Abstract
The effective time window of any therapeutic in an experimental stroke model is limited by the rate of injury progression. Intracerebral hemorrhage in rodents is commonly induced by striatal injection of either autologous blood or bacterial collagenase, which digests local blood vessels. During time window studies of the heme oxygenase-1 inducer hemin, which is protective when administered within 1-3 hours in both models, the rate of perihematomal injury was directly compared after striatal blood or collagenase injection. Surprisingly, about 80% of the loss of perihematomal cell viability as measured by MTT reduction assay occurred within 6 hours of collagenase injection. In contrast, significant viability loss was not observed at this time point after autologous blood injection, but rather it progressed over the subsequent four days to a level similar to that produced by collagenase. Consistent with these observations, systemic hemin therapy reduced blood-brain barrier disruption and perihematomal cell injury when initiated at 6 hours after striatal injection of blood but not collagenase. These results indicate that the rate of early cell injury differs markedly in the collagenase and blood injection ICH models, which may contribute to inconsistent results in time window studies. The blood injection model may be more appropriate for prolonged time window studies of a neuroprotective agent.
Keywords: Heme, heme oxygenase, preconditioning, stroke, stroke models
1. Introduction
Spontaneous intracerebral hemorrhage accounts for about 10-15% of all strokes, and is associated with death and disability rates that have remained persistently high over the past two decades. Preclinical testing of novel therapies for this devastating disease process is usually conducted in rodents, inducing a parenchymal hematoma by stereotactic injection of autologous blood or bacterial collagenase. The advantages and limitations of these methods have not been precisely defined, and relevance to clinical ICH remains a source of ongoing controversy. While the hematoma produced either by blood or collagenase injection may appear grossly similar on histological sections in the days after hemorrhage, initial events are in fact quite distinct. Blood injection creates a hematoma over minutes from a single point source, compressing adjacent tissue; however, the pressure increase is usually not sufficient to produce early ischemia even when relatively large volumes are infused (Qureshi et al., 1999). The catalytic activity of collagenase disrupts the microvessels it contacts, resulting in multiple punctate hemorrhages that coalesce over hours to give the appearance of a single hematoma (Klebe et al., 2018). The effect of this microvessel destruction on early tissue perfusion and viability is unknown.
Although both ICH models have been well-characterized in the days to weeks after hemorrhage induction, little is known of their effect on the viability of neural cells in the initial hours after hemorrhage. This information deficit is largely due to quantification of perihematomal cell loss with histological endpoints that often have a delayed appearance. After ischemic stroke in rodents, morphological changes are not consistently detected until 12-24 hours after vessel occlusion (Hakim et al., 1992). However, when injury is quantified by 2,3,5-triphenyltetrazolium (TTC) staining, cell death as defined by the staining deficit is apparent within a few hours (Bose et al., 1988). The TTC method has not been extensively used in ICH models, perhaps due to difficulty in distinguishing its red formazan reduction product from an acute hematoma. This limitation can be easily avoided by using a tetrazolium salt with a product of a different color and absorbance wavelength, such as MTT (3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide), a marker of cell viability utilized in vitro for decades.
Quantification of cell injury in the hyperacute phase after ICH may be quite relevant to model selection for time window studies of potential pharmacotherapies. We have previously reported that the heme oxygenase-1 (HO-1) inducer hemin protected perihematomal cells when administered early (1-3 hours) after hemorrhage in both ICH models (Lu et al., 2014). While extending time window experiments, the progression of striatal injury was directly compared after stereotactic blood or collagenase injection, selecting outcome measures that are sensitive at early time points.
2. Results
A total of 193 mice (98 males and 95 females) were used in this study. Four mice died (2 males and 2 females), all within 24 hours of experimental ICH. Two had been injected with collagenase and randomized to the vehicle group and two had been injected with autologous blood but had not yet received hemin or vehicle. Male and female mice sustained similar loss of cell viability and blood-brain barrier disruption, consistent with our prior studies using these models to assess the effects of HO-1 overexpression (Chen-Roetling et al., 2015; Chen-Roetling et al., 2017).
