Abstract
Background
Negative symptoms are frequent in patients with schizophrenia and are associated with marked impairments in social functioning. The efficacy of drug-based treatments and psychological interventions on primary negative symptoms remains limited. The Positive Emotions Programme for Schizophrenia (PEPS) is designed to improve pleasure and motivation in schizophrenia patients by targeting emotion regulation and cognitive skills relevant to apathy and anhedonia. The main hypothesis of this study is that patients who attend 8 one-hour sessions of PEPS and treatment as usual (TAU) will have lower total apathy-avolition and anhedonia-asociality composite scores on the Scale for the Assessment of Negative Symptoms (SANS) than patients who attend only TAU.
Methods
Eighty participants diagnosed with schizophrenia or schizoaffective disorder were randomized to receive either TAU or PEPS + TAU. The participants were assessed by independent evaluators before randomization (T0), in a post-test after 8 weeks of treatment (T1) and at a 6-month follow-up (T2).
Results
The post-test results and 6-month follow-up assessments according to an intention-to-treat analysis showed that the apathy and anhedonia composite scores on the SANS indicated statistically greater clinical improvements in PEPS participants than in non-PEPS participants. In the post-test, anhedonia but not apathy was significantly improved, thus favouring the PEPS condition. These results were sustained at the 6-month follow-up.
Conclusions
PEPS is an effective intervention to reduce anhedonia in schizophrenia. PEPS is a short, easy-to-use, group-based, freely available intervention that is easy to implement in a variety of environments (ClinicalTrials.gov ID: NCT02593058).
Keywords: Anhedonia, Apathy, Schizophrenia, Positive psychology, Randomized controlled trial, Negative symptoms
Background
Negative symptoms in schizophrenia are characterized by marked reductions in goal-directed behaviour, which can include speech, non-verbal behaviour and social behaviour [1]. This reduction in goal-directed behaviour hampers daily life functioning and interferes with the recovery process [2, 3, 4, 5]. The distinction between primary negative symptoms, a core aspect of the illness, and secondary negative symptoms bears important therapeutic implications [6]. Primary negative symptoms comprise the core features intrinsic to schizophrenia. Secondary negative symptoms are transient and attributable to the effects of factors such as unrelieved positive symptoms, depression, extrapyramidal side effects of antipsychotic drugs or social isolation. Primary and secondary negative symptoms may be similar in clinical expression, despite their contrasting aetiologies [7]. Secondary negative symptoms often diminish with the resolution of their causative factors, but primary negative symptoms are likely to persist despite treatment with either conventional or second-generation antipsychotics. Indeed, the efficacy of drug-based treatments and psychological interventions against primary negative symptoms remains limited [8, 9, 10]. Thus, there is a clear clinical need to develop treatments for primary negative symptoms.
The recent literature has distinguished the negative symptoms associated with a diminished capacity to experience (apathy, anhedonia) from symptoms that are associated with a limited capacity for expression (emotional blunting, alogia) [11, 12, 13, 14]. The apathy-anhedonia syndrome tends to be associated with a poorer prognosis than symptoms related to diminished expression, suggesting that the former is the severer facet of the psychopathology of schizophrenia [13]. Few psychosocial interventions have been developed with the specific intention of treating negative symptoms of schizophrenia. Cognitive behavioural therapy for negative symptoms is based on the premise that negative symptoms are maintained by dysfunctional beliefs that contribute to apathy-anhedonia syndrome [15]. The cognitive techniques used in this approach aim to modify these dysfunctional beliefs. However, this intervention improves functional outcomes but has no significant effect on anhedonia. An alternative psychological intervention is to maximize positive emotions [16, 17]. The field of affective science [18, 19] has described emotion regulation strategies that increase the frequency, intensity and duration of positive emotional experiences. These strategies include anticipating or remembering enjoyment, expressing emotions via non-verbal behaviours, directing controlled attention towards positive experiences when they occur and sharing positive experiences with others. Several studies indicate that individuals with negative symptoms of schizophrenia exhibit impaired positive emotion regulation strategies. For example, these individuals present a reduced capacity to anticipate pleasurable activities and envision the future [20, 21]. They report lower levels of pleasure in savouring past, present and future events than normal controls [22]. They also manifest a reduced ability to express and maintain positive emotions [23, 24]. Finally, they tend to avoid interpersonal interactions and anticipate a lower amount of pleasure in such interactions [25, 26].
The Positive Emotions Programme for Schizophrenia (PEPS) was developed in response to these issues [27]. PEPS is an intervention consisting of 8 one-hour sessions applied to groups of 5–10 participants. The aim of the programme is to increase cognitive control of positive emotions, including the anticipation and maintenance of those emotions. The programme uses visual and audio materials as part of a PowerPoint presentation of slides projected onto a screen. A pilot study was conducted with participants who met the ICD-10 criteria for schizophrenia or schizoaffective disorders [28]. Thirty-one participants completed the programme. Those who dropped out did not differ significantly from those who completed the programme. Participation in the programme was accompanied by statistically significant reductions in the total scores for avolition-apathy and anhedonia-asociality on the Scale for the Assessment of Negative Symptoms (SANS), with moderate effect sizes. Furthermore, there was a statistically significant reduction in depression on the Calgary Depression Scale for Schizophrenia (CDSS), with a large effect size. Emotional blunting and alogia remained stable during the intervention.
From this pilot study, the present randomized controlled clinical trial was designed (ClinicalTrials.gov ID: NCT02593058) to compare PEPS and treatment as usual (TAU). The goal of the study was to establish whether PEPS is clinically effective using a randomized, controlled, assessor-blind trial. The main hypothesis was that 8 sessions of PEPS added to TAU would significantly reduce the composite SANS scores for apathy/avolition and anhedonia/asociality compared with TAU only. This randomized clinical trial also tested whether the expected change was sustained at the 6-month follow-up.
Materials and Methods
Trial Design
This randomized controlled trial compares the PEPS combined with TAU (PEPS + TAU) to TAU alone. Participants diagnosed with a schizophrenia spectrum disorder underwent one of these interventions for 8 weeks. Tests were administered to evaluate individuals' current psychopathology and ability to savour pleasure at the time of inclusion, at the end of the 8-week intervention and at the 6-month follow-up. Participants were evaluated at baseline (T0) and then randomized to either TAU or PEPS + TAU. Randomization was completed in blocks of 6, 8, 10 or 12 participants depending on the number of available candidates to keep a short delay between the first evaluation and the start of the intervention. Once a block was recruited, an independent researcher (S.R.) received participant numbers and performed randomization using http://www.randomization.com/. Participants were randomly assigned to two parallel groups in a 1: 1 ratio. They were then informed of their allocation by the main investigator (J.F.) and the local investigators (A.I., A.B., G.T.) and asked to not disclose their allocation to the independent raters (C.F. and J.C.). Data were independently managed by a research assistant (J.P.). The intervention consisted of 8 weekly 1-h sessions of PEPS. At the end of the intervention (i.e., 8 weeks later), participants were again assessed (T1) by raters who were maintained unaware of group allocation. A third assessment (T2) was performed 6 months later to measure the stability of changes.
Subjects
To be included in the study, participants were required to meet the ICD-10 criteria for diagnosis of schizophrenia or a schizoaffective disorder (F20, F25), to present a score of at least 2 on the overall SANS anhedonia scale, to be between 18 and 65 years of age, to be able to read and understand French and to demonstrate capacity for consent according to the San Diego Brief Assessment of Capacity to Consent [29]. This tool measures a patient's understanding of an information sheet. If the potential participant was unable to respond correctly to the questions asked after reading the sheet, the patient was excluded. The procedure can be conducted a maximum of two times. Exclusion criteria were evidence of an organic brain disease, a clinically significant concurrent medical illness or a learning disability that could interfere with participation in group sessions.
