The first-in-class, once-daily Bruton tyrosine kinase (BTK) inhibitor ibrutinib is one of the preferred standards of care for patients with relapsed or refractory (R/R) mantle cell lymphoma (MCL), which has a median overall survival (OS) of 4-5 years.1–3 We previously reported a pooled analysis of 370 patients with R/R MCL treated with ibrutinib in three studies (phase II PCYC-1104 and SPARK, phase III RAY) with a median 2-year follow up.4 Here, we present extended 3.5-year follow up. Patients treated with ibrutinib in second line and those achieving a complete response (CR) gained the greatest benefit from ibrutinib, without new long-term safety concerns.
Analysis included patients enrolled between 2011 and 2013 who received oral ibrutinib 560 mg QD. Tumor response was evaluated using revised response criteria for malignant lymphoma.5 Positron emission tomography (PET) scans were not conducted routinely but were required to confirm CR suspected on computed tomography scan. TP53 mutational status was determined by DNA sequencing using formalin-fixed paraffin-embedded slides. Progression-free survival (PFS), duration of response (DOR), and OS were analyzed by number of prior lines of therapy and best tumor response; DOR and PFS were further analyzed for second-line ibrutinib patients with a time to next treatment (TTNT) ≥2 years in front-line treatment, with TTNT serving as an approximation for DOR. Multivariate analysis was performed to identify predictors of PFS and OS. Grade ≥3 or serious treatment-emergent adverse events (TEAEs) were analyzed over time and by number of prior lines of therapy. All analyses are descriptive.
Baseline characteristics for the pooled dataset were published previously.2,6,7 Nearly one-third (31.8%) of the 370 patients had high-risk disease per simplified Mantle Cell Lymphoma International Prognostic Index (sMIPI), 48.9% had bulky disease (≥5 cm), 11.9% had blastoid variant, and 73.2% had received ≥2 prior lines of therapy (median 2; range, 1-9). Baseline cardiac risk factors included hypertension in 176 (47.6%) and history of atrial fibrillation (AF) or arrhythmia in 53 (14.3%) patients.
Eighty-seven (23.5%) patients benefiting from ibrutinib after completion of the original study rolled over to an open-label access study (CAN3001), 58.6% of whom were receiving ibrutinib at the time of this analysis. Across the overall population, estimated median duration of follow up was 41.4 months [95% confidence interval (CI): 37.3-43.9 months] and median treatment exposure 11.1 months (range, 0.03-76.9 months). Approximately one-third (n=115, 31.1%) of patients received ibrutinib for ≥2 years, including 83 (22.4%) and 62 (16.8%) patients receiving treatment for ≥3 and ≥4 years, respectively. Treatment discontinuation rates due to disease progression, AEs, and death were 59.2%, 10.3%, and 5.1%, respectively.
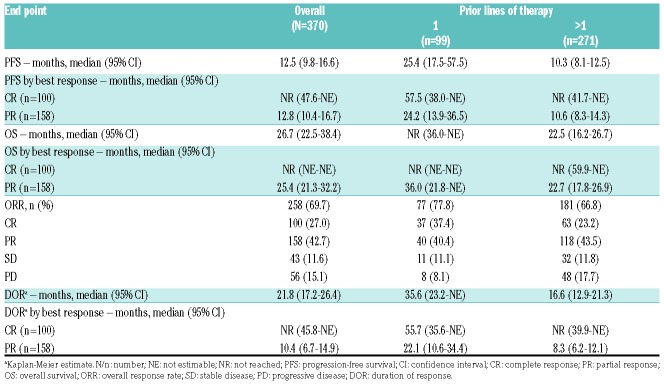
Overall, median PFS and OS were 12.5 (95%CI: 9.8-16.6) and 26.7 (95%CI: 22.5-38.4) months, respectively (Table 1). Response rates, DOR, PFS, and OS, overall and by subgroups, are summarized in Table 1.
Table 1.
Progression-free survival, overall survival, best tumor response, and duration of response.
