Abstract
With modern chemotherapy, approximately 90% of patients with pediatric acute lymphoblastic leukemia are now cured. However, subsets of patients can be identified who remain at very high risk of relapse with expected 4-year disease-free survival rates <80%; such patients are appropriate candidates for intensive therapeutic strategies designed to improve survival. The AALL1131 trial was designed to determine, in a randomized fashion, whether substitution with cyclophosphamide/etoposide (experimental arm 1) would improve the 4-year disease-free survival of children, adolescents, and young adults with very high-risk B-cell acute lymphoblastic leukemia compared to a modified Berlin-Frankfurt-Münster regimen (control arm). Patients 1-30 years of age with newly diagnosed very high-risk B-cell acute lymphoblastic leukemia were randomized after induction in a 1:2 fashion to the control arm or experimental arm 1 in which they were given cyclophosphamide (440 mg/m2 days 1-5)/etoposide (100 mg/m2 days 1-5) during part 2 of consolidation and delayed intensification. Prospective interim monitoring rules for efficacy and futility were included where futility would be determined for a one-sided P-value ≥0.7664. The study was stopped for futility as the interim monitoring boundary was crossed [hazard ratio 0.606 (95% confidence interval: 0.297 - 1.237)] and the very high-risk arm of AALL1131 was closed in February 2017. Using data current as of December 31, 2017, 4-year disease-free survival rates were 85.5±6.8% (control arm) versus 72.3±6.3% (experimental arm 1) (P-value = 0.76). There were no significant differences in grade 3/4 adverse events between the two arms. Substitution of this therapy for very high-risk B-cell acute lymphoblastic leukemia patients on the Children’s Oncology Group AALL1131 trial (NCT02883049) randomized to cyclophosphamide/etoposide during part 2 of consolidation and delayed intensification did not improve disease-free survival.
Introduction
With modern chemotherapy regimens, approximately 90% of patients with pediatric B-cell acute lymphoblastic leukemia (B-ALL) are now cured.1,2 However, subsets of patients remain at very high-risk (VHR) of relapse with an expected 4-year disease-free survival (DFS) rate <80%. Current post-induction intensification strategies, which have focused on optimizing the use of drugs commonly administered in ALL therapy, have delivered sub-optimal results for these VHR B-ALL patients. In the absence of a specific targeted intervention (such as Abl-tyrosine kinase inhibitors in Philadelphia chromosome-positive ALL), intensive chemotherapy continues to be the mainstay of treatment. We hypothesized that further optimization or intensification of the dose and schedule of established agents or combination regimens typically used to treat newly diagnosed ALL patients would probably not improve outcomes further for VHR B-ALL patients, and therefore novel or targeted therapies should be investigated. Given that there was not a molecularly targeted agent available for this population of patients at the time the study was conceived, this trial was designed to test the use of different consolidation strategies, based on drugs not commonly used in frontline ALL trials, including fractionated cyclophosphamide and etoposide.
The Children’s Oncology Group (COG) AALL1131 trial thus aimed to determine, in a randomized fashion, whether replacing cyclophosphamide, cytarabine, and 6-mercaptopurine during consolidation or cyclophosphamide, cytarabine, and 6-thioguanine during delayed intensification with cyclophosphamide and etoposide (experimental arm 1) during the consolidation and reconsolidation phases of COG augmented Berlin-Frankfurt-Münster therapy (control arm)3 would improve the 4-year DFS of children, adolescents, and young adults with VHR B-ALL. The cyclophosphamide/etoposide combination was well tolerated in prior relapse B-ALL studies4,5 and a similar combination of ifosfamide/etoposide yielded 40% complete remission rates in children with refractory ALL,6 making cyclophosphamide/etoposide an encouraging combination to study.
