Abstract
Objective: To facilitate decision-making support for individual patients, development and external validation of a nomogram was undertaken to reveal prognostic factors and predict the value of concurrent chemoradiotherapy (CCRT) compared with radiotherapy (RT) for stage-II nasopharyngeal carcinoma (NPC) patients.
Methods: Clinical data of 419 and 309 patients with American Joint Committee on Cancer (2017) stage-II NPC in two institutions in China were collected retrospectively. Overall survival (OS) and progression-free survival were compared using Kaplan–Meier estimates. Cox regression analysis was used to identify the prognostic factors for building the nomogram. Predictive accuracy and discriminative ability were measured using the Concordance Index.
Results: Finally, there were 24 and 20 deaths in the development and validation group, respectively. Patients with stage T2N1, N1 stage, involvement of retropharyngeal and unilateral cervical lymph nodes, and who had RT alone had worse OS (P=0.019, 0.035, 0.003 and 0.010, respectively; log-rank test) than patients with stage T1N1 and T2N0, N0 stage, involvement of retropharyngeal or unilateral cervical lymph nodes, and CCRT, respectively. After multivariate analysis of the training set, age, neutrophil-to-lymphocyte ratio, therapy type, and pretreatment plasma concentration of Epstein–Barr virus DNA were independent prognostic factors of OS. A nomogram was established externally by involving all the factors stated above. The Concordance Index for the established nomogram to predict the OS of the training set was 0.793 (95% CI 0.689–0.897), and 0.803 (95% CI 0.696–0.910) in the validation set.
Conclusion: These data suggest that the nomogram was validated externally, could predict long-term outcome accurately, and enable accurate stratification of risk groups for stage-II NPC. Our model facilitated individualized care of NPC patients.
Keywords: chemotherapy, nasopharyngeal carcinoma, nomogram, prognosis, radiotherapy
Introduction
Nasopharyngeal carcinoma (NPC) has unique epidemiologic features (regional, racial, and familial) in southern China,1 where the highest incidence of NPC was found to be 1.9–2.8 per 100,000 person-years.2 Increasing attention has focused on advanced NPC, which causes the highest incidence of death-related recurrence and distant metastasis. The prognosis of patients with stage I–II NPC is, in general, favorable upon radiotherapy (RT) initiation. Overall survival (OS) is 84–90% for early stage NPC with RT alone.3
While, treatment of stage-II NPC is controversial. A retrospective study4 revealed that addition of concurrent chemoradiotherapy (CCRT) did not improve survival significantly but increased the prevalence of acute-toxicity reactions in patients with stage-II NPC. A meta-analysis5 of 2138 patients with stage-II NPC concluded that intensity-modulated radiotherapy (IMRT) alone was superior to CCRT with equivalent survival outcomes and fewer grade-3–4 acute-toxicity reactions. Either survival or complications, there were many studies in favor of RT alone. Chemotherapy for stage-II NPC is sometimes not recommended because an advantage of CCRT compared with RT alone has not been shown.
Patients with early stage NPC and positive lymph nodes are likely to develop distant metastasis and have poor OS.6 The role of adjunctive chemotherapy for stage-II NPC has been defined in a phase-III randomized trial.7 Results showed that chemotherapy improved 5-year OS (P=0.007), progression-free survival (PFS) (P=0.017), and distant metastasis-free survival (DMFS) (P=0.007) significantly; the CCRT group experienced more acute toxic effects (P=0.001) and the prevalence of late toxic effects did not increase significantly. A meta-analysis6 comprising 16 studies (3038 patients) compared conventional RT alone with CCRT. It demonstrated that CCRT could improve the prognosis significantly in terms of OS, PFS, and LRFS for stage-II NPC, but not DMFS whereas, with IMRT, patients with stage-II NPC did not benefit from the addition of chemotherapy. Nevertheless, a subgroup of patients with T2N1 disease carried a higher risk of regional recurrence and distant metastasis. In addition, the National Comprehensive Cancer Network has recommended CCRT for stage-II NPC, but the evidence for its efficacy is weak, which may attribute to the subgroups of various stages and other prognostic factors.
In recent years, a “nomogram” has been shown to be a reliable model for prognosis prediction for people suffering from cancer.8,9 Some nomograms have validated the prognostic factors in advanced NPC.10,11 However, a nomogram has not been developed for early stage NPC.
