Ocean warming events, such as the recent 2015 El Niño in the California current ecosystem, and demanding life stages, such as reproduction, can cause chronic stress in sea lions. We demonstrate that stress hormones are associated with metabolism and immune markers in chronically stressed animals.
Keywords: California sea lion, capture stress, El Niño, thyroid, immunoglobulin, glucocorticoid
Abstract
Wildlife is exposed to a diverse set of extrinsic and intrinsic stressors, such as climatic variation or life history constraints, which may impact individual health and fitness. El Niño and climatic anomalies between 2013 and 2016 had major ecological impacts on the California Current ecosystem. As top marine predators, California sea lions (CSL) experienced decreased prey availability and foraging success, impacting their nutritional state. We hypothesize that chronic stress to juvenile CSL increased during the 2015–2016 El Niño and that breeding represents a period of chronic stress for adults, which impact a variety of physiological processes. We opportunistically captured and sampled juvenile CSL (female, n = 29; male, n = 38) in central California and adult male CSL (n = 76) in Astoria, Oregon and quantified a suite of analytes in serum as indicators of acute stress markers, metabolism and thyroid function, and adaptive immune response. We found that stress hormones and glucose were decreased in juvenile CSL during 2016 relative to 2015 and in adult male CSL after the breeding season, which may indicate chronic stress downregulating HPA (hypothalamic-pituitary-adrenal) axis sensitivity with associated metabolic impacts. Conversely, thyroid hormones for both juvenile and adult male CSL were increased, suggesting greater energetic requirements resulting from increased foraging activity during suboptimal conditions in juveniles and breeding tenure in adult males. Immunoglobulin IgG was elevated in juveniles in 2016 but reduced in adult males post-breeding. This suggests that juveniles may face immunostimulatory pressure during anomalously warm ocean environments; however, for adult males, breeding is a significant energetic cost resulting in reductions to immune function. Our results indicate that environmental conditions and life history stage may influence physiological responses in an important marine predator and a sentinel species of changing ocean ecosystems.
Introduction
All organisms are exposed to a variety of stressors over the course of their lives, and some of these stressors can have an important impact on homeostasis. Extrinsic stressors arise from chemical, physical or biological factors in an animal’s external environment (Acevedo and Duffus, 2009), all of which can be influenced by climatic variation. Intrinsic stressors are often the sequela of tradeoffs inherent to specific life history strategies. Understanding how wildlife species physiologically adapt to and cope with acute and long-term disturbances to endocrine and immune systems is critical to determine the fitness consequences of natural stressors (Wingfield et al., 1997, Wikelski and Cooke, 2006, Dantzer et al., 2014, McCormick and Romero, 2017).
Sustained responses to chronic stressors, such as extended periods of food limitation or reproduction, are adaptive if the stressor is cyclic, predictable and there has been sufficient time for selection to shape the response to the stressor (Boonstra, 2013). For example, glucocorticoid (GC) hormones released by the hypothalamic-pituitary-adrenal (HPA) axis can be stimulatory by increasing gluconeogenesis (conversion of non-carbohydrates, such as lactate, into glucose), metabolic rate and growth (McMahon et al., 1988, Sapolsky et al., 2000). If unpredictable (e.g. major short-term environmental disturbance), continuous exposure to a stressor can result in a decrease in both basal corticosteroids and ability to cope with future or additional stressors (Rich and Romero, 2005, Romero et al., 2009). It is unclear, however, how these cumulative effects manifest in wildlife species and if these sustained responses can be considered maladaptive (Boonstra, 2013).
Repeated or sustained stress can have downstream consequences on important biological functions (McEwen and Stellar, 1993).Chronically elevated GCs can alter the hypothalamus-pituitary-thyroid (HPT) axis through a variety of mechanisms (Charmandari et al., 2005, Cooper and Ladenson, 2011), which over time, will suppress thyroid function and reduce energy expenditure (Danforth and Burger, 1984, Helmreich et al., 2005, Chrousos, 2009). Chronic stress can also down-regulate immune response (McEwen et al., 1997), facilitating an energetic trade-off between immune function and other metabolic processes (Sheldon and Verhulst, 1996, Råberg et al., 1998). The adaptive immune response, represented in part by immunoglobulin (Ig) production, is highly specialized to specific pathogens and requires substantial biochemical and/or energetic reserves with high developmental costs (McDade et al., 2016).
Anomalous weather and climatic events can be major drivers for shifts and disturbances to marine ecosystems (Hughes et al., 2005). Short-term warming, such as the onset of an El Niño-Southern Oscillation (ENSO) event, has been extensively documented as intense local stressors to many ecologically important marine species, such as coral reefs (Claar et al., 2018), seabirds (Wingfield et al., 2018), marine iguanas (Amblyrhynchus cristatus,Romero and Wikelski, 2001) and pinnipeds (seals and sea lions, Trillmich et al., 1991). Increases in sea surface temperature (SST) can reduce the distribution of nutrients via a decrease in local upwelling and consequently, a local decrease in productivity of primary producers (Trenberth, 1997) and shifts in distribution and quantity of prey species (Enfield, 2001). For pinnipeds, unpredictable perturbations from ENSO events cause alterations to diving/foraging (Crocker et al., 2006, Weise and Harvey, 2008), reproductive behaviours (Soto et al., 2006, Melin et al., 2008), increases in stranding events due to malnutrition (Greig et al., 2005, Melin et al., 2009, Barcenas-de la Cruz et al., 2018) and overall mortality due to reduced pup development and survival (Trillmich and Limberger, 1985, Ono et al., 1987, DeLong et al., 2017). Although pinnipeds are widespread and ecologically important marine predators, few studies have measured the effect that sustained exposure to intrinsic or unpredictable stressors may have on endocrine and immune responses.
Between 2013 and 2016, there were multiple disruptions to primary productivity within the marine environment of the California Current ecosystem, including two ENSO events (Leising et al., 2014, Leising et al., 2015, Jacox et al., 2016). Starting in 2014, a large-scale warm water anomaly within the Pacific Ocean known as `the Blob’ was associated with delays to the start of coastal upwelling, resulting in an increased duration of heightened SST (Kintisch, 2015, Peterson et al., 2015). An El Niño was then declared in March 2015 with peak departures from normal monthly SST occurring around December (NOAA National Centers for Environmental Information, 2015). These combined climatic events led to a collapse and distribution shift of forage fish for an abundant top predator, the California sea lion (CSL, Zalophus californianus; MacCall et al., 2016, McClatchie et al., 2016). Foraging patterns for CSL were altered and animals were likely driven farther offshore to feed (Elorriaga-Verplancken et al., 2016). When forced to increase foraging effort, nursing adult female sea lions have difficulty in allocating resources towards pup development (Ono et al., 1987). Additionally, body condition and glucose-dependent immune markers, IgG and IgA (Palmer et al., 2015), were reduced in dependent CSL pups born during anomalous conditions of high SST in 2015 (Banuet-Martínez et al., 2017), and also showed abnormalities to erythrocytes, which can further impact nutrient uptake (Flores-Morán et al., 2017). This extended period presented a unique opportunity to investigate the effects of sustained stressors on CSL facing abnormally high energetic demands.
Juvenile and adult male CSL represent critical and understudied life history stages for this species. Juvenile CSL exhibit lower diving capacity compared to mature adults (Weise et al., 2010, Kuhn and Costa, 2014), and likely must modulate or increase diving effort (i.e. greater duration, longer distance), or change prey types during suboptimal conditions (Costa et al., 1991). During the peak reproductive season (June and July), adult males of various body size and social rank cease foraging to participate in territorial defence throughout a long breeding tenure, which can last several weeks (Riedman, 1990). Breeding therefore carries high energetic costs, and the combined timing of breeding and suboptimal climatic conditions caused by El Niño may lead to allostatic overload and reduced energy for coping (Wingfield and Ramenofsky, 2011).
Our objective was to examine how markers of stress, metabolism and immune defence may be impacted by inter-annual differences in environmental conditions in juvenile CSL and life history stage (i.e. pre- and post-breeding) in adult male CSL. We use the 2015 El Niño event as a proxy for a variety of documented changes in primary productivity and prey availability, which likely caused chronic stress in CSL. In this study, we assessed a suite of analytes during capture (an acute stressor) in free-ranging juvenile CSL during the fall months of 2015 and 2016 in central California and adult males during the 2016 spring (pre-breeding) and late summer (post-breeding) seasons in Astoria, Oregon (Fig. 1). Specifically, we measured serum concentrations of stress hormones (total cortisol, corticosterone and aldosterone); thyroid hormones (total T4, T3 and rT3) to evaluate thyroid function; glucose and lactate as indicators of carbohydrate metabolism; and two important immune markers, IgM (novel or recent infections) and IgG (strong secondary immune response after repeat exposures to pathogens). We hypothesize that the cumulative effects of anomalous conditions surrounding the 2015 El Niño, one of the strongest on record (Jacox et al., 2016), carried into 2016. Therefore, we predicted that sustained stress from energetically costly events, such as increased foraging effort and reproduction, would cause downregulation of the HPA axis in both age groups, with subsequent suppression of corticosteroids, glucose levels, thyroid hormones and immune markers across years and seasons.
