests”) of the subjunctional folds, while sodium channels are concentrated at the bottom of the subjunctional folds. A synaptic basal lamina is found in the synaptic cleft and extends into each of the subjunctional folds. The morphology of the gutter and the subjunctional folds varies among different vertebrates: In some cases, the gutter is a deep invagination; also, the subjunctional folds can be extremely extensive in some vertebrates.
Agnathan (Jawless) Fish
The agnathans include the hagfish and lampreys. They have two basic kinds of muscle fibers: slow fibers with relatively slow contractions and twitch fibers with propagation of action potentials and faster contractions (Morgan and Proske 1984). In the segmental blocks of body musculature, called myotomes, slow fibers are smaller, and twitch fibers are larger (Jansen et al. 1963; Morgan and Proske 1984); in the lamprey myotomes, “parietal” and “central” fibers are considered to be the slow and twitch fibers, respectively (Teräväinen 1971). In the hagfish, Myxine glutinosa, the NMJs on twitch fibers (also called “white” fibers) form an endplate (“en plaque”) at the end of the muscle fiber (Jansen et al. 1963). In contrast, innervation of slow fibers (“red” fibers) is from fine, varicose nerve branches running along the muscle fiber. The pattern may be similar in lampreys, but is not well understood; also, it appears that some of the central twitch fibers may not receive direct innervation and instead are electrically coupled to other central fibers (Teräväinen 1971). In the hagfish, typically both kinds of fibers have NMJs with deeply indenting presynaptic terminals; these rarely show any deep subjunctional folds, and at most, one or two are seen (Korneliussen 1973a, b). In the slow fiber NMJs, the terminal often has numerous dense-cored vesicles in addition to smaller, clear ones (the former believed to contain monoamines like serotonin). Most interestingly, some of the slow fiber NMJs have deeply invaginating presynaptic terminals in which the top half or more of the invagination is filled with a “plug-like” Schwann cell (Fig. 9a, b). In contrast, the micrograph of a lamprey slow fiber NMJ terminal, shown in Teräväinen (1971), does not appear to be indenting into the fiber; however, images of twitch fiber NMJs do show that the presynaptic terminal can be deeply indenting. Definitive subjunctional folds seem to be absent from both kind of muscle NMJs in lampreys. However, in both hagfish and lampreys, images of twitch fiber NMJs often show numerous, deep “tube-like invaginations” (Korneliussen 1973c) that are postsynaptic and close to the synapse; but these open on the muscle surface membrane to the side of the NMJ and are associated with the myotendinous junction (since these NMJs form on the end of the muscle fiber where it is joined to the tendon or myoseptum; Teräväinen 1971; Korneliussen 1973a, c). Note that the myoseptum, which is the connective tissue between myotomes, functions like a myotendinous junction between muscle and bone (Charvet et al. 2011). These myotendinous/myoseptal junctions are believed to be mechanical attachments, but they look curiously like NMJ subjunctional folds, so perhaps they have some effect on the current generated at the NMJ.
Fig. 9.
NMJs of fish. a, b NMJs of the hagfish, Myxine glutinosa, tend to have indenting/invaginating terminals with Schwann cells that largely fill the top of the invagination. These two examples are from figures of red fibers NMJs in Korneliussen 1973a. a Modified from figure 7 in the latter study (reprinted with permission from Springer); this is an NMJ on a parietal (myotomal) muscle fiber (mf). Note the deep indention that is filled largely by the Schwann cell (S; nucl = nucleus). The presynaptic terminal is at the deep end and so is invaginated completely within the indention; it is filled with small, clear vesicles, as well as some mitochondria and dense-cored vesicles (dv). b Drawing based on figure 9 in the latter study; this is an NMJ on a craniovelar (branchial) muscle fiber. Note how the terminal and Schwann cell (pink) are both enclosed in a deep invagination into the muscle fiber. c The NMJs of dogfish sharks vary; the example shows one of the complex white/twitch fiber NMJs with dual presynaptic terminals (a + b) deeply indenting/invaginating into the muscle fiber (myotomal). Note the extensive subjunctional folds on the left; also, note that some similar surface folds on the right, and extending directly from the muscle fiber surface, are probably myoseptal invaginations (modified from figure 9 in Bone 1972; reprinted with permission from the Company of Biologists Ltd.). Scale bar is 1 micrometer. d Innervation of a myotomal, white muscle fiber close to the myoseptum (upper left of image), from the conger eel, which has better developed subjunctional folds than similar NMJs of most other bony fish (modified from figure 6 in Best and Bone 1973; reprinted with permission from Springer). Arrows show dense-cored vesicles (Color figure online)
Sharks (Chondrichthyes)
In the myotomes of the axial muscles of sharks, red fibers can be distinguished from white fibers by the area taken up by myofibrils (<60 vs. >90%), mitochondria (>30 vs. <1%), and glycogen (a few percent vs. little or none) (there also are some intermediate fibers; Kryvi 1977). The myotomes of the dogfish shark, Scyliorhinus canicula, have a central core of fast, white, twitch fibers surrounded by some slow, red fibers (Bone 1972). The twitch fibers are innervated on an end of a fiber, while the slow fibers are innervated along their length. Slow fiber NMJ terminals are moderately indented, while some twitch fiber terminals appear to be more deeply invaginating into the fiber. This is especially true for twitch fiber NMJ images showing two terminals in the invagination (Fig. 9c); at least one of the terminals appears to be fully within the invagination; in those with two terminals, one of the two terminals has abundant dense-cored vesicles along with smaller, clear vesicles; in these cases, the terminals appear in the images to be separated from each other in the invagination by slender glial processes. Slow fiber terminals show only a few subjunctional folds, while some of the twitch fiber terminals have very extensive, and often branching, subjunctional folds. Since the latter are on the end of the fiber, images of the twitch NMJs also show adjacent invaginations from the myoseptum.
Bony Fish (Osteichthyes)
The muscles of bony fish can include slow and twitch fibers, as well as some kinds with intermediate properties (Morgan and Proske 1984). However, there generally seems to be little variation in NMJ structure. Thus, the NMJs of red (slow) and white (twitch) muscle fibers of the pectoral fin muscles of the snake fish, Ophiocephalus argus (now Channa argus), show few differences; both have relatively shallow indentations and lack definitive subjunctional folds (Nakajima 1969). Similarly, the NMJs of the red muscle fibers (in this case, these are twitch fibers) of the dorsal fins of the seahorse, Hippocampus hudsonius, also have shallow indentations and lack subjunctional folds (Bergman 1967). This also is true for NMJs of extraocular eye muscles (Reger 1961; Marotte and Mark 1970; Kordylewski 1974). Interestingly, both Reger (1961) and Kordylewski (1974) describe some prominent SR structures in the subjunctional sarcoplasm. Best and Bone (1973) describe a wider variety of NMJ structures at the ends of muscle fibers of the segmental myotomes of various fish (note: this is where the NMJs are often associated with myoseptal invaginations). In some fish in this study, the presynaptic terminals are moderately indenting into the fiber and lack subjunctional folds. But the NMJs of the catfish, Ameiurus nebulosus, have a few small subjunctional folds and the presynaptic terminal sometimes is “...almost entirely embedded in depressions of the sarcolemma surface…” Finally, the authors note that the NMJs of the conger eel, Conger conger, can have large, well-developed subjunctional folds (Fig. 9d).
Amphibians (Frogs, Salamanders, Newts)
The frog twitch muscle fiber NMJs (Fig. 10a) extend as several long branches parallel to the long axis of the fiber (Birks et al. 1960; Slater 2008), while the slow muscle fiber NMJs are formed from multiple groups of small terminals (Page 1965; Hess 1970). The presynaptic terminals of the frog NMJ tend to form only shallow indentions into the surface of the muscle fiber; this is similar in twitch (fast; Birks et al. 1960; Hess 1967) and slow (Page 1965; Hess 1967) muscle fiber NMJs. In the twitch fiber NMJs, the covering Schwann cell often extends processes (“Schwann fingers”) between the pre- and postsynaptic membranes of the NMJs (Birks et al. 1960; Matthews-Bellinger and Salpeter 1978); these glial processes may “…separate one presynaptic density from the next and define a series of ‘synaptic units.”’ (Heuser and Reese 1973). Subjunctional folds are well developed at twitch fiber NMJs (Birks et al. 1960; Hess 1967), but they are rare or absent at slow fiber NMJs (Page 1965; Hess 1967). Studies on the NMJs of myotomes in the segmental trunk musculature of larval frogs and salamanders (swimming stage) show only shallow indentions and at most, a few, short subjunctional folds (Best and Bone 1973; Kullberg et al. 1977).
Fig. 10.
NMJs in frogs and toads. Examples of NMJs with indenting terminals from the skeletal muscle of the frog (a1, a2) and from the smooth muscle of the intestines of the toad, Bufo marinus (b). a1 This is a simple cross section through the very long endplate on a twitch fiber, showing the shallow/moderate indention of the terminal and close enwrapping of the Schwann cell (Birks et al. 1960; Heuser and Reese 1973; Matthews-Bellinger and Salpeter 1978). a2 This is an enlarged portion of a longitudinal section through the endplate (cutaneous pectoris muscle of the frog, Rana pipiens; Matthews- Bellinger and Salpeter 1978). Note how the Schwann fingers (s) define a synaptic unit with an active zone (red arrow) in between; these units run along the length of the endplate. f, Subjunctional folds. b Note how this terminal is entirely below the rim of the indention that also contains a Schwann cell process (s; Rogers and Burnstock 1966). The postsynaptic membrane is lined with flask-like caveolar structures, and the terminal vesicles include a few small clear ones and some larger granular ones (Color figure online)
Reptiles and Birds
In the lizard, Anolis sp. (Robertson 1956; Walrond and Reese 1985) and garter snake, Thamnophis sirtalis (Hess 1965), the twitch fibers have a large end plate terminal structure (“en plaque”), while the slow or tonic fibers have multiple terminals in groups (“en grappe”) along the muscle fiber. In both animals, the presynaptic terminals of the NMJs are indenting more in the twitch NMJs compared to the slow NMJs, and some of these in the lizard are deep enough to be invaginations; Robertson (1956) notes how the “troughs…partially envelope the terminal…” In both animals, the twitch fiber NMJs have well-developed subjunctional folds, while the slow fiber NMJs have at most, a few short subjunctional folds.
In the chicken, the pectoralis and posterior part of the latissimus dorsi muscle are made up mostly of twitch fibers while the anterior part of the latissimus dorsi is made up mostly of slow fibers (Hess 1967; Atsumi 1977; Kwong and Gauthier 1987). As in reptiles, the twitch fibers have a single en plaque endplate, while the slow fibers have multiple en grappe endplates. Both twitch and slow NMJs have at most a shallow indention for the presynaptic terminal; twitch fiber NMJs have a few to a moderate number of subjunctional folds, while slow fiber NMJs usually have few to none (but occasionally a moderate number). Similar ultrastructure is seen in the NMJs of the Japanese quail, Coturnix japonica (Rosser et al. 1987).
Mammals
Most mammalian skeletal muscles are made up of twitch fibers (Padykula and Gauthier 1970; Ellisman et al. 1976; Pette and Staron 1997; Schiaffino and Reggiani 2011). Slow or tonic fibers are found in only a few special muscles including extraocular and some ear muscles (Teräväinen 1968a, b; Fernand and Hess 1969; Pette and Staron 1997; Schiaffino and Reggiani 2011). Broadly speaking, mammalian twitch fibers are classified further as fast twitch and slow twitch, but fiber classification is actually very complicated and beyond the scope of this review (see reviews of Pette and Staron 1997; Schiaffino and Reggiani 2011). For example, the extensor digitorum longum (EDL) muscle of the rat contains mostly or all fast twitch fibers, while the rat soleus muscle is made of mostly slow twitch fibers (Ellisman et al. 1976). In this study, fast twitch fibers tend to have larger NMJ endplates than slow twitch fibers; and the former tend to have more regular, larger, and more closely packed subjunctional folds (Fig. 11a, b). The fast twitch NMJ has a series of “bulblike terminal expansions” separated by outgrowths of sarcoplasm, so that many of these terminal expansions are invaginations into the fiber. Another example of invaginating presynaptic terminals is in the NMJs of the fibularis longus muscle of 3-month-old mice; Boaro et al. (1998) note that the presynaptic terminals are in “deep synaptic gutters”; in one figure, the terminal is surrounded up to it neck in well-developed subjunctional folds.
True slow (slow/tonic; not slow twitch) fibers are found in a few specialized muscles. Fernand and Hess (1969) describe two types of fibers in the ear muscles of the cat, including the tensor tympani and stapedius. They define these as twitch and slow, as we have described in other classes of vertebrates. The twitch fibers have a large endplate and slow fibers have a chain of small terminals along the fiber. However, more recent studies indicate that there may be few or no true slow fibers in the stapedius of various animals (Veggetti et al. 1982; van den Berge and Wirtz 1989; Jung et al. 2004; Schiaffino and Reggiani 2011). In any case, both kinds of fibers in these two muscles have partially indenting terminals, with well-developed subjunctional folds at the twitch fiber NMJs (Fernand and Hess 1969; van den Berge and Wirtz 1989) and a few small or no subjunctional folds at the slow fiber NMJs (Fernand and Hess 1969). The extraocular muscles of the rat also have twitch and slow fibers with large endplates or multiple endings, respectively (Teräväinen 1968a, b) (Fig. 11c, d); recent studies confirm that extraocular muscles contain both twitch and slow fibers (reviewed in Pette and Staron 1997; Schiaffino and Reggiani 2011). Subjunctional folds are better developed in the twitch fiber NMJs and are irregular and less well developed in the slow fibers. The slow fiber NMJs can include occasional invaginating terminals; for one figure, the author notes that the “... sarcoplasm protrudes towards the terminal and covers it almost totally by thin projections.” (Teräväinen 1968a) (Fig. 11d). Also, the twitch fiber NMJs can have a well-developed SR just below the subjunctional folds (Teräväinen 1968a, b).
Other Kinds of Vertebrate NMJs
Muscle spindles are proprioceptive receptor organs sensitive to muscle stretch, to monitor skeletal muscle length. They contain several kinds of “intrafusal” muscle fibers; their functional types vary and in mammals at least seem to be some combination of twitch and slow/tonic fibers (reviewed in Pette and Staron 1997; Schiaffino and Reggiani 2011). The terminals of NMJs of those in frogs (Katz 1961), snakes (Ichiki et al. 1976), and chickens (Ovalle et al. 1999) tend to be only slightly indenting with few, short subjunctional folds, but a few in the snake are more deeply indenting. Similarly, in mammals, NMJ terminals vary from little or no indention or subjunctional folds to those more deeply indenting and with a moderate number of subjunctional folds (Arbuthnott et al. 1982; Kucera 1984; Kucera and Walro 1986). In many of these, the top of the terminal lies flush with the surface of the muscle fiber.
Like skeletal muscle fibers, cardiac (heart) muscle fibers are striated; in mammals, the sarcomeres are associated with large T-tubules that contact single tubules of SR to form dyads instead of triads (Page 1968; Mescher 2016). In contrast, the T-tubule system appears to be absent from the cardiac muscle fibers of fish, amphibians, reptiles, and birds (Page 1968; Sommer and Johnson 1969; Lemanski et al. 1975). Also, T-tubules often are lacking from mammalian Purkinje fibers, which are specialized cardiac muscle fibers involved in impulse conduction through the heart (Sommer and Johnson 1968; Jensen et al. 1978; but see Hong and Shaw 2017). The heart is innervated by the autonomic nervous system. In some cases, motor nerve terminals form close contacts with cardiac muscle fibers, while they may lack direct contact in other cases. In the frog heart, Woods (Rana temporaria; 1970) reports that motor terminals can come into close contact with the muscle fibers but there is no specialization; Thaemert (R. pipiens; 1966) illustrates terminals in low to moderately deep indentions of the muscle fibers, but there also is little or no specialization in the subsynaptic sarcoplasm except for occasional vesicles. Jensen et al. (1978) report that motor terminals associated with Purkinje fibers in one area (moderator band) of the heart of the ox (Bos taurus) and goat (Capra aegagrus) typically remain about 0.3–0.8 micrometers from the fiber surface. In contrast, Hirano and Ogawa (1967) examine the innervation of presumed Purkinje fibers from another area (interventricular septum) of the guinea pig heart and find some deeply invaginating presynaptic terminals; although not described in detail, images appear to show some irregular SR structures and possible membrane infoldings in the surrounding sarcoplasm. Deeply invaginating presynaptic terminals also have been found at the innervations of specialized cardiac muscle fibers of the pacemaker region (trout—Yamauchi and Burnstock 1968; rabbit—Trautwein and Uchizono 1963) and the conductive tissue in the atrioventricular node (rat—Mochet et al. 1975). In some cases, the adjacent sarcoplasm has some SR and vesicles including some vesicles opening onto the sarcolemma, described as “in- pocketings” (Yamauchi and Burnstock 1968) or “pinocytic vesicles” Mochet et al. 1975). Finally, in the heart atrium of fish, Saetersdal et al. (1974) describes presynaptic terminals that are partly invaginating into muscle fibers due to extensions of “sarcoplasmic protrusions” of the muscle fiber, as well as invaginating into endocardial cells that line the inside of the atrium. The subsynaptic sarcoplasm is vesiculated, including apparent inpocketings; the figure of an invagination into an endocardial cell has a wide separation between the endocardial cell surface and the terminal, which is surrounded by a Schwann cell process.