Disparate effect of hemin in blood and collagenase injection ICH models.
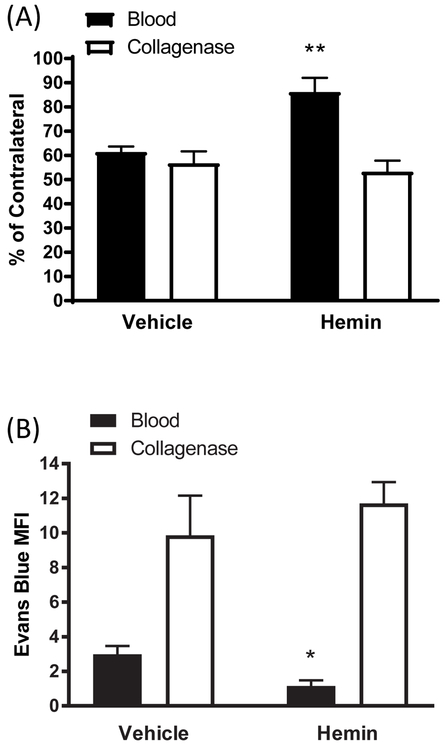
In a prior study we reported that the HO-1 inducer hemin, administered i.p. beginning 1-3 hours after striatal collagenase or blood injection, increased perihematomal cell viability while reducing blood-brain barrier disruption and neurological deficits (Lu et al., 2014). When hemin administration was delayed for 6 hours after ICH induction, a significant protective effect on striatal cell viability and barrier function persisted in the blood injection model (Fig. 1). However, no effect was observed in the collagenase model when treatment was initiated at the same time point. The protective effect of systemic hemin therapy was lost in the blood injection model when treatment was delayed for 12 hours (data not shown).
Figure 1.
Effect of delayed hemin treatment on cell viability and blood-brain barrier disruption. Mice received striatal injections of autologous blood or collagenase, followed 6 hours later by hemin 4 mg/kg i.p., repeated daily for a total of 4 doses. A) striatal cell viability as quantified by reduction of MTT to formazan, expressed as a percentage of that in contralateral striata; B) Evans blue mean fluorescence intensity (MFI, arbitrary units). Bars represent mean ± S.E.M, n = 7-9 mice for cell viability experiments and 7-8 for Evans blue experiments. *P < 0.05, **P < 0.01 v. vehicle group.
Rapid loss of perihematomal cell viability after collagenase injection.
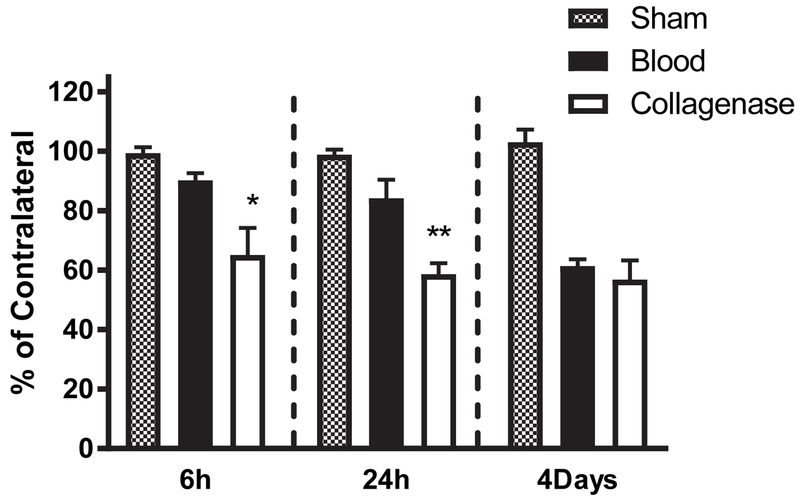
Based on the disparate response to hemin therapy begun at 6 hours in the blood injection and collagenase models, and the similar response when begun at 1-3 hours (Lu et al., 2014), we hypothesized that perihematomal cell injury progressed more rapidly after collagenase injection. Consistent with this hypothesis, cell viability loss in the hyperacute phase after hemorrhage induction differed significantly between models. Surprisingly, the MTT signal was already reduced to 65±9% of that in the contralateral striatum by 6 hours after collagenase treatment, compared with a minimal and statistically insignificant reduction after blood injection (Fig. 2). These differences persisted at 24 hours but had resolved by four days. At the latter time point, striatal viability loss approximated 40-50% in both models, consistent with our prior reported results at this or later time points using these models (Chen-Roetling et al., 2017; Chen et al., 2011; Lu et al., 2014).
