Abstract
Toxoplasma gondii (T. gondii) as an obligate intracellular protozoan with a worldwide distribution can infect virtually all warm-blooded animals and humans. This study aims to provide a summary of the available data on genotypes of T. gondii in human. Five databases including MEDLINE in PubMed, Scopus, Science Direct, Web of Science and Google Scholar were searched for the T. gondii genotyping in human during 1995–August 2017. Next, we screened all the articles based on the inclusion and exclusion criteria. Overall, 26 studies were eligible regarding genotyping T. gondii in human samples. In clonal genotyping, 167 out of 286 cases (58%) were infected with type II. Genetic characterisation of T. gondii isolates displayed that type II was the most predominant genotype in human with the prevalence of 64.3%, 62.1% and 41.7% in patients with AIDS, congenital and ocular toxoplasmosis, respectively. In ToxoDB genotyping, most individuals were infected with genotypes #9 and #65 (21.2%). Based on these results, genotype profile of T. gondii isolates is different throughout the world. The strains in Asian and African countries are characterised by low genetic diversity, while in North and South America a wide diversity of this parasite is found. In countries without any data (e.g. Australia, Western and Southern Africa and Western Asia), identification of T. gondii genotypes might discover higher genetic diversity.
Key words: AIDS, congenital, genotype, ocular, Toxoplasma gondii
Introduction
Toxoplasma gondii as an obligate intracellular protozoan with a worldwide distribution can infect virtually all warm-blooded animals and human. One-third of the human population is assumed to be infected with T. gondii [1]. In immunocompetent individuals, clinical symptoms of toxoplasmosis present as mild and self-limiting, including fever, malaise and lymphadenopathy. However, infection is usually more severe in immunocompromised people and pregnant women. In these groups, the infection is accompanied by more complications, such as encephalitis, retinochoroiditis, foetus abortion, splenomegaly and pneumonitis [2].
The number and genetic diversity of parasites play an important role in pathogenesis of T. gondii [3]. Identifying the genotypes of T. gondii using molecular technologies is invaluable in epidemiological studies. These techniques include multilocus polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP), microsatellite analysis, random amplified polymorphic (RAPD), multilocus sequence typing (MLST) and high-resolution melting (HRM) analysis [4].
The first genotyping studies on T. gondii strains in France and the United States of America demonstrated a clonal population structure with three main lineages namely type I, II and III [5]. Despite the sexual phase and global distribution of T. gondii, the population structure of this parasite is explained as highly clonal with low genetic diversity [6]. This was found in genetic researches on isolates from Europe and USA which classified the isolates as three major genotypes of type I, II and III (approximately 90%) [7].
More genetic variation of T. gondii, except for the three main lineages, was found in the other continents. These ‘new’ genotypes were designated as atypical, exotic, recombinant or non-archetypal genotypes [8, 9]. Multilocus PCR-RFLP analysis of 10 gene markers in 1457 T. gondii isolates worldwide revealed 189 different genotypes. The data of these genotypes are displayed in ToxoDB [10].
Numerous studies were performed on T. gondii genotyping in different groups of patients and healthy individuals. However, there is no exhaustive documented data regarding this subject. Therefore, this study aims to provide a summary of the available data on genotypes of T. gondii in human. This study was carried out to evaluate the predominant genotypes in different continents of the world. Moreover, the prevalence of genotypes is assessed in various groups of human toxoplasmosis, namely immunocompromised, congenital, ocular toxoplasmosis, cancer and immunocompetent.
Methods
This review followed the preferred reporting items for systematic reviews (PRISMA) guidelines [11].
Literature search, study selection and data extraction
We searched MEDLINE in PubMed, Scopus, Science Direct, Web of Science and Google Scholar during 1995–August 2017. The utilised keywords entailed Toxoplasma gondii, toxoplasmosis, genotype, genotyping, molecular characterisation, human, individuals, patients, pregnancy, women, congenital, cancer, AIDS, HIV, immunocompromised and ocular toxoplasmosis. We searched the mentioned keywords alone and in combination with the others.
In this systematic review, the exclusion criteria encompassed not describing the T. gondii genotypes in human, in addition to using genetic characterisation methods with less than five gene markers. To collect precise information, a comprehensive search was completed on all published and unpublished articles including full texts, abstracts and parasitology congresses summaries. All the data were collected from English language articles. We defined a protocol for data extraction and two authors assessed the obtained data independently. Afterwards the disagreements between the comments were discussed and resolved.
The data extracted from studies encompassed year, first author, country and continent, total sample size, methods, genotypes of T. gondii, type of patients and molecular markers used for genotyping in the study.
The genotypes were classified as classical types I, II, III, mix/recombinant (i.e. I&III, I&II, II&III/exotic and unknown) atypical, Africa I and ToxoDB genotype. A protocol of this systematic review with CRD 42017070501 code is available in international prospective register of systematic reviews (PROSPERO, http://www.crd.york.ac.uk/prospero) [12].