Figure 1.
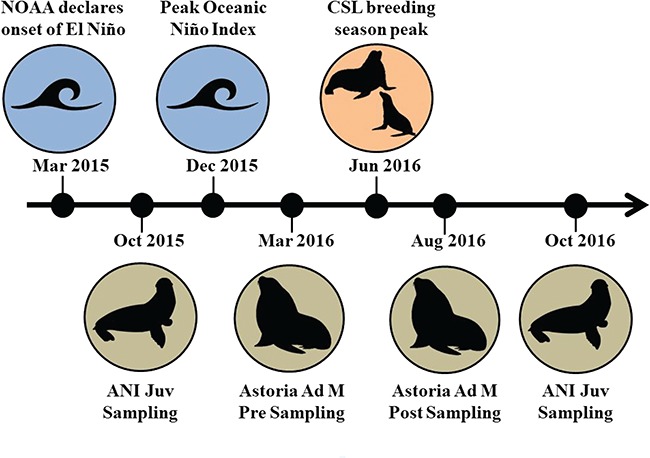
Timeline of events relevant to the CSL sampling periods during this study. During March 2015, an El Niño event was declared, with peak departures from normal monthly SST (Oceanic Niño Index) occurring around December 2015. Juvenile (Juv) CSL were sampled on Año Nuevo Island (ANI) during October 2015 and October 2016. Adult male (Ad M) CSL were sampled in Astoria, Oregon in March 2016 and late August 2016, both pre- and post-breeding season. NOAA data taken from State of the Climate, Global Climate Report (https://www.ncdc.noaa.gov/sotc/global/201513).
Materials and methods
Study subject and capture procedure
We sampled individual juvenile CSL in October 2015 (female, n = 16; male, n = 17) and October 2016 (female, n = 13; male, n = 21) at Año Nuevo Island (ANI) in San Mateo County, California (37.1086°N, −122.3362°W) and adult male CSL in March (pre-breeding, n = 34) and late August–September 2016 (post-breeding, n = 42) in Astoria, Oregon (46.19447°N, −123.80292°W) as part of a larger health assessment study. Juvenile CSL at ANI were individually captured with modified hoop nets by several personnel who approached multiple animals along the beach. Once captured, CSL were placed into separate carriers (Petmate Vari Kennel, dimensions 48" L X 32" W X 35" H) ~25 m from the sampling area. Single CSL were successively removed, recaptured, physically restrained, anesthetized with isoflurane gas and sampled. Adult CSL at Astoria voluntarily hauled out on and confined in a floating cage trap, i.e. a modified large, metal capture cage with sliding guillotine doors set open. Individual CSL were ultimately led into a squeeze cage into which animals were moved for sedation with a combination of midazolam (0.1 mg/kg) and butorphanol (0.1 mg/kg) and anaesthesia using isoflurane gas. The methodology for adult captures is described in greater detail by Wright et al. (2010) and Melin et al. (2013).
Once anaesthesia had begun, blood was collected as soon as safely possible from the caudal gluteal vein via Vacutainer needles into 10 ml serum Vacutainer tubes. Times of capture, anaesthesia start and blood collection were recorded, as were the sex and standard length (SL). SL was measured as a straight length from the tip of the nose to the tip of the tail. Ages of juveniles were estimated to be between 1.5 and 4.5 years old based on methods with known age individuals using a combination of body and tooth size, and lack of secondary sexual characteristics (Greig et al., 2005, Laake et al., 2016). Adult males were identified and aged based on size, pelage colour and presence of a fully developed sagittal crest. Due to logistical difficulties, we were unable to measure body mass in all animals in this study and instead used SL as a proxy for body size. Before release, all CSL were given a numerical front flipper tag (Allflex USA, Inc.) to identify individuals; and there was no repeated sampling of individuals.
Sample analysis
Once collected, blood samples were allowed to clot and centrifuged that day at 1534 g (LW Scientific Portafuge E7, USA) for 15 minutes to separate serum. Samples were frozen and stored at −80°C until analysis. We quantified total concentrations of all analytes in duplicate using commercially available assay platforms. Mean concentrations for each analyte separated by age class and sampling period are found in Supplementary Tables 1 and 2. Cortisol, total T3 (TT3), total T4 (TT4) (MP Biomedicals, Orangeburg, NY, USA), rT3 (ALPCO, Salem, NH, USA) and aldosterone (IBL International, Hamburg, Germany) were measured using hormone specific I125 radioimmunoassay (RIA) kits. Corticosterone was measured using enzyme-linked immunosorbent assay (Cayman Chemical Company, Ann Arbor, Michigan, USA). IgG and IgM were measured using antibody-sensitized microsphere microagglutination assays (Thermo Fisher Scientific, Waltham, MA, USA). Metabolites glucose and lactate were measured using a YSI 2300 analyzer to yield quantitative (i.e. mM) rather than relative values. GCs and aldosterone were measured in all serum samples (n = 143), and thyroid hormones, Igs and metabolic markers were measured in a subset of animals depending on available sample volume (TT3, n = 137; rT3, n = 108; and TT4, n = 115; IgG and IgM, n = 102; lactate/glucose, n = 103).
Figure 2.
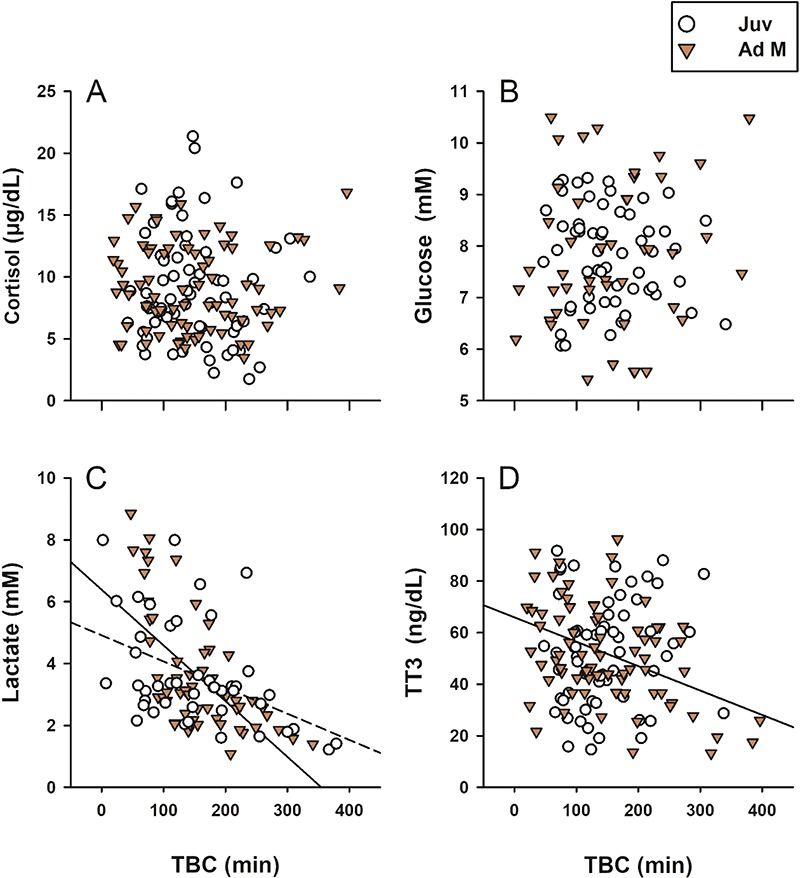
Relationships between length of time between capture and blood collection (TBC) and stress and metabolism markers in juvenile (Juv) and adult male (Ad M) sea lions. Separate lines of fit (Juv, dotted; Ad M, solid) are derived from GLM leverage plots when age class was included as a fixed effect. TBC exhibited no significant linear relationships with cortisol (a) or glucose (b) concentrations (P > 0.05). Lactate (c) was initially high but significantly decreased with TBC in both age classes (Juv, R2 = 0.40, P < 0.0001; Ad M, R2 = 0.20, P = 0.002), and TT3 (d) decreased with time in adult males only (R2 = 0.19, P < 0.0001).
Initial values for serum cortisol, aldosterone, TT3 and rT3 were on the standard curve provided with manufacturer’s standards. Other analytes were diluted according to the following methods to be brought onto the standard curve: corticosterone samples diluted 1:10 with EIA buffer, TT4 samples diluted 1:3 with zero calibrator and both IgM and IgG diluted 1:240 with manufacturer provided dilution buffer. All assay platforms for GCs, aldosterone, thyroid hormones and Igs were validated for use in CSL. Serially diluted samples for each analyte yielded curves that were parallel to the standard curves (Supplementary Figures 1 and 2). We assessed accuracy and calculated % mean recovery of three added standards for each assay platform (cortisol = 96.5%, corticosterone = 96.3%, aldosterone = 103.1%, TT3 = 97.1%, rT3 = 98.9%, TT4 = 101.2%, IgG = 97.2%, IgM = 102.1%). Mean intra-assay coefficient of variation (CV) was calculated for each analyte (cortisol = 2.43%, corticosterone = 2.67%, aldosterone = 2.28%, TT3 = 1.88%, rT3 = 3.13%, TT4 = 1.6%, IgG = 5.61%, IgM = 3.43%, lactate = 1.33%, glucose = 1.56%). All assays were performed in single runs except rT3. Mean inter-assay CV for rT3 was 3.38%.
