Capsule Summary:
Plasma tryptase elevation during aspirin-induced respiratory reactions occurs in 50% of reactions despite CysLT1R antagonist prophylaxis and identifies lung function decline and extra-respiratory symptoms in aspirin-exacerbated respiratory disease (AERD).
Keywords: Aspirin-exacerbated respiratory disease (AERD), Tryptase, Mast Cell, Aspirin, Lung function, CysLT1R antagonist
To the editor:
Mast cell activation in the respiratory tract is a well-recognized feature of aspirin-induced respiratory reactions in patients with aspirin-exacerbated respiratory disease (AERD). This activation is reflected by release of tryptase and production of lipid mediators, which can result in substantial bronchoconstriction. The use of drugs that target cysteinyl leukotriene (cysLT) synthesis or that block the type 1 cysLT receptor (CysLT1R) can attenuate aspirin-induced bronchoconstriction but generally does not prevent manifestations of nasal congestion, sneezing and rhinorrhea during formal aspirin challenges.(1, 2) Hence, CysLT1R antagonists are used commonly to increase the safety of diagnostic aspirin challenges and therapeutic desensitization procedures. Nevertheless, some patients with AERD manifest substantial reductions in forced expiratory volume in one second (FEV1) despite prophylaxis with CysLT1R antagonists, in some cases associated with extra-respiratory symptoms of gastrointestinal distress, rash(3, 4) and rarely laryngospasm or hypotension. Serum or plasma tryptase levels increase in a subset of patients with AERD during their reaction to aspirin challenge. We hypothesized that mast cell activation, marked by an increase in plasma tryptase, drives lower airway narrowing (and potentially extra-respiratory symptoms) in subjects with AERD undergoing oral aspirin challenge in the setting of prophylaxis with montelukast, a CysLT1R antagonist.
To test this hypothesis, subjects with AERD were recruited to undergo a standardized one-day oral aspirin challenge protocol at the Brigham and Women’s Hospital AERD Center. All subjects were pretreated with the cysLT1R antagonist montelukast for 4 weeks prior to challenge. All subjects had well-controlled asthma in the previous 6 months with a baseline FEV1% predicted ≥ 70%. Aspirin administration started at a dose of 40 mg followed by doubling-dose increases every 90 minutes until the onset of an upper and/or lower respiratory reaction. Respiratory reactions were characterized by a decline in forced expiratory volume in one second (FEV1) of ≥15% and/or an increase in nasal symptom scores of ≥2 as assessed by the total nasal symptom score(5). Blood, urine, total nasal symptom scores (TNSS), and lung function were collected at baseline and during the 3-hour period following the onset of a respiratory reaction (Supplemental Table 1). Urinary eicosanoids, platelet-leukocyte aggregates, platelet activation markers, and plasma total tryptase were assessed as previously described.(6) Mast cell activation was defined as an increase in plasma total tryptase of >10% from baseline 1 hour after the onset of a respiratory reaction. Mean and standard deviation are reported and were compared using the Student’s unpaired or paired T-test. Correlations were assessed with Spearman’s correlation coefficient. All analyses were performed using GraphPad Prism version 7.03 for Windows, GraphPad Software, La Jolla California USA.
Of 39 subjects who completed our oral aspirin challenge protocol and met criteria for an aspirin-induced reaction, 19 demonstrated mast cell activation with a >10% increase in serum tryptase 1 hour after the onset of a reaction (denoted as MChigh) with the remaining 20 demonstrating less than a 10% rise in tryptase (MClow, Figure 1A), 16 of whom actually had a decline in tryptase. There were no differences in sex, age, or baseline peripheral blood eosinophil counts, FEV1% predicted, TNSS, urinary LTE4, prostaglandin D metabolite (PGD-M), or thromboxane B2 levels between the MChigh and the MClow groups (Supplemental Table 2). Numbers of platelet-adherent leukocytes and the extent of platelet activation in blood (percent of CD61+ platelets that were CD62P+) also did not differ between groups (data not shown). However, the baseline plasma tryptase level was slightly higher in the MClow subjects compared to the MChigh subjects (4.1 ± 1.8 vs 3.0 ± 1.5 ng/mL, P=0.043, Supplemental Table 2). The MChigh subjects with an increase in plasma tryptase >10% exhibited greater declines in FEV1 than did subjects in the MClow group (−16.8 ± 10.2% vs −6.2 ±12.8%, Figure 1B) without a corresponding difference in sino-nasal symptom scores (Figure 1C). Independent of group assignment, the change in plasma tryptase (%) inversely correlated with the FEV1% change (Figure 1D). Six subjects reported gastrointestinal symptoms during their aspirin-induced reaction, all of whom were in the MChigh group (data not shown). The provocative dose of aspirin needed to elicit symptoms of a reaction did not differ between subjects in the MChigh vs MClow groups.