Results
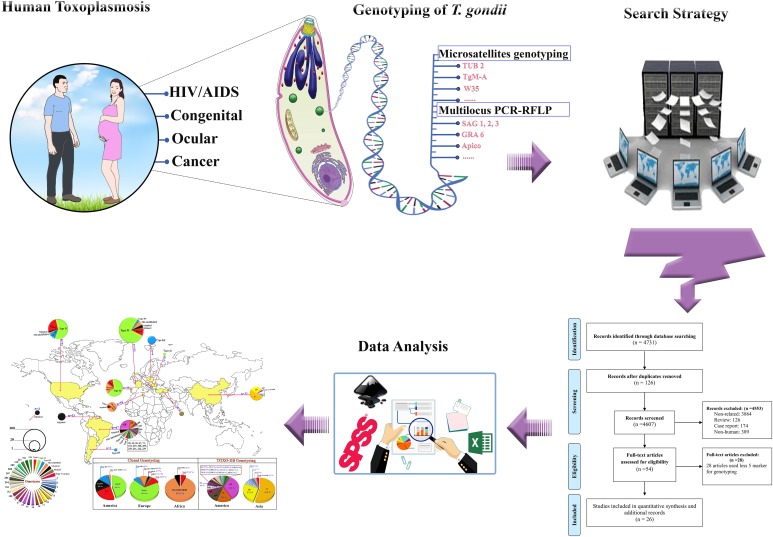
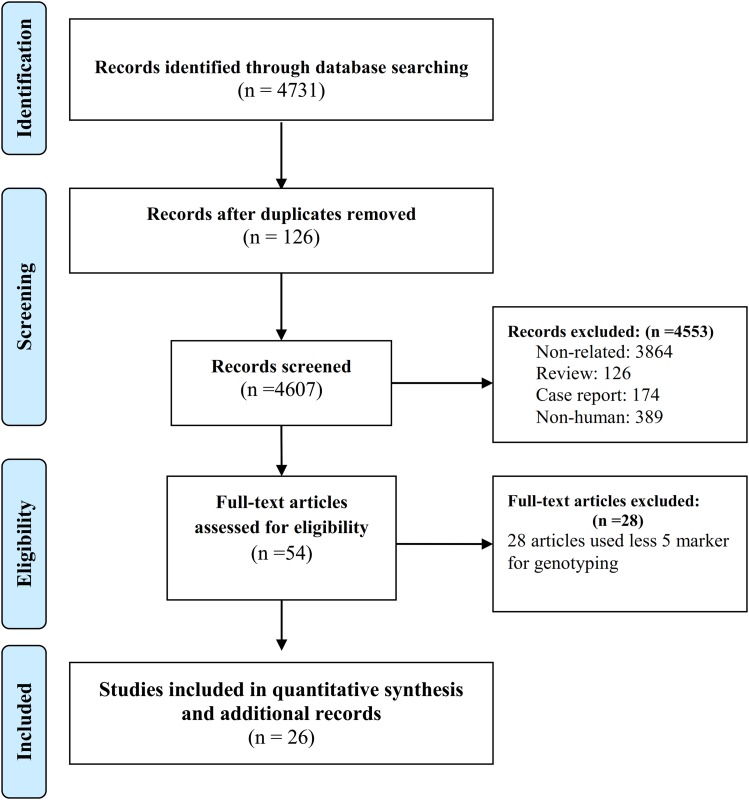
A graphic summary of this study on genetic diversity of T. gondii is shown in Figure 1. Overall, we selected 4731 studies for this systematic review. After screening, 26 papers were eligible as genotyping studies on T. gondii in human samples (Table 1 and Fig. 2). From these papers, we collected 371 individual genotyping data, 286 of which being about clonal genotyping (I, II, III, mix/recombinant, Africa I and atypical) and 85 about ToxoDB genotyping (genotypes #1–#231).
Fig. 1.
Graphic summary of the study on genetic diversity of T. gondii in human.
Table 1.
Overview of the included studies regarding genotyping T. gondii isolates from human and summary of the main findings
| No. | Author | Patients group | Methods for genotyping | Molecular markers for genotyping | No. of isolates | No. (%) genotype |
|---|---|---|---|---|---|---|
| 1 | Howe and Sibley [13] | AIDS/congenital toxoplasmosis | PCR-RFLP | SAG1, SAG2, L328, ROP1, 850, 62 | 68 | 16 (23.5) I, 43 (63.2) II, 9 (13.3) III |
| 2 | Grigg et al. [9] | Ocular toxoplasmosis | PCR-RFLP | B1, SAG1, SAG2, SAG3, SAG4 | 12 | 3 (25) I, 3 (25) II, 1 (8.4) III, 5 (41.6) I&III |
| 3 | Ajzenberg et al. [14] | Congenital toxoplasmosis | PCR of MS markers | TUB2, W35, TG-MA, w35487, N60608, N82375, N83021, N61191, AA519150 | 86 | 73 (84.8) II, 7 (8.1) I, 2 (2.3) III, 4 (4.6) atypical |
| 4 | De melo Ferreira [15] | Congenital toxoplasmosis | PCR-RFLP | SAG1, SAG2, SAG3, B1, cB21-4, cS10-A6, GRA6, L363 | 4 | 4 (100) atypical |
| 5 | Khan et al. [16] | Ocular toxoplasmosis | PCR-RFLP | 5′SAG2, 3′SAG2, BTUB, GRA6, SAG3 | 11 | 3 (27.3) I, 2 (18.1) II, 3 (27.3) III, 3 (27.3) I&III |
| 6 | Nowakowske et al. [17] | Newborns | PCR-RFLP | 3′SAG2, 5′SAG2, SAG3, BTUB, GRA6 | 9 | 9 (100) III |
| 7 | Djurković-Djaković et al. [18] | Congenital toxoplasmosis | PCR-RFLP | 5′SAG2, 3′SAG2, BTUB, SAG3, GRA6 | 1 | 1 (100) II |
| 8 | Genot et al. [19] | AIDS | PCR of MS markers | TUB2, TgM-A, W35, B17, B18 | 1 | 1 (100) I&III |
| 9 | Demar et al. [20] | Congenital immunocompetent | PCR of MS markers | TUB2, W35, TgM-A, B18, B17 | 11 | 11 (100) atypical |
| 10 | Zhou et al. [21] | ND | Mn-PCR-RFLP | SAG1, SAG2, SAG3, BTUB, GRA6, c22-8, c29-2, L358, PK1, Apico | 3 | 1 (33.3) #1, 1 (33.3) #3, 1 (33.3) #4 |
| 11 | Boughattes et al. [22] | Congenital toxoplasmosis | PCR-RFLP | 3′SAG2, 5′SAG2, SAG3, BTUB, GRA6, APICO | 14 | 1 (7.14) I, 7 (50) I&III, 3 (21.4) I&II, 3 (21.4) I&II + I&III |
| 12 | Ferreira et al. [23] | AIDS, congenital, pregnant, ocular, acute toxoplasmosis | Mn-PCR-RFLP | SAG1, SAG2, SAG3, BTUB, GRA6, C22-2, C29-2, L358, PK1, APICO | 20 | 18 (90) #65, 1 (5) #71, 1 (5) #6 |