Methods
COG AALL1131 (NCT02883049), a phase III trial for patients aged 1-30 years with newly diagnosed high-risk B-ALL opened to enrollment on February 27, 2012 and the VHR randomization closed on February 15, 2017. Eligibility criteria included: 1-9 years of age inclusive with a presenting white blood cell count ≥50×109/L; ≥10 to <31 years of age with any white blood cell count; >1 to <31 years of age with testicular leukemia, central nervous system leukemia (CNS3; ≥5/μL white blood cells and cytospin positive for blasts in the cerebral spinal fluid and/or clinical signs of CNS leukemia), or steroid pre-treatment in patients <10 years of age for whom no pre-steroid white blood cell count was obtained.7 At the end of induction therapy, patients were fur ther classified as VHR if they had any of the following criteria: ≥13 years of age; CNS3 leukemia at diagnosis; day 29 bone marrow minimal residual disease ≥0.01% determined by flow cytometry;7,8 induction failure [>25% bone marrow blasts (M3) on induction day 29], severe hypodiploidy (DNA index <0.81 and/or <44 chromosomes); intrachromosomal amplification of chromosome 21, or lysine methyltransferase 2A (KMT2A, formerly mixed lineage leukemia, MLL) rearrangement. In addition, patients with National Cancer Institute standard-risk B-ALL, enrolled on the COG study AALL0932 (NCT01190930) for standard-risk B-ALL (≥1 to <10 years of age with a white blood cell count <50x109/L), were classified as VHR following induction if they met any of the above VHR criteria or if they had day 29 bone marrow minimal residual disease ≥0.01% in the absence of favorable cytogenetics (no trisomies of chromosomes 4 and 10 and no ETV6/RUNX1 fusion). Patients with Down syndrome were not eligible for the VHR stratum given the concern of increased toxicity of the regimen. Toxicities were graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.0. The study was approved by the National Cancer Institute, the Pediatric Central Institutional Review Board, and institutional review boards at each participating COG institution.
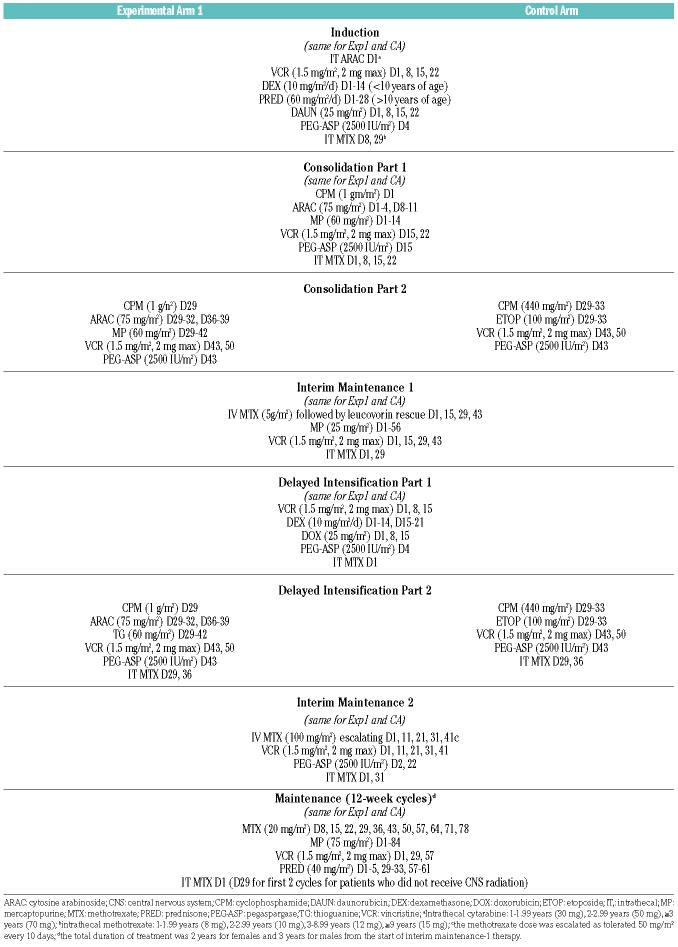
