ABSTRACT
Background and objective
This study aimed to investigate whether quantitative lung vessel morphology determined by a new fully automated algorithm is associated with functional indices in idiopathic pulmonary fibrosis (IPF).
Methods
A total of 152 IPF patients had vessel volume, density, tortuosity and heterogeneity quantified from computed tomography (CT) images by a fully automated algorithm. Separate quantitation of vessel metrics in pulmonary arteries and veins was performed in 106 patients. Results were evaluated against readouts from lung function tests.
Results
Normalized vessel volume expressed as a percentage of total lung volume was moderately correlated with functional indices on univariable linear regression analysis: forced vital capacity (R2 = 0.27, P < 1 × 10−6), diffusion capacity for carbon monoxide (DLCO; R2 = 0.12, P = 3 × 10−5), total lung capacity (TLC; R2 = 0.45, P < 1 × 10−6) and composite physiologic index (CPI; R2 = 0.28, P < 1 × 10−6). Normalized vessel volume was correlated with vessel density but not with vessel heterogeneity. Quantitatively derived vessel metrics (and artery and vein subdivision scores) were not significantly linked with the transfer factor for carbon monoxide (KCO), and only weakly with DLCO.
On multivariable linear regression analysis, normalized vessel volume and vessel heterogeneity were independently linked with DLCO, TLC and CPI indicating that they capture different aspects of lung damage. Artery–vein separation provided no additional information beyond that captured in the whole vasculature.
Conclusion
Our study confirms previous observations of links between vessel volume and functional measures of disease severity in IPF using a new vessel quantitation tool. Additionally, the new tool shows independent linkages of normalized vessel volume and vessel heterogeneity with functional indices. Quantitative vessel metrics do not appear to reflect vasculopathic damage in IPF.
Keywords: interstitial lung disease, lung fibrosis, radiology and other imaging, respiratory structure and function
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrosing lung disease associated with a median survival from diagnosis of 3–5 years.1, 2 Pulmonary function tests (PFT) including forced vital capacity (FVC)2 and diffusion capacity for carbon monoxide (DLCO)3 as well as visual analysis of parenchymal features on computed tomography (CT) images, including the extent of interstitial lung disease (ILD)4 and honeycombing,1, 5 form the basis of determining baseline disease severity in IPF.
Recent advances in computer technology have resulted in the development of tools capable of classifying and quantifying parenchymal features on CT data sets.6, 7 Whilst the majority of automated parenchymal features reflect patterns scored visually by radiologists, there has been increasing focus on novel CT patterns that computer tools can recognize, but which cannot be quantified visually. An example is the vessel‐related structures (VRS) readout determined by CALIPER,7 which was able to powerfully predict outcome in patients with IPF.8, 9 However, until now, CALIPER has been the only tool used to evaluate vasculature in patients with lung fibrosis.
Our study therefore aimed to use a new vessel quantitation tool to determine the volume of pulmonary vessels in patients with IPF. Extra segmentation features of the computer tool allowed pulmonary arteries and veins to be distinguished as well as new vessel parameters including vessel density, tortuosity and heterogeneity to be quantified. Computer‐derived vessel metrics were evaluated against PFT in a structure–function analysis.
METHODS
Study design
IPF patients presenting to the Royal Brompton Hospital and diagnosed by a multidisciplinary team using established guidelines10 were retrospectively identified. Patients for whom a complete non‐contrast volumetric CT scan was available were included in the study population. PFTs were considered if obtained within 3 months of the CT scan and included forced expiratory volume in the first second (FEV1), FVC, total lung capacity (TLC), DLCO, transfer factor for carbon monoxide (KCO) and the composite physiologic index (CPI).11 CT and pulmonary function protocols are included in Appendix S1 (Supplementary Information). Approval for this study of clinically indicated CT and pulmonary function data with a waiver for consent was obtained from the Institutional Ethics Committee of the Royal Brompton Hospital.
Computer analysis of CT imaging
Image pre‐processing with a 1‐voxel wide median filter and segmentation of lung and airways was performed on the whole CT images using the Chest Imaging Platform.12 The vessel segmentation was performed with in‐house developed software. A detailed description and validation of the automatic vessel extraction algorithm is presented by Payer et al.13 Briefly, a multi‐scale vessel enhancement filter produces images with a high response for tubular structures as well as the respective radius and an estimate for the tube orientation. Optimized vessel paths with sub‐voxel accuracy are generated from regularly spaced maxima of the vesselness response following the tubular structures. The vessel trees are reconstructed from these paths and subsequently separated at the bifurcations into individual vessel segments. Only segments with diameters between 2 and 10 mm are included. Finally, arteries and veins are labelled by exploiting that arteries and veins are roughly uniformly distributed in the lung and that bronchi run approximately parallel and in close proximity to the arteries. The algorithm results in properly labelled and morphologically characterized vessel segments in most subjects.13
Validation of vessel segmentations
Overlay colour maps of the vessels captured by the computer algorithm were superimposed on the CT images and were visually assessed by a radiologist (J.J.) to check the adequacy of pulmonary vessel extraction. Quality control of vessel extraction was evaluated using two metrics scored to the nearest 5%: (i) proportion of labelled structures that were not vascular in origin and (ii) proportion of vessels that were not labelled at all by the computer tool. For both metrics, a threshold of >10% error was used to exclude subjects. In addition, pulmonary artery/vein separation was evaluated by the same radiologist and subjects where >20% of the vessels were mislabelled as arteries or veins were excluded from any artery or vein sub‐analysis.
