Abstract
Sarcopenia, age‐associated involuntary loss of muscle and strength, can progress to clinically relevant functional decline. Resistance exercise attenuates muscle and strength loss but may not be feasible for some older adults. Aerobic exercise training (AET) improves cardiopulmonary health; however, effects on protein turnover, muscle mass, and strength are less clear. We aimed to determine whether AET improves basal myofibrillar protein synthesis (MPS) and capillarization, promoting hypertrophy and strength. We hypothesized that AET improves strength with increased MPS and capillarization. Older adults were randomized to non‐exercise (NON; n = 11, 71.4 ± 4.18 years) or exercise (EX; n = 12, 73.7 ± 4.05 years). EX completed 24 weeks of AET (walking 3×/week, 45 minutes, 70% heart rate reserve); NON remained sedentary. A stable isotope tracer was infused. MPS and capillarization were analyzed from vastus lateralis muscle biopsies. Strength was measured via isokinetic dynamometry. Lean mass was determined with dual‐energy X‐ray absorptiometry. Basal MPS increased in EX (+50.7%, P = 0.01) along with capillary density (+66.4%, P = 0.03), peak oxygen consumption (+15.8%, P = 0.01), quadriceps strength (+15.1%, P = 0.01), and muscle quality (peak torque divided by leg lean mass, +15.5%, P = 0.01). Lean mass did not change (P > 0.05). AET increases muscle protein turnover and capillarization in older adults, improving muscle quality.
Keywords: capillarization, protein turnover, sarcopenia, strength, walking exercise
1. INTRODUCTION
Skeletal muscle is responsible for force production and locomotion. However, aging promotes progressive skeletal muscle atrophy and subsequent strength decline via a myriad of biological mechanisms that have not yet been fully elucidated. Muscle protein anabolic resistance to exercise may at least partially contribute to the gradual atrophic phenomenon via impaired anabolic response to mechanical loading compared to younger adults.1 This involuntary loss of skeletal muscle and strength, termed sarcopenia, can progress to a clinically relevant decline in physical function associated with disability, physical dependence, mortality, and an increased healthcare burden.2, 3, 4, 5, 6, 7
Skeletal muscle mass is regulated by protein turnover, undulating between a net accretion and net loss of muscle protein.8 When the synthesis and breakdown rates of muscle protein are in balance, muscle mass is maintained; however, in many conditions—such as fasting and aging—a net negative protein balance may occur in the absence of an anabolic intervention, contributing to skeletal muscle wasting. Conversely, when synthesis exceeds breakdown, myofiber hypertrophy is facilitated.9
Resistance exercise training (RET) upregulates muscle protein synthesis, promoting a net positive protein balance, and effectively attenuates sarcopenia when performed with sufficient intensity and volume over time.10 Although ideal, RET may not be feasible for many older adults due to inadequate access to facilities with safe equipment, lack of proper instruction, and/or cost limitations. Aerobic exercise training (AET) is well established to improve cardiorespiratory health; however, the effects of AET on protein turnover, skeletal muscle mass, and strength remain less clear with varying modes and intensities producing conflicting results.11
Most studies that have investigated protein turnover and the hypertrophic response to AET have utilized lower body cycling as the selected mode of exercise. Like RET, cycling participation may be restricted to those older adults with the financial means to access proper equipment and/or facilities. Walking is a more feasible AET option for a greater number of older adults at risk of sarcopenia and associated frailty. The aim of this study was to determine whether moderate‐intensity AET—specifically walking—improves basal myofibrillar protein synthesis (MPS) and capillarization, thereby promoting skeletal muscle hypertrophy and increased strength. We hypothesized that AET would improve muscle quality (ie, strength per unit muscle mass) in conjunction with an increase in basal MPS and enhanced capillarization.
2. METHODS
The study protocol was approved by the University of Texas Medical Branch (UTMB) Institutional Review Board (IRB) and registered at ClinicalTrials.gov (NCT00872911), and additional data from the larger cohort have recently been published.12 All subjects provided written informed consent before commencing study participation.
2.1. Subjects
Healthy, nonobese, non‐frail, sedentary to low‐active (<7500 steps/day) older adults (65‐82 years) were recruited and randomized into the experimental exercise group (EX; n = 12, 73.7 ± 4.05 years) or non‐exercise control group (NON; n = 11, 71.4 ± 4.18 years). Exclusion criteria included diabetes, cancer, uncontrolled high blood pressure, tobacco use, cardiopulmonary and kidney disease, dietary protein intake below the estimated average requirement or above 1.5 g/kg/day, and orthopedic conditions precluding exercise participation. Thorough medical screening was performed at the Institute for Translational Sciences Clinical Research Center (ITS‐CRC) to assess inclusion of each subject. Refer to Table 1 for subject characteristics.
Table 1.
Subject characteristics
| Exercise | Non‐exercise | |||
|---|---|---|---|---|
| N | 12 (4 males, 8 females) | 11 (3 males, 8 females) | ||
| Age, y | 73.67 ± 4.05 | 71.36 ± 4.18 | ||
| Height, cm | 166.20 ± 8.99 | 164.40 ± 7.06 | ||
| Pre | Post | Pre | Post | |
| Weight, kg | 71.41 ± 10.56 | 71.10 ± 10.37 | 69.48 ± 11.59 | 70.8 ± 12.07a |
| BMI (kg/m2) | 25.84 ± 3.00 | 25.70 ± 2.82 | 25.58 ± 2.73 | 26.00 ± 2.88a |
| Body fat, % | 36.97 ± 8.78 | 36.40 ± 9.01 | 38.01 ± 7.73 | 38.60 ± 7.41 |
| Fat, kg | 25.36 ± 7.33 | 24.8 ± 7.59 | 25.55 ± 6.83 | 26.3 ± 6.81a |
| Lean mass, kg | 42.75 ± 7.50 | 42.90 ± 7.55 | 41.21 ± 8.57 | 41.70 ± 9.08 |
| VO2 peak, mL/kg/min | 23.20 ± 5.53 | 26.90 ± 8.54a | 22.80 ± 3.86 | 23.5 ± 5.09 |
Data are mean ± SD.
