Abstract
Cachexia is an old disease but a new research area that has recently been vigorously investigated. The diagnostic and staging criteria for cancer-associated cachexia have been established through an international consensus report (CR) published in 2011, which may greatly influence the designs and interventions of future clinical trials. However, no standard treatment has been established so far. This may be partially due to the lack of a widely accepted common endpoint for clinical trials. This review aimed to summarize designs and endpoints of 65 randomized controlled trials for cancer cachexia in the past 16 years and seek clinically relevant patient-centered outcomes for future clinical trials. Compared with trials before the CR, trials after the report revealed that the study populations tended to be at the earlier stage of cachexia and included patients with precachexia or those at risk for cachexia. Nonpharmacological interventions have been widely tested, and functional endpoints have increasingly been selected in combination with standard endpoints of body mass or lean body mass. Disability-free survival has recently been used as a functional endpoint in clinical trials in several research fields. It might be also a suitable patient-centered outcome responsive to multiple physical changes in cancer cachexia, and patients might find it more acceptable than other classical endpoints. More efforts would be needed to identify an optimal measurable endpoint and establish a better combination of pharmacological and nonpharmacological interventions to improve the functional prognosis for patients with cancer cachexia.
Keywords: Cancer cachexia, disability, endpoint, functional prognosis, patient-centered outcome

Introduction
Wasting conditions associated with various inflammatory diseases have been known since ancient time. In Greece, Hippocrates precisely described the core pathogenesis of cachexia, wherein “the flesh is consumed and becomes water” and considered cachexia as a sign of death.[1] In ancient China, a similar wasting condition, limpness, was described in an old textbook and reported to be induced by chronic inflammatory diseases in various organs.[2] According to the account, the patient's muscles in the trunk and lower limbs had atrophy, which resulted in difficulties in standing up or walking. The unfavorable impact of cachexia on functional prognosis has been more emphasized than that on survival prognosis. In the Middle East, Avicenna, a medieval Arab scholar, also stated the importance of avoiding a cachectic condition for the maintenance of healthy and active life, especially in the elderly population.[3] Despite this long history of cachexia in humans, there is limited understanding of such condition. This may be partial because cachexia is a functional disorder and its primary cause is macroscopically invisible.
Based on recent advances in medicine, the pathophysiology of cachexia is known to involve multiple organs including skeletal muscles, adipose tissues, and the digestive, immune, or central nervous system.[4] However, this may only be the initial step in identifying the core mechanism shared by many pro-cachectic diseases, including pulmonary, cardiac, malignant, rheumatic, and renal disease.[5] With regard to cancer-associated cachexia, it was a pivotal step to achieve consensus about specific diagnostic and staging criteria.[6] Through that consensus report (CR), cancer cachexia can be easily diagnosed using a few anthropometric measurements and quick interview. Furthermore, the CR has taught that cachexia could develop not only in the terminal phase but also in the very early phase of the cancer trajectory. Cachexia can insidiously start and progress immediately after or even before the diagnosis of cancer. In addition, the CR proposed the development of a multimodal intervention by combining nutritional, physical, and psychosocial interventions. These framework and future direction of care possibly can greatly impact the designs and interventions of future clinical trials. However, the ultimate goal of cancer cachexia care and clinical trials remains to be elucidated. There are discrepancies in recognition of clinically relevant outcomes among researchers, pharmaceutical companies, and regulatory authorities. One of the newly developed ghrelin receptor agonists, anamorelin, has constantly been associated with a significant increase in lean body mass, weight, and appetite among patients with advanced nonsmall cell lung cancer (NSCLC) in two large-scale randomized phase III studies.[7] However, the drug was refused for marketing authorization by the European Medicines Agency (EMA) because of the potential risks outweigh the benefits.[8] The EMA concluded that the efficacy of anamorelin was not established because there was only a marginal effect on lean body mass and no reliable and clinically relevant effect on patient functioning or quality of life (QOL).
Therefore, there is an urgent need to reconsider the clinically relevant endpoints of clinical trials for cancer cachexia that simultaneously meet the demands from patients, researchers, and regulatory authorities. This review aimed to explore changes in designing clinical trials after the publication of the CR and discuss optimal patient-centered outcomes in future clinical trials for cancer cachexia.
Methods
Randomized controlled trials (RCTs) were identified by searching the PubMed using the following keywords: (cachexia [tiab] OR cachectic [tiab] OR malnutrition [Mesh] OR malnutrition [tiab] OR “muscle wasting” [tiab] OR “muscular wasting” [tiab] OR “muscle weakness”[Mesh] OR “muscle weakness” [tiab] OR “muscular weakness” [tiab] OR sarcopenia [tiab] OR “wasting syndrome” [MeSH: noexp] OR “wasting syndrome” [tiab] OR “weight loss” [tiab]) AND (neoplasms [MeSH] OR cancer [tiab] OR tumor [tiab] OR tumour [tiab] OR neoplas [tiab] OR malignan [tiab] OR carcinoma [tiab] OR adenocarcinoma [tiab] OR choricarcinoma [tiab] OR leukemia [tiab] OR leukaemia [tiab] OR metastat [tiab] OR sarcoma [tiab] OR teratoma [tiab]). The prespecified inclusion criteria were articles in the English language, articles published between January 2003 and September 2018, studies involving adults, and RCTs. Studies on hematologic malignancies, surgically operable cancers, cancer survivors, or noncancer populations were excluded. RCTs published within 8 years before the CR (2003–2010) were classified as the pre-CR group, whereas those published within 8 years after the CR (2011–2018) were classified as the post-CR group. Entry criteria, the cachectic status of participants, concurrent treatments, and type of intervention were compared between the groups. Data on primary, secondary, and exploratory endpoints of each study were collected and compared between the groups.
