Abstract
Background
Evidence shows that adequate calcium intake during pregnancy reduces the risk of hypertensive disorders of pregnancy. In most low‐ and middle‐income countries (LMICs) the daily calcium intake is well below recommendations. Mapping calcium intake during pregnancy worldwide and identifying populations with low calcium intake will provide the evidence base for more targeted actions to improve calcium intake.
Objective
To assess dietary calcium intake during pregnancy worldwide.
Search strategy
MEDLINE and EMBASE (from July 2004 to November 2017).
Selection criteria
Cross‐sectional, cohort, and intervention studies reporting calcium intake during pregnancy.
Data collection and analysis
Five reviewers working in pairs independently performed screening, extraction, and quality assessment. We reported summary measures of calcium intake and calculated the weighted arithmetic mean for high‐income countries (HICs) and LMICs independently, and for geographic regions, among studies reporting country of recruitment, mean intake, and total number of participants. When available, inadequate intakes were reported.
Main results
From 1880 citations 105 works met the inclusion criteria, providing data for 73 958 women in 37 countries. The mean calcium intake was 948.3 mg/day (95% CI 872.1–1024.4 mg/day) for HICs and 647.6 mg/day (95% CI 568.7–726.5 mg/day) for LMICs. Calcium intakes below 800 mg/day were reported in five (29%) countries from HICs and in 14 (82%) countries from LMICs.
Conclusion
These results are consistent with a lack of improvement in calcium dietary intake during pregnancy and confirm the gap between HICs and LMICs, with alarmingly low intakes recorded for pregnant women in LMICs. From the public health perspective, in the absence of specific local data, calcium supplementation of pregnant women in these countries should be universal.
Tweetable abstract
Despite dietary recommendations, women in LMICs face pregnancy with diets low in calcium.
Keywords: Calcium, dietary, high‐income countries, hypertension, low‐ and middle‐income countries, pre‐eclampsia, pregnancy, systematic review
Tweetable abstract
Despite dietary recommendations, women in LMICs face pregnancy with diets low in calcium.
Introduction
Hypertensive disorders of pregnancy cause around 46 000 maternal deaths and 1.5–2.0 million neonatal deaths annually.1 Over 99% of these deaths occur in less developed countries.1 Although maternal mortality has decreased overall, the percentage of maternal deaths resulting from hypertension has remained stagnant, with 9.71% (36 497 deaths) in 1990 and 9.99% (29 275 deaths) in 2013.2 Evidence has shown that calcium supplementation during pregnancy prevents the development of hypertensive disorders of pregnancy.3
Calcium is a mineral required for normal physiological functioning. Requirements increase in specific periods of life, especially during pregnancy.4 There is no consensus regarding the recommended intake during pregnancy. Calcium recommendations for individuals over 19 years of age vary from 700 to 1000 mg, depending on the reference guidelines.5,6 Although most guidelines acknowledge the increased demand for calcium during pregnancy, some guidelines increase recommendations during pregnancy up to 1300 mg/day to achieve a positive balance, whereas others state that metabolic adaptations during pregnancy compensate for the increased demand for calcium.5,7–12
Previous studies show that in most low‐ and middle‐income countries (LMICs) the daily calcium intake is well below recommendations; however, low intakes are also observed in particular age groups, such as adolescents in high‐income countries (HICs).13–15 Current World Health Organization (WHO) guidelines recommend that in populations where the calcium intake is low, women should receive calcium supplementation after 20 weeks of gestation as part of antenatal care for the prevention of pre‐eclampsia, particularly among those at higher risk of developing hypertension.16 In addition, results from a recent randomised trial suggest that the beneficial effects of calcium supplementation on the reduction of pre‐eclampsia/eclampsia in women with low dietary calcium intake is greatest when supplementation is commenced before and continued throughout pregnancy.17 The mechanism by which calcium may have an effect on blood pressure is not well established; one hypothesis is that low calcium intakes increase the levels of parathyroid hormone and 1,25‐dihydroxy vitamin D (DHVD), which are required to maintain specific calcium concentrations in extracellular fluids. Higher levels of parathyroid hormone and DHVD stimulate calcium influx into different cell types and increase intracellular calcium into the vascular smooth muscle cell, and consequently increased muscle reactivity, peripheral vascular resistance, and thus higher blood pressure.18,19
In order to effectively implement the WHO recommendations and reduce the risk of hypertensive disorders of pregnancy in the most vulnerable women, it is essential to identify the specific populations or subpopulations at higher risk. This would allow the evidence‐based priorisation of calcium supplementation, and would inform the development of policies, strategies, and actions, including a population‐specific quantity assessment of the additional calcium intake required. A systematic review was conducted and published in 2005 to assess calcium dietary intake in pregnant women globally, including studies published from 1991 to July 2004.13 We aimed to update the 2005 review and assess calcium intake during pregnancy worldwide from 2004 onwards. This update will map calcium intake during pregnancy worldwide and identify populations with low calcium intake, which will provide the evidence base for more targeted actions in order to improve calcium intakes.
Methods
We followed the reporting recommendations of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement and the Meta‐analysis of Observational Studies in Epidemiology (MOOSE) group.20,21 The protocol of this review was published in PROSPERO (the international prospective register of systematic reviews).22 This systematic review did not include primary data collection, and there was no patient and public involvement in the development of the protocol for the review.
Criteria for considering studies for this review
The studies included in this review required the following criteria.
Type of study
The studies included were: cross‐sectional, cohort or intervention studies reporting calcium intake at any time during pregnancy and using any methodology (e.g. 24‐hour recall, food frequency questionnaires, weight records, food records). In the case of clinical trials, we included baseline data or data from the placebo arm. We excluded case reports and case–control studies. We only included studies reporting the country of recruitment.
Type of participants
The participants included pregnant women of any age, ethnic group, parity, education, and socio‐economic status. We excluded studies reporting on women with specific conditions (e.g. women with systemic lupus, erythematosus lupus, rheumatoid arthritis, diabetes, bariatric surgery, twin pregnancies, and vegetarian diet); however, we included studies that only reported data for women who were obese or overweight.
Data collection period
We included all studies collecting data after July 2004, which is the date of the most recent data included in the previous systematic review.
Search strategy for the identification of studies
The search strategy was developed with the assistance of a librarian experienced in electronic search strategies for systematic reviews from the Institute of Clinical Effectiveness (IECS), and was tested by a second expert in search strategies from the WHO. We searched MEDLINE (from July 2004 to 7 November 2017) and EMBASE (from July 2004 to 7 November 2017) using a combination of medical subject headings, keyword terms, and word variants for pregnancy and calcium.
This review had no language restrictions. Appendix S1 presents the search strategy developed for this systematic review.
We checked the reference lists of systematic reviews and of primary studies selected for full‐text evaluation for additional potentially relevant articles not identified by the electronic search. Authors of relevant papers were contacted regarding any further published or unpublished work. Authors of manuscripts reporting incomplete information were contacted to provide the missing information.
Process of study identification, selection, and data extraction
We used the software package covidence (Veritas Health Innovation Ltd, Melbourne, Victoria, Australia) for the selection of studies and data extraction. Five reviewers (GC, APB, CFL, IR, and EH) independently and in duplicate screened the titles and abstracts to select potentially relevant citations for full‐text evaluation. Discrepancies were resolved through discussion and consensus. When citations were considered relevant or when information in the title or abstract was insufficient for decision on inclusion or exclusion criteria, the full text was retrieved and evaluated.
After full‐text evaluation, three reviewers (GC, IR, and CFL) independently extracted data from the included studies using a template created in covidence. Data extracted from the studies included: study identification information (study title, authors, country, year); study characteristics (study design, data collection period); participants (sample size, study inclusion criteria, age of the women, country or United Nations region, gestational age at assessment); outcome (dietary assessment methodology, food chemical composition table used, daily calcium intake as mean or median in mg/day, percentage of inadequate intake as a percentage, and confidence interval and methodology for assessing inadequate intake).
