Abstract
The question of whether the anterior and posterior hippocampus serve different or complementary functional roles during episodic memory processing has been motivated by noteworthy findings in rodent experiments and from noninvasive studies in humans. Researchers have synthesized these data to postulate several models of functional specialization, However, the issue has not been explored in detail using direct brain recordings. We recently published evidence that theta power increases during episodic memory encoding occur in the posterior hippocampus in humans. In our current investigation we analyzed an expanded data set of 32 epilepsy patients undergoing stereo EEG seizure mapping surgery with electrodes precisely targeted to the anterior and posterior hippocampus simultaneously who performed an episodic memory task. Using a repeated measures design, we looked for an interaction between encoding versus retrieval differences in gamma oscillatory power and anterior versus posterior hippocampal location. Our findings are consistent with a recently articulated model (the HERNET model) favoring posterior hippocampal activation during retrieval related processing. We also tested for encoding versus retrieval differences in the preferred gamma frequency band (high versus low gamma oscillations) motivated by published rodent data.
Evidence for functional specialization along the longitudinal axis of the the hippocampus has been synthesized in a number of recent high–profile reviews of human and rodent data (Moser and Moser, 1998; Fanselow and Dong, 2010; Poppenk et al., 2013; Strange et al., 2014). Noteworthy experimental findings include gene expression differences along this axis, and smaller place cell fields in the dorsal hippocampus of rodents (Kjelstrup et al., 2008; Dong et al., 2009). In humans, spatial memory retrieval may be more reliant on the posterior hippocampus (Ryan et al., 2010; Poppenk et al., 2013) and, famously, anatomical differences between London cabdrivers and controls, seemingly related to extensive spatial learning, predominate in posterior hippocampal regions (Woollett and Maguire, 2011), with some analogous findings in rodent data (Fanselow and Dong, 2010). Several efforts have been made to synthesize these diverse human and animal findings into a unified model of anterior versus posterior hippocampal function (see for example Strange et al. (2014), Poppenk et al. (2013), and Fanselow and Dong (2010)). The latter authors suggested preferential representation of affective information in ventral hippocampus, while Strange et al. (2014) and Poppenk et al. (2014) postulate more general differences in the nature of the information represented in anterior versus posterior regions (discussed below).
More controversially, meta–analytic examinations of human noninvasive data have led to the hypothesis that the relevant functional difference in terms of memory along the hippocampal axis is between encoding and re-trieval (Lepage, Habib, and Tulving, 1998), with greater posterior hippocampal activation during item retrieval (termed the “HERNET” model by Kim, 2015). However, multiple contradictory findings have also been reported (see Poppenk et al., 2013, Box 1). We recently published evidence that, during the successful encoding of single words, enhanced theta power is confined to the posterior hippocampus (Lin et al., 2017). In that analysis, we did not observe strong evidence of anterior/posterior differences in theta power during retrieval; indeed we found little evidence of sustained retrieval–related theta power increases in any hippocampal region.
Here, we further examine the possibility of a dissociation within the hippocampus between the loci of encoding–related and retrieval–related activity by comparing changes in gamma power along the longitudinal axis of the human hippocampus. To accomplish this, we took advantage of an expanded stereo EEG database that included 32 subjects (23 of whom were included in our previous analysis (Lin et al., 2017). In all of these subjects we recorded signal from the anterior and posterior hippocampus concurrently, permitting the effect of recording location along the hippocampal axis to be examined within subjects. We further employed this expanded data set to contrast low versus high gamma power contributions to processing during encoding versus retrieval. This goal was motivated by findings from animal studies indicating that memory retrieval is accompanied by enhanced coherence across hippocampal subfields in low gamma oscillations that accompany slow wave ripples, possibly part of an overall shift from faster to slower gamma rhythms during item retrieval (Carr, Karlsson, and Frank, 2012; Colgin, 2012).
