Abstract
Acute gastroenteritis (AGE) remains a common condition in both low- and high-income countries. In Belgium, however, there is currently a lack of information on the societal health and economic impact of AGE. We conducted a retrospective study using mortality and cause-of-death data, hospital data, primary care data, health interview survey data and other published data. We estimated the burden of illness during a 5-year period (2010–2014) in Belgium in terms of deaths, patients admitted to hospitals, patients consulting their general practitioner (GP) and cases occurring in the community. We further quantified the health impact in terms of disability-adjusted life years (DALYs) and the economic impact in terms of cost-of-illness estimates. We estimated 343 deaths, 27 707 hospitalised patients, 464 222 GP consultations and 10 058 741 episodes occurring in the community (0.91 cases/person) on average per year. AGE was associated with 11 855 DALYs per year (107 DALY per 100 000 persons). The economic burden was estimated to represent direct costs of €112 million, indirect costs of €927 million (90% of the total costs) and an average total cost of €103 per case and €94 per person. AGE results in a substantial health and economic impact in Belgium, justifying continued mitigation efforts.
Key words: Burden of disease, cost-of-illness, disability-adjusted life years, gastroenteritis
Introduction
Acute gastroenteritis (AGE) is a common condition causing a significant disease burden worldwide. The Global Burden of Disease study estimated that diarrhoea was a leading cause of death with 1.7 million deaths in 2016 [1]. Although in high-income countries mortality due to diarrhoeal diseases is low, AGE gives rise to numerous episodes, general practitioner (GP) consultations and hospitalisations. The number of deaths, hospitalised patients, GP consultations and cases occurring in the community can be described in a disease pyramid, allowing for a more complete picture of the burden of illness [2, 3]. The disease pyramid further provides a basis for quantifying the health and economic impact of AGE [4, 5].
Different data sources provide information on the AGE disease pyramid in Belgium, albeit with varying degrees of completeness. All deaths and hospitalisations are registered and classified according to ICD coding. Systematic collection of data on AGE from GPs is however more limited. Furthermore, AGE cases occurring in the community are difficult to estimate as not all cases seek health care, especially when the disease is mild and self-limiting.
The aim of this study was to assess the health and economic impact of AGE in Belgium using available data sources.
Materials and methods
Reference period and population
We performed a retrospective analysis of routinely collected health information from different sources targeting the Belgian population for the years 2010–2014. This time period provided the most complete coverage across databases. First, we reconstructed the AGE disease pyramid by estimating the number of episodes, GP consultations, hospitalisations and deaths occurring annually in Belgium. Then, we quantified the health impact in terms of disability-adjusted life years (DALYs) and the economic impact in terms of direct and indirect costs.
Reconstruction of the disease pyramid using different data sources
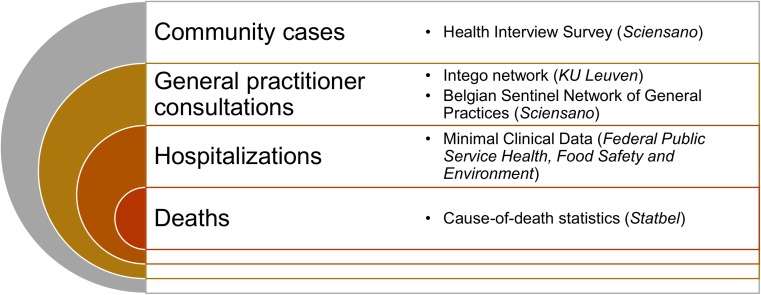
Figure 1 summarises the different steps in the AGE disease pyramid and the data sources mapped to each step. In what follows, we describe the mapping for each step in more detail.
Fig. 1.
Data sources used to reconstruct the acute gastroenteritis disease pyramid in Belgium.
Mortality and cause-of-death data
Statbel, the Belgian national institute of statistics, is responsible for the compilation of mortality and ICD-10 coded cause-of-death data based on death certificates. In the current study, only the underlying cause of death was taken into account. The underlying cause of death is defined in accordance with the encoding rules of the World Health Organization (WHO), as the diseases or the injuries which initiate a chain reaction of morbidities that finally led to death [6]. Cause-of-death data can be extracted through the Standardized Procedures for Mortality Analysis website (SPMA), available online through https://spma.wiv-isp.be/. We extracted data for 2010–2014 from SPMA to establish the number of deaths with the ICD-10 codes A00-09 (‘Diarrhoea and gastroenteritis of presumed infectious origin’) as the underlying cause of death.