Based on a large cohort in our center, we aimed to establish a nomogram for survival prediction of individual patients with stage-II NPC. In addition, a cohort of patients were also included for external validation to test if this nomogram could be applied to predict their survival. Also, we stratified risk groups according to the factors which may help select individualized care of patients with stage-II NPC.
Methods
Inclusion criteria
The inclusion criteria for our study were (i) diagnosed with primary undifferentiated non-keratinized carcinoma according to pathology; (ii) stage-II NPC according to American Joint Committee on Cancer (AJCC) guidelines (8th edition); (iii) adequate clinical information in medical records; (iv) acquiring the standard and complete treatment: received radiotherapy with or without chemotherapy.
Patients
This study protocol was approved by the Research Ethics Committee of Sun Yat-sen University Cancer Center and The First People’s Hospital of Foshan, Guangzhou, China. Owing to the retrospective study design and analysis of clinical data, all data were anonymized; therefore, informed consent was formally waived by the Ethics Committee. All patient information is ensured to be confidential. All the procedures in this study are in accordance with the Helsinki Declaration.
Data from the training set were obtained from the medical records of 419 patients with NPC treated between 5 January 2010 and 14 October 2013 at Sun Yat-sen University Cancer Center. A total of 309 patients in the validation set were obtained from The First People’s Hospital of Foshan between January 2010 and August 2013. Demographic data (age, sex, smoking status, blood-test results [baseline laboratory data: plasma Epstein–Barr virus (EBV) DNA concentration, absolute neutrophil count, lymphocytes, and plasma fibrinogen], stage and treatment) were obtained from the electronic records of the hospital. All patients were restaged according to the 8th version of the AJCC staging system. Staging work-up comprised direct fiber-optic nasopharyngoscopy, magnetic resonance imaging of the nasopharynx and neck, abdominal ultrasound, chest radiography, whole-body scintigraphy, and/or positron emission tomography-computed tomography, and plasma EBV DNA concentration.
Treatment
The treatment strategies for all patients were based on National Comprehensive Cancer Network Guidelines. All patients received IMRT with or without chemotherapy. IMRT involved fractions of 2.12–2.24 Gy daily for 5 days per week, up to a total of 68–72 Gy to the primary tumor, 60–66 Gy to involved areas of the neck, and 54–56 Gy to uninvolved areas. CCRT consisted of cisplatin or nedaplatin administered triweekly or weekly until the end of RT.
Quantification of plasma EBV DNA concentration
The pretreatment plasma EBV DNA concentration was measured using real-time quantitative polymerase chain reaction (qRT-PCR). This assay was developed for detection of the plasma EBV DNA concentration and targets the BamHI-W fragment region of the EBV genome. qRT-PCR was carried out using 2× TaqMan™ Reagent (Roche, Basel, Switzerland), the amplification primers W-44F (50-AGTCTCTGCCTCAGGGCA-30) and W-119R (50-ACAGAGGGCCTGTCCACCG-30) and the dual-labeled fluorescent probe W-67T (50-[FAM]-CACTGTCTGTAAAGTCCAGCCTCC-[TAMRA]-30). At both institutions, a plasma EBV DNA concentration of <103 copies/mL was defined as “undetectable”. The cutoff value for EBV DNA was defined by analysis of receiver operator characteristic curves.
Follow-up
The primary endpoint was OS, measured from the date of the first NPC diagnosis to the date of death or loss to follow-up. The secondary endpoint was PFS (to relapse, distant metastasis, patient censorship or death at final follow-up). All patients were followed up routinely after therapy: every 3 months during the first 2 years, every 6 months during years 3–5, and annually thereafter. The surveillance work-up comprised normal routine assessments, imaging evaluation as well as measurement of the plasma EBV DNA concentration.
Statistical analyses
Statistical analyses were done using IBM v22.0 (IBM, Armonk, NY, USA). Survival outcomes were estimated using the Kaplan–Meier method and compared with the log-rank test. All analyses were two-sided; the level of significance was set at P<0.05. Significant variables (P<0.05) were entered into a Cox proportional hazards multivariate model to identify independent prognostic factors via forward stepwise procedures (P<0.05). Based on multivariate analyses, nomograms were generated to provide visualized risk prediction using the “survival” and “rms” packages of R 2.14.1 (www.r-project.org). Nomograms were subjected to bootstrap resampling (n=1000) for internal and external validation to correct the Concordance Index and explain variance with respect to over-optimism. During external validation, the nomogram point scores were calculated for individual patients, and then Cox regression analysis was undertaken using total point scores as predictors in the validation set.