Risk of bias assessment
Three reviewers (GC, IR, and FL) independently assessed the quality of each included study, discrepancies were discussed, and if consensus was not reached a third reviewer was consulted (AC). We assessed the quality of the data in each included study using an adapted version of the Newcastle–Ottawa Scale (NOS) for non‐randomised studies in meta‐analyses, Robins (Risk Of Bias in Non‐randomized Studies – of Interventions), and the Scottish Intercollegiate Guidelines Network (SIGN).23,24 We evaluated the risk of bias by assessing the eligibility criteria, sample size, representativeness (whether a sampling methodology was used appropriately to produce an estimate representative of the target population or if a convenience sample or special group was selected), response rate, data collection tool, clarity of the questions/statements and definition of the outcome, clarity of the objective, ethical considerations, and consistency between research question and data reported (Appendix S2).25 We present the evaluation results for each question for each included study. The potential risk of bias was characterised as follows: eligibility criteria (low risk, defined eligibility; high risk, not defined); sample size (low risk, 100 subjects or more; high risk, <100 or not explained); data collection tool (low risk, described with references; moderate risk, described without references; high risk, not described); definition of the outcome (low risk, well defined; high risk, improperly defined); clarity of results data (low risk, reported with numerators and denominators; high risk, not clearly reported), and representativeness (low risk, representative; moderate risk, not described or somehow representative; high risk, selected group).26 Study quality was characterised as follows: the clarity of the objective was assessed as clear, not clear, or not described; ethical consideration was defined as reporting committee approval and informed consent (either of these or none); consistency of results and conclusion were scored as yes or no; and the response rate was scored as ≥70% or <70%.
Strategy for analysis and data synthesis
We reported summary measures of calcium intake for each included study. For studies reporting mean intake, standard deviation (SD), and total number of participants we calculated the standard error of the mean (SE). If we found similar values of means and medians reported in some studies we would assume a normal distribution. Following the Cochrane Handbook recommendations, for studies with a sample size of more than 100 and reporting the median and the interquartile, we calculated the adjusted SD by subtracting the interquartile and dividing it by 1.35, the suggested width of one standard deviation.24 We then performed meta‐analyses by World Bank Classification of country income, country, United Nations region, and trimester of gestational age using stats direct.27 We present the random‐effects model results of the meta‐analysis. Stratum weights were calculated as the inverse of the variance for the mean. The pooled estimate was calculated as a weighted mean (sum of weights for each stratum divided by the sum of the weights). The inconsistency of results across studies was summarised as the I² statistic, which is the percentage of variation across studies resulting from heterogeneity rather than chance. We tested group differences between HIC and LMIC using The Cochrane Manager Reviewer (revman 5).28
As recommended by the Institute of Medicine (IOM), we used the Estimated Average Requirement (EAR) of 800 mg of calcium/day as the cut‐off point to assess the adequacy of calcium intake in populations.29
Funding for this review was obtained from the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), and World Health Organization.
Results
The search strategy retrieved a total of 1880 articles, 1867 of which were screened after excluding 13 duplicates. A total of 406 were selected for full‐text evaluation and finally 105 articles were included in the review (Figure 1).30–128 The summary of characteristics of the included studies are shown in Table 1.
Figure 1.
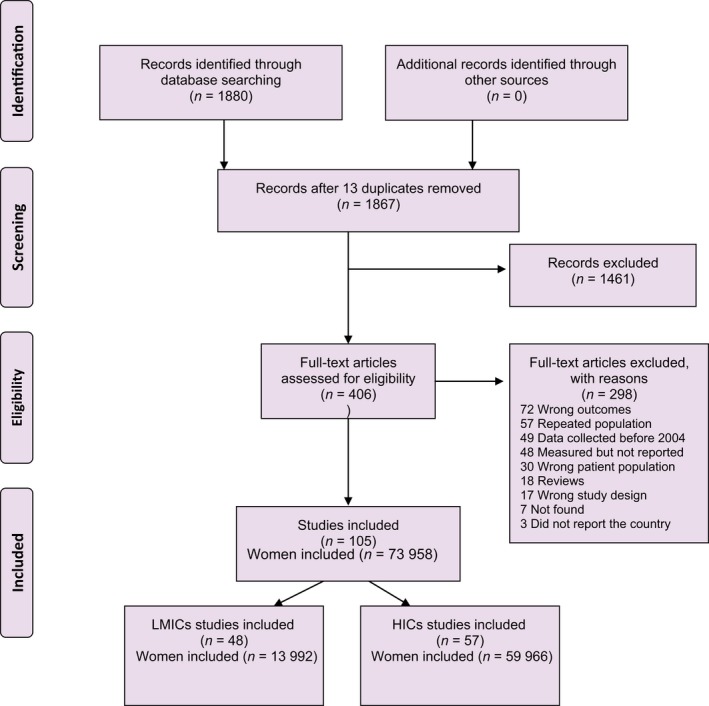
Study selection process for systematic review. PRISMA 2009 flow diagram.
Table 1.
General characteristics of the 105 included studies with 73 958 women
| Study characteristic | Number of studies | % | Number of women | % |
|---|---|---|---|---|
| Country income level | ||||
| High income | 57 | 54.3 | 59 966 | 81.1 |
| Upper middle income | 27 | 25.7 | 8582 | 11.6 |
| Lower middle income | 19 | 18.1 | 4851 | 6.6 |
| Low income | 2 | 1.9 | 559 | 0.8 |
| Country region | ||||
| Western Europe and others | 47 | 44.8 | 55 646 | 75.2 |
| Asia Pacific | 37 | 35.2 | 12 638 | 17.1 |
| Latin America and Caribbean | 11 | 10.5 | 3009 | 4.1 |
| Africa | 6 | 5.7 | 1902 | 2.6 |
| Eastern Europe | 4 | 3.8 | 763 | 1.0 |
| Study design | ||||
| Cross‐sectional | 52 | 49.5 | 16 540 | 22.4 |
| Cohort | 28 | 26.7 | 53 014 | 71.7 |
| Randomised controlled trial | 14 | 13.3 | 2936 | 4.0 |
| Quasi‐experimental | 3 | 2.9 | 300 | 0.4 |
| Case–control | 1 | 1.0 | 40 | 0.1 |
| N/A | 7 | 6.7 | 1128 | 1.5 |
| Population | ||||
| Adult women only | 61 | 58.1 | 61 161 | 82.7 |
| Adolescents only | 3 | 2.9 | 529 | 0.7 |
| Adults and adolescents | 33 | 31.4 | 10 932 | 14.8 |
| N/A | 8 | 7.6 | 1336 | 1.8 |
| Sample size | ||||
| <100 | 39 | 37.1 | 1951 | 2.6 |
| 100–1000 | 56 | 53.3 | 18 222 | 24.6 |
| >1000 | 10 | 9.5 | 53 785 | 72.7 |
| Time of dietary assessment | ||||
| Trimester 1 | 15 | 14.3 | 4608 | 6.2 |
| Trimester 2 | 13 | 12.4 | 44 909 | 60.7 |
| Trimester 3 | 24 | 22.9 | 8239 | 11.1 |
| All | 31 | 29.5 | 11 467 | 15.5 |
| Each trimester reported | 7 | 6.7 | 1796 | 2.4 |
| N/A | 15 | 14.3 | 2939 | 4.0 |
| Data collection methodology | ||||
| 24‐h recall | 41 | 39.0 | 20 427 | 27.6 |
| FFQ | 31 | 29.5 | 44 861 | 60.7 |
| Food records | 16 | 15.2 | 3300 | 4.5 |
| Diet history | 4 | 3.8 | 2990 | 4.0 |
| Other | 7 | 6.7 | 1616 | 2.2 |
| N/A | 6 | 5.7 | 764 | 1.0 |
This review included 73 958 women from 37 countries, of which 19 are HICs (Table S1) and 18 are LMICs (Table S2). Fifty‐seven studies were from HICs, although they represented 81.1% of the women included in the review (59 966). Forty‐eight studies were from LMICs representing 18.9% of the women (13 992). Regions were unevenly represented: 47 studies in Western Europe (55 646 women), 37 studies in the Asia Pacific Group (12 638 women), 11 studies in Latin America (3009 women), six studies in Africa (1902 women), and only four studies in Eastern Europe (763 women). Around two‐thirds of the studies in LMICs were conducted in four countries: India (10 studies, 2137 women), Iran (seven studies, 1949 women), Brazil (eight studies, 2514 women), and China (seven studies, 2763 women). Half of the studies in high‐income countries were conducted in four countries: USA (15 studies, 3795 women), Ireland (six studies, 2522 women), Australia (five studies, 1058 women), and Greece (four studies, 817 women).
