Abstract
Standard first-line therapy of chronic myeloid leukemia is treatment with imatinib. In the randomized German Chronic Myeloid Leukemia-Study IV, more potent BCR-ABL inhibition with 800 mg (‘high-dose’) imatinib accelerated achievement of a deep molecular remission. However, whether and when a de-escalation of the dose intensity under high-dose imatinib can be safely performed without increasing the risk of losing deep molecular response is unknown. To gain insights into this clinically relevant question, we analyzed the outcome of imatinib dose reductions from 800 mg to 400 mg daily in the Chronic Myeloid Leukemia-Study IV. Of the 422 patients that were randomized to the 800 mg arm, 68 reduced imatinib to 400 mg after they had achieved at least a stable major molecular response. Of these 68 patients, 61 (90%) maintained major molecular remission on imatinib at 400 mg. Five of the seven patients who lost major molecular remission on the imatinib standard dose regained major molecular remission while still on 400 mg imatinib. Only two of 68 patients had to switch to more potent kinase inhibition to regain major molecular remission. Importantly, the lengths of the intervals between imatinib high-dose treatment before and after achieving major molecular remission were associated with the probabilities of maintaining major molecular remission with the standard dose of imatinib. Taken together, the data support the view that a deep molecular remission achieved with high-dose imatinib can be safely maintained with standard dose in most patients. Study protocol registered at clinicaltrials.gov 00055874.
Introduction
Approved first-line therapies of chronic myeloid leukemia (CML) are the tyrosine kinase inhibitors (TKIs) imatinib, dasatinib, nilotinib and bosutinib.1–5 Imatinib led to distinctively improved progression-free and overall survival of chronic phase CML patients as compared with previous conventional treatment standards in CML.6,7 Second-generation TKIs, such as nilotinib and dasatinib, but also a higher dose of imatinib (800 mg/day), induce deep molecular response (MR) faster8,9 and in a larger proportion of patients.10,11 As a consequence, deep molecular remission (an essential eligibility criterion for TKI discontinuation) can be achieved earlier and in more patients when compared to imatinib standard dose.12,13 However, the benefit of pursuing highly-potent BCR-ABL-kinase inhibition once deep MR has been achieved is less clear. Moreover, for those patients in deep MR, which (for whatever reason) require long-term treatment, the tolerability and prevention of organ damage through clinically relevant and potentially irreversible side effects, such as pulmonary hypertension, diabetes, hypercholesterinemia, and cardiovascular morbidity become the most important priority.14–17 Thus, if high-potency BCR-ABL inhibition is not needed to sustain remission or improve survival, then the risk of potentially harmful side effects from second- or third-generation TKI must be weighed against the long-term safety of using imatinib,18–20 especially when also considering that generic imatinib is more cost effective. By analyzing the outcome of 800 mg to 400 mg imatinib dose reductions performed in at least stable major molecular remission (MMR) within the randomized German CML-Study IV,8,21 we aimed to address the clinically important questions of in which patients and at what time after initiation of strong BCR-ABL inhibition with 800 mg imatinib less potent BCR-ABL inhibition with standard dose imatinib is sufficient to maintain stable MMR.
Methods
Patients and Chronic Myeloid Leukemia-Study IV protocol
All patients investigated in this study were treated within the randomized German CML-Study IV.8,21 Imatinib monotherapy at 800 mg/day was one of the five arms in this trial. The study protocol was registered at clinicaltrials.gov 00055874. Randomization took place from July 2002 through March 2012. During a pilot-phase of 3 years, only high-risk patients according to the Euro score22 were randomized to imatinib 800 mg/day. In 2005, imatinib 800 mg/day was started as a full study arm.
To avoid selection bias towards high-risk patients, in this retrospective analysis, only patients randomized from 2005 were evaluated.
