Abstract
Obesity is the strongest risk factor for endometrial cancer (EC). To inform targeted screening and prevention strategies, we assessed the impact of obesity and subsequent bariatric surgery‐induced weight loss on endometrial morphology and molecular pathways implicated in endometrial carcinogenesis. Blood and endometrial tissue were obtained from women with class III–IV obesity (body mass index ≥40 and ≥50 kg/m2, respectively) immediately prior to gastric bypass or sleeve gastrectomy, and at two and 12 months’ follow up. The endometrium underwent pathological examination and immunohistochemistry was used to quantify proliferation (Ki‐67), oncogenic signaling (PTEN, pAKT, pERK) and hormone receptor (ER, PR) expression status. Circulating biomarkers of insulin resistance, reproductive function and inflammation were also measured at each time point. Seventy‐two women underwent bariatric surgery. At 12 months, the mean change in total and excess body weight was −32.7 and −62.8%, respectively. Baseline endometrial biopsies revealed neoplastic change in 10 women (14%): four had EC, six had atypical hyperplasia (AH). After bariatric surgery, most cases of AH resolved (5/6) without intervention (3/6) or with intrauterine progestin (2/6). Biomarkers of endometrial proliferation (Ki‐67), oncogenic signaling (pAKT) and hormone receptor status (ER, PR) were significantly reduced, with restoration of glandular PTEN expression, at 2 and 12 months. There were reductions in circulating biomarkers of insulin resistance (HbA1c, HOMA‐IR) and inflammation (hsCRP, IL‐6), and increases in reproductive biomarkers (LH, FSH, SHBG). We found an unexpectedly high prevalence of occult neoplastic changes in the endometrium of women undergoing bariatric surgery. Their spontaneous reversal and accompanying down‐regulation of PI3K‐AKT–mTOR signaling with weight loss may have implications for screening, prevention and treatment of this disease.
Keywords: obesity, bariatric surgery, weight loss, endometrial cancer, atypical endometrial hyperplasia
Short abstract
What's new?
Obesity is a major risk factor for endometrial cancer (EC). In this study, the authors found that, in obese women, bariatric surgery‐induced weight loss resulted in significant, beneficial changes in circulating biomarkers of insulin resistance, inflammation, and reproductive hormones, in endometrial morphology, and in molecular pathways that are implicated in endometrial carcinogenesis. The latter included changes in Ki‐67 expression and activation of the PI3K‐AKT‐mTOR oncogenic signaling pathway, including PTEN and pAKT. These results may have important implications for screening, prevention and treatment of EC.
Introduction
Endometrial cancer (EC) is the fourth most common cancer affecting women in the United Kingdom and its incidence is rising.1 Obesity is the strongest risk factor for type 1 EC2 and its precursor lesion, atypical hyperplasia (AH),3 with every 5 kg/m2 increase in body mass index (BMI) conferring a 1.6‐fold higher risk of EC. Consequently, women with class III‐IV obesity (BMI ≥40 kg/m2 and ≥50 kg/m2, respectively) have an almost tenfold higher lifetime risk of EC compared to women of normal weight,2 with an estimated 40% of all EC directly attributable to obesity.4 Furthermore, women with class III‐IV obesity who sustain long‐term weight loss after bariatric surgery show enduring reduction in EC risk in both retrospective studies5, 6 and the prospective Swedish Obesity Subjects study.7 The strength and consistency of the above relationships suggest that the obesity epidemic and the steep rise in EC rates are causally linked. Despite these epidemiological associations, the mechanisms linking obesity and EC are incompletely understood, but are likely to include the proliferative effects of excess estrogen unopposed by progesterone in noncycling premenopausal and postmenopausal endometrium, insulin resistance and the proinflammatory state observed with excess adiposity.8, 9
It is not known whether screening women with class III‐IV obesity for occult endometrial cancer is beneficial or whether weight loss induces predictable molecular changes in the endometrium. An explicit understanding of the molecular ‘cause and effect’ of obesity‐driven endometrial carcinogenesis would enable the design of prevention strategies to reduce the number of women dying from EC. We hypothesized that women with class III‐IV obesity are at high risk of endometrial malignancy, and that bariatric surgery‐induced weight loss instigates molecular changes that restore the endometrium to a low risk state. In our study, we used bariatric surgery as a model of EC risk reduction to gain insights into the endometrial response to acute weight loss.
Methods
Study design
This was a prospective cohort study of women undergoing bariatric surgery by laparoscopic gastric bypass or sleeve gastrectomy. We recruited women with a BMI ≥40 kg/m2 undergoing bariatric surgery assessment at a specialist weight management service in Greater Manchester, United Kingdom. Women who had a gastric band (two women), gastric balloon (one woman) or underwent medically supervised dietary calorie restriction instead of bariatric surgery (eight women) were excluded from the primary analyses. All participants gave written, informed consent. Approval was obtained from the North West Research Ethics Committee (12/NW/0050) and the study was prospectively registered on the UK clinical trial database (ISRCTN17241389).
Endometrial sampling and clinical management
Clinical measurements, blood and endometrial samples were acquired at baseline, 2 and 12 months after bariatric surgery. At baseline, we recorded the last menstrual period (LMP), menstrual bleeding pattern, contraceptive use and cervical cytology history. We defined postmenopausal status as last menses occurring >1 year before baseline; the remainder were defined as premenopausal. Height was measured using a stadiometer; weight was measured using electronic scales after removal of bulky clothing, and BMI was derived (kg/m2). Cervical screening was performed, if indicated, in accordance with the National Cervical Screening Program. Endometrial sampling was performed using a Pipelle© (Carefusion, UK) or MedGyn Endosampler© (MedGyn, IL). We rotated the device through 360° whilst sampling the full length of the uterine cavity to obtain a large and representative sample of the endometrium. The premenopausal participants were sampled in the late proliferative phase where possible, apart from baseline samples, which were obtained under general anesthesia during bariatric surgery.
Abnormal cervical cytology was managed according to the National Cervical Screening Guidelines.10 Women with endometrial pathology were discussed within the regional Gynecological Oncology Multidisciplinary Team (MDT). Women with EC were offered total laparoscopic hysterectomy and bilateral salpingo‐oophorectomy followed by adjuvant treatment if indicated. Women with AH had repeat endometrial sampling at 6–8 weeks. Persistent abnormalities were treated with 6 months of intrauterine progestin (Mirena coil). All women with resolved endometrial abnormalities were followed by 3‐ to 6‐monthly endometrial surveillance.