We wrote to the corresponding authors of 22 studies; six authors replied. We included five of those six studies, as authors of four studies confirmed the data collection dates,34,40,104,129 and one study confirmed the total number of included subjects.66 The sixth study was excluded as the data collection date was before 2004.109
The characteristics informing the risk of bias assessment are summarised in Figure S1 and Table S3. Most studies clearly defined their objective (91, 86.7%) and the eligibility criteria (70, 66.7%), had a sample of at least 100 individuals or a sample size calculation (66, 62.9%), used a data collection tool defined with references to the sources (43, 41.0%), or without references (57, 54.3%), had a well‐defined outcome (87, 82.9%), presented the results clearly (77, 73.3%), and demonstrated consistency between the research question and outcome reporting (83, 79.0%). On the other hand, only 33 (31.4%) had a representative sample of the population included in the study. The summary of risk of bias is presented in Figure S2.
Most studies had a cross‐sectional (52, 49.5%) or cohort (28, 26.7%) design (Table 1). Sample size ranged from nine to 32 653 pregnant women. The results of some studies were presented by population subgroups, mainly by age of the pregnant women and gestational age (Tables S1 and S2). Sixty‐one studies (58.1%) only included adult women, although they represented 82.7% of the women included in this review; 33 (31.4%) studies included both adults and adolescents. Three (2.9%) studies only reported adolescent pregnancies, representing 0.7% of the women in the review (529). Dietary assessment was performed during the third trimester in 24 studies (22.9%) or at any trimester in 31 studies (29.5%). The methodology most frequently used was 24‐hour recall in 41 (39.0%) studies and food frequency questionnaire (FFQ) in 31 (29.5%) studies (Table 1). Although 31 (29.5%) studies reported the percentage of inadequate intakes, only 22 (20.9%) reported the cut‐off point used, which are different, thus making it difficult to compare studies. Only three studies fully reported the methodology used to obtain the inadequate intake values.21,51,63
Included in the meta‐analysis were 91 studies that reported mean intakes with standard deviation, standard error, or confidence interval, or median values with confidence intervals. Figures 2 and 3 show the mean calcium intakes and approximate 95% CIs for each of the 91 studies included in the meta‐analyses: 50 studies with 58 subgroups for HICs and 41 studies with 44 subgroups for LMICs, respectively. The mean calcium intake was 948.3 mg/day (95% CI 872.1–1024.4 mg/day) for HICs and 647.6 mg/day (95% CI 568.7–726.5 mg/day) for LMICs. There was a statistically significant difference between LMICs and HICs (P < 0.00001), I² = 96.4%. The meta‐analysis by UN regions showed 621.7 mg/day (95% CI 400.4–843.0 mg/day for Latin America (ten studies), 566.0 mg/day (95% CI 408.1–723.9 mg/day) for Africa (five studies), 652.7 mg/day (95% CI 583.8–721.6 mg/day) for Asia Pacific (32 studies), and 988.5 mg/day (95% CI 920.8–10756.1 mg/day) for Western Europe and others group (43 studies); only one study reported on the calcium intake for Eastern Europe, with 1235 mg/day (95% CI 1102.0–1367.9 mg/day; Figure S3).
Figure 2.
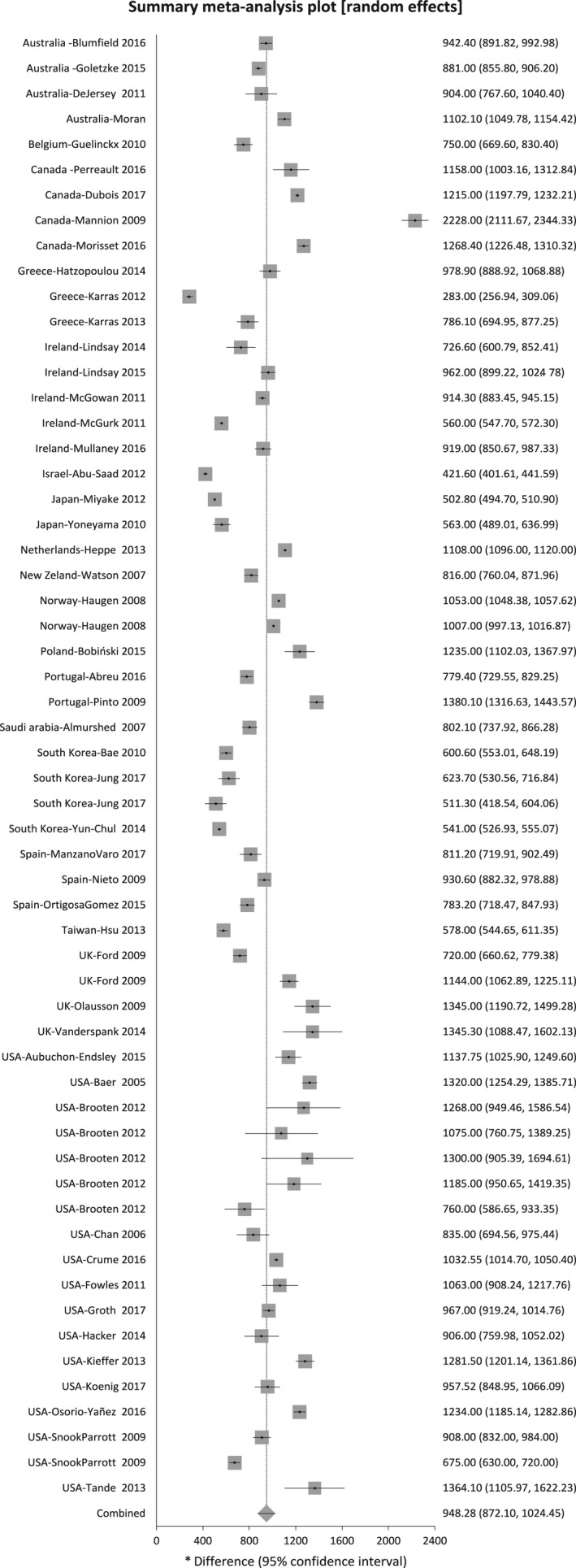
Mean calcium intake and approximate 95% CIs in studies from high‐income countries.
Figure 3.
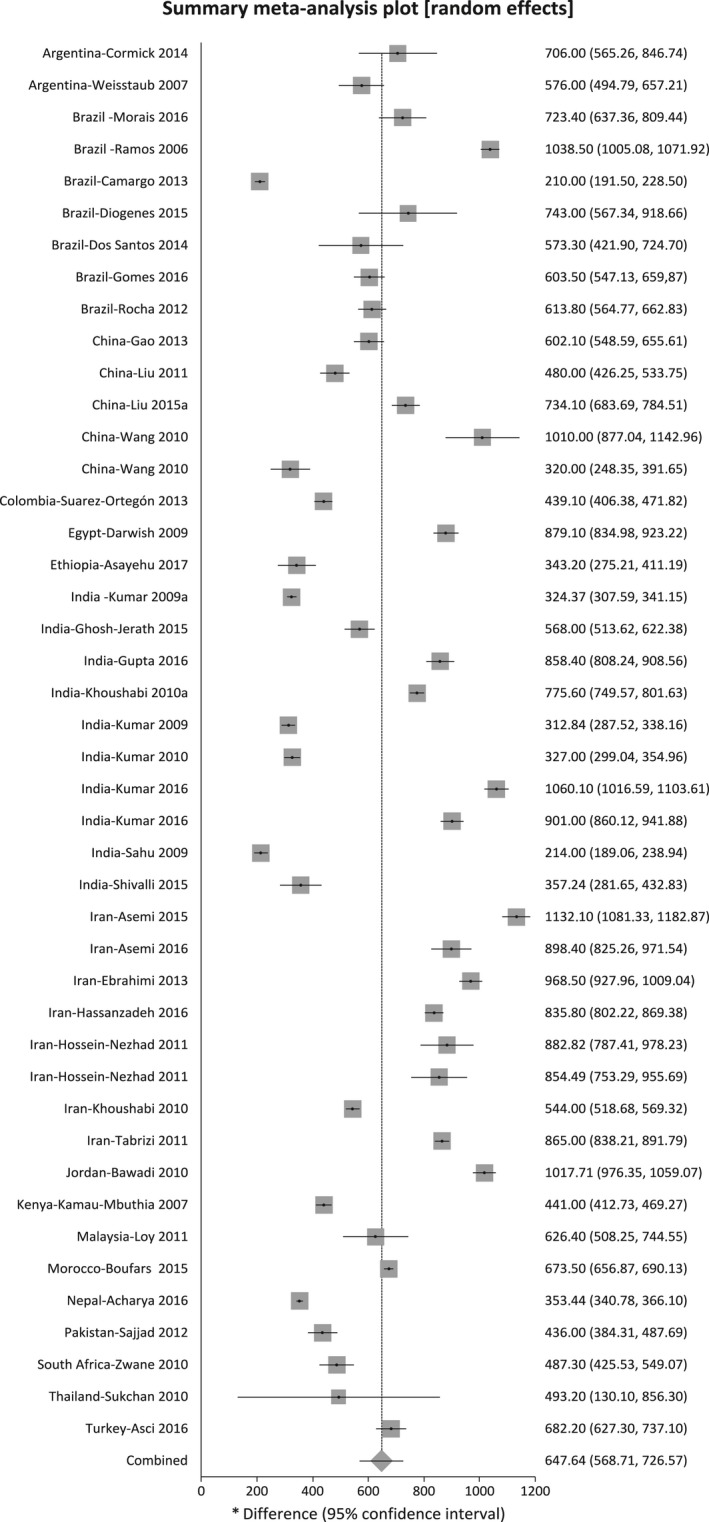
Mean calcium intake and approximate 95% CIs in studies from low‐ and middle‐income countries.