In brief, subjects performed a free recall procedure after undergoing seizure mapping surgery (stereo EEG electrode implantation). The free recall procedure included testing sessions comprising 25 lists, each comprising of 12 common nouns. Words were presented for 1600 msec, separated by an inter–stimulus interval of 400 msec when the monitor was blanked (jittered). After all items had been presented on a given list and a mathematical distractor task had been performed for 30 seconds, subjects were given 30 seconds to freely recall as many of the items as possible from the immediately preceding list in any order (Lin et al., 2017; Burke et al., 2014). Words recalled from preceding lists (intrusions) were considered errors and excluded from analysis. We excluded subjects in whom the seizure onset zone was directly within the hippocampus; otherwise all subjects who had both anterior and posterior hippocampal electrodes inserted and who successfully completed the behavioral paradigm were included in the analysis (32 subjects). Signal from electrodes with frequent interictal activity (as determined by expert neurology review) or excessive noise was removed as part of a standard processing pipeline (Lin et al., 2017; Lega, Germi, and Rugg, 2017). Electrodes were either AdTech (19 participants, model RD10R) or PMT (13 participants, model 2102) stereo EEG depth electrodes with cylindrical contacts spaced between 3 and 4 mm apart. For each subject, the same type of electrode was used for all hippocampal recordings. Electrodes were targeted separately to the anterior and posterior hippocampus (lateral insertion) by the operating surgeon using a robotic stereotactic surgical technique (Gonzalez-Martinez et al., 2013). The uncal notch was used to demarcate anterior versus posterior hippocampal location (Poppenk et al., 2013). Electrode location within the anterior/posterior hippocampus was confirmed with expert neuroradiology review; MNI coordinates were determined by co–registering patient brain data with a standard MNI atlas in FSL software. Artifact was removed from the signal using a kurtosis algorithm (threshold of 4), and power was extracted using Morlet wavelets (Burke et al., 2014). For the present analysis, we sought to directly compare oscillatory power during successful encoding to successful retrieval. Therefore we normalized power by reference to a 400 ms prestimulus period during encoding of non–recalled study words, and then averaged power across an 1800 msec epoch (Lin et al., 2017) following each study event (for encoding power) and across a 1000 msec epoch preceding each correct recall response (for retrieval, excluding incorrect responses). This was done separately for each region (anterior vs. posterior) and frequency band (low gamma, 30–70 Hz, high gamma 80–150 Hz) for each hippocampal electrode. All analyses were conducted at the subject level as a paired comparison between anterior and posterior activity. In cases in which more than one electrode contact was present in either the anterior of posterior hippocampus of a given subject, signal from all contacts was averaged to yield a single a single power value (in each frequency band) for anterior and posterior locations for each subject during encoding and retrieval (average of 1.8 electrodes per subject). We did not select electorates for analysis on the basis of functional or other criteria, rather, all electrodes that were neuroradiologically confirmed as located within the hippocampus were included in the analysis. The data were analyzed using a repeated measures ANOVA (treating subject as a random effect), with the a priori goal to test for an interaction between memory condition (encoding versus retrieval) and hippocampal location (anterior versus posterior). Our full model included the additional factor of frequency band (high versus low gamma) to test for the second order interaction predicted on the basis of the animal findings described above (Carr, Karlsson, and Frank, 2012; Colgin, 2012). Significance levels for post hoc analyses (t–tests and bivariate correlations) were subjected to Bonferroni correction for multiple comparisons. This analysis differs from that described in our previous publication (Lin et al., 2017) in that we included both encoding and retrieval power and anterior versus posterior location in a repeated measures design, with the goal of investigating how power may shift between encoding and retrieval within an individual subject’s hippocampus. Our data were unbalanced in terms of hemisphere (22 of 32 subjects’ recordings from the dominant hemisphere), so we collapsed across this variable for the purposes of the present analysis.
