Abstract
Survivorship Care Plans (SCPs) may facilitate long-term care for cancer survivors, but their effectiveness has not been established in hematopoietic cell transplantation recipients. We evaluated the impact of individualized SCPs on patient-reported outcomes among transplant survivors. Adult (≥18 years at transplant) survivors who were 1-5 years post transplantation, proficient in English, and without relapse or secondary cancers were eligible for this multicenter randomized trial. SCPs were developed based on risk-factors and treatment exposures using patient data routinely submitted by transplant centers to the Center for International Blood and Marrow Transplant Research and published guidelines for long-term follow up of transplant survivors. Phone surveys assessing patient-reported outcomes were conducted at baseline and at 6 months. The primary end point was confidence in survivorship information, and secondary end points included cancer and treatment distress, knowledge of transplant exposures, health care utilization, and health-related quality of life. Of 495 patients enrolled, 458 completed a baseline survey and were randomized (care plan=231, standard care=227); 200 (87%) and 199 (88%) completed the 6-month assessments, respectively. Patients’ characteristics were similar in the two arms. Participants on the care plan arm reported significantly lower distress scores at 6 months and an increase in the Mental Component Summary quality of life score assessed by the Short Form 12 (SF-12) instrument. No effect was observed on the end point of confidence in survivorship information or other secondary outcomes. Provision of individualized SCPs generated using registry data was associated with reduced distress and improved mental domain of quality of life among 1-5 year hematopoietic cell transplantation survivors. Trial registered at clinicaltrials.gov 02200133.
Introduction
It is estimated that there will be 250,000 hematopoietic cell transplantation (HCT) survivors in the US by 2020.1 Patients who survive the period of early complications and disease relapse (generally 1-2 years after transplantation) can expect a high probability of subsequent long-term survival.2–7 Although potentially cured of their underlying disease, HCT survivors continue to be at risk for late complications that can cause substantial morbidity, mortality, and functional deficits, and contribute to psychosocial and quality of life impairments.8–23 Established survivorship guidelines provide a pragmatic approach to the long-term follow up of autologous and allogeneic HCT survivors by recommending a minimum set of screening and preventive evaluations that need to be performed periodically post-transplantation.24,25
Hematopoietic cell transplantation survivors frequently do not receive or adhere to preventive care guidelines.26–28 Many barriers contribute to the inadequate provision of co-ordinated patient-centric survivorship care in this patient population.29–31 Among these, a lack of awareness of exposures and risks by patients is strongly associated with a lower likelihood of adherence to preventive care recommendations.26 In addition, both transplant and non-transplant providers identify lack of knowledge of risks of late complications and of awareness of guidelines as barriers to providing adequate preventive care.32 Finally, capacity limitations at transplant centers may impede provision and co-ordination of preventive care to HCT survivors.29,33–35 Interventions to enhance patient awareness of preventive care could potentially enhance appropriate healthcare utilization and adherence to survivorship guidelines, although this approach has not been previously tested.
A treatment summary and Survivorship Care Plan (SCP) is a tool that provides cancer survivors with information on their cancer type, treatments and potential conse quences, and recommendations regarding follow up and preventive care. SCPs are generally accepted as an important component of cancer survivorship care.36 Randomized trials of SCPs in cancer patients have primarily focused on providing information through in-person visits with patients or educating primary care providers, and evidence of their efficacy in enhancing various aspects of cancer survivorship care is generally negative.37 They are also frequently underused. This may be due to a variety of reasons, including insufficient resources for their generation and implementation, and a paucity of evidence regarding an impact on patient outcomes.38–42 The use and the dissemination of SCPs in HCT survivors are hampered by similar challenges, and many transplant centers do not routinely provide patients with this tool. Furthermore, these barriers may be accentuated because of the highly complex nature and unique exposures associated with the transplant procedure and the challenges involved in providing co-ordinated survivorship care.29
We hypothesized that a patient-centered approach with a personalized SCP based on published guidelines for the prevention of HCT-related late complications,24,25 and generated using patient data routinely submitted by US transplant centers to an international clinical outcomes registry [Center for International Blood and Marrow Transplant Research (CIBMTR)], would increase patient awareness of recommended preventive care, which in turn would reduce distress, promote healthy behaviors, enhance healthcare utilization for appropriate preventive care, and improve health-related quality of life (HRQOL). By using existing CIBMTR data, this approach would overcome several system-level barriers to providing survivorship care through transplant centers. Furthermore, it could serve as a template for a general, efficient mechanism for providing a patient-centric SCP to long-term HCT survivors who frequently are no longer under the care of transplant centers and are particularly vulnerable to gaps in preventive care. In a multicenter randomized controlled trial (RCT), we evaluated the efficacy of such an individualized SCP instrument generated using registry data and mailed to patients versus standard care on improving patient-reported outcomes in adult HCT survivors 1-5 years after their transplant.