Figure S4 shows the mean and 95% CI calcium intake for each trimester in LMICs and HICs separately. The first trimester showed a mean calcium intake of 656.9 mg/day (95% CI 578.9–735.0 mg/day) in LMICs (eight countries) and 923.0 mg/day (95% CI 734.4–1111.7 mg/day) in HICs (11 countries); the second trimester showed a mean calcium intake of 565.9 mg/day (95% CI 358.9–772.9 mg/day) in LMICs (four countries) and 928.9 mg/day (95% CI 871.4–986.5 mg/day) in HICs (13 countries); and the third trimester showed a mean calcium intake of 826.6 mg/day (95% CI 739.8–913.4 mg/day) in LMICs (15 countries) and 931.5 mg/day (95% CI 756.2–1106.8 mg/day) in HICs (12 countries).
Tables S1 and S2 show daily calcium intakes of each included study for HICs and LMICs, respectively. Calcium intake in HICs ranged from 283 to 2228 mg/day, whereas in LMICs the intake ranged from 210 to 1631 mg/day. Within the HICs there were two studies with extreme values compared with the other studies in that region. Mannion et al. interviewed 264 women who agreed to participate out of 1000 who were attending a prenatal course in Alberta, Canada.96 They calculated calcium intake using an FFQ via telephone and included the use of calcium supplements and antacids with calcium. They acknowledge the difficulty of the methodology used to estimate antacid intake and state that a 500‐mg/day dose was used when information was missing. Karras et al. report very low calcium intake in northern Greece; however, the data are published in abstract format and do not provide details of the sample nor methodology used.78
Considering an estimated average requirement of 800 mg/day, which is the suggested value to estimate the prevalence of nutrient inadequacy at a population level, five (27.8%) countries from HICs report calcium intakes below this value at least in one subgroup, whereas from LMICs 15 (88.2%) countries reported calcium intakes below this value (Figure S5).29,130
Table S4 shows the 39 subgroups of 30 studies (12 HICs and 18 LMICs) that reported percentages of inadequate intakes and the cut‐off point used. The proportion of the population below the estimated average intake ranged from above 90% in Israel, Ethiopia, Kenya, and Indonesia, to below 10% in Greece and Canada.
Discussion
Main findings
This systematic review shows that about 27.8% (five out of 18) of the countries from HICs have a calcium intake below the 800‐mg/day recommended for this during pregnancy, whereas in LMICs the vast majority are below these values (88.2% of included countries from LMICs). The differences in mean calcium intake between LMICs and HICs is around 300 mg/day, and this difference remains throughout all trimesters of pregnancy, although they lessen in the third trimester.
Our results corroborate the results of the previous review, which included studies from 1991 to 2004, where calcium intake was below 600 mg/day in LMICs and above 1000 mg/day in HICs.13 The data gathered in our review is consistent with a lack of improvement in the dietary intake of calcium in pregnant women, and confirms the gap between higher and lower income countries.
Strengths and limitations
The strengths of this review include the broad search strategy to capture the largest number of publications with data from July 2004 and the great number of articles included (a two‐fold increase in the number of articles included compared with the previous review), showing the growing scientific and epidemiological interest in this issue. We tried to reduce bias by screening and extracting the information in duplicate using a data‐extraction form specifically designed for this review.
Our review has several limitations. None of the studies were nationally representative and thus the dietary intake estimates reported cannot be extrapolated to the entire country. About two‐thirds of the studies were not representative of their included population, even if not nationally representative, or their assessment about the representativeness was uncertain. In addition, the variability in the methodology used to collect the data on micronutrient intake limits the comparability. We found very few studies reporting inadequate intakes, with also diverse methodologies and cut‐off points. Data were available only from a limited number of countries (18 HICs and 19 LMICs), and information and estimates from the most deprived populations and from many LICs is lacking.
Interpretation
Taking in consideration that LMICs have a mean intake of around 600 mg, an increase in the population mean intake of around 400–500 mg/day of calcium intake may help to attain values close to current calcium intake recommendations for pregnancy. We observed considerable variability, however, and thus setting‐specific in‐depth analysis and action is warranted.
The attainment of calcium recommendations before and during pregnancy would involve a substantial reduction in the incidence of pregnancy hypertension, as pre‐eclampsia is one of the three major causes of maternal mortality globally.1,132
Strategies to increase calcium intake at the population level in populations with low calcium intake would be of relevance. This is in line with the observation that originated the hypothesis about the relationship between calcium intake and pregnancy hypertension, such as the association of population calcium intake and prevalence of pre‐eclampsia/eclampsia.131
There are three broad approaches to improve dietary intake: one is behavioral interventions that, although ideal, rely on personal habits and ability; the second is supplementation that targets individuals; and the third is fortification, which aims to improve the dietary intake of the whole population.132
Recommendations to improve dietary calcium by increasing the consumption of calcium‐rich foods and taking calcium supplements have been around for many years; however, data from this systematic review show a current low intake and almost no change from the previous similar review in LMICs.6 Supplementation strategies are difficult to achieve in LMICs as women in these countries do not always attend antenatal care early in pregnancy.133 Additionally, depending on the dose, complete adherence to supplementation is difficult to achieve, even if women come for Antenatal care, because of the texture of the calcium tablets or some minor side effects. Logistic and cost are also barriers in some low‐resource settings.
There are several successful food fortification strategies that have been used in both HICs and LMICs, such as fortification with iron, iodine, vitamin A, vitamin B complex, folic acid, zinc, vitamin D, and vitamin B12. These have contributed to improvements in health and the lowering of the incidence of goiter, beriberi, pellagra, anaemia, and neural tube defects.134–136 In order to carefully develop this strategy and decide the vehicle to fortify, and the amount of calcium required, however, more detailed information is needed regarding the dietary intakes of calcium that are representative of populations from LMICs so as not to cause harm through excessive intake in any population group. Any food fortification strategy should be preceded with intake simulations to ensure that none of the population groups exceeds the upper limit of intake recommendations. This review shows that a strategy to increase calcium intake by around 400–500 mg/day in populations of low calcium intake would take these populations closer to the recommendations, decreasing the gap between HICs.
Conclusion
This study confirms the gap between HICs and LMICs with alarming low calcium intakes in pregnant women in LMICs. From the public health perspective, in the absence of specific local data, calcium supplementation of pregnant women in these countries should be universal.
Disclosure of interests
None declared. Completed disclosure of interests form available to view online as supporting information..
Contribution to authorship
APB and AMG conceived the idea. GC, APB, IR, and CFL carried out data extraction. GC, APB, AC and JB carried out the analysis. All authors carried out the interpretation of the data, revised the article critically for intellectual content, and approved the final draft for publication.
Details of ethics approval
Not required for this review.
Funding
Funding for this review was obtained from the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), and World Health Organization.
Supporting information
Figure S1. Risk of bias graph: author's judgment about each risk of bias item.
Figure S2. Risk of bias summary.
Figure S3. Means and 95% CIs for regions according to United Nations classification.
Figure S4. Means and 95% CIs for trimester in low‐ and middle‐income countries and in high‐income countries.
Figure S5. Means and 95% CIs for 35 included countries.
Table S1. Reported daily calcium intakes from HICs.
Table S2. Reported daily calcium intakes from LMICs.
Table S3. Summary of risk of bias.
Table S4. Studies reporting percentage of the population with inadequate intakes as percentages below the estimated average intake (EAR) order.
Appendix S1. Search strategy.
Appendix S2. Risk of bias and quality assessment prompts for the included studies.