The AALL1131 study was originally designed to investigate the addition of clofarabine to cyclophosphamide/etoposide as experimental arm 2 versus cyclophosphamide/etoposide (experimental arm 1) versus the control arm in a 2:2:1 randomization for patients with VHR B-ALL. The study design was later amended to a 2:1 randomization between experimental arm 1 and the control arm, retaining those patients initially randomized to experimental arm 1 and the control arm, after the clofarabine arm (experimental arm 2) was closed because of unacceptable toxicity (September 2014).7 Patients classified as VHR were subsequently randomized after induction in a 1:2 fashion to cyclophosphamide (1 g/m2 day 29)/cytarabine (75 mg/m2 days 29-33 and 36-40)/6-mercaptopurine (60 mg/m2 days 29-42 consolidation) or thioguanine (60 mg/m2 days 29-42 during part 2 of delayed intensification) (control arm) or cyclophosphamide (440 mg/m2, days 29-33)/etoposide (100 mg/m2, days 29-33) (experimental arm 1) during part 2 of consolidation and delayed intensification. Both arms included the same dose and schedule of pegaspargase (2,500 IU/m2) on day 43 and vincristine (1.5 mg/m2) on days 43 and 50 of consolidation and delayed intensification. The delayed intensification also included intrathecal methotrexate on days 29 and 36 on all arms. Patients with CNS3 leukemia received 1800 cGy of cranial irradiation during the first month of maintenance therapy. Any patient with testicular leukemia at diagnosis that did not resolve by the end of induction received 2400 cGy testicular irradiation during consolidation. The remainder of the VHR therapy was identical between the two arms.7 The complete AALL1131 VHR treatment regimen is shown in Table 1. The study did not capture detailed information on patients who underwent hematopoietic stem cell transplantation off protocol therapy. The VHR randomization was powered (80%) to compare a 4-year DFS of 70% versus 79% (HR=0.661) using a two-sided log-rank test (α=5%). DFS was defined as the time from post-induction randomization to first event (death in remission, relapse, or second malignant neoplasm) or date of last contact for those who remained event-free. Survival rates were estimated using the method of Kaplan-Meier with standard errors of Peto et al.9,10 Interim monitoring for efficacy utilized an αt2 spending function and futility monitoring was based on the method