Methods
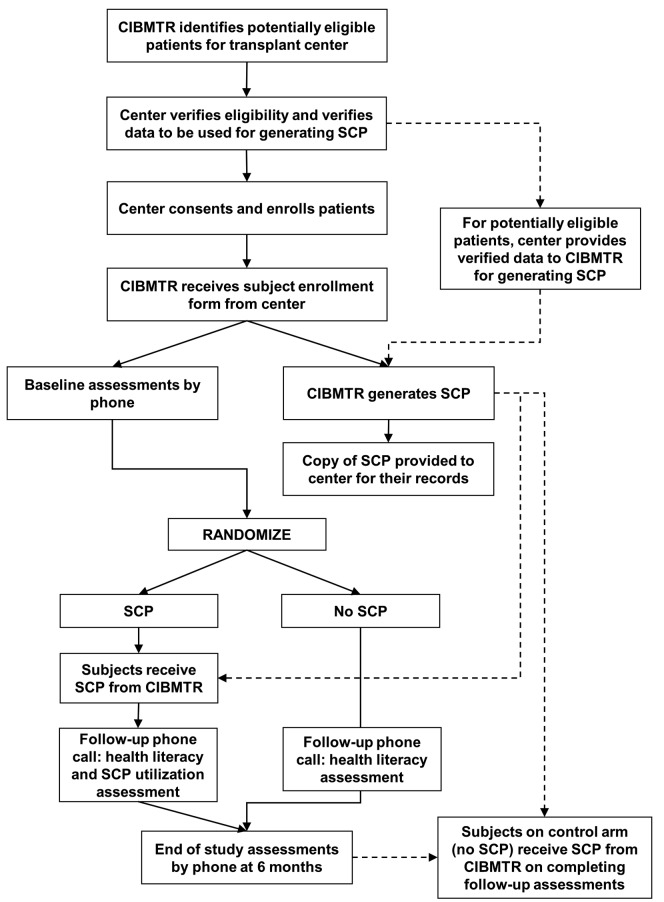
Potentially eligible patients from 17 participating US centers were identified from the CIBMTR database, and paper-based SCPs personalized to HCT specific exposures were generated using CIBMTR data for patients who consented and enrolled on the study (see Online Supplementary Methods).43 Patient eligibility criteria were kept broad and included patients who were 1-5 years post transplant, ≥18 years at the time of HCT, with no evidence of disease relapse/progression or second cancers, and fluency in English; patients were eligible irrespective of transplant type (autologous or allogeneic), diagnosis, donor source or conditioning regimen. None of the participating centers had an existing mechanism for routinely providing SCPs to their patients. The RCT used a multi-center design with patient-level randomization to treatment (Figure 1), and was approved by Institutional Review Boards at the National Marrow Donor Program (NMDP) and each participating site. A random order list of survivors was generated and released in blocks to centers, who confirmed patient survival and accuracy of SCP-related data. Centers contacted patients and obtained their consent to the study, and then informed the CIBMTR, who proceeded with the rest of the study procedures. The CIBMTR Survey Research Group (SRG) conducted a phone assessment within 30 days of the patient receiving the participant enrollment form. Patients were randomized 1:1 to the SCP or control arm (with delayed SCP). Patients randomized to the SCP arm received an informative letter by express post and their printed SCP while patients on the control arm only received an informative letter. SRG then contacted all enrolled patients by phone between 7-28 days of mailing study materials to conduct a health literacy assessment using the Newest Vital Sign.44 During this contact, patients on the SCP arm were given the opportunity to address any questions about the content or use of their SCP. No further contact was made till the 6-month phone survey. The Confidence in Survivorship Information (CSI) was the primary end point (Online Supplementary Table S1).45 Secondary end points focused on Cancer and Treatment Distress (CTXD),20,46 as well as measures of Knowledge of Transplant Exposures, Health Care Utilization,26 and HRQOL using the SF-12.47 Patients on the intervention arm also received a 12-item assessment for qualitative feedback on SCP utilization. Sample size calculations were performed using a standard error formula that allowed for possible variability in treatment effect across centers and considered dropouts from baseline to 6 months. Our enrollment goal was 495 patients, which yielded adequate power to detect standardized effect sizes of ≥0.3, which are considered to be clinically meaningful, and anticipated a 10% drop-off from baseline to 6 months. An intention-to-treat approach was followed for analysis. A mixed model with center-level random effects and a fixed treatment effect was used to test whether there was a change in baseline and 6-month response between the treatment and control groups for the primary and secondary end points. The 6-month assessment was used as a response variable and the baseline assessment was used as an explanatory variable in the regression models. If a treatment effect was observed, we further evaluated whether the effect was modified by demographic variables or any interactions between variables.