Calculation of morphological readouts
Readouts were analysed for the whole lung (left and right lungs combined) and individually for three zones of equal volume (upper zones, middle zones and lower‐zones). The normalized vessel volumes for arteries, veins and all vessels were calculated by normalizing the cumulative volumes of the segmented arteries, veins and the combined vessel trees, respectively, to the subject's quantitatively derived total lung volume (for total lung scores) and zonal volume (for zonal scores). The number of vessel segments was also normalized to the respective subject's total/zonal lung volume calculating the vessel density. The tortuosity of the vessel segments was determined using the distance metric.14, 15 This is calculated as the ratio between the length of a vessel segment along its centre line and the Euclidean distance between its end points. The median distance metric was used as measure of vessel tortuosity. The distribution width between the 15th and the 85th percentile of distance metric values was considered as measure for vessel heterogeneity. Additionally, the mean lung attenuation was calculated as the average X‐ray attenuation of the lung parenchyma (lung segmentation after extraction of vessels and airways).
Statistical analysis
Statistical analyses were performed with SPSS (IBM SPSS Statistics for Macintosh, Version 20.0 (IBM Corp., Armonk, NY, USA). Data are given as medians with ranges, means with SD or numbers of patients as appropriate. Group differences were examined using Student's t‐test for continuous variables, chi‐square test for categorical variables and the Mann–Whitney U‐test for non‐normally distributed median values. Univariable and multivariable linear regression analyses were performed to explore relationships amongst various quantitative CT metrics and between CT metrics and PFT.
RESULTS
Baseline data
The study population comprised 176 IPF patients. Of the 176 CT, 4 (2%) could not be segmented due to motion artefacts. Twenty further subjects were excluded for either having >10% of the lung vessels not segmented or >10% of structures labelled as vessels representing non‐vascular (often coarse fibrotic) artefact. The 24 excluded patients had more severe baseline disease than the 152 patients included in the study (Table S1, Supplementary Information). Patients with >20% artery/vein misclassification (n = 46) were excluded from all artery and vein analyses. Demographic and baseline functional and quantitative results for the 152 study subjects are shown in Table 1. Representative CT images and a 3D rendering of the labelled vessels are presented in Figure 1.
Table 1.
Patient demographics and mean and SD of pulmonary function tests and quantitative CT data
| Variable (n = 152 unless stated) Units are percentage unless stated |
Value |
|---|---|
| Median age (years, range) | 67 (38–86) |
| Male/female | 122/30 |
| Never/ever/current smokers | 50/100/2 |
| Alive/dead | 51/101 |
| GAP index (1/2/3) (n = 136) | 44/73/19 |
| FEV1% predicted (n = 138) | 73.7 ± 19.4 |
| FVC % predicted (n = 138) | 72.7 ± 20.7 |
| DLCO % predicted (n = 141) | 38.9 ± 12.9 |
| KCO % predicted (n = 141) | 69.9 ± 19.0 |
| TLC % predicted (n = 132) | 67.2 ± 16.5 |
| CPI (n = 137) | 52.2 ± 11.8 |
| Computed metrics | |
| Total lung volume (L) | 3.9 ± 1.1 |
| Normalized vessel volume (%) | 3.6 ± 0.8 |
| Normalized arterial volume (%;n = 106) | 2.1 ± 0.5 |
| Normalized venous volume (%; n = 106) | 1.6 ± 0.4 |
| Vessel density (vess/L) | 361.5 ± 106.1 |
| Arterial density (vess/L; n = 106) | 198.9 ± 53.9 |
| Venous density (vess/L; n = 106) | 167.6 ± 48.7 |
| Vessel tortuosity (1) | 1.029 ± 0.003 |
| Arterial tortuosity (1; n = 106) | 1.029 ± 0.004 |
| Venous tortuosity (1; n = 106) | 1.029 ± 0.003 |
| Vessel heterogeneity (1) | 0.058 ± 0.006 |
| Arterial heterogeneity (1; n = 106) | 0.057 ± 0.007 |
| Venous heterogeneity (1; n = 106) | 0.058 ± 0.006 |
CPI, composite physiologic index; CT, computed tomography; DLCO, diffusion capacity for carbon monoxide; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; GAP, gender age physiology; KCO, transfer factor for carbon monoxide; TLC, total lung capacity.
Figure 1.
