Abstract
Over the past two decades, immunotherapy has emerged as a promising treatment option for patients with cancer. However, newer versions of immunotherapy, such as checkpoint inhibitors, may be associated with unusual adverse effects (AEs) that can range in severity from mild to life-threatening. Unlike common AEs of conventional chemotherapy, which have a predictable nadir or cyclic pattern after administration, AEs of these newer immunotherapies are variable, depending on the type of immunotherapy, route of administration, and mechanism of action. The onset and resolution of these AEs may be present at any time, during administration of treatment, a few weeks after administration of treatment, or several months after completion of treatment. Therefore, improving outcomes in patients undergoing oncologic immunotherapy requires oncology nurses’ knowledge and understanding of various immunotherapy agents, as well as early recognition and management of potential AEs, especially AEs associated with checkpoint inhibitors and other therapies that manipulate T-cell activation causing autoimmune toxicity. This article draws upon current evidence from systematic reviews, meta-analyses, and expert consensus guidelines to provide a brief overview of common immunotherapies used in cancer and management of their associated AEs.
Keywords: Adverse events, cancer, immunotherapy, management

Introduction
Over the past two decades, the Food and Drug Administration (FDA) has approved several different types of immunotherapies as treatment options for patients with cancer, secondary to reports of improved survival, and complete remissions in some cancers.[1,2,3,4,5,6,7] Immunotherapy uses the body's immune system to combat cancer; specifically, it stimulates the production of specific antibodies or counteracts malignant cells’ production of signals or pathways that suppress immune responses.[8] However, stimulating the immune system may cause unusual adverse events (AEs), especially with checkpoint inhibitors and other therapies that manipulate T-cell activation causing autoimmune toxicity.[9] Occurring in any system of the body, these AEs range from mild to life-threating in severity, depending on the type of immunotherapy, route of administration, and mechanism of action.[10,11] Unlike the AEs of conventional chemotherapy, which have a predictable nadir or cyclic pattern after administration, AEs related to these newer versions of immunotherapy are variable in regard to their onset and resolution and may be present at any time, from a period of a few weeks during administration of treatment to several months after completion of treatment.[12] Therefore, improving outcomes in patients undergoing oncologic immunotherapy requires oncology nurses’ knowledge and understanding of the various immunotherapy agents, as well as early recognition and management of potential AEs, especially AEs associated with checkpoint inhibitors and other agents that manipulate T-cell activation causing autoimmune toxicity. This article draws upon current evidence from systemic reviews, meta-analyses, and expert consensus guidelines to provide a brief overview of common immunotherapies used in cancer and management of their associated AEs.
Categories of Immunotherapy
The major oncologic immunotherapies involve cancer vaccines, monoclonal antibodies (mAbs), chimeric antigen receptor (CAR) T-cell therapy, cytokines, oncolytic viral immunotherapy, and immune checkpoint inhibitors.[11] Given the variability in mechanism of action of the different immunotherapies and the heterogeneity of AEs, it is imperative that oncology nurses become familiar with the current version of The National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE v. 5.0), which is a standardized list of AE terms commonly found in oncology. The CTCAE is available in detail at https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. The CTCAE serves as a universal tool for oncology nurses to properly gauge or measure the severity of the AE, track the progress of the AE, document the AEs in standardize terminology, and help oncology nurses to initiate the proper treatment for the AEs based on the CTCAE grade and the established guidelines and algorithms.
Cancer vaccines
Cancer vaccines stimulate or restore the immune system's ability to target peptides or antigens on cancer cells.[13] Generally, these biological response modifiers are categorized as cell-based, peptide-based, tumor-cell-based, or immune- or dendritic-cell-based vaccines.[10] Currently, sipuleucel-T (Provenge; Dendreon Pharmaceuticals) is the only therapeutic dendritic-cell-based vaccine that has received FDA approval for the treatment of hormone-refractory prostate cancer.[14] Sipuleucel-T uses the patient's own cells to induce an immune response against prostatic acid phosphatase (PAP), which is found in 95% of prostate adenocarcinomas and is specific to prostate tissue.[15] Sipuleucel-T is made by harvesting the patient's peripheral blood mononuclear cells using leukapheresis. The cells are then sent to the laboratory, where they are cultured in vitro for 36–44 h with a fusion protein, composed of recombinant PAP and granulocyte-macrophage-colony-stimulating factor (GM-CSF), and then reinfused back into the patient.[14] Normally, this process is replicated every 2 weeks for a total of three doses.[15]
Generally, sipuleucel-T is well tolerated; however, common AEs experienced by patients participating in sipuleucel-T clinical trials include chills (44.0%–57.8%), pyrexia (29.3%–36.2%), headache (16.0%–23.3%), myalgia (9.8%–21.6%), influenza-like illness (9.8%–13.8%), and hypertension (7.4%–11.2%).[15] One clinical trial reported groin pain (5%), vomiting (10.9%), dyspnea (10.9%), asthenia (5.3%–14.3%), and hyperhidrosis.[15] Other reported AEs include stroke, myocardial infarction, and increased risk of deep vein thrombosis.[16]
However, most AEs associated with sipuleucel-T are infusion related which are caused by a release of cytokines.[17] Usually, infusion-related AEs are self-limiting and resolve within 24–48 h after vaccine infusion.[10] To minimize infusion-related AEs, the European Society for Medical Oncology clinical practice guidelines recommends premedication with acetaminophen and diphenhydramine and adjustment in the infusion rate of sipuleucel-T [Table 1].[15,17,18,19,20,21,22,23,24,25]
Table 1.