Finally, the predictive accuracy for OS was validated by calculating the Concordance Index of the nomogram in the validation set. The value of the Concordance Index ranged from 0.5 to 1.0, which denotes a random chance to a perfect ability to correctly discriminate between the outcome and model, respectively. The 3-, 5-, and 6-year OS was calibrated by comparing predicted and observed survival.
Results
Clinicopathologic characteristics of patients in the primary cohort
A total of 409 patients with stage-II NPC who had undergone RT or CCRT in the primary set were eligible for the final analysis. There were 24 deaths at a median follow-up of 65 (range, 7.567–99.233) months. The predominant histology type was World Health Organization type III. All patients received IMRT with or without platin-based chemotherapy. An independent external validation set of 309 patients was recruited, with 20 events at a median follow up of 65.433 (range, 13.7–99.0) months. The clinicopathologic features of patients in the training set and external validation set are summarized in Table 1.
Table 1.
Clinical features of patients with nasopharyngeal carcinoma in the training set and validation set
| Characteristic | Training set | Validation set |
|---|---|---|
| n (%) | n (%) | |
| Total | 419 (100) | 309 (100) |
| Sex | ||
| Male | 393 (93.8) | 292 (94.5) |
| Female | 26 (6.2) | 17 (5.5) |
| Age (years) | ||
| <45 | 216 (51.6) | 165 (53.4) |
| ≥45 | 203 (48.4) | 144 (46.6) |
| Smoking | ||
| Yes | 113 (27.0) | 82 (26.5) |
| No | 306 (73.0) | 227 (73.5) |
| Stage (8th edition) | ||
| T2N0 | 77 (18.3) | 54 (17.5) |
| T1N1 | 198 (47.3) | 151 (48.9) |
| T2N1 | 144 (34.4) | 104 (33.6) |
| Lymph node site | ||
| Site1 | 94 (22.4) | 72 (23.3) |
| Site2 | 163 (38.9) | 117 (37.9) |
| Site3 | 162 (38.7) | 120 (38.8) |
| N category | ||
| N0 | 77 (18.3) | 54 (17.5) |
| N1 | 342 (81.7) | 255 (82.5) |
| Treatment | ||
| RT | 109 (26.0) | 80 (25.9) |
| CCRT | 310 (74.0) | 229 (74.1) |
| CT regimen | ||
| Triweekly | 257 (61.3) | 159 (51.5) |
| Weekly | 53 (12.7) | 70 (22.6) |
| NLR group | ||
| <2.8 | 263 (62.8) | 188 (60.8) |
| ≥2.8 | 156 (37.2) | 121 (39.2) |
| Fibrinogen (mg/mL) | ||
| <3.3 | 274 (65.4) | 203 (65.7) |
| ≥3.3 | 145 (34.6) | 106 (34.3) |
| EBV DNA (copies/mL) | ||
| <3000 | 237 (56.6) | 175 (56.6) |
| ≥3000 | 182 (43.4) | 134 (43.4) |
| Outcomes | ||
| Death | 24 | 20 |
| Distant metastasis | 20 | 14 |
| Localregional relapse | 6 | 5 |
Abbreviations: RT, radiotherapy; CCRT, concurrent chemoradiotherapy; CT, chemotherapy; NLR, neutrophil–lymphocyte ratio; EBV DNA, EBV (Epstein–Barr virus) DNA concentrations.