Significantly different from baseline, P < 0.05.
2.2. Study design
Subjects completed a metabolic study with muscle biopsy, body composition assessment, strength testing, and aerobic fitness measurement before and after 24 weeks of AET (EX group) or lack of participation in a structured exercise program (NON group). Refer to Figure 1 for study schematic.
Figure 1.
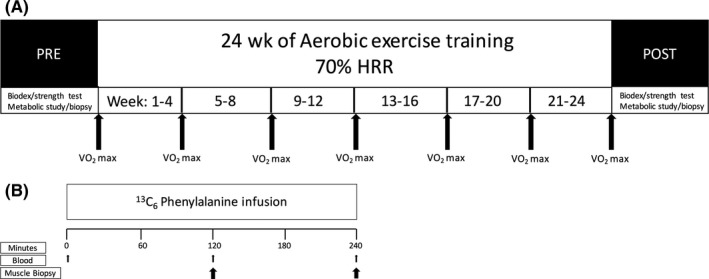
Study flow schematic. A, Training schematic. Prior to and following the 24‐wk period, muscle strength testing, VO 2 max testing, and a metabolic study were conducted. The non‐exercise group remained sedentary during the 24‐wk period, while the exercise group participated in supervised treadmill walking 3×/wk. For the exercise group, VO 2 max was reassessed every 4 wk to maintain training intensity at 70% heart rate reserve. B, Infusion study schematic. Prior to 24 wk of the aerobic exercise intervention or no exercise training, after an overnight fast, arterialized background blood was collected from a hand or wrist vein followed by continuous 13C6 phenylalanine infusion through the antecubital vein of the opposite arm. Blood and vastus lateralis biopsies were taken at 120 and 240 min. For both groups, the same protocol was repeated after 24 wk
2.3. Aerobic exercise training
The EX group completed 24 weeks of treadmill walking 3 days per week for 45 minutes at 70% heart rate reserve (HRR), while the NON group did not participate in structured exercise. VO2 max testing was repeated every 4 weeks to accommodate increasing aerobic fitness and adjust target heart rate to preserve the 70% HRR intensity. All AET sessions were supervised to ensure completion at the prescribed intensity with treadmill speed and incline being adjusted to accommodate maintenance of the target heart rate (±5%). A heart rate monitor was worn for the duration of each session (Polar FS1, Polar Electro, Kempele, Finland).
2.4. Body composition
Dual‐energy X‐ray absorptiometry (Lunar iDXA, GE Healthcare, Chicago, IL) was used to measure body composition immediately before and after the 24‐week AET period. All scans were analyzed by a single‐blinded technician, and the DXA was calibrated prior to each scan.
2.5. Aerobic fitness
VO2 peak was measured by the modified Bruce incremental treadmill test at the start and end of the 24‐week period, along with repeated measurements every 4 weeks for the EX group.13
2.6. Strength and muscle quality
Strength assessments were completed 10‐14 days before the first metabolic study and repeated 7‐14 days before the final metabolic study. An isokinetic dynamometer was used to assess leg peak strength (Biodex System 4, Shirley, NY). Subjects completed a warm‐up followed by an isokinetic (120°/s) peak torque strength test on the right leg. Muscle quality was defined as strength per unit muscle mass (ie, isokinetic peak torque of the right leg divided by right leg lean mass measured by DXA).
2.7. Metabolic study
To determine basal MPS, a metabolic study with a stable isotope tracer infusion and muscle and blood samples was performed as previously described.12 The night before the metabolic study, subjects were admitted to the ITS‐CRC where they were given a standardized dinner (10 kcal/kg; 60% carbohydrate, 20% fat, and 20% protein) and snack at 22:00, after which they were given only water ad libitum. In the morning following the overnight fast, a retrograde catheter was placed in a wrist vein, and the wrist was heated to facilitate arterialized blood sampling. Additionally, a catheter was placed in the antecubital vein of the other arm to infuse the stable isotope tracer. Background blood samples were collected, followed by a primed continuous infusion of l‐[ring13C6]phenylalanine with a priming dose of 2 μmol/kg and an infusion rate of 0.05 μmol/kg/min. At 120 minutes after commencement of the infusion, the first muscle biopsy was collected from the vastus lateralis (time 120) followed by the second biopsy from the same leg 240 minutes after the start of the infusion (time 240). Blood samples were collected at frequent intervals throughout the study, including concurrent sampling with each muscle biopsy.
2.8. Muscle biopsies
All muscle biopsies were sampled from the vastus lateralis with a 5‐mm Bergström needle using aseptic procedures and local lidocaine injection. Any visible adipose tissue was removed from muscle biopsy samples. Muscle tissue was quickly aliquoted, frozen in liquid nitrogen, and stored at −80°C until analysis. For measurement of MPS as the fractional synthetic rate (FSR), muscle samples were immediately rinsed with ice‐cold saline and blotted to remove blood before freezing in liquid nitrogen. For immunohistochemical analyses, approximately 20 mg of muscle was oriented and embedded in Tissue Tek at optical cutting temperature (Thermo Fisher Scientific, Waltham, MA) onto a cork and frozen in isopentane cooled in liquid nitrogen.
2.9. Myofibrillar protein fraction isolation
Muscle samples were processed to obtain the myofibrillar protein fraction as previously described.14 In summation, about 50 mg of frozen muscle tissue was placed in buffer with protease and phosphatase inhibitors at a 1:9 weight‐to‐volume ratio.15 Samples were homogenized in buffer and centrifuged at 4°C and 3400 g for 10 minutes. Supernatant was removed and saved for Western blotting. The pellet was suspended in isolation buffer and centrifuged at 4°C and 700 g for 10 minutes. The remaining pellet was again suspended in a PBS buffer and centrifuged for 3 cycles with removal of supernatant and resuspension each time, followed by agitation on ice for 20 minutes and sonication at 4°C. This was followed by centrifugation and removal of the supernatant containing the nuclear extract; the resulting pellet was suspended in double distilled water and centrifuged again. Then, the pellet was resuspended in 1 mL 0.3 mol/L NaOH and heated for 30 minutes at 50°C to precipitate myofibrillar proteins. The pellet in 0.3 mol/L NaOH was centrifuged, the supernatant was removed, and the pellet was again suspended in 1 mL 0.3 mol/L NaOH and heated for 10 minutes at 37°C. After centrifugation, the supernatant was collected, and the pellet containing collagen was saved and frozen. Then, 1 mL perchloric acid was added to the supernatant to precipitate myofibrillar proteins followed by centrifugation. The resulting pellet was washed with 70% ethanol before hydrolyzing overnight in 1.5 mL 6 mol/L HCl.