Other Immunotherapy agents
| Immunotherapy agent | Drug and company | Target | Indication | Common selected AEs | Management |
|---|---|---|---|---|---|
| CAR T-cell | |||||
| Axicabtagene ciloleucel (Yescarta) KITE Pharma, Inc. |
CD19 | Adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy. | • Cytokine release syndrome (CSR) (Fever (100.4 °F/38 °C or higher), hypotension, tachycardia, hypoxia, and chills). • Immune effector cell-associated neurotoxicity syndrome (ICANS) (delirium, encephalopathy, aphasis, lethargy, difficulty concentrating, agitation, tremor, and seizures). • Neutropenia • Anemia • Fatigue • Anorexia • Diarrhea • Nausea/vomiting • Constipation • Cardiac arrhythmias |
CSR • Grade 1- Supportive care for fever, headache, fatigue, myalgia, and malaise. • Grade 2- Administer tocilizumab intravenously. Repeat tocilizumab every 8 hours as needed if not responsive to intravenous fluids or increasing supplemental oxygen. Limit of 3 doses of tocilizumab in a 24-hour period. Administer corticosteroids if no improvement within 24 h • Grade 3- Give tocilizumab as per grade 2. Administer methylprednisone 1 mg/kg every 6 hours, continue until the event is grade 1, then taper over 3 days. • Grade 4- Same as per grade 2. Administer methylprednisolone 1000 mg intravenously per day for 3 days. |
|
| Tisageniecleucel (Kymriah) Novartis |
CD19 | Adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy. Pediatric and young adults B-cell acute lymphoblastic leukemia. | ICANS • Grade 2 with concurrent CRS. Administer tocilizumab as per grade 2 CRS. If no improvement within 24 hours, start dexamethasone 10 mg every 6 hours until event returns to grade 1. If no concurrent CRS, administer dexamethasone 10 mg every 6 hours until event is grade 1 or less, taper of 3 days. • Grade 3 with concurrent CRS. Administer tocilizumab as per grade 2 CRS, and start dexamethasone with first dose of tocilizumab, repeat dexamethasone every 6 hours until event is grade 1, then taper over 3 days. If no concurrent CRS, administer dexamethasone every 6 hours until grade 1, taper of 3 days. Consider adding prophylaxis non-sedating anti-seizure medication. • Grade 4 with concurrent CRS. Start tocilizumab as per grade 2 CRS and methylprednisolone 1000 mg per day with the first dose of tocilizumab. Continue methylprednisolone for 2 more days. If no concurrent CRS, administer methylprednisolone 1000 mg per day for 3 days. General • Monitor for hypersensitivity reactions during infusion. • Monitor for signs and symptoms of infection, treat as needed. • Monitor complete blood counts frequently. • Encourage patients to avoid driving and engaging in hazardous occupations or activities for at least 8 weeks post treatment. |
||
| Cytokines | |||||
| IFN alpha-2b (Intron A) Merck |
No specific target. Binds to type 1 interferon receptors and activates tyrosine kinase which produces antiproliferative and immunomodulatory effects. |
Carcinoid tumors Melanoma Renal cell carcinoma Cutaneous T-cell lymphoma Hairy cell leukemia Follicular lymphoma Chronic myeloid leukemia |
• Injection site reaction • Alopecia • Anorexia • Nausea/vomiting • Dry mouth • Increased liver enzymes • Arthralgia • Myalgia • Asthenia • Flu-like symptoms • Diarrhea • Chills • Vomiting • Rash • Bilirubinemia • Thrombocytopenia • Nausea • Confusion • Increased serum creatinine • Capillary leak syndrome (peripheral edema, hypotension, pleural effusion, dyspnea, ascites, oliguria, weight gain, fever, pulmonary edema) |
• Assess complete blood counts, thyroid function studies, and liver enzymes. • Assess for depression and suicidal risk. Start antidepressant therapy at the earliest sign of depression. • Assess for autoimmune disorders and discontinue treatment as needed. |
|
| Aldesleukin (IL-2: Proleukin) Novartis |
No specific target. Inhibits tumor growth by stimulating growth and activity of T cells and B lymphocytes. | Metastatic renal cell carcinoma Metastatic melanoma |
• Assess baseline pulmonary, cardiac, hepatic, renal, and neurological function prior to starting treatment. • Monitor for signs and symptoms of infection, treat as needed. • Assess for baseline pre-existing autoimmune disease and inflammatory disorders. • Monitor blood glucose levels throughout treatment. • Monitor vital signs, urine output, and weight. • Assess complete blood counts, electrolytes, renal, and hepatic function daily while receiving treatment. • Treat symptoms with steroids, intravenous immune globulin (IVIG), β2 agonist, and volume replacements as per individual facility guidelines. |
||
| Vaccine | |||||
| Sipuleucel-T (Provenge) Dendreon |
Prostatic acid phosphatase (PAP) | Hormone-refractory prostate cancer | • Infusion related reactions • Chills • Fatigue • Fever • Back pain • Nausea • Arthralgias • Headache |
• Monitor for infusion related reactions. • Consider premedication with acetaminophen and diphenhydramine. • Use universal precautions when handling to limit potential exposure to infectious diseases. |
|
| Viral therapy | |||||
| Talimogene laherparepvec (Imlygic or T-VEC) Amgen |
No specific target. Designed to mediate tumor regression via replication within and lysis of tumor cells | Advanced melanoma | • Fever and chills • Fatigue • Nausea • Flu-like symptoms • Injection site reaction (pain, erythema, swelling) |
Assess for injection site reaction. Consider premedication with acetaminophen or indomethacin. Monitor for signs and symptoms of infection, treat as needed. |
Monoclonal antibodies
mAbs are cell-derived, laboratory-generated substances that target specific antigens on tumors.[8] mAbs – which may be murine (made from mice), chimeric (part mouse and part human), humanized (mouse antibodies attached to human antibodies), or fully human (human antibodies) – hinder tumor growth by inhibiting tumor cells’ survival cascades, interfering with tumor angiogenesis, and enabling malignant cells to avoid programmed cell death (PD) and evade immune checkpoints.[26] To date, the FDA has approved several mAbs for the treatment of cancer [Table 2].[17,26,27,28,29,30,31,32,33]
Table 2.
Monoclonal antibodies (mAbs)
| Monoclonal antibodies (mAbs) | Company | Target | Indication | Common selected AEs | Management |
|---|---|---|---|---|---|
| Bevacizumab (Avastin) | Genentech | VEGF | Metastatic colorectal cancer Non-small cell lung cancer Renal cell cancer Cervical, ovarian, fallopian tube, and peritoneal cance Recurrent glioblastoma |
Epistaxis Headache Hypertension Rhinitis Proteinuria Taste alteration Dry skin Rectal hemorrhage Lacrimation disorder Back pain Exfoliative dermatitis |
Hypertension • Evaluate risk and maintain blood pressure within normal range. • Treat with angiotensin-converting enzyme (ACE) inhibitors, beta blockers, calcium channel blockers, and diuretics. Proteinuria • Assess urinary protein excretion assessment before every cycle of anti-VEGF using a urine dipstick. • If urine dipstick >2+, order 24-h urine collection for protein. • Hold treatment if 24-hour urine protein levels are >2 grams and restart treatment when levels are <2 grams. • Discontinue treatment for 24-hour urine protein >3.5 grams. • Angiotensin II receptors and ACE inhibitors may reduce the severity of proteinuria and end-stage renal disease. Hemorrhage • Prior to starting an anti-VEGF, assess for risk factors or any signs of bleeding. Wound healing • Discontinue treatment at least 28 days prior to surgery and reinitiate at 28 days after surgery or when wound is completely healed. |
| Blinatumomab (Blincyto) | Amgen | CD19 | Philadelphia- chromosome - negative relapsed or refractory B-cell precursor acute lymphoblastic leukemia | Infection Headache Neutropenia, Thrombocytopenia Fever Anemia Infusion reaction |
• Assess for signs and symptoms of infection. • Monitor for signs and symptoms of neurotoxic AEs. • Assess infusion related AEs • Pre-medicate with dexamethasone. |