The participants were outpatients and were recruited in nursing homes and rehabilitation workshops in the cantons of Fribourg and Vaud (Fondation HorizonSud in Marsens, SISP SA in Lausanne, Fondation Stanislas in Montherod, Unit of Rehabilitation of the Community Psychiatry Service, Department of Psychiatry, Lausanne). Potential participants were invited to presentations that provided an overview of the trial. During these meetings, the principal investigator and the person responsible for the site explained the aims of the study and the extent and nature of participation in the study, including randomization, a description of the control and experimental interventions, and a description of the three evaluations (pre-, post- and follow-up evaluations). The patients were also informed about the confidentiality of the data and their right to withdraw from participation at any time without giving any explanation. The patients received a written description of the study. Once the participants expressed their interest, their understanding of the protocol of the study was verified with the University of California San Diego Brief Assessment of Capacity to Consent, an instrument that measures decisional capacity [29]. In cases of failure to understand the study goal, voluntary participation, right to withdraw, randomization or number of assessment points, patients were excluded. A research assistant assessed the participants for eligibility in the study and met the case manager of the participant. An experienced clinician verified the diagnosis. This study was approved by the Board of Human Research Ethics Committee of the Canton de Vaud (Board Name: Commission cantonale d'éthique de la recherche sur l'être humain, CER-VD; Approval No.: 446/15). This committee is affiliated with the Swiss Ethics Committees on research involving humans. All participants signed a written informed consent form.
Interventions
Treatment as Usual
TAU consists of psychiatric management by a clinical team composed of at least one psychiatrist, a social worker and/or a psychiatric nurse, with additional access to community treatment or hospital admission. Treatment involves medication, regular office-based or community contacts with the clinical team for treatment monitoring, and socialization groups, therapy and psycho-educational groups. No attempts were made to standardize this treatment because TAU is tailored to each patient's specific needs. Patients receiving only TAU were invited to participate freely in PEPS after the trial.
Positive Emotions Programme for Schizophrenia
PEPS is an intervention meant to reduce anhedonia and apathy. The programme teaches skills to help overcome defeatist thinking and to increase the anticipation and maintenance of positive emotions. J.F. and A.N. conceived the programme. The intervention development and the programme description have been published [27]. The French version of PEPS can be downloaded for free at www.seretablir.net/peps/. PEPS involves 8 one-hour group sessions, administered using visual and audio materials and presented as PowerPoint presentation slides projected onto a screen. The programme uses a collaborative, egalitarian approach. Group facilitators participate in sessions just as the participants do by doing the exercises, sharing their experiences and carrying out the given tasks. This style of animation allows group leaders to act as models and promote participant involvement [30]. The pedagogical concept underpinning PEPS was designed according to Kolb and Kolb's model [31] of experiential learning. This model sees the learning process as the transformation of an experience into personal knowledge. The sequential organization of the learning activity starts with the learner going through an experience (the concrete experience phase). Next, the learner is invited to describe it and to give it a meaning (the reflective observation phase). Distancing oneself from the experience broadens the learner's understanding, generalizing and developing concepts through more abstract thought (the abstract conceptualization phase). The learner then initiates an experimental approach to validate the newly acquired knowledge through reality tests (the active experimentation phase). The learning activities for each skill taught in PEPS always proceed via these four steps. The skills content includes changing defeatist performance beliefs, savouring pleasant experiences, expressing emotions by increasing behavioural expression, sharing pleasant experiences with others and anticipating pleasant moments in the future. To familiarize the participant with the training materials, each session starts with a brief relaxation exercise using slowed breathing and mental focalization on savouring a pleasant experience. Group facilitators received a day's training before leading a group themselves and were supervised for 2 one-hour periods during a cycle of 8 sessions. Group leaders were clinicians working in the different sites (social workers, nurses, peer practitioners). The groups were led by 2–3 group leaders. Supervision was provided by experienced clinicians and teachers (A.N. and L.F.).
Outcomes
Main Outcome
The main outcome is the reduction of the composite scores of apathy/avolition and anhedonia/asociality on the SANS (hereafter called the apathy and anhedonia SANS composite score). This outcome aims to measure diminished experience syndrome. It is the weighted sum of the 3 items of the avolition and apathy subscale and the 4 items of the anhedonia and social withdrawal subscale of the SANS. The change is expected to remain at the 6-month follow-up. The secondary outcomes are depression, anticipatory and consummatory pleasure, beliefs about savouring and social anhedonia. To control for secondary negative symptom delusions, hallucinations, extrapyramidal side effects and depression are assessed at T0.
Measures
The following data and scales were used at T0 (pre-test), T1 (post-test) and T2 (6-month follow-up) as part of standardized interviews with an independent evaluator trained in their administration. The average time needed to complete the scales with the participants was 1 h.
The SANS [32] measures schizophrenia's deficit symptoms within the framework of schizophrenic disorders. It comprises 25 items, scored from 0 to 5. A definition of each item, including examples, facilitates a better understanding of the scale's content. The rating system is ordinal, from 0 (absent) to 5 (severe). The 25 items are grouped into five subscales of withdrawal or emotional poverty, alogia (lack of speech), avolition and apathy (lack of energy, lack of initiative), anhedonia and social withdrawal (loss of interests), and attention, and a score is assigned to each component. The scale was translated into French with acceptable validity [33, 34].
The CDSS [35] includes 9 items: depression, hopelessness, self-depreciation, guilty ideas of reference, pathological guilt, morning depression, early wakening, suicide and observed depression. This scale has been validated in French [36].
The Temporal Experience of Pleasure Scale (TEPS) contains 18 items included in two subscales: anticipatory pleasure (10 items) and consummatory pleasure (8 items) [37, 38]. Responses to items fall on a 6-point Likert scale from 1 (very false for me) to 6 (very true for me). Both the total anticipatory and consummatory scores of the TEPS are used. This scale has also been validated in French [39].
The Anticipatory and Consummatory Interpersonal Pleasure Scale (ACIPS) [40] is designed to assess one's ability to experience pleasure in the interpersonal domain. It is a 17-item self-report questionnaire measuring three constructs, including intimate social interactions, group social interactions and social bonding. The ACIPS is scored on a 6-point Likert scale, ranging from 1 (very false for me) to 6 (very true for me). The ACIPS focuses on interpersonal pleasure. This scale is validated in French [41].
The Savoring Beliefs Inventory (SBI) is a self-assessment questionnaire composed of 24 items divided into three temporal orientations, past, present and future, each represented by 8 items [42]. Half of the items are positively formulated, while the other half are negatively framed. Each item is rated on a 7-point Likert scale ranging from “strongly disagree” to “strongly agree.” The SBI has been translated to and validated in French. The French version of the SBI is a valid scale for measuring attitudes such as the ability to savour positive experiences, including anticipation, reminiscence or the present moment [43].
At the pre-test (T0), the following instruments are also used:
Collection of sociodemographic and clinical data filled in with the patient's case manager and psychiatrist: age, sex, marital status, educational level, main source of income, living arrangements (e.g., nursing home, with family), psychiatric diagnosis, duration of illness, and actual treatment.
The Psychotic Symptom Rating Scales [44], French version [45], is a 17-item multidimensional measure of delusions and auditory hallucinations. Symptoms are rated over the past 2 weeks. Two scales exist for auditory hallucinations (11 items) and delusions (6 items). A 5-point ordinal scale is used to rate symptom scores (0–4).
The Simpson-Angus Scale [46], French version [47], is a 10-item rating scale that has been used widely for the assessment of extrapyramidal side effects in both clinical practice and research settings [48]. Each item of the 10-item Simpson-Angus Scale is rated on a 5-point scale (0–4), and the mean score is obtained by adding the items and dividing by 10.
