Abstract
Objective
To assess the effects of onabotulinumtoxinA treatment for chronic migraine (CM) on comorbid symptoms of depression, anxiety, fatigue and poor sleep quality.
Methods
The Chronic Migraine OnabotulinuMtoxinA Prolonged Efficacy open-Label (COMPEL) study is a multicentre, open-label, prospective study assessing the long-term safety and efficacy of onabotulinumtoxinA 155 U over nine treatments (108 weeks) in adults with CM. The Patient Health Questionnaire (PHQ-9) and Generalised Anxiety Disorder (GAD-7) scales were used to assess the effects of onabotulinumtoxinA on comorbid symptoms of depression and anxiety, respectively. A clinically meaningful improvement was assessed by the percentage of patients experiencing a ≥1 severity category reduction in PHQ-9 and GAD-7. The effects of onabotulinumtoxinA on associated sleep quality and fatigue were assessed using the Pittsburgh Sleep Quality Index and Fatigue Severity Scale, respectively.
Results
OnabotulinumtoxinA treatment was associated with sustained reduction in headache days and PHQ-9 and GAD-7 scores in the analysis population (n=715) over 108 weeks. PHQ-9 and GAD-7 scores were significantly reduced at all time points in patients with clinically significant symptoms of depression and/or anxiety at baseline. By week 108, 78.0% and 81.5% had clinically meaningful improvement in depression and anxiety symptoms, respectively. Sleep quality and symptoms of fatigue also improved; however, less is understood about clinically meaningful changes in these measures. No new safety concerns were identified.
Conclusion
In addition to reducing headache frequency, onabotulinumtoxinA treatment for CM was associated with clinically meaningful reduction in symptoms of depression and anxiety, and improved associated symptoms of poor sleep quality and fatigue.
Trial registration number
Keywords: anxiety, comorbidities, depression, onabotulinumtoxinA, sleep, fatigue
Introduction
Migraine, a common disabling neurological disease, can be episodic (EM; <15 headache days/month) or chronic (CM; ≥15 headache days/month for >3 months).1 Globally, migraine prevalence is 14.7%.2 Approximately 8% of people with migraine have CM,3 but CM is more burdensome than EM in terms of migraine-related disability, health-related quality of life,4 utilisation of healthcare resources and most headache treatments,5 and involvement of comorbid conditions.6
Depression and anxiety are often comorbid with migraine,6 7 and even more so with CM.4–6 Comorbid depression and anxiety are associated with negative outcomes,8 including increased rates of CM onset or progression to CM,9 reduced quality of life and increased overall disease burden,4 and can make migraine treatment more challenging.10 Studies have suggested a shared aetiology or underlying pathway for depression and migraine,11 12 which is likely bidirectional.13
Sleep disturbances are also associated with headache severity,14 appear to be related to progression to CM, have a bidirectional relationship with migraine15–17 and are more common in CM than EM.18 19 Less is known about fatigue and migraine; however, in those with migraine, fatigue is correlated with symptoms of depression and headache intensity measures.20
Given the likely associations among depression, anxiety, fatigue, poor sleep quality and increased migraine-related burden and risk of migraine disease progression, it is critical to understand the impact of migraine treatment on the manifestation and management of these comorbidities. Potential treatments for CM that also reduce psychiatric comorbidities, improve sleep quality and reduce associated fatigue symptoms would be valuable.
OnabotulinumtoxinA is approved for headache prevention in adults with CM.21 The safety and efficacy of onabotulinumtoxinA for CM treatment were first established in the Phase III REsearch Evaluating Migraine Prophylaxis Therapy (PREEMPT) trials.22 Compared with placebo, onabotulinumtoxinA significantly improved the frequency of headache days, Headache Impact Test (HIT-6) scores and Migraine-Specific Quality of Life scores at the end of the 24-week, double-blind treatment period, with further improvement observed after a 32-week, open-label phase.22
The Chronic migraine OnabotulinuMtoxinA Prolonged Efficacy open-Label (COMPEL) study was designed to extend the understanding of the long-term management of CM by evaluating the efficacy and safety of onabotulinumtoxinA after nine treatments (108 weeks). In the COMPEL study, in patients who persisted with treatment, onabotulinumtoxinA 155 U was found to be safe and effective for CM treatment over 108 weeks, reducing headache day frequency and HIT-6 scores in people with CM regardless of the presence of symptoms of comorbid disease.23
This analysis of the COMPEL study evaluates the effects of onabotulinumtoxinA CM treatment on clinically significant symptoms of depression and anxiety, as well as associated poor sleep quality and symptoms of fatigue. Additional exploratory analyses were conducted among those with depression or anxiety at baseline. These assessments compared clinically meaningful reductions in comorbid symptoms among those who responded to onabotulinumtoxinA treatment with clinically meaningful reductions in headache days with those who did not.
Methods
Study design
The COMPEL study was an international, multicentre, open-label, prospective study evaluating the long-term safety and efficacy of onabotulinumtoxinA 155 U (fixed-dose, fixed-site treatment paradigm) every 12 weeks for nine treatment cycles (108 weeks). Study methodology has been described previously23 and is summarised briefly here for context.
Study participants
Adults with CM and stable comorbidities were included in the study.23 Patients could be receiving oral preventive treatment for migraine (stable dose for >4 weeks prior to study and to remain unchanged up to week 24), but were excluded if they had previously been administered onabotulinumtoxinA, had severe major depressive disorder (Beck Depression Inventory-II score >24), reported suicidal ideation, as determined by endorsement of a non-zero response to item 9 (‘Thoughts that you would be better off dead, or hurting yourself in some way’) on the Patient Health Questionnaire (PHQ-9), or were pregnant or planning a pregnancy.23 There were no specific exclusion criteria relating to the degree of anxiety.