2.10. Myofibrillar protein synthesis
Following extraction of bound myofibrillar proteins and muscle intracellular free amino acids from vastus lateralis biopsy samples, gas chromatography‐mass spectrometry (6890 Plus CG, 5973N MSD, 7683 autosampler, Agilent Technologies, Palo Alto, CA) was used to analyze bound tracer enrichments for l‐[ring‐13C6]phenylalanine as previously described.16 Basal myofibrillar protein synthesis was calculated as the FSR by measuring incorporation of the tracer into proteins (change in protein‐bound enrichment over time) and using the precursor‐product method16:
FSR = (ΔE p/t)/{[E M(1) + E M(2)]/2} · 60 · 100
where ΔE p is the difference in protein‐bound enrichment between the first and second biopsy and t is the time between the two biopsies. E M(1) + E M(2) represent the phenylalanine tracer enrichments in the free intracellular pool from the first and second biopsy, respectively. FSR is calculated as percent per hour (%/h).
2.11. Western blot analysis
Western blot analysis was completed as previously described.17 The following antibodies were used: MuRF1 (MP3401; ECM Biosciences, Versailles, KY), Atrogin‐1 (AP2041; ECM Biosciences), Beclin1 (3738; Cell Signaling Technology, Danvers, MA), and LC3B (2775; Cell Signaling). Densitometry measurements from all proteins were normalized to β‐tubulin (2146; Cell Signaling) and a rodent loading control loaded on all gels. Data are expressed as arbitrary units (AU).
2.12. Immunohistochemical analysis
For immunohistochemical analysis, approximately 20 mg of the muscle biopsy was mounted at resting length as described above. Samples were cryosectioned to 7‐μm‐thick sections (HM525X; Thermo Fisher Scientific), and sections were air‐dried for 1 hour prior to storage at −20°C. Staining was conducted as previously described18:
For capillary/laminin staining, slides were fixed in ice‐cold acetone for 10 minutes and then blocked in 2.5% normal horse serum (no. S‐2012; Vector Laboratories, Inc., Burlingame, CA) for 1 hour. Slides were incubated overnight in Rhodamine‐labeled Ulex Europaeus Agglutinin I (no. RL‐1062; Vector Laboratories)—an endothelial cell marker19and the following primary antibody: anti‐laminin (no. L9393; Sigma Aldrich, St. Louis, MO). The following day, slides were washed and incubated for 1 hour in secondary antibody: goat anti‐rabbit IgG AF647 (no. A21245; Invitrogen, Grand Island, NY). Slides were then washed and mounted in fluorescent mounting media.
For CD56 (neural cell adhesion molecule, NCAM)/fiber type/laminin staining, slides were fixed for 10 minutes in ice‐cold acetone and incubated in anti‐laminin (no. L9393; Sigma Aldrich) and anti‐MyHC type 1 antibodies (no. BA.D5; IgG2b, DSHB) for 1 hour at room temperature followed by overnight incubation at 4°C. On the next day, slides were incubated in secondary antibodies for 1 hour at room temperature: goat anti‐rabbit AF488 (no. A11034; Invitrogen) and goat anti‐mouse IgG2b AF647 (no. A21242; Invitrogen). Slides were then blocked for 1 hour in 2.5% normal horse serum (no. S‐2012; Vector Laboratories) and incubated overnight in anti‐CD56/NCAM primary antibody (no. 555514; BD Biosciences, San Jose, CA), followed by incubation in goat anti‐mouse IgG1 AF555 (no. A21127; Invitrogen). Slides were washed and mounted in fluorescent mounting media.
2.13. Image acquisition and analysis
Images were captured at 10× and 20× magnification at room temperature on a Zeiss upright microscope (AxioImager M1; Zeiss, Oberkochen, Germany). AxioVision Rel software (v. 4.9) was used to analyze all images. Capillaries were measured as Ulex Europaeus agglutinin‐positive cellular structures that resided outside the laminin border of muscle fibers. Capillary density was quantified as capillaries normalized to fiber number (caps/fiber) and capillaries normalized to area of the muscle section (caps/mm2). NCAM+ denervated fibers were determined by expression of CD56/NCAM throughout the fiber and were normalized to type I myofiber number and total myofiber number.
2.14. Citrate synthase activity
The citrate synthase (CS) activity assay was conducted as previously described.20 Briefly, maximal CS activity was measured spectrophotometrically. Muscle homogenate was diluted in a 110 mmol/L Tris‐HCl buffer (pH 8.1) with 300 μmol/L acetyl‐CoA and 100 μmol/L of 5,5′‐dithiobis‐2‐nitrobenzoic acid (DTNB) in wells on a 96‐well plate. Oxaloacetate was quickly added to each well at a 500 μmol/L concentration immediately before placing the plate into the microplate reader (Bio‐Rad, Hercules, CA). Absorbance was recorded at 412 nmol/L at 30‐second intervals for 5 minutes at room temperature. The change in light absorbance resulting from the reaction of DTNB with free thiol groups of coenzyme A produced by condensation of oxaloacetate and acetyl‐CoA was used to calculate CS activity. CS activity was normalized to the total protein content of muscle lysates measured using a Bradford assay and expressed as μmol/min/μg protein.
2.15. Plasma endothelin‐1 concentration
Plasma concentrations of endothelin‐1 were measured in duplicate with an enzyme‐linked immunosorbent assay (ELISA) kit per manufacturer instructions (Endothelin‐1 Immunoassay Quantikine ELISA, R&D Systems, MN) using a microplate reader (Bio‐Rad).