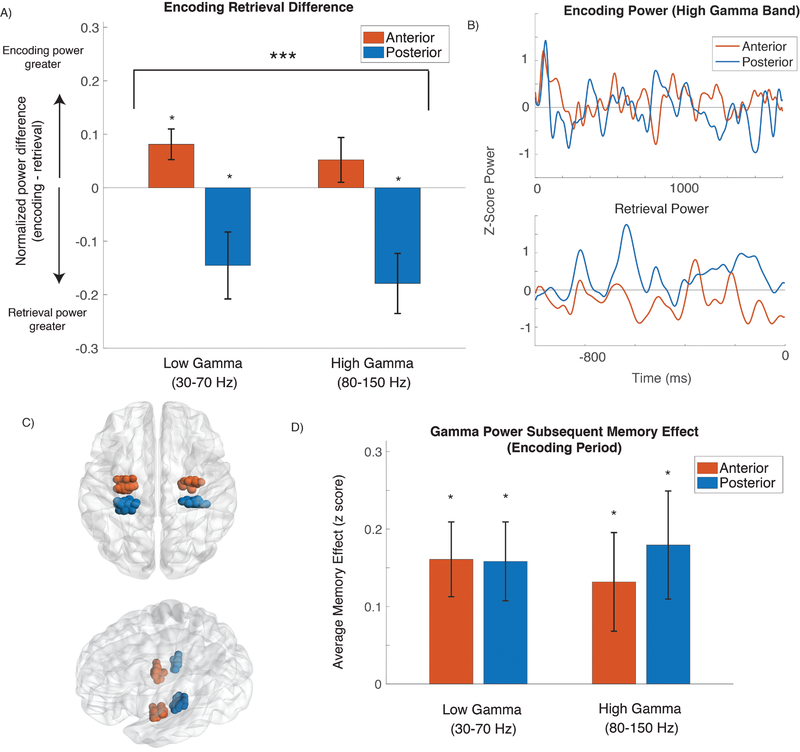
Results from the ANOVA described above revealed a significant interaction between successful encoding/retrieval power differences and location along the hippocampal axis (F(1,31) = 13.15, p = 0.001, partial η2 = 0.30) that was unmodified by the factor of gamma band. Results are shown in Figure 1. In a convergent analysis approach, we also correlated the encoding/retrieval power difference with the longitudinal location of each electrode as measured by Y coordinate value in MNI space for each electrode. This correlation was significant (r = 0.32, corrected p = 0.01), also consistent with a gradient favoring retrieval related activation in the posterior hippocampus. Finally, we tested whether the encoding/retrieval gamma power difference was independently significant within each hippocampal region (anterior and posterior location). We applied a t–test to the distribution of power differences across subjects, separately for the high and low gamma bands (resulting in 4 tests). Results are shown in Figure 1. Low gamma power was significantly greater (corrected p < 0.05, tested against a zero null hypothesis) for encoding in the anterior hippocampus, and both low and high gamma power were significantly greater in the posterior hippocampus during retrieval.
Figure 1: A. Difference between encoding and retrieval oscillatory power.
Bar plot of difference in oscillatory power between encoding and retrieval for anterior (red) and posterior (blue) electrodes *** denotes significant interaction effect between encoding/retrieval and anterior/posterior hippocampal location across both gamma frequency bands. * denotes significant encoding versus retrieval gamma power difference across subjects with each band/hippocampal location (t–test, Bonferroni corrected). B. Example of encoding and retrieval high gamma power from an individual subject. Normalized power during encoding and retrieval from anterior (red) and posterior (blue) electrodes from a single subject. Normalized power smoothed with 50 msec moving average. C. Aggregate plot of electrode locations. Electrodes targeted based upon location of the uncal apex, MNI Y coordinate ~ −21. D. Encoding subsequent memory effect. * denotes significantly greater power following presentation of successfully remembered items.
Consistent with previous reports (Lega, Jacobs, and Kahana, 2011; Lin et al., 2017; Burke et al., 2014) and our a priori expectations, both high and low gamma power in the anterior and posterior hippocampus were significantly greater during successful than unsuccessful encoding (gamma band subsequent memory effect). We contrasted power directly between epochs following presentation of later recalled versus non–recalled items within each electrode and then tested the resulting distribution of test statistics (at the subject level) against a zero null hypothesis (t(31) > 2.1, p < 0.02 for all regions/bands (see Figure 1D). We also compared the SME distributions directly between anterior and posterior hippocampal locations; the comparisons were not significant for either the low or high gamma bands (t(31) < 0.8, p > 0.20). Finally, at the subject level, we examined the correlation between the anterior–posterior difference in the SME and the anterior–posterior encoding/retrieval power difference. This was not significant (r = 0.24, p > 0.15). These analyses indicate that while a hippocampal gamma SME was present in our dataset as expected, longitudinal differences in the SME did not drive the anterior–posterior difference in gamma power that we observed between encoding and retrieval.