Hospital data
The Minimal Clinical Data (MCD) is a compulsory hospital data registration system, providing summarised clinical and demographic information for all persons admitted to Belgian hospitals. Participation to the MCD has been mandatory for all hospitals since 1991, and is managed by the Federal Public Service Health, Food Safety and Environment. Hospital data are available for hospitalised episodes of AGE as a primary (main) or secondary (any non-primary) diagnosis, coded by ICD-9 until 2014. In 2015, the database shifted to ICD-10 coding, but data were not available for the year 2015. We obtained the annual number of hospitalised AGE cases during 2010–2014, by age, from MCD as the number of persons admitted to hospital with AGE defined by ICD-9 codes 001-009 as a primary or secondary diagnosis during their hospital stay.
Primary care data
The two major sources of primary care data in Belgium are the Belgian Sentinel Network of General Practices (SNGP) [7], managed by Sciensano, the Belgian institute for health, and the Intego network, managed by the Katholieke Universiteit Leuven (KU Leuven) [8].
The Belgian SNGP comprises approximately 150 general practices with one or more sentinel GPs who purposively record routine clinical care data for the surveillance of different health problems. The network covers between 1.4% and 1.8% of the Belgian population throughout all regions. AGE was included in the SNGP network surveillance in 2002. For each case, a questionnaire was completed containing patient characteristics and specific questions about the symptoms (number of loose stools/day, fever, blood in stool, vomiting and dehydration), antibiotic prescription and if a stool sample was requested. Information on the age and gender distribution of the sentinel population is lacking, therefore the same age and gender distribution as in the complete Belgian population is assumed when calculating the incidence rates. For the SNGP, a case of AGE was defined as any episode with at least four loose stools/day or loose stool/vomiting in combination of at least two other symptoms (fever or blood in stool).
The Intego network, operational since 1994, is an electronic patient record (EPR)-based network of 54 voluntarily participating GP practices in Flanders, the northern region of the country, which all use the same EPR software. The network is coordinated by the Academic Centre for General Practice at the KU Leuven and covers approximately 2% of the Flemish population. The Intego database primarily uses the International Classification of Primary Care (ICPC) coding system to register GP diagnoses. In this coding system, AGE may be classified as ICPC code D70 (‘gastrointestinal infection’) or D73 (‘gastroenteritis presumed infection’).
To estimate the number of GP consultations for AGE, we first extracted from the Intego database the number of GP registrations in Flanders with ICPC codes D70 or D73, by age, for the period 2010–2014. We then used the SNGP data for 2002 to extrapolate the number of consultations to the other regions (i.e. the Walloon and Brussels Capital Region), based on the proportion of AGE-related consultations per region in 2002.
Health interview survey data
The Belgian Health Interview Survey (BHIS) has, to date, taken place in 1997, 2001, 2004, 2008 and 2013 [9]. The main objective of the BHIS is to describe in a representative manner the health status, health behaviour and use of health services of the Belgian population. The sampling design is a stratified clustered multi-stage with municipalities as primary, households as secondary and individuals as the tertiary sampling units. Questions on AGE have only been included in the BHIS 2001. In 2001, a total of 12 111 people were interviewed. The AGE-specific questions asked if the participant had had an episode of AGE in the last 2 weeks prior to interview. Cases of AGE in the BHIS 2001 were defined as every person who reported three or more loose stools in a 24 h period during the 14 days prior to the interview, without having reported having chronic bowel problems during the past 6 months.
The number of community cases was estimated from the BHIS 2001 considering an average duration of symptoms of 1–5 days [10]. In a baseline scenario, we estimated the number of community cases by multiplying the number of positive respondents with 365/17, considering an average duration of symptoms of 4 days (17 = 14 days + [4–1] days of symptoms). In alternative incidence scenarios, we estimated the minimum number of cases considering a 5-day duration of symptoms (number of positive responses × 365/18), and the maximum number assuming the minimum duration of 1 day for all cases (number of positive responses × 365/14). In 2006, a rotavirus vaccine came on the market in Belgium, and quickly achieved high uptake (mean uptake in 2012: 89%) [11]. We therefore further corrected the community incidence for children 0–9 years old by applying the post-/pre-vaccination proportionate decrease (76.9%) in AGE hospitalisations for this age group, as estimated previously [12] and assuming that the decrease in community incidence would be the same as in hospitalisations.
