Abstract
Magnesium sulfate is the standard therapy for prevention and treatment of eclampsia. Two standard dosing regimens require either continuous intravenous infusion or frequent, large‐volume intramuscular injections, which may preclude patients from receiving optimal care. This project sought to identify alternative, potentially more convenient, but similarly effective dosing regimens that could be used in restrictive clinical settings. A 2‐compartment population pharmacokinetic (PK) model was developed to characterize serial PK data from 92 pregnant women with preeclampsia who received magnesium sulfate. Body weight and serum creatinine concentration had a significant impact on magnesium PK. The final PK model was used to simulate magnesium concentration profiles for the 2 standard regimens and several simplified alternative dosing regimens. The simulations suggest that intravenous regimens with loading doses of 8 g over 60 minutes followed by 2 g/h for 10 hours and 12 g over 120 minutes followed by 2 g/h for 8 hours (same total dose as the standard intravenous regimen but shorter treatment duration) would result in magnesium concentrations below the toxic range. For the intramuscular regimens, higher maintenance doses given less frequently (4 g intravenously + 10‐g intramuscular loading doses with maintenance doses of 8 g every 6 hours or 10 g every 8 hours for 24 hours) or removal of the intravenous loading dose (eg, 10 g intramusculary every 8 hours for 24 hours) may be reasonable alternatives. In addition, individualized dose adjustments based on body weight and serum creatinine were proposed for the standard regimens.
Keywords: alternative dosing regimens, magnesium sulfate, modeling and simulation, population pharmacokinetics, preeclampsia
Preeclampsia is a disorder occurring during pregnancy characterized by high blood pressure and proteinuria, affecting between 2% and 8% of pregnancies worldwide.1, 2, 3, 4 Preeclampsia is associated with multiple organ system dysfunction that may include thrombocytopenia, impaired liver function, acute renal dysfunction, pulmonary edema, and visual disturbances. If left untreated, this progressive syndrome may lead to eclampsia with the occurrence of seizures. Preeclampsia and eclampsia are the leading causes of maternal and perinatal mortality and morbidity globally.
Two commonly used dosing regimens of magnesium sulfate, proposed by Zuspan and Pritchard, are widely accepted as standard treatments for preeclampsia and eclampsia.5 A systematic review of 15 randomized trials showed that magnesium sulfate usage is associated with reduced mortality.6 Several international organizations, including the World Health Organization (WHO), recommend magnesium sulfate as the anticonvulsant of choice in cases of severe preeclampsia or eclampsia.7 Despite this global endorsement, a putative therapeutic concentration range has been identified empirically (serum concentration between 2 and 3.5 mmol/L), because the mechanism of action for magnesium sulfate in eclampsia prophylaxis is poorly understood.8 A serum magnesium concentration of at least 2 mmol/L has been generally cited as the potential minimum therapeutic concentration range,8 with exposure‐related toxicity starting to occur at concentrations of 3.5 to 5 mmol/L.9 The high incidence of complications from preeclampsia and eclampsia in low‐resource countries10 may reflect a lack of access to magnesium sulfate treatment in these regions. Moreover, regimens with lower total daily dose and/or shorter treatment duration that might be easier to implement compared with the standard Zuspan and Pritchard regimens have been reported in these regions.10 The need for trained health‐care providers to handle the logistics of the standard regimens (continuous intravenous or intravenous combined with intramuscular administration) with different loading and maintenance doses likely contributes to the use of these variations from the standard regimens.7 Recently, concerns about adverse events with the use of standard regimens and coverage limitations posed by health resource requirements in low‐income settings have renewed interest in identifying the minimum effective dose of magnesium sulfate for preventing and treating eclampsia. In response, the WHO has embarked on a research project to identify a simpler magnesium sulfate regimen based on the minimum dose required to achieve clinical efficacy.
As outlined in Table 1, the Zuspan regimen includes a loading dose of 4 g over 20 minutes followed by a maintenance regimen of 1 g per hour for 24 hours; and the Pritchard regimen includes a loading dose of 4 g intravenously combined with 10 g intramuscularly (divided and given as 2 separate injections, one in each buttock), followed by a maintenance regimen of 5 g intramuscularly every 4 hours for 5 doses. The goal of this analysis was to identify simplified alternative dosing regimen(s) of magnesium sulfate that would be more practically implementable (eg, less frequent injections, lower total dose, or shorter duration of administration) without attenuation of efficacy or increased risk of adverse events that may be related to high serum concentrations of magnesium. Achieving this goal could potentially increase access to optimal treatment for preeclampsia or eclampsia globally.
Table 1.