2.16. Statistical analysis
All statistical analyses were performed with GraphPad Prism version 7.00 for Mac OS X (GraphPad Software, La Jolla, CA). Independent t tests were used to compare baseline participant characteristics between groups. We used a two‐factor ANOVA with repeated measures on the time factor to assess time * training/group differences. Tukey's post hoc paired t test was conducted when interaction or main effects were detected. For Western blots, independent t tests were used to compare fold change from baseline between groups, and paired t tests assessed EX and NON raw value changes. Data are reported as means ± standard deviations (SDs) with P < 0.05 considered significant for all tests.
3. RESULTS
3.1. Body composition
Baseline body composition was not different between the EX and NON groups (Table 1). There were no changes in body composition after 24 weeks of AET for the EX group, including no change in total lean mass (Table 1) or leg lean mass. The NON group increased fat mass (P = 0.05).
3.2. Aerobic fitness
VO2 peak was not different between groups at baseline (Table 1). After 24 weeks, VO2 peak increased by 15.95% in the EX group (P = 0.01) but was unchanged in the NON group.
3.3. Basal myofibrillar protein synthesis
The FSR of the myofibrillar protein fraction in the basal state was not different between groups at baseline (Figure 2). There was no significant time * training interaction; however, there was a main effect of time (P = 0.002) with the EX group significantly increasing myofibrillar FSR from 0.0396%/h to 0.0597% (P = 0.01), while the NON group did not change basal FSR after the 24‐week period.
Figure 2.
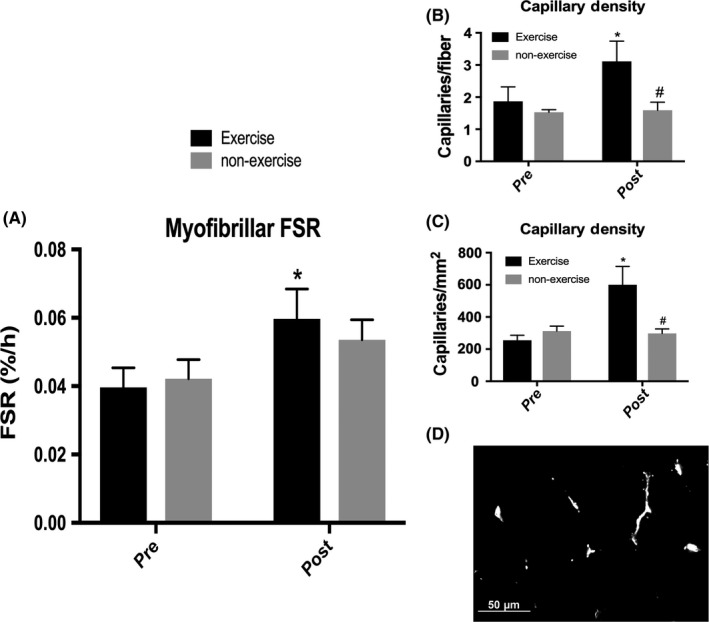
Basal myofibrillar protein synthesis and capillary density before and after 24 wk of exercise or non‐exercise. A, Myofibrillar fractional synthetic rate. No significant time * training interaction. Main effect of time (P = 0.002). *Significantly different from baseline for exercise group (P = 0.01). B, Capillaries per myofiber. Trending to significant time * training interaction (P = 0.07). *Significantly different from baseline for exercise group (P = 0.03). #Significantly different from exercise group at post (P = 0.04). C, Capillaries per mm2. Significant time * training interaction (P = 0.0220). Main effect of time (P = 0.03). *Significantly different from baseline for exercise group (P = 0.01). #significantly different from exercise group at post (P = 0.01). n = 8 with 4 in each group. D, Representative image demonstrating capillaries (white)
3.4. Capillary density
Immunohistochemical samples were not available for all subjects included in other analyses but were taken from a subset of subjects (n = 8; EX: n = 4, NON: n = 4). At baseline, there was no difference between groups for either measure of capillary density (Figure 2). Regarding capillaries per fiber, there was a trend toward a significant time * training interaction (P = 0.07) with the EX group increasing from 1.87 capillaries/fiber to 3.11 capillaries/fiber (P = 0.03). The EX and NON groups were significantly different after the 24‐week period (P = 0.04) with the NON group exhibiting no change in capillaries/fiber. Regarding capillaries per area of the muscle section, there was a significant time * training interaction (P = 0.02) with only the EX group increasing capillaries/mm2 from 254.98 to 600.15 (P = 0.01). The EX and NON groups showed a significantly different number of capillaries/mm2 after the 24‐week period (P = 0.01).
3.5. Strength and muscle quality
Leg lean mass was not different between groups and did not change in either group (Figure 3). Quadriceps peak torque was not different between groups at baseline, and after the 24‐week period, there was a trend toward a time * training interaction (P = 0.07) with a main effect of time (P = 0.04). The EX group demonstrated a significant 15.07% increase in quadriceps peak torque from 64.633 Nm to 74.375 Nm (P = 0.01). Muscle quality was not different between groups at baseline (Figure 3). There was a trend toward a significant time * training interaction (P = 0.06) with a main effect of time (P = 0.02). Only the EX group significantly increased muscle quality from baseline with a 15.51% increase from 8.375 to 9.677 Nm/kg (P = 0.01).
Figure 3.