Efficacy and safety outcomes
The primary efficacy measure in the COMPEL study was change from baseline in headache days per 28-day period (headache frequency) at 108 weeks as assessed by patients’ daily diaries.23 Exploratory outcome measures included change in PHQ-9, Generalised Anxiety Disorder (GAD-7), Pittsburgh Sleep Quality Index (PSQI) and Fatigue Severity Scale (FSS) scores from baseline. Safety and tolerability were assessed at each visit for patients who received ≥1 onabotulinumtoxinA treatment.23 Patients were withdrawn from the study and referred for appropriate follow-up care if they became pregnant or reported or showed signs of suicidal ideation.
Assessment of comorbidities
The PHQ-9, querying the presence of nine diagnostic criteria for major depressive disorder24 based on the Diagnostic and Statistical Manual for Mental Disorders Fourth Edition (DSM-IV),25 was used to assess depressive symptoms. Respondents indicated how frequently they had each of the nine depression criteria over the preceding 2 weeks on a 4-point (0–3) scale, where 0=‘not at all’, 1=‘several days’, 2=‘more than half the days’ and 3=‘nearly every day’. The total scores range from 0 to 27 (none to most severe), distributed as 0–4 (minimal depressive symptoms), 5–9 (mild), 10–14 (moderate), 15–19 (moderately severe) and 20–27 (severe). Using the validated scoring system, a PHQ-9 sum score ≥15 is indicative of major depression.24
Similarly, the total GAD-7 score, consisting of seven anxiety criteria from the DSM-IV, was used to provide a measure of anxiety severity.26 Respondents rated how frequently they experienced seven anxiety symptoms over the preceding 2 weeks on a 4-point (0–3) scale. The total GAD-7 scores range from 0 to 21 (none to most severe), distributed as 0–4 (minimal symptoms of anxiety), 5–9 (mild), 10–14 (moderate) and 15–21 (severe). Using the validated scoring system, a GAD-7 score ≥10 indicates generalised anxiety disorder.
Sleep quality was assessed using the PSQI, a self-administered assessment of sleep disturbances that may affect sleep quality, including sleep apnoea.27 The total score for the seven components is usually used, with a higher score indicating more severe sleep disturbances. Poor sleep quality is associated with a PSQI global score >5 (range 0–21).28
Fatigue was assessed using the FSS, a validated measure assessing fatigue severity and its impact on daily living, scored on a 7-point (1–7) scale ranging from disagree (1) to agree (7).29 30 A higher score indicates more severe fatigue, with scores ≥36 considered significant fatigue (range 9–63).
Statistical analyses
Changes in symptoms of depression and anxiety in patients with CM with clinically significant depressive or anxiety symptoms at baseline were assessed after onabotulinumtoxinA treatment and were based on a null hypothesis of no improvement. The PHQ-9 was completed at screening for exclusion of suicidal ideation per the protocol, and the PHQ-9 and GAD-7 were completed at baseline and at weeks 12, 24, 48, 72 and 108. Changes in the PHQ-9 and GAD-7 scores from baseline were assessed in the total population and in those with clinically significant baseline depressive or anxiety symptoms. Consistent with other studies in which a decrease of ≥5 points for individuals with pretreatment PHQ-9 scores ≥8 was considered clinically relevant,31 32 a ≥1 severity category improvement in the PHQ-9 or GAD-7 score from baseline (eg, from moderate [PHQ-9 or GAD-7 score of 10–14] to mild [PHQ-9 or GAD-7 score of 5–9]) was considered clinically meaningful improvement.
A subgroup analysis was undertaken in patients who experienced ≥50% or ≥25% reductions in headache days at week 24 after onabotulinumtoxinA treatment compared with those who did not. The hypothesis was that if effects on depressive symptoms were a consequence of increased headache frequency, then depressive symptoms would be reduced only in those with clinically meaningful reductions in headache frequency. Alternatively, if there is a shared biology for CM and depression and anxiety for which onabotulinumtoxinA treatment has therapeutic benefit, then depression symptom reduction would be seen in those with or without a reduction in headache frequency. Therefore, changes in PHQ-9 and GAD-7 scores from baseline and percentages of patients with ≥1 severity category improvement in PHQ-9 and GAD-7 scores from baseline were assessed in those with ≥50% reductions in headache days at week 24, the traditionally defined responder rate, and also those with only ≥25% reductions.33
Analyses were also undertaken to determine onabotulinumtoxinA effects on sleep quality and fatigue symptom in the analysis population and for sleep quality in those with poor sleep quality (PSQI >5) at baseline. The PSQI and FSS were completed at baseline and at weeks 24, 60, 84 and 108. A ≥3-point decrease in the total PSQI score was considered to be a clinically meaningful improvement28; however, this definition has not been validated in clinical trials. No clinically meaningful improvement was defined for FSS scores as there was no validated or published definition available in the literature; however, patients with a ≥20% or ≥30% decrease in FSS score from baseline were assessed.
The main analyses for the changes in comorbid symptoms were based on observed data; per-protocol sensitivity analyses were undertaken using last observation carried forward for missing data. Tests of statistical significance were only carried out for the main analysis population and the subgroup analyses comparing outcomes in headache day reduction responders versus non-responders.
Results
Patient demographics and disposition
A total of 716 patients were enrolled in the study (safety population) and 715 patients received ≥1 dose of onabotulinumtoxinA; of these, 373 patients (52.1%) completed the study.23 The most common reasons for study discontinuation were withdrawal of consent (n=92, 12.8%) and loss to follow-up (n=82, 11.5%), with 4.9% (35/716) of patients discontinuing because of lack of efficacy and 3.5% (25/716) because of adverse events; 4.1% (29/716) discontinued the study after the final treatment and before the final follow-up visit.