Statistical analysis
To account for the large variability in handling duration, we first evaluated the effect of time between initial capture and blood collection (hereafter TBC) on each analyte using a general linear model (GLM) with TBC and age class as independent variables. TBC was included as a covariate in subsequent models when significant relationships were found for the respective analyte. Model R2 values for TBC were derived by plotting the GLM effect leverage pairs for each analyte accounting for the effect of age (y = partial residuals, x = regressor shrinkage). We then created scatterplot matrices and investigated significant associations between measured analytes using multivariate Pearson correlations for each age class (Supplementary Figure 3 and 4). Finally, because of differences in capture techniques, geographic locations and time periods for adults and juveniles, we did not attempt to make specific comparisons for temporal effects between the age classes. We instead used two separate GLMs to independently examine how analytes changed with year, sex and body size in juvenile CSL, and between pre- and post-breeding seasons and with body size in adult male CSL. For juveniles, sex, year and SL were included as fixed effects. Season and SL were included for adult males. When significant differences between groups were detected, post hoc comparisons were performed using Student’s t-tests. For all tests, model residuals were visually assessed for normality and homoscedascity, and results were considered significant when P < 0.05. Data analyses were performed with JMP Pro 13 (SAS Institute, Cary, NC, USA).
Results
Response to capture
TBC was highly variable for juveniles on ANI (mean = 116 minutes ±65 SD) and adult males in Astoria (mean=172 minutes ±88 SD). However, times between anaesthesia start and blood collection were short by comparison for both locations (ANI, mean = 7 minutes ±5 SD; Astoria, mean = 13 minutes ±4 SD). Controlling for age class, TBC was not associated with cortisol (Fig. 2a, juveniles, P = 0.57; adults, P = 0.83), corticosterone (juveniles, P = 0.86; adults, P = 0.47), aldosterone (juveniles, P = 0.29; adults, P = 0.41), glucose (Figure 2b, juveniles, P = 0.36; adults, P = 0.29), IgM (juveniles, P = 0.86; adults, P = 0.69) or IgG (juveniles, P = 0.99; adults, P = 0.52). We therefore excluded TBC in subsequent GLM analyses for these analytes due to lack of significance and ability to explain model variance. Controlling for age class, we did, however, find a significant negative association of TBC and lactate (Fig. 2c, juveniles, R2 = 0.40, F1,55 = 36.8, P < 0.0001; adults, R2 = 0.20, F1,44 = 11.1, P = 0.002). Lactate was initially high in animals sampled quickly after capture but significantly lower in those sampled later. TBC did affect thyroid hormones differently between age classes. TBC did not affect TT4 (juveniles, P = 0.32; adults, P = 0.75) or rT3 (juveniles, P = 0.53; adults, P = 0.64) for either age class; however, TBC was negatively associated with TT3 in adult males (Fig. 2d, R2 = 0.19, F1, 73 = 16.8, P < 0.0001) but not in juveniles (P = 0.35).
Associations between hormones, immune markers and metabolites
We highlight significant relationships of varying directions between cortisol and other analytes (Fig. 3). Several correlations were shared for both age classes. Serum cortisol-corticosterone (juveniles, r = 0.86, P < 0.0001; adults, r = 0.85, P < 0.0001) and cortisol-aldosterone (juveniles, r = 0.83, P < 0.0001; adults, r = 0.67, P < 0.0001) were strongly and positively correlated with each other in both age classes (Fig. 3a, b). Cortisol was positively correlated with glucose (Fig. 3c, juveniles, r = 0.39, P = 0.0009; adults, r = 0.67, P = 0.0009) and negatively correlated with TT3 in both age classes (Fig. 3d, juveniles, r = −0.14, P = 0.048; adults, r = −0.27, P = 0.044). Controlling for time, cortisol was negatively associated with lactate in adult males only (r = −0.48, P = 0.017; juveniles, P > 0.05). Cortisol was positively correlated with rT3 (r = 0.45, P = 0.0007) and TT4 (r = 0.51, P = 0.0091) in juveniles only (Fig. 3e). We found no correlations between any corticosteroids and IgM or IgG for either age class (Supplementary Figure 3, P > 0.05). IgG and IgM were negatively correlated in adult males (r = −0.20, P = 0.011) and approaching significance for juveniles (r = −0.24, P = 0.08). Glucose was positively correlated with IgG for adult males (r = 0.40, P < 0.0001).
Figure 3.
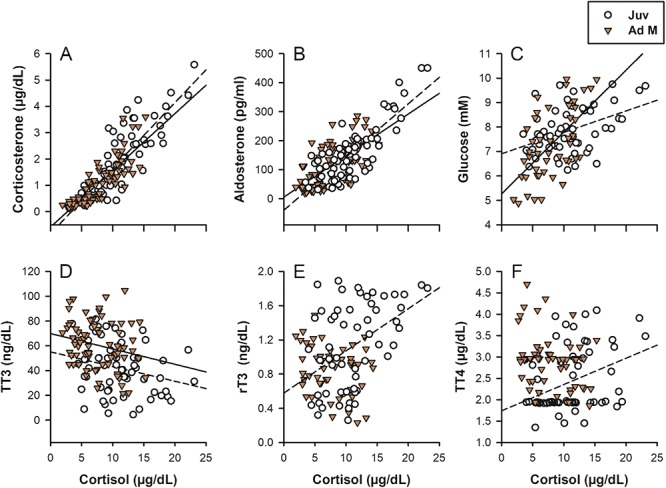
Relationships between markers for stress and metabolism in juvenile (Juv) and adult male (Ad M) sea lions. Separate lines of fit (Juv, dotted; Ad M, solid) are derived from Pearson’s correlations using multivariate comparisons for each age class. Cortisol was significantly positively associated with corticosterone (a, Juv, r = 0.86, P < 0.0001; Ad M, r = 0.85, P < 0.0001), aldosterone (b, Juv, r = 0.83, P < 0.0001; Ad M, r = 0.67, P < 0.0001) and glucose (c, Juv, r = 0.39, P = 0.0009; Ad M, r = 0.67, P = 0.0009), and negatively associated with TT3 (D, Juv, r = −0.14, P = 0.0133; Ad M, r = −0.27, P = 0.0044). rT3 (E, r = 0.45, P = 0.0007) and TT4 (F, r = 0.51, P = 0.009) were positively associated with cortisol in juvenile CSL only.
Annual, sex and body size differences in juveniles
Several strong effects of year and sex were evident for most measured analytes in juvenile CSL (Table 1). Year greatly affected all corticosteroids measured, with ~40% reductions to cortisol and ~50% reductions in corticosterone and aldosterone during 2016 compared to 2015 (Fig. 4a, b, c). Similarly, thyroid hormones rT3 and TT4 (Fig. 4e, f) and glucose (Fig. 5c) were significantly lower in 2016 compared to 2015. Conversely, we observed ~40% increases to TT3 (Fig. 4d) and IgG (Fig. 5a) in 2016 compared to 2015. IgM did not change between years (Fig. 5b). Sex only affected cortisol, TT3, TT4 and IgM (Table 1), each being significantly higher in juvenile females than males (Student’s t-test, P < 0.05). SL in juveniles only affected IgM, with smaller juveniles exhibiting elevated IgM (Table 1). As previously mentioned, lactate was greatly influenced by TBC, and year, sex and SL did not significantly affect lactate when TBC was included in the model (P > 0.05).
Table 1.
Results from GLM examining effects of year, sex and standard length (SL) on analytes in juvenile CSLs
| Den df | Year | F | P | Sex | F | P | SL | F | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cortisol | 63 | 2016 < 2015 | 23.1 | <0.0001 | F > M | 4.89 | 0.031 | 0.87 | 0.35 | |
| Corticosterone | 63 | 2016 < 2015 | 19.7 | <0.0001 | 0.37 | 0.55 | 0.13 | 0.72 | ||
| Aldosterone | 63 | 2016 < 2015 | 15.3 | <0.0001 | 2.14 | 0.15 | 0.001 | 0.98 | ||
| Total T4 | 52 | 2016 < 2015 | 4.23 | 0.045 | F > M | 5.48 | 0.023 | 1.36 | 0.25 | |
| Total T3 | 58 | 2016 > 2015 | 22.8 | <0.0001 | F > M | 22.7 | <0.0001 | 0.23 | 0.63 | |
| Reverse T3 | 53 | 2016 < 2015 | 23.3 | <0.0001 | 0.03 | 0.86 | 2.97 | 0.09 | ||
| IgG | 48 | 2016 > 2015 | 7.06 | 0.0107 | 0.50 | 0.48 | 0.06 | 0.80 | ||
| IgM | 48 | 0.13 | 0.72 | F > M | 3.12 | 0.0437 | Neg | 7.6 | 0.0081 | |
| Glucose | 53 | 2016 < 2015 | 6.54 | 0.0135 | 3.67 | 0.06 | 0.04 | 0.83 | ||
| Lactate* | 52 | 1.1 | 0.29 | 0.68 | 0.41 | 2.14 | 0.15 |
Significant results are in bold; inequalities denote directionality of differences via post-hoc Student’s t-tests. Denominator degrees of freedom (den df) were calculated via GLM output. *We controlled for time of blood collection (TBC) for lactate.