Figure 1: Tryptase, FEV1 and Total Nasal symptom score stratified by level of plasma tryptase increase during aspirin-induced reaction.
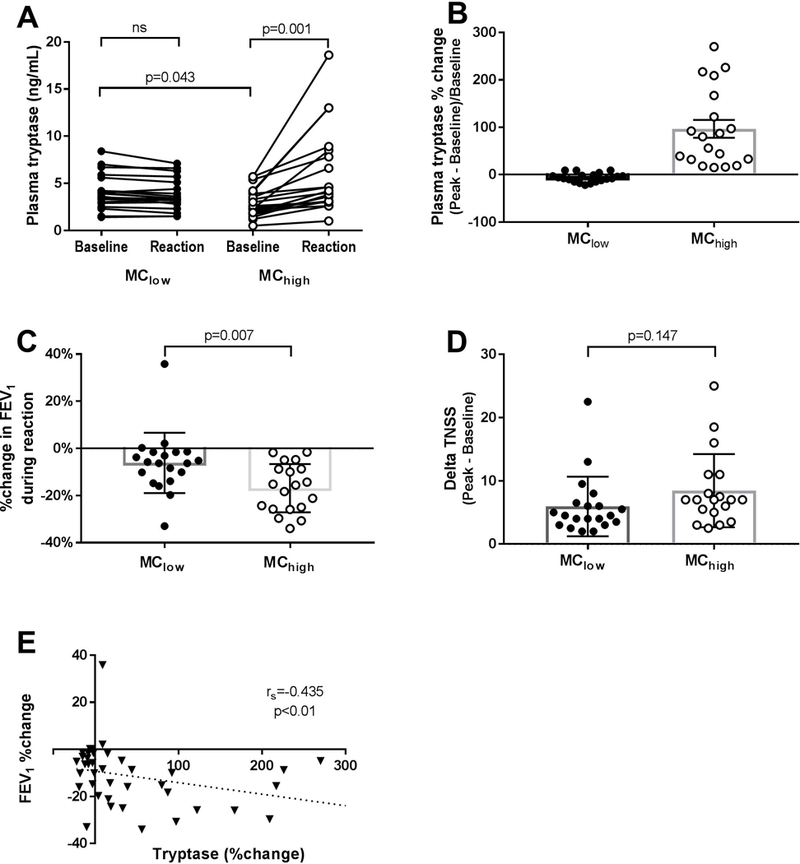
(MClow = ≤10% increase in plasma tryptase from baseline, MChigh = >10% increase in plasma tryptase from baseline). A, Plasma tryptase change from baseline during aspirin-induced reaction shown as ng/mL tryptase (left panel) and percent change in tryptase (right panel). B, Maximal percentage change in FEV1 during aspirin-induced reaction. C, Maximal change in total nasal symptom score (TNSS) during aspirin-induced reaction. D, Correlation between change in plasma tryptase and change in FEV1 during aspirin-induced reaction.
Compared with subjects in the MClow group, subjects in the MChigh group displayed higher peak urinary levels of LTE4 (1.75 ± 3.80 vs 15.71 ± 17.35 ng/mg creatinine, P=0.001) and PGD-M (2.69 ± 2.66 vs 16.27 ± 21.80 ng/mg creatinine, P=0.009), but did not differ in baseline or trough PGE-M or change in TXB2 (Figure 2A–D). The change in plasma tryptase (%) correlated with the peak urinary LTE4 and peak PGD-M levels across all 39 subjects (Figure 2E). As we have previously reported,(3) peak urinary LTE4 and PGD-M levels strongly correlated with each other and with the FEV1% change observed (data not shown). No difference in platelet-leukocyte aggregates or platelet activation as assessed by %CD62P positive during reaction were observed between groups (data not shown).