Figure 2.
Rapid loss of perihematomal cell viability in collagenase ICH model. Bars represent mean striatal cell viability loss, quantified by MTT assay, at indicated time points after stereotactic blood (7-8/condition) or collagenase (7-10/condition) injection. Sham mice (4/condition) were subjected to anesthesia and needle insertion only. *P < 0.05, **P < 0.01 v. value in blood injection group.
Rapid blood-brain barrier disruption after collagenase injection.
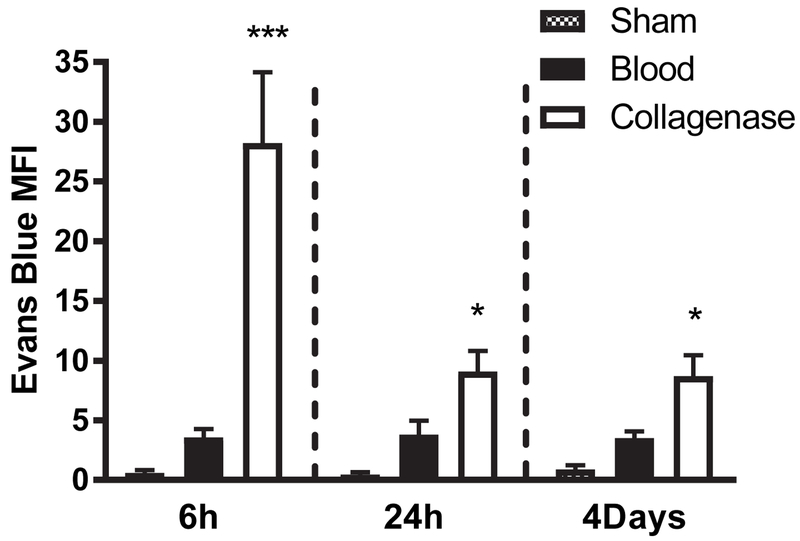
Blood-brain barrier breakdown is a more prominent feature of the collagenase ICH model than the blood injection model (MacLellan et al., 2008). Consistent with those observations, Evans blue leakage into the striatal parenchyma was significantly greater in collagenase-injected mice at each time point. Differences were most pronounced at 6 hours, when the striatal Evans blue signal in the collagenase-injected mice exceeded that of blood-injected mice about eightfold (Fig. 3).
Figure 3.
Time course of blood-brain barrier disruption in collagenase and blood injection models. Bars represent Evans blue mean fluorescence intensity (MFI, ± S.E.M) at indicated time points in striata of mice receiving stereotactic injection of collagenase or blood (n = 7/condition). Sham mice (4/condition) received anesthesia and needle insertion only. *P < 0.05, **P < 0.001 v. mean value in blood injection group.
Effect of delayed hemin therapy on neurological outcome.