Disability-adjusted life years
The burden of AGE was evaluated in terms of DALYs, a summary measure of population health that is widely used to quantify burden of disease. DALYs were calculated as previously described [13] by summing years of life lost (YLLs) and years lived with disability (YLDs). Estimations for YLLs were performed using the standard life expectancy table from the Global Burden of Disease study [1]. For the estimations of YLDs, we stratified AGE cases into mild, moderate and severe and matched these strata with the levels across the disease pyramid (i.e. cases occurring in the community, GP visits and hospitalisations, respectively). To ensure that each case would be categorised in one part of the disease pyramid only we excluded the number of severe cases from the moderate cases and the number of moderate and severe cases from the number of mild cases. In a baseline scenario, we used the disability weights from Salomon et al. [14] – i.e. 0.074, 0.188 and 0.247, for mild, moderate and severe AGE, respectively. The duration of symptoms was set as 3 days for mild AGE (community cases), 10 days for moderate AGE (when a GP was consulted) and 14 days for severe AGE (hospitalisation cases) [15]. In an alternative disability weight scenario, we used the annual profile disability weights from Haagsma et al. [15] – i.e. 0.000, 0.015 and 0.041 for mild, moderate and severe AGE. DALYs are presented as the total number in Belgium per year, as number of DALYs per 100 000 persons and as number of DALYs per 100 000 cases.
Cost-of-illness
The economic impact of AGE was calculated considering direct medical costs, direct non-medical costs and indirect costs. We estimated the total cost-of-illness from a societal perspective using data on the total number of AGE cases per age group, the volumes (number of consultations, hospitalisations, medication packages) for use of resources and the unit costs of each of these items. As such, the average costs per case in a certain age group was calculated and subsequently multiplied by the total number of cases in each age group [5, 16, 17]. Then, the total absolute cost-of-illness of AGE in Belgium and per case was calculated by summing costs across all age groups. Our cost-of-illness estimates were in line with the Belgian guidelines for economic evaluations and budget impact analyses, developed by the Belgian Healthcare Knowledge Centre (KCE) [18].
Direct costs
We estimated the total direct medical costs as the sum of the costs for consultations at GPs and/or specialists, hospital admissions, prescribed and over-the-counter medication, and laboratory stool tests for diagnostic purposes. Unit costs for GP or specialist consultations as well as for hospitalisations were obtained from the National Institute of Health and Disability Insurance (NIHDI) for 2016 (www.inami.fgov.be). The direct cost per GP consultation was €24.48. For consultation of gastroenterologist, a cost of €36.74 was used, and in the absence of reliable data, we assumed that a 14% fraction of the people that visited a GP consulted a specialist [19]. We used a mean duration of 4.4 days per hospitalisation and a mean cost of €2800 per hospitalisation day as estimated by NIHDI (https://tct.fgov.be/webetct/etct-web/). For the prescribed and the over-the-counter medications, we considered three main categories: antidiarrhoeal drugs (AD, domperidone; €7.2 per package); oral rehydration solutions (ORS; €9 per package); and antibiotics (AB, amoxicillin and clavulanic acid; €10.41 per package) (prices derived from the Belgian Center for Pharmacotherapeutic Information). The proportions of episodes in which these drugs were used, i.e. 4.3% for AD, 7.4% for ORS and 6.5% for AB, were taken from a previous study [5]. For the diagnostic stool tests, we assumed that 13.5% of the GP visits resulted in the prescription of diagnostic stool tests with a unit cost of €60 per test [5].
For the direct non-medical costs, we considered travel costs to and from the GP and the hospital as previously estimated in the Netherlands [5]. The average distance from a Belgian household to their GP was set to be 1.8 km as in the Dutch study, considering the same population density. We further assumed that half of the patients used a car (or other paid transport), while the other half used a bicycle or went on foot [5]. The average distance from a Belgian household to the nearest general hospital was set to be 7.0 km as in the Dutch study; we further assumed that all patients used paid transport to go to the hospital [5]. For all paid transports, we used a cost of €0.30/ km [18] and no parking costs were included. We did not consider costs for additional cleaning material and diapers for patients in the absence of reliable data for the estimations. For the pharmacy, we did not estimate any additional costs as we considered that usually people went on foot or bought the medication on the way to the GP.
Indirect costs
We associated indirect costs with losses due to absence from work using two scenarios. According to the baseline scenario, we assumed that in 37% of adult episodes (18–64), and in 18.6% of the episodes in children (0–17) and seniors (65+), an employed adult had to be absent from work due to AGE, as previously described in a recent survey in the Netherlands [20]. The average number of working days that an employee was absent from work due to gastroenteritis (3.5 days) was retrieved from Securex, one of the largest human resource companies in Belgium. The number of absenteeism episodes was multiplied with the average number of absence days from work per episode and then multiplied with the average daily gross salary of €257 (2010 average) [18] per working day to estimate the total costs.
