Abstract
EEXPAND (phase Ib, dose-finding study) evaluated the starting dose of ruxolitinib in patients with myelofibrosis with baseline platelet counts of 50-99×109/L. The study consisted of dose-escalation and safety-expansion phases. Based on the baseline platelet counts, patients were assigned to stratum 1 (75-99×109/L) or stratum 2 (50-74×109/L), with the primary objective of determining the maximum safe starting dose (MSSD); key secondary objectives included safety and efficacy. At week 48 data cutoff (stratum 1, n=44; stratum 2, n=25), 24.6% (17 out of 69) of patients were still receiving treatment. The MSSD was established as ruxolitinib 10 mg twice daily in both strata. Thrombocytopenia [grade 4 (stratum 1, n=1; stratum 2, n=2)] was the only reported dose-limiting toxicity (study drug related) at 10 mg twice daily. In the MSSD cohort (stratum 1, n=20; stratum 2, n=18), adverse events (regardless of study drug relationship) led to treatment discontinuation in 15.0% and 33.3% of patients in stratum 1 and stratum 2, respectively, and dose adjustment/interruption in 45.0% and 66.7% of patients in stratum 1 and stratum 2, respectively. Three cases of on-treatment deaths were reported at the MSSD. Spleen response was achieved at week 48 in 33.3% and 30.0% of patients in stratum 1 and stratum 2, respectively. Improvements in the Total Symptom Score were also observed. In this study, ruxolitinib demonstrated acceptable tolerability in both the strata at the MSSD of 10 mg twice daily. (Registered at: clinicaltrials.gov identifier: 01317875).
Introduction
Myelofibrosis (MF) is a rare, chronic, Philadelphia chromosome-negative myeloproliferative neoplasm caused by clonal proliferation of pluripotent hematopoietic stem cells.1,2 The common clinical presentations associated with MF include splenomegaly due to extramedullary hematopoiesis, progressive bone marrow fibrosis with cytopenias, and debilitating constitutional symptoms (e.g., fatigue, night sweats, and fever), which substantially diminish the quality of life.3–6 The dysregulated activation of the Janus kinase (JAK)/signal transducer and activator of transcription pathway is the hallmark of MF and can result from mutations in JAK2, in the cytokine receptor, or in other components of the signaling pathway.7,8
Ruxolitinib, a potent and selective oral JAK1/JAK2 inhibitor, was approved for the treatment of intermediate- and high-risk patients with MF based on two randomized, phase III studies: COMFORT-I (n=309; clinicaltrials.gov identifier: 00952289) and COMFORT-II (n=219; clinicaltrials.gov identifier: 00934544).9–12 In both COMFORT studies, ruxolitinib demonstrated marked and sustained clinical benefits in spleen size and improvement in symptom burden, and was generally well tolerated.9–12
Patients with MF may present with thrombocytopenia (platelet counts, <100×109/L) owing to the nature of the disease.13,14 Across various studies, approximately 16-26% of patients with MF were found to be thrombocytopenic at diagnosis.15–19 In addition, patients with MF may also experience treatment-emergent thrombocytopenia while on ruxolitinib therapy (owing to its mechanism of action).9,12,20 The Study 251 (n=153; clinicaltrials.gov identifier: 00509899) identified thrombocytopenia as a dose-limiting toxicity (DLT) for ruxolitinib.21 Patients in the COMFORT studies had baseline platelet counts ≥100×109/L, limiting the safety and efficacy data in patients with lower platelet counts.14,18,21
To date, only a few treatment options have been evaluated for patients with MF and thrombocytopenia. The safety and efficacy of JAK inhibitors in thrombocytopenic patients with MF have also not been adequately explored.15,22 Evidence from clinical trials evaluating the use of ruxolitinib in patients with MF with baseline thrombocytopenia (platelet counts <100×109/L) is limited.14,23–25
The Study 258 (n=50; clinicaltrials.gov identifier: 01348490) evaluated the efficacy and safety of low-dose ruxolitinib [5 mg twice daily (bid)] with subsequent dose escalation in patients with low platelet counts (50 to <100×109/L).14 Ruxolitinib was generally well tolerated and provided efficacy benefits, suggesting that a starting dose of 5 mg bid with escalation to 10 mg bid may be suitable for the low platelet count population.14
The JUMP study (n=2233; clinicaltrials.gov.identifier: 01493414), a phase IIIb expanded-access study, was amended to enroll patients with baseline platelet counts ≥50×109/L to gather additional safety and efficacy data in patients with low platelet counts.23–25 In the JUMP study, the safety profile of ruxolitinib in the low-platelet patient cohort was consistent with that observed in patients with platelet counts ≥100×109/L. Spleen and symptom responses achieved with low-dose ruxolitinib (5 mg bid) were within the expected range based on the COMFORT studies.23
The recommended starting dose of ruxolitinib (prescribing information) is based on the platelet count.26 The maximum recommended starting dose in patients with platelet counts between 50×109/L and 100×109/L is 5 mg bid, and the dose should be titrated with caution.26,27 However, the findings from the COMFORT-I and the Study 258 demonstrated that the final titrated doses of ≥10 mg bid resulted in larger improvements in spleen volume and MF-related symptoms compared to titrated doses of ≤5 mg bid.14,28
The purpose of the EXPAND study (clinicaltrials.gov identifier: 01317875: open-label, phase Ib, dose-finding study) was to establish the maximum safe starting dose (MSSD) of ruxolitinib in patients with MF with baseline platelet counts between 50×109/L and 100×109/L. The study also intended to assess the safety and tolerability of ruxolitinib in this patient population.