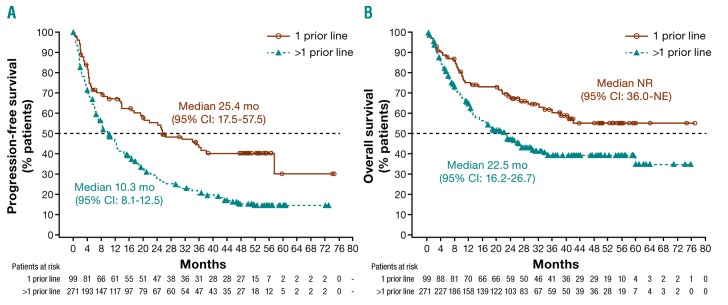
Patients receiving ibrutinib in second line had better outcomes than those treated in later lines (>1 prior line): median PFS and OS were 25.4 months (95%CI: 17.5-57.5) and not reached [NR; 95%CI: 36.0-not estimable (NE)], respectively (Table 1 and Figure 1). Overall response (ORR; 77.8%) and CR (37.4%) rates were higher in second versus later lines, and the median DOR of 35.6 months was twice as long with earlier versus later use (Table 1). Multivariate analyses of PFS and OS were consistent with univariate and multivariate analyses reported previously;4 number of prior lines of therapy was an independent predictor of PFS (HR 1.64; 95%CI: 1.197-2.248; P=0.002).
Figure 1.
Kaplan-Meier curves of progression-free survival (PFS) and overall survival (OS) in pooled population including the CAN3001 study. (A) Patients with 1 prior line of therapy before ibrutinib treatment had longer median PFS than those with >1 prior line: 25.4 (95%CI: 17.5-57.5) vs. 10.3 (95%CI: 8.1-12.5) months. (B) Patients with 1 prior line of therapy before ibrutinib treatment had longer median overall survival than those with >1 prior line: NR (95%CI: 36.0-NE) versus 22.5 (95%CI: 16.2-26.7) months. CI: confidence interval; mo: months; NE: not estimable; NR: not reached.
For patients achieving a CR with ibrutinib, median PFS (95%CI: 47.6 months-NE), OS (95%CI: NE-NE), and DOR (95%CI: 45.8 months-NE) were not reached; durability of response was consistent regardless of extent of prior treatment (Table 1). For patients with a partial response (PR), however, DOR was longer with ibrutinib use in second versus later lines (22.1 vs. 8.3 months).
We further evaluated ibrutinib outcomes in subgroups with traditionally better or worse prognosis, namely patients with chemosensitive disease or mutated TP53. For 55 of 99 patients receiving ibrutinib in second line, time from initiation of front-line chemoimmunotherapy to start of ibrutinib on study was ≥2 years (median 42 months; range, 35.2-48.0 months). ORR, CR rate, and median PFS and DOR for second-line ibrutinib in these 55 patients were 87.3%, 47.3%, and 57.5 (95%CI: 25.2-NE) and 55.7 (95%CI: 33.1-NE) months, respectively. Of 144 patients in the pooled dataset with known TP53 mutation status, 20 (13.9%) had mutated TP53 (3 with blastoid disease and 12 with >1 prior line of therapy). In ibrutinib-treated patients with mutated and wild-type TP53, respectively, median PFS was 4.0 (95%:CI: 2.1-8.3) and 12.0 (95%CI: 7.1-15.6) months, and median OS was 10.3 (95%CI: 2.5-12.6) and 33.6 (95%CI: 18.3-NE) months; ORR in patients with mutated and wild-type TP53 was 55.0% (11 PRs; response data missing for 3 out of 20 patients) and 70.2% (31 CRs; 56 PRs), respectively.