| 13 | Boughattes et al. [24] | Congenital toxoplasmosis | PCR-RFLP | 3′SAG2, 5′SAG2, SAG3, AK69, APICO, UPRT1 | 1 | 1 (100) I&III |
| 14 | Boughattes et al. [25] | Pregnant and diabetic | PCR-RFLP | 3′SAG2, 5′SAG2, SAG2, SAG3, GRA6, BTUB, APICO, PK1, KT850, UPRT1 | 1 | 1 (100) atypical |
| 15 | Fekkar et al. [26] | Ocular toxoplasmosis | PCR of MS markers | TUB2, W35, TG-MA, B18, B17 | 13 | 10 (76.9) II, 1 (7.7) II&III, 2 (15.4) Africa I |
| 16 | Carneiro et al. [27] | Newborns | Mn-PCR-RFLP | 3′SAG2, 5′SAG2, SAG1, SAG3, SAG2, GRA6, L356, APICO, C29-2, C22-9, PK1 | 25 | 7 (28) #11, 1 (4) #8, 3 (12) #206, 2 (8) #41, 2 (8) #108, 1 (4) #67, 1 (4) #207, 1 (4) #162, 1 (4) #208, 1 (4) #36, 1 (4) #209, 1 (4) #210, 1 (4) #211, 1 (4) #212, 1 (4) (mix/recombinant) |
| 18 | Wang et al. [28] | AIDS/cancer | Mn-PCR-RFLP | SAG1, SAG2, SAG3, BTUB, GRA6, C22-2, C29-2, L358, PK1, APICO | 4 | 1 (25) #1, 1 (25) #9, 1 (25) #10, 1 (25) #204 |
| 19 | Costache et al. [29] | Congenital toxoplasmosis | PCR of MS markers | TUB2, W35, TG-MA, w35487, N60608, N82375, N83021, N61191, AA519150 | 1 | 1 (100) II |
| 20 | Döşkaya et al. [30] | Congenital toxoplasmosis | PCR of MS markers | TUB-2, W35, TgM-A, B18, B17, M33, IV.1, XI.1, M48, M102, N60, N82, AA, N61, N83 | 2 | 2 (100) Africa I |
| 21 | Pardini et al. [31] | Congenital toxoplasmosis | PCR-RFLP | SAG2, SAG3, BTUB, GRA6, c29-2, c22-8, L358, PK1, Apico | 1 | 1 (100) III |
| 22 | Higa et al. [32] | Congenital toxoplasmosis | Mn-PCR-RFLP | SAG1, SAG2, SAG3, BTUB, GRA6, C22-2, C29-2, L358, PK1, APICO | 3 | 3 (100) #166 |
| 23 | Silva et al. [33] | Congenital toxoplasmosis | Mn-PCR-RFLP | SAG1, 5′SAG2, 3′SAG2, SAG2 alt, SAG3, BTUB, GRA6, c22-8, c29-2, L358, PK1, Apico | 4 | 2 (50) #108, 1 (25) # 206, 1 (25) # 229 |
| 24 | Yeraa et al. [34] | Congenital toxoplasmosis | PCR of MS markers | TUB2, W35, TgM-A, B18, B17, M33, IV.1, XI.1, M48, M102, N60, N82, AA, N61, N83 | 2 | 2 (100) atypical |
| 25 | Wang et al. [35] | Cancer | Mn- PCR-RFLP | SAG1, SAG2, SAG3, BTUB, GRA6, C22-2, C29-2, L358, PK1, APICO | 9 | 9 (100) #9 |
| 26 | Cong et al. [36] | Cancer | Mn-PCR-RFLP | SAG1, SAG2, SAG3, BTUB, GRA6, C22-2, C29-2, L358, PK1, APICO | 17 | 8 (47.1) #10, 8 (47.1) #9, 1 (5.8) (type I variant) |
| 27 | Virales et al. [37] | Congenital toxoplasmosis | PCR-RFLP/PCR of MS markers | SAG2, TUB, TgM, W35, B17, B18 | 48 | 6 (12.5) I, 32 (66.6) II, 6 (12.5) I&II, 4 (8.3) I&III |
MS, microsatellite Marker; ND, not determined; Mn, multi locus nested-PCR-RFLP (10 markers).
Fig. 2.
PRISMA flow diagram describing the study design.