| Brentuximab vedotin (Adcetris) | SeattleGenetics | CD30 | Hodgkin lymphoma Systemic anaplastic large cell lymphoma |
Neutropenia Peripheral sensory neuropathy Fatigue Nausea/vomiting Anemia Upper respiratory infection Diarrhea Pyrexia Rash Thrombocytopenia Cough |
• Assess complete blood count and treat for grade 3 or higher neutropenia. • Assess for signs and symptoms of infection. • Assess and grade peripheral neuropathy and treat as needed. |
| Cetuximab (Erbitux) | Lilly | EGFR | Metastatic colorectal cancer Head and neck cancer |
Acne-like rash Fatigue Growth of eyelashes Dry skin Allergic reaction, Myocardial infarction Diarrhea, Hypomagnesemia Paronychia |
Electrolytes • Monitor electrolyte imbalances (magnesium, calcium, potassium) and replete as needed. Treatment of cutaneous AEs • Rash ✓ Grade 1- Apply emollients regularly. ✓ Grade 2- Oral or topical application of tetracycline antibiotics, corticosteroids, moisturizers, and sunscreen. Refer to dermatologist. ✓ Grade 3- Continue topical and oral regimen and refer to dermatologist. Hold EGFR therapy. • Paronychia ✓ Grade 1- Warm water or white vinegar soaks. ✓ Grade 2- Topical corticosteroids and antimicrobials, consult a dermatologist and podiatrist as needed. ✓ Grade 3- Refer to dermatologist or podiatrist, continue topical steroids, antifungals, antibiotics, antiseptics, and silver nitrate. Gastrointestinal AEs • Diarrhea ✓ Assess for infection versus drug related. ✓ Grade 1-2- loperamide, hydrate. ✓ Grade 3-4- In addition to loperamide, add codeine for a short-term basis. ✓ Obtain stool cultures and hospitalization for intravenous fluids as needed. ✓ Referral to gastroenterologist if diarrhea does not resolve. |
| Daratumumab (Darzalex) | Janssen | CD38 | Multiple myeloma | Fatigue Nausea/vomiting, Diarrhea Constipation Muscle spasms Back pain Fever, chills Dizziness Insomnia Dyspnea Cough Edema Neuropathy Arthralgias Cold-like symptoms |
Fatigue • Assess treatable contributing factors. • Assess psychosocial factors. • Treatment may include physical activity, psychosocial interventions, mind-body interventions, and pharmacologic interventions. • Assess for signs and symptoms of infection. |
| Dinutuximab (Unituxin) | United Therapeutics | GD2 gangloside | Neuroblastoma (in children) | Pain Fever Thrombocytopenia Lymphopenia Infusion reactions Hypotension Hypertension Hyponatremia |
• Assess for signs and symptoms of infection. • Monitor blood pressure and treat as per guidelines. Discontinue treatment for severe or uncontrolled hypertension. • Assess infusion related AEs |
| Elotuzumab (Empliciti) | Bristol-Myers Squibb | SLAMF7 | Multiple myeloma | Infusion reaction Fever, chills Hypertension |
• Assess for signs and symptoms of infection. • Monitor blood pressure and treat as per guidelines. Discontinue treatment for severe or uncontrolled hypertension. • Assess infusion related AEs. |
| Gemtuzumab Ozogamicin (Mylotarg) | Pfizer Inc. | CD33 | Newly-Diagnosed Acute myeloid leukemia (AML) Relapsed or refractory AML |
Nausea/vomiting, Diarrhea Constipation Headache Dizziness Anxiety Depression Cytopenia Elevated liver enzymes |
• Assess complete blood counts and metabolic panels three times per week. • Assess for signs and symptoms of infection. • Assess infusion related AEs. Discontinue for severe infusion reactions. |
| Ibritumomab tiuxetan (Zevalin) | Biogen Idec, Inc | CD20 | Non-Hodgkin lymphoma | Cytopenia Fatigue Nasopharyngitis Nausea Abdominal pain Asthenia Cough Diarrhea Pyrexia |
• Assess for signs and symptoms of infection. • Assess infusion related AEs. Discontinue for severe infusion reactions. • Monitor complete blood counts and platelet count weekly after administration of drug. |
| Necitumumab (Portrazza) | Lilly | EGFR | Non-small cell lung cancer | Hypomagnesemia Hypokalemia Vomiting Diarrhea Acne Weight loss Mucositis Hemoptysis |
Electrolytes Monitor electrolyte imbalances (magnesium, calcium, potassium) and replete as needed. Treatment of cutaneous AEs • Rash ✓ Grade 1- Apply emollients regularly. ✓ Grade 2- Oral or topical application of tetracycline antibiotics, corticosteroids, moisturizers, and sunscreen. Refer to dermatologist. ✓ Grade 3- Continue topical and oral regimen and refer to dermatologist. Hold EGFR therapy. • Paronychia ✓ Grade 1- Warm water or white vinegar soaks. ✓ Grade 2- Topical corticosteroids and antimicrobials, consult a dermatologist and podiatrist as needed. ✓ Grade 3- Refer to dermatologist or podiatrist, continue topical steroids, antifungals, antibiotics, antiseptics, and silver nitrate. Gastrointestinal AEs • Diarrhea ✓ Assess for infection versus drug related. ✓ Grade 1-2- Ioperamide, hydrate. ✓ Grade 3-4- In addition to loperamide, add codeine for a short-term basis. ✓ Obtain stool cultures and hospitalization for intravenous fluids as needed. ✓ Referral to gastroenterologist if diarrhea does not resolve. • Stomatitis and Mucositis ✓ Grade 1- Continue EGFR, oral rinses, non-alcoholic mouthwashes. Consider prophylaxis with antifungal, antiviral, and antibacterial. ✓ Grade 2- Hold EGFR, topical analgesics, mucosal coating agents, and benzydamine HCL may be administered as needed. Treat infections with topical or systemic antibacterials. ✓ Grade 3- Discontinue EGFR, hospitalization for supportive care. |
| Obinutuzumab (Gazyva) | Genentech | CD20 | Chronic myeloid leukemia Follicular lymphoma |
Infusion reaction Neutropenia Thrombocytopenia, Anemia Fever Cough Nausea Diarrhea |
• Monitor for infusion related AEs and treat as per guidelines. • Monitor for signs and symptoms of infection. • Monitor complete blood counts frequently. |
| Ofatumumab (Arzerra) | Novartis | CD20 | Chronic lymphocytic leukemia | Infusion reaction Neutropenia Pneumonia Fever Cough Diarrhea Anemia Fatigue Dyspnea Rash Nausea Bronchitis Upper respiratory tract infection |
• Pre-medicate with oral or intravenous antihistamine, acetaminophen, and intravenous corticosteroid to minimize infusion reaction. • Monitor complete blood counts and assess neurologic function. |
| Olaratumab (Lartruvo) | Lilly | PDGF R alpha | Soft tissue sarcoma | Nausea/vomiting Fatigue Myalgias Mucositis Alopecia Diarrhea Anorexia Abdominal pain Neuropathy Headache Lymphopenia Neutropenia Thrombocytopenia Hyperglycemia Elevated activated Partial thromboplastin time Hypokalemia, Hypophosphatemia |
• Monitor electrolyte imbalances and replete as needed. • Monitor for signs and symptoms of infection. • Monitor complete blood counts frequently. • Monitor for infusion related AEs and treat as per guidelines. |
| Panitumumab (Vectibix) | Amgen | EGFR | Wild-type RAS metastatic colorectal caner | Acneiform dermatitis Pruritus Erythema Rash Skin exfoliation Paronychia Dry skin Skin fissures Diarrhea, Nausea/vomiting Fatigue Abdominal pain Overgrowth of eyelashes |
Electrolytes Monitor electrolyte imbalances (magnesium, calcium, potassium) and replete as needed. Treatment of cutaneous AEs • Rash ✓ Grade 1- Apply emollients regularly. ✓ Grade 2- Oral or topical application of tetracycline antibiotics, corticosteroids, moisturizers, and sunscreen. Refer to dermatologist. ✓ Grade 3- Continue topical and oral regimen and refer to dermatologist. Hold EGFR therapy. • Paronychia ✓ Grade 1- Warm water or white vinegar soaks. ✓ Grade 2- Topical corticosteroids and antimicrobials, consult a dermatologist and podiatrist as needed. ✓ Grade 3- Refer to dermatologist or podiatrist, continue topical steroids, antifungals, antibiotics, antiseptics, and silver nitrate. Gastrointestinal AEs • Diarrhea ✓ Assess for infection versus drug related. ✓ Grade 1-2- Ioperamide, hydrate. ✓ Grade 3-4- In addition to loperamide, add codeine for a short-term basis. ✓ Obtain stool cultures and hospitalization for intravenous fluids as needed. ✓ Referral to gastroenterologist if diarrhea does not resolve. |