Figure 4.
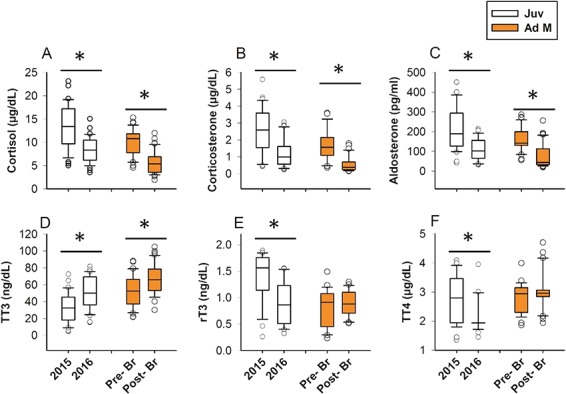
Box plots depicting stress and thyroid hormone concentrations across the years 2015 and 2016 in juveniles (Juv) and the 2016 summer breeding season in adult males (Ad M). Pre- Br: pre-breeding season; Post- Br: post-breeding season. Central lines in boxes are medians, and whiskers are 95% confidence intervals with outliers. *grouped by a horizontal line denotes significant differences between years or seasons separately for each age class according to post-hoc Student’s t-tests (P < 0.05).
Figure 5.
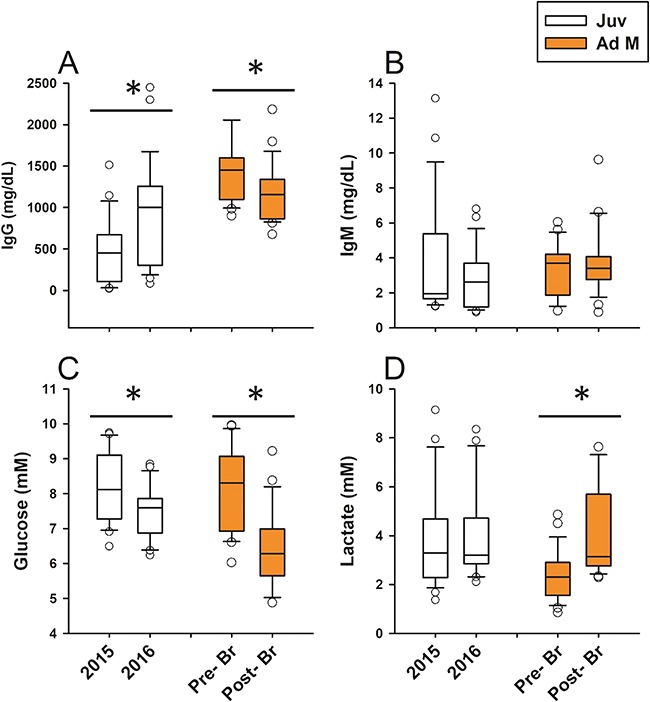
Box plots depicting Ig and glucose/lactate concentrations across the years 2015 and 2016 in juveniles (Juv) and the 2016 summer breeding season in adult males (Ad M). Pre- Br: pre-breeding season; Post- Br: post-breeding season. Central lines in boxes are medians, and whiskers are 95% confidence intervals with outliers. *grouped by a horizontal line denotes significant differences between years for Juv or seasons for Ad M according to post-hoc Student’s t-tests (P < 0.05).
Seasonal and body size differences in adult males
We also observed strong seasonal effects on several measured analytes in adult male CSL (Table 2). Season greatly affected all corticosteroids measured, with 45%, 67% and 55% reductions in cortisol, corticosterone and aldosterone, respectively, post- versus pre-breeding season (Fig. 4a, b, C). IgG (Fig. 5a) and glucose (Fig. 5c) were also reduced post- versus pre-breeding seasons. Conversely, when we controlled for TBC, TT3 was significantly higher post-breeding than pre-breeding (Fig. 4d, Student’s t-test, P < 0.05). Season had no effect on IgM, rT3 or TT4 (P > 0.05). SL only significantly affected cortisol and corticosterone, with smaller adult males exhibiting increased concentrations of GCs during capture. When we controlled for TBC, season (F2, 43 = 9.52, P = 0.0036) but not SL (P = 0.32) affected lactate, with lactate being higher post-breeding compared to the pre-breeding season (Student’s t-test, P < 0.05).
Table 2.
Results from GLM examining effects of season and standard length (SL) on analytes in adult male CSLs
| Den df | Season | F | P | SL | F | P | |
|---|---|---|---|---|---|---|---|
| Cortisol | 73 | Pre > Post | 68.6 | <0.0001 | Neg | 8.33 | 0.0051 |
| Corticosterone | 73 | Pre > Post | 57.0 | <0.0001 | Neg | 7.67 | 0.0071 |
| Aldosterone | 73 | Pre > Post | 37.2 | <0.0001 | - | 0.27 | 0.61 |
| Total T4 | 55 | - | 2.77 | 0.10 | - | 0.20 | 0.66 |
| Total T3* | 72 | Pre < Post | 4.6 | 0.0362 | - | 0.01 | 0.91 |
| Reverse T3 | 48 | - | 0.98 | 0.32 | - | 1.85 | 0.18 |
| IgG | 47 | Pre > Post | 7.06 | 0.0107 | - | 0.0004 | 0.98 |
| IgM | 47 | - | 0.88 | 0.35 | - | 0.41 | 0.52 |
| Glucose | 47 | Pre > Post | 6.7 | 0.013 | - | 0.15 | 0.71 |
| Lactate* | 42 | Pre < Post | 9.5 | 0.0036 | - | 1.03 | 0.32 |
Significant results are in bold; inequalities denote directionality of differences via post-hoc Student’s t-tests. Den df were calculated via GLM output. *We controlled for TBC for total T3 and lactate.
Discussion
We found strong inter-annual and seasonal variation in several physiological markers measured after capture and prolonged restraint (a proxy for an acute stress event) in free-ranging CSL. These changes manifested as large reductions to stress hormones between sampling periods and varying directions of markers for metabolism and immune function depending on age class. The ambiguity of multiple causes of anomalous oceanographic conditions during the current study period makes the specific timeline and duration of these effects difficult to measure. Both study years (2015 and 2016) were likely stressful to CSL; therefore, we lack information on baseline stress responses but are able to compare 2 years that reflect a continuation of chronically stressful oceanographic conditions characterized by impacts to sea lion nutritional resources (McClatchie et al., 2016). Nevertheless, we observed clear inter-annual variability in several aspects of the physiology of juvenile sea lions between the start of a recently declared El Niño event in 2015 and again 1 year later after exposure to increasingly high SST. We similarly observed marked changes to several markers measured in adult male CSL preceding and immediately following the reproductive season of 2016 (approximately a 6-month period). We should note that although adult sea lions in this study likely engaged in breeding behaviour; they also in general had the opportunity to forage. Adult male CSL demonstrate behavioural shifts to foraging and diving effort during El Niño conditions (Weise et al., 2006) as well. Therefore, we cannot rule out the potential for temporal effects of climate variability between breeding seasons exacerbating natural life history variability for this age group.
Handling effects on hormones and metabolism
In our study, sea lions experienced a capture event and were subsequently held in enclosures for highly variable lengths of time before being quickly recaptured and sampled. We assume here that capture acts as an acute stressor such that we expect to see physiological responses typical of short-term challenges (DeRango et al., 2018, Chinn et al., 2018) while recognizing that we could not standardize the timing or magnitude of the stressor. Stress hormones released during acute stressors can quickly impact metabolic pathways (Charmandari et al., 2005, Champagne et al., 2012, Champagne et al., 2018). Therefore, within the relatively brief time frame of capture in our study, we expected increases in serum concentrations of corticosteroids, glucose and lactate post-capture, but not in thyroid hormones, which have not shown short-term changes in response to acute stress in previous studies on pinnipeds (McCormley et al., 2018). Serum corticosteroids measured throughout capture via serial sampling in Guadalupe fur seals begin to decline from peak values at around 60 minutes post-capture (DeRango et al., 2018). Although we found no linear relationships between holding time and corticosteroids in the current study, capture likely influenced GCs and aldosterone measured at the time of sampling. Both GCs were highly associated with aldosterone, which provides further evidence of the regulation of aldosterone secretion in association with the HPA axis in otariids as reported in other pinnipeds (Ensminger et al., 2014, Champagne et al., 2015, Jelincic et al., 2017, DeRango et al., 2018, McCormley et al., 2018). We also found that TT3 was significantly lower in adult male CSL, which were held for long holding periods before sampling. Adult male elephant seals during the breeding and moulting season alter thyroid hormones during sustained exposure to GCs within 2 hours via adrenocorticotropic hormone (ACTH) challenges (Ensminger et al., 2014). Therefore, the 4–6 hour holding period for some adult male CSL in our study may alter the HPT axis via sustained stress.