Figure 2: Change in urinary eicosanoids stratified by level of plasma tryptase change during aspirin-induced reaction.
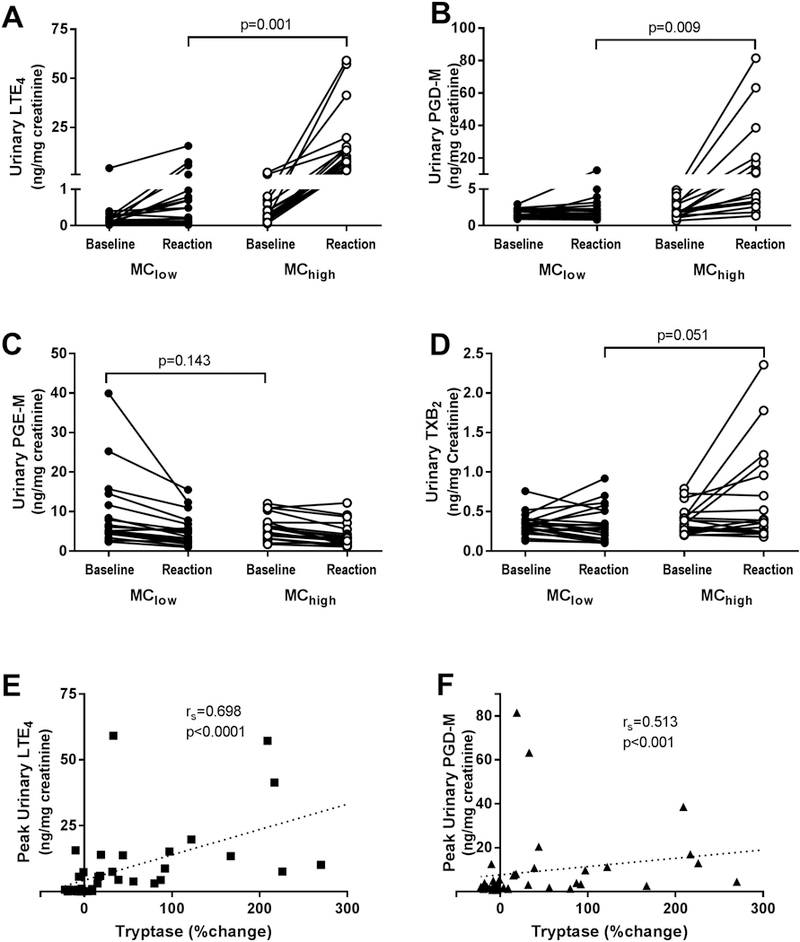
(MClow = ≤10% increase in plasma tryptase from baseline, MChigh = >10% increase in plasma tryptase from baseline). A, Baseline and peak leukotriene E4 (LTE4). B, Baseline and peak prostaglandin D metabolite (PGD-M). C, Baseline and nadir prostaglandin M metabolite (PGD-M). D, Baseline and peak thromboxane B2 (TXB2). For A-D, n=19 paired sets for MClow and n=19 paired sets for MChigh subjects. Correlations between change in plasma tryptase and peak urinary LTE4 (E) and PGD-M levels (F) are shown.
In a prior study of 17 subjects with AERD undergoing oral aspirin challenge, Bosso and colleagues reported that three subjects displayed increases in serum tryptase, as well as histamine.(4) Notably, all three subjects displayed reductions in FEV1 of >30%, moderate-to-severe naso-ocular symptoms, and two of the three developed gastrointestinal symptoms. Eicosanoids were not quantified. Importantly, this study pre-dated the availability of CysLT1R antagonists, which blunt cysLT-induced bronchoconstriction and effectively “shift” dominant symptoms from the lower to the upper respiratory tract.(1) Our study, conducted with subjects who were all on montelukast prophylaxis, demonstrates that 50% of subjects with AERD display an increase of >10% in plasma tryptase using a more sensitive assay than that used in the prior study.(6) The fact that the changes in tryptase correlated inversely with FEV1% change supports the bronchoconstrictive role of PGD2 and other smooth muscle-active mediators derived from mast cells, as well as potential functions of cysLTs at receptors other than CysLT1R. A prior study reported that endogenous cysLTs may drive mast cell activation in AERD, based on suppression of tryptase release by treatment with the 5-lipoxygenase inhibitor zileuton during an oral aspirin challenge.(7) It is unclear whether montelukast similarly alters mast cell function, or whether the effect of zileuton reflected involvement of CysLT2R and/or CysLT3R, as is suggested by murine models.(8) One subject demonstrated a >30% fall in FEV1 without an associated increase in tryptase, urinary LTE4 or PGD-M (Figure 1B) suggesting additional mast cell-independent pathways are possible. No baseline clinical differences between MChigh subjects and MClow subjects were observed, and the only biochemical difference noted at baseline was that MClow subjects had slightly higher plasma tryptase levels (Supplemental Table 2). This could suggest that the mast cells from the MClow patients actually start out with a baseline level of increased stimulation, though this is not supported by the urinary eicosanoid data, as neither urinary LTE4 or PGD-M are increased at baseline in the MClow group. Thus, there are still no biomarkers to date that can prospectively identify those subjects at risk for severe lower respiratory and systemic reactions during aspirin challenge.