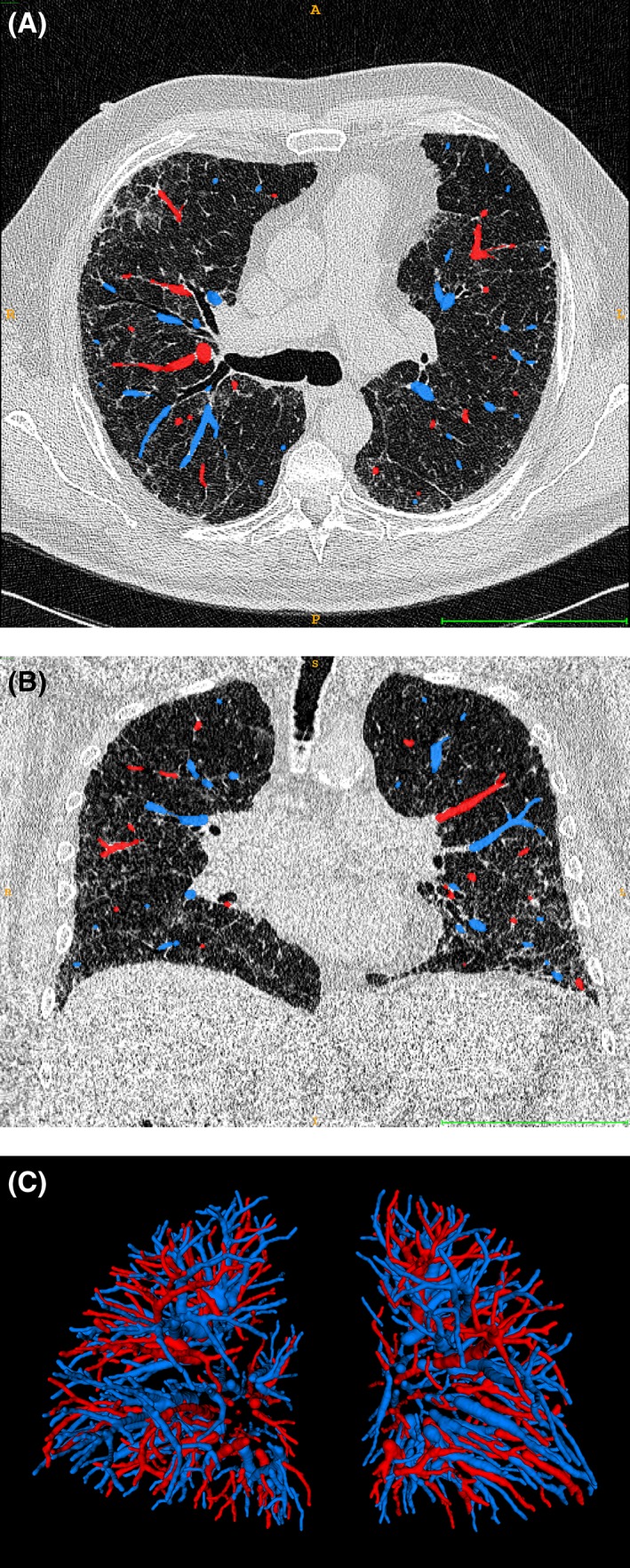
Transversal (A) and coronal (B) thoracic computed tomography (CT) images of a representative patient with overlays of the arteries (blue) and veins (red) and 3D rendering of the arterial and venous vessel trees in this patient (C). The bar at the bottom of (A) and (B) is 10 cm wide.
Relationships between quantitative variables
Examination of interrelationships between the quantitative vessel metrics (normalized vessel volume, vessel density, vessel tortuosity and vessel heterogeneity) demonstrated strong correlations between normalized vessel volume and vessel density, and between vessel tortuosity and vessel heterogeneity. No significant correlations were identified between normalized vessel volume and vessel tortuosity or vessel heterogeneity (Table S2, Supplementary Information).
Mean lung attenuation demonstrated a moderate negative correlation with computer‐derived total lung volume (R2 = 0.44, P < 1 × 10−6). Mean lung attenuation was strongly correlated with normalized vessel volume and moderately with vessel density but showed weak relationships with tortuosity and vessel heterogeneity (Table S3, Supplementary Information). Total lung volume showed significant correlations with vessel volume expressed in millilitres (R2 = 0.40, P < 1x10−6) and normalized to the total lung volume (R2 = 0.16, P < 1 × 10−6). Total lung volume was significantly associated with vessel density but correlations with vessel tortuosity and heterogeneity were weak (Table S3, Supplementary Information).
Structure–function correlations
Relationships between quantitative CT features and functional indices are shown in Tables 2, 3, 4. Both total lung volume and mean lung attenuation measured quantitatively were correlated with FEV1, FVC, DLCO, TLC and CPI, reiterating the previously identified strong links between both measures (Table S3, Supplementary Information).
Table 2.