Figure 1.
Study schema. CIBMTR: Center for International Blood and Marrow Transplant Research; SCP: Survivorship Care Plan.
Further details are available in the Online Supplementary Appendix.
Results
Patients’ characteristics
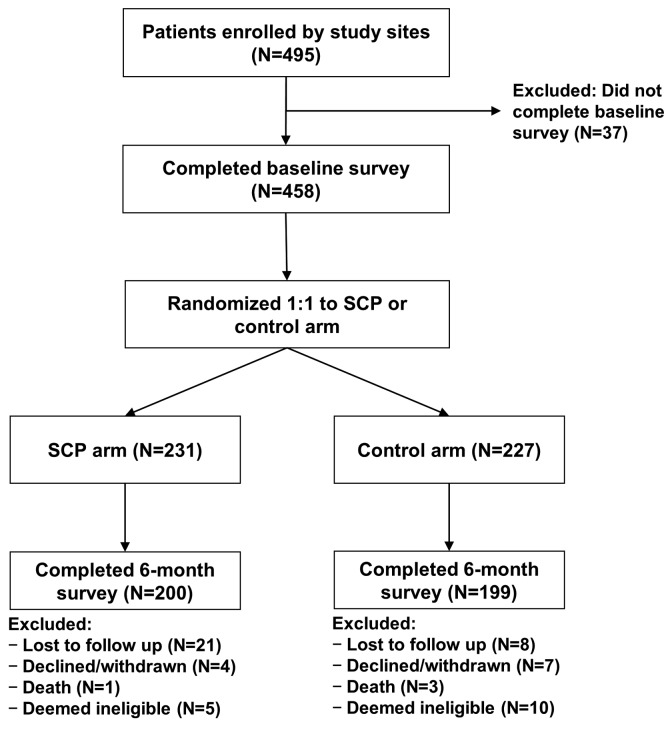
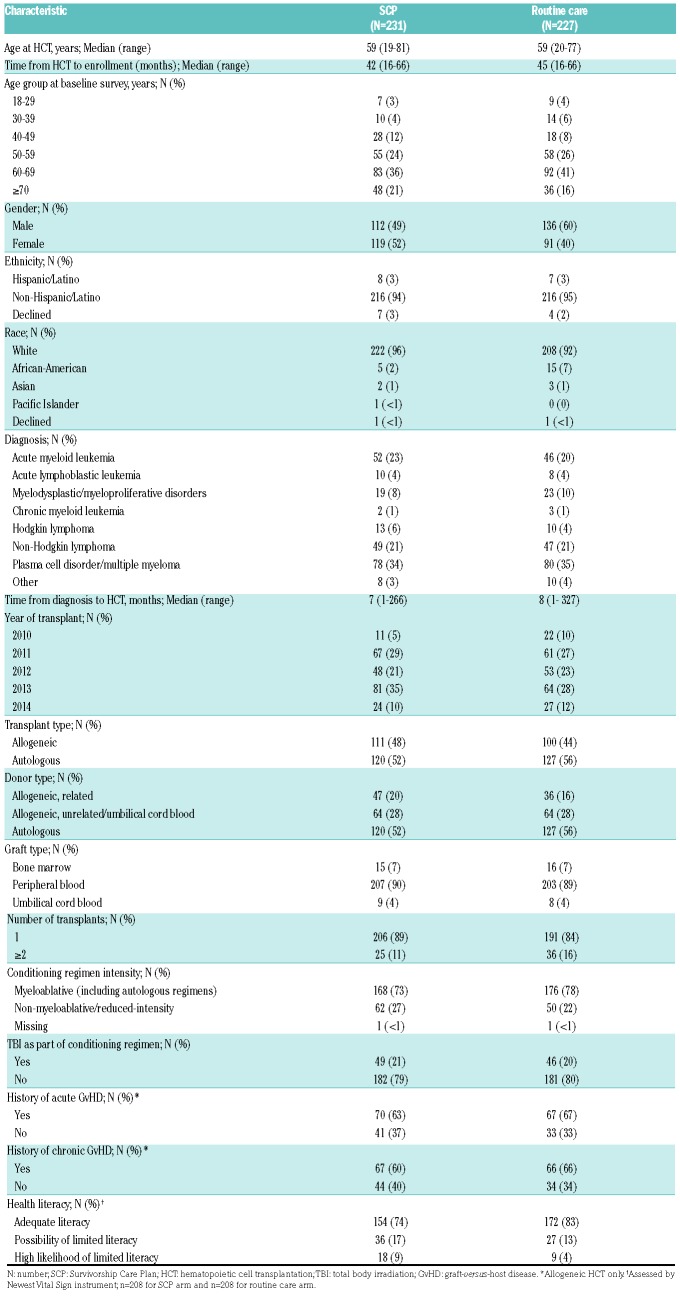
Among the 495 patients enrolled, 458 completed the baseline survey and were randomized (SCP=231, control=227); 200 (87%) and 199 (88%) completed 6-month assessments, respectively (Figure 2). The main reasons for dropout were loss to follow up or patients not eligible for follow-up assessment due to interim disease relapse or progression. A greater proportion of patients who completed the 6-month assessment were White and reported higher health literacy scores; otherwise there were no significant differences in the demographic characteristics between patients who did and those who did not complete the 6-month assessments (Online Supplementary Table S2). Patients’ and transplant characteristics (including health literacy scores) were well balanced between the two arms, except for gender (49% males in SCP compared to 60% in controls; P=0.01) (Table 1). Median age was 59 years in both arms and enrolled patients were predominantly White (96% SCP and 92% controls). In the SCP and control arms, 48% and 44% had received allogeneic HCT; among allogeneic HCT recipients 63% and 67% had a history of acute GvHD, and 60% and 66% had a history of chronic GvHD, respectively.
