Abstract
Background
The influence of pre- or postprandial administration on pharmacokinetics of cyclosporine is supposed to be less in gel-based formulations than in microemulsions. This study was designed to investigate the influence of a high-fat meal on the pharmacokinetic profile of the two cyclosporine containing formulations Ciclosporin Pro (gel-based emulsion) and Sandimmun®Optoral (microemulsion) in renal transplant recipients.
Methods
A randomized, open-label, repeated-measurement, comparative phase IV trial was conducted with two sequence groups for nutrition condition (fasting→fed, fed→fasting) and two treatment phases (Sandimmun® Optoral → Ciclosporin Pro), each covering both nutrition conditions. Primary pharmacokinetic variable of interest was the reduction of bioavailability due to high-fat food compared to fasting conditions measured by the difference D of ln-transformed bioavailability variables (AUCSS, τ, Css, max, und Css, min).
Results
A nutrition effect was found for both study medications with respect to the parameters AUCSS, τ and CSS, max, but not to CSS, min. The reduction of bioavailability caused by high-fat food was not significantly different for Sandimmun®Optoral and Ciclosporin Pro.
Conclusions
An effect of high-fat breakfast prior to the morning dose on AUCSS, τ and CSS, max was found for Sandimmun® Optoral and for Ciclosporin Pro. Trough level monitoring did not capture ingestion-related variability. Conversion to Ciclosporin Pro seems to be safe with regard to intra-individual pharmacokinetic variability.
Trial registration
EudraCT No. 2009–011354-18 (29th April 2019)
Electronic supplementary material
The online version of this article (10.1186/s12882-019-1340-z) contains supplementary material, which is available to authorized users.
Keywords: Cyclosporine, Microemulsion, Gel-based emulsion, Food intake, Bioavailability
Background
Solid organ transplantation has become successful with the development of immunosuppressive therapy. Today, several immunosuppressive substances are available for establishing an individually adapted lifelong immunosuppressive therapy.
Cyclosporine is a powerful immunosuppressant that acts specifically on lymphocytes, mainly T-helper cells. lt inhibits the activation of calcineurin, an important step in the production of lymphokines including interleukin-2, thus resulting in depression of cell-mediated immune response. Cyclosporine has a narrow therapeutic window and is not only used in transplantation, but also for treatment of nephrotic syndrome and various autoimmune diseases. Absorption of conventional formulations of cyclosporine from the gastrointestinal tract is variable and incomplete. An oral microemulsifying formulation, Sandimmun® Optoral, with improved absorption characteristics is available and is more rapidly and completely absorbed, with peak concentrations achieved about 1.5 to 2 h after dose intake (Sandimmun® Optoral Produktmonographie, Novartis Pharma GmbH, Nuremberg 2001).
Ciclosporin Pro formulation is a gel emulsion preconcentrate (GEM) developed by TEVA, registered and marketed in Germany; it is also marketed in Austria as Neoimmune and as Equoral® in different other counties (e.g. Poland, Bulgaria, Latvia, Lithuania, Estonia, Romania, Chile, Mexico, Russia). The formulation is described in publications as GEM 101 [1]. Contrary to the microemulsifying formulation of Sandimmun® Optoral, the addition of small amounts of water to the GEM forms a gel, which can be demonstrated by increasing viscosity of the preparation. This gel with excessive water breaks into particles with different shapes, followed by a rapid decline in viscosity of the created dispersion. The particle diameter is much larger than in microemulsions (100 μm vs. < 150 nm in microemulsions) [1]. It has been postulated that particle size plays a major role in the bioequivalence of cyclosporine [2]. However, a study has suggested that in contrast to previous assumptions, conventional formulations of cyclosporine are quite well absorbed, and that the low bioavailability is due to extensive cytochrome-mediated metabolism in the gut wall. If this was the case, the improved bioavailability seen with the microemulsifying formulation can be presumably less been ascribed to improved absorption than to protection from such metabolism [3]. Moreover, the bioavailability of the GEM formulation has been shown to be bioequivalent to the reference product (cyclosporine microemulsifying formulation) in healthy subjects as well as in renal transplant recipients [1, 3, 4].
The influence of preprandial or postprandial administration on the pharmacokinetics of various cyclosporine formulations has been demonstrated in various studies [5–8]. Two Czech pharmacokinetic studies in healthy volunteers [9, 10] led to generation of the hypothesis that the rate and extent of absorption of the gel-based emulsion Ciclosporin Pro is less influenced by meals than this of the microemulsion.
Strict 12 h dosing intervals are set for patients after transplantation. Administration after fasting is truly only possible in the morning. A formulation which releases cyclosporine independently from or less influenced by food uptake would therefore be preferred. This study was designed to further investigate the influence of a high-fat meal intake compared to fasting conditions on the pharmacokinetic profile of the two cyclosporine containing formulations Ciclosporin Pro (gel-based emulsion) and Sandimmun®Optoral (microemulsion) in stable renal transplant recipients.
Methods
Study design
A single-centre randomized, open-label, repeated-measurement, comparative phase IV trial on the bioavailability of Ciclosporin Pro versus Sandimmun® Optoral under fasting versus fed conditions in patients with stable renal transplants was conducted between June 2010 and December 2012.
Ethics approval
This study was conducted in compliance with Good Clinical Practices (GCP) and the Declaration of Helsinki, and in accordance with applicable legal and regulatory requirements. The study protocol was approved by the ethics commission of the medical faculty of the University Duisburg-Essen and registered in the European Union Clinical Trials Register (EudraCT No. 2009–011354-18).
Inclusion and exclusion criteria
Detailed inclusion and exclusion criteria can be found in Additional file 1: Figure S1.
Study procedure
Patients were under stable Sandimmun® Optoral treatment (reference drug) when entering the study. The patients were assigned to the following sequence groups in conformity with two gender-specific randomization lists:
Group A (fasting → fed):
| PK-profile | 1 | after 10 h fasting (Sandimmun® Optoral, day 28); |
| PK-profile | 2 | after high-fat breakfast (Sandimmun® Optoral, day 29); |
| PK-profile | 3 | after 10 h fasting (Ciclosporin Pro, day 57); |
| PK-profile | 4 | after high-fat breakfast (Ciclosporin Pro, day 58); |
Group B (fed → fasting):
| PK-profile | 1 | after high-fat breakfast (Sandimmun® Optoral, day 28) |
| PK-profile | 2 | after 10 h fasting (Sandimmun® Optoral, day 29); |
| PK-profile | 3 | after high-fat breakfast (Ciclosporin Pro, day 57); |
| PK-profile | 4 | after 10 h fasting (Ciclosporin Pro, day 58). |
The randomization list was prepared using the software RANCODE 3.6 (IDV Gauting, Germany) by staff of medicomp GmbH who was not involved in the study conduct. The allocation ratio was 1:1. Randomization took place in blocks aiming for a well-balanced group distribution at each recruitment time.