Definition of high-dose imatinib treatment
Imatinib at a dose of 800 mg/day for at least 6 months was classified as high-dose therapy. Six months was chosen because the presence of MMR after 6 months significantly increased the probabilities of patients going on to achieve deep MR later.8 A high-dose treatment interval began with the first dose of 800 mg/day and ended at the time of imatinib dose reduction to 400 mg/day. An intermittent 600 mg/day interval which directly preceded or followed a high-dose treatment interval with 800 mg/day was still considered high-dose treatment because the effective median dose of imatinib in the 800 mg arm was seen to be only 600 mg in the CML-Study IV.8
The molecular analyses are described in the Online Supplementary Appendix.
Statistical analysis
Survival without loss of MMR was defined as the time between the start of reduced imatinib therapy with 400 mg/day either until loss of MMR or until the date of the last evaluation of MR status with the date linkable to the reduction period, as defined in the Online Supplementary Methods. Probabilities of molecular relapse-free survival (RFS) were estimated by the Kaplan-Meier method. The association between a variable and molecular RFS was assessed by Cox regression.23 For identification of cutoffs, the minimal P-value approach was used while assuming that the smallest group should contain at least 10% of patients.24 Bootstrap resampling and kernel density estimation were carried out to assess the stability of a cutoff.25,26
Point estimates are given together with their 95% confidence intervals (95%CI). In the case of the hazard ratios (HR), for estimation of the 95%CI, the profile likelihood was used and P-values were calculated from the likelihood ratio test. All analyses are descriptive and exploratory. Apart from the minimal P-value approach, the significance level of the two-sided P-value was 0.05 for all statistical tests. Analyses were carried out with SAS v.9.4 or R v.3.4.3.
Ethical approval
The CML-Study IV was performed in accordance with the Declaration of Helsinki, and was approved by the central ethics committee of the Medizinische Fakultaet Mannheim and the local ethics committees of all participating centers. Written informed consent was obtained from all patients prior to entering the CML-Study IV.
Results
Imatinib dose reduction in the 800 mg cohort of the Chronic Myeloid Leukemia-Study IV
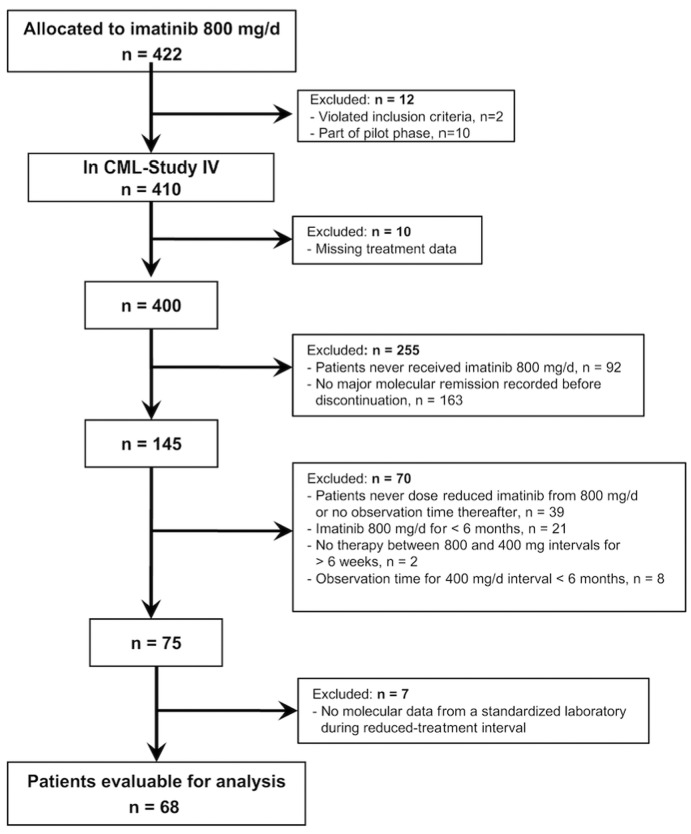
Of 1551 patients with newly diagnosed chronic phase CML, 422 were randomly assigned to 800 mg imatinib per day. Of these, two patients violated CML-Study IV inclusion criteria, ten were part of the pilot study, and a further ten were excluded from this analysis due to missing treatment data (see the CONSORT flow diagram in Figure 1).