Most knowledge of gluconeogenesis in pinnipeds comes from studies of fasting physiology in the northern elephant seal (Mirounga angustirostris,Champagne et al., 2013). In elephant seals there was no relationship between cortisol concentrations after restraint and rates of glucose production (Champagne et al., 2012); however, it remains unknown how capture stress impacts carbohydrate metabolism in other pinniped species or otariids. In our study, glucose remained relatively high and stable (mean = 7.8 ± 1.2 mM), with no association between time to blood collection after capture. Endogenous glucose production increases with prolonged activity (Mourtzakis et al., 2006); however, juvenile sea lions in this study were quickly captured after a brief but intense chase period and then remained in carriers, and adult were confined in a cage with conspecifics until sampling. Interestingly, lactate exhibited a strong relationship to handling time, where lactate was very high in animals that were sampled quickly after capture and then significantly lower in animals that were held longer. Therefore, it is likely that high lactate concentrations after the intense chase or agitation period are converted to glucose via gluconeogenesis during the rest period to keep glucose levels stable.
Climatic and life history contexts
Lower corticosteroids measured in response to capture often reflect reduced HPA sensitivity after sustained stressors (Rich and Romero, 2005). Reductions in corticosteroids and glucose during 2016 in juvenile CSL may be consistent with suppression of the HPA axis via negative feedback secondary to chronic stress. A comprehensive analysis of Galápagos sea birds found that, generally, species showed the strongest response to induced acute stressors when their prey sources were more affected by El Niño (Wingfield et al., 2018), suggesting that nutrient limitation likely plays a role in reducing HPA sensitivity. Similarly, reductions in GC release during capture in sea otters were strongest in reproductive, lactating females and those living in prey-limited populations (Enhydra lutris, Chinn et al., 2018). We propose that the prolonged energetic stress of increased foraging effort or reduced food intake in juveniles and breeding tenure in adult male CSL may have lessened the animals’ ability to mount a normal stress response to handling (Cockrem and Silverin, 2002), evident by decreased corticosteroids measured in both age classes. Furthermore, both corticosteroids and glucose measured during capture were lower in juvenile females and smaller adult CSL. To our knowledge, only Pedernera-Romano et al. (2010) have measured serum GCs in free-ranging CSL, and they reported similar sex and body size effects on cortisol release in pups. As a result of decreased GCs, we expected numerous effects to downstream metabolic and immune processes.
As previously mentioned, chronically high concentrations of GCs will suppress thyroid (Ferguson and Peterson, 1992) and modulate energy reserves during stressful events. Although the effects of chronic stress on thyroid hormones are largely unexplored within CSL, we expected suppression of thyroid after chronic elevation of GCs, such as across years during anomalous conditions or after the breeding season. Low magnitude cortisol responses to handling consistent with chronic stress have been associated with a reduction in thyroid hormones in fur seals (DeRango et al., 2018). Similarly, suppression of thyroid TT3 was observed after several days of repeated ACTH stimulation in fasting elephant seals (McCormley et al., 2018). Because we are suggesting that reduced GCs after capture may reflect sustained stress exposure and reduced HPA sensitivity, we predicted a positive relationship between GCs and thyroid hormones. Contrary to our prediction, cortisol after capture in this study was negatively associated with TT3, and TT3 production increased across what we considered energetically costly time periods in both age classes. The observed elevation of TT3 and reductions to the reservoir hormone TT4 and inactive rT3 between years may reflect the high rates of energy expenditure found in these animals. Within pinnipeds, some life history stages may require elevation of active thyroid function during stress to support energy intensive behaviours, such as the development of foraging (Somo et al., 2015) or breeding (Crocker et al., 2012a) while fasting. Physiological limitations due to smaller body size may restrict juvenile CSL in our study area to small home range sizes (McHuron et al., 2018a), and the spatial abundance of forage fish during the study period was limited (McClatchie et al., 2016). Foraging otariids continuously maintain high field metabolic rates (Costa, 1991), and confinement to areas of reduced prey availability leads to increases in energy requirements and metabolic rates (McHuron et al., 2017) and physiological challenges to juvenile CSL. Although we were not able to determine exact age or breeding success within individual adult males in this study, we also found increases in thyroid hormones measured directly after the conclusion of the breeding season. Investigations into seasonal effects on thyroid hormones in Steller sea lions (Eumatopias jubatus) similarly found that animals had increased concentrations during the reproductive months of July to late summer (Myers et al., 2010). Adult male elephant seals when breeding maintain high rates of energy expenditure despite fasting during a long reproductive tenure (Crocker et al., 2012b). The ability to raise T3 over the breeding period was associated with high rates of energy expenditure and breeding success (Crocker et al., 2012a). Our results suggest that adult male CSL may upregulate T3 in a similar fashion as they fast over the duration of the breeding tenure and engage in energetically costly behaviours. It is unclear whether maintaining increased thyroid levels long term while constrained by anomalous conditions or additional unpredictable stressors has the potential to be deleterious or active an ‘emergency life history state’ (Romero and Wingfield, 2015) in these animals.
The relationship between immune function and stress related to environmental or life history variables can be complex and likely mediated by a variety of factors. IgG production is mediated by glucose, through T-cell activated class switching (Palmer et al., 2015); therefore, adaptive immune responses are known to be compromised by nutrient limitation (Martin et al., 2007). Reproduction is one of the most energetically expensive processes in pinnipeds (Trillmich, 1990), and reduced IgG and compromises to immune function have been found in lactating elephant seals (Peck et al., 2016) and harbour seals (Phoca vitulina,Ross et al., 1993). Although it is difficult to distinguish the breeding costs of adult male and female pinnipeds, our results suggest similar trends, which reflect physiological stress-induced immunosuppression characteristic of reproduction and energetic limitations in males. Although we found no correlations between cortisol and immune markers, adult male CSL exhibited reduced cortisol, glucose and IgG after energetically costly events during the breeding season. Adult males during colonial breeding engage in strenuous fighting with conspecifics and often inflict wounds, potentially associated with high pathogen exposure. These obligate interactions and repeat exposures to the same pathogens may create an energetic trade-off moving resources away from the adaptive immune response and towards reproduction during the breeding season.
Banuet-Martínez et al. (2017) hypothesized that El Niño conditions would drive nutrient limitation and trade-offs to immune function in CSL. Contrarily, IgG measured in juvenile CSL was elevated during 2016, even with observed reductions to glucose during this year. A previous study demonstrated that closely related Galápagos sea lion (Zalophus wollebaeki) juveniles living near human-impacted colonies had elevated IgG responses, likely due to increased pathogen exposure from a myriad of environmental sources (Brock et al., 2013a). Our pattern similarly may reflect greater immunostimulatory pressure with individuals mounting a large immune response during 2016 despite nutrient limitation. Although we did not specifically measure pathogen burden, exposure to parasites or other pathogens may increase during El Niño events (Marcogliese, 2008), and increased SST can broadly affect how pinnipeds may clear pathogens (Seguel et al., 2018).
IgM was the only parameter to show an association with body length measured in juvenile CSL only. For pinnipeds, large body mass and adipose reserves are important for allocating increased energy stores towards heightened adaptive immune responses (Peck et al., 2016). Here we find that shorter SL (a proxy for body size and age) is associated with heightened IgM. This is striking, as CSL pups in another study born during anomalous conditions in 2015 exhibited decreased body condition and both IgG and IgM responses (Banuet-Martínez et al., 2017). Similarly, Galápagos sea lion pups with low body condition also had decreased IgG responses (Brock et al., 2013b). This species lives in areas characterized by low productivity environments and pups may invest in immunity and growth according to scarce resources provided by their mother. The authors propose that, if resources are low, evident by low body condition, pups will likely allocate energy towards somatic growth over immune function. Because length and body condition do not always correlate, it is possible that larger SL in our study may actually be reflective of older age. Our data may suggest that older animals apportion some energetic reserves towards costly immune processes, such as IgM production, and fight off pathogens early during exposure rather than continue growth. Furthermore, it may be equally likely that older juveniles have prior exposure to many circulating pathogens, and thus would elevate IgM in response to novel pathogens. Available energy reserves in developing juvenile sea lions thus likely impact the allocation of energy towards immune response at the beginning of exposure to pathogens where the developmental costs of immune responses are high. Controlling for annual effects, we also found that females had higher IgM than males along with increased stress and thyroid hormones. As a sexually dimorphic species, it is likely that, even at an early stage, juvenile males are allocating greater resources towards somatic growth than females and may be disproportionally affected by energetic trade-offs to stress and immune responses.