This was an open-label study, thus the investigational team, the patient, and the clinical study monitor knew the sequence group (fasting→fed or fed→fasting) during the study. Nevertheless, data management personnel and the statistician were unaware of the patient’s assignment to Sequence Group A or Sequence Group B during the trial. The laboratory analyzing the blood samples was blinded as well. The database was unblinded on 19 Nov 2013, following completion of data cleaning, data quality control, and database lock. The allocation of patients to the per-protocol set was performed prior to unblinding, i.e. without knowledge of the patient’s sequence group.
Additional file 2: Figure S2 illustrates study procedures.
All patients came to the study center fasting (10 h). Patients either received a standardized normal breakfast or a high-fat breakfast. For high-fat breakfast patients were allowed to choose one of three high-fat breakfasts. Each breakfast consisted of 728–765 kcal of which 55–58% were based on fat. Compositions are shown in detail in Additional file 3: Figure S3. Breakfast was taken at study side under supervision. Patients received standardized meals five hours (lunch) and ten hours (dinner) after morning dose administration. Patients who took the medication under fasting conditions received a standardized breakfast 1 h after morning dose administration. The evening dose was also taken under control of a nurse or medical doctor.
Adherence
Adherence concerning fasting condition and complete breakfast intake was assessed at the time of each PK profile including the timing of start of breakfast relative to the intake of the study medication. Breakfasts and study medications were taken at study side under supervision. For profiles after high-fat breakfast the time between intake of the morning dose and start of breakfast was planned to be between − 50 and − 10 min (boundaries included), which corresponds to 30 ± 20 min. For profiles after 10 h fasting the desired value was at least 40 min, which corresponds to 60–20 min.
Four pharmacokinetic profiles were captured at day 28 and day 29 (Sandimmun® Optoral) and day 57 and day 58 (Ciclosporin Pro). These pharmacokinetic profiles consisted of plasma concentrations for cyclosporine during τ = 12 h observation starting at morning medication intake and were summarized to the following analysis variables:
AUCSS, τ area under the cyclosporine-concentration-time curve during one dosing interval τ at steady state
CSS, max maximal cyclosporine concentration during one dosing interval τ at steady state
CSS, min minimal cyclosporine concentration during one dosing interval τ at steady state.
Primary pharmacokinetic variable of interest was the reduction of bioavailability due to high-fat food compared to fasting conditions measured by the difference D of the natural logarithm (ln)-transformed bioavailability variables (AUCSS, τ, Css, max, und Css, min). The primary objective was to examine differences in the nutrition-dependent reduction of bioavailability between Ciclosporin Pro and Sandimmun® Optoral.
The two secondary pharmacokinetic variables used to examine nutrition effects on the bioavailability of Ciclosporin Pro and Sandimmun® Optoral as well as bioequivalence included:
TSS, max time of maximal cyclosporine concentration during one dosing interval at steady state
PTF% peak-trough-fluctuation at steady state [%].
Study medication
Both cyclosporine A formulations, Sandimmun® Optoral and Ciclosporin Pro are approved drugs against rejection following allogenic organ transplantation. In healthy subjects as well as in renal transplant patients bioequivalence of Ciclosporin Pro could be demonstrated in comparison to the originator product Sandimmun® Optoral [4]. The current study concentrates on the aspect of oral nutrition on pharmacokinetic profiles. The study was not primarily designed to evaluate the bioequivalence of two cyclosporine formulations. The exploration of bioequivalence in the current study was performed in a biased subset of patients who had stable C0 levels during the treatment phase, rather than in a randomly selected patient cohort.
Since this was an open-label study with all patients receiving the same medication in identical sequence order, and both medications, test drug and reference drug, have a marketing authorization in Germany, commercially available medication was used for the study. Teva GmbH provided the quantity of Ciclosporin Pro required for the clinical trial. Study medication was dispensed to the patients according to the treatment phase.
Dosing
Study medication dosage was individualized for each patient.
Dosing was guided by cyclosporine A blood concentrations (C0 level). Intended drug levels depend on concomitant medication against graft rejection and metabolization which shows high inter-individual differences [11]. Therefore, dosing varied between 0.6 and 2.4 mg/kg body weight.
Patients were to receive the same dose (milligram:milligram) of the study medication as the cyclosporine dose the patient had been taken for the past 2 months. Dosage was planned to remain the same again when switching study medication on day 30. To ensure patient safety, cyclosporine blood concentrations (C0 levels) were controlled 7 days after study start (Visit 2) and 7 days after switch of medication from Sandimmun® Optoral to Ciclosporin Pro on day 36 (Visit 5). The medication dose may have been adjusted after these visits if necessary. In case of a dose adjustment, the patient should have come to another control visit 7 days later.
The individual dose of trial medication had to be taken twice daily, at 8.00 o’clock in the morning and at 20.00 o’clock in the evening.
Concomitant medication
Concomitant medication was recorded in every study visit (see Additional file 2: Figure S2). A potentially relevant protocol violation was the use of medication that might have had an effect on the outcome measures. This could have included but was not limited to the use of other calcineurin inhibitors like tacrolimus and the concomitant use of commercially available formulations of cyclosporine. The administered pre-study and concomitant medication were medically reviewed with a particular focus on medication which was initiated and/or for which the dose was changed less than 5 days (which corresponds to about ten times the half life of cyclosporine) prior to a PK profile. During this review it was decided whether individual violations were minor or major. Medications that were stopped at least 5 days before PK profile 1, started after end of study, or started at least 5 days before PK profile 1 and stopped at or after last visit date were not reviewed as these were not considered candidates for major violations.
Concomitant medication is listed in detail in Additional file 4: Figure S4.