Acknowledgements
We would like to acknowledge Elizabeth Hauser for her help with the initial screening. We thank Ines Banjari, Taniya S. Nagpal, Dorothy Brooten, Anne‐Sophie Morisset, Janet C. King, Carmen Marino Donangelo, and Shashi Kant for providing the requested information for their articles.
Cormick G, Betrán AP, Romero IB, Lombardo CF, Gülmezoglu AM, Ciapponi A, Belizán JM. Global inequities in dietary calcium intake during pregnancy: a systematic review and meta‐analysis. BJOG 2019; 126:444–456.
Systematic review registration: Prospero Centre for Reviews and Dissemination, University of York: PROSPERO 2018 CRD42018087485.
Linked article This article is commented on by GJ Hofmeyr, p. 457 in this issue. To view this mini commentary visit https://doi.org/10.1111/1471-0528.15543.
References
- 1. von Dadelszen P, Magee LA. Preventing deaths due to the hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2016;36:83–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kassebaum NJ, Bertozzi‐Villa A, Coggeshall MS, Shackelford KA, Steiner C, Heuton KR, et al. Global, regional, and national levels and causes of maternal mortality during 1990‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 2014;384:980–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hofmeyr GJ, Lawrie TA, Atallah AN, Duley L, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev 2014;(6):CD001059. [DOI] [PubMed] [Google Scholar]
- 4. Heaney RP. Calcium intake and disease prevention. Vol. 50, Arquivos Brasileiros de Endocrinologia & Metabologia. scielo; 2006. p. 685–93. [DOI] [PubMed]
- 5. Ross AC, Taylor CL, Yaktine AL. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: US National Academies Press; 2010. [PubMed] [Google Scholar]
- 6. WHO . Calcium Supplementation in Pregnant Women: Guideline. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 7. FAO, WHO . Human Vitamin and Mineral Requirements: Report of a Joint FAO‐WHO Expert Consultation. Bangkok: Food and Agricultural Organization; World Health Organization; 2001. [Google Scholar]
- 8. Dietary reference values for food energy and nutrients for the United Kingdom . Report of the panel on dietary reference values of the committee on medical aspects of food policy. Rep Health Soc Subj (Lond) 1991;41:1–210. [PubMed] [Google Scholar]
- 9. Communities C of the E . Nutrient and Energy Intakes for the European Community. Luxembourg; 1993. (Reports of the Scientific Committee for Food). [DOI] [PubMed]
- 10. Australian Government Publishing Service . Recommended Dietary Intakes for Use in Australia. Canberra: Australian Government Publishing Service; 1991. [Google Scholar]
- 11. Anon. New reference values for calcium. Ann Nutr Metab 2013;63:186–92. [DOI] [PubMed] [Google Scholar]
- 12. Canadian Government Publishing Services . Scientific Review Committee Authority of the Minister of National Health and Welfare. Nutrition Recommendations. Ottawa: Canadian Government Publishing Services; 1990. [Google Scholar]
- 13. Merialdi M, Mathai M, Ngoc NTN, Purwar M, Campodonico L, Abdel‐Aleem H, et al. World Health Organization systematic review of the literature and multinational nutritional survey of calcium intake during pregnancy. Fetal Matern Med Rev 2005;16:97. [Google Scholar]
- 14. Lee SE, Talegawkar SA, Merialdi M, Caulfield LE. Dietary intakes of women during pregnancy in low‐ and middle‐income countries. Public Health Nutr 2013;16:1340–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Institute of Medicine . Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: The National Academies Press; 1997. [PubMed] [Google Scholar]
- 16. WHO . WHO Recommendations for Prevention and Treatment of Pre‐eclampsia and Eclampsia. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 17. Hofmeyr GJ, Betrán AP, Singata‐Madliki M, Cormick G, Munjanja SP, Fawcus S, et al. Pre‐pregnancy and early pregnancy calcium supplementation among women at high risk of pre‐eclampsia: a multicentre, double‐blind randomised, placebo‐controlled trial. Lancet (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Repke JT, Villar J, Anderson C, Pareja G, Dubin N, Belizan JM. Biochemical changes associated with blood pressure reduction induced by calcium supplementation during pregnancy. Am J Obstet Gynecol 1989;160:684–90. [DOI] [PubMed] [Google Scholar]
- 19. Cormick G, Ciapponi A, Cafferata ML, Belizan JM. Calcium supplementation for prevention of primary hypertension. Cochrane Database Syst Rev 2015;(6):CD010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 22. Cormick G, Betrán AP, Lombardo CF, Romero IB, Perez S, Ciapponi A. Systematic review on calcium intake during pregnancy: PROSPERO 2018 CRD42018087485. 2018.
- 23. Wells G, Shea B, O'Connell D, Peterson j, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non‐Randomized Studies in Meta‐Analysis. 2000.
- 24. SIGN . Critical Appraisal Notes and Checklists. Edinburgh: Scottish Intercollegiate Guidelines Network; 2014. [Google Scholar]
- 25. Long Q, Kingdon C, Yang F, Renecle MD, Jahanfar S, Bohren MBA. Prevalence of and factors for preferences for caesarean section in China: a mixed‐methods systematic review. PLoS Med 2018;15:e1002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Vol. 2011, The Cochrane Collaboration. 2011.
- 27. StatsDirect Ltd . StatsDirect. 3.1.15. Cambridge: StatsDirect Ltd; 2017. [Google Scholar]
- 28. The Nordic Cochrane Centre . Review Manager RevMan (5th edn). Copenhagen: The Nordic Cochrane Centre; 2014. [Google Scholar]
- 29. WHO . WHO Meeting on Estimating Appropriate Levels of Vitamins and Minerals for Food Fortification Programmes. Geneva: World Health Organization; 2010. [Google Scholar]
- 30. Abreu S, Santos PC, Montenegro N, Mota J. Relationship between dairy product intake during pregnancy and neonatal and maternal outcomes among Portuguese women. Obes Res Clin Pract 2017;11:276–86. [DOI] [PubMed] [Google Scholar]
- 31. Abu‐Saad K, Shahar DR, Fraser D, Vardi H, Friger M, Bolotin A, et al. Adequacy of usual dietary intake and nutritional status among pregnant women in the context of nutrition transition: the DEPOSIT Study. Br J Nutr 2012;108:1874–83. [DOI] [PubMed] [Google Scholar]
- 32. Bae HS, Kim SY, Ahnv HS, Cho YK. Comparison of nutrient intake, life style variables, and pregnancy outcomes by the depression degree of pregnant women. Nutr Res Pract 2010;4:323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baer HJ, Blum RE, Rockett HRH, Leppert J, Gardner JD, Suitor CW, et al. Use of a food frequency questionnaire in American Indian and Caucasian pregnant women: a validation study. BMC Public Health 2005;5:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Banjari I, Kenjerić D, Mandić M. Nutritional intake and iron blood status in first trimester of pregnancy. Ann Nutr Metab 2011;58:174. [Google Scholar]
- 35. Bawadi HA, Al‐Kuran O, Al‐Bastoni LAA, Tayyem RF, Jaradat A, Tuuri G, et al. Gestational nutrition improves outcomes of vaginal deliveries in Jordan: an epidemiologic screening. Nutr Res 2010;30:110–7. [DOI] [PubMed] [Google Scholar]
- 36. Blumfield ML, Schreurs M, Rollo ME, MacDonald‐Wicks LK, Kokavec A, Collins CE. The association between portion size, nutrient intake and gestational weight gain: a secondary analysis in the WATCH study 2006/7. J Hum Nutr Diet 2016;29:271–80. [DOI] [PubMed] [Google Scholar]
- 37. Bobiński R, Mikulska M, Mojska H, Ulman‐Włodarz I. The dietary composition of women who delivered healthy full‐term infants, preterm infants, and full‐term infants who were small for gestational age. Biol Res Nurs 2015;17:495–502. [DOI] [PubMed] [Google Scholar]