Vertebrate smooth muscles are the involuntary, nonstriated muscles found in various internal organs, innervated by autonomic nerve fibers; they lack a T-tubule system and the SR is poorly developed (Smith 1966; Mescher 2016). In many cases, such as in the iris of the rabbit (Richardson 1964), the coral fish stomach (Tan and Wong 1980), and the intestines of mice and rats (Lane and Rhodin 1964; Taxi 1964; Wong 1977), NMJs are unspecialized and sometimes poorly defined, and there are no indentations or subjunctional folds. In contrast, presynaptic terminals of NMJs in the vas deferens from mice (Lane and Rhodin 1964) and rats (Taxi 1964) (Fig. 11e) can lie in deep indentations and sometimes appear to be almost completely invaginated; Taxi (1964) notes that the nerve terminals sometimes may occupy “...profondes galleries intracellulaires...á l’intérieur des cellules musculaires lisses...” (deep intracellular galleries inside the smooth muscle cells). Taxi (1964) also notes that SR seems to be well developed in the subjunctional area. In addition, there are moderately deep indentions at NMJs of smooth muscle of the intestine of the frog, Rana pipiens (Thaemert 1966), and toad, Bufo marinus (Rogers and Burnstock 1966) (Fig. 10b). These NMJs are particularly interesting because they have subjunctional “caveolae intracellulares.” These structures are flask-shaped structures often over 100 nm long and apparently opening onto the postsynaptic membrane. In some cases, there appears to be a partial second layer of these flasks or vesicles, and they appear to be budding off of the ones in the first layer (Thaemert 1966).
Motor Endings on Other Kinds of Cells
Motor nerve endings also innervate non-muscle cells, especially some types of exocrine and endocrine gland cells; they are secretomotor nerve endings, affecting gland cell secretion (Fig. 12). As for smooth muscles, the terminals are derived from the autonomic nervous system in most cases. These synaptic contacts of gland cells often have well-developed synaptic densities and distinctive presynaptic vesicles (Coupland 1965; Bargmann et al. 1967; Ju and Zhang 1992). In the synapses on acinar cells of the rat parotid gland, there is a distinctive subsurface cistern ~10–15 nm from the cell membrane at its junction with the indenting nerve terminal; it has ribosomes on the side opposite to the junction and is continuous with the adjacent, regular RER in the cell (Hand 1970). At least some of these terminals appear to be covered over almost completely by processes extending from the cell and so can be considered invaginating terminals. Indenting terminals also are found on acinar cells of the chicken pancreas (Dahl 1973), and fully invaginated terminals can occur in cells of the intercalated duct of the bat pancreas (Watari 1968). Indenting terminals also are common at the motor nerve endings on brown fat cells of mammals (Bargmann et al. 1968).
Fig. 12.
Motor nerve endings in endocrine (a-c) and exocrine (d, e) glands. a An axon fascicle is invaginated in a rat adrenal cortical (glomerulosa) cell (g). The lower right process in the fascicle is a synaptic terminal with small, clear vesicles, dense-cored vesicles (arrows) and mitochondria (asterisks). Modified from figure 5B of Kleitman and Holzwarth (1985; reprinted with permission from Springer). b Invaginating terminal (arrow) in an epithelial cell in the pars intermedia of the cat pituitary. Modified from figure 5 in Bargmann et al. (1967; reprinted with permission from Springer). c Drawing of an invaginating terminal in a corticotrope cell of the pars distalis of the dog anterior pituitary. Arrow indicates the thickened postsynaptic density (Ju and Zhang 1990). d Drawing of an axon synapsing on an intercalary cell of the conducting duct of the salivary/silk gland of the mite, Bakericheyla chanayi (Filimonova and Amosova 2015). Note the five active zones (small arrows) formed in a row on the cell surface, followed by an invaginating terminal. e Drawing of an invaginating double terminal in a cell of the labral gland of the water flea, Daphnia obtusa (Zeni and Zaffagni 1988). Arrow, active zone
Some deeply invaginating terminals can innervate adrenal and pancreatic endocrine cells. The indentions of terminals on adrenal cortical cells sometimes can be deep (sheep—Robinson et al. 1977; rat—Kleitman and Holzwarth 1985); in one figure in Kleitman and Holzwarth (1985), a small nerve fascicle is invaginated completely within a glomerulosa cell and one of the processes appears to be forming a synapse (containing presynaptic small clear vesicles and some large dense-cored ones) (Fig. 12a). In the rat adrenal medulla, terminals contain small clear and large dense-cored vesicles and show thickenings of the pre- and postsynaptic membrane; their contact structure with the chromaffin cell varies from flat on the surface (“plane bouton”), indenting, or as a finger-like process that fully invaginates into the cell (Coupland 1965; also a figure in Woods 1970). Presynaptic terminals innervating cells in the Islets of Langerhans in the cat pancreas are indenting or deeply invaginating and include types with various combinations of small, clear vesicles and large or small densecored vesicles (Legg 1967; Esterhuizen et al. 1968); and this innervation includes both cholinergic and adrenergic types (Esterhuizen et al. 1968). No distinct synaptic specializations or subsynaptic structures are evident in the published micrographs in these studies, except for occasional tubulovesicular structures. In the Islets of the toad (Bufo vulgaris formosus), Kobayashi (1966) shows a terminal partly indenting between three Islet cells, but again there are no obvious subsynaptic specializations. Indenting terminals on Islet cells also are described in the dog (Watari 1968; Kobayashi and Fujita 1969) and chicken (Watanabe and Yasuda 1977).
Complex endocrine structures with indenting/invaginating terminals include the pituitary and lung neuroepithelial bodies. The pituitary gland receives some innervation from the autonomic nervous system (acetylcholine, noradrenaline), as well as from several other sources of innervation that may utilize substance P (substance P-like immunoreactive; SP-LI), GABA, serotonin, calcitonin gene-related peptide, and/or galanin (Ju and Zhang 1990, 1992). Ju and Zhang (1990) found SP-LI reactive terminals on all the gland cell types in the pars distalis of the dog anterior pituitary; typically, they have small clear vesicles and some large dense-cored vesicles, and most have asymmetric densities. While some form synapses flat on the surface and others are in indentions, some invaginate completely into the cell (Fig. 12c). Similarly, presynaptic terminals can invaginate into the gland cells in the pars tuberalis of the rat and mouse anterior pituitary (Unsicker 1971), and in the cat pars intermedia (Bargmann et al. 1967; Baumgarten et al. 1972) (Fig. 12b). Finally, innervation of neuroepithelial bodies, which are sensory structures found in the airways of lungs and sense low oxygen, high carbon dioxide, and stretching, includes both afferent and efferent terminals (Adriaensen et al. 2003; Cutz et al. 2013). Both kinds of terminals can extend between the neuroendocrine cells and sometimes derive from a common nerve fiber, as noted in various animals (salamander—Goniakowska-Witalinska et al. 1992; rabbit—Lauweryns and van Lommel 1987). Both kinds typically indent partially into the surface of the cell, and often they form between two columnar neuroepithelial body cells and so one varicosity will indent both cells.
There also are some examples of invaginating motor endings in invertebrates, particularly for salivary glands. Terminals can invaginate completely in the salivary gland cells of the snail, Helix pomatia; examples show either a mixture of small, clear vesicles and large, granular (dense or dense cored) vesicles, or mostly large granular vesicles, and the RER of the gland cell is apposed closely to the postsynaptic membrane (Elekes and Ude 1994). Interestingly, this study looked at distribution of the neuropeptide, FMRFamide, and found FMRFamide-like labeling in indenting/invaginating terminals on both salivary gland cells and at NMJs in other organs, as we described in an earlier section. In the salivary gland tubules of Octopus vulgaris, axons, either in groups or singular, and often without an accompanying satellite cell, are invaginated deep within the basal infoldings of the epithelial cells; many of these invaginated axons profiles contain numerous vesicles, and they also can have active zones with thickened PSDs (Ducros 1972). In the unusual salivary/silk glands of a mite, Bakericheyla chanayi, an axon can make an invaginating synapse in an intercalary cell (a kind of cell found at the base of the conducting duct); Filimonova and Amosova 2015). A micrograph shows an axon with five synaptic active zones in a row on the cell surface, followed by a sixth active zone inside a deep invagination into the cell, which has vesiculated structures and RER around the invaginating terminal (Fig. 12d). It is curious that this innervation includes so many active zones in a row and that the last one is invaginating; it perhaps reflects the need for precise control at the duct of a complicated gland complex that may have multiple functions. The labral glands of the water flea, Daphnia obtusa (Crustacea), may be a type of salivary gland, but their function is unclear; the gland cells can be invaginated by double axon terminals that form distinctive active zones inside the cell (Zeni and Zaffagnini 1988) (Fig. 12e). Both terminals contain a similar mixture of clear and granular vesicles, and in the cell cytoplasm, the terminal is surrounded by vesiculate structures including RER.
Most studies that we have found on invertebrate endocrine cell innervation do not show invaginating or distinctively indenting terminals. The dorsal body gland of the snail, Megalobulimus abbreviatus, adjacent to the brain, is an endocrine gland that secretes a female reproductive hormone (Moraes et al. 2010). Axon terminals often appear to be deeply indenting to fully invaginating in the gland cells; in the latter case, the cell extends processes that surround the terminal. The terminals contain dense or dense-cored vesicles of various sizes, but there does not appear to be distinctive synaptic specializations or special postsynaptic structures. In larvae of the greater wax moth, Galleria mellonella, neurosecretory fiber terminals, filled mostly with neurosecretory granules (120–150-nm granular vesicles), can form distinct indentions on cells of the prothoracic glands (secretes molting hormone; Benedeczky et al. 1980).
Is There Any Special Advantage to an Invaginating Presynaptic Terminal?
There are perhaps three possible advantages to invaginat- ing presynaptic terminals: mechanical, biochemical, or electrical. Mechanical is the simplest explanation, and in particular, this could explain the common occurrence of indenting or invaginating motor terminals in muscle cells; of course, the muscle cell is contracting and relaxing, often rapidly, and a nerve contact might need to be anchored to the muscle fiber. But indenting or invaginating terminals are about equally common in motor endings of exocrine and endocrine cells that presumably would not be subjected to the level of mechanical stress of many muscle fibers. Nor would this explain most of the numerous other examples of invaginating terminals that we have described for other cell types. On the other hand, some unique aspects of the vertebrate motor terminals, such as the basal lamina that binds between the pre- and postsynaptic membranes and extends deep into the subjunctional folds, may provide a mechanical advantage, especially in those vertebrates in which the subjunctional folds are well developed. Thus, “... sites of synaptic contact are tightly adhesive...” (Marques et al. 2000) and “…maturation of the basal lamina binds the pre- and postsynaptic cells together strongly enough to withstand up to a century of constant use.” (Sanes and Lichtman 1999). Furthermore, Marques et al. (2000) suggest that the deepening of the indention (gutter) of the mammalian NMJ that occurs during development may be due to a combination of 1) the required maintenance of nerve-muscle adhesion during muscle growth and 2) a more rapid growth of the muscle surface compared to the nerve terminal; thus, the nerve terminal becomes embedded in the muscle fiber.
It is likely that the invagination improves the conduc- tion/transmission of biochemical and/or electrical signals between the pre- and postsynaptic processes. We have discussed this previously in detail for invaginating postsynaptic spines (Petralia et al. 2016), and many of these ideas may apply to invaginating presynaptic terminals. Broadly speaking, the invagination provides an enclosed, secluded environment to contain and isolate certain biochemical signals and processes and to exclude other ones that can affect the signaling system. For example, large terminals such as the hippocampal mossy terminals are invaginated by large spine processes called excrescences and within the invagination are numerous active zones (Petralia et al. 2016). This creates a continuous synaptic cleft region that is isolated from glial processes, allowing for extensive spillover of glutamate that can be channeled to reach various pre- and postsynaptic receptors within this space; this can shape the specialized response of these synapses. Other substances can be exchanged readily in this continuous cleft; for example, the synapses also release zinc that affects synaptic plasticity, and the enclosed area between the invaginated pre- and postsynaptic processes channels the zinc and slows its loss from the area.
It seems likely that the indenting/invaginating terminal serves to sequester and isolate chemicals/substances associated with signaling for electrical current exchange and plasticity between the pre- and postsynaptic processes as we have described previously for invaginating spine synapses. As for the latter, the presynaptic invaginating terminals can show a continuous cleft area between the pre- and postsynaptic membranes within the cleft, without glia or other invading processes. But there are exceptions; there may be small profiles (presumably glial) wedged in this area that often are not described by the authors. Glial processes typically cover the top of the invagination and this is best known for the vertebrate NMJ, in which a Schwann cell envelopes the terminal. The most dramatic modification of this arrangement is found in the hagfish NMJ, in which more than the top half of the invagination is filled with and plugged by the Schwann cell (Korneliussen 1973a, b). In other cases, glial processes can lie close to the active zones. In the shark, NMJ invaginations with two terminals appear to have glial processes between the terminals (Bone 1972), and in the frog NMJ, Schwann cell “fingers” run between the active zones in the indention, to organize the NMJ into a series of synaptic units (Birks et al. 1960; Heuser and Reese 1973). Some arrangements of glia in indenting/invaginating terminals in arthropod NMJs are like those of vertebrates. Thus, while glial processes associated with indenting or invaginating NMJ terminals sometimes seem to be excluded from the invagination, in other cases, they are associated intimately with the synaptic active zones in the invagination. This is evident, for example, in NMJs in the horseshoe crab (Fourtner and Sherman 1973) and various crustaceans (Sherman and Atwood 1972; Jahromi and Atwood 1974; Pearce et al. 1985). Glial processes can extend partly along the sides of the indentions in NMJs of wasps (Edwards et al. 1958a) and sphinx moths (Rheuben and Reese 1978). Also, a similar arrangement occurs in deeply invaginating terminals of NMJs in eyestalk muscles of the sentinel crab (Hoyle and McNeill 1968). But it is likely that such glial process extensions occur commonly inside the indentions; thus, such processes seem to be absent in electron micrographs of the invaginating NMJ of the Drosophila larvae, but light microscope studies show intermittent extension of glial processes deep within the invaginations (Fuentes- Medel et al. 2009).
Is There an Advantage of Invaginating Terminals Versus Invaginating Spines?
But even if invagination of the postsynaptic spine has biochemical/electrical advantages, why would the opposite arrangement occur, i.e., invagination of the presynaptic process into a postsynaptic cell? Perhaps there is just a simple, mechanical explanation: The smaller process is the one that invaginates into the larger one. This explains at least some of the common examples of terminals that form invaginating synapses on cell bodies such as muscle fibers and gland cells, as well as unusual examples such as the indenting/invaginating contact of large terminals of the vestibular nerve on neuron somas in the larval lamprey (Stefanelli and Caravita 1970) and the rat (Sotelo and Palay 1968, 1970). Note, however, that in some cases, somal spines can invaginate into presynaptic terminals, as we have noted here and in Petralia et al. (2016), so this is not an absolute rule. The large basal invaginations containing multiple afferent and efferent processes, found in vertebrate retinal photoreceptor cells (rods and cones), as well as in some hair cells (in a cnidarian nematocyte—Holt- mann and Thurm 2001a, b, and a urochordate coronal organ—Burighel et al. 2003), represent a special situation. In this case, it appears that most of the processes in the invagination are postsynaptic, but there are some presynaptic processes as well, perhaps to allow feedback control as we discuss for the retinal processes. These last three examples fit well in our suggested simple explanation, i.e., the large presynaptic process (hair cell base or large cone pedicle/rod spherule) with several small, invaginating, mainly postsynaptic processes. In contrast, the size relationship is somewhat different in the electroreceptor synapse, which has both a large presynaptic sensory cell base and a large postsynaptic process; in this case, the major invaginating process is the presynaptic terminal (J0rgensen 2005). In addition, the sides of the postsynaptic processes that surround the presynaptic invagination often appear to invaginate in the opposite direction; and since they may contain vesicles, they could be an example of invaginating efferents. So maybe in this case, equal-sized processes foster reciprocal invaginations. We have described in an earlier section a similar arrangement in the developing auditory endbulbs of Held. But perhaps this simple explanation is just circular reasoning, and there probably are other causal factors underlying a preference for presynaptic versus postsynaptic invaginations in particular circumstances. Certainly, in the case of the cone and rod synapses (as well as some similar synaptic arrangements in invertebrates), the invagination serves as an enclosure to integrate signals between the presynaptic cone/rod cell and several pre- and postsynaptic processes in the invagination, most notably for the mechanism of inhibition of the photoreceptor terminal; such a function may be difficult to accomplish in another way. This also is true for NMJs; while some simple animals, mostly relatively slow moving, evolved cell processes that extend out to form an NMJ with sometimes distant nervous tissue elements, as we have described in an earlier section, most animals have NMJs directly on the muscle fiber, and these commonly are indenting or invaginating into the muscle fiber.