Figure 1.
Flow diagram: patients of the Chronic Myeloid Leukemia-Study IV considered for final analysis.
Of the remaining 400 patients randomized to 800 mg imatinib, 92 patients had never received imatinib 800 mg/day and 163 patients never achieved MMR within the imatinib 800 mg/day interval. Of the remaining 145 patients, 39 had never reduced the 800 mg/day dose or had no observation time after dose reduction. A further 21 had an 800 mg/day interval of less than 6 months (i.e. not high-dose imatinib by our definition). Two patients were excluded because they had more than 6 weeks without any therapy between ending 800 mg/day imatinib and recommencing 400 mg/day. Eight patients were not considered because treatment with 400 mg/day lasted for less than 6 months. After exclusion of a further seven patients who had not been monitored by the central molecular diagnostic laboratory of the CML-Study IV, 68 patients were evaluable for our analyses.
Data entry was closed on July 21, 2015.
Patients’ characteristics are shown in Online Supplementary Table S1. Median age was 52 years and 71% of the 68 patients were male.
Treatment course of patients with imatinib dose reduction to 400 mg
Twenty-five of the 68 patients on high-dose imatinib (37%) started their primary treatment directly with 800 mg/day (first treatment interval). Forty patients (59%) increased to 800 mg/day after a first period of 400 mg imatinib. Three patients only started the 800 mg imatinib dose later.
Median time on high-dose imatinib therapy was 31 months (range: 6-98 months) for the 68 patients who later reduced imatinib treatment to 400 mg (Online Supplementary Table S1). In this cohort, the median duration of treatment with 400 mg/day after dose reduction was 34 months (range: 6-78 months). For 53 out of 68 patients (78%), this was the last reported treatment and dose. Five patients (7%) eventually stopped any TKI therapy following imatinib dose reduction to 400 mg. In one patient, no information regarding treatment after dose reduction to 400 mg was available. The remaining 9 out of 68 patients (13%) received a more potent ABL-kinase inhibition: 600 mg imatinib (n=1), 800 mg imatinib (n=5), nilotinib (n=2), or dasatinib (n=1).
Molecular relapse-free survival after imatinib dose reduction to 400 mg
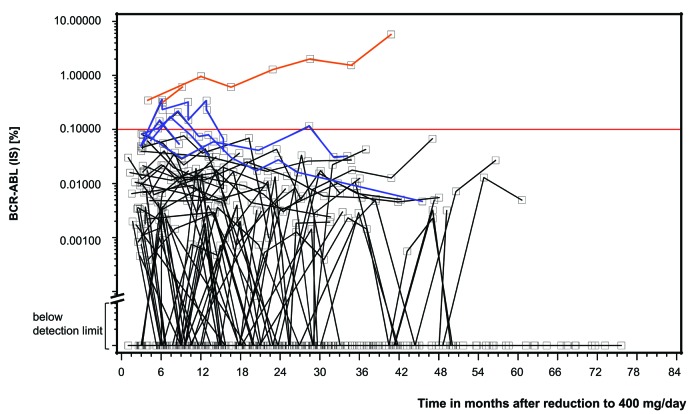
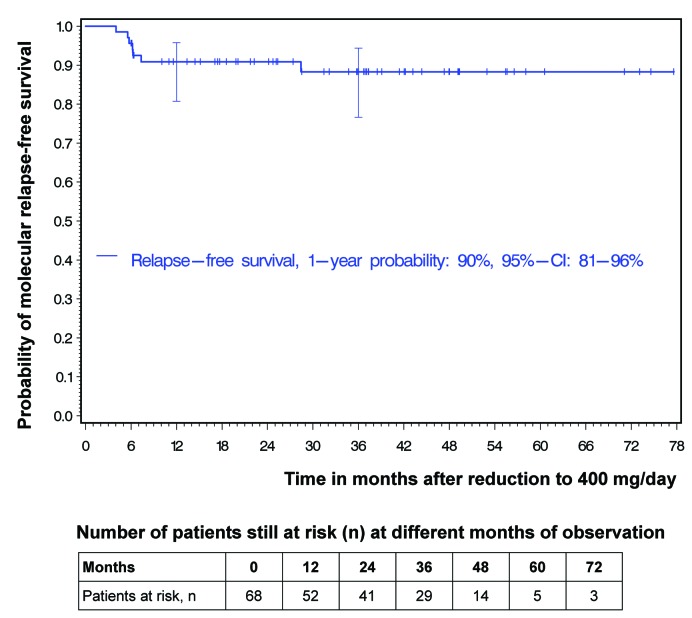