Standard and Alternative Dosing Regimens of Magnesium Sulfate
| Loading Regimen | Maintenance Regimen | Total | ||||
|---|---|---|---|---|---|---|
| Intravenous Regimens | Dose (g) | Duration (min) | Dose (g/h) | Duration (h) | Dose | Rationale for Evaluation |
| 4 g in 20 min, 1 g/h × 24 h (Zuspan regimen) | 4 | 20 | 1 | 24 | 28 | Standard regimen, widely utilized |
| 4 g in 20 min, 2 g/h × 24 h | 4 | 20 | 2 | 24 | 52 | Common regimen—higher maintenance dose |
| 6 g in 20 min, 2 g/h × 24 h | 6 | 20 | 2 | 24 | 54 | Common regimen—higher loading and maintenance doses |
| 12 g in 120 min 3 g/h × 12 h | 12 | 120 | 3 | 12 | 48 | Hypothetical regimen—higher loading and maintenance doses, shorter duration |
| 12 g in 120 min 2 g/h × 8 h | 12 | 120 | 2 | 8 | 28 | Hypothetical regimen—same daily dose, shorter duration |
| 8 g in 60 min, 2 g/h × 10 h | 8 | 60 | 2 | 10 | 28 | Hypothetical regimen—same daily dose, shorter duration |
| 4 g in 20 min, 1 g/h × 12 h | 4 | 20 | 1 | 12 | 16 | Less common regimen—shorter duration |
| 4 g in 20 min, 1 g/h × 8 h | 4 | 20 | 1 | 8 | 12 | Less common regimen—shorter duration |
| 6 g in 20 min | 6 | 20 | 0 | 0 | 6 | Less common regimen—shorter duration |
| Loading Regimen | Maintenance Regimen | Total | ||||
|---|---|---|---|---|---|---|
| Regimens That Include Intramuscular Dosing | Dose (g) | IV Duration (min) or No. IM Dosesa | IM Dose (g) | Frequency/No. IM Injections | Dose (g/24 h) | Rationale for Evaluation |
| 4 g IV/10 g IM, 5 g q4h × 5 (Pritchard regimen) |
4 IV 10 IM |
20 1 |
5 | q4h × 5 | 39 | Standard regimen, widely utilized |
| 4 g IV/10 g IM, 8 g q6h × 3 |
4 IV 10 IM |
20 1 |
8 | q6h × 3 | 38 | Hypothetical regimens—fewer injections |
| 4 g IV/10 g IM, 10 g q8h × 2 |
4 IV 10 IM |
20 1 |
10 | q8h × 2 | 34 | Hypothetical regimens—fewer injections |
| 4 g IV/10 g IM, 5 g q4h × 2 |
4 IV 10 IM |
20 1 |
5 | q4h × 2 | 24 | Hypothetical regimens —fewer injections |
| 4 g IV/10 g IM |
4 IV 10 IM |
20 1 |
0 | 0 | 14 | Less common regimen—no maintenance dose |
| 10 g IM | 10 IM | 1 | 0 | 0 | 10 | Less common regimen—no IV loading dose, no maintenance dose |
| 10 g IM q12h × 2 | 10 IM | 1 | 10 | q12h × 1 | 20 | Hypothetical regimen—no IV loading dose, single maintenance dose |
| 10 g IM q8h × 3 | 10 IM | 1 | 10 | q8h × 2 | 30 | Hypothetical regimen—no IV loading dose, 2 maintenance doses |
| 4 g IV/6 g IM |
4 IV 6 IM |
15–20 1 |
0 | 0 | 10 | Less common regimen—no maintenance dose |
g, Gram; h, hour; IM, intramuscular; IV, intravenous; q, every.
Doses are typically divided and given as 2 separate injections, one in each buttock.
Modeling and simulation is an approach to quantitatively assess the feasibility of alternative dosing regimens that may be untested. In this article, we present a population pharmacokinetic (PK) model of magnesium sulfate developed using a subset of existing PK data from women treated for preeclampsia.11 Using this model, simulations were performed to compare the standard and alternative regimens that were considered simpler and/or more convenient to identify alternatives that provide PK exposure within a generally accepted therapeutic range for serum magnesium concentrations. Because the PK driver for magnesium sulfate efficacy is unclear, area under concentration‐time curve (AUC) was used arbitrarily to compare alternative regimens with the standard regimens.
Methods
All patients signed informed consent documents that were approved by an independent ethics committee or institutional review board (the Stanford University Institutional Review Board), and the studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.11
Source Data
The source data were obtained from a previously published study that characterized the PK and placental transfer of magnesium sulfate in pregnant women.11 Pregnant women were prescribed magnesium sulfate for either preeclampsia, preterm labor tocolysis, or neuroprotection of the extremely preterm fetus. A total of 111 pregnant women were studied in the original publication, and a cohort of 92 pregnant women with preeclampsia were used in this analysis (the nonpreeclampsia cohort in the original study was not used).
Pharmacokinetic Data and Covariates
All women with preeclampsia received an intravenous infusion loading dose of 4 g magnesium sulfate (MgSO4·7H2O) over 20 minutes, followed by a continuous intravenous infusion maintenance dose of 2 g/h of magnesium sulfate. Magnesium sulfate was continued for 24 hours after delivery, but the duration of magnesium administration prior to delivery was variable depending on when the diagnosis of preeclampsia was made relative to delivery. Maternal blood samples were obtained at baseline before the administration of magnesium sulfate at 0.5, 1, 2, 4, and every 6 hours thereafter during administration and 1, 3, 6, 9, and 12 hours after magnesium sulfate was discontinued. The number of samples available for each woman was dependent on clinical characteristics, which dictated the duration of magnesium treatment. Serum magnesium concentrations were measured at a local laboratory with a Dimension RxL Max Integrated Chemistry System (Siemens, Berlin, Germany). All magnesium concentrations used for this analysis were above the lower limit of quantification of 0.2 mmol/L (magnesium concentrations were reported in units of mg/L in the data set, and these units were used in the modeling).
Demographic characteristics were used to assess their influence on the PK of magnesium. The available characteristics that were included in the analysis were: age, weight, height, body mass index, and gestational age of the fetus at baseline, serum creatinine concentration at baseline and during treatment, and antepartum or postpartum status during treatment. The last observation carried forward was applied to impute missing serum creatinine concentrations. In the case of a missing creatinine concentration at baseline, the first postdose serum creatinine concentration was carried backward.
Population Pharmacokinetic Analysis
Software
The population PK model development and simulations were performed using NONMEM software (version 7.3; ICON plc, Hanover, Maryland), using the first‐order conditional estimation with eta‐epsilon interaction method for model estimation. Simulated data sets were created in SAS (version 9.4; SAS Institute Inc., Cary, North Carolina), and plots were created in R (version 3.3.2; The R Project for Statistical Computing, www.r-project.org).
Model Structure
The population PK analysis was performed using the nonlinear mixed‐effects modeling approach for change from baseline in magnesium concentration. Both 1‐ and 2‐compartment models with linear elimination from the central compartment were evaluated, and MgSO4 was set to be dosed into the central compartment for intravenous administration. Mixed‐effects models describe the influence of both fixed effects and random effects on the dependent variable. Fixed effects are factors that do not vary across individuals, whereas random effects are stochastic components accounting for interindividual variability (IIV), interoccasion variability (IOV), and residual unexplained variability.12 Consistent with most biological systems, population PK parameters (clearance [CL] and volume) were set to follow a log‐normal distribution and to be nonnegative and were defined as shown below:
where Pi is the parameter for individual i, TVP is the typical population value of the parameter, ηPi represents the deviation for the ith subject's parameter value from TVP and κij is the deviation for the ith subject on the jth occasion. Antepartum and postpartum status were considered as different occasions with potential IOV as women continued their treatment after delivery.