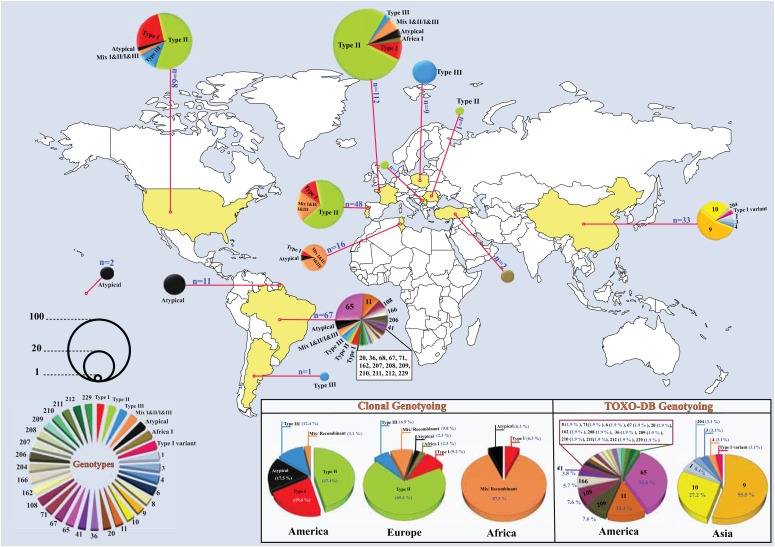
Out of 286 cases, in clonal genotyping using PCR-RFLP based on multilocus typing (i.e. more than five gene markers) and microsatellite markers, 166 (58%), 36 (12.6%), 34 (11.9%), 24 (8.4%), 22 (7.7%) and 4 (1.4%) were infected with types II, I, mix/recombinant, III, atypical and Africa I type, respectively (Table 2). The global prevalence rate of T. gondii clonal genotypes in different continents is indicated in Figure 3. The most prevalent types were I and III reported from America (19/97, 19.6% and 12/97, 12.4%, respectively) and type II from Europe (120/173, 69.4%) (Table 3).
Table 2.
Clonal genotyping of T. gondii based on multilocus PCR-RFLP and microsatellite in different groups
| Group | Author | Country | No. of isolates | Type I No. (%) |
Type II No. (%) |
Type III No. (%) |
Mix/recombinant, Africa I and atypical No. (%) |
|---|---|---|---|---|---|---|---|
| Congenital | Howe and Sibley [13] | USA | 41 | 10 (24.4) | 25 (61) | 6 (14.6) | |
| Ajzenberg et al. [14] | France | 86 | 7 (8.1) | 73 (84.9) | 2 (2.3) | 4 (4.6) atypical | |
| Ferreira et al. [23] | Brazil | 4 | 4 (100) atypical | ||||
| Nowakowske et al. [17] | Poland | 9 | 9 (100) | ||||
| Djurković-Djaković et al. [18] | Serbia | 1 | 1 (100) | ||||
| Demar et al. [20] | French Guiana | 3 | 3 (100) atypical | ||||
| Boughattes et al. [22] | Tunisia | 14 | 1 (7.1) | 13 (50) mix/recombinant | |||
| Boughattes et al. [24] | Tunisia | 1 | 1 (100) mix/recombinant | ||||
| Boughattes et al. [24] | Tunisia | 1 | 1 (100) atypical | ||||
| Costache et al. [29] | Romania | 1 | 1 (100) | ||||
| Pardini et al. [31] | Argentina | 1 | 1 (100) | ||||
| Döşkaya et al. [30] | Turkey | 2 | 2 (100) Africa I | ||||
| Yera et al. [34] | French Polynesia | 2 | 2 (100) atypical | ||||
| Vilares et al. [37] | Portugal | 48 | 6 (12.5) | 32 (66.7) | 10 (20.8) mix/recombinant | ||
| Total | 214 | 24 (11.2) | 133 (62.1) | 17 (7.9) | 24 (11.2) mix/recombinant, 14 (6.5) atypical, 2 (0.9) Africa I | ||
| AIDS | Howe and Sibley [13] | USA | 27 | 6 (20) | 18 (70) | 3 (10) | |
| Genot et al. [19] | France | 1 | 1 (100) mix/recombinant | ||||
| Total | 28 | 6 (21.4) | 18 (64.3) | 3 (10.7) | 1 (3.6) mix/recombinant | ||
| Ocular | Grigg et al. [9] | France | 12 | 3 (25) | 3 (25) | 1 (8.4) | 5 (41.7) mix/recombinant |
| Khan et al. [16] | Brazil | 11 | 3 (27.2) | 2 (18.2) | 3 (27.2) | 3 (27.2) mix/recombinant | |
| Fekkar et al. [26] | France | 13 | 10 (77) | 1 (7.7) mix/recombinant, 2 (15.4) Africa I | |||
| Total | 36 | 6 (16.7) | 15 (41.7) | 4 (11.1) | 9 (22.2) mix/recombinant, 2 (5.6) Africa I | ||
| Immunocompetent | Demar et al. [20] | French Guiana | 8 | 8 (100) atypical | |||
| Total | 286 | 36 (12.6) | 166 (58) | 24 (8.4%) | 34 (11.9) mix/recombinant, 22 (7.7) atypical, 4 (1.4) Africa I | ||
Fig. 3.
Geographical distribution of T. gondii strains in the world; sizes of pie charts correlate with the total number of isolates for each country; colours indicate different genotypes.
Table 3.