| Pertuzumab (Perjeta) | Genentech | HER-2 | Metastatic breast cancer Neoadjuvant treatment of breast cancer | Nausea Diarrhea Fatigue Dry skin Alopecia Dermatitis Pruritus Neuropathy Cold symptoms |
• Assess for signs and symptoms of infection. • Assess left ventricular ejection function and monitor for cardiac failure or dysfunction |
| Ramucirumab (Cyramza) | Amgen | VEGFR2 | Non-small cell lung cancer Gastric cancer or GE Junction Colorectal cancer |
Hypertension Diarrhea Headache, Hyponatremia Neutropenia Intestinal obstruction Blood clots Epistaxis |
Hypertension • Evaluate risk and maintain blood pressure within normal range. • Treat with angiotensin-converting enzyme (ACE) inhibitors, beta blockers, calcium channel blockers, and diuretics. • Discontinue treatment for severe or uncontrolled hypertension. Hemorrhage • Prior to starting an anti-VEGF, assess for risk factors or any signs of bleeding. Proteinuria • Assess urine protein levels protein levels before each cycle. |
| Rituxin (Rituximab | Genentech | CD20 | Low grade or follicular lymphoma Non-Hodgkin lymphoma Chronic lymphocytic leukemia |
Infusion reactions Infections Myalgias Fatigue Nausea |
• Assess for infusion related AEs • Premedicate with acetaminophen and antihistamine prior to each infusion. • Assess complete blood counts and renal function. |
| Trastuzumab (Herceptin) | Roche | HER-2 | Adjuvant and Metastatic breast cancer Metastatic Gastric cancer |
Fever Nausea/vomiting Infusion reactions Diarrhea Infections Increased cough Headache Fatigue Dyspnea Rash Neutropenia Anemia Myalgia Cardiomyopathy |
• Assess for signs and symptoms of infection. • Assess left ventricular ejection function and monitor for cardiac failure or dysfunction. |
| Trastuzumab Emtansine (Kadcyla) | Genentech | HER-2 | Metastatic breast cancer | Thrombocytopenia Elevated liver enzymes Hypokalemia Myalgia Arthralgia Anemia Neutropenia Fatigue Nausea Cardiomyopathy |
• Assess liver enzymes at baseline and prior to each dose. • Monitor electrolytes and replete as needed. • Monitor for signs and symptoms of infection. • Assess left ventricular function at baseline and during treatment. • Monitor complete blood counts frequently. |
AEs associated with mAbs are specific to the pharmacologic mechanism of action [Table 1], and their management depends on the mechanism of action and the route of administration [Table 1].[10,26,27,28,29] For example, mAb-related AEs can range from a mild headache, diarrhea, transient pruritus, and dermatitis to potentially serious or life-threatening AEs such as anaphylaxis, cardiovascular AEs, thromboembolic AEs, cytokine release syndrome (CRS), hepatitis, pulmonary AEs, hemorrhage, and cytopenias.[30,34] While the mechanism behind some mAbs AEs such as cytopenias is unclear, AEs such as Stevens–Johnson syndrome, urticaria, serum sickness, and anaphylaxis are generally mediated by the immune system.[33] The mechanism behind pulmonary AEs such as interstitial pneumonitis, acute respiratory distress syndrome, hypersensitivity pneumonitis, or bronchiolitis obliterans organizing pneumonia is a result of activation of cytotoxic T-lymphocytes, which leads to alveolar and vascular damage, cytokine release, and likely cross-reaction between lung and tumor antigens.[33] In contrast, cardiac AEs are believed to result from the inhibition of a growth factor (neuregulin 1) which is needed for cardiac development and maintenance.[33] Similarly, AE such as acneiform rash which occurs in 50%–100% of patients receiving cetuximab and panitumumab is a result of the inhibition of epidermal growth factor receptor (EGFR) which initiates the alteration and rupture of the epithelial barrier, which in turn facilitates bacterial access and proliferation.[33] AEs (hypertension, hemorrhage, and thromboembolism) associated with mAbs that target vascular endothelial growth factor (VEGF) and VEGF receptor are a result of the disruption of physiological processes involved in wound healing, blood pressure regulation, coagulation, renal filtration, and vascular homeostasis.[31]
Other frequently reported AEs of mAbs are infusion related and a result of antigen–antibody interactions precipitating cytokine release.[17,30] Infusion-related AEs can occur within 30 min to 2 h after the infusion or 24 h later and are described as pruritus, chills, fever, asthenia, dyspnea, nausea, rash, or headache.[30,34] Severe and potentially fatal infusion-related AEs may occur in 0.3% of patients and present as angioedema, hypotension, bronchospasm, and cardiac arrest.[30,34] Furthermore, the incidence of infusion-related AEs varies among different mAbs. For example, rituximab is 77%, trastuzumab is 40%, cetuximab is 15%–20%, bevacizumab is <3%, and panitumumab is 3%.[33] Management of infusion-related AEs is based on well-established clinical practice guidelines by the European Society for Medical Oncology.[17]
Chimeric antigen receptor T-cells
CAR T-cells are genetically engineered T-cells reprogrammed to produce CARs on the cell membrane.[8,35] Once these cells have been collected from the patient's blood, reprogrammed, and injected back into the patient, tumor-specific recognition occurs, and then, T-cell memory enables the T-cells to proliferate, destroy tumor cells, and conduct surveillance.[36] The FDA has approved two CAR T-cell therapies: (1) axicabtagene ciloleucel (Yescarta: Kite, Santa Monica, CA) for adult patients with diffuse large B-cell lymphoma who have relapsed after two other kinds of treatment and (2) tisagenlecleucel (Kymriah: Novartis, Switzerland) for children and young adults with B-cell acute lymphoblastic leukemia.[37]
A common AE associated with CAR T-cell therapy is CRS, with incidence rates of 43%–100% in adult and pediatric patients with relapsed and refractory acute lymphoblastic leukemia.[18,38,39,40] CSR occurs when T-cells engage with a target antigen, multiply in the body, and release cytokines that cause an inflammatory response.[18] The onset and severity of CRS depend on the type of CAR T-cell therapy and the degree of immune cell activation.[19] Typically, CRS symptoms, if they occur, develop days to weeks after infusion of CAR T-cell therapy.[19] Patients with CRS may experience constitutional symptoms such as fever, rigors, malaise, myalgias, arthralgias, fatigue, nausea, vomiting, and headache, while other patients may develop severe symptoms such as hypotension, tachycardia, capillary leak syndrome, and multiorgan dysfunction.[18,19,38,39,40,41] In addition, patients may experience neurotoxicity, also known as immune effector cell-associated neurotoxicity syndrome, which can occur concurrently with or after CSR, and vary from mild, expressive aphasia to confusion, lethargy, agitation, delirium, difficulty concentrating, seizures, encephalopathy, and infrequently, cerebral edema.[19,42]
In a trial conducted by Schuster et al., in which 28 patients with diffuse large B-cell lymphoma or follicular lymphoma that had relapsed or was refractory to previous treatment were treated with CAR T-cell therapy, CRS occurred in 16 patients and neurotoxicity (ranging from mild cognitive disturbance to global encephalopathy) occurred in 11 patients.[42] In this study, CRS and neurotoxicity were self-limiting in all patients but one, who was given tocilizumab (Actemra: Genentech, South San Francisco, CA, USA), an anti-interleukin (IL)-6 antibody that reversed the symptoms of CRS within a few hours.[42] A similar study of 161 patients (133 patients completed the toxicity assessment) with acute lymphoblastic leukemia, chronic lymphocytic leukemia, and non-Hodgkin's lymphoma treated with CAR T-cell therapy reported that CRS developed in 71% of patients and that neurotoxicity was observed in 40% of patients.[43] In this study, CRS and neurotoxicity were reversible except six patients who died.[43]