Seven of 68 patients experienced a loss of MMR during the reduction interval (Figure 2). This resulted in a 1-year molecular relapse-free survival (RFS) of 90% (95%CI: 81-96%). With only one MMR loss occurring later than 12 months after dose reduction, the 3-year molecular RFS was 88% (95%CI: 77-94%) (Figure 3). However, MMR loss was only temporary in five of the seven patients; these patients regained MMR while still on the lower 400 mg imatinib dose. Only two patients with MMR loss were switched to more potent ABL-inhibition with nilotinib or 600 mg imatinib to regain MMR (Figure 2). It is worthy of note that, at the time of stopping high-dose treatment, 43 of 68 patients were at least in MR4, 33 of them even at least in MR4.5. Of the 43 patients, 10 lost MR4 at some point; none lost MMR.
Figure 2.
Courses of BCR-ABL (IS) in 68 patients with imatinib dose reduction. Results below the horizontal red line represent at least major molecular response (MMR). Sixty-one patients have never lost MMR (courses with black lines). Five patients with loss of MMR regained MMR while continuing with reduced imatinib dose at 400 mg/day (blue lines). Two patients with loss of MMR did not regain MMR while on the lower imatinib dose and were switched to nilotinib or imatinib at 600 mg/day, respectively (orange lines).
Figure 3.
Probabilities of molecular relapse-free survival after dose reduction to imatinib at 400 mg/day. At 12 and 36 months, horizontal crossbars indicate the upper and lower limit of the 95% confidence interval (CI) for the estimated probability.
Clinical variables and high-dose imatinib treatment durations prior to and after achieving major molecular response were associated with probabilities of relapse-free survival
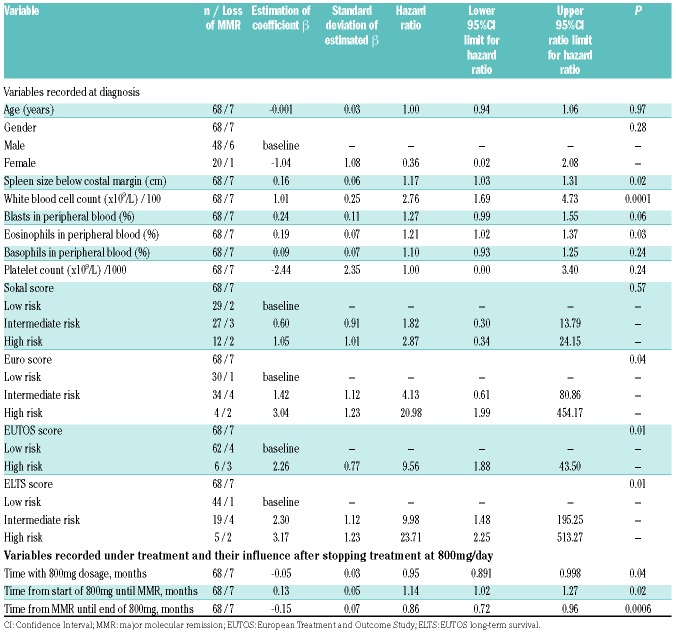
Of the clinical variables evaluated at diagnosis, larger spleen size below costal margin (HR: 1.17, 95%CI: 1.03-1.31; P=0.02), higher white blood cell count (WBC) (HR: 2.76, 95%CI: 1.69-4.73; P=0.0001), and a higher percentage of eosinophils (HR: 1.21, 95%CI: 1.02-1.37; P=0.03) were significantly associated with probabilities of lower RFS (Table 1). Furthermore, the high-risk groups according to the Euro and the European Treatment and Outcome Study (EUTOS) scores, as well as the intermediate- and high-risk groups according to the EUTOS long-term survival (ELTS) score, suggested significantly worse molecular RFS than their corresponding low-risk groups.