Figure 2.
CONSORT diagram. N: number; SCP: Survivorship Care Plan.
Table 1.
Baseline characteristics of patients enrolled on the study.
Analyses of primary and secondary end points
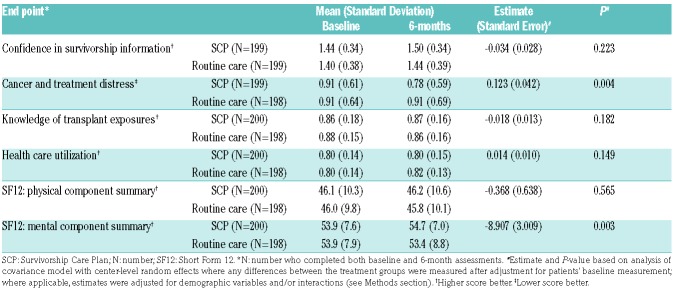
Of the 458 patients randomized to the two arms, 399 completed 6-month assessments, including 398 who completed pre- and post-measurements for the primary end point (Table 2). We did not find any association between the SCP intervention and change in CSI scores from baseline to 6-months (P=0.223), even after assessing for the effect of demographic factors and interactions. However, we did observe a significant decrease in CTXD scores (P=0.004) and an increase in HRQOL Mental Component Summary (MCS) scores as assessed by SF-12 (P=0.003) among patients randomized to the SCP arm. There was no association between the SCP intervention and other secondary end points.
Table 2.
Analysis for primary and secondary end points.
We further assessed the effect of demographic variables and interactions for the end points of CTXD and SF-12 MCS, where a significant treatment effect was observed. Age was significantly associated with CTXD scores (regression estimate −0.006, standard error 0.002; P=0.001), with lower distress among older patients. However, there was no significant interaction between age and SCP intervention and adjustment for age did not modify the treatment effect. The decrease in CTXD score for the SCP arm was independent of gender, health literacy, diagnosis, transplant type, and GvHD status (including acute and chronic GvHD). We also found a similar effect of age on MCS scores, with older patients reporting significantly higher scores (estimate 0.03, standard error 0.034; P<0.001), and there was a significant interaction between age and SCP intervention (P=0.012). However, increase in mean MCS score in the SCP arm was independent of gender, health literacy, diagnosis, transplant type, and GvHD status.
Utilization of Survivorship Care Plans
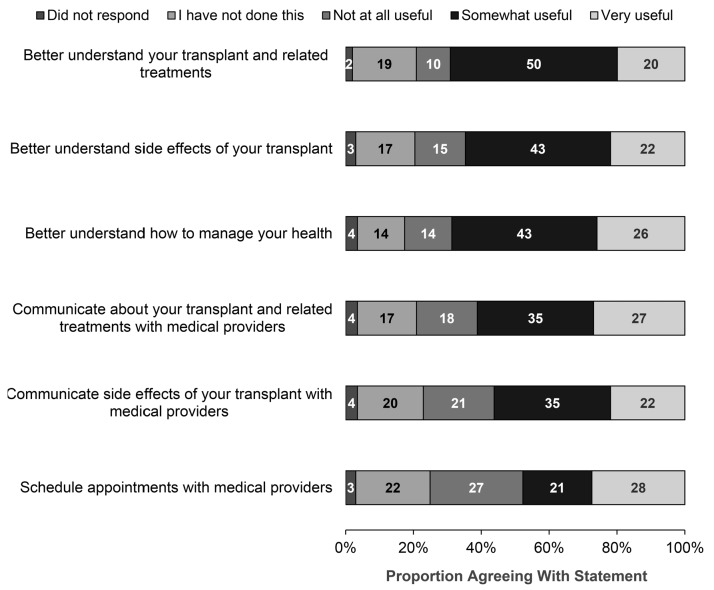
At their 6-month end-of-study assessments, patients on the intervention arm were asked questions about the usefulness of the SCP for their survivorship care (Figure 3). A relatively large proportion of survivors reported that they found the SCP somewhat or very useful for helping them better understand their HCT and related treatments (70%), side effects of HCT (65%), and managing their health (69%). The SCP helped survivors better communicate about HCT and its side effects with their medical providers. The 6-month interview included an open-ended question about patients’ experience with the SCP; dominant themes identified on qualitative analyses included patients reporting that the SCP helped survivors focus on their overall health, supported them in making care decisions with providers, and supported emotional health and coping.