Random effects ηPi and κij were assumed to be normally distributed, with mean 0 and estimated variance ω2, and were reported as percent coefficient of variation (%CV):
The shrinkage of the random effects (η‐shrinkage) and residual variability (ε‐shrinkage) were assessed using the following13:
where ω is the standard deviation of the random effect, SD(ηEBE) the standard deviation of the individual parameter estimates (EBE, empirical Bayesian estimates) of random effect η, IWRES the individual weighted residual, σ the standard deviation of the residual variability, and yij and ŷij the jth observed and predicted dependent variable for the ith individual. When shrinkage is high (eg, above 30%), the individual parameter estimates deviate from the true individual parameters and approach the typical population value.13
Various residual error structures were evaluated, including additive, proportional, and combined (additive + proportional) residual errors, as described below:
where yij and represent the jth observed and predicted dependent variable, respsectively, for the ith individual and εprop,ij and εadd,ij are the proportional and additive residual error, respectively. Model selection was based on the likelihood ratio test (LRT), goodness‐of‐fit plots, and scientific plausibility. The best structural model (base model) was the starting point for the covariate assessment.
Covariate Analysis
The continuous covariates of age, body weight, gestational age, and serum creatinine concentration and categorical covariate antepartum or postpartum status were evaluated for their effects on the population PK model parameters. The continuous covariates were included as a power function centered on the median covariate value. Allometric scaling factors for body weight were fixed to values of 0.75 and 1 for CL and volume of distribution (Vd), respectively. The categorical covariate was included as an indicator variable, with a value of 1 for antepartum status and 0 for postpartum status. Given the small number of covariates to be evaluated, the covariates were individually tested, and those with a P < .01 based on the LRT were included in the final model.
Model Simulations
Simulations were performed for the standard Zuspan and Pritchard regimens, as well as a series of simplified alternative intravenous and intramuscular dosing regimens that were more practical and/or convenient compared with the standard regimens. These alternative regimens were selected (in part) based on the results of an international survey and their previous application in research contexts for eclampsia prophylaxis, tocolysis, or neuroprotection and were specified by the coauthors prior to the simulation analysis.10, 14 Features of the alternative simplified regimens included elimination of the loading dose, reducing the frequency of injections, and/or reducing the treatment duration compared with the standard regimens. The specific characteristics of the standard and alternative regimens used in the simulations are provided in Table 1. Simulations were performed for specific subgroups of women based on different combinations of body weight and serum creatinine concentration: low (60 kg), middle (85 kg), and high (110 kg) maternal weight and low (0.5 mg/dL), middle (0.8 mg/dL), and high (1.2 mg/dL) maternal serum creatinine. Magnesium concentration profiles were evaluated in the context of concentrations of 1.5 to 2.5 mmol/L as the putative therapeutic range and 3.5 mmol/L as a concentration associated with toxicity. Baseline magnesium concentration was assumed to be 0.74 mmol/L (18 mg/L) in the simulations, equal to the observed median baseline magnesium value (Table 2).
Table 2.
Characteristics of the 92 Women With Preeclampsia Included in Population Pharmacokinetic Model
| Parameter | Statistic | Value |
|---|---|---|
| Age (years) | Mean (SD) | 30.0 (7.3) |
| Min–Max | 19–44 | |
| Weight (kg) | Mean (SD) | 90.3 (20.2) |
| Min–Max | 57–157 | |
| Height (cm) | Mean (SD) | 160.8 (7.2) |
| Min–Max | 147–183 | |
| BMI (kg/m2) | Mean (SD) | 34.8 (6.5) |
| Min–Max | 20.9–52.3 | |
| Serum magnesium at baseline (mg/L) | Mean (SD) | 18.3 (2.2) |
| Min–Max | 14–25 | |
| Serum creatinine (mg/dL) | Mean (SD) | 0.82 (0.29) |
| Min–Max | 0.4–2.1 | |
| Gestational age at baseline (weeks) | Mean (SD) | 34.73 (4.31) |
| Min–Max | 21.0–40.3 |
BMI, body mass index; SD, standard deviation.
In addition, for the standard Zuspan and Pritchard treatment regimens, maintenance dose adjustments were derived for a range of body weights (65‐105 kg) and serum creatinine concentrations (0.5‐1.2 mg/dL) to achieve magnesium exposure similar to a typical patient with a body weight of 85 kg and serum creatinine concentration of 0.8 mg/dL. Maintenance doses were adjusted to achieve average serum magnesium concentration of 1.6 mmol/L in 24 hours for the Zuspan regimen and 1.8 mmol/L in 24 hours for the Pritchard regimen. These target concentrations represented the predicted average magnesium concentration in 24 hours for a typical woman with a body weight of 85 kg and serum creatinine of 0.8 mg/dL receiving either the Zuspan or Pritchard regimen.
Because all patients in the source PK data set received intravenous treatment, the PK parameters specific for intramuscular dosing, absorption rate constant (Ka) and absolute bioavailability (F), could not be estimated. Therefore, Ka and F values obtained from the literature14 were used to simulate magnesium concentration‐time profiles for the intramuscular dosing regimen: Ka = 0.317 h‐1 and F = 0.862.
Results
Subjects and Data for Analysis
The analysis data set included 623 blood samples from 92 women with preeclampsia, with 370 of the samples (59.4%) drawn during intravenous treatment and 253 (40.6%) after treatment was discontinued. The number of samples (%) drawn before and after birth were 270 (43.3%) and 353 (56.4%), respectively. The postpartum samples included 8 samples taken at delivery. Patient characteristics are summarized in Table 2.
Pharmacokinetic Modeling
Serum magnesium concentration, expressed as change from baseline, was adequately described by a 2‐compartment PK structural model. In the final population PK model, all structural PK parameters, CL, central volume of distribution (Vc), peripheral volume of distribution (Vp), and intercompartment clearance (Q), were allometrically scaled by maternal weight, and CL was further adjusted for serum creatinine level and antepartum or postpartum status. The residual error was best described by a combined residual error structure. Effects of age and gestational age on CL were not statistically significant. Additional supporting information, including the details of the model development, is provided in Supplemental Table S1.