Univariable analysis of various quantitative CT features with baseline FEV1 (top) and FVC (bottom)
| Pulmonary function test | CT pattern | Number of subjects | Beta coefficient | 95% CI | P‐value | R2 |
|---|---|---|---|---|---|---|
| FEV1 | Total lung volume (L) | 136 | 6.54 | 3.87, 9.20 | 3 × 10−6 | 0.15 |
| Mean lung attenuation (HU) | 136 | −0.16 | −0.21, −0.12 | <1 × 10−6 | 0.26 | |
| Normalized vessel volume (%) | 136 | −8.27 | −12.00, −4.54 | 2 × 10−5 | 0.12 | |
| Normalized arterial volume (%) | 96 | −6.09 | −13.87, 1.69 | 0.12 | 0.03 | |
| Normalized venous volume (%) | 96 | −8.88 | −18.16, 0.40 | 0.06 | 0.04 | |
| Vessel density (vess/L) | 136 | −0.05 | −0.08, −0.01 | 0.005 | 0.06 | |
| Arterial density (vess/L) | 96 | −0.03 | −0.10, 0.04 | 0.35 | 0.01 | |
| Venous density (vess/L) | 96 | −0.03 | −0.11, 0.04 | 0.40 | 0.01 | |
| Vessel heterogeneity (1) | 136 | −164.60 | −718.64, 389.44 | 0.56 | 0.00 | |
| Arterial heterogeneity (1) | 95 | 217.28 | −321.16, 755.95 | 0.43 | 0.01 | |
| Venous heterogeneity (1) | 95 | 58.42 | −550.02, 666.86 | 0.85 | 0.00 | |
| FVC | Total lung volume (L) | 136 | 10.59 | 8.08, 13.11 | <1 × 10−6 | 0.34 |
| Mean lung attenuation (HU) | 136 | −0.24 | −0.28, −0.19 | <1 × 10−6 | 0.47 | |
| Normalized vessel volume (%) | 136 | −13.14 | −16.77, −9.51 | <1 × 10−6 | 0.27 | |
| Normalized arterial volume (%) | 96 | −16.18 | −23.69, −8.68 | 5 × 10−5 | 0.16 | |
| Normalized venous volume (%) | 96 | −20.29 | −29.22, −11.36 | 2 × 10−5 | 0.18 | |
| Vessel density (vess/L) | 136 | −0.08 | −0.11, −0.05 | 1 × 10−6 | 0.16 | |
| Arterial density (vess/L) | 96 | −0.12 | −0.19 ‐0.05 | 0.001 | 0.11 | |
| Venous density (vess/L) | 96 | −0.12 | −0.20, −0.05 | 0.003 | 0.10 | |
| Vessel heterogeneity (1) | 136 | −471.88 | −1060.19, 116.44 | 0.12 | 0.02 | |
| Arterial heterogeneity (1) | 96 | −88.94 | −650.98, 473.11 | 0.75 | 0.00 | |
| Venous heterogeneity (1) | 96 | −396.40 | −1024.20, 232.07 | 0.21 | 0.02 |
CT, computed tomography; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; HU, Hounsfield Unit.
Table 3.
Univariable analysis of various quantitative CT features with baseline DLCO (top) and KCO (bottom)
| Pulmonary function test | CT pattern | Number of subjects | Beta coefficient | 95% CI | P‐value | R2 |
|---|---|---|---|---|---|---|
| DLCO | Total lung volume (L) | 139 | 3.46 | 1.68, 5.25 | 0.0002 | 0.10 |
| Mean lung attenuation (HU) | 139 | −0.11 | −0.14, −0.08 | <1 × 10−6 | 0.25 | |
| Normalized vessel volume (%) | 139 | −5.43 | −7.90, −2.96 | 3 × 10−5 | 0.12 | |
| Normalized arterial volume (%) | 96 | −9.48 | −14.74, −4.23 | 0.001 | 0.12 | |
| Normalized venous volume (%) | 96 | −8.06 | −14.65, −1.47 | 0.02 | 0.06 | |
| Vessel density (vess/L) | 139 | −0.05 | −0.07, −0.03 | <1 × 10−6 | 0.17 | |
| Arterial density (vess/L) | 96 | −0.08 | −0.13, −0.03 | 0.001 | 0.11 | |
| Venous density (vess/L) | 96 | −0.08 | −0.13, −0.02 | 0.008 | 0.07 | |
| Vessel heterogeneity (1) | 139 | −397.93 | −755.89, −39.97 | 0.03 | 0.03 | |
| Arterial heterogeneity (1) | 96 | −221.35 | −599.12, 156.42 | 0.25 | 0.01 | |
| Venous heterogeneity (1) | 96 | −203.23 | −629.48, 223.02 | 0.35 | 0.01 | |
| KCO | Total lung volume (L) | 139 | 3.85 | −6.53, −1.17 | 0.005 | 0.06 |
| Mean lung attenuation (HU) | 139 | 0.02 | −0.03, 0.07 | 0.47 | 0.00 | |
| Normalized vessel volume (%) | 139 | 1.99 | −1.86, 5.85 | 0.31 | 0.01 | |
| Normalized arterial volume (%) | 96 | 0.95 | −7.41, 9.31 | 0.82 | 0.01 | |
| Normalized venous volume (%) | 96 | 5.74 | −4.33, 15.82 | 0.26 | 0.01 | |
| Vessel density (vess/L) | 139 | −0.02 | −0.05, 0.01 | 0.24 | 0.01 | |
| Arterial density (vess/L) | 96 | −0.00 | −0.08, 0.07 | 0.96 | 0.00 | |
| Venous density (vess/L) | 96 | −0.00 | −0.08, 0.09 | 0.94 | 0.00 | |
| Vessel heterogeneity (1) | 139 | −136.95 | −671.13, 397.24 | 0.61 | 0.00 | |
| Arterial heterogeneity (1) | 96 | −125.38 | −692.96, 442.20 | 0.66 | 0.00 | |
| Venous heterogeneity (1) | 96 | 219.99 | −417.98, 857.96 | 0.69 | 0.01 |
CT, computed tomography; DLCO, diffusion capacity for carbon monoxide; HU, Hounsfield Unit; KCO, transfer factor for carbon monoxide.
Table 4.