- 38. Bojar I, Owoc A, Humeniuk E, Wierzba W, Fronczak A. Inappropriate consumption of vitamins and minerals by pregnant women in Poland. Ann Agric Environ Med 2012;19:263–6. [PubMed] [Google Scholar]
- 39. Boufars A, Belghiti H, Guerinech H, Bouaiti EA, Razine R, Mrabet M. Energy intake in a population of pregnant women. Eur J Epidemiol 2015;30:824. [Google Scholar]
- 40. Brooten D, Youngblut JM, Golembeski S, Magnus MH, Hannan J. Perceived weight gain, risk, and nutrition in pregnancy in five racial groups. J Am Acad Nurse Pract 2012;24:32–42. [DOI] [PubMed] [Google Scholar]
- 41. Camargo EB, Moraes LFS, Souza CM, Akutsu R, Barreto JM, da Silva EMK, et al. Survey of calcium supplementation to prevent preeclampsia: the gap between evidence and practice in Brazil. BMC Pregnancy Childbirth 2013;13:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Acharya O, Zotor FB, Chaudhary P, Deepak K, Amuna P, Ellahi B. Maternal nutritional status, food intake and pregnancy weight gain in Nepal. J Health Manag 2016;18:1–12. [Google Scholar]
- 43. Chan GM, McElligott K, McNaught T, Gill G. Effects of dietary calcium intervention on adolescent mothers and newborns: a randomized controlled trial. Obstet Gynecol 2006;108:565–71. [DOI] [PubMed] [Google Scholar]
- 44. Cheng Y, Dibley MJ, Zhang X, Zeng L, Yan H. Assessment of dietary intake among pregnant women in a rural area of western China. BMC Public Health 2009;9:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cormick G, Zhang NN, Andrade SP, Quiroga MJ, Di Marco I, Porta A, et al. Gaps between calcium recommendations to prevent pre‐eclampsia and current intakes in one hospital in Argentina. BMC Res Notes 2014;7:920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Crume TL, Brinton JT, Shapiro A, Kaar J, Glueck DH, Siega‐Riz AM, et al. Maternal dietary intake during pregnancy and offspring body composition: The Healthy Start Study. Am J Obstet Gynecol 2016;215:609.e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Darwish AM, Mohamad SN, Gamal Al‐Din HR, Elsayed YA, Ahmad SI. Prevalence and predictors of deficient dietary calcium intake during the third trimester of pregnancy: the experience of a developing country. J Obstet Gynaecol Res 2009;35:106–12. [DOI] [PubMed] [Google Scholar]
- 48. De Jersey SJ, Ross LJ, Himstedt K, McIntyre HD, Callaway LK. Weight gain and nutritional intake in obese pregnant women: some clues for intervention. Nutr Diet 2011;68:53–9. [Google Scholar]
- 49. Diogenes MEL, Bezerra FF, Rezende EP, Donangelo CM. Calcium plus vitamin d supplementation during the third trimester of pregnancy in adolescents accustomed to low calcium diets does not affect infant bone mass at early lactation in a randomized controlled trial. J Nutr 2015;145:1515–23. [DOI] [PubMed] [Google Scholar]
- 50. Dos SQ, Sichieri R, Marchioni DML, Verly Junior E. Brazilian pregnant and lactating women do not change their food intake to meet nutritional goals. BMC Pregnancy Childbirth 2014;14:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dubois L, Diasparra M, Bedard B, Colapinto CK, Fontaine‐Bisson B, Morisset AS, et al. Adequacy of nutritional intake from food and supplements in a cohort of pregnant women in Québec, Canada: the 3D Cohort Study (Design, Develop, Discover). Am J Clin Nutr 2017;106:541–8. [DOI] [PubMed] [Google Scholar]
- 52. Ebrahimi F, Shariff ZM, Rezaeian M, Tabatabaei SZ, Mun CY, Tajik E. Socioeconomic status and intake of energy and sodium are associated with calcium intake among pregnant women in Rafsanjan city. Iran. J Obstet Gynaecol Res 2013;39:146–53. [DOI] [PubMed] [Google Scholar]
- 53. Alevizos AG, Stamatiou KN, Lacroix RE, Natzar MA, Mihas CC, Bovis KD, et al. Dietary intake in immigrant Arabian pregnant women. Saudi Med J 2006;27:1019–21. [PubMed] [Google Scholar]
- 54. Elmacioglu F, Surucu B, Alper T, Ozenoglu A, Ugurlu S. Is adequate and balanced nutrition during pregnancy more effective than iron and folic acid supplements? Cent Eur J Med 2010;5:235–42. [Google Scholar]
- 55. Essley B, McIntyre A, Cooper B, McNanley T, Kent T, Witter F, et al. PTH increases and serum NTX is associated with maternal bone loss in pregnant adolescents. J Bone Miner Res 2010;25:S139. [Google Scholar]
- 56. Ford FA, Mouratidou T, Wademan SE, Fraser RB. Effect of the introduction of Healthy Start on dietary behaviour during and after pregnancy: early results from the before and after Sheffield study. Br J Nutr 2009;101:1828–36. [DOI] [PubMed] [Google Scholar]
- 57. Fowles ER, Murphey C. Nutrition and mental health in early pregnancy: a pilot study. J Midwifery Womens Health 2009;54:73–7. [DOI] [PubMed] [Google Scholar]
- 58. Fowles ER, Murphey C, Ruiz RJ. Exploring relationships among psychosocial status, dietary quality, and measures of placental development during the first trimester in low‐income women. Biol Res Nurs 2011;13:70–9. [DOI] [PubMed] [Google Scholar]
- 59. Gao H, Stiller CK, Scherbaum V, Biesalski HK, Wang Q, Hormann E, et al. Dietary intake and food habits of pregnant women residing in urban and rural areas of Deyang city, Sichuan Province, China. Nutrients 2013;5:2933–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ghosh‐Jerath S, Devasenapathy N, Singh A, Shankar A, Zodpey S. Ante natal care (ANC) utilization, dietary practices and nutritional outcomes in pregnant and recently delivered women in urban slums of Delhi, India: an exploratory cross‐sectional study. Reprod Health 2015;12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Goletzke J, Buyken AE, Louie JCY, Moses RG, Brand‐Miller JC. Dietary micronutrient intake during pregnancy is a function of carbohydrate quality1,2. Am J Clin Nutr 2015;102:626–32. [DOI] [PubMed] [Google Scholar]
- 62. Gomes CB, Malta MB, Corrente JE, Benicio MH, Carvalhaes MA. High prevalence of inadequate calcium and vitamin D dietary intake in two cohorts of pregnant women. Cad Saude Publica 2016;32:e00127815. [DOI] [PubMed] [Google Scholar]
- 63. Groth SW, Stewart PA, Ossip DJ, Block RC, Wixom N, Fernandez ID. Micronutrient intake is inadequate for a sample of pregnant African‐American women. J Acad Nutr Diet 2017;117:589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Almurshed KS, Bani IA, Al‐Kanhal MA, Al‐Amri MA. A study of maternal dietary intake during pregnancy in Riyadh, Saudi Arabia. J Family Community Med 2007;14:9–13. [PMC free article] [PubMed] [Google Scholar]
- 65. Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. Am J Clin Nutr 2010;91:373–80. [DOI] [PubMed] [Google Scholar]
- 66. Gupta A, Kant S, Pandav CS, Gupta SK, Rai SK, Misra P. Dietary calcium intake, serum calcium level, and their association with preeclampsia in rural North India. Indian J Community Med 2016;41:223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hacker A, King J, Van Loan M, Gertz E, Adams M, Sawyer A, et al. Calcium supplementation during pregnancy improves tibial bone density at one year post‐partum in racially diverse women. FASEB J 2014;28 (Suppl 1). [Google Scholar]
- 68. Hassanzadeh A, Paknahad Z, Khoigani MG. The relationship between macro‐ and micro‐nutrients intake and risk of preterm premature rupture of membranes in pregnant women of Isfahan. Adv Biomed Res 2016;5:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hatzopoulou K, Filis V, Grammatikopoulou MG, Kotzamanidis C, Tsigga M. Greek pregnant women demonstrate inadequate micronutrient intake despite supplement use. J Diet Suppl 2014;11:155–65. [DOI] [PubMed] [Google Scholar]
- 70. Haugen M, Brantsæter AL, Alexander J, Meltzer HM. Dietary supplements contribute substantially to the total nutrient intake in pregnant Norwegian women. Ann Nutr Metab 2008;52:272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Heppe DHM, Medina‐Gomez C, Hofman A, Franco OH, Rivadeneira F, Jaddoe VWV. Maternal first‐trimester diet and childhood bone mass: the Generation R Study. Am J Clin Nutr 2013;98:224–32. [DOI] [PubMed] [Google Scholar]