A specific hypothesis advanced by Kim, 2015 was that the amygdala should exhibit the same encoding/retrieval dissociation as the anterior hippocampus. Our dataset allowed us to test this prediction, because a subset of subjects (18) had electrodes targeted independently to the amygdala and posterior hippocampus. We employed the same repeated measures ANOVA as described above, substituting measures of gamma power from the amygdala for those from the anterior hippocampus. The interaction between encoding/retrieval power differences and anatomical location (amygdala versus posterior hippocampus) was no longer significant F(1,17) = 0.186).
Our analysis model included both high and low gamma power to allow us to test for evidence of a shift between low and high gamma power according to whether items were being encoded or retrieved. As discussed above, the motivation for this analysis was animal data indicating that low gamma (rather than high gamma) oscillations are coherent across CA regions during memory retrieval (Carr, Karlsson, and Frank, 2012; Colgin, 2012). However, we observed neither a high/low gamma power shift in encoding versus retrieval (F(1,31) = 1.233, p = 0.275, partial η2 = 0.01) or an interaction between this effect and hippocampal location (F(1,31) = 0.016).
We analyzed a unique dataset that provided the opportunity to directly examine the interaction for gamma power between location along the hippocampal axis, frequency band, and phase of mnemonic processing. The present findings provide limited support for differences in processing during encoding versus retrieval along the hippocampal axis. The data directly inform the interpretation of experimental data and models of mnemonic processing developed across a diversity of human and animal paradigms. Early models of long–axis hippocampal specialization based upon lesion experiments suggested that the relevant functional distinction between anterior and posterior hippocampus favors affective processing in anterior regions (based partially upon amygdala and hypothalamic input), while memory processing without an affective component favors posterior areas (Moser and Moser, 1998; Fanselow and Dong, 2010). As the affective content of the stimuli used in our experiment was not directly manipulated, our data do not directly support or contradict this view. However our observation of an encoding–retrieval gradient in hippocampal activity for items without strong affective qualities suggests that an affective–centered model alone is at best incomplete, a conclusion consistent with more recent experiments in which specific hippocampal regions were selectively inactivated or lesioned (see Fanselow and Dong, 2010, discussing observations of Rudy and Matus-Amat, 2005). Similarly, the memory task we used does not explicitly manipulate the novelty of individual items, so it is difficult to postulate whether the encoding/retrieval differences we observed are attributable to this factor (Poppenk et al., 2008; Poppenk et al., 2013). On the one hand, the memory items were commonly used nouns with meanings easily familiar to subjects. On the other hand, novelty effects in the hippocampus are held to be contextually dependent. Thus, it can be argued that the item–context associations formed during encoding would be more novel than when the same representation is later retrieved, and that this may have favored preferential anterior activation during encoding (see for example Thakral, Wang, and Rugg, 2015).
Poppenk et al., 2013 postulated that the posterior hippocampus supports fine-grained, detail–level representations, while the anterior hippocampus is implicated in more abstract, higher level (‘gist–like’) representations. Our paradigm does not provide direct evidence allowing evaluation of this model; notably the ‘grain–size’ of the features of the words supporting recall cannot be determined, and in addition no control was effected over encoding strategy. This model does however neatly fit extent observations from animal studies, including findings of greater place cell specificity in the posterior hippocampus (Kjelstrup et al., 2008; Royer et al., 2010), and the greater ratio of DG to CA areas in the dorsal hippocampus (the DG being implicated more strongly in pattern–discrimination processing necessary for fine–grained representation, see Poppenk et al., 2013). Using human fMRI data, Duncan, Tompary, and Davachi, 2014 report that DG/CA3 hippocampal subregions are more active during memory retrieval than encoding. However, in the absence of microelectrode recordings precisely localized to hippocampal subfields, we cannot state whether our data support this hypothesis, which remains an important topic for future investigation.