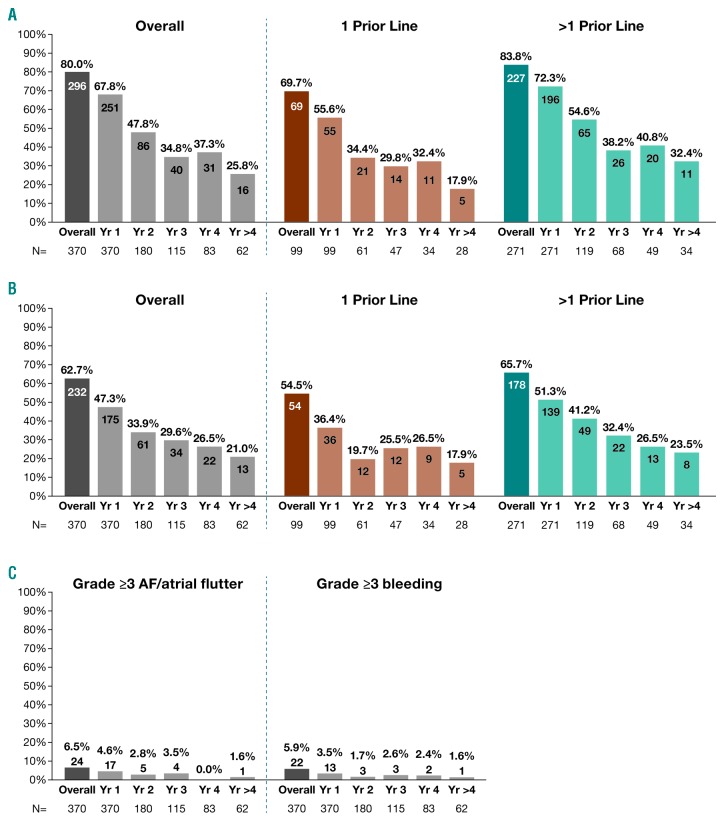
Grade ≥3 TEAEs occurred in 296 (80.0%) patients, most frequently (≥5% of patients) neutropenia (17.0%), thrombocytopenia (12.4%), pneumonia (12.7%), anemia (10.0%), AF (AF/atrial flutter; 6.2%), and hypertension (5.1%). Serious (any grade) TEAEs occurred in 232 (62.7%) patients, most frequently (≥5% of patients) pneumonia (12.4%), and AF (5.4%). Incidence of grade ≥3 and serious TEAEs generally decreased with continued ibrutinib treatment (Figure 2A and B). Ibrutinib use in second versus later lines was associated with lower rates of grade ≥3 (69.7% vs. 83.8%) and serious TEAEs (54.5% vs. 65.7%), including AF. Second primary malignancies (mostly non-melanoma skin cancers) occurred in 10.8% of patients, consistent with an incidence of 8.2% (median follow up 31 months) in a US population-based study.8
Figure 2.
Incidence of treatment-emergent adverse events (TEAEs) over time. (A) Grade ≥3 TEAEs generally decreased over time with lower rates associated with ibrutinib use in second versus later lines. (B) Any-grade serious TEAEs generally decreased over time with lower rates associated with ibrutinib use in second versus later lines. (C) Incidence of grade ≥3 AF and bleeding was highest in year 1 and decreased thereafter. Overall population is the full ibrutinib cohort enrolled in the original three studies (N=370), including those who continued on to the CAN3001 study. Number of patients with adverse events shown on bars. Recurrent events per patient were counted only once per year. If recurrent events occurred in a single patient in separate years, these were counted separately in each year (once only per year). N: number; AF: atrial fibrillation; Yr: year.
Forty-two (11.4%) patients had any-grade AF event on study. Of 53 patients with AF or arrhythmia history, 37 (70%) had no recurrence on ibrutinib. Incidence of grade ≥3 AF and bleeding was highest in year 1 and decreased thereafter (Figure 2C). All-grade AF led to dose reductions in two (0.5%) patients and no treatment discontinuations; grade ≥3 bleeding led to dose reduction in one (0.3%) and treatment discontinuation in three (0.8%) patients. Thirty-three of 42 (78.6%) patients with any-grade AF on study received anticoagulant and/or antiplatelet (AC/AP) medications including, among others, warfarin and novel oral anticoagulants. One patient with AF on study had a major hemorrhage but had not received AC/AP medications within 2 years.
Extended 3.5-year median follow up from this large multistudy cohort of R/R MCL patients confirmed that ibrutinib is highly active and provides durable responses, with some patients remaining on therapy for ≥4 years. CRs were highly durable regardless of the extent of prior treatment, and depth of response continued to improve over time.4 Multivariate analysis demonstrated that number of prior lines of therapy independently predicted PFS, as did certain characteristics constituting high-risk disease. These studies enrolled a challenging patient population, demonstrated by the high proportions of patients with multiple prior treatments and high-risk or bulky disease.
Clinical outcomes were best for patients receiving ibrutinib in second line, in whom median PFS exceeded 2 years and CR rate by Cheson 2007 criteria exceeded 35%; an improvement of nearly 15% versus later lines. In addition, grade ≥3 TEAEs, including AF, were less common with earlier ibrutinib use, and incidence generally decreased over time with continued treatment.