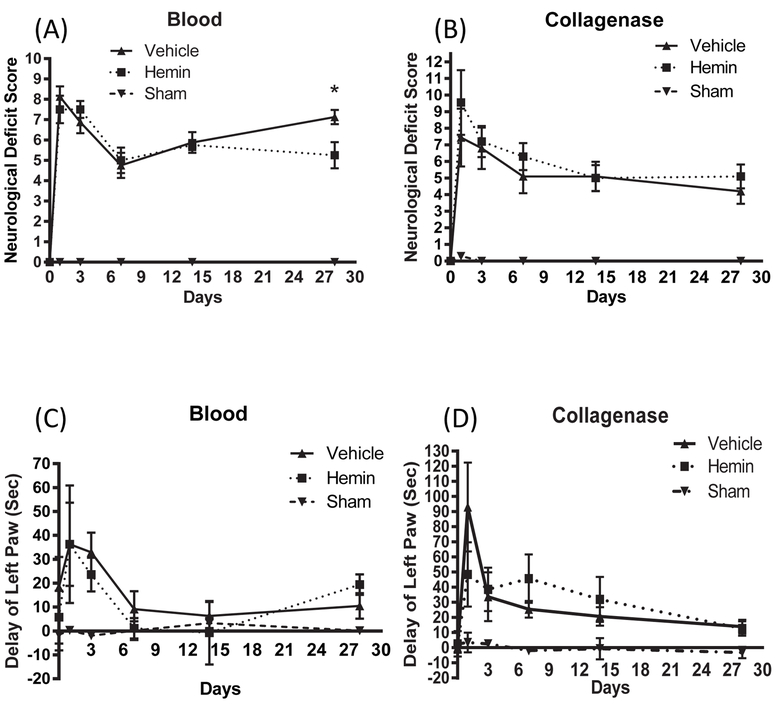
In contrast to the robust benefit we previously reported when hemin therapy was begun at 1-3 hours (Lu et al., 2014), delayed hemin administration had a modest effect on behavioral outcome. Significant improvement in neurological deficit scoring was observed in the blood injection model, but only at a late time point after hemorrhage (Fig. 4). No significant differences between vehicle and hemin groups were observed in collagenase-treated mice over the 4 weeks after hemorrhage.
Figure 4.
Effect of hemin therapy initiated at 6 hours after striatal blood or collagenase injection on neurological outcome, as measured by 24-point neurological deficit score (A, B) and adhesive removal test (C, D). *P < 0.05 v. vehicle, n = 6-13/condition.
3. Discussion
This study addresses two aims. First, we present evidence that systemic hemin therapy reduces perihematomal cell injury in the blood injection ICH model with a time window consistent with therapeutic feasibility, but this finding could not be confirmed in the collagenase model. Second, we report that perihematomal cell death, as defined by loss of metabolic activity needed to reduce a tetrazolium salt, proceeds very rapidly after collagenase-induced ICH. This novel observation likely accounts for the inefficacy of hemin when begun 6 hours after ICH in that model, and suggests that the blood injection model may be preferable for extended time course studies of putative neuroprotectants.
The beneficial effect of hemin is at least partly mediated by induction of HO-1 in responsive cell populations via the combined effects on Nrf2 transcription factor activation and Bach1-mediated transcription suppression (Reichard et al., 2007). When administered systemically, most HO-1 induction occurs in perivascular astrocytes (Chen-Roetling and Regan, 2017), in agreement with observations after systemic treatment with other Nrf2 activators (Alfieri et al., 2013; Jazwa et al., 2011). The benefit of astrocyte HO-1 after ICH has been confirmed in genetically modified mouse models, where it consistently protects the blood-brain barrier while reducing neurological deficits and mortality (Chen-Roetling et al., 2017; Lu et al., 2014). The time needed for HO-1 induction may contribute to its shorter effective time window after ICH when compared with that of agents providing direct cytoprotection, such as iron chelators (Cui et al., 2015). This limitation may account for its rather weak impact on neurological outcome in the blood injection model when treatment was initiated at 6 hours.
Blood-brain barrier disruption was most severe at 6 hours after collagenase injection, the earliest time point tested. This result is consistent with prior observations by Rosenberg et al. using [14C] sucrose efflux (Rosenberg et al., 1993), and Chiu et al. using gadolinium-enhanced MRI (Chiu et al., 2017). Very early BBB injury is not a surprising consequence of the breakdown of collagen type IV, a key stabilizing component of the basement membrane (Baeten and Akassoglou, 2011). The weaker Evans blue signal observed at all time points after autologous blood injection is in agreement with a prior study that directly compared BBB integrity in the collagenase and blood injection models (MacLellan et al., 2008), and highlights the very distinct injuries produced by striatal collagenase and blood injection.
