Piezo1 is a mechanosensitive ion channel that is believed to be expressed in red blood cells (RBCs), mainly supported by the findings that mutations of the PIEZO1 gene are associated with the RBC disease hereditary xerocytosis.1,2 So far, several mutations (e.g. R2456H, T2127M and E2496ELE) have been reported to exhibit a partial gain-of-function phenotype with generation of mechanically activated currents that inactivate more slowly than wild type.1–5 However, characterization of the mutated ion channel has almost exclusively been performed based on heterologous expression in cell lines, and recordings in RBCs were only occasional.6 Here, we present a patient with a novel PIEZO1 mutation (R2110W) and a patch clamp based high-throughput screening assay for Piezo1 activity. It is the first electrophysiological single-cell based screening ever performed on RBCs, demonstrating the Piezo1 gain-of-function mutation directly on RBCs. Thus, we provide a putative routine approach for detecting functional (Piezo1) channel mutations as the molecular cause of rare anaemia that can offer a standard method in specialized hematologic centers.
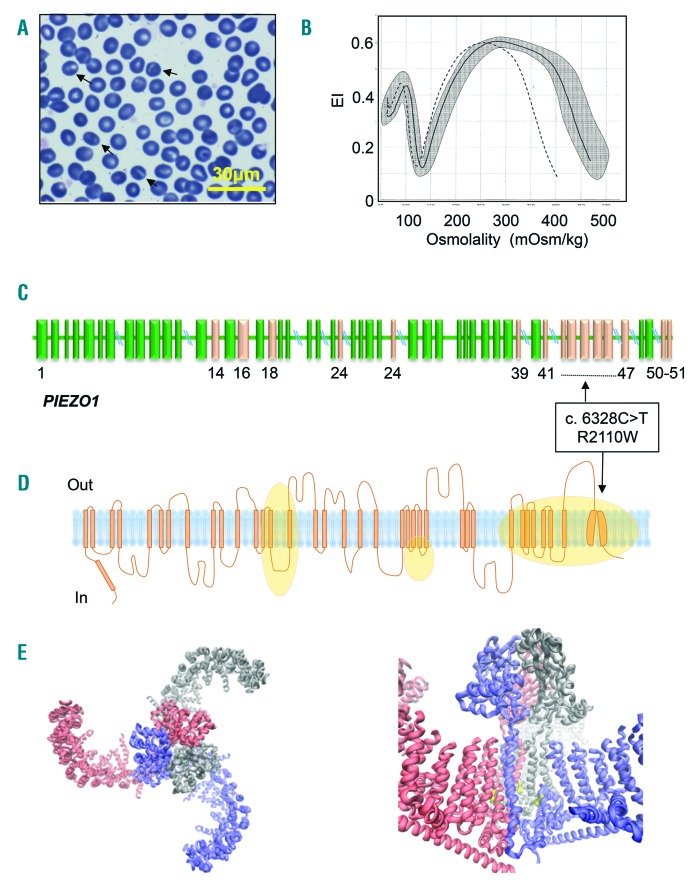
The patient, an Italian man, born from unrelated parents, with no evidence of jaundice, anemia, splenomegaly, or gallstones, was studied at the age of 43 years for compensated hemolysis and evidence suggestive of iron overload. Patient’s data are summarized in Online Supplementary Table S1. Due to abnormal RBC morphology, characterized by presence of stomatocytes (Figure 1A) and the observation of a marked leftward shift osmotic gradient ektacytometry (LoRRca Osmoscan curve evaluation, Mechatronics, Hoorn, the Netherlands) (Figure 1B), a mild form of hereditary dehydrated stomatocytosis was suspected. After receiving the patient’s informed consent, the DNA sample of the patient was analyzed on a next-generation sequencing (NGS)-targeted panel containing 40 genes associated with congenital hemolytic anemia (see Online Supplementary Appendix and Online Supplementary Table S2 for details). A PIEZO1 c.6328C>T missense mutation, R2110W, was identified as the only causative mutation (Figure 1C and D). The new variant (rs776531529) had not previously been reported, minor allele frequency (MAF) was not available with either EXAC and 1000G, and it was predicted to be pathogenetic by in silico analysis as deleterious by PolyPhen2 (score 0.990) and SIFT (score 0.00), and as possibly damaging by M-CAP (score 0.847). The same variant has been recently reported within a series of hemolytic patients studied by NGS but lacking functional information.7 Residue R2110 is located in the ‘anchor’ region (Figure 1E) that together with ‘beam’ are thought to transmit membrane tension-induced conformational changes into channel gating by lever-like motions.8 Although it has been shown that single mutations can interfere with channel gating transitions,2 further investigations are required to elucidate the underlying structural mechanism of this mutation.