Time-to-event curves were generated using the Kaplan–Meier method. Time-to-event was calculated as the time from the entry of the study to the date of the event, or last visit of the patients for whom the date of the event could not be confirmed. The database of a previous prospective observational study that recruited 60 elderly patients with newly diagnosed locally advanced or metastatic NSCLC who were to start chemotherapy and/or radiotherapy (trial registration no. UMIN000009768,[9]) was used, in which the physical parameters of the patients such as weight, shuttle walking distance, and handgrip strength were regularly measured without any nutritional or exercise interventions. The event of cancer cachexia was defined as having ≥5% loss in the body weight within 6 months before participating in the study. The event of walking disturbance was defined as having a ≥10% decline in the incremental shuttle walking distance from the baseline value. The event of muscle weakness was defined as having ≥10% decline in handgrip strength from the baseline value. A disabling event was defined as having ≥10 points decline in the Barthel index from the baseline value.
Results
Overview of randomized controlled trials for cancer cachexia
A total of 65 RCT articles published between January 2003 and August 2018 were identified in PubMed; the reference list is shown in Supplementary Table 1. Twenty-one trials comprised the pre-CR group, and 44 trials comprised the post-CR group [Table 1]. According to the definitions in the CR,[6] 46 (71%) studies were designed to include patients with cancer cachexia. Among these studies, 12 (18%) potentially included patients with precachexia. Patients with refractory cachexia were excluded in most of the studies, while performance status or expected life expectancy was used as exclusion criteria. Approximately 19 (29%) studies recruited patients at risk for cachexia regardless of the presence of anorexia or weight loss. These were patients who underwent radiotherapy with or without chemotherapy for treatment of locally advanced head and neck, thoracic, or gynecological cancer, or patients with metastatic cancers who received palliative chemotherapy or care. In the post-CR group, the number of studies for patients with cachexia decreased, while that for patients with precachexia or patients at risk for cachexia increased.
Supplementary Table 1.
Reference list of selected publications of randomized controlled trials
| No. | Randomized controlled trial published during 8 years after the consensus report (from 2011 to 2018) | ||
|---|---|---|---|
| Publications | Cachectic status (sample size) | Study populations and interventions (1: cancer type, 2: intervention, 3: concurrent cancer treatment) | |
| 1. | Kouchaki B, et.al. Support Care Cancer. 2018; 26 (7):2479-2489. | Cachexia (90) | Gastrointestinal cancer Megestrol acetate + celecoxib vs megestrol acetate alone Not specified or combined |
| 2. | Jatoi A, et.al. Ann Oncol. 2017; 28 (8):1957-1963. | Cachexia (263) | Mixed cancer Creatine vs placebo Not specified or combined |
| 3. | Currow D, et.al. Ann Oncol. 2017; 28 (8):1949-1956. | Cachexia (513) | Non-small-cell lung cancer Anamorelin vs placebo Not specified or combined |
| 4. | Werner K, et.al. Lipids Health Dis. 2017; 16 (1):104. | Cachexia (60) | Pancreatic cancer marine phospholipids vs fish oil Not specified or combined |
| 5. | Kapoor N, et.al. Integr Cancer Ther. 2017; 16 (1):74-84. | Cachexia (63) | Adult female cancer Improved Atta + usual care vs usual care Palliative care alone |
| 6. | Leedo E, et.al. Nutr Cancer. 2017; 69 (3):444-453. | Cachexia (40) | Lung cancer Home Delivery Meal Service of Energy- and Protein-Rich Meals vs usual care Not specified or combined |
| 7. | Lin JX, et.al. Medicine (Baltimore). 2017; 96 (26):e7373. | Cachexia (110) | Colorectal cancer multidisciplinary team approach for nutritional interventions vs usual carer Palliative chemotherapy |
| 8. | Takayama K, et.al. Support Care Cancer. 2016; 24 (8):3495-505. | Cachexia (181) | Non-small-cell lung cancer Anamorelin vs placebo Palliative chemotherapy |
| 9. | Temel JS, et.al. Lancet Oncol. 2016; 17 (4):519-531. | Cachexia (484) | Non-small-cell lung cancerr Anamorelin vs placebor Not specified or combined |
| 10. | Jatoi A, et.al. Support Care Cancer. 2016; 24 (9):3739-46. | Cachexia (141) | Mixed cancer white wine vs ONS Not specified or combined |
| 11. | Focan C, et.al. Anticancer Res. 2015; 35 (11):6311-5. | Cachexia (53) | Mixed cancerr Mindfulness alternating dietetic and psychological approaches vs usual carer Not specified or combined |
| 12. | De Waele E, et.al. Appetite. 2015; 91:298-301. | Cachexia (20) | Mixed cancerr Tight Caloric Control (TiCaCo) vs usual carer Not specified or combined |
| 13. | Kanat O, et.al. Tumori. 2013; 99 (2):229-33. | Cachexia (62) | Mixed cancerr 1) MA plus meloxicam; 2) MA plus meloxicam plus oral EPA-enriched nutritional supplement; or 3) meloxicam plus oral EPA-enriched nutritional supplementr Not specified or combined |