No prior study had assessed cell viability with a functional assay in the hyperacute phase after CNS blood or collagenase injection. In the initial characterization of the collagenase model, Rosenberg et al. reported that brain cells maintained an intact appearance at 4 hours when examined after standard hematoxylin and eosin or Nissl staining, despite extensive hemorrhage at that time point; morphological changes were not observed until 24 hours (Rosenberg et al., 1990). However, ischemic stroke studies have demonstrated that morphology is a not a sensitive marker of early cell injury in the CNS. In permanent middle cerebral artery and common carotid artery occlusion models, histological changes were weak or absent for at least six hours and for up to 24 hours (Bose et al., 1988; Hakim et al., 1992). In contrast, staining with TTC, a tetrazolium salt similar to MTT, demonstrated decreased cell viability in the same regions within two hours that was fully developed by 6 hours (Bose et al., 1988). At time points three days or later after striatal hemorrhage, cell viability assay results do correlate well with neuronal cell counts in mouse ICH models (Chen-Roetling et al., 2017; Chen et al., 2011; Lu et al., 2014). The present results suggest that the time course of cell death in the collagenase model resembles that of ischemic stroke models.
The present study was not designed to detect differences in response to hemin in male and female animals. Consistent with our prior observations (Chen-Roetling et al., 2015; Chen-Roetling et al., 2017), no trend toward any difference was identified in either model. Although both sexes were used in all experiments, comparisons were made between groups with equal numbers of males and females to control for any potential bias. Further investigation using male and female cohorts seems a worthy topic for future investigation. Sex differences in outcome after intraparenchymal hemorrhage have been reported in animal models, but the effect has been quite variable, with both worse and better outcomes observed in males (Lekic et al., 2008; Nakamura et al., 2004). Considerable variability has also been observed in clinical studies, although the majority have reported no sex-related differences in outcome (Gokhale et al., 2015).
The blood and collagenase doses were selected because they consistently produce loss of about half of striatal cells, a process that is largely complete by four days in both models (Chen-Roetling et al., 2014; Chen-Roetling et al., 2017; Chen et al., 2011). Although the collagenase dose used was lower than in most rodent ICH studies (Klebe et al., 2018; MacLellan et al., 2008; Rosenberg et al., 1990), it is possible that an even lower dose would produce less microvascular destruction and more gradual viability loss. The rate of cell injury progression may be an important consideration for interventional ICH studies. The slower rate provided by autologous blood injection may be preferable for time course studies of neuroprotective compounds, while rapid completion of the hemorrhagic injury may be advantageous for testing regenerative therapies such as stem cell transplantation. A functional tetrazolium-based assay is an efficient and inexpensive method to quantify cell loss in the initial hours after experimental ICH and may be of value in selecting the appropriate model.
4. Experimental Procedure
Animals.
Swiss Webster mice (7-10 weeks old, 30-40 g, males and females) were purchased from Taconic Laboratories. They were housed in a dedicated facility that was fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All mice were provided with continuous access to food and water, in a temperature and humidity-controlled environment with a 12-hour cycle of light and darkness. All procedures adhered to protocols that had been approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University and were carried out in accordance with EC Directive 86/609/EEC for animal experiments. All direct comparisons were made between groups with equal numbers of males and females to control for any bias due to sex differences.
ICH models.