The preliminary findings from the dose-escalation and safety-expansion phases of EXPAND at the preplanned interim analysis [day 168 (week 24)] were previously reported.29 Guided by the occurrence of protocol-defined DLTs during the first cycle of treatment (28 days), 15 mg bid was initially declared as the MSSD for patients enrolled in stratum 1 (S1; platelet counts: 75-99×109/L) of the study, whereas 10 mg bid was declared as the MSSD for patients in stratum 2 (S2; platelet counts: 50-74×109/L). However, based on the safety and efficacy findings from the interim analysis, the MSSD for S1 was subsequently lowered to 10 mg bid (as per the protocol amendment). Here, we present the results from the 48-week follow up of EXPAND for the MSSD cohorts.
Methods
Patient population
Eligible patients: i) were aged ≥18 years; ii) had been diagnosed with intermediate-1, intermediate-2, or high-risk MF (primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF);30 iii) had a palpable spleen (≥5 cm from the costal margin); and iv) fulfilled the platelet count criteria at screening or study day 1 (S1: <100×109/L and ≥75×109/L; S2: <75×109/L and ≥50×109/L). An Eastern Oncology Cooperative Group performance status of ≤2 was required at screening. The key exclusion criteria included: i) patients with any history of platelet counts <45×109/L within 30 days prior to screening; ii) platelet transfusion within 14 days prior to screening; iii) history or predisposition to clinically significant bleeding; iv) history of platelet dysfunction and/or bleeding diathesis; and v) regular use of drugs inhibiting platelet function.
Study design
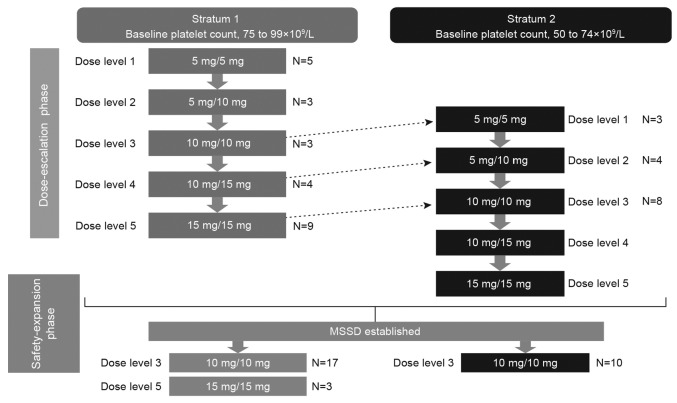
EXPAND was a phase Ib, open-label, multicenter, dose-finding study of ruxolitinib in patients with intermediate- or high-risk primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF who had baseline platelet counts between ≥50×109/L and <100×109/L. The study design is shown in Figure 1.
Figure 1.
Study design. Dark arrows represent escalation from a given dose level to the following one, only if both that dose level and the previous one have been deemed safe. Dotted arrows represent each dose level in stratum 2, which will open to patients only if both that dose level and the following one have been deemed safe in stratum 1. Per protocol amendment, new patients enrolled in stratum 1 in the safety-expansion phase will be given the 10 mg twice-daily (bid) dose instead of the 15 mg bid dose level previously evaluated as the maximum safe starting dose (MSSD). The MSSD cohort (10 mg bid) in stratum 1 included 3 patients from the dose-escalation and 17 patients from the safety-expansion phases. In stratum 2, the MSSD cohort included 8 patients from the dose-escalation and 10 patients from the safety-expansion phases.