Blood biomarker quantification
We measured circulating biomarkers of a) reproductive function (luteinizing hormone, LH; follicle stimulating hormone, FSH; sex hormone binding globulin, SHBG; testosterone; free androgen index, FAI; estradiol; progesterone), b) insulin resistance (glucose and insulin to derive Homeostasis Model Assessment: Insulin Resistance, HOMA‐IR11; glycosylated hemoglobin A1c, HbA1c), c) adiposity (adiponectin, leptin) and d) inflammation (high sensitivity C‐reactive protein, hsCRP; interleukin‐6, IL‐6) under fasting conditions. With the exception of adiponectin, leptin, IL‐6 and hsCRP, all analytes were measured using standard automated clinical service protocols in the hospital Clinical Biochemistry Laboratory. Adiponectin, leptin and IL‐6 were measured using a DuoSet ELISA development kit (R&D Systems, Abingdon, UK). hsCRP was measured by an in‐house antibody sandwich ELISA technique with antihuman CRP primary antibodies from Abcam (Cambridge, UK).
Individuals were classified as having diabetes if they reported a history of this diagnosis, had a fasting glucose ≥7.0 mmol/l or HbA1c ≥ 48 mmol/l12; and were classified as insulin resistant if HOMA‐IR > 2.7.11
Histopathology and tissue biomarker quantification
Endometrial tissue was formalin‐fixed, paraffin embedded, sectioned and stained with hematoxylin and eosin. Morphology was assessed by a Specialist Gynecological Histopathologist using the WHO classification system.13, 14 Abnormalities were confirmed by second expert review. Tissue sections (4 μm) were baked for 30 min at 70 °C. The automated Ventana BenchMark Ultra IHC Staining Module (Ventana Co., Tucson, AZ) was used together with the Ultraview 3,3′‐diaminobenzidine (DAB) v3 detection system (Ventana Co.). Tissue sections were deparaffinized and incubated in EZPrep Volume Adjust (Ventana Co.). A heat‐induced antigen retrieval protocol was carried out using a TRIS–ethylenediamine tetracetic acid (EDTA)–boric acid pH 8 buffer, Cell Conditioner 1(CC1). The sections were incubated with ultraviolet inhibitor blocking solution for 4 min, then with an optimized concentration of antibody (Supporting Information Table S1). This was followed by incubation with horseradish peroxidase‐linked secondary antibody, DAB chromogen and copper. Counterstain (hematoxylin II) was applied for 12 min before a 4‐min incubation with bluing reagent. Slides were dehydrated through three steps of 99% IMS and two changes of Xylene. Sections were coverslipped using ClearVue Mount XYL (Thermo Scientific). Negative (isotype control) and positive tissue controls were used for quality assurance.
The Ki‐67 score was the proportion of glandular cells with positive nuclear staining. An overview of the whole slide allowed three representative fields to be chosen, including any abnormal areas, as identified by the study pathologists. The Ki‐67 score was determined from >1,000 nuclei scored in 3 representative high‐powered fields (x20); scanty samples were scored in their entirety.15 ER and PR staining was assessed by a modified H‐score (0–18), the product of area score (proportion of positively stained tissue, scored 0–6) and intensity of staining score (0 = none, 1 = mild, 2 = moderate, 3 = strong).16 Phosphorylated (p)AKT and phosphorylated (p)ERK staining was scored using the percentage of positively stained tissue [H = (3 × % strong staining) + (2 × % moderate staining) + (% weak staining)] to account for within tissue heterogeneity. Glandular and stromal positivity were scored separately for pERK. PTEN status was scored visually as ‘PTEN null’ if any endometrial glands not expressing PTEN protein were present, in the presence of immediately adjacent PTEN positive stroma. Slides were scored as ‘PTEN positive’ if all endometrial glands examined expressed PTEN.17
All scoring was performed manually by two independent scorers who were blinded to time point. Discrepant scores (>10% or disagreement as to PTEN status) were reviewed and resolved by consensus agreement. The Interobserver Intraclass Correlation Coefficients (ICCs) were greater than 0.95 (Cronbach's alpha for average measures = 0.976 (0.944–0.990) for Ki‐67 scoring). In menstruating women, the time of sampling relative to cycle phase was recognized as a potential confounder. Thus, we performed a planned subgroup analysis of women whose biopsies were taken at within‐individual matched menstrual cycle phases over time; who were not taking exogenous hormones; and who did not develop endometrial pathology during follow‐up.
Statistical analyses
The study was powered to observe a 5% reduction in Ki‐67 after bariatric surgery.18 Assuming a 50% correlation between biopsies timed for menstrual cycle phase, a sample size of 51 would have 80% power at α = 0.05 to detect a 5% change in Ki‐67. We enrolled 72 women to allow for 30% attrition due to protocol violation and loss to follow up.
Statistical analyses were performed using Graphpad Prism 5.0b for Mac (GraphPad Software, San Diego) and SPSS 23.0 for Mac (IBM Corp, Armonk, NY). Descriptive statistics included mean and standard deviation (SD) for normally distributed, and median and interquartile range (IQR), for nonparametric data. Within‐individual changes over time were compared using paired t‐test and Wilcoxon signed‐rank test for parametric and nonparametric data, respectively. To account for multiple testing, a conservative p value of less than 0·01 indicated statistical significance.
Results
Baseline characteristics
Our study population comprised 72 women with a mean age of 42 (range 24–65) years (Table 1, Fig. 1); 55 (76%) were premenopausal. The median weight, BMI and excess body weight at baseline were 134.8 kg, 52.1 kg/m2 and 76.6 kg, respectively. Overall, 24 (33%) had type 2 diabetes (T2DM), of whom three were diagnosed after study enrolment (baseline HbA1c levels ≥48 mmol/l), and a further 22 (28%) were insulin resistant (HOMA‐IR > 2.7). Thus, 46 (64%) of the total cohort were either insulin resistant or had T2DM. Only 22 (31%) women reported normal menstrual cycles; 33 (46%) had oligomenorrhea or amenorrhea and 20 (29%) had heavy menstrual or intermenstrual bleeding. Three of the 18 postmenopausal women reported bleeding, none of whom had histologically abnormal endometrium.
Table 1.