The final model structure is provided below, and parameter estimates are provided in Table 3.
where CL is the typical population value of clearance, CLi is clearance for subject i, Q is typical population value of intercompartmental clearance, Qi is intercompartmental clearance for subject i, Vc is typical population value of volume of distribution of the central compartment, Vci is volume of distribution of the central compartment for subject i, Vp is the typical population value of distribution of the peripheral compartment, Vpi is the volume of distribution of the peripheral compartment for subject i, WTi is the weight in kilograms for subject i, Cri is serum creatinine in mg/dL for subject i, η CL is IIV on CL with mean 0 and variance , η Vc is IIV on Vc with mean 0 and variance , η IOV is IOV on CL with mean 0 and variance , and APij is indicator variable for the jth observation of patient i, with a value of 1 for antepartum status and 0 for postpartum status.
Table 3.
Parameter Estimates of the Final Population Pharmacokinetic Model
| PK Parameter | Estimate | % RSE | – |
|---|---|---|---|
| CL (L/h) | 3.72 | 3.5% | – |
| Vc (L) | 15.4 | 11.6% | – |
| Q (L/h) | 3.66 | 24.5% | – |
| Vp (L) | 17.0 | 9.8% | – |
| Serum creatinine exponent for CL, θ | −0.731 | 14.2% | – |
| WT exponent for CL and Q | 0.75 (fixed) | – | – |
| WT exponent for Vc and Vp | 1 (fixed) | – | – |
| Random Effect | Estimate (CV%) | % RSE | Shrinkage |
|---|---|---|---|
| IIV on CL, | 0.0749 (27.9%) | 21.2% | 14.3% |
| IIV on Vc, | 0.241 (52.2%) | 30.7% | 33.3% |
| IOV on CL, | 0.056 (23.9%) | 42.2% | 48.9% |
| Residual Error | Estimate | % RSE | Shrinkage |
|---|---|---|---|
| Additive (mg/L) | 4.97 | 7.2% | 11.3% |
| Proportional | 0.12 | 13.5% |
CL, clearance; CV, coefficient of variation; IIV, interindividual variability; IOV, interoccasion variability (antepartum vs postpartum); Q, intercompartmental clearance; RSE, relative standard error; Vc, central volume of distribution; Vp, peripheral volume of distribution; WT, body weight; ω2, variance.
Evaluation of the Final Population Pharmacokinetic Model
Figure 1 shows the goodness‐of‐fit plots of the population prediction (A) and individual predictions (B) versus the observed change from baseline magnesium concentrations. The predictions generally appeared to scatter randomly around the line of unity, indicating an adequate fit of the data. Diagnostic plots (C, D) confirmed that the residuals were acceptable and indicated no structural bias in the final model.
Figure 1.
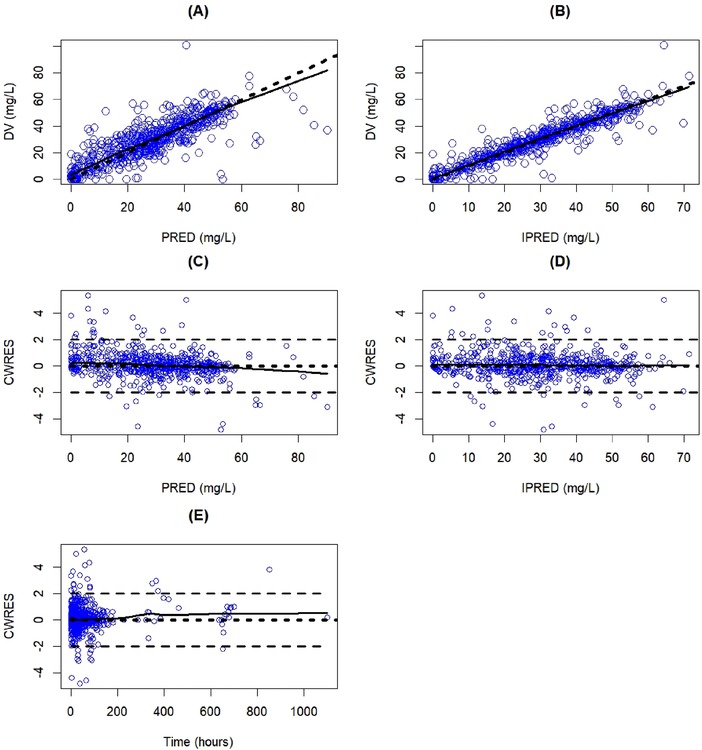
Goodness‐of‐fit plots of the final population pharmacokinetic model. The dashed lines represent the identity line in (A) and (B). The horizontal lines at −2, 0, and 2 represent the CWRES in (C), (D), and (E). The solid lines represent a Loess smoothed line. Blue circles indicate model‐predicted values. CWRES, conditional weighted residual; DV, dependent variable (observed change from baseline magnesium concentration); PRED, population‐predicted change from baseline magnesium concentration; IPRED, individual‐predicted change from baseline magnesium concentration.
A comparison of interindividual variation of CL and Vc between the base model and the final model shows that adjustment for CL and Vc with body weight and creatinine concentration was justified because the interindividual variability of CL and Vc was significantly reduced by introducing weight and creatinine as covariates in the final model (see Supplemental Figure S1). An overlay of observed versus model‐predicted magnesium concentration profiles along with infusion rate and duration of infusion for individual women in the data set is shown in Figure S2.
Simulations Based on the Final Population Pharmacokinetic Model
Using the fixed‐effect parameter estimates (thetas) from final PK model, magnesium concentration profiles over a 36‐hour period were predicted for a typical woman with preeclampsia every 6 minutes who received the 2 standard regimens with varying body weight and serum creatinine values: the Zuspan intravenous regimen is shown in Figure 2, and the Pritchard intramuscular regimen is shown in Figure 3.
Figure 2.