The enrolled population had a mean (SD) age of 43.0 (11.3) years and were primarily female (n=607, 84.8%) and Caucasian (n=582, 81.3%). Mild or worse depressive symptoms (PHQ-9 ≥5) were present in 529 patients (74.5% of those with PHQ-9 scores at baseline) and clinically significant anxiety (GAD-7 ≥10) in 175 patients (24.6% of those with GAD-7 scores). At baseline, the mean (SD) PHQ-9 score was 11.4 (4.6), indicating moderate depressive symptoms, and the mean (SD) GAD-7 score was 14.0 (3.3), indicating moderate anxiety symptoms.
Mild or worse depressive symptoms and clinically significant anxiety were endorsed by 174 patients (24.5%). Patients with clinically significant baseline depressive or anxiety symptoms had similar demographic characteristics to the overall population. Poor sleep quality, as assessed by PSQI >5, was reported in almost all patients (n=707, 99.4%), with a mean (SD) PSQI score of 13.3 (3.7). The mean (SD) FSS score was 38.0 (14.5), indicative of significant fatigue.29
Overall efficacy results
In the analysis population, statistically significant mean (SD) reductions in headache days from a baseline of 22.0 (4.8) days were observed from the first assessment (–7.4 [6.2] days at week 24) and were sustained and further improved throughout the 108-week period (–10.7 [6.4] days).
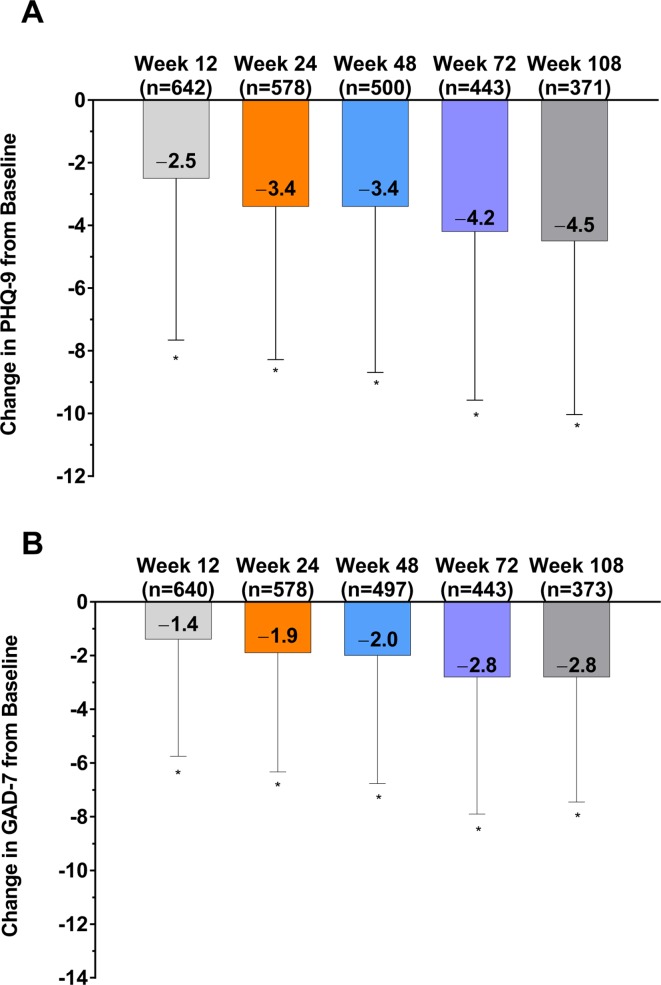
Based on observed data only, onabotulinumtoxinA treatment was also associated with significantly decreased (improved) mean sum PHQ-9 and GAD-7 scores; change from baseline was significant at all time points for both assessments (figure 1A,B). Similarly, in per-protocol analyses using last observation carried forward for missing data points, the reduction in PHQ-9 and GAD-7 scores was significant at all time points (online supplementary figure 1). The percentage of patients who experienced ≥1 severity category improvement on PHQ-9 or GAD-7 (ie, clinically meaningful improvement) increased over the study. By week 108, 53.4% (198/371) of the analysis population had a clinically meaningful improvement in symptoms of depression and 37.3% (139/373) in symptoms of anxiety (online supplementary figure 2).
Figure 1.
Change in (A) PHQ-9 scores and (B) GAD-7 scores in the analysis population after treatment with onabotulinumtoxinA. *p<0.0001 compared with baseline. GAD-7, 7-Item Generalised Anxiety Disorder; PHQ-9, 9-Item Patient Health Questionnaire.
jnnp-2018-319290supp001.pdf (44.5KB, pdf)
Effects on comorbid symptoms
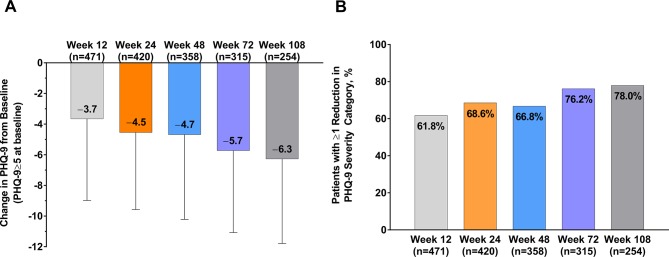
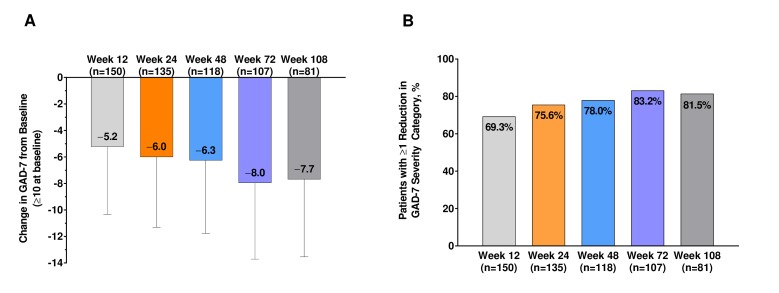
In the 529 patients with mild or worse depressive symptoms at baseline (PHQ-9 ≥5), the mean (SD) PHQ-9 scores were reduced from baseline at week 12 by 4.5 (5.0), at week 48 by 4.7 (5.5) and at week 108 by 6.3 (5.5) (figure 2A). PHQ-9 scores decreased over the duration of the study, the magnitude of which was related to the degree of depressive symptoms at baseline (online supplementary figure 3). Of those with mild symptoms of depression, 69.6% had PHQ-9 scores <5 by week 108, as did 51.6% of those with moderate symptoms, 47.9% of those with moderately severe symptoms and 53.8% of those with severe symptoms. At week 12, 61.8% had a clinically meaningful improvement in depression symptoms, increasing to 66.8% at week 48 and 78.0% at week 108 (figure 2B). Among 175 patients with clinically significant baseline anxiety (GAD ≥10), the mean (SD) GAD-7 scores were reduced from baseline at week 12 by 6.0 (5.3), at week 48 by 6.3 (5.5) and at week 108 by 7.7 (5.8) (figure 3A). GAD-7 scores decreased over the duration of the study; the magnitude was related to the degree of symptoms of anxiety at baseline (online supplementary figure 4). At week 12, 69.3% had clinically meaningful improvements in anxiety symptoms, increasing to 78.0% at week 48 and 81.5% at week 108 (figure 3B). Of those with mild symptoms of anxiety, 65.8% had GAD-7 scores <5 by week 108, as did 43.7% of those with moderate symptoms and 40.6% of those with severe symptoms.