Cumulative ocean stressors
As large marine predators, sea lions respond dynamically to changes within their environment and provide clues about the current state of marine ecosystems (Simeone et al., 2015). Our study highlights the importance of sea lions as sentinel species for changing marine ecosystems and provides reference ranges for several markers for acute stress, metabolism and immune function during anomalous conditions for both developing and mature sea lions. Here we expand stress data for this model species, which may importantly provide future comparisons and environmental context, and inform marine mammal conservation decisions (Atkinson et al., 2015, Ocean Studies Board, 2017). The marine environment contains a multitude of stressors with which sea lions must physiologically cope. Our results may be equally confounded by documented naturally occurring toxic algal blooms, which also increase with SST (Van Dolah, 2000) and are known to cause domoic acid toxicosis and subsequent mortality in CSL (Gulland et al., 2012). Chronic exposure to domoic acid will reduce endocrine function by potentially altering the HPA pathway, thereby reducing cortisol release (Gulland et al., 2012). Natural stressors can be compounded by anthropogenic sources, such as exposure to marine debris, noise, contaminants and habitat modification or degradation (Kovacs et al., 2012, Maxwell et al., 2013), all of which can manifest as population-level disturbances to sea lions (McHuron et al., 2018b). Thus, the ambiguity of multiple stressors within the marine ecosystem can make it difficult to disentangle the exact cause and the cumulative effects observed in our study animals. Although reactive stress responses to normal life history events or severe weather are likely adaptive (Boonstra, 2013), it is unclear how these responses may impact fitness and life history trade-offs (Zera and Harshman, 2001) for individual sea lions. Large-scale warming anomalies within the California Current ecosystem will likely be persistent (Peterson et al., 2015); and as El Niño events are predicted to become more frequent (Cai et al., 2014), individuals may not be able to effectively respond to increased frequencies of disturbances in novel or changing conditions (Wingfield, 2013). The United States population of CSL is currently deemed stable; however, recent models show that incremental increases in SST likely drastically reduce future populations (Laake et al., 2018). Our results suggest that chronic stress due to oceanographic anomalies and nutritional limitations may alter physiological processes and has the potential to exacerbate natural stressors due to life history events.
Supplementary Material
Acknowledgements
All animal procedures were performed under protocols approved by the University of California Los Angeles, Institutional Animal Care and Use Committee (ARC # 2012-035-21) and National Marine Fisheries Service permits No. 17115-04 and No. 16087-01. We thank the many veterinarians, biologists and volunteers of the Marine Mammal Laboratory (Alaska Fisheries Science Center), Oregon and Washington Departments of Fish and Wildlife, The Marine Mammal Center, University of California Santa Cruz (especially P. Robinson, G. Oliver and P. Morris) and R. Greene for providing access to field sites and expertise for safe capture of the animals. Similarly, we thank staff from Año Nuevo State Park for logistical support. We are also grateful for the helpful review of the manuscript by F. Trillmich. Graphics used in Fig. 1 are attributed to http://www.getdrawings.com NC 4.0 license.
Funding
This work was supported by grants provided by the National Science Foundation Biological Oceanography Program (grant number OCE-1335657); United States Department of Defense, Strategic Environmental Research and Development Program (grant number RC01-020 to K.C.P.); and Office of Naval Research (grant number N000141410393 to D.E.C.).
References
- Acevedo-Whitehouse K, Duffus AL (2009) Effects of environmental change on wildlife health. Philos Trans R Soc Lond B Biol Sci 364: 3429–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson S, Crocker D, Houser D, Mashburn K (2015) Stress physiology in marine mammals: how well do they fit the terrestrial model. J Comp Physiol B 185: 463–486. [DOI] [PubMed] [Google Scholar]
- Banuet-Martínez M, Espinosa-de Aquino W, Elorriaga-Verplancken FR, Flores-Morán A, García OP, Camacho M, Acevedo-Whitehouse K (2017) Climatic anomaly affects the immune competence of California sea lions. PloS One 12: e0179359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcenas-De la Cruz D, DeRango E, Johnson SP, Simeone CA (2018) Evidence of anthropogenic trauma in marine mammals stranded along the central California coast, 2003–2015. Mar Mammal Sci 34: 330–346. [Google Scholar]
- Boonstra R. (2013) Reality as the leading cause of stress: rethinking the impact of chronic stress in nature. Funct Ecol 27: 11–23. [Google Scholar]
- Brock P, Hall A, Goodman S, Cruz M, Acevedo-Whitehouse K (2013a) Applying the tools of ecological immunology to conservation: a test case in the Galápagos sea lion. Anim Conserv 16: 19–31. [Google Scholar]
- Brock PM, Hall AJ, Goodman SJ, Cruz M, Acevedo-Whitehouse K (2013b) Immune activity, body condition and human-associated environmental impacts in a wild marine mammal. PloS One 8: e67132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Borlace S, Lengaigne M, Van Rensch P, Collins M, Vecchi G, Timmermann A, Santoso A, McPhaden MJ, Wu L (2014) Increasing frequency of extreme El Niño events due to greenhouse warming. Nat Clim Chang 4: 111. [Google Scholar]
- Champagne CD, Houser DS, Costa DP, Crocker DE (2012) The effects of handling and anesthetic agents on the stress response and carbohydrate metabolism in northern elephant seals. PloS One 7: e38442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne CD, Boaz SM, Fowler MA, Houser DS, Costa DP, Crocker DE (2013) A profile of carbohydrate metabolites in the fasting northern elephant seal. Comp Biochem Physiol Part D Genomics Proteomics 8: 141–151. [DOI] [PubMed] [Google Scholar]
- Champagne C, Tift M, Houser D, Crocker D (2015) Adrenal sensitivity to stress is maintained despite variation in baseline glucocorticoids in moulting seals. Conserv Physiol 3: doi: 10.1093/conphys/cov004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne CD, Kellar NM, Trego ML, Brendan D, Rudy B, Wasser SK, Booth RK, Crocker DE, Houser DS (2018) Comprehensive endocrine response to acute stress in the bottlenose dolphin from serum, blubber, and feces. Gen Comp Endocrinol 266: 178–193. doi: 10.1016/j.ygcen. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Tsigos C, Chrousos G (2005) Endocrinology of the stress response. Annu Rev Physiol 67: 259–284. [DOI] [PubMed] [Google Scholar]
- Chinn SM, Monson DH, Tinker MT, Staedler MM, Crocker DE (2018) Lactation and resource limitation affect stress responses, thyroid hormones, immune function, and antioxidant capacity of sea otters (Enhydra lutris). Ecol Evol 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. (2009) Stress and disorders of the stress system. Nat Rev Endocrinol 5: 374–381. [DOI] [PubMed] [Google Scholar]
- Claar DC, Szostek L, McDevitt-Irwin JM, Schanze JJ, Baum JK (2018) Global patterns and impacts of El Niño events on coral reefs: a meta-analysis. PloS One 13: e0190957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockrem J, Silverin B (2002) Variation within and between birds in corticosterone responses of great tits (Parus major). Gen Comp Endocrinol 125: 197–206. [DOI] [PubMed] [Google Scholar]
- Cooper D, Ladenson P (2011) Chapter 7. The thyroid gland In DG Gardner, D Shoback, eds, Greenspan’s Basic & Clinical Endocrinology. McGraw-Hill Education, New York, NY. [Google Scholar]
- Costa DP. (1991) Reproductive and foraging energetics of high latitude penguins, albatrosses and pinnipeds: implications for life history patterns. Am Zool 31: 111–130. [Google Scholar]
- Costa D, Antonelis G, DeLong R (1991) Effects of El Niño on the foraging energetics of the California sea lion In Pinnipeds and El Niño. Springer, Berlin, Heidelberg, pp. 156–165. [Google Scholar]
- Crocker DE, Costa DP, Le Boeuf BJ, Webb PM, Houser DS (2006) Impact of El Niño on the foraging behavior of female northern elephant seals. Mar Ecol Prog Ser 309: 1–10. [Google Scholar]
- Crocker DE, Houser DS, Webb PM (2012a) Impact of body reserves on energy expenditure, water flux, and mating success in breeding male northern elephant seals. Physiol Biochem Zool 85: 11–20. [DOI] [PubMed] [Google Scholar]
- Crocker DE, Ortiz RM, Houser DS, Webb PM, Costa DP (2012b) Hormone and metabolite changes associated with extended breeding fasts in male northern elephant seals (Mirounga angustirostris). Comp Biochem Physiol A Mol Integr Physiol 161: 388–394. [DOI] [PubMed] [Google Scholar]
- Danforth E, Burger A (1984) The role of thyroid hormones in the control of energy expenditure. Clin Endocrinol Metab 13: 581–595. [DOI] [PubMed] [Google Scholar]