As has been previously noted by others (Kim, 2015; Poppenk et al., 2013), anterior versus posterior distinctions in encoding and retrieval activity map onto proposed distinctions between networks supporting item and relational (especially spatial) memory, namely the anterior temporal (AT)/posterior temporal (PT) distinction pro- posed by Ranganath and Ritchey (2012). According to this perspective, interacting with parahippocampal cortex, the posterior hippocampus supports relational representations necessary for successful recollection (retrieval of items plus context), something necessary for free recall and other episodic memory paradigms. By contrast, the anterior hippocampus (interacting with the perirhinal cortex) is more heavily implicated in representations of item information (AT system). The PT network integrates with default mode network (DMN) regions thought to be preferentially active during ‘introspective’ attention that may be consistent with memory retrieval (Foster et al., 2013; Kim, 2015), and these same regions preferentially connect with the anterior rather than posterior hippocampus (Foster et al., 2013). Related to these differences in anatomical connectivity, Kim (in the HERNET model) pro- posed that by virtue of the strong anatomical connectivity between the two regions, the amygdala would behave analogously to the anterior hippocampus, although this is not what we observed (2015). It should be noted that overall the memory effects of the amygdala have not been described in detail using this paradigm, nor do amygdala lesions impair recall performance, (Zola-Morgan, Squire, and Amaral, 1989).
We did not observe a shift in the preferred gamma frequency band between encoding and retrieval or a significant interaction between this factor, the preferred gamma band and anterior versus posterior location. Certainly, one must be careful not to over interpret a negative result as a positive finding might rely strongly upon choosing a specific and narrow frequency range, or may only be present in a fraction of electrodes, requiring a different analytical strategy from the one we employed. It is also possible (and, indeed, likely) that observing a high to low gamma power transition between encoding and retrieval will require microelectrode recordings precisely localized to hippocampal subfields (Carr, Karlsson, and Frank, 2012; Duncan, Tompary, and Davachi, 2014). This question leads in to the wider issue of whether slow wave ripple activity (and replay) exists in humans, evidence for which is currently very limited (Axmacher, Elger, and Fell, 2008).
In conclusion, we present first of its kind human data that examines how neural activity differs along the hippocampal axis during memory encoding versus retrieval in an episodic memory task. Our data suggest that the posterior hippocampus exhibits greater gamma frequency activity during memory retrieval, and our findings inform the emerging literature focused on the functional specialization of different regions within the hippocampus. We expect that these findings will motivate follow–up investigations with specific experimental manipulations to further explicate this important question.
Acknowledgments:
Funding in part via NIH R21 NS095094–01A1, the Texas Health Resources Disease Oriented Clinical Scholars program, and DARPA Restoring Active Memory project (Cooperative Agreement N66001–14-2–4032). We thank Blackrock Microsystems for providing neural recording and stimulation equipment. The views, opinions, and/or findings contained in this material are those of the authors and should not be interpreted as representing the official views or policies of the Department of Defense or the U.S. Government.
Footnotes
The authors declare no competing conflicts of interest.
References
- Axmacher Nikolai, Elger Christian E, and Fell Juergen (2008). Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain 131(7): 1806–1817. [DOI] [PubMed] [Google Scholar]
- Burke JF, Long NM, Zaghloul KA, Sharan AD, Sperling MR, and Kahana MJ (2014). Human intracranial high-frequency activity maps episodic memory formation in space and time. NeuroImage 85 Pt. 2: 834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr Margaret F, Karlsson Mattias P, and Frank Loren M (2012). Transient slow gamma synchrony underlies hippocampal memory replay. Neuron 75(4): 700–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin Laura Lee (2012). Slow gamma takes the reins in replay. Neuron 75(4): 549–550. [DOI] [PubMed] [Google Scholar]