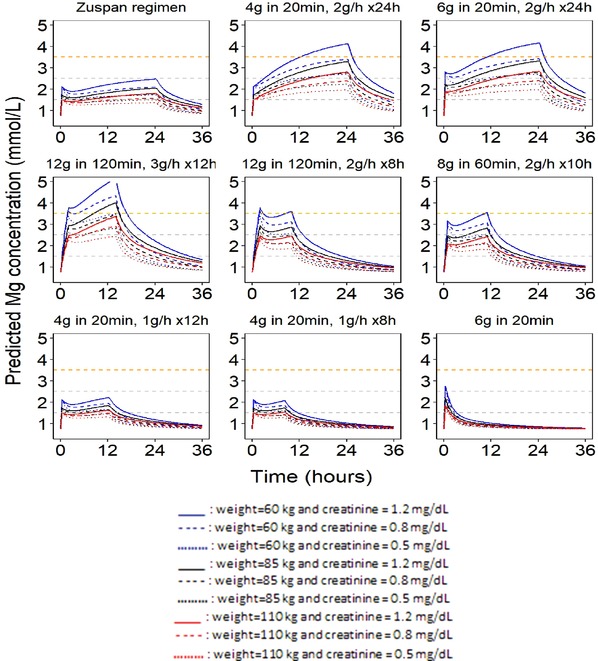
Simulated magnesium profiles for standard (Zuspan) and alternative intravenous regimens. The dashed horizontal gray lines at 1.5 and 2.5 mmol represent the magnesium concentration range considered the putative therapeutic concentration range. The dashed orange horizontal line at 3.5 mmol/L represents the lower‐bound safety margin. The baseline magnesium concentration was assumed to be 0.74 mmol/L (18 mg/L) in the prediction of concentration‐time profiles.
Figure 3.
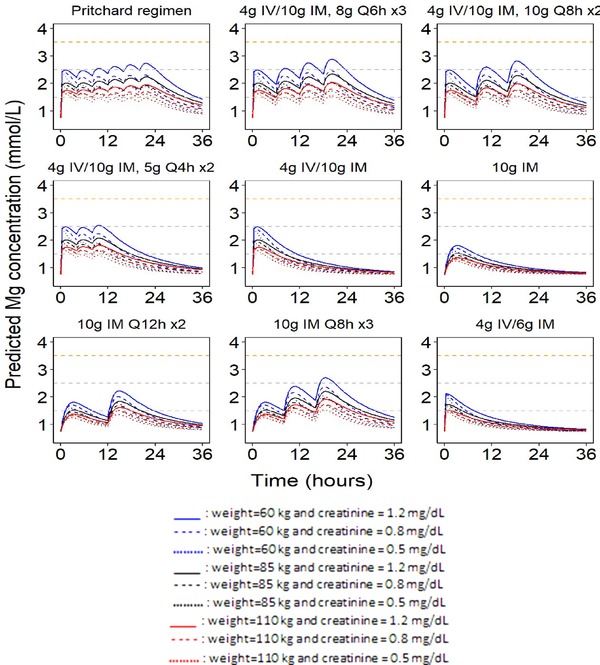
Simulated magnesium profiles for standard (Pritchard) and alternative intramuscular regimens. The dashed horizontal gray lines at 1.5 and 2.5 mmol represent the magnesium concentration range considered the putative therapeutic concentration range. The dashed orange horizontal line at 3.5 mmol/L represents the lower‐bound safety margin. The baseline magnesium concentration was assumed to be 0.74 mmol/L (18 mg/L) in the prediction of the concentration‐time profiles.
Selected body weight and serum creatinine conditions were based on the median and 5th and 95th percentiles of the data set in Table 2.
For both the Zuspan intravenous and Pritchard intramuscular regimens, magnesium concentrations quickly increased to 1.5 to 2.5 mmol/L after the loading dose and remained in this region throughout the maintenance dose period, which was below the range of the generally accepted safety limit of 3.5 mmol/L.
Using the same final model, magnesium concentration profiles were also predicted for the alternative regimens (Table 1) and are shown in Figure 2 (intravenous dosing) and Figure 3 (intramuscular dosing), together with the corresponding Zuspan and Pritchard regimens. The intravenous regimens that used loading doses of 4 or 6 g, but increased maintenance doses to 2 g/h reached potentially toxic magnesium concentrations during the latter half of a 24‐hour dosing interval for patients with low and median body weight and high creatinine values. However, regimens with loading doses of 8 g over 60 minutes followed by maintenance doses of 2 g/h for 10 hours and 12 g over 120 minutes followed by 2 g/h for 8 hours rapidly achieved potentially therapeutic magnesium concentrations without approaching the toxic range. These regimens delivered the same total daily dose as the Zuspan regimen, but with a shorter treatment duration. With higher concentrations at the end of the infusion compared with the Zuspan regimen, these regimens should provide protection comparable to or better than the Zuspan regimen after completion of administration. Reducing the duration of the Zuspan regimen maintenance dose from 24 hours to 12 or 8 hours or eliminating the maintenance dose resulted in a shorter period of magnesium exposure within the therapeutic range.
The intramuscular regimens with higher maintenance doses given less frequently (8 g every 6 hours or 10 g every 8 hours) would be reasonable alternative regimens with fewer injections compared with the standard Pritchard regimen; however, they resulted in larger fluctuations in magnesium concentration during active maintenance treatment, as shown in Figure 3. Peak‐to‐trough ratios ranged from 1.3 to 1.7 for the standard Pritchard regimen, which increased to 1.7–2.2 for 8 g every 6 hours (8 g every 6 hours × 3) and 2.0–2.9 for 10 g every 8 hours (10 g every 8 hours × 3) as maintenance doses across the simulations for various typical women with varying values for body weight and serum creatinine. As expected, fewer maintenance intramuscular injections reduced the duration of magnesium exposure within the putative therapeutic range, and removal of the intravenous component of the loading doses delayed the time to reach potentially therapeutic magnesium concentrations.