- Dantzer B, Fletcher QE, Boonstra R, Sheriff MJ (2014) Measures of physiological stress: a transparent or opaque window into the status, management and conservation of species. Conserv Physiol 2: doi: 10.1093/conphys/cou023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong RL, Melin SR, Laake JL, Morris P, Orr AJ, Harris JD (2017) Age-and sex-specific survival of California Sea lions (Zalophus californianus) at San Miguel island, California. Mar Mammal Sci 33: 1097–1125. [Google Scholar]
- DeRango EJ, Greig DJ, Gálvez C, Norris TA, Barbosa L, Elorriaga-Verplancken FR, Crocker DE (2018) Response to capture stress involves multiple corticosteroids and is associated with serum thyroid hormone concentrations in Guadalupe fur seals (Arctocephalus philippii townsendi). Mar Mammal Sci. doi: 10.1111/mms.12517. [DOI] [Google Scholar]
- Elorriaga-Verplancken FR, Sierra-Rodríguez GE, Rosales-Nanduca H, Acevedo-Whitehouse K, Sandoval-Sierra J (2016) Impact of the 2015 El Niño-southern oscillation on the abundance and foraging habits of Guadalupe fur seals and California sea lions from the San Benito archipelago, Mexico. PloS One 11: e0155034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enfield DB. (2001) Evolution and historical perspective of the 1997–1998 El Niño–southern oscillation event. Bull Mar Sci 69: 7–25. [Google Scholar]
- Ensminger DC, Somo DA, Houser DS, Crocker DE (2014) Metabolic responses to adrenocorticotropic hormone (ACTH) vary with life-history stage in adult male northern elephant seals. Gen Comp Endocrinol 204: 150–157. [DOI] [PubMed] [Google Scholar]
- Ferguson DC, Peterson ME (1992) Serum free and total iodothyronine concentrations in dogs. Am J Vet Res 53: 1636–1640. [PubMed] [Google Scholar]
- Flores-Morán A, Banuet-Martínez M, Elorriaga-Verplancken FR, García-Ortuño LE, Sandoval-Sierra J, Acevedo-Whitehouse K (2017) Atypical red blood cells are prevalent in California sea lion pups born during anomalous sea surface temperature events. Physiol Biochem Zool 90: 564–574. [DOI] [PubMed] [Google Scholar]
- Greig DJ, Gulland FM, Kreuder C (2005) A decade of live California sea lion (Zalophus californianus) strandings along the central California coast: causes and trends, 1991-2000. Aquat Mamm 31: 11–22. [Google Scholar]
- Gulland FM, Hall AJ, Greig DJ, Frame ER, Colegrove KM, Booth RK, Wasser SK, Scott-Moncrieff JC (2012) Evaluation of circulating eosinophil count and adrenal gland function in California sea lions naturally exposed to domoic acid. J Am Vet Med Assoc 241: 943–949. [DOI] [PubMed] [Google Scholar]
- Helmreich DL, Parfitt DB, Lu XY, Akil H, Watson SJ (2005) Relation between the hypothalamic-pituitary-thyroid (HPT) axis and the hypothalamic-pituitary-adrenal (HPA) axis during repeated stress. Neuroendocrinology 81: 183–192. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Bellwood DR, Folke C, Steneck RS, Wilson J (2005) New paradigms for supporting the resilience of marine ecosystems. Trends Ecol Evol 20: 380–386. [DOI] [PubMed] [Google Scholar]
- Jacox MG, Hazen EL, Zaba KD, Rudnick DL, Edwards CA, Moore AM, Bograd SJ (2016) Impacts of the 2015–2016 El Niño on the California current system: early assessment and comparison to past events. Geophys Res Lett 43: 7072–7080. [Google Scholar]
- Jelincic J, Tift M, Houser D, Crocker D (2017) Variation in adrenal and thyroid hormones with life history stage in juvenile northern elephant seals (Mirounga angustirostris). Gen Comp Endocrinol 252: 111–118. [DOI] [PubMed] [Google Scholar]
- Kintisch E. (2015) ‘The blob’ invades pacific, flummoxing climate experts. Science 348: 17–18. [DOI] [PubMed] [Google Scholar]
- Kovacs KM, Aguilar A, Aurioles D, Burkanov V, Campagna C, Gales N, Gelatt T, Goldsworthy SD, Goodman SJ, Hofmeyr GJ (2012) Global threats to pinnipeds. Mar Mammal Sci 28: 414–436. [Google Scholar]
- Kuhn CE, Costa DP (2014) Interannual variation in the at-sea behavior of California sea lions (Zalophus californianus). Mar Mammal Sci 30: 1297–1319. [Google Scholar]
- Laake JL, Melin SR, Orr AJ, Greig DG, Prager KC, DeLong RL, Harris JD (2016) California sea lion sex- and age-specific morphometry. US Department of Commerce, National Oceanic and Atmospheric Administration Administration Tech. Memo. NMFS-AFSC-312, 21 p United States Department of Commerce, Springfield, VA. doi: 10.7289/V5/TM-AFSC-312. [DOI] [Google Scholar]
- Laake JL, Lowry MS, DeLong RL, Melin SR, Carretta JV (2018) Population growth and status of California sea lions. J Wildl Manage 82: 583–595. [Google Scholar]
- Leising AW, Schroeder ID, Bograd SJ, Bjorkstedt EP, Field J, Sakuma K, Abell J, Robertson RR, Tyburczy J, Peterson WT (2014) State of the California current 2013–14: El Niño looming. In California Cooperative Oceanic Fisheries Investigations Reports, Vol. 55California Cooperative Oceanic Fisheries Investigations, La Jolla, CA, pp. 51–87. [Google Scholar]
- Leising AW, Schroeder ID, Bograd SJ, Abell J, Durazo R, Gaxiola-Castro G, Bjorkstedt EP, Field J, Sakuma K, Robertson RR et al. (2015) State of the California Current 2014–15: impacts of the warm-water "blob" In California Cooperative Oceanic Fisheries Investigations Reports, Vol. 56 California Cooperative Oceanic Fisheries Investigations, La Jolla, CA: pp. 31–68. [Google Scholar]
- MacCall AD, Sydeman WJ, Davison PC, Thayer JA (2016) Recent collapse of northern anchovy biomass off California. Fish Res 175: 87–94. [Google Scholar]
- Marcogliese D. (2008) The impact of climate change on the parasites and infectious diseases of aquatic animals. Rev Sci Tech 27: 467–484. [PubMed] [Google Scholar]
- Martin LB II, Navara KJ, Weil ZM, Nelson RJ (2007) Immunological memory is compromised by food restriction in deer mice, Peromyscus maniculatus. Am J Physiol Regul Integr Comp Physiol 292: R316–R320. [DOI] [PubMed] [Google Scholar]
- Maxwell SM, Hazen EL, Bograd SJ, Halpern BS, Breed GA, Nickel B, Teutschel NM, Crowder LB, Benson S, Dutton PH (2013) Cumulative human impacts on marine predators. Nat Commun 4: 2688. [DOI] [PubMed] [Google Scholar]
- McClatchie S, Field J, Thompson AR, Gerrodette T, Lowry M, Fiedler PC, Watson W, Nieto KM, Vetter RD (2016) Food limitation of sea lion pups and the decline of forage off central and southern California. R Soc Open Sci 3: 150628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick SD, Romero LM (2017) Conservation endocrinology. Bioscience 67: 429–442. [Google Scholar]
- McCormley MC, Champagne CD, Deyarmin JS, Stephan AP, Crocker DE, Houser DS, Khudyakov JI (2018) Repeated adrenocorticotropic hormone administration alters adrenal and thyroid hormones in free-ranging elephant seals. Conserv Physiol 6: doi: 10.1093/conphys/coy040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Georgiev AV, Kuzawa CW (2016) Trade-offs between acquired and innate immune defenses in humans. Evol Med Public Health 2016: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Stellar E (1993) Stress and the individual: mechanisms leading to disease. Arch of Intern Med 153: 2093–2101. [PubMed] [Google Scholar]
- McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL (1997) The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res Rev 23: 79–133. [DOI] [PubMed] [Google Scholar]
- McHuron E, Mangel M, Schwarz L, Costa D (2017) Energy and prey requirements of California sea lions under variable environmental conditions. Mar Ecol Prog Ser 567: 235–247. [Google Scholar]
- McHuron EA, Block BA, Costa DP (2018a) Movements and dive behavior of juvenile California sea lions from Año Nuevo Island. Mar Mammal Sci 34: 238–249. [Google Scholar]
- McHuron EA, Schwarz LK, Costa DP, Mangel M (2018b) A state-dependent model for assessing the population consequences of disturbance on income-breeding mammals. Ecol Modell 385: 133–144. [Google Scholar]
- McMahon M, Gerich J, Rizza R (1988) Effects of glucocorticoids on carbohydrate metabolism. Diabetes Metab Rev 4: 17–30. [DOI] [PubMed] [Google Scholar]
- Melin S, DeLong R, Siniff D (2008) The effects of El Niño on the foraging behavior of lactating California sea lions (Zalophus californianus californianus) during the nonbreeding season. Can J Zool 86: 192–206. [Google Scholar]
- Melin SR, Orr AJ, Harris JD, Laake JL, Delong RL, Gulland F, Stoudt S (2009) Unprecedented mortality of California sea lion pups associated with anomalous oceanographic conditions along the central California coast in 2009. In California Cooperative Oceanic Fisheries Investigations Reports, Vol. 51 California Cooperative Oceanic Fisheries Investigations, La Jolla, CA, pp. 182–194. [Google Scholar]
- Melin SR, Haulena M, Bonn W, Tennis MJ, Brown RF, Harris JD (2013) Reversible immobilization of free-ranging adult male California sea lions (Zalophus californianus). Mar Mammal Sci 29: E529–E536. [Google Scholar]
- Mourtzakis M, Saltin B, Graham T, Pilegaard H (2006) Carbohydrate metabolism during prolonged exercise and recovery: interactions between pyruvate dehydrogenase, fatty acids, and amino acids. J Appl Physiol 100: 1822–1830. [DOI] [PubMed] [Google Scholar]
- Myers MJ, Litz B, Atkinson S (2010) The effects of age, sex, season and geographic region on circulating serum cortisol concentrations in threatened and endangered Steller Sea lions (Eumetopias jubatus). Gen Comp Endocrinol 165: 72–77. [DOI] [PubMed] [Google Scholar]
- NOAA National Centers for Environmental Information 2015. , State of the Climate: National Climate Report for Annual, https://www.ncdc.noaa.gov/sotc/national/201513. Published online on January 2016; retrieved on 10 September 2018.