- Dong Hong-Wei, Larry W Swanson Lin Chen, Fanselow Michael S, and Toga Arthur W (2009). Genomic–anatomic evidence for distinct functional domains in hippocampal field ca1. Proceedings of the National Academy of Sciences 106(28): 11794–11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan Katherine, Tompary Alexa, and Davachi Lila (2014). Associative encoding and retrieval are predicted by functional connectivity in distinct hippocampal area ca1 pathways. Journal of Neuroscience 34(34): 11188–11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow Michael S and Dong Hong-Wei (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65(1): 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster Brett L, Kaveh Anthony, Dastjerdi Mohammad, Miller Kai J, and Parvizi Josef (2013). Human retrosplenial cortex displays transient theta phase locking with medial temporal cortex prior to activation during autobiographical memory retrieval. The Journal of Neuroscience 33(25): 10439–10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Martinez Jorge, Bulacio Juan, Alexopoulos Andreas, Jehi Lara, Bingaman William, and Najm Imad (2013). Stereoelectroencephalography in the “difficult to localize” refractory focal epilepsy: early experience from a north american epilepsy center. Epilepsia 54(2): 323–330. [DOI] [PubMed] [Google Scholar]
- Kim Hongkeun (2015). Encoding and retrieval along the long axis of the hippocampus and their relationships with dorsal attention and default mode networks: the hernet model. Hippocampus 25(4): 500–510. [DOI] [PubMed] [Google Scholar]
- Kjelstrup Kirsten Brun, Solstad Trygve, Vegard Heimly Brun Torkel Hafting, Leutgeb Stefan, Witter Menno P, Moser Edvard I, and Moser May-Britt (2008). Finite scale of spatial representation in the hippocampus. Science 321(5885): 140–143. [DOI] [PubMed] [Google Scholar]
- Lega BC, Jacobs J, and Kahana MJ (2011). Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus 22(4): 748–761. [DOI] [PubMed] [Google Scholar]
- Lega Bradley, Germi James, and Rugg Michael (2017). Modulation of oscillatory power and connectivity in the human posterior cingulate cortex supports the encoding and retrieval of episodic memories. Journal of Cognitive Neuroscience. [DOI] [PubMed] [Google Scholar]
- Lepage Martin, Habib Reza, and Tulving Endel (1998). Hippocampal pet activations of memory encoding and retrieval: the hiper model. Hippocampus 8(4): 313–322. [DOI] [PubMed] [Google Scholar]
- Lin Jui Jui, Rugg Michael, Das Sandhitsu, Stein Joel, Rizzuto Daniel, Kahana Michael, and Lega Bradley (2017). Theta band power increases in the posterior hippocampus predict successful episodic memory encoding in humans. Hippocampus 27: 1040–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB and Moser EI (1998). Functional differentiation in the hippocampus. Hippocampus 8(6): 608–619. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Walia G, McIntosh AR, Joanisse MF, Klein D, and Köhler S(2008). Why is the meaning of a sentence better remembered than its form? an fmri study on the role of novelty-encoding processes. Hippocam-pus 18(9): 909–918. [DOI] [PubMed] [Google Scholar]
- Poppenk Jordan, Evensmoen Hallvard R, Moscovitch Morris, and Nadel Lynn (2013). Long-axis specialization of the human hippocampus. Trends in cognitive sciences 17(5): 230–240. [DOI] [PubMed] [Google Scholar]
- Ranganath Charan and Ritchey Maureen (2012). Two cortical systems for memory-guided behaviour. Nature Reviews Neuroscience 13(10): 713–726. [DOI] [PubMed] [Google Scholar]
- Royer Sébastien, Sirota Anton, Patel Jagdish, and Buzsáki György (2010). Distinct representations and theta dynamics in dorsal and ventral hippocampus. The Journal of Neuroscience 30(5): 1777–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy Jerry W and Matus-Amat Patricia (2005). The ventral hippocampus supports a memory representation of context and contextual fear conditioning: implications for a unitary function of the hippocampus. Behavioral neuroscience 119(1): 154. [DOI] [PubMed] [Google Scholar]
- Ryan Lee, Lin Chun-Yu, Ketcham Katie, and Nadel Lynn (2010). The role of medial temporal lobe in retrieving spatial and nonspatial relations from episodic and semantic memory. Hippocampus 20(1): 11–18. [DOI] [PubMed] [Google Scholar]
- Strange Bryan A, Witter Menno P, Ed Lein S, and Moser Edvard I (2014). Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience 15(10): 655–669. [DOI] [PubMed] [Google Scholar]
- Thakral Preston P, Wang Tracy H, and Rugg Michael D (2015). Cortical reinstatement and the confidence and accuracy of source memory. NeuroImage 109: 118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollett Katherine and Maguire Eleanor A (2011). Acquiring “the knowledge” of london’s layout drives structural brain changes. Current biology 21(24): 2109–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan Stuart, Squire Larry R, and Amaral David G (1989). Lesions of the amygdala that spare adjacent cortical regions do not impair memory or exacerbate the impairment following lesions of the hippocampal formation. Journal of Neuroscience 9(6): 1922–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]