Global prevalence rate of clonal genotypes of T. gondii based on microsatellite and multilocus PCR-RFLP by continent
| Continent | No. of isolates | Type I No. (%) |
Type II No. (%) |
Type III No. (%) |
Mix/recombinant No. (%) |
Atypical No. (%) |
Africa I No. (%) |
|---|---|---|---|---|---|---|---|
| America | 97 | 19 (19.6) | 46 (47.4) | 12 (12.4) | 3 (3.1) | 17 (17.5) | – |
| Africa | 16 | 1 (6.3) | – | – | 14 (87.5) | 1 (6.3) | – |
| Europe | 173 | 16 (9.2) | 120 (69.4) | 12 (6.9) | 17 (9.8) | 4 (2.3) | 4 (2.3) |
| Total | 286 | 36 (12.6) | 166 (58) | 24 (8.4) | 34 (11.9) | 22 (7.7) | 4 (1.4) |
In ToxoDB genotyping using PCR-RFLP based on multilocus typing, most individuals were infected with genotypes #9 and #65 (21.2%). It should be noted that genotype #65 was isolated from America, and genotype #9 from Asia (Table 4 and Fig. 3).
Table 4.
Genetic characterisation of T. gondii based on multilocus PCR-RFLP (ToxoDB) in different groups
| Group | Author | Country | Type of sample | No. of isolates | No. (%) ToxoDB genotype |
|---|---|---|---|---|---|
| AIDS | Ferreira et al. [23] | Brazil | CSF | 9 | 7 (#65), 1(#71), 1 (#6) |
| Wang et al. [28] | China | Blood | 2 | 1 (#9), 1 (#10) | |
| Total | 11 | 7 (63.6) #65, 1 (9.1) #71, 1 (9.1) #6, 1 (9.1) #9, 1 (9.1) #10 | |||
| Congenital | Ferreira et al. [23] | Brazil | Amniotic fluid | 1 | 1 (100) #65 |
| Ferreira et al. [23] | Brazil | Blood | 1 | 1 (100) #65 | |
| Carneiro et al. [27] | Brazil | Blood | 25 | 7 (28) #11, 1 (4) #8, 3 (12) #206, 2 (8) #41, 2 (8) #108, 1 (4) #67, 1 (4) #207, 1 (4) #162, 1 (4) #208, 1 (4) #36, 1 (4) #209, 1 (4) #210, 1 (4) #211, 1 (4) #212, 1 mix/recombinant | |
| Silva et al. [33] | Brazil | Blood | 4 | 2 (50) #108, 1 (25) # 206, 1 (25) #229 | |
| Higa et al. [32] | Brazil | Blood | 3 | 3 (100) #166 | |
| Total | 34 | 7 (20.5) #11, 4 (11.7) #108, 4 (11.7) #206, 3 (8.8) #166, 2 (5.8) #65, 2 (5.8) #41, 1 (2.9) # 229, 1 (2.9) #8, 1 (2.9) #67, 1 (2.9) #207, 1 (2.9) #162, 1 (2.9) #208, 1 (2.9) #36, 1 (2.9) #209, 1 (2.9) #210 1 (2.9) #211, 1 (2.9) #212, 1 (2.9) mix/recombinant | |||
| Ocular | Ferreira et al. [23] | Brazil | Blood | 7 | 7 (100) #65 |
| Cancer | Wang et al. [28] | China | Blood | 2 | 1 (50) #204, 1 (50) #1 |
| Cong et al. [36] | China | Blood | 17 | 8 (47.1) #9, 8 (47.1) #10, 1 (5.8) type I variant | |
| Wang et al. [35] | China | Blood | 9 | 9 (100) #9 | |
| Total | 28 | 17 (60.7) #9, 8 (28.5) #10, 1 (3.7) #204, 1 (3.7) #1, 1 (3.7) type I variant | |||
| Unknown | Zhou et al. [21] | China | ND | 3 | 1 (33.3) #1, 1 (33.3) #3, 1 (33.3) #4 |
| Acute toxoplasmosis | Ferreira et al. [23] | Brazil | Blood/CSF | 2 | 2 (100) #65 |
| Total | 85 | 18 (21.1) # 9, 18 (21.1) #65, 9 (10.5) #10, 7 (8.2) #11, 4 (4.7) #206, 4 (4.7) #108, 3 (3.5) #166, 2 (2.3) #41, 3 (2.3) #1, 2 (2.3) #204, 1 (1.1) #8, 1 (1.1) #71, 1 (1.1) #6, 1 (1.1) #67, 1 (1.1) #20, 1 (1.1) #162, 1 (1.1) #208, 1 (1.1) #36, 1 (1.1) #209, 1 (1.1) #210, 1 (1.1) #211, 1 (1.1) #212, 1 (1.1) #229, 1 (1.1) #3, 1 (1.1) #4, 1 (1.1) type I variant | |||
ND, not determined.
In addition, the genotyping data of T. gondii were divided into several groups, including AIDS, congenital, ocular, acute toxoplasmosis, cancer and immunocompetent. Genetic characterisation of T. gondii isolates by PCR-RFLP based on multilocus and microsatellite markers (more than five gene markers) revealed that type II was the most predominant genotype among all groups of human toxoplasmosis (64.3%, 62.1% and 41.7% in AIDS patients, congenital form and ocular toxoplasmosis, respectively). On the other hand, the atypical type (100%) was the most common genotype in the immunocompetent group (Table 2).
Furthermore, our analysis showed that ToxoDB genotype #65 was the most prevalent in ocular (100%) and AIDS (63.6%) groups. In addition, genotypes #11 and #9 were more prevalent in the congenital (20.5%) and other groups (54.5%) (Table 3). Genetic diversity of T. gondii isolates in different groups of human and patients is demonstrated in Table 5.