Figure 3.
Patient-reported assessment of usefulness of Survivorship Care Plan (SCP) intervention. N=201 respondents on SCP arm who completed 6-month end-of-study assessments.
Discussion
In this large multicenter RCT of HCT survivors 1-5 years post transplantation, we demonstrate that SCPs generated using a centralized clinical registry (CIBMTR), individualized to patient exposures, and without clinician contact to interpret or personalize their content and recommendations, are feasible and have desirable outcomes, including lower treatment-related distress and improved mental health domain of HRQOL. Our results support further research towards broader implementation of our SCP instrument to facilitate care of HCT survivors, and provides evidence to support a patient-centered approach towards administration of SCPs. SCPs have been endorsed as a tool for facilitating the care of cancer survivors with the goal of improving patient outcomes by promoting coordination of care, shared-decision making, self-management, and adherence to treatment recommendations.36,48 Evidence on their efficacy in impacting patients’ outcomes is mixed, and SCPs have not been universally adopted due to other barriers, such as the lack of standardized templates, the need for extensive resources and time for their generation, and the lack of reimbursement for their implementation.42,48–50 Transplant centers face similar challenges, and many programs have capacity limitations that frequently prevent provision of personalized comprehensive SCPs to their patients. Our SCP procedure provides several advantages to patients and transplant centers. It uses data that centers routinely submit electronically to the CIBMTR and will provide a resource-effective mechanism for centers to generate the SCP for their recipients. Instead of receiving a generic SCP, patients can receive one that is specific to their treatment exposures. Our approach of facilitating patient ownership of survivorship care is different from the prevalent non-transplant cancer literature where SCPs have largely been tested in a context in which clinicians provide them to their patients.37 Our SCP instrument was in a paper-based format and was mailed to patients; more general dissemination would require its translation into an electronic format. Hence, further research is still needed to guide its implementation. An ongoing project funded by the National Cancer Institute is investigating its use in combination with an online health informatics platform to facilitate a self-management program for selected late complications among HCT survivors (clinicaltrials.gov identifier: 03125070; Syrjala, Baker and Majhail).
Of note, we did not observe any impact of the SCP intervention on our primary end point of CSI. Our study population consisted of HCT survivors who had been transplanted relatively recently (1-5 years) and enrolled by centers with an interest in providing survivorship care to their transplant recipients; it is possible our instrument may be more effective in enhancing knowledge and confidence about follow-up care among patients who underwent transplantation among patients further out from transplantation or those who are not followed primarily or closely by their transplant centers. The CSI instrument has been validated in cancer survivors but not among HCT recipients, and it is also possible that it did not adequately measure the underlying construct in our patient population. The 6-month pre- and post-intervention follow-up period was most likely too short to detect any significant associations with changes in healthcare adherence or utilization. We did not observe any interaction of GvHD with the intervention or study outcomes. This was most likely due to our study population being relatively further out from transplantation and the short duration of the intervention. Furthermore, it is likely that patients with GvHD were under the active care of transplant centers and this may have impacted patient-reported outcomes assessed in our study (e.g. greater confidence in recommended care, less distress, etc.). These same factors were probably responsible for some patients not finding the SCP tool to be useful for various aspects of survivorship care (see Figure 3; “I have not done this” and “Not at all useful” responses on SCP utilization survey administered as part of end-of-study assessments for the intervention arm).
The concordant findings of a reduction in CTXD scores and an improvement in SF-12 MCS scores cross-validate the overall effect of SCP on reducing distress and improving HRQOL in our study population of HCT survivors. It is important to note that these effects occurred over a relatively short period of time and did not require any additional clinical contact or intervention to facilitate the use of the SCP. Interestingly, we found an independent association between older age and lower CTXD scores, which is consistent with other literature where older adults are less distressed about cancer and survivorship.51–54 The SCP provided concise information on previous treatments and potential late effects, and practical guidance regarding recommended preventive care that survivors could easily understand and share, which may have empowered them in the CTXD domains (e.g. uncertainty, health burden and medical demands) and MCS domains (e.g. mental health, social functioning, role-emotional), leading to the improvement in those areas.55
Some limitations of our study must be acknowledged. First, the treatment summary portion of our SCP primarily included HCT-related and post-transplant events and did not have detailed information on pre-transplant exposures as those data are not captured comprehensively by the CIBMTR. However, transplant centers have the option to add information about those exposures to the basic template of the SCP. Participants who completed 6-month assessments were more likely White and had higher health literacy, which may limit the extent to which our findings can be generalized. However, this is reflective of the prevailing healthcare disparities in HCT, and research on other interventions to facilitate SCP use in this under-served population is needed.56 Notwithstanding these limitations, the pragmatic nature of our study eligibility criteria and schema will make our results broadly applicable to transplant centers in the US.