of Anderson and High,11 with the first interim analysis scheduled for when 20% of the expected DFS events had been observed. Prospective interim monitoring rules for efficacy and futility were included where futility would be determined for a one-sided P-value ≥0.7664. Cumulative incidence rates were computed using the cumulative incidence function for competing risks, and comparisons were made using the K-sample test.12 Proportions between the two arms were compared using a χ2 test or Fisher exact test. A P value <0.05 was considered statistically significant for all comparisons. All analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA). Graphics were generated with R version 2.13.1 (http://www.r-project.org).
Table 1.
AALL1131 very high-risk treatment regimen.
Results
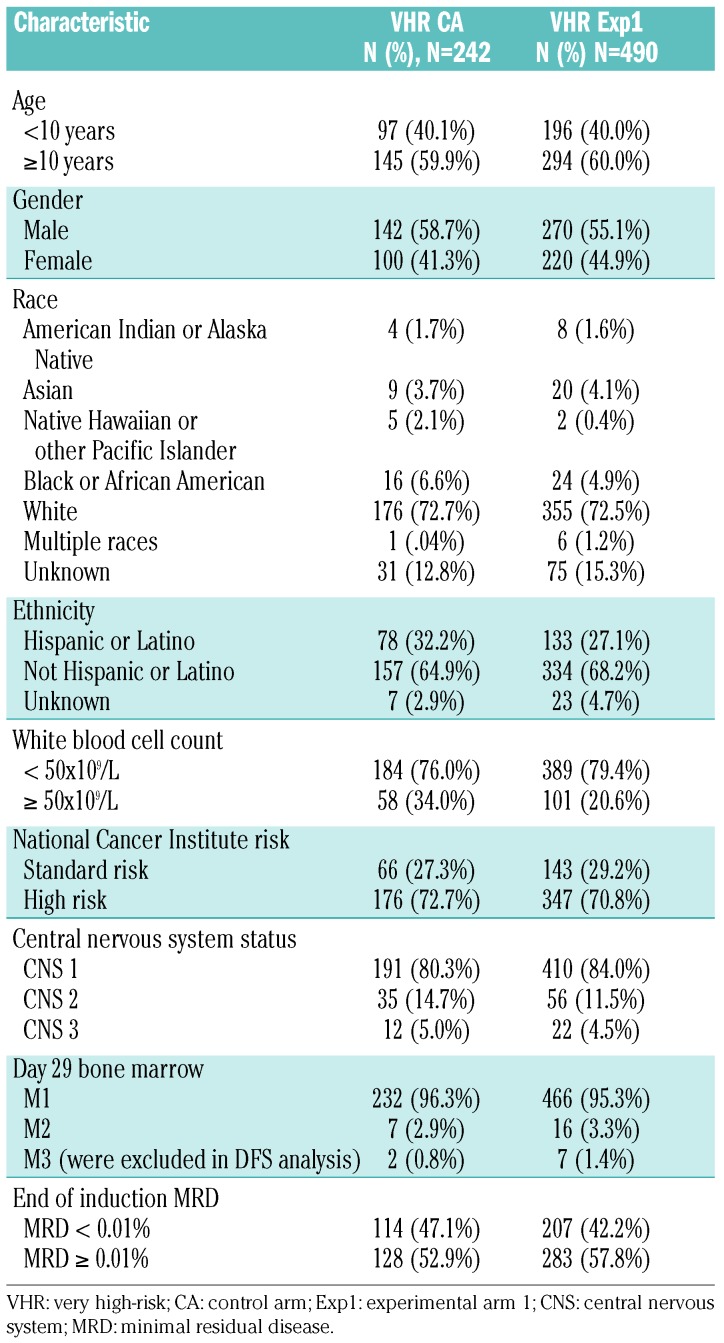
A total of 732 eligible, evaluable patients were enrolled in the VHR part of AALL1131 and randomized to either the control arm (n=242) or experimental arm 1 (n=490) as of the data freeze for this report (December /31, 2017). The Consolidated Standards of Reporting Trials (CONSORT) diagram for the study is shown in Online Supplementary Figure S1. Two patients on the control arm and seven on experimental arm 1 had induction failures and were excluded from analyses, resulting in 240 and 483 patients, respectively, on the two arms included in this report. There were no significant differences in patients’ characteristics between the two arms (Table 2), including no difference in the proportion of patients with minimal residual disease <0.01% at the end of consolidation between those in the control arm (87.4%) and those in experimental arm 1 (87.2%). As of the data cutoff date of December 31, 2016, 20% (n=41) of expected DFS events had occurred, triggering a scheduled interim monitoring for efficacy and futility. The 3-year DFS rates were 88.3±6.3% (control arm) versus 81.2±5.6% (experimental arm 1) (P=0.92). The study was stopped for futility as the interim monitoring boundary was crossed, indicating non-superiority of experimental arm 1 [hazard ratio 0.606 (95% confidence interval: 0.297-1.237)]. As a result, the VHR sub-study of AALL1131 was permanently closed in February 2017.
Table 2.
Patients’ characteristics.