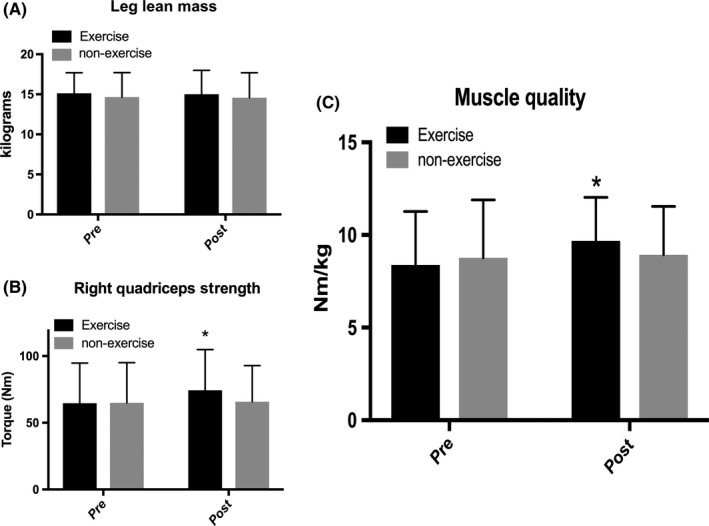
Muscle quality determinants before and after 24 wk of exercise or non‐exercise. A, Leg lean mass. B, Right quadriceps strength. Trending to significant time * training interaction (P = 0.07). Main effect of time (P = 0.04). *Significantly different from baseline for exercise group (P = 0.01). C, Muscle quality defined as right quadriceps peak torque divided by right leg lean mass. Trending to significant time * training interaction (P = 0.06). Main effect of time (P = 0.02). *Significantly different from baseline for exercise group (P = 0.01). Data are mean ± SD
3.6. CD56/neural cell adhesion molecule
NCAM, a marker of denervation of skeletal muscle fibers known to be elevated with aging and decreased by resistance training,21 was not significantly different between groups at baseline (Figure 4). Due to the heterogeneity in number of NCAM+ myofibers between individuals, our study was underpowered to detect a statistically significant change. However, the EX group exhibited a 42% decrease in number of NCAM+ fibers per MHC type I myofibers and a 16% decrease in NCAM+ fibers per total myofiber number, indicating fewer denervated myofibers after 24 weeks of AET.
Figure 4.
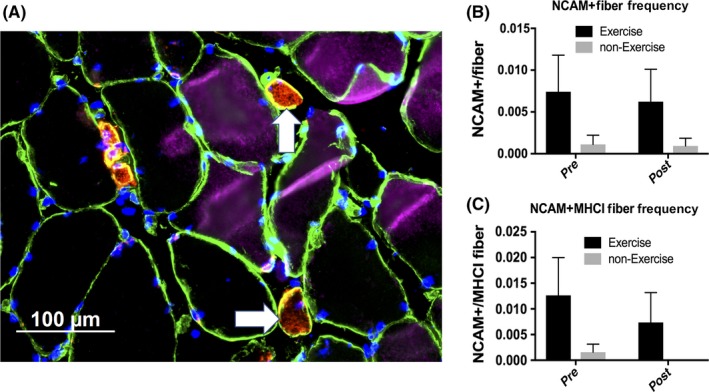
CD56/NCAM+ fiber frequency before and after 24 wk of exercise or non‐exercise. A, Representative image demonstrating laminin (green), MHC type I (pink), NCAM/CD56 (red), and DAPI (blue). White arrows indicate NCAM+ fibers. B, NCAM+ fiber frequency. Main effect of training (P = 0.02). C, NCAM+ MHCI fiber frequency. Main effect of training (P = 0.04) [Colour figure can be viewed at wileyonlinelibrary.com]
3.7. E3 Ubiquitin ligase and autophagy protein expression
Muscle RING‐finger protein‐1 (MuRF1) and Atrogin‐1 are E3 ubiquitin ligases that mediate ubiquitination of myofibrillar proteins to be degraded via the ubiquitin‐proteasome pathway.22, 23 Beclin1 and LC3B are proteins involved in autophagy, a secondary pathway for degrading proteins, often during nutrient stress.24 We speculate that upregulated skeletal muscle breakdown in the basal state may accompany increased basal myofibrillar protein synthesis after AET to promote enhanced protein turnover without skewing the net protein balance (concurrent with no hypertrophy). In order to provide some evidence for our speculation, we investigated the expression of proteins associated with these degradation pathways in the absence of a more direct breakdown measure on the metabolic study day. We found that there was no significant change in basal expression of any of the autophagy proteins (Table 2). Atrogin‐1 was not significantly altered; however, basal expression of MuRF1 was upregulated on average by 64% in EX, exhibiting a trend toward a difference from pre‐training to post‐training in EX only (P = 0.08) and in fold change between EX and NON (P = 0.11).
Table 2.
Total protein levels before and after 24 wk of exercise or non‐exercise
| Exercise | Non‐exercise | |||||
|---|---|---|---|---|---|---|
| Pre | Post | Change,% | Pre | Post | Change,% | |
| MuRF1 (AU) | 1.48 ± 1.33 | 2.28 ± 2.34a | 63.68 ± 91.50& | 2.27 ± 1.72 | 2.19 ± 2.51 | −1.69 ± 69.48 |
| Atrogin‐1 (AU) | 1.01 ± 0.74 | 0.87 ± 0.43 | −1.04 ± 30.90 | 1.21 ± 0.76 | 1.08 ± 0.62 | −0.78 ± 29.79 |
| Beclin1 (AU) | −2.25 ± 2.51 | −1.98 ± 3.18 | −4.09 ± 92.33 | 0.44 ± 2.52 | −0.40 ± 2.51 | ‐27.74 ± 78.73 |
| LC3B (AU) | 38.95 ± 56.31 | 33.87 ± 39.07 | 8.15 ± 41.02 | 37.67 ± 31.58 | 30.32 ± 22.77 | 10.41 ± 34.76 |
| Endothelin‐1 (pg/mL) | 1.20 ± 0.73 | 1.26 ± 1.16 | 0.99 ± 0.43 | 0.93 ± 0.37 | ||
| Citrate synthase activity (μmol/min/μg protein) | 28.25 ± 13.15 | 30.81 ± 8.42 | 30.71 ± 10.67 | 31.15 ± 3.96 | ||
Data are mean ± SD.
Significantly different from baseline.
Trending toward significantly different from baseline (P = 0.08). &trending toward significantly different from NON (P = 0.11).
4. DISCUSSION
The primary finding from our study is that moderate‐intensity AET—specifically walking—can enhance skeletal muscle quality in previously sedentary, healthy older adults via improved quadriceps strength. This occurred in conjunction with an increase in myofibrillar protein synthesis and enhanced muscle capillarization.