| 14. | Del Fabbro E, et.al. J Clin Oncol. 2013; 31 (10):1271-6. | Cachexia (48) | Lung or gastrointestinal cancerr Melatonin vs placebor Not specified or combined |
| 15. | Garcia JM, et.al. Support Care Cancer. 2013; 21 (1):129-37. | Cachexia (16) | Mixed cancerr Anamorelin vs placebor Not specified or combined |
| 16. | Yeh KY, et.al. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013; 116 (1):41-8. | Cachexia (68) | Head and neck cancerr Omega-3 fatty acid-, micronutrient-, and probiotic-enriched nutrition vs usual carer Not specified or combined |
| 17. | Uster A, et.al. Nutrition. 2013; 29 (11-12):1342-9. | Cachexia (58) | Mixed cancerr Nutritional counseling vs usual carer Not specified or combined |
| 18. | Wen HS, et.al. Chemotherapy. 2012; 58 (6):461-7. | Cachexia (102) | Mixed cancerr MA+thalidomide vs MAr Not specified or combined |
| 19. | Yennurajalingam S, et.al. J Palliat Med. 2012; 15 (10):1059-64. | Cachexia (31) | Mixed cancerr Thalidomide vs placebor Palliative care alone |
| 20. | Madeddu C, et.al. Clin Nutr. 2012; 31 (2):176-82. | Cachexia (60) | Mixed cancerr carnitine+celecoxib±MAr Not specified or combined |
| 21. | Macciò A, et.al. Gynecol Oncol. 2012; 124 (3):417-25. | Cachexia (104) | Gynecological tumorsr MA+L-carnitine, celecoxib, and antioxidants vs MA aloner Not specified or combined |
| 22. | Turcott JG, et.al. Support Care Cancer. 2018; 26 (9):3029-3038. | Pre-cachexia and cachexia (47) | Non-small-cell lung cancerr Nabilone vs placebor Palliative chemotherapy |
| 23. | Wright TJ, et.al. J Cachexia Sarcopenia Muscle. 2018; 9 (3):482-496. | Pre-cachexia and cachexia (28) | Mixed cancerr Testosterone or placebor Not specified or combined |
| 24. | Ziętarska M, et.al. Nutrients. 2017; 9 (10). | Pre-cachexia and cachexia (114) | Colorectal cancerr high protein ONS vs usual carer Palliative chemotherapy |
| 25. | Jeon JH, et.al. Integr Cancer Ther. 2017; 16 (1):118-125. | Pre-cachexia and cachexia (16) | metastatic cancerr true vs sham moxibustionr Palliative care alone |
| 26. | Sukaraphat N, et.al. J Med Assoc Thai. 2016; 99 (12):1283-90. | Pre-cachexia and cachexia (50) | locally advanced unresectable or metastatic cancerr dietary counseling vs usual carer Palliative chemotherapy |
| 27. | Cong MH, et.al. Chin Med J (Engl). 2015; 128 (22):3003-7. | Pre-cachexia and cachexia (50) | Esophageal cancerr Interdisciplinary nutrition support vs usual carer Radiotherapy±chemotherapy |
| 28. | Dobs AS, et.al. Lancet Oncol. 2013; 14 (4):335-45. | Pre-cachexia and cachexia (159) | Mixed cancerr Enobosarm vs placebor Palliative chemotherapy |
| 29. | Finocchiaro C, et.al. Br J Nutr. 2012; 108 (2):327-33. | Pre-cachexia and cachexia (33) | Non-small-cell lung cancerr n-3 fatty acids (EPA+DHA) vs placebor Palliative chemotherapy |
| 30. | Baldwin C, et.al. J Hum Nutr Diet. 2011; 24 (5):431-40. | Pre-cachexia and cachexia (358) | Mixed cancerr dietary advice, a nutritional supplement or dietary advice plus supplement vs usual carer Palliative chemotherapy |
| 31. | Golan T, et.al. J Cachexia Sarcopenia Muscle. 2018 Jul [ePub] | High risk for cachexia (125) | Pancreatic cancerr Antimyostatin antibody (LY2495655) vs placebor Palliative chemotherapy |
| 32. | Solís-Martínez O, et.al. Nutr Cancer. 2018;70 (4):663-670. | High risk for cachexia (32) | Head and neck cancerr High-protein ONS with EPA vs ONS without EPAr Not specified or combined |
| 33. | Cereda E, et.al. Radiother Oncol. 2018; 126 (1):81-88. | High risk for cachexia (159) | Head and neck cancerr ONS+Nutritional counseling vs Nutritional counselingr Radiotherapy±chemotherapy |
| 34. | Solheim TS, et.al. J Cachexia Sarcopenia Muscle. 2017; 8 (5):778-788. | High risk for cachexia (46) | Lung or pancreatic cancer (Pre-MENAC study)r Multimodal intervention vs usual carer Palliative chemotherapy |
| 35. | Zdenkowski N, et.al. Support Care Cancer. 2017; 25 (6):1963-1971. | High risk for cachexia (80) | Pancreatic cancer (PICNIC trial)r Pancreatic extract vs placebo.r Not specified or combined |
| 36. | Sandmael JA, et.al. Cancer. 2017; 123 (22):4440-4448. | High risk for cachexia (41) | Head and neck cancerr Resistance training+ONS during vs after radiotherapyr Radiotherapy±chemotherapy |
| 37. | Hajdú SF, et.al. Acta Oncol. 2017; 56 (2):354-359. | High risk for cachexia (69) | Head and neck cancerr Swallowing therapy and progressive resistance training vs usual carer Radiotherapy±chemotherapy |
| 38. | Roussel LM, et.al. Eur Arch Otorhinolaryngol. 2017; 274 (2):977-987. | High risk for cachexia (87) | Head and neck cancerr Intensive nutritional care vs usual carer Radiotherapy±chemotherapy |
| 39. | Ishikawa T, et.al. Oncol Rep. 2016; 36 (2):1093-100. | High risk for cachexia (33) | Esophageal cancerr Amino acid-rich elemental diet Elental® vs controlr Radiotherapy±chemotherapy |
| 40. | Kiss N, et.al. Nutr Cancer. 2016; 68 (6):958-67. | High risk for cachexia (24) | Lung cancerr Early and Intensive Dietary Counseling vs usual carer Radiotherapy±chemotherapy |