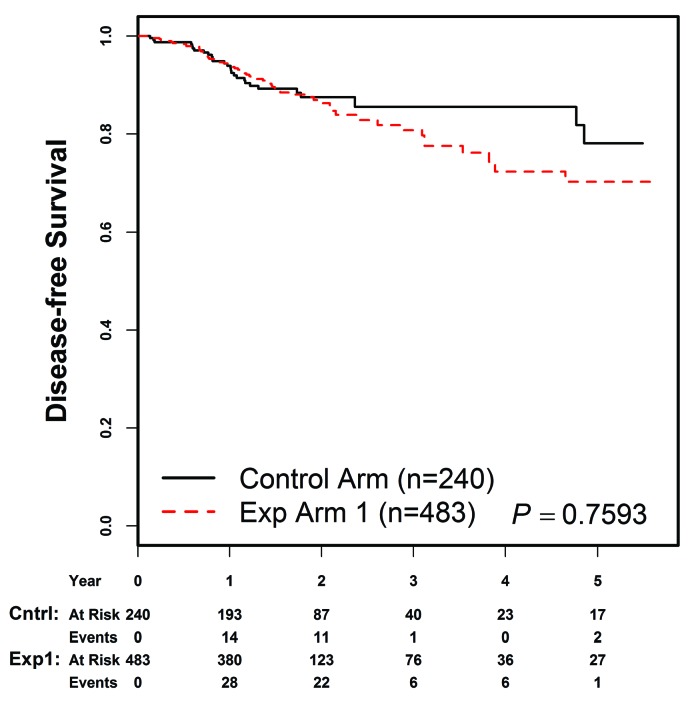
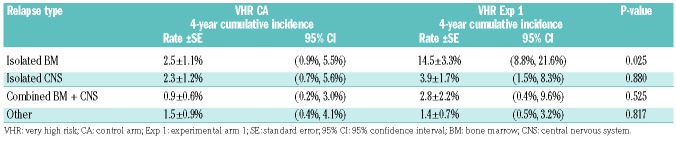
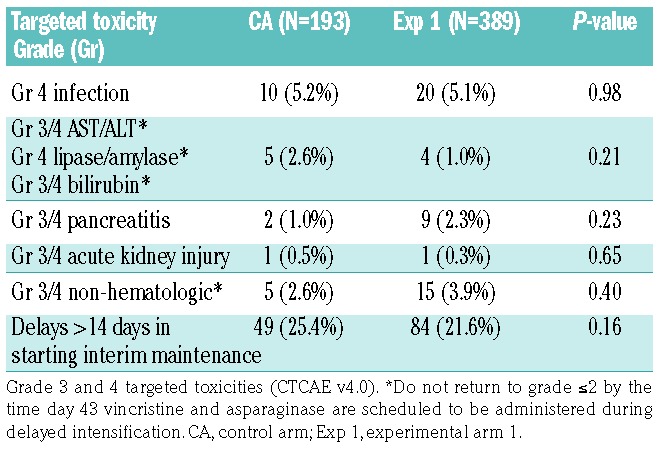
As of December 31, 2017, the date of freezing the data, the 4-year DFS rates were 85.5±6.8% (control arm) versus 72.3±6.3% (experimental arm 1) (P=0.76) (Figure 1). Table 3 gives the distribution of DFS events by arm. The 4-year cumulative incidence rates for each type of relapse are summarized in Table 4, by regimen. The cumulative incidence of isolated bone marrow relapses was significantly different between the control arm and experimental arm 1 (2.5±1.1% versus 14.5±3.3%, P=0.025). Grade 5 toxicity rates were 5.0% on the control arm (n=12) and 2.9% on experimental arm 1 (n=14). Of the 12 grade 5 toxicities reported among patients on the control arm, six occurred on therapy. These six deaths were attributed to infection (n=4) and multi-organ failure (n=2). Of the 14 deaths reported among patients on experimental arm 1, five occurred while on therapy and were attributed to infection (n=3), thrombosis (n=1) and multi-organ failure (n=1). A total of 235 subjects on the control arm and 477 on experimental arm 1 completed part 2 of consolidation and had toxicity data submitted. There were no significant differences in grade 3/4 adverse events or delays in starting the interim maintenance-1 phase of therapy after consolidation between the control arm and experimental arm 1 (Table 5A). In addition, a total of 193 subjects on the control arm and 389 on experimental arm 1 completed part 2 of delayed intensification and had toxicity data submitted. There were no significant differences in grade 3/4 adverse events or delays in starting the interim maintenance-1 phase of therapy after delayed intensification between the control arm and experimental arm 1 (Table 5B).
Figure 1.
Outcomes based on information in the database at December 31, 2017 with additional follow-up, Four-year disease-free survival rates were 85.5±6.8% in the control arm (Contr) versus 72.3±6.3% in experimental arm 1 (Exp 1) (P=0.76).
Table 3.
Summary of disease-free survival events by randomization arm.