The study period consisted of 2 phases: dose escalation and safety expansion. The successive cohorts of newly enrolled patients received increasing doses of ruxolitinib until the MSSD was determined in the dose-escalation phase. The MSSD was defined as the dose level most closely associated with a posterior probability of DLT between 16% and 33% that did not also have >25% probability of excessive toxicity. A DLT was defined as the occurrence of any treatment-related toxicity occurring through study day 28 (Online Supplementary Table S1). A preplanned interim analysis was conducted when the last patient enrolled in the dose-escalation phase completed week 24.29
An adaptive Bayesian logistic regression model guided by escalation with overdose control was used to allocate patients into each cohort (5 dose levels) in the dose-escalation phase (Treatment Dose Levels) (Online Supplementary Appendix). The patients in the dose-determining set (DDS) enrolled in the dose-finding part of the study were assessed to determine the MSSD. The DDS consisted of all patients from the safety set who met the minimum exposure criterion and had sufficient safety evaluations or who experienced a DLT. The safety set consisted of all patients who received at least 1 dose of ruxolitinib. The DDS definition, minimum exposure criterion, and planned enrollment are provided in the Online Supplementary Appendix.
The safety-expansion phase was conducted after determination of the MSSD to further evaluate the safety and tolerability of the MSSD, and establish that the dose was suitable for use in patients with MF with low platelet counts. Per protocol amendment, 10 mg bid was evaluated as the starting dose for all new patients enrolled in S1 in the safety-expansion phase (Online Supplementary Appendix). Patients who were already receiving the 15 mg bid dose continued to take their assigned dose.
The end of the study will occur after all study patients complete their last assessment as per protocol (follow-up visit 30 days after the end of the treatment visit) (Online Supplementary Appendix). Details of the statistical analyses are presented in the Online Supplementary Appendix.
The study was approved by the institutional review boards of the respective institutions prior to patient enrollment and was conducted in accordance with the principles of the Declaration of Helsinki. All patients provided written informed consent. The trial is registered at clinicaltrials.gov identifier: 01317875.
Assessments
In the dose-escalation phase, the primary objective was to determine the MSSD (incidence rate of DLTs) of ruxolitinib. The key secondary objectives included safety [frequency, duration, and severity of adverse events (AEs) and serious AEs] and efficacy (spleen response: proportion of patients achieving ≥50% of reduction in palpable measurement of spleen length at week 48 data cutoff relative to day 1). The key exploratory objectives included patient-reported outcomes [change in the Total Symptom Score (TSS) as assessed by the modified Myelofibrosis Symptom Assessment Form (MFSAF) v.2.0 diary].31–33
Results
Results from the interim analysis (week 24 data cutoff: January 20, 2015) of the study have been presented at the 2015 American Society of Hematology meeting.29 At that data cutoff, 46 patients (S1, n=27; S2, n=19) had received treatment.
Overall study cohort
At week 48 data cutoff (December 7, 2017), the final enrollment for EXPAND included 69 patients (S1, n=44; S2, n=25) (Online Supplementary Table S2). Overall, 31.8% (14 out of 44) of patients in S1 and 12.0% (3 out of 25) of patients in S2 were still receiving ruxolitinib treatment (Online Supplementary Table S3). The median exposure to ruxolitinib was 51.4 weeks (range, 0.9-210.0 weeks) in S1 and 67.4 weeks (range, 4.4-161.1 weeks) in S2.
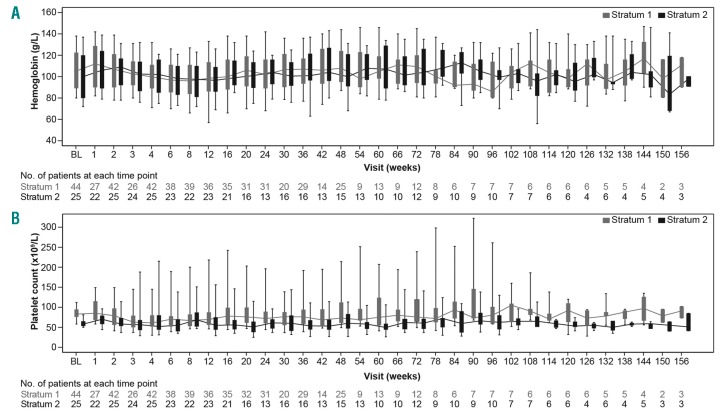
The AEs (in ≥20% of patients in either stratum, regardless of study drug relationship) reported in the overall cohort are presented in Online Supplementary Table S4. Reasons for on-treatment death included acute myeloid leukemia (1 patient), cardiac arrest (1 patient), and unknown (1 patient, not suspected to be related to study drug) in S1 and complications following gastrointestinal ulcer (1 patient) and multiple organ failure (1 patient) in S2 (Online Supplementary Table S5). Hemoglobin levels and platelet counts over time are presented in Figure 2. An initial decrease in the blood count parameters was observed in the first few weeks; however, the parameters stabilized with time. Spleen response at week 48 was achieved in 7 out of 22 patients [31.8% (95%CI: 13.9, 54.9)] in S1 and 5 out of 14 patients [35.7% (95%CI: 12.8, 64.9)] in S2. A spleen response at any time point was observed in 22 out of 43 patients [51.2% (95%CI: 35.5, 66.7)] in S1 and 17 out of 25 patients [68.0% (95%CI: 46.5, 85.1)] in S2 (Online Supplementary Figure S1).