Anesthesia was induced and maintained with 2% isoflurane in oxygen. Temperature was monitored via rectal probe and kept at 37±0.5°C throughout the course of the surgical procedure. Using aseptic technique, the skin was incised, and a small burr hole was made at the following location relative to bregma: 2.5 mm right lateral, 0.5 mm anterior. Injection depth was 3 mm below the skull surface into the striatum. A 30-gauge Hamilton needle with attached glass syringe was then used to inject 25 μl autologous blood obtained from a tail vein. Alternatively, ICH was induced by injection of sterile collagenase (Sigma-Aldrich Product C9572). Due to some variability in potency of collagenase lots, its dose was adjusted to that producing about 50% loss of cell viability by four days in otherwise untreated mice, which is the time point approximating maximal striatal viability loss after murine ICH (Chen-Roetling et al., 2014; Chen-Roetling et al., 2017). Collagenase dose ranged from 0.03-0.06 units, and all direct comparisons were made with mice receiving the same dose. The method used to dilute collagenase solutions while minimizing activity loss has previously been detailed (Chen-Roetling et al., 2013). Surgical control mice were subjected to needle insertion only. After the wound was closed with sutures, animals recovered from anesthesia while closely monitored in a temperature-controlled environment.
Hemin treatment.
A random number generator (www.random.org) was used to assign all mice to treatment groups. Hemin (Frontier Scientific, Logan UT) was prepared as a 1 mM solution under reduced light immediately prior to use. It was diluted in sterile physiologic saline, and injected i.p. at defined time points in a total volume of 500 μl, with repeat doses daily for a total of four doses. The control group received an equal volume of sterile saline solution.
Outcome Measures:
All personnel conducting these tests were blinded to treatment group.
Cell viability assay.
After euthanasia under deep isoflurane anesthesia, hemorrhagic and contralateral striata were quickly excised and placed into Hanks Balanced Salt Solution supplemented with 27.5 mM glucose, 20.5 mM sucrose and 4.2 mM sodium bicarbonate. Tissue was then dissociated by gentle trituration to provide all cells with rapid access to the methylthiazolyldiphenyl-tetrazolium (MTT) solution, which was added to produce a final concentration of 0.125 mg/ml. After 4 minutes incubation in a 37°C water bath, cells were collected by low speed centrifugation. The supernatant was immediately removed, and the purple formazan reaction product was extracted by vortexing in 2 ml of 91% isopropanol. The absorbance of this solution at 562 nm was determined and was scaled to that in the contralateral striatum (= 100). Prior studies have demonstrated close correlation of results of this assay with cell counts of striatal neurons at four days or later after hemorrhage, and with fluorescence measurements in mice expressing the red fluorescent protein variant dTomato selectively in central neurons (Chen-Roetling et al., 2013; Chen-Roetling et al., 2017; Qu et al., 2007).
Blood-brain barrier permeability assay.
Following a previously described protocol, mice were injected with 4 ml/kg of a solution of 2% Evans blue in sterile saline. After 3 hours, they were anesthetized with isoflurane as described above and then perfused via the left ventricle with 25 ml sterile saline. Striata were rapidly excised under a dissecting microscope in a dry Petri dish, and Evans blue was extracted in a 50% trichloroacetic acid solution, following the method of Uyama et al. (Uyama et al., 1988). The fluorescence (ex:620 nm, em:680 nm) of the supernatant solution was then quantified in a Molecular Devices Spectramax plate reader. The fluorescence of the control contralateral extract solution was subtracted from that of the injected striatum to yield the signal produced by ICH.
Behavioral testing.
Sensorimotor deficits were quantified using the adhesive removal test (Chen et al., 2011). Three-millimeter diameter adhesive circles were punched from electrical tape and placed onto the left or right forepaw. The difference in mean time needed for removal of the adhesive on left v. right forepaw was calculated. In addition, a neurological disability score was calculated following the method of Glushakov et al. (Glushakov et al., 2013), which is based on observations of motor function including body symmetry, gait, circling behavior, and limb symmetry. Scores range from 0 for normal function to 24 for greatest impairment.