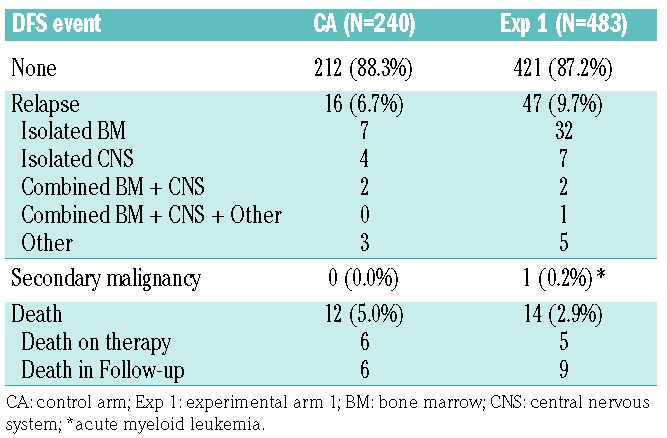
Table 4.
Cumulative incidence rates for types of relapse by randomization arm.
Table 5A.
Toxicities in patients completing part 2 of consolidation.
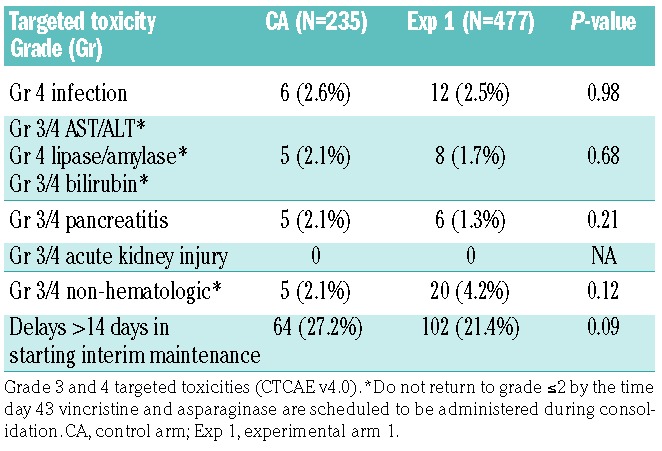
Table 5B.
Toxicities in patients completing part 2 of the delayed intensification.
One-hundred and seven patients went off therapy during consolidation or interim maintenance-1, with the reason stated being “physician determines it is in the best interest of the patient” (n=86; 32 in the control arm and 54 in experimental arm 1) or “refusal of further protocol therapy by patient/parent/guardian” (n=21; 3 in the control arm and 18 in experimental arm 1): Some of these patients may have proceeded to hematopoietic stem cell transplantation. Long-term study outcomes may be published in the future when follow-up data are more mature.
Discussion
The 5-year event-free survival rate for patients with high-risk B-ALL enrolled in the COG AALL0232 trial (2004 – 2011) randomized to high-dose methotrexate during interim maintenance-1 was 79.6% compared to 75.2% for the patients in the control arm (Capizzi methotrexate) (P=0.008) of that study.3 Patients identified as having VHR B-ALL are predicted to fare worse than high-risk patients overall and, depending on the specific VHR risk factors, their 4-year DFS rates can range anywhere from 40 to 80%.8,13–22 Based on the relatively poor outcomes for these VHR patients, they are candidates for investigation of novel, more intensive, yet potentially more toxic, therapeutic strategies designed to improve DFS. Based on this hypothesis, and without mature data from the AALL0232 study available at the time of study development, the COG high-risk B-ALL study AALL1131 was designed to further intensify cytotoxic chemotherapy during the consolidation and delayed intensification phases of therapy to improve the DFS in the VHR subgroup. Two intensification strategies were tested in AALL1131 and compared to the control arm. The first intensification strategy included the combination of clofarabine with cyclophosphamide/etoposide, a promising combination in relapsed ALL23,24 (experimental arm 2) and cyclophosphamide/etoposide without clofarabine (experimental arm 1). This began as a 1:2:2 randomization between the control arm and experimental arms 1 and 2, respectively. Experimental arm 2, testing clofarabine, was found to be too toxic and not feasible when given in this combination to newly diagnosed patients with VHR B-ALL, and this arm of AALL1131 was, therefore, closed to further accrual in September 2014.7 AALL1131 thus continued as a two-arm study comparing the control arm with experimental arm 1 in a 1:2 randomized fashion. This randomization was later stopped for futility when the interim monitoring boundary was crossed, identifying non-superiority of DFS when consolidation and delayed intensification included cyclophosphamide/etoposide compared to standard VHR therapy (modified augmented Berlin-Frankfurt-Münster regimen). With additional follow-up after closure of the randomization, there was even stronger evidence that experimental arm 1 would never be superior to the control arm with the reported DFS being 85.5±6.8% for the control arm compared to 72.3±6.3% for experimental arm 1 (P=0.76). The 4-year DFS of 85.5±6.8% reported for the control arm of this study was higher than the 70% we originally predicted based on data available for patients with VHR features treated in the preceding B-ALL studies for standard-risk (AALL0331) and high-risk (AALL0232) patients. Many patients in these earlier studies did not receive high-dose methotrexate during interim maintenance-1 which may have resulted in the differences in the DFS rates we report. Additionally, the definitions of VHR were expanded in AALL1131 to include groups of patients at least 13 years of age as well as lower minimal residual disease thresholds which may also have contributed to differences in the DFS we observed.