- 72. Hossein‐Nezhad A, Mirzaei K, Maghbooli Z, Najmafshar A, Larijani B. The influence of folic acid supplementation on maternal and fetal bone turnover. J Bone Miner Metab 2011;29:186–92. [DOI] [PubMed] [Google Scholar]
- 73. Hsu W‐Y, Wu C‐H, Hsieh CT‐C, Lo H‐C, Lin J‐S, Kao M‐D. Low body weight gain, low white blood cell count and high serum ferritin as markers of poor nutrition and increased risk for preterm delivery. Asia Pac J Clin Nutr 2013;22:90–9. [DOI] [PubMed] [Google Scholar]
- 74. Jia HX, Han JH, Li HZ, Liang D, Deng TT, Chang SY. Mineral intake in urban pregnant women from base diet, fortified foods, and food supplements: focus on calcium, iron, and zinc. Biomed Environ Sci 2016;29:898–901. [DOI] [PubMed] [Google Scholar]
- 75. Asayehu TT, Lachat C, De Henauw S, Gebreyesus SH. Dietary behaviour, food and nutrient intake of women do not change during pregnancy in Southern Ethiopia. Matern Child Nutr 2017;13:e12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jung YM, Choi MJ. Nutrient intake according to weight gain during pregnancy, job status, and household income. Clin Nutr Res 2017;6:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kamau‐Mbuthia E, Elmadfa I. Diet quality of pregnant women attending an antenatal clinic in Nakuru, Kenya. Ann Nutr Metab 2007;51:324–30. [DOI] [PubMed] [Google Scholar]
- 78. Karras SN, Shah I, Petroczi A, Goulis DG, Bili H, Papadopoulou F, et al. An observational study reveals that neonatal vitamin D is primarily determined by maternal contributions: implications of a new assay on the roles of vitamin D forms. Nutr J 2013;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Karras S, Bili H, Goulis D, Papadopoulou F, Harizopoulou V, Kintiraki E, et al. High prevalence of vitamin D deficiency among pregnant women at term and their neonates in Thessaloniki, Northern Greece.Bone 2012;50:S104. [Google Scholar]
- 80. Khoushabi F, Saraswathi G. Association between maternal nutrition status and birth weight of neonates in selected hospitals in Mysore city, India. Pakistan J Nutr 2010;9:1124–30. [Google Scholar]
- 81. Khoushabi F, Saraswathi G. Impact of nutritional status on birth weight of neonates in Zahedan City, Iran. Nutr Res Pract 2010;4:339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kieffer EC, Welmerink DB, Sinco BR, Welch KB, Schumann CY, Uhley V. Periconception diet does not vary by duration of us residence for Mexican immigrant women. J Acad Nutr Diet 2013;113:652–8. [DOI] [PubMed] [Google Scholar]
- 83. Koenig MD, McFarlin BL, Steffen AD, Tussing‐Humphreys L, Giurgescu C, Engeland CG, et al. Decreased nutrient intake is associated with premature cervical remodeling. J Obstet Gynecol Neonatal Nurs 2017;46:123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kolarzyk E, Pietrzycka A, Stȩpniewski M, Łyszczarz J, Mendyk A, Ostachowska‐Ga̧sior A. Micronutrients and macronutrients and parameters of antioxidative ability in saliva of women: inhabitants of Krakow (Poland) in the course of uncomplicated singleton pregnancy. Biol Trace Elem Res 2006;114:73–84. [DOI] [PubMed] [Google Scholar]
- 85. Kumar A, Agarwal K, Devi SG, Gupta RK, Batra S. Hypocalcemia in pregnant women. Biol Trace Elem Res 2010;136:26–32. [DOI] [PubMed] [Google Scholar]
- 86. Aşcı Ö, Rathfisch G. Effect of lifestyle interventions of pregnant women on their dietary habits, lifestyle behaviors, and weight gain: a randomized controlled trial. J Heal Popul Nutr 2016;35:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kumar A, Devi SG, Batra S, Singh C, Shukla DK. Calcium supplementation for the prevention of pre‐eclampsia. Int J Gynecol Obstet 2009;104:32–6. [DOI] [PubMed] [Google Scholar]
- 88. Kumar A, Meena M, Gyaneshwori Devi S, Gupta RK, Batra S. Calcium in midpregnancy. Arch Gynecol Obstet 2009;279:315–9. [DOI] [PubMed] [Google Scholar]
- 89. Kumar KJ, Chavan A, Shushma K, Murthy S. Comparison of vitamin D status between urban and rural south Indian mothers and their newborns. J Nepal Paediatr Soc 2016;36:243–9. [Google Scholar]
- 90. Lindsay K, Heneghan C, McNulty B, Brennan L, McAuliffe F. Lifestyle and dietary habits of an obese pregnant cohort. Matern Child Heal J 2015;19:25–32. [DOI] [PubMed] [Google Scholar]
- 91. Lindsay KL, Gibney ER, McNulty BA, McAuliffe FM. Pregnant immigrant nigerian women: an exploration of dietary intakes. Public Health 2014;128:647–53. [DOI] [PubMed] [Google Scholar]
- 92. Liu FL, Zhang YM, Pares GV, Reidy KC, Zhao WZ, Zhao A, et al. Nutrient intakes of pregnant women and their associated factors in eight cities of china: a cross‐sectional study. Chin Med J (Engl) 2015;128:1778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Liu J, Sánchez B, Ettinger A, Cantoral A, Jones A, Tellez‐Rojo M, et al. Impact of maternal prenatal mineral intake on pubertal onset in Mexican children. FASEB J 2015;29: (Suppl 1). [Google Scholar]
- 94. Liu Z, Qiu L, Chen YM, Su YX. Effect of milk and calcium supplementation on bone density and bone turnover in pregnant Chinese women: a randomized controlled trail. Arch Gynecol Obstet 2011;283:205–11. [DOI] [PubMed] [Google Scholar]
- 95. Loy SL, Marhazlina M, Nor Azwany Y, Hamid Jan JM. Development, validity and reproducibility of a food frequency questionnaire in pregnancy for the Universiti Sains Malaysia birth cohort study. Malays J Nutr 2011;17:1–18. [PubMed] [Google Scholar]
- 96. Mannion CA, Lindop RJ. Vitamin/mineral supplements and calcium‐based antacids increase maternal calcium intake. J Am Coll Nutr 2009;28:362–8. [DOI] [PubMed] [Google Scholar]
- 97. Asemi Z, Esmaillzadeh A. The effect of multi mineral‐vitamin d supplementation on pregnancy outcomes in pregnant women at risk for pre‐eclampsia. Int J Prev Med 2015;6:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Manzano VC, Garcia‐Algar O, Mur SA, Ferrer CR, Carrascosa LA, Yeste FD, et al. Plasma 25‐OH vitamin D concentrations in cord blood after summer months, Spain. Rev Esp Salud Publica 2017;91:pii: e201701009. [PubMed] [Google Scholar]
- 99. McGowan C, Byrne J, Walsh J, McAuliffe F. Maternal dietary patterns during pregnancy and associated nutrient intakes. Ann Nutr Metab 2011;58:177. [Google Scholar]
- 100. McGurk P, Hill AJ, McCance DR. An investigation of dietary intake of pregnant women in the third trimester in Northern Ireland. J Hum Nutr Diet 2011;24:293–4. [Google Scholar]
- 101. Miyake Y, Tanaka K, Okubo H, Sasaki S, Arakawa M. Dairy food, calcium and vitamin D intake and prevalence of allergic disorders in pregnant Japanese women. Int J Tuberc Lung Dis 2012;16:255–61. [DOI] [PubMed] [Google Scholar]
- 102. Morais CA, De Sousa FLP, Bandoni DH, De Souza EA, Momentti AC, De Assis NR, et al. Calcium supplementation as part of the adequate calcium intake in prenatal care for the prevention of preeclampsia. Pregnancy Hypertens 2016;6:200. [Google Scholar]
- 103. Moran LJ, Sui Z, Cramp CS, Dodd JM. A decrease in diet quality occurs during pregnancy in overweight and obese women which is maintained post‐partum. Int J Obes 2013;37:704–11. [DOI] [PubMed] [Google Scholar]
- 104. Morisset A‐S, Weiler HA, Dubois L, Ashley‐Martin J, Shapiro GD, Dodds L, et al. Rankings of iron, vitamin D, and calcium intakes in relation to maternal characteristics of pregnant Canadian women. Appl Physiol Nutr Metab 2016;41:749–57. [DOI] [PubMed] [Google Scholar]
- 105. Mullaney L, Cawley S, Kennedy R, O'Higgins AC, McCartney D, Turner MJ. Maternal nutrient intakes from food and drinks consumed in early pregnancy in Ireland. J Public Health (Oxf) 2017;39:754–62. [DOI] [PubMed] [Google Scholar]