An ideal mechanism to provide SCPs to HCT survivors would involve a dynamic, adaptable, and patient-specific shared-decision making approach between patients, their transplant centers, and other providers. However, several challenges prevent centers from providing this tool to facilitate survivorship care for their patients, and SCPs that can be generated efficiently and without requiring significant center resources would have an impact on patient care. Our study supports further implementation of an individualized SCP generated using CIBMTR data in a population of HCT survivors that is at significant risk for late morbidity and mortality. Future research will examine the role of the SCP instrument in preventing specific late complications, in facilitating co-ordination of care, and will serve as a platform for investigating novel methods for survivorship care delivery and implementation.
Supplementary Material
Acknowledgments
Key to the success of this patient-centered outcomes research study were the many contributions and engagement of our more than 40 stakeholder group representatives including: transplant recipients, caregivers, community hematology/oncology physicians, and transplant physicians, nurses, social workers, patient health educators, and patient advocates. We are deeply indebted to the National Marrow Donor Program’s Patient Services Advisory Group, which includes representation from many of these stakeholder groups, of whom several members participated in the protocol team for this study or provided feedback and guidance on study design and interpretation of results. The study investigators would like to the following transplant centers for enrolling patients on this study (site investigators are listed in parentheses): Baylor University, Dallas, TX (Jana Reynolds); Cleveland Clinic, Cleveland, OH (Navneet Majhail); Fred Hutchinson Cancer Research Center, Seattle, WA (K Scott Baker), Karmanos Cancer Institute, Detroit, MI (Abhinav Deol); Loyola University, Chicago, IL (Patrick Stiff); Mayo Clinic, Rochester, MN (Shahrukh Hashmi); Mayo Clinic, Scottsdale, AZ (Nandita Khera); Ohio State University, Columbus, OH (Samantha Jaglowski); Roswell Park Comprehensive Cancer Center, Buffalo, NY (Theresa Hahn); UMass Memorial Medical Center, Worcester, MA (Jan Cerny); University of Florida, Gainesville, FL (John R. Wingard); University of Kansas, Kansas City, KS (Joseph McGuirk); University of Minnesota, Minneapolis, MN (Shernan Holtan); University of North Carolina, Chapel Hill, NC (William Wood); University of Pennsylvania, Philadelphia, PA (Alison Loren); Vanderbilt University, Nashville, TN (Bipin Savani). We are especially grateful to study coordinators at all sites who worked tirelessly and often outside regular working hours to contact and enroll patients on our trial. We would also like to thank members of CIBMTR’s Health Services Research (HSR) Program and Resource for Clinical Investigation in Blood and Marrow Transplantation (RCI BMT), including their Survey Research Group, who helped with study conduct, data collection and management, and performed patient interviews.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/5/1084
Funding
This study was funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (CD-12-11-4062). The views in this publication are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee. K. Scott Baker, Navneet Majhail and Karen Syrjala are partially supported by a grant from the National Cancer Institute (R01-CA215134). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Majhail NS, Tao L, Bredeson C, et al. Prevalence of hematopoietic cell transplant survivors in the United States. Biol Blood Marrow Transplant. 2013;19(10):1498–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majhail NS, Bajorunaite R, Lazarus HM, et al. High probability of long-term survival in 2-year survivors of autologous hematopoietic cell transplantation for AML in first or second CR. Bone Marrow Transplant. 2011;46(3):385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majhail NS, Bajorunaite R, Lazarus HM, et al. Long-term survival and late relapse in 2-year survivors of autologous haematopoiet ic cell transplantation for Hodgkin and non-Hodgkin lymphoma. Br J Haematol. 2009;147(1):129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin PJ, Counts GW, Jr, Appelbaum FR, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010; 28(6):1011–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia S, Robison LL, Francisco L, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105(11):4215–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun CL, Francisco L, Baker KS, Weisdorf DJ, Forman SJ, Bhatia S. Adverse psychological outcomes in long-term survivors of hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study (BMTSS). Blood. 2011;118(17):4723–4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armenian SH, Sun CL, Kawashima T, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS). Blood. 2011;118(5):1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood. 2010; 116(17):3129–3139; quiz 3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker KS, Ness KK, Weisdorf D, et al. Late effects in survivors of acute leukemia treated with hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Leukemia. 2010; 24(12):2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majhail NS, Ness KK, Burns LJ, et al. Late effects in survivors of Hodgkin and non-Hodgkin lymphoma treated with autologous hematopoietic cell transplantation: a report from the bone marrow transplant survivor study. Biol Blood Marrow Transplant. 2007;13(10):1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majhail NS, Douglas Rizzo J. Surviving the cure: long term followup of hematopoietic cell transplant recipients. Bone Marrow Transplant. 2013;48(9):1145–1151. [DOI] [PubMed] [Google Scholar]