| 41. | Poulsen GM, et.al. Clin Nutr. 2014; 33 (5):749-53. | High risk for cachexia (61) | Mixed cancerr Nutritional counseling vs usual carer Not specified or combined |
| 42. | Lønbro S, et.al. Radiother Oncol. 2013; 108 (2):314-9. | High risk for cachexia (41) | Head and neck cancerr Early vs delayed progressive resistance trainingr Radiotherapy±chemotherapy |
| 43. | Silander E, et.al. Eur J Clin Nutr. 2013; 67 (1):47-52. | High risk for cachexia (134) | Head and neck cancerr Prophylactic percutaneous endoscopic gastrostomy vs usual carer Radiotherapy±chemotherapy±surgery |
| 44. | Kraft M, et.al. Nutr J. 2012; 11:52. | High risk for cachexia (72) | Pancreatic cancer (CARPAN study)r L-Carnitine vs placebor Not specified or combined |
| Randomized controlled trial published during 8 years before the consensus report (from 2003 to 2010) | |||
| Publications | Cachectic potential (sample size) | Study populations and interventions (1: cancer type, 2: intervention, 3: concurrent cancer treatment) | |
| 45. | Mantovani G. Oncologist. 2010;15 (2):200-11. | Cachexia (332) | Mixed cancerr MA vs EPA vs Carnitine vs Thalidomide vs Combinationr Not specified or combined |
| 46. | Hasenberg T, et.al. Colorectal Dis. 2010; 12 (10 Online):e190-9. | Cachexia (82) | Colorectal cancerr Early parenteral nutrition vs usual carer Palliative chemotherapy |
| 47. | Wiedenmann B, et.al. J Support Oncol. 2008; 6 (1):18-25. | Cachexia (89) | Pancreatic cancerr Infliximab vs usual carer Palliative chemotherapy |
| 48. | Strasser F, et.al. Br J Cancer. 2008; 98 (2):300-8. | Cachexia (21 with crossover) | Mixed cancerr Ghrelin (iv) vs placebor Palliative care alone |
| 49. | Jatoi A, et.al. Cancer. 2007; 110 (6):1396-403. | Cachexia (63) | Mixed cancerr Etanercept vs placebor Not specified or combined |
| 50. | Lai V, et.al. Head Neck. 2008; 30 (1):67-74. | Cachexia (11) | head and neck or gastrointestinal cancerr Celecoxib vs placebor Palliative care alone |
| 51. | Fearon KC, et.al. J Clin Oncol. 2006; 24 (21):3401-7. | Cachexia (580) | Gastrointestinal or lung cancerr EPA 2g vs EPA 4g vs placebor Palliative care alone |
| 52. | Cannabis-In-Cachexia-Study-Group, Strasser F, et.al. Clin Oncol. 2006; 24 (21):3394-400. | Cachexia (243) | Mixed cancerr Cannabis extract vs delta-9-tetrahydrocannabinol vs placebor Not specified or combined |
| 53. | Gordon JN, et.al. Gut. 2005; 54 (4):540-5. | Cachexia (50) | Pancreatic cancerr Thalidomide vs placebor Palliative care alone |
| 54. | Persson C, et.al. Nutrition. 2005; 21 (2):170-8. | Cachexia (24) | Gastrointestinal cancerr Fish oil vs melatoninr Not specified or combined |
| 55. | Jatoi A, et.al. J Clin Oncol. 2004; 22 (12):2469-76. | Cachexia (421) | Mixed cancerr EPA vs MA vs EPA+MA Not specified or combined |
| 56. | Fearon KC, et.al. Gut. 2003; 52 (10):1479-86. | Cachexia (200) | Pancreatic cancerr protein and energy dense supplement enriched with n-3 fatty acids and antioxidants vs control supplementr Palliative care alone |
| 57. | Bruera E, et.al. J Clin Oncol. 2003; 21 (1):129-34. | Cachexia (60) | Mixed cancerr Fish oil vs placebor Not specified or combined |
| 58. | Berk L, et.al. Support Care Cancer. 2008; 16 (10):1179-88. | Pre-cachexia and cachexia (472) | Mixed cancer beta-hydroxyl beta-methyl butyrate, glutamine, and arginine mixture vs isocaloric control mixture Not specified or combined |
| 59. | Lundholm K, et.al. Clin Cancer Res. 2007; 13 (9):2699-706. | Pre-cachexia and cachexia (138) | Mixed cancer Palliative support (indomethacin, recombinant erythropoietin, nutritional care+home parenteral nutrition) vs palliative support+insulin Not specified or combined |
| 60. | Lundholm K, et.al. Cancer. 2004; 100 (9):1967-77. | Pre-cachexia and cachexia (309) | Mixed cancer Nutritional support + indomethacin + erythropoietin vs indomethacin + erythropoietin Palliative care alone |
| 61. | Hopkinson JB, et.al. J Pain Symptom Manage. 2010; 40 (5):684-95. | High risk for cachexia (50) | Mixed cancer Psychosocial intervention for weight- and eating-related distress (the Macmillan Approach to Weight and Eating, MAWE) vs usual care Not specified or combined |
| 62. | Jatoi A, et.al. Lung Cancer. 2010; 68 (2):234-9. | High risk for cachexia (61) | Non-small-cell lung cancer Infliximab vs placebo Palliative chemotherapy |
| 63. | Maddocks M, et.al. J Pain Symptom Manage. 2009; 38 (6):950-6. | High risk for cachexia (16) | Non-small-cell lung cancer neuromuscular electrical stimulation vs usual care Not specified or combined |
| 64. | Rabinovitch R, et.al. Head Neck. 2006; 28 (4):287-96. | High risk for cachexia (1073) | Head and neck cancer Nutritional support before vs during vs after radiotherapy Radiotherapy±chemotherapy |
| 65. | Isenring EA, et.al. Br J Cancer. 2004; 91 (3):447-52. | High risk for cachexia (60) | Gastrointestinal or head and neck cancer Early and intensive nutritional support vs usual care Radiotherapy±chemotherapy |
Table 1.