For patients with a TTNT of ≥2 years (median 3.5 years) in front line, in whom retreatment with chemoimmunotherapy may be considered in clinical practice, second-line ibrutinib provided highly durable responses with a median duration >4.5 years, notably longer than TTNT in front line. This compares favorably with bendamustine-rituximab in the relapsed setting, which was associated with a DOR of 1.6 years, albeit in a population with a median 2 prior lines of therapy.9
Among patients with mutated TP53, who generally have a more aggressive disease course and poor outcomes with traditional chemoimmunotherapy,10 responses with ibrutinib monotherapy were less favorable than in the overall population (no CRs). Notably, in two phase II trials, ibrutinib combined with the BCL-2 inhibitor venetoclax (n=24) or with lenalidomide and rituximab (n=50) achieved CR rates of 50-64% in patients with mutated TP53,11,12 suggesting that ibrutinib combination therapy may improve outcomes in this setting. Allogeneic hematopoietic cell transplant may be used to consolidate any response to ibrutinib, although the effectiveness for mutated TP53 disease requires investigation.13
Some patients had cardiac risk factors that could predispose them to AF and arrhythmias: nearly half were hypertensive and 14% had a history of AF or arrhythmia. Among the latter, AF did not recur in the majority (70%) when treated with ibrutinib. Symptomatic AF, which occurred in 6.5% of patients with extended follow up, was manageable, causing no ibrutinib discontinuations and dose reductions in only two patients. Importantly, no major bleeds occurred in patients with AF receiving AC/AP medications for stroke prophylaxis.
Overall, these favorable outcomes with second-line ibrutinib treatment for MCL support its use as a standard treatment in this setting, both for patients who relapse early after front-line treatment and require novel non-chemotherapy approaches, and those with a durable front-line remission. Several ongoing studies, including a phase III trial of ibrutinib plus venetoclax, are evaluating ibrutinib-based combinations to further improve outcomes in this rare, aggressive lymphoma, particularly for patients with high-risk disease requiring innovative therapies.
Supplementary Material
Acknowledgments
The authors would like to thank Mark Wildgust, Jessica Vermeulen, and Todd Henninger from Janssen for their support in the long-term reporting for this analysis. The authors would also like to thank the patients who participated in this trial, and their families, as well as the investigators, study co-ordinators, study teams, and nurses.
Footnotes
Funding: this study was sponsored by Janssen Research & Development. Writing assistance was provided by Liqing Xiao and Natalie Dennis of PAREXEL and was funded by Janssen Global Services, LLC.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Herrmann A, Hoster E, Zwingers T, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009; 27(4):512–518. [DOI] [PubMed] [Google Scholar]
- 2.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013; 369(6):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dreyling M, Campo E, Hermine O, et al. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017; 28(suppl_4):iv62–iv71. [DOI] [PubMed] [Google Scholar]
- 4.Rule S, Dreyling M, Goy A, et al. Outcomes in 370 patients with mantle cell lymphoma treated with ibrutinib: a pooled analysis from three open-label studies. Br J Haematol. 2017;179(3):430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. [DOI] [PubMed] [Google Scholar]
- 6.Wang M, Goy A, Martin P, et al. Efficacy and safety of single-agent ibrutinib in patients with mantle cell lymphoma who progressed after bortezomib therapy. Blood. 2014;124(21):4471. [Google Scholar]
- 7.Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387(10020):770–778. [DOI] [PubMed] [Google Scholar]
- 8.Shah BK, Khanal A. Second primary malignancies in mantle cell lymphoma: a US population-based study. Anticancer Res. 2015; 35(6):3437–3440. [PubMed] [Google Scholar]
- 9.Czuczman MS, Goy A, Lamonica D, Graf DA, Munteanu MC, van der Jagt RH. Phase II study of bendamustine combined with rituximab in relapsed/refractory mantle cell lymphoma: efficacy, tolerability, and safety findings. Ann Hematol. 2015;94(12):2025–2032. [DOI] [PubMed] [Google Scholar]
- 10.Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130(17):1903–1910. [DOI] [PubMed] [Google Scholar]
- 11.Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018; 378(13):1211–1223. [DOI] [PubMed] [Google Scholar]
- 12.Jerkeman M, Eskelund CW, Hutching M, et al. Ibrutinib, lenalidomide, and rituximab in relapsed or refractory mantle cell lymphoma (PHILEMON): a multicentre, open-label, single-arm, phase 2 trial. Lancet Haematol. 2018;5(3):e109–e116. [DOI] [PubMed] [Google Scholar]
- 13.Dreger P, Michallet M, Bosman P, et al. Ibrutinib for bridging to allo-geneic hematopoietic cell transplantation in patients with chronic lymphocytic leukemia or mantle cell lymphoma: a study by the EBMT Chronic Malignancies and Lymphoma Working Parties. Bone Marrow Transplant. 2018. May 4 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.