For all patients, the symptom-rating assessments were administered by clinicians trained to reliably administer these scales. Regular tests of interrater reliability were conducted. Intraclass correlations for the different scales were above 0.85. To assure the blinding of the raters, appointments were organized by the main investigator (J.F.) and the local investigators (A.I., A.B., G.T.). Independent evaluators were present only at assessment times. They did not participate in clinical meetings or supervision and had different research meetings focusing on data collection. Participants were taught not to disclose their allocation to the evaluators before T1 and T2. A test for blinding was conducted after each assessment. Raters were asked to guess the group allocation of the participant and indicate the certainty of their guess on a form while describing any hints they received if they were certain of the participant's group allocation. At T1, 4 PEPS participants and 2 TAU participants gave an involuntary hint disclosing their allocation during the interview with evaluators, and a nurse disclosed the allocation of 1 TAU participant by mistake. Three participants were wrongly allocated (2 TAU participants identified as PEPS participants and 1 PEPS participant as a TAU participant). Blinding was maintained at T2, and no newer disclosure of the allocation was highlighted.
Statistical Analysis
All analyses were conducted using the IBM SPSS Statistics package version 22. All statistical tests were two tailed, and significance was set at the 0.05 level. From the pilot study [28], the sample size was calculated with an α set at 0.05, a β set conservatively at 0.95 and an effect size f of 0.44 for the apathy and anhedonia SANS composite score. Using an a priori computation for an ANCOVA, the proposed trial required a total sample size of 70 participants for the two arms. With an estimated dropout rate of 16%, 84 participants were estimated to be needed, and approximately 110 potential participants were screened. Intention-to-treat analysis was used for each outcome. The multiple imputation method was performed with 50 imputations using the fully conditional specification method to impute missing data for the patients who completed the baseline but neither the post-test nor the 6-month follow-up assessment. The multiple imputation process was stratified by treatment arm and involved regression of the relevant outcome variables with missing information (dropouts with no end of treatment scores) on all the non-missing values of the baseline outcome measures (all variables in the first column of Table 2) with the addition of age and gender. A multiple regression approach to ANCOVA was used to allow adequate pooling of the estimates. Differences between post-test and pre-test as well as differences between 6-month follow-up and pre-test scores were treated as dependent variables, treatment condition was a fixed factor, and pretreatment scores were covariates. The improvement and deterioration of patients in the two groups were evaluated on the main outcome with the participants who were assessed at the post-test. Using the standard error of the difference and an α-level of 0.05, patients who scored 0.208 points lower or higher at the post-test were considered to have significantly improved or deteriorated.
Table 2.
Between-group differences at the post-test (T1) and pre-test (T0) on ANCOVA (means and SD in parentheses)
| TAU (n = 40) |
TAU + PEPS (n = 40) |
Differences (T1–T0)a |
B group effect | p | d | ||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | TAU | PEPS + TAU | ||||
| Main outcome | |||||||||
| SANS composite score apathy and anhedonia | 4.08 (1.89) | 3.81 (1.84) | 3.76 (2.10) | 2.96 (2.01) | – 0.23 (1.07) | – 0.83 (1.09) | – 0.598 | 0.014 | – 0.55 |
| SANS apathy score | 5.05 (3.47) | 4.77 (3.32) | 4.65 (3.19) | 3.80 (3.32) | – 0.23 (2.26) | – 0.91 (2.38) | – 0.685 | 0.192 | – 0.30 |
| SANS anhedonia score | 9.58 (\3.86) | 8.89 (4.11) | 8.85 (5.01) | 6.78 (4.57) | – 0.60 (2.79) | – 2.16 (2.71) | – 1.557 | 0.012 | – 0.57 |
| Secondary outcomesSANS affective flattening score | 7.63 (6.35) | 7.06 (7.32) | 8.88 (7.82) | 6.22 (6.84) | – 0.75 (5.14) | – 2.47 (4.95) | – 1.720 | 0.123 | – 0.34 |
| SANS alogia score | 3.15 (3.24) | 3.34 (3.21) | 3.93 (3.83) | 3.57 (3.54) | 0.03 (2.69) | – 0.18 (2.80) | – 0.208 | 0.737 | – 0.08 |
| SANS attention score | 2.98 (2.29) | 2.89 (2.91) | 3.40 (2.90) | 3.01 (2.46) | – 0.17 (2.37) | – 0.31 (2.11) | – 0.132 | 0.791 | – 0.06 |
| SANS sum of subscores | 28.38 (l5.04) | 26.95 (15.30) | 29.70 (18.20) | 23.38 (15.76) | – 1.57 (8.51) | – 6.18 (8.38) | – 4.612 | 0.015 | – 0.55 |
| CDSS | 5.78 (5.19) | 4.31 (3.65) | 5.45 (4.10) | 3.46 (3.23) | – 1.37 (2.97) | – 2.09 (2.87) | – 0.725 | 0.261 | – 0.25 |
| TEPS anticipatory | 39.48 (11.61) | 39.82 (10.61) | 43.68 (9.51) | 46.17 (9.11) | 0.40 (7.32) | 3.24 (7.06) | 3.635 | 0.025 | 0.51 |
| TEPS consummatory | 32.13 (7.77) | 32.41 (6.75) | 36.58 (7.00) | 38.24 (6.67) | – 0.80 (5.92) | 2.75 (5.70) | 3.552 | 0.007 | 0.61 |
| ACIPS intimate social interactions | 34.33 (8.05) | 34.66 (8.31) | 35.30 (7.80) | 35.58 (7.91) | 0.13 (6.70) | 0.48 (6.63) | 0.345 | 0.818 | 0.05 |
| ACIPS group social interactions | 17.63 (4.84) | 17.56 (5.94) | 18.38 (5.47) | 19.16 (4.80) | – 0.16 (4.12) | 0.87 (4.07) | 1.031 | 0.263 | 0.25 |
| ACIPS social bonding | 22.50 (5.68) | 21.78 (6.06) | 22.15 (4.82) | 23.03 (5.45) | – 0.67 (4.42) | 0.83 (4.36) | 1.498 | 0.128 | 0.34 |
| SBI total | 16.33 (28.70) | 21.10 (26.42) | 18.73 (21.36) | 29.20 (26.05) | 4.33 (21.09) | 10.92 (20.88) | 6.594 | 0.157 | 0.31 |
| SBI anticipating pleasure | 6.03 (10.83) | 7.36 (9.95) | 7.30 (9.05) | 10.49 (11.30) | 1.07(9.07) | 3.46 (8.92) | 2.385 | 0.228 | 0.27 |
| SBI present-moment pleasure | 3.90 (11.46) | 5.46 (11.33) | 4.43 (8.00) | 8.78 (9.04) | 1.44 (8.77) | 4.47 (8.74) | 3.029 | 0.123 | 0.35 |
| SBI reminiscing pleasure | 6.40 (10.37) | 8.27 (9.95) | 7.00 (8.54) | 9.94 (9.15) | 1.74 (8.03) | 3.07 (7.87) | 1.326 | 0.457 | 0.17 |
TAU, treatment as usual; PEPS, Positive Emotions Programme for Schizophrenia; SD, standard deviation; SANS, Scale for Assessment of Negative Symptoms; CDSS, Calgary Depression Scale for Schizophrenia; TEPS, Temporal Experience of Pleasure Scale; ACIPS, Anticipatory and Consummatory Interpersonal Pleasure Scale; SBI, Savouring Beliefs Inventory – French version.
Estimated means obtained from ANCOVA after controlling for baseline.