Management of CRS depends on the grading as outlined in consensus guidelines established by a CAR T-cell therapy-associated toxicity working group and the American Society for Blood and Marrow Transplant [Table 1].[20,44] Opinions vary on the need for hospitalization; Neelapu et al. recommended hospitalization and close monitoring of patients for a period of 7 days after CAR T-cell infusion, whereas Teachey et al. posited that patients can receive CAR T-cells in the outpatient setting and be admitted to the hospital only if the patient develops a fever.[20,45]
Cytokines
Cytokines, which are small protein molecules naturally produced by the body, regulate the differentiation, migration, activation, and suppression of leukocytes.[13] Of the several different varieties of cytokines, recombinant interferon alpha-2b (IFN-alpha-2b) and IL-2 are the most widely used cytokines in cancer treatment.[21] Recombinant IFN-alpha-2b has been approved for non-Hodgkin's lymphoma, hairy cell leukemia, and melanoma.[46] Recombinant IFN-alpha-2b is associated with flu-like symptoms, such as chills, fever, headache, and myalgia,[10,22] which are generally controlled with nonsteroidal anti-inflammatory drugs.[47] Other potential AEs include anorexia, depression, fatigue, hepatic dysfunction, thyroid dysfunction, autoimmune hemolytic anemia/thrombocytopenia, and immune-mediated nephritic syndrome.[10,48] Patients with grade 2–3 fatigue may require a break from treatment or a dose reduction, and patients with depression may require prophylactic antidepressants.[47] Generally, AEs associated with IFN-alpha-2b tend to reverse rapidly when therapy is discontinued.[10]
IL-2 is a T-cell growth factor that promotes the antitumor activity of natural killer cells, enhances the growth and proliferation of regulatory T-cells (Tregs), and induces lymphokine-activated killer cells that mediate antitumor effects.[13] IL-2 has been approved for the treatment of metastatic melanoma and metastatic renal cell cancer and can be administered intravenously or subcutaneously.[49] Common AEs associated with IL-2 include chills, fatigue, fever, nausea, diarrhea, vomiting, hypotension, transaminitis, dyspnea, oliguria, and hyperbilirubinemia.[10,23] In a retrospective analysis of 243 patients with advanced melanoma who received high-dose IL-2, the following AEs were reported: oliguria (14%–58%), hypotension (14%–39%), and tachycardia (10%–21%).[50] Given that these AEs can be severe or life-threatening, most patients are administered high-dose IL-2 on an inpatient unit with cardiac monitoring at institutions that have healthcare providers who have experience in recognizing and managing these AEs using specific institutional guidelines and standing orders [Table 1].[10,23,24]
Oncolytic viral therapy
Oncolytic viral therapy, which increases a patient's immune response to cancer without harming normal tissue, uses a modified virus that can force tumor cells to self-destruct and release antigens.[13] In 2015, talimogene laherparepvec (T-VEC) (Imlygic: Amgen, Thousand Oaks, CA, USA), a second-generation oncolytic herpes simplex type 1, was engineered to express human GM-CSF, received FDA approval for use in patients with advanced melanoma.[51] The mechanism of action of T-VEC is unknown; however, it is thought that T-VEC uses the herpes virus entry mediator, glycoproteins, and nectins on the cell surface to enter cancer cells and trigger cell lysis.[52] Common AEs associated with T-VEC are fever (42.8%), chills (48.6), fatigue (50.3%), nausea (35.6%), vomiting (21.2%), headache (18.8%), and erythema, pain, and cellulitis (27.7%) at the injection site.[51,52] Management of AEs is mainly supportive; for example, acetaminophen or indomethacin can be given for pain, chills, or fever, and ice bags can be applied to the injection site 5–10 min before T-VEC injection to minimize pain at the injection site [Table 1].[25]
Immune checkpoint inhibitors
Immune checkpoint inhibitors, which enhance the immune system's preexisting antitumor responses, target molecules that switch immune responses on and off.[8] For instance, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) is normally expressed on the surface of naive effector T-cells and Tregs, which inhibit autoimmunity, and promotes tolerance to self-antigens.[53] Similarly, PD-1 is an immune inhibitory receptor which negatively regulates T-cell functions through the engagement of programmed death ligand 1 (PD-L1), which is found on varies malignant cells.[53] Hence, checkpoint inhibitors disrupt the signaling pathways that allow cancer cells to evade T-cell-mediated death by preventing CTLA-4 and PD-1 from binding with specific ligands, thus enhancing the immune system's ability to attack malignant cells.[54] The FDA has approved several checkpoint inhibitors that have shown clinical efficacy in the treatment of a number of cancers [Table 3].[54,55,56,57]
Table 3.
Checkpoint inhibitors
| Checkpoint inhibitors[54,55,56,57,58,59,60,61,64,65] | Company | Target | Indication | Common selected irAEs | Management[58,60,61,64,65] |
|---|---|---|---|---|---|
| Atezolizumab (Tecentriq) | Roche/Genentech Ltd | PD-L1 | Metastatic nonsmall cell lung cancer Advanced urothelial cancer |
Fatigue Diarrhea Fever Myalgias Hepatitis Pruritus Pneumonitis Dermatitis |
Baseline Perform a baseline assessment of thyroid studies, complete blood counts, liver function, and metabolic panels, grade, and document them prior to starting each treatment, and at intervals of 6-12 weeks for the first 6 months after completing treatment Document any co-morbid conditions Evaluate baseline radiological examinations Assess for history of autoimmune disease, which may worsen with starting a checkpoint inhibitor Inform patients and caregivers of potential irAEs before treatment initiation Infusion irAEs Assess for infusion related AEs Interrupt or slow the rate of infusion for grade 1 or 2 For grade 3 or 4, permanently, discontinue the treatment General management Assess for irAEs and manage according to grade, clinical guidelines or algorithms Fatigue irAEs Assess adenocorticotropic hormone, cortisol, and testosterone Assess treatable contributing factors (gastrointestinal, hepatic, and pulmonary irAEs) Assess psychosocial factors. Treatment may include physical activity, psychosocial interventions, mind-body interventions, and pharmacologic interventions Dermatologic irAEs Grade 1- Treat with topical emollients, oral antihistamines, and mild strength topical corticosteroids Grade 2- Topical emollients, oral antihistamines, median to high strength topical steroids Grade 3- Hold treatment, treat with topical emollients, oral antihistamines, high strength topical steroids or systemic corticosteroids depending on the severity of symptoms Grade 4- Hold treatment, hospital admission, dermatologist referral, intravenous methylprednisolone Gastrointestinal irAEs Assess complete blood count, serum electrolyte panel, stool analysis for enteropathogens, and Clostridium difficile Grade 1- Consider holding treatment, hydration, loperamide Grade 2- Hold treatment, intravenous methylprednisolone, consider infliximab if no response to steroids. If refractory to infliximab, consider vedolizumab Grade 3- Discontinue anti-CTLA-4, consider resuming anti-PD1/anti-PD-L1 after symptoms have resolved. Consider Gastrointestinal referral Grade 4- Discontinue treatment permanently, hospitalization Endocrine irAEs Thyroid 1. Check thyroid panel at baseline and prior to each treatment 2. Hormone replacement therapy for symptomatic hypothyroidism or TSH >10 3. Beta-blockers for symptomatic hyperthyroidism Diabetes mellitus Monitor blood glucose levels with each dose Lifestyle and diet modification as needed Endocrine referral if symptomatic or uncontrolled blood glucose levels Hepatic irAEs Evaluate liver function tests prior to starting every cycle of treatment Grade 2- Hold treatment, monitor liver function tests twice weekly If grade 2 lasts longer than 1-2 weeks, check for disease related causes, concomitant drug or alcohol administration, and infectious diseases Treat with corticosteroids Grade 3- Treatment should be permanently discontinued Start corticosteroids If no response to steroids, mycophenolate mofetil should be started Consider hepatologist referral and liver biopsy Pulmonary irAEs Grade 1- Hold treatment; re-evaluate in 1-2 weeks, repeat chest imaging after 3-4 weeks as needed Grade 2- Assess for infection, monitor every 3-7 days, if no improvement in 48-72 hours, start methylprednisolone Grade 3-4- Discontinue treatment permanently, hospital admission, referral to infectious disease Musculoskeletal irAEs Mild- Continue treatment, NSAIDS Moderate- Consider holding treatment, prednisone, consider rheumatology referral if symptoms do not improve Severe- Consider discontinuation of treatment, methylprednisolone and infliximab, and rheumatology referral |