Figure 2.
Blood parameters over time. (A) Hemoglobin levels. (B) platelet counts. BL: baseline.
Maximum safe starting dose cohort
Patients’ characteristics
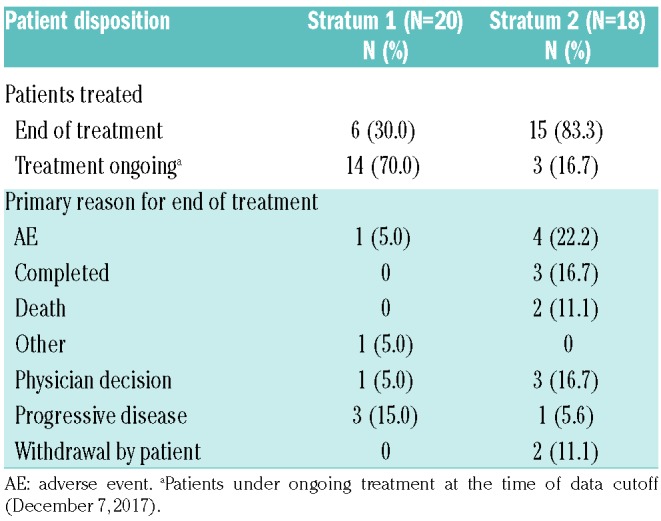
Baseline patients’ characteristics (S1, n=20; S2, n=18) were indicative of an advanced disease stage (Online Supplementary Table S6). In the MSSD cohort, 70.0% (14 out of 20) of patients in S1 and 16.7% (3 out of 18) of patients in S2 were still receiving ruxolitinib treatment (Table 1). The primary reasons for the end of treatment included AEs [S1, n=1 (5.0%); S2, n=4 (22.2%)], treatment duration completed [S1, n=0; S2, n=3 (16.7%]), physician decision [S1, n=1 (5.0%); S2, n=3 (16.7%)], disease progression [S1, n=3 (15.0%); S2, n=1 (5.6%)], and death [S1, n=0; S2, n=2 (11.1%)].
Table 1.
Patient disposition (week 48 analysis; maximum safe starting dose cohort).
Dosing and exposure
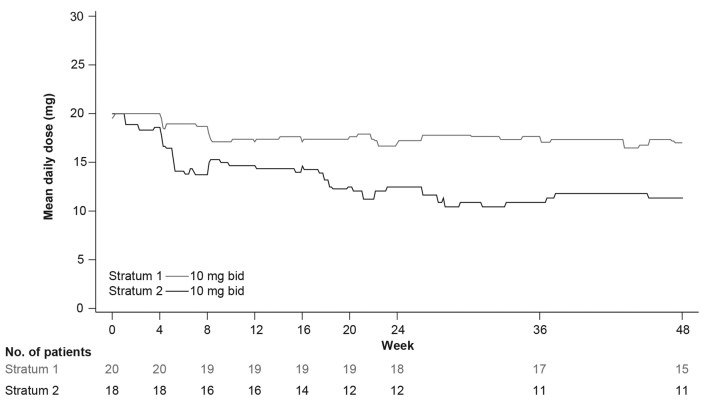
The median exposure to ruxolitinib was 54.8 weeks (range, 4.3-210.0 weeks) in S1 and 83.2 weeks (range, 4.4-161.1 weeks) in S2. Overall, 45.0% (9 out of 20) of patients in S1 and 88.9% (16 out of 18) of patients in S2 had at least 1 dose reduction/interruption (Online Supplementary Table S7). The mean total daily dose over time plot by stratum at MSSD (10 mg bid in both S1 and S2) is shown in Figure 3. The mean dose intensity was 17.96 mg/day [standard deviation (SD)=3.055] in S1 and 13.27 mg/day (SD=5.030) in S2.
Figure 3.
Mean daily dose over time by stratum at maximum safe starting dose. bid: twice daily.
Dose modifications were observed during the first 12 weeks of treatment in some patients; 30.0% (6 out of 20) of patients in S1 and 61.1% (11 out of 18) of patients in S2 had at least 1 dose reduction/interruption (Online Supplementary Table S8). Among these patients with a dose down-titration, 3 of 6 patients in S1 and 10 of 11 patients in S2 did not resume the initial 10 mg bid dose. Thrombocytopenia was the most frequent AE leading to an early dose titration.