Because body weight and serum creatinine were significant covariates affecting magnesium CL and Vd, maintenance dose titration for patients across a range of body weights (65, 75, 85, 95, and 105 kg) and creatinine concentrations (0.5, 0.8, and 1.2 mg/dL) were suggested using the final population PK model. Although only these selected values were simulated, they may be informative to customize maintenance doses for patients, especially those with marked deviation from a body weight of 85 kg and a creatinine concentration of 0.8 mg/dL. For example, patients with a body weight above 85 kg or serum creatinine concentration below 0.8 mg/dL would require higher intravenous maintenance doses to achieve the target efficacious magnesium concentration of 1.6 mmol/L in 24 hours. Predictions of maintenance doses by body weight and serum creatinine concentration for the standard dosing regimens that achieve an average serum magnesium concentration in 24 hours similar to that for a typical patient with a body weight of 85 kg and serum creatinine concentration of 0.8 mg/dL are presented in Table 4. With the goal of providing a relatively simple table for dosing guidance, the numbers of simulated weights and serum creatinine concentrations were limited to illustrate when maintenance dose adjustments could be considered. As such, interpolations may be necessary for weights and creatinine concentrations that fall between the simulated values. Furthermore, clinicians are likely to round off the intramuscular dose adjustments to the nearest gram (Table 4).
Table 4.
Predicted Magnesium Maintenance Doses to Achieve Typical Magnesium Concentrations Comparable to the Standard Regimens
| Predicted Maintenance Dosea by Body Weight and Serum Creatinine Level | |||
|---|---|---|---|
| Zuspan Intravenous Regimen | CREA (0.5 mg/dL) | CREA (0.8 mg/dL) | CREA (1.2 mg/dL) |
| WT (65 kg) | 1.0 g/h | 0.8 g/h | 0.6 g/h |
| WT (75 kg) | 1.2 g/h | 0.9 g/h | 0.7 g/h |
| WT (85 kg) | 1.3 g/h | 1.0 g/h | 0.8 g/h |
| WT (95 kg) | 1.5 g/h | 1.1 g/h | 0.9 g/h |
| WT (105 kg) | 1.6 g/h | 1.2 g/h | 1.0 g/h |
| Predicted Maintenance Dosea by Body Weight and Serum Creatinine Level | |||
|---|---|---|---|
| Pritchard Intramuscular Regimen | CREA (0.5 mg/dL) | CREA (0.8 mg/dL) | CREA (1.2 mg/dL) |
| WT (65 kg) | 5.3 g q4h | 3.2 g q4h | 1.7 g q4h |
| WT (75 kg) | 6.4 g q4h | 4.1 g q4h | 2.6 g q4h |
| WT (85 kg) | 7.6 g q4h | 5.0 g q4h | 3.4 g q4h |
| WT (95 kg) | 8.6 g q4h | 5.9 g q4h | 4.1 g q4h |
| WT (105 kg) | 9.8 g q4h | 6.8 g q4h | 4.9 g q4h |
IM, intramuscular; IV, intravenous; CREA, serum creatinine; WT, body weight.
Note: The predicted average magnesium concentration in 24 hours for a typical patient with body weight of 85 kg and serum creatinine of 0.8 mg/dL receiving the Zuspan regimen (a 4‐g loading dose over 20 minutes followed by a 1‐g/h maintenance dose for 24 hours) was 1.6 mmol/L. The predicted average magnesium concentration in 24 hours for a typical patient with body weight of 85 kg and serum creatinine of 0.8 mg/dL receiving the Pritchard regimen (a 4‐g intravenous loading dose over 20 minutes and a 10‐g intramuscular loading dose followed by 5 intramuscular maintenance doses of 5 g every 4 hours for 24 hours) was 1.8 mmol/L.
Maintenance dose of MgSO4·7H2O in grams/hour for intravenous infusion or in grams per every 4 hours for intramuscular injection.
Discussion
The population PK of magnesium has been evaluated in several independent studies using either 1‐ or 2‐compartment structural models. The choice of a 1‐ or 2‐compartment model seemed to be dependent on the source data and sampling scheme, with a 1‐compartment model generally used for sparse PK sampling (eg, 2 or 3 samples throughout treatment and the posttreatment period) and a 2‐compartment model used for serial PK sampling (samples were collected at baseline, every 30 minutes or 1 hour at the beginning of treatment, and then every few hours until the end of posttreatment). In this population PK analysis of 92 preeclamptic women with serial PK data, a 2‐compartment model provided the best fit to the PK data, with body weight and serum creatinine as statistically significant covariates. The 2‐compartment model decreased the objective function value by about 65 units compared with the 1‐compartment model. The population PK analysis of the original full data set (including 92 preeclamptic and 19 nonpreeclamptic women), previously published by Brookfield et al,11 used a 1‐compartment model, with preeclampsia as the only covariate that significantly impacted CL. The CL estimate in the Brookfield analysis was approximately 32% lower for preeclamptic women, with an estimate of 3.98 L/h (4.5% RSE), which was similar to the estimate from our model of 3.72 L/h (3.5% RSE). Estimates for Vd were generally comparable (22.5 L/70 kg for the 1‐compartment model and 32.4 L/85 kg for the 2‐compartment model). The model‐estimated PK parameters from the current analysis were also generally similar to other literature estimates for 2‐compartment models.15, 16
There were several potential limitations to this analysis. First, the presence of endogenous magnesium prior to administration of magnesium sulfate had to be accounted for in the PK model. Therefore, the change from baseline magnesium concentration was used as the dependent variable, rather than the absolute magnesium concentration. The underlying hypothesis of this approach was that the change from baseline was solely attributable to the magnesium sulfate administration and that the baseline remained constant. For simulation purposes, the predicted magnesium change from baseline was added to the baseline concentration (taking a population average for magnesium baseline levels) to describe the time course of total magnesium concentration over time. Second, fixed values of F and Ka that were obtained from literature were used with our model for simulating magnesium profiles of the intramuscular dosing regimen because of a lack of PK data for intramuscular regimens. Thus, it was possible that these absorption parameters would have deviated when estimated within our current model.