Advantages of NMJ Invaginations, Subjunctional Folds, and SSR
Typically, the vertebrate NMJ is a highly organized structure that appears to be designed to maximize the efficiency of nervous control of muscle contraction. The subjunctional folds of twitch fiber NMJs tend to be aligned perpendicular to the long axis of the muscle fiber; this is seen definitively in the elongate NMJs of frogs (Birks et al. 1960; Matthews-Bellinger and Salpeter 1978; Martin 1994). In mice, this perpendicular orientation of the subjunctional folds can be found for NMJ terminal branches that are lined up either parallel or perpendicular to the long axis of the muscle fiber (Marques et al. 2000). Comparison of twitch fibers in frog, rat/mouse, and human reveals an inverse relationship between the complexity of the subjunctional folds and NMJ area, with frogs having the largest NMJ area relative to fiber size (i.e., amount of fiber surface covered) and the least area of subjunctional folds (surface area of NMJ increased 2×); humans are the opposite with the smallest NMJ area and the most area of subjunctional folds (surface area increased 8×), with rats/mice in between (Slater 2008). This design then, which seems to be perfected in humans, allows the acetylcholine to bind to the receptors on the crests of the subjunctional folds, to generate currents that can spread to and activate the sodium channels at the bottom of the folds, while the electrical resistance of the folded membrane reduces the spread of the current across the folded membrane (Mat- thews-Bellinger and Salpeter 1978; Martin 1994; Slater 2008). The influence of the acetylcholine coming from a single release site is controlled tightly by several factors, including the amount (quanta) and probability of release of acetylcholine, the distribution of the receptors, and the ratio of receptors to acetylcholinesterase (about 10:1 around the crests of the folds). The result is that a human NMJ is very efficient, requiring only a small amount of acetylcholine release, since the effect is amplified strongly by the extensive subjunctional folds.
While there seems to be a definitive role for an increase in subjunctional folds for efficient muscle function, is there a role for the indention (gutter/primary fold)/invagination itself? It seems likely that a deeper invagination is related directly to the complicated subjunctional folds. Thus, twitch muscle NMJs have only a relatively shallow indention in the frog, with its relatively modest subjunctional folds, while the presynaptic terminals in many mammals can be deeply indenting or invaginating into the muscle cell, with the opening of the subjunctional folds wrapping around three sides of the terminal (Boaro et al. 1998; Slater 2008). Thus, the depth of the invagination may depend on the extent of subjunctional fold formation; this may hold also for the NMJs of twitch fibers in sharks, which also can have deep indentions or invaginations and extensive subjunctional folds (Bone 1972). But the jawless fish NMJs seem to be an exception to this rule, since they can have deeply indenting/invaginating terminals but with few or no subjunctional folds. However, an invagination can increase the area of synaptic surface, so maybe this has some equivalence to the frog NMJ with its shallow indention and moderately developed subjunctional folds. This also could explain the presence of invaginating terminals in various invertebrates that we have discussed in an earlier section, such as a chaetognath, snail, sea urchin, and many kinds of arthropods including Drosophila. We discussed in an earlier section how the invaginating terminal in Drosophila NMJs forms an enclosed environment for numerous signaling pathways between the terminal and muscle fiber, and that these involve clear and dense-cored vesicles, as well as exosomes released into this cleft.
In some invertebrates, the NMJ indention/invagination is associated with some kind of elaborate SSR, including in chaetognaths, crustaceans, and insects; this has been studied best in the fully invaginating terminals of Drosophila larval NMJs. But the function of this SSR is different from that of the vertebrate subjunctional folds, because the SSRs actually are internal membranes of the sarcoplasm, even though they can open at the postsynaptic membrane (Prokop 2006). Also, unlike the vertebrate twitch muscle, which has a high concentration of voltage-gated sodium channels at the base of the subjunctional folds, and can propagate an action potential through the muscle fiber, muscles of Drosophila larvae have no voltage-gated sodium channels, and they function via graded contractions (Nguyen and Stewart 2016). They do have voltage-gated calcium channels, but their role in Drosophila muscle contraction is not clear. Nguyen and Stewart (2016) suggest that: “...it seems less likely that the Drosophila SSR acts as an amplifier, but rather since these nerve terminals are high-output terminals, the SSR may act as a filter to dampen the synaptic response.” Thus, the SSR may provide a longitudinal resistance that can modulate synaptic effectiveness.
Invaginations for Ephaptic Conduction
While the function of invaginating presynaptic terminals may be to improve chemical neurotransmission or associated exchanges of signaling substances, it also is possible that the invaginations mainly mediate ephaptic conduction (electrical conduction between close cell membranes). This has been studied best for the vertebrate retinal horizontal cell processes that are part of the complex of mainly postsynaptic processes in the base of cone photoreceptor cell (Kamermans and Fahrenfort 2004; Gardner et al. 2015; Kramer and Davenport 2015). Yet Gardner et al. (2015) note that their model of the ephaptic effect in these processes “... can be applied to any neuronal circuit where dendritic spines are invaginating in presynaptic terminals or boutons.” As we described in an earlier section, lateral inhibition via horizontal processes may utilize GABA, protons, or ephaptic transmission, but the latter seems to be the best supported mechanism, with the others being subsidiary mechanisms (and perhaps depending on species). Ephaptic conduction might explain any case where the surface membranes of processes from two cells come into close contact without an obvious active zone site containing presynaptic vesicles. We have described several possible cases in invertebrates. These include the invaginating knob-like structures in the sponge, Tethya lyncurium (Pavans de Ceccatty 1966), the enwrapping of outer nerve ring axons by processes of sphincter muscles of the jellyfish, Polyorchis penicillatus (Spencer 1979), and the tunnel fibers of octopi and finger twigs of octopi and squid, both invaginating into the photoreceptor cell terminals. But in addition, perhaps an ephaptic effect plays a role in communication for many kinds of invaginating pre- and postsynaptic processes, as suggested by Gardner et al. (2015). This could be especially true for the various examples (some of which we have noted earlier) where the active zones are not very distinctive.
The Invaginating Presynaptic Terminal of NMJs in Development/Aging and Disease
There is little or no information about development/aging and disease for many of these invaginating presynaptic terminals. We already discussed in an earlier section the occurrence of indenting/invaginating terminals in the development of the vertebrate auditory and vestibular system. And previously we have discussed broadly, aging retinal synapses and NMJs (Petralia et al. 2014). Also, previously we have discussed diseases of the invaginating synapses of vertebrate cones and rods in detail (Petralia et al. 2016). Since we concentrate on retinal invaginating horizontal cell processes in this review, it might be of use here to consider horizontal cell processes in aging and disease. Ectopic sprouting of horizontal cell processes is common in aging animals and in retinal diseases. For example, horizontal cell processes extend more peripherally, into the outer nuclear layer (ONL), in aging humans (Eliasieh et al. 2007) and mice (Terzibasi et al. 2009; Samuel et al. 2011; but not Liets et al. 2006). These processes also extend into the ONL in several diseases such as retinitis pigmentosa (Fariss et al. 2000) and the vitreo- retinopathy associated with retinal detachment (Sethi et al. 2005). In most of these studies, ectopic bipolar neuron processes also extend into the ONL and may form synapses together with the horizontal processes (Eliasieh et al. 2007), but some studies found evidence of ectopic invaginated synapses in the ONL with only postsynaptic bipolar neuron processes (aging, Terzibasi et al. 2009; macular degeneration, Sullivan et al. 2007). In either case of ectopic synapses, with or without horizontal cell processes, it seems likely that the normal feedback mechanisms mediated by horizontal cells would be altered in aging and diseased retinas. This has been indicated at least for some retinal diseases (Arden and Hogg 1985; Arden et al. 1988); however, the horizontal cell feedback examined in these latter studies is between rods and cones, rather than direct feedback within a single invagination. Functional changes also likely take place in the connections between horizontal and bipolar neuron processes (Varela et al. 2003).
Several studies have examined the development, aging, and disease of NMJs, especially on changes in the indention/invagination and associated subjunctional folds; these have been studies best in mice/rats, Drosophila, and lobsters. In mice and rats, the NMJ is a simple, flat contact in the late embryo, has formed an indention and subjunctional folds by the first postnatal week, and these deepen in the second postnatal week, finally forming the deep indention and elaborate subjunctional folds by the end of the second or third postnatal week (Teräväinen 1968b; Sanes and Lichtman 1999; Marques et al. 2000). During chicken development, the indentions typically do not get very deep and have, at most, a moderate development of subjunctional folds, which start to appear either in the late embryo (in ovo; Hirano 1967) or posthatching (Kwong and Gauthier 1987). Aging mice, 21 (Boaro et al. 1998) or 29 (Fahim and Robbins 1982) months old, show several changes including signs of degeneration and intrusion of Schwann cell processes into the indention. In the NMJ images shown for the extensor digitorum longus muscle, Fahim and Robbins (1982) show a shallow indention at 7 months, while the example at 29 months is a double terminal with greater branching of the subjunctional folds (both changes noted frequently at this age); the image shows the muscle membrane and folds wrapping the sides of the NMJ completely so that the terminal is almost completely invaginated. We described the development of the NMJs in Drosophila in an earlier section: Basically, the terminal sits in a shallow groove in the embryo, and by the third instar larval stage, it is completely invaginated and has a highly developed SSR; then the invagination becomes reduced somewhat in the adult fly. Flies can live to just over 80 days; Beramendi et al. (2007) and Wagner et al. (2015) examined NMJs from different abdominal muscles, comparing old adults (56–75 days) with various younger adult ages. Both studies noted an increase in terminal size, and this was associated with a greater accumulation of various organelles and vesicles, including alterations in the endosomal and autophagy systems, probably concomitant with aging. Beramendi et al. (2007) also note that there is a reduction in SSR thickness, and in some cases, the enlarged terminals appear to protrude out of the invagination on the top, so that only a basal lamina may cover them here in these cases. Several studies have examined development and aging in lobster leg muscle NMJs (Govind and Pearce 1981; Govind and Derosa 1983; Pearce et al. 1985; Govind 1992), especially examining first-stage larvae (<0.1 g; 1 day old), young, sexually mature animals (0.5 kg; 5–7 years old), and older animals (~5–10 kg; maybe up to 30–50 years old; lobsters can live ~100 years). Generally, axonal branching, and synapse number and size all increase with age, apparently throughout life. Most micrographs from these different ages show some indention of the presynaptic terminal into the NMJ. In one pair of images of NMJs from a 0.5- and 5-kg animal, the small terminal of the 0.5-kg animal is partly indenting, while the large terminal of the 5-kg animal is deeply indenting, covered on more than three sides with active zones (Pearce et al. 1985). Unlike rodents and flies, lobsters may maintain fully functional NMJs well into “old age,” with little signs of aging (Pearce et al. 1985; Petralia et al. 2014).
Various muscle diseases can affect the indention/in- vagination of NMJs. Duchenne dystrophy is an X-linked recessive form of muscular dystrophy that causes muscle degeneration and premature death. People with this disease can have some normal-looking NMJs, with shallow to moderately deep indentions and well-developed subjunctional folds. But many NMJs show an atrophy of subjunctional folds, and often these NMJs tend to look flattened out with at most, a shallow indention (Jerusalem et al. 1974). NMJs of the mutant mouse model (mdx; absence of dystrophin protein) of this disease show a similar reduction in subjunctional folds; and deeply indenting terminals, seen in normal mice, seem to be lacking (Torres and Duchen 1987; Nagel et al. 1990). NMJs from people with myasthenia gravis (MG) tend to have a reduction in the size of the terminal as well as a reduction in subjunctional folds, while depth of indention of the terminals shown in figures varies widely (Engel and Santa 1971). NMJ size in people with limb-girdle myasthenia (LGM) is reduced to about half, and there is a reduction in subsynaptic folds; the images shown include a deeply indenting control terminal and a terminal from an LGM patient with little or no indention (Slater 2008). In contrast to these two diseases, NMJs in myasthenic syndrome (Lambert—Eaton) show an extreme increase in the subjunctional folds and consequently, the terminal often appears almost completely invaginated due to the expansion of the folded sarcoplasm over the terminal (Engel and Santa 1971; Santa et al. 1972)! This syndrome is an autoimmune disorder like MG, but antibodies are made against presynaptic P/Q-type voltage-gated calcium channels, while antibodies in MG are made against postsynaptic acetylcholine receptors (Mahadeva et al. 2008).
Conclusions
It seems likely that invaginating presynaptic terminals serve to enhance or modify neurotransmission or other types of cell signaling by providing a restricted, enclosed space to isolate specific chemical interactions. In addition, the invagination may be an optimal structural arrangement for propagation of local electrical field effects to modulate signal transmission; we suggest that future studies will show that this ephaptic conduction is a widespread function of invaginating spines and terminals (see Gardner et al. 2015). Evidence already supports dual chemical and electrical feedback mechanisms in the invaginations of photoreceptor cell terminals. Perhaps some presynaptic terminals are invaginating to add an extra level of electrical modulation over the basic chemical communication; both mechanisms might be optimized by the invagination.
Acknowledgements
This work was supported by the Intramural Research Programs of NIH/NIDCD and NIH/NIA. The code and animal protocol for the Advanced Imaging Core of NIDCD is ZIC DC000081 and 1167-16, respectively.