According to an alternative scenario for estimating indirect costs, we assumed that only people who asked for medical advice, i.e. the moderate or severe cases, were absent from work. Indeed, in Belgium, a medical certificate is needed to justify absence from work. We estimated the workdays lost by multiplying the sum of people that visited a GP, a specialist or had been hospitalised with the average number of working days that an employee was absent from work due to gastroenteritis (3.5 days) as described above. We considered age group-specific employment rates using Eurostat data for 2012 – i.e. 25.3% for ages 18–24, 79.3% for ages 25–54 and 39.5% for ages 55–64. We assumed that for children (0–17) and elderly people (65+), at least one productive adult was taking care of them and was absent from work for 1 day in case of a moderate episode and for 2 days in case of a severe episode [17].
Results
Disease pyramid
The disease pyramid of AGE in Belgium is presented in Table 1. We estimated an average of 10 058 741 community cases per year in Belgium during the study period, corresponding to 0.91 cases/person per year (range 0.86–1.1 cases/person per year) in an average population of 11 052 385. The alternative incidence scenarios yielded a minimum and a maximum estimate of 9.5 and 12.2 million community cases, respectively. Cause-of-death data yielded an average of 343 (217–408) AGE deaths per year, corresponding to 3.1 deaths/100 000 persons, or 2.9 deaths/100 000 community cases (Supplementary Table S1). Hospital data yielded an average of 27 707 (26 312–29 217) AGE hospitalisations per year, corresponding to 251 hospitalisations/100 000 persons or 275 hospitalisations/100 000 community cases. Based on primary care data, we estimated 464 222 GP consultations due to AGE on average per year (460 187–466 602), corresponding to 4200 consultations/100 000 persons, or 4450 consultations/100 000 community cases.
Table 1.
Estimated disease pyramid of acute gastroenteritis cases in Belgium, 2010–2014
| Component | Number of cases | |||||
|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | Average | |
| Deaths | 408 | 362 | 217 | 387 | 339 | 343 |
| Hospitalisations | 27 699 | 26 652 | 28 654 | 29 217 | 26 312 | 27 707 |
| GP consultations | 460 187 | 463 267 | 465 081 | 465 975 | 466 602 | 464 222 |
| Community cases, baseline estimate | 9 920 001 | 10 010 579 | 10 075 302 | 10 121 939 | 10 165 885 | 10 058 741 |
| Community cases, minimum estimate | 9 368 890 | 9 454 436 | 9 515 563 | 9 559 610 | 9 601 114 | 9 499 922 |
| Community cases, maximum estimate | 12 045 716 | 12 155 704 | 12 234 296 | 12 290 926 | 12 344 289 | 12 214 186 |
GP, general practitioner.
Disability-adjusted life years
During 2010–2014, the fatal AGE cases resulted in an annual average of 3509 YLLs. This corresponds to an annual rate of 32 YLLs per 100 000 persons and 35 YLLs per 100 000 cases (Table 2). In our baseline disability weights scenario, 8346 years of life were lost on average per year due to disability in Belgium, corresponding to 83 YLDs per 100 000 cases/year and 76 YLDs per 100 000 persons/year. Mild AGE cases contributed the most to the YLD estimate (70%), followed by moderate (27%) and severe cases (3%) (Table 2). In total, AGE was responsible for 11 855 DALYs per year in Belgium during 2010–2014, corresponding to 107 DALYs per 100 000 persons and 118 DALYs per 100 000 cases.
Table 2.
Estimated annual disease burden of acute gastroenteritis (AGE) in Belgium, 2010–2014 (baseline scenario)
| Health state | Cases | YLDs | YLLs | DALYs | DALYs/1000 casesa | DALYs/100 000 total cases | DALYs/100 000 persons |
|---|---|---|---|---|---|---|---|
| AGE, mild | 9 594 519 | 5836 | N/A | 5836 | 0.61 | 58 | 53 |
| AGE, moderate | 436 515 | 2248 | N/A | 2248 | 5.15 | 22 | 20 |
| AGE, severe | 27 707 | 262 | N/A | 262 | 9.47 | 2.61 | 2.37 |
| AGE, deaths | 343 | N/A | 3509 | 3509 | 10 229 | 35 | 32 |
| TOTAL | N/A | 8346 | 3509 | 11 855 | N/A | 118 | 107 |
DALYs per 1000 mild, moderate or severe cases, respectively.