Survival outcomes according to the 8th edition of the AJCC staging system in the training set
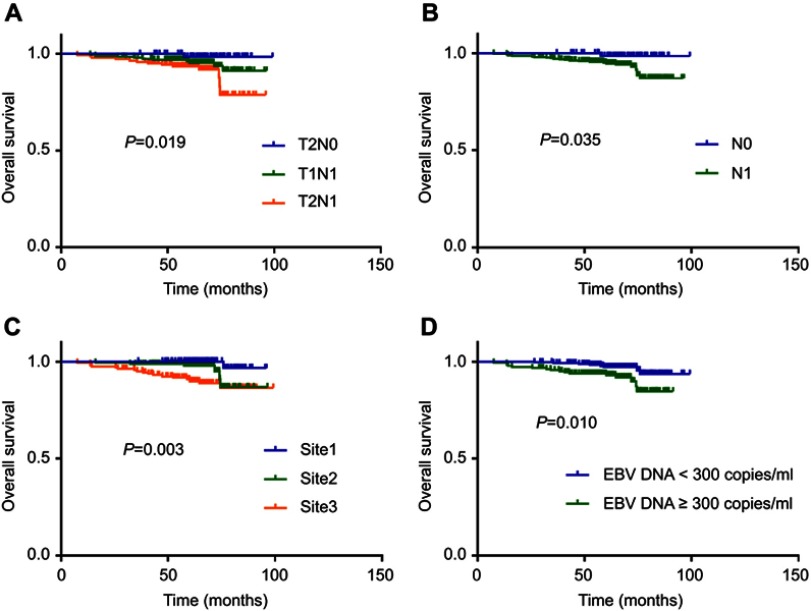
Five-year OS and PFS for the training set was 95.3% and 95.2%, respectively. We restaged patients according to the AJCC staging system (8th version), and compared survival outcomes for T stage, N stage, lymph-node site and category of plasma EBV DNA concentration. Stage T2N1 vs T1N1 vs T2N0 showed worse OS (P=0.019, Figure 1A). Stage N1 expressed worse OS than N0 (P=0.035, Figure 1B). Involvement of retropharyngeal or unilateral cervical lymph nodes resulted in better OS than both sites (P=0.003, Figure 1C). Patients with a higher EBV DNA concentration (≥3000 copies/mL) had worse OS (P=0.010; Figure 1D).
Figure 1.
Kaplan–Meier overall survival curves for all 419 patients with NPC in training set stratified by stage (A), N category (B), lymph node site (C), and EBV DNA concentration (D).
Abbreviations: NPC, nasopharyngeal carcinoma
Survival outcomes according to therapeutic regimen
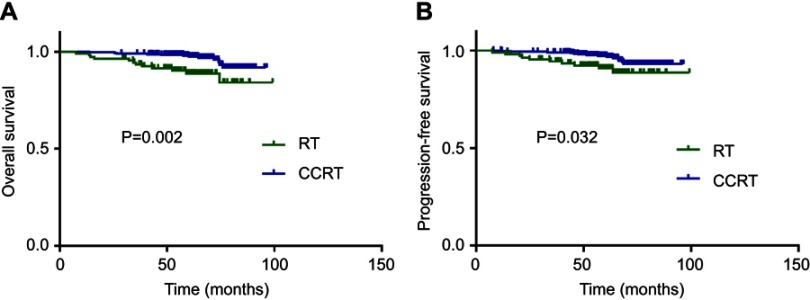
Compared with RT alone, patients who received CCRT had longer OS (P=0.002; Figure 2A) and PFS (P=0.032; Figure 2B).
Figure 2.
Effect of therapy regimen on survival outcomes. Kaplan–Meier overall survival (A) and progression-free survival (B) curves for all 419 patients with NPC in training set stratified as RT and CCRT group.
Abbreviations: RT, radiotherapy; CCRT, concurrent chemoradiotherapy;
Independent prognostic factors in the training set
Data from the training set were used to identify prognostic factors and build the model. The results of the univariate analysis are listed in Table 2. A poor prognosis was associated with: age >45 years; stage T2N1; N1 stage; involvement of retropharyngeal and unilateral cervical lymph nodes; increased neutrophil-to-lymphocyte ratio (NLR) (≥2.8); increased plasma fibrinogen level (≥3.30 mg/dL); higher plasma EBV DNA concentration (≥3000 copies/mL); RT alone. Variables considered to be significant in the univariate analysis were entered into the Cox multivariate analysis. Age, NLR, plasma EBV DNA concentration and therapy were shown to be independent in the multivariate Cox regression model and were incorporated in the nomogram according to the algorithm.
Table 2.
Cox’s proportional hazards regression model of overall survival for the 419 patients with nasopharyngeal carcinoma in training set
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (years) | ||||
| ≥45 vs <45 | 2.656 (1.101–6.404) | 0.030 | 2.454 (1.002–6.007) | 0.049 |
| Stage | ||||
| T2N0 | 1 | 0.043 | ||
| T1N1 vs T2N0 | 4.865 (0.621–38.109) | 0.132 | ||
| T2N1 vs T2N0 | 9.650 (1.253–74.315) | 0.030 | ||
| N category | ||||
| N1 vs N0 | 3.318 (1.940–5.677) | 0.035 | ||
| Lymph node site | ||||
| Site 1 | 1 | 0.013 | ||
| Site 2 vs Site 1 | 4.455 (0.534–37.157) | 0.167 | ||
| Site 3 vs Site 1 | 11.586 (1.541–87.111) | 0.017 | ||
| NLR group | ||||
| ≥2.80 vs <2.80 | 3.254 (1.421–7.451) | 0.005 | 2.425 (1.024–5.739) | 0.044 |
| Fibrinogen (mg/mL) | ||||
| ≥3.30 vs <3.30 | 1.151 (1.008–1.314) | 0.038 | ||
| EBV DNA (copies/mL) | ||||
| ≥3000 vs <3000 | 2.896 (1.238–6.774) | 0.014 | 2.434 (1.009–5.871) | 0.048 |
| Therapy | ||||
| RT vs CCRT | 3.315 (1.487–7.388) | 0.003 | 2.716 (1.168–6.317) | 0.020 |
Notes: Lymph node site: lymph node location. Site 1= retropharyngeal lymph node, Site 2= unilateral cervical lymph node, Site 3= retropharyngeal and unilateral cervical lymph nodes.