Glossary
- Active zone
The area of the presynaptic membrane where presynaptic vesicles release neurotransmitter. In the structures described in this review, the active zones are located in the portion of the presynaptic terminal that lies within the postsynaptic invagination
- Gutter
A postsynaptic indention or shallow invagination on the surface of some muscle fibers; in these cases, the presynaptic terminal runs parallel to the surface of the muscle cell and forms the neuromuscular junction along the gutter (e.g., Fig. 10a)
- Indenting terminals
Presynaptic terminals that are enclosed partially within a postsynaptic invagination (indention); see definitions of invaginating/invaginated terminals and postsynaptic invagination
- Invaginating/invaginated terminals
Presynaptic terminals that are enclosed only partially or entirely within a postsynaptic invagination; if only partially, then the postsynaptic structure can be described as indented. The term “invaginate” is based here on standard definitions, including “to fold up or enclose in a sheath-like or pouch-like structure” (Wiktionary) and “to insert or receive, as into a sheath” (Dictionary.com)
- Motor ending
Presynaptic terminal junctions formed on muscle or gland cells
- Postsynaptic invagination
An infolding of the postsynaptic membrane to form a cavity (pouch, sheath) that contains the invaginated structure; it can contain the entire presynaptic terminal, or part of the terminal (postsynaptic indention) or an invaginated projection or protrusion from the presynaptic terminal
- Protrusions
The Merriam-Webster Dictionary (online) describes extensions beyond the normal surface of a structure as projections, protrusions, protuberances, and bulges. A protrusion is an extension that seems to be a deformity that is thrust out from the surface. More specifically for this review, we define typically wide, invaginating extensions of presynaptic terminals, containing active zones within the extensions, as types of invaginating protrusions (see Fig. 1b3)
- Sarcoplasmic reticulum (SR) and subsynaptic reticulum (SSR)
SR denotes the endoplasmic reticulum (ER) found in the sarcoplasm, i.e., the cytoplasm of a muscle cell. SSR refers to SR, often elaborate, associated with the postsynaptic membrane in many arthropod neuromuscular junctions
- Subjunctional folds
Multiple postsynaptic invaginations, often deep and convoluted, found in many kinds of vertebrate neuromuscular junctions. Typically, these lack any extensions of the presynaptic membrane, but they do contain extensions of the basal lamina from the synaptic cleft
References
- Achim K, & Arendt D (2014). Structural evolution of cell types by step-wise assembly of cellular modules. Current Opinion in Genetics & Development, 27, 102–108. [DOI] [PubMed] [Google Scholar]
- Acsády L, Katona I, Martínez-Guijarro FJ, Buzsáki G, & Freund TF (2000). Unusual target selectivity of perisomatic inhibitory cells in the hilar region of the rat hippocampus. Journal of Neuroscience, 20(18), 6907–6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriaensen D, Brouns I, Van Genechten J, & Timmermans JP (2003). Functional morphology of pulmonary neuroepithelial bodies: Extremely complex airway receptors. The Anatomical Record Part A, Discoveries in Molecular, Cellular, and Evolutionary Biology, 270(1), 25–40. [DOI] [PubMed] [Google Scholar]
- Ammermüller J, & Kolb H (1996). Functional architecture of the turtle retina. Progress in Retinal and Eye Research, 15(2), 393–433. [Google Scholar]
- Andres KH, von During M, & Petrasch E (1988). The fine structure of ampullary and tuberous electroreceptors in the South American blind catfish Pseudocetopsis spec. Anatomy and Embryology (Berlin), 177(6), 523–535. [DOI] [PubMed] [Google Scholar]
- Arama J, Abitbol K, Goffin D, Fuchs C, Sihra TS, Thomson AM, et al. (2015). GABAA receptor activity shapes the formation of inhibitory synapses between developing medium spiny neurons. Frontiers in Cellular Neuroscience, 9, #290 (291–218). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuthnott ER, Ballard KJ, Boyd IA, Gladden MH, & Sutherland FI (1982). The ultrastructure of cat fusimotor endings and their relationship to foci of sarcomere convergence in intrafusal fibres. Journal of Physiology, 331, 285–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden GB, Gorin MB, Polkinghorne PJ, Jay M, & Bird AC (1988). Detection of the carrier state of X-linked retinoschisis. American Journal of Ophthalmology, 105(6), 590–595. [DOI] [PubMed] [Google Scholar]
- Arden GB, & Hogg CR (1985). Rod-cone interactions and analysis of retinal disease. British Journal of Ophthalmology, 69(6), 404–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsumi S (1977). Development of neuromuscular-junctions of fast and slow muscles in chick-embryo—Light and electron-microscopic Study. Journal of Neurocytology, 6(6), 691–709. [DOI] [PubMed] [Google Scholar]
- Attwell D, Barbour B, & Szatkowski M (1993). Nonvesicular release of neurotransmitter. Neuron, 11(3), 401–407. [DOI] [PubMed] [Google Scholar]
- Atwood HL, & Johnston HS (1968). Neuromuscular synapses of a crab motor axon. Journal of Experimental Zoology, 167, 457–470. [Google Scholar]
- Atwood HL (1976). Organization and synaptic physiology of crustacean neuromuscular systems. Progress in Neurobiology, 7(Pt 4), 291–391. [DOI] [PubMed] [Google Scholar]
- Atwood HL, & Morin WA (1970). Neuromuscular and axoaxonal synapses of the crayish opener muscle. Journal of Ultrastructure Research, 32(3), 351–369. [DOI] [PubMed] [Google Scholar]
- Babai N, & Thoreson WB (2009). Horizontal cell feedback regulates calcium currents and intracellular calcium levels in rod photoreceptors of salamander and mouse retina. Journal of Physiology, 587(Pt 10), 2353–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Venkatesan A, & Bhat MA (2017). Neurexin, Neuroligin and Wishful Thinking coordinate synaptic cytoarchitecture and growth at neuromuscular junctions. Molecular and Cellular Neuroscience, 78, 9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber VC, & Graziadei P (1967). The fine structure of cephalopod blood vessels. 3. Vessel innervation. Zeitschrift für Zellforschung und mikroskopische Anatomie, 77(2), 162–174. [DOI] [PubMed] [Google Scholar]
- Bargmann W, Lindner E, & Andres KH (1967). Uber Synapsen an Endokrinen Epithelzellen Und Die Definition Sekretorischer Neuron - Untersuchungen Am Zwischenlappen Der Katzenhypophyse. Zeitschrift für Zellforschung und mikroskopische Anatomie, 77(2), 282–298. [PubMed] [Google Scholar]
- Bargmann W, von Hehn G, & Lindner E (1968). On the cells of the brown fatty tissue and their innervation. Zeitschrift für Zellforschung und mikroskopische Anatomie, 85(4), 601–613. [PubMed] [Google Scholar]
- Barlow RB, Hitt JM, & Dodge FA (2001). Limulus vision in the marine environment. Biological Bulletin, 200(2), 169–176. [DOI] [PubMed] [Google Scholar]
- Baumgarten HG, Björklund A, Holstein AF, & Nobin A (1972). Organization and ultrastructural identification of catecholamine nerve terminals in neural lobe and pars intermedia of rat pituitary. Zeitschrift für Zellforschung und mikroskopische Anatomie, 126(4), 483–517. [DOI] [PubMed] [Google Scholar]
- Beccari N (1911). La costituzione, i nuclei terminali e le vie di connessione del nervo acustico nella Lacerta muralis. Arch ital Anat Embryol, 10, 646–698. [Google Scholar]
- Benedeczky I, Csoknya M, Fekete E, & Halasy K (1990). Ultrastructure of the nerve-muscle junction in the pharynx of the earthworm, Lumbricus terrestris. Zoomorphology, 109(6), 337–341. [Google Scholar]
- Benedeczky I, Mala J, & Sehnal F (1980). Ultrastructural-study on the innervation of prothoracic glands in Galleria mellonella. General and Comparative Endocrinology, 41(3), 400–407. [DOI] [PubMed] [Google Scholar]
- Beramendi A, Peron S, Casanova G, Reggiani C, & Cantera R (2007). Neuromuscular junction in abdominal muscles of Drosophila melanogaster during adulthood and aging. Journal of Comparative Neurology, 501(4), 498–508. [DOI] [PubMed] [Google Scholar]
- Bergman RA (1967). Motor nerve endings of twitch muscle fibers in Hippocampus hudsonius. Journal of Cell Biology, 32(3), 751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthold CH, Kellerth JO, & Conradi S (1979). Electron microscopic studies of serially sectioned cat spinal alphamotoneurons. I. Effects of microelectrode impalement and intracellular staining with the fluorescent dye “Procion Yellow”. Journal of Comparative Neurology, 184(4), 709–740. [DOI] [PubMed] [Google Scholar]
- Best ACG, & Bone Q (1973). Terminal neuromuscular junctions of lower chordates. Zeitschrift für Zellforschung und mikroskopische Anatomie, 143(4), 495–504. [DOI] [PubMed] [Google Scholar]
- Biazik J, Ylä-Anttila P, Vihinen H, Jokitalo E, & Eskelinen EL (2015). Ultrastructural relationship of the phagophore with surrounding organelles. Autophagy, 11(3), 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R, Huxley HE, & Katz B (1960). The fine structure of the neuromuscular junction of the frog. Journal of Physiology-London, 150(1), 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagburn JM, Alexopoulos H, Davies JA, & Bacon JP (1999). Null mutation in shaking-B eliminates electrical, but not chemical, synapses in the Drosophila giant fiber system: A structural study. Journal of Comparative Neurology, 404(4), 449–458. [PubMed] [Google Scholar]
- Boaro SN, Soares JC, & König B Jr. (1998). Comparative structural analysis of neuromuscular junctions in mice at different ages. Annals of Anatomy, 180(2), 173–179. [DOI] [PubMed] [Google Scholar]
- Bone Q (1972). The dogfish neuromuscular junction: Dual innervation of vertebrate striated muscle fibres? Journal of Cell Science, 10(3), 657–665. [DOI] [PubMed] [Google Scholar]
- Bone Q, & Ryan KP (1974). On the structure and innervation of the muscle bands of Doliolum (Tunicata: Cyclomyaria). Proceedings of the Royal Society of London, Series B: Biological Sciences, 187(1088), 315–327. [DOI] [PubMed] [Google Scholar]
- Bosch E (1990). Ultrastructure of the electrotonic and chemical components of the lateral-to-motor and medial-to-motor synapses in crayfish nerve cord. Journal of Comparative Neurology, 299(4), 446–461. [DOI] [PubMed] [Google Scholar]
- Bradacs H, Cooper R, Msghina M, & Atwood H (1997). Differential physiology and morphology of phasic and tonic motor axons in a crayfish limb extensor muscle. Journal of Experimental Biology, 200(Pt 4), 677–691. [DOI] [PubMed] [Google Scholar]
- Brenowitz S, & Trussell LO (2001). Maturation of synaptic transmission at end-bulb synapses of the cochlear nucleus. Journal of Neuroscience, 21(23), 9487–9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V, Zhong Y, & Wu CF (1990). Morphological plasticity of motor axons in Drosophila mutants with altered excitability. Journal of Neuroscience, 10(11), 3754–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burighel P, Lane NJ, Fabio G, Stefano T, Zaniolo G, Carnevali MD, et al. (2003). Novel, secondary sensory cell organ in ascidians: In search of the ancestor of the vertebrate lateral line. Journal of Comparative Neurology, 461(2), 236–249. [DOI] [PubMed] [Google Scholar]
- Burton PM (2008). Insights from diploblasts; the evolution of mesoderm and muscle. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 310(1), 5–14. [DOI] [PubMed] [Google Scholar]
- Carr CE, & Boudreau RE (1996). Development of the time coding pathways in the auditory brainstem of the barn owl. Journal of Comparative Neurology, 373(4), 467–483. [DOI] [PubMed] [Google Scholar]
- Case NM, Gray EG, & Young JZ (1972). Ultrastructure and synaptic relations in the optic lobe of the brain of Eledone and Octopus. Journal of Ultrastructure Research, 39(1), 115–123. [DOI] [PubMed] [Google Scholar]
- Castejón OJ, & Villegas GM (1964). Fine structure of the synaptic contacts in the stellate ganglion of the squid. Journal of Ultrastructure Research, 10, 585–598. [DOI] [PubMed] [Google Scholar]
- Cavey MJ, & Cloney RA (1976). Ultrastructure and differentiation of ascidian muscle. I. Caudal musculature of the larva of Diplosoma macdonaldi. Cell and Tissue Research, 174(3), 289–313. [DOI] [PubMed] [Google Scholar]
- Chang FL, & Greenough WT (1984). Transient and enduring morphological correlates of synaptic activity and efficacy change in the rat hippocampal slice. Brain Research, 309(1), 35–46. [DOI] [PubMed] [Google Scholar]
- Charvet B, Malbouyres M, Pagnon-Minot A, Ruggiero F, & Le Guellec D (2011). Development of the zebrafish myoseptum with emphasis on the myotendinous junction. Cell and Tissue Research, 346(3), 439–449. [DOI] [PubMed] [Google Scholar]
- Chien P, & Koopowitz H (1972). The ultrastructure of neuromuscular systems in Notoplana acticola, a free-living polyclad flatworm. Zeitschrift für Zellforschung und mikroskopische Anatomie, 133(2), 277–288. [DOI] [PubMed] [Google Scholar]
- Clément P (1977). Ultrastructural research on rotifers. Archiv f Hydrobiologie Beih, 8, 270–297. [Google Scholar]
- Cobb JL (1978). An ultrastructural study of the dermal papulae of the starfish, Asterias rubens, with special reference to innervation of the muscles. Cell and Tissue Research, 187(3), 515–523. [DOI] [PubMed] [Google Scholar]
- Cobb JLS, & Laverack MS (1966). Lantern of Echinus esculentus (L). 3. Fine structure of lantern retractor muscle and its innervation. Proceedings of the Royal Society Series B-Biological Sciences, 164(997), 651–658. [Google Scholar]
- Cohen AI (1973). An ultrastructural analysis of the photoreceptors of the squid and their synaptic connections. 3. Photoreceptor terminations in the optic lobes. Journal of Comparative Neurology, 147(3), 399–426. [DOI] [PubMed] [Google Scholar]
- Conradi S, & Skoglund S (1969). Observations on the ultrastruture and distribution of neuronal and glial elements on the motoneuron surface in the lumbosacral spinal cord of the cat during postnatal development. Acta Physiologica Scandinavica. Supplementum, 333, 5–52. [PubMed] [Google Scholar]
- Coupland RE (1965). Electron microscopic observations on structure of rat adrenal medulla.2. Normal innervation. Journal of Anatomy, 99, 255–272. [PMC free article] [PubMed] [Google Scholar]
- Cousin CE, & Dorsey CH (1991). Nervous system of Schistosoma mansoni cercaria: Organization and fine structure. Parasitology Research, 77(2), 132–141. [DOI] [PubMed] [Google Scholar]
- Cutz E, Pan J, Yeger H, Domnik NJ, & Fisher JT (2013). Recent advances and contraversies on the role of pulmonary neuroepithelial bodies as airway sensors. Seminars in Cell & Developmental Biology, 24(1), 40–50. [DOI] [PubMed] [Google Scholar]
- Dahl E (1973). The fine structure of the pancreatic nerves of the domestic fowl. Zeitschrift für Zellforschung und mikroskopische Anatomie, 136(4), 501–510. [DOI] [PubMed] [Google Scholar]
- Deshpande M, & Rodal AA (2016). The crossroads of synaptic growth signaling, membrane traffic and neurological disease: Insights from Drosophila. Traffic, 17(2), 87–101. [DOI] [PubMed] [Google Scholar]
- Desmond NL, & Levy WB (1983). Synaptic correlates of associative potentiation/depression: An ultrastructural study in the hippocampus. Brain Research, 265(1), 21–30. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Li W, & Saszik S (2006). Parallel processing in two transmitter microenvironments at the cone photoreceptor synapse. Neuron, 50(5), 735–748. [DOI] [PubMed] [Google Scholar]
- Dilly PN, Gray EG, & Young JZ (1963). Electron microscopy of optic nerves and optic lobes of Octopus and Eledone. Proceedings of the Royal Society of London, Series B: Biological Sciences, 158, 446–456. [DOI] [PubMed] [Google Scholar]
- Dolder H (1975). An ultrastructural and cytochemical study of neuromuscular junctions in echinoderms. Histochemistry, 44(4), 313–322. [DOI] [PubMed] [Google Scholar]
- Dowling JE, & Boycott BB (1966). Organization of the primate retina: Electron microscopy. Proceedings of the Royal Society of London, Series B: Biological Sciences, 166(1002), 80–111. [DOI] [PubMed] [Google Scholar]
- Dowling JE, & Werblin FS (1969). Organization of retina of the mudpuppy, Necturus maculosus. I. Synaptic structure. Journal of Neurophysiology, 32(3), 315–338. [DOI] [PubMed] [Google Scholar]