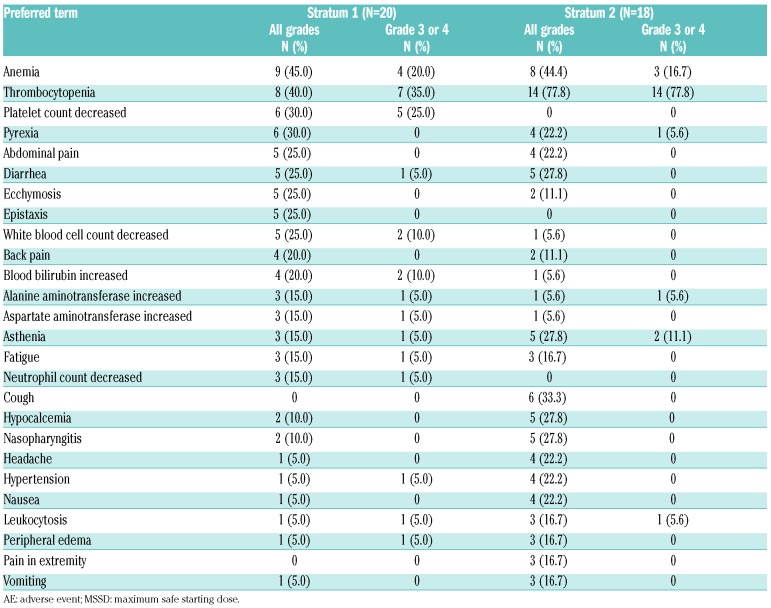
Safety in the maximum safe starting dose cohort
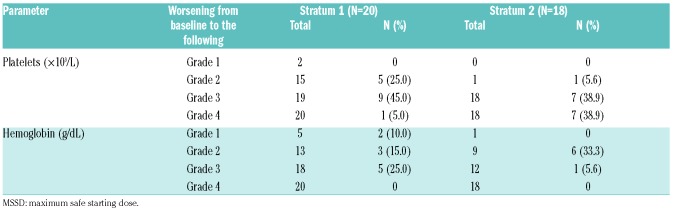
Adverse events (in ≥15% of patients in either stratum, regardless of study drug relationship) in the MSSD cohort are presented in Table 2. As observed in the interim analysis, anemia and thrombocytopenia (all grades) were the most common hematologic AEs in both strata [anemia: S1, n=9 (45.0%); S2, n=8 (44.4%) and thrombocytopenia: S1, n=8 (40.0%); S2, n=14 (77.8%)]. Grade 3 or 4 AEs were reported in 70.0% (14 out of 20) of patients in S1 and 88.9% (16 out of 18) of patients in S2. The AEs [regardless of study drug relationship; any system organ class (SOC)] led to treatment discontinuation in 15.0% (3 out of 20) of patients in S1 and 33.3% (6 out of 18) of patients in S2, with thrombocytopenia being reported as the most common reason for treatment discontinuation [S1, n=1 (5.0%); S2, n=3 (16.7%)]. Dose adjustment or study drug interruption due to AEs [regardless of study drug relationship (any SOC)] was observed in 45.0% (9 out of 20) of patients in S1 and 66.7% (12 out of 18) of patients in S2. Thrombocytopenia was the primary reason for dose adjustment/interruption in both strata [S1, n=4 (20.0%); S2, n=12 (66.7%)]. Overall, 25% (5 out of 20) of patients in S1 and 38.9% (7 out of 18) of patients in S2 experienced a serious AE [regardless of study drug relationship (any SOC)]. Thrombocytopenia (grade 4, related to study drug) was the only DLT reported in both strata at 10 mg bid [S1, n=1; S2, n=2 (1 DLT in S2 was reported at the interim analysis)]. Grade 4 worsening from baseline in platelet count was observed in 1 patient (5.0%) in S1 and 7 patients (38.9%) in S2 (Table 3). No grade 4 worsening from baseline was reported in either stratum for hemoglobin levels. Reasons for on-treatment death (in the MSSD cohort) included acute myeloid leukemia (1 patient) and cardiac arrest (1 patient) in S1, and multiple organ failure (1 patient) in S2 (Online Supplementary Table S5).
Table 2.
All grade adverse events, regardless of study drug relationship, in ≥15% of patients in either stratum (week 48 analysis; maximum safe starting dose cohort).
Table 3.
New or worsened hematologic abnormalities (week 48 analysis; maximum safe starting dose cohort).
Efficacy in the maximum safe starting dose cohort
Spleen response
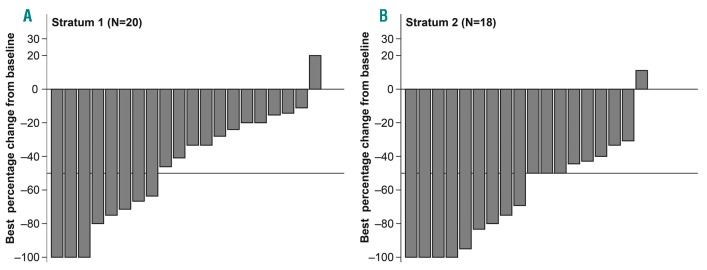
At week 48, spleen response was achieved in 5 out of 15 patients [33.3% (95%CI: 11.8, 61.6)] in S1 and 3 out of 10 patients [30.0% (95%CI: 6.7, 65.2)] in S2. A spleen response at any time point was observed in 8 out of 20 patients [40.0% (95%CI: 19.1, 63.9)] in S1 and 12 out of 18 patients [66.7% (95%CI: 41.0, 86.7)] in S2 (Figure 4).