Abbreviations: HR, hazard ratio; CI, confidence interval; NLR, Neutrophil/Lymphocyte Ratio; RT, Radiotherapy; CCRT, Concurrent chemoradiotherapy.
Prognostic nomogram for OS prediction
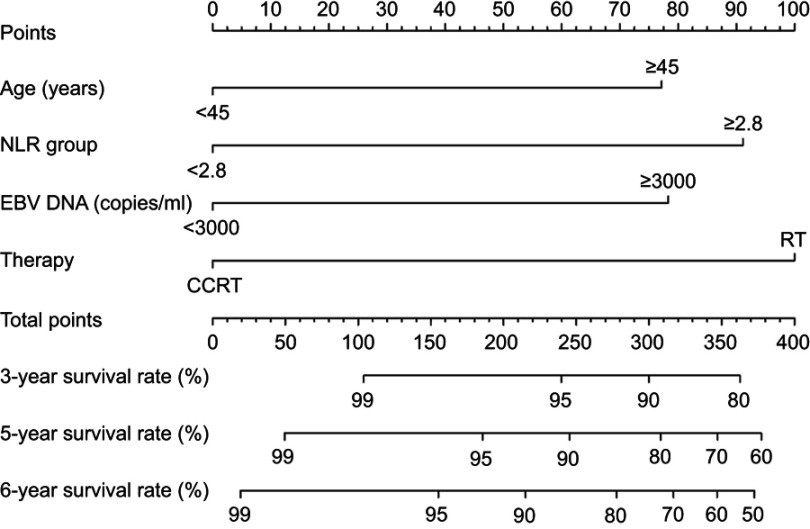
Using the data of patients in the training set, a nomogram was constructed for OS prediction (Figure 3). Longer lines indicate a greater prognostic impact of specific variables, and larger points in the nomogram indicate a shorter OS. Therapy had the greatest impact on OS, followed by the NLR, plasma EBV DNA concentration, and age. Each subtype within the variables stated above was assigned a score on the point scale. By addition of the total score and locating it on the total point scale, we could draw a straight line down to determine the estimated probability of survival. It could predict the 3-, 5-, and 6-year OS of NPC patients.
Figure 3.
A nomogram predicts the overall survival (OS) of patients with NPC. This nomogram was based on age, the NLR (neutrophil–lymphocyte ratio), EBV (Epstein–Barr virus) DNA concentration and therapy regimen. The total score of each patient was the sum of the points identified at the top of the scale for each factor and was then identified on the total points scale to determine the probability of 3-year, 5-year, and 6-year OS.
Validation of the nomogram
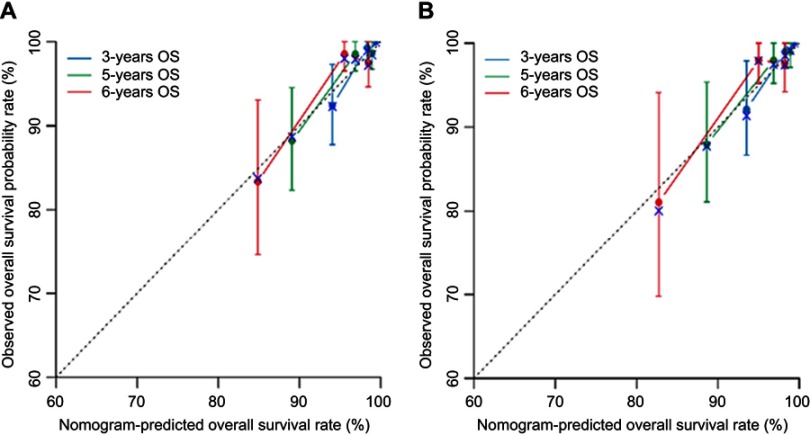
Data from the external validation set were used to validate the model. The calibration plot based on the data from the external validation set for the probability of OS at 3, 5, and 6 years demonstrated excellent agreement between the prediction according to the nomogram and actual observation in training and validation sets (Figure 4). The Concordance Index for the established nomogram to predict the OS of the training set was 0.793 (95% confidence interval, 0.689–0.897) and 0.803 (0.696–0.910) in the validation set (Table 3).