- Doyle WL (1967). Vesiculated axons in haemal vessels of an holothurian Cucumaria frondosa. Biological Bulletin, 132(3), 329–336. [Google Scholar]
- Ducros C (1972). Ultrastructural study of the innervation of the posterior salivary glands in Octopus vulgaris. II. Muscle innervation in the ducts and the glands. Zeitschrift für Zellforschung und mikroskopische Anatomie, 132(1), 51–65. [PubMed] [Google Scholar]
- Duvert M, & Barets AL (1983). Ultrastructural studies of neuromuscular junctions in visceral and skeletal muscles of the chaetognath Sagitta setosa. Cell and Tissue Research, 233(3), 657–669. [DOI] [PubMed] [Google Scholar]
- Duvert M, & Savineau JP (1986). Ultrastructural and physiological studies of the contraction of the trunk musculature of Sagitta setosa (chaetognath). Tissue and Cell, 18(6), 937–952. [DOI] [PubMed] [Google Scholar]
- Eakin RM, & Brandenburger JL (1971). Fine structure of the eyes of jumping spiders. Journal of Ultrastructure Research, 37(5), 618–663. [DOI] [PubMed] [Google Scholar]
- Edwards GA (1959). The fine structure of a multiterminal innervation of an insect muscle. The Journal of Biophysical and Biochemical Cytology, 5(2), 241–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards GA, Ruska H, & De Harven E (1958a). Electron microscopy of peripheral nerves and neuromuscular junctions in the wasp leg. The Journal of Biophysical and Biochemical Cytology, 4(1), 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards GA, Ruska H, & De Harven E (1958b). Neuromuscular junctions in flight and tymbal muscles of the cicada. The Journal of Biophysical and Biochemical Cytology, 4(3), 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elekes K, & Ude J (1994). Peripheral connections of FMRFamide-like immunoreactive neurons in the snail, Helix pomatia—An immunogold electron-microscopic study. Journal of Neurocytology, 23(12), 758–769. [DOI] [PubMed] [Google Scholar]
- Eliasieh K, Liets LC, & Chalupa LM (2007). Cellular reorganization in the human retina during normal aging. Investigative Ophthalmology & Visual Science, 48(6), 2824–2830. [DOI] [PubMed] [Google Scholar]
- Ellisman MH, Rash JE, Staehelin LA, & Porter KR (1976). Studies of excitable membranes. II. A comparison of specializations at neuromuscular junctions and nonjunctional sarcolemmas of mammalian fast and slow twitch muscle fibers. Journal of Cell Biology, 68(3), 752–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endeman D, Fahrenfort I, Sjoerdsma T, Steijaert M, Ten Eikelder H, & Kamermans M (2012). Chloride currents in cones modify feedback from horizontal cells to cones in goldfish retina. Journal of Physiology, 590(22), 5581–5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AG, & Santa T (1971). Histometric analysis of the ultrastructure of the neuromuscular junction in myasthenia gravis and in the myasthenic syndrome. Annals of the New York Academy of Sciences, 183, 46–63. [DOI] [PubMed] [Google Scholar]
- Esterhuizen AC, Spriggs TL, & Lever JD (1968). Nature of islet-cell innervation in the cat pancreas. Diabetes, 17(1), 33–36. [DOI] [PubMed] [Google Scholar]
- Fahim MA, & Robbins N (1982). Ultrastructural studies of young and old mouse neuromuscular junctions. Journal of Neurocytology, 11(4), 641–656. [DOI] [PubMed] [Google Scholar]
- Fahrenbach WH (1994). Microscopic anatomy of Pycnogonida: I. Cuticle, epidermis, and muscle. Journal of Morphology, 222(1), 33–48. [DOI] [PubMed] [Google Scholar]
- Fariss RN, Li ZY, & Milam AH (2000). Abnormalities in rod photoreceptors, amacrine cells, and horizontal cells in human retinas with retinitis pigmentosa. American Journal of Ophthalmology, 129(2), 215–223. [DOI] [PubMed] [Google Scholar]
- Farnesi RM, & Vagnetti D (1975). The fine structure of the myoneural junctions in the body wall muscles in Branchiobdella pentodonta Whit. (Annelida, Oligochaeta). Anatomical Record, 182(1), 91–102. [DOI] [PubMed] [Google Scholar]
- Fedorenko GM, & Uzdensky AB (2009). Ultrastructure of neuroglial contacts in crayfish stretch receptor. Cell and Tissue Research, 337(3), 477–490. [DOI] [PubMed] [Google Scholar]
- Fernand VS, & Hess A (1969). The occurrence, structure and innervation of slow and twitch muscle fibres in the tensor tympani and stapedius of the cat. Journal of Physiology, 200(2), 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filimonova SA, & Amosova LI (2015). Peculiar salivary glands in a silk-producing mite Bakericheyla chanayi (Cheyletidae). Journal of Morphology, 276(7), 772–786. [DOI] [PubMed] [Google Scholar]
- Fischer FP (1992). Quantitative analysis of the innervation of the chicken basilar papilla. Hearing Research, 61(1–2), 167–178. [DOI] [PubMed] [Google Scholar]
- Fisher SK, & Boycott BB (1974). Synaptic connections made by horizontal cells within the outer plexiform layer of the retina of the cat and the rabbit. Proceedings of the Royal Society of London, Series B: Biological Sciences, 186(1085), 317–331. [DOI] [PubMed] [Google Scholar]
- Florey E (1969). Ultrastructure and function of cephalopod chromatophores. American Zoologist, 9(2), 429–442. [DOI] [PubMed] [Google Scholar]
- Fourtner CR, & Sherman RG (1973). Chelicerate skeletal neuromuscular systems. American Zoologist, 13(2), 271–289. [Google Scholar]
- Freifeld L, Clark DA, Schnitzer MJ, Horowitz MA, & Clandinin TR (2013). GABAergic lateral interactions tune the early stages of visual processing in Drosophila. Neuron, 78(6), 1075–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M, & Léránth C (1986). The cholinergic innervation of the rat fascia dentata: Identification of target structures on granule cells by combining choline acetyltransferase immunocytochemistry and Golgi impregnation. Journal of Comparative Neurology, 243(1), 58–70. [DOI] [PubMed] [Google Scholar]
- Fuentes-Medel Y, Ashley J, Barria R, Maloney R, Freeman M, & Budnik V (2012). Integration of a retrograde signal during synapse formation by glia-secreted TGF-beta ligand. Current Biology, 22(19), 1831–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Medel Y, Logan MA, Ashley J, Ataman B, Budnik V, & Freeman MR (2009). Glia and muscle sculpt neuromuscular arbors by engulfing destabilized synaptic boutons and shed presynaptic debris. PLoS Biology, 7(8), e1000184. (1000181–1000115). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner CL, Jones JR, Baer SM, & Crook SM (2015). Driftdiffusion simulation of the ephaptic effect in the triad synapse of the retina. Journal of Computational Neuroscience, 38(1), 129–142. [DOI] [PubMed] [Google Scholar]
- Glantz RM, & Bartels A (1994). The spatiotemporal transfer function of crayfish lamina monopolar neurons. Journal of Neurophysiology, 71(6), 2168–2182. [DOI] [PubMed] [Google Scholar]
- Glantz RM, Miller CS, & Nässel DR (2000). Tachykinin-related peptide and GABA-mediated presynaptic inhibition of crayfish photoreceptors. Journal of Neuroscience, 20(5), 1780–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniakowska-Witalinska L, Lauweryns JM, Zaccone G, Fasulo S, & Tagliafierro G (1992). Ultrastructure and immunocyto-chemistry of the neuroepithelial bodies in the lung of the tiger salamander, Ambystoma tigrinum (Urodela, Amphibia). Anatomical Record, 234(3), 419–431. [DOI] [PubMed] [Google Scholar]
- Govind CK (1992). Age-related remodeling of lobster neuromuscular terminals. Experimental Gerontology, 27(1), 63–74. [DOI] [PubMed] [Google Scholar]
- Govind CK, Atwood HL, & Pearce J (1995). Inhibitory axoaxonal and neuromuscular synapses in the crayfish opener muscle: Membrane definition and ultrastructure. Journal of Comparative Neurology, 351(3), 476–488. [DOI] [PubMed] [Google Scholar]
- Govind CK, & Derosa RA (1983). Fine structure of comparable synapses in a mature and larval lobster muscle. Tissue and Cell, 15(1), 97–106. [DOI] [PubMed] [Google Scholar]
- Govind CK, & Pearce J (1981). Remodeling of multiterminal innervation by nerve terminal sprouting in an identifiable lobster motoneuron. Science, 212(4502), 1522–1524. [DOI] [PubMed] [Google Scholar]
- Govind CK, Stephens PJ, & Eisen JS (1985). Polyneuronal innervation of an adult and embryonic lobster muscle. Journal of Embryology and Experimental Morphology, 87, 13–26. [PubMed] [Google Scholar]
- Graziadei P (1966). The ultrastructure of the motor nerve endings in the muscles of cephalopods. Journal of Ultrastructure Research, 15(1), 1–13. [DOI] [PubMed] [Google Scholar]
- Greb H, Hermann S, Dirks P, Ommen G, Kretschmer V, Schultz K, et al. (2017). Complexity of gap junctions between horizontal cells of the carp retina. Neuroscience, 340, 8–22. [DOI] [PubMed] [Google Scholar]
- Greferath U, Grünert U, Müller F, & Wässle H (1994). Localization of GABAA receptors in the rabbit retina. Cell and Tissue Research, 276(2), 295–307. [DOI] [PubMed] [Google Scholar]
- Gur M, Purple RL, & Whitehead R (1972). Ultrastructure within the lateral plexus of the Limulus eye. Journal of General Physiology, 59(3), 285–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner GS (1974). The ultrastructure of retinula cell endings in the compound eye of the crayfish. Journal of Neurocytology, 3(3), 295–311. [DOI] [PubMed] [Google Scholar]
- Haghighat N, Cohen RS, & Pappas GD (1984). Fine structure of squid (Loligo pealei) optic lobe synapses. Neuroscience, 13(2), 527–546. [DOI] [PubMed] [Google Scholar]
- Hama K (1961). Some observations on the fine structure of the giant fibers of the crayfishes (Cambarus virilus and Cambarus clarkii) with special reference to the submicroscopic organization of the synapses. Anatomical Record, 141, 275–293. [DOI] [PubMed] [Google Scholar]
- Hámori J, & Horridge GA (1966). Lobster optic lamina. 2. Types of synapse. Journal of Cell Science, 1(2), 257–270. [DOI] [PubMed] [Google Scholar]
- Hand AR (1970). Nerve-acinar cell relationships in the rat parotid gland. Journal of Cell Biology, 47(2), 540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J, & Lowy J (1957). Structure of smooth muscles. Nature, 180(4592), 906–909. [DOI] [PubMed] [Google Scholar]
- Häring P, Stähli C, Schoch P, Takács B, Staehelin T, & Möhler H (1985). Monoclonal-antibodies reveal structural homogeneity of gamma-aminobutyric acid benzodiazepine receptors in different brain-areas. Proceedings of the National Academy of Sciences USA, 82(14), 4837–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart RJ, Beadle DJ, & Botham RP (1980). The ultrastructure of somatic neuromuscular junctions in the tick Amblyomma variegatum (Fabr.). Cell and Tissue Research, 206(3), 505–508. [DOI] [PubMed] [Google Scholar]
- Harvey DM, & Calkins DJ (2002). Localization of kainate receptors to the presynaptic active zone of the rod photoreceptor in primate retina. Visual Neuroscience, 19(5), 681–692. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Grünert U, & Wässle H (2000). The cone pedicle, a complex synapse in the retina. Neuron, 27(1), 85–95. [DOI] [PubMed] [Google Scholar]
- He S, Weiler R, & Vaney DI (2000). Endogenous dopaminergic regulation of horizontal cell coupling in the mammalian retina. Journal of Comparative Neurology, 418(1), 33–40. [DOI] [PubMed] [Google Scholar]
- Heitler WJ, Cobb JL, & Fraser K (1985). Ultrastructure of the segmental giant neuron of crayfish. Journal of Neurocytology, 14(6), 921–941. [DOI] [PubMed] [Google Scholar]
- Hering H, Lin CC, & Sheng M (2003). Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. Journal of Neuroscience, 23(8), 3262–3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Nicaise ML (1973). The nervous system of ctenophores. III. Ultrastructure of synapses. Journal of Neurocytology, 2(3), 249–263. [DOI] [PubMed] [Google Scholar]
- Hernandez-Nicaise ML, Mackie GO, & Meech RW (1980). Giant smooth muscle cells of Beroe. Ultrastructure, innervation, and electrical properties. Journal of General Physiology, 75(1), 79–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A (1965). The sarcoplasmic reticulum, the T system, and the motor terminals of slow and twitch muscle fibers in the garter snake. Journal of Cell Biology, 26(2), 467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A (1967). The structure of vertebrate slow and twitch muscle fibers. Investigative Ophthalmology, 6(3), 217–228. [PubMed] [Google Scholar]
- Hess A (1970). Vertebrate slow muscle fibers. Physiological Reviews, 50(1), 40–62. [DOI] [PubMed] [Google Scholar]
- Heuser JE, & Reese TS (1973). Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. Journal of Cell Biology, 57(2), 315–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RB, & Sanger JW (1974). Anatomy of innervation and neuromuscular-junctions of radular protractor muscle of whelk, Busycon canaliculatum (L). Biological Bulletin, 147(2), 369–385. [Google Scholar]
- Hinojosa R (1973). Synaptic ultrastructure in the tangential nucleus of the goldfish (Carassius auratus). American Journal of Anatomy, 137(2), 159–186. [DOI] [PubMed] [Google Scholar]
- Hinojosa R, & Robertson JD (1967). Ultrastructure of the spoon type synaptic endings in the nucleus vestibularis tangentialis of the chick. Journal of Cell Biology, 34(2), 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano H (1967). Ultrastructural study on the morphogenesis of the neuromuscular junction in the skeletal muscle of the chick. Zeitschrift für Zellforschung und mikroskopische Anatomie, 79(2), 198–208. [PubMed] [Google Scholar]
- Hirano AA, Brandstätter JH, & Brecha NC (2005). Cellular distribution and subcellular localization of molecular components of vesicular transmitter release in horizontal cells of rabbit retina. Journal of Comparative Neurology, 488(1), 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano AA, Liu X, Boulter J, Grove J, de Sevilla Perez, Müller L, et al. (2016). Targeted deletion of vesicular GABA transporter from retinal horizontal cells eliminates feedback modulation of photoreceptor calcium channels. eNeuro, 3(2), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano H, & Ogawa K (1967). Ultrastructural localization of cholinesterase activity in nerve endings in the guinea pig heart. Journal of Electron Microscopy (Tokyo), 16(4), 313–321. [PubMed] [Google Scholar]
- Holmberg K (1970). The hagfish retina: Fine structure of retinal cells in Myxine glutinosa, L., with special reference to receptor and epithelial cells. Zeitschrift für Zellforschung und mikroskopische Anatomie, 111(4), 519–538. [DOI] [PubMed] [Google Scholar]
- Holmberg K (1971). Hagfish retina—Electron microscopic study comparing receptor and epithelial cells in pacific hagfish, Polistotrema stouti, with those in Atlantic hagfish, Myxine glutinosa. Zeitschrift für Zellforschung und mikroskopische Anatomie, 121(2), 249–269. [DOI] [PubMed] [Google Scholar]
- Holmberg K, & Öhman P (1976). Fine-structure of retinal synaptic organelles in lamprey and hagfish photoreceptors. Vision Research, 16(3), 237–239. [DOI] [PubMed] [Google Scholar]
- Holtmann M, & Thurm U (2001a). Variations of concentric hair cells in a cnidarian sensory epithelium (Coryne tubulosa). Journal of Comparative Neurology, 432(4), 550–563. [PubMed] [Google Scholar]
- Holtmann M, & Thurm U (2001b). Mono- and oligo-vesicular synapses and their connectivity in a Cnidarian sensory epithelium (Coryne tubulosa). Journal of Comparative Neurology, 432(4), 537–549. [DOI] [PubMed] [Google Scholar]
- Hombach S, Janssen-Bienhold U, Söhl G, Schubert T, Büssow H, Ott T, et al. (2004). Functional expression of connexin57 in horizontal cells of the mouse retina. European Journal of Neuroscience, 19(10), 2633–2640. [DOI] [PubMed] [Google Scholar]
- Hong T, & Shaw RM (2017). Cardiac T-Tubule Microanatomy and Function. Physiological Reviews, 97(1), 227–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horridge GA (1965). Non-motile sensory cilia and neuromuscular junctions in a ctenophore independent effector organ. Proceedings of the Royal Society Series B-Biological Sciences, 162(988), 333–337. [Google Scholar]
- Hoyle G, & Mcneill PA (1968). Correlated physiological and ultrastructural studies on specialised muscles. Ic. Neuromuscular junctions in eyestalk levator muscles of Podophthalmus vigil (Weber). Journal of Experimental Zoology, 167(4), 523–550. [Google Scholar]
- Ichiki M, Nakagaki I, Konishi A, & Fukami Y (1976). The innervation of muscle spindles in the snake, Elaphe quadrivirgata. Journal of Anatomy, 122(Pt 1), 141–167. [PMC free article] [PubMed] [Google Scholar]