To our knowledge, this is the first study to investigate both capillarization and myofibrillar protein synthesis adaptations in response to a walking AET program in older adults. Capillary density increased in our subjects, comparable to other AET studies.25, 26 We believe this not only facilitated enhanced oxygen delivery and increased VO2 peak to improve exercise capacity, but also likely supported the myofibrillar protein synthetic adaptations. Increased capillarization of skeletal muscle may promote enhanced delivery of nutrients and removal of waste products, potentially driving elevated basal myofibrillar protein synthesis and improved muscle quality.
Muscle contraction intensity can regulate muscle protein synthesis. For example, very light load contractions (ie, 16% one‐repetition maximum) are inadequate to elicit an increase in myofibrillar protein synthesis that is observed with higher load contractions.27 As intensity of muscle contractions involved in aerobic exercise may vary greatly, previous work has demonstrated inconsistent results regarding the impact of acute aerobic exercise and AET on muscle protein synthesis and subsequent hypertrophy and strength. Low‐intensity walking acutely increased mixed‐muscle FSR in older adults very transiently with no increase from basal at 1 hour post‐exercise,28 while a single bout of moderate‐intensity cycling was insufficient to elevate myofibrillar FSR at 24 hours post‐exercise.29 Yet, high‐intensity interval cycling did elicit an increase in FSR at 1 and 2 days after the acute bout, although not to the same extent as a bout of resistance exercise.29 These results highlight the differential impact of various modes and intensities of aerobic exercise; however, our study did not investigate the acute effects of a single bout of aerobic exercise but instead the impact of moderate‐intensity AET on the basal state and how this may impact chronic adaptations.
Like the acute exercise studies described above, AET studies have utilized varying modes, intensities, and durations of exercise which makes comparison challenging. Short et al30 observed that 4 months of cycle training 3‐4 days per week at 80% peak heart rate could enhance mixed‐muscle FSR in young and older adults in the basal state by 22%. These results are comparable to our myofibrillar protein synthesis data as we utilized a similar exercise intensity, although we employed walking instead of cycling. Demonstrating further similarity to our work, Short et al's AET regimen did not produce any change in lean mass; however, strength measurements were not reported, so muscle quality cannot be assessed from this study. Long‐term aerobic exercise (at least 10 years) of varying modes and intensities (cycling, rowing, running, swimming, and skiing) effectively attenuates the strength decline of aging and, in pooled age groups, is associated with greater lean mass compared to sedentary individuals. However, higher lean body mass in pooled active participants was largely driven by young and middle‐aged participants. In aerobically active older adults, there is not a clear difference in lean mass despite enhanced maintenance of strength.31
In contrast, Mikkelsen et al32 clearly reported enhanced maintenance of muscle mass in older adult life‐long endurance runners compared to their sedentary counterparts. Although basal muscle protein synthesis was not measured in that study, our data support the efficacy of aerobic exercise at a lower intensity (walking) to promote protein synthesis which may help to conserve muscle mass in older adults. Other groups have observed increased quadriceps volume and MHC type I cross‐sectional area with 3 months of cycle training 3‐4 days per week at 60%‐75% HRR33, 34 and increased mid‐thigh muscle area after 6 months of walking/running training 5 days per week with intensity increasing progressively up to 85% HRR.35 The Trappe laboratory also found significant strength improvement concurrent with the observed hypertrophy resultant from cycle training.33 Why did hypertrophy occur in these studies but not in the current study? Possible explanations include additional resistance provided to the quadriceps via variable settings on the cycle ergometer and the eccentric loading associated with recovery from the flight phase of running compared to walking only. We were interested in walking specifically as it does not require equipment and may be more feasible for many older adults whose orthopedic limitations preclude running.
Although initially surprising, the lack of an increase in lean mass in our study after 24 weeks of moderate‐intensity AET stimulated further investigation. We then postulated that neuromuscular alterations along with upregulated muscle protein breakdown may partially explain the improved muscle strength without the increase in size observed in cycle training and running studies and typically associated with resistance exercise training. First, we employed immunohistochemical techniques to visualize CD56/NCAM on muscle sections from a small subset of subjects. CD56/NCAM is indicative of denervation of myofibers and is elevated in old muscle compared to young, highlighting progressive denervation with aging. Resistance exercise decreases NCAM+ fibers, suggesting reinnervation of myofibers contributing to strength improvement, even in the absence of hypertrophy.21 However, the impact of AET on denervation of myofibers has been less explored. Unfortunately, we did not have immunohistochemistry samples from all subjects which limited our capacity to precisely determine a role of innervation on enhanced muscle quality. From our small sample, we observed a 42% decrease in NCAM+ MHC type I myofibers and a 16% decrease in total NCAM+ myofibers. Although not statistically significant, these data suggest a clinically relevant decrease in denervation that may contribute to augmented quadriceps strength by improving the capacity for motor unit recruitment. To strengthen this assertion, a larger sample size is needed, but these data merit further exploration when additional samples are available.