Figure 4.
Waterfall plot of best response in spleen length by stratum at maximum safe starting dose.
The waterfall plot for the best response in spleen length for patients treated at the MSSD, with or without dose titration, within the first 12 weeks is presented in Online Supplementary Figure S2. A decrease in best percentage change from baseline in spleen length was evident in 100.0% of patients with dose down-titration in both S1 (n=3) and S2 (n=10). In patients without a dose down-titration, a decrease in best percentage change from baseline in spleen length was seen in 94.1% (16 out of 17) of patients in S1 and 87.5% (7 out of 8) of patients in S2.
Symptom response
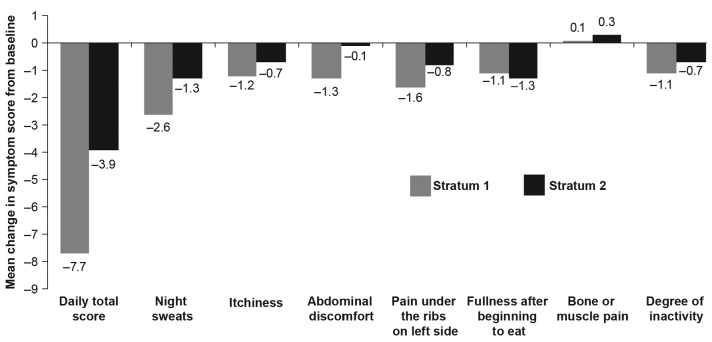
An improvement (i.e. decrease) in TSS was observed at the MSSD in both strata. The mean change in TSS from baseline at week 24 was −7.7 (SD=9.70) in S1 and −3.9 (SD=11.36) in S2. Compared to the baseline symptom score, the mean individual symptom scores decreased (improved) for all categories except bone/muscle pain (slight worsening) at week 24 (Figure 5). A trend in symptom improvement was also observed in patients with early dose titration (Online Supplementary Table S9 and Online Supplementary Figure S3).
Figure 5.
Change in total symptom score and individual symptom scores of Myelofibrosis Symptom Assessment Form diary from baseline to week 24 by stratum at maximum safe starting dose.
Discussion
Only a few clinical trials are currently evaluating the treatment options for patients with MF with thrombocytopenia (platelet counts <100×109/L), highlighting the need for conducting this analysis. The JAK inhibitors that have been evaluated in this setting include ruxolitinib, pacritinib, momelotinib, and fedratinib;34–36 however, only ruxolitinib is currently approved for the treatment of patients with MF. Ruxolitinib was approved for the treatment of intermediate- and high-risk patients with MF based on the COMFORT studies in patients with normal platelet counts (≥100×109/L).9–12,28 Findings from a post hoc analysis of COMFORT-I showed that patients with cytopenias at baseline could be effectively managed with ruxolitinib dose adjustments and that doses of ≥10 mg bid yielded clinically meaningful reductions in spleen volume and symptom improvement.28 These findings support the use of ruxolitinib as a therapeutic option for patients with MF with low baseline platelet counts (<100×109/L).
The preliminary observations based on toxicity during the first cycle of treatment indicated ruxolitinib 15 mg and 10 mg bid as MSSDs for S1 and S2, respectively. However, observations from the interim analysis showed that the majority of patients receiving the 15 mg bid MSSD dose in S1 experienced thrombocytopenia, thus requiring dose reductions after the first cycle. These patients subsequently continued study treatment at the 10 mg bid or lower dose. Clinical benefit was observed across all starting dose levels, including in those patients who started treatment at the 10 mg bid dose level. Based on these observations, the initially stated MSSD of 15 mg bid for S1 was revised to 10 mg bid as per protocol amendment. Based on the results from the interim and 48-week analyses of EXPAND, 10 mg bid was established as the MSSD for both strata (S1: platelet count=75-99×109/L; S2: platelet count=50-74×109/L).