Table 1.
Univariate Cox regression estimating the influence on relapse-free survival after reduction to imatinib at 400 mg.
The longer the total treatment time at 800 mg/day, the higher were the probabilities of RFS (HR: 0.95, 95%CI: 0.891-0.998; P=0.04). However, as in the EURO-SKI study, which analyzed TKI discontinuation, the main focus was to investigate treatment time after dividing this time interval into the time of high-dose treatment before and after achievement of MR.27
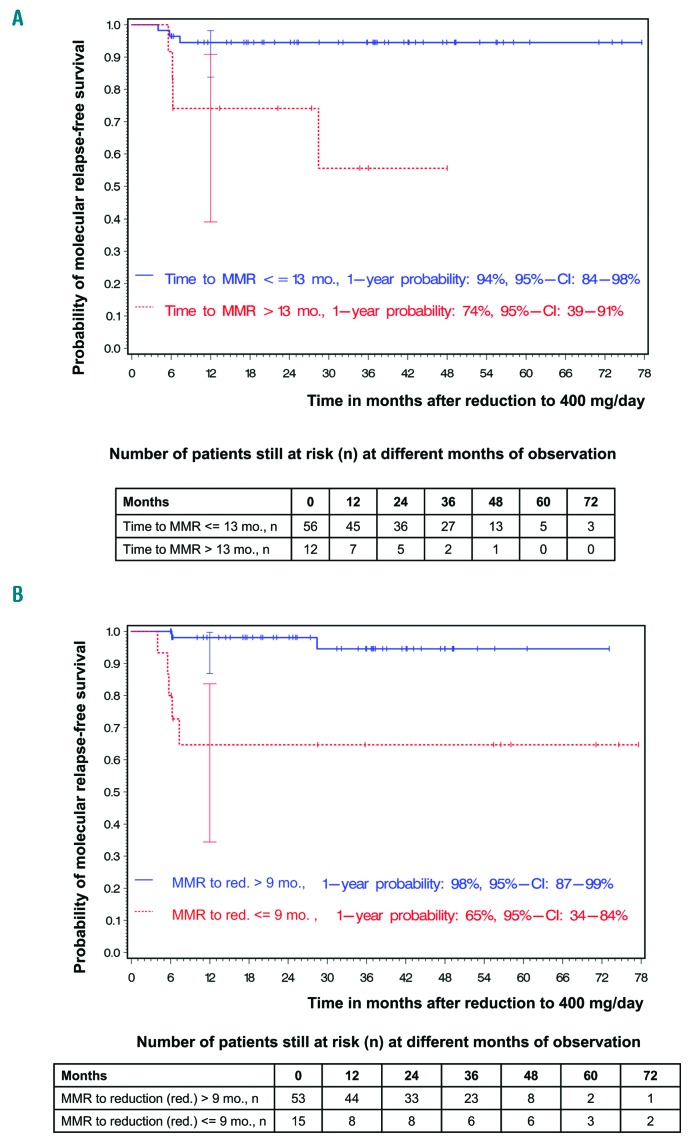
Four of the 68 patients had already achieved MMR with 400 mg imatinib/day prior to the high-dose treatment interval. The median time to achieving MMR was 5 months (range: 0-23 months) (Online Supplementary Table S1). The longer the time with treatment at 800 mg/day until achieving MMR, the lower were the probabilities of RFS (HR: 1.14, 95%CI: 1.02-1.27; P=0.02). Using the minimum P-value approach, with the prerequisite that the smallest group contained at least 10% of the patients, a cutoff of 13 months was observed (Padjusted=0.007). This cutoff was confirmed with bootstrap resampling: in 1000 bootstrap samples, the cutoff of 13 months was most frequently chosen. For the 56 patients who had an MMR within 13 months while on treatment at 800 mg/day, the probability of molecular RFS 12 months after stopping high-dose treatment was 94% (95%CI: 84-98%), whereas it was 74% [95%CI: 39-91%; HR: 7.39 (95%CI: 1.62-37.74)] for the 12 patients who had an MMR after more than 13 months of high-dose treatment (Figure 4A).