Baseline characteristics of study participants
| All participants (n = 72) | Cycle‐matched participants (n = 25) | |
|---|---|---|
| Mean age (SD), years | 42 (11.5) | 44 (9.3) |
| White British | 66 (91.7) | 24 (96) |
| Asian | 3 | 0 |
| Black | 2 | 0 |
| Other | 1 | 1 |
| Median BMI (IQR), kg/m2 | 52.1 (46.8–56.9) |
54.6 (46.8–59.7) |
| Median weight (IQR), kg | 134.8 (117.0–152.6) |
143.3 (124.4–169.4) |
| Median Waist Hip Circumference Ratio (IQR) | 0.87 (0.79–0.92) |
0.9 (0.82–0.92) |
| Median parity (IQR) | 2 (0–2) | 2 (1–3) |
| Menopausal status, n (%) | ||
| Premenopausal | 55 (76.4) | 20 (80) |
| Postmenopausal | 17 (23.6) | 5 (20) |
| Menstrual cycle, n (%) | ||
| Amenorrhoeic | 30 (41.7) | 4 (16) |
| Regular | 23 (31.9) | 7 (28) |
| Irregular | 19 (26.4) | 9 (36) |
| Abnormal bleeding, n (%) | ||
| HMB | 17 (23.6) | 5 (20) |
| IMB | 3 | 1 |
| PMB | 3 | 0 |
| PCB | 3 | 0 |
| Exogenous hormones, n (%) | ||
| HRT | 3 (4.2) | 0 |
| Progestins | 9 (12.5) | 0 |
| PCOS | 13 (18.1) | 7 (28) |
| Yes | 24 (33.3) | 8 (32) |
| No | 48 (66.6) | 17 (68) |
| Metformin | 20 (27.8) | 7 (28) |
| Insulin | 7 (9.7) | 4 (16) |
| Other1 | 8 (11.1) | 0 |
IQR interquartile range; SD standard deviation. Values in parentheses are percentages unless otherwise specified. Percentages not shown if numerator less than 5.
*HMB Heavy Menstrual Bleeding, IMB Intermenstrual Bleeding, PMB Postmenopausal Bleeding; §Progestin contraceptive (progesterone‐only pill, injectable);
Other medications (liraglutide, glibenclamide, gliclazide).
Figure 1.
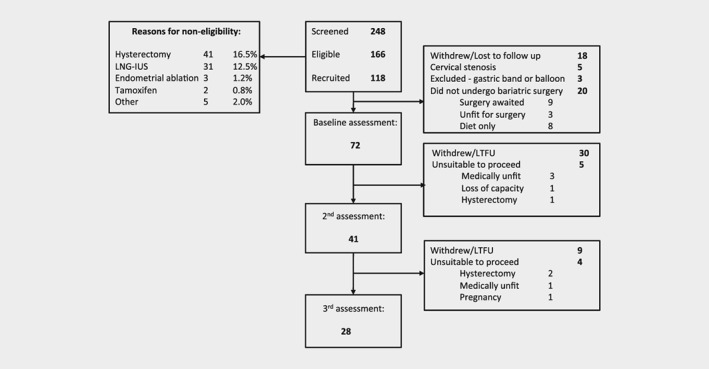
Accrual and retention of participants
Cervical screening was overdue in 36 (50%) women, and was performed opportunistically. Negative cytology was found in 31 women (86%), 2 (6%) had high risk HPV negative borderline cytology and one woman had HSIL with suspicion for invasion and subsequently underwent radical trachelectomy and laparoscopic bilateral pelvic lymphadenectomy for a stage 1b1 squamous cell carcinoma of the cervix.
Bariatric surgery and weight changes
In total, 53 women had a laparoscopic gastric bypass (74%) and 19 had a laparoscopic sleeve gastrectomy (26%). At 12 months, mean change in total and excess body weight was −32.7 and −62.8%, respectively (Table 2 ).
Table 2.
Weight change after bariatric surgery
| Baseline (T0) | 2 months (T1) | 12 months (T2) | Mean difference (95% CI) | ||
|---|---|---|---|---|---|
| T1 vs. T0 | T2 vs. T0 | ||||
| n | 72 | 41 | 28 | ||
| Mean weight (SD), kg | 144.4 (24.4) | 122.8 (23.3) | 97.7 (22.6) | −21.8 (−24.8 to −18.7) p < 0.0001 | −47.2 (−53.3 to −41.1) p < 0.0001 |
| Mean BMI (SD), kg/m2 | 53.6 (7.7) | 45.6 (7.3) | 36.1 (7.2) | −8.21 (−9.3 to −7.1) p < 0.0001 | −18.76 (−20.8 to −16.7) p < 0.0001 |
| Mean change in total body weight (SD), % | −15.2 (5.9) | −32.7 (9.9) | |||
| Mean change in excess body weight (SD), % | −20.6 (37.4) | −62.8 (20.3) | |||
| Mean change in BMI (SD), kg/m2 | −8.1 (3.4) | −18.8 (4.6) | |||
SD: standard deviation. CI: confidence interval.
Endometrial pathology changes
Of the 72 women, 10 (14%) had an endometrial abnormality at baseline; four had EC and six had AH (Table 3 ). All women with EC underwent hysterectomy. Women with and without endometrial pathology were of similar age (42.6 vs. 42.9 years) and BMI (53 vs. 52 kg/m2). Of the 10 women with endometrial pathology, eight were premenopausal and eight had known or undiagnosed T2DM or insulin resistance (HOMA‐IR > 2.7).
Table 3.