| Avelumab (Bavencio) | Merck | PD-L1 | Metastatic Merkel cell cancer | Fatigue Myalgias Colitis Infusion reaction Dermatitis, Hypothyroidism Hyperthyroidism Hyperglycemia Nephritis Hepatitis |
|
| Durvalumab (Imfinzi) | Astra Zeneca |
PD-L1 | Unresectable stage III non-small cell lung cancer Urothelial cancer |
Fatigue Colitis Fever Myalgias |
|
| Ipilimumab (Yervoy) | Bristol- Myers Squibb Co | CTLA-4 | Melanoma Combined with Nivolumab for treatment of advanced renal cell cancer, and microsatellite instability high or mismatch repair deficient metastatic colorectal cancer |
Fatigue Diarrhea Colitis Pruritus Myalgias Dermatitis Hepatitis | |
| Nivolumab (Opdivo) | Bristol- Myers Squibb Co | PD-1 | Metastatic melanoma Metastatic non-small cell lung cancer Advanced renal cell cancer Metastatic urothelial cancer Classical Hodgkin lymphoma Recurrent/metastatic squamous cell cancer of the head and neck Hepatocellular cancer |
Fatigue Myalgias Dermatitis Diarrhea Hypothyroidism Colitis Hepatitis, Pneumonitis |
|
| Pembrolizumab (Keytruda) | Merck and Co Inc | PD-1 | Advanced nonsmall cell lung cancer Classical Hodgkin lymphoma Advanced gastric cancer Advanced melanoma Microsatellite instability-High cancer Advanced cervical cancer Head and neck squamous cell cancer Advanced urothelial bladder cancer Primary mediastinal B-cell lymphoma |
Fatigue Dermatitis Arthralgias Cough Hyperglycemia Hepatitis Pruritus |
irAEs: Immune-related adverse events, CTLA-4: Cytotoxic T-lymphocyte-associated protein 4, NSAIDS: Nonsteroidal anti-inflammatory drugs, PD-1: Programmed cell death-1, TSH: Thyroid-stimulating hormone
The AEs associated with checkpoint inhibitors are referred to as immune-related AEs (irAEs).[56] These irAEs are secondary to the infiltration of activated T-cells – which are also involved in autoimmunity – into normal tissue.[10] These irAEs can affect any organ or multiple organs simultaneously or at different time points. The areas most commonly affected are skin, gastrointestinal tract, endocrine, lungs, thyroid, pituitary, adrenal glands, and musculoskeletal system and less commonly affected are nervous, renal, hematologic, ocular, and cardiovascular system.[47,56,58,59,60,61] For example, in a retrospective study of 50 patients with nonsmall cell lung cancer, who were treated with an immune checkpoint inhibitor, the most frequent irAEs were fatigue (42%), rash (22%), nausea (20%), and fever (20%).[62] Similarly, a retrospective analysis to assess the safety profile of nivolumab in 576 patients with advanced melanoma found that 71% of patients experienced irAEs, with the most common irAEs being fatigue (25%), pruritus (17%), diarrhea (13%), and rash (13%).[63]
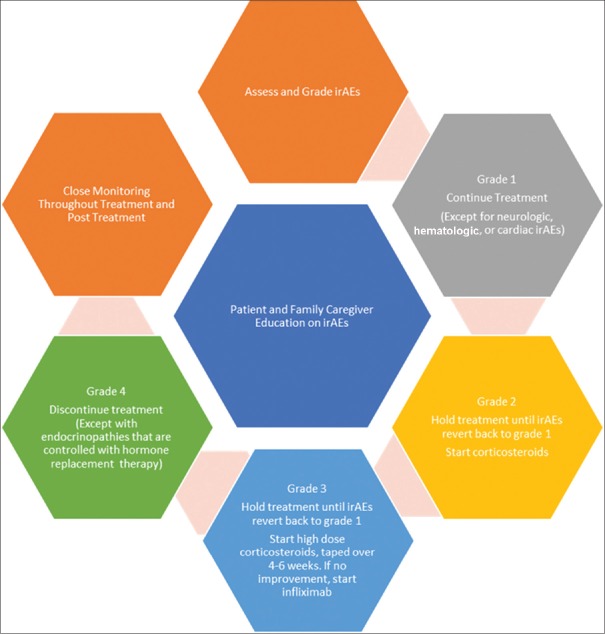
The management of irAEs is based on well-established clinical practice guidelines, such as the National Comprehensive Cancer Network, the American Society of Clinical Oncology, the European Society for Medical Oncology, and the Society for Immunotherapy of Cancer.[58,60,64,65] For patients with grade 1 irAEs that are not cardiac, hematologic, or neurologically related, they continue checkpoint inhibitors with close monitoring. For patients with grade 2 irAEs, the checkpoint inhibitor should be put on hold and corticosteroids may be given; the checkpoint inhibitor may be resumed when the patient's symptoms and/or laboratory values return to grade 1 or less. For patients with grade 3 irAEs, the checkpoint inhibitor should be placed on hold and high-dose corticosteroids should be administered and tapered over 4–6 weeks; if symptoms do not improve within 48–72 h, administer infliximab; however, if symptoms and/or laboratory values return to grade 1 or less, the checkpoint inhibitor may be resumed with caution. For patients with grade 4 irAEs – except for endocrinopathies that are controlled with hormone replacement – the checkpoint inhibitor should be permanently discontinued [Figure 1].[58]
Figure 1.
Clinical steps for assessing and managing immune-related adverse events[58]
Since check inhibitors can cause irAEs to occur in any organ of the body, potentiate autoimmune diseases, or aggravate other comorbid diseases, patients should be thoroughly screened and examined before starting an immune checkpoint inhibitor.[60] Furthermore, patients and caregivers should be educated in early recognition and management of irAEs to minimize serious or life-threatening complications.[66] A complete patient educational guide on irAEs can be accessed at https://www.esmo.org/Patients/Patient-Guides/Patient-Guide-on-Immunotherapy-Side-Effects.
Combination immunotherapy
Although immunotherapy has changed the landscape of cancer treatment, one of the biggest challenges of this type of treatment is that many patients do not benefit from it (i.e. they have primary resistance) and some patients relapsed after a period of response (i.e., they develop acquired resistance).[67] To overcome this challenge, researchers are using strategies such as combining anti-PD-1 or PD-L1 agents with other immunotherapy agents, molecular targeted therapy, vaccines, chemotherapies, radiotherapy, or chemoradiotherapies.[57] As of September 2017, over 1, 105 combination immunotherapy clinical trials were in progress; however, only one checkpoint inhibitor combination, nivolumab (Opdivo) with ipilimumab (Yervoy), has been approved for clinical use.[57]
As checkpoint inhibitors are combined with other immunotherapy agents or other treatment modalities, the likelihood of more severe or newer AEs occurring increases.[66] For example, a systematic review that assessed the clinical, epidemiological, humanistic, and economic burden of gastrointestinal AEs due to combination checkpoint inhibitors in advance melanoma reported that patients who received combination of ipilimumab plus nivolumab experienced more AEs than patients who received monotherapy checkpoint inhibitors.[68] Similarly, an observational study of patients with nonsmall cell lung cancer receiving nivolumab plus an EGFR-tyrosine kinase inhibitor (TKI) reported higher incidents of interstitial pneumonitis for nivolumab in combination with EGFR-TKI versus treatment with either drug alone.[69]
Conclusion
Because of the variability in the mechanism of action among the major categories of oncologic immunotherapy treatments, and because of the heterogeneity of AEs, it is imperative that oncology nurses become familiar with the different AEs so that they can initiate appropriate management and referrals to specialist to improve patient outcomes. Oncology nurses need to be on the forefront of assessing and documenting AEs and the long-term impact on patients, which may lead to a better understanding of why some patients develop AEs and how they can be predicted and alleviated in patients with cancer.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
I would like to thank Laura Russell for the scientific editing of this manuscript.