In summary, intensification of cytotoxic chemotherapy by substituting either clofarabine/cyclophosphamide/etoposide or cyclophosphamide/etoposide for cyclophosphamide/cytarabine/6-mercaptopurine (or 6-thioguanine) during part 2 of consolidation and delayed intensification did not improve DFS compared to that of patients receiving standard COG VHR therapy in this study. Future therapeutic studies for pediatric patients with VHR B-ALL could pursue immunologically and/or molecularly targeted therapies which may have more potential to improve outcomes than further intensification of cytotoxic chemotherapy.25–27 In this regard, the COG is currently investigating the tyrosine kinase inhibitor dasatinib for newly diagnosed high-risk patients with Philadelphia-like B-ALL harboring ABL-class lesions (AALL1131, NCT02883049) and ruxolitinib for newly diagnosed NCI-HR patients with Philadelphia-like BALL harboring CRLF2 rearranged and/or JAK pathway mutated B-ALL (AALL1521, NCT02723994). Additionally, the COG plans to bring both inotuzumab ozogamicin (humanized monoclonal antibody against CD22) and blinatumomab (anti-CD19/CD3 bispecific T-cell engager antibody) into the next generation of upfront studies for high-risk and standard-risk B-ALL, respectively, anticipated to open in 2019.
Supplementary Material
Acknowledgments
This trial was supported by grants U10 CA98543, U10 CA98413, U10 CA180886, U10 CA180899 from the National Institutes of Health and supported by St. Baldrick’s Foundation. LG is the Ergen Family Chair in Pediatric Oncology at the Children’s Hospital of Colorado. EAR is a KiDS of NYU Foundation Professor at NYU Langone Health. MLL is the UCSF Benioff Chair of Children’s Health and Deborah and Arthur Ablin Endowed Chair in Pediatric Molecular Oncology. SPH is the Jeffrey E. Perelman Distinguished Chair in the Department of Pediatrics at The Children’s Hospital of Philadelphia.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/5/986
References
- 1.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30(14): 1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pui CH, Yang JJ, Hunger SP, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. 2015;33(27):2938–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsen EC, Devidas M, Chen S, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: a report from children’s oncology group study AALL0232. J Clin Oncol. 2016;34(20):2380–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raetz EA, Borowitz MJ, Devidas M, et al. Reinduction platform for children with first marrow relapse of acute lymphoblastic leukemia: a Children’s Oncology Group Study [corrected]. J Clin Oncol. 2008;26(28):3971–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raetz EA, Cairo MS, Borowitz MJ, et al. Re-induction chemoimmunotherapy with epratuzumab in relapsed acute lymphoblastic leukemia (ALL): phase II results from Children’s Oncology Group (COG) study ADVL04P2. Pediatr Blood Cancer. 2015;62(7):1171–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crooks GM, Sato JK. Ifosfamide and etoposide in recurrent childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 1995;17(1):34–38. [DOI] [PubMed] [Google Scholar]