Results
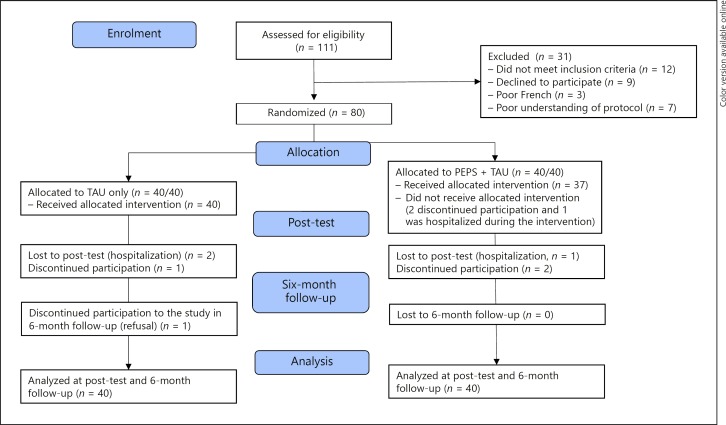
Figure 1 presents the Consolidated Standards of Reporting Trials (CONSORT) flow diagram indicating that 111 participants were interviewed to determine their eligibility for the randomized clinical trial. Thirty-one participants were excluded: 12 did not meet the inclusion criteria, 9 declined participation, 7 failed the San Diego Brief Assessment of Capacity to Consent, and 3 were not fluent in French. Eighty participants were randomized into the two groups (i.e., TAU or PEPS + TAU; screening-to-inclusion ratio: 72%), 40 in each group. Four participants later declined participation during the intervention, 2 in the PEPS + TAU group, 1 in the TAU-only group, and 1 during the 6-month follow-up in the TAU-only condition. Three participants were hospitalized, 1 in each group for psychiatric hospitalization and 1 in the TAU group for a chirurgical hospitalization. These 3 participants were not available at post-test assessment points and were considered dropouts. This resulted in a dropout rate of 8.75% for the entire sample. The entire sample comprised 31 female and 49 male participants. Their mean age was 39.90 years (SD 10.90), and their mean duration of illness was 16.08 years (SD 9.67). Sixty-six participants met the ICD-10 criteria for schizophrenia, and 14 those for schizo-affective disorders. Their mean scores were 5.61 (SD 4.65) for depression on the CDSS, 0.27 (SD 0.41) for extrapyramidal side effects on the Angus-Simpson Scale, 4.95 (SD 10.43) for hallucinations and 3.50 (SD 5.68) for delusions on the Psychotic Symptom Rating Scales. These mean scores indicate that the sample had low values for depression, hallucination, delusion and extrapyramidal symptoms (Table 1). In PEPS + TAU, 25 participants attended 8 sessions of PEPS; 6 participants 7 sessions; 1 participant 6 sessions; 1 participant 5 sessions; 2 participants 4 sessions; 1 participant 3 sessions; and 1 participant 2 sessions. On average, PEPS + TAU participants attended 90% of the programme.
Fig. 1.
CONSORT 2010 flow diagram.
Table 1.
Baseline characteristics of the TAU and PEPS + TAU groups
| TAU(n = 40) | PEPS + TAU (n = 40) | |
|---|---|---|
| Sociodemographic characteristics | ||
| Sex female/male | 12/28 | 19/21 |
| Age (SD), years | 39.83 (10.27) | 39.98 (11.62) |
| Marital status: currently married | 4 | 2 |
| Educational level: secondary | 8 | 8 |
| Main source of income: state aid | 38 | 40 |
| Living situation: independent living | 17 | 15 |
| Clinical variables | ||
| ICD-10 diagnosis | ||
| Schizophrenia | 34 | 32 |
| Schizoaffective disorders | 6 | 8 |
| Duration of illness (SD), years | 15.55 (10.18) | 16.60 (9.23) |
| Actual treatment | ||
| Chlorpromazine equivalent (SD) | 281 (198) | 383 (352) |
| Fluoxetine equivalent (SD) | 23.65 (34.56) | 36.75 (78.85) |
| Main outcome variable | ||
| Composite score: apathy and anhedonia (SD) | 4.08 (1.89) | 3.76 (2.10) |
| Calgary Depression Scale for Schizophrenia (SD) | 5.78 (5.19) | 5.45 (4.10) |
| Simpson-Angus Scale (SD) | 0.30 (0.48) | 0.23 (0.34) |
| PSYRATS Hallucination (SD) | 4.03 (9.92) | 5.88 (10.96) |
| PSYRATS Delusions (SD) | 3.90 (6.05) | 3.10 (5.33) |
TAU, treatment as usual; PEPS, Positive Emotions Programme for Schizophrenia; SD, standard deviation; PSYRATS, Psychotic Symptom Rating Scales.
All participants were recruited between January 2016 and February 2017. The first group started in February 2016, and the last one ended in May 2017; the last post-test follow-up was in November 2017. No adverse events were reported during the entire trial.
Main Outcome
Apathy and anhedonia SANS composite scores decreased more in the PEPS + TAU group than in the TAU-only group between pre-test (T0) and post-test (T1) assessments with a medium effect size (B = −0.598, p = 0.014, d = −0.55) (Table 2). This difference remained at the 6-month follow-up (T2) with a medium to large effect size in favour of the PEPS + TAU condition (B = −1.156, p = 0.001, d = −0.76) (Table 3). When looking at individual elements of the composite score, the SANS anhedonia/asociality score improved with a medium effect size in favour of the PEPS + TAU condition (B = −1.557, p = 0.012, d = −0.57) but not the SANS apathy/avolition score (B = − 0.685, p = 0.19, d = −0.30) (Table 2). At the 6-month follow-up, the anhedonia/asociality score improved with a medium to large effect size (B = − 3.102, p = 0.001, d = −0.78), and the apathy/avolition score improved with a small to medium effect size (B = 1.188, p = 0.045, d = −0.45) (Table 3).
Table 3.
Between-group differences at the 6-month follow-up (T2) and pre-test (T0) on ANCOVA
| TAU(n = 40) |
TAU + PEPS (n = 40) |
Differences (T2–T0)a |
B group effect | p | d | ||||
|---|---|---|---|---|---|---|---|---|---|
| T0 mean (SD) |
T2 mean (SD) |
T0 mean (SD) |
T2 mean (SD) |
TAU mean (SD) |
PEPS + TAU mean (SD) | ||||
| Main outcome | |||||||||
| SANS composite score apathy | |||||||||
| and anhedonia | 4.08 (1.89) | 3.73 (1.94) | 3.76 (2.10) | 2.40 (1.86) | – 0.28 (1.54) | – 1.43 (1.52) | – 1.156 | 0.001 | – 0.76 |
| SANS apathy score | 5.05 (3.47) | 4.35 (3.38) | 4.65 (3.19) | 2.96 (2.85) | – 0.61 (2.64) | – 1.79 (2.63) | – 1.188 | 0.045 | – 0.45 |
| SANS anhedonia score | 9.58 (\3.86) | 9.14 (4.44) | 8.85 (5.01) | 5.65 (4.81) | – 0.26 (4.00) | – 3.37 (3.98) | – 3.102 | 0.001 | – 0.78 |
| Secondary outcomes | |||||||||
| SANS affective flattening | |||||||||
| score | 7.63 (6.35) | 7.28 (6.97) | 8.88 (7.82) | 5.68 (5.36) | – 0.63 (4.95) | – 2.91 (4.83) | – 2.280 | 0.038 | – 0.47 |
| SANS alogia score | 3.15 (3.24) | 2.88 (2.93) | 3.93 (3.83) | 2.29 (2.64) | – 0.49 (2.37) | – 1.42 (2.30) | – 0.929 | 0.075 | – 0.40 |
| SANS attention score | 2.98 (2.29) | 2.18 (2.42) | 3.40 (2.90) | 2.15 (2.55) | – 0.91 (2.26) | – 1.13 (2.18) | – 0.220 | 0.659 | – 0.10 |
| SANS sum of subscores | 28.38 (l5.04) | 25.84 (15.54) | 29.70 (18.20) | 18.74 (12.95) | – 2.80 (10.22) | – 10.70 (10.07) | – 7.903 | < 0.001 | – 0.78 |
| CDSS | 5.78 (5.19) | 3.66 (5.04) | 5.45 (4.10) | 2.01 (2.46) | – 2.02 (3.69) | – 3.54 (3.39) | – 1.525 | 0.054 | – 0.43 |
| TEPS anticipatory | 39.48 (11.61) | 40.27 (11.58) | 43.68 (9.51) | 45.51 (8.27) | – 0.08 (8.29) | 2.72 (7.79) | 2.801 | 0.118 | 0.35 |
| TEPS consummatory | 32.13 (7.77) | 33.68 (6.65) | 36.58 (7.00) | 38.67 (5.78) | 0.41 (5.41) | 3.23 (4.93) | 2.822 | 0.016 | 0.55 |
| ACIPS intimate social | |||||||||
| interactions | 34.33 (8.05) | 35.13 (7.66) | 35.30 (7.80) | 37.67 (8.79) | 0.61 (6.63) | 2.57 (7.03) | 1.966 | 0.210 | 0.29 |
| ACIPS group social | |||||||||
| interactions | 17.63 (4.84) | 17.98 (4.74) | 18.38 (5.47) | 20.13 (4.07) | 0.18 (3.77) | 1.93 (3.63) | 1.757 | 0.033 | 0.48 |
| ACIPS social bonding | 22.50 (5.68) | 22.74 (5.54) | 22.15 (4.82) | 23.50 (6.37) | 0.32 (5.21) | 1.27 (5.43) | 0.946 | 0.419 | 0.18 |
| SBI total | 16.33 (28.70) | 20.16 (28.93) | 18.73 (21.36) | 31.55 (24.01) | 3.51 (19.33) | 13.15 (19.21) | 9.643 | 0.026 | 0.50 |
| SBI anticipating pleasure | 6.03 (10.83) | 6.81 (11.25) | 7.30 (9.05) | 10.95 (11.34) | 0.60 (8.96) | 3.85 (8.97) | 3.252 | 0.110 | 0.36 |
| SBI present-moment pleasure | 3.90 (11.46) | 5.26 (10.91) | 4.43 (8.00) | 10.74 (7.96) | 1.24 (8.01) | 6.44 (7.89) | 5.196 | 0.003 | 0.65 |
| SBI reminiscing pleasure | 6.40 (10.37) | 8.08 (12.29) | 7.00 (8.54) | 9.85 (9.06) | 1.57 (9.37) | 2.96 (8.66) | 1.397 | 0.488 | 0.15 |
TAU, treatment as usual; PEPS, Positive Emotions Programme for Schizophrenia; SD, standard deviation; SANS, Scale for Assessment of Negative Symptoms; CDSS, Calgary Depression Scale for Schizophrenia; TEPS, Temporal Experience of Pleasure Scale; ACIPS, Anticipatory and Consummatory Interpersonal Pleasure Scale; SBI, Savouring Beliefs Inventory – French version.