Figure 1.
A novel mutation of PIEZO1 (R2110W). (A) Patient red blood cell (RBC) peripheral blood smear (100x objective); arrows indicate stomatocytes. (B) LoRRca Osmoscan profile of the patient (dotted line), daily normal control (black line), compared with 150 normal controls (gray area). (C) Schematic representation of the PIEZO1 gene and position of R2110W mutation. Exons in which mutations have already been reported are indicated in pink. (D) Schematic representation of Piezo1 channel, orange areas represent regions affected by mutations already reported. (E, left panel) Structural illustration of the mPiezo1 channel based on pdb file 5Z108 indicating the trimeric structure of the protein from top view. (E, right panel) Residue R2126 corresponding to R2110 in humans is highlighted in yellow. It is located in the ‘anchor’ region, that together with ‘beam’ are thought to transmit membrane tension-induced conformational changes into channel gating by lever-like motions.8
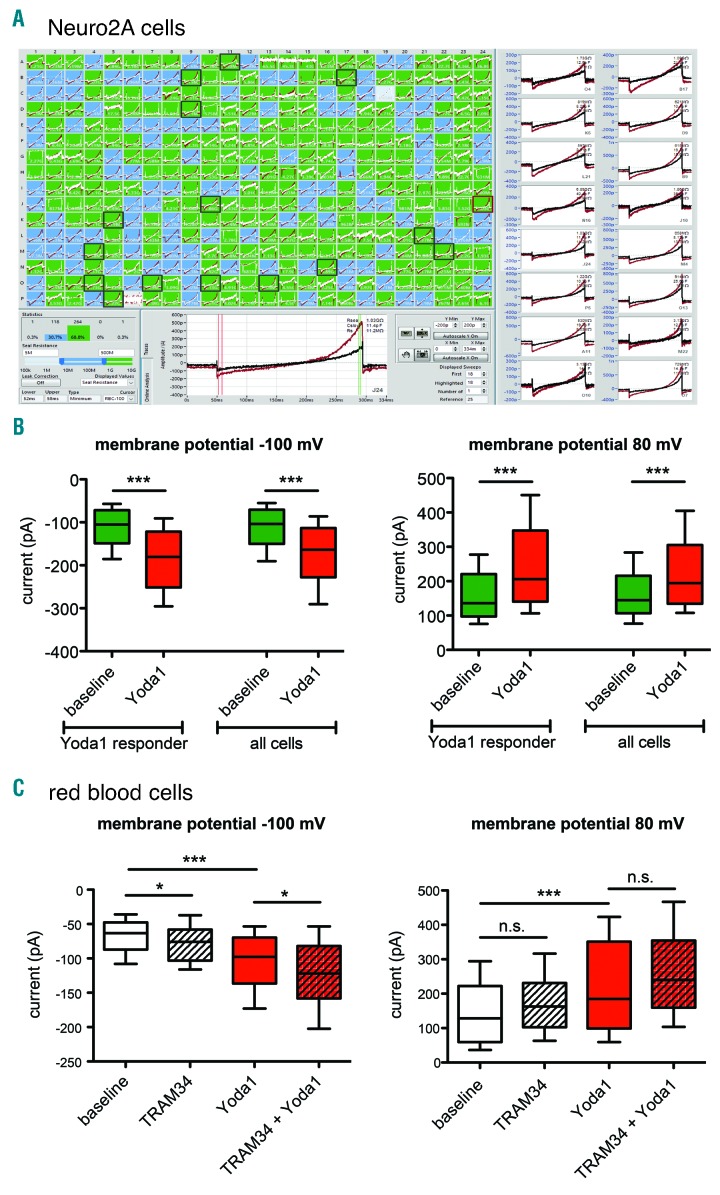
The finding of a new mutation always raises the question as to whether there is a functional impairment of the affected proteins. This is particularly true in the case of PIEZO1 mutations where a large number of VUS (variants of unknown significance) is observed. For channelopathies in general, like cystic fibrosis, erythromelalgia or hereditary xerocytosis, the patch clamp technique is the most direct method to test channel activity. However, due to the difficulties in patch clamping RBCs,9 the characterization of channelopathies effecting RBCs has not often been reported.6 Here, we present the first development of a high-throughput patch clamp assay for RBCs, based on a 384-well planar patch clamp approach (SyncroPatch 384PE, Nanion Technologies, Munich, Germany), which goes significantly beyond our previous work that first showed the suitability of planar patch clamp for RBC measurements.9,10 We developed the high-throughput assay to record Piezo1 activity in RBCs upon chemical activation using Yoda1,11 as low abundance of Piezo1 in RBCs requires large sample sizes and reliable channel activation for high assay validity. Initial assay development was performed on mouse neuroblastoma cells (Neuro2A) due to larger abundance of Piezo1 in these cells (Figure 2A). The experimental procedures are described in the Online Supplementary Appendix (see Online Supplementary Table S3 for the quality criteria applied and Online Supplementary Figure S1 for the results of their implementation). This strict quality filtering provided the basis on which to divide the Piezo1 response upon Yoda1 from Neuro2A cells into Yoda1 responders and non-responders, reflecting the biological variability of Piezo1 currents expressed in Neuro2A cells (Figure 2A).