References
- 1.Mayor S. Immunotherapy improves overall survival in pancreatic cancer. Lancet Oncol. 2015;16:e58. doi: 10.1016/S1470-2045(15)70017-3. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Xiao-Zhong G, Qi XS. Clinical outcomes of specific immunotherapy in advanced pancreatic cancer: A systematic review and meta-analysis. J Immunol Res. 2017;2017:8282391. doi: 10.1155/2017/8282391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Printz C. Immunotherapy drug improves survival of patients with squamous non-small cell lung cancer. Cancer. 2015;121:3562–3. doi: 10.1002/cncr.29700. [DOI] [PubMed] [Google Scholar]
- 4.Gao D, Li C, Xie X, Zhao P, Wei X, Sun W, et al. Autologous tumor lysate-pulsed dendritic cell immunotherapy with cytokine-induced killer cells improves survival in gastric and colorectal cancer patients. PLoS One. 2014;9:e93886. doi: 10.1371/journal.pone.0093886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780–8. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 6.Karbach J, Gnjatic S, Biskamp M, Atmaca A, Weidmann E, Brandt K, et al. Long-term complete remission following radiosurgery and immunotherapy in a melanoma patient with brain metastasis: Immunologic correlates. Cancer Immunol Res. 2014;2:404–9. doi: 10.1158/2326-6066.CIR-13-0200. [DOI] [PubMed] [Google Scholar]
- 7.Weng J, Lai P, Qin L, Lai Y, Jiang Z, Luo C, et al. A novel generation 1928zT2 CAR T cells induce remission in extramedullary relapse of acute lymphoblastic leukemia. J Hematol Oncol. 2018;11:25. doi: 10.1186/s13045-018-0572-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanculeanu DL, Daniela Z, Lazescu A, Bunghez R, Anghel R. Development of new immunotherapy treatments in different cancer types. J Med Life. 2016;9:240–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2015;33:2092–9. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ventola CL. Cancer immunotherapy, part 2: Efficacy, safety, and other clinical considerations. P T. 2017;42:452–63. [PMC free article] [PubMed] [Google Scholar]
- 11.Alatrash G, Jakher H, Stafford PD, Mittendorf EA. Cancer immunotherapies, their safety and toxicity. Expert Opin Drug Saf. 2013;12:631–45. doi: 10.1517/14740338.2013.795944. [DOI] [PubMed] [Google Scholar]
- 12.Conn Y. Recent developments in oncology immunotherapy, implications for NPs part 1. J Nurse Pract. 2018;14:251–8.e1. [Google Scholar]
- 13.Ventola CL. Cancer immunotherapy, part 1: Current strategies and agents. PT. 2017;42:375–83. [PMC free article] [PubMed] [Google Scholar]
- 14.Hu R, George DJ, Zhang T. What is the role of sipuleucel-T in the treatment of patients with advanced prostate cancer? An update on the evidence. Ther Adv Urol. 2016;8:272–8. doi: 10.1177/1756287216645314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graff JN, Chamberlain ED. Sipuleucel-T in the treatment of prostate cancer: An evidence-based review of its place in therapy. Core Evid. 2015;10:1–10. doi: 10.2147/CE.S54712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwok KK, Vincent EC, Gibson JN. 36 - antineoplastic drugs. In: Dowd FJ, Johnson BS, Mariotti AJ, editors. Pharmacology and Therapeutics for Dentistry. 7th ed. St. Louis, Missouri: Mosby; 2017. pp. 530–62. [Google Scholar]
- 17.Roselló S, Blasco I, García Fabregat L, Cervantes A, Jordan K. ESMO Guidelines Committee. Management of infusion reactions to systemic anticancer therapy: ESMO clinical practice guidelines. Ann Oncol. 2017;28:iv100–18. doi: 10.1093/annonc/mdx216. [DOI] [PubMed] [Google Scholar]
- 18.Frey N. Cytokine release syndrome: Who is at risk and how to treat. Best Pract Res Clin Haematol. 2017;30:336–40. doi: 10.1016/j.beha.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–95. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy – Assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waldmann TA. Cytokines in cancer immunotherapy. Cold Spring Harb Perspect Biol. 2018;10 doi: 10.1101/cshperspect.a028472. pii: a028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, Li J, Chen J, Liu ZJ. Effect of adjuvant interferon therapy on hepatitis b/c virus-related hepatocellular carcinoma after curative therapy – Meta-analysis. Adv Clin Exp Med. 2015;24:331–40. doi: 10.17219/acem/29760. [DOI] [PubMed] [Google Scholar]
- 23.Dutcher JP, Schwartzentruber DJ, Kaufman HL, Agarwala SS, Tarhini AA, Lowder JN. High dose interleukin-2 (Aldesleukin) - expert consensus on best management practices-2014. J Immuno Ther Cancer. 2014;2:26. doi: 10.1186/s40425-014-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barber FD. Recent developments in oncology immunotherapy, adverse effects part 2. J Nurse Pract. 2018;14:259–66. [Google Scholar]
- 25.Rehman H, Silk AW, Kane MP, Kaufman HL. Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J Immunother Cancer. 2016;4:53. doi: 10.1186/s40425-016-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarboe J, Gupta A, Saif W. Therapeutic human monoclonal antibodies against cancer. Methods Mol Biol. 2014;1060:61–77. doi: 10.1007/978-1-62703-586-6_4. [DOI] [PubMed] [Google Scholar]
- 27.Singh S, Kumar NK, Dwiwedi P, Charan J, Kaur R, Sidhu P, et al. Monoclonal antibodies: A review. Curr Clin Pharmacol. 2018;13:85–99. doi: 10.2174/1574884712666170809124728. [DOI] [PubMed] [Google Scholar]
- 28.Sherbenou DW, Mark TM, Forsberg P. Monoclonal antibodies in multiple myeloma: A new wave of the future. Clin Lymphoma Myeloma Leuk. 2017;17:545–54. doi: 10.1016/j.clml.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 29.Tobias A, O’brien MP, Agulnik M. Olaratumab for advanced soft tissue sarcoma. Expert Rev Clin Pharmacol. 2017;10:699–705. doi: 10.1080/17512433.2017.1324295. [DOI] [PubMed] [Google Scholar]
- 30.Guan M, Zhou YP, Sun JL, Chen SC. Adverse events of monoclonal antibodies used for cancer therapy. Biomed Res Int. 2015;2015:428169. doi: 10.1155/2015/428169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandes AA, Bartolotti M, Tosoni A, Poggi R, Franceschi E. Practical management of bevacizumab-related toxicities in glioblastoma. Oncologist. 2015;20:166–75. doi: 10.1634/theoncologist.2014-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofheinz RD, Deplanque G, Komatsu Y, Kobayashi Y, Ocvirk J, Racca P, et al. Recommendations for the prophylactic management of skin reactions induced by epidermal growth factor receptor inhibitors in patients with solid tumors. Oncologist. 2016;21:1483–91. doi: 10.1634/theoncologist.2016-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baldo BA. Adverse events to monoclonal antibodies used for cancer therapy: Focus on hypersensitivity responses. Oncoimmunology. 2013;2:e26333. doi: 10.4161/onci.26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gharwan H, Groninger H. Kinase inhibitors and monoclonal antibodies in oncology: Clinical implications. Nat Rev Clin Oncol. 2016;13:209–27. doi: 10.1038/nrclinonc.2015.213. [DOI] [PubMed] [Google Scholar]