Cyclosporine a assay
Cyclosporine A was measured by the antibody-conjugated magnetic immunoassay (ACMIA) method [12] (Siemens Healthcare Diagnostics Inc., Newark, USA): patient whole blood in EDTA is lysed and present cyclosporine A conjugated with a monoclonal mouse antibody is sequestered by β-galactosidase. Free antibody-enzyme conjugates are separated by added magnetic particles coated with cyclosporine A. The remaining supernatant containing the antibody-enzyme complex is then mixed with the substrate and β-galactosidase catalyses the hydrolysis of chlorphenol red β-galatopyranoside (CPRG, yellow) to chlorphenol red (red). Photometry is performed at 577 nm (700 nm). The assay ranges from 25 to 500 ng/ml with a functional sensitivity (at 20% coefficient of variation) of 30 ng/ml. For further details on precision, accuracy, and specificity see manufacturer information [13].
Data acquisition
The full analysis set (FAS) included all randomized patients who received at least one dose of study medication. The per-protocol analysis set (PP) included all patients from the FAS for whom the following criteria were met:
All four pharmacokinetic profiles evaluable
all of the major inclusion criteria, none of the major exclusion criteria fulfilled
absence of relevant protocol violations
absence of diarrhoea or vomiting at the day of each PK-profile
sufficient compliance concerning the application of the study medication and concerning the breakfast intake
absence of dosage adaptations between the pharmacokinetic profiles.
Safety analysis was performed by documentation of adverse events, performance of blood pressure and heart rate measurements, 12-lead ECG, laboratory assessments (creatinine, creatinine clearance, haematology, urine analysis) and cyclosporine C0-levels for monitoring the therapy.
The contract research organization medicomp GmbH (Munich, Germany), who was not involved in the study conduct, was responsible for monitoring, data management, and statistical analysis (data safety and monitoring board, DSMB). Monitoring checked case report forms at time of completion. Quality control included initiation visit and regular monitoring visits for check of completeness, formal and medical plausibility and consistency of the data recorded in case report forms as well as compliance with the protocol including source documentation verification. 100% source data verification was done for 100% of patients and was documented on respective forms. After source data verification, the completed case report forms and other patient documents were collected by the monitor and were forwarded to data management where monitored data were entered and underwent additionally completeness, consistency and plausibility checks.
DSMB advised sponsor with regard to drug safety. Sponsor was allowed to disclose patient data for drug safety reasons.
Sample size calculation
There were two studies with healthy male volunteers values (Equoral® 100 mg vs. Neoral® 100 mg) which provides estimates for Cmax- and AUC-values under fasting conditions [9] and under fed conditions [10]. The values for Cmax and AUC from these two studies were used to estimate the difference D of ln-transformed values of bioavailability under fed conditions to bioavailability under fasting conditions (Ciclosporin Pro: DCmax = − 0.098 and DAUC = − 0.042; Sandimmun Optoral: DCmax = − 0.330 and DAUC = − 0.218). Estimates for the variances of ln-transformed values were derived using:
- the empirical coefficients of variation of the non-transformed values per treatment:
- ○ Study under fasting conditions:
- AUC: CV%Ciclosporin, fasting = 36.79% and CV%Sandimmun, fasting = 50.38%;
- Cmax: CV%Ciclosporin, fasting = 27.58% and CV%Sandimmun, fasting = 18.08%
- ○ Study under fed conditions:
- AUC: CV%Ciclosporin, fed = 24.22% and CV%Sandimmun, fed = 21.44%;
- Cmax: CV%Ciclosporin, fed = 32.99% and CV%Sandimmun, fed = 24.99%
different assumptions for within patient correlations.
Sample size calculations were performed using nQuery Advisor 6.0 for a paired t-test, a one-sided α-level of 2.5% and a power of 80%. Calculations based on two studies with healthy male volunteers led to the conclusion that a sample size of 30 evaluable patients, i.e. 30 patients with 4 evaluable pharmacokinetic profiles, should be planned for this study.
Statistical analysis
The statistical analysis was performed using the software package SAS version 9.3 (SAS Institute Inc., Cary, NC 27513, USA). Primary variables were checked for normal distribution by Kolmogorov-Smirnov test. Statistical analysis was performed by using paired, one-sided t-tests. A one-sided t-test (left-sided) was chosen, as it was assumed that high-fat food reduces the bioavailability of Ciclosporin Pro as well as of Sandimmun® Optoral, and that an increase of bioavailability is neither observed for Ciclosporin Pro or Sandimmun® Optoral, respectively. The two sequence groups were pooled for analysis of primary endpoints. To control for multiplicity in the analysis of primary endpoints hierarchical testing was applied. An overall significance level of 2.5% for primary pharmacokinetic endpoints and 5% for secondary pharmacokinetic endpoints was used. To explore the robustness of results, two analyses of variance (ANOVA) were calculated for each pharmacokinetic parameter of primary interest by using a mixed model with a two-sided significance level of 5%, respectively.
Results
Demographic data and baseline characteristics
31 patients were randomized in this single-centre study. All 31 patients received at least one dosage of study medication and were included in the full analysis set (Group A: fasting→fed: 15; Group B: fed→fasting: 16). Demographic data and baseline anamnestic characteristics for the FAS are presented in Table 1. All patients had undergone one kidney transplantation.
Table 1.