Characteristics of 65 randomized controlled trials for patients with cachexia before and after the international consensus report on cancer cachexia
| Characteristics of study population | Total | Pre-CR group | Post-CR group |
|---|---|---|---|
| Publication year | 2003-2018 | 2003-2010 | 2011-2018 |
| Number of studies* | 65 | 21 | 44 |
| Cachectic status, n (%)† | |||
| Cachexia | 34 (52) | 13 (62) | 21 (48) |
| Precachexia or cachexia | 12 (18) | 3 (14) | 9 (20) |
| High risk for cachexia‡ | 19 (29) | 5 (24) | 14 (32) |
| Cancer type, n (%) | |||
| Lung | 9 (14) | 2 (10) | 7 (16) |
| Head and neck | 9 (14) | 1 (5) | 8 (18) |
| Pancreatic | 7 (11) | 3 (14) | 4 (9) |
| Colorectal | 3 (5) | 1 (5) | 2 (5) |
| Esophageal | 2 (3) | 0 | 2 (5) |
| Mixed | 35 (54) | 14 (67) | 21 (48) |
| Concurrent treatment, n (%) | |||
| Palliative care | 9 (14) | 6 (29) | 3 (8) |
| Palliative chemotherapy | 13 (20) | 3 (14) | 10 (23) |
| Radiotherapy ± chemotherapy | 10 (15) | 1 (5) | 9 (23) |
| Combined or not specified | 33 (51) | 11 (52) | 22 (50) |
| Type of intervention, n (%) | |||
| Pharmacological | 37 (57) | 15 (71) | 22 (50) |
| ω3-PUFAs or fish oil | 7 (11) | 3 (14) | 4 (9) |
| Ghrelin or Ghrelin analogue | 5 (8) | 1 (5) | 4 (9) |
| Anti-TNF | 3 (5) | 3 (14) | 0 |
| Thalidomide | 2 (3) | 1 (5) | 1 (2) |
| Melatonin | 2 (3) | 1 (5) | 1 (2) |
| SARMs | 2 (3) | 0 | 2 (5) |
| Cannabinoids | 2 (3) | 1 (5) | 1 (2) |
| Anti-myostatin | 1 (2) | 0 | 1 (2) |
| NSAIDs | 1 (2) | 1 (5) | 0 |
| Combination | 9 (14) | 4 (19) | 5 (11) |
| Others | 3 (5) | 0 | 3 (7) |
| Nonpharmacological | 28 (43) | 6 (29) | 22 (50) |
| Nutritional intervention¦ | 21 (32) | 4 (19) | 17 (39) |
| Exercise intervention | 3 (5) | 1 (5) | 2 (5) |
| Psychosocial intervention | 2 (3) | 1 (5) | 1 (2) |
| Combined interventions | 1 (2) | 0 | 1 (2) |
| Others | 1 (2) | 0 | 1 (2) |
*Studies for patients with nonsolid tumor, hormone-sensitive tumor, pediatric cancer, or indication for curative surgery were excluded (see Supplement Table 1 for complete references). †Assessed according to the consensus report, ‡Studies that did not require weight loss or presence of anorexia and included patients with solid tumor with high cachectic potential, such as those who received palliative chemotherapy or cervical, thoracic, or abdominal radiotherapy with or without chemotherapy, §Nutritional counseling, oral nutritional supplements, and/or artificial nutrition. CR: Consensus report for cancer cachexia published in 2011, PUFAs: Polyunsaturated fatty acids, TNF: Tumor necrosis factor, SARMs: Selective androgen receptor modulators, NSAIDs: Nonsteroidal anti-inflammatory drugs
With regard to cancer types and concurrent treatment modalities, most studies included a mixed population. After the CR, the proportion of studies for patients with specific cancer types receiving active cancer treatment was increasing. The major concurrent cancer treatments included palliative chemotherapy (20%) and radiotherapy with or without chemotherapy (15%). A total of 37 (57%) pharmacological interventions were tested, including single or combined use of omega-3 fatty acids, megestrol acetate, thalidomide, L-carnitine, anti-cytokines, nonsteroidal anti-inflammatory drugs, cannabinoids, ghrelin or its analogs, selective androgen receptor modulators, and others. Nonpharmacological interventions were tested in 28 (43%) studies, mostly in the post-CR group. Among these interventions, nutritional counseling with or without the use of oral nutritional supplements was the most common, and the proportion of studies involving such had doubled after the CR.
Common endpoints in randomized controlled trials for cancer cachexia
Endpoints tested in the listed studies are summarized in Table 2. They were classified into eight components as follows: body mass, nutritional status, physical function, symptoms, QOL, prognosis, use of medical resources, tolerance to cancer treatment, and biomarkers. The three most commonly assessed endpoints were body weight or body mass index (49 studies, 75%), global QOL (43 studies, 66%), and lean body mass (31 studies, 48%). Lean body or skeletal muscle mass was measured using dual-energy X-ray absorptiometry, bioelectrical impedance analysis, or computed tomography. The major questionnaires used for the assessment of global QOL were the European Organisation for Research and Treatment of Cancer QOL Questionnaire-Core 30 (40 studies) and the Functional Assessment of Anorexia/Cachexia Therapy (14 studies). Nutritional status was assessed in 23 (35%) studies, and the major endpoints included the amount of food intake and nutritional assessment tools such as the Patient-Generated Subjective Global Assessment. Physical function was assessed in 21 (32%) studies, and the major endpoints included performance status and handgrip strength. Recently, assessments for walking capacity have been increasingly adopted as endpoints, including field walking tests, performance tests for the lower limbs, and physical activity measured by pedometers/accelerometers.
Table 2.