Characteristics of women found to have endometrial pathology
| ID | Age (years) | Baseline BMI (Kg/m2) | Bleeding pattern at baseline | Bariatric surgery procedure | % Total body weight loss at 12 months | T0 Baseline | T1 6–8 weeks | T2 12 months | Management of endometrial abnormality (Final diagnosis) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Morphology | Ki‐67 (%) | Glandular PTEN staining | Morphology | Ki‐67 (%) | Glandular PTEN staining | Morphology | Ki‐67 (%) | Glandular PTEN staining | |||||||
| 1 | 42 | 46.1 | HMB Regular |
Bypass | −24.3 | Grade 1 EEC | 5.2 | positive | Grade 2 EEC |
54.8 | positive | N/A | Hysterectomy (3a G2 EEC)1 |
||
| 2 | 52 | 51.9 | None | Bypass | N/A | Grade 1 EEC | 10.73 | null | Grade 1 EEC | 5.6 | positive | N/A | Hysterectomy (1a G1 EEC) |
||
| 3 | 55 | 54.6 | None | Bypass | N/A | Grade 1 EEC | 45.51 | null | Grade 1 EEC | 12.7 | positive | N/A | Hysterectomy (1a G1 EEC) |
||
| 4 | 24 | 59.7 | HMB Oligomenorrhoea |
Bypass | −13.8 | Grade 1 EEC | 24.1 | positive | Grade 1 EEC | 4.2 | positive | Grade 1 EEC | 2.71 | positive | LNG‐IUS Hysterectomy (1a G1 EEC) |
| 5 | 44 | 57.1 | Amenorrhea | Sleeve | −33.0 | Squamous metaplasia | 22.5 | positive | Normal | 20.6 | positive | Grade 1 EEC | 34.8 | null | Hysterectomy (1a G1 EEC) |
| 6 | 35 | 69.9 | Normal | Bypass | −34.1 | AH, concerning for carcinoma | 50.3 | null | AH | 49.4 | positive | Normal | 9.8 | positive | LNG‐IUS 6/12 |
| 7 | 43 | 47.5 | HMB Oligomenorrhoea |
Sleeve | −30.1 | AH | 46.6 | null | AH | 49.4 | null | AH BOT2 |
20.6 | positive | Hysterectomy (tiny focus AH) |
| 8 | 39 | 56.9 | HMB Oligomenorrhoea |
Sleeve | −39.6 | AH | 60.8 | positive | Normal | 0.53 | positive | Normal | 21.8 | positive | No intervention |
| 9 | 45 | 57.0 | IMB | Bypass | −42.5 | AH | 65.4 | null | AH | 46.2 | positive | Normal | 0.43 | positive | LNG‐IUS 6/12 |
| 10 | 40 | 41.6 | Oligomenorrhoea | Bypass | −15.8 | AH | 27.8 | null | Normal | 0.13 | ‐ | Normal | 23.3 | null | No intervention |
| 11 | 51 | 46.8 | HMB Oligomenorrhoea |
Bypass | −21.5 | AH | 37.5 | null | Normal | 32.6 | positive | Normal | 15.0 | positive | No intervention |
| 12 | 49 | 54.9 | Normal HMB at T2 |
Sleeve | −34.0 | Normal | 5.0 | null | Normal | 35.5 | positive | Squamous metaplasia4 | 27.5 | null | No intervention |
AH atypical hyperplasia, BOT borderline ovarian tumor, EEC type 1 (endometrioid) endometrial carcinoma, HMB heavy menstrual bleeding, IMB intermenstrual bleeding; LNG‐IUS levonorgestrel intrauterine system.
Recurrence (omental nodules) at 18 months; all other women remain in remission from EC; 2Final histology at hysterectomy and bilateral salpingo‐oophorectomy at 5 months post‐bariatric surgery; 3Sample taken in luteal phase; 4Mild abnormality suspicious but not diagnostic for AH, with squamous metaplasia.
Of the six women with AH, resolution occurred in three women within 2 months of bariatric surgery after weight loss of between 9 and 22% of their total body weight, and in two other women within 6 months with intrauterine progestin. In four of the six, resolution of AH was accompanied by restoration of normal menstrual cycles. No recurrence of AH has been detected in the five women whose endometrium was managed nonsurgically at up to 4 years of follow up. Another woman had squamous metaplasia on her first biopsy. Her subsequent outpatient hysteroscopy and biopsy were normal, as was her repeat biopsy 2 months later. Her 12‐month biopsy, however, showed grade 1 endometrioid EC and she was treated by hysterectomy. A further case of squamous metaplasia was detected on the 12‐month biopsy of a woman with previously normal histology at baseline and 2 months, which normalized spontaneously after further weight loss.
Changes in endometrial tissue biomarkers
Baseline Ki‐67 varied with menopausal status, endometrial morphology and menstrual cycle phase. In histologically normal endometrium, Ki‐67 scores were lower in postmenopausal [median: 5 (interquartile range, IQR: 1, 13)%] than premenopausal women [median: 30 (IQR: 9, 44)%]. As expected, higher Ki‐67 scores were seen in both EC and AH compared to normal endometrium [median: 36 (IQR: 21, 48)% vs. 23 (IQR: 5, 43)% p = 0.17]. Ki‐67 scores were higher in the proliferative [median: 33 (IQR: 15, 43)%] than secretory menstrual cycle phase [median: 20 (IQR: 4, 52)%]. There was no significant correlation between baseline Ki‐67 and BMI or index of insulin resistance.
Compared to baseline, median Ki‐67 score was 15.1% lower at 2 months (p = 0.009) and 15.8% lower at 12 months after bariatric surgery (p = 0.034) (Fig. 2 a and b). Analysis of a subgroup of women who had follow‐up samples matched for menstrual cycle phase showed significant reductions in Ki‐67 scores. The median Ki‐67 score was 29.3%, 18.4% and 12.3% at baseline, 2 and 12 months, respectively, and the mean difference in Ki‐67 was −11.24% (95% CI ‐20.67, −1.81; p = 0.0028) and − 17.79% (95% CI ‐34.42, −1.17; p = 0.016) at 2 and 12 months post bariatric surgery, respectively (Fig. 2 c and d).
Figure 2.
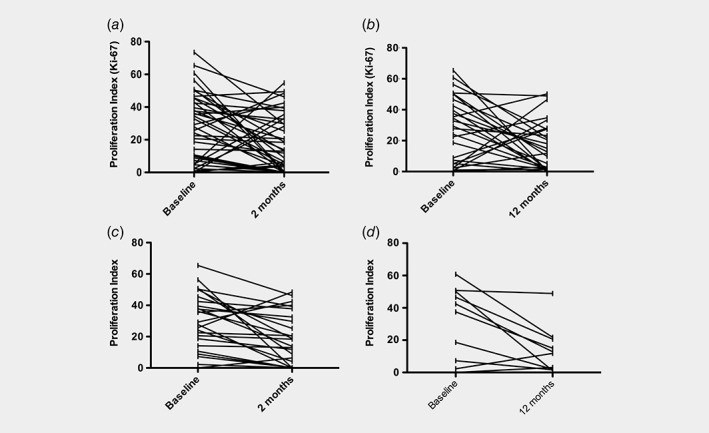
The effect of weight loss on Ki‐67 expression in the endometrium. Compared with baseline, at 2 months a lower Ki‐67 was observed in 29/41 (71%) women (range −1.3% to −60.3%); the remaining 11 (34%) showed static or higher Ki‐67 levels (range 2.5% to 49.6%). Women whose Ki‐67 increased during follow up (n = 11) either developed an abnormal endometrium (n = 3), underwent sampling during a different phase of the menstrual cycle (n = 2), or established regular menstruation after >6 months of amenorrhea (n = 2). Four cases were unexplained. (a) Ki‐67 scores in the bariatric surgery cohort at baseline and 2 months (−15.1% at 2 months, p = 0.009), and (b) at baseline and 12 months (−15.8% at 12 months, p = 0.034); (c) Ki‐67 scores in the menstrual cycle controlled bariatric surgery subgroup at baseline and 2 months (−11.24% at 2 months, p = 0.0028), and (d) at baseline and 12 months (−17.79% at 12 months, p = 0.016).