Our population PK model was used to simulate serum magnesium profiles for the standard intravenous (Zuspan) and intramuscular (Pritchard) regimens of magnesium sulfate, as well as profiles for a series of alternative regimens that were selected for evaluation as potential simplifications to the standard regimens. The Zuspan and Pritchard dosing regimens have shown widespread clinical efficacy, such that modeling and simulation are a valuable approach to assess these regimens for comparisons and to identify promising alternative regimens. As the precise relationship between magnesium exposure and efficacy is not well defined, the minimum effective serum magnesium concentration and the PK driver for eclampsia prophylaxis are unknown, likely because of insufficient data for women with preeclampsia progressing to eclampsia to draw reliable conclusions. In the absence of knowledge of the relationship between PK and efficacy, the proposed dosing regimens are based solely on PK simulations, which have their limitations, and confirmatory clinical studies may be necessary. Given that the incidence of eclamptic seizures was approximately 2% in the placebo group in the Magpie trial,1 prohibitively large clinical trials would be required for studies to compare various dosing regimens.4
A serum magnesium concentration of at least 2 mmol/L has generally been cited as the potential minimum therapeutic concentration.8 However, a systematic review of 28 studies investigating magnesium sulfate in women with preeclampsia and eclampsia concluded that the minimum effective serum magnesium concentration for eclampsia prophylaxis may be lower than 2 mmol/L.8 Using our model, simulated magnesium profiles for the standard Zuspan and Pritchard regimens demonstrated slightly lower magnesium concentrations, especially for women with higher body weight and lower creatinine. These simulations were consistent with the findings of a recently completed randomized trial that aimed to compare intravenous and intramuscular regimens of magnesium with 300 women at risk for eclampsia.14 Although a consensus of therapeutic levels was lacking, there were exposure‐related toxicities at the upper end of the therapeutic range. For example, loss of the patella tendon reflex was reported to occur at concentrations of 3.5 to 5 mmol/L, and alteration in cardiac conduction and possible cardiac arrest may occur at concentrations of >7.5 and 12.5 mmol/L, respectively.8 Respiratory depression has also been reported at concentrations of approximately 12 mmol/L. Our simulations indicated that the magnesium concentrations of the standard Zuspan and Pritchard regimens were below the safety margin of 3.5 mmol/L.
The simulated magnesium profiles were evaluated using criteria of achieving a therapeutic concentration (1.5 to 2.5 mmol/L), while not exceeding a potentially toxic concentration (3.5 mmol/L). The intravenous regimens with loading doses of 8 g over 60 minutes followed by 2 g/h for 10 hours and 12 g over 120 minutes, with maintenance doses of followed by 2 g/h for 8 hours rapidly achieved potentially therapeutic magnesium concentrations without exceeding the toxic range. If magnesium efficacy is related to area under the concentration‐time curve (AUC), rather than time above a therapeutic concentration, then either of these regimens with higher loading doses may be promising alternatives for intravenous administration because the total daily dose is the same as the standard Zuspan regimen (which delivers a total of 28 g of magnesium sulfate over 24 hours). Although these regimens may be promising alternatives, a loading dose for 1 to 2 hours is not typically used in clinical practice based on the personal clinical experience of the authors. In addition, the use of intravenous‐based treatments remains a challenge in low‐resource settings, for which intravenous administration is only available in specific facilities.
For the intramuscular regimens, higher maintenance doses given less frequently (8 g every 6 hours or 10 g every 8 hours) may be reasonable alternative dosing regimens, although the impact of the greater fluctuations in serum magnesium concentrations on efficacy is not known. Despite the use of higher maintenance doses, maximum magnesium concentrations were comparable to the Pritchard regimen and were maintained below the toxic range of 3.5 mmol/L. These less frequent intramuscular dosing regimens with associated fewer injections not only reduce potential suffering, but also may improve treatment compliance and are recommended to be evaluated in clinical trials. As expected, fewer maintenance intramuscular injections reduced the duration of magnesium exposure within the putative therapeutic range, and removal of the intravenous component of the loading doses delayed the time to reach potentially therapeutic magnesium concentrations. Although the regimen with only a 10‐g intramuscular loading dose seems unlikely to be successful, it is quite popular in low‐resource settings and was associated with improved maternal and fetal survival in a study in northern Nigeria.17 Systematic reviews of randomized studies (n = 4)18 and nonrandomized studies (n = 5)19 of various lower‐dose or loading dose‐only regimens in women with either preeclampsia or eclampsia were unable to identify differences in maternal and perinatal outcomes compared with standard regimens. However, definitive conclusions from either review could not be drawn because of the relatively sparse data evaluated. Considering the importance of intramuscular regimens in low‐resource settings, regimens without an intravenous loading dose (eg, 10 g every 8 hours) should be evaluated in clinical trials, even if there is a theoretical risk of compromised efficacy.
Based on the final population PK model, maintenance dose titrations of the standard Zuspan and Pritchard regimens were simulated for women across a range of body weights (65‐105 kg) and creatinine levels (0.5‐1.2 mg/dL). These simulations may be useful to clinicians who want to customize dosing for women based on their demographic characteristics to maximize therapeutic benefit and minimize toxicity. These adjusted maintenance doses increase the probability of maintaining magnesium concentrations within a safe and efficacious range, especially for women at the extreme values of body weight and/or serum creatinine.
In conclusion, model‐based simulations characterized magnesium serum concentration profiles for the standard Zuspan and Prichard regimens, as well as alternative intravenous and intramuscular dosing scenarios in women with preeclampsia. The simulation analysis enabled comparisons between these regimens and among individual women with varying demographics. For intramuscular regimens, higher maintenance doses given less frequently (8 g every 6 hours or 10 g every 8 hours) or removal of intravenous loading dose (eg, 10 g every 8 hours) may provide reasonable alternative dosing regimens, especially for restrictive clinical settings, although the impact of the greater fluctuations in serum magnesium concentrations on efficacy is not known. In addition, adjustment for maintenance dose to the standard Zuspan and Pritchard regimens for a range of body weights and serum creatinine values was proposed to deliver more consistent magnesium concentrations. This robust population PK model may enable estimations of magnesium exposure for other studies with limited or no PK sampling and development of an exposure‐response model for studying an optimal dosing regimen in women with preeclampsia. This is the subject of another analysis, which will be published separately.