Ruxolitinib was generally well tolerated at all dose levels, including the MSSDs, and no new safety signal was observed. However, as expected, a higher frequency of thrombocytopenia was observed, which was managed by dose reduction/interruption. Evidently, the 10 mg bid dose was better tolerated in S1 versus S2; 3 out of 6 patients in S1 versus 10 out of 11 patients in S2 did not resume the 10 mg bid dose after the initial dose reduction (first 12 weeks). A medically meaningful spleen size response was observed with ruxolitinib treatment at the 10 mg bid dose. In the MSSD cohort, at least 50% of reduction in the spleen length was observed in 33.3% of patients in S1 and 30.0% of patients in S2 at week 48, whereas a spleen response at any time point was achieved by 40.0% of patients in S1 and 66.7% of patients in S2. A decrease in the best percentage change from baseline was observed at the MSSD in 95.0% of patients in S1 and 94.4% of patients in S2. An improvement in symptom response (decrease in the MFSAF-TSS) was also observed at the MSSD in both strata.
During the first 12 weeks of treatment, the ruxolitinib dose was reduced below 10 mg bid in some patients, mostly due to ruxolitinib-associated hematologic toxicity. However, spleen and symptom benefit was observed in these patients despite the early dose titration, and the treatment was continued at the reduced dose, suggesting that a starting dose of 10 mg bid may still be effective in the long-term (as was also observed in the COMFORT-I study).28
In the subgroup with dose down-titration in S1 (3 out of 6 patients), a decrease in the best percentage change from baseline in spleen length was observed; however, none of these patients achieved an at least 50% reduction in spleen length. All patients in S2 (n=10) who had a dose down-titration achieved a decrease in the best percentage change from baseline in spleen length; 7 out of 10 patients achieved an at least 50% reduction in spleen length (Online Supplementary Figure S2). A trend in symptom improvement was also observed in patients with early dose titration at the MSSD; this effect was more pronounced in S1 than in S2 (Online Supplementary Table S9 and Online Supplementary Figure S3).
The observations from the EXPAND study are consistent with the findings from other clinical trials evaluating the use of ruxolitinib in patients with MF with baseline thrombocytopenia. In the Study 258, the median percentage change from baseline in spleen length at week 24 in the 30 evaluable patients was −29.7% (range, −100.0% to 58.3%). In the EXPAND study, the median percentage change from baseline in spleen length at week 24 for the overall population (n=69) was −36.9% (range, −100.0% to 55.6%). As expected (owing to baseline patients’ characteristics and the mechanism of action of ruxolitinib), thrombocytopenia was frequent in both the Study 258 and the EXPAND study (64.0% vs. 68.1%, respectively). Low-dose ruxolitinib was shown to be generally well tolerated and efficacious in patients with MF with low platelet counts in JUMP.23
The findings to date from the 48-week follow-up analysis of the EXPAND study provide evidence to support a starting dose of ruxolitinib at 10 mg bid for patients with MF with low baseline platelet counts of 75-99×109/L (S1) but are less conclusive for baseline platelet counts of 50-74×109/L (S2). The reported AEs were consistent with the known safety profile of ruxolitinib, with the exception of thrombocytopenia in S2, which was expected. Ruxolitinib treatment was generally well tolerated and provided spleen size reduction and symptom response benefit. The tolerability of ruxolitinib in this previously unstudied patient population with MF with low platelet counts at baseline was acceptable at doses of 10 mg bid in both strata. The study is ongoing, and further evaluations will be performed at the end of the study to confirm the safety and efficacy of ruxolitinib in the study cohorts.
Supplementary Material
Acknowledgments
The authors would like to thank Archana Rai and Ambrin Fatima, PhD (Novartis Healthcare Pvt Ltd) for providing medical writing assistance.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/5/947
References
- 1.Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med. 2006;355(23): 2452–2466. [DOI] [PubMed] [Google Scholar]
- 2.Le Bousse-Kerdiles MC. Primary myelofibrosis and the “bad seeds in bad soil” concept. Fibrogenesis Tissue Repair. 2012; 5(Suppl 1):S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdel-Wahab OI, Levine RL. Primary myelofibrosis: update on definition, pathogenesis, and treatment. Annu Rev Med. 2009;60:233–245. [DOI] [PubMed] [Google Scholar]
- 4.Barosi G. Myelofibrosis with myeloid metaplasia: diagnostic definition and prognostic classification for clinical studies and treatment guidelines. J Clin Oncol. 1999; 17(9):2954–2970. [DOI] [PubMed] [Google Scholar]
- 5.Mesa RA, Schwager S, Radia D, et al. The Myelofibrosis Symptom Assessment Form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk Res. 2009;33(9):1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118(2):401–408. [DOI] [PubMed] [Google Scholar]
- 7.Ihle JN, Gilliland DG. Jak2: normal function and role in hematopoietic disorders. Curr Opin Genet Dev. 2007;17(1):8–14. [DOI] [PubMed] [Google Scholar]