Baseline characteristics (FAS). The sequence groups did not show any relevant differences in terms of gender, age, height, or weight in the FAS or the PP analysis set
| fasting→fed | fed→fasting | Total | ||
|---|---|---|---|---|
| (n = 15) | (n = 16) | (n = 31) | ||
| Gender | ||||
| Male | n (%) | 10 (66.7%) | 10 (62.5%) | 20 (64.5%) |
| Female | n (%) | 5 (33.3%) | 6 (37.5%) | 11 (35.5%) |
| Race | 15 (100%) | 16 (100%) | 31 (100%) | |
| Caucasian | n (%) | |||
| Age [years] | Mean (SD) | 48.5 (12.4) | 49.9 (14.0) | 49.3 (13.1) |
| Range | 19–67 | 22–77 | 19–77 | |
| Weight [kg] | Mean (SD) | 77.8 (14.3) | 78.8 (11.3) | 78.3 (12.6) |
| Range | 55–102 | 59–102 | 55–102 | |
| Height [cm] | Mean (SD) | 174.3 (11.6) | 173.9 (14.6) | 174.1 (13.0) |
| Range | 160–200 | 143–200 | 143–200 | |
| BMI [kg/m2] | Mean (SD) | 25.5 (3.4) | 26.3 (4.0) | 25.9 (3.7) |
| Range | 21.3–30.2 | 19.8–32.2 | 19.8–32.2 | |
| Smoking habits | 9 (60.0%) | 13 (81.3%) | 22 (71.0%) | |
| Non-smoker | n (%) | |||
| Ex-smoker | n (%) | 3 (20.0%) | – | 3 (9.7%) |
| Smoker | n (%) | 1 (6.7%) | 3 (18.8%) | 4 (12.9%) |
| Unknown | n (%) | 2 (13.3%) | – | 2 (6.5%) |
| Time since last renal transplantation [years] | Median (Range) | 6.0 (2–28) | 6.5 (1–24) | 6.0 (1–28) |
| Number of previous rejection episodes | ||||
| 0 | n (%) | 13 (86.7%) | 11 (68.8%) | 24 (77.4%) |
| 1 | n (%) | 1 (6.7%) | 5 (31.3%) | 6 (19.4%) |
| 2 | n (%) | 1 (6.7%) | – | 1 (3.2%) |
| Time since end of last rejection episode [months] | Median (Range) | 36.1 (33–39) | 23.7 (6–63) | 36.1 (6–63) |
| [n = 2] | [n = 4]a | [n = 6]a | ||
| Duration of last rejection episode [days] | Median (Range) | 3.5 (3–4) | 4.0 (3–7) | 4.0 (3–7) |
| [n = 2] | [n = 4]a | [n = 6]a | ||
| Last daily ciclosporin dose applied before baseline [mg/kg/day] | Mean (SD) | 2.43 (0.707) | 2.31 (0.560) | 2.37 (0.628) |
a For one patient only year was given for start and end date of last rejection episode (1989). As a result, time since end and duration of last rejection were not assessable for this patient
Overall, there were no clinically relevant differences between sequence groups with respect to the anamnestic characteristics presented in Table 1.
Kidney function was stable in all patients with a mean baseline serum creatinine of 1.82 mg/dl (min. 1.07 mg/dl; max. 2.61 mg/dl) and unchanged during study duration as was eGFR (MDRD) which was gathered at baseline, day 29 (PK 2) and day 58 (PK 4) (Table 2).
Table 2.
eGFR (MDRD). eGFR was gathered at indicated time points
| eGFR (MDRD) [ml/min/1.73 m2] | A: fasting→fed | B: fed→fasting | Total |
|---|---|---|---|
| Baseline | 38.8 ± 8.8 | 38.7 ± 11.0 | 38.7 ± 9.7 |
| Day 29 (PK2) | 38.7 ± 10.9 | 43.5 ± 9.1 | 41.2 ± 10.0 |
| Day 58 (PK4) | 39.8 ± 8.7 | 40.4 ± 11.4 | 40.1 ± 10.1 |
Concomitant medication
Use of prohibited concomitant therapy was regarded as a major violation in one patient, who reported concomitant use of commercially available formulations of cyclosporine and was therefore excluded from the PP analysis set (see Additional file 5: Figure S5).
Dosing
The mean initial daily dose of Sandimmun® Optoral was 2.4 mg/kg/day, the mean initial daily dose of Ciclosporin Pro was 2.2 mg/kg/day. Five (23.8%) of the 21 PP patients had at least one change in daily dose (mg) of Sandimmun® Optoral. Three (14.3%) of the 21 PP patients had at least one change in daily dose (mg) of Ciclosporin Pro. None of the study patients had a change in daily dose (mg) at the time of switch from Sandimmun® Optoral to Ciclosporin Pro. Both PK-profiles for the Sandimmun® Optoral and Ciclosporin Pro groups respectively were performed with the same (morning) dose in all 21 PP patients.
Adherence
All patients were adherent concerning fasting conditions and complete breakfast intake on the day of each PK profile.
Deviations from boundaries found in three patients were classified as major protocol violations and patients were excluded from the per protocol analysis set (see Additional file 5: Figure S5).
Exclusion from PP analysis set
Ten of the 31 FAS patients (32.3%) were excluded from the PP analysis set. Four patients discontinued the study prematurely. One patient terminated the study due to “patient’s request to withdraw”, three patients were discontinued due to “intolerable adverse events” (pancreatitis, severe leg pain, relapse of ulcerative colitis). Relation to the study medication was medically extremely unlikely for all observed intolerable adverse events, but prohibited completion of the remaining scheduled visits. Furthermore, 6 more patients were excluded from the PP set due to reasons summarized in Additional file 5: Figure S5. Therefore, the PP analysis comprised 21 patients treated with study medication (Group A: fasting→fed: 11 patients; Group B: fed→fasting: 10 patients). In the PP set all patients completed the study entirely in each sequence group. Results of the pharmacokinetic study are reported for the PP set only. It was planned to enroll sufficient patients so that 30 patients would be available with four evaluable pharmacokinetic profiles. However, the study was stopped prematurely after 30 month and the enrolment of 31 patients due to recruitment difficulties within a reasonable time frame. This reduced the power of statistical testing.
Descriptive statistics for the primary pharmacokinetic parameters and evaluation of differences
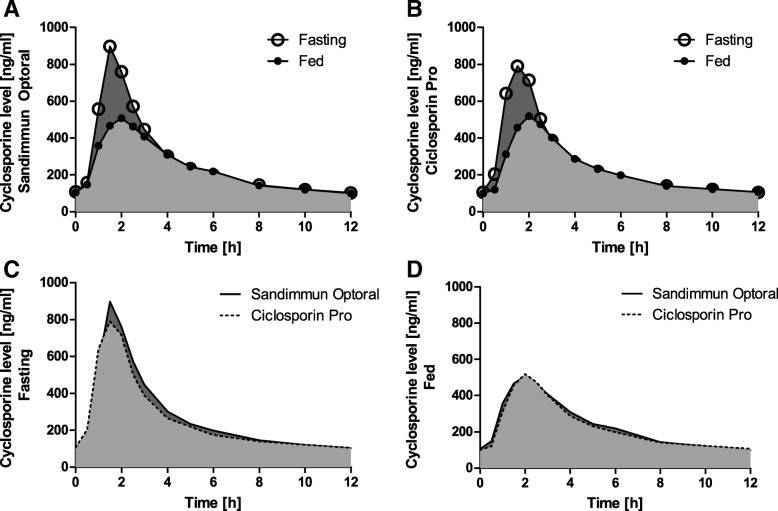
The pharmacokinetic parameters AUCSS, τ, CSS, max, and CSS, min measured under both nutrition conditions under Ciclosporin Pro as well as under Sandimmun® Optoral are displayed in Table 3 and Fig. 1.
Table 3.