Classification of endpoints in randomized controlled trials for patients with cachexia
| Endpoints | Total | Pre-CR group | Post-CR group |
|---|---|---|---|
| Publication year | 2003-2018 | 2003-2010 | 2011-2018 |
| Number of studies | 65 | 21 | 44 |
| Body mass, n (%) | |||
| Body weight or BMI | 49 (75) | 15 (71) | 34 (77) |
| Lean body massa | 31 (48) | 12 (57) | 19 (43) |
| Anthropometricsb | 6 (9) | 4 (19) | 2 (5) |
| Nutritional status, n (%) | |||
| Food intake | 23 (35) | 8 (38) | 15 (34) |
| Resting energy expenditurec | 9 (14) | 5 (24) | 4 (9) |
| Assessment toold | 8 (12) | 1 (5) | 7 (16) |
| Physical function, n (%) | |||
| Performance statuse | 19 (29) | 8 (38) | 11 (25) |
| Hand-grip strength | 15 (23) | 2 (10) | 13 (30) |
| Physical activityf | 7 (11) | 3 (14) | 4 (9) |
| Field walking testsg | 6 (9) | 2 (10) | 4 (9) |
| Performance testsh | 5 (8) | 0 | 5 (11) |
| Lower limb strengthi | 3 (5) | 1 (5) | 2 (5) |
| Cardiopulmonary exercise test | 2 (3) | 2 (10) | 0 |
| Symptoms, n (%) | |||
| Anorexiaj | 24 (37) | 11 (52) | 13 (30) |
| Fatiguek | 17 (26) | 4 (19) | 13 (30) |
| Psychosociall | 4 (6) | 1 (5) | 3 (7) |
| QOL, n (%) | |||
| Global scalem | 43 (66) | 16 (76) | 27 (61) |
| Specific modulen | 9 (14) | 0 | 9 (20) |
| Prognosis, n (%) | |||
| Overall survival | 21 (32) | 9 (43) | 12 (27) |
| Progression-free survival | 3 (5) | 1 (5) | 2 (5) |
| Use of medical resources, n (%) | |||
| Length of hospital stay | 3 (5) | 0 | 3 (7) |
| Medical cost | 1 (2) | 0 | 1 (2) |
| Cancer treatment, n (%) | |||
| Toxicity | 7 (11) | 3 (14) | 4 (9) |
| Treatment delivery | 5 (8) | 1 (5) | 4 (9) |
| Treatment efficacy | 4 (6) | 2 (10) | 2 (5) |
| Biomarkers, n (%) | |||
| Inflammatoryo | 25 (38) | 10 (48) | 15 (34) |
| Nutritionalp | 21 (32) | 6 (29) | 15 (34) |
| Metabolicq | 13 (20) | 5 (24) | 8 (18) |
| Endocrinologicalr | 5 (8) | 1 (5) | 4 (9) |
aLean body mass, fat-free mass, or lumbar skeletal muscle mass measured by dual-energy X-ray absorptiometry, bioelectrical impedance analysis, or computed tomography, bArm muscle area or triceps skinfold thickness, cMeasured or estimated by calorimetry or an accelerometer, dAssessed by PG-SGA or NRS-2002, eKarnofsky or Eastern Cooperative Oncology Group performance status, fMeasured by a pedometer/accelerometer or questionnaire, gAssessed by the 6-min walk test or shuttle walking test, hAssessed by the stair climb test, 30-s chair stand test, or 10-min walk speed test, iMuscle strength in knee flexors, knee extensors, or quadriceps, jAssessed by a visual analogue scale, symptom scale of the EORTC QLQ-Core 30 questionnaire, the National Central Cancer Treatment Group (NCCTG) anorexia questionnaire, or others, kAssessed by the FACIT-F, MFSI-SF, symptom scale of the EORTC QLQ-Core 30, BFI, or others, lAssessments for depression, anxiety, insomnia, or weight-/eating-related distress, mAssessed by the EORTC QLQ-Core 30, FAACT, EQ5D, or others, nAssessed by the EORTC QLQ-H and N35 (head and neck cancer module) or EORTC QLQ-PAN26 (pancreatic cancer module), FACT-L, or others, oNutritional biomarkers included serum albumin, prealbumin, transferrin, hemoglobin, lymphocyte count, and others, pInflammatory biomarkers included C-reactive protein, Glasgow prognostic score, cytokines and their receptors, and others, qMetabolic biomarkers included lipids, fatty acids, reactive oxygen species, bone metabolic markers, and others, rEndocrinological biomarkers included growth hormone, ghrelin, leptin, insulin-like growth factor 1, insulin-like growth factor-binding protein 3, and others. CR: Consensus report for cancer cachexia published in 2011, BMI: Body mass index, PG-SGA: Patientgenerated subjective global assessment, NRS: Nutritional risk screening, EORTC QLQ: European organisation for research and treatment of cancer QOL questionnaire, BFI: Brief fatigue inventory, FACIT-F: Functional assessment of chronic illness therapy-fatigue, FAACT: Functional assessment of anorexia/cachexia therapy, FACT-L: Functional assessment of cancer therapy-Lung, EQ5D: EuroQol 5-Dimension, MFSI-SF: Multidimensional fatigue symptom inventory-short form, QOL: Quality of life
Overall survival was selected as a prognostic indicator in 21 (32%) studies but rarely had positive results even in studies that showed improvements in weight or lean body mass.[10,11,12] Studies focusing on the use of medical resources have recently emerged (3 studies, 5%). Length of hospital stay and medical costs were assessed.[10,13,14] Moreover, the protective effects of interventions on the toxicity of radiotherapy or chemotherapy were assessed in several studies. Inflammatory and nutritional biomarkers were assessed in 25 (38%), and 21 (32%) studies and the major items were C-reactive protein, albumin, prealbumin, interleukin-6, and tumor necrosis factor-alpha.
Discussion
Functional prognosis and disability-free survival
The ultimate goal of care for cancer cachexia has not been established. The selection of endpoints, measuring scales, or statistical analysis varies with the hypothesis or preference of researchers, pharmaceutical companies, and regulatory authorities.[15] These variations in the selection of endpoints might decrease the comparability of the results of clinical trials and impede the development of effective treatment. Although concomitant improvement in skeletal muscle mass, physical function, QOL, and overall survival may be the ideal goal, these parameters do not always correlate with each other. For example, gain in lean body mass was not always associated with improvement in physical function[7,11,16,17,18] or QOL[11,18,19] among the listed studies.
This review proposes the model of the sequential relationship between physical events in patients with advanced lung cancer [Figure 1]. This model was developed by the results of a previous prospective observational study that recruited elderly patients with advanced NSCLC.[9] In this aspect, sequential physical losses may begin early during cancer trajectory and continue until death. The earliest event among the patients was weight loss. More than half of the patients demonstrated ≥ 5% weight loss at the time of the study, suggesting that cachexia may start before the diagnosis of advanced cancer. In the following months, walking capacity and muscle strength declined. These physical losses finally resulted in disabling events, which were defined as a 10-point decrease in the Barthel index from baseline and occurred at a median of 13 months from baseline.
Figure 1.