- Ocean Studies Board (2017) Approaches to understanding the cumulative effects of stressors on marine mammals. Division on Earth and Life Studies, National Academies of Sciences, Engineering, and Medicine National Academies Press, Washington, D.C. [Google Scholar]
- Ono KA, Boness DJ, Oftedal OT (1987) The effect of a natural environmental disturbance on maternal investment and pup behavior in the California sea lion. Behav Ecol Sociobiol 21: 109–118. [Google Scholar]
- Palmer CS, Ostrowski M, Balderson B, Christian N, Crowe SM (2015) Glucose metabolism regulates t cell activation, differentiation, and functions. Front Immunol 6: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck HE, Costa DP, Crocker DE (2016) Body reserves influence allocation to immune responses in capital breeding female northern elephant seals. Funct Ecol, PICES Press, Sidney, British Columbia, Canada: 30: 389–397. [Google Scholar]
- Pedernera-Romano C, Aurioles-Gamboa D, Valdez R, Brousset D, Romano M, Galindo F (2010) Serum cortisol in California sea lion pups (Zalophus californianus californianus). Anim Welf 19: 275–280. [Google Scholar]
- Peterson W, Robert M, Bond N (2015) The Warm Blob Continues to Dominate the Ecosystem of the Northern California Current, Vol 23, PICES Press, Canada, p. 44. [Google Scholar]
- Råberg L, Grahn M, Hasselquist D, Svensson E (1998) On the adaptive significance of stress-induced immunosuppression. Proc R Soc Lond B Biol Sci 265: 1637–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich EL, Romero LM (2005) Exposure to chronic stress downregulates corticosterone responses to acute stressors. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 288: 1628–1636. [DOI] [PubMed] [Google Scholar]
- Riedman M. (1990) The Pinnipeds: Seals, Sea Lions, and Walruses. University of California Press, Berkeley and Los Angeles. [Google Scholar]
- Romero LM, Wikelski M (2001) Corticosterone levels predict survival probabilities of Galápagos marine iguanas during El Niño events. Proc Natl Acad Sci 98: 7366–7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero LM, Dickens MJ, Cyr NE (2009) The reactive scope model—a new model integrating homeostasis, allostasis, and stress. Horm Behav 55: 375–389. [DOI] [PubMed] [Google Scholar]
- Romero LM, Wingfield JC (2015) Tempests, Poxes, Predators, and People: Stress in Wild Animals and How They Cope, Oxford University Press, New York, NY. [Google Scholar]
- Ross PS, Pohajdak B, Bowen WD, Addison RF (1993) Immune function in free-ranging harbor seal (Phoca vitulina) mothers and their pups during lactation. J Wildl Dis 29: 21–29. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21: 55–89. [DOI] [PubMed] [Google Scholar]
- Seguel M, Montalva F, Perez-Venegas D, Gutiérrez J, Paves HJ, Müller A, Gottdenker N (2018) Immune-mediated hookworm clearance and survival of a marine mammal decrease with warmer ocean temperatures. eLife 7: e38432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon BC, Verhulst S (1996) Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11: 317–321. [DOI] [PubMed] [Google Scholar]
- Simeone CA, Gulland FM, Norris T, Rowles TK (2015) A systematic review of changes in marine mammal health in North America, 1972-2012: the need for a novel integrated approach. PloS One 10:e0142105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somo DA, Ensminger DC, Sharick JT, Kanatous SB, Crocker DE (2015) Development of dive capacity in northern elephant seals (Mirounga angustirostris): reduced body reserves at weaning are associated with elevated body oxygen stores during the postweaning fast. Physiol Biochem Zool 88: 471–482. [DOI] [PubMed] [Google Scholar]
- Soto KH, Trites AW, Arias-Schreiber M (2006) Changes in diet and maternal attendance of south American sea lions indicate changes in the marine environment and prey abundance. Mar Ecol Prog Ser 312: 277–290. [Google Scholar]
- Trenberth KE. (1997) The definition of El Niño. Bull Am Meteorol Soc 78: 2771–2778. [Google Scholar]
- Trillmich F. (1990) The behavioral ecology of maternal effort in fur seals and sea lions. Behaviour 114: 3–20. [Google Scholar]
- Trillmich F, Limberger D (1985) Drastic effects of El Niño on Galápagos pinnipeds. Oecologia 67: 19–22. [DOI] [PubMed] [Google Scholar]
- Trillmich F, Ono K, Costa D, DeLong R, Feldkamp S, Francis J, Gentry RL, Heath C, LeBoeuf B, Majluf P (1991) The effects of El Niño on pinniped populations in the eastern Pacific In Pinnipeds and El Niño. Springer, Berlin, Heidelberg: pp. 247–270. [Google Scholar]
- Van Dolah FM. (2000) Marine algal toxins: origins, health effects, and their increased occurrence. Environ Health Perspect 108: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise MJ, Costa DP, Kudela RM (2006) Movement and diving behavior of male California Sea lion (Zalophus californianus) during anomalous oceanographic conditions of 2005 compared to those of 2004. Geophys Res Lett 33. [Google Scholar]
- Weise MJ, Harvey JT (2008) Temporal variability in ocean climate and California sea lion diet and biomass consumption: implications for fisheries management. Mar Ecol Prog Ser 373: 157–172. [Google Scholar]
- Weise MJ, Harvey JT, Costa DP (2010) The role of body size in individual-based foraging strategies of a top marine predator. Ecology 91: 1004–1015. [DOI] [PubMed] [Google Scholar]
- Wikelski M, Cooke SJ (2006) Conservation physiology. Trends Ecol Evol 21: 38–46. [DOI] [PubMed] [Google Scholar]
- Wingfield JC. (2013) The comparative biology of environmental stress: behavioural endocrinology and variation in ability to cope with novel, changing environments. Anim Behav 85: 1127–1133. [Google Scholar]
- Wingfield JC, Hunt K, Breuner C, Dunlap K, Fowler GS, Freed L, Lepson J (1997) Environmental stress, field endocrinology, and conservation biology In Behavioral Approaches to Conservation in the Wild, Cambridge University Press, Cambridge, pp. 95–131 [Google Scholar]
- Wingfield JC, Ramenofsky M (2011) Hormone-behavior interrelationships of birds in response to weather. In Advances in the study of behavior, Vol 43: Elsevier, Academic Press, Cambridge, MA, pp. 93–188. [Google Scholar]
- Wingfield JC, Hau M, Boersma PD, Romero LM, Hillgarth N, Ramenofsky M, Wrege P, Scheibling R, Kelley JP, Walker B (2018) Effects of El Niño and La Niña southern oscillation events on the adrenocortical responses to stress in birds of the Galápagos islands. Gen Comp Endocrinol 259: 20–33. [DOI] [PubMed] [Google Scholar]
- Wright BE, Tennis MJ, Brown RF (2010) Movements of male California sea lions captured in the Columbia River. Northwest Sci 84: 60–72. [Google Scholar]
- Zera AJ, Harshman LG (2001) The physiology of life history trade-offs in animals. Annu Rev Ecol Syst 32: 95–126. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