Figure 4.
The calibration curve for predicting overall survival (OS) at 3-, 5-, and 6-year in the training set (A) and validation set (B). Nomogram-predicted OS is plotted on the x-axis; actual rates of OS are plotted on the y-axis. The dashed lines along the 45-degree line through the origin represent the perfect calibration models in which the predicted probabilities are identical to the actual probability.
Table 3.
The concordance index values for performance of the multivariate model for prediction of OS in the training set and validation set
| Model | Concordance Index | Concordance Index 95%CI | Z | P-value | n | |
|---|---|---|---|---|---|---|
| Training set | 0.793 | 0.689 | 0.897 | 5.53 | <0.001 | 419 |
| Validation set | 0.803 | 0.696 | 0.910 | 5.53 | <0.001 | 309 |
Nomograms for risk stratification
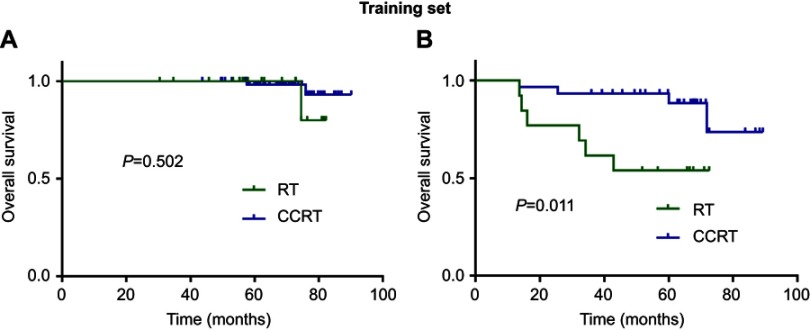
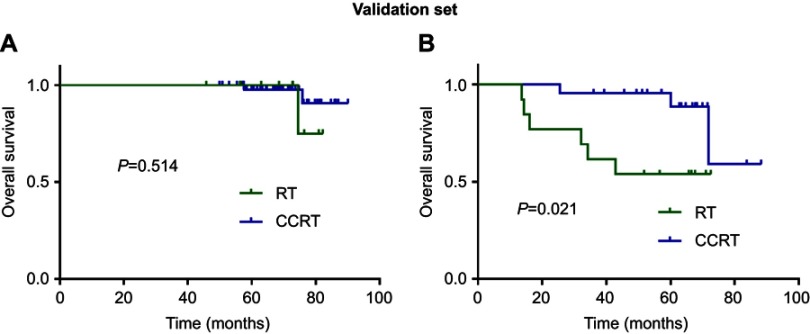
We stratified patients in the training set and validation set into two risk groups according to scores generated by the nomogram. A “low risk” group was determined to be one with age <45 years, NLR <2.80 and plasma EBV DNA concentration <3000 copies/mL. A “high risk” group was determined to be with age ≥45 years, NLR ≥2.80 and plasma EBV DNA concentration ≥3000 copies/mL. Higher OS was seen with CCRT compared with RT alone in the “high risk” group of the training set (P=0.011; Figure 5B), but a significant difference was not observed in the “low risk” group (P=0.502; Figure 5A). With regard to the validation set, CCRT also achieved higher OS in the “high risk” group (P=0.021; Figure 6B), but not in the “low risk” group (P=0.514; Figure 6A).
Figure 5.
Kaplan–Meier overall survival curves for comparing CCRT vs RT in training set stratified by “low risk” (A) and “high risk” (B).
Figure 6.
Kaplan–Meier overall survival curves for comparing CCRT vs RT in validation set stratified by “low risk” (A) and “high risk” (B).