Estimated means obtained from ANCOVA after controlling for baseline.
Adding the Psychotic Symptom Rating Scales, CDSS and Simpson-Angus Scale at the pre-test as covariates did not change the pattern of the results. To evaluate the robustness of our findings, a complete case analysis was also performed on the main outcomes. The same pattern of findings could be obtained with either the original data or the pooled results of the 50 imputed data sets. A second analysis was also performed excluding those people whose allocation had been accidentally revealed. The same pattern of findings was obtained with the exception of the SANS apathy/avolition score at the 6-month follow-up, which was no longer significant (p = 0.146 instead of p = 0.045). Table 4 compares the changes in the main outcome of the participants. Forty-nine percent of patients in the TAU group had significantly improved scores, 11% had unchanged scores, and 41% had deteriorated scores. In the TAU + PEPS group, 68% of participants improved, 19% remained unchanged, and 14% worsened. A series of logistic regressions was performed to verify whether deterioration could be predicted by any of the sociodemographic or baseline data. Only age was significantly associated with a higher likelihood of deterioration during the TAU + PEPS intervention (odds ratio = 1.115, p = 0.039). While the mean age was 39.98 years in this group, the patients who deteriorated were 63, 60, 55, 40 and 37 years old.
Table 4.
Participants who exhibited improved, unchanged or worsened main outcomes on the post-test
| TAU + PEPS, % (n = 37) | TAU, % (n = 37) | |
|---|---|---|
| Improved | 68 (n = 25) | 49 (n = 18) |
| Unchanged | 19 (n = 7) | 11 (n = 4) |
| Worsened | 14 (n =5) | 41 (n = 15) |
Secondary Outcomes
Concerning the secondary outcomes, the TEPS anticipatory and consummatory scores were significantly improved with a medium effect size at the post-test (T1) (Table 2). At the 6-month follow-up (T2), the TEPS consummatory score showed sustained improvement with a medium effect size. The ACIPS group social interactions score and the SBI total score were significantly improved with a medium effect size at the 6-month follow-up. The SBI was improved mainly on the present-moment pleasure scale (Table 3).
The other subscales of the SANS were not significantly improved at the post-test for affective flattening, alogia and attention, or at 6 months for alogia and attention. Only affective flattening is improved at the 6-month follow-up. The sum of the five subscales of the SANS was statistically decreased with a medium effect size at the post-test (Table 2) and a large effect size at the 6-month follow-up (Table 3) favouring the experimental condition.
Discussion
The aim of this randomized controlled trial was to investigate the effect of a short group intervention (PEPS) on apathy and avolition in schizophrenia. PEPS led to moderate to large improvement in the primary outcome of apathy and anhedonia SANS composite score and moderate improvement in secondary outcomes of anticipatory and consummatory pleasure, as well as social interactions compared with the control group. Effects were maintained at the 6-month follow-up for the main outcome. There were no differences between groups on measures of alogia and attention. Participation in the entire program was high, while attrition was low, and no adverse events were reported. The results indicate that 8 sessions of PEPS intervention effectively reduced the diminished capacity to experience syndrome in a clinical sample suffering from schizophrenia.
In the present study, between-group effects on apathy/avolition and anhedonia/asociality at the post-test (d = −0.55) and 6-month follow-up (d = −0.76) were similar to the effects on apathy/avolition (d = −0.66) of an 18-month recovery-oriented cognitive therapy programme to improve psychosocial functioning and negative symptoms in low-functioning patients with schizophrenia [15]. However, when splitting the composite score, PEPS appears to be mainly effective in reducing the SANS anhedonia/asociality score. The benefit on the SANS apathy/avolition score obtained at the 6-month follow-up was lost when participants whose group allocation had been accidentally revealed were excluded. The significant reduction in the SANS anhedonia/asociality score in the PEPS + TAU group compared with the TAU-only arm is particularly promising because the intervention is specifically intended to maximize positive emotion experiences. The results suggest that anhedonia involves psychological processes such as emotion regulation skills that can be trained, indicating that anhedonia does not constitute a mere experiential abnormality. Patients could learn positive emotion regulation skills despite potential cognitive impairments in working memory and long-term memory. The fact that the SANS apathy/avolition score did not significantly improve is not surprising because PEPS is a very short intervention and focuses on positive emotion regulation training. Since apathy and anhedonia are frequently considered overlapping symptoms resulting from the same underlying process [49], it was expected that improving pleasure would increase goal-oriented behaviours. However, the results suggest that improving pleasure is not enough to improve motivation. Research evidence indicates that patients with schizophrenia have a reduced capacity for decision making related to reward; particularly, patients with schizophrenia exhibit slowed ability to modulate behaviour to seek rewards [50]. In the present study, an improvement in apathy would probably have required adjunctive training in instrumental and social skills.
At the post-test (T1), the anticipatory and consummatory scales of the TEPS were significantly improved, but the other self-report scales were not changed. The improvement was sustained at the 6-month follow-up for the consummatory scale, but not the anticipatory scale of the TEPS. The present-moment scale of the SBI showed an improvement at the 6-month follow-up. The ACIPS group social interaction score was also significantly improved, favouring PEPS + TAU. The results of these self-report scales are in line with the reduction in the anhedonia-asociality score of the SANS, showing that PEPS improves current pleasure for the TEPS and the SBI. Anticipatory and actual pleasure were distinguished in an attempt to explain the fact that patients engage in fewer reward-seeking behaviours despite a seemingly intact capacity to experience pleasure. Studies have indicated that individuals with schizophrenia predict that future events will result in less pleasure than their control counterparts [38, 39, 51]. In a replication study using the TEPS with another cohort of schizophrenia subjects, the opposite result was obtained. Schizophrenia subjects self-reported a difference in consummatory pleasure but not anticipatory pleasure compared with controls [52]. In another study, there were no significant differences in either consummatory or anticipatory pleasure between schizophrenia and healthy control groups, except for patients with high levels of amotivation who were impaired on both dimensions [53]. Frost and Strauss [54] advocated that scales with a hypothetical self-report format do not rely on experiential emotion but rather on semantic emotion knowledge and cannot measure consummatory pleasure or anticipatory pleasure. They suggested that a better measure would involve asking participants how they feel in the moment when directly exposed to a situation. The present study shows that a reduction in the anhedonia-asociality scale score of the SANS measured by independent raters is associated with improved current pleasure self-ratings by patients. The specific improvement in the ACIPS group social interaction may be directly related to the group format of PEPS, in which patients could have experienced pleasure together.