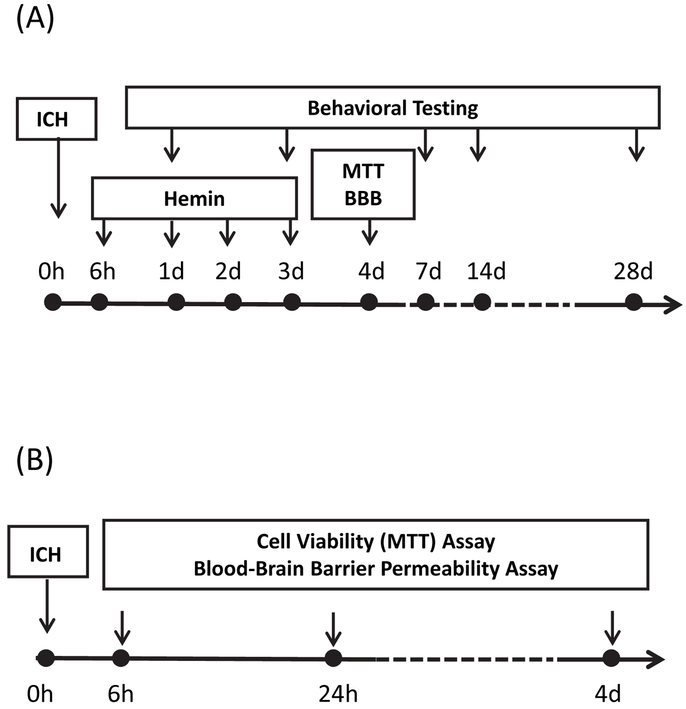
Time lines of hemin treatment and injury time course experiments are provided in Fig. 5.
Figure 5.
Time line of experiments testing: A) effect of hemin therapy initiated at 6 hours after induction of ICH on striatal cell viability (MTT assay), blood-brain barrier (BBB) disruption (Evans blue assay), and behavioral deficits; B) time course of cell viability loss and blood-brain barrier disruption after ICH induction by striatal injection of either collagenase or autologous blood.
Statistics.
Differences in the mean values between three or more groups were analyzed with one-way ANOVA and the Bonferroni multiple comparisons test, and differences between two groups with an unpaired t-test.
Highlights.
Most cell viability loss occurred within 6 hours in the collagenase ICH model.
Cell viability loss progressed over days in the blood injection ICH model.
Hemin therapy protected with a 6 hour window in the blood injection model only.
The blood injection model may be preferred for ICH time window studies.
Acknowledgement
This work was supported by the National Institutes of Health (grant numbers R01NS079500 and R01NS095205).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfieri A, Srivastava S, Siow RC, Cash D, Modo M, Duchen MR, Fraser PA, Williams SC, Mann GE, 2013. Sulforaphane preconditioning of the Nrf2/HO-1 defense pathway protects the cerebral vasculature against blood-brain barrier disruption and neurological deficits in stroke. Free Radic Biol Med 65, 1012–22. [DOI] [PubMed] [Google Scholar]
- Baeten KM, Akassoglou K, 2011. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev Neurobiol 71, 1018–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose B, Jones SC, Lorig R, Friel HT, Weinstein M, Little JR, 1988. Evolving focal cerebral ischemia in cats: spatial correlation of nuclear magnetic resonance imaging, cerebral blood flow, tetrazolium staining, and histopathology. Stroke. 19, 28–37. [DOI] [PubMed] [Google Scholar]
- Chen-Roetling J, Lu X, Regan KA, Regan RF, 2013. A rapid fluorescent method to quantify neuronal loss after experimental intracerebral hemorrhage. J Neurosci Methods. 216, 128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Roetling J, Cai Y, Regan RF, 2014. Neuroprotective effect of heme oxygenase-2 knockout in the blood injection model of intracerebral hemorrhage. BMC Res Notes. 7, 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Roetling J, Song W, Schipper HM, Regan CS, Regan RF, 2015. Astrocyte overexpression of heme oxygenase-1 improves outcome after intracerebral hemorrhage. Stroke. 46, 1093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Roetling J, Kamalapathy P, Cao Y, Song W, Schipper HM, Regan RF, 2017. Astrocyte heme oxygenase-1 reduces mortality and improves outcome after collagenase-induced intracerebral hemorrhage. Neurobiol Dis 102, 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Roetling J, Regan RF, 2017. Targeting the Nrf2-Heme Oxygenase-1 Axis after Intracerebral Hemorrhage. Current Pharmaceutical Design. 