Table 5.
Genetic diversity of T. gondii isolates in different groups of patients
| Category | Groups of patients | ToxoDB genotypes |
|---|---|---|
| All genotypes | Ocular | #65 |
| AIDS | #6, #9, #10, #65, #71 | |
| Congenital | #8, #11, #36, #41, #65, #67, #108, #162, #166, # 206, #207, #208, #209, #210, #211, #212, #229 | |
| Cancer | #9, #10, #204, #1, type I variant | |
| Common genotype ToxoDB | All groups | #65 |
| Ocular and AIDS | #65 | |
| Ocular and congenital | #65 | |
| AIDS and congenital | #65 | |
| AIDS and cancer | #9 | |
| Specific genotypes | Ocular | – |
| AIDS | #6, #71 | |
| Congenital | #8, #11, #36, #41, #67, #108, #162, #166, #206, #207, #208, #209, #210, #211, #212, #229 | |
| Cancer | – |
Discussion
This systematic review is beneficial for understanding the epidemiology of T. gondii genotypes in high-risk groups of human. The present study determined the global prevalence rate of T. gondii genotypes in AIDS, congenital, ocular toxoplasmosis and other groups of patients, as well as healthy individuals. We used the documented data from the literature collected from different countries.
After searching all the databases, 26 articles were included in this study. According to the results, type II was the most predominant genotype in human toxoplasmosis during 1995–August 2017. Type I lineages are uniformly lethal with absolute lethal dose (LD100) of 1 in mice. On the other hand, the type II and III lineages are significantly less virulent with LD100⩾103. Moreover, type I or type I-like atypical isolates are more likely to cause intensive retinochoroiditis in patients and the atypical isolates often result in severe acute or disseminated toxoplasmosis in immunocompromised patients [5, 13].
Various analyses indicate that the prevalence of T. gondii genotypes varies in different continents. In current study, the highest and lowest prevalence of T. gondii type I strain were reported from American (19.6%) and African continents (6.3%). As mentioned, type I strains of T. gondii with LD100 of almost one can cause lethal infections in all species of laboratory mice even at low dose inoculations. Therefore, practicing the basic measures seems to be necessary for controlling the disease, especially in the American continent.
According to the literature, type II strains of T. gondii are the predominant among human samples in America, and Europe (USA, France, Portugal, etc.). Type II strains of T. gondii are of low virulence and high cyst-forming [5, 14, 38, 39]. We found in our analysis that Europe has the highest prevalence of T. gondii genotype type II (69.4%).
Similar to type II, type III strains of T. gondii are low virulence with LD100⩾103 [5]. In this review, the highest prevalence of type III strains of T. gondii was observed in America (12/97, 12.4%). On the other hand, the lowest prevalence was reported from the European continent (12/173, 6.9%).
According to our study, in the AIDS/HIV group, genotyping of T. gondii was performed on 28 isolates using multilocus markers. Our analysis showed that type II strains (18/28, 64.3%) were the most common, followed by type I (6/28, 21.4%), type III (3/28, 10.7%) and mix/recombinant type (1/28, 3.6%). Toxoplasmosis in immunocompromised patients is often due to reactivation of tissue cysts usually leading to severe and life-threatening diseases, toxoplasmic encephalitis and death [40]. The predominance of type II in the patients may simply reflect the prevalence of type II infection in general population.
In order to determine the genotypes in congenital toxoplasmosis group, 214 T. gondii isolates were examined. Totally, 133 (62.1%), 24 (11.2%), 24 (11.2%), 17 (7.9%), 14 (6.5%) and 2 (0.9%) isolates were classified as types II, I, mix/recombinant, III, atypical and Africa I, respectively.
Congenital toxoplasmosis may result in intracerebral calcification, hydrocephalus, mental retardation and retinochoroiditis, which may be present at birth or develop later in life [41]. The role of genotypes in congenital toxoplasmosis is still controversial. However, it was shown in the study performed by Hutson et al. that congenital infections with type II strain are frequently associated with hydrocephalus characteristic patterns [42].
Rico-Torres et al. (2016) in a systematic review study demonstrated that neonatal cases infected with type II strain of T. gondii during the first half of pregnancy present severe clinical symptoms. The latter result suggests a crucial role for immature state and response of the foetus in susceptibility to disease [43]. Among the 26 papers included in this systematic review, only one study had determined the genotype of T. gondii in immunocompetent individuals and all the eight samples were infected with the atypical type.
In the ocular toxoplasmosis group, 36 isolates were genotyped and the obtained results showed that type II strains were predominant (15/36, 41.7%), followed by mix/recombinant type (9/36, 25%), type I (6/36, 16.7%), type III (4/36, 11.1%) and Africa I type (2/36, 5.6%) in this group. The previous studies in different countries revealed that ocular toxoplasmosis is associated with type I strain of T. gondii.
According to the studies conducted using less than five gene markers, type I strain has the ability for extracellular migration and decreases conversion to the bradyzoite form, in comparison with types II and III [44]. Our systematic review by more than five gene markers demonstrates that type II is the most prevalent in these patients. The reason for this difference can be attributed to application of more gene markers causing the genotyping to be more precise.
In recent years, researchers have used different markers (about 10–12 markers) of multilocus PCR-RFLP and microsatellites for epidemiology and genotyping studies [44]. Findings of these studies have indicated a higher genetic diversity among the different groups of patients and healthy individuals. In ToxoDB, 1457 human and animal samples were registered that revealed different ToxoDB genotypes (#1–#231). At present, major genotypes such as Chinese 1 (#9), type Br I (#6), type Br II (#11), type Br III (#8), type IV (#17) and type 12 (#4, #5) were added to the previous list (type I, II, III lineages and atypical or exotic genotypes) [38, 45].