Declaration of Conflicting Interests
All authors declare no conflicts of interest. Yan Xu and Elizabeth Migoya were employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, at the time the work was being conducted. Medical writing was coordinated and supported by Certara Strategic Consulting and Medical Writing Innovations, LLC.
Supporting information
Supporting Figure S1
Supporting Figure S2
Supporting Table S1
Acknowledgments
The authors acknowledge Priya Agrawal (Merck & Co., Inc., Kenilworth, New Jersey), Jeffrey Jacobs (Merck for Mothers), A. Metin Gülmezoglu (UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction [HRP], Department of Reproductive Health and Research, World Health Organization, Geneva, Switzerland). The authors thank the women who participated in the clinical trial and the investigators and their staff at the clinical study site for their valuable contributions.
Funding
This study was sponsored by Merck for Mothers (a philanthropic initiative of Merck & Co., Inc., Kenilworth, N.J., USA, known outside the United States as Merck Sharpe & Dohme [MSD]) and the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research, World Health Organization, Geneva, Switzerland.
Data Sharing
The authors are unable to share the data supporting the results of this study.
Author Contributions
Lihong Du contributed to protocol design, population PK modeling, data interpretation, manuscript writing, and statistical analysis. L.W. supervised L.D. E.M. contributed to protocol design and overall analysis planning. Y.X. contributed to protocol design, analysis plan, and manuscript review. B.C. contributed PK raw data and data interpretation. K.B. contributed PK raw data and data interpretation. H.W. developed models and manuscript outline. R.D. supervised H.W.. P.L. contributed with manuscript review and data interpretation. U.S. contributed with manuscript review and data interpretation. V.T. contributed with manuscript review and data interpretation. Q.L. contributed to project coordination, simulation scenarios, and data interpretation. O.O. contributed to project leadership and coordination, simulation scenarios, and data interpretation. All authors reviewed and approved the final version of this manuscript.
References
- 1. Magpie Trial Collaborative Group . Do women with pre‐eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo‐controlled trial. Lancet. 2002;359(9321):1877–1890. [DOI] [PubMed] [Google Scholar]
- 2. Al‐Jameil N, Aziz KF, Fareed KM, Tabassum H. A brief overview of preeclampsia. J Clin Med Res. 2014;6(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eiland E, Nzerue C, Faulkner M. Preeclampsia 2012. J Pregnancy. 2012;2012:586578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early‐ versus late‐onset disease. Am J Obstet Gynecol. 2013;209(6):544. [DOI] [PubMed] [Google Scholar]
- 5. Zuspan FP. Problems encountered in the treatment of pregnancy‐induced hypertension. A point of view. Am J Obstet Gynecol. 1978;131(6):591–597. [DOI] [PubMed] [Google Scholar]
- 6. Duley L, Gülmezoglu AM, Henderson‐Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with pre‐eclampsia. Cochrane Database Syst Rev. 2010;11:CD000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Program for Appropriate Technology in Health (PATH) . Delivery of Magnesium Sulfate. Technology Opportunity Assessment Prepared for the Merck for Mothers Program. http://sites.path.org/mnhtech/files/2013/03/Magnesium_Sulfate_18March2013_FINAL.pdf. Published February 2012. Accessed July 1, 2018.
- 8. Okusanya BO, Oladapo OT, Long Q, et al. Clinical pharmacokinetic properties of magnesium sulphate in women with pre‐eclampsia and eclampsia. BJOG. 2016;123(3):356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nick J. Deep tendon reflexes, magnesium, and calcium: assessments and implications. J Obstet Gynecol Neonatal Nurs. 2004;33(2):221–230. [DOI] [PubMed] [Google Scholar]
- 10. Long Q, Oladapo OT, Leathersich S, et al. Clinical practice patterns on the use of magnesium sulphate for treatment of pre‐eclampsia and eclampsia: a multi‐country survey. BJOG. 2017;124(12):1883–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brookfield KF, Su F, Elkomy MH, Drover DR, Lyell DJ, Carvalho. Pharmacokinetics and placental transfer of magnesium sulfate in pregnant women. Am J Obstet Gynecol. 2016;214(6):737.e1–e9. [DOI] [PubMed] [Google Scholar]
- 12. Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model‐based drug development‐part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol. 2013;2(4):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Savic RM, Karlsson MO. Importance of shrinkage in empirical bayes estimates for diagnostics: problems and solutions. AAPS J. 2009;11:558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salinger D, Mundle S, Regi A, et al. Magnesium sulphate for prevention of eclampsia: are intramuscular and intravenous regimens equivalent? A population pharmacokinetic study. BJOG. 2013;120(7):894–900. [DOI] [PubMed] [Google Scholar]
- 15. Lu JF, Pfister M, Ferrari P, Chen G, Sheiner L. Pharmacokinetic‐pharmacodynamic modelling of magnesium plasma concentration and blood pressure in preeclamptic women. Clin Pharmacokinet. 2002;41(13):1105–1113. [DOI] [PubMed] [Google Scholar]
- 16. Chuan FS, Charles BG, Boyle RK, Rasiah RL. Population pharmacokinetics of magnesium in preeclampsia. Am J Obstet Gynecol. 2001;185(3):593–599. [DOI] [PubMed] [Google Scholar]
- 17. Ishaku SM, Ahonsi BAO, Tukur J, Oginni A. Attrition from care after the critical phase of severe pre‐eclampsia and eclampsia: insights from an intervention with magnesium sulphate in a primary care setting in northern Nigeria. Health. 2013;5(9):1461–1466. [Google Scholar]
- 18. Duley L, Matar HE, Almerie MQ, Hall DR. Alternative magnesium sulphate regimens for women with preeclampsia and eclampsia. Cochrane Database Syst Rev. 2010;(8):CD007388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pratt JJ, Niedle PS, Vogel JP, et al. Alternative regimens of magnesium sulfate for treatment of preeclampsia and eclampsia: a systematic review of nonrandomized studies. Acta Obstet Gynecol Scand. 2016;95(2):144–156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure S1
Supporting Figure S2
Supporting Table S1