23, 2226–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhang X, Chen-Roetling J, Regan RF, 2011. Increased striatal injury and behavioral deficits after intracerebral hemorrhage in hemopexin knockout mice. J Neurosurg 114, 1159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CD, Yao NW, Guo JH, Shen CC, Lee HT, Chiu YP, Ji HR, Chen X, Chen CC, Chang C, 2017. Inhibition of astrocytic activity alleviates sequela in acute stages of intracerebral hemorrhage. Oncotarget 8, 94850–94861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui HJ, He HY, Yang AL, Zhou HJ, Wang C, Luo JK, Lin Y, Tang T, 2015. Efficacy of deferoxamine in animal models of intracerebral hemorrhage: a systematic review and stratified meta-analysis. PLoS One. 10, e0127256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glushakov AV, Robbins SW, Bracy CL, Narumiya S, Dore S, 2013. Prostaglandin F2alpha FP receptor antagonist improves outcomes after experimental traumatic brain injury. J Neuroinflammation. 10, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhale S, Caplan LR, James ML, 2015. Sex differences in incidence, pathophysiology, and outcome of primary intracerebral hemorrhage. Stroke. 46, 886–92. [DOI] [PubMed] [Google Scholar]
- Hakim AM, Hogan MJ, Carpenter S, 1992. Time course of cerebral blood flow and histological outcome after focal cerebral ischemia in rats. Stroke. 23, 1138–43; discussion 1143–4. [DOI] [PubMed] [Google Scholar]
- Jazwa A, Rojo AI, Innamorato NG, Hesse M, Fernandez-Ruiz J, Cuadrado A, 2011. Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid Redox Signal. 14, 2347–60. [DOI] [PubMed] [Google Scholar]
- Klebe D, Iniaghe L, Burchell S, Reis C, Akyol O, Tang J, Zhang JH, 2018. Intracerebral Hemorrhage in Mice. Methods in Molecular Biology. 1717, 83–91. [DOI] [PubMed] [Google Scholar]
- Lekic T, Tang J, Zhang JH, 2008. Rat model of intracerebellar hemorrhage. Acta Neurochir Suppl 105, 131–4. [DOI] [PubMed] [Google Scholar]
- Lu X, Chen-Roetling J, Regan RF, 2014. Systemic hemin therapy attenuates blood-brain barrier disruption after intracerebral hemorrhage. Neurobiol Dis 70C, 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLellan CL, Silasi G, Poon CC, Edmundson CL, Buist R, Peeling J, Colbourne F, 2008. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J Cereb Blood Flow Metab 28, 516–25. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Xi G, Hua Y, Schallert T, Hoff JT, Keep RF, 2004. Intracerebral hemorrhage in mice: model characterization and application for genetically modified mice. J Cereb Blood Flow Metab 24, 487–94. [DOI] [PubMed] [Google Scholar]
- Qu Y, Chen-Roetling J, Benvenisti-Zarom L, Regan RF, 2007. Attenuation of oxidative injury after induction of experimental intracerebral hemorrhage in heme oxygenase-2 knockout mice. J Neurosurg 106, 428–35. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Wilson DA, Hanley DF, Traystman RJ, 1999. No evidence for an ischemic penumbra in massive experimental intracerebral hemorrhage. Neurology. 52, 266–72. [DOI] [PubMed] [Google Scholar]
- Reichard JF, Motz GT, Puga A, 2007. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic Acids Res 35, 7074–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M, 1990. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 21, 801–807. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Estrada E, Kelley RO, Kornfeld M, 1993. Bacterial collagenase disrupts extracellular matrix and opens blood-brain barrier in rat. Neuroscience Letters. 160, 117–9. [DOI] [PubMed] [Google Scholar]
- Uyama O, Okamura N, Yanase M, Narita M, Kawabata K, Sugita M, 1988. Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using Evans blue fluorescence. J Cereb Blood Flow Metab 8, 282–4. [DOI] [PubMed] [Google Scholar]