Figure 2.
Assay development to investigate Piezo1 activity based on activation with Yoda1. Piezo1 channels endogenously expressed in Neuro2A cells were investigated on the SyncroPatch 384PE. (A) Screenshot of the data acquisition and analysis software of the SyncroPatch 384PE. (Upper left) Display of 384 parallel recordings, color-coded based on seal resistance (green: >500 MΩ, blue: 50 - 500 MΩ, gray: <50 MΩ or disabled). At the end of the experiment, 178 out of 384 patches (46.4%) exceeded 1 GΩ, and 87 out of 384 patches (22.7%) exceeded 3 GΩ. (Bottom center) Raw data trace of a single well recorded in external solution (black trace) and in the presence of 10 μM Yoda1 (red trace). Currents were elicited at room temperature by a 300 ms ramp voltage protocol from −100 to 80 mV, at a holding potential of −60 mV. The red and green vertical cursors in the trace indicate the regions of interest for the analysis, respectively currents at −100 mV and 80 mV. (Right) Raw data traces of 16 selected wells. (B) Statistical analysis of the currents at −100mV (left) and at 80 mV (right). 140 out of 384 Neuro2A cells (37%) passed the quality criteria and 85 cells (60% of the valid cells) were considered as Yoda1 responders. The current amplitudes did not show a Gaussian distribution and were, therefore, presented as median and box plots (25-75%) with whiskers (10-90%). There was a highly significant increase (P<0.0001; Mann-Whitney test) whenever 10 μM Yoda1 was applied in both Yoda1-responding cells and the entire cell population at membrane potentials of 80 mV and −100 mV. (C) Transfer of the assay to human red blood cells (RBC) and investigation of a putative Gardos channel contribution to the Yoda1-activated current in RBC. Currents were recorded before and after 10 μM Yoda1 addition and two different experiments were conducted, respectively, in the absence and presence of 500 nM TRAM-34, a specific Gardos channel blocker. At positive membrane potentials, there was no significant change induced by TRAM-34, whereas at negative membrane potential, there was a slight significant increase in the absolute current amplitude. Since this change is opposite to a putative contribution of the Gardos channel, a participation of the Gardos channel to the measured currents can be excluded.