- 7.Salzer WL, Burke MJ, Devidas M, et al. Toxicity associated with intensive postinduction therapy incorporating clofarabine in the very high-risk stratum of patients with newly diagnosed high-risk B-lymphoblastic leukemia: a report from the Children’s Oncology Group study AALL1131. Cancer. 2018;124(6):1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borowitz MJ, Wood BL, Devidas M, et al. Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children’s Oncology Group study AALL0232. Blood. 2015;126(8):964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35(1):1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 11.Anderson JR, High R. Alternatives to the standard Fleming, Harrington, and O’Brien futility boundary. Clin Trials. 2011;8(3): 270–276. [DOI] [PubMed] [Google Scholar]
- 12.Gray R. A Class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 13.Schultz KR, Pullen DJ, Sather HN, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG). Blood. 2007;109(3): 926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pui CH, Crist WM, Look AT. Biology and clinical significance of cytogenetic abnormalities in childhood acute lymphoblastic leukemia. Blood. 1990;76(8):1449–1463. [PubMed] [Google Scholar]
- 15.Pui CH, Gaynon PS, Boyett JM, et al. Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet. 2002;359(9321):1909–1915. [DOI] [PubMed] [Google Scholar]
- 16.Pui CH, Carroll AJ, Raimondi SC, et al. Clinical presentation, karyotypic characterization, and treatment outcome of childhood acute lymphoblastic leukemia with a near-haploid or hypodiploid less than 45 line. Blood. 1990;75(5):1170–1177. [PubMed] [Google Scholar]
- 17.Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a Children’s Oncology Group study. J Clin Oncol. 2009;27(31):5175–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pui CH, Chessells JM, Camitta B, et al. Clinical heterogeneity in childhood acute lymphoblastic leukemia with 11q23 rearrangements. Leukemia. 2003;17(4):700–706. [DOI] [PubMed] [Google Scholar]
- 19.Heerema NA, Nachman JB, Sather HN, et al. Hypodiploidy with less than 45 chromosomes confers adverse risk in childhood acute lymphoblastic leukemia: a report from the Children’s Cancer Group. Blood. 1999; 94(12):4036–4045. [PubMed] [Google Scholar]
- 20.Silverman LB, Gelber RD, Young ML, Dalton VK, Barr RD, Sallan SE. Induction failure in acute lymphoblastic leukemia of childhood. Cancer. 1999;85(6):1395–1404. [DOI] [PubMed] [Google Scholar]
- 21.van Dongen JJ, Seriu T, Panzer-Grumayer ER, et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998;352(9142):1731–1738. [DOI] [PubMed] [Google Scholar]
- 22.Dworzak MN, Froschl G, Printz D, et al. Prognostic significance and modalities of flow cytometric minimal residual disease detection in childhood acute lymphoblastic leukemia. Blood. 2002;99(6):1952–1958. [DOI] [PubMed] [Google Scholar]
- 23.Hijiya N, Thomson B, Isakoff MS, et al. Phase 2 trial of clofarabine in combination with etoposide and cyclophosphamide in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. Blood. 2011;118(23):6043–6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Locatelli F, Testi AM, Bernardo ME, et al. Clofarabine, cyclophosphamide and etoposide as single-course re-induction therapy for children with refractory/multiple relapsed acute lymphoblastic leukaemia. Br J Haematol. 2009;147(3):371–378. [DOI] [PubMed] [Google Scholar]
- 25.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gokbuget N, Zugmaier G, Klinger M, et al. Long-term relapse-free survival in a phase 2 study of blinatumomab for the treatment of patients with minimal residual disease in B-lineage acute lymphoblastic leukemia. Haematologica. 2017;102(4):e132–e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weston BW, Hayden MA, Roberts KG, et al. Tyrosine kinase inhibitor therapy induces remission in a patient with refractory EBF1-PDGFRB-positive acute lymphoblastic leukemia. J Clin Oncol. 2013;31(25):e413–416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.