Reduced denervation of myofibers resulting from AET is a probable contributor to enhanced muscle quality but provides an incomplete picture of the complex physiological interplay. Considering this and with the aim to offer a more complete picture, we speculated that skeletal muscle protein breakdown may be upregulated concurrently with the observed increase in basal myofibrillar protein synthesis, as observed with no change in lean mass in young adults by Pikosky et al36 after 4 weeks of group run/walk training. Muscle protein synthesis and breakdown rates fluctuate in response to external stimuli; however, when the two rates are balanced, neither hypertrophy nor atrophy occurs and skeletal muscle mass is maintained.8, 37 A net positive protein balance (ie, higher rates of muscle protein synthesis as compared to breakdown) or net negative protein balance (ie, higher rates of muscle protein breakdown as compared to synthesis) triggers hypertrophy or atrophy, respectively. Therefore, overall protein turnover may be amplified without a change in net protein balance if both synthesis and breakdown are simultaneously upregulated. Our data confirm upregulation of basal myofibrillar protein synthesis (measured by FSR) in response to AET, but in the current study, we did not have a clear metabolic measure of muscle protein breakdown, like fractional breakdown rate (FBR). As a proxy, we chose to investigate expression levels of proteins associated with the two protein degradation pathways in muscle: the ubiquitin‐proteasome pathway and autophagy. We found no change in basal expression of any of the measured autophagy proteins following AET which was similar to Fry et al38 who found no increase in autophagy‐associated proteins despite a 16% increase in FBR in young and older adults after an acute exercise bout. This is not altogether surprising as the autophagosomal‐lysosomal system necessarily degrades myofibrillar proteins during nutrient stress to provide amino acids for vital cellular functions with autophagy flux being essential for survival and maintenance of muscle mass.39 These subjects had not been fasting for a sufficient period to reach significant nutrient stress, particularly during resting conditions. The lack of upregulated autophagy as a contributor to the conjectured elevation of overall muscle protein breakdown following AET was not unexpected as excessive autophagy produces extreme muscle atrophy and is typically dependent on nutrient stress and/or wasting diseases.40
Since MuRF1 expression is upregulated in mice after AET,41 we further speculated that the ubiquitin‐proteasome pathway was more likely to be enhanced and support our postulation of elevated basal muscle protein breakdown (alongside elevated myofibrillar protein synthesis) as this pathway is the primary mechanism of protein degradation in mammals. Our group has previously provided support of MuRF1 as a proxy for muscle protein breakdown as increased expression of MuRF1 was associated with a 16% elevation of FBR after resistance exercise in young and older adults.38 Subsequently, we did observe a slight trend for an increase in MuRF1 basal levels following AET. MuRF1 is a muscle‐specific E3 ubiquitin ligase important for ubiquitin‐proteasome pathway‐mediated proteolysis in skeletal muscle, and its overexpression is associated with muscle wasting.23, 42 However, with simultaneous upregulation of myofibrillar protein synthesis, the ubiquitin‐proteasome pathway may serve to degrade damaged or misfolded myofibrillar proteins while properly folded, better functioning replacement proteins are concomitantly synthesized, promoting enriched muscle quality. Since lean mass did not change, upregulation of myofibrillar protein synthesis and proteolysis likely represents skeletal muscle remodeling as opposed to bulk accretion. The data supporting our speculation of upregulated muscle protein breakdown concurrent with muscle protein synthesis are not conclusive; however, these data support further exploration of this idea in subsequent AET clinical trials utilizing a metabolic measure of muscle protein breakdown, such as FBR.
5. PERSPECTIVES
We report that AET—specifically moderate‐intensity walking—is a viable intervention to combat the functional strength decline associated with aging. This is achieved with upregulated basal myofibrillar protein synthesis supported by enhanced capillarization, but these improvements are not accompanied by an increase in lean mass. Therefore, the quality of the existing skeletal muscle mass is improved as older adults become stronger per unit of muscle. Ideally, older adults should also participate in RET as this mode is well established to promote hypertrophy and strength gain, mitigating the sarcopenic process. However, RET may not be practical or feasible for many older adults at high risk of functional decline and frailty. These older adults should be advised that walking training at a feasible intensity (70% HRR) performed only 3 days per week is sufficient to elicit strength improvements that translate into improved muscle quality and function which should help to alleviate the risk of falls.
CONFLICT OF INTEREST
The authors have no conflicts of interests to disclose.
ACKNOWLEDGEMENTS
These results are without fabrication, falsification, or data manipulation. We gratefully recognize the contributions made by study coordinators: Jennifer Timmerman, Paula Skinkis, and Roxana Hirst, the nursing staff at UTMB ITS‐Clinical Research Center, and by Samantha Lane, Kelli Faaitiiti, and Amanda Randolph. This work was supported by National Institutes of Health/National Institute on Aging (grant numbers R01AG030070, R01AG030070S1, R56AG051267, P30AG024832, UL1TR001439, and T32AG000270).
Brightwell CR, Markofski MM, Moro T, et al. Moderate-intensity aerobic exercise improves skeletal muscle quality in older adults. Transl Sports Med. 2019;2:109‐119. 10.1002/tsm2.70
REFERENCES
- 1. Fry CS, Drummond MJ, Glynn EL, et al. Aging impairs contraction‐induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle. 2011;1(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deer RR, Volpi E. Protein intake and muscle function in older adults. Curr Opin Clin Nutr Metab Care. 2015;18(3):248‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim T, Choi K. Sarcopenia: definition, epidemiology, and pathophysiology. J Bone Metab. 2013;20(1):109‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. von Haehling S, Morley J, Anker S. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1(2):129‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Landi F, Cruz‐Jentoft AJ, Liperoti R, et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing. 2013;42(2):203‐209. [DOI] [PubMed] [Google Scholar]
- 7. Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52(1):80‐85. [DOI] [PubMed] [Google Scholar]
- 8. Rennie M, Wackerhage H, Spangenburg E, Booth F. Control of the size of the human muscle mass. Annu Rev Physiol. 2004;66:799‐828. [DOI] [PubMed] [Google Scholar]