YLDs, years lived with disability; YLLs, years of life lost; DALYs, disability-adjusted life years; N/A, not applicable.
Using the alternative disability weights scenario, we estimated 6548 YLDs for the moderate cases and 1136 YLDs for the severe cases of AGE, while this approach resulted in zero YLDs for mild cases. Consequently, the total estimated DALYs were 11 192, corresponding to 101 DALYs per 100 000 persons and 111 DALYs per 100 000 cases (Supplementary Table S2).
Cost-of-illness
We estimated the direct medical costs at €112 million, or €11 per case and €10 per person, accounting for 11% of the total costs (Table 3). Nearly 70% of direct medical costs were due to hospitalisation (€78 million), while another 15% was due to the medication (€17 million). The direct non-medical costs (i.e. the patients’ transport costs) were lower, contributing only €402 144. We estimated the indirect costs as €927 million accounting for 90% of the total costs according to baseline scenario, or €85 per case and €84 per person. In the alternative indirect cost scenario, we estimated an indirect cost of €98 million or 47% of total costs, corresponding to €10 per case and €9 per person (Table 3).
Table 3.
Estimated annual direct and indirect costs of acute gastroenteritis in Belgium, 2010–2014
| Unit costa | Average estimated units used per year | Total costs | |
|---|---|---|---|
| TOTAL COSTS | 1 039 451 369 | ||
| Direct costs | 112 167 401 | ||
| Direct medical costs | 111 765 256 | ||
| Consultation with a general practitioner | 24.48 | 464 222 | 11 364 155 |
| Consultation with a specialist | 36.74 | 64 991 | 2 387 772 |
| Hospitalisation | 2800 | 27 707 | 77 579 600 |
| Antibiotics | 10.41 | 653 974 | 6 807 868 |
| Oral rehydration solutions | 9 | 747 399 | 6 726 588 |
| Antidiarrhoeals | 7.2 | 435 983 | 3 139 075 |
| Stool tests | 60 | 62 670 | 3 760 198 |
| Direct non-medical costs | 402 144 | ||
| Transport from and to the doctor | 1.08 | 264 607 | 285 775 |
| Transport from and to the hospital | 4.2 | 27 707 | 116 369 |
| Indirect costs | 927 283 968 | ||
| Productivity losses – children | 257 | 272 795 | 70 108 224 |
| Productivity losses – adults | 257 | 3 335 314 | 857 175 744 |
Prices in euros.
In Belgium, the annual total costs for AGE under the baseline scenario amounted to €1 billion on average during the study period. This is equivalent to an average of €103 per case and €94 per person (Table 3). Using the alternative indirect cost scenario, we estimated the total costs at €210 million, or €21 per case and €19 per person (Table 4).
Table 4.
Estimated annual indirect and total costs of acute gastroenteritis in Belgium, 2010–2014 (alternative scenario)
| Unit costa | Average estimated units used per year | Total costs | |
|---|---|---|---|
| TOTAL COSTS | 210 060 243 | ||
| Indirect costs | 97 892 842 | ||
| Productivity losses 0–17 | 257 | 100 528 | 25 835 696 |
| Productivity losses 18–24 | 257 | 16 714 | 4 295 498 |
| Productivity losses 25–54 | 257 | 197 472 | 50 750 304 |
| Productivity losses 55–64 | 257 | 13 510 | 3 472 070 |
| Productivity losses 65+ | 257 | 52 682 | 13 539 274 |
Prices in euros.
Discussion
We performed to our knowledge the first assessment of the health and economic burden of all-cause AGE in Belgium. During 2010–2014, we estimated 0.91 AGE cases per person per year, resulting in 12 000 DALYs and a total cost of €1 billion, corresponding to €103 per AGE case when productivity losses were included. The estimated costs when productivity losses related to time off from work were excluded were considerably less, namely €112 million at an average cost per case of €11.
As in other high-income countries, AGE is common and represents a significant burden to society in Belgium. Our AGE incidence (0.91 cases/person per year) is at the lower end of the range of AGE incidences estimated from retrospective surveys in other high-income countries [21–24]. A review of estimates of the incidence and prevalence of AGE from 33 studies from high-income countries has shown a range from 0.1 to 3.5 episodes per person year [25]. However, our estimates for the episodes occurring in the community are based on the BHIS conducted nearly 20 years ago and it is unknown if the incidence has changed through these years. Although our estimates were similar to most other countries, more recent data are necessary to confirm the current burden of AGE at community level.