Compared to baseline, glandular phosphorylated AKT (pAKT) was 15% lower at 2 months (p = 0.03) and 11.7% lower at 12 months (p = 0.002) after bariatric surgery. Global ER and PR expression were lower after bariatric surgery [ER mean difference in H‐score at 2 months −1.5 (95% CI ‐3, 0.1), p = 0.025 and at 12 months −2.3 (95% CI ‐4, −0.3), p = 0.04; PR mean difference in H‐score at 2 months −1.4 (95% CI ‐3, 0.2), p = 0.031 and at 12 months −3.5 (95% CI ‐5.8, −1.1), p = 0.004)]. There was no change in pERK expression over time.
In total, 12/72 (24%) baseline biopsies were glandular PTEN null, including 7/10 of the women with baseline endometrial abnormalities, of whom 6/7 regained glandular PTEN expression as their endometrial abnormalities resolved (Table 3). Five women (8%) with histologically normal endometrium were PTEN null at baseline and 4/5 were PTEN positive by 2 or 12 months, whilst four women went from PTEN positive to PTEN null over the same time period, including both women who developed endometrial abnormalities during follow up. Loss of PTEN expression coincided with development of their endometrial neoplastic lesions.
Changes in circulating biomarkers
As expected, there were significant reductions in circulating biomarkers of insulin resistance (HbA1c, HOMA‐IR) and inflammation (hsCRP, IL‐6), and significant increases in LH, FSH, and SHBG at two and 12 months after the considerable weight loss resulting from bariatric surgery (Table 4).
Table 4.
Changes in markers of insulin resistance, adiposity, inflammation and reproductive function at 2 and 12 months after bariatric surgery
| T0 (n = 72) | T1 vs. T0 (n = 41) | T2 vs. T0 (n = 27) | |||
|---|---|---|---|---|---|
| Baseline mean values (SD) | Mean difference (95% CI) | p Value | Mean difference (95% CI) | p Value | |
| Glucose, mmol/L | 7.4 (2.8) | −2.4 (−3.6, −1.3) | 0.0003 | −3.3 (−4.2, −2.2) | <0.0001 |
| Insulin, mU/L | 17.3 (13.1) | −6.7 (−11.8, −1.6) | 0.02 | −12.4 (−18.6, −6.1) | <0.0001 |
| HOMA‐IR | 6.6 (7) | −4.5 (−7.4, −1.5) | 0.0032 | −6.8 (−10.0, −3.6) | <0.0001 |
| HbA1c, mmol/mol | 47.7 (19.9) | −10.5 (−16.0, −4.9) | <0.0001 | −11.3 (−17.9, −4.7) | <0.0001 |
| Adiponectin, mg/L | 1.7 (1.7) | 0.47 (0.17, 1.1) | <0.0001 | 1.3 (0.97, 1.7) | <0.0001 |
| Leptin, ng/mL | 56.3 (27.1) | −8.1 (−13.2, −2.9) | 0.001 | −25.3 (−34.0, −16.6) | <0.0001 |
| hsCRP, mg/L | 7.4 (6.3) | −0.05 (−1.7, −1.6) | 0.35 | −4.3 (−6.3, −2.3) | <0.0001 |
| IL‐6, pg/ml | 6 (6.9) | −1.5 (−6.8, −3.8) | <0.0001 | −5.7 (−8.9, −2.6) | <0.0001 |
| Estradiol, pmol/L | 273 (218) | 76.8 (−15, 168) | 0.15 | 9.7 (−14.1, 161) | 0.55 |
| Progesterone, ng/ml | 5.8 (6.9) | −1.5 (−5.5, 2.6) | 0.011 | 1.5 (−3.8, 6.9) | 0.53 |
| Testosterone, nmol/L | 1 (0.4) | −0.2 (−0,4, 0.0) | 0.06 | −0.3 (−0.8, 0.4) | 0.003 |
| SHBG, nmol/L | 31.4 (22.1) | 26.7 (17.5, 35.9) | <0.0001 | 44.0 (30.5, 57.5) | <0.0001 |
| FAI | 4.1 (3.2) | −1.9 (−2.9, −0.9) | 0.0002 | −2.1 (−3.2, −1.1) | 0.0010 |
| LH, IU/L | 8.6 (7) | 6.4 (2.1, 10.8) | 0.015 | 5.2 (1.4, 9.0) | 0.0051 |
| FSH, IU/L | 10.1 (12.1) | 5.6 (1.4, 9.8) | 0.0008 | 8.8 (2.9, 14.8) | 0.0005 |
SD: standard deviation. CI: confidence interval.
Discussion
Main findings
This prospective study found a high prevalence of endometrial pathology in women with class III‐IV obesity selected for gastric bypass or sleeve gastrectomy at a regional specialist weight management service. Bariatric surgery resulted not only in significant weight loss amounting to a 20% fall in BMI, and 63% excess body weight loss, but also consequent serial improvements in circulating biomarkers of insulin resistance, reproductive function and inflammation, and perhaps most striking of all, sustained resolution of AH. This latter finding provides compelling evidence for a causal relationship between obesity and endometrial neoplasia, with implications not only for its treatment but also for the detection of molecular intermediary biomarkers of risk. We observed reductions in endometrial Ki‐67, pAKT, hormone receptors and restoration of glandular PTEN expression with weight loss, both in the whole cohort and the subgroup of women matched for menstrual cycle phase.