Figure 4.
Factors influencing the probabilities of molecular relapse-free survival after imatinib dose reduction to 400 mg/day. (A) Impact of time to major molecular response (MMR) and molecular relapse-free survival. (B) Impact of interval between MMR and imatinib dose reduction and molecular relapse-free survival. At 12 months (mo), horizontal crossbars indicate the upper and lower limit of the 95% confidence interval (CI) for the estimated probability.
The median time from achievement of MMR until dose reduction to 400 mg was 23 months (range: 0-93 months) (Online Supplementary Table S1). The longer the time with MMR while on treatment at 800 mg/day, the higher were the probabilities of RFS (HR: 0.86, 95%CI: 0.72-0.96; P=0.0006). Using the minimum P-value approach, with the prerequisite that the smallest group contained at least 10% of the patients, a cutoff of 8.5 months was observed (Padjusted=0.011). This cutoff was confirmed with bootstrap resampling: in 1000 bootstrap samples, the cutoff of 8.5 months was most frequently chosen. For the 53 patients who were more than 9 months on high-dose treatment after achievement of MMR, the probability of molecular RFS 12 months after stopping high-dose treatment was 98% (95%CI: 87-99%), whereas it was 65% [95%CI: 34-84%; HR: 0.096 (95%CI: 0.014-0.449)] for the 15 patients who were only on high-dose treatment for 9 months or less after achieving MMR (Figure 4B).
Discussion
The concept of starting CML therapy upfront with more potent BCR-ABL inhibition than is achievable with 400 mg imatinib has been introduced to prevent early disease progression and induce deep MR faster and more effectively.2 However, it is not known in which patients and when after the initiation of a more potent BCR-ABL kinase inhibition (second/third-generation TKI or 800 mg imatinib) a deep MR can be maintained after de-escalation to 400 mg imatinib.
Trials investigating dose reductions are rare. In the DESTINY study, the dose of second-generation TKIs was reduced to half the respective standard dose.28 However, in terms of BCR-ABL inhibitory potency, even reduced second-generation TKI doses such as those used in the DESTINY trial demonstrate significantly more BCR-ABL inhibition than 400 mg imatinib. To our knowledge, a controlled switch from highly potent BCR-ABL kinase inhibition with 800 mg imatinib or second/third-generation TKI to 400 mg/day imatinib has never been performed prospectively.
On the other hand, a reduction of imatinib treatment intensity to 400 mg is frequently required in patients who achieve a deep MR but experience toxicities or acquire/have worsening comorbidities of a type that prevents the further use of second-generation TKI. Furthermore, those patients with deep MR who relapse after TKI cessation or who do not wish to discontinue their TKI, and therefore require life-long TKI therapy, are all candidates for a dose de-escalation to imatinib 400 mg.
Here, we studied the stability of a deep MR in patients of the German CML-Study IV who had MMR or better response for at least 6 months when they reduced imatinib from 800 mg to 400 mg per day. We wished to gain insight into whether treatment duration with 800 mg imatinib has an impact on subsequent maintenance of deep MR with imatinib at the 400 mg standard dose. We also searched for clinical variables associated with maintenance of at least MMR after dose reduction of imatinib.