- Jahromi SS, & Atwood HL (1974). Three-dimensional ultrastructure of the crayfish neuromuscular apparatus. Journal of Cell Biology, 63(2 Pt 1), 599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James RE, Hoover KM, Bulgari D, McLaughlin CN, Wilson CG, Wharton KA, et al. (2014). Crimpy enables discrimination of presynaptic and postsynaptic pools of a BMP at the Drosophila neuromuscular junction. Developmental Cell, 31(5), 586–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J, Andersen P, & Jansen JKS (1963). On the structure and innervation of the parietal muscle of the hagfish (Myxine glutinosa). Acta Morphologica Neerlando-Scandinavica, 5, 329–338. [PubMed] [Google Scholar]
- Jensen H, Holtet L, & Hoen R (1978). Nerve-Purkinje fiber relationship in the moderator band of bovine and caprine heart. Cell and Tissue Research, 188(1), 11–18. [DOI] [PubMed] [Google Scholar]
- Jerusalem F, Engel AG, & Gomez MR (1974). Duchenne dystrophy. II. Morphometric study of motor end-plate fine structure. Brain, 97(1), 123–130. [DOI] [PubMed] [Google Scholar]
- Jhaveri S, & Morest DK (1982). Sequential alterations of neuronal architecture in nucleus magnocellularis of the developing chicken: An electron microscope study. Neuroscience, 7(4), 855–870. [DOI] [PubMed] [Google Scholar]
- Johnston JB (1902). The brain of Petromyzon. Journal of Comparative Neurology, 12(1), 1–82. [Google Scholar]
- Jones EG, & Powell TP (1969). Morphological variations in the dendritic spines of the neocortex. Journal of Cell Science, 5(2), 509–529. [DOI] [PubMed] [Google Scholar]
- Jørgensen JM (2005). Morphology of electroreceptive sensory organs In Bullock TH, Hopkins CD, Popper AN, & Fay RR (Eds.), Electroreception (pp. 47–67). New York: Springer. [Google Scholar]
- Ju G, & Zhang X (1990). An electron-microscopic study on substance P-like immunoreactive nerve-fibers in the pars-distalis of the adenohypophysis in the dog. Neuroscience, 38(2), 503–513. [DOI] [PubMed] [Google Scholar]
- Ju G, & Zhang X (1992). An electron-microscopic study of calcitonin gene-related peptide-like immunoreactive innervation of the anterior-pituitary in the dog. Journal of Comparative Neurology, 326(1), 101–111. [DOI] [PubMed] [Google Scholar]
- Jung HH, Han SH, Nam SY, Kim YH, & Kim JL (2004). Myosin heavy chain composition of rat middle ear muscles. Acta Oto-Laryngologica, 124(5), 569–573. [DOI] [PubMed] [Google Scholar]
- Kamermans M, & Fahrenfort I (2004). Ephaptic interactions within a chemical synapse: Hemichannel-mediated ephaptic inhibition in the retina. Current Opinion in Neurobiology, 14(5), 531–541. [DOI] [PubMed] [Google Scholar]
- Kamimura K, Ueno K, Nakagawa J, Hamada R, Saitoe M, & Maeda N (2013). Perlecan regulates bidirectional Wnt signaling at the Drosophila neuromuscular junction. Journal of Cell Biology, 200(2), 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B (1961). Terminations of afferent nerve fibre in muscle spindle of frog. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences, 243(703), 221–240. [Google Scholar]
- Kerr KS, Fuentes-Medel Y, Brewer C, Barria R, Ashley J, Abruzzi KC, et al. (2014). Glial wingless/Wnt regulates glutamate receptor clustering and synaptic physiology at the Drosophila neuromuscular junction. Journal of Neuroscience, 34(8), 2910–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MJ, Atwood HL, & Govind CK (1996). Structural features of crayfish phasic and tonic neuromuscular terminals. Journal of Comparative Neurology, 372(4), 618–626. [DOI] [PubMed] [Google Scholar]
- Klaassen LJ, Sun Z, Steijaert MN, Bolte P, Fahrenfort I, Sjoerdsma T, et al. (2011). Synaptic transmission from horizontal cells to cones is impaired by loss of connexin hemichannels. PLoS Biology, 9(7), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitman N, & Holzwarth MA (1985). Catecholaminergic innervation of the rat adrenal-cortex. Cell and Tissue Research, 241(1), 139–147. [DOI] [PubMed] [Google Scholar]
- Klooster J, & Kamermans M (2016). An ultrastructural and immunohistochemical analysis of the outer plexiform layer of the retina of the European Silver Eel (Anguilla anguilla L). PLoS ONE, 11(3), e0152967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klooster J, Nunes Cardozo B, Yazulla S, & Kamermans M (2004). Postsynaptic localization of gamma-aminobutyric acid transporters and receptors in the outer plexiform layer of the goldfish retina: An ultrastructural study. Journal of Comparative Neurology, 474(1), 58–74. [DOI] [PubMed] [Google Scholar]
- Kobayashi K (1966). Electron microscope studies of the Langerhans Islets in the toad pancreas. Archivum Histologicum Japonicum, 26(5), 439–482. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, & Fujita T (1969). Fine structure of mammalian and avian pancreatic islets with special reference to D cells and nervous elements. Zeitschrift für Zellforschung und mikroskopische Anatomie, 100(3), 340–363. [DOI] [PubMed] [Google Scholar]
- Kolb H (1977). The organization of the outer plexiform layer in the retina of the cat: Electron microscopic observations. Journal of Neurocytology, 6(2), 131–153. [DOI] [PubMed] [Google Scholar]
- Kolb H, & Jones J (1984). Synaptic organization of the outer plexiform layer of the turtle retina: An electron microscope study of serial sections. Journal of Neurocytology, 13(4), 567–591. [DOI] [PubMed] [Google Scholar]
- Kordylewski L (1974). The anatomy and the fine structure of extraocular muscles of the gudgeon, Gobio gobio (Linnaeus). Acta Anatomica (Basel), 87(4), 597–614. [DOI] [PubMed] [Google Scholar]
- Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, et al. (2009). Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell, 139(2), 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkut C, Li Y, Koles K, Brewer C, Ashley J, Yoshihara M, et al. (2013). Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron, 77(6), 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H, Sotelo C, & Bennett MVL (1977). Lateral vestibular nucleus of toadfish Opsanus tau—Ultrastructural and electrophysiological observations with special reference to electrotonic transmission. Neuroscience, 2(6), 851–884. [Google Scholar]
- Korn H, Sotelo C, & Crepel F (1973). Electronic coupling between neurons in the rat lateral vestibular nucleus. Experimental Brain Research, 16(3), 255–275. [PubMed] [Google Scholar]
- Korneliussen H (1973a). Ultrastructure of motor nerve terminals on different types of muscle fibers in the Atlantic hagfish (Myxine glutinosa, L.). Occurrence of round and elongated profiles of synaptic vesicles and dense-core vesicles. Zeitschrift für Zellforschung und mikroskopische Anatomie, 147(1), 87–105. [DOI] [PubMed] [Google Scholar]
- Korneliussen H (1973b). Dense-core vesicles in motor nerve terminals. Monoaminergic innervation of slow non-twitch muscle fibers in the Atlantic hagfish (Myxine glutinosa, L.)? Zeitschrift für Zellforschung und mikroskopische Anatomie, 140(3), 425–432. [PubMed] [Google Scholar]
- Korneliussen H (1973c). Ultrastructure of myotendinous junctions in Myxine and rat. Specializations between the plasma membrane and the lamina densa. Z Anat Entwicklungsgesch, 142(1), 91–101. [DOI] [PubMed] [Google Scholar]
- Kramer RH, & Davenport CM (2015). Lateral inhibition in the vertebrate retina: The case of the missing neurotransmitter. PLoS Biology, 13(12), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryvi H (1977). Ultrastructure of the different fibre types in axial muscles of the sharks Etmopterus spinax and Galeus melastomus. Cell and Tissue Research, 184(3), 287–300. [DOI] [PubMed] [Google Scholar]
- Kucera J (1984). Ultrastructure of extrafusal and intrafusal terminals of a (dynamic) skeletofusimotor axon in cat tenuissimus muscle. Brain Research, 298(1), 181–186. [DOI] [PubMed] [Google Scholar]
- Kucera J, & Walro JM (1986). Factors that determine the form of neuromuscular junctions of intrafusal fibers in the cat. American Journal of Anatomy, 176(1), 97–117. [DOI] [PubMed] [Google Scholar]
- Kullberg RW, Lentz TL, & Cohen MW (1977). Development of the myotomal neuromuscular junction in Xenopus laevis: An electrophysiological and fine-structural study. Development Biology, 60(1), 101–129. [DOI] [PubMed] [Google Scholar]
- Kwong WH, & Gauthier GF (1987). Neuromuscular junctions in adult and developing fast and slow muscles. Anatomical Record, 219(4), 409–419. [DOI] [PubMed] [Google Scholar]
- Lacalli TC (1990). Structure and organization of the nervous-system in the actinotroch larva of Phoronis vancouverensis. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences, 327(1244), 655–685. [Google Scholar]
- Lacalli TC (2002). The dorsal compartment locomotory control system in amphioxus larvae. Journal of Morphology, 252(3), 227–237. [DOI] [PubMed] [Google Scholar]
- Lane BP, & Rhodin JA (1964). Cellular interrelationships and electrical activity in two types of smooth muscle. Journal of Ultrastructure Research, 10, 470–488. [DOI] [PubMed] [Google Scholar]
- Lang F (1972). Ultrastructure of neuromuscular junctions in Limulus heart. Zeitschrift für Zellforschung und mikroskopische Anatomie, 130(4), 481–488. [DOI] [PubMed] [Google Scholar]
- Lasansky A (1973). Organization of the outer synaptic layer in the retina of the larval tiger salamander. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 265(872), 471–489. [DOI] [PubMed] [Google Scholar]
- Lauweryns JM, & Van Lommel A (1987). Ultrastructure of nerve endings and synaptic junctions in rabbit intrapulmonary neuroepithelial bodies: A single and serial section analysis. Journal of Anatomy, 151, 65–83. [PMC free article] [PubMed] [Google Scholar]
- Legg PG (1967). Fine structure and innervation of beta and delta cells in Islet of Langerhans of cat. Zeitschrift für Zellforschung und mikroskopische Anatomie, 80(3), 307–321. [DOI] [PubMed] [Google Scholar]
- Leitch B (1992). Ultrastructure of electrical synapses: Review. Electron Microsc Rev, 5(2), 311–339. [DOI] [PubMed] [Google Scholar]
- Leitch B, Cobb JL, Heitler WJ, & Pitman RM (1989). Postembryonic development of rectifying electrical synapses in the crayfish: Ultrastructure. Journal of Neurocytology, 18(6), 749–761. [DOI] [PubMed] [Google Scholar]
- Leitch B, Pitman RM, Heitler WJ, & Cobb JL (1992). Structural and functional post-embryonic development of a nonrectifying electrical synapse in the crayfish. Journal of Neurocytology, 21(2), 120–128. [DOI] [PubMed] [Google Scholar]
- Lemanski LF, Fitts EP, & Marx BS (1975). Fine structure of the heart in the Japanese Medaka, Oryzias latipes. Journal of Ultrastructure Research, 53(1), 37–65. [DOI] [PubMed] [Google Scholar]
- Léránth C, & Frotscher M (1986). Synaptic connections of cholecystokinin-immunoreactive neurons and terminals in the rat fascia dentata: A combined light and electron microscopic study. Journal of Comparative Neurology, 254(1), 51–64. [DOI] [PubMed] [Google Scholar]
- Li YC, Li YN, Cheng CX, Sakamoto H, Kawate T, Shimada O, et al. (2005). Subsurface cisterna-lined axonal invaginations and double-walled vesicles at the axonal-myelin sheath interface. Neuroscience Research, 53(3), 298–303. [DOI] [PubMed] [Google Scholar]
- Li W, Ochalski PA, Brimijoin S, Jordan LM, & Nagy JI (1995). C-terminals on motoneurons: Electron microscope localization of cholinergic markers in adult rats and antibody-induced depletion in neonates. Neuroscience, 65(3), 879–891. [DOI] [PubMed] [Google Scholar]
- Liets LC, Eliasieh K, van der List DA, & Chalupa LM (2006). Dendrites of rod bipolar cells sprout in normal aging retina. Proceedings of the National Academy of Sciences USA, 103(32), 12156–12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linberg KA, & Fisher SK (1986). An ultrastructural study of interplexiform cell synapses in the human retina. Journal of Comparative Neurology, 243(4), 561–576. [DOI] [PubMed] [Google Scholar]
- Linberg KA, & Fisher SK (1988). Ultrastructural evidence that horizontal cell axon terminals are presynaptic in the human retina. Journal of Comparative Neurology, 268(2), 281–297. [DOI] [PubMed] [Google Scholar]
- Lissmann HW, & Mullinger AM (1968). Organization of ampullary electric receptors in Gymnotidae (Pisces). Proceedings of the Royal Society of London, Series B: Biological Sciences, 169(1017), 345–378. [DOI] [PubMed] [Google Scholar]
- Liu X, Hirano AA, Sun X, Brecha NC, & Barnes S (2013). Calcium channels in rat horizontal cells regulate feedback inhibition of photoreceptors through an unconventional GABA- and pH-sensitive mechanism. Journal of Physiology, 591(13), 3309–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhao JW, Du JL, & Yang XL (2005). Functional GABA(B) receptors are expressed at the cone photoreceptor terminals in bullfrog retina. Neuroscience, 132(1), 103–113. [DOI] [PubMed] [Google Scholar]
- Macrae EK (1963). Observations on the fine structure of pharyngeal muscle in the planarian Dugesia tigrina. Journal of Cell Biology, 18, 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadeva B, Phillips LH 2nd, & Juel VC (2008). Autoimmune disorders of neuromuscular transmission. Seminars in Neurology, 28(2), 212–227. [DOI] [PubMed] [Google Scholar]
- Manni L, Mackie GO, Caicci F, Zaniolo G, & Burighel P (2006). Coronal organ of ascidians and the evolutionary significance of secondary sensory cells in chordates. Journal of Comparative Neurology, 495(4), 363–373. [DOI] [PubMed] [Google Scholar]
- Marotte LR, & Mark RF (1970). The mechanism of selective reinnervation of fish eye muscle. II. Evidence from electronmicroscopy of nerve endings. Brain Research, 19(1), 53–62. [DOI] [PubMed] [Google Scholar]
- Marques MJ, Conchello JA, & Lichtman JW (2000). From plaque to pretzel: Fold formation and acetylcholine receptor loss at the developing neuromuscular junction. Journal of Neuroscience, 20(10), 3663–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AR (1994). Amplification of neuromuscular transmission by postjunctional folds. Proceedings of the Royal Society of London B: Biological Sciences, 258(1353), 321–326. [DOI] [PubMed] [Google Scholar]
- Matthews-Bellinger J, & Salpeter MM (1978). Distribution of acetylcholine receptors at frog neuromuscular-junctions with a discussion of some physiological implications. Journal of Physiology-London, 279, 197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe BD, Marques G, Haghighi AP, Fetter RD, Crotty ML, Haerry TE, et al. (2003). The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron, 39(2), 241–254. [DOI] [PubMed] [Google Scholar]
- McKenna OC, & Rosenbluth J (1973). Myoneural and intermuscular junctions in a molluscan smooth muscle. Journal of Ultrastructure Research, 42(5), 434–450. [DOI] [PubMed] [Google Scholar]
- Melamed J, & Trujillo-Cenóz O (1971). Innervation of the retinal muscles in wolf spiders (Araneae-Lycosidae). Journal of Ultrastructure Research, 35(3), 359–369. [DOI] [PubMed] [Google Scholar]
- Mescher AL (2016). Junqueira’s Basic Histology Text and Atlas (14th ed.). New York: McGraw Hill Education. [Google Scholar]
- Mill PJ, & Knapp MF (1970a). Neuromuscular junctions in the body wall muscles of the earthworm, Lumbricus terrestris Linn. Journal of Cell Science, 7(1), 263–271. [DOI] [PubMed] [Google Scholar]
- Mill PJ, & Knapp MF (1970b). The fine structure of obliquely striated body wall muscles in the earthworm, Lumbricus terrestris Linn. Journal of Cell Science, 7(1), 233–261. [DOI] [PubMed] [Google Scholar]
- Miller T, & Adams ME (1974). Ultrastructure and electrical properties of the hyperneural muscle of Periplaneta americana. Journal of Insect Physiology, 20(10), 1925–1936. [DOI] [PubMed] [Google Scholar]
- Mitchell N, Petralia RS, Currier DG, Wang YX, Kim A, Mattson MP, et al. (2012). Sonic hedgehog regulates presynaptic terminal size, ultrastructure and function in hippocampal neurons. Journal of Cell Science, 125(Pt 18), 4207–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochet M, Moravec J, Guillemot H, & Hatt PY (1975). The ultrastructure of rat conductive tissue; an electron microscopic study of the atrioventricular node and the bundle of His. Journal of Molecular and Cellular Cardiology, 7(12), 879–889. [DOI] [PubMed] [Google Scholar]