Strengths and limitations
There were three key strengths to our study. First, we successfully assessed baseline prevalence of endometrial pathology in women with class III‐IV obesity undergoing bariatric surgery. Previous studies have been limited in this regard by small sample size/high technical failure rates, lower average BMI, a high proportion of women on combined hormonal contraception, and women excluded because of abnormal bleeding. We were successful in achieving endometrial sampling at 2 and 12 months follow up without anesthesia, whereas other studies have found this to be clinically impossible for a significant minority of women. Second, we successfully controlled for menstrual cycle phase in most follow‐up samples, which impacts blood (hormone) and tissue (Ki‐67, proliferation pathways, hormone receptor status) biomarkers more than does obesity and weight loss, and found that our hypothesis of reduced Ki‐67 after bariatric surgery was upheld. Third, serial follow‐up sampling at two and 12 months distinguished the more immediate metabolic consequences of bariatric surgery from longer‐term loss of adiposity.
A limitation of our work was the small sample size, and the lack of a weight‐stable BMI matched control group. The comparatively high rates of loss to follow‐up were not unexpected, in part reflecting discomfort associated with the biopsy procedure, but despite this attrition the per protocol analysis was sufficiently powered to show a significant Ki‐67 reduction. The women who followed a medically supervised low calorie diet (n = 8) failed to achieve or maintain weight loss at 12 months and were excluded from the primary analyses; however, we observed no significant changes over time in their circulating or tissue biomarkers of endometrial cancer risk (data not shown). The prevalence of endometrial pathology in our study may be greater than that in the general obese population because our participants had presented for bariatric surgery and subsequently met stringent UK eligibility criteria, meaning there was a high prevalence of diabetes and high median BMI. We chose the late proliferative phase (day 12) for endometrial sampling to avoid reliance on ovulation (which was highly variable in our cohort), however, we could not always control for cycle phase due to irregular periods and the date chosen for bariatric surgery.
In the context of what is known, several comments can be made. Previous studies19, 20, 21 found rates and severity of endometrial pathology lower than ours, probably because in these other studies, progestin therapy was frequent, women with abnormal bleeding were excluded and failed or inadequate biopsies were common. Our participants were more obese and insulin resistant than women in previously reported cohorts,22 which may be causally important.23, 24 The high prevalence of insulin resistance /T2DM in EC patients is well established.25, 26 Elevated circulating insulin and insulin‐like growth factor (IGF)‐I are thought to drive endometrial proliferation through PI3K‐AKT–mTOR pathway activation.9 In our study, bariatric surgery precipitated changes in systemic metabolic parameters, inflammatory markers and reproductive hormone profiles, which mirrored published work,21, 27, 28 however the role of these systemic changes in mediating tissue effects remains unclear.
Molecular biomarkers of endometrial cancer risk
Besides occult AH and EC in a proportion of our participants at baseline, class III‐IV obesity was associated with high Ki‐67, high pAKT and loss of PTEN expression in phenotypically normal glands. This pro‐proliferative milieu sets the scene for mutational events that instigate endometrial carcinogenesis.29 Further, the combined expression profile of these endometrial biomarkers provides a putative molecular signature that denotes the ‘at risk’ endometrium, and thus an opportunity for targeted screening, risk reducing interventions and primary prevention of EC. In women with endometrial abnormalities, glandular PTEN loss was common at baseline but restoration of PTEN expression accompanied resolution of AH. In women with morphologically normal endometrium, PTEN null glands at baseline seldom persisted through weight loss, suggesting that bariatric surgery may be effective at clearing these latent endometrial precursor lesions. Thus, glandular PTEN loss may be a reversible feature of early endometrial carcinogenesis and its restoration may indicate the transition to low risk endometrium.30 The clearance of PTEN null glands has been described in women treated with intrauterine progestin,30 but not after weight loss in obese women. PTEN expression was inversely correlated to pAKT expression, consistent with previous work.31
Clinical implications
Standard management of postmenopausal AH is hysterectomy because not only is there a risk of coexisting EC, but also the risk of progression to EC over 10 years is around 20%.32, 33, 34 With rising rates of AH observed among young women, there is a need for fertility sparing treatment. Weight loss is associated with a reduced risk of EC in epidemiological studies35, 36 and our results demonstrate for the first time that bariatric surgery is capable of not only reversing AH but also sustaining normal morphology. AH lesions detected at baseline in three of six women had resolved at 6–8 weeks postbaseline sampling, and had not relapsed out to 4 years. Two further women responded to intrauterine progestin and remained disease‐free out to 4 years. The mechanisms of EC risk reduction after bariatric surgery may be two‐fold: early acute metabolic changes with improved insulin sensitivity, followed by enduring hormonal changes due to loss of adiposity. In our study, however, there were no significant changes in serum estradiol or progesterone levels, raising uncertainty as to their role in AH resolution. Recurrence of AH is common after cessation of progestin therapy.37, 38 However, risk modification through sustained improvements in insulin resistance and reduction of BMI may reduce the risk of relapse.39, 40
A Ki‐67 drop was observed at 2 months postbariatric surgery that was sustained at 12 months. This drop in Ki‐67 was observed in most women with endometrial pathology who underwent repeat sampling, and coincided with disease regression. By contrast, a Ki‐67 rise was observed in participants who developed EC or showed progressive disease on repeat sampling (Table 3). Ki‐67 is a known prognostic biomarker in EC.41, 42, 43 We hypothesize that a falling Ki‐67 is associated with reduced EC risk, and that women who do not demonstrate a Ki‐67 drop might be best served by longer‐term surveillance.
The high prevalence of endometrial pathology in our study cohort suggests that women referred for bariatric surgery should undergo both cervical and endometrial screening. Because screening by endometrial biopsy is technically challenging in the outpatient setting (7% failure rate in the current study), there is now a clear need for effective noninvasive screening tests for EC, and studies to address this are underway.
In the UK, current clinical practice guidelines do not consider risk factors when determining which women with abnormal bleeding should be referred for investigation. Retrospective data demonstrates that obesity is more predictive of endometrial abnormality than age.44 Our study suggests that abnormal bleeding in premenopausal women with class III‐IV obesity should be taken seriously as a ‘red flag’ symptom for cancer and investigated appropriately.