- 106. Naqvi N, Fatima J, Bano F, Haque QS, Jawad K. Effect of minerals on pregnant women suffering from pre‐eclampsia and eclampsia. Biomed Res 2010;21:419–22. [Google Scholar]
- 107. Nieto A, Herrera JA, Villar J, Matorras R, López de la Manzanara C, Arribas I, et al. Association between calcium intake, parathormone levels and blood pressure during pregnancy. Colomb Med 2009;40:185–93. [Google Scholar]
- 108. Ashman AM, Collins CE, Brown LJ, Rae KM, Rollo ME. A brief tool to assess image‐based dietary records and guide nutrition counselling among pregnant women: an evaluation. JMIR mHealth uHealth 2016;4:e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. O'Brien EC, Kilbane MT, McKenna MJ, Segurado R, Geraghty AA, McAuliffe FM. Calcium intake in winter pregnancy attenuates impact of vitamin D inadequacy on urine NTX, a marker of bone resorption. Eur J Nutr 2018;57:1015–23. [DOI] [PubMed] [Google Scholar]
- 110. Olausson H, Ann Laskey M, Goldberg GR, Prentice A. Changes in bone mineral status and bone size during pregnancy and the influences of body weight and calcium intake. Obstet Gynecol Surv 2009;64:86–7. [DOI] [PubMed] [Google Scholar]
- 111. Ortigosa Gomez S, Garcia‐Algar O, Mur Sierra A, Ferrer Costa R, Carrascosa Lezcano A, Yeste FD. Sociodemographic factors related to plasma concentrations of 25‐OH vitamin D and PTH in cord blood. Rev Esp Salud Publica 2015;89:75–83. [DOI] [PubMed] [Google Scholar]
- 112. Osorio‐Yañez C, Gelaye B, Miller RS, Enquobahrie DA, Baccarelli AA, Qiu C, et al. Associations of maternal urinary cadmium with trimester‐specific blood pressure in pregnancy: role of dietary intake of micronutrients. Biol Trace Elem Res 2016;174:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Perreault M, Xu VYY, Hamilton S, Wright D, Foster W, Atkinson SA. Validation of a food frequency questionnaire for bone nutrients in pregnant women. Can J Diet Pract Res 2016;77:133–9. [DOI] [PubMed] [Google Scholar]
- 114. Pinto E, Barros H, Santos Silva ID. Dietary intake and nutritional adequacy prior to conception and during pregnancy: a follow‐up study in the north of Portugal. Public Health Nutr 2009;12:922–31. [DOI] [PubMed] [Google Scholar]
- 115. Ramos JGL, Brietzke E, Martins‐Costa SH, Vettorazzi‐Stuczynski J, Barros E, Carvalho C. Reported calcium intake is reduced in women with preeclampsia. Hypertens Pregnancy 2006;25:229–39. [DOI] [PubMed] [Google Scholar]
- 116. Rocha VS, Lavanda I, Nakano EY, Ruano R, Zugaib M, Colli C. Calcium and magnesium status is not impaired in pregnant women. Nutr Res 2012;32:542–6. [DOI] [PubMed] [Google Scholar]
- 117. Sahu M, Bhatia V, Aggarwal A, Rawat V, Saxena P, Pandey A, et al. Vitamin D deficiency in rural girls and pregnant women despite abundant sunshine in northern India. Clin Endocrinol (Oxf) 2009;70:680–4. [DOI] [PubMed] [Google Scholar]
- 118. Sajjad R, Khan A. Nutrient intakes of pregnant women in comparison to the reference intake. Pakistan J Nutr 2012;11:166–71. [Google Scholar]
- 119. Aubuchon‐Endsley NL, Kennedy TS, Gilchrist M, Thomas DG, Grant S. Relationships among socioeconomic status, dietary intake, and stress in breastfeeding women. J Acad Nutr Diet 2015;115:939–946.e1. [DOI] [PubMed] [Google Scholar]
- 120. Sato APS, Fujimori E, Szarfarc SC, Borges ALV, Tsunechiro MA. Food consumption and iron intake of pregnant and reproductive aged women. Rev Latino‐Americana Enferm 2010;18:247–54. [DOI] [PubMed] [Google Scholar]
- 121. Shipala EK, Wafula SW, Ettyang GA, Were EO. Nutrient intake among pregnant teenage girls attending ante‐natal clinics in two health facilities in Bungoma south district, Western Kenya. East Afr Med J 2012;89:94–9. [PubMed] [Google Scholar]
- 122. Shivalli S, Srivastava RK, Singh GP. Trials of improved practices (TIPs) to enhance the dietary and iron‐folate intake during pregnancy‐ A quasi experimental study among rural pregnant women of Varanasi, India. PLoS ONE 2015;10:e0137735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Snook Parrott M, Bodnar LM, Simhan HN, Harger G, Markovic N, Roberts JM. Maternal cereal consumption and adequacy of micronutrient intake in the periconceptional period. Public Health Nutr 2009;12:1276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Suarez‐Ortegón MF, Mosquera M, Caicedo DM, De Plata CA, Méndez F. Nutrients intake as determinants of blood lead and cadmium levels in Colombian pregnant women. Am J Hum Biol 2013;25:344–50. [DOI] [PubMed] [Google Scholar]
- 125. Sukchan P, Liabsuetrakul T, Chongsuvivatwong V, Songwathana P, Sornsrivichai V, Kuning M. Inadequacy of nutrients intake among pregnant women in the deep south of Thailand. BMC Public Health 2010;10:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Sun JJ, Tur SJ, Lu YQ, Wang XJ, Ma MT. Investigation on dietary structure in 318 Uigur pregnant women in Urumchi, Yining and Atushi. Chinese J Clin Rehabil 2006;10:22–4. [Google Scholar]
- 127. Tabrizi FM, Saraswathi G. Maternal nutrient intake and maternal serum micronutrients and their relation to birth weight‐A longitudinal study. Int J Collab Res Intern Med Public Heal 2011;3:617–32. [Google Scholar]
- 128. Tande DL, Ralph JL, Johnson LK, Scheett AJ, Hoverson BS, Anderson CM. First trimester dietary intake, biochemical measures, and subsequent gestational hypertension among nulliparous women. J Midwifery Women's Heal 2013;58:423–30. [DOI] [PubMed] [Google Scholar]
- 129. Vanderspank D, Bernier SM, Sopper MM, Watson P, Mottola MF. Activity restriction increases deoxypyridinoline excretion in hospitalized high‐risk pregnant women. Biol Res Nurs 2014;16:7–15. [DOI] [PubMed] [Google Scholar]
- 130. Carriquiry AL. Assessing the prevalence of nutrient inadequacy. Public Health Nutr 1999;2:23–33. [DOI] [PubMed] [Google Scholar]
- 131. Belizan JM, Villar J. The relationship between calcium intake and edema‐, proteinuria‐, and hypertension‐getosis: an hypothesis. Am J Clin Nutr 1980;33:2202–10. [DOI] [PubMed] [Google Scholar]
- 132. Organization WH . e‐Library of Evidence for Nutrition Actions (eLENA). Department of Nutrition for Health and Development (NHD).
- 133. Hofmeyr GJ, Duley L, Atallah A. Dietary calcium supplementation for prevention of pre‐eclampsia and related problems: a systematic review and commentary. BJOG 2007;114:933–43. [DOI] [PubMed] [Google Scholar]
- 134. Bishai D, Nalubola R. The history of food fortification in the United States: its relevance for current fortification efforts in developing countries. Econ Dev Cult Change 2002;51:37–53. [Google Scholar]
- 135. Dwyer JT, Wiemer KL, Dary O, Keen CL, King JC, Miller KB, et al. Fortification and health: challenges and opportunities. Adv Nutr 2015;6:124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Tulchinsky TH. The key role of government in addressing the pandemic of micronutrient deficiency conditions in Southeast Asia. Nutrients 2015;7:2518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Risk of bias graph: author's judgment about each risk of bias item.
Figure S2. Risk of bias summary.
Figure S3. Means and 95% CIs for regions according to United Nations classification.
Figure S4. Means and 95% CIs for trimester in low‐ and middle‐income countries and in high‐income countries.
Figure S5. Means and 95% CIs for 35 included countries.
Table S1. Reported daily calcium intakes from HICs.
Table S2. Reported daily calcium intakes from LMICs.
Table S3. Summary of risk of bias.
Table S4. Studies reporting percentage of the population with inadequate intakes as percentages below the estimated average intake (EAR) order.
Appendix S1. Search strategy.
Appendix S2. Risk of bias and quality assessment prompts for the included studies.