Discussion
RT remains the cornerstone of management of early stage and locoregionally advanced disease. However, systemic chemotherapy has been shown to improve survival in patients with advanced disease significantly.5,12,13 Whether patients with stage-II disease should also receive chemotherapy is controversial. Lee and colleagues showed that the prevalence of local relapse, regional relapse, and distant metastasis was 17%, 3% and 28%, respectively, in stage-II NPC patients treated with RT alone.14 The primary pattern of failure was distant metastasis and local relapse, which was due mostly to the involvement of cervical lymph nodes. T1–2/N1 patients may need CCRT. To a certain extent, this therapeutic controversy may have been contributed by the stage migration of patients due to the discrepancies between various staging systems, as well as changes in N-staging classifications by the AJCC in their updates, especially with the recent changes in the 8th staging system (where there were large differences in the criteria for T and N stages). Thus, we restaged patients according to the newest AJCC staging system. The related prognostic factors (including therapeutic regimen) were analyzed and validated further externally by a nomogram. We explored the true role of CCRT for early stage NPC in the IMRT era.
We demonstrated that CCRT improved OS and PFS in patients with stage-II NPC. Our result is in contrast to a systematic review and meta-analysis15 that compared the outcomes for 1302 patients with stage-II NPC which showed that addition of CCRT to IMRT led to no survival benefit and more acute-toxicity reactions. Another meta-analysis6 showed that, compared with conventional RT alone, CCRT could improve the prognosis significantly in terms of OS, PFS, and LRFS for stage-II NPC, but not DMFS whereas, with IMRT, patients with stage-II NPC did not benefit from chemotherapy addition.
Recently, Xu et al5 conducted a systemic review and meta-analysis involving 2138 patients with stage-II NPC and concluded that CCRT was better than two-dimensional RT alone with a significant benefit in locoregional recurrence-free survival. IMRT alone was superior to CCRT with equivalent survival outcomes and fewer grade-3–4 acute-toxicity reactions.
In the IMRT era, Tham et al16 found no significant difference in treatment outcome in patients treated with or without chemotherapy of any schedule, and with acceptable toxicity. Those data may be due to advances in RT methods that offer a more satisfactory balance between target dose coverage and sparing of adjacent organs at risk. Such advances would lead to satisfactory therapeutic effects in patients with stage-II NPC treated with IMRT alone,4,16,17 and this may explain the non-significant difference in survival outcomes between CCRT and IMRT alone. Zhang et al18 conducted analyses of matching of propensity scores of cisplatin-based CCRT in low risk-NPC in the IMRT era and found no survival benefit.
OS for patients with early-stage NPC was approximately 80–90% with RT alone. The outcomes for this group after RT alone have been moderately satisfactory. Several studies have shown that addition of chemotherapy to IMRT can improve survival in these patients. The first randomized clinical trial to compare CCRT with RT alone in early-stage NPC was by Chen et al.7 They found that addition of cisplatin-based chemotherapy to RT resulted in a 10.9% increase in the prevalence of 5-year DMFS, which suggests that CCRT with cisplatin has systemic cytotoxicity in addition to radiosensitization. The large reduction in the prevalence of distant metastasis with CCRT using cisplatin could translate into substantial improvements in OS. The randomized controlled trial maybe the reason of the different survival results compared with systematic review and meta-analysis.
The retrospective studies, prospective studies, and meta-analysis detailed above suggest that uncertainty remains. Improved IMRT methods, increased toxicities of CCRT, and other related prognostic factors may be the reasons. Our observation that CCRT improved survival in patients with stage-II NPC is encouraging. Patients with T2N1M0 NPC had worse OS than those with T1N1 or T2N0 disease. Patients with a higher NLR (which denotes inflammation status) and a higher plasma EBV DNA concentration also had worse OS. Thus, we can hypothesize that patients with those risks may benefit considerably from CCRT. The external validation in our study verified the unfavorable risk factors.
Furthermore, we selected patients with the highest scores as the high-risk group, and CCRT achieved better OS that RT alone in the high-risk group in the training and validation sets. While the limitation was the complications of the therapy has not been studied, this may be attributed to the retrospective records of uncertainty compared to the prospective trial. Thus, these models facilitated decision-making support in daily clinical practice and can be used for patient counseling and shared decision-making to select patients who would benefit most from CCRT.
Conclusions
In our study, we developed and validated a novel nomogram for patients with stage-II NPC. This nomogram provides an accurate and precise prediction for OS. Assessment of patients with stage-II NPC with precise population stratification may increase the benefits of CCRT considerably. This nomogram could help clinicians with decision-making, especially for guiding patients to have acquiring RT or CCRT.