- 8.Parganas E, Wang D, Stravopodis D, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93(3):385–395. [DOI] [PubMed] [Google Scholar]
- 9.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–798. [DOI] [PubMed] [Google Scholar]
- 10.Harrison CN, Vannucchi AM, Kiladjian JJ, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2016;30(8):1701–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verstovsek S, Mesa RA, Gotlib J, et al. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J Hematol Oncol. 2017;10(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balduini A, Badalucco S, Pugliano MT, et al. In vitro megakaryocyte differentiation and proplatelet formation in Ph-negative classical myeloproliferative neoplasms: distinct patterns in the different clinical phenotypes. PLoS One. 2011;6(6):e21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talpaz M, Paquette R, Afrin L, et al. Interim analysis of safety and efficacy of ruxolitinib in patients with myelofibrosis and low platelet counts. J Hematol Oncol. 2013;6(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Ali HK, Vannucchi AM. Managing patients with myelofibrosis and low platelet counts. Ann Hematol. 2017; 96(4):537–548. [DOI] [PubMed] [Google Scholar]
- 16.Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30(33):4098–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29(4):392–397. [DOI] [PubMed] [Google Scholar]
- 18.Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. 2007;109(1):68–76. [DOI] [PubMed] [Google Scholar]
- 19.Tefferi A, Lasho TL, Jimma T, et al. One thousand patients with primary myelofibrosis: the Mayo Clinic experience. Mayo Clin Proc. 2012;87(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verstovsek S, Mesa RA, Gotlib J, et al. Efficacy, safety and survival with ruxolitinib in patients with myelofibrosis: results of a median 2-year follow-up of COMFORT-I. Haematologica. 2013;98(12):1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363(12):1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison CN, Gisslinger H, Miller CB, et al. EXPAND: A phase 1b, open-label, dose-finding study of ruxolitinib in patients with myelofibrosis and baseline platelet counts between 50×109/L and 99×109/L. Blood. 2012;120(21):177. [Google Scholar]
- 23.Griesshammer M, Vannucchi AM, le Coutre P, et al. Safety and efficacy of ruxolitinib in patients with low platelets enrolled in a phase 3b expanded-access study in myelofibrosis (MF). Blood. 2014; 124(21):1859. [Google Scholar]
- 24.Tavares R, Palumbo GA, Le Coutre P, et al. Safety and efficacy of ruxolitinib in an 1869-patient cohort of JUMP: an open-label, multicenter, single-arm, expanded-access study in patients with myelofibrosis. Blood. 2015;126(23):2799. [Google Scholar]
- 25.Al-Ali HK, Griesshammer M, le Coutre P, et al. Safety and efficacy of ruxolitinib in an open-label, multicenter, single-arm phase 3b expanded-access study in patients with myelofibrosis: a snapshot of 1144 patients in the JUMP trial. Haematologica. 2016; 101(9):1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.JAKAFI® (ruxolitinib) prescribing information USFDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202192s015lbl.pdf Accessed March 21, 2018.
- 27.JAKAVI® (ruxolitinib) Summary of product characteristics. http://www.emaeuropa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002464/WC500133223.pdf Accessed March 21, 2018.
- 28.Verstovsek S, Gotlib J, Gupta V, et al. Management of cytopenias in patients with myelofibrosis treated with ruxolitinib and effect of dose modifications on efficacy outcomes. Onco Targets Ther. 2013;7:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vannucchi AM, Gisslinger H, Harrison CN, et al. EXPAND: A phase 1b, open-label, dose-finding study of ruxolitinib in patients with myelofibrosis (MF) and low platelet counts (50×109/L to 99×109/L) at baseline. Blood. 2015;126(23):2817. [Google Scholar]
- 30.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–2901. [DOI] [PubMed] [Google Scholar]
- 31.Mukuria C, Rowen D, Brazier JE, et al. Deriving a preference-based measure for myelofibrosis from the EORTC QLQ-C30 and the MF-SAF. Value Health. 2015;18(6):846–855. [DOI] [PubMed] [Google Scholar]
- 32.Mesa RA, Schwager S, Radia D, et al. The Myelofibrosis Symptom Assessment Form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leukemia Res. 2009;33(9):1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mesa RA, Kantarjian H, Tefferi A, et al. Evaluating the serial use of the Myelofibrosis Symptom Assessment Form for measuring symptomatic improvement: performance in 87 myelofibrosis patients on a JAK1 and JAK2 inhibitor (INCB018424) clinical trial. Cancer. 2011; 117(21):4869–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrison CN, Vannucchi AM, Platzbecker U, et al. Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): a randomised, open-label, phase 3 trial. Lancet Haematol. 2018;5(2):e73–e81. [DOI] [PubMed] [Google Scholar]
- 35.Pardanani A, Harrison C, Cortes JE, et al. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol. 2015;1(5):643–651. [DOI] [PubMed] [Google Scholar]
- 36.Verstovsek S, Komrokji RS. A comprehensive review of pacritinib in myelofibrosis. Future Oncol. 2015;11(20):2819–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.