Interestingly, the implied proportion of cases consulting a GP (5%) was at the lower end of the range of reported estimates from other countries, i.e. 4–33% [5, 21, 26, 27]. Nevertheless, the GP consulting rate was expected to be high in Belgium as a GP certificate is generally needed to justify absence from work. These discrepancies might imply an overestimation of the number of community cases in our study, or an underestimation of the number of GP consultations, or both. The discrepancies could also be the result of differences in study design or case definitions across studies, or of different healthcare systems and healthcare-seeking behaviour across countries.
DALY calculations complement information on disease incidence and mortality. Our estimate of 118 DALYs per 100 000 cases, which represents the patient burden, is very low in comparison with most of the infectious diseases occurring in several countries in EU and globally [28–30]. However, the total burden of the disease per 100 000 persons, driven by the large number of episodes, is higher than each of the 31 communicable diseases in a recent European study, including influenza, tuberculosis and HIV/AIDS [28]. Our results further indicated that the DALYs associated with mortality were only a small fraction of the total DALYs (30%), while the morbidity impact accounted for 70%. This is a reflection of the low case fatality ratio of AGE in high-income countries, particularly compared with low-income countries, where mortality remains the dominant contributor to the AGE disease burden [30].
Studies on the economic burden of AGE conducted in other countries mainly focused on only health care costs [31, 32], gastrointestinal disease (acute and chronic) [33–35], foodborne gastroenteritis [36–38] or specific pathogens [31, 39, 40]. Therefore, their results are difficult to compare with ours. The estimated economic burden (including direct and indirect costs) of gastrointestinal infections or foodborne illnesses in high-income countries varies between €14 in Australia and €1305 in the USA per case [31]. The estimates of a study conducted in the Netherlands in 2004 yielded estimates comparable with ours – i.e. a total cost of €77 per case and indirect costs representing 82% of the total costs [5].
Our study has several limitations. First and foremost, our disease pyramid estimates are limited by the inherent limitations of each of the applied data sources. For the community cases, the only available database was the BHIS performed in 2001. Since this database has a very short recall period (14 days) and probably overestimates the real incidence of the disease [26], we corrected the estimates by inserting the disease duration in the calculations. Our scenario analyses based on a minimum and a maximum duration of symptoms of 0 and 5 days showed that our estimate was relatively robust against this assumption. For the GP consultations, the only available nationally representative data (SNGP) were for the year 2002, while the most recent data (Intego) were only available for Flanders. Furthermore, it is possible that one AGE event resulted in more than one GP consultation; however, Intego data show that for the vast majority of events, only one consultation was registered. Finally, our death estimates were derived from cause-of-death data considering only the underlying cause. Nonetheless, AGE could have contributed to the death without being listed as the underlying cause. Over the 5-year study period, in addition to the 1713 AGE deaths, another 297 (59 per year) had ICD-10 codes A00-09 listed in the chain of events leading to death, but without being the underlying cause of death. Including these deaths would have resulted in a 17% increase in the number of AGE deaths, and a consequent increase in the number of YLLs and DALYs. However, it is common practice in burden of disease studies to assign deaths only to the underlying cause of death, thus avoiding double-counting when deaths would be assigned to more than one cause.
In addition to the inherent biases in the individual data sources, across data sources, case definitions were not constant. The exact impact of this limitation is difficult to ascertain. Furthermore, we used a 5-year reference period, 2010–2014, to average out possible aberrant temporal variations. The study period was further driven by the availability of data across data sources. In particular, the last available MCD data according to ICD-9 classification were from 2014; in 2015, MCD data were not available due to transforming to ICD-10 coding.
For the DALY calculations, we used two different approaches for defining morbidity-associated health losses, in line with current major studies [14, 40]. Interestingly these two estimations were only 9% different, suggesting that the overall estimates are robust. However, the YLD estimates by severity level did differ significantly.
Our cost-of-illness estimates were generally limited by a lack of recent and nationally representative data on healthcare use and productivity losses, and should therefore be interpreted with caution. The current estimates are therefore partially based on data from other countries [5], data from studies regarding different diseases [17, 19] or expert advices. Data were particularly lacking for estimating indirect costs. We considered two scenarios for estimating the extent of absenteeism among patients and caregivers, which resulted in widely different estimates, ranging from €927 million in our baseline scenario to €98 million in a more conservative alternative scenario. The important contribution of productivity losses clearly warrants further nationally representative surveys to address this gap. A key data gap was the absenteeism associated with AGE in older adults (65+), who account for an important proportion of the AGE cases. As most surveys focus on AGE in children and their parents, little is known about the consequences for older adults and their caregivers. In both scenarios, we therefore assumed the same levels of absenteeism for caregivers of children and elderly adults, which may have been an overestimation of the indirect cost. Furthermore, we excluded certain cost components due to general lack of data, such as non-specified over-the-counter medication, taxi use, parking expenses or additional cleaning material and diapers. We also did not consider the possible use of leftovers from previously bought medication from the patients with AGE, which led to an overestimation in our cost estimates; however, the effect of these aspects on the total cost estimates are not likely to be substantial.