- 35.Callahan C, Baniewicz D, Ely B. CAR T-cell therapy: Pediatric patients with relapsed and refractory acute lymphoblastic leukemia. Clin J Oncol Nurs. 2017;21:22–8. doi: 10.1188/17.CJON.S2.22-28. [DOI] [PubMed] [Google Scholar]
- 36.Bayer V, Amaya B, Baniewicz D, Callahan C, Marsh L, McCoy AS, et al. Cancer immunotherapy: An evidence-based overview and implications for practice. Clin J Oncol Nurs. 2017;21:13–21. doi: 10.1188/17.CJON.S2.13-21. [DOI] [PubMed] [Google Scholar]
- 37.Zheng PP, Kros JM, Li J. Approved CAR T cell therapies: Ice bucket challenges on glaring safety risks and long-term impacts. Drug Discov Today. 2018;23:1175–82. doi: 10.1016/j.drudis.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123–38. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics. 2016;3:16011. doi: 10.1038/mto.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–54. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turtle CJ, Hay KA, Gust J, Hanafi L, Li D, Liles WC, et al. Cytokine release syndrome (CRS) and neurotoxicity (NT) after CD19-specific chimeric antigen receptor-(CAR-) modified T cells. J Clin Oncol. 2017;35(15 Suppl):3020. doi: 10.1200/JCO.2017.35.15_suppl.3020. [Google Scholar]
- 44.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASBMT consensus grading for cytokine release syndrome and neurological toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2018 Dec;25 doi: 10.1016/j.bbmt.2018.12.758. pii: S1083-8791(18)31691-4. doi: 10.1016/j. bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 45.Teachey DT, Bishop MR, Maloney DG, Grupp SA. Toxicity management after chimeric antigen receptor T cell therapy: One size does not fit ’ALL’. Nature Rev Clin Oncol. 2018;15:218. doi: 10.1038/nrclinonc.2018.19. [DOI] [PubMed] [Google Scholar]
- 46.Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Pérez-Gracia JL, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120:6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weber JS, Postow M, Lao CD, Schadendorf D. Management of adverse events following treatment with anti-programmed death-1 agents. Oncologist. 2016;21:1230–40. doi: 10.1634/theoncologist.2016-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talpaz M, Hehlmann R, Quintás-Cardama A, Mercer J, Cortes J. Re-emergence of interferon-α in the treatment of chronic myeloid leukemia. Leukemia. 2013;27:803–12. doi: 10.1038/leu.2012.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang T, Zhou C, Ren S. Role of IL-2 in cancer immunotherapy. Oncoimmunology. 2016;5:e1163462. doi: 10.1080/2162402X.2016.1163462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davar D, Ding F, Saul M, Sander C, Tarhini AA, Kirkwood JM, et al. High-dose interleukin-2 (HD IL-2) for advanced melanoma: A single center experience from the university of pittsburgh cancer institute. J Immunother Cancer. 2017;5:74. doi: 10.1186/s40425-017-0279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson DB, Puzanov I, Kelley MC. Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma. Immunotherapy. 2015;7:611–9. doi: 10.2217/imt.15.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corrigan PA, Beaulieu C, Patel RB, Lowe DK. Talimogene laherparepvec: An oncolytic virus therapy for melanoma. Ann Pharmacother. 2017;51:675–81. doi: 10.1177/1060028017702654. [DOI] [PubMed] [Google Scholar]
- 53.Rafique I, Kirkwood JM, Tarhini AA. Immune checkpoint blockade and interferon-α in melanoma. Semin Oncol. 2015;42:436–47. doi: 10.1053/j.seminoncol.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dine J, Gordon R, Shames Y, Kasler MK, Barton-Burke M. Immune checkpoint inhibitors: An innovation in immunotherapy for the treatment and management of patients with cancer. Asia Pac J Oncol Nurs. 2017;4:127–35. doi: 10.4103/apjon.apjon_4_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thallinger C, Füreder T, Preusser M, Heller G, Müllauer L, Höller C, et al. Review of cancer treatment with immune checkpoint inhibitors: Current concepts, expectations, limitations and pitfalls. Wien Klin Wochenschr. 2018;130:85–91. doi: 10.1007/s00508-017-1285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gordon R, Kasler MK, Stasi K, Shames Y, Errante M, Ciccolini K, et al. Checkpoint inhibitors: Common immune-related adverse events and their management. Clin J Oncol Nurs. 2017;21:45–52. doi: 10.1188/17.CJON.S2.45-52. [DOI] [PubMed] [Google Scholar]
- 57.Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol. 2018;29:84–91. doi: 10.1093/annonc/mdx755. [DOI] [PubMed] [Google Scholar]
- 58.Brahmer JR, Lacchetti C, Thompson JA. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline summary. J Oncol Pract. 2018;14:247–9. doi: 10.1200/JOP.18.00005. [DOI] [PubMed] [Google Scholar]
- 59.Nagai H, Muto M. Optimal management of immune-related adverse events resulting from treatment with immune checkpoint inhibitors: A review and update. Int J Clin Oncol. 2018;23:410–20. doi: 10.1007/s10147-018-1259-6. [DOI] [PubMed] [Google Scholar]
- 60.Puzanov I, Diab A, Abdallah K, Bingham CO, 3rd, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the society for immunotherapy of cancer (SITC) toxicity management working group. J Immunother Cancer. 2017;5:95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015:76–83. doi: 10.14694/EdBook_AM.2015.35.76. doi: 10.14694/EdBook_AM.35.76. [DOI] [PubMed] [Google Scholar]
- 62.Hsu JC, Lin JY, Hsu MY, Lin PC. Effectiveness and safety of immune checkpoint inhibitors: A retrospective study in Taiwan. PLoS One. 2018;13:e0202725. doi: 10.1371/journal.pone.0202725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785–92. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 64.Thompson JA. New NCCN guidelines: Recognition and management of immunotherapy-related toxicity. J Natl Compr Canc Netw. 2018;16:594–6. doi: 10.6004/jnccn.2018.0047. [DOI] [PubMed] [Google Scholar]
- 65.Haanen JB, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–42. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 66.Pennock GK, Chow LQ. The evolving role of immune checkpoint inhibitors in cancer treatment. Oncologist. 2015;20:812–22. doi: 10.1634/theoncologist.2014-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–23. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mearns ES, Bell JA, Galaznik A, Puglielli SM, Cichewicz AB, Boulanger T, et al. Gastrointestinal adverse events with combination of checkpoint inhibitors in advanced melanoma: A systematic review. Melanoma Manag. 2018;5:MMT01. doi: 10.2217/mmt-2017-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oshima Y, Tanimoto T, Yuji K, Tojo A. EGFR-TKI-associated interstitial pneumonitis in nivolumab-treated patients with non-small cell lung cancer. JAMA Oncol. 2018;4:1112–5. doi: 10.1001/jamaoncol.2017.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]