Figure 2.
Change in (A) PHQ-9 scores from baseline and (B) the percentage of patients experiencing a ≥1 reduction in PHQ-9 severity category after treatment with onabotulinumtoxinA in patients with mild or worse depressive symptoms at baseline (PHQ-9 >5). PHQ-9, 9-Item Patient Health Questionnaire.
Figure 3.
Change in (A) GAD-7 scores from baseline and (B) the percentage of patients experiencing a ≥1 reduction in GAD-7 severity category in patients with symptoms of anxiety at baseline after treatment with onabotulinumtoxinA. GAD-7, 7-Item Generalised Anxiety Disorder.
In the analysis population, sleep quality was observed to improve with onabotulinumtoxinA treatment; the mean (SD) PSQI score significantly decreased from a baseline of 13.3 (3.7) to 11.0 (3.7) at week 108 (p<0.0001; table 1). A total of 40.9% had minimal clinically important responses (ie, ≥3-point decrease in PSQI28) at week 108. The results were similar in patients with poor sleep quality at baseline (data not shown). Similarly, symptoms of fatigue improved. The mean (SD) FSS scores significantly decreased from a baseline of 38.0 (14.5) to 30.1 (14.6) at week 108 (p<0.0001). At week 108, 43.8% of patients had a ≥20% decrease in FSS score, and 31.2% of patients had a ≥30% decrease in FSS score.
Table 1.
Summary of change in sleep quality and fatigue symptoms after onabotulinumtoxinA treatment
| Index measure | Analysis population (n=715) |
| Pittsburgh Sleep Quality Index, mean (SD) | |
| Total score at baseline | 13.3 (3.7) |
| Total score at week 60 | 11.7 (3.6)* |
| Total score at week 108 | 11.0 (3.7)* |
| Fatigue Severity Scale, mean (SD) | |
| Total score at baseline | 38.0 (14.5) |
| Total score at week 60 | 32.8 (14.6)* |
| Total score at week 108 | 30.1 (14.6)* |
*Indicates p<0.0001 compared with baseline.
Effect in ≥50% or ≥25% headache day reduction responders
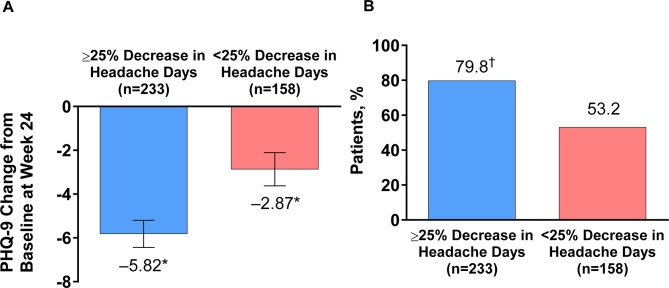
In patients with baseline depressive symptoms, a significant improvement (reduction) in mean PHQ-9 scores was observed at week 24 in patients with or without a ≥50% decrease in headache day frequency (≥50% headache day reduction responders or non-responders). The magnitude of change from baseline in PHQ-9 score was greater in ≥50% headache day reduction responders (−6.3) than in non-responders (–3.6). A significantly higher percentage of ≥50% headache day reduction responders had clinical improvements in depression symptoms at week 24 (83.7%) compared with non-responders (60.3%). The results were slightly less pronounced in those with a ≥25% reduction in headache days (≥25% headache day reduction responders). PHQ-9 scores changed by –5.8 in ≥25% headache day reduction responders vs –2.9 in non-responders (figure 4A); 79.8% of ≥25% headache day reduction responders had a clinical improvement in depression symptoms at week 24 vs 53.2% of non-responders (figure 4B).
Figure 4.
Mean change in (A) PHQ-9 and (B) the percentage of patients with a reduction of ≥1 severity category in PHQ-9 scores in those with depressive symptoms (PHQ-9 ≥5) at baseline, with or without a ≥25% decrease in headache days after treatment with onabotulinumtoxinA. *Significant change compared with baseline, p<0.0001. †Significant difference compared with non-responder group, p<0.001. PHQ-9, 9-Item Patient Health Questionnaire.