The assay was then transferred to RBCs (Figure 2B and Online Supplementary Figure S1). Previous reports described a direct activation of the Gardos channel consecutive to RBC mechanical stimulation, likely to be associated with the activation of Piezo1.12 Given this, we tested whether the assay measures Gardos channel activity instead of or in addition to Piezo1 openings. Comparing measurements in the presence and absence of the Gardos channel inhibitor TRAM-34 did not support this: TRAM-34 neither decreased the recorded current at positive nor at negative membrane potentials (Figure 2B), excluding an involvement of Gardos channel activity in the assay. Likewise for the RBCs, we applied strict quality criteria and set higher seal resistance thresholds (Online Supplementary Table S3) in order to separate Yoda1 responders from non-responders (Online Supplementary Figure S1). This indicates the importance of recording a large number of cells to allow for a thorough statistical analysis. However, utilizing the SyncroPatch 384/768PE (Nanion Technologies, Munich, Germany) and considering the quality criteria and success rate (Online Supplementary Table S3 and Online Supplementary Figure S1), one or two runs of the assay, corresponding to a time of 20-40 minutes, are sufficient to obtain statistical data on more than 100 RBCs. It is worthwhile to notice that manual patch clamp would require 2 weeks of continuous lab work, which would not meet the requirement to work on ‘fresh’ cells (see below).
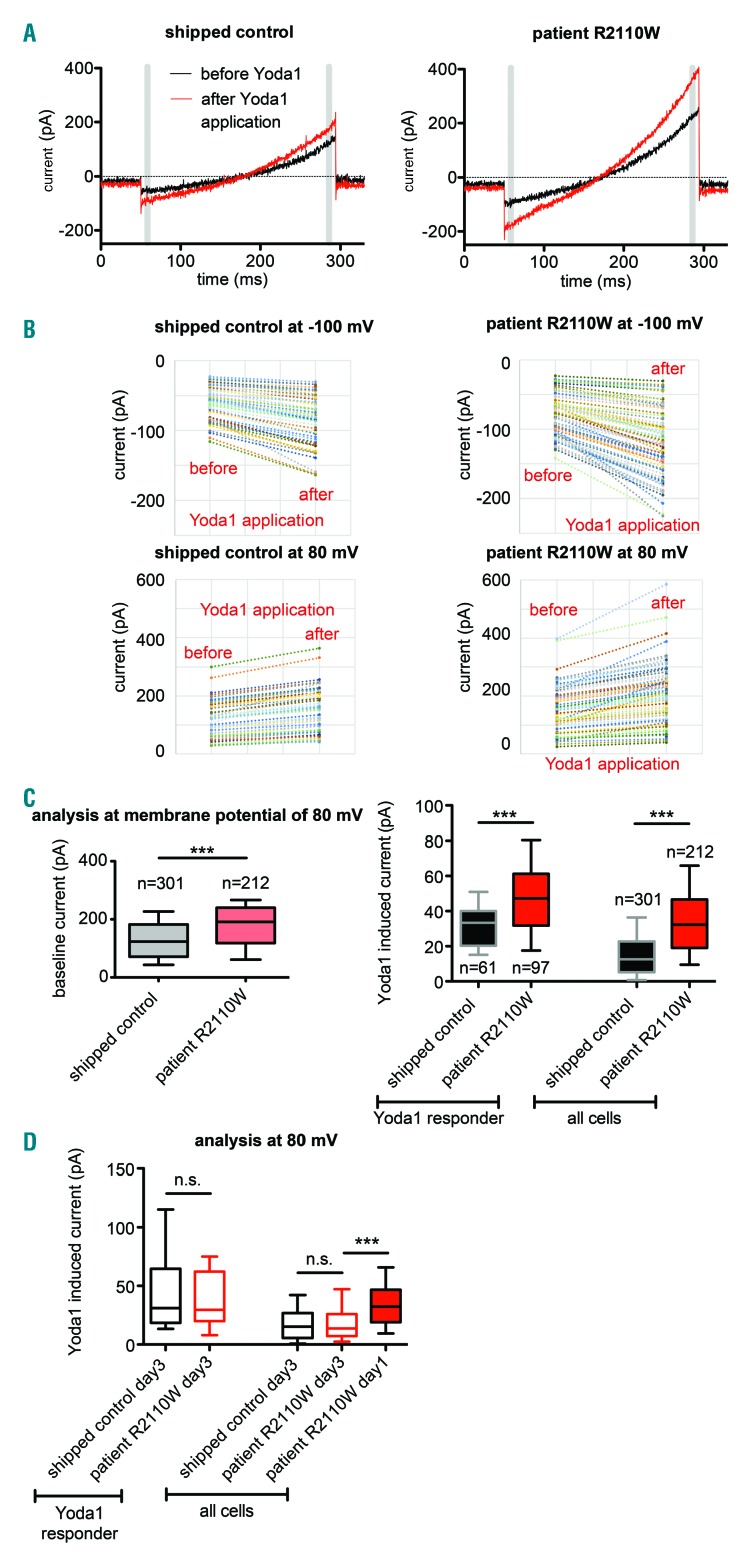
Finally, we characterized the electrophysiological properties from healthy and patient RBCs carrying the novel PIEZO1 R2110W mutation (Figure 3A). Please note that Figure 3B contains exclusively responding cells, while the statistics in Figure 3C contain all cells meeting the quality criteria depicted in Online Supplementary Table S3, independent of their response to Yoda1. The higher current amplitude at positive potentials compared to negative ones can be clearly explained by the