Despite these limitations, our results indicate major health and economic losses associated with AGE morbidity in Belgium. To support risk management, further studies are needed to unravel the relative contribution of specific pathogens to the AGE burden, as well as the role of community-acquired vs. hospital-acquired AGE.
Conclusions
AGE results in a substantial health and economic impact in Belgium, justifying continued mitigation efforts. Nationally representative surveys are needed for addressing several of the identified data gaps, while further research is needed to identify the aetiologies underlying AGE in Belgium to support appropriate interventions.
Acknowledgements
This study was conducted within the framework of the Belgian Burden of Infectious Disease Study (BeBOID) and the Belgian National Burden of Disease Study (BeBOD), initiatives launched by Sciensano, the Belgian institute for health.
Author ORCIDs
Brecht Devleesschauwer, 0000-0002-2867-6892
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S095026881900044X.
click here to view supplementary material
References
- 1.GBD 2016 Causes of Death Collaborators (2017) Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet 390, 1151–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lake RJ et al. (2010) The disease pyramid for acute gastrointestinal illness in New Zealand. Epidemiology and Infection 138, 1468–1471. [DOI] [PubMed] [Google Scholar]
- 3.Wheeler JG et al. (1999) Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. The Infectious Intestinal Disease Study Executive. BMJ 318, 1046–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doorduyn Y, Van Pelt W and Havelaar AH (2012) The burden of infectious intestinal disease (IID) in the community: a survey of self-reported IID in the Netherlands. Epidemiology and Infection 140, 1185–1192. [DOI] [PubMed] [Google Scholar]
- 5.van den Brandhof WE et al. (2004) Costs of gastroenteritis in The Netherlands. Epidemiology and Infection 132, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization (2016) International Statistical Classification of Diseases and Related Health Problems, Tenth Revision Geneva: World Health Organization. [PubMed] [Google Scholar]
- 7.Boffin N et al. (2017) Four sexually transmitted infections (STIs) in Belgian general practice: first results (2013–2014) of a nationwide continuing surveillance study. BMJ Open 7, e012118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Truyers C et al. (2014) The Intego database: background, methods and basic results of a Flemish general practice-based continuous morbidity registration project. BMC Medical Informatics and Decision Making 14, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demarest S et al. (2017) Sample substitution can be an acceptable data-collection strategy: the case of the Belgian Health Interview Survey. International Journal of Public Health 62, 949–957. [DOI] [PubMed] [Google Scholar]
- 10.Graves NS (2013) Acute gastroenteritis. Primary Care 40, 727–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Standaert B et al. (2013) Impact of rotavirus vaccination on hospitalisations in Belgium: comparing model predictions with observed data. PLoS ONE 8, e53864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabbe M et al. (2016) Sustained low rotavirus activity and hospitalisation rates in the post-vaccination era in Belgium, 2007 to 2014. Eurosurveillance 21(27):pii=30273. doi: 10.2807/1560-7917.ES.2016.21.27.30273. [DOI] [PubMed] [Google Scholar]
- 13.Devleesschauwer B et al. (2014) DALY calculation in practice: a stepwise approach. International Journal of Public Health 59, 571–574. [DOI] [PubMed] [Google Scholar]
- 14.Salomon JA et al. (2015) Disability weights for the Global Burden of Disease 2013 study. The Lancet Global Health 3, e712–e723. [DOI] [PubMed] [Google Scholar]
- 15.Haagsma JA et al. (2008) Disability adjusted life years and minimal disease: application of a preference-based relevance criterion to rank enteric pathogens. Population Health Metrics 6, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilcke J et al. (2012) The health and economic burden of chickenpox and herpes zoster in Belgium. Epidemiology and Infection 140, 2096–2109. [DOI] [PubMed] [Google Scholar]
- 17.Bilcke J et al. (2008) The health and economic burden of rotavirus disease in Belgium. European Journal of Pediatrics 167, 1409–1419. [DOI] [PubMed] [Google Scholar]
- 18.Cleemput N et al. (2012) Belgian Guidelines for Economic Evaluations and Budget Impact Analyses, 2nd Edn Brussel, Belgium: Federal Knowledge Centre for Health Care (KCE) KCE reports 183C (D/2012/10.273/54). https://kce.fgov.be/sites/default/files/page_documents/KCE_183C_economic_evaluations_second_edition.pdf (accessed 13 September 2018). [Google Scholar]