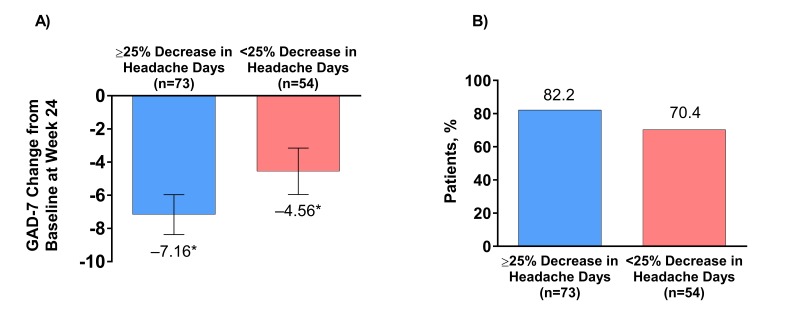
In patients with clinically significant baseline anxiety (GAD ≥10), a significant improvement (reduction) in mean change in GAD-7 score was observed at week 24 in ≥50% headache day reduction responders and non-responders. The magnitude of GAD-7 score change from baseline was greater in ≥50% headache day reduction responders (–7.6) than in non-responders (–5.1); 86.0% of ≥50% headache day reduction responders had clinical improvements in anxiety symptoms at week 24 vs 71.4% of non-responders. The results were similar in ≥25% headache day reduction responders. GAD-7 scores changed by –7.2 in ≥25% headache day reduction responders vs –4.6 in non-responders (figure 5A). There was a non-significant difference in the percentage of ≥25% headache day reduction responders who had clinical improvement in anxiety symptoms (82.2%) compared with non-responders (70.4%; figure 5B).
Figure 5.
Mean change in (A) GAD-7 scores from baseline and (B) the percentage of patients with a reduction of ≥1 severity category in GAD-7 scores in those with clinically significant anxiety (GAD-7 ≥10) at baseline, with or without a ≥25% decrease in headache days after treatment with effect of onabotulinumtoxinA. *Significant change compared with baseline, p<0.0001. GAD-7, 7-Item Generalised Anxiety Disorder.
Safety and tolerability
OnabotulinumtoxinA was well tolerated (table 2), with treatment-emergent adverse events reported by 436 patients (60.9%) and treatment-related adverse events (TRAEs) by 131 patients (18.3%). TRAEs occurring in ≥2% of patients included neck pain, eyelid ptosis, musculoskeletal stiffness and injection site pain (table 2). Despite extensive screening throughout the study, there were few reports of suicidal ideation; four patients (0.6%) discontinued from the study due to suicidal ideation.
Table 2.
Summary of adverse events in patients receiving ≥1 onabotulinumtoxinA treatment
| AE, n (%) | Safety population (N=716) |
| Any AE | |
| ≥1 AE | 436 (60.9) |
| Serious AE | 75 (10.5) |
| AE in those who discontinued treatment | 32 (4.5) |
| Treatment-related AE | |
| ≥1 treatment-related AE | 131 (18.3) |
| Serious treatment-related AE | 1 (0.1) |
| Treatment-related AE in those who discontinued treatment | 13 (1.8) |
| Treatment-related AE with incidence ≥1% | |
| Neck pain | 29 (4.1) |
| Eyelid ptosis | 18 (2.5) |
| Musculoskeletal stiffness | 17 (2.4) |
| Injection site pain | 14 (2.0) |
| Headache | 12 (1.7) |
| Muscular weakness | 10 (1.4) |
| Facial paresis | 9 (1.3) |
| Migraine | 7 (1.0) |
| Skin tightness | 7 (1.0) |
Reproduced from Blumenfeld AM, et al,23 available under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
AE, adverse event.
Discussion
The COMPEL study demonstrated that onabotulinumtoxinA 155 U administered over nine fixed-site, fixed-dose treatment cycles for CM was associated with clinically significant reductions in symptoms of depression and anxiety, suggesting additional possible beneficial effects beyond headache frequency reduction.23 These findings are also consistent with those from a small, open-label study in which onabotulinumtoxinA use was associated with decreased depression and anxiety symptoms in addition to reducing headache day frequency.34 In our analysis, beneficial onabotulinumtoxinA effects on depressive and anxiety symptoms occurred even with limited improvements in headache days.
The changes in symptoms of depression and anxiety all reached statistical significance, even in patients with only mild symptoms of depression or anxiety at baseline. However, for outcomes to be meaningful for patient and healthcare professionals, an assessment against clinically relevant change must be demonstrated. In this analysis, reduction by ≥1 severity category was defined as clinically relevant. This definition is consistent with the recommended definition of clinically relevant change of improvement in PHQ-9 score ≥5 points for individuals with pretreatment PHQ-9 scores of ≥8.31 At week 24, 68.6% of those with mild or worse depressive symptoms and 79.8% of ≥25% headache day reduction responders had a clinically meaningful change in PHQ-9 scores. Importantly, and although less robust, onabotulinumtoxinA treatment was associated with clinically significant change in PHQ-9 scores in ≥50% and ≥25% headache day reduction non-responders, suggesting that the effect may be independent of the effect on headache day frequency.
Similarly, the GAD-7 questionnaire has been validated as a screening tool in patients with migraine,35 assessing anxiety severity and monitoring change over time.26 In this analysis, reduction by ≥1 severity category was defined as clinically relevant, largely consistent with a definition of clinically relevant change of change in GAD-7 score from ≥10 to<10, or a reduction by ≥50% of the pretreatment GAD-7.32 At week 24, 75.6% of patients with clinically significant anxiety and 82.2% of ≥25% headache day reduction responders had a clinically meaningful change in GAD-7 scores. As with depression, onabotulinumtoxinA treatment was associated with a clinically relevant change in anxiety symptoms whether or not patients had a reduction in headache days.