AUCSS, τ [h*ng/ml], CSS, max [ng/ml], and CSS, min [ng/ml] for each treatment and nutrition condition (PP; n = 21)
| Ciclosporin Pro | Sandimmun Optoral | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fasting | high-fat | fasting | high-fat | |||||||||
| Geom. mean | 95% CI | CV % | Geom. mean | 95% CI | CV % | Geom. mean | 95% CI | CV % | Geom. mean | 95% CI | CV % | |
| AUC | 3006.5 | [2652.7;3407.5] | 28.0 | 2652.7 | [2334.5;3014.2] | 28.6 | 3228.6 | [2857.8;3647.6] | 27.3 | 2711.2 | [2357.7;3117.7] | 31.4 |
| Cmax | 844.6 | [702.4;1015.7] | 42.2 | 570.9 | [463.6;703.2] | 48.3 | 940.6 | [802.7;1102.3] | 35.9 | 560.2 | [454.3;690.8] | 48.6 |
| Cmin | 97.9 | [87.1;110.1] | 26.2 | 91.4 | [81.1;103.0] | 26.7 | 100.0 | [88.8;112.5] | 26.4 | 97.7 | [86.9;109.9] | 26.2 |
Fig. 1.
Pharmacokinetic profiles. Graphs show pharmacokinetic profiles of Sandimmun®Optoral (a) and Ciclosporin Pro (b) taken after 10 h of fasting or high-fat breakfast. Parts (c) and (d) illustrate the difference between Sandimmun®Optoral and Ciclosporin Pro in these conditions
A difference in bioavailability due to high-fat nutrition of Ciclosporin Pro compared to Sandimmun® Optoral in terms of the difference D of ln-transformed bioavailability measured by the primary variables AUCSS, τ, CSS, max, and CSS, min was not observed (Table 4). For the pharmacokinetic parameter CSS, max the p-value of the one-sided t-test (α = 0.025) was 0.1820 (n = 21). The corresponding p-values for the other two parameters were > 0.025, respectively. Nevertheless, for the two pharmacokinetic parameters AUCSS, τ and CSS, max the arithmetic mean of the differences of ln-transformed nutrition effects between Ciclosporin Pro and Sandimmun® Optoral, i.e. DCiclosporinPro – DSandimmun Optoral was slightly greater than zero, meaning that the reduction of bioavailability of cyclosporine caused by the ingestion of high-fat food immediately before medication intake appeared on average numerically less pronounced under Ciclosporin Pro than under Sandimmun® Optoral.
Table 4.
Difference between Ciclosporin Pro and Sandimmun® Optoral in ln-transformed nutrition effects (PP, n = 21)
| PK parameter | DCiclosporin Pro – DSandimmun Optoral Mean [SD] | DCiclosporin Pro – DSandimmun Optoral [97.5% CI] | Testing of H0* | |
|---|---|---|---|---|
| t-value | p-value** | |||
| CSS, max | 0.1266 [0.6248] | [−0.1578; Infinity] | 0.93 | 0.1820 |
| AUCSS, τ | 0.0494 [0.2850] | [−0.0803; Infinity] | 0.79 | 0.2180 |
| CSS, min | −0.0464 [0.3271] | [−0.1953; Infinity] | − 0.65 | 0.7384 |
Bold entries are significant
* Testing of H0 (0 ≥ DCiclosporin Pro - DSandimmun Optoral) by means of the one-sided paired t-test (α = 0.025)
** lowest (one-sided) significance level, for which the null hypothesis could be rejected
To explore the robustness of results, two analyses of variance (ANOVA) were calculated for each pharmacokinetic parameter by using a mixed model with a two-sided significance level of 5%, respectively. Both robustness analyses confirmed the results of the one-sided t-test (Additional file 6: Figure S6 and Additional file 7: Figure S7).
Nutrition effect on the bioavailability of Ciclosporin pro and Sandimmun® Optoral
The extent of the impact of nutrition on the bioavailability of Ciclosporin Pro and (separately) on the bioavailability of Sandimmun® Optoral was measured by the differences DCiclosporin Pro and DSandimmun Optoral (Table 5). According to the results of two-sided t-tests with a significance level of α = 0.05, for both study medications the null hypothesis, that there is no nutrition effect on the bioavailability of the respective medication can be rejected with respect to the pharmacokinetic parameters AUCSS, τ and CSS, max (two-sided p-values < 0.05). This means a nutrition effect was found for both study medications with respect to the parameters AUCSS, τ and CSS, max, but not with respect to CSS, min.
Table 5.
Difference of ln-transformed PK variables between high-fat versus fasting condition (PP; n = 21) for Ciclosporin Pro and Sandimmun® Optoral
| PK parameter | DCiclosporin Pro Mean [SD] |
DCiclosporin Pro [95% CI] |
Testing of H0* | |
| t-value | p-value** | |||
| AUCSS, τ | −0.1252 [0.2008] | [−0.2166; − 0.0338] | −2.86 | 0.0097 |
| CSS, max | −0.3916 [0.4371] | [− 0.5906; − 0.1927] | −4.11 | 0.0005 |
| CSS, min | − 0.0690 [0.2094] | [− 0.1644; 0.0263] | −1.51 | 0.1465 |
| PK parameter | DSandimmun Optoral Mean [SD] |
DSandimmun Optoral [95% CI] |
Testing of H0* | |
| t-value | p-value** | |||
| AUCSS, τ | −0.1747 [0.1443] | [−0.2403; − 0.1090] | −5.55 | <.0001 |
| CSS, max | −0.5183 [0.3148] | [− 0.6616; − 0.3750] | −7.55 | <.0001 |
| CSS, min | − 0.0227 [0.1789] | [− 0.1041; 0.0588] | −0.58 | 0.5681 |
* Testing of H0 (DCiclosporin Pro = 0) by means of the two-sided paired t-test
** lowest (two-sided) significance level, for which the null hypothesis could be rejected
Bioequivalence of Ciclosporin pro and Sandimmun® Optoral under fasting conditions
The bioequivalence of Ciclosporin Pro and Sandimmun® Optoral under fasting conditions and separately under fed conditions was explored by calculation of geometric means of the ratios of non-transformed pharmacokinetic variables including corresponding 90% confidence intervals (Table 6). The 90% confidence intervals based on AUCSS, τ were within the acceptance interval [0.80;1.25] under fasting and high-fat conditions. However, with the exception of the PP set under high-fat condition, they were not within the more stringent acceptance interval [0.90; 1.11] for narrow therapeutic index drugs. Furthermore, the 90% confidence intervals based on CSS, max were not included in any acceptance interval. Therefore, bioequivalence of Ciclosporin Pro and Sandimmun® Optoral (in the sense of similarity in AUCSS, τ and CSS, max) – which had been proven in other studies - could neither be documented for fasting nor for high-fat conditions.