Univariable analysis of various quantitative CT features with baseline TLC (top) and the CPI (bottom)
|
Pulmonary function test |
CT pattern | Number of subjects | Beta coefficient | 95% CI | P‐value | R2 |
|---|---|---|---|---|---|---|
| TLC | Total lung volume (L) | 131 | 9.69 | 7.89, 11.49 | <1 × 10−6 | 0.46 |
| Mean lung attenuation (HU) | 131 | −0.21 | −0.024, −0.18 | <1 × 10−6 | 0.61 | |
| Normalized vessel volume (%) | 131 | −13.25 | −15.77, −10.72 | <1 × 10−6 | 0.45 | |
| Normalized arterial volume (%) | 90 | −22.73 | −28.31, −17.15 | <1 × 10−6 | 0.42 | |
| Normalized venous volume (%) | 90 | −25.11 | −31.94, −18.29 | <1 × 10−6 | 0.38 | |
| Vessel density (vess/L) | 131 | −0.09 | −0.11, −0.07 | <1 × 10−6 | 0.31 | |
| Arterial density (vess/L) | 90 | −0.17 | −0.22, −0.12 | <1 × 10−6 | 0.31 | |
| Venous density (vess/L) | 90 | −0.17 | −0.23, −0.10 | 6 × 10−6 | 0.24 | |
| Vessel heterogeneity (1) | 131 | −485.65 | −968.88, −2.42 | 0.049 | 0.03 | |
| Arterial heterogeneity (1) | 90 | −235.98 | −739.71, 267.75 | 0.35 | 0.01 | |
| Venous heterogeneity (1) | 90 | −539.40 | −1093.49, 14.69 | 0.06 | 0.04 | |
| CPI | Total lung volume (L) | 136 | −5.49 | −6.99, −4.00 | <1 × 10−6 | 0.28 |
| Mean lung attenuation (HU) | 136 | 0.14 | 0.12, 0.16 | <1 × 10−6 | 0.50 | |
| Normalized vessel volume (%) | 136 | 7.66 | 5.58, 9.74 | <1 × 10−6 | 0.28 | |
| Normalized arterial volume (%) | 95 | 12.51 | 8.23, 16.80 | 1 × 10−6 | 0.26 | |
| Normalized venous volume (%) | 95 | 12.75 | 7.29, 18.22 | 1 × 10−5 | 0.19 | |
| Vessel density (vess/L) | 136 | 0.06 | 0.04, 0.08 | <1 × 10−6 | 0.27 | |
| Arterial density (vess/L) | 95 | 0.10 | 0.07, 0.14 | 1 × 10−5 | 0.23 | |
| Venous density (vess/L) | 95 | 0.10 | 0.06, 0.15 | 5 × 10−5 | 0.16 | |
| Vessel heterogeneity (1) | 136 | 453.44 | 124.82, 782.07 | 0.007 | 0.05 | |
| Arterial heterogeneity (1) | 95 | 267.12 | −67.44, 601.68 | 0.12 | 0.03 | |
| Venous heterogeneity (1) | 95 | 360.57 | −13.48, 735.36 | 0.06 | 0.04 |
CPI, composite physiologic index; CT, computed tomography; HU, Hounsfield Unit; TLC, total lung capacity.
Of the vessel metrics, normalized vessel volume demonstrated the strongest linkages with FEV1, FVC, TLC and CPI (Tables 2, 4). Vessel density was weakly but significantly linked with these functional parameters. Vessel tortuosity and vessel heterogeneity were not strongly linked with any functional indices. As the correlations were similarly weak for vessel tortuosity and heterogeneity, only results for the latter are shown in Tables 2, 3, 4. None of the quantitative vessel metrics was significantly linked to KCO. Normalized vessel volume (R2 = 0.12, P = 3 × 10−5) and vessel density (R2 = 0.16, P = 1 × 10−6) showed weak links with DLCO, but vessel tortuosity or heterogeneity did not. Therefore, the readouts of macrovascular morphology showed at best only weak correlations with the two functional parameters that may contain information on small vessel disease.
When vessel metrics were subdivided according to zonal location in the z‐axis, lower zone metrics correlated weakly with FVC, DLCO and CPI compared to upper zone or middle zone metrics. Middle zone metrics demonstrated slightly stronger functional correlations than upper zone metrics for DLCO but were equivalent for CPI and weaker for FVC (Table S4, Supplementary Information).
On multivariable linear regression analyses, in models adjusted for patient age, male gender, smoking status and CT slice thickness (0.7 or 1.0 mm), normalized vessel volume and vessel heterogeneity were independently linked to DLCO, TLC and CPI (Table 5). Vessel heterogeneity in the middle zones showed stronger independent links with DLCO and CPI than upper or lower zone heterogeneity (Table S5, Supplementary Information). Vessel density was not examined in the multivariable models due to strong collinearity with normalized vessel volume. Vessel tortuosity was not correlated with any functional indices in the multivariable analysis.
Table 5.