According to new nomenclature, identified genotypes are Type I (#10), Type II (#1 clonal, #3 variant) and Type III (#2), kinds of atypical or exotic genotypes, Chinese 1 (#9, #10), Type Br I (#6), Type Br II (#11), Type Br III (#8), Type IV (#17) and Type 12 (#4, #5).
Out of the 26 studies included in the current review, 11 studies used multilocus gene markers for genotyping (ToxoDB) and determination of genetic diversity. Our analysis demonstrated that genotype #65 is predominant among all the different groups of human. Moreover, genotype #65 is the most prevalent genotype in AIDS and ocular toxoplasmosis groups. However, in the congenital toxoplasmosis group, genotype #11 was the most common. Genotypes #6 and #71 were observed only in the AIDS group. On the other hand, 16 different ToxoDB genotypes, including #8, #11, #36, #41, #67, #108, #162, #166, #206, #207, #207, #209, #210, #211, #212 and #229 were found in the congenital toxoplasmosis group. It seems that the cause of high-genetic diversity in congenital toxoplasmosis may be due to more sampling from the different regions of the world.
This review indicated that genetic diversity of T. gondii was low in Asian and African countries, contrary to the ones in North and South America.
Conclusion
The current study showed that new techniques with more genetic markers for T. gondii genotyping, might lead to different results regarding genetic diversity. This is true especially in countries that do not have any data concerning genotyping of this parasite, such as Australia, Western and Southern Africa and Western Asia. Identification of the specific genotype in each area of the world could be valuable in developing new strategies for treatment, vaccination, diagnosis, control and prevention of T. gondii in human.
Acknowledgements
The authors would like to acknowledge the Toxoplasmosis Research Center (TRC) of Mazandaran University of Medical Sciences for their support with the grant number of 10296.
Conflict of interest
The authors of the current study declare no conflicts of interests.
Author ORCIDs
J. Javidnia http://orcid.org/0000-0001-9336-2518
A. Daryani http://orcid.org/0000-0001-8571-5803
References
- 1.Dubey JP (2016) Toxoplasmosis of Animals and Humans: Boca Raton, Florida, USA: CRC press. [Google Scholar]
- 2.Tenter AM, Heckeroth AR and Weiss LM (2000) Toxoplasma gondii: from animals to humans. International Journal for Parasitology 30, 1217–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blader IJ and Saeij JP (2009) Communication between Toxoplasma gondii and its host: impact on parasite growth, development, immune evasion, and virulence. Apmis 117, 458–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su C, Zhang X and Dubey J (2006) Genotyping of Toxoplasma gondii by multilocus PCR-RFLP markers: a high resolution and simple method for identification of parasites. International Journal for Parasitology 36, 841–848. [DOI] [PubMed] [Google Scholar]
- 5.Dardé M-L (2008) Toxoplasma gondii ‘new’ genotypes and virulence. Parasite 15, 366–371. [DOI] [PubMed] [Google Scholar]
- 6.Sibley LD et al. (2009) Genetic diversity of Toxoplasma gondii in animals and humans. Philosophical Transactions of the Royal Society of London B: Biological Sciences 364, 2749–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sibley LD et al. (2002) Genetic approaches to studying virulence and pathogenesis in Toxoplasma gondii. Philosophical Transactions of the Royal Society B: Biological Sciences 357, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ajzenberg D et al. (2004) Genetic diversity, clonality and sexuality in Toxoplasma gondii. International Journal for Parasitology 34, 1185–1196. [DOI] [PubMed] [Google Scholar]
- 9.Grigg ME et al. (2001) Unusual abundance of atypical strains associated with human ocular toxoplasmosis. The Journal of Infectious Diseases 184, 633–639. [DOI] [PubMed] [Google Scholar]
- 10.Switaj K et al. (2006) Association of ocular toxoplasmosis with type I Toxoplasma gondii strains: direct genotyping from peripheral blood samples. Journal of Clinical Microbiology 44, 4262–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D et al. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine 18, 264–269. [DOI] [PubMed] [Google Scholar]
- 12.PROSPERO (2017) The global status of genetic diversity of Toxoplasma gondii isolates from human: a systematic review. CRD42017072730.