- 14.Battiwalla M, Hashmi S, Majhail N, Pavletic S, Savani BN, Shelburne N. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: Developing Recommendations to Improve Survivorship and Long-Term Outcomes. Biol Blood Marrow Transplant. 2017;23(1):6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Battiwalla M, Tichelli A, Majhail NS. Long-Term Survivorship after Hematopoietic Cell Transplantation: Roadmap for Research and Care. Biol Blood Marrow Transplant. 2017;23(2):184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majhail NS. Long-term complications after hematopoietic cell transplantation. Hematol Oncol Stem Cell Ther. 2017;10(4):220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Syrjala KL, Artherholt SB, Kurland BF, et al. Prospective neurocognitive function over 5 years after allogeneic hematopoietic cell transplantation for cancer survivors compared with matched controls at 5 years. J Clin Oncol. 2011;29(17):2397–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirchhoff AC, Leisenring W, Syrjala KL. Prospective predictors of return to work in the 5 years after hematopoietic cell transplantation. J Cancer Surviv. 2010;4(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Syrjala KL, Langer SL, Abrams JR, Storer BE, Martin PJ. Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. J Clin Oncol. 2005;23(27):6596–6606. [DOI] [PubMed] [Google Scholar]
- 20.Syrjala KL, Langer SL, Abrams JR, et al. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. JAMA. 2004;291(19):2335–2343. [DOI] [PubMed] [Google Scholar]
- 21.Majhail NS, Brazauskas R, Rizzo JD, et al. Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood. 2011;117(1):316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majhail NS. Secondary cancers following allogeneic haematopoietic cell transplantation in adults. Br J Haematol. 2011;154(3):301–310. [DOI] [PubMed] [Google Scholar]
- 23.Sun CL, Kersey JH, Francisco L, et al. Burden of morbidity in 10+ year survivors of hematopoietic cell transplantation: report from the bone marrow transplantation survivor study. Biol Blood Marrow Transplant. 2013;19(7):1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Bone Marrow Transplant. 2012;47(3):337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(3):348–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khera N, Chow EJ, Leisenring WM, et al. Factors associated with adherence to preventive care practices among hematopoietic cell transplantation survivors. Biol Blood Marrow Transplant. 2011;17(7):995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bishop MM, Lee SJ, Beaumont JL, et al. The preventive health behaviors of long-term survivors of cancer and hematopoietic stem cell transplantation compared with matched controls. Biol Blood Marrow Transplant. 2010;16(2):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armenian SH, Sun CL, Francisco L, et al. Health behaviors and cancer screening practices in long-term survivors of hematopoietic cell transplantation (HCT): a report from the BMT Survivor Study. Bone Marrow Transplant. 2012;47(2):283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashmi SK, Bredeson C, Duarte RF, et al. National Institutes of Health Blood and Marrow Transplant Late Effects Initiative: The Healthcare Delivery Working Group Report. Biol Blood Marrow Transplant. 2017;23(5):717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majhail NS. Optimizing Quality and Efficiency of Healthcare Delivery in Hematopoietic Cell Transplantation. Curr Hematol Malig Rep. 2015;10(3):199–204. [DOI] [PubMed] [Google Scholar]
- 31.Khera N, Martin P, Edsall K, et al. Patient-centered care coordination in hematopoietic cell transplantation. Blood Adv. 2017; 1(19):1617–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mani S, Rybicki L, Carraway H, Moore H, Vakharia N, Majhail NS. Primary care physician preferences and perspectives on long-term care of survivors of hematologic malignancies and hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(3 (Suppl)):S85–86. [Google Scholar]