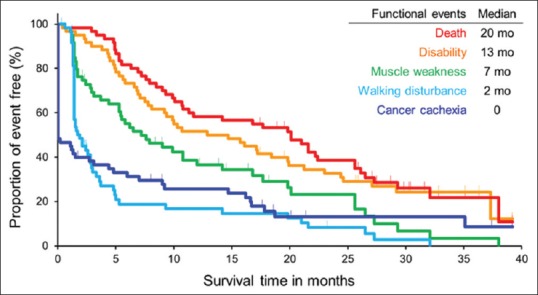
Sequential functional events in cancer trajectory in elderly patients with advanced lung cancer. Blue curve: event of cancer cachexia. Aqua curve: event of walking disturbance. Green curve: event of muscle weakness. Orange curve: disabling event. Red curve: overall survival
The disability-free survival (DFS) curve occurred slightly below the overall survival curve with a slightly high-pitched slope. This study proposes DFS as a new measurable indicator of functional prognosis in cancer cachexia because disabling events reflect the multiple physical losses due to cancer cachexia and are independent of a death event.[20] Due to the advances in medical fields and changes in lifestyle, humans are now able to lead a physically vigorous life until shortly before the biologically fixed lifespan end. People increasingly wish for an active long life despite the presence or absence of incurable diseases.[21] DFS originally corresponds to the disability-free life expectancy in epidemiological studies.[22] Recently, DFS has been introduced as a relevant patient-centered outcome of clinical trials in the fields of perioperative,[23,24] geriatric,[25] and pediatric medicine.[26] However, it is rarely selected as an endpoint of oncological clinical trials. A previous study reported that the presence of cancer cachexia at baseline was strongly associated with short DFS and long postdisability survival in elderly patients with advanced cancer.[27] In addition, this endpoint was associated with increased use of medical resources, including frequent unplanned visits, longer hospital stay, and higher cumulative medical costs. All of these measurements were considered important by their caregivers and healthcare providers.
Future direction of clinical trials for cancer cachexia
A new nonpharmacological multimodal intervention for cancer cachexia called the Nutrition and Exercise Treatment for Advanced Cancer (NEXTAC) program, is being developed. It combined nutritional counseling, low-intensity home-based resistance training, and promotive counseling of physical activity and was designed to prevent disability in elderly patients at risk for cachexia who are newly diagnosed with advanced NSCLC or pancreatic cancer and are to start systemic chemotherapy. Results of the Phase I feasibility study of this new intervention (NEXTAC-ONE) have been reported elsewhere.[28] A total of 30 participants showed excellent attendance (96.7%) and compliance for each intervention (≥90%) in the program, with only one dropout. The majority of patients was also adhered to the health education that was conducted and changed their health-related behavior such as increasing indoor or outdoor activity.[29] No severe adverse event occurred. Consequently, a prospective, multicenter, randomized Phase II clinical trial (NEXTAC-TWO, trial registration no. UMIN000028801) is being conducted to improve DFS in elderly patients with advanced cancer. A total of 130 patients are planned to be randomized to usual care or usual care plus NEXTAC in a 1:1 ratio. It was hypothesized that the NEXTAC prolongs 4 months of DFS from the usual care with 80% power. If this program could be proved to be both feasible and effective, it will be combined with newly emerging pharmacological interventions for cachexia to further improve functional prognosis and socioeconomic outcomes in elderly patients with advanced cancer.
Limitations
This review has several limitations. First, the literature search was carried out using only PubMed. A single reviewer (TN) carried out the selection of articles for inclusion. These drawbacks may result in a potential selection bias in the establishment of a reference list for RCTs. Second, not only the primary endpoint but also secondary and exploratory endpoints were included, without weighing the evidence that was shown in each study. Finally, the database used for the time-to-event curves included small samples from a single institution in Japan. Based on these limitations, we should pay careful attention while interpreting the results.
Conclusion
Clinical trials evaluating treatments for cancer cachexia are increasing. After the international consensus on the diagnostic and staging criteria for cancer cachexia, most clinical trials selected study populations in the earlier stage of the disease and the intervention tended to start earlier, concurrent with active cancer treatment. The true endpoint of these studies may be the expansion of active life with better QOL. Although the classical endpoint of increasing body mass would be an important outcome, it may not always contribute as a true endpoint. Thus, an optimal measurable endpoint should be identified, and a better combination of pharmacological and nonpharmacological interventions be established to improve functional prognosis in patients with this long-standing disease.
Financial support and sponsorship
This study (UMIN000023207) was supported by the Japan Agency for Medical Research and Development (AMED) under grant number JP18ck0106212.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The author would like to thank the patients and their families for taking part in this study, as well as the investigators and staffs in the division of Thoracic Oncology in the Shizuoka Cancer Center, Japan. The author would like to acknowledge Toshiaki Takahashi, MD, Koichi Takayama, MD, and Kazuo Tamura, MD for their generous instruction and support in conducting research for cancer cachexia.
References
- 1.Katz AM, Katz PB. Diseases of the heart in the works of Hippocrates. Br Heart J. 1962;24:257–64. doi: 10.1136/hrt.24.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unschuld PU. Berkeley, Los Angeles: University of California Press; 2011. Huang Di Neijing Su Wen. [Google Scholar]
- 3.Nimrouzi M, Zare M. Principles of nutrition in Islamic and traditional Persian medicine. J Evid Based Complementary Altern Med. 2014;19:267–70. doi: 10.1177/2156587214542006. [DOI] [PubMed] [Google Scholar]
- 4.Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cachexia and sarcopenia: Mechanisms and potential targets for intervention. Curr Opin Pharmacol. 2015;22:100–6. doi: 10.1016/j.coph.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 5.von Haehling S, Anker SD. Prevalence, incidence and clinical impact of cachexia: Facts and numbers-update 2014. J Cachexia Sarcopenia Muscle. 2014;5:261–3. doi: 10.1007/s13539-014-0164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12:489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 7.Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): Results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016;17:519–31. doi: 10.1016/S1470-2045(15)00558-6. [DOI] [PubMed] [Google Scholar]
- 8.European Medicines Agency. Adlumiz: EPAR – Refusal Public Assessment Report. [Last accessed on 2018 Oct 14]. Available from: https://www.ema.europa.eu/medicines/human/EPAR/adlumiz .