Only 14% of participants who received PEPS deteriorated on the main outcome at the post-test compared with 41% in the TAU-only group. The deterioration in the PEPS arm was associated with the age of the participants. While this finding must be replicated, PEPS intervention may not be as effective in older patients.
Important strengths of this study were the randomized design, high retention rate, controlled sources of secondary negative symptoms and high intervention acceptability. The study retention rate was high, and 91.25% of participants remained in the study until its end. The study was sufficiently powered to test the hypothesis according to the sample size calculation and the minimal dropout rate. The interrater reliability was good (intraclass coefficient correlation > 0.85), and the blindness of the assessors was maintained for 91% of the participants until the 6-month follow-up by a clear separation between the clinical and assessment teams. The study also controlled for sources of secondary negative symptoms such as depression, psychosis and extrapyramidal side effects. Participants who were randomized to the PEPS + TAU group attended 90% of the sessions. This high attendance rate is attributable to the fact that the participants described the programme as attractive. The participants appreciated the involvement of the group leaders in the exercises and valued the emphasis on positive aspects of their lives rather than on symptoms and deficits.
The main limitation of this study is the absence of an active control group. Certainly, group psychotherapeutic treatments can improve negative symptoms in the treatment of schizophrenia compared with TAU and appear to be non-specific and positively related to group intensity [55]. The collaborative, egalitarian approach used in the programme may have played an important role in the improvement of patients, making the patients more committed to treatment [56]. However, the selective effect on anhedonia suggests that this placebo effect is not sufficient for other negative symptoms, except potentially for affective flattening at the six-month follow-up, suggesting a specific effect of PEPS on anhedonia. Contrary to what is usually presumed, a meta-analysis showed that the negative symptoms of schizophrenia tend to improve significantly and consistently in outpatient settings, with a greater reduction found on the SANS than on the PANSS [57]. In the present study, the improvement in the SANS sum of subscores in the TAU arm (within-group Cohen's d = 0.166) matches the small improvement observed in the non-drug intervention of this meta-analysis, suggesting that TAU acts as a consistent control group. The SANS sum of subscores in the PEPS + TAU arm (within-group Cohen's d = 0.940) is superior to those reported in the non-drug studies reviewed in this meta-analysis.
Although traditional interventions have focused predominantly on ways to reduce negative emotions, interventions to improve positive emotions have been very recently developed to treat psychiatric disorders [58, 59]. A shift from approaches focusing on disease to well-being is increasingly advocated [60]. For example, well-being therapy [61] was developed in the 1990s as an adjunctive treatment to increase the level of recovery and has been found to be effective in depression and cyclothymic disorders and to promote psychological well-being in educational settings. The use of well-being therapy to improve functional outcomes as an additional component to psychological treatments in psychotic disorders was postulated in 2004 [62]. Along this line of thought, the PEPS is a specific positive emotion regulation skills training intervention for patients with schizophrenia. The applications issued from positive psychology interventions with patients suffering from schizophrenia are infrequent [63]. The main interventions that have been tested with patients suffering from schizophrenia or related psychosis are mindfulness [64], acceptance and commitment therapy [65], love kindness meditation [66] and compassion therapy [67]. These interventions are difficult to compare with the intervention in the current study; although they involve emotion regulation techniques, PEPS focuses on learning positive emotion regulation skills. To our knowledge, the only intervention comparable to the PEPS is a positive intervention called WELLFOCUS PPT, which has been tested in a controlled study on patients with psychosis [63]. The programme aims to increase positive experiences, amplify strengths, foster positive relationships and create a more meaningful self-narrative. The results showed an improvement in the Brief Psychiatric Rating Scale and depression. However, the study did not distinguish positive from negative symptoms. Additionally, the effect sizes for these outcomes are small to moderate.
This paradigm should be repeated in other environments to confirm the results. It would also be useful to develop a tailored individual version of the therapy for patients with severer anhedonia complemented with other emotion regulation strategies to improve positive emotions, such as those described in the model of Quoidbach et al. [68]. Clinical practice requires interventions that can be applied directly to the patient's bed. PEPS is freely available on the Internet, but translation to other languages and development of an interactive e-learning platform to train group leaders are necessary.
Further studies are needed to more precisely assess the impact of emotion regulation strategies on endocrine and autonomic stress in schizophrenia because maladaptive emotion regulation strategies may render people vulnerable to mental health problems in general [69]. Impaired subjective well-being is also associated with reduced anterior cingulate activity during reward processing, which may lead to a reduction in the integration of environmental stimuli and reward outcomes [70]. Future research should scrutinize the impact of the intervention on biological variables.
In summary, the present study shows that a short, easy-to-use, group-based intervention such as PEPS may be efficacious in reducing anhedonia in patients with schizophrenia. Considering the relatively brief and group-based nature of the intervention, PEPS could be a cost-effective intervention to implement for patients with negative symptoms of schizophrenia as an adjunctive treatment to social skills training [71] or other psychosocial interventions.
Disclosure Statement
The authors have no financial conflict of interest to declare.
Funding Sources
The Swiss National Science Foundation supported this work (grant No.: 105319_163355).