Regarding our exploratory analyses, findings demonstrated that depression and anxiety symptoms were reduced even in ≥25% headache day reduction non-responders. Thus, it seems possible that onabotulinumtoxinA could be having an independent effect on depression and anxiety symptoms, as has been observed by others for depressive symptoms.36 37 In addition to the effect on migraine, onabotulinumtoxinA could improve mood through social feedback mechanisms as a result of the individual’s improved appearance after relaxation of muscles in the glabellar region (the ‘facial feedback hypothesis’).36 In addition, through modulation of proprioceptive input, the peripheral effects of onabotulinumtoxinA may have a secondary central effect by dampening the peripheral sensory afferents travelling to the trigeminal nucleus, and thus modulating the central nuclei of the limbic system.38 39 Alternatively, and not possible to determine by these exploratory analyses, the effects could be an indirect consequence of onabotulinumtoxinA reducing headache day severity with or without reducing headache day frequency, leading to improvement in depression and anxiety symptoms, or they could be due to the healthcare professional providing standard pharmacological and/or non-pharmacological care for depression and anxiety throughout the study. Further, the majority of our sample was overusing headache medication at baseline. It is well recognised that medication overuse has a close association with headache as well as comorbid depression and anxiety.40 As previously reported, headache days were reduced in this subgroup.23 It is possible that the reduction in depression and anxiety symptoms that were observed was related to the beneficial effect of onabotulinumtoxinA, the possibility that as patients feel better their headaches improve and their depression and anxiety improve, or that patient medication strategies improve by removing overused medications, and consequently their headaches improve or they receive a preventive medication, which also leads to improvement in depression and anxiety symptoms.
In this analysis, >40% of all patients experienced a clinically significant improvement in sleep patterns (ie, a change in PSQI global score of ≥3 points28); the results were similar in 99.4% of patients with poor sleep quality at baseline. Approximately one-third of all patients experienced a ≥30% reduction in FSS score. It has long been recognised that poor sleep quality and fatigue are associated with psychiatric disorders. Indeed, insomnia/hypersomnia and fatigue are two of the nine hallmark symptoms of depression.41 As with the symptoms of depression and anxiety, poor sleep quality also negatively influences headache severity,14 and when occurring with depressive symptoms in people with migraine may result in cognitive decline.42 Given the adverse consequences of comorbid psychiatric symptoms in individuals with migraine,43 the role of onabotulinumtoxinA on these symptoms warrants further investigation.
Over nine treatment cycles, onabotulinumtoxinA was well tolerated, with mild and transient adverse events and no unexpected adverse events. There were few reports of suicidal ideation.
Study limitations
The limitations associated with a non-randomised, open-label study with long-term follow-up have been previously outlined and include unintentional bias and low persistency rates.23 However, because the efficacy and safety of onabotulinumtoxinA in people with CM have been clearly established, an open-label design was considered appropriate for this extended-duration study (108 weeks) to enable the assessment of effectiveness in individuals motivated to stay on treatment for nine cycles. In our study over a period of 2 years, 11.5% of patients were lost to follow-up compared with 5.5% in the shorter 52-week PREEMPT studies.22 A further 12.8% of patients withdrew consent over the 2-year study duration. It was not expected that patients who did not benefit from onabotulinumtoxinA would stay on treatment. The results from the PREEMPT trials indicated approximately 50% of patients experienced a ≥50% reduction in headache day frequency after two treatment cycles and approximately 70% after five treatment cycles.22 In our study, 56.1% of patients remained on onabotulinumtoxinA treatment for nine treatment cycles, but not all of those attended the final follow-up visit, possibly due to a lack of motivation to attend a visit where no treatment was being administered. Further, an analysis of the 282 patients who completed all study visits demonstrated that onabotulinumtoxinA reduced headache days to a similar level seen in the overall population (week 108: –11.8 [7.3] vs –10.7 [6.4] days, respectively), suggesting limited bias from low persistency rates.
In our study, we also permitted the concomitant use of a single oral preventive medication, with medication remaining stable until at least week 24. Preventive medications could potentially affect symptoms of depression and anxiety; however, as preventive medications were only added in 6.1% (n=44) of patients, it is unlikely that the addition of these medications had a clinically relevant effect on our results.
The definitions of depression and anxiety were not based on a clinical diagnosis but on the self-reported validated PHQ-9 and GAD-7 questionnaire sum scoring methods, respectively, chosen for their widespread acceptance and simplicity to administer in a clinical trial situation. The sum score algorithm does not directly follow the clinical algorithm from the DSM-IV; the primary criteria necessary for a clinical diagnosis based on the DSM-IV criteria do not need to be endorsed in the sum score method for an individual to be classified as having clinically significant depression or anxiety. Similarly, the definitions of poor sleep quality and fatigue were based on PSQI and FSS self-assessments. The exclusion of patients with severe depression and suicidal ideation means the effect of onabotulinumtoxinA on patients with the most severe baseline depression symptoms was not captured. If those with the highest scores had been included, it is possible that outcomes may have varied. In this study, cut points of PHQ-9 ≥5 (for depression), GAD-7 ≥10 (for anxiety) and PSQI >5 (for sleep quality) were selected. Different cut points could have resulted in different outcomes.
Furthermore, as previously discussed, our definition of clinically relevant change in depressive and anxiety symptoms differed slightly from others.31 32 Finally, the exploratory nature of the current analyses may limit the widespread generalisability of the results. Nonetheless, the study adds to our understanding of CM management in patients with comorbid depression, anxiety, fatigue and poor sleep quality. The change in depressive symptoms in patients with CM is particularly impressive, with >80% of the ≥25% headache day reduction responders experiencing clinically meaningful reduction in depressive symptoms. Further research should be undertaken to determine whether changes in headache frequency and severity after onabotulinumtoxinA treatment are driving improvements in the comorbidity or whether improvements in comorbid disease symptoms are an independent effect of onabotulinumtoxinA.
Conclusions
Data from the COMPEL study support the efficacy and safety of onabotulinumtoxinA for the treatment of CM in people with symptoms of depression and anxiety for up to 108 weeks. Depression and anxiety symptoms were significantly reduced even in patients who did not have meaningful reduction in headache days after onabotulinumtoxinA. OnabotulinumtoxinA treatment was also associated with improvement in sleep quality and reduced fatigue symptoms across the analysis population. Together, these data suggest that onabotulinumtoxinA may have beneficial effects beyond those of headache frequency reduction, particularly in people with CM with psychiatric comorbidities.