While AERD is a highly distinctive syndrome, this work highlights that there are multiple endotypes of AERD. The difference in plasma tryptase may well reflect the extent of mast cell activation in the lung (and in some instances in the intestine), which comprise much larger mucosal surfaces (and many more mast cells) than the sinonasal mucosa. This difference in mast cell distribution and burden in the lungs vs the sinonasal mucosa may account for why the change in tryptase was more closely correlated to the change in FEV1 in our study. These differences provide one explanation for why some have respiratory tract-restricted disease and others demonstrate a systemic disease, and for why some exhibit lower respiratory tract involvement despite CysLT1R antagonist prophylaxis. How and whether these differences impact therapeutic response to daily high-dose aspirin therapy and novel biologic therapies available and in development for the treatment of moderate-to-severe asthma and nasal polyposis is not known. As we seek precision medicine, the contribution of mast cells needs to be considered.
Supplementary Material
Acknowledgments
Funding Source:
This work was supported by the National Institutes of Health (Grants K23HL111113, K23AI118804, R01HL128241, U19 AI095219 and T32AI007306–32), and by generous contributions from the Kaye and Vinik families.
References
- 1.White A, Ludington E, Mehra P, Stevenson DD, Simon RA. Effect of leukotriene modifier drugs on the safety of oral aspirin challenges. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2006;97(5):688–93. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson DD, Simon RA, Mathison DA, Christiansen SC. Montelukast is only partially effective in inhibiting aspirin responses in aspirin-sensitive asthmatics. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2000;85(6 Pt 1):477–82. [DOI] [PubMed] [Google Scholar]
- 3.Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D(2): a dominant mediator of aspirin-exacerbated respiratory disease. The Journal of allergy and clinical immunology. 2015;135(1):245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosso JV, Schwartz LB, Stevenson DD. Tryptase and histamine release during aspirin-induced respiratory reactions. The Journal of allergy and clinical immunology. 1991;88(6):830–7. [DOI] [PubMed] [Google Scholar]
- 5.Laidlaw TM, Cahill KN, Cardet JC, Murphy K, Cui J, Dioneda B, et al. A trial of P2Y12 receptor inhibition with prasugrel identifies a potentially distinct endotype of patients with aspirin-exacerbated respiratory disease. The Journal of allergy and clinical immunology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz LB, Bradford TR, Rouse C, Irani AM, Rasp G, Van der Zwan JK, et al. Development of a new, more sensitive immunoassay for human tryptase: use in systemic anaphylaxis. J Clin Immunol. 1994;14(3):190–204. [DOI] [PubMed] [Google Scholar]
- 7.Fischer AR, Rosenberg MA, Lilly CM, Callery JC, Rubin P, Cohn J, et al. Direct evidence for a role of the mast cell in the nasal response to aspirin in aspirin-sensitive asthma. The Journal of allergy and clinical immunology. 1994;94(6 Pt 1):1046–56. [DOI] [PubMed] [Google Scholar]
- 8.Liu T, Barrett NA, Kanaoka Y, Yoshimoto E, Garofalo D, Cirka H, et al. Type 2 Cysteinyl Leukotriene Receptors Drive IL-33-Dependent Type 2 Immunopathology and Aspirin Sensitivity. J Immunol. 2018;200(3):915–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