References
- 1.Aleman A, Lincoln TM, Bruggeman R, Melle I, Arends J, Arango C, et al. Treatment of negative symptoms: where do we stand, and where do we go? Schizophr Res. 2017 Aug;186:55–62. doi: 10.1016/j.schres.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Fervaha G, Foussias G, Agid O, Remington G. Impact of primary negative symptoms on functional outcomes in schizophrenia. Eur Psychiatry. 2014 Sep;29((7)):449–55. doi: 10.1016/j.eurpsy.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Marder SR. Clinician perceptions, expectations, and management of negative symptoms in schizophrenia. J Clin Psychiatry. 2013 Jan;74((1)):e01. doi: 10.4088/JCP.12045tx4c. [DOI] [PubMed] [Google Scholar]
- 4.Ventura J, Wood RC, Hellemann GS. Symptom domains and neurocognitive functioning can help differentiate social cognitive processes in schizophrenia: a meta-analysis. Schizophr Bull. 2013 Jan;39((1)):102–11. doi: 10.1093/schbul/sbr067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsang HW, Leung AY, Chung RC, Bell M, Cheung WM. Review on vocational predictors: a systematic review of predictors of vocational outcomes among individuals with schizophrenia: an update since 1998. Aust N Z J Psychiatry. 2010 Jun;44((6)):495–504. doi: 10.3109/00048671003785716. [DOI] [PubMed] [Google Scholar]
- 6.Mucci A, Merlotti E, Üçok A, Aleman A, Galderisi S. Primary and persistent negative symptoms: Concepts, assessments and neurobiological bases. Schizophr Res. 2017 Aug;186:19–28. doi: 10.1016/j.schres.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Möller HJ. Clinical evaluation of negative symptoms in schizophrenia. Eur Psychiatry. 2007 Sep;22((6)):380–6. doi: 10.1016/j.eurpsy.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Turkington D, Morrison AP. Cognitive therapy for negative symptoms of schizophrenia. Arch Gen Psychiatry. 2012 Feb;69((2)):119–20. doi: 10.1001/archgenpsychiatry.2011.141. [DOI] [PubMed] [Google Scholar]
- 9.Erhart SM, Marder SR, Carpenter WT. Treatment of schizophrenia negative symptoms: future prospects. Schizophr Bull. 2006 Apr;32((2)):234–7. doi: 10.1093/schbul/sbj055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fusar-Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, et al. Treatments of Negative Symptoms in Schizophrenia: Meta-Analysis of 168 Randomized Placebo-Controlled Trials. Schizophr Bull. 2015 Jul;41((4)):892–9. doi: 10.1093/schbul/sbu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006 Apr;32((2)):238–45. doi: 10.1093/schbul/sbj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann MN, Hager OM, Reimann AV, Chumbley JR, Kirschner M, Seifritz E, et al. Apathy but not diminished expression in schizophrenia is associated with discounting of monetary rewards by physical effort. Schizophr Bull. 2015 Mar;41((2)):503–12. doi: 10.1093/schbul/sbu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strauss GP, Horan WP, Kirkpatrick B, Fischer BA, Keller WR, Miski P, et al. Deconstructing negative symptoms of schizophrenia: avolition-apathy and diminished expression clusters predict clinical presentation and functional outcome. J Psychiatr Res. 2013 Jun;47((6)):783–90. doi: 10.1016/j.jpsychires.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foussias G, Remington G. Negative symptoms in schizophrenia: avolition and Occam's razor. Schizophr Bull. 2010 Mar;36((2)):359–69. doi: 10.1093/schbul/sbn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant PM, Huh GA, Perivoliotis D, Stolar NM, Beck AT. Randomized trial to evaluate the efficacy of cognitive therapy for low-functioning patients with schizophrenia. Arch Gen Psychiatry. 2012 Feb;69((2)):121–7. doi: 10.1001/archgenpsychiatry.2011.129. [DOI] [PubMed] [Google Scholar]
- 16.Strauss GP. Translating basic emotion research into novel psychosocial interventions for anhedonia. Schizophr Bull. 2013 Jul;39((4)):737–9. doi: 10.1093/schbul/sbt082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favrod J, Rexhaj S, Nguyen A, Cungi C, Bonsack C, Projecting oneself into the future, an intervention for improving pleasure in patients with anhedonia . Anhedonia: A Comprehensive Handbook Volume I: Conceptual Issues And Neurobiological Advances. In: Ritsner MS, editor. Dordrecht, Springer Science+Business Media Dordrecht. vol Volumes I. 2014. pp. pp 95–104. Conceptual issues and neurobiological advances. [Google Scholar]
- 18.Quoidbach J, Berry EV, Hansenne M, Mikolajczak M. Positive emotion regulation and well-being: comparing the impact of eight savoring and dampening strategies. Pers Individ Dif. 2010;49((5)):368–73. [Google Scholar]
- 19.Bryant FB, Veroff J. Savoring: A New Model of Positive Experience. Mahwah (NJ) 2007:32. [Google Scholar]
- 20.Raffard S, Esposito F, Boulenger JP, Van der Linden M. Impaired ability to imagine future pleasant events is associated with apathy in schizophrenia. Psychiatry Res. 2013 Oct;209((3)):393–400. doi: 10.1016/j.psychres.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Serper M, Payne E, Dill C, Portillo C, Taliercio J. Allocating effort and anticipating pleasure in schizophrenia: relationship with real world functioning. Eur Psychiatry. 2017 Oct;46:57–64. doi: 10.1016/j.eurpsy.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Cassar R, Applegate E, Bentall RP. Poor savouring and low self-efficacy are predictors of anhedonia in patients with schizophrenia spectrum disorders. Psychiatry Res. 2013 Dec;210((3)):830–4. doi: 10.1016/j.psychres.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Kring AM, Germans Gard M, Gard DE. Emotion deficits in schizophrenia: timing matters. J Abnorm Psychol. 2011 Feb;120((1)):79–87. doi: 10.1037/a0021402. [DOI] [PubMed] [Google Scholar]
- 24.Mote J, Stuart BK, Kring AM. Diminished emotion expressivity but not experience in men and women with schizophrenia. J Abnorm Psychol. 2014 Nov;123((4)):796–801. doi: 10.1037/abn0000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Worswick E, Dimic S, Wildgrube C, Priebe S. Negative Symptoms and Avoidance of Social Interaction: A Study of Non-Verbal Behaviour. Psychopathology. 2018;51((1)):1–9. doi: 10.1159/000484414. [DOI] [PubMed] [Google Scholar]
- 26.Campellone TR, Kring AM. Anticipated pleasure for positive and negative social interaction outcomes in schizophrenia. Psychiatry Res. 2018 Jan;259:203–9. doi: 10.1016/j.psychres.2017.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen A, Frobert L, McCluskey I, Golay P, Bonsack C, Favrod J. Development of the Positive Emotions Program for Schizophrenia: An Intervention to Improve Pleasure and Motivation in Schizophrenia. Front Psychiatry. 2016 Feb;7:13. doi: 10.3389/fpsyt.2016.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Favrod J, Nguyen A, Fankhauser C, Ismailaj A, Hasler JD, Ringuet A, et al. Positive Emotions Program for Schizophrenia (PEPS): a pilot intervention to reduce anhedonia and apathy. BMC Psychiatry. 2015 Sep;15((1)):231. doi: 10.1186/s12888-015-0610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeste DV, Palmer BW, Appelbaum PS, Golshan S, Glorioso D, Dunn LB, et al. A new brief instrument for assessing decisional capacity for clinical research. Arch Gen Psychiatry. 2007 Aug;64((8)):966–74. doi: 10.1001/archpsyc.64.8.966. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen A, Favrod J, Frobert L, Pellet J. Impact of self-disclosure of professionals on empowerment of patients: a conversational analysis. Eur Arch Psychiatry Clin Neurosci. 2017;267:S89. [Google Scholar]
- 31.Kolb AY, Kolb DA. The Learning Way. Simul Gaming. 2008;40((3)):297–327. [Google Scholar]
- 32.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989 Nov;155((7)):49–58. [PubMed] [Google Scholar]
- 33.Dollfus S, Langlois S, Assouly-Besse F, Petit M. [Depressive symptoms and negative symptoms during schizophrenia] Encephale. 1995 Jun;21((Spec No 3)):23–7. [PubMed] [Google Scholar]
- 34.Lecrubier Y, Boyer P. Fiche descriptive et traduction française de la SANS. Psychiatr Psychobiol. 1987:414–23. [Google Scholar]
- 35.Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry Suppl. 1993 Dec;163((22)):39–44. [PubMed] [Google Scholar]
- 36.Reine G, Bernard D, Auquier P, Le Fur B, Lançon C. [Psychometric properties of French version of the Calgary depression scale for schizophrenics (CDSS)] Encephale. 2000 Jan-Feb;26((1)):52–61. [PubMed] [Google Scholar]