Acknowledgments
Editorial support for the development of this manuscript was provided by Lee B Hohaia, PharmD, of Complete Healthcare Communications (North Wales, Pennsylvania), a CHC Group company.
Footnotes
Contributors: AMB, SJT, LDR, AMA, DCB and SDS conceived and developed these post hoc analyses of the COMPEL study data. AO conducted the statistical analyses. All authors discussed the results and contributed to the final manuscript and approved it for submission.
Funding: The study was sponsored by Allergan (Dublin, Ireland). Allergan funded the editorial and writing support, and provided support for the study design, and the collection, analysis and interpretation of the data. Other than in their role as authors, employees of Allergan did not have a role in the final decision of which data to include in the manuscript and on the decision to submit the manuscript for publication.
Competing interests: AMB has served on advisory boards for Allergan, Amgen, Alder, Teva, Supernus, Promius, Eaglet and Lilly, and has received funding for speaking from Allergan, Amgen, Pernix, Supernus, Depomed, Avanir and Promius. He holds patents for onabotulinumtoxinA in migraine assigned to Allergan. SJT is an employee of the Dartmouth-Hitchcock Medical Center and receives a salary from the American Headache Society (AHS). He also serves as a consultant for Acorda, Alder, Alexsa, Allergan, Amgen, ATI, BioVision, Cefaly, Charleston Laboratories, DeepBench, Dr Reddy’s, ElectroCore, Eli Lilly, eNeura, GLG, Guidepoint Global, Impax, Neurolief, Novartis, Scion Neurostim, Slingshot Insights, Supernus, Teva and Zosano, and has received royalties for books published by Springer. His employer receives research grants from Alder, Allergan, Amgen, ATI, Dr Reddy's, Scion Neurostim, Teva and Zosano. LDR has served as a speaker for Avanir, Pernix and Merck. AMA and AO are employees of Allergan, and AMA holds stock in the company. DCB has received grant support and honoraria from Allergan, Avanir, Amgen, Eli Lilly and Company, and Promius, and for work on the editorial board of Current Pain and Headache Reports. SDS has served as a consultant and/or advisory panel member and received honoraria from Alder BioPharmaceuticals, Allergan, Amgen, Avanir Pharmaceuticals, eNeura, ElectroCore Medical, Eli Lilly and Company, Medscape and Teva Pharmaceuticals, and grants from Amgen and Teva Pharmaceuticals. His employer has received research support from Allergan, Amgen, Cumberland Pharmaceuticals, ElectroCore Medical, Eli Lilly and Company, and Troy Healthcare.
Patient consent for publication: Not required.
Ethics approval: The central IRB for the COMPEL study was Quorum Review IRB in Seattle, Washington.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Headache Classification Committee of the International Headache Society The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- 2. Vos T, Flaxman AD, Naghavi M, et al. . Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet 2012;380:2163–96. 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buse DC, Manack AN, Fanning KM, et al. . Chronic migraine prevalence, disability, and sociodemographic factors: results from the American Study. Headache 2012;52:1456–70. 10.1111/j.1526-4610.2012.02223.x [DOI] [PubMed] [Google Scholar]
- 4. Blumenfeld AM, Varon SF, Wilcox TK, et al. . Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS). Cephalalgia 2011;31:301–15. 10.1177/0333102410381145 [DOI] [PubMed] [Google Scholar]
- 5. Ferrari A, Leone S, Vergoni AV, et al. . Similarities and differences between chronic migraine and episodic migraine. Headache 2007;47:65–72. 10.1111/j.1526-4610.2006.00629.x [DOI] [PubMed] [Google Scholar]
- 6. Buse DC, Manack A, Serrano D, et al. . Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry 2010;81:428–32. 10.1136/jnnp.2009.192492 [DOI] [PubMed] [Google Scholar]
- 7. Ramage-Morin PL, Gilmour H. Prevalence of migraine in the Canadian household population. Health Rep 2014;25:10–16. [PubMed] [Google Scholar]
- 8. Saunders K, Merikangas K, Low NC, et al. . Impact of comorbidity on headache-related disability. Neurology 2008;70:538–47. 10.1212/01.wnl.0000297192.84581.21 [DOI] [PubMed] [Google Scholar]
- 9. Ashina S, Serrano D, Lipton RB, et al. . Depression and risk of transformation of episodic to chronic migraine. J Headache Pain 2012;13:615–24. 10.1007/s10194-012-0479-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamelsky SW, Lipton RB. Psychiatric comorbidity of migraine. Headache 2006;46:1327–33. 10.1111/j.1526-4610.2006.00576.x [DOI] [PubMed] [Google Scholar]
- 11. Yang Y, Zhao H, Heath AC, et al. . Shared genetic factors underlie migraine and depression. Twin Res Hum Genet 2016;19:341–50. 10.1017/thg.2016.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dikmen P, Yavuz B, Aydinlar E. The relationships between migraine, depression, anxiety, stress, and sleep disturbances. Acta Neurol Belg 2015;115:6. [DOI] [PubMed] [Google Scholar]
- 13. Breslau N, Lipton RB, Stewart WF, et al. . Comorbidity of migraine and depression: investigating potential etiology and prognosis. Neurology 2003;60:1308–12. 10.1212/01.WNL.0000058907.41080.54 [DOI] [PubMed] [Google Scholar]
- 14. Houle TT, Butschek RA, Turner DP, et al. . Stress and sleep duration predict headache severity in chronic headache sufferers. Pain 2012;153:2432–40. 10.1016/j.pain.2012.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scher AI, Lipton RB, Stewart WF. Habitual snoring as a risk factor for chronic daily headache. Neurology 2003;60:1366–8. 10.1212/01.WNL.0000055873.71552.51 [DOI] [PubMed] [Google Scholar]