recently reported voltage dependence of Piezo1.13 A highly significantly increased whole-cell current (P<0.0001; Mann-Whitney test) was identified for baseline currents and Yoda1-induced currents, considering both Yoda1-responding cells and the entire cell population at membrane potentials of 80 mV (Figure 3C) and −100 mV (Online Supplementary Figure S2A). The percentage of RBCs responding to Yoda1 is higher in the patient (46%) than the control (20%), probably due to an augmented cationic non-selective permeability of Piezo1-mutated RBCs. Although there is a significant difference between control and patient RBCs already at baseline conditions (Figure 3C, left), this difference is hardly indicative of a specific mutation. Only the differences after application of Yoda1 can account for differences in Piezo1 activity. In line with previous reports of PIEZO1 mutations in RBCs,1–5 and considering both baseline currents and Yoda1-induced currents (Figure 3), also R2110W represents a gain-of-function mutation. The structural localization of the residue in a gating-sensitive region of the channel protein would support this hypothesis. Our high-throughput patch clamp assay allows such evidence to be understand directly from RBC performed patch clamp recordings without the need to generate heterologously over-expressing cell lines. Heterologous studies represent a valuable method to assess amino acid changing variants in polymorphic proteins such as Piezo1. However, investigating heterologous cell lines is expensive and time consuming. Our approach is suited to characterize the functional electrophysiological impairment of RBCs from hereditary xerocytosis patients in less than 30 min, presenting a useful complementation of gene sequencing. We like to stress that we noticed a disappearance of the difference in the Yoda1-induced current over time (within 3 days) in storage (Figure 3D). Thus assays are recommended to be performed in laboratories, where samples can be processed within 24 hours after blood withdrawal.
Figure 3.