Table 6.
Ratio of non-transformed PK variables of Ciclosporin Pro to Sandimmun® Optoral under fasting condition (top) and under high-fat conditions (bottom) (PP; n = 18)
| BioavailabilityCiclo Pro/BioavailabilityOptoral Geometric mean |
BioavailabilityCiclo Pro/BioavailabilityOptoral [90% CI] |
|
|---|---|---|
| PK parameter (fasting) | ||
| AUCSS, τ | 0.940 | [0.868; 1.018] a |
| CSS, max | 0.863 | [0.745; 0.999] |
| CSS, min | 1.011 | [0.922; 1.108] b |
| PK parameter (high-fat) | ||
| AUCSS, τ | 1.034 | [0.972; 1.099] b |
| CSS, max | 1.082 | [0.930; 1.260] |
| CSS, min | 0.955 | [0.881; 1.035] a |
a CI included in [0.80; 1.25], but not in [0.90; 1.11]
b CI included in [0.90; 1.11]
Safety evaluation
A total of 26 adverse events (AE) occurred in 14/31 patients (45.2%).
At first sight, the proportion of patients with AEs occurring under Ciclosporin Pro appeared higher than the proportion of patients with AEs occurring under Sandimmun® Optoral. However, in three patients bradycardia was documented as the only adverse event. Bradycardia was diagnosed based on the ECG at the final visit. Therefore, by definition the AEs were considered to have started under treatment with Ciclosporin Pro, although no ECG was performed after treatment with Sandimmun® Optoral and the lowest heart rate was already observed by Visit Day 29 (PK2) for all three patients. However, if the bradycardia events diagnosed using the ECG at the final visit are not counted as AEs with onset during Ciclosporin Pro, the number of individual events remains still slightly higher for the Ciclosporin Pro treatment period with no clear pattern.
There was only one adverse drug reaction (urinary tract infection, observed under treatment with Ciclosporin Pro) and no AE led to discontinuation or reduction of study drug. No death occurred during the study. Two serious adverse events were reported for two patients under treatment with Ciclosporin Pro (pancreatitis and relapse of ulcerative colitis). The causality was assessed as “no reasonable possibility” for both events as a relation to the study medication is medically extremely unlikely. Three AEs were related to laboratory parameters (increases in creatinine, gamma-glutamyltransferase and alkaline phosphatase). However, there was no laboratory parameter for which abnormalities were reported as an AE by more than one patient. Tolerability of both study drugs was assessed as very good or good by both the patients and investigators for all patients for whom an assessment was available.
Discussion
Nutrition effects on bioavailability were found for the gel-based cyclosporine formulation Ciclosporin Pro as well as for the microemulsion Sandimmun® Optoral with respect to AUCSS, τ and CSS, max. Effects were not captured by CSS, min. A significant difference in reduction of bioavailability due to high-fat nutrition between the two formulations could not be proven in the current trial (For p-values see Table 4).
Calcineurin inhibitors are immunosuppressive drugs with a relatively narrow therapeutic range. Overdosing results in acute toxicity not limited to the kidney, while underdosing is associated with allograft rejection [14]. Therapeutic drug monitoring is conventionally based on measurement of trough levels, although targeting C2 (the 2 h post-dose blood cyclosporine concentration) values might go along with reduced incidence of acute renal allograft rejection [15, 16]. High intra-individual variability of the second commonly used calcineurin inhibitor tacrolimus has been shown to go along with inferior allograft survival [17, 18] and development of de novo donor-specific antibodies [19]. Dietary compliance has been identified as a major problem in this setting [18]. Increased intra-individual variability of cyclosporine trough levels was associated with a higher incidence of acute rejection episodes, reduced long-term renal function and shortened graft survival [20]. Therefore, interventions to minimize the causes of high variability, not only of trough levels, but possibly on whole pharmacokinetic profiles, are essential to improve long-term outcomes following renal transplantation.
The influence of preprandial or postprandial administration on the pharmacokinetics of various cyclosporine formulations has been shown in various studies. Mueller et al. showed the influence of a high fat meal on cyclosporine bioavailability from the first marketed formulation (Sandimmun®) vs the microemulsion (Neoral®) in healthy volunteers. The food intake had minor influence on cyclosporine uptake from the microemulsion [5]. In contrast to our results, Kees et al. showed a higher AUC after a fat rich breakfast compared to the fasting AUC for a gel-based capsule formulation (Neoimmun®) in healthy volunteers. Cmax of the fed and the fasting pharmacokinetic profiles were comparable with an increase after food intake in male subjects only [6]. Two Japanese studies in patients with psoriasis showed a consistently lower Cmax when Neoral® (microemulsion) was taken postprandial compared to the preprandial administration (p not given and p < 0.05, respectively) [7, 8]. As administration after fasting is truly only possible in the morning and patient compliance concerning dietary restrictions is limited, a formulation which releases cyclosporine independently from food uptake going along with increased stability of pharmacokinetic profiles would be preferred.
Both cyclosporine formulations analysed in the current trial showed a nutrition effect resulting in a decreased AUCSS, τ and CSS, max, while the usually recorded CSS, min appeared unchanged reflecting the problem of trough level monitoring [21]. As administration after fasting twice daily is not practicable intra-individual differences in AUCSS, τ and CSS, max have to be assumed. This questions the whole concept of trough level monitoring. The development of a functional enzyme activity monitoring might be a future goal for optimizing therapy.
In the patient population analyzed, bioequivalence of Ciclosporin Pro and Sandimmun® Optoral (in the sense of similarity in both, AUCSS, τ and CSS, max) could not be documented neither under fasting conditions nor under high-fat conditions. However, this study was not designed to evaluate bioequivalence. It has to be kept in mind that bioequivalence was assessed as an exploratory secondary endpoint and that the examined patient population for the evaluation of bioequivalence was not randomly selected but was a biased subset of patients who had stable C0 levels during the treatment phase, i.e. patients who required a change in the morning dose after switch of medication from Sandimmun® Optoral to Ciclosporin Pro were excluded from the bioequivalence evaluation.