- 9. Marcotte GR, West DW, Baar K. The molecular basis for load‐induced skeletal muscle hypertrophy. Calcif Tissue Int. 2015;96(3):196‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moro T, Brightwell CR, Deer RR, et al. Muscle protein anabolic resistance to essential amino acids does not occur in healthy older adults before or after resistance exercise training. J Nutr. 2018;148(6):900‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Santos‐Parker JR, LaRocca TJ, Seals DR. Aerobic exercise and other healthy lifestyle factors that influence vascular aging. Adv Physiol Educ. 2014;38(4):296‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Markofski MM, Jennings K, Timmerman KL, et al. Effect of aerobic exercise training and essential amino acid supplementation for 24 weeks on physical function, body composition and muscle metabolism in healthy, independent older adults: a randomized clinical trial. J Gerontol A Biol Sci Med Sci. 2018. [Epub ahead of print]. 10.1093/gerona/gly109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Franklin B, Whaley M, Howley E, Balady G. ACSM's Guidelines for Exercise Testing and Prescription, 6th edn Philadelphia, PA: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 14. Reidy PT, Walker DK, Dickinson JM, et al. Soy‐dairy protein blend and whey protein ingestion after resistance exercise increases amino acid transport and transporter expression in human skeletal muscle. J Appl Physiol. 2014;116(11):1353‐1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E‐BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576(Pt 2):613‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. Hoboken, NJ: Wiley‐Liss; 2005. [Google Scholar]
- 17. Drummond MJ, Fry CS, Glynn EL, et al. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol (1985). 2011;111(1):135‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arentson‐Lantz EJ, English KL, Paddon‐Jones D, Fry CS. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle‐aged adults. J Appl Physiol (1985). 2016;120(8):965‐975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holthofer H, Virtanen I, Kariniemi AL, Hormia M, Linder E, Miettinen A. Ulex europaeus I lectin as a marker for vascular endothelium in human tissues. Lab Invest. 1982;47(1):60‐66. [PubMed] [Google Scholar]
- 20. Porter C, Hurren NM, Cotter MV, et al. Mitochondrial respiratory capacity and coupling control decline with age in human skeletal muscle. Am J Physiol Endocrinol Metab. 2015;309(3):E224‐E232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Messi ML, Li T, Wang ZM, Marsh AP, Nicklas B, Delbono O. Resistance training enhances skeletal muscle innervation without modifying the number of satellite cells or their myofiber association in obese older adults. J Gerontol A Biol Sci Med Sci. 2016;71(10):1273‐1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin‐1, a muscle‐specific F‐box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA. 2001;98(25):14440‐14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Palma L, Marinelli M, Pavan M, Orazi A. Ubiquitin ligases MuRF1 and MAFbx in human skeletal muscle atrophy. Joint Bone Spine. 2008;75(1):53‐57. [DOI] [PubMed] [Google Scholar]
- 24. Kaushik S, Singh R, Cuervo AM. Autophagic pathways and metabolic stress. Diabetes Obes Metab. 2010;12(suppl 2):4‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prior SJ, Blumenthal JB, Katzel LI, Goldberg AP, Ryan AS. Increased skeletal muscle capillarization after aerobic exercise training and weight loss improves insulin sensitivity in adults with IGT. Diabetes Care. 2014;37(5):1469‐1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gavin TP, Kraus RM, Carrithers JA, Garry JP, Hickner RC. Aging and the skeletal muscle angiogenic response to exercise in women. J Gerontol A Biol Sci Med Sci. 2015;70(10):1189‐1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holm L, van Hall G, Rose AJ, et al. Contraction intensity and feeding affect collagen and myofibrillar protein synthesis rates differently in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298(2):E257‐E269. [DOI] [PubMed] [Google Scholar]
- 28. Sheffield‐Moore M, Yeckel CW, Volpi E, et al. Postexercise protein metabolism in older and younger men following moderate‐intensity aerobic exercise. Am J Physiol Endocrinol Metab. 2004;287(3):E513‐E522. [DOI] [PubMed] [Google Scholar]
- 29. Bell KE, Seguin C, Parise G, Baker SK, Phillips SM. Day‐to‐day changes in muscle protein synthesis in recovery from resistance, aerobic, and high‐intensity interval exercise in older men. J Gerontol A Biol Sci Med Sci. 2015;70(8):1024‐1029. [DOI] [PubMed] [Google Scholar]
- 30. Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004;286(1):E92‐E101. [DOI] [PubMed] [Google Scholar]
- 31. Crane JD, Macneil LG, Tarnopolsky MA. Long‐term aerobic exercise is associated with greater muscle strength throughout the life span. J Gerontol A Biol Sci Med Sci. 2013;68(6):631‐638. [DOI] [PubMed] [Google Scholar]
- 32. Mikkelsen UR, Couppe C, Karlsen A, et al. Life‐long endurance exercise in humans: circulating levels of inflammatory markers and leg muscle size. Mech Ageing Dev. 2013;134(11–12):531‐540. [DOI] [PubMed] [Google Scholar]
- 33. Harber MP, Konopka AR, Douglass MD, et al. Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1452‐R1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harber MP, Konopka AR, Undem MK, et al. Aerobic exercise training induces skeletal muscle hypertrophy and age‐dependent adaptations in myofiber function in young and older men. J Appl Physiol (1985). 2012;113(9):1495‐1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schwartz RS, Shuman WP, Larson V, et al. The effect of intensive endurance exercise training on body fat distribution in young and older men. Metabolism. 1991;40(5):545‐551. [DOI] [PubMed] [Google Scholar]
- 36. Pikosky MA, Gaine PC, Martin WF, et al. Aerobic exercise training increases skeletal muscle protein turnover in healthy adults at rest. J Nutr. 2006;136(2):379‐383. [DOI] [PubMed] [Google Scholar]
- 37. McGlory C, Phillips SM. Exercise and the regulation of skeletal muscle hypertrophy In: Progress in Molecular Biology and Translational Science. Amsterdam, The Netherlands: Elsevier; 2015;135:153-173. [DOI] [PubMed] [Google Scholar]
- 38. Fry CS, Drummond MJ, Glynn EL, et al. Skeletal muscle autophagy and protein breakdown following resistance exercise are similar in younger and older adults. J Gerontol A Biol Sci Med Sci. 2013;68(5):599‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Masiero E, Agatea L, Mammucari C, et al. Autophagy is required to maintain muscle mass. Cell Metab. 2009;10(6):507‐515. [DOI] [PubMed] [Google Scholar]
- 40. Sandri M. Protein breakdown in muscle wasting: role of autophagy‐lysosome and ubiquitin‐proteasome. Int J Biochem Cell Biol. 2013;45(10):2121‐2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cunha TF, Moreira JB, Paixao NA, et al. Aerobic exercise training upregulates skeletal muscle calpain and ubiquitin‐proteasome systems in healthy mice. J Appl Physiol (1985). 2012;112(11):1839‐1846. [DOI] [PubMed] [Google Scholar]
- 42. Wall BT, Dirks ML, Snijders T, Senden JM, Dolmans J, van Loon LJ. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol (Oxf). 2014;210(3):600‐611. [DOI] [PubMed] [Google Scholar]