- 37.Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: A scale development study. J Res Pers. 2006;40((6)):1086–102. [Google Scholar]
- 38.Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007 Jul;93((1-3)):253–60. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Favrod J, Ernst F, Giuliani F, Bonsack C. [Validation of the Temporal Experience of Pleasure Scale (TEPS) in a French-speaking environment] Encephale. 2009 Jun;35((3)):241–8. doi: 10.1016/j.encep.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Gooding DC, Pflum MJ. Further validation of the ACIPS as a measure of social hedonic response. Psychiatry Res. 2014 Mar;215((3)):771–7. doi: 10.1016/j.psychres.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Chaix J, Golay P, Fankhauser C, Nguyen A, Gooding DC, Favrod J. Confirmatory Factor Analysis of the French Version of the Anticipatory and Consummatory Interpersonal Pleasure Scale. Front Psychol. 2017 Jul;8:1296. doi: 10.3389/fpsyg.2017.01296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bryant FB. Savoring Beliefs Inventory (SBI): A scale for measuring beliefs about savouring. J Ment Health. 2003;12((2)):175–96. [Google Scholar]
- 43.Golay P, Thonon B, Nguyen A, Fankhauser C, Favrod J. Confirmatory Factor Analysis of the French Version of the Savoring Beliefs Inventory. Front Psychol. 2018 Feb;9:181. doi: 10.3389/fpsyg.2018.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haddock G, McCarron J, Tarrier N, Faragher EB. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS) Psychol Med. 1999 Jul;29((4)):879–89. doi: 10.1017/s0033291799008661. [DOI] [PubMed] [Google Scholar]
- 45.Favrod J, Rexhaj S, Ferrari P, Bardy S, Hayoz C, Morandi S, et al. French version validation of the psychotic symptom rating scales (PSYRATS) for outpatients with persistent psychotic symptoms. BMC Psychiatry. 2012 Sep;12((1)):161. doi: 10.1186/1471-244X-12-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212(S212):11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 47.Lejoyeux M, Gorwood P, Stalla-Bourdillon A, Adès J. [Translation and application of the Simpson and Angus Scale of Extrapyramidal Symptoms] Encephale. 1993 Jan-Feb;19((1)):17–21. [PubMed] [Google Scholar]
- 48.Janno S, Holi MM, Tuisku K, Wahlbeck K. Validity of Simpson-Angus Scale (SAS) in a naturalistic schizophrenia population. BMC Neurol. 2005 Mar;5((1)):5. doi: 10.1186/1471-2377-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaiser S, Lyne J, Agartz I, Clarke M, Mørch-Johnsen L, Faerden A. Individual negative symptoms and domains - Relevance for assessment, pathomechanisms and treatment. Schizophr Res. 2017 Aug;186:39–45. doi: 10.1016/j.schres.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 50.Taylor N, Hollis JP, Corcoran S, Gross R, Cuthbert B, Swails LW, et al. Impaired reward responsiveness in schizophrenia. Schizophr Res. 2018 Sep;199:46–52. doi: 10.1016/j.schres.2018.02.057. [DOI] [PubMed] [Google Scholar]
- 51.Chan RC, Wang Y, Huang J, Shi Y, Wang Y, Hong X, et al. Anticipatory and consummatory components of the experience of pleasure in schizophrenia: cross-cultural validation and extension. Psychiatry Res. 2010 Jan;175((1-2)):181–3. doi: 10.1016/j.psychres.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 52.Strauss GP, Wilbur RC, Warren KR, August SM, Gold JM. Anticipatory vs. consummatory pleasure: what is the nature of hedonic deficits in schizophrenia? Psychiatry Res. 2011 May;187((1-2)):36–41. doi: 10.1016/j.psychres.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Da Silva S, Saperia S, Siddiqui I, Fervaha G, Agid O, Daskalakis ZJ, et al. Investigating consummatory and anticipatory pleasure across motivation deficits in schizophrenia and healthy controls. Psychiatry Res. 2017 Aug;254:112–7. doi: 10.1016/j.psychres.2017.04.040. [DOI] [PubMed] [Google Scholar]
- 54.Frost KH, Strauss GP. A Review of Anticipatory Pleasure in Schizophrenia. Curr Behav Neurosci Rep. 2016 Sep;3((3)):232–47. doi: 10.1007/s40473-016-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orfanos S, Banks C, Priebe S. Are Group Psychotherapeutic Treatments Effective for Patients with Schizophrenia? A Systematic Review and Meta-Analysis. Psychother Psychosom. 2015;84((4)):241–9. doi: 10.1159/000377705. [DOI] [PubMed] [Google Scholar]
- 56.Fava GA, Guidi J, Rafanelli C, Rickels K. The Clinical Inadequacy of the Placebo Model and the Development of an Alternative Conceptual Framework. Psychother Psychosom. 2017;86((6)):332–40. doi: 10.1159/000480038. [DOI] [PubMed] [Google Scholar]
- 57.Savill M, Banks C, Khanom H, Priebe S. Do negative symptoms of schizophrenia change over time? A meta-analysis of longitudinal data. Psychol Med. 2015 Jun;45((8)):1613–27. doi: 10.1017/S0033291714002712. [DOI] [PubMed] [Google Scholar]
- 58.Fava GA, Ruini C, Rafanelli C, Finos L, Salmaso L, Mangelli L, et al. Well-being therapy of generalized anxiety disorder. Psychother Psychosom. 2005;74((1)):26–30. doi: 10.1159/000082023. [DOI] [PubMed] [Google Scholar]
- 59.Berking M, Ebert D, Cuijpers P, Hofmann SG. Emotion regulation skills training enhances the efficacy of inpatient cognitive behavioral therapy for major depressive disorder: a randomized controlled trial. Psychother Psychosom. 2013;82((4)):234–45. doi: 10.1159/000348448. [DOI] [PubMed] [Google Scholar]
- 60.Hasler G. Well-Being: An Important Concept for Psychotherapy and Psychiatric Neuroscience. Psychother Psychosom. 2016;85((5)):255–61. doi: 10.1159/000447268. [DOI] [PubMed] [Google Scholar]
- 61.Fava GA. Well-Being Therapy: Current Indications and Emerging Perspectives. Psychother Psychosom. 2016;85((3)):136–45. doi: 10.1159/000444114. [DOI] [PubMed] [Google Scholar]
- 62.Penn DL, Mueser KT, Tarrier N, Gloege A, Cather C, Serrano D, et al. Supportive therapy for schizophrenia: possible mechanisms and implications for adjunctive psychosocial treatments. Schizophr Bull. 2004;30((1)):101–12. doi: 10.1093/oxfordjournals.schbul.a007055. [DOI] [PubMed] [Google Scholar]
- 63.Schrank B, Brownell T, Jakaite Z, Larkin C, Pesola F, Riches S, et al. Evaluation of a positive psychotherapy group intervention for people with psychosis: pilot randomised controlled trial. Epidemiol Psychiatr Sci. 2016 Jun;25((3)):235–46. doi: 10.1017/S2045796015000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khoury B, Lecomte T, Gaudiano BA, Paquin K. Mindfulness interventions for psychosis: a meta-analysis. Schizophr Res. 2013 Oct;150((1)):176–84. doi: 10.1016/j.schres.2013.07.055. [DOI] [PubMed] [Google Scholar]
- 65.Bach P, Hayes SC. The use of acceptance and commitment therapy to prevent the rehospitalization of psychotic patients: a randomized controlled trial. J Consult Clin Psychol. 2002 Oct;70((5)):1129–39. doi: 10.1037//0022-006x.70.5.1129. [DOI] [PubMed] [Google Scholar]
- 66.Johnson DP, Penn DL, Fredrickson BL, Kring AM, Meyer PS, Catalino LI, et al. A pilot study of loving-kindness meditation for the negative symptoms of schizophrenia. Schizophr Res. 2011 Jul;129((2-3)):137–40. doi: 10.1016/j.schres.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 67.Tabak NT, Horan WP, Green MF. Mindfulness in schizophrenia: associations with self-reported motivation, emotion regulation, dysfunctional attitudes, and negative symptoms. Schizophr Res. 2015 Oct;168((1-2)):537–42. doi: 10.1016/j.schres.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quoidbach J, Mikolajczak M, Gross JJ. Positive interventions: an emotion regulation perspective. Psychol Bull. 2015 May;141((3)):655–93. doi: 10.1037/a0038648. [DOI] [PubMed] [Google Scholar]
- 69.Krkovic K, Moritz S, Lincoln TM. Neurocognitive deficits or stress overload: why do individuals with schizophrenia show poor performance in neurocognitive tests? Schizophr Res. 2017 May;183:151–6. doi: 10.1016/j.schres.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 70.Gilleen J, Shergill SS, Kapur S. Impaired subjective well-being in schizophrenia is associated with reduced anterior cingulate activity during reward processing. Psychol Med. 2015 Feb;45((3)):589–600. doi: 10.1017/S0033291714001718. [DOI] [PubMed] [Google Scholar]
- 71.Turner DT, McGlanaghy E, Cuijpers P, van der Gaag M, Karyotaki E, MacBeth A. A Meta-Analysis of Social Skills Training and Related Interventions for Psychosis. Schizophr Bull. 2018 Apr;44((3)):475–91. doi: 10.1093/schbul/sbx146. [DOI] [PMC free article] [PubMed] [Google Scholar]