Strengths and limitations
Following sample size calculations, it was planned to enroll sufficient patients so that 30 patients would be available with four evaluable pharmacokinetic profiles. However, the study was stopped prematurely after 30 month and the enrolment of 31 patients due to recruitment difficulties within a reasonable time frame. The resulting number of evaluable patients, i.e. patients with four evaluable pharmacokinetic profiles was 24 in the FAS and 21 in the PP analysis set. This reduced the power of statistical testing.
This was an open-label study, thus the investigational team, the patient, and the clinical study monitor knew the sequence group during the study. Nevertheless, data management personnel and the statistician were unaware of the patient’s assignment to sequence group. The laboratory was blinded as well. The database was unblinded following completion of data cleaning, data quality control, and database lock. The allocation of patients to the per-protocol set was performed prior to unblinding, i.e. without knowledge of the patient’s sequence group. Therefore, influence on study results due to knowledge of assignment to study groups should be negligible.
Inclusion and exclusion criteria were defined with emphasis on patient safety. Therefore, as every change in immunosuppressive therapy is a risk factor for adverse events, especially stable transplant function was of interest. No changes in transplant function or rejection episodes in connection with the study were reported.
Conclusions
High-fat food leads to changes in bioavailability of cyclosporine, not captured by trough level monitoring.
The reduction of bioavailability of cyclosporine caused by the ingestion of high-fat food immediately before medication intake was not different under Ciclosporin Pro compared to Sandimmun® Optoral. Conversion to Ciclosporin Pro seems to be safe with regard to intra-individual pharmacokinetic variability.
Additional files
Figure S1. Inclusion and exclusion criteria. (DOCX 27 kb)
Figure S2. Diagram of study procedures. (DOCX 39 kb)
Figure S3. Composition of breakfasts. (DOCX 32 kb)
Figure S4. Concomitant medication during study. (DOCX 27 kb)
Figure S5. Reasons for exclusion from the per-protocol analysis set. (DOCX 20 kb)
Figure S6. ANOVA for ln-transformed nutrition effects (top) and Geometric Least Squares Means for nutrition effects and Ratios of Geometric Least Squares Means. (DOCX 28 kb)
Figure S7. ANOVA for ln-transformed pharmacokinetic parameters (top) and Geometric Least Squares Means for pharmacokinetic parameters. (DOCX 32 kb)
Acknowledgements
The authors thank all participating patients and Raffaela Kirchmeyer for her excellent assistance. The authors thank Carla-Maria Brandes for her support and collaboration throughout the study period. The authors thank the contract research organization medicomp GmbH for monitoring, data management and statistical analysis. We acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen.
Funding
The study was supported by Teva Pharmaceuticals BV. Ciclosporin Pro [25 mg, 50 mg and 100 mg soft capsules] was provided by Teva GmbH.
Teva was neither involved in the design of the study nor in collection, analysis, and interpretation of data or in writing the manuscript. Teva approved the final manuscript.
Availability of data and materials
Datasets analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AE
Adverse event
- ANOVA
Analysis of variance
- FAS
Full analysis set
- GCP
Good clinical practices
- GEM
Gel emulsion preconcentrate
- HEENT
Head, eyes, ears, nose, throat
- PK
Pharmacokinetic
- PP
Per protocol analysis set
Authors’ contributions
AK and OW designed research/study, AG, SD, AM, AK, OW performed research/study, AM, AK, OW collected data, AG, SD, HR, JK, BW, UE, AM, AK, OW analyzed and interpreted data, AG and OW wrote the paper, SD, HR, JK, BW, UE, AM, AK substantively revised the work. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
This study was conducted in compliance with Good Clinical Practices (GCP) and the Declaration of Helsinki, and in accordance with applicable legal and regulatory requirements. All patients gave informed consent. The study protocol was approved by the ethics commission of the medical faculty of the University Duisburg-Essen (10–4300) and registered in the European Union Clinical Trials Register (EudraCT No. 2009–011354-18).
Consent for publication
Not applicable.
Competing interests
AG received travel grants, speaker’s fees and research support from Amgen, Amicus, Alexion, Astellas and Astute. SD received travel grants and/or honoraria from Astellas, Novartis and Roche. HG received travel grants from Astellas, Chiesi and Novartis. JK received travel grants from Astellas, Novartis and Roche. BW has received travel grants from Alexion, Astellas, Biohope, Chiesi, Chemocentryx, Cytosorbents, Novartis, Pfizer, Roche, Sanofi, Teva and Wyeth. UE received research support and/or honoraria from Astellas, Astute, Amgen, Novartis and Teva. AM reports no conflicts of interest. AK received honoraria from Novartis. OW received research funds and/or honoraria from Alexion, Astellas, Bristol-Myers Squibb, Chiesi, Janssen-Cilag, MSD, Novartis, Pfizer, Roche and Shire.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anja Gäckler, Phone: (49) 201-723 6551, Email: anja.gaeckler@uk-essen.de.
Sebastian Dolff, Email: sebastian.dolff@uk-essen.de.
Hana Rohn, Email: hana.rohn@uk-essen.de.
Johannes Korth, Email: johannes.korth@uk-essen.de.
Benjamin Wilde, Email: benjamin.wilde@uk-essen.de.
Ute Eisenberger, Email: ute.eisenberger@uk-essen.de.
Anna Mitchell, Email: annamitchell@gmx.de.
Andreas Kribben, Email: andreas.kribben@uk-essen.de.
Oliver Witzke, Email: oliver.witzke@uk-essen.de.