- 19.Bilcke J, Coenen S and Beutels P (2014) Influenza-like-illness and clinically diagnosed flu: disease burden, costs and quality of life for patients seeking ambulatory care or no professional care at all. PLoS ONE 9, e102634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mughini-Gras L et al. (2016) Societal burden and correlates of acute gastroenteritis in families with preschool children. Scientific Reports 6, 22144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuusi M et al. (2003) Incidence of gastroenteritis in Norway – a population-based survey. Epidemiology and Infection 131, 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoogenboom-Verdegaal AM et al. (1994) Community-based study of the incidence of gastrointestinal diseases in The Netherlands. Epidemiology and Infection 112, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansdotter FIR et al. (2015) The incidence of acute gastrointestinal illness in Sweden. Scandinavian Journal of Public Health 43, 540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herikstad H et al. (2002) A population-based estimate of the burden of diarrhoeal illness in the United States: FoodNet, 1996–7. Epidemiology and Infection 129, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy SL, Scallan E and Beach MJ (2006) The rate of acute gastrointestinal illness in developed countries. Journal of Water and Health 4(Suppl 2), 31–69. [DOI] [PubMed] [Google Scholar]
- 26.Van Cauteren D et al. (2012) Burden of acute gastroenteritis and healthcare-seeking behaviour in France: a population-based study. Epidemiology and Infection 140, 697–705. [DOI] [PubMed] [Google Scholar]
- 27.Roberts JA et al. (2003) The study of infectious intestinal disease in England: socio-economic impact. Epidemiology and Infection 130, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cassini A et al. (2018) Impact of infectious diseases on population health using incidence-based disability-adjusted life years (DALYs): results from the Burden of Communicable Diseases in Europe study, European Union and European Economic Area countries, 2009 to 2013. Eurosurveillance 23(16):pii=17-00454. doi: 10.2807/1560-7917.ES.2018.23.16.17-00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Lier A et al. (2016) Disease burden of 32 infectious diseases in the Netherlands, 2007–2011. PLoS ONE 11, e0153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirk MD et al. (2015) World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Medicine 12, e1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmutz C et al. (2017) Estimating healthcare costs of acute gastroenteritis and human campylobacteriosis in Switzerland. Epidemiology and Infection 145, 627–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barker SF et al. (2018) Cost of gastroenteritis in Australia: a healthcare perspective. PLoS ONE 13, e0195759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin M et al. (2016) Healthcare costs of rotavirus and other types of gastroenteritis in children in Norway. The Pediatric Infectious Disease Journal 35, e97–e101. [DOI] [PubMed] [Google Scholar]
- 34.Sowmyanarayanan TV et al. (2012) Direct costs of hospitalization for rotavirus gastroenteritis in different health facilities in India. The Indian Journal of Medical Research 136, 68–73. [PMC free article] [PubMed] [Google Scholar]
- 35.Tichopád A et al. (2016) Cost burden of severe community-acquired rotavirus gastroenteritis requiring hospitalization in the Czech Republic, Slovakia, Poland, and Hungary: a retrospective patient chart review. Value in Health Regional Issues 10, 53–60. [DOI] [PubMed] [Google Scholar]
- 36.Scott WG et al. (2000) Economic cost to New Zealand of foodborne infectious disease. The New Zealand Medical Journal 113, 281–284. [PubMed] [Google Scholar]
- 37.Scharff RL (2015) State estimates for the annual cost of foodborne illness. Journal of Food Protection 78, 1064–1071. [DOI] [PubMed] [Google Scholar]
- 38.Minor T et al. (2015) The per case and total annual costs of foodborne illness in the United States. Risk Analysis 35, 1125–1139. [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann S, Batz MB and Morris JG Jr. (2012) Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. Journal of Food Protection 75, 1292–1302. [DOI] [PubMed] [Google Scholar]
- 40.Mangen MJ et al. (2015) Cost-of-illness and disease burden of food-related pathogens in the Netherlands, 2011. International Journal of Food Microbiology 196, 84–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S095026881900044X.
click here to view supplementary material