Acknowledgments
The study was supported by the Natural Science Foundation of Guangdong Province, China [grant number 2016A020215083]. The funding agency had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer. 2011;30(2):114–119. doi:10.5732/cjc.010.10377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jia WH, Huang QH, Liao J, et al. Trends in incidence and mortality of nasopharyngeal carcinoma over a 20–25 year period (1978/1983-2002) in Sihui and Cangwu counties in southern China. BMC Cancer. 2006;6:178. doi: 10.1186/1471-2407-6-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chua DT, Sham JS, Kwong DL, Au GK. Treatment outcome after radiotherapy alone for patients with stage I-II nasopharyngeal carcinoma. Cancer. 2003;98(1):74–80. doi: 10.1002/cncr.11485 [DOI] [PubMed] [Google Scholar]
- 4.Su Z, Mao YP, Tang J, Lan XW, OuYang PY, Xie FY. Long-term outcomes of concurrent chemoradiotherapy versus radiotherapy alone in stage II nasopharyngeal carcinoma treated with IMRT: a retrospective study. Tumour Biol. 2016;37(4):4429–4438. doi: 10.1007/s13277-015-4266-5 [DOI] [PubMed] [Google Scholar]
- 5.Xu C, Zhang LH, Chen YP, et al. Chemoradiotherapy versus radiotherapy alone in stage II nasopharyngeal carcinoma: a systemic review and meta-analysis of 2138 patients. J Cancer. 2017;8(2):287–297. doi: 10.7150/jca.17317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S, Li S, Shen L. Combined chemoradiation vs radiation therapy alone in stage-II nasopharyngeal carcinoma: a meta-analysis of the published literature. Curr Prob Cancer. 2018;42(3):302–318. doi: 10.1016/j.currproblcancer.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 7.Chen QY, Wen YF, Guo L, et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J National Cancer Inst. 2011;103(23):1761–1770. doi: 10.1093/jnci/djr432 [DOI] [PubMed] [Google Scholar]
- 8.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–1370. doi: 10.1200/JCO.2007.12.9791 [DOI] [PubMed] [Google Scholar]
- 9.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi: 10.1158/1078-0432.CCR-04-0713 [DOI] [PubMed] [Google Scholar]
- 10.Liang W, Shen G, Zhang Y, et al. Development and validation of a nomogram for predicting the survival of patients with non-metastatic nasopharyngeal carcinoma after curative treatment. Chin J Cancer. 2016;35(1):98. doi: 10.1186/s40880-016-0160-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Xia L, Wang Y, et al. Development and external validation of nomograms to predict the risk of skeletal metastasis at the time of diagnosis and skeletal metastasis-free survival in nasopharyngeal carcinoma. BMC Cancer. 2017;17(1):628. doi: 10.1186/s12885-017-3630-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan AT, Teo PM, Ngan RK, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol. 2002;20(8):2038–2044. doi: 10.1200/JCO.2002.08.149 [DOI] [PubMed] [Google Scholar]
- 13.Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee On Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005;23(27):6730–6738. doi: 10.1200/JCO.2005.16.790 [DOI] [PubMed] [Google Scholar]
- 14.Lee AW, Poon YF, Foo W, et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976–1985: overall survival and patterns of failure. Int J Radiat Oncol Biol Phys. 1992;23(2):261–270. [DOI] [PubMed] [Google Scholar]
- 15.Liu F, Jin T, Liu L, et al. The role of concurrent chemotherapy for stage II nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: a systematic review and meta-analysis. PLoS One. 2018;13(3):e0194733. doi: 10.1371/journal.pone.0194733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tham IW, Lin S, Pan J, Han L, Lu JJ, Wee J. Intensity-modulated radiation therapy without concurrent chemotherapy for stage IIb nasopharyngeal cancer. Am J Clin Oncol. 2010;33(3):294–299. doi: 10.1097/COC.0b013e3181d2edab [DOI] [PubMed] [Google Scholar]
- 17.Tham IW, Lu JJ. Controversies and challenges in the current management of nasopharyngeal cancer. Expert Rev Anticancer Ther. 2010;10(9):1439–1450. doi: 10.1586/era.10.97 [DOI] [PubMed] [Google Scholar]
- 18.Zhang LN, Gao YH, Lan XW, et al. Propensity score matching analysis of cisplatin-based concurrent chemotherapy in low risk nasopharyngeal carcinoma in the intensity-modulated radiotherapy era. Oncotarget. 2015;6(41):44019–44029. doi: 10.18632/oncotarget.5806 [DOI] [PMC free article] [PubMed] [Google Scholar]