- Moraes GD, Achaval M, Piva MMD, Faccioni-Heuser MC, Wassermann GF, & Zancan DM (2010). Ultrastructural analysis of the dorsal body gland of the terrestrial snail Megalobulimus abbreviatus (Becquaert, 1948). Brazilian Journal of Biology, 70(2), 341–350. [DOI] [PubMed] [Google Scholar]
- Morgan DL, & Proske U (1984). Vertebrate slow muscle: Its structure, pattern of innervation, and mechanical properties. Physiological Reviews, 64(1), 103–169. [DOI] [PubMed] [Google Scholar]
- Msghina M, Govind CK, & Atwood HL (1998). Synaptic structure and transmitter release in crustacean phasic and tonic motor neurons. Journal of Neuroscience, 18(4), 1374–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnaini E, Walberg F, & Brodal A (1967). Mode of termination of primary vestibular fibres in the lateral vestibular nucleus. An experimental electron microscopical study in the cat. Experimental Brain Research, 4(3), 187–211. [DOI] [PubMed] [Google Scholar]
- Mullinger AM (1969). The organization of ampullary sense organs in the electric fish, Gymnarchus niloticus. Tissue Cell, 1(1), 31–52. [DOI] [PubMed] [Google Scholar]
- Murakami M, Shimoda Y, Nakatani K, Miyachi E, & Watanabe S (1982). GABA-mediated negative feedback from horizontal cells to cones in carp retina. Japanese Journal of Physiology, 32(6), 911–926. [DOI] [PubMed] [Google Scholar]
- Murray M, Zimmer J, & Raisman G (1979). Quantitative electron-microscopic evidence for re-Innervation in the adult-rat interpeduncular nucleus after lesions of the fasciculus retroflexus. Journal of Comparative Neurology, 187(2), 447–468. [DOI] [PubMed] [Google Scholar]
- Nagel A, Lehmann-Horn F, & Engel AG (1990). Neuromuscular transmission in the mdx mouse. Muscle and Nerve, 13(8), 742–749. [DOI] [PubMed] [Google Scholar]
- Nägerl UV, Willig KI, Hein B, Hell SW, & Bonhoeffer T (2008). Live-cell imaging of dendritic spines by STED microscopy. Proceedings of the National Academy of Sciences USA, 105(48), 18982–18987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JI, Bautista W, Blakley B, & Rash JE (2013). Morphologically mixed chemical-electrical synapses formed by primary afferents in rodent vestibular nuclei as revealed by immunofluorescence detection of connexin36 and vesicular glutamate transporter-1. Neuroscience, 252, 468–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y (1969). Fine structure of red and white muscle fibers and their neuromuscular junctions in the snake fish (Ophiocephalus argus). Tissue and Cell, 1(2), 229–246. [DOI] [PubMed] [Google Scholar]
- Neises GR, Mattox DE, & Gulley RL (1982). The maturation of the end bulb of Held in the rat anteroventral cochlear nucleus. Anatomical Record, 204(3), 271–279. [DOI] [PubMed] [Google Scholar]
- Nguyen CT, & Stewart BA (2016). The influence of postsynaptic structure on missing quanta at the Drosophila neuromuscular junction. BMC Neuroscience, 17, 53 (51–11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunzi MG, & Franzini Armstrong C (1981). The structure of smooth and striated portions of the adductor muscle of the valves in a scallop. Journal of Ultrastructure Research, 76(2), 134–148. [DOI] [PubMed] [Google Scholar]
- O’Connor AK, O’Brien RD, & Salpeter MM (1965). Pharmacology and fine structure of peripheral muscle innervation in the cockroach Periplaneta americana. Journal of Insect Physiology, 11(10), 1351–1358. [DOI] [PubMed] [Google Scholar]
- Oberdorfer MD (1977). The neural organization of the first optic ganglion of the principle eyes of jumping spiders (Salticidae). Journal of Comparative Neurology, 174(1), 95–118. [DOI] [PubMed] [Google Scholar]
- Ohkawa T, Satake S, Yokoi N, Miyazaki Y, Ohshita T, Sobue G, et al. (2014). Identification and characterization of GABA(A) receptor autoantibodies in autoimmune encephalitis. Journal of Neuroscience, 34(24), 8151–8163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Økland S (1980). The heart ultrastructure of Lepidopleurus asellus (Spengler) and Tonicella marmorea (Fabricius) (Mollusca, Polyplacophora). Zoomorphology, 96(1–2), 1–19. [Google Scholar]
- Oleskevich S, Youssoufian M, & Walmsley B (2004). Presynaptic plasticity at two giant auditory synapses in normal and deaf mice. Journal of Physiology, 560(Pt 3), 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omiya Y, Uchigashima M, Konno K, Yamasaki M, Miyazaki T, Yoshida T, et al. (2015). VGluT3-expressing CCK-positive basket cells construct invaginating synapses enriched with endocannabinoid signaling proteins in particular cortical and cortex-like amygdaloid regions of mouse brains. Journal of Neuroscience, 35(10), 4215–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne MP (1967). Fine Structure of Neuromuscular Junctions in Segmental Muscles of Blowfly Larva. Journal of Insect Physiology, 13(6), 827–833. [Google Scholar]
- Ovalle WK, Dow PR, & Nahirney PC (1999). Structure, distribution and innervation of muscle spindles in avian fast and slow skeletal muscle. Journal of Anatomy, 194(Pt 3), 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padykula HA, & Gauthier GF (1970). The ultrastructure of the neuromuscular junctions of mammalian red, white, and intermediate skeletal muscle fibers. Journal of Cell Biology, 46(1), 27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page SG (1965). A comparison of fine structures of frog slow and twitch muscle fibres. Journal of Cell Biology, 26(2), 477–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S (1968). Structure of the sarcoplasmic reticulum in vertebrate muscle. British Medical Bulletin, 24(2), 170–173. [DOI] [PubMed] [Google Scholar]
- Pan SC (1980). The fine structure of the miracidium of Schistosoma mansoni. Journal of Invertebrate Pathology, 36(3), 307–372. [DOI] [PubMed] [Google Scholar]
- Parks TN (1981). Morphology of axosomatic endings in an avian cochlear nucleus: Nucleus magnocellularis of the chicken. Journal of Comparative Neurology, 203(3), 425–440. [DOI] [PubMed] [Google Scholar]
- Pattnaik B, Jellali A, Sahel J, Dreyfus H, & Picaud S (2000). GABAC receptors are localized with microtubule-associated protein 1B in mammalian cone photoreceptors. Journal of Neuroscience, 20(18), 6789–6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavans de Ceccatty M (1966). Ultrastructures et rapports des cellules mesenchymateuses de type nerveux de l’eponge Tethya lyncurium Link. Annales des Sciences Naturelles—Zoologie et Biologie Animale, 8, 577–614. [Google Scholar]
- Pearce J, Govind CK, & Meiss DE (1985). Growth-related features of lobster neuromuscular terminals. Brain Research, 353(2), 215–228. [DOI] [PubMed] [Google Scholar]
- Peichl L, & González-Soriano J (1994). Morphological types of horizontal cell in rodent retinae: A comparison of rat, mouse, gerbil, and guinea pig. Visual Neuroscience, 11(3), 501–517. [DOI] [PubMed] [Google Scholar]
- Pentreath VW, & Cobb JL (1972). Neurobiology of echinodermata. Biological Reviews of the Cambridge Philosophical Society, 47(3), 363–392. [DOI] [PubMed] [Google Scholar]
- Peters A, & Kaiserman-Abramof IR (1970). The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. American Journal of Anatomy, 127(4), 321–356. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Mattson MP, & Yao PJ (2014). Communication breakdown: The impact of ageing on synapse structure. Ageing Research Reviews, 14, 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, & Peusner KD (1990). Ultrastructural study of synapses at the time of neuronal migration and early differentiation in the tangential vestibular nucleus of the chick embryo in vivo. Journal of Comparative Neurology, 292(2), 231–245. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Mattson MP, & Yao PJ (2015). Structure, distribution, and function of neuronal/synaptic spinules and related invaginating projections. Neuromolecular Medicine, 17(3), 211–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Mattson MP, & Yao PJ (2016). The diversity of spine synapses in animals. NeuroMolecular Medicine, 18, 497–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pette D, & Staron RS (1997). Mammalian skeletal muscle fiber type transitions. International Review of Cytology, 170, 143–223. [DOI] [PubMed] [Google Scholar]
- Peusner KD (1984). The development of synapses and “spoon” synaptic terminal space in the tangential vestibular nucleus: A quantitative electron microscope study. Journal of Comparative Neurology, 230(3), 372–385. [DOI] [PubMed] [Google Scholar]
- Peusner KD, & Giaume C (1994). The first developing “mixed” synapses between vestibular sensory neurons mediate glutamate chemical transmission. Neuroscience, 58(1), 99–113. [DOI] [PubMed] [Google Scholar]
- Piscopo S, Moccia F, Di Cristo C, Caputi L, Di Cosmo A, & Brown ER (2007). Pre- and postsynaptic excitation and inhibition at octopus optic lobe photoreceptor terminals; implications for the function of the ‘presynaptic bags’. European Journal of Neuroscience, 26(8), 2196–2203. [DOI] [PubMed] [Google Scholar]
- Popova E (2014). Role of dopamine in distal retina. Journal of Comparative Physiology A, 200(5), 333–358. [DOI] [PubMed] [Google Scholar]
- Prokop A (1999). Integrating bits and pieces: Synapse structure and formation in Drosophila embryos. Cell and Tissue Research, 297(2), 169–186. [DOI] [PubMed] [Google Scholar]
- Prokop A (2006). Organization of the efferent system and structure of neuromuscular junctions in Drosophila. International Review of Neurobiology, 75, 71–90. [DOI] [PubMed] [Google Scholar]
- Prokop A, & Meinertzhagen IA (2006). Development and structure of synaptic contacts in Drosophila. Seminars in Cell & Developmental Biology, 17(1), 20–30. [DOI] [PubMed] [Google Scholar]
- Pucci I, & Afzelius BA (1962). Electron microscope study of sarcotubules and related structures in the leech muscle. Journal of Ultrastructure Research, 7, 210–224. [Google Scholar]
- Puthussery T, Percival KA, Venkataramani S, Gayet-Primo J, Grünert U, & Taylor WR (2014). Kainate receptors mediate synaptic input to transient and sustained OFF visual pathways in primate retina. Journal of Neuroscience, 34(22), 7611–7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola E, & Gilula NB (1975). Intramembrane organization of specialized contacts in the outer plexiform layer of the retina. A freeze-fracture study in monkeys and rabbits. Journal of Cell Biology, 65(1), 192–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger JF (1961). The fine structure of neuromuscular junctions and the sarcoplasmic reticulum of extrinsic eye muscles of Fundulus heteroclitus. The Journal of Biophysical and Biochemical Cytology, 10(4), 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger JF (1965). Fine Structure of Neuromuscular Junctions and Contact Zones between Body Wall Muscle Cells of Ascaris lumbricoides (Var Suum). Zeitschrift für Zellforschung und mikroskopische Anatomie, 67(2), 196–210. [DOI] [PubMed] [Google Scholar]
- Reger JF (1969). Studies on the fine structure of muscle fibres and contained crystalloids in basal socket muscle of the entoproct, Barentsia gracilis. Journal of Cell Science, 4(2), 305–325. [DOI] [PubMed] [Google Scholar]
- Rheuben MB (1985). Quantitative comparison of the structural features of slow and fast neuromuscular junctions in Manduca. Journal of Neuroscience, 5(7), 1704–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheuben MB, & Reese TS (1978). Three-dimensional structure and membrane specializations of moth excitatory neuromuscular synapse. Journal of Ultrastructure Research, 65(2), 95–111. [DOI] [PubMed] [Google Scholar]
- Richardson KC (1964). The fine structure of the albino rabbit iris with special reference to the identification of adrenergic and cholinergic nerves and nerve endings in its intrinsic muscles. American Journal of Anatomy, 114, 173–205. [DOI] [PubMed] [Google Scholar]
- Rieger RM, & Rieger GE (1975). Fine structure of the pharyngeal bulb in Trilobodrilus and its phylogenetic significance within Archiannelida. Tissue and Cell, 7(2), 267–279. [DOI] [PubMed] [Google Scholar]
- Rivlin PK, St Clair RM, Vilinsky I, & Deitcher DL (2004). Morphology and molecular organization of the adult neuromuscular junction of Drosophila. Journal of Comparative Neurology, 468(4), 596–613. [DOI] [PubMed] [Google Scholar]
- Robertson JD (1956). The ultrastructure of a reptilian myoneural junction. The Journal of Biophysical and Biochemical Cytology, 2(4), 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PM, Perry RA, Hardy KJ, Coghlan JP, & Scoggins BA (1977). Innervation of adrenal-cortex in sheep, Ovis ovis. Journal of Anatomy, 124, 117–129. [PMC free article] [PubMed] [Google Scholar]
- Roelandse M, Welman A, Wagner U, Hagmann J, & Matus A (2003). Focal motility determines the geometry of dendritic spines. Neuroscience, 121(1), 39–49. [DOI] [PubMed] [Google Scholar]
- Rogers DC (1969). Fine structure of smooth muscle and neuromuscular junctions in the foot of Helix aspersa. Zeitschrift für Zellforschung und mikroskopische Anatomie, 99(3), 315–335. [DOI] [PubMed] [Google Scholar]
- Rogers DC, & Burnstock G (1966). Muliaxonal autonomic junctions in intestinal smooth muscle of the toad (Bufo marinus). Journal of Comparative Neurology, 126(4), 625–652. [PubMed] [Google Scholar]
- Ronnevi LO (1977). Spontaneous phagocytosis of boutons on spinal motoneurons during early postnatal-development—Electron microscopical study in cat. Journal of Neurocytology, 6(5), 487–504. [DOI] [PubMed] [Google Scholar]
- Ronnevi LO (1979). Spontaneous phagocytosis of C-type synaptic terminals by spinal alpha-motoneurons in newborn kittens. An electron microscopic study. Brain Research, 162(2), 189–199. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J (1965). Ultrastructure of somatic muscle cells in Ascaris lumbricoides. II. Intermuscular junctions, neuromuscular junctions, and glycogen stores. Journal of Cell Biology, 26(2), 579–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth J (1972). Myoneural junctions of two ultrastructurally distinct types in earthworm body wall muscle. Journal of Cell Biology, 54(3), 566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth J (1973). Postjunctional membrane specialization at cholinergic myoneural junctions in the leech. Journal of Comparative Neurology, 151(4), 399–406. [DOI] [PubMed] [Google Scholar]
- Rosser BWC, George JC, & Frombach SK (1987). Architecture of the pectoralis-muscle of the Japanese-Quail (Coturnix japonica)—Histochemical and ultrastructural characterization, and distribution of muscle-fiber types. Canadian Journal of Zoology-Revue Canadienne De Zoologie, 65(1), 63–71. [Google Scholar]
- Roth A, & Tscharntke H (1976). Ultrastructure of the ampullary electroreceptors in lungfish and Brachiopterygii. Cell and Tissue Research, 173(1), 95–108. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Montey KL, Wright AL, Bennett ML, & Pongstaporn T (2006). Postnatal development of a large auditory nerve terminal: The endbulb of Held in cats. Hearing Research, 216–217, 100–115. [DOI] [PubMed] [Google Scholar]
- Saetersdal TS, Justesen NP, & Krohnstad AW (1974). Ultrastructure and innervation of the teleostean atrium. Journal of Molecular and Cellular Cardiology, 6(5), 415–437. [DOI] [PubMed] [Google Scholar]
- Sakai H, & Naka KI (1983). Synaptic organization involving receptor, horizontal and on- and off-center bipolar cells in the catfish retina. Vision Research, 23(4), 339–351. [DOI] [PubMed] [Google Scholar]
- Sakai HM, & Naka K (1986). Synaptic organization of the cone horizontal cells in the catfish retina. Journal of Comparative Neurology, 245(1), 107–115. [DOI] [PubMed] [Google Scholar]
- Samuel MA, Zhang Y, Meister M, & Sanes JR (2011). Age-related alterations in neurons of the mouse retina. Journal of Neuroscience, 31(44), 16033–16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, & Lichtman JW (1999). Development of the vertebrate neuromuscular junction. Annual Review of Neuroscience, 22, 389–442. [DOI] [PubMed] [Google Scholar]
- Santa T, Engel AG, & Lambert EG (1972). Histometric study of neuromuscular junction ultrastructure. II. Myasthenic syndrome. Neurology, 22(4), 370–376. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, & Reggiani C (2011). Fiber types in mammalian skeletal muscles. Physiological Reviews, 91(4), 1447–1531. [DOI] [PubMed] [Google Scholar]