Our study emphasizes the potential for nonsurgical treatment strategies, specifically bariatric surgery‐induced weight loss and hormone therapy, for women with AH/EC, since hysterectomy is not without risk and renders premenopausal women infertile. Intrauterine progestin shows significant promise in this regard45 but response is variable.46 The additional benefit of weight loss achieved through dietary intervention in obese women with AH/EC treated with intrauterine progestin is being assessed in the ANZGOG feMMe study.47 Our data support the notion that AH is reversible and that its subsequent course may be influenced by obesity and weight loss. Bariatric surgery alone or in combination with intrauterine progestin is an attractive treatment option for obese women with AH that warrants further study.
In conclusion, our study has demonstrated that occult endometrial abnormalities are common in women with class III‐IV obesity undergoing bariatric surgery. Furthermore, the acute and profound weight loss that occurs after bariatric surgery exerts highly significant changes in the endometrium. Not only is weight loss associated with resolution of AH, but the molecular changes that ensue even in morphological normal endometrium herald the transition from high to low risk endometrium. In the context of the current global obesity crisis and consequent emerging epidemic of EC,48, 49 this may have important implications for the screening, prevention and treatment of EC. The high Ki‐67‐high pAKT‐PTEN null molecular signature in phenotypically normal endometrium may identify a woman as at high risk of future EC and thus suitable for targeted screening and primary prevention strategies. The success of these strategies may in turn be judged against the persistence or clearance of the molecular signature. Accurate risk prediction combined with development of clinically effective risk reducing interventions is fundamental to reversing the upward trajectory of new diagnoses and corresponding deaths from EC in those parts of the world where obesity is the major health challenge facing women today.
Authors’ contribution
EJC was Principal Investigator, obtained funding for the study and is its guarantor. MLM recruited to the study and performed study procedures, data interpretation and wrote the first draft of the manuscript. EJC and HCK conceptualized the study, supervised study execution, contributed to data interpretation and wrote the first draft of the manuscript. AGR contributed to data interpretation and edited the first draft manuscript. AED, AAS, BJA and BA contributed to surgical recruitment, patient sampling and weight management and RJM, CLH, MNA, JB, MK and PWP performed pathological interpretation and serum analyses. BHK contributed to statistical interpretation. All authors provided critical comment and approved the final version of the manuscript.
Supporting information
Table S1 Immunohistochemical antibodies and protocols
Acknowledgements
We acknowledge the generous gift of time and samples from the women in our study. We would like to thank Hayley Ammori, Joe Woods, Tina Pritchard, Donna Roberts, Adele Poole and Linsey Nelson for their help with patient recruitment, data acquisition, data management and obtaining regulatory approvals. We are grateful for the invaluable assistance of Gill Hesketh who sadly passed away before our study was completed. This article presents independent research funded by the National Institute for Health Research (NIHR), supported by the NIHR Manchester Biomedical Research Centre and facilitated by the Greater Manchester Local Clinical Research Network. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the study; and decision to submit the study for publication.
Conflicts of interest: The authors report no conflicts of interest.
References
- 1.Uterine cancer statistics [Internet]. Cancer Research UK. 2015. [cited 2018 Jan 7]. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer
- 2. Crosbie E, Zwahlen M, Kitchener H, et al. Body mass index, hormone replacement therapy and endometrial cancer risk: a meta analysis. Cancer Epidemiol Biomarkers Prev 2010;19:3119–30. [DOI] [PubMed] [Google Scholar]
- 3. Linkov F, Edwards R, Balk J, et al. Endometrial hyperplasia, endometrial cancer and prevention: gaps in existing research of modifiable risk factors. Eur J Cancer 2008;44:1632–44. [DOI] [PubMed] [Google Scholar]
- 4. Arnold M, Pandeya N, Byrnes G, et al. Global burden of cancer attributable to high body‐mass index in 2012: a population‐based study. Lancet Oncol 2015;16:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adams TD, Stroup AM, Gress RE, et al. Cancer incidence and mortality after gastric bypass surgery. Obesity 2009;17:796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ward KK, Roncancio AM, Shah NR, et al. Bariatric surgery decreases the risk of uterine malignancy. Gynecol Oncol 2014;133:63–6. [DOI] [PubMed] [Google Scholar]
- 7. Sjöström L, Gummesson A, Sjöström CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish obese subjects study): a prospective, controlled intervention trial. Lancet Oncol 2009;10:653–62. [DOI] [PubMed] [Google Scholar]
- 8. Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer 2015;15:484–98. [DOI] [PubMed] [Google Scholar]
- 9. MacKintosh M, Crosbie E. Obesity‐driven endometrial cancer: is weight loss the answer? BJOG 2013;120:791–4. [DOI] [PubMed] [Google Scholar]
- 10.Cervical screening: programme and colposcopy management ‐ GOV.UK [Internet]. [cited 2018 Jan 15]. Available from: https://www.gov.uk/government/publications/cervical-screening-programme-and-colposcopy-management
- 11. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 12. WHO . Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus, Abbreviated Report of a WHO Consultation. 2011. [PubMed]
- 13. Emons G, Beckmann MW, Schmidt D, et al. Uterus commission of the gynecological oncology working group (AGO). New WHO classification of endometrial Hyperplasias. Geburtshilfe Frauenheilkd 2015;75:135–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kurman RJ, Carcangiu M, Herrington CS, et al. WHO classification of tumours of female reproductive organs, 4th ed. Lyon: IARC, 2014. [Google Scholar]
- 15. Dowsett M, Nielsen TO, A'Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the international Ki67 in breast cancer working group. JNCI J Natl Cancer Inst 2011;103:1656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Libby EF, Azrad M, Novak L, et al. Obesity is associated with higher 4E‐BP1 expression in endometrial cancer. Curr Biomark Find 2014;2014:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ørbo A, Rise CE, Mutter GL. Regression of latent endometrial precancers by progestin infiltrated intrauterine device. Cancer Res 2006;66:5613–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Villavicencio A, Aguilar G, Argüello G, et al. The effect of overweight and obesity on proliferation and activation of AKT and ERK in human endometria. Gynecol Oncol 2010;117:96–102. [DOI] [PubMed] [Google Scholar]