Current response of patient cells with the novel PIEZO1 mutation (R2110W) compared to healthy red blood cells (RBCs). (A) Raw data traces of Piezo1 activation by Yoda1 (red traces) recorded at room temperature in exemplary control (left) and patient (right) RBCs. The gray bars depict the time points (equal to membrane potential) at which the analyzed currents were measured. (B) Scatter charts comparing Yoda1 responder cells in control and patient conditions. In control condition, 61 out of 301 RBCs (20% of the valid cells) were considered as Yoda1 responders. In patient condition, 97 out of 212 (46% of the valid cells) were considered as Yoda1 responders. Peak current amplitude values were recorded for each cell in control and patient conditions before and after Yoda1 administration. (C) Statistical analysis of all measured cells, independent of their response to Yoda1. The voltage protocol was the same as described in Figure 2A, except the holding potential was set to −30 mV. Because the current amplitudes did not show a Gaussian distribution, they were presented as median and box plots (25-75%) with whiskers (10-90%) in patient cells compared to controls. The numbers adjacent to the boxes refer to the numbers of cells measured. Here analysis of positive 80 mV membrane potential is shown exclusively. The analysis at negative membrane potentials reveals the same results and is depicted in Online Supplementary Figure S1A. The baseline values (left) were already indicating a highly significant difference between patient and control. This difference persisted after application of Yoda1, when considering both Yoda1-responding cells and the entire cell population at membrane potentials of 80 mV and −100 mV. (D) Statistical analysis of cells 3 days after blood withdrawal. Shipped control day3 Yoda1 responders=15; patient R2110W day3 Yoda1 responders=19; shipped control day3 all cells=89; patient R2110W day3 all cells=98; patient R2110W day1 all cells=212. The difference between patient and control cells disappeared.
Patch clamp based screening assays may extend to other RBC-related channelopathies, like Gardos Channelopathy10 or hereditary anemias that present an increased channel activity as a secondary effect, like NMDA-receptor activity in sickle cell disease.14 This is of particular importance since membrane leak of Ca2+, which is mostly mediated by ion channels, was proposed to be a common component in the molecular regulation of anemias.15 However, due to the technical investment required, such patch clamp assays might be limited to application in research laboratories or hematologic centers.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Zarychanski R, Schulz VP, Houston BL, et al. Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood. 2012;120(9):1908–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae C, Gnanasambandam R, Nicolai C, Sachs F, Gottlieb PA. Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc Nat Acad Sci U S A. 2013;110(12):E1162–E1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albuisson J, Murthy SE, Bandell M, et al. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat Commun. 2013;4:1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andolfo I, Alper SL, De Franceschi L, et al. Multiple clinical forms of dehydrated hereditary stomatocytosis arise from mutations in PIEZO1. Blood. 2013;121(19):3925–3935. [DOI] [PubMed] [Google Scholar]
- 5.Archer NM, Shmukler BE, Andolfo I, et al. Hereditary xerocytosis revisited. Am J Hematol. 2014;89(12):1142–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaestner L. Channelizing the red blood cell: molecular biology competes with patch-clamp. Front Mol Biosci. 2015;2:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo R, Andolfo I, Manna F, et al. Multi-gene panel testing improves diagnosis and management of patients with hereditary anemias. Am J Hematol. 2018;93(5):672–682. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Q, Zhou H, Chi S, et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature. 2018;554(7693):487–492. [DOI] [PubMed] [Google Scholar]
- 9.Minetti G, Egée S, Mörsdorf D, et al. Red cell investigations: Art and artefacts. Blood Rev. 2013;27(2):91–101. [DOI] [PubMed] [Google Scholar]
- 10.Fermo E, Bogdanova A, Petkova-Kirova P, et al. “Gardos Channelopathy”: a variant of hereditary Stomatocytosis with complex molecular regulation. Sci Rep. 2017;7(1):1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Syeda R, Xu J, Dubin AE, et al. Chemical activation of the mechanotransduction channel Piezo1. Elife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyrda A, Cytlak U, Ciuraszkiewicz A, et al. Local membrane deformations activate Ca2+-dependent K+ and anionic currents in intact human red blood cells. PLoS One. 2010;5(2):e9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moroni M, Servin-Vences MR, Fleischer R, Sánchez-Carranza O, Lewin GR. Voltage gating of mechanosensitive PIEZO channels. Nat Commun. 2018;9(1):1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogdanova A, Makhro A, Kaestner L. Calcium Handling in Red Blood Cells of Sickle Cell Disease Patients. In: Sickle Cell Disease. Hauppauge, NY: Nova Science Publishers; 2015. pp. 29–59. [Google Scholar]
- 15.Hertz L, Huisjes R, Llaudet-Planas E, et al. Is Increased Intracellular Calcium in Red Blood Cells a Common Component in the Molecular Mechanism Causing Anemia? Front Physiol. 2017;8:673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.