- 9.Naito T, Okayama T, Aoyama T, Ohashi T, Masuda Y, Kimura M, et al. Skeletal muscle depletion during chemotherapy has a large impact on physical function in elderly Japanese patients with advanced non-small-cell lung cancer. BMC Cancer. 2017;17:571. doi: 10.1186/s12885-017-3562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraft M, Kraft K, Gartner S, Mayerle J, Simon P, Weber E, et al. L-Carnitine-supplementation in advanced pancreatic cancer (CARPAN)-a randomized multicentre trial. Nutr J. 2012;11:52. doi: 10.1186/1475-2891-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon JN, Trebble TM, Ellis RD, Duncan HD, Johns T, Goggin PM. Thalidomide in the treatment of cancer cachexia: A randomised placebo controlled trial. Gut. 2005;54:540–5. doi: 10.1136/gut.2004.047563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jatoi A, Rowland K, Loprinzi CL, Sloan JA, Dakhil SR, MacDonald N, et al. An eicosapentaenoic acid supplement versus megestrol acetate versus both for patients with cancer-associated wasting: A North Central Cancer Treatment Group and National Cancer Institute of Canada collaborative effort. J Clin Oncol. 2004;22:2469–76. doi: 10.1200/JCO.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 13.De Waele E, Mattens S, Honore PM, Spapen H, De Greve J, Pen JJ. Nutrition therapy in cachectic cancer patients. The Tight Caloric Control (TiCaCo) pilot trial. Appetite. 2015;91:298–301. doi: 10.1016/j.appet.2015.04.049. [DOI] [PubMed] [Google Scholar]
- 14.Cong MH, Li SL, Cheng GW, Liu JY, Song CX, Deng YB, et al. An interdisciplinary nutrition support team improves clinical and hospitalized outcomes of esophageal cancer patients with concurrent chemoradiotherapy. Chin Med J (Engl) 2015;128:3003–7. doi: 10.4103/0366-6999.168963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fearon K, Argiles JM, Baracos VE, Bernabei R, Coats A, Crawford J, et al. Request for regulatory guidance for cancer cachexia intervention trials. J Cachexia Sarcopenia Muscle. 2015;6:272–4. doi: 10.1002/jcsm.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takayama K, Katakami N, Yokoyama T, Atagi S, Yoshimori K, Kagamu H, et al. Anamorelin (ONO-7643) in Japanese patients with non-small cell lung cancer and cachexia: Results of a randomized phase 2 trial. Support Care Cancer. 2016;24:3495–505. doi: 10.1007/s00520-016-3144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maccio A, Madeddu C, Gramignano G, Mulas C, Floris C, Sanna E, et al. A randomized phase III clinical trial of a combined treatment for cachexia in patients with gynecological cancers: Evaluating the impact on metabolic and inflammatory profiles and quality of life. Gynecol Oncol. 2012;124:417–25. doi: 10.1016/j.ygyno.2011.12.435. [DOI] [PubMed] [Google Scholar]
- 18.Dobs AS, Boccia RV, Croot CC, Gabrail NY, Dalton JT, Hancock ML, et al. Effects of enobosarm on muscle wasting and physical function in patients with cancer: A double-blind, randomised controlled phase 2 trial. Lancet Oncol. 2013;14:335–45. doi: 10.1016/S1470-2045(13)70055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantovani G, Maccio A, Madeddu C, Serpe R, Massa E, Dessi M, et al. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist. 2010;15:200–11. doi: 10.1634/theoncologist.2009-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. 2010;362:1173–80. doi: 10.1056/NEJMoa0909087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med. 1980;303:130–5. doi: 10.1056/NEJM198007173030304. [DOI] [PubMed] [Google Scholar]
- 22.Katz S, Branch LG, Branson MH, Papsidero JA, Beck JC, Greer DS. Active life expectancy. N Engl J Med. 1983;309:1218–24. doi: 10.1056/NEJM198311173092005. [DOI] [PubMed] [Google Scholar]
- 23.Shulman MA, Myles PS, Chan MT, McIlroy DR, Wallace S, Ponsford J. Measurement of disability-free survival after surgery. Anesthesiology. 2015;122:524–36. doi: 10.1097/ALN.0000000000000586. [DOI] [PubMed] [Google Scholar]
- 24.Myles PS, Bellomo R, Corcoran T, Forbes A, Peyton P, Story D, et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378:2263–74. doi: 10.1056/NEJMoa1801601. [DOI] [PubMed] [Google Scholar]
- 25.McNeil JJ, Woods RL, Nelson MR, Reid CM, Kirpach B, Wolfe R, et al. Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med. 2018;379:1499–508. doi: 10.1056/NEJMoa1800722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osborn DA, Schindler T, Jones LJ, Sinn JK, Bolisetty S. Higher versus lower amino acid intake in parenteral nutrition for newborn infants. Cochrane Database Syst Rev. 2018;3:CD005949. doi: 10.1002/14651858.CD005949.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naito T, Okayama T, Aoyama T, Ohashi T, Masuda Y, Kimura M, et al. Unfavorable impact of cancer cachexia on activity of daily living and need for inpatient care in elderly patients with advanced non-small-cell lung cancer in Japan: A prospective longitudinal observational study. BMC Cancer. 2017;17:800. doi: 10.1186/s12885-017-3795-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naito T, Mitsunaga S, Miura S, Tatematsu N, Inano T, Mouri T, et al. Feasibility of early multimodal interventions for elderly patients with advanced pancreatic and non-small-cell lung cancer. J Cachexia Sarcopenia Muscle. 2018;18 doi: 10.1002/jcsm.12351. doi: 10.1002/jcsm.12351. [Epub ahead of print] PMID: 30334618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mouri T, Naito T, Morikawa A, Tatematsu N, Miura S, Okayama T, et al. Promotion of behavioral change and the impact on quality of life in elderly patients with advanced cancer: A physical activity intervention of the multimodal nutrition and exercise treatment for advanced cancer program. Asia Pac J Oncol Nurs. 2018;5:383–90. doi: 10.4103/apjon.apjon_21_18. [DOI] [PMC free article] [PubMed] [Google Scholar]