References
- 1.Murdan S, Andrýsek T, Son D. Novel gels and their dispersions--oral drug delivery systems for ciclosporin. Int J Pharm. 2005;300(1–2):113–124. doi: 10.1016/j.ijpharm.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Tarr B, Yalkowsky S. Enhanced intestinal absorption of cyclosporine in rats through the reduction of emulsion droplet size. Pharm Res. 1989;6(1):40–43. doi: 10.1023/A:1015843517762. [DOI] [PubMed] [Google Scholar]
- 3.Andrysek T, Masri M, Jegorov A, Veselsky Z, Matha V. Equoral, new cyclosporine drug delivery system, versus neoral: a bioequivalence study in healthy volunteers. Transplant Proc. 2003;35(1):207–209. doi: 10.1016/S0041-1345(02)03924-6. [DOI] [PubMed] [Google Scholar]
- 4.Masri M, Haberal M, Rizvi A, Stephan A, Bilgin N, Nagvi A, Barbari A, Kamel G, Zafar N, Emiroglu R, et al. Switchability of neoral and equoral according to Food and Drug Administration rules and regulations. Transplant Proc. 2005;37(7):2988–2993. doi: 10.1016/j.transproceed.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 5.Mueller E, Kovarik J, van Bree J, Grevel J, Lücker P, Kutz K. Influence of a fat-rich meal on the pharmaocokinetics of a new oral formulation of cyclosporine in a crossover comparison with the market formulation. Pharm Res. 1994;11(1):151–155. doi: 10.1023/A:1018922517162. [DOI] [PubMed] [Google Scholar]
- 6.Kees F, Bucher M, Schweda F, Gschaidmeier H, Faerber L, Seifert R. Neoimmun versus Neoral: a bioequivalence study in healthy volunteers and influence of a fat-rich meal on the bioavailability of Neoimmun. Naunyn Schmiedeberg’s Arch Pharmacol. 2007;375(6):393–399. doi: 10.1007/s00210-007-0169-3. [DOI] [PubMed] [Google Scholar]
- 7.Hashizume H, Ito T, Yagi H, Takigawa M, Kageyama H, Furukawa F, Hata M, Shirahama S, Tanaka M, Higashishiba T, et al. Efficacy and safety or preprandial versus postprandial administraion of low-dose cyclosporin microemulsion (Neoral) in patients with psoriasis vulgaris. J Dermatol. 2007;34(7):430–434. doi: 10.1111/j.1346-8138.2007.00305.x. [DOI] [PubMed] [Google Scholar]
- 8.Umezawa Y, Mabuchi T, Ozawa A. Preprandial vs. postprandial pharmocokinetics of cyclosporine in patients with psoriasis. Int J Dermatol. 2007;46(8):880–882. doi: 10.1111/j.1365-4632.2007.03134.x. [DOI] [PubMed] [Google Scholar]
- 9.Frederik Vinet B. Clinical study report MDS 010421. Opava: Data on file, IVAX Pharmaceuticals; 2001. [Google Scholar]
- 10.Frederik Vinet B. Clinical study report MDS 010422[29] Opava: Data on file, IVAX Pharmaceuticals; 2001. [Google Scholar]
- 11.Albring A, Wendt L, Harz N, Engler H, Wilde B, Kribben A, Lindemann M, Schedlowski M, Witzke O. Relationship between pharmacokinetics and pharmacodynamics of calcineurin inhibitors in renal transplant patients. Clin Transpl. 2015;29(4):294–300. doi: 10.1111/ctr.12504. [DOI] [PubMed] [Google Scholar]
- 12.Huet E, Morand K, Blanchet B, Astier A, Hulin A. Evaluation of the new heterogeneous ACMIA immunoassay for the determination of whole-blood cyclosporine concentrations in bone marrow, kidney, heart, and liver transplant recipients. Transplant Proc. 2004;36(5):1317–1320. doi: 10.1016/j.transproceed.2004.05.062. [DOI] [PubMed] [Google Scholar]
- 13.Cyclosporine a - instructions for use/package inserts. https://doclib.healthcare.siemens.com/document/531626. Acccessed 31 Dec 2018.
- 14.Coto E, Tavira B. Pharmacogenetics of calcineurin inhibitors in renal transplantation. Transplantation. 2009;88(3S):S62–S67. doi: 10.1097/TP.0b013e3181afe9e7. [DOI] [PubMed] [Google Scholar]
- 15.Stoves J, Baker R, Newstead C. Measurement of cyclosporin exposure in renal transplant recipients during the early post-operative period: is C2 alone sufficient? Nephrol Dial Transplant. 2005;20(4):854–855. doi: 10.1093/ndt/gfh725. [DOI] [PubMed] [Google Scholar]
- 16.di Paolo S, Teutonico A, Stallone G, Infante B, Schena A, Grandaliano G, Battaglia M, Schena P. Cyclosporin exposure correlates with 1 year graft function and histological damage in renal transplanted patients. Nephrol Dial Transplant. 2004;19(8):2107–2112. doi: 10.1093/ndt/gfh344. [DOI] [PubMed] [Google Scholar]
- 17.Goodall D, Willicombe M, McLean A, Taube D. High intrapatient variability of tacrolimus levels and outpatient clinic nonattendance are associated with inferior outcomes in renal transplant patients. Transplant Direct. 2017;3(8):e192. doi: 10.1097/TXD.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whalen H, Glen J, Harkins V, Stevens K, Jardine A, Geddes C, Clancy M. High intrapatient tacrolimus variability is associated with worse outcomes in renal transplantation using a low-dose tacrolimus immunosuppressive regime. Transplantation. 2017;101(2):430–436. doi: 10.1097/TP.0000000000001129. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigo E, Segundo D, Fernández-Fresnedo G, López-Hoyos M, Benito A, Ruiz J, de Cos M, Arias M. Within-patient variability in tacrolimus blood levels predicts kidney graft loss and donor-specific antibody development. Transplantation. 2016;100(11):2479–2485. doi: 10.1097/TP.0000000000001040. [DOI] [PubMed] [Google Scholar]
- 20.Waiser J, Slowinski T, Brinker-Paschke A, Budde K, Schreiber M, Böhler T, Hauser I, Neumayer H. Impact of the variability of cyclosporin a trough levels on long-term renal allograft function. Nephrol Dial Transplant. 2002;17(7):1310–1317. doi: 10.1093/ndt/17.7.1310. [DOI] [PubMed] [Google Scholar]
- 21.Einecke G, Mai I, Fritsche L, Slowinski T, Waiser J, Neumayer H, Budde K. The value of C2 monitoring in stable renal allograft recipients on maintenance immunosuppression. Nephrol Dial Transplant. 2004;19(1):215–222. doi: 10.1093/ndt/gfg434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Inclusion and exclusion criteria. (DOCX 27 kb)
Figure S2. Diagram of study procedures. (DOCX 39 kb)
Figure S3. Composition of breakfasts. (DOCX 32 kb)
Figure S4. Concomitant medication during study. (DOCX 27 kb)
Figure S5. Reasons for exclusion from the per-protocol analysis set. (DOCX 20 kb)
Figure S6. ANOVA for ln-transformed nutrition effects (top) and Geometric Least Squares Means for nutrition effects and Ratios of Geometric Least Squares Means. (DOCX 28 kb)
Figure S7. ANOVA for ln-transformed pharmacokinetic parameters (top) and Geometric Least Squares Means for pharmacokinetic parameters. (DOCX 32 kb)
Data Availability Statement
Datasets analysed during the current study are available from the corresponding author on reasonable request.