Multivariable linear regression analyses demonstrating relationships between pulmonary functional indices and total vessel volume and vessel heterogeneity metrics
| Dependent variable | Independent variable | Beta coefficient | 95% CI | P‐value | R2 |
|---|---|---|---|---|---|
| FEV1 | Normalized vessel volume (%) | −6.78 | −10.68, −2.87 | 0.001 | 0.21 |
| Vessel heterogeneity (1) | −126.45 | −518.05, 770.95 | 0.70 | ||
| FVC | Normalized vessel volume (%) | −11.61 | −15.40, −7.82 | <1 × 10−6 | 0.35 |
| Vessel heterogeneity (1) | −214.05 | −839.19, 411.08 | 0.50 | ||
| DLCO | Normalized vessel volume (%) | −5.58 | −8.16, −3.501 | 4 × 10−5 | 0.21 |
| Vessel heterogeneity (1) | −680.13 | −1103.32, −256.93 | 0.002 | ||
| KCO | Normalized vessel volume (%) | 1.13 | −2.83, 5.08 | 0.57 | 0.15 |
| Vessel heterogeneity (1) | −548.55 | −1198.28, 101.18 | 0.10 | ||
| TLC | Normalized vessel volume (%) | −12.63 | −15.28, −9.99 | <1 × 10−6 | 0.51 |
| Vessel heterogeneity (1) | −528.32 | −968.25, −88.39 | 0.01 | ||
| CPI | Normalized vessel volume (%) | 7.46 | 5.28, 9.65 | <1 × 10−6 | 0.34 |
| Vessel heterogeneity (1) | 558.28 | 231.26, 945.30 | 0.001 |
All models were adjusted for patient age, male gender, smoking status (never vs ever) and CT slice thickness (0.7 vs 1.0 mm).
CPI, composite physiologic index; CT, computed tomography; DLCO, diffusion capacity for carbon monoxide; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; KCO, transfer factor for carbon monoxide; TLC, total lung capacity.
Artery and vein separation
Correlations were strong between normalized arterial and venous volume (R2 = 0.70, P < 1 × 10−6), arterial and venous density (R2 = 0.83, P < 1 × 10−6), arterial and venous tortuosity (R2 = 0.68, P < 1 × 10−6) and arterial and venous heterogeneity (R2 = 0.54, P < 1 × 10−6). Strong links between normalized vessel volume and vessel density and between vessel tortuosity and vessel heterogeneity were maintained when arteries and veins were examined separately (Table S2, Supplementary Information). All functional linkages for normalized vessel volume and density were maintained, although weakly, when separately examined in arteries and veins (Tables 2, 3, 4).
DISCUSSION
Our study has used a novel vessel quantitation tool to confirm previous observations of correlations between the normalized volume of pulmonary vessels and lung function indices in IPF patients. The number of vessels per lung volume (vessel density), a new quantitative vessel parameter, was strongly correlated with the normalized vessel volume and also correlated to measures of lung function. The vessel heterogeneity (distribution width of tortuosity) was independently linked with DLCO, TLC and CPI. No vessel metric was significantly correlated with KCO whilst there were weak linkages with DLCO, suggesting that macrovascular morphology is poorly linked to microvascular disease. Vessel metrics distant to areas of fibrosis demonstrated stronger functional correlations than metrics in the lower lung zones. Separation of arteries and veins demonstrated no additional functional information beyond that explained by the whole vasculature.
The importance of the pulmonary vessels as prognostic indices in IPF has primarily been considered in relation to pulmonary hypertension. The main pulmonary artery diameter,16 the pulmonary artery/aorta ratio17 and quantitatively derived vessel tortuosity15 have been shown to predict the likelihood of pulmonary hypertension and of exacerbations in COPD.18 However, quantitation of the combined pulmonary arteries and veins (excluding hilar vessels) by a computer tool, CALIPER, linked strongly to functional indices7 and mortality,8 but only weakly with indirect measures of pulmonary hypertension. The current analysis shows that further readouts of vessel morphology correlate with lung function parameters and may therefore also be of relevance as prognostic markers in IPF.
We identified relatively stronger inverse relationships between lung function indices and vessel volume in the upper/middle zones compared to the lower lung zones. These results are in line with previous reports of stronger links between FVC decline and mortality in baseline upper/middle zone VRS when compared to lower zone VRS in patients with IPF.9 MRI studies have demonstrated that fibrotic tissues show delayed contrast enhancement when compared to morphologically normal appearing lung in lung fibrosis patients.19, 20 A local increase in pulmonary vascular resistance in fibrotic areas may reduce regional pulmonary blood flow due to both hypoxic vasoconstriction and proliferative remodelling caused by inflammation. This may cause increased pulmonary arterial pressure and increased vessel size in unaffected vessels in regions of normal lung parenchyma which may therefore act as a surrogate marker of both pulmonary hypertension and the extent of interstitial disease.
In addition to vessel tortuosity, we evaluated a new metric of vessel heterogeneity that captured variations in vessel tortuosity which would be expected to differ between regions of fibrosis and normal lung parenchyma. Examining vessel heterogeneity was motivated by the histopathological description of IPF as spatially and temporally heterogeneous disease.21 The relationship of vessel heterogeneity with lung function indices was strongest in the lung mid‐zones which contain the largest mix of normal and fibrotic tissue in IPF lungs and therefore the widest range of vascular morphology. Further work is necessary to determine how vessel tortuosity is linked with the development and progression of fibrosis and how this might influence local changes in perfusion.
Linkages were identified between quantitatively derived total lung volume and mean lung attenuation reflecting the increase in parenchymal collagen deposition and functional airspace loss as well as the concomitant shrinkage of the functional lung volume as a result of lung fibrosis. Mean lung attenuation, which captures a global picture of fibrosis extent/severity in the lung, was strongly correlated with normalized vessel volume and vessel density. The association of normalized vessel volume and vessel density with overall fibrosis extent was also captured in the strong links for both variables with CPI which reflects the morphological extent of lung fibrosis on a CT scan.11 The observed linkages all go to confirm previous reports describing marked associations between increases in normalized vessel volume with increased total extent of ILD.7 Intriguingly, vessel heterogeneity did not demonstrate linkages with mean lung attenuation, total lung volume or total vessel volume, yet was independently linked to TLC and CPI, suggesting that vessel heterogeneity might reflect an independent facet of lung damage.