We found that, if BCR-ABL-monitoring once every three months is ensured, imatinib dose reduction from 800 mg to 400 mg/day in patients with stable MMR did not compromise efficacy or risk sustained MMR in most patients, as only two of seven patients who had lost MMR on 400 mg imatinib required a rescue treatment with more potent BCR-ABL-kinase inhibitors. This also suggests that if standard dose imatinib treatment and BCR-ABL monitoring are ensured, a single loss of MMR might not require immediate re-intensification of TKI treatment.29 Secondly, despite only 7 events, statistical modeling suggested that achieving an MMR within 13 months under 800 mg imatinib, as well as staying on 800 mg imatinib for at least 9 months after achievement of MMR, are both good predictors of a successful continuous MMR maintenance under the standard imatinib dose of 400 mg.
Interestingly, with the exception of WBC count, all other prognostic markers identified for a successful imatinib dose reduction have previously also been reported as predictors for treatment-free remission (TFR).7,22,27,30 Based on this, it is tempting to speculate that the biology of TFR and rapid MR are mechanistically linked. For example, immuno-biological features such as CD86+ plasmacytoid dendritic cell counts were recently shown to be associated with both TFR rate and rapid, deep MR under TKI therapy.31,32
With 68 patients and 7 events only, the cutoffs and our prognostic analyses remain exploratory and should be confirmed independently. In summary, here we show that if MMR was achieved within 13 months on 800 mg imatinib, a reduction of treatment intensity to 400 mg imatinib is feasible with a high probably that MMR will be maintained. From these results, we speculate that a switch from a second-generation TKI to 400 mg imatinib is unlikely to lead to a loss of MMR. This is no trivial speculation because there are no data to support it, and a prospective clinical trial investigating a switch from second-generation TKI to imatinib will probably never be performed.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/5/955
Funding
This work was supported by the “Deutsche Forschungsgemeinschaft, DFG, Klinische Forschergruppe 210 “Genetics of Drug resistance in Cancer”, and the Deutsche José Carreras Leukämiestiftung (AR12/12), the “Anneliese Pohl Stiftung” and the EUTOS 2016 program (Novartis) to AB. The CML-Study IV has been supported by the German Government (BMBF 01GI0270); Deutsche Krebshilfe (Nr. 106642); Deutsche José-Carreras Leukämiestiftung (DJCLS Roche, Grenzach-Wyhlen; and Essex, Munich, Germany. We H09/f, H06/04v, H03/01, R05/23, AH06.01); European thank E Matzat, R Pleil-Lösch, I Stalljann, G Bartsch, C Union (LSHC-CT-2004–503216); Novartis Oncology, Sodan-Boyer, M Meckesheimer, U Böhm, A Gil and J Hehlmann Nürnberg (Drs G Gerhard, S Schaffert, A Jacob and U Haus); for assistance.
References
- 1.Hehlmann R, Lauseker M, Saussele S, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017;31(11):2398–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochhaus A, Larson RA, Guilhot F, et al. Long-Term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376(10):917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: The dasatinib versus imatinib study in treatment-naive chronic myeloid leukemia patients trial. J Clin Oncol. 2016; 34(20):2333–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30(5):1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: Results from the randomized BFORE trial. J Clin Oncol. 2018;36(3):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. [DOI] [PubMed] [Google Scholar]
- 7.Pfirrmann M, Baccarani M, Saussele S, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 2016;30(1):48–56. [DOI] [PubMed] [Google Scholar]
- 8.Hehlmann R, Muller MC, Lauseker M, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol. 2014;32(5):415–423. [DOI] [PubMed] [Google Scholar]
- 9.Cortes JE, Baccarani M, Guilhot F, et al. Phase III, randomized, open-label study of daily imatinib mesylate 400 mg versus 800 mg in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase using molecular end points: tyrosine kinase inhibitor optimization and selectivity study. J Clin Oncol. 2010;28(3):424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010; 362(24):2260–2270. [DOI] [PubMed] [Google Scholar]