- 16. Zhu Z, Fan X, Li X, et al. . Prevalence and predictive factors for poor sleep quality among migraineurs in a tertiary hospital headache clinic. Acta Neurol Belg 2013;113:229–35. 10.1007/s13760-012-0159-1 [DOI] [PubMed] [Google Scholar]
- 17. Aguggia M, Cavallini M, Divito N, et al. . Sleep and primary headaches. Neurol Sci 2011;32(S1):51–4. 10.1007/s10072-011-0524-5 [DOI] [PubMed] [Google Scholar]
- 18. Rains JC. Chronic headache and potentially modifiable risk factors: screening and behavioral management of sleep disorders. Headache 2008;48:32–9. 10.1111/j.1526-4610.2007.00972.x [DOI] [PubMed] [Google Scholar]
- 19. Calhoun AH, Ford S, Finkel AG, et al. . The prevalence and spectrum of sleep problems in women with transformed migraine. Headache 2006;46:604–10. 10.1111/j.1526-4610.2006.00410.x [DOI] [PubMed] [Google Scholar]
- 20. Seo J-G, Park S-P. Significance of fatigue in patients with migraine. J Clin Neurosci 2018;50:69–73. 10.1016/j.jocn.2018.01.032 [DOI] [PubMed] [Google Scholar]
- 21. Botox® (onabotulinumtoxinA for injection, for intramuscular, intradetrusor, or intradermal use) Full prescribing Information. Irvine, CA: Allergan, 2016. [Google Scholar]
- 22. Aurora SK, Winner P, Freeman MC, et al. . OnabotulinumtoxinA for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache 2011;51:1358–73. 10.1111/j.1526-4610.2011.01990.x [DOI] [PubMed] [Google Scholar]
- 23. Blumenfeld AM, Stark RJ, Freeman MC, et al. . Long-term study of the efficacy and safety of OnabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J Headache Pain 2018;19:13 10.1186/s10194-018-0840-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. American Psychiatric Association Diagnostic and statistical manual of mental disorders. Washington DC, 1994. [Google Scholar]
- 26. Spitzer RL, Kroenke K, Williams JB, et al. . A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 27. Buysse DJ, Reynolds CF, Monk TH, et al. . The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 28. Hughes CM, McCullough CA, Bradbury I, et al. . Acupuncture and reflexology for insomnia: a feasibility study. Acupunct Med 2009;27:163–8. 10.1136/aim.2009.000760 [DOI] [PubMed] [Google Scholar]
- 29. Valko PO, Bassetti CL, Bloch KE, et al. . Validation of the fatigue severity scale in a Swiss cohort. Sleep 2008;31:1601–7. 10.1093/sleep/31.11.1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krupp LB, LaRocca NG, Muir-Nash J, et al. . The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989;46:1121–3. [DOI] [PubMed] [Google Scholar]
- 31. McMillan D, Gilbody S, Richards D. Defining successful treatment outcome in depression using the PHQ-9: a comparison of methods. J Affect Disord 2010;127(1-3):122–9. 10.1016/j.jad.2010.04.030 [DOI] [PubMed] [Google Scholar]
- 32. Robinson E, Titov N, Andrews G, et al. . Internet treatment for generalized anxiety disorder: a randomized controlled trial comparing clinician vs. technician assistance. PLoS One 2010;5:e10942 10.1371/journal.pone.0010942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Silberstein S, Tfelt-Hansen P, Dodick DW, et al. . Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia 2008;28:484–95. 10.1111/j.1468-2982.2008.01555.x [DOI] [PubMed] [Google Scholar]
- 34. Boudreau GP, Grosberg BM, McAllister PJ, et al. . Prophylactic onabotulinumtoxinA in patients with chronic migraine and comorbid depression: an open-label, multicenter, pilot study of efficacy, safety and effect on headache-related disability, depression, and anxiety. Int J Gen Med 2015;8:79–86. 10.2147/IJGM.S70456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seo J-G, Park S-P. Validation of the Generalized Anxiety Disorder-7 (GAD-7) and GAD-2 in patients with migraine. J Headache Pain 2015;16:97 10.1186/s10194-015-0583-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Magid M, Finzi E, Kruger T, et al. . Treating depression with botulinum toxin: a pooled analysis of randomized controlled trials. Pharmacopsychiatry 2015;48:205–10. 10.1055/s-0035-1559621 [DOI] [PubMed] [Google Scholar]
- 37. Wollmer MA, Kalak N, Jung S, et al. . Agitation predicts response of depression to botulinum toxin treatment in a randomized controlled trial. Front Psychiatry 2014;5:36 10.3389/fpsyt.2014.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aoki KR. Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache 2003;43(s1):9–15. 10.1046/j.1526-4610.43.7s.3.x [DOI] [PubMed] [Google Scholar]
- 39. Whitcup SM, Turkel CC, DeGryse RE, et al. . Development of onabotulinumtoxinA for chronic migraine. Ann N Y Acad Sci 2014;1329:67–80. 10.1111/nyas.12488 [DOI] [PubMed] [Google Scholar]
- 40. Lampl C, Thomas H, Tassorelli C, et al. . Headache, depression and anxiety: associations in the Eurolight project. J Headache Pain 2016;17:59 10.1186/s10194-016-0649-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. American Psychiatric Association Diagnostic and statistical manual of mental disorders. 5th edn Arlington VA, 2013. [Google Scholar]
- 42. Lee SH, Kang Y, Cho S-J. Subjective cognitive decline in patients with migraine and its relationship with depression, anxiety, and sleep quality. J Headache Pain 2017;18:77 10.1186/s10194-017-0779-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Buse DC, Silberstein SD, Manack AN, et al. . Psychiatric comorbidities of episodic and chronic migraine. J Neurol 2013;260:1960–9. 10.1007/s00415-012-6725-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2018-319290supp001.pdf (44.5KB, pdf)