Functional correlations of quantitative artery and vein metrics were similar or weaker than total vessel metrics. The challenges associated with performing accurate artery/vein separation are considerable and our findings suggest that quantifying total lung vessels might yet be a more pragmatic approach to evaluating functionally important measures in IPF.
There were limitations to the current study. Patients excluded from our analysis had significantly more severe disease than study patients, which may have introduced bias into our analysis. However, the mean DLCO in our study cohort (39.1%) was similar to the mean DLCO (43.7%) in the landmark Pirfenidone trial,22 indicating that our study population constituted an acceptable degree of baseline disease severity. We had no right heart catheterization data to examine links between quantitative vessel metrics and measures of pulmonary hypertension. Data sets of patients with concomitant CT imaging and right heart catheterization are likely to be encumbered by selection bias of their own and are rare in practice. Yet, such analyses remain a goal for future work.
In conclusion, we have shown that vessel metrics quantified by a novel algorithm for lung vessel analysis link strongly to lung function in IPF validating previously presented results using a different vessel quantification tool. Quantitative vessel metrics demonstrated weak overall linkages with DLCO and KCO, suggesting that metrics of macrovessels do not reflect functional microvasculopathy. Whilst total upper zone vessel metrics better predicted functional indices than lower zone metrics, separation of vessel metrics into pulmonary artery and vein subdivisions did not enhance functional correlations when compared to equivalent total vessel scores.
Disclosure statement
J.J. reports personal fees from Boehringer Ingelheim unrelated to the submitted work. A.D. reports personal fees from Boehringer Ingelheim and Roche unrelated to the submitted work. H.O. reports personal fees from participating in advisory boards and speaking at symposia from Actelion, Astra Zeneca, Bayer, Bellerophon, Boehringer Ingelheim, Chiesi, GSK, Menarini, MSD, Novartis and Pfizer, and received research grants from Actelion, Boehringer Ingelheim, Roche and Inventiva unrelated to the submitted work. A.U.W. receives personal fees for participating in advisory boards and speaking at symposia from Boehringer Ingelheim, Intermune, Roche and Bayer, and for participating in advisory boards from Gilead, MSD and speaker fees from Chiesi.
Abbreviations
- CPI
composite physiologic index
- CT
computed tomography
- DLCO
diffusion capacity for carbon monoxide
- FEV1
forced expiratory volume in the first second
- FVC
forced vital capacity
- HU
Hounsfield Unit
- ILD
interstitial lung disease
- IPF
idiopathic pulmonary fibrosis
- KCO
transfer factor for carbon monoxide
- MRI
magnetic resonance imaging
- PFT
pulmonary function test
- TLC
total lung capacity
- VRS
vessel‐related structure
Supporting information
Appendix S1 Additional methods.
Table S1 Group differences between patients included and excluded from the study population.
Table S2 Relationships between quantitative vessel metrics.
Table S3 Relationships between total lung volume and mean lung attenuation quantified by computer analysis, with computer‐derived vessel metrics.
Table S4 Relationships between pulmonary functional indices and total vessel metrics subdivided according to three equal sized lung zones.
Table S5 Multivariable linear regression relationships between pulmonary functional indices and zonal total vessel volume and vessel heterogeneity metrics.
Acknowledgements
The authors thank Nicola Giuliani for his excellent technical assistance. J.J. was supported by Wellcome Trust Clinical Research Career Development Fellowship 209553/Z/17/Z. C.P. was supported by the Austrian Science Fund (FWF): P28078‐N33.
Jacob, J , Pienn, M , Payer, C , et al. Quantitative CT‐derived vessel metrics in idiopathic pulmonary fibrosis: A structure–function study. Respirology. 2019;24:445–452. 10.1111/resp.13485
(Associate Editor: Helen Whitford; Senior Editor: Lutz Beckert)
REFERENCES
- 1. Flaherty KR, Thwaite EL, Kazerooni EA, Gross BH, Toews GB, Colby TV, Travis WD, Mumford JA, Murray S, Flint A et al Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax 2003; 58: 143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schwartz DA, Helmers RA, Galvin JR, van Fossen DS, Frees KL, Dayton C, Burmeister LF, Hunninghake GW. Determinants of survival in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 1994; 149(2 Pt 1): 450–4. [DOI] [PubMed] [Google Scholar]
- 3. Nathan SD, Shlobin OA, Weir N, Ahmad S, Kaldjob JM, Battle E. Long‐term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest 2011; 140: 221–9. [DOI] [PubMed] [Google Scholar]
- 4. Lynch DA, Godwin JD, Safrin S, Null N. High‐resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am. J. Respir. Crit. Care Med. 2005; 172: 488–93. [DOI] [PubMed] [Google Scholar]
- 5. Sumikawa H, Johkoh T, Colby TV, Ichikado K, Suga M, Taniguchi H, Kondoh Y, Ogura T, Arakawa H, Fujimoto K et al Computed tomography findings in pathological usual interstitial pneumonia: relationship to survival. Am. J. Respir. Crit. Care Med. 2008; 177: 433–9. [DOI] [PubMed] [Google Scholar]