- 33.Denzen EM, Majhail NS, Stickney Ferguson S, et al. Hematopoietic cell transplantation in 2020: summary of year 2 recommendations of the National Marrow Donor Program’s System Capacity Initiative. Biol Blood Marrow Transplant. 2013;19(1):4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majhail NS, Mau LW, Chitphakdithai P, et al. National Survey of Hematopoietic Cell Transplantation Center Personnel, Infrastructure, and Models of Care Delivery. Biol Blood Marrow Transplant. 2015;21(7):1308–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Majhail NS, Murphy EA, Denzen EM, et al. The National Marrow Donor Program’s Symposium on Hematopoietic Cell Transplantation in 2020: a health care resource and infrastructure assessment. Biol Blood Marrow Transplant. 2012;18(2):172–182. [DOI] [PubMed] [Google Scholar]
- 36.McCabe MS, Bhatia S, Oeffinger KC, et al. American Society of Clinical Oncology statement: achieving high-quality cancer survivorship care. J Clin Oncol. 2013;31(5):631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobsen PB, DeRosa AP, Henderson TO, et al. Systematic Review of the Impact of Cancer Survivorship Care Plans on Health Outcomes and Health Care Delivery. J Clin Oncol. 2018;36(20):2088–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salz T, Oeffinger KC, McCabe MS, Layne TM, Bach PB. Survivorship care plans in research and practice. CA Cancer J Clin. 2012;62(2):101–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stricker CT, Jacobs LA, Risendal B, et al. Survivorship care planning after the institute of medicine recommendations: how are we faring? J Cancer Surviv. 2011; 5(4):358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobs LA, Shulman LN. Follow-up care of cancer survivors: challenges and solutions. Lancet Oncol. 2017;18(1):e19–e29. [DOI] [PubMed] [Google Scholar]
- 41.Klemanski DL, Browning KK, Kue J. Survivorship care plan preferences of cancer survivors and health care providers: a systematic review and quality appraisal of the evidence. J Cancer Surviv. 2016; 10(1):71–86. [DOI] [PubMed] [Google Scholar]
- 42.Brennan ME, Gormally JF, Butow P, Boyle FM, Spillane AJ. Survivorship care plans in cancer: a systematic review of care plan outcomes. Br J Cancer. 2014;111(10):1899–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denzen EM, Preussler JM, Murphy EA, et al. Tailoring a survivorship care plan: Patient and provider preferences for recipients of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2018; S1083-8791(18)30614–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. 2005;3(6):514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palmer SC, Jacobs LA, Mao J, Stricker CT. Are cancer survivors confident in their knowledge about their disease and care? Poster presented at the 6th Biennial Cancer Survivorship Research: Translating Science to Care, June 14, 2012 Available from: https://smhs.gwu.edu/cancercontroltap/sites/cancercontroltap/files/CSI%20description.pdf?src=TAPResource. [Google Scholar]
- 46.Syrjala KL, Chapko ME. Evidence for a biopsychosocial model of cancer treatment-related pain. Pain. 1995;61(1):69–79. [DOI] [PubMed] [Google Scholar]
- 47.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996; 34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 48.Mayer DK, Nekhlyudov L, Snyder CF, Merrill JK, Wollins DS, Shulman LN. American Society of Clinical Oncology clinical expert statement on cancer survivorship care planning. J Oncol Pract. 2014;10(6):345–351. [DOI] [PubMed] [Google Scholar]
- 49.Blanch-Hartigan D, Forsythe LP, Alfano CM, et al. Provision and discussion of survivorship care plans among cancer survivors: results of a nationally representative survey of oncologists and primary care physicians. J Clin Oncol. 2014;32(15):1578–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hewitt ME, Bamundo A, Day R, Harvey C. Perspectives on post-treatment cancer care: qualitative research with survivors, nurses, and physicians. J Clin Oncol. 2007; 25(16):2270–2273. [DOI] [PubMed] [Google Scholar]
- 51.Acquati C, Kayser K. Predictors of psychological distress among cancer patients receiving care at a safety-net institution: the role of younger age and psychosocial problems. Support Care Cancer. 2017; 25(7):2305–2312. [DOI] [PubMed] [Google Scholar]
- 52.Head BA, Schapmire TJ, Keeney CE, et al. Use of the Distress Thermometer to discern clinically relevant quality of life differences in women with breast cancer. Qual Life Res. 2012;21(2):215–223. [DOI] [PubMed] [Google Scholar]
- 53.Jacobsen PB, Shibata D, Siegel EM, et al. Evaluating the quality of psychosocial care in outpatient medical oncology settings using performance indicators. Psychooncology. 2011;20(11):1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zebrack B, Kayser K, Sundstrom L, et al. Psychosocial distress screening implementation in cancer care: an analysis of adherence, responsiveness, and acceptability. J Clin Oncol. 2015;33(10):1165–1170. [DOI] [PubMed] [Google Scholar]
- 55.Syrjala KL, Sutton SK, Jim HS, et al. Cancer and treatment distress psychometric evaluation over time: A BMT CTN 0902 secondary analysis. Cancer. 2017;123(8):1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Majhail NS, Nayyar S, Santibanez ME, Murphy EA, Denzen EM. Racial disparities in hematopoietic cell transplantation in the United States. Bone Marrow Transplant. 2012;47(11):1385–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.