- Sethi CS, Lewis GP, Fisher SK, Leitner WP, Mann DL, Luthert PJ, et al. (2005). Glial remodeling and neural plasticity in human retinal detachment with proliferative vitreoretinopathy. Investigative Ophthalmology & Visual Science, 46(1), 329–342. [DOI] [PubMed] [Google Scholar]
- Shao M, Hirsch JC, Giaume C, & Peusner KD (2004). Spontaneous synaptic activity in chick vestibular nucleus neurons during the perinatal period. Neuroscience, 127(1), 81–90. [DOI] [PubMed] [Google Scholar]
- Shaw SR (1975). Retinal resistance barriers and electrical lateral inhibition. Nature, 255(5508), 480–483. [DOI] [PubMed] [Google Scholar]
- Shepherd GM (2004). The Synaptic Organization of the Brain (5th ed.). New York: Oxford University Press. [Google Scholar]
- Sherman RG (1973). Ultrastructural features of cardiac muscle cells in a tarantula spider. Journal of Morphology, 140(2), 215–241. [DOI] [PubMed] [Google Scholar]
- Sherman RG, & Atwood HL (1972). Correlated electrophysiological and ultrastructural studies of a crustacean motor unit. Journal of General Physiology, 59(5), 586–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman RG, & Fourtner CR (1972). Ultrastructural features of synaptic regions in walking leg muscles of the horseshoe crab, Limulus polyphemus (L.). Journal of Ultrastructure Research, 40(1), 44–54. [DOI] [PubMed] [Google Scholar]
- Sherman RG, & Luff AR (1971). Structural features of tarsal claw muscles of spider Eurypelma marxi Simon. Canadian Journal of Zoology, 49(12), 1549–1556. [Google Scholar]
- Shi L, Fu AKY, & Ip NY (2012). Molecular mechanisms underlying maturation and maintenance of the vertebrate neuromuscular junction. Trends in Neurosciences, 35(7), 441–453. [DOI] [PubMed] [Google Scholar]
- Slater CR (2008). Structural factors influencing the efficacy of neuromuscular transmission. Myasthenia Gravis and Related Disorders: 11th International Conference, 1132, 1–12. [DOI] [PubMed] [Google Scholar]
- Smith DS (1960). Innervation of the fibrillar flight muscle of an insect: Tenebrio molitor (Coleoptera). The Journal of Biophysical and Biochemical Cytology, 8(2), 447–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DS (1966). The organization and function of the sarcoplasmic reticulum and T-system of muscle cells. Progress in Biophysics and Molecular Biology, 16, 107–142. [DOI] [PubMed] [Google Scholar]
- Smith DS (1971). On the significance of cross-bridges between microtubules and synaptic vesicles. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 261(839), 395–405. [DOI] [PubMed] [Google Scholar]
- Sommer JR, & Johnson EA (1968). Cardiac muscle. A comparative study of Purkinje fibers and ventricular fibers. Journal of Cell Biology, 36(3), 497–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer JR, & Johnson EA (1969). Cardiac muscle. A comparative ultrastructural study with special reference to frog and chicken hearts. Zeitschrift für Zellforschung und mikroskopische Anatomie, 98(3), 437–468. [PubMed] [Google Scholar]
- Sotelo C, & Palay SL (1968). The fine structure of the lateral vestibular nucleus in the rat. I. Neurons and neuroglial cells. Journal of Cell Biology, 36(1), 151–179. [PubMed] [Google Scholar]
- Sotelo C, & Palay SL (1970). The fine structure of the later vestibular nucleus in the rat. II. Synaptic organization. Brain Research, 18(1), 93–115. [DOI] [PubMed] [Google Scholar]
- Spencer AN (1979). Neurobiology of Polyorchis. II. Structure of effector systems. Journal of Neurobiology, 10(2), 95–117. [DOI] [PubMed] [Google Scholar]
- Stefanelli A (1937). Il sistema statico dei Petromizonti (sistema laterale, sistema vestibolare, cervelletto). I: Centri nervosi e vie centrali. Archivio Zoologico italiano, 24, 209–273. [Google Scholar]
- Stefanelli A, & Caravita S (1970). Ultrastructural features of the synaptic complex of the vestibular nuclei of Lampetra planeri (Bloch). Zeitschrift für Zellforschung und mikroskopische Anatomie, 108(2), 282–296. [DOI] [PubMed] [Google Scholar]
- Steinmetz PR, Kraus JE, Larroux C, Hammel JU, Amon-Hassenzahl A, Houliston E, et al. (2012). Independent evolution of striated muscles in cnidarians and bilaterians. Nature, 487(7406), 231–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P, & Demb JB (2004). Retina. In Shepherd GM (Ed.), The Synaptic Organization of the Brain (5th ed., pp. 217–269). New York: Oxford University Press. [Google Scholar]
- Sterling P, & Matthews G (2005). Structure and function of ribbon synapses. Trends in Neurosciences, 28(1), 20–29. [DOI] [PubMed] [Google Scholar]
- Sulbarán G, Alamo L, Pinto A, Márquez G, Méndez F, Padrón R, et al. (2015). An invertebrate smooth muscle with striated muscle myosin filaments. Proceedings of the National Academy of Sciences USA, 112(42), E5660–E5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RK, Woldemussie E, & Pow DV (2007). Dendritic and synaptic plasticity of neurons in the human age-related macular degeneration retina. Investigative Ophthalmology & Visual Science, 48(6), 2782–2791. [DOI] [PubMed] [Google Scholar]
- Suwa H, Gilland E, & Baker R (1999). Otolith ocular reflex function of the tangential nucleus in teleost fish. Annals of the New York Academy of Sciences, 871, 1–14. [DOI] [PubMed] [Google Scholar]
- Suzuki H, & Tasaki K (1983). Inhibitory retinal efferents from dopaminergic cells in the optic lobe of the octopus. Vision Research, 23(4), 451–457. [DOI] [PubMed] [Google Scholar]
- Szamier RB, & Bennett MV (1973). Rapid degeneration of ampullary electroreceptor organs after denervation. Journal of Cell Biology, 56(2), 466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szamier RB, & Bennett MVL (1980). Ampullary electroreceptors in the fresh-water ray. Potamotrygon. Journal of Comparative Physiology, 138(3), 225–230. [Google Scholar]
- Szamier RB, & Wachtel AW (1969). Special cutaneous receptor organs of fish.3. Ampullary organs of Eigenmannia. Journal of Morphology, 128(3), 261–290. [DOI] [PubMed] [Google Scholar]
- Szpir MR, Sento S, & Ryugo DK (1990). Central projections of cochlear nerve fibers in the alligator lizard. Journal of Comparative Neurology, 295(4), 530–547. [DOI] [PubMed] [Google Scholar]
- Takasaka T, & Smith CA (1971). The structure and innervation of the pigeon’s basilar papilla. Journal of Ultrastructure Research, 35(1), 20–65. [DOI] [PubMed] [Google Scholar]
- Tan X, Beurg M, Hackney C, Mahendrasingam S, & Fettiplace R (2013). Electrical tuning and transduction in short hair cells of the chicken auditory papilla. Journal of Neurophysiology, 109(8), 2007–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CK, & Wong WC (1980). Innervation of the muscularis externa in the stomach of a coral fish, Chelmon rostratus Cuvier. Journal of Anatomy, 130(Pt 2), 289–303. [PMC free article] [PubMed] [Google Scholar]
- Tatsukawa T, Hirasawa H, Kaneko A, & Kaneda M (2005). GABA-mediated component in the feedback response of turtle retinal cones. Visual Neuroscience, 22(3), 317–324. [DOI] [PubMed] [Google Scholar]
- Taxi J (1964). Electron microscope study of the innervation of intestinal smooth muscle, compared to that of some other mammalian smooth muscles. Archives de Biologie (Liege), 75, 301–328. [PubMed] [Google Scholar]
- Teeter JH, Szamier RB, & Bennett MVL (1980). Ampullary electroreceptors in the sturgeon Scaphirhynchus platorynchus (Rafinesque). Journal of Comparative Physiology, 138(3), 213–223. [Google Scholar]
- Teräväinen H (1968a). Electron microscopic and histochemical observations on different types of nerve endings in the extraocular muscles of the rat. Zeitschrift für Zellforschung und mikroskopische Anatomie, 90(3), 372–388. [DOI] [PubMed] [Google Scholar]
- Teräväinen H (1968b). Development of the myoneural junction in the rat. Zeitschrift für Zellforschung und mikroskopische Anatomie, 87(2), 249–265. [DOI] [PubMed] [Google Scholar]
- Teräväinen H (1971). Anatomical and physiological studies on muscles of lamprey. Journal of Neurophysiology, 34(6), 954–973. [DOI] [PubMed] [Google Scholar]
- Terzibasi E, Calamusa M, Novelli E, Domenici L, Strettoi E, & Cellerino A (2009). Age-dependent remodelling of retinal circuitry. Neurobiology of Aging, 30(5), 819–828. [DOI] [PubMed] [Google Scholar]
- Teuchert G (1977). Ultrastructure of marine gastrotrich Turbanella cornuta Remane (Macrodasyoidea) and its functional and phylogenetical importance. Zoomorphologie, 88(3), 189–246. [Google Scholar]
- Thaemert JC (1966). Ultrastructure of cardiac muscle and nerve contiguities. Journal of Cell Biology, 29(1), 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Babai N, & Bartoletti TM (2008). Feedback from horizontal cells to rod photoreceptors in vertebrate retina. Journal of Neuroscience, 28(22), 5691–5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, & Mangel SC (2012). Lateral interactions in the outer retina. Progress in Retinal and Eye Research, 31(5), 407–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titmus MJ (1981). Ultrastructure of identified fast excitatory, slow excitatory and inhibitory neuromuscular junctions in the locust. Journal of Neurocytology, 10(3), 363–385. [DOI] [PubMed] [Google Scholar]
- Torres LF, & Duchen LW (1987). The mutant mdx: Inherited myopathy in the mouse. Morphological studies of nerves, muscles and end-plates. Brain, 110(Pt 2), 269–299. [DOI] [PubMed] [Google Scholar]
- Torroja L, Packard M, Gorczyca M, White K, & Budnik V (1999). The Drosophila beta-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction. Journal of Neuroscience, 19(18), 7793–7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwein W, & Uchizono K (1963). Electron microscopic and electrophysiologic study of the pacemaker in the sino-atrial node of the rabbit heart. Zeitschrift für Zellforschung und mikroskopische Anatomie, 61, 96–109. [DOI] [PubMed] [Google Scholar]
- Trujillo-Cenóz O, & Melamed J (1967). The fine structure o the visual system of Lycosa (Araneae: Lycosidae). II. Primary visual centers. Zeitschrift für Zellforschung und mikroskopische Anatomie, 76(3), 377–388. [DOI] [PubMed] [Google Scholar]
- Unsicker K (1971). Innervation of mammalian endocrine glands (anterior pituitary and parathyroids). Zeitschrift für Zellforschung und mikroskopische Anatomie, 121(2), 283–291. [DOI] [PubMed] [Google Scholar]
- Van den Berge H, & Wirtz P (1989). Detailed morphology of the stapedius muscle of the rat. An integrated light microscopical, morphometrical, histochemical, immunohistochemical and electron microscopical study in relation to function. Journal of Anatomy, 166, 157–169. [PMC free article] [PubMed] [Google Scholar]
- Van Vactor D, & Sigrist SJ (2017). Presynaptic morphogenesis, active zone organization and structural plasticity in Drosophila. Current Opinion in Neurobiology, 43, 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi N, Morigiwa K, Wang TL, Shi YJ, & Sterling P (1998). Neurochemistry of the mammalian cone ‘synaptic complex’. Vision Research, 38(10), 1359–1369. [DOI] [PubMed] [Google Scholar]
- Vardi N, & Sterling P (1994). Subcellular localization of GABAA receptor on bipolar cells in macaque and human retina. Vision Research, 34(10), 1235–1246. [DOI] [PubMed] [Google Scholar]
- Varela C, Igartua I, De la Rosa EJ, & De la Villa P (2003). Functional modifications in rod bipolar cells in a mouse model of retinitis pigmentosa. Vision Research, 43(8), 879–885. [DOI] [PubMed] [Google Scholar]
- Veggetti A, Mascarello F, & Carpene E (1982). A comparative histochemical study of fibre types in middle ear muscles. Journal of Anatomy, 135(Pt 2), 333–352. [PMC free article] [PubMed] [Google Scholar]
- Wagner N, Laugks U, Heckmann M, Asan E, & Neuser K (2015). Aging Drosophila melanogaster display altered pre- and postsynaptic ultrastructure at adult neuromuscular junctions. Journal of Comparative Neurology, 523(16), 2457–2475. [DOI] [PubMed] [Google Scholar]
- Walrond JP, & Reese TS (1985). Structure of axon terminals and active zones at synapses on lizard twitch and tonic muscle fibers. Journal of Neuroscience, 5(5), 1118–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Wenthold RJ, Ottersen OP, & Petralia RS (1998). Endbulb synapses in the anteroventral cochlear nucleus express a specific subset of AMPA-type glutamate receptor subunits. Journal of Neuroscience, 18(3), 1148–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, & Yasuda M (1977). Electron microscopic study on the innervation of the pancreas of the domestic fowl. Cell and Tissue Research, 180(4), 453–465. [DOI] [PubMed] [Google Scholar]
- Watari N (1968). Fine structure of nervous elements in the pancreas of some vertebrates. Zeitschrift für Zellforschung und mikroskopische Anatomie, 85(3), 291–314. [DOI] [PubMed] [Google Scholar]
- Weckström M, & Laughlin S (2010). Extracellular potentials modify the transfer of information at photoreceptor output synapses in the blowfly compound eye. Journal of Neuroscience, 30(28), 9557–9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler R, & Wagner HJ (1984). Light-dependent change of cone-horizontal cell-Interactions in carp retina. Brain Research, 298(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Westfall JA, Elliott CF, & Carlin RW (2002). Ultrastructural evidence for two-cell and three-cell neural pathways in the tentacle epidermis of the sea anemone Aiptasia pallida. Journal of Morphology, 251(1), 83–92. [DOI] [PubMed] [Google Scholar]
- Westfall JA, Kinnamon JC, & Sims DE (1980). Neuro-epitheliomuscular cell and neuro-neuronal gap junctions in Hydra. Journal of Neurocytology, 9(6), 725–732. [DOI] [PubMed] [Google Scholar]
- Westfall JA, Yamataka S, & Enos PD (1971). Ultrastructural evidence of polarized synapses in the nerve net of Hydra. Journal of Cell Biology, 51(1), 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VJ, & Wylie RM (1970). A short-latency labyrinthine input to the vestibular nuclei in the pigeon. Science, 168(3927), 124–127. [DOI] [PubMed] [Google Scholar]
- Wolf H, & Harzsch S (2002). Evolution of the arthropod neuromuscular system. 2. Inhibitory innervation of the walking legs of a scorpion: Vaejovis spinigerus (Wood, 1863), Vaejovidae, Scorpiones, Arachnida. Arthropod Structure & Development, 31(3), 203–215. [DOI] [PubMed] [Google Scholar]
- Wong WC (1977). Ultrastructural localization of adrenergic nerve terminals in the circular muscle layer and muscularis mucosae of the rat duodenum after acute treatment with 6-hydroxydopamine. Journal of Anatomy, 124(Pt 3), 637–642. [PMC free article] [PubMed] [Google Scholar]
- Woods RI (1970). The innervation of the frog’s heart. 3. Electronmicroscopy of the autonomic nerve fibres and their vesicles. Proceedings of the Royal Society of London, Series B: Biological Sciences, 176(1042), 63–68. [DOI] [PubMed] [Google Scholar]
- Yaksta-Sauerland BA, & Coggeshall RE (1973). Neuromuscular junctions in the leech. Journal of Comparative Neurology, 151(1), 85–100. [DOI] [PubMed] [Google Scholar]
- Yamauchi A, & Burnstock G (1968). An electron microscopic study on the innervation of the trout heart. Journal of Comparative Neurology, 132(4), 567–588. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Studholme KM, Vitorica J, & de Blas AL (1989). Immunocytochemical localization of GABAA receptors in goldfish and chicken retinas. Journal of Comparative Neurology, 280(1), 15–26. [DOI] [PubMed] [Google Scholar]
- Ylä-Anttila P, Vihinen H, Jokitalo E, & Eskelinen EL (2009). Monitoring autophagy by electron microscopy in Mammalian cells. Methods in Enzymology, 452, 143–164. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Uchigashima M, Yamasaki M, Katona I, Yamazaki M, Sakimura K, et al. (2011). Unique inhibitory synapse with particularly rich endocannabinoid signaling machinery on pyramidal neurons in basal amygdaloid nucleus. Proceedings of the National Academy of Sciences USA, 108(7), 3059–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebe E, & Rathmayer W (1968). Elektronenmikroskopische untersuchungen an spinnenmuskeln. Zeitschrift für Zellforschung und mikroskopische Anatomie, 92, 377–387. [PubMed] [Google Scholar]
- Zeni C, & Zaffagnini F (1988). Occurrence of innervation in labral glands of Daphnia obtusa (Crustacea, Cladocera). Journal of Morphology, 198(1), 43–48. [DOI] [PubMed] [Google Scholar]