- 6. Kim HJ, Brown MS, Chong D, Gjertson DW, Lu P, Kim HJ, Coy H, Goldin JG. Comparison of the quantitative CT imaging biomarkers of idiopathic pulmonary fibrosis at baseline and early change with an interval of 7 months. Acad. Radiol. 2015; 22: 70–80. [DOI] [PubMed] [Google Scholar]
- 7. Jacob J, Bartholmai B, Rajagopalan S, Kokosi M, Nair A, Karwoski R, Raghunath SM, Walsh SL, Wells AU, Hansell DM. Automated quantitative CT versus visual CT scoring in idiopathic pulmonary fibrosis: validation against pulmonary function. J. Thorac. Imaging 2016; 31: 304–11. [DOI] [PubMed] [Google Scholar]
- 8. Jacob J, Bartholmai B, Rajagopalan S, Kokosi M, Nair A, Karwoski R, Walsh S, Wells AU, Hansell DM. Mortality prediction in idiopathic pulmonary fibrosis: evaluation of automated computer tomographic analysis with conventional severity measures. Eur. Respir. J. 2016; 49: 1601011. [DOI] [PubMed] [Google Scholar]
- 9. Jacob J, Bartholmai BJ, Rajagopalan S, van Moorsel CHM, van Es HW, van Beek FT, Struik MHL, Kokosi M, Egashira R, Brun AL et al Predicting outcome in idiopathic pulmonary fibrosis using automated CT analysis. Am. J. Respir. Crit. Care Med. 2018; 198: 767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA et al; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis . An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis—evidence‐based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011; 183: 788–824. [DOI] [PMC free article] [PubMed]
- 11. Wells AU, Desai SR, Rubens MB, Goh NS, Cramer D, Nicholson AG, Colby TV, Du Bois RM, Hansell DM. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am. J. Respir. Crit. Care Med. 2003; 167: 962–9. [DOI] [PubMed] [Google Scholar]
- 12. Estepar RSJ, Ross JC, Harmouche R, Onieva J, Diaz AA, Washko GR. Chest imaging platform: an open‐source library and workstation for quantitative chest imaging. C66 Lung Imaging II: New Probes and Emerging Technologies, Brigham and Womens Hospital, Boston, MA, 2015; A4975‐A.
- 13. Payer C, Pienn M, Balint Z, Shekhovtsov A, Talakic E, Nagy E, Olschewski A, Olschewski H, Urschler M. Automated integer programming based separation of arteries and veins from thoracic CT images. Med. Image Anal. 2016; 34: 109–22. [DOI] [PubMed] [Google Scholar]
- 14. Bullitt E, Gerig G, Pizer SM, Lin WL, Aylward SR. Measuring tortuosity of the intracerebral vasculature from MRA images. IEEE Trans. Med. Imaging 2003; 22: 1163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Helmberger M, Pienn M, Urschler M, Kullnig P, Stollberger R, Kovacs G, Olschewski A, Olschewski H, Bálint Z. Quantification of tortuosity and fractal dimension of the lung vessels in pulmonary hypertension patients. PLoS One 2014; 9: e87515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chin M, Johns C, Currie BJ, Weatherley N, Hill C, Elliot C, Rajaram S, Wild JM, Condliffe R, Bianchi S et al Pulmonary artery size in interstitial lung disease and pulmonary hypertension: association with interstitial lung disease severity and diagnostic utility. Front. Cardiovasc. Med. 2018; 5: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ng CS, Wells AU, Padley SP. A CT sign of chronic pulmonary arterial hypertension: the ratio of main pulmonary artery to aortic diameter. J. Thorac. Imaging 1999; 14: 270–80. [DOI] [PubMed] [Google Scholar]
- 18. Wells JM, Washko GR, Han MK, Abbas N, Nath H, Mamary AJ, Regan E, Bailey WC, Martinex FJ, Westfall E et al Pulmonary Arterial Enlargement and Acute Exacerbations of COPD. N Engl J Med. 2012; 367: 913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lavelle LP, Brady D, McEvoy S, Murphy D, Gibney B, Gallagher A, Butler M, Shortt F, McMullen M, Fabre A et al Pulmonary fibrosis: tissue characterization using late‐enhanced MRI compared with unenhanced anatomic high‐resolution CT. Diagn. Interv. Radiol. 2017; 23: 106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mirsadraee S, Tse M, Kershaw L, Semple S, Schembri N, Chin C, Murchison JT, Hirani N, van Beek EJR. T1 characteristics of interstitial pulmonary fibrosis on 3T MRI—a predictor of early interstitial change? Quant. Imaging Med. Surg. 2016; 6: 42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Travis WD, King TE, Bateman ED, Lynch DA, Capron F, Center D, Colby TV, Cordier JF, Du Bois RM, Galvin JR et al ATS/ERS international consensus classification of idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2001; 165: 277–304. [Google Scholar]
- 22. King TE, Bradford WZ, Castro‐Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L et al A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014; 370: 2083–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Additional methods.
Table S1 Group differences between patients included and excluded from the study population.
Table S2 Relationships between quantitative vessel metrics.
Table S3 Relationships between total lung volume and mean lung attenuation quantified by computer analysis, with computer‐derived vessel metrics.
Table S4 Relationships between pulmonary functional indices and total vessel metrics subdivided according to three equal sized lung zones.
Table S5 Multivariable linear regression relationships between pulmonary functional indices and zonal total vessel volume and vessel heterogeneity metrics.