- 19. Argenta P, Kassing M, Truskinovsky A, et al. Bariatric surgery and endometrial pathology in asymptomatic morbidly obese women: a prospective, pilot study. BJOG Int J Obstet Gynaecol 2013;120:795–800. [DOI] [PubMed] [Google Scholar]
- 20. Kaiyrlykyzy A, Freese KE, Elishaev E, et al. Endometrial histology in severely obese bariatric surgery candidates: an exploratory analysis. Surg Obes Relat Dis Off J Am Soc Bariatr Surg 2015;11:653–8. [DOI] [PubMed] [Google Scholar]
- 21. Modesitt SC, Hallowell PT, Slack‐Davis JK, et al. Women at extreme risk for obesity‐related carcinogenesis: baseline endometrial pathology and impact of bariatric surgery on weight, metabolic profiles and quality of life. Gynecol Oncol 2015;138:238–45. [DOI] [PubMed] [Google Scholar]
- 22. Byers T, Sedjo RL. Does intentional weight loss reduce cancer risk? Diabetes Obes Metab 2011;13:1063–72. [DOI] [PubMed] [Google Scholar]
- 23. Hernandez AV, Pasupuleti V, Benites‐Zapata VA, et al. Insulin resistance and endometrial cancer risk: a systematic review and meta‐analysis. Eur J Cancer Oxf Engl 1990 2015;51:2747–58. [DOI] [PubMed] [Google Scholar]
- 24. Nead KT, Sharp SJ, Thompson DJ, et al. Evidence of a causal association between Insulinemia and endometrial cancer: a Mendelian randomization analysis. J Natl Cancer Inst 2015;107. pii: djv178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burzawa JK, Schmeler KM, Soliman PT, et al. Prospective evaluation of insulin resistance among endometrial cancer patients. Am J Obstet Gynecol 2011;204:355.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sivalingam VN, Kitson S, McVey R, et al. Measuring the biological effect of presurgical metformin treatment in endometrial cancer. Br J Cancer 2016;114:281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 2013;310:2416–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Linkov F, Maxwell GL, Felix AS, et al. Longitudinal evaluation of cancer‐associated biomarkers before and after weight loss in RENEW study participants: implications for cancer risk reduction. Gynecol Oncol 2012;125:114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanderson PA, Critchley HOD, Williams ARW, et al. New concepts for an old problem: the diagnosis of endometrial hyperplasia. Hum Reprod Update 2017;23:232–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ørbo A, Arnes M, Lyså LM, et al. Expression of PAX2 and PTEN correlates to therapy response in endometrial hyperplasia. Anticancer Res 2015. Dec;35:6401–9. [PubMed] [Google Scholar]
- 31. Kanamori Y, Kigawa J, Itamochi H, et al. Correlation between loss of PTEN expression and Akt phosphorylation in endometrial carcinoma. Clin Cancer Res 2001;7:892–5. [PubMed] [Google Scholar]
- 32. Kurman R, Kaminski P, Norris H. The behavior of endometrial hyperplasia. A longterm study of “untreated” hyperplasia in 170 patients. Cancer 1985;56:403–12. [DOI] [PubMed] [Google Scholar]
- 33. Lacey JV, Ioffe OB, Ronnett BM, et al. Endometrial carcinoma risk among women diagnosed with endometrial hyperplasia: the 34‐year experience in a large health plan. Br J Cancer 2008;98:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lacey JV, Sherman ME, Rush BB, et al. Absolute risk of endometrial carcinoma during 20‐year follow‐up among women with endometrial hyperplasia. J Clin Oncol 2010;28:788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Renehan AG. Bariatric surgery, weight reduction, and cancer prevention. Lancet Oncol 2009;10:640–1. [DOI] [PubMed] [Google Scholar]
- 36. Luo J, Chlebowski RT, Hendryx M, et al. Intentional weight loss and endometrial cancer risk. J Clin Oncol Off J Am Soc Clin Oncol 2017;35:1189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gallos ID, Ganesan R, Gupta JK. Prediction of regression and relapse of endometrial hyperplasia with conservative therapy. Obstet Gynecol 2013;121:1165–71. [DOI] [PubMed] [Google Scholar]
- 38. Gallos ID, Krishan P, Shehmar M, et al. Relapse of endometrial hyperplasia after conservative treatment: a cohort study with long‐term follow‐up. Hum Reprod Oxf Engl 2013;28:1231–6. [DOI] [PubMed] [Google Scholar]
- 39. Gallos I. Risk of relapse of endometrial hyperplasia is high and long‐term treatment and follow up are recommended. BJOG 2016;123:1520–0. [DOI] [PubMed] [Google Scholar]
- 40. Ørbo A, Arnes M, Vereide AB, et al. Relapse risk of endometrial hyperplasia after treatment with the levonorgestrel‐impregnated intrauterine system or oral progestogens. BJOG 2016;123:1512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Salvesen HB, Iversen OE, Akslen LA. Identification of high‐risk patients by assessment of nuclear Ki‐67 expression in a prospective study of endometrial carcinomas. Clin Cancer Res 1998;4:2779–85. [PubMed] [Google Scholar]
- 42. Salvesen HB, Iversen OE, Akslen LA. Prognostic significance of angiogenesis and Ki‐67, p53, and p21 expression: a population‐based endometrial carcinoma study. J Clin Oncol Off J Am Soc Clin Oncol 1999;17:1382–90. [DOI] [PubMed] [Google Scholar]
- 43. Kitson S, Sivalingam VN, Bolton J, et al. Ki‐67 in endometrial cancer: scoring optimization and prognostic relevance for window studies. Mod Pathol Off J U S Can Acad Pathol Inc 2017;30:459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol 2016;215:598.e1–8. [DOI] [PubMed] [Google Scholar]
- 45. Gunderson CC, Fader AN, Carson KA, et al. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol 2012;125:477–82. [DOI] [PubMed] [Google Scholar]
- 46. Derbyshire A, Ryan N, Crosbie E. Biomarkers needed to predict progestin response in endometrial cancer. BJOG 2017;124:1584–4. [DOI] [PubMed] [Google Scholar]
- 47. Hawkes AL, Quinn M, Gebski V, et al. Improving treatment for obese women with early stage cancer of the uterus: rationale and design of the levonorgestrel intrauterine device ± metformin ± weight loss in endometrial cancer (feMME) trial. Contemp Clin Trials 2014;39:14–21. [DOI] [PubMed] [Google Scholar]
- 48. Crosbie E, Morrison J. The emerging epidemic of endometrial cancer: time to take action In: The Cochrane Collaboration , ed Cochrane database of systematic reviews. Chichester, UK: John Wiley & Sons, Ltd, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Immunohistochemical antibodies and protocols