- 13.Howe DK and Sibley LD (1995) Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. Journal of Infectious Diseases 172, 1561–1566. [DOI] [PubMed] [Google Scholar]
- 14.Ajzenberg D et al. (2002) Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis, and correlation with clinical findings. The Journal of Infectious Diseases 186, 684–689. [DOI] [PubMed] [Google Scholar]
- 15.de Melo Ferreira A et al. (2006) Genetic analysis of natural recombinant Brazilian Toxoplasma gondii strains by multilocus PCR-RFLP. Infection, Genetics and Evolution 6, 22–31. [DOI] [PubMed] [Google Scholar]
- 16.Khan A et al. (2006) Genetic divergence of Toxoplasma gondii strains associated with ocular toxoplasmosis, Brazil. Emerging Infectious Diseases 12, 942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nowakowska D et al. (2006) Genotyping of Toxoplasma gondii by multiplex PCR and peptide-based serological testing of samples from infants in Poland diagnosed with congenital toxoplasmosis. Journal of Clinical Microbiology 44, 1382–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Djurković-Djaković O et al. (2006) A human origin type II strain of Toxoplasma gondii causing severe encephalitis in mice. Microbes and Infection 8, 2206–2212. [DOI] [PubMed] [Google Scholar]
- 19.Genot S et al. (2007) Severe Toxoplasma gondii I/III recombinant-genotype encephalitis in a human immunodeficiency virus patient. Journal of Clinical Microbiology 45, 3138–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demar M et al. (2007) Fatal outbreak of human toxoplasmosis along the Maroni River: epidemiological, clinical, and parasitological aspects. Clinical Infectious Diseases 45, e88–e95. [DOI] [PubMed] [Google Scholar]
- 21.Zhou P et al. (2009) Genetic characterization of Toxoplasma gondii isolates from China. Parasitology International 58, 193–195. [DOI] [PubMed] [Google Scholar]
- 22.Boughattas S et al. (2010) Direct genotypic characterization of Toxoplasma gondii strains associated with congenital toxoplasmosis in Tunisia (North Africa). The American Journal of Tropical Medicine and Hygiene 82, 1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira IMR et al. (2011) Toxoplasma gondii isolates: multilocus RFLP-PCR genotyping from human patients in Sao Paulo State, Brazil identified distinct genotypes. Experimental Parasitology 129, 190–195. [DOI] [PubMed] [Google Scholar]
- 24.Boughattas S et al. (2011) An atypical strain associated with congenital toxoplasmosis in Tunisia. Microbiologica-Quarterly Journal of Microbiological Sciences 34, 413. [PubMed] [Google Scholar]
- 25.Boughattas S et al. (2011) Case of fatal congenital toxoplasmosis associated with I/III recombinant genotype. Tropical Biomedicine 28, 615–619. [PubMed] [Google Scholar]
- 26.Fekkar A et al. (2011) Direct genotyping of Toxoplasma gondii in ocular fluid samples from 20 patients with ocular toxoplasmosis: predominance of type II in France. Journal of Clinical Microbiology 49, 1513–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carneiro ACAV et al. (2013) Genetic characterization of Toxoplasma gondii revealed highly diverse genotypes for isolates from newborns with congenital toxoplasmosis in southeastern Brazil. Journal of Clinical Microbiology 51, 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L et al. (2013) Genotypes and mouse virulence of Toxoplasma gondii isolates from animals and humans in China. PLoS ONE 8, e53483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costache CA et al. (2013) First isolation and genetic characterization of a Toxoplasma gondii strain from a symptomatic human case of congenital toxoplasmosis in Romania. Parasite 20, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Döşkaya M et al. (2013) Isolation of Toxoplasma gondii strains similar to Africa 1 genotype in Turkey. Parasitology International 62, 471–474. [DOI] [PubMed] [Google Scholar]
- 31.Pardini L et al. (2014) First isolation and molecular characterization of Toxoplasma gondii from a human placenta in Argentina. Parasitology International 63, 470–472. [DOI] [PubMed] [Google Scholar]
- 32.Higa LT et al. (2014) Toxoplasma gondii genotypes isolated from pregnant women with follow-up of infected children in southern Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene 108, 244–246. [DOI] [PubMed] [Google Scholar]
- 33.Silva LA et al. (2014) Overlapping Toxoplasma gondii genotypes circulating in domestic animals and humans in southeastern Brazil. PLoS ONE 9, e90237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yera H et al. (2014) New description of Toxoplasma gondii genotypes from French Polynesia. Acta Tropica 134, 10–12. [DOI] [PubMed] [Google Scholar]
- 35.Wang L et al. (2015) Seroprevalence and genetic characterization of Toxoplasma gondii in cancer patients in Anhui Province, Eastern China. Parasites & Vectors 8, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cong W et al. (2015) Toxoplasma gondii infection in cancer patients: prevalence, risk factors, genotypes and association with clinical diagnosis. Cancer Letters 359, 307–313. [DOI] [PubMed] [Google Scholar]
- 37.Vilares A et al. (2017) Molecular and virulence characterization of Toxoplasma gondii strains isolated from humans in Portugal. Parasitology Research 116, 979–985. [DOI] [PubMed] [Google Scholar]
- 38.Sharif M et al. (2017) Genetic diversity of Toxoplasma gondii isolates from ruminants: a systematic review. International Journal of Food Microbiology 3, 38–49. [DOI] [PubMed] [Google Scholar]
- 39.Alruhaili M (2016) Genetic diversity of African isolates of Toxoplasma gondii (dissertation/PhD thesis). University of Salford, Salford. [Google Scholar]
- 40.Ajzenberg D et al. (2016) Performance testing of PCR assay in blood samples for the diagnosis of toxoplasmic encephalitis in AIDS patients from the French Departments of America and genetic diversity of Toxoplasma gondii: a prospective and multicentric study. PLoS Neglected Tropical Diseases 10, e0004790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McAuley JB (2014) Congenital toxoplasmosis. Journal of the Pediatric Infectious Diseases Society 3, S30–S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hutson SL et al. (2015) Patterns of hydrocephalus caused by congenital Toxoplasma gondii infection associate with parasite genetics. Clinical Infectious Diseases 61, 1831–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rico-Torres CP, Vargas-Villavicencio JA and Correa D (2016) Is Toxoplasma gondii type related to clinical outcome in human congenital infection? Systematic and critical review. European Journal of Clinical Microbiology & Infectious Diseases: Official Publication of the European Society of Clinical Microbiology 35, 1079–1088. [DOI] [PubMed] [Google Scholar]
- 44.Liu Q et al. (2015) Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasites & Vectors 8, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shwab EK et al. (2014) Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology 14, 453–461. [DOI] [PubMed] [Google Scholar]