- 11.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–2259. [DOI] [PubMed] [Google Scholar]
- 12.Saussele S, Richter J, Hochhaus A, Mahon FX. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia. 2016;30(8):1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hochhaus A, Masszi T, Giles FJ, et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia. 2017;31(7):1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steegmann JL, Baccarani M, Breccia M, et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016; 30(8):1648–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hochhaus A, Baccarani M, Deininger M, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22(6):1200–1206. [DOI] [PubMed] [Google Scholar]
- 16.Hughes TP, Lipton JH, Spector N, et al. Deep molecular responses achieved in patients with CML-CP who are switched to nilotinib after long-term imatinib. Blood. 2014;124(5):729–736. [DOI] [PubMed] [Google Scholar]
- 17.Gambacorti-Passerini C, Brummendorf TH, Kim DW, et al. Bosutinib efficacy and safety in chronic phase chronic myeloid leukemia after imatinib resistance or intolerance: Minimum 24-month follow-up. Am J Hematol. 2014;89(7):732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Efficace F, Baccarani M, Breccia M, et al. Chronic fatigue is the most important factor limiting health-related quality of life of chronic myeloid leukemia patients treated with imatinib. Leukemia. 2013;27(7):1511–1519. [DOI] [PubMed] [Google Scholar]
- 19.Guerin A, Chen L, Ionescu-Ittu R, et al. Impact of low-grade adverse events on health-related quality of life in adult patients receiving imatinib or nilotinib for newly diagnosed Philadelphia chromosome positive chronic myelogenous leukemia in chronic phase. Curr Med Res Opin. 2014;30(11):2317–2328. [DOI] [PubMed] [Google Scholar]
- 20.Flynn KE, Atallah E. Quality of life and long-term therapy in patients with chronic myeloid leukemia. Curr Hematol Malig Rep. 2016;11(2):80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hehlmann R, Lauseker M, Jung-Munkwitz S, et al. Tolerability-adapted imatinib 800 mg/d versus 400 mg/d versus 400 mg/d plus interferon-alpha in newly diagnosed chronic myeloid leukemia. J Clin Oncol. 2011;29(12):1634–1642. [DOI] [PubMed] [Google Scholar]
- 22.Hasford J, Pfirrmann M, Hehlmann R, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst. 1998;90(11):850–858. [DOI] [PubMed] [Google Scholar]
- 23.Therneau TM, Grambsch PM. Modeling survival data: Extending the Cox model. New York: Springer, 2000. [Google Scholar]
- 24.Altmann DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “Optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst. 1994;86(11):829–835. [DOI] [PubMed] [Google Scholar]
- 25.Davison AC, Hinkley DV. Bootstrap methods and their application. Cambridge: Cambridge University Press, 1997. [Google Scholar]
- 26.Silverman B. Density estimation for statistics and data analysis. London: Chapman and Hall, 1986. [Google Scholar]
- 27.Saussele S, Richter J, Guilhot J, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19(6):747–757. [DOI] [PubMed] [Google Scholar]
- 28.Clark RE, Polydoros F, Apperley JF, et al. De-escalation of tyrosine kinase inhibitor dose in patients with chronic myeloid leukaemia with stable major molecular response (DESTINY): an interim analysis of a non-randomised, phase 2 trial. Lancet Haematol. 2017;4(7):e310–e316. [DOI] [PubMed] [Google Scholar]
- 29.Fassoni AC, Baldow C, Roeder I, Glauche I. Reduced tyrosine kinase inhibitor dose is predicted to be as effective as standard dose in chronic myeloid leukemia: A simulation study based on phase 3 trial data. Haematologica. 2018;103(11):1825–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasford J, Baccarani M, Hoffmann V, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011; 118(3):686–692. [DOI] [PubMed] [Google Scholar]
- 31.Schutz C, Inselmann S, Sausslele S, et al. Expression of the CTLA-4 ligand CD86 on plasmacytoid dendritic cells (pDC) predicts risk of disease recurrence after treatment discontinuation in CML. Leukemia. 2017; 31(4):829–836. [DOI] [PubMed] [Google Scholar]
- 32.Inselmann S, Wang Y, Saussele S, et al. Development, function and clinical significance of plasmacytoid dendritic cells in chronic myeloid leukemia. Cancer